
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 10 May 2022
Sec. T Cell Biology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.878457
 Liang Zeng1†
Liang Zeng1† Shu-Hua Li2†
Shu-Hua Li2† Shuo-Yu Xu3,4†
Shuo-Yu Xu3,4† Kai Chen1
Kai Chen1 Liang-Jun Qin1
Liang-Jun Qin1 Xiao-Yun Liu5
Xiao-Yun Liu5 Fang Wang5
Fang Wang5 Sha Fu6
Sha Fu6 Ling Deng5
Ling Deng5 Feng-Hua Wang7
Feng-Hua Wang7 Lei Miao8
Lei Miao8 Le Li7
Le Li7 Na Liu9*
Na Liu9* Ran Wang10*
Ran Wang10* Hai-Yun Wang1,8*
Hai-Yun Wang1,8*Background: Infiltrating immune cells have been reported as prognostic markers in many cancer types. We aimed to evaluate the prognostic role of tumor-infiltrating lymphocytes, namely CD3+ T cells, CD8+ cytotoxic T cells and memory T cells (CD45RO+), in neuroblastoma.
Patients and Methods: Immunohistochemistry was used to determine the expression of CD3, CD8 and CD45RO in the tumor samples of 244 neuroblastoma patients. We then used digital pathology to calculate the densities of these markers and derived an immunoscore based on such densities.
Results: Densities of CD3+ and CD8+ T cells in tumor were positively associated with the overall survival (OS) and event-free survival (EFS), whereas density of CD45RO+ T cells in tumor was negatively associated with OS but not EFS. An immunoscore with low density of CD3 and CD8 (CD3-CD8-) was indictive of a greater risk of death (hazard ratio 6.39, 95% confidence interval 3.09-13.20) and any event (i.e., relapse at any site, progressive disease, second malignancy, or death) (hazard ratio 4.65, 95% confidence interval 2.73-7.93). Multivariable analysis revealed that the CD3-CD8- immunoscore was an independent prognostic indicator for OS, even after adjusting for other known prognostic indicators.
Conclusions: The new immunoscore based on digital pathology evaluated densities of tumor-infiltrating CD3+ and CD8+ T cells contributes to the prediction of prognosis in neuroblastoma patients.
Emerging evidence has demonstrated that tumor microenvironment (TME) could modulate cancer progression whereas the characteristics of TME may be used for molecular classification to predict treatment response and cancer survival (1, 2). Among different cell types observed in TME, tumor-infiltrating lymphocytes (TILs), including macrophages, dendritic cells, mast cells, natural killer cells, naive and memory lymphocytes, B cells, and effector T cells, are suggested as the main players in modulating cancer progression (3–5). Recent studies have indeed shown an association of TILs with the prognosis of several cancer types (5–7). For instance, a high density of infiltrating CD3+ T cells, CD8+ cytotoxic T cells and CD45RO+ memory T cells was reported to indicate a long overall survival (OS) and progression-free survival among patients with gastric, liver, lung, nasopharyngeal, and colorectal cancers (1, 8–11). Contradicting findings do also exist. For instance, one study reported that high density of CD8+ cytotoxic T cells was associated with larger tumor size in glioblastoma patients (12). Regardless, the existing literature does seem to suggest that TILs might have a generic role in the progression of many solid tumors.
Neuroblastoma (NB) is a common tumor deriving from sympathoadrenal progenitor cells within the neural crest and has substantially varying clinical courses ranging from spontaneous regression to widespread metastasis despite intensive therapy (13, 14). The International Neuroblastoma Staging System (INSS) is currently used to determine treatment strategy and assessment of NB prognosis. NB patients are also commonly classified as at high-, intermediate-, or low- risk of death according to the Children’s Oncology Group (COG) risk classification (15). The different risk groups might demonstrate specific genetic features. TERT rearrangement and inactivating mutations in ATRX have for instance been found predominantly among patients with high-risk NB (16). However, neither the staging systems nor the genetic variations seem to explain completely the extremely divergent clinical course of NB.
With the wide application of cancer immunotherapies, it becomes increasingly important to understand the immunological landscape of the TME to understand the divergent course of NB and to predict treatment response. A recent study showed that CD4+ T cells and macrophages are involved in the oncogenesis of NB (17). Marco et al. showed that tumor-infiltrating T cells, mainly CD3+, CD4+, and CD8+ T cells, improved clinical outcomes of patients with therapy-resistant NB (18) whereas Riyue et al. demonstrated that patients with high-risk NB showed a better survival when the tumor was T cell inflamed (19). A detailed description of the phenotypes of all infiltrating T cells, especially CD3+ T cells, CD8+ cytotoxic T cells, and CD45RO+ memory T cells, using a large collection of NB specimens is however currently not available.
To this end, we investigated the densities of tumor-infiltrating CD3+, CD8+ and CD45RO+ T cells in a large sample of NB patients using digital pathology and developed a new immunoscore, summarizing such densities, to assess its role in predicting OS and event-free survival (EFS) of NB patients.
All tumor specimens of 726 NB patients collected between January 2012 and December 2020 were identified in two academic institutions, the Guangzhou Women and Children’s Medical Center (GWCMC) and the First Affiliated Hospital of Sun Yat-sen University (SYSU), in Guangdong Province, China. Among these, we included 244 NB patients with a newly diagnosed NB during this period and at an age <15 years (Figure 1). Only specimens with a tumor content greater than 50% were included in the study. All diagnoses were pathologically confirmed and restaged according to the INSS (20). The COG risk classification was performed for each patient according to medical records. This study is reported according to the Reporting Recommendations for Tumor Marker Prognostic Studies criteria (21). An Institutional Review Board approved the ethical, legal, and social implications of this project. Parents of all NB patients provided written informed consent to the participation of the study, mostly at the time of admission to the two academic institutions.
