- 1State Key Laboratory of Trauma, Burn and Combined Injury, Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 2Chongqing Key Laboratory for Disease Proteomics, Chongqing, China
For the skin immune system, γδ T cells are important components, which help in defensing against damage and infection of skin. Compared to the conventional αβ T cells, γδ T cells have their own differentiation, development and activation characteristics. In adult mice, dendritic epidermal T cells (DETCs), Vγ4 and Vγ6 γδ T cells are the main subsets of skin, the coordination and interaction among them play a crucial role in wound repair. To get a clear overview of γδ T cells, this review synopsizes their derivation, development, colonization and activation, and focuses their function in acute and chronic wound healing, as well as the underlining mechanism. The aim of this paper is to provide cues for the study of human epidermal γδ T cells and the potential treatment for skin rehabilitation.
Introduction
γδ T cells (according to their γδ TCR) were first identified as a novel T-cell subset in the mid-1980s (1). As a gap between innate and adaptive immune response, they participate in regulating carcinoma (2), maintaining antimicrobial barrier (3), wound healing (4), psoriasis (5) and graft rejection (6). γδ T cells represent less than 5% of peripheral lymphocyte population in mice, human and rat (7, 8), whereas it constitutes a relatively large fraction of T lymphocytes in chicken, sheep, cattle and pig (15–50%) (8). In adult mice, γδ T cells are unequally distributed (9); there are less than 5% of total T cells in the lung, approximately 20–40% of the intraepithelial T cells of intestinal, approximately 10–20% of total T cells in the reproductive tracks, approximately 50–70% of skin dermal T cells and approximately 95% of epidermal T cells. In addition, they are divided into Vγ1-7 γδ T subsets according to the γ chain (10). Almost all γδ T cells in epidermis are dendritic epidermal T cells (DETCs: named by its dendritic morphology), expressing an invariant Vγ5Vδ1 TCR (according to Tonegawa’s nomenclature, which is adopted in this paper), equal to Vγ3Vδ1 TCR (according to Garman’s nomenclature) (11, 12). They maintain a homeostatic population by self-renew and can secrete growth factors such as IGF-1 (Insulin-like growth factor 1) and KGF-1/KGF-2 (keratinocyte growth factor 1/2) etc. (13) Most γδ T cells in dermis are Vγ4 T and Vγ6 Cells, they can secrete IL-17A (interleukin-17A), IFN-γ (interferon-γ) and the growth factors (4).
In humans, γδ T cells are classified based on the presented Vδ gene segment. Until now, there exists three true Vδ genes: Vδ1-3; and seven functional Vγ gene segments: Vγ2-5, Vγ8, Vγ9, and Vγ11 (14). Vδ1 γδ T cells primarily colonized in the dermis, and a small population is distributed in the epidermis, whereas Vδ2 TCRs are mainly distributed in peripheral blood and dermal (15, 16). Human epidermal γδ T cells play a functionally similar role as DETCs in promoting wound healing via secreting insulin-like growth factor 1 (IGF-1) and regulating cutaneous carcinoma (17, 18). However, they are not called DETCs as they do not possess dendritic morphology and take different molecular mechanisms in epidermis homing, antigen recognition and activation.
The skin, which is essential in defencing against external pathogens and environmental factors such as the microbes attack, ultraviolet radiation and heat injury (15, 19), serves as the largest interface between the body and the external environment. On one side, skin needs enough defending power to maintain homeostasis; on the other side, it needs fast and effective responses to repair the injury and restore the integrity upon injury or inflammation. Wound repair mainly contains four overlapping stages, which includes hemostasis, inflammation, proliferation and remodeling (20). Immune cells manage wound repair by secreting cytokines and chemokines to induce inflammatory microenvironment and promote re-epithelialization. DETCs, Vγ4 T cells and Vγ6 T cells are the main subsets of skin T lymphocytes and the equilibrium, coordination and interaction among them significantly affect their effectiveness in wound repair. This review primarily focuses on the discussion the rodent and murine γδ T cells, including their development, differentiation, colonization, activation, their functions and the underlining mechanism in wound healing. In addition, by consolidating the recent research breakthrough in the field, perhaps this article may also provide potential cues for the study of human skin γδ T cells and the potential treatment for skin rehabilitation.
The Development and Colonization of γδ T Cells
γδ T cells and αβ T cells originate from the same progenitor in the thymus. When bone marrow-derived hematopoietic stem cells (HSC) migrate into the thymus, Notch receptor 1 (Notch 1) and Delta-like 4 (DLL-4) signaling leads to the generation of T cell progenitors called double-negative cells expressing CD4- and CD8- (DNs, CD4- and CD8-) (19, 21, 22), which commit them to the T-cell fate. Then these immature thymocytes pass through four developmental stages, from DN1 to DN4 (23, 24). DN1 cells are uniformly bipotent, they can give rise to both αβ and γδT cells (25); the next DN2 stage initiates the divergence of αβ and γδ T cells, and in this stage, cells expressing IL-7R and SOX13 (one high mobility group (HMG) box TF) and other unknown factors exhibiting the tendency to γδT cells fate (26, 27). TCR δ, γ and β start to rearrange stochastically (somatic recombination of the V, D, and J genes encoding the V domain of the corresponding TCR proteins) (28–30), and then weak signal strength boosts the divergence of αβ lineage (preTCR: consisting of the invariant pTαchain paired with a full-length β chain), while the strong signal enhances the γδT cells and selectively promotes the precisely rearranged and paired γδ chain (TCR γδ) (28, 29, 31–33), DETCs, IFN-γ-producing V γ1 cells and IL-17A-producing V γ6 cells are markedly depleted in mice with attenuated TCR signaling of their own (34, 35), this process is called the positive selection. The invalidly rearranged cells or validly rearranged cells without sufficient activation signaling from ligand undergo apoptosis similar to the death of the αβ T cells without useful TCR. Whether this phenomenon leads to the successive development characteristic of γδ T cells has to be verified. Partial cells of this stage retain bipotency, whereas other cells just give rise only to αβ or γδ T cells (36). The divergence of αβ and γδ lineage is completed at the DN3 stage, and by this stage, almost all of the cells complete lineage commitment, with a major population exhibiting αβ lineage restriction (25). But the precursor cells with type of TCR (preTCR or γδ TCR) can’t dictate the lineage choice, as the γδ TCR and αβ TCR can generate αβ and γδ lineage cells under some special circumstances, respectively (37–39); transitioning into the DN4 stage, the TCRα chain gene-rearrangement begins, which generates double positive(CD4+, CD8+) αβ T cells (DP αβ T cells) marking the point of irreversible commitment to the αβ lineage (36, 40). Then the DP αβ cells commit the positive and negative selection and get matured (41). While the subset of immature γδ T cells will develop the effector commitment, the relatively weaker signals enhance the IL-17–producing γδ T cell subset, and progressively stronger signals promote IFN-γ–producing and innate γδ T cells (24). However, there has no direct evidence whether the stronger or weaker signal leads to higher productions of IFN-γ- or IL-17A- V γ4 T cells, respectively. CD24 or heat-stable antigen (HSA) is recognized as the marker of γδ T cell lineage for irreversible commitment. The expression of CD24+CD73+ indicates that these cells are unable to switch to the αβ T cells (19, 42). Therefore, the TCR signaling operates in sequential developmental windows with distinct outcomes, and it determines the lineage and effector commitment successively (10). In addition, TCR γδ-independent factors are crucial in γδ T cells differentiation, such as the miRNAs, Sox4/Sox13/RORγ axis (SRY-box-containing gene 4/13/retinoid-related orphan receptor γ axis), and Notch signaling (13, 43, 44). Thus, every subset has its own development characteristic.
The development of the γδ subset occurs step by step as follows: T cell commitment–αβ/γδ lineage commitment–γδ subset commitment–effector commitment (Figure 1); therefore, the same factor can take different functions during disparate stages. This theory can reconcile some inconsistent research results. For instance, IL-7 and the transcription factor SOX13 promote the survival and development of early precursor cells and are absolutely required for TCRγ gene rearrangement. However, at the later stage, their function mainly promotes the IL-17-producing cells (26, 27, 45, 46). Besides, the same factor can give rise to an identical or a different function for various subsets at the same cross-section in time, just like the PLZF and Egr2/3/id3; the former promotes the development of the Vγ1+ and Vγ6+ cells (47, 48), while the later one takes an opposite function in IL-17- and IFN-γ-producing cells (10).
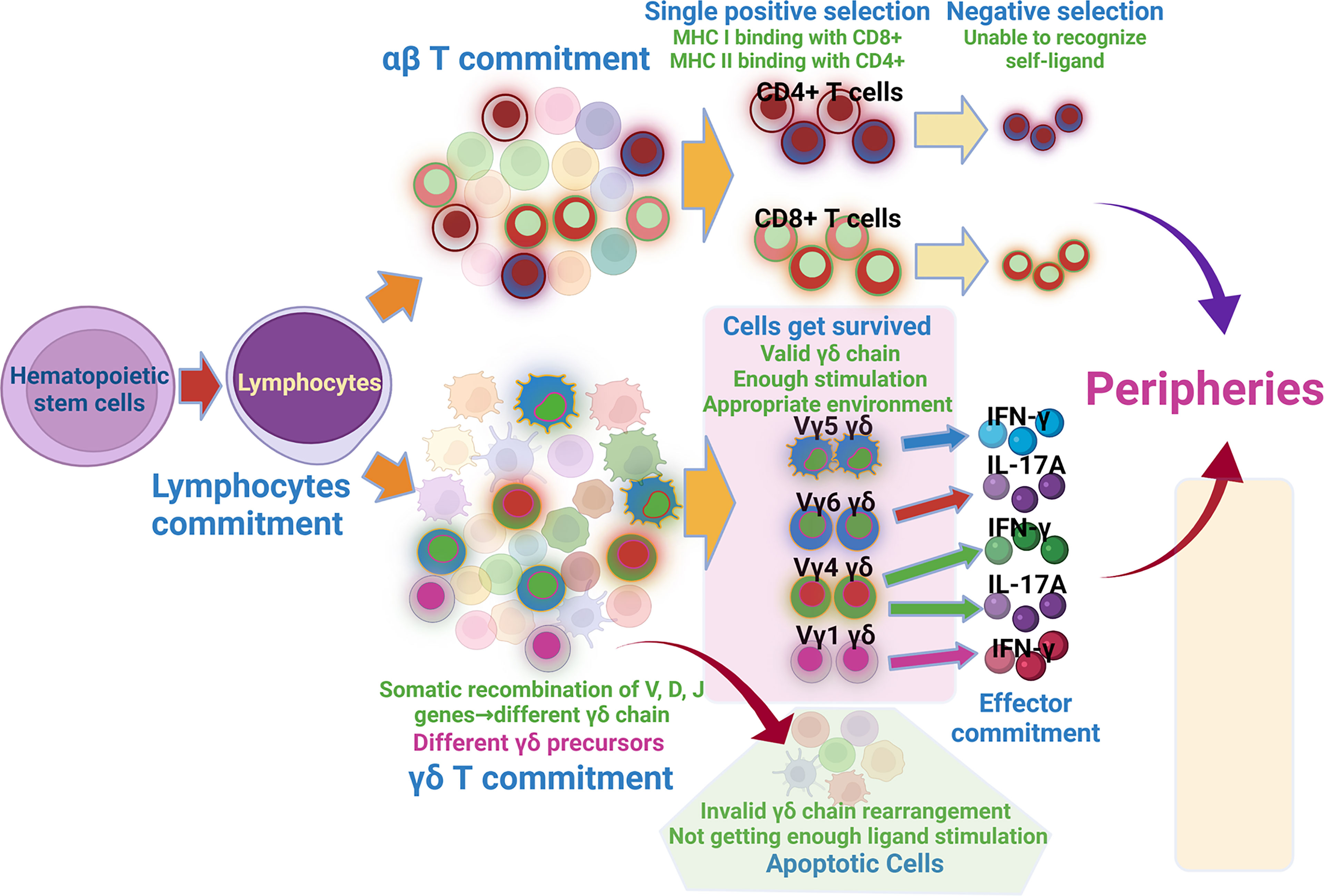
Figure 1 Development of αβ and γδ T cells. Hematopoietic stem cells migrating into thymus get lymphocytes commitment, the lymphocytes then get αβ commitment and γδ commitment. αβ cells passing through sequential single positive selection and negative selection get matured. Somatic recombination of V, D, J genes forms different γδ chain, which produces varied γδ precursors. Among them, cells with valid γδ chain, getting enough stimulation and appropriate environment get survived, cells with invalid γδ chain and getting insufficient ligand stimulation get apoptosis. Survived γδ T cells then undertake effector commitment and get matured.
DETCs expressing a canonical Vγ5Vδ1 TCR are a restricted antigen repertoire and act exclusively as resident T cells in the murine epidermis (12). They derive from DETC progenitors which are restrictedly generated in the embryonic thymus at day 13 to 17 (49), and at E16 and E18 (50), DETCs egress from the thymus and move to the epidermal layer where they self-renew. Existing research have confirmed that the development of DETCs can be influenced by ERK-Egr-Id3 axis (35), Lck (51), Syk (52), ZAP-70 (53), IL-7R/JAK/STAT pathway (54, 55), RunX3 (regulating CD103 and CD122) (56), miRNAs (downregulating CD122/IL-2Rβ and CD45RB expression) (43) and Skint-1 (promoting the selective development of Vγ5+ DETC) in the thymus (35, 57); their skin-homing are affected by the ITK (through promoting CCR10 and S1PR1 expression) (58, 59), SIPR1 (sphingosine-1-phosphate receptor 1, involved in thymic egress) (60), GPR15 (orphan G protein-linked chemoattractant receptor 15, regulating the recruitment of γδT cells to skin) (61), CD103 (62), E, P-selectins ligands (63) (Expressed on DETCs, binding to selectins expressed on the endothelium), CCR10 (64) and CCR4 (63) (binding to CCL27/28 expressed by keratinocytes), Vγ5 T cells have low expression in CCR9 and CCR7, so they will not migrate into lymphoid organ and spleen. Matured DETCs express the markers including CD27–, CD69+, T-bet+, NKG2D+, JAML+, CD100+, and CD103+ (15).
