
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 14 June 2022
Sec. Microbial Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.880196
This article is part of the Research Topic Immune Interactions with Pathogenic and Commensal Fungi View all 17 articles
Human disseminated protothecosis is a rare infection caused by members of the genus Prototheca, an achlorophyllic algae always associated with debilitated hosts. The presence of non-budding cells and large, spherical cells (sporangia) with endosporulation (morula) in histology is proof of Prototheca infection. Regrettably, due to the lack of specificity of clinical features and low awareness among clinicians, protothecosis is always underestimated and misdiagnosed. The available data on a species-specific analysis of this infection are limited. In this review, we summarize the etiological, epidemiological, and clinical aspects of disseminated protothecosis. The potential pathogenicity and clinical differences between P. zopfii and P. wickerhamii were observed. Additionally, the skin not only became the main invasion site but also the most involved organ by the pathogen. With the increasing numbers of immunocompromised individuals throughout the world, the incidence of disseminated infection caused by Prototheca is bound to increase, and disseminated protothecosis that accompanies skin symptoms should be taken into account by clinicians.
In humans, disorders caused by Prototheca species can be cutaneous, olecranon bursitis, systemic, or disseminated (1). In contrast to the former two types of infection, disseminated protothecosis is mainly associated with immunocompromised hosts, such as patients under immunosuppressive therapy or with longstanding intravascular catheters, cancer, AIDS, diabetes mellitus or solid organ transplantation (2). This infection type had the worst prognosis, with only 33% cure or improvement, and 56% death (1). Thus, characterized by high mortality rates and with the increasing numbers of immunocompromised individuals throughout the world, disseminated protothecosis has aroused a gradual interest in the study of the various aspects of this infection and its causative microorganism.
Regrettably, due to the lack of specificity of clinical features and the lack of awareness among clinicians, up to date, only around 200 cases of Prototheca infection have been reported worldwide and systemic infection cases account for about 9% of the cases (1). Moreover, even after the start of the phycology working group in ISHAM (The International Society for Human & Animal Mycology) in 2017, little is known about Prototheca spp. or the disease caused by these algae, especially the disseminated type (1). Also, whether there are possible differences in pathogenicity within or between Prototheca species compared to other types of infection? This review provides a summary of the literature addressing etiological, epidemiological, and clinical aspects of disseminated protothecosis. According to a state defined by the presence of an achlorophyllic alga pathogen in the blood (algaemia) and/or any other sterile deep-tissues, organs, or there are more than 2 lesions in the whole body (1, 3), to our knowledge, 37 cases have been described in the literature from 1970 to 2019 with disseminated protothecosis.
We searched the PubMed database by using the terms “disseminated protothecosis”, “systemic protothecosis”, “human”, and “Prototheca”. We only reviewed cases with sufficient clinical information and laboratory/sequence data to identify the alga in the period 1970–2019. Of these, 35 articles presenting 37 cases of human disseminated/systemic protothecosis were included (Table 1) (3–37).
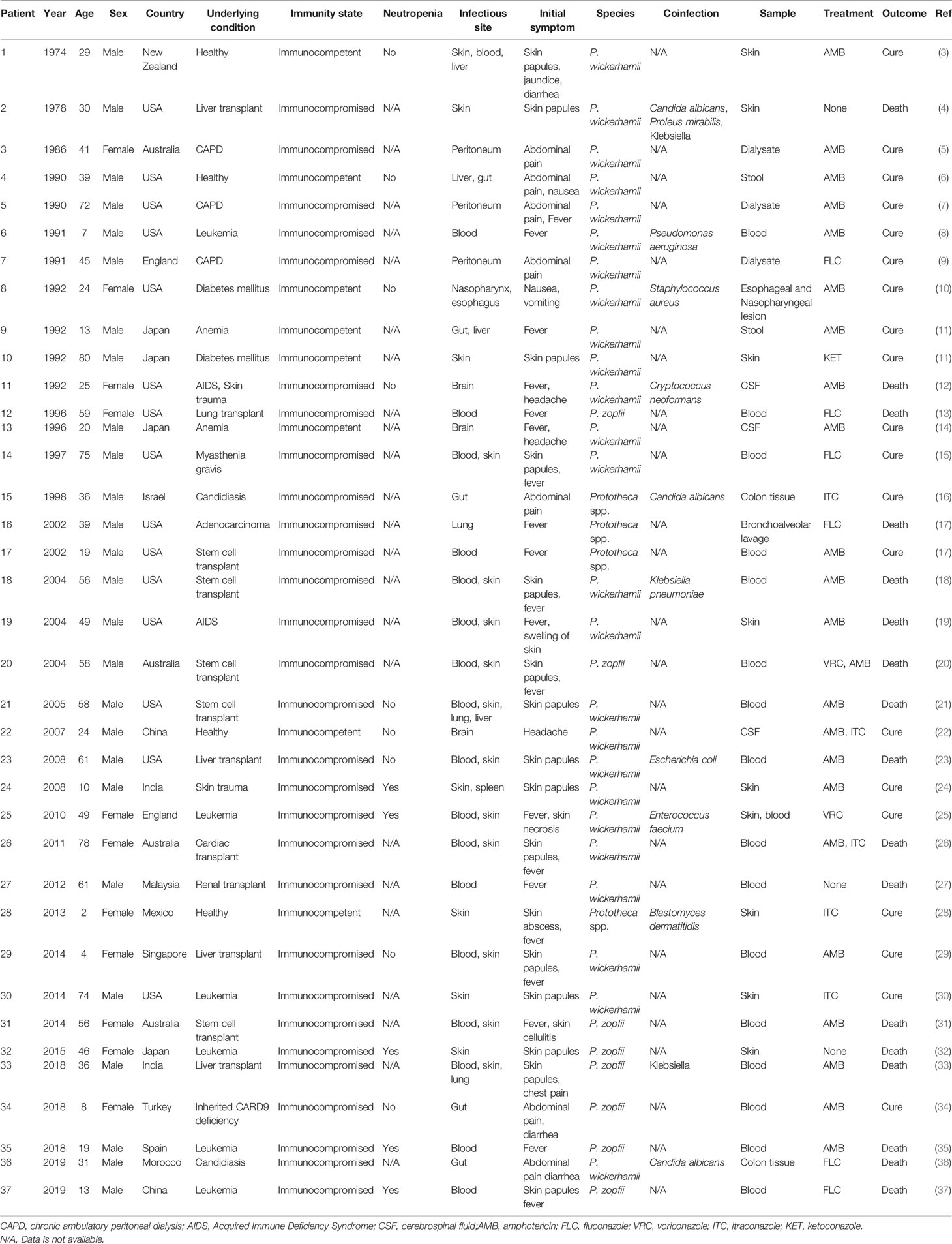
Table 1 Clinical description of patients with disseminated protothecosis collected during the study period.
