- 1Department of Inflammation and Immunity, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, United States
- 2Department of Biological, Geological, and Environmental Sciences, Cleveland State University, Cleveland, OH, United States
Introduction
Interest in antibody-mediated injury to transplants was renewed with discovery that deposition of the complement split product C4d on capillaries of renal transplants was a marker of poor outcomes (1, 2). Subsequent adoption of C4d and circulating donor specific antibodies (DSA) as two criteria in the Banff classification for antibody-mediated rejection (AMR) strengthened the concept that complement was a major contributor to graft injury (3). Since then, several clinical tests have been introduced to assess complement activation by DSA (4–6). Similarly, therapeutic interventions targeting various components of the complement cascade have been evaluated for preventing ischemia reperfusion injury or treating antibody-mediated rejection (7). These approaches are based on voluminous evidence for the inflammatory effects of complement activation starting in 1895 with Jules Bordet’s discovery that serum components we now know as complement could cause lysis of bacteria (8). However, in the last 30 years evidence has accumulated that some complement components can prevent escalation of complement activation to inflammation. A prime example is C1q, a subcomponent of C1, the first component of the classical pathway of complement. Many clinical reports link deficiencies in C1q with increased inflammatory responses, autoantibodies and severe autoimmune disease resembling lupus (9, 10). Furthermore, experimental deletion of C1q in mice resulted in autoimmune phenotypes with increased autoantibody titers (11). Rejection of cardiac allografts was also accelerated in C1q deficient mice compared to normal controls (12). The accelerated rejection was associated with increased antibody titers in the circulation, IgG deposition in capillaries and neutrophil infiltrates in the cardiac allografts.
C1q Functions as Part of the C1 Complex and Independently as a Pattern Recognition Receptor
C1 is a complex of 3 subcomponents: C1q, C1r and C1s. When Lepow and colleagues first isolated these 3 subcomponents, they reported that all 3 were required for initiation of the classical cascade (13). C1q was found to bind to antibody-antigen complexes, whereas C1r and C1s provided the protease activity to cleave subsequent complement components. In this context, C1q binds to the Fc portion of antibody. C1q has 6 globular heads to engage antibodies and optimal binding to IgG occurs when 6 IgG antibodies are arrayed in a hexamer formation (14). C1r and C1s stabilize C1q binding to IgG in addition to cleaving the downstream complement components C4 and C2 (15).
However, C1q has critical functions independent of antibodies or C1r and C1s. The independent functions of C1q predate both antibodies or C1r and C1s in evolutionary time (16). In lampreys, a primitive vertebrate that lacks immunoglobulin, the orthologue of mammalian C1q binds N-acetylglucosamine (17). In mammals, C1q retains pattern recognition receptor (PRR) functions (18). Isolated C1q binds directly to apoptotic cells when phosphatidylserine, calreticulin, DNA and other molecules are exteriorized (19–21). This PRR function of C1q facilitates ingestion of apoptotic bodies by macrophages (22). Systemic C1q causes efficient clearance of apoptotic bodies in the spleen by marginal zone macrophages (23). Importantly, C1q opsonized apoptotic bodies polarize macrophages towards non-inflammatory profiles (24). More recent studies have demonstrated that C1q also inhibits maturation of monocytes into dendritic cells and metabolically regulates T cells (25).
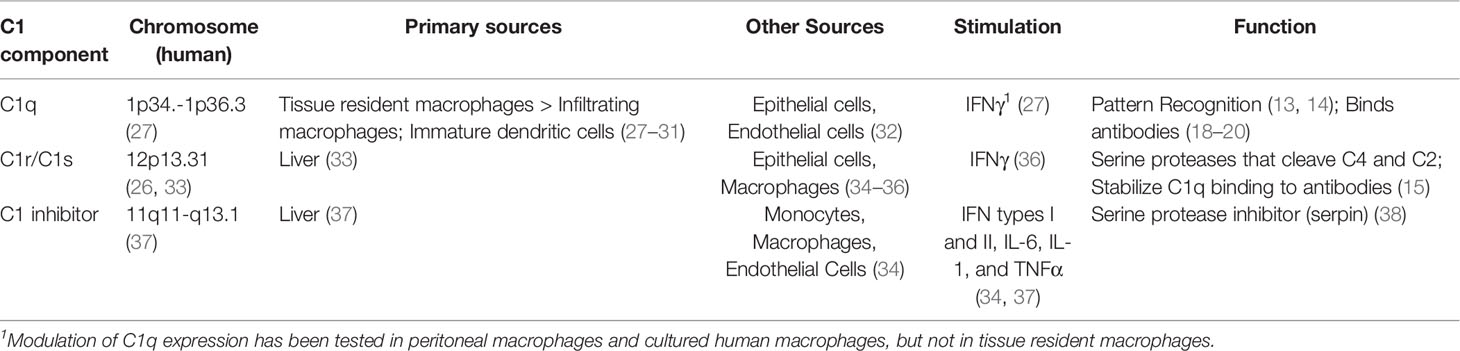
The disparate evolutionary origins and functions of C1q and the two C1 proteases are further underscored by the fact that these molecules are encoded by genes on different chromosomes (26, 27). The primary sources of C1q and the two C1 proteases are also different (Table 1). The majority of C1r and C1s is produced by hepatocytes (33), whereas C1q is primarily produced by macrophages and dendritic cells (34). Although macrophages can produce some C1r and C1s as well as C1q, early immunohistological studies suggested that C1q, C1r and C1s are frequently produced by different macrophages (35). This early observation is provocative in the context of new data about macrophage heterogeneity.