Vγ4 T cells appearing at the late fetal stage(from E16)and afterward (49), are the dominant subset of murine peripheral γδ cells. In addition, Vγ4 T cells exist in peripheral lymphoid organs, blood, liver, lung, spleen and dermis (65). They are divided into two main subsets: IL-17A+Vγ4 T cells (CCR6+CD27-), and IFN-γ+Vγ4 T cells (CCR6-CD27+) (66). The majority of γδ T cells in lymph node are IL-17A γδT cells, whereas a large population in splenic is IFN-γ γδ T cells (67); the mechanism leading to this biased distribution is unclear. The development of IL-17A producing cells is also regulated by the comprehensive factors, such as Sox4/Sox13/RORγt/IL-17 axis (68), Notch signaling/Hes-1 axis (44, 69), Wnt signaling pathway/TCF1 and Lef1 axis (70), TGF-β (71), Blk (B lymphoid kinase, a Src family kinase) (72) and IL-7 (45). Moreover, CCR6 is recognized to be critical for their homing to skin, CCR6-deficiency reduced the number of both Vγ4 and Vγ6+ cells in the skin (73). Other research reports that thymic Vγ4 requires extrathymic environment for skin homing, such as getting activated or obtaining CCR6 expression (74). Matured IL-17 producing Vγ4 T cells (thymus-derived) contain variable δ chain. Most of them express CD3+, CD4-, CD8-, CD44+, CD69+, RORγt+, CCR6+, CD25+, CD27-, Scart2+, CD45RB-, CD122-, CD27-, NK1.1-, T-bet-, IL-23R (31, 66, 75–80). Recent research found that some IL-17 producing γδ T cells are bone derived, and they often just have δ4 chain. In addition, they express CCR2+ and require IL-23 and IL-1β for their reprogramming from CD27+ γδ T cells (81, 82). In addition, IFN-γ-producing γδT cells are affected by ERK-Egr-Id3 axis (10, 34), ThPOK/PLZF/T-bet axis (83), researches have reported thymic γδ T cells with antigen-experience or binding antigen have high affinity in producing IFN-γ (67), matured IFN-γ producing Vγ4 T cells have variable δ chain. Their expression characteristics are CD3+, CD4-, CD8-, CD44+, T-bet+, NK1.1+, CCR6-, CD27+, CD45RB+, CD122+(IL-2/IL-15 receptor β chain) (31, 66, 75–80).
Vγ6 T cells, which exclusively express the Vδ1 TCR chain (74), are generated solely in the thymic second wave around embryonic day E14 (up to the birth) (49). In mice, about half of the dermal γδ T cells are the Vγ6 T cells, while the rest mainly express Vγ4 TCR (4, 74). Vγ6 T cells also localize to uterine epithelia, tongue and meninges, enthesis, pLNs, testis (79, 84–86). Conventionally, dermal Vγ6 T cells are considered bona fide tissue-resident cells that do not recirculate out of the skin and their generation is restricted to the confined window of fetal development. Furthermore, Vγ6 T cells cannot be induced in adult animals with the phenomenon that Vγ6+ γδ T cells become rare in the adult thymus (87, 88). But recent research confirmed that they have a high mobility and can travel between pLNs and tissues (79); however, whether the proliferated Vγ6+ in pLNs or thymus refill the pool of terminally differentiated skin Vγ6 remains to be tested. Their development is affected by IL-7 (45), TGF-β (71), Blk (72), PLZF (47). Matured Vγ6 cells exhibit the expression characteristics of CD27–, IL-23R+, RORγt+, CCR6+, CD69+, CD44+, Scart1+, cMAF+, PLZF+, PD-1 receptor and CCR2 (15, 79).
γδ T Cells in Maintaining Skin Homeostasis
Skin comprises two major compartments, the epidermis and the dermis. The epidermis is mainly composed of keratinocytes (~95%) and residing immune cells (~5%, mainly are Langerhans cells (LC) and T cells) (89). The immune cell composition is subject to species specific differences. In naïve wild type (WT) mice, DETCs dominate the epidermal T cell compartment(~95%). Human epidermis is home to both γδ and αβ T cells, while resident T cells in epidermis show effector functions very similar to that of DETC (90).
The DETCs proliferate and maintain a homeostatic population by themselves, which cannot be reconstituted with bone marrow cells or fetal thymocytes (88). Aryl hydrocarbon receptor (AhR) and Linker for activation of T cells (LAT) are recognized to be the important factors in maintaining DETCs proliferative expansion and self-renewal (91). AHR-KO mice and LAT–deficient mice lack peripheral DETCs neither through affecting the DETCs generation nor skin homing (92). DETCs are characterized with lots of dendrites; most of the dendrites anchor to the apical epidermis where they are immobilized at distal. The remaining dendrites are positioned within the basal epidermis and are highly mobile (93). PALPs (containing prominent co-clusters of TCR and proteins phosphorylated on tyrosine residues) (94) of the apically oriented dendrites contribute the anchoring of DETCs to the squamous keratinocyte junctions, E-cadherin receptor integrin αEβ7(CD103) highly enriched at the ends of apical dendrites modulates the dendrite anchoring, which binds with E-cadherin expressed by keratinocytes. This structure allows the frequent contact of DETCs with the neighbouring cells as well as continuous scanning for antigens in the skin surface (94). Although healthy skin does not appear to express DETC TCR ligand detectable by soluble Vγ5Vδ1 TCR tetramers (95), low grade stresses from outside environment might sustain a basal expression of ligands sufficient for TCR activation but below the sensitivity of currently existed detection method. This presence of agonistic TCR-proximal signals make the DETCs to be a semi-activated state via Lck-dependent TCR activation (94), these semi-activated DETCs establish a polarized conduit system for transepithelial cargo transport, which contributes to the accumulation of matured lysosomes and the probe of the epidermal molecular composition (96). Normally, semi-activated DETCs express CD122 and CD69 (marker of pre-activation/semi-activation), their autocrine cytokines can help maintaining steady state of themselves and other cells (93), including IL-13, IGF-1, GM-CSF (Table 1). IL-13 plays an important role in regulating epithelial cells homeostasis and maintaining skin integrity through promoting EC (Epithelial cells) maturation and transiting through epidermis, the mice lacking canonical DETCs or IL-13 shows a higher degree of water loss, a poorer barrier function and a declined tolerance to damage compared to the WT skin (97); IGF-1 can protect themselves and keratinocytes from apoptosis (98), while GM-CSF is crucial for LC maturation (92). In turn, the paracrine cytokines by neighboring keratinocytes, fibroblasts and other cells are crucial in keeping the homeostasis of DETCs (96, 99). IL-7 secreted by keratinocytes and fibroblast mesenchymal cells serves as a growth factor for DETCs (100); IL-15 secreted by epithelial cells helps the survival and proliferation of DETCs via binding IL-15Ra (CD215) expressed on DETCs (101).
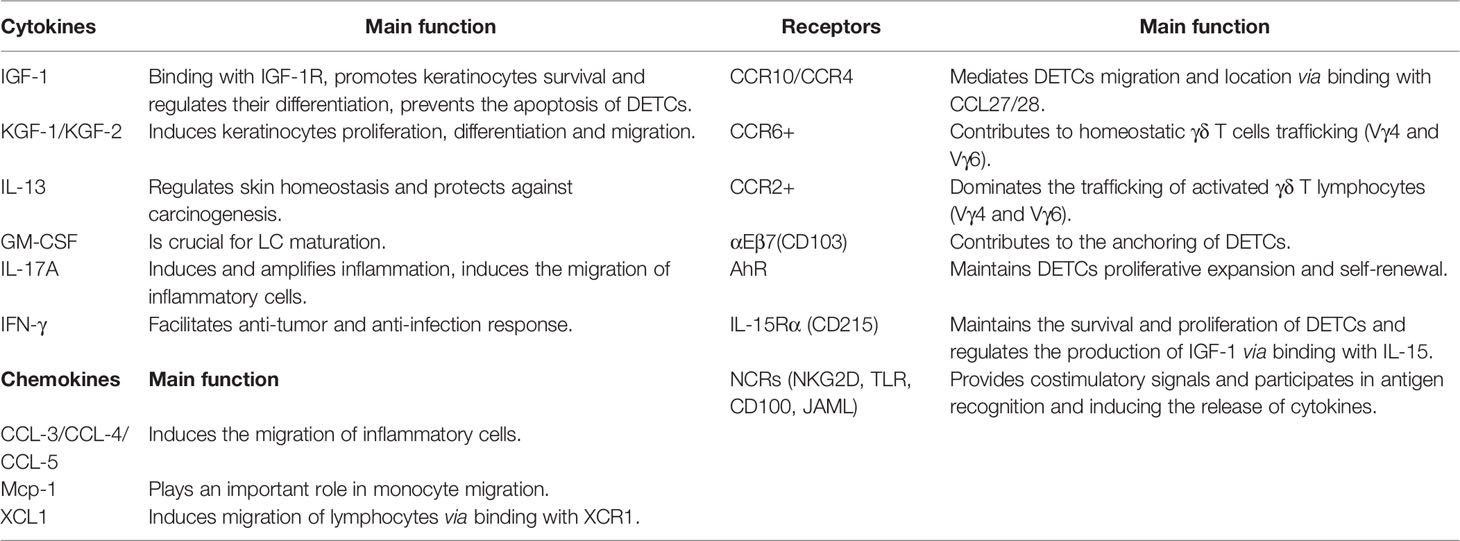
Table 1 Main cytokines, chemokines, and receptors of DETCs, Vγ4 and Vγ6 T cells in skin homeostasis and wound healing.
The immune cells residing in the dermis under homeostasis include dermal subsets of dendritic cells (DCs), mast cells, T cells (αβ and γδ T cells), innate lymphoid cells (ILC), B cells, macrophages and NK cells (102). γδ T cells of dermis mainly comprised of Vγ4 and Vγ6 γδ T cells. Vγ6 γδ T cells represent virtually 100% of the dermal γδ T cells in newborn mice, but comprise only about 40% in adult mice, as the Vγ4 γδ T cells in the dermis gradually increase over time (103). The majority of Vγ6+ γδ T cells display tissue residency, but may retain the capability to circulate between tissues, while the Vγ4 T cells display the recirculating characteristic. Recent researches have indicated that both dermal Vγ4 and Vγ6 T subsets are radioresistant (74, 104). Under homeostasis conditions, both subsets can traffic between tissues and lymph nodes at a slow but steady rate (79, 87, 105, 106); a substantial flux of γδ T cells through the skin to draining LNs is observed through analysis of skin-draining lymph in cattle (107). It is proposed that CCR6-dependent manner contributes to homeostatic γδT17 cell trafficking, CCR6 can bind with CCL20 expressed in mucocutaneous sites and subcapsular region of primate LNs (108), while CCR2-dependent manner dominates the activated trafficking (73), this trafficking characteristic facilitates their immune surveillance function. Upon activated by ligands such as the specific ligands triggered by the imiquimod treatment, the migration will significantly increase. However, it seems that the Vγ4+ dermal cells are able to migrate more efficiently than the Vγ6+ γδT cells (103, 109). For the resident Vγ6γδT cells, they usually act as persistent effector cells in the skin, high expressions of the anti-apoptotic BCL2A1 protein protects them from activation-induced cell death (79). However, whether the resident Vγ6+ T cells can be refilled by the Vγ6 T cells from pLN and thymus is uncertain, and interesting to be tested. For the Vγ4 cells, they can be reconstituted by thymic Vγ4+ cells and bone marrow, but they need to go to the periphery and mature before migrating to the dermis (74, 81). The CCR6 expressed on their surface and the CCL20 expressed by epidermal keratinocytes, endothelial cells, and dendritic cells are crucial for their recruitment (82).
Collectively, DETCs exist in epidermis, they maintain a homeostatic population by self-renewal. Under homeostasis, they secrete IL-13, IGF-1 and GM-CSF to help in epithelial cells maturation and proliferation. IL-7 and IL-15 secreted by epithelial cells contribute to the survival and proliferation of DETCs, PALPs of the apically oriented dendrites contribute to the anchoring of DETCs to the keratinocyte junctions. Vγ4 and Vγ6 T are main subsets in the dermis, they traffic between tissues and lymph nodes at a slow but steady rate under homeostasis, CCR6 expressed on their surface combining with the CCL20 expressed in mucocutaneous sites and subcapsular region of primate LNs is an important pathway (Figure 2).
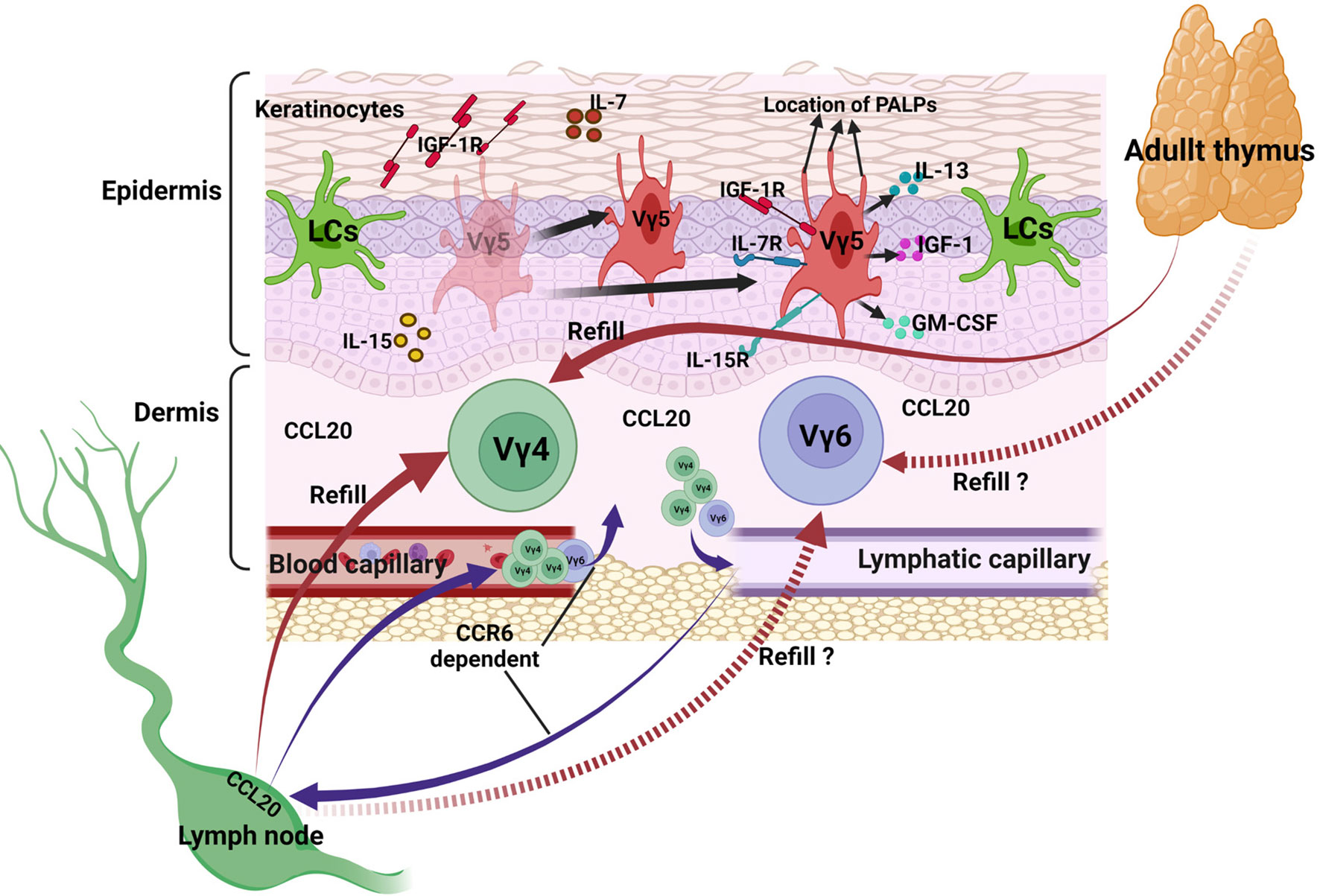
Figure 2 γδ T cells in maintaining skin homeostasis. DETCs in epidermis proliferate and maintain a homeostatic population by themselves, they secrete IL-13, IGF-1 and GM-CSF to help keeping steady state of themselves and other cells. IL-7 and IL-15 secreted by epithelial cells contribute to the survival and proliferation of DETCs, PALPs of the apically oriented dendrites contribute to the anchoring of DETCs to the keratinocyte junctions. Vγ4 and Vγ6 T subsets in the dermis traffic between tissues and lymph nodes at a slow but steady rate, CCR6 expressed on their surface combining with the CCL20 expressed in mucocutaneous sites and subcapsular region of primate LNs is an important pathway.