Charts were reviewed for clinical details. Data collected included demographics, location, risk factors, affected site, causative species, diagnostic investigations, management, and outcomes. The χ2 test was used for comparisons of categorical variables with a level of significance of.05, and the Fisher exact test was used in the analysis of contingency tables when the sample sizes were small. All analyses were conducted with SPSS version 22.0 (IBM Corp., Armonk, New York).
Originally, Prototheca was described in 1894 as a yeast-like fungus recovered from slime flux in trees (38). However, in 1913, and on the basis of ultra-structure, life cycle, and reproduction system, the genus was recognized as algae (39). It was thought to be a mutant of the genus Chlorella that is unable to produce chlorophyll and lacks photosynthesis. Currently, Prototheca is accepted as a separate genus in the family Chlorellaceae and comprises unicellular algae that reproduce asexually via sporangia with sporangiospores. Eight species are known, i.e., P. blaschkeae, P. cutis, P. miyajii, P. wickerhamii, P. zopfii, P. stagnora, P. ulmea, and the recently described P. tumulicola (40). The former five have been associated with human and animal infection P. wickerhamii was the predominate one. Only P. zopfii and P. wickerhamii have been reported to cause disseminated infection in humans.
Out of the 37 cases of disseminated protothecosis reported in the literature, 25 were caused by P. wickerhamii (67.6%), 8 by P. zopfii (21.6%), and for the remaining four cases (10.8%), the cause was not identified to the species level. Unlike P. wickerhamii, which can cause skin, blood, cerebrospinal, and gastrointestinal tract infections, P. zopfii appears to be primarily detected in the blood (6/8), with higher mortality (7/8, 87.5%) than P. wickerhamii (9/25, 36.0%) (P <.05) (Table 2). Whether this is related to the potential virulence or pathogenicity of different species is not yet known. A previous study has indeed demonstrated that P. zopfii is lethal for immunosuppressed mice compared to P. wickerhamii, which could not induce infection (2, 41). Thus, the scant number of P. zopfii infections might in fact be due to the lower abundance of this species in the environment rather than the low pathogenic potential of the species. Nevertheless, ecological studies on the distribution and abundance of Prototheca species have not been conducted so far, and other factors that might contribute to the lower frequency of P. zopfii infections could not be excluded.
Prototheca species are saprophytes, occupying niches with decaying organic matter in moist environments, such as tree slime flux, animal manure, and sewage. They can also be found on plant and food item surfaces, in soil, or in water. In mammals, species can be present as transient gastrointestinal flora or as asymptomatic colonizers of skin and nails (2, 39, 42). From being considered non-pathogenic to causing disseminated infections in humans, we are rediscovering the mysterious algae ubiquitous in nature.
Prototheca infection could spread through exogenous or endogenous routes. The former is related to defects in the skin and mucosa (such as postoperative wounds). Compared to traumatic inoculation with the algae from the environment, dermal barrier destruction caused by hospital-acquired cases, including surgical operations and catheter-related procedures, are main exogenous route of invasion. Prototheca spp. may survive chlorination by forming biofilms (43) and be returned to the environment via sewage effluent and household waste. Cows with mastitis caused by P. zopfii (genotypes 1) may be a source for infection in humans, with immunocompromised farmers at the highest risk (44). Although the possibility of human-to-human transmission was raised with the outbreak of P. wickerhamii algaemia and sepsis in a tertiary care chemotherapy oncology unit recently (45), although contrary to our traditional understanding, the report cannot be ignored because of the public safety concerns. Endogenous colonization during prolonged immunosuppression in the gut followed by translocation resulting in algaemia and sepsis is suspected to be the cause of the outbreak.
Disseminated protothecosis is common in patients with underlying immunosuppression or several underlying diseases (2). Our review indicated that there were still four patients (10.8%) with normal immune status, but all of them were successfully treated and had a good prognosis, and most of the cases were associated with P. wickerhamii (3/4, 75.0%) (Table 3). Among the other 33 immunocompromised cases, organ transplantation was the most common, with twelve (32.4%) cases of solid organs and stem cell transplantation, followed by six (16.2%) cases of leukemia, and three (8.1%) cases of chronic ambulatory peritoneal dialysis (Table 2). Intestinal neutropenia does not appear to be an important risk factor for protothecosis. In our review, only 5 of the 14 patients for whom neutrophil counts were available showed neutropenia. Additionally, only two cases of AIDS were found, which is consistent with previous studies and suggests that a type of immunodeficiency other than AIDS contributes to susceptibility to protothecosis (12, 19). Interestingly, the case of CARD9 deficiency caused by P. zopfii provides a new insight into the mechanism of anti-Prototheca immunity (34). All cases of AIDS and diabetes are related to P. wickerhamii, whereas transplantation and leukemia do not seem to be associated with the species type. However, more data are needed to prove the relevance of this association (Table 3).
In addition to one traumatic implant, 22 (59.5%) patients had a history of surgical and instrument injury related to defects in the skin and mucosa. Although glucocorticoid therapy might be considered the highest risk factor for Prototheca infection, it was not found among the patients reviewed (2). We believe this situation still exists because of the high proportion of organ transplantation cases and the one case of myasthenia gravis (15), in which large doses of glucocorticoids must be used for long periods. A likely explanation for the glucocorticoids as predisposing factors to infection may include exogenous or endogenous aspects. It may be on one hand to shrink and thin the epidermis to weaken the barrier function of the skin and, on the other hand, may suppress lymphocyte activation and impair PMNs and macrophages to increase endogenous colonization (46).
Protothecosis occurs globally and has been reported on every continent except Antarctica (2). In this review, the world distribution of 37 disseminated cases is shown in Figure 1. It predominated in the USA (fifteen, 40.5%), where it is prevalent in the southeast, followed by Australia and Japan (four each, 10.8%). Two of the cases were from England (5.4%), two from India (5.4%), two from China (5.4%), and one each from Israel, Malaysia, Singapore, Spain, Mexico, Turkey, Morocco, and New Zealand (2.7%). In general, Prototheca species seem to have a preference for warm and humid climates, which matches the epidemiology of the disease in both humans and animals. Similar to what has been reported in previous studies, we found that males are more affected (26 cases, 70.3%) compared to females (11 cases, 29.7%). Disseminated protothecosis can occur in individuals of any age, but mostly in patients between 30 and 60 years old. Median = 39 years (Table 2).