Resident Macrophages as Sources of C1q
Techniques for cell lineage tracking have distinguished macrophages that infiltrate inflamed tissues from resident macrophages (28), and single cell RNA sequencing has provided transcriptional signatures for different macrophage populations in quiescent and inflamed organs (28–30). Various functions have been proposed for tissue resident macrophages, among which is clearance of tissue debris (30, 39). In this context it is notable that C1q has been identified as a marker of resident macrophages in kidney, heart and lung (28–30, 40, 41). Less data has been reported for C1r and C1s expression, but Pinto, et al. found in contrast to C1q transcripts, C1r and C1s transcripts were expressed at low levels in resident macrophages of hearts (30). In the quiescent kidney, C1r is primarily expressed by epithelial cells of distal tubules rather than macrophages, and activation of complement and recruitment of macrophages to inflamed kidneys is decreased greatly in C1r knockout mice (36).
Effects of Transplantation on Resident Macrophages and C1q
Although upregulation of C1 expression has been reported in renal allo- and isografts in rats within hours after transplantation (42) as well as in biopsies from human renal transplants after perfusion is reestablished (43), the source of C1 and the kinetics of different subcomponents has not been established completely. In biopsies from a few renal transplants, Malone et al. (29) leveraged single nucleotide variation to demonstrate resident macrophages persisted up to 28 days after transplantation in non-rejecting human kidneys and these macrophages expressed high levels of C1q transcripts. In contrast, biopsies from grafts undergoing rejection contained only infiltrating macrophages that expressed low levels of C1q and high levels of CD68 and Fcγreceptor 3a. Similar findings have been reported for experimental heart allografts in mice (31). In this model, numbers of donor macrophages decreased within 14 days in allografts to mice without immunosuppression, but a subpopulation of donor macrophages, which was identified as CCR2-, persisted undiminished in recipients treated with immunosuppression. In contrast CCR2+ macrophages decreased regardless of immunosuppression. Donor CCR2- macrophages expressed higher levels of C1q than CCR2+ macrophages and only CCR2- macrophages were found to be essential for graft survival. Expression of C1r or C1s by these subpopulations of resident macrophages was not reported. Moreover, it has not been established whether C1q expression is modulated in resident macrophages after transplantation, although IFNγ has been demonstrated to stimulate C1q promoter activity in cultured macrophages derived from human blood (27). Similarly, IFNγ, IL-10 and dexamethasone increase expression of C1q by human monocytes isolated from blood (44), but the effects of these mediators on production of C1q and C1r or C1s by resident macrophages in tissues is not known.
Production of C1r by tubular epithelial cells is notable because expression of C1r and C1s transcipts have been reported to increase in human renal transplants during antibody or cell-mediated rejection (45, 46); particularly in conjunction with tubulitis (46). In a mouse model, C1q expression increases in proximal tubular epithelium during rejection (32). These data suggest that C1q is produced by subsets of resident macrophages while they persist in the graft, whereas both C1q and the C1 proteases are produced by tubular epithelial cells stimulated by IFNγ.
C1 Inhibitor Eliminates C1r and C1s but Preserves C1q Function
Another component that determines the balance of C1q functions is C1 inhibitor (C1inh). C1inh regulates complement activation by covalently binding C1r and C1s and removing these proteases from C1q. By removing C1r and C1s from the C1 complex, structural studies indicate that C1inh increases the flexibility of C1q and enhances the PRR function of C1q (18, 38). C1inh is primarily produced in the liver, but the production of C1inh by other cells including macrophages and endothelial cells can be increased by IFNγ (37). Little is known about the production of C1inh by resident or infiltrating macrophages following transplantation. However, transcripts for C1inh have been reported to increase during chronic antibody-mediated rejection (47).
Connecting C1q, Damaged Cells, Autoantibodies and Alloantibodies in Transplants
Most transplanted organs are retrieved from brain dead or non-heart beating donors and subjected to various periods of warm and cold ischemia. All these conditions increase injury to parenchymal cells and may alter resident macrophage functions (48). Experimental models have linked ischemic injury to increased inflammatory infiltrates and release of extracellular vesicles (49, 50). Although the immunogenicity of different subtypes of extracellular vesicles has not been fully established, proteomic assays have demonstrated some extracellular vesicles contain autoantigens and alloantigens (49, 51). At least some types of extracellular vesicles can deliver their antigenic content to antigen presenting cells (52). C1q could be a critical variable in determining the immune response to the antigenic content of extracellular vesicles that express phosphatidylserine and other potential ligands for C1q. In particular, B cell responses may be modulated by C1q as evidenced by increased auto- and alloantibody production in C1q deficient mice and humans (9–12). Even though antibodies to MHC antigens are more conclusively linked to graft injury and poor outcomes, there is increasing evidence that autoantibodies can contribute to graft damage (53). The range of autoantibodies implicated in graft injury include angiotensin II type I receptor, endothelin A, k-alpha tubulin, collagen V, vimentin and perlecan. Recently, antibody-mediated rejection has been associated with increases in antibodies associated with autoimmune diseases such as lupus, including IgG anti-Ro/Sjögren syndrome-antigen A (SS-A) and anti-major centromere autoantigen (CENP)-B (54).
Discussion