The Activation of γδ T Cells
γδ TCRs have the ability for both innate and adaptive ligand recognition via either germline-encoded regions of the receptor, resemble the PRRs or adaptive antigen binding via the CDRs, this pattern seems to be distinguished from αβ TCRs (102). Most αβ TCRs bind to MHC I/II (major histocompatibility complexes I/II) which presents small peptide fragments derived from pathogens or pathological tissues. Together with co-receptor engagement of CD4 or CD8 and co-stimulation through CD28, this elicits αβ T-cell activation (110). Similar to αβ T cells, the activation of γδ T cells may require the engagement of both γδ TCR and co-receptors, including junctional adhesion molecule-like protein (JAML) (111), Toll-like receptor (TLR) (112), the semaphorin CD100 (113) and C-type lectin-like stimulatory receptor-natural killer group 2D (NKG2D) (114). As no general restricting molecule could be identified, no effective methods can assess whether the recognition of certain antigens by γδ TCRs is generalized, and the affinity of TCRs to their antigens is typically low, the antigens activating the γδ TCR or γδT cells have not yet been clearly identified up to now. Recent years, many studies have been conducted to explore the antigens. The antigens activating the γδ T cells can be divided into 4 categories (115): First of all, MHC or MHC-like recognition antigen includes MHC-Ib molecule T10/T22 (116), MART-1 (117), MHC-related protein 1 (MR-1) (118). Secondly, there are IG-like recognition of antigens, including Annexin A2 (119), ephrin receptor A2 (EphA2) (120), the human DNA mismatch repair protein MutS-Homologue 2 (hMSH2) (121), heat shock protein (HSP) 60 (122), PE(phycoerythrin) (123). Thirdly, this group contains Phosphoantigen, including 4-hydroxy-3-methyl-but-2- enylpyrophosphate (HMBPP), Isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (124). Lastly, there are B7 receptor family-like proteins, including BTNLs (BTNL1 and BTNL6 in mice, BTNL3 and 8 in human) (125, 126). Furthermore, the antigens can be categorized into DAMPs and PAMPs (damage associated molecular patterns and pathogen-associated molecular patterns) according to their derivation, the former ones are generated in cell necrosis (often associated with tissue injury), whereas the controlled cell death, or apoptosis, does not lead to the generation of DAMPs, the latter ones are elicited by pathogens (127). In addition, some papers divide the ligands into self ligands and non-self ligands (128).
Shortly after wounding or inflammation, damaged keratinocytes closely adjacent to the lesion quickly and transiently upregulate related stress antigen. The γδT cells of epidermis and dermis get complete activation via recognizing the antigens by TCR and co-stimulatory receptors. Activated epidermal γδT cells retract their dendrites and round up within 24 h after wounding (129). Within 48 h, epidermal γδ T cells secrete cytokines and growth factors to regulate inflammation and proliferation, such as KGF-1, KGF-2, IL-13, IFN-γ, TNF-α, IGF-1, IL-2, and IL-17 (Table 1), epidermal γδ T cells restore their dendritic morphology 5 days post wounding (4, 129). For the Vγ4 T cells, they are most commonly found early post wounding, accounting for half of the IL-17A+ cells on the third day (130), firstly, they get activated, proliferate and secrete IL-17A, IFN-γ, IL-17F, IL-22 and other cytokines to regulate the inflammation promptly. Secondly, the keratinocytes close to the lesion upregulate the production of CCL20, which increases the epidermal infiltration of dermal γδ T cells by binding their CCR6 (130, 131), in the absence of CCR6, fewer γδ T cells is observed at the wound site leading to 4-day delay in wound closure, this indicates a key role for CCR6 in efficient wound repair (132). The CCL20–CCR6 axis of dermal T cell recruitment occurs similarly in the human epidermis, resulting in Th17 cell infiltration (133). Thirdly, the migration of resident γδ T cells into the local draining lymph nodes increases, the traffic manner is CCR7-independent (105), and Vγ4+ cells homing from inflamed skin to sLNs during psoriasis predominantly lack CCR6 expression (109). It likely occurs via afferent lymph draining from dermis, but the definite pathway involved is undetermined. Fourthly, the γδ T cells specific expressing Vγ4Vδ4 in lymph nodes selectively expand promptly (105, 109), the reason leading to the selective expansion is uncertain, cytokines may play a crucial role in this process. Lastly, general γδ T cells and expanded Vγ4Vδ4 γδ T cells infiltrate back into inflammatory skin via S1P1 and CCR2 (82, 134), however, whether CCR2 up-regulation promotes the recruitment of thymus-derived Vγ4 T cells to inflamed tissue is unclear. Importantly, the re-filtrated Vγ4 Vδ4 T cells persist for months and respond more rapidly like the memory-like cells in the imiquimod (IMQ)-induced mice model (82). Activated Vγ6 T cells show very similar traits with Vγ4 T cells, CCR2 and CCR6 expressed on their surface are also crucial for the migration in homeostasis and inflammation state (73); however, it seems like their efficiency is lower than the Vγ4 cells (135).
Taken together, the antigens activating the γδ T cells can be divided into 4 categories: MHC-like recognition antigens, IG-like recognition of antigen, phosphoantigen and B7 receptor family-like proteins; they can also be categorized into DAMPs and PAMPs. The binding of these antigens with the γδ TCR and co-stimulatory receptors helps in the complete activation of γδ T cells. Activated γδ T cells secrete chemokines, cytokines and growth factors to regulate inflammation and proliferation. Activated Vγ4 T cells migrate to epidermis via CCR6-CCL20 pathway, in addition, the traffic of Vγ4 and Vγ6 T subsets between skin and lymph nodes increases, the traffic from skin to lymph nodes is CCR6/CCR7-independent, while that from lymph nodes to skin is CCR2-dependent (Figure 3).
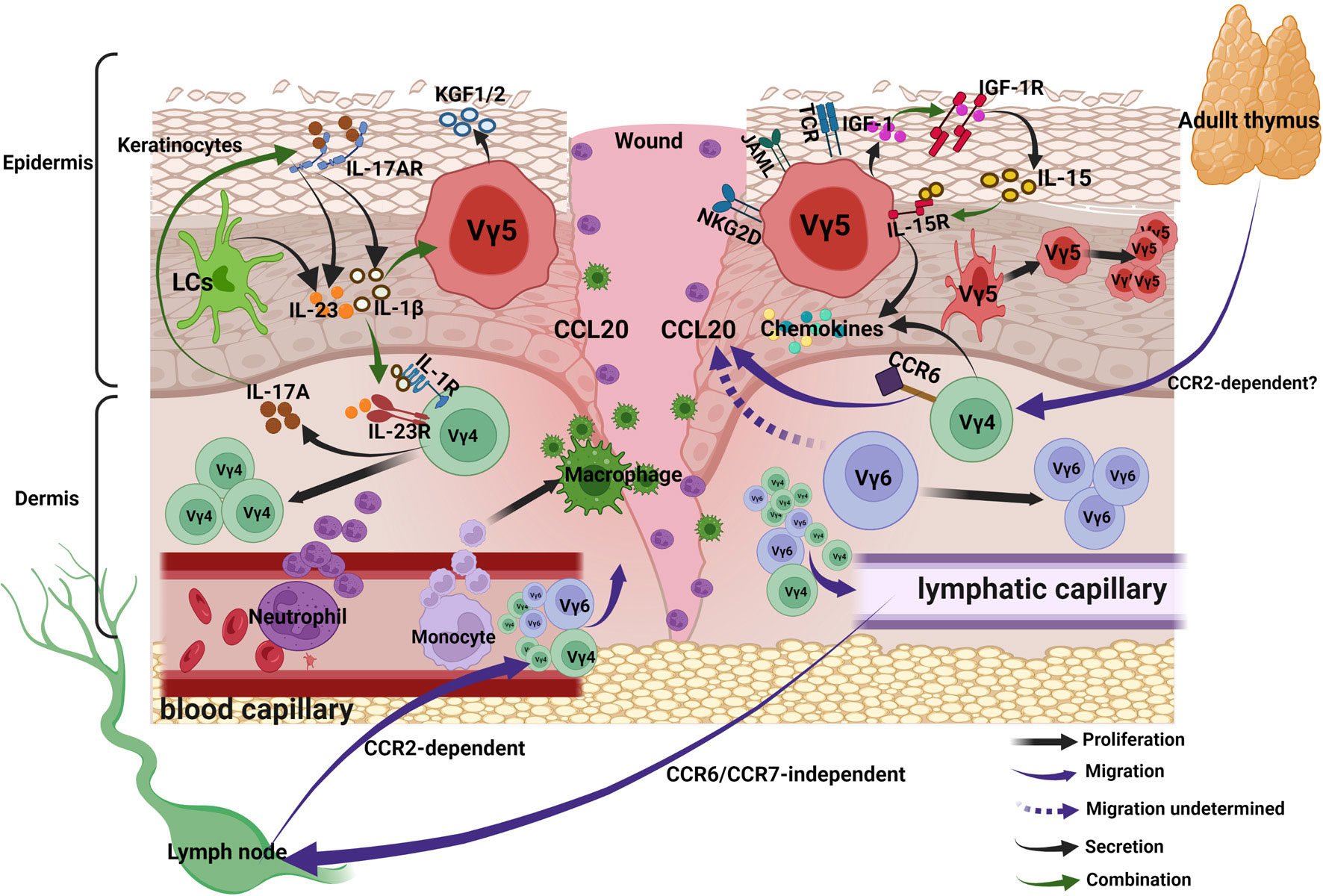
Figure 3 γδ T cells in acute wound healing. Upon activation, DETCs and Vγ4 T cells secrete chemokines to recruit neutrophils and macrophages into lesion site. Activated Vγ4 T cells migrate to epidermis via CCR6-CCL20 pathway, in addition, the traffic of Vγ4 and Vγ6 T subsets between skin and lymph nodes increases, the traffic from skin to lymph nodes is CCR6/CCR7-independent, while that from lymph nodes to skin is CCR2-dependent. Keratinocytes-derived IL-15 and DETCs-derived IGF-1 forms a positive feedback loop and promotes re-epithelialization. The positive feedback loop between wound-derived IL-1β/IL-23 and Vγ4-derived IL-17 can amplify the local inflammation, the IL-1β/IL-23 suppresses IGF-1 production of DETCs.
γδ T Cells in Acute Wound Healing
The skin, the largest organ by surface area is susceptible to injury in shielding our internal tissues from microbial infection, temperature variation, radiation and mechanical damage (136). Recognizing the mechanism underlining the wound healing is valuable for regulating the healing effectiveness. Theoretically, both cells residing in skin and cells capable of trafficking to the skin as the keratinocytes, neutrophils, macrophages, T lymphocytes, mast cells, dendritic cells, endothelial cells, fibroblasts, myofibroblasts and epidermal stem cells, can influence the healing result (137–139). To observe their functions, a great number of surgically constructed models of skin injury in rodents have been established. In particular, murine models are used most often. It is well-established that appropriate inflammation and vigorous re-epithelization are crucial in wound healing, immune cells are essential in constructing inflammatory microenvironment and regulating re-epithelization (140). γδT cells as the major immune cells of skin, we sought to discuss their significant functions, and the related mechanism in wound healing below.
Recruitment of Inflammatory Cells
Efficient Infiltration of inflammatory cells including neutrophils and macrophages are crucial for wound repair. Neutrophils are usually recruited as “first responders” from the bone marrow in response to “find me” signals on the day following injury, they clean debris and bacteria to provide a good environment for wound healing, as well as to modulate inflammation by producing ROS, chemokines (CXCL2, CXCL8) and MCP-1 (monocyte chemoattractant protein 1), different cytokines (IL-6, IL-1β, IL-10) (141). The accumulation of macrophages is usually seen within the 24-48 h at the site of injury, and their local accumulation actively participates in all stages of wound healing, including facilitating phagocytosis of bacteria and damage tissue, determining the duration of inflammation and promoting keratinocyte migration and ECM synthesis (142). Studies have confirmed that depletion, deletion, or excessive infiltration of these cells can result in delayed wound healing, keloids or hypertrophic scars (137, 143–146). γδ T cells participate in the recruitment of inflammatory cells in skin wounding. γδ TCR-deficient (δTCR-/-) C57 male mice exhibit reduction in the cellular infiltration upon injury, including macrophages, αβ T lymphocytes, neutrophils (104, 147, 148). Activated γδ T cells, including DETCs and Vγ4 T cells express CCL-3 (MIP-1α), CCL-4 (MIP-1β), CCL5 (Rantes), MCP-1, and XCL1 (lymphocyte chemokines), IL-17, which induce the migration of inflammatory cells (19, 106, 149–152). In addition, they indirectly affect cells infiltration via regulating other cells, such as DETCs-induced hyaluronan production by epithelial cells increases the migration of macrophages (153).
Wound-Derived IL-1β/IL-23 and Vγ4-Derived IL-17 Loop for Inflammatory Responses
As the first line of defense, keratinocytes can recognize ligand by pattern-recognition receptors (PRRs) (154), which lead to the subsequent activation of distinct signaling pathways and the production of different cytokines and chemokines (138). TLR (Toll-like receptor) activation is a critical element in initiating and amplifying inflammation after skin injury, including TLR-1, -2, -3, -4, -5, -6, and -9, which are upregulated in wounds (155), The activation of keratinocytes increases the production of IL-1β, IL-23, IL-15, IL-1α, TNF-α, IL-8, CCL2 (156). Together with the IL-1β produced by platelets, neutrophils and macrophages (157, 158), as well as the IL-23 produced by LCs and DCs (159), the IL-23 and IL-1β induce the resident and infiltrated Vγ4 T cells secreting IL-17A (160, 161), which can bind with the up-regulated IL-17RA expressed on the keratinocytes. The binding enhances the production of epidermal IL-1β and IL-23 (130). Thus, this process creates a positive feedback that the IL-1β/IL-23-IL-17 loop amplifies local inflammation after skin injury. IL-17A, mainly produced by the immune cells, including γδT cells and Th17 cells, is required for efficient skin wound healing. IL-17a-/- mice exhibit defects in wound repair (3); however, Rodero et al. reported that blocking IL-17A with an IL-17A-neutralizing antibody significantly promotes skin wound repair (162). To reconcile this conflicting result, Li et al. confirmed that different IL-17A levels play a distinct role in wound healing; both low and excessive levels of IL-17A have a negative impact on skin wound repair, while a moderate level of IL-17A is required for efficient skin wound healing (130). They concluded that Vγ4-derived IL-17A indirectly delayed the wound healing through upregulating of IL-1β and IL-23 by keratinocytes, which inhibits IGF-1 production by DETCs through NF-κB signal pathway (130). However, the underlining reason of different levels of IL-17A leading to variant effectiveness was not distinctly explicated in their study.