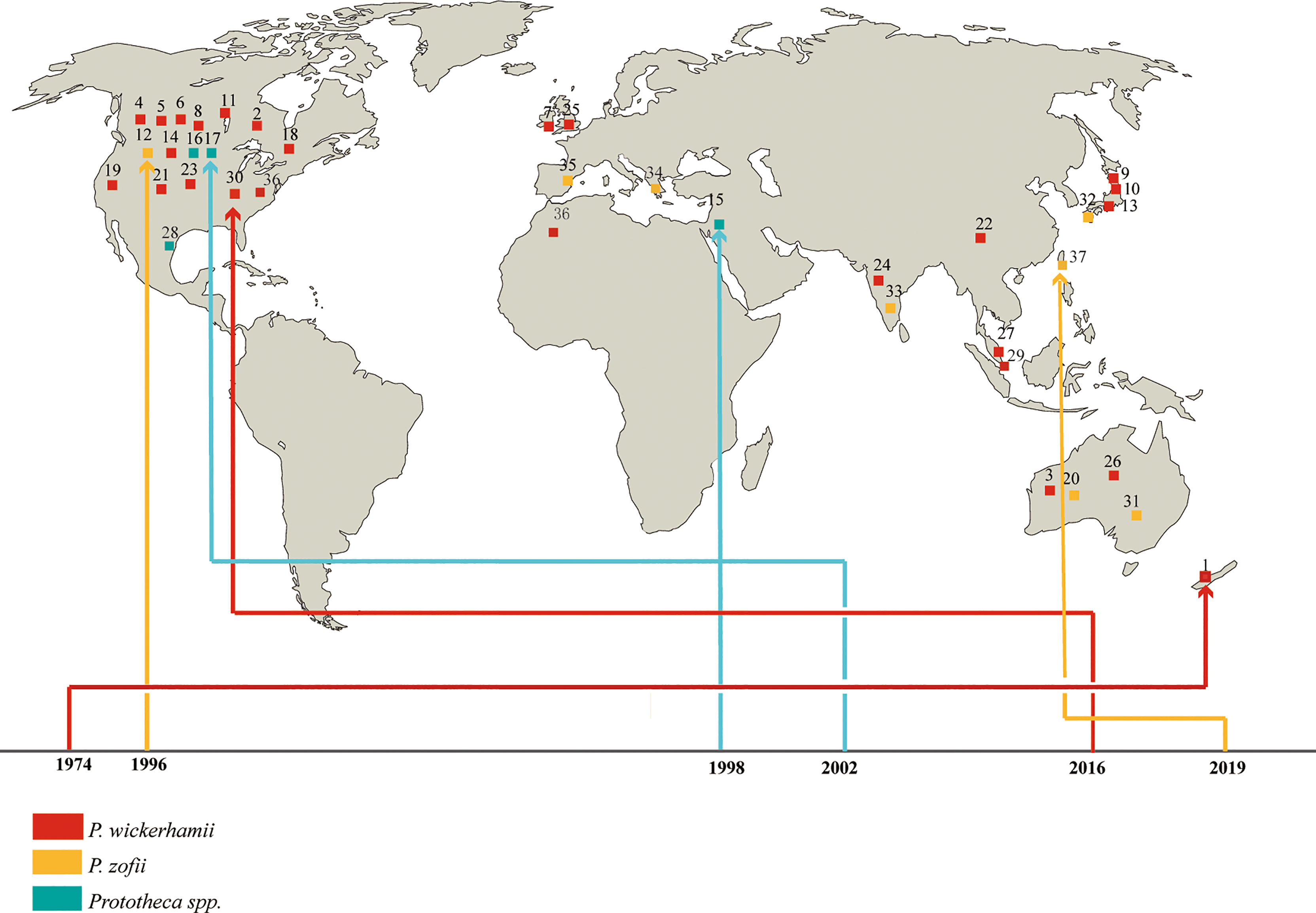
Figure 1 Geographic distribution of patients with disseminated protothecosis collected during the study period.
No specific clinical features were noted. Clinical initial signs of disseminated protothecosis in humans can include fever, skin lesions, abdominal pain, diarrhea, and headache, which are associated with the infectious sites. Similar to what has been reported in previous studies (2), the organs most commonly affected in dissemination are the skin (19 cases, 51.4%) and blood (17 cases, 45.9%), followed by the gut (5 cases, 13.5%) and liver (4 cases, 10.8%), then the lungs (3 cases, 8.1%), peritoneum (3 cases, 8.1%), and brain (3 cases, 8.1%) (Figure 2). There are various forms of skin lesions without specificity that can be manifested as erythematous papules, plaques, nodules, ulcers, papules, necrotic crusts, pustules, and bullae with purulent discharge, even presenting as an eczematoid eruption (4, 15, 19, 25, 31). At least three patients had lesions at the site of catheter implantation in this review (23, 25, 37). Unfortunately, even though skin lesions are the most common initial signs of this disseminated type, the mortality of patients with visual lesions was not lower than that of other factors, suggesting that atypical lesions were indeed overlooked or misdiagnosed by clinicians. In addition, algaemia, which represents blood involvement, is more easily covered up and ignored by bacteremia and fungaemia (45), especially under the condition of administration of prophylactic systemic antifungals, patient discharge, death, or transfer to another clinical unit. In addition, cholestatic jaundice and hepatitis are also considered the typical clinical presentations of systemic protothecosis (2). The algae can even invade the brain, but interestingly, out of a total of three patients with brain infection, only one patient with AIDS died, and the remaining two patients had a good prognosis. One immunocompetent patient has been asymptomatic but alga has tested positive for cerebrospinal fluid for 7 years (14). In all but those three cases, the species involved was P. wickerhamii.
Disseminated protothecosis is rare but has a high mortality (17/37, 45.9%). The majority of patients are over 30 years of age or elderly (higher mortality, fourteen, 60.9%). Cases with organ transplants (10/12, 83.3%) have higher mortality compared to leukemia (3/6, 50.0%) and other diseases (4/19, 21.1%) (P <.05). Among all, P. zopfii patients showed a higher rate of mortality (7/8, 87.5%) than patients infected with P. wickerhamii (9/25, 36.0%), leading to 100% of deaths among transplant and leukemia patients (Table 3). We hypothesized that the cause of the high mortality in those groups might be associated with the central venous catheter or Hickman catheter, thereby increasing the opportunity for biofilm formation and contributing to hard-to-treat characteristics. In many cases of protothecosis, co-occurrence with other pathogens was found, such as Candida, Staphylococci, herpes simplex virus, Enterococci, Leuconostoc, Klebsiella, Cryptococcus, Blastomyces dermatitidis, Pseudomonas, or Escherichia (4, 8, 10, 12, 16, 23, 25, 27, 33, 36). Our data showed that there were eleven (6/11, 54.5%) co-infected patients with slightly higher mortality than those (11/26, 42.3%) who were not co-infected.