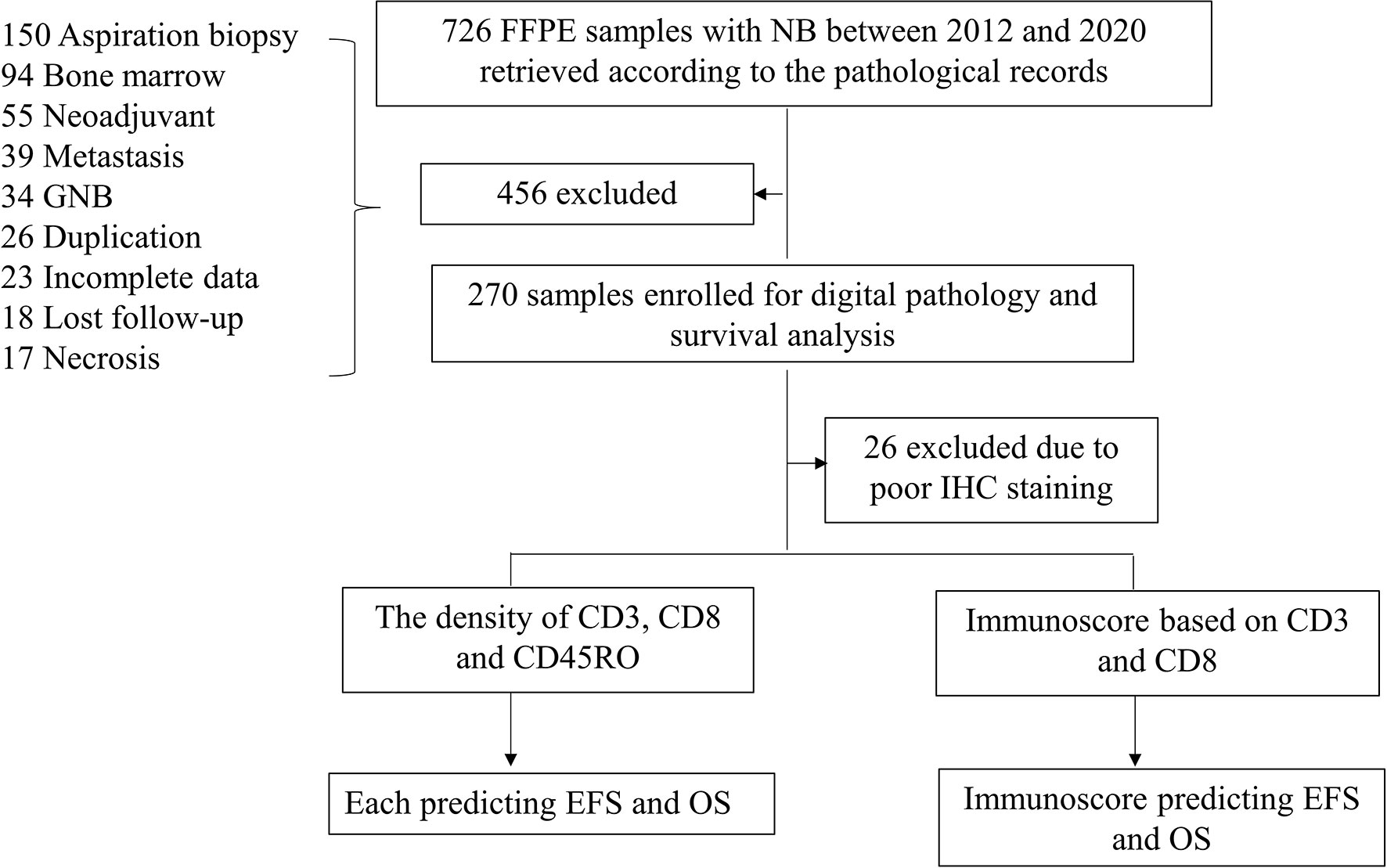
Figure 1 Workflow of the present study. Abbreviations: GNB, ganglioneuroblastoma; NB, neuroblastoma; IHC, immunohistochemistry; EFS, event-free survival; OS, overall survival.
Pathological sequential slides of 4 µm thickness were sliced up from the formalin-fixed, paraffin-embedded (FFPE) tumor blocks and used for immunohistochemistry (IHC) analysis. Primary antibodies used were CD3 (Catalog #ab16669, 1:800; Abcam, Cambridge, UK), CD8 (Catalog #ab4055, 1:800; Abcam, Cambridge, UK), and CD45RO (clone UCHL1, 1:1600; Cell Signaling Technology, CST, Beverly, Massachusetts, USA). IHC was conducted as previously described (22). Supplementary Figure 1 shows example images of IHC staining with high, low, and negative expressions of all three markers (online only).
Physical glass slides stained with CD3, CD8, and CD45RO were first reviewed by two experienced pathologists (L.Z and K.C) to ensure good staining quality for downstream analysis. All slides were then digitized at x200 magnification (Pannaromic Scan 150, 3DHistech, Hungary). Tumor areas with tumor nests and surrounding stroma areas, as well as necrosis areas, were manually annotated by a pathologist (K.C), using the QuPath software (version 0.2.3, University of Edinburgh, Scotland) (Figure 2A). Regions-of-interest (ROI) was defined as annotated tumor areas excluding necrosis areas. The density of positively stained cells in the ROIs was quantified in the following steps. Stain deconvolution (23) was first performed to separate the original images into haematoxylin channel and DAB channel images. Nuclei were segmented from the haematoxylin channel images using a Mask-RCNN based deep learning method as described in a previous study (24). The positively stained areas were identified from the DAB channel images using a pre-defined cutoff value obtained according to the control slides. A positive cell was identified if the positively stained areas were found to overlap with the segmented nucleus region for CD3 and CD8 stained images or with the ring-shape surrounding nucleus region for CD45RO stained images. Finally, the numbers of CD3+, CD8+ and CD45RO+ T cells were quantified from the ROIs of each image and recorded as density per mm2. Figures 2B–M show example images with high and low numbers of CD3+, CD8+, and CD45RO+ T cells.