As we all know, IL-17A participates in inflammation through different pathways (163), we propose that the IL-17A—IL-1β/IL-23—IGF pathway impedes wound healing; whereas the IL-17A—β-defensin3/S100A8/Reg3γ/AMP (3, 164) and other pathways [through driving the production of VEGF by epithelial and fibroblastic cells to stimulate angiogenesis (165, 166)] promote wound healing. Under an excessive expression, the impeding pathway is markedly activated; therefore, IL-17A hinders the wound repair. Similarly, in the IL-17A-depleted mice, the promoting pathway is severely retarded, thus the wound healing is delayed. However, under a moderate expression, the promoting pathway is noticeably activated, IL-17A hence accelerates wound healing. It is worthy to explore these related molecular mechanisms for the details.
Moreover, we deliberate that these dual roles coexist at the same time, depending on the concentration gradient between the central injury tissue and the surrounding wounding tissue, reminiscent of the oxygen gradient in the wounding site (167). Moderate accumulation of IL-17A in the peripheries is beneficial for wound closure; while excessive accumulation of IL-17A at the excessive level in the center of injury leads to delayed repair, which leaves adequate time for inflammatory cells to create a good repair microenvironment. This process confirms the sequential order in repair, from the bottoms up and from the peripheries to the center (168). Further research is needed to justify this inference.
DETCs-Derived IGF-1 and KGF-1-2 for Re-Epithelialization
During homeostasis, DETCs constitutively generate IGF-1, which binds to IGF-1R (IGF-1 receptor) expressed on “keratinocytes and DETCs” and triggers phosphoinositide 3-kinase and mitogen-activated protein kinase pathways to prevent them from apoptosis (98, 169). Meanwhile, keratinocytes secrete IL-15, which helps the survival and proliferation of DETCs (170). Upon injury, the production of IL-15 is upregulated by activated keratinocytes and Langerhans cells (170, 171), increased IL-15 enhances the IGF-1 production of DETCs through binding to their IL-15R (IL-15 receptor). The up-regulated IGF-1 causes an increase in phosphorylated IGF-1R levels at wound margins 24 h after injury (98). This in addition protects keratinocytes from apoptosis in damaged areas (98), also directly stimulates keratinocytes to produce more IL-15, partly through the mTOR-dependent pathway (172). This positive feedback loop of keratinocytes-derived IL-15 and DETCs-derived IGF-1 contributes to the significant accumulation of IGF-1, which exhibits a significant function in promoting re-epithelialization. Impaired epidermal to DETCs signaling slows wound repair (173), and it has been found that the insufficient activation of DETCs upon injury leads to abnormal wound healing in diabetic mice, the insufficient activation partly attributes to the impaired production of IGF-1. Exogenous supplement of IL-15 can rescue the defective IGF-1 expression (93). Whether there is another feedback loop between DETCs and other cells such as LCs, or other signaling deeply involved in the regulation of IL-15 expression is still unknown.
In addition to IGF-1, activated DETCs aid in skin repair by secreting KGF within 24 hours of injury, including KGF-1 and KGF-2 (174). However, they don’t secrete KGFs under homeostasis (129). When binding to the KGF receptor (KGFR) expressed on keratinocytes, KGF accelerates the migration and proliferation of keratinocytes by activating the downstream signaling pathways, including mTOR, ERK-MAPK, P13K/Akt (87, 96). KGF plays a commendable function in regulating keratinocytes, but since DETCs do not express KGFR, no positive feedback loop has been identified.
Taken together, upon activation, DETCs and Vγ4 T cells secrete chemokines to recruit neutrophils and macrophages into lesion site. Keratinocytes-derived IL-15 and DETCs-derived IGF-1 forms a positive feedback loop and promotes re-epithelialization. The positive feedback loop between wound-derived IL-1β/IL-23 and Vγ4-derived IL-17 can amplify the local inflammation, whereas the IL-1β/IL-23 suppresses IGF-1 production of DETCs (Figure 3).
γδ T Cells in Chronic Wound Healing
Common features of chronic non-healing wounds include repeated infection, tissue necrosis, continuous exudation, defective re-epithelization, reduced angiogenesis and overproduction of ROS (175, 176). They are usually observed in elderly people suffering from pathological conditions, like obesity, diabetes mellitus and vascular disease (177). Chronic wound healing is characterized by the prolonged presence of myeloid cell populations, such as macrophages, neutrophils and monocytes. In the late stage of inflammation (137), incessantly activated γδ T cells participate in the chronic wound healing through inducing persistent inflammatory microenvironment via the main pathways mentioned above. For re-epithelialization, the robust activation of EPSCs (Epidermal stem cells) and efficient recruitment of their progeny towards an epidermal lineage are crucial, a stage which facilitates the re-establishment of an intact keratinocyte layer during wound healing (178, 179). For this process, the balance of proliferation of pluripotent EPSCs and their differentiation into terminally differentiated cells are pivotal (Figure 4A) (168, 180). In chronic or refractory wound, persistent inflammatory condition leads to excessive proliferation and differentiation, with the sacrifice of subsequent loss of the stem cell reservoir (181–183) and the balance is broken (Figure 4B). Supplementing sufficient EPSCs for restoring balance is the effective method to accelerate the wound healing (184–186). Our previous study found that DETCs-derived IGF-1 promotes the proliferation of EPSCs (187), while the IGF-1 secretion is regulated by Vγ4-derived IL-17A (130). So, we therefore hypothesize that the γδ T cells participate in regulating the differentiation and proliferation balance of EPSCs in refractory wound, the potential mechanism seems to be the continuous secretion of IL-17A by Vdifleads sustained inflammation which promotes the excessive differentiation, while suppresses the level of IGF-1 produced by DETCs beneficial for the proliferation of EPSCs (Figure 4C). Further research needs to be conducted in this regard.
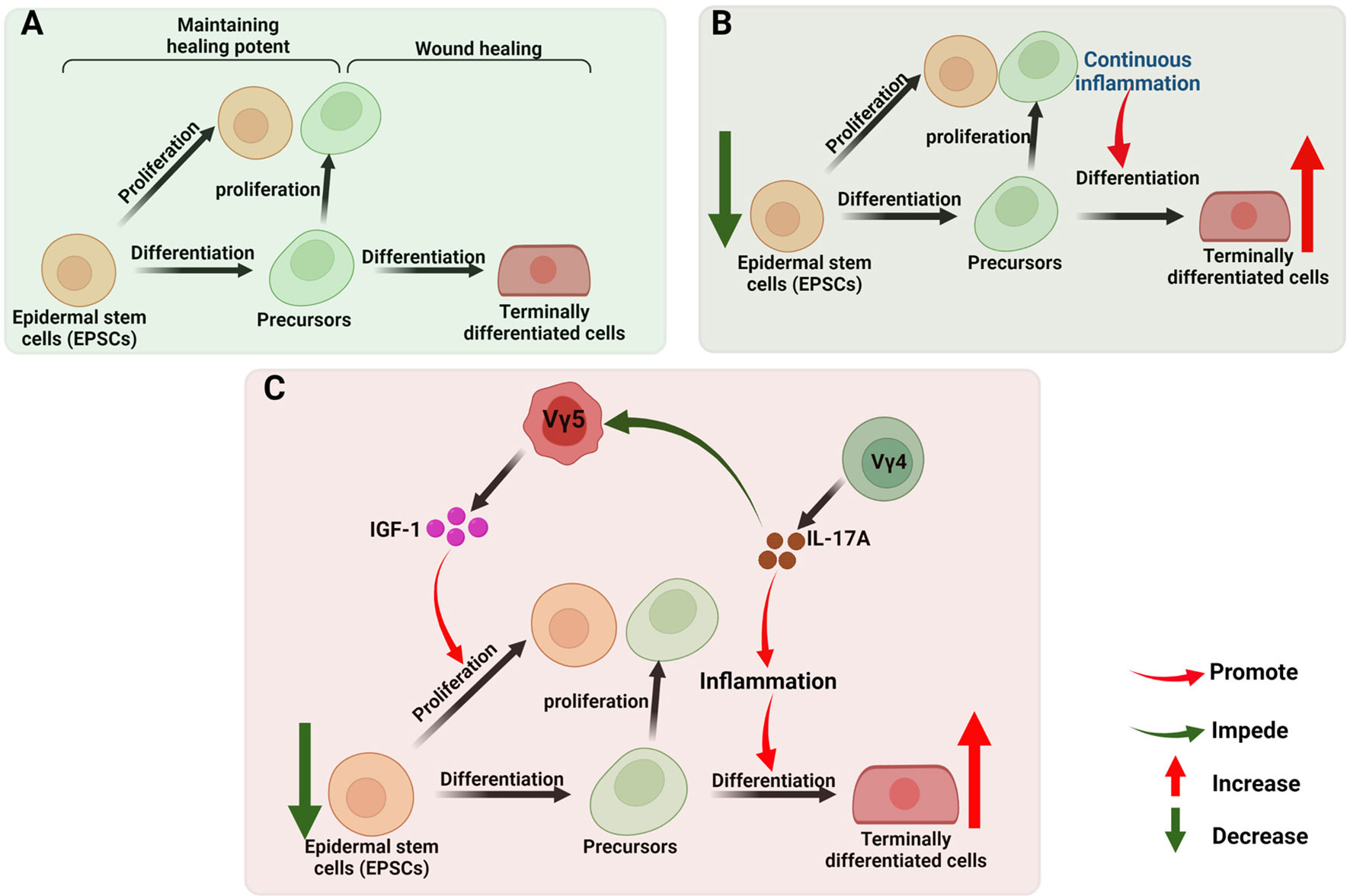
Figure 4 γδ T cells in chronic wound healing. (A) The robust activation of EPSCs and efficient recruitment of their progeny towards an epidermal lineage are crucial in the re-establishment of an intact keratinocyte layer during wound healing. The balance of proliferation of pluripotent EPSCs (maintaining healing potent) and their differentiation into terminally differentiated cells (wound healing) are pivotal; (B) In chronic or refractory wound, persistent inflammatory condition leads to excessive proliferation and differentiation, with the sacrifice of subsequent loss of the stem cell reservoir. (C) In chronic or refractory wound, continuous secretion of IL-17A by Vγ4 leads sustained inflammation which promotes the excessive differentiation, while suppresses the level of IGF-1 produced by DETCs beneficial for the proliferation of EPSCs, this inference is worthy to be tested.
Collectively, the differentiation and proliferation balance of EPSCs is crucial in wound healing, disordered immune microenvironment constructed by lymphcytes will break this balance in chronic and refractory wound. Given that the isolation and ex vivo expansion of various γδ T cell subsets is feasible (188), upon the molecular and cellular interations between γδ T cells and EPSCs being elucidated, precisely supplementing or clearing certain γδ T cell subsets, cytokines or chemokines in local will be an effective method to restore balanced microenvironment, which is expected to improve the effectiveness of clinical treatments for refractory wounds.
Role of γδ T Cells in Other Skin Diseases
Fibrosis is essential for wound healing and tissue repair, which is characterized by the accumulation of extracellular matrix (ECM) components mainly produced by myofibroblasts. T lymphocytes, macrophages and other inflammation cells cooperatively regulate fibrotic process (189).
Studies have found γδ T cells play critical roles in fibrosis and fibrotic diseases of many tissues, including hepatic, lung, kidney and heart. IL-17/IL-22 producing γδ T cells can protect the liver from excessive fibrosis via inducing HSCs (hepatic stellate cells) apoptosis (190). Besides, IFNγ-producing γδ T cells also show protective effect in liver fibrosis, these cells have direct cytotoxicity against activated HSCs (191). For lung, Vγ6Vδ1 γδ T cells protect it from pulmonary fibrosis by secreting IL-22 (192). However, some researches demonstrate γδ T cells accumulation tends to promote fibrosis, IL-17-producing γδ T cells induces myofibroblast activation and ECM deposition in kidney injury model and myocardial infarction model of mice (193, 194). So, it is more likely that their function in regulating fibrosis is tissue-specific.
Up to now, researches related to the γδ T cells in skin fibrosis is inadequate, Ohtsuka found the human skin fibroblasts stimulated by γδ T cells supernatant showed elevated proliferation and collagen synthesis (195), another study demonstrated the activated γδ T cells in systemic sclerosis (SSc) play an important role on fibrosis (196). In addition, Meyer demonstrated epidermal γδ T cells induces profibrotic response of fibroblasts via mice in chronic inflammation, this phenotype of mice lacking fibroblast growth factor bears continuous inflammatory response (197). Recently, Shook (198) found CD301b-expressing macrophages activated the proliferation of wound bed adipocyte precursors (APs) through IGF-1, these Aps become fibrotic after injury. DETCs secreted sufficient IGF-1 upon skin injury, whether they can play equivalent effect deserves further study.
For immune-mediated skin diseases, psoriasis, atopic dermatitis (AD) and contact dermatitis (CD) are all chronic and prevalent (15). The prevalence of psoriasis is about 2% to 3% (199), γδ17 T cells have been proved to be critical in imiquimod- (IMQ) or IL-23-induced psoriasis of mice, both Vγ6 and Vγ4 are clearly pathogenic in these models (131), memory-like dermal Vγ4 γδ17 T cells accumulated in inflamed skin and peripheral lymph nodes lead to faster and stronger responses upon secondary challenge (82). STAT 3 and STAT 4 facilitate the complete effector functions of γδ17 T cells (200). PD-1 and CD109 exert protective role in psoriasis (201, 202), while LAT1 and CD69 exert opposite function (203). In humans, patients with psoriasis also display increased accumulation of γδ T cells (Vγ9Vδ2) in the skin, effective therapy can decrease the numbers, indicating their role in the disease (204). AD is a T cell-mediated chronic skin disease, affecting up to 20% of children worldwide, its onset is associated with skin barrier dysfunction and immune disorder (205), it is characterized by highly expanded dermal αβ T cells which produce IL-17 and IL-22 (206), patients suffered from AD also present decreased proportion of γδ T cells (207). However, children with AD display higher frequency of Vγ9Vδ2 T cells (208). So the specific role and underlined mechanism of γδ T cells in AD is worthy to investigate. CD is the most frequent immune-mediated skin disease, its prevalence is about 95%, which is caused by chemical and allergens (209). The role of DETCs in CD is controversial (15), IL-17 secreted by Vn CD is controversialsed by chemicalproinflammatory role (106), however, their respective role in CD needs to be evaluated in depth.
Discussion and Conclusion
γδ T cells are important components of the skin immune system and DETCs(Vγ5), Vγ4 and Vγ6 T cells are their major subsets. DETCs are particularly generated in the embryonic thymus and implanted in the epidermis where they maintain a homeostatic population by themselves. Vγ4 T cells appearing in the late fetal stage can be generated in the adult thymus, and they possess the recirculating characteristic which can be refilled by newly generated Vγ4 cells from thymus and pLN. Vγ6 T cells are generated solely in the thymic second wave around embryonic day E14 (up to the birth), and they mainly display tissue residency, but retain circulating capability, whether they can be refilled by circulating cells is uncertain. The development and differentiation of γδ T cells are regulated by both TCRγδ-dependent and TCRγδ-independent factor. The combined effect of various factors leads to the differentiation of γδ T cells. Their functional development is accomplished step by step as follows: T cell commitment–αβ/γδ lineage commitment–γδ subset commitment–effector commitment.