Prototheca remains a diagnostic and therapeutic enigma. For the diagnosis, combining histopathological and microbiological tests is recommended for cases where protothecosis is suspected. Histopathologic examination of infected tissue may be accomplished using the PAS, GMS, or Gridley fungus stain to visualize the endosporulating sporangia (morula form) of Prototheca spp. (Figure 3). In addition to the size differences noted previously, the two species of Prototheca differ in that P. wickerhamii tends to form symmetrical morula forms, whereas these forms are rare in P. zopfii, which exhibits more random internal segmentation (2). However, in the absence of these morphological features, the organism may resemble other fungi such as Blastomyces, Coccidioides, Cryptococcus, Emergomyces, Paracoccidioides, Pneumocystis, Rhinosporidium, and chromoblastomycosis agents (2). Of note, diseases caused by these fungi differ clinically from protothecosis in the presence of respiratory symptoms since the infection is mainly acquired by inhalation. However, chromoblastomycosis is an implantation mycosis but has a chronic nature and is characterized by the presence of darkly pigmented muriform cells in the infected tissue.
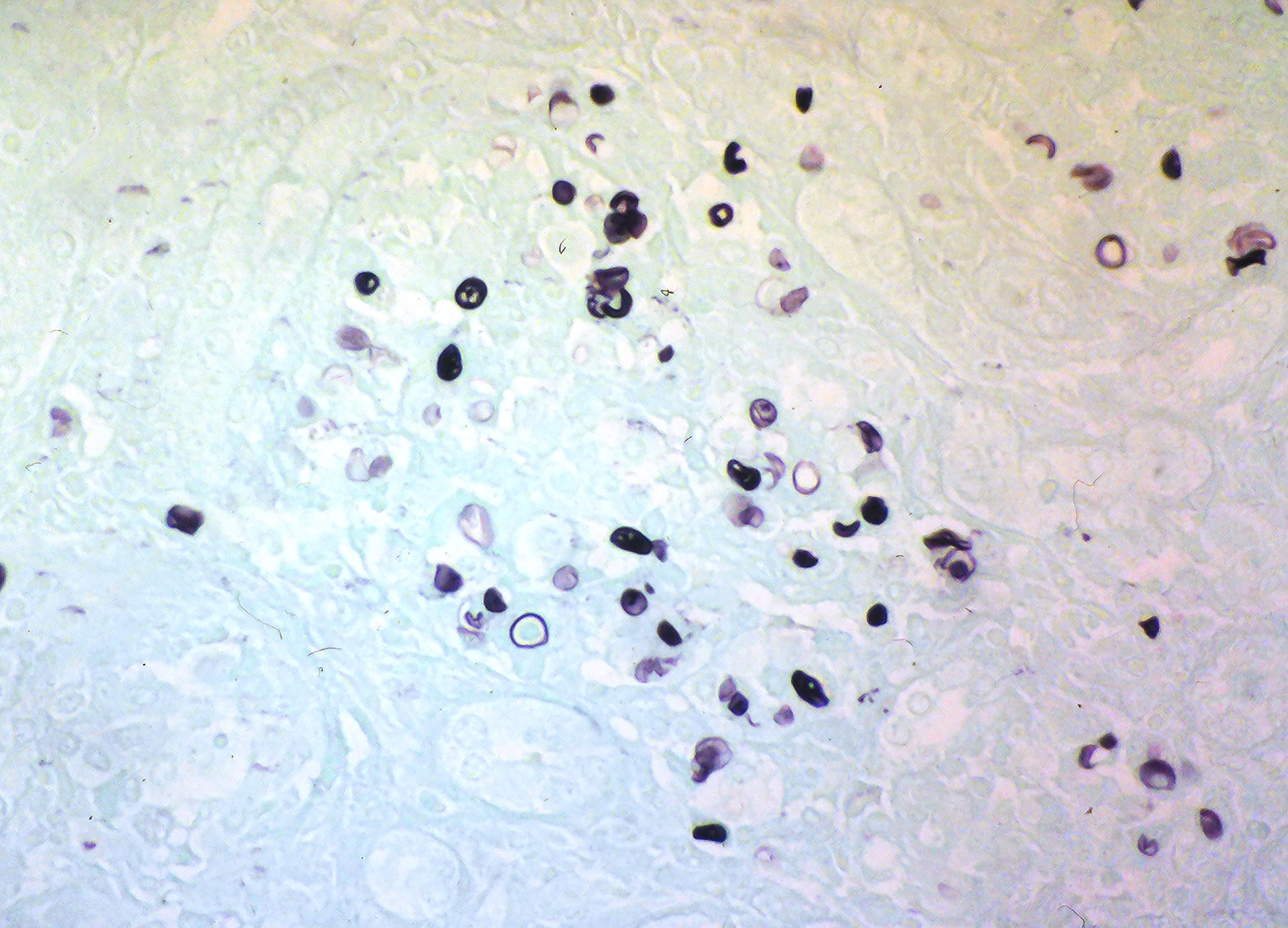
Figure 3 Grocott’s methenamine silver stain of histology section showing Prototheca sporangia and spores (courtesy of Prof. Henrik Jensen).
The inflammatory response in protothecosis is predominantly granulomatous but can consist of lymphocytes, plasma cells, eosinophils, neutrophils, macrophages, epithelioid cells, and giant cells. Prototheca species grow rapidly on routine laboratory media such as Sabouraud’s glucose agar, blood culture bottles, and blood agar (47). For confirmation, the presence of unicellular organisms, 3–30 µm in diameter, and sporangium containing autospores is indicative of Prototheca infection (Figure 4). Nevertheless, situations such as contamination of growth by co-infecting yeasts or bacteria, or growth of a single colony on solid media, often lead to missed diagnosis (45).