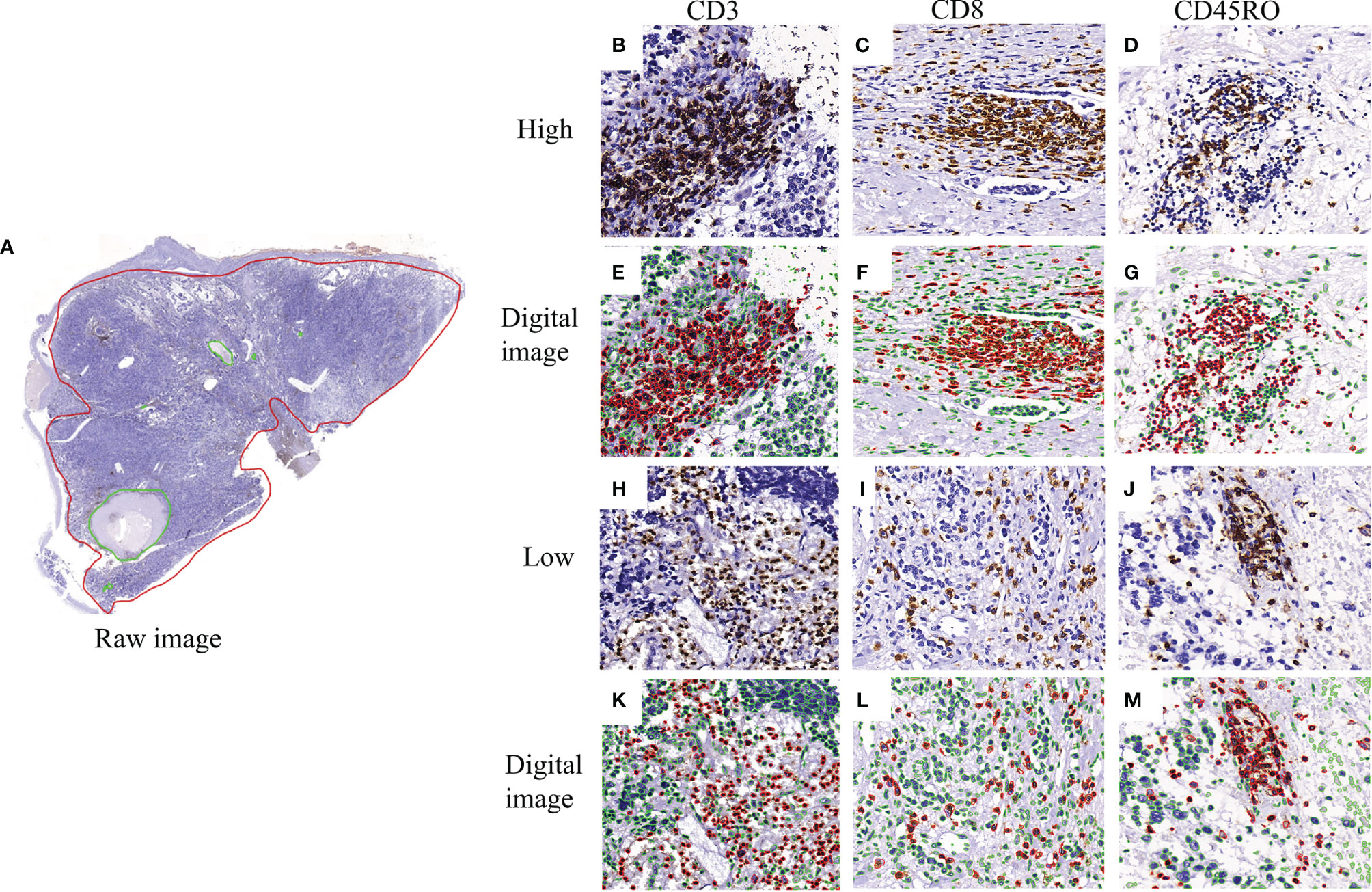
Figure 2 Representative image of CD3+, CD8+, and CD45RO+ expression and digital pathology. (A) a full view of immunohistochemistry staining labeled by the red circle as the tissue areas including tumorous and stromal areas and green circle as the necrosis areas; (B–D) the high expression of CD3+, CD8+ and CD45RO+ T cells; (E–G) the corresponding visual nucleus segmentation images in red color as positive and in green color as negative; (H–J) the low expression of CD3+, CD8+ and CD45RO+ T cells; (K–M) the corresponding visual nucleus segmentation images in red color as positive and in green color as negative.
We used the ‘Survminer’ package in R software (version 4.1.2) to calculate optimal cut-off values to define a high or low density of CD3+, CD8+ and CD45RO+ T cells, using OS as the outcome. Based on such cutoff values, each patient was given a binary score, 0 for low density indicated as (-) and 1 for high density indicated as (+), for each cell type (CD3+, CD8+ and CD45RO+).
Descriptive statistics were provided for clinical characteristics of the NB patients. The primary outcome was OS and secondary outcome was EFS, as identified through medical records. OS was calculated from the date of cancer diagnosis to the date of death from any cause. EFS was determined from the date of cancer diagnosis to the first occurrence of any event (i.e., relapse at any site, progressive disease, second malignancy, or death). Patients without an event were censored on the date of last contact (25).
The correlations between the expression levels of CD3, CD8, and CD45RO and NB clinical variables were analyzed using the χ2 test or Fisher’s exact test. We used Kaplan-Meier curves with the log-rank test method to estimate the differences in OS and EFS between patients with high and low densities of CD3+, CD8+ or CD45RO+ T cells. We also compared patients with different value of an immunoscore created based upon these individual markers. The decision of creating an immunoscore was made a priori given previous studies of similar kind using digital pathology (11). Hazard ratios (HRs) with 95% confidence intervals (CI) were first calculated using a univariate Cox regression analysis to show the associations of different prognostic indicators, including the densities of CD3+, CD8+, and CD45RO+ T cells and the combined immunoscore, with the risk of death or any event. Multivariable Cox regression analysis with backward selection was then used to test the prognostic roles of these different factors, mutually adjusted for one another. Only factors showing a statistically significant association (P < 0.05) in the univariable analysis were included in the multivariable analysis. We analyzed CD3+, CD8+, and CD45RO+ T cells separately in the first multivariable model and as a combined immunoscore in the second multivariable model.
Statistics analyses were performed using R (version 4.1.2) and Stata version 15.1 (Texas, USA). All statistical tests were two sided and considered significant when the P value was less than 0.05.
Table 1 displays the patient characteristics. We evaluated the numbers of infiltrating total T cells (CD3+), cytotoxic T cells (CD8+), and memory T cells (CD45RO+) per mm2 in the NB tissue blocks. A wide spectrum of infiltrating immune cells in the tissue blocks were identified using CD3, CD8, and CD45RO antibodies. The highest density was found for CD3 (median: 156, range: 0-2100/mm2) followed by CD8 (median: 90, range: 0-864/mm2) and CD45RO (median: 14, range: 0-949/mm2) (P<0.001; Supplementary Figure 2A). The densities by age, pathological grade, MYCN status, risk classification, INSS, and gender are shown in Supplementary Figures 2B–G (online only). The 3-year OS and EFS were 77.1% (70.8-82.3%) and 63.0% (56.2-69.1%), whereas the 5-year OS and EFS were 70.1% (62.4-76.6%) and 60.5% (53.2-66.9%) in the 244 NB patients.