Under homeostasis, γδT cells participate in maintaining skin integrity with the help of paracrine and autocrine factors, traffiking between tissues and lymph nodes of Vγ4 and Vγ6 T cells at a slow rate in the steady state which plays an important role in immune surveillance. Besides, these cells are radioresistant, for mice receiving lethal irradiation, 100% of DETCs (V0%+) remained of host origin, while 90% of Vγ5-γδ T cells in dermal remained host-derived (104). Upon injury or inflammation, antigens including MHC-like recognition antigens, IG-like recognition of antigen, Phosphoantigen or B7 receptor family-like proteins are upregulated. The binding of these antigens with the γδTCR and co-stimulatory receptors helps in the complete activation of γδT cells. Initially, activated γδT cells secrete chemokines to recruit the inflammatory cells, including neutrophils and macrophages etc. Subsequently, they secrete IGF-1, KGF-1/KGF-2, IL-17 to regulate inflammation and re-epithelialization. Injury provide an opportunity for microorganisms to enter into the wound tissues, including microorganisms constituting the skin microbiota and residing in the environment.
It is noteworthy to mention that the positive feedback loop of DETCs-derived IGF-1 and keratinocytes-derived IL-15 leads to the accumulation of IGF-1 in wound bed, on one hand, it protects keratinocytes and epidermal γδ T cells from apoptosis, on the other hand, it exhibits a significant function in promoting re-epithelialization, γδ T cells in the epidermal of both mice and humans show equivalent function. In the dermal, the wound-derived IL-1β/IL-23 and Vγ4-derived IL-17 feedback loop can amplify the local inflammation. IL-17A participates in regulating wound healing by either promoting pathway (like the IL-17A—IL-1β/IL-23—IGF pathway) or impeding pathway (like the IL-17A—β-defensin3/S100A8/Reg3γ/AMP pathway). Different doses affect each pathway to different degrees, both low and excessive levels of IL-17A have a negative impact on skin wound repair, while a moderate level of IL-17A is required for efficient skin wound healing, suggesting that IL-17A plays a varied role in wound healing. For chronic and refractory wounds, they provide a lot of opportunities for microorganisms to enter into the wound tissues (210), including commensal microbiota residing in the skin and microorganisms existed in the environment, pathogenic interaction of microorganisms with the skin cells will induce pathogenic immune response (177, 211). In this process, abnormal accumulated γδ T cells or their disordered function contribute to unbalanced immune microenvironment, which breaks the differentiation and proliferation balance of EPSCs, restoring balanced microenvironment is expected to improve the effectiveness of clinical treatments for refractory wounds. Further research needs to be conducted in this regard.
In addition, γδ T cells play critical roles in fibrosis and fibrotic diseases of many tissues, their protective or deleterious function in fibrosis is more likely tissue-specific. Up to now, researches related to the γδ T cells in skin fibrosis is inadequate, investigating their role in keloids and hypertrophic scars forming is valuable. For immune-mediated skin diseases, both Vγ6 and Vγ4 are clearly pathogenic in imiquimod-induced psoriasis, their function in atopic dermatitis and contact dermatitis needs to be evaluated in depth.
Author Contributions
WGH and RS wrote the manuscript. JY and CC participated in the project discussion. ZL made some valuable suggestions about manuscript structure. GPL helped to design the manuscript structure and edited the language. WFH and GXL evaluated and reviewed manuscript structure, ideas and science. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by funds from the National Natural Sciences Foundation of China (No. 31872742 and 82172232 to WFH, No. 81630055 and No. 81920108022 to GXL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all members of WFH and GXL laboratories for valuable discussion. The authors would like to thank the full support of the core facilities at the Institute of Burn Research, Southwest Hospital, Third Military Medical University (Army Medical University).
References
1. Brenner MB, McLean J, Dialynas DP, Strominger JL, Smith JA, Owen FL, et al. Identification of a Putative Second T-Cell Receptor. Nature (1986) 322(6075):145–9. doi: 10.1038/322145a0
2. Raverdeau M, Cunningham SP, Harmon C, Lynch L. Gammadelta T Cells in Cancer: A Small Population of Lymphocytes With Big Implications. Clin Transl Immunol (2019) 8(10):e01080. doi: 10.1002/cti2.1080
3. MacLeod AS, Hemmers S, Garijo O, Chabod M, Mowen K, Witherden DA, et al. Dendritic Epidermal T Cells Regulate Skin Antimicrobial Barrier Function. J Clin Invest (2013) 123(10):4364–74. doi: 10.1172/JCI70064
4. Munoz LD, Sweeney MJ, Jameson JM. Skin Resident Gammadelta T Cell Function and Regulation in Wound Repair. Int J Mol Sci (2020) 21(23):9286. doi: 10.3390/ijms21239286
5. Shiromizu CM, Jancic CC. Gammadelta T Lymphocytes: An Effector Cell in Autoimmunity and Infection. Front Immunol (2018) 9:2389. doi: 10.3389/fimmu.2018.02389
6. Li Y, Huang Z, Yan R, Liu M, Bai Y, Liang G, et al. Vgamma4 Gammadelta T Cells Provide an Early Source of IL-17A and Accelerate Skin Graft Rejection. J Invest Dermatol (2017) 137(12):2513–22. doi: 10.1016/j.jid.2017.03.043
7. Lawetzky A, Tiefenthaler G, Kubo R, Hunig T. Identification and Characterization of Rat T Cell Subpopulations Expressing T Cell Receptors Alpha/Beta and Gamma/Delta. Eur J Immunol (1990) 20(2):343–9. doi: 10.1002/eji.1830200217
8. Paul S, Singh AK, Lal G. Phenotypic and Functional Plasticity of Gamma-Delta (Gammadelta) T Cells in Inflammation and Tolerance. Int Rev Immunol (2014) 33(6):537–58. doi: 10.3109/08830185.2013.863306
9. Chien YH, Meyer C, Bonneville M. Gammadelta T Cells: First Line of Defense and Beyond. Annu Rev Immunol (2014) 32:121–55. doi: 10.1146/annurev-immunol-032713-120216
10. Munoz-Ruiz M, Sumaria N, Pennington DJ, Silva-Santos B. Thymic Determinants of Gammadelta T Cell Differentiation. Trends Immunol (2017) 38(5):336–44. doi: 10.1016/j.it.2017.01.007
11. Garman RD, Doherty PJ, Raulet DH. Diversity, Rearrangement, and Expression of Murine T Cell Gamma Genes. Cell (1986) 45(5):733–42. doi: 10.1016/0092-8674(86)90787-7
12. Heilig JS, Tonegawa S. Diversity of Murine Gamma Genes and Expression in Fetal and Adult T Lymphocytes. Nature (1986) 322(6082):836–40. doi: 10.1038/322836a0
13. Xu Y, Dimitrion P, Cvetkovski S, Zhou L, Mi QS. Epidermal Resident Gammadelta T Cell Development and Function in Skin. Cell Mol Life Sci (2021) 78(2):573–80. doi: 10.1007/s00018-020-03613-9
14. Adams EJ, Gu S, Luoma AM. Human Gamma Delta T Cells: Evolution and Ligand Recognition. Cell Immunol (2015) 296(1):31–40. doi: 10.1016/j.cellimm.2015.04.008
15. Castillo-Gonzalez R, Cibrian D, Sanchez-Madrid F. Dissecting the Complexity of Gammadelta T-Cell Subsets in Skin Homeostasis, Inflammation, and Malignancy. J Allergy Clin Immunol (2021) 147(6):2030–42. doi: 10.1016/j.jaci.2020.11.023
16. Xiang J, Qiu M, Zhang H. Role of Dendritic Epidermal T Cells in Cutaneous Carcinoma. Front Immunol (2020) 11:1266. doi: 10.3389/fimmu.2020.01266
17. Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, et al. A Role for Human Skin-Resident T Cells in Wound Healing. J Exp Med (2009) 206(4):743–50. doi: 10.1084/jem.20081787
18. Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the Complexity of Gammadelta T-Cell Subsets in Mouse and Human. Immunology (2012) 136(3):283–90. doi: 10.1111/j.1365-2567.2012.03582.x
19. Giri S, Lal G. Differentiation and Functional Plasticity of Gamma-Delta (Gammadelta) T Cells Under Homeostatic and Disease Conditions. Mol Immunol (2021) 136:138–49. doi: 10.1016/j.molimm.2021.06.006
20. Reinke JM, Sorg H. Wound Repair and Regeneration. Eur Surg Res (2012) 49(1):35–43. doi: 10.1159/000339613
21. Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, et al. Delta-Like 4 Is the Essential, Nonredundant Ligand for Notch1 During Thymic T Cell Lineage Commitment. J Exp Med (2008) 205(11):2515–23. doi: 10.1084/jem.20080829
22. Kreslavsky T, Gleimer M, Garbe AI, von Boehmer H. Alphabeta Versus Gammadelta Fate Choice: Counting the T-Cell Lineages at the Branch Point. Immunol Rev (2010) 238(1):169–81. doi: 10.1111/j.1600-065X.2010.00947.x
23. Godfrey DI, Kennedy J, Suda T, Zlotnik A. A Developmental Pathway Involving Four Phenotypically and Functionally Distinct Subsets of CD3-CD4-CD8- Triple-Negative Adult Mouse Thymocytes Defined by CD44 and CD25 Expression. J Immunol (1993) 150(10):4244–52.
24. Fahl SP, Coffey F, Wiest DL. Origins of Gammadelta T Cell Effector Subsets: A Riddle Wrapped in an Enigma. J Immunol (2014) 193(9):4289–94. doi: 10.4049/jimmunol.1401813
25. Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zuniga-Pflucker JC. Stage-Specific and Differential Notch Dependency at the Alphabeta and Gammadelta T Lineage Bifurcation. Immunity (2006) 25(1):105–16. doi: 10.1016/j.immuni.2006.05.010
26. Kang J, Volkmann A, Raulet DH. Evidence That Gammadelta Versus Alphabeta T Cell Fate Determination Is Initiated Independently of T Cell Receptor Signaling. J Exp Med (2001) 193(6):689–98. doi: 10.1084/jem.193.6.689
27. Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, et al. Regulation of Gammadelta Versus Alphabeta T Lymphocyte Differentiation by the Transcription Factor SOX13. Science (2007) 315(5809):230–3. doi: 10.1126/science.1135344
28. Livak F, Tourigny M, Schatz DG, Petrie HT. Characterization of TCR Gene Rearrangements During Adult Murine T Cell Development. J Immunol (1999) 162(5):2575–80.
29. Pereira P, Boucontet L. Rates of Recombination and Chain Pair Biases Greatly Influence the Primary Gammadelta TCR Repertoire in the Thymus of Adult Mice. J Immunol (2004) 173(5):3261–70. doi: 10.4049/jimmunol.173.5.3261
30. Xiong N, Raulet DH. Development and Selection of Gammadelta T Cells. Immunol Rev (2007) 215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x
31. Ciofani M, Zuniga-Pflucker JC. Determining Gammadelta Versus Alphass T Cell Development. Nat Rev Immunol (2010) 10(9):657–63. doi: 10.1038/nri2820
32. Fahl SP, Kappes DJ, Wiest DL. TCR Signaling Circuits in Alphabeta/Gammadelta T Lineage Choice. In: Soboloff J, Kappes DJ, editors. Signaling Mechanisms Regulating T Cell Diversity and Function. Boca Raton FL: CRC Press/Taylor & Francis, (2018). p. 85–104.
33. Hayes SM, Li L, Love PE. TCR Signal Strength Influences Alphabeta/Gammadelta Lineage Fate. Immunity (2005) 22(5):583–93. doi: 10.1016/j.immuni.2005.03.014
34. Munoz-Ruiz M, Ribot JC, Grosso AR, Goncalves-Sousa N, Pamplona A, Pennington DJ, et al. TCR Signal Strength Controls Thymic Differentiation of Discrete Proinflammatory Gammadelta T Cell Subsets. Nat Immunol (2016) 17(6):721–7. doi: 10.1038/ni.3424
35. Turchinovich G, Hayday AC. Skint-1 Identifies a Common Molecular Mechanism for the Development of Interferon-Gamma-Secreting Versus Interleukin-17-Secreting Gammadelta T Cells. Immunity (2011) 35(1):59–68. doi: 10.1016/j.immuni.2011.04.018
36. Narayan K, Kang J. Molecular Events That Regulate Alphabeta Versus Gammadelta T Cell Lineage Commitment: Old Suspects, New Players and Different Game Plans. Curr Opin Immunol (2007) 19(2):169–75. doi: 10.1016/j.coi.2007.01.002
37. Terrence K, Pavlovich CP, Matechak EO, Fowlkes BJ. Premature Expression of T Cell Receptor (TCR)alphabeta Suppresses TCRgammadelta Gene Rearrangement But Permits Development of Gammadelta Lineage T Cells. J Exp Med (2000) 192(4):537–48. doi: 10.1084/jem.192.4.537
38. Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T Cell Receptor-Instructed Alphabeta Versus Gammadelta Lineage Commitment Revealed by Single-Cell Analysis. J Exp Med (2008) 205(5):1173–86. doi: 10.1084/jem.20072425
39. Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, et al. Attenuation of gammadeltaTCR Signaling Efficiently Diverts Thymocytes to the Alphabeta Lineage. Immunity (2005) 22(5):595–606. doi: 10.1016/j.immuni.2005.04.003
40. Carpenter AC, Bosselut R. Decision Checkpoints in the Thymus. Nat Immunol (2010) 11(8):666–73. doi: 10.1038/ni.1887
41. Starr TK, Jameson SC, Hogquist KA. Positive and Negative Selection of T Cells. Annu Rev Immunol (2003) 21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107
42. Coffey F, Lee SY, Buus TB, Lauritsen JP, Wong GW, Joachims ML, et al. The TCR Ligand-Inducible Expression of CD73 Marks Gammadelta Lineage Commitment and a Metastable Intermediate in Effector Specification. J Exp Med (2014) 211(2):329–43. doi: 10.1084/jem.20131540
43. Yao Y, Liu Q, Martin C, Yin C, Dong Z, Mi QS, et al. Embryonic Fate Mapping Uncovers the Critical Role of microRNAs in the Development of Epidermal Gammadelta T Cells. J Invest Dermatol (2018) 138(1):236–9. doi: 10.1016/j.jid.2017.08.023
44. Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, et al. Notch-Hes1 Pathway Is Required for the Development of IL-17-Producing Gammadelta T Cells. Blood (2011) 118(3):586–93. doi: 10.1182/blood-2011-02-334995
45. Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL-7) Selectively Promotes Mouse and Human IL-17-Producing Gammadelta Cells. Proc Natl Acad Sci USA (2012) 109(43):17549–54. doi: 10.1073/pnas.1204327109
46. Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, et al. STAT3 and NF-kappaB Signal Pathway Is Required for IL-23-Mediated IL-17 Production in Spontaneous Arthritis Animal Model IL-1 Receptor Antagonist-Deficient Mice. J Immunol (2006) 176(9):5652–61. doi: 10.4049/jimmunol.176.9.5652
47. Lu Y, Cao X, Zhang X, Kovalovsky D. PLZF Controls the Development of Fetal-Derived IL-17+Vgamma6+ Gammadelta T Cells. J Immunol (2015) 195(9):4273–81. doi: 10.4049/jimmunol.1500939
48. Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, et al. Development of Promyelocytic Zinc Finger and ThPOK-Expressing Innate Gamma Delta T Cells Is Controlled by Strength of TCR Signaling and Id3. J Immunol (2010) 184(3):1268–79. doi: 10.4049/jimmunol.0903218
49. Havran WL, Allison JP. Developmentally Ordered Appearance of Thymocytes Expressing Different T-Cell Antigen Receptors. Nature (1988) 335(6189):443–5. doi: 10.1038/335443a0
50. Havran WL, Allison JP. Origin of Thy-1+ Dendritic Epidermal Cells of Adult Mice From Fetal Thymic Precursors. Nature (1990) 344(6261):68–70. doi: 10.1038/344068a0
51. Kawai K, Kishihara K, Molina TJ, Wallace VA, Mak TW, Ohashi PS. Impaired Development of V Gamma 3 Dendritic Epidermal T Cells in P56lck Protein Tyrosine Kinase-Deficient and CD45 Protein Tyrosine Phosphatase-Deficient Mice. J Exp Med (1995) 181(1):345–9. doi: 10.1084/jem.181.1.345
52. Mallick-Wood CA, Pao W, Cheng AM, Lewis JM, Kulkarni S, Bolen JB, et al. Disruption of Epithelial Gamma Delta T Cell Repertoires by Mutation of the Syk Tyrosine Kinase. Proc Natl Acad Sci USA (1996) 93(18):9704–9. doi: 10.1073/pnas.93.18.9704
53. Kadlecek TA, van Oers NS, Lefrancois L, Olson S, Finlay D, Chu DH, et al. Differential Requirements for ZAP-70 in TCR Signaling and T Cell Development. J Immunol (1998) 161(9):4688–94.