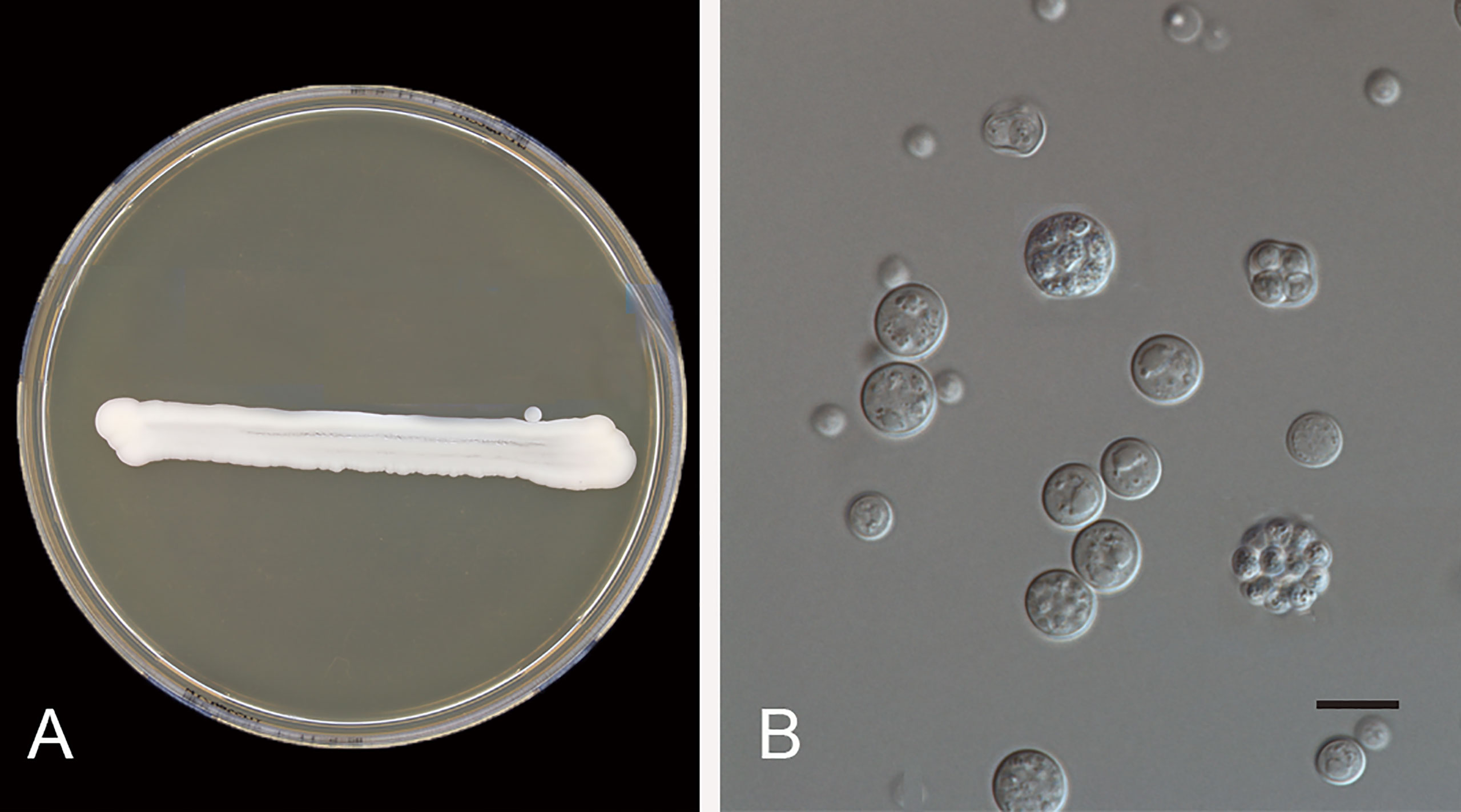
Figure 4 Prototheca wickerhamii (CBS 608.66). (A) Colonies on MEA 5 days at 24°C; (B) yeast-like organisms, the mature sporangium contains 2–20 or more autospores, and ruptures to release the daughter cells. Scale bars: 10 μm.
There are possible differences in pathogenicity and treatment between P. wickerhamii and P. zopfii, so the identification of these algae to species level has become an inevitable trend. In addition to the traditional morphological methods, commercial physiological systems such as API 20C or API 20C-AUX and the database of VITEK 2 have been developed (48). Currently, rapid automated identification of Prototheca is possible using matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS) (49). In addition, the sequencing of 18S and 28S rDNA has been applied for the identification and genotyping of species (50, 51). Recently, the mitochondrial cytb gene has been proved effective for discrimination and suggested as the gold standard for the identification of the Prototheca microalgae (40) (Figure 5).
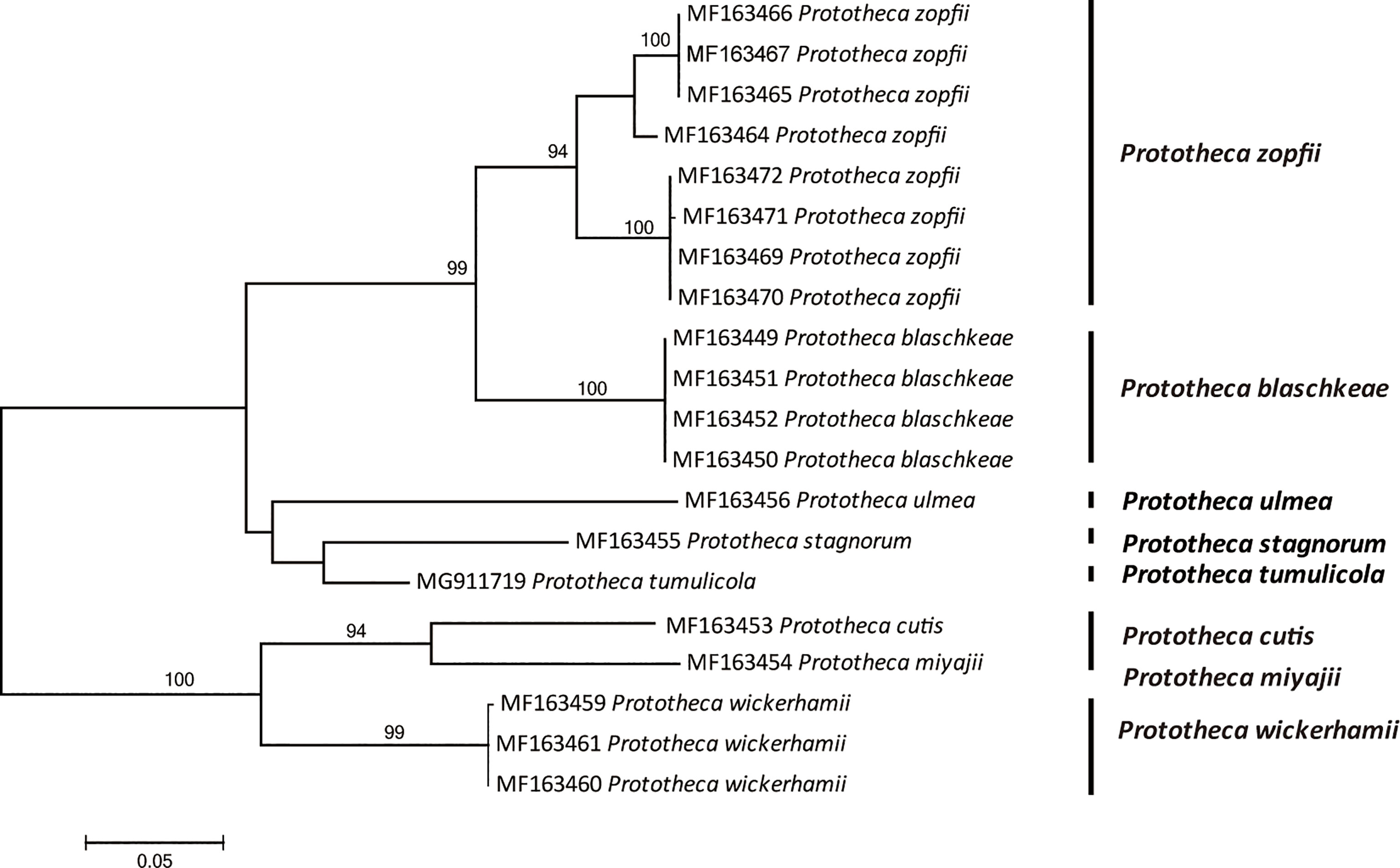
Figure 5 Phylogenetic tree constructed through maximum likelihood analysis based on cytb sequences. The bootstrap values obtained by the analysis are marked at the nodes.