We next analyzed the prognostic values of CD3, CD8, and CD45RO in NB. A low density of CD3+ T cells was associated with a lower OS (P<0.0001; Figure 3A) and a higher risk of death (HR 4.46, 95% CI 2.44-8.17) (Supplementary Table 1, online only). Similarly, a low density of CD8+ T cells was also associated with a lower OS (P<0.0001; Figure 3B) and a higher risk for death (HR 4.25, 95% CI 2.29-7.90) (Supplementary Table 1). Similar results were found for EFS (P<0.0001 for both CD3+ and CD8+ T cells; Figures 3D, E) and the risk of any event (HR 3.78, 95% CI 2.38-6.02 for CD3+ T cells and HR 3.06, 95% CI 1.94-4.84 for CD8+ T cells (Supplementary Table 1). A low density of CD45RO+ T cells was associated with a greater OS (P=0.0410; Figure 3C) and a lower risk of death (HR 0.55, 95% CI 0.31-0.95) (Supplementary Table 1) but not any event (Figure 3F and Supplementary Table 1).
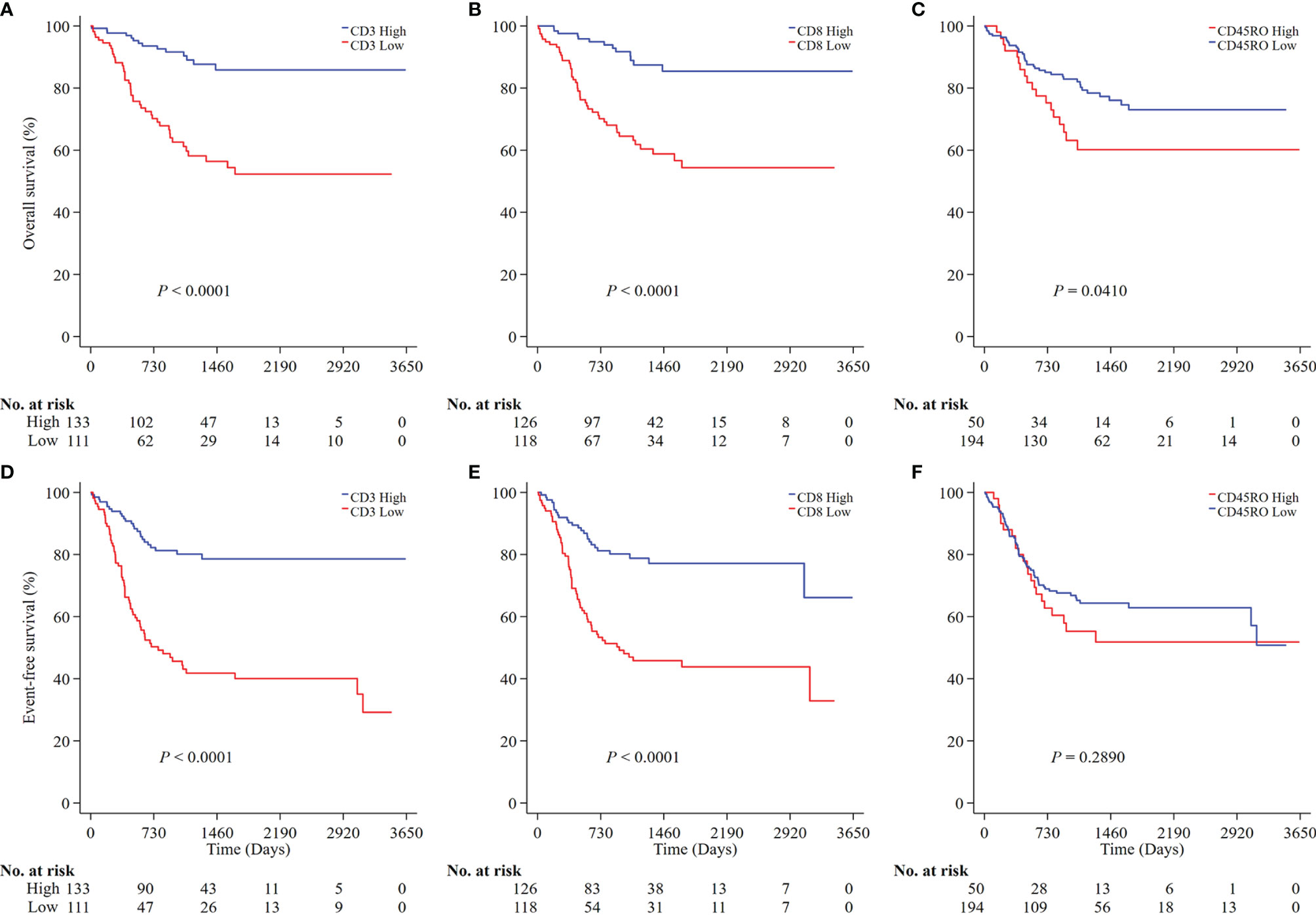
Figure 3 Survival analysis of CD3, CD8 and CD45RO expression in all NB patients. Kaplan-Meier survival curves were performed according to the high and low densities of CD3, CD8 and CD45RO (A–C for OS; D–F for EFS). P values were calculated by Log-rank test. NB, neuroblastoma; OS, overall survival; EFS, event-free survival.
To understand the joint prognostic value of these immune markers, we developed an immunoscore (IS) summarizing the individual markers. As CD45RO+ T cells was not associated with EFS in the above analysis, we classified all NB patients into four groups according to the densities of CD3 and CD8, namely high densities of both CD3 and CD8 (CD3+CD8+), high density of CD3 but low density of CD8 (CD3+CD8-), low density of CD3 but high density of CD8 (CD3-CD8+) and low densities of both CD3 and CD8 (CD3-CD8-). The immunoscore of CD3-CD8- was associated with a lower OS (P<0.0001 for both OS and EFS; Figures 4A, B) and a higher risk of both death and any event (HR 6.39, 95% CI 3.09-13.20 for death and HR 4.65, 95% CI 2.73-7.93 for any event (Supplementary Table 1).