54. Witherden DA, Havran WL. Molecular Aspects of Epithelial Gammadelta T Cell Regulation. Trends Immunol (2011) 32(6):265–71. doi: 10.1016/j.it.2011.03.005
55. Kang J, DiBenedetto B, Narayan K, Zhao H, Der SD, Chambers CA. STAT5 Is Required for Thymopoiesis in a Development Stage-Specific Manner. J Immunol (2004) 173(4):2307–14. doi: 10.4049/jimmunol.173.4.2307
56. Woolf E, Brenner O, Goldenberg D, Levanon D, Groner Y. Runx3 Regulates Dendritic Epidermal T Cell Development. Dev Biol (2007) 303(2):703–14. doi: 10.1016/j.ydbio.2006.12.005
57. Salim M, Knowles TJ, Hart R, Mohammed F, Woodward MJ, Willcox CR, et al. Characterization of a Putative Receptor Binding Surface on Skint-1, a Critical Determinant of Dendritic Epidermal T Cell Selection. J Biol Chem (2016) 291(17):9310–21. doi: 10.1074/jbc.M116.722066
58. Andreotti AH, Schwartzberg PL, Joseph RE, Berg LJ. T-Cell Signaling Regulated by the Tec Family Kinase, Itk. Cold Spring Harb Perspect Biol (2010) 2(7):a002287. doi: 10.1101/cshperspect.a002287
59. Xia M, Qi Q, Jin Y, Wiest DL, August A, Xiong N. Differential Roles of IL-2-Inducible T Cell Kinase-Mediated TCR Signals in Tissue-Specific Localization and Maintenance of Skin Intraepithelial T Cells. J Immunol (2010) 184(12):6807–14. doi: 10.4049/jimmunol.1000453
60. Xiong N, Kang C, Raulet DH. Positive Selection of Dendritic Epidermal Gammadelta T Cell Precursors in the Fetal Thymus Determines Expression of Skin-Homing Receptors. Immunity (2004) 21(1):121–31. doi: 10.1016/j.immuni.2004.06.008
61. Lahl K, Sweere J, Pan J, Butcher E. Orphan Chemoattractant Receptor GPR15 Mediates Dendritic Epidermal T-Cell Recruitment to the Skin. Eur J Immunol (2014) 44(9):2577–81. doi: 10.1002/eji.201444628
62. Schon MP, Schon M, Parker CM, Williams IR. Dendritic Epidermal T Cells (DETC) Are Diminished in Integrin Alphae(CD103)-Deficient Mice. J Invest Dermatol (2002) 119(1):190–3. doi: 10.1046/j.1523-1747.2002.17973.x
63. Jiang X, Campbell JJ, Kupper TS. Embryonic Trafficking of Gammadelta T Cells to Skin Is Dependent on E/P Selectin Ligands and CCR4. Proc Natl Acad Sci USA (2010) 107(16):7443–8. doi: 10.1073/pnas.0912943107
64. Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 Is Important for the Development of Skin-Specific gammadeltaT Cells by Regulating Their Migration and Location. J Immunol (2010) 185(10):5723–31. doi: 10.4049/jimmunol.1001612
65. Parker ME, Ciofani M. Regulation of Gammadelta T Cell Effector Diversification in the Thymus. Front Immunol (2020) 11:42. doi: 10.3389/fimmu.2020.00042
66. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 Is a Thymic Determinant of the Balance Between Interferon-Gamma- and Interleukin 17-Producing Gammadelta T Cell Subsets. Nat Immunol (2009) 10(4):427–36. doi: 10.1038/ni.1717
67. Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic Selection Determines Gammadelta T Cell Effector Fate: Antigen-Naive Cells Make Interleukin-17 and Antigen-Experienced Cells Make Interferon Gamma. Immunity (2008) 29(1):90–100. doi: 10.1016/j.immuni.2008.04.022
68. Malhotra N, Narayan K, Cho OH, Sylvia KE, Yin C, Melichar H, et al. A Network of High-Mobility Group Box Transcription Factors Programs Innate Interleukin-17 Production. Immunity (2013) 38(4):681–93. doi: 10.1016/j.immuni.2013.01.010
69. Germar K, Dose M, Konstantinou T, Zhang J, Wang H, Lobry C, et al. T-Cell Factor 1 Is a Gatekeeper for T-Cell Specification in Response to Notch Signaling. Proc Natl Acad Sci USA (2011) 108(50):20060–5. doi: 10.1073/pnas.1110230108
70. Weber BN, Chi AW, Chavez A, Yashiro-Ohtani Y, Yang Q, Shestova O, et al. A Critical Role for TCF-1 in T-Lineage Specification and Differentiation. Nature (2011) 476(7358):63–8. doi: 10.1038/nature10279
71. Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, et al. Cutting Edge: Spontaneous Development of IL-17-Producing Gamma Delta T Cells in the Thymus Occurs via a TGF-Beta 1-Dependent Mechanism. J Immunol (2010) 184(4):1675–9. doi: 10.4049/jimmunol.0903539
72. Laird RM, Laky K, Hayes SM. Unexpected Role for the B Cell-Specific Src Family Kinase B Lymphoid Kinase in the Development of IL-17-Producing Gammadelta T Cells. J Immunol (2010) 185(11):6518–27. doi: 10.4049/jimmunol.1002766
73. McKenzie DR, Kara EE, Bastow CR, Tyllis TS, Fenix KA, Gregor CE, et al. IL-17-Producing Gammadelta T Cells Switch Migratory Patterns Between Resting and Activated States. Nat Commun (2017) 8:15632. doi: 10.1038/ncomms15632
74. Cai Y, Xue F, Fleming C, Yang J, Ding C, Ma Y, et al. Differential Developmental Requirement and Peripheral Regulation for Dermal Vgamma4 and Vgamma6T17 Cells in Health and Inflammation. Nat Commun (2014) 5:3986. doi: 10.1038/ncomms4986
75. Khairallah C, Chu TH, Sheridan BS. Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front Immunol (2018) 9:2636. doi: 10.3389/fimmu.2018.02636
76. Barros-Martins J, Schmolka N, Fontinha D, Pires de Miranda M, Simas JP, Brok I, et al. Effector Gammadelta T Cell Differentiation Relies on Master But Not Auxiliary Th Cell Transcription Factors. J Immunol (2016) 196(9):3642–52. doi: 10.4049/jimmunol.1501921
77. Sumaria N, Grandjean CL, Silva-Santos B, Pennington DJ. Strong TCRgammadelta Signaling Prohibits Thymic Development of IL-17a-Secreting Gammadelta T Cells. Cell Rep (2017) 19(12):2469–76. doi: 10.1016/j.celrep.2017.05.071
78. Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, et al. CCR6 and NK1.1 Distinguish Between IL-17A and IFN-Gamma-Producing Gammadelta Effector T Cells. Eur J Immunol (2009) 39(12):3488–97. doi: 10.1002/eji.200939922
79. Tan L, Sandrock I, Odak I, Aizenbud Y, Wilharm A, Barros-Martins J, et al. Single-Cell Transcriptomics Identifies the Adaptation of Scart1(+) Vgamma6(+) T Cells to Skin Residency as Activated Effector Cells. Cell Rep (2019) 27(12):3657–3671 e3654. doi: 10.1016/j.celrep.2019.05.064
80. Fitzpatrick S, Lausch R, Barrington RA. CCR6-Positive Gammadelta T Cells Provide Protection Against Intracorneal HSV-1 Infection. Invest Ophthalmol Vis Sci (2019) 60(12):3952–62. doi: 10.1167/iovs.19-27810
81. Papotto PH, Goncalves-Sousa N, Schmolka N, Iseppon A, Mensurado S, Stockinger B, et al. IL-23 Drives Differentiation of Peripheral Gammadelta17 T Cells From Adult Bone Marrow-Derived Precursors. EMBO Rep (2017) 18(11):1957–67. doi: 10.15252/embr.201744200
82. Ramirez-Valle F, Gray EE, Cyster JG. Inflammation Induces Dermal Vgamma4+ Gammadeltat17 Memory-Like Cells That Travel to Distant Skin and Accelerate Secondary IL-17-Driven Responses. Proc Natl Acad Sci USA (2015) 112(26):8046–51. doi: 10.1073/pnas.1508990112
83. Park K, He X, Lee HO, Hua X, Li Y, Wiest D, et al. TCR-Mediated ThPOK Induction Promotes Development of Mature (CD24-) Gammadelta Thymocytes. EMBO J (2010) 29(14):2329–41. doi: 10.1038/emboj.2010.113
84. Wilharm A, Tabib Y, Nassar M, Reinhardt A, Mizraji G, Sandrock I, et al. Mutual Interplay Between IL-17-Producing gammadeltaT Cells and Microbiota Orchestrates Oral Mucosal Homeostasis. Proc Natl Acad Sci USA (2019) 116(7):2652–61. doi: 10.1073/pnas.1818812116
85. Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdorfer L, et al. Interleukin-23-Dependent Gamma/Delta T Cells Produce Interleukin-17 and Accumulate in the Enthesis, Aortic Valve, and Ciliary Body in Mice. Arthritis Rheumatol (2016) 68(10):2476–86. doi: 10.1002/art.39732
86. Wilharm A, Brigas HC, Sandrock I, Ribeiro M, Amado T, Reinhardt A, et al. Microbiota-Dependent Expansion of Testicular IL-17-Producing Vgamma6(+) Gammadelta T Cells Upon Puberty Promotes Local Tissue Immune Surveillance. Mucosal Immunol (2021) 14(1):242–52. doi: 10.1038/s41385-020-0330-6
87. Gray EE, Suzuki K, Cyster JG. Cutting Edge: Identification of a Motile IL-17-Producing Gammadelta T Cell Population in the Dermis. J Immunol (2011) 186(11):6091–5. doi: 10.4049/jimmunol.1100427
88. Gentek R, Ghigo C, Hoeffel G, Jorquera A, Msallam R, Wienert S, et al. Epidermal Gammadelta T Cells Originate From Yolk Sac Hematopoiesis and Clonally Self-Renew in the Adult. J Exp Med (2018) 215(12):2994–3005. doi: 10.1084/jem.20181206
89. Fuchs E. Epithelial Skin Biology: Three Decades of Developmental Biology, a Hundred Questions Answered and a Thousand New Ones to Address. Curr Top Dev Biol (2016) 116:357–74. doi: 10.1016/bs.ctdb.2015.11.033
90. Nielsen MM, Witherden DA, Havran WL. Gammadelta T Cells in Homeostasis and Host Defence of Epithelial Barrier Tissues. Nat Rev Immunol (2017) 17(12):733–45. doi: 10.1038/nri.2017.101
91. Zhang B, Wu J, Jiao Y, Bock C, Dai M, Chen B, et al. Differential Requirements of TCR Signaling in Homeostatic Maintenance and Function of Dendritic Epidermal T Cells. J Immunol (2015) 195(9):4282–91. doi: 10.4049/jimmunol.1501220
92. Kadow S, Jux B, Zahner SP, Wingerath B, Chmill S, Clausen BE, et al. Aryl Hydrocarbon Receptor Is Critical for Homeostasis of Invariant Gammadelta T Cells in the Murine Epidermis. J Immunol (2011) 187(6):3104–10. doi: 10.4049/jimmunol.1100912
93. Li Y, Wu J, Luo G, He W. Functions of Vgamma4 T Cells and Dendritic Epidermal T Cells on Skin Wound Healing. Front Immunol (2018) 9:1099. doi: 10.3389/fimmu.2018.01099
94. Chodaczek G, Papanna V, Zal MA, Zal T. Body-Barrier Surveillance by Epidermal Gammadelta TCRs. Nat Immunol (2012) 13(3):272–82. doi: 10.1038/ni.2240
95. Komori HK, Witherden DA, Kelly R, Sendaydiego K, Jameson JM, Teyton L, et al. Cutting Edge: Dendritic Epidermal Gammadelta T Cell Ligands Are Rapidly and Locally Expressed by Keratinocytes Following Cutaneous Wounding. J Immunol (2012) 188(7):2972–6. doi: 10.4049/jimmunol.1100887
96. Chen C, Meng Z, Ren H, Zhao N, Shang R, He W, et al. The Molecular Mechanisms Supporting the Homeostasis and Activation of Dendritic Epidermal T Cell and Its Role in Promoting Wound Healing. Burns Trauma (2021) 9:tkab009. doi: 10.1093/burnst/tkab009
97. Dalessandri T, Crawford G, Hayes M, Castro Seoane R, Strid J. IL-13 From Intraepithelial Lymphocytes Regulates Tissue Homeostasis and Protects Against Carcinogenesis in the Skin. Nat Commun (2016) 7:12080. doi: 10.1038/ncomms12080
98. Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic Epidermal T Cells Regulate Skin Homeostasis Through Local Production of Insulin-Like Growth Factor 1. Nat Immunol (2005) 6(1):73–9. doi: 10.1038/ni1152
99. Moller P, Bohm M, Czarnetszki BM, Schadendorf D. Interleukin-7. Biology and Implications for Dermatology. Exp Dermatol (1996) 5(3):129–37. doi: 10.1111/j.1600-0625.1996.tb00107.x
100. Takashima A, Matsue H, Bergstresser PR, Ariizumi K. Interleukin-7-Dependent Interaction of Dendritic Epidermal T Cells With Keratinocytes. J Invest Dermatol (1995) 105(1 Suppl):50S–3S. doi: 10.1111/1523-1747.ep12315288
101. De Creus A, Van Beneden K, Stevenaert F, Debacker V, Plum J, Leclercq G. Developmental and Functional Defects of Thymic and Epidermal V Gamma 3 Cells in IL-15-Deficient and IFN Regulatory Factor-1-Deficient Mice. J Immunol (2002) 168(12):6486–93. doi: 10.4049/jimmunol.168.12.6486