Therapeutically, in addition to the fact that algae in general have low susceptibility to antimicrobial agents, there has been no consistency in the clinical responses. Treatment of protothecal infections remains a challenge (32). Antifungals such as amphotericin B and itraconazole form the mainstay of treatment, although Prototheca is susceptible to voriconazole, miconazole, clotrimazole, tetracycline, gentamicin, amikacin, and polymyxin B (29). Disseminated patients treated with an antifungal regimen that included amphotericin B were more likely to survive than those treated with a triazole alone. Amphotericin B and its lipid-based formulations provide broad spectrum cover, however, treatment failures even with combination antifungal therapy with amphotericin B have been reported (46). Our data also suggest that amphotericin B is effective in only 56.5% (13/23) of the disseminated patients. Antifungal treatment needs to be reassessed in cases of no clinical improvement. Furthermore, catheter removal should be the first consideration in treating a catheter-related Prototheca infection.
Human Disseminated Protothecosis is an emerging environmental algal disease with high mortality that typically occurs in immunocompromised individuals. Under the condition of immune deficiency, the destruction of the skin barrier caused by surgery and catheter is highly considered to be associated with this type. Organ transplantation is the most common risk factor, followed by leukemia. P. wickerhamii and P. zopfii are the dominant species that cause disseminated infection. The former has a significantly lower mortality than the latter, but is associated with brain infections. Low susceptibility to antimicrobial agents and Prototheca biofilms contributes to the hard-to-treat character of this algal infection.
Significantly, our study confirmed that disseminated protothecosis most frequently involves the skin, which is indeed different from other opportunistic biofilm-forming fungi prevalent in intensive care units (ICUs) and transplant patients. Candida auris, for example, is usually associated with bloodstream infections rather than skin infections (52). The difference in clinical manifestations could be related to the biological behavior of the species. The dominant species P. wickerhamii, which causes disseminated protothecosis, has an optimum growth temperature between 25 and 37°C and cannot grow above 40°C (53), however, C. auris could grow well at 42°C (52). Based on the difference in this thermal tolerance property, it is hypothesized that the skin is a window through which Prototheca spp. diffuses back into the environment to escape the powerful thermoregulatory immunity of the body. Since this study has limitations due to the small sample, more research work, especially the association of each species type with distinct profiles of clinical manifestation and response to treatment and epidemiological patterns, should be launched.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
XW and YR carried out the literature search and participated in the data analysis and drafted the manuscript. YaJ designed this project, phylogenetic tree construction, participated in the data analysis, and revised the manuscript. SJ and XL carried out the statistical analysis. SA and YiJ contributed to the discussion and revision of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by the District Science Foundation Program (NSFC No. 81960368) from the National Natural Science Foundation of China, the Guizhou Provincial Natural Science Foundation [ZK (2021) zhongdian030], and the Doctoral Cultivating Fund of Guizhou Medical University (gyfybsky-2021-56).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to the members of the Center of Expertise in Mycology of Radboud University Medical for fruitful discussions. We also thank Professor Henrik Elvang Jensen from the Department of Veterinary and Animal Sciences, University of Copenhagen, for providing the histological images of Prototheca.
CARD9, caspase recruitment domain-containing protein 9; AIDS, Acquired Immune Deficiency Syndrome; PMN, polymorphonuclear; PAS, periodic acid Schiff reaction; GMS, Gomori methenamine silver stain; MALDI-TOF MS, Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry.