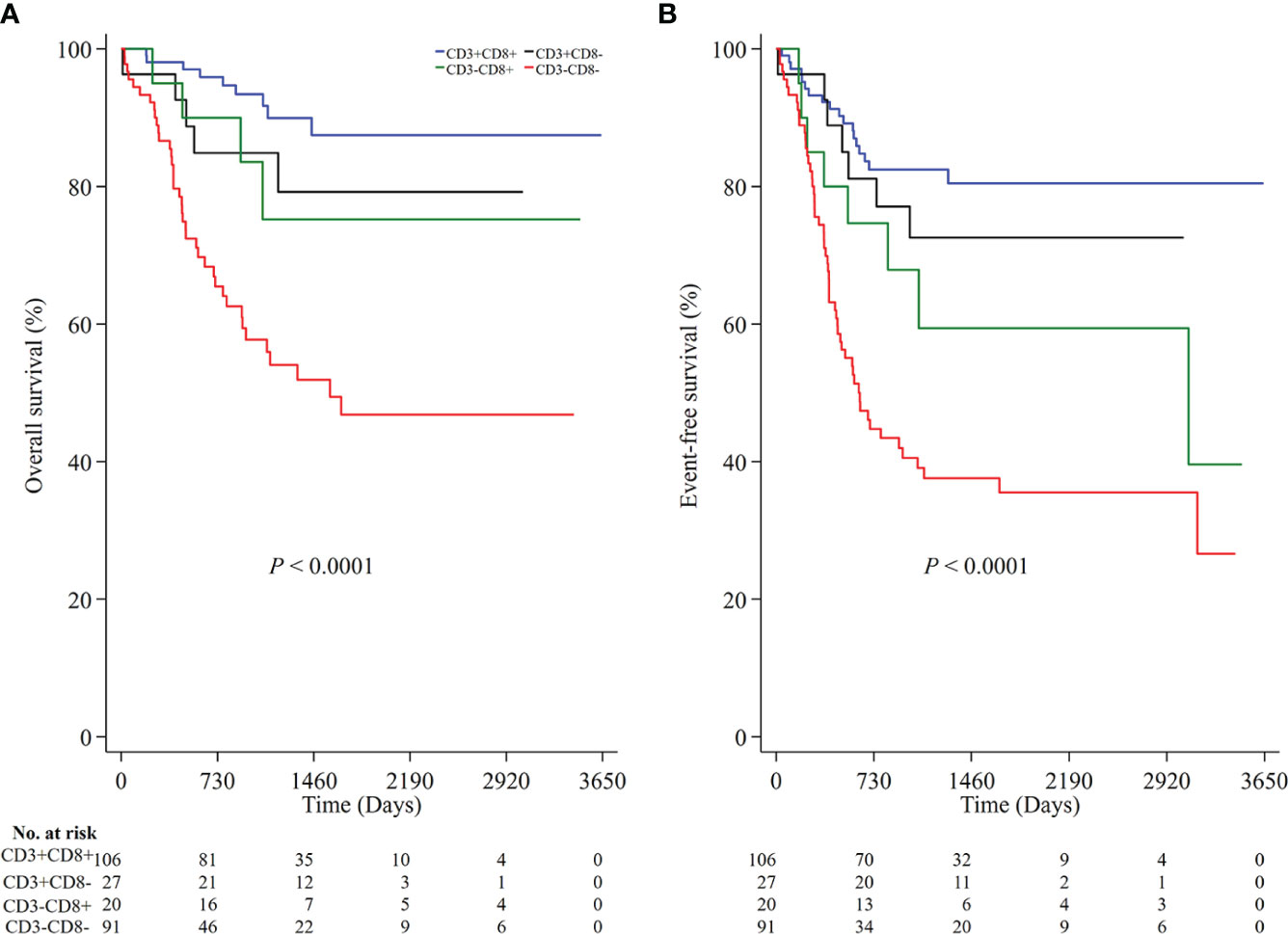
Figure 4 The prognostic value of the immunoscore based on the high and low densities of CD3 and CD8 in all NB patients. Kaplan-Meier survival analysis with the log-rank test was performed according to the immunoscore (A, P < 0.0001 for OS; B, P < 0.0001 for EFS). P values were calculated by Log-rank test. NB, neuroblastoma; OS, overall survival; EFS, event-free survival.
As age, MYCN status, INSS, and COG risk were statistically significantly associated with OS and EFS in the univariable analysis (Supplementary Table 1), we performed multivariate Cox regression analysis to assess the roles of the individually markers as well as the immunoscore after adjustment for age, MYCN status, INSS, and COG risk in two separate models. In the model for individual markers, we found similar associations between CD3+ and CD8+ T cells with the risk of death or any event, as in the univariable analysis (Supplementary Table 2, online only). In the model for immunoscore, we found that the immunoscore of CD3-CD8- was still associated with a higher risk of death and any event after adjustment for all other prognostic factors (HR 6.04, 95% CI 2.83-12.86, P < 0.0001 for death; HR 4.45, 95% CI 2.55-7.76, P<0.0001 for any event) (Table 2).
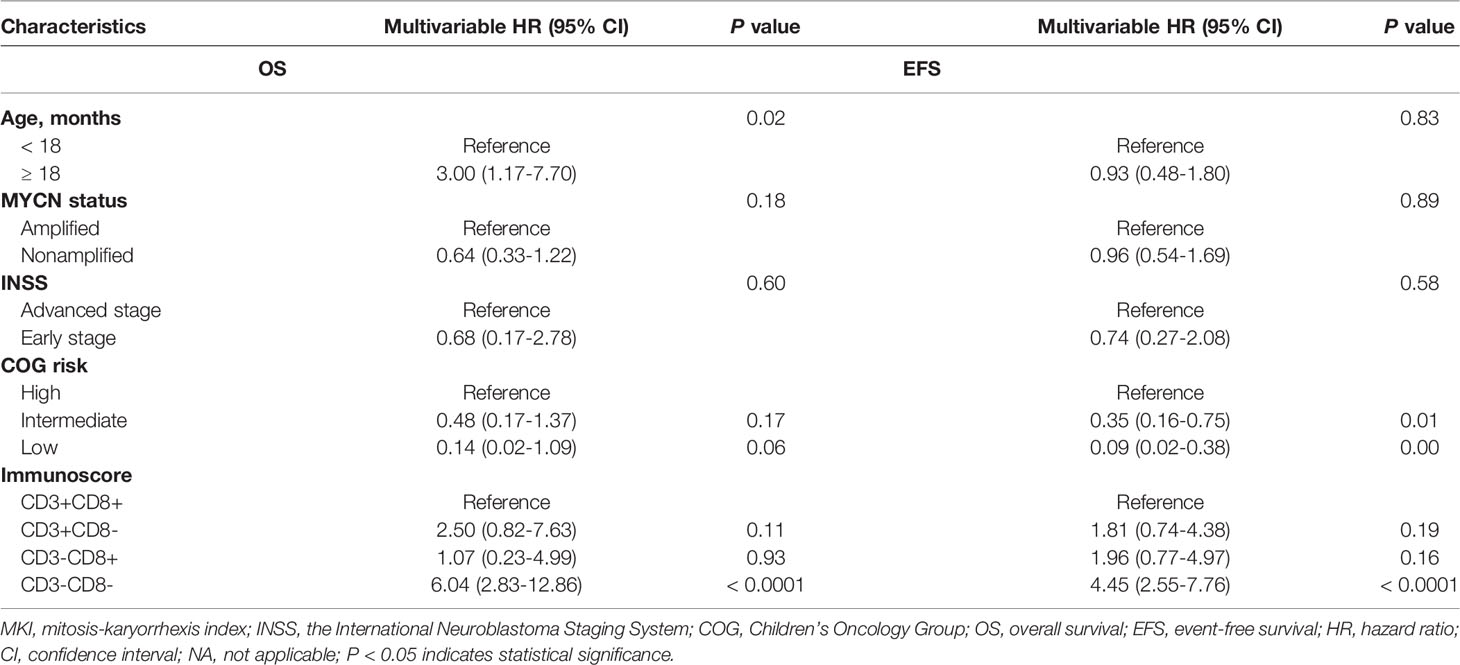
Table 2 Multivariable Cox regression analysis of risk factors associated with OS and EFS in NB patients.