102. Belkaid Y, Tamoutounour S. The Influence of Skin Microorganisms on Cutaneous Immunity. Nat Rev Immunol (2016) 16(6):353–66. doi: 10.1038/nri.2016.48
103. O’Brien RL, Born WK. Dermal Gammadelta T Cells–What Have We Learned? Cell Immunol (2015) 296(1):62–9. doi: 10.1016/j.cellimm.2015.01.011
104. Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, et al. Cutaneous Immunosurveillance by Self-Renewing Dermal Gammadelta T Cells. J Exp Med (2011) 208(3):505–18. doi: 10.1084/jem.20101824
105. Nakamizo S, Egawa G, Tomura M, Sakai S, Tsuchiya S, Kitoh A, et al. Dermal Vgamma4(+) Gammadelta T Cells Possess a Migratory Potency to the Draining Lymph Nodes and Modulate CD8(+) T-Cell Activity Through TNF-Alpha Production. J Invest Dermatol (2015) 135(4):1007–15. doi: 10.1038/jid.2014.516
106. Jiang X, Park CO, Geddes Sweeney J, Yoo MJ, Gaide O, Kupper TS. Dermal Gammadelta T Cells Do Not Freely Re-Circulate Out of Skin and Produce IL-17 to Promote Neutrophil Infiltration During Primary Contact Hypersensitivity. PloS One (2017) 12(1):e0169397. doi: 10.1371/journal.pone.0169397
107. Van Rhijn I, Rutten V, Charleston B, Smits M, van Eden W, Koets AP. Massive, Sustained Gammadelta T Cell Migration From the Bovine Skin In Vivo. J Leukoc Biol (2007) 81(4):968–73. doi: 10.1189/jlb.0506331
108. Zhang Y, Roth TL, Gray EE, Chen H, Rodda LB, Liang Y, et al. Migratory and Adhesive Cues Controlling Innate-Like Lymphocyte Surveillance of the Pathogen-Exposed Surface of the Lymph Node. Elife (2016) 5:18156. doi: 10.7554/eLife.18156
109. Gray EE, Ramirez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, et al. Deficiency in IL-17-Committed Vgamma4(+) Gammadelta T Cells in a Spontaneous Sox13-Mutant CD45.1(+) Congenic Mouse Substrain Provides Protection From Dermatitis. Nat Immunol (2013) 14(6):584–92. doi: 10.1038/ni.2585
110. Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial Lymphocytes: Exploring the Third Way in Immunology. Nat Immunol (2001) 2(11):997–1003. doi: 10.1038/ni1101-997
111. Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, et al. The Junctional Adhesion Molecule JAML Is a Costimulatory Receptor for Epithelial Gammadelta T Cell Activation. Science (2010) 329(5996):1205–10. doi: 10.1126/science.1192698
112. Shimura H, Nitahara A, Ito A, Tomiyama K, Ito M, Kawai K. Up-Regulation of Cell Surface Toll-Like Receptor 4-MD2 Expression on Dendritic Epidermal T Cells After the Emigration From Epidermis During Cutaneous Inflammation. J Dermatol Sci (2005) 37(2):101–10. doi: 10.1016/j.jdermsci.2004.11.006
113. Witherden DA, Watanabe M, Garijo O, Rieder SE, Sarkisyan G, Cronin SJ, et al. The CD100 Receptor Interacts With Its Plexin B2 Ligand to Regulate Epidermal Gammadelta T Cell Function. Immunity (2012) 37(2):314–25. doi: 10.1016/j.immuni.2012.05.026
114. Yoshida S, Mohamed RH, Kajikawa M, Koizumi J, Tanaka M, Fugo K, et al. Involvement of an NKG2D Ligand H60c in Epidermal Dendritic T Cell-Mediated Wound Repair. J Immunol (2012) 188(8):3972–9. doi: 10.4049/jimmunol.1102886
115. Deseke M, Prinz I. Ligand Recognition by the Gammadelta TCR and Discrimination Between Homeostasis and Stress Conditions. Cell Mol Immunol (2020) 17(9):914–24. doi: 10.1038/s41423-020-0503-y
116. Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, et al. A Population of Murine Gammadelta T Cells That Recognize an Inducible MHC Class Ib Molecule. Science (2000) 287(5451):314–6. doi: 10.1126/science.287.5451.314
117. Russano AM, Agea E, Corazzi L, Postle AD, De Libero G, Porcelli S, et al. Recognition of Pollen-Derived Phosphatidyl-Ethanolamine by Human CD1d-Restricted Gamma Delta T Cells. J Allergy Clin Immunol (2006) 117(5):1178–84. doi: 10.1016/j.jaci.2006.01.001
118. Le Nours J, Gherardin NA, Ramarathinam SH, Awad W, Wiede F, Gully BS, et al. A Class of Gammadelta T Cell Receptors Recognize the Underside of the Antigen-Presenting Molecule MR1. Science (2019) 366(6472):1522–7. doi: 10.1126/science.aav3900
119. Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S, et al. Sensing of Cell Stress by Human Gammadelta TCR-Dependent Recognition of Annexin A2. Proc Natl Acad Sci USA (2017) 114(12):3163–8. doi: 10.1073/pnas.1621052114
120. Silva-Santos B, Schamel WW, Fisch P, Eberl M. Gammadelta T-Cell Conference 2012: Close Encounters for the Fifth Time. Eur J Immunol (2012) 42(12):3101–5. doi: 10.1002/eji.201270101
121. Dai Y, Chen H, Mo C, Cui L, He W. Ectopically Expressed Human Tumor Biomarker MutS Homologue 2 Is a Novel Endogenous Ligand That Is Recognized by Human Gammadelta T Cells to Induce Innate Anti-Tumor/Virus Immunity. J Biol Chem (2012) 287(20):16812–9. doi: 10.1074/jbc.M111.327650
122. Born W, Hall L, Dallas A, Boymel J, Shinnick T, Young D, et al. Recognition of a Peptide Antigen by Heat Shock–Reactive Gamma Delta T Lymphocytes. Science (1990) 249(4964):67–9. doi: 10.1126/science.1695022
123. Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA, et al. Gammadelta T Cells Recognize a Microbial Encoded B Cell Antigen to Initiate a Rapid Antigen-Specific Interleukin-17 Response. Immunity (2012) 37(3):524–34. doi: 10.1016/j.immuni.2012.06.011
124. Liuzzi AR, McLaren JE, Price DA, Eberl M. Early Innate Responses to Pathogens: Pattern Recognition by Unconventional Human T-Cells. Curr Opin Immunol (2015) 36:31–7. doi: 10.1016/j.coi.2015.06.002
125. Vantourout P, Laing A, Woodward MJ, Zlatareva I, Apolonia L, Jones AW, et al. Heteromeric Interactions Regulate Butyrophilin (BTN) and BTN-Like Molecules Governing Gammadelta T Cell Biology. Proc Natl Acad Sci USA (2018) 115(5):1039–44. doi: 10.1073/pnas.1701237115
126. Riano F, Karunakaran MM, Starick L, Li J, Scholz CJ, Kunzmann V, et al. Vgamma9Vdelta2 TCR-Activation by Phosphorylated Antigens Requires Butyrophilin 3 A1 (BTN3A1) and Additional Genes on Human Chromosome 6. Eur J Immunol (2014) 44(9):2571–6. doi: 10.1002/eji.201444712
127. Schwacha MG, Rani M, Nicholson SE, Lewis AM, Holloway TL, Sordo S, et al. Dermal Gammadelta T-Cells Can Be Activated by Mitochondrial Damage-Associated Molecular Patterns. PloS One (2016) 11(7):e0158993. doi: 10.1371/journal.pone.0158993
128. Hayday AC. Gammadelta T Cells and the Lymphoid Stress-Surveillance Response. Immunity (2009) 31(2):184–96. doi: 10.1016/j.immuni.2009.08.006
129. Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, et al. A Role for Skin Gammadelta T Cells in Wound Repair. Science (2002) 296(5568):747–9. doi: 10.1126/science.1069639
130. Li Y, Wang Y, Zhou L, Liu M, Liang G, Yan R, et al. Vgamma4 T Cells Inhibit the Pro-Healing Functions of Dendritic Epidermal T Cells to Delay Skin Wound Closure Through IL-17a. Front Immunol (2018) 9:240. doi: 10.3389/fimmu.2018.00240
131. Mabuchi T, Singh TP, Takekoshi T, Jia GF, Wu X, Kao MC, et al. CCR6 Is Required for Epidermal Trafficking of Gammadelta-T Cells in an IL-23-Induced Model of Psoriasiform Dermatitis. J Invest Dermatol (2013) 133(1):164–71. doi: 10.1038/jid.2012.260
132. Anderson LS, Yu S, Rivara KR, Reynolds MB, Hernandez AA, Wu X, et al. CCR6(+) Gammadelta T Cells Home to Skin Wounds and Restore Normal Wound Healing in CCR6-Deficient Mice. J Invest Dermatol (2019) 139(9):2061–2064 e2062. doi: 10.1016/j.jid.2019.02.032
133. Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, Skorcheva I, et al. Th17 Cytokines Stimulate CCL20 Expression in Keratinocytes In Vitro and In Vivo: Implications for Psoriasis Pathogenesis. J Invest Dermatol (2009) 129(9):2175–83. doi: 10.1038/jid.2009.65
134. Maeda Y, Seki N, Kataoka H, Takemoto K, Utsumi H, Fukunari A, et al. IL-17-Producing Vgamma4+ Gammadelta T Cells Require Sphingosine 1-Phosphate Receptor 1 for Their Egress From the Lymph Nodes Under Homeostatic and Inflammatory Conditions. J Immunol (2015) 195(4):1408–16. doi: 10.4049/jimmunol.1500599
135. Akitsu A, Ishigame H, Kakuta S, Chung SH, Ikeda S, Shimizu K, et al. IL-1 Receptor Antagonist-Deficient Mice Develop Autoimmune Arthritis Due to Intrinsic Activation of IL-17-Producing CCR2(+)Vgamma6(+)gammadelta T Cells. Nat Commun (2015) 6:7464. doi: 10.1038/ncomms8464
136. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev (2019) 99(1):665–706. doi: 10.1152/physrev.00067.2017
137. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of Acute and Chronic Wound Healing. Biomolecules (2021) 11(5):700. doi: 10.3390/biom11050700
138. Piipponen M, Li D, Landen NX. The Immune Functions of Keratinocytes in Skin Wound Healing. Int J Mol Sci (2020) 21(22):8790. doi: 10.3390/ijms21228790
139. Xu X, Gu S, Huang X, Ren J, Gu Y, Wei C, et al. The Role of Macrophages in the Formation of Hypertrophic Scars and Keloids. Burns Trauma. (2020) 8:tkaa006. doi: 10.1093/burnst/tkaa006
140. Han CM, Cheng B, Wu P. Clinical Guideline on Topical Growth Factors for Skin Wounds. Burns Trauma (2020) 8:tkaa035. doi: 10.1093/burnst/tkaa035
141. Su Y, Richmond A. Chemokine Regulation of Neutrophil Infiltration of Skin Wounds. Adv Wound Care (New Rochelle). (2015) 4(11):631–40. doi: 10.1089/wound.2014.0559
142. Yanez DA, Lacher RK, Vidyarthi A, Colegio OR. The Role of Macrophages in Skin Homeostasis. Pflugers Arch (2017) 469(3-4):455–63. doi: 10.1007/s00424-017-1953-7
143. Nguyen KT, Seth AK, Hong SJ, et al. Deficient Cytokine Expression and Neutrophil Oxidative Burst Contribute to Impaired Cutaneous Wound Healing in Diabetic, Biofilm-Containing Chronic Wounds. Wound Repair Regen (2013) 21(6):833–41. doi: 10.1111/wrr.12109
144. Butzelaar L, Schooneman DP, Soykan EA, Talhout W, Ulrich MM, van den Broek LJ, et al. Inhibited Early Immunologic Response Is Associated With Hypertrophic Scarring. Exp Dermatol (2016) 25(10):797–804. doi: 10.1111/exd.13100
145. Kwon SH, Gurtner GC. Is Early Inflammation Good or Bad? Linking Early Immune Changes to Hypertrophic Scarring. Exp Dermatol (2017) 26(2):133–4. doi: 10.1111/exd.13167
146. Mirza R, DiPietro LA, Koh TJ. Selective and Specific Macrophage Ablation Is Detrimental to Wound Healing in Mice. Am J Pathol (2009) 175(6):2454–62. doi: 10.2353/ajpath.2009.090248
147. Daniel T, Thobe BM, Chaudry IH, Choudhry MA, Hubbard WJ, Schwacha MG. Regulation of the Postburn Wound Inflammatory Response by Gammadelta T-Cells. Shock (2007) 28(3):278–83. doi: 10.1097/shk.0b013e318034264c
148. Rani M, Zhang Q, Scherer MR, Cap AP, Schwacha MG. Activated Skin Gammadelta T-Cells Regulate T-Cell Infiltration of the Wound Site After Burn. Innate Immun (2015) 21(2):140–50. doi: 10.1177/1753425913519350
149. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte Chemoattractant Protein 1 Acts as a T-Lymphocyte Chemoattractant. Proc Natl Acad Sci USA (1994) 91(9):3652–6. doi: 10.1073/pnas.91.9.3652
150. Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. Chemokine Expression by Intraepithelial Gamma Delta T Cells. Implications for the Recruitment of Inflammatory Cells to Damaged Epithelia. J Immunol (1996) 157(3):985–92.