1. Todd JR, Matsumoto T, Ueno R, Murugaiyan J, Britten A, King JW, et al. Medical Phycology 2017. Med Mycol (2018) 56(suppl1):S188–204. doi: 10.1093/mmy/myx162
2. Lass-Florl C, Mayr A. Human Protothecosis. Clin Microbiol Rev (2007) 20(2):230–42. doi: 10.1128/CMR.00032-06
3. Cox GE, Wilson JD, Brown P. Protothecosis: A Case of Disseminated Algal Infection. Lancet.(1974) (7877) 2:379–82. doi: 10.1016/s0140-6736(74)91760-7
4. Dagher FJ, Smith AG, Pankoski D, Ollodart RM. Skin Protothecosis in a Patient With Renal Allograft. South Med J (1978) 71(2):222–4. doi: 10.1097/00007611-197802000-00039
5. O'Connor JP, Nimmo GR, Rigby RJ, Petrie JJ, Hardie IR, Strong RW. Algal Peritonitis Complicating Continuous Ambulatory Peritoneal Dialysis. Am J Kidney Dis (1986) 8(2):122–3. doi: 10.1016/s0272-6386(86)80123-8
6. Chan JC, Jeffers LJ, Gould EW, Hutson D, Martinez OV, Reddy KR, et al. Visceral Protothecosis Mimicking Sclerosing Cholangitis in an Immunocompetent Host: Successful Antifungal Therapy. Rev Infect Dis (1990) 12(5):802–7. doi: 10.1093/clinids/12.5.802
7. Sands M, Poppel D, Brown R. Peritonitis Due to Prototheca Wickerhamii in a Patient Undergoing Chronic Ambulatory Peritoneal Dialysis. Rev Infect Dis (1991) 13(3):376–8. doi: 10.1093/clinids/13.3.376
8. Heney C, Greeff M, Davis V. Hickman Catheter-Related Protothecal Algaemia in an Immunocompromised Child. J Infect Dis (1991) 163(4):930–1. doi: 10.1093/infdis/163.4.930
9. Gibb AP, Aggarwal R, Swainson CP. Successful Treatment of Prototheca Peritonitis Complicating Continuous Ambulatory Peritoneal Dialysis. J Infect (1991) 22(4):183–5. doi: 10.1016/0163-4453(91)91679-r
10. Iacoviello VR, DeGirolami PC, Lucarini J, Sutker K, Williams ME, Wanke CA. Protothecosis Complicating Prolonged Endotracheal Intubation: Case Report and Literature Review. Clin Infect Dis (1992) 15(6):959–67. doi: 10.1093/clind/15.6.959
11. Matsuda T, Matsumoto T. Protothecosis: A Report of Two Cases in Japan and a Review of the Literature. Eur J Epidemiol (1992) 8(3):397–406. doi: 10.1007/BF00158575
12. Kaminski ZC, Kapila R, Sharer LR, Kloser P, Kaufman L. Meningitis Due to Prototheca Wickerhamii in a Patient With AIDS. Clin Infect Dis (1992) 15(4):704–6. doi: 10.1093/clind/15.4.704
13. Kwok N, Stanley N, Schwartz. Prototheca Sepsis in a Lung Transplant Patient. Clin Microbiol Newsl (1996) 18(4):34–25. doi: 10.1016/0196-4399(96)84101-8
14. Takaki K, Okada K, Umeno M, Tanaka M, Takeda T, Ohsaki K, et al. Chronic Prototheca Meningitis. Scand J Infect Dis (1996) 28(3):321–3. doi: 10.3109/00365549609027183
15. Mohabeer AJ, Kaplan PJ, Southern PM Jr., Gander RM. Algaemia Due to Prototheca Wickerhamii in a Patient With Myasthenia Gravis. J Clin Microbiol (1997) 35(12):305–7. doi: 10.1128/JCM.35.12.3305-3307.1997
16. Raz R, Rottem M, Bisharat N, Sakran W, Nussinson E, Trougouboff P, et al. Intestinal Protothecosis in a Patient With Chronic Mucocutaneous Candidiasis. Clin Infect Dis (1998) 27(2):399–400. doi: 10.1086/514651
17. Torres HA, Bodey GP, Tarrand JJ, Kontoyiannis DP. Protothecosis in Patients With Cancer: Case Series and Literature Review. Clin Microbio Infect (2003) 9(8):786–92. doi: 10.1046/j.1469-0691.2003.00600.x
18. Khoury JA, Dubberke ER, Devine SM. Fatal Case of Protothecosis in a Hematopoietic Stem Cell Transplant Recipient After Infliximab Treatment for Graft-Versus-Host Disease. Blood. (2004) 104(10):3414–5. doi: 10.1182/blood-2004-07-2720
19. Pascual JS, Balos LL, Baer AN. Disseminated Prototheca Wickerhamii Infection With Arthritis and Tenosynovitis. J Rheumatol (2004) 31(9):1861–5.
20. Lass-Florl C, Fille M, Gunsilius E, Gastl G, Nachbaur D. Disseminated Infection With Prototheca Zopfii After Unrelated Stem Cell Transplantation for Leukemia. J Clin Microbiol (2004) 42(10):4907–8. doi: 10.1128/JCM.42.10.4907-4908.2004
21. Hariprasad SM, Prasad A, Smith M, Shah GK, Grand MG, Shepherd JB, et al. Bilateral Choroiditis From Prototheca Wickerhamii Algaemia. Arch Ophthalmol (2005) 123(8):1138–41. doi: 10.1001/archopht.123.8.1138
22. Zhang QQ, Zhu LP, Weng XH, Li L, Wang JJ. Meningitis Due to Prototheca Wickerhamii: Rare Case in China. Med Mycol (2007) 45(1):85–8. doi: 10.1080/13693780601003835
23. Narita M, Muder RR, Cacciarelli TV, Singh N. Protothecosis After Liver Transplantation. Liver Transplant (2008) 14(8):1211–5. doi: 10.1002/lt.21565
24. Mathew LG, Pulimood S, Thomas M, Acharya MA, Raj PM, Mathews MS. Disseminated Protothecosis. Indian J Pediatr (2010) 77(2):198–9. doi: 10.1007/s12098-009-0251-6
25. Gaur S, Marrin C, Barnes RA. Disseminated Protothecosis Following Traumatic Hickman Line Removal in a Patient With Leukaemia. Med Mycol (2010) 48(2):410–2. doi: 10.1080/13693780903188698
26. McMullan B, Muthiah K, Stark D, Lee L, Marriott D. Prototheca Wickerhamii Mimicking Yeast: A Cautionary Tale. J Clin Microbiol (2011) 49(8):3078–81. doi: 10.1128/JCM.00487-11
27. Mohd Tap R, Sabaratnam P, Salleh MA, Razak MF, Ahmad N. Characterization of Prototheca Wickerhamii Isolated From Disseminated Algaemia of Kidney Transplant Patient From Malaysia. Mycopathologia (2012) 173(2-3):173–8. doi: 10.1007/s11046-011-9469-8
28. Tello-Zavala MC, Mercado-Lara A, Gómez-Hernández N, Recio-Martinez V. Disseminated Protothecosis in a Mexican Child. Pediatr Infect Dis (2013) 32(12):e476–477. doi: 10.1097/INF.0b013e3182a2cfb2
29. Tan RM, Aw MM, Quak SH, Chan SM. Pulmonary Protothecosis in a Pediatric Liver Transplant Patient. J Pediatr Infect Dis Soc (2014) 3(3):e31–34. doi: 10.1093/jpids/pit034