In a study of 244 incident patients with NB, we performed the IHC analysis and digital pathology to assess the prognostic values of the densities of tumor-infiltrating CD3+, CD8+ and CD45RO+ T cells in NB survival. We found a high density of CD3+ and CD8+ T cells to indicate longer OS and EFS whereas an immunoscore of low densities of both CD3 and CD8 to indicate the highest risk of death or any progression event.
A previous study has reported that TME within tumor regions, including tumor interior and invasive margins, may be defined by the type, functional orientation, density, and location of immune cells (26). Therefore, tumor-infiltrating immune cells have obtained considerable research interest, especially in terms of their prognostic values in treatment response and caner survival (27). Several studies have shown that the density and distribution of TILs based on H&E slides are valuable prognostic makers in breast (28), non-small cell lung (29) and gastric (30) cancers. Moreover, studies have shown that the density of TILs is independently associated with treatment response of neoadjuvant chemotherapy in triple-negative breast cancer with HER2-overexpressing disease and homologous recombination deficient status (31, 32).
Galon et al. reported however that using H&E slides to evaluate density of TILs was not reproducible and highly subjective, and suggested that immunoscore through quantifying specific T cells such as CD3+, CD8+ and CD45RO+ T cells might be more reproducible and objective (1). Such immunoscore has indeed been reported as prognostic indicators in many cancers. Frank et al. showed for instance that CD3+, CD8+ and CD45RO+ T cells were correlated with cancer recurrence and OS in patients with rectal cancer and an immunoscore integrating the three was a powerful prognostic tool and might supplement the TNM staging system (33). In this study, we used digital pathology and showed that CD3+ T cells and CD8+ T cells mirrored each other as previously shown in non-small cell lung cancer and colorectal cancer (1, 10), similarly suggesting that high densities of CD3+ and CD8+ T cells were associated with a longer OS in NB.
High densities of TILs appear to decrease the risk of cancer recurrence, however, the driving forces for the differential densities remain unknown. It has been speculated that both host and tumor factors might contribute to the density of TILs. Therefore the densities of CD3+, CD8+, and CD45RO+ T cells in NB may also differ by clinical variables. In our study, we found that the density of CD3+ T cells was higher than that of CD8+ and CD45RO+ T cells. Further, density of CD3+ T cells was higher among NB patients with MYCN nonamplification and male, density of CD8+ T cells was higher among NB patients with differentiated pathology and male, whereas density of CD45RO+ T cells was higher among NB patients diagnosed above 18 months of age. These suggest that CD3+, CD8+ and CD45RO+ T cells might have different roles in the prognosis of NB, namely that CD3+ T cells are mostly strongly associated with OS and EFS of NB, followed by CD8+ T cells, as suggested in a previous study (18), whereas CD45RO+ T cells might have little or a harmful effect on NB survival. In general, a high density of CD45RO has been related to good clinical outcomes of several solid tumors, including melanoma, head and neck cancer, lung cancer, and colorectal cancer (34). The contradictory finding of NB and the previous studies might suggest that the role of CD45RO might be specific to tumor type and TME. Indeed, in contrast to other solid tumors, NB is a pediatric cancer with low mutation burden, little infiltrating immune cells, and poor response to immune checkpoint inhibitors (35, 36).
It is known that the tumor microenvironment is both diverse and complicated as different immune cells are capable of infiltrating tumor tissues, indicating that a synergistic view of different immune cells might provide better understanding of the immune microenvironment. For example, one study reported that the number of CD8+ TILs alone could not predict survival of patients with glioma, whereas the combination of having both low density of CD8+ and high density of CD4+ predicted survival (33). In the present study, we classified the NB patients into four groups based on the densities of CD3+ and CD8+ T cells and found that NB patients with CD3-CD8- had the highest risk of death and progression event, compared with other patients. Our results add therefore important new knowledge to existing literature suggesting a beneficial effect of cytotoxic T lymphocytes in tumors of diverse origin (11, 26, 31).
Digital pathology is gaining momentum in the analysis of pathologic tissue samples due to its accuracy and quantification of the full-view slides, providing a tool for more automatic and objective evaluation (37). Another advantage is the possibility to facilitate complex spatial patterns and standard metrics (38). In our study, we conducted digital pathology to evaluate the densities of CD3+, CD8+ and CD45RO+ T cells in NB tumor tissues. Due to the lower overall densities of infiltrating immune cells in NB tumors, compared to other cancers, we evaluated the densities of these markers in the indicated slides as a whole. Franck et al. have also used digital pathology and presented an immunoscore based on the densities of CD3+ and CD8+ T cells measured in the core and invasive margins of tumors, and found it to predict the survival of patients with rectal cancer (33). Therefore, immunoscore using digital pathology made it possible to determine the immunotype for each NB patient, strongly indicative of prognosis.
Novel tumor classification has also been proposed based on the immunoscore, as hot (high immunoscore), altered (intermediate immunoscore), and cold (low immunoscore) tumors (39). Immunotherapies are the most rapidly growing field in cancer treatment and have a major impact in clinical outcomes of cancer patients. A consensus was made that effector T cells play the central role in antitumor responses (40). Several studies have reported that hot tumors show a fertile ground for effective immune checkpoint inhibitors (ICIs) based on monotherapy or combination therapies, whereas cold tumors featured by low immunoscore should be treated with combined priming therapy, which may enhance T cell responses to convert an immune cold tumor to an immune hot tumor (41). NB is in general characterized as low immunogenicity due to the lower tumor mutation burden and lower infiltrating of immune cells (42), impeding the effective engagement during immunotherapy (43). Thus, a different or more extensive biomarker should be developed for successful implementation of novel immunotherapies in NB patients to improve survival.