151. Dong P, Zhang S, Cai M, Kang N, Hu Y, Cui L, et al. Global Characterization of Differential Gene Expression Profiles in Mouse Vgamma1+ and Vgamma4+ Gammadelta T Cells. PloS One (2014) 9(11):e112964. doi: 10.1371/journal.pone.0112964
152. Hasegawa M, Higashi K, Matsushita T, Hamaguchi Y, Saito K, Fujimoto M, et al. Dermokine Inhibits ELR(+)CXC Chemokine Expression and Delays Early Skin Wound Healing. J Dermatol Sci (2013) 70(1):34–41. doi: 10.1016/j.jdermsci.2013.01.007
153. Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. Gammadelta T Cell-Induced Hyaluronan Production by Epithelial Cells Regulates Inflammation. J Exp Med (2005) 201(8):1269–79. doi: 10.1084/jem.20042057
154. Chen YE, Fischbach MA, Belkaid Y. Skin Microbiota-Host Interactions. Nature (2018) 553(7689):427–36. doi: 10.1038/nature25177
155. Miller LS, Modlin RL. Toll-Like Receptors in the Skin. Semin Immunopathol (2007) 29(1):15–26. doi: 10.1007/s00281-007-0061-8
156. Bodnar RJ. Chemokine Regulation of Angiogenesis During Wound Healing. Adv Wound Care (New Rochelle). (2015) 4(11):641–50. doi: 10.1089/wound.2014.0594
157. Ellis S, Lin EJ, Tartar D. Immunology of Wound Healing. Curr Dermatol Rep (2018) 7(4):350–8. doi: 10.1007/s13671-018-0234-9
158. Phillipson M, Kubes P. The Healing Power of Neutrophils. Trends Immunol (2019) 40(7):635–47. doi: 10.1016/j.it.2019.05.001
159. Li Z, Lamb R, Coles MC, Bennett CL, Ambler CA. Inducible Ablation of CD11c(+) Cells to Determine Their Role in Skin Wound Repair. Immunology (2021) 163(1):105–11. doi: 10.1111/imm.13312
160. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 Induce Innate IL-17 Production From Gammadelta T Cells, Amplifying Th17 Responses and Autoimmunity. Immunity (2009) 31(2):331–41. doi: 10.1016/j.immuni.2009.08.001
161. Sutton CE, Mielke LA, Mills KH. IL-17-Producing Gammadelta T Cells and Innate Lymphoid Cells. Eur J Immunol (2012) 42(9):2221–31. doi: 10.1002/eji.201242569
162. Rodero MP, Hodgson SS, Hollier B, Combadiere C, Khosrotehrani K. Reduced Il17a Expression Distinguishes a Ly6c(lo)MHCII(hi) Macrophage Population Promoting Wound Healing. J Invest Dermatol (2013) 133(3):783–92. doi: 10.1038/jid.2012.368
163. Blauvelt A, Chiricozzi A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin Rev Allergy Immunol (2018) 55(3):379–90. doi: 10.1007/s12016-018-8702-3
164. MacLeod AS, Mansbridge JN. The Innate Immune System in Acute and Chronic Wounds. Adv Wound Care (New Rochelle). (2016) 5(2):65–78. doi: 10.1089/wound.2014.0608
165. Liu L, Sun H, Wu S, Tan H, Sun Y, Liu X, et al. IL17A Promotes CXCR2dependent Angiogenesis in a Mouse Model of Liver Cancer. Mol Med Rep (2019) 20(2):1065–74. doi: 10.3892/mmr.2019.10310
166. Majumder S, McGeachy MJ. IL-17 in the Pathogenesis of Disease: Good Intentions Gone Awry. Annu Rev Immunol (2021) 39:537–56. doi: 10.1146/annurev-immunol-101819-092536
167. Younis I. Role of Oxygen in Wound Healing. J Wound Care (2020) 29(Sup5b):S4–S10. doi: 10.12968/jowc.2020.29.Sup5b.S4
168. Fujiwara H, Tsutsui K, Morita R. Multi-Tasking Epidermal Stem Cells: Beyond Epidermal Maintenance. Dev Growth Differ (2018) 60(9):531–41. doi: 10.1111/dgd.12577
169. Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal Homeostasis: The Role of the Growth Hormone and Insulin-Like Growth Factor Systems. Endocr Rev (2003) 24(6):737–64. doi: 10.1210/er.2002-0021
170. Edelbaum D, Mohamadzadeh M, Bergstresser PR, Sugamura K, Takashima A. Interleukin (IL)-15 Promotes the Growth of Murine Epidermal Gamma Delta T Cells by a Mechanism Involving the Beta- and Gamma C-Chains of the IL-2 Receptor. J Invest Dermatol (1995) 105(6):837–43. doi: 10.1111/1523-1747.ep12326630
171. Romano E, Rossi M, Ratzinger G, de Cos MA, Chung DJ, Panageas KS, et al. Peptide-Loaded Langerhans Cells, Despite Increased IL15 Secretion and T-Cell Activation In Vitro, Elicit Antitumor T-Cell Responses Comparable to Peptide-Loaded Monocyte-Derived Dendritic Cells In Vivo. Clin Cancer Res (2011) 17(7):1984–97. doi: 10.1158/1078-0432.CCR-10-3421
172. Liu Z, Liang G, Gui L, Li Y, Liu M, Bai Y, et al. Weakened IL-15 Production and Impaired mTOR Activation Alter Dendritic Epidermal T Cell Homeostasis in Diabetic Mice. Sci Rep (2017) 7(1):6028. doi: 10.1038/s41598-017-05950-5
173. Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, et al. Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell (2016) 167(5):1323–1338 e1314. doi: 10.1016/j.cell.2016.10.052
174. Werner S, Peters KG, Longaker MT, Fuller-Pace F, Banda MJ, Williams LT. Large Induction of Keratinocyte Growth Factor Expression in the Dermis During Wound Healing. Proc Natl Acad Sci USA (1992) 89(15):6896–900. doi: 10.1073/pnas.89.15.6896
175. Larouche J, Sheoran S, Maruyama K, Martino MM. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv Wound Care (New Rochelle). (2018) 7(7):209–31. doi: 10.1089/wound.2017.0761
176. Wang Z, Shi C. Cellular Senescence Is a Promising Target for Chronic Wounds: A Comprehensive Review. Burns Trauma (2020) 8:tkaa021. doi: 10.1093/burnst/tkaa021
177. Tomic-Canic M, Burgess JL, O’Neill KE, Strbo N, Pastar I. Skin Microbiota and Its Interplay With Wound Healing. Am J Clin Dermatol (2020) 21(Suppl 1):36–43. doi: 10.1007/s40257-020-00536-w
178. Yang R, Liu F, Wang J, Chen X, Xie J, Xiong K. Epidermal Stem Cells in Wound Healing and Their Clinical Applications. Stem Cell Res Ther (2019) 10(1):229. doi: 10.1186/s13287-019-1312-z
179. Garcin CL, Ansell DM. The Battle of the Bulge: Re-Evaluating Hair Follicle Stem Cells in Wound Repair. Exp Dermatol (2017) 26(2):101–4. doi: 10.1111/exd.13184
180. Xiao T, Yan Z, Xiao S, Xia Y. Proinflammatory Cytokines Regulate Epidermal Stem Cells in Wound Epithelialization. Stem Cell Res Ther (2020) 11(1):232. doi: 10.1186/s13287-020-01755-y
181. Stojadinovic O, Pastar I, Nusbaum AG, Vukelic S, Krzyzanowska A, Tomic-Canic M. Deregulation of Epidermal Stem Cell Niche Contributes to Pathogenesis of Nonhealing Venous Ulcers. Wound Repair Regen (2014) 22(2):220–7. doi: 10.1111/wrr.12142
182. Arnold I, Watt FM. C-Myc Activation in Transgenic Mouse Epidermis Results in Mobilization of Stem Cells and Differentiation of Their Progeny. Curr Biol (2001) 11(8):558–68. doi: 10.1016/S0960-9822(01)00154-3
183. Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated Expression of C-Myc Depletes Epidermal Stem Cells. Nat Genet (2001) 28(2):165–8. doi: 10.1038/88889
184. Bauer JW, Koller J, Murauer EM, De Rosa L, Enzo E, Carulli S, et al. Closure of a Large Chronic Wound Through Transplantation of Gene-Corrected Epidermal Stem Cells. J Invest Dermatol (2017) 137(3):778–81. doi: 10.1016/j.jid.2016.10.038
185. Jimenez F, Garde C, Poblet E, Jimeno B, Ortiz J, Martinez ML, et al. A Pilot Clinical Study of Hair Grafting in Chronic Leg Ulcers. Wound Repair Regen (2012) 20(6):806–14. doi: 10.1111/j.1524-475X.2012.00846.x
186. Li Z, Maitz P. Cell Therapy for Severe Burn Wound Healing. Burns Trauma. (2018) 6:13. doi: 10.1186/s41038-018-0117-0
187. Zhu HJ, Chen C, Zhang XR, Hu XH, Huang Y, Yang JC, et al. [Mechanism Study of Dendritic Epidermal T Lymphocytes in Promoting Healing of Full-Thickness Skin Defects Wound on Mice by Regulating the Proliferation and Differentiation of Epidermal Stem Cells in Mice]. Zhonghua Shao Shang Za Zhi (2020) 36(10):905–14. doi: 10.3760/cma.j.cn501120-20200623-00324
188. Kobayashi H, Tanaka Y. Gammadelta T Cell Immunotherapy-A Review. Pharm (Basel) (2015) 8(1):40–61. doi: 10.3390/ph8010040
189. Zhang M, Zhang S. T Cells in Fibrosis and Fibrotic Diseases. Front Immunol (2020) 11:1142. doi: 10.3389/fimmu.2020.01142
190. Hammerich L, Bangen JM, Govaere O, Zimmermann HW, Gassler N, Huss S, et al. Chemokine Receptor CCR6-Dependent Accumulation of Gammadelta T Cells in Injured Liver Restricts Hepatic Inflammation and Fibrosis. Hepatology (2014) 59(2):630–42. doi: 10.1002/hep.26697
191. Liu M, Hu Y, Yuan Y, Tian Z, Zhang C. gammadeltaT Cells Suppress Liver Fibrosis via Strong Cytolysis and Enhanced NK Cell-Mediated Cytotoxicity Against Hepatic Stellate Cells. Front Immunol (2019) 10:477. doi: 10.3389/fimmu.2019.00477
192. Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. Gammadelta T Cells Protect Against Lung Fibrosis via IL-22. J Exp Med (2010) 207(10):2239–53. doi: 10.1084/jem.20100061
193. Peng X, Xiao Z, Zhang J, Li Y, Dong Y, Du J. IL-17A Produced by Both Gammadelta T and Th17 Cells Promotes Renal Fibrosis via RANTES-Mediated Leukocyte Infiltration After Renal Obstruction. J Pathol (2015) 235(1):79–89. doi: 10.1002/path.4430
194. Yan X, Shichita T, Katsumata Y, Matsuhashi T, Ito H, Ito K, et al. Deleterious Effect of the IL-23/IL-17A Axis and gammadeltaT Cells on Left Ventricular Remodeling After Myocardial Infarction. J Am Heart Assoc (2012) 1(5):e004408. doi: 10.1161/JAHA.112.004408
195. Ohtsuka T. Effect of Gammadelta T Cell Supernatant on Human Skin Fibroblast Proliferation and Collagen Production–Possible Role of Transforming Growth Factor-Beta and Basic Fibroblast Growth Factor. Int J Dermatol (2008) 47(11):1135–40. doi: 10.1111/j.1365-4632.2008.03805.x
196. Ueda-Hayakawa I, Hasegawa M, Hamaguchi Y, Takehara K, Fujimoto M. Circulating Gamma/Delta T Cells in Systemic Sclerosis Exhibit Activated Phenotype and Enhance Gene Expression of Proalpha2(I) Collagen of Fibroblasts. J Dermatol Sci (2013) 69(1):54–60. doi: 10.1016/j.jdermsci.2012.10.003
197. Meyer M, Muller AK, Yang J, Sulcova J, Werner S. The Role of Chronic Inflammation in Cutaneous Fibrosis: Fibroblast Growth Factor Receptor Deficiency in Keratinocytes as an Example. J Investig Dermatol Symp Proc (2011) 15(1):48–52. doi: 10.1038/jidsymp.2011.1
198. Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, Lopez-Giraldez F, Dash BC, et al. Myofibroblast Proliferation and Heterogeneity Are Supported by Macrophages During Skin Repair. Science (2018) 362(6417):6417. doi: 10.1126/science.aar2971
199. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet (2021) 397(10281):1301–15. doi: 10.1016/S0140-6736(20)32549-6
200. Agerholm R, Rizk J, Vinals MT, Bekiaris V. STAT3 But Not STAT4 Is Critical for Gammadeltat17 Cell Responses and Skin Inflammation. EMBO Rep (2019) 20(11):e48647. doi: 10.15252/embr.201948647
201. Imai Y, Ayithan N, Wu X, Yuan Y, Wang L, Hwang ST. Cutting Edge: PD-1 Regulates Imiquimod-Induced Psoriasiform Dermatitis Through Inhibition of IL-17a Expression by Innate Gammadelta-Low T Cells. J Immunol (2015) 195(2):421–5. doi: 10.4049/jimmunol.1500448
202. Zhang H, Carnevale G, Polese B, Simard M, Thurairajah B, Khan N, et al. CD109 Restrains Activation of Cutaneous IL-17-Producing Gammadelta T Cells by Commensal Microbiota. Cell Rep (2019) 29(2):391–405 e395. doi: 10.1016/j.celrep.2019.09.003
203. Cibrian D, Saiz ML, de la Fuente H, Sanchez-Diaz R, Moreno-Gonzalo O, Jorge I, et al. CD69 Controls the Uptake of L-Tryptophan Through LAT1-CD98 and AhR-Dependent Secretion of IL-22 in Psoriasis. Nat Immunol (2016) 17(8):985–96. doi: 10.1038/ni.3504
204. Laggner U, Di Meglio P, Perera GK, Hundhausen C, Lacy KE, Ali N, et al. Identification of a Novel Proinflammatory Human Skin-Homing Vgamma9Vdelta2 T Cell Subset With a Potential Role in Psoriasis. J Immunol (2011) 187(5):2783–93. doi: 10.4049/jimmunol.1100804
205. Boguniewicz M, Leung DY. Atopic Dermatitis: A Disease of Altered Skin Barrier and Immune Dysregulation. Immunol Rev (2011) 242(1):233–46. doi: 10.1111/j.1600-065X.2011.01027.x
206. Spidale NA, Malhotra N, Frascoli M, Sylvia K, Miu B, Freeman C, et al. Neonatal-Derived IL-17 Producing Dermal Gammadelta T Cells Are Required to Prevent Spontaneous Atopic Dermatitis. Elife (2020) 9:51188. doi: 10.7554/eLife.51188
207. Schauer U, Dippel E, Gieler U, Brauer J, Jung T, Heymanns J, et al. T Cell Receptor Gamma Delta Bearing Cells Are Decreased in the Peripheral Blood of Patients With Atopic Diseases. Clin Exp Immunol (1991) 86(3):440–3. doi: 10.1111/j.1365-2249.1991.tb02950.x
208. Cairo C, Arabito E, Landi F, Casati A, Brunetti E, Mancino G, et al. Analysis of Circulating Gammadelta T Cells in Children Affected by IgE-Associated and Non-IgE-Associated Allergic Atopic Eczema/Dermatitis Syndrome. Clin Exp Immunol (2005) 141(1):116–21. doi: 10.1111/j.1365-2249.2005.02813.x
209. Bains SN, Nash P, Fonacier L. Irritant Contact Dermatitis. Clin Rev Allergy Immunol (2019) 56(1):99–109. doi: 10.1007/s12016-018-8713-0
210. Zeeuwen PL, Boekhorst J, van den Bogaard EH, de Koning HD, van de Kerkhof PM, Saulnier DM, et al. Microbiome Dynamics of Human Epidermis Following Skin Barrier Disruption. Genome Biol (2012) 13(11):R101. doi: 10.1186/gb-2012-13-11-r101
Keywords: γδT cells, wound healing, DETCs, Vγ4, Vγ6, homeostasis
Citation: Hu W, Shang R, Yang J, Chen C, Liu Z, Liang G, He W and Luo G (2022) Skin γδ T Cells and Their Function in Wound Healing. Front. Immunol. 13:875076. doi: 10.3389/fimmu.2022.875076
Received: 13 February 2022; Accepted: 21 March 2022;
Published: 11 April 2022.
Edited by:
Guangchao Cao, Jinan University, ChinaReviewed by:
Zhongyang Liu, Zhengzhou University, ChinaPhillip Scott, University of Pennsylvania, United States
Xiuli Wu, Jinan University, China
Copyright © 2022 Hu, Shang, Yang, Chen, Liu, Liang, He and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangping Liang, MTA3ODMyMDc4NEBxcS5jb20=; Weifeng He, d2hlNzYxMjExQGhvdG1haWwuY29t; Gaoxing Luo, bG9neHdAeWFob28uY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share corresponding authorship
 Wengang Hu
Wengang Hu Ruoyu Shang1,2†
Ruoyu Shang1,2† Weifeng He
Weifeng He Gaoxing Luo
Gaoxing Luo