30. Takano M, Hoshi S, Nagai K, Ishidaira H, Onozaki M, Satoh K, et al. The First Case of Human Protothecosis Caused by Prototheca Zopfii in Japan. J Infect Chemother (2014) 20(10):647–9. doi: 10.1016/j.jiac.2014.06.009
31. Macesic N, Fleming S, Kidd S, Madigan V, Chean R, Ritchie D, et al. Protothecosis in Hematopoietic Stem Cell Transplantation: Case Report and Review of Previous Cases. Transpl Infect Dis (2014) 16(3):490–5. doi: 10.1111/tid.12223
32. Kovalyshyn I, Brankov N, Fernandez AP. Erythematous and Edematous Plaques on the Bilateral Extremities in an Immunocompromised Patient. Protothecosis Int J Dermatol (2016) 55(2):e59–61. doi: 10.1111/ijd.13068
33. Rao PV, Sethuraman N, Ramanathan Y, Gopalakrishnan R. Disseminated Protothecosis Caused by Prototheca Zopfii in a Liver Transplant Recipient. J Glob Infect Dis (2018) 10(4):228–9. doi: 10.4103/jgid.jgid_55_17
34. Sari S, Dalgic B, Muehlenbachs A, DeLeon-Carnes M, Goldsmith CS, Ekinci O, et al. Prototheca Zopfii Colitis In Inherited CARD9 Deficiency. J Infect Dis (2018) 218(3):485–9. doi: 10.1093/infdis/jiy198
35. Fernandez NB, Taverna CG, Vivot M, Córdoba S, Paravano L. First Bloodstream Infection Due to Prototheca Zopfii Var. Hydrocarbonea in an Immunocompromised Patient. Med Myo Case Rep (2019) 24(5):9–12. doi: 10.1016/j.mmcr.2019.02.003
36. Konzi K, Assiad A, Zouaidia F, Beggar H, Ouazzani L, Lyagoubi M, et al. A Case of Intestinal Protothecosis. Med Mal Infect (2019) 4(8):621–3. doi: 10.1016/j.medmal.2019.09.002
37. Sim JY, Hsueh PR, Chuang PC, Lee PL. Fatal Disseminated Infection Caused by Prototheca Zopfii in a Child With Leukemia. Microbiol Immunol Infect (2019) 52(5):833–5. doi: 10.1016/j.jmii.2019.05.001
39. Huerre M, Ravisse P, Solomon H, Ave P, Briquelet N, Maurin S, et al. [Human Protothecosis and Environment]. Bull Soc Pathol Exot (1993) 8(5 pt 2):484–8.
40. Jagielski T, Gawor J, Bakuła Z, Decewicz P, Maciszewski K, Karnkowska A. Cytb as a New Genetic Marker for Differentiation of Prototheca Species. J Clin Microbiol (2018) 56(10):e00584–18. doi: 10.1128/JCM.00584-18
41. Phair JP, Williams E. Bassaris HP, Zeiss CR, Morlock BA. Phagocytosis and Algicidal Activity of Human Polymorphonuclear Neutrophils Against Prototheca Wickerhamii. J Infect Dis (1981) 144(1):72–7. doi: 10.1093/infdis/144.1.72
42. Nosanchuk J, Greenberg R. Protothecosis of the Olecranon Bursa Caused by Achloric Algae. Am J Clin Pathol (1973) 59(4):567–73. doi: 10.1093/ajcp/59.4.567
43. Kwiecinski J. Biofilm Formation by Pathogenic Prototheca Algae. Lett Appl Microbiol (2015) 61(6):511–7. doi: 10.1111/lam.12497
44. Muhammad S, Paloma AC, Cameron G, Barkema HW, Han B, Gao J, et al. Murine and Human Cathelicidins Contribute Differently to Hallmarks of Mastitis Induced by Pathogenic Prototheca Bovis Algae. Front Cell Infect Microbiol (2020) 10:31. doi: 10.3389/fcimb.2020.00031
45. Khan ID, Sahni AK, Sen S, Gupta RM, Basu A. Outbreak of Prototheca Wickerhamii Algaemia and Sepsis in a Terti are Chemotherapy Oncology Unit. Med J Armed Forces India (2018) 74(4):358–64. doi: 10.1016/j.mjafi.2017.07.012
46. Torres HA, Bodey GP, Tarrand JJ, Kontoyiannis DP. Protothecosis in Patients With Cancer: Case Series and Literature Review. Clin Microbiol Infect (2003) 9(8):786–92. doi: 10.1046/j.1469-0691.2003.00600.x
47. Mayorga J, Barba-Gomez JF, Verduzco-Martinez AP, Verduzco-Martínez AP, Muñoz-Estrada VF, Welsh O. Protothecosis. Clin Dermatol (2012) 30(4):432–6. doi: 10.1046/j.1469-0691.2003.00600.x
48. Mayorga J, Barba-Gomez JF, Verduzco-Martinez AP, Munoz-Estrada VF, Welsh O. Establishment of a Matrix-Assisted Laser Desorption Ionization Time-Of-Flight Mass Spectrometry Database for Rapid Identification of Infectious Achlorophyllous Green Micro-Algae of the Genus Prototheca. Clin Microbiol Infect (2012) 1(5):461–7. doi: 10.1111/j.1469-0691.2011.03593.x
49. Kapica L. First Case of Human Protothecosis in Canada: Laboratory Aspects. Mycopathologia. (1981) 73(1):43–8. doi: 10.1007/BF00443013
50. Wang X, Fu YF, Wang RY, Li L, Cao YH, Chen YQ, et al. Identification of Clinically Relevant Fungi and Prototheca Species by rRNA Gene Sequencing and Multilocus PCR Coupled With Electrospray Ionization Mass Spectrometry. PloS One (2014) 9(5):e98110. doi: 10.1371/journal.pone.0098110
51. Satoh K, Ooe K, Nagayama H, Makimura K. Prototheca Cutis Sp. Nov, a Newly Discovered Pathogen of Protothecosis Isolated From Inflamed Human Skin. Int J Syst Evol Microbiol (2010) 60(5):1236–40. doi: 10.1099/ijs.0.016402-0
52. Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida Auris: Epidemiology, Biology, Antifungal Resistance, and Virulence. PloS Pathog (2020) 16(10):e1008921. doi: 10.1371/journal.ppat.1008921
Keywords: humans, immunosuppression, skin diseases, diagnostic errors, Prototheca
Citation: Wang X, Ran Y, Jia S, Ahmed S, Long X, Jiang Y and Jiang Y (2022) Human Disseminated Protothecosis: The Skin is the “Window”? Front. Immunol. 13:880196. doi: 10.3389/fimmu.2022.880196
Received: 21 February 2022; Accepted: 12 May 2022;
Published: 14 June 2022.
Edited by:
Wenjie Fang, Shanghai Changzheng Hospital, ChinaReviewed by:
Mirian Nacagami Sotto, University of São Paulo, BrazilCopyright © 2022 Wang, Ran, Jia, Ahmed, Long, Jiang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanping Jiang, amlhbmd5YW5waW5nMTE5QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.