There are several limitations in this study. First, we did not evaluate TILs density using H&E slides. However, it remains interesting to incorporate information on the presence of tertiary lymphoid structures (TLSs) and TILs to the immunoscore, which will likely lead to better understanding of the functional role of immune contexture in NB. Another limitation is the lack of independent validation. Future studies of independent samples are therefore needed to validate our findings.
In conclusion, our work shows that high densities of CD3+ and CD8+ T cells are indicative of longer OS and EFS in NB patients. Additionally, an immunoscore based on the densities of CD3+ and CD8+ T cells may help to classify patients with NB into different prognostic profiles. Together, these findings might pave the way for a novel implementation of immunotherapy in NB patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institute Research Ethics Committee of Guangzhou Women and Children’s Medical Center [2021]078A01. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
H-YW and NL performed study concept and design. LZ, S-HL, KC, and L-JQ conducted experiment. LZ and KC reviewed IHC slides. S-YX conducted digital pathology. H-YW, LZ, X-YL, S-YX, and RW performed data analysis and interpretation. FW, SF, S-HL, F-HW, LL, and LM retrieved clinical data. H-YW, LZ, and X-YL wrote manuscript drafting. All authors have reviewed and approved the final manuscript for publication.
This work was partially supported by the start-up program of Guangzhou Women and Children’s Medical Center (No. 3001151-04) and the National Natural Science Foundation of China (81602468).
Author S-YX was employed by Bio-totem Pte. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SW declared a shared affiliation with the authors X-YL, FW, LD, and NL to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Senior engineer Wei Wang (Cellsvision [Guangzhou]) Medical Technology Inc. China) for the professional image scanning for this study. We also would like to thank Fang Fang (Karolinska Institutet, Stockholm, Sweden) for reviewing this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.878457/full#supplementary-material
NB, Neuroblastoma; INRG, International Neuroblastoma Risk Group; TME, tumor microenvironment; INSS, International Neuroblastoma Staging System; IHC, immunohistochemistry; FFPE, formalin-fixed, paraffin-embedded; OS, overall survival; EFS, event-free survival; COG, Children’s Oncology Group.
1. Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International Validation of the Consensus Immunoscore for the Classification of Colon Cancer: A Prognostic and Accuracy Study. Lancet (2018) 391:2128–39. doi: 10.1016/S0140-6736(18)30789-X
2. Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res (2019) 79:4557–66. doi: 10.1158/0008-5472.CAN-18-3962
3. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated Regulation of Myeloid Cells by Tumours. Nat Rev Immunol (2012) 12:253–68. doi: 10.1038/nri3175
4. Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell (2010) 141:39–51. doi: 10.1016/j.cell.2010.03.014
5. Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In Situ Cytotoxic and Memory T Cells Predict Outcome in Patients With Early-Stage Colorectal Cancer. J Clin Oncol (2009) 27:5944–51. doi: 10.1200/JCO.2008.19.6147
6. Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-Based Prognostic Factors of Colorectal Cancers Are Associated With the State of the Local Immune Reaction. J Clin Oncol (2011) 29:610–8. doi: 10.1200/JCO.2010.30.5425
7. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science (2006) 313:1960–4. doi: 10.1126/science.1129139
8. Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z, et al. Intratumoral CD3 and CD8 T-Cell Densities Associated With Relapse-Free Survival in HCC. Cancer Immunol Res (2016) 4:419–30. doi: 10.1158/2326-6066.CIR-15-0110
9. Wen T, Wang Z, Li Y, Li Z, Che X, Fan Y, et al. A Four-Factor Immunoscore System That Predicts Clinical Outcome for Stage II/III Gastric Cancer. Cancer Immunol Res (2017) 5:524–34. doi: 10.1158/2326-6066.CIR-16-0381
10. Donnem T, Kilvaer TK, Andersen S, Richardsen E, Paulsen EE, Hald SM, et al. Strategies for Clinical Implementation of TNM-Immunoscore in Resected Nonsmall-Cell Lung Cancer. Ann Oncol (2016) 27:225–32. doi: 10.1093/annonc/mdv560
11. Wang YQ, Chen L, Mao YP, Li YQ, Jiang W, Xu SY, et al. Prognostic Value of Immune Score in Nasopharyngeal Carcinoma Using Digital Pathology. J Immunother Cancer (2020) 8:1–10. doi: 10.1136/jitc-2019-000334
12. Orrego E, Castaneda CA, Castillo M, Bernabe LA, Casavilca S, Chakravarti A, et al. Distribution of Tumor-Infiltrating Immune Cells in Glioblastoma. CNS Oncol (2018) 7:CNS21. doi: 10.2217/cns-2017-0037
13. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet (2007) 369:2106–20. doi: 10.1016/S0140-6736(07)60983-0
14. Maris JM. Recent Advances in Neuroblastoma. N Engl J Med (2010) 362:2202–11. doi: 10.1056/NEJMra0804577
15. Irwin MS, Naranjo A, Zhang FF, Cohn SL, London WB, Gastier-Foster JM, et al. Revised Neuroblastoma Risk Classification System: A Report From the Children's Oncology Group. J Clin Oncol (2021) 39:3229–41. doi: 10.1200/JCO.21.00278
16. Peifer M, Hertwig F, Roels F, Dreidax D, Gartlgruber M, Menon R, et al. Telomerase Activation by Genomic Rearrangements in High-Risk Neuroblastoma. Nature (2015) 526:700–4. doi: 10.1038/nature14980
17. Van de Velde LA, Allen EK, Crawford JC, Wilson TL, Guy CS, Russier M, et al. Neuroblastoma Formation Requires Unconventional CD4 T Cells and Arginase-1-Dependent Myeloid Cells. Cancer Res (2021). doi: 10.1101/2021.02.08.430292
18. Mina M, Boldrini R, Citti A, Romania P, D'Alicandro V, De Ioris M, et al. Tumor-Infiltrating T Lymphocytes Improve Clinical Outcome of Therapy-Resistant Neuroblastoma. Oncoimmunology (2015) 81:5047–59 4:e1019981. doi: 10.1080/2162402X.2015.1019981
19. Bao R, Spranger S, Hernandez K, Zha Y, Pytel P, Luke JJ, et al. Immunogenomic Determinants of Tumor Microenvironment Correlate With Superior Survival in High-Risk Neuroblastoma. J Immunother Cancer (2021) 9:1–15. doi: 10.1136/jitc-2021-002417
20. Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, et al. The International Neuroblastoma Risk Group (INRG) Staging System: An INRG Task Force Report. J Clin Oncol (2009) 27:298–303. doi: 10.1200/JCO.2008.16.6876
21. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al Statistics Subcommittee of the, Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK). J Natl Cancer Inst (2005) 97:1180–4. doi: 10.1093/jnci/dji237
22. Wang HY, Sun BY, Zhu ZH, Chang ET, To KF, Hwang JS, et al. Eight-Signature Classifier for Prediction of Nasopharyngeal [Corrected] Carcinoma Survival. J Clin Oncol (2011) 29:4516–25. doi: 10.1200/JCO.2010.33.7741
23. Ruifrok AC, Katz RL, Johnston DA. Comparison of Quantification of Histochemical Staining by Hue-Saturation-Intensity (HSI) Transformation and Color-Deconvolution. Appl Immunohistochem Mol Morphol (2003) 11:85–91. doi: 10.1097/00129039-200303000-00014
24. Jung H, Lodhi B, Kang J. An Automatic Nuclei Segmentation Method Based on Deep Convolutional Neural Networks for Histopathology Images. BMC BioMed Eng (2019) 1:24. doi: 10.1186/s42490-019-0026-8
25. Park JR, Kreissman SG, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA (2019) 322:746–55. doi: 10.1001/jama.2019.11642
26. Angell H, Galon J. From the Immune Contexture to the Immunoscore: The Role of Prognostic and Predictive Immune Markers in Cancer. Curr Opin Immunol (2013) 25:261–7. doi: 10.1016/j.coi.2013.03.004
27. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab Plus Ipilimumab in Lung Cancer With a High Tumor Mutational Burden. N Engl J Med (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
28. Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, et al. Tumour-Infiltrating Lymphocytes in Advanced HER2-Positive Breast Cancer Treated With Pertuzumab or Placebo in Addition to Trastuzumab and Docetaxel: A Retrospective Analysis of the CLEOPATRA Study. Lancet Oncol (2017) 18:52–62. doi: 10.1016/S1470-2045(16)30631-3
29. Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol (2016) 34:1223–30. doi: 10.1200/JCO.2015.63.0970
30. Kang BW, Seo AN, Yoon S, Bae HI, Jeon SW, Kwon OK, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Epstein-Barr Virus-Associated Gastric Cancer. Ann Oncol (2016) 27:494–501. doi: 10.1093/annonc/mdv610
31. Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor Infiltrating Lymphocytes are Prognostic in Triple Negative Breast Cancer and Predictive for Trastuzumab Benefit in Early Breast Cancer: Results From the FinHER Trial. Ann Oncol (2014) 25:1544–50. doi: 10.1093/annonc/mdu112
32. Telli ML, Chu C, Badve SS, Vinayak S, Silver DP, Isakoff SJ, et al. Association of Tumor-Infiltrating Lymphocytes With Homologous Recombination Deficiency and BRCA1/2 Status in Patients With Early Triple-Negative Breast Cancer: A Pooled Analysis. Clin Cancer Res (2020) 26:2704–10. doi: 10.1158/1078-0432.CCR-19-0664
33. Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, et al. Prognostic and Predictive Values of the Immunoscore in Patients With Rectal Cancer. Clin Cancer Res (2014) 20:1891–9. doi: 10.1158/1078-0432.CCR-13-2830
34. Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector Memory T Cells, Early Metastasis, and Survival in Colorectal Cancer. N Engl J Med (2005) 353:2654–66. doi: 10.1056/NEJMoa051424
35. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational Heterogeneity in Cancer and the Search for New Cancer-Associated Genes. Nature (2013) 499:214–8. doi: 10.1038/nature12213
36. Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, et al. Nivolumab in Children and Young Adults With Relapsed or Refractory Solid Tumours or Lymphoma (ADVL1412): A Multicentre, Open-Label, Single-Arm, Phase 1-2 Trial. Lancet Oncol (2020) 21:541–50. doi: 10.1016/S1470-2045(20)30023-1
37. Baxi V, Edwards R, Montalto M, Saha S. Digital Pathology and Artificial Intelligence in Translational Medicine and Clinical Practice. Mod Pathol (2022) 35:23–32. doi: 10.1038/s41379-021-00919-2
38. Heindl A, Sestak I, Naidoo K, Cuzick J, Dowsett M, Yuan Y. Relevance of Spatial Heterogeneity of Immune Infiltration for Predicting Risk of Recurrence After Endocrine Therapy of ER+ Breast Cancer. J Natl Cancer Inst (2018) 110:1–10. doi: 10.1093/jnci/djx137
39. Galon J, Bruni D. Approaches to Treat Immune Hot, Altered and Cold Tumours With Combination Immunotherapies. Nat Rev Drug Discov (2019) 18:197–218. doi: 10.1038/s41573-018-0007-y
40. Fu C, Jiang A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front Immunol (2018) 9:3059. doi: 10.3389/fimmu.2018.03059
41. Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front Immunol (2019) 10:168. doi: 10.3389/fimmu.2019.00168
42. Grobner SN, Worst BC, Weischenfeldt J, Buchhalter I, Kleinheinz K, Rudneva VA, et al. The Landscape of Genomic Alterations Across Childhood Cancers. Nature (2018) 555:321–7. doi: 10.1038/nature25480
Keywords: neuroblastoma, prognosis, immunology, digital pathology, CD3/CD8 T cells
Citation: Zeng L, Li S-H, Xu S-Y, Chen K, Qin L-J, Liu X-Y, Wang F, Fu S, Deng L, Wang F-H, Miao L, Li L, Liu N, Wang R and Wang H-Y (2022) Clinical Significance of a CD3/CD8-Based Immunoscore in Neuroblastoma Patients Using Digital Pathology. Front. Immunol. 13:878457. doi: 10.3389/fimmu.2022.878457
Received: 18 February 2022; Accepted: 11 April 2022;
Published: 10 May 2022.
Edited by:
Silvia Deaglio, University of Turin, ItalyReviewed by:
Thomas Kilvær, University Hospital of North Norway, NorwayCopyright © 2022 Zeng, Li, Xu, Chen, Qin, Liu, Wang, Fu, Deng, Wang, Miao, Li, Liu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Yun Wang, d2FuZ2h5MjlAbWFpbDMuc3lzdS5lZHUuY24=; Ran Wang, d2FuZ3I1NUBtYWlsLnN5c3UuZWR1LmNu; Na Liu, bGl1bjFAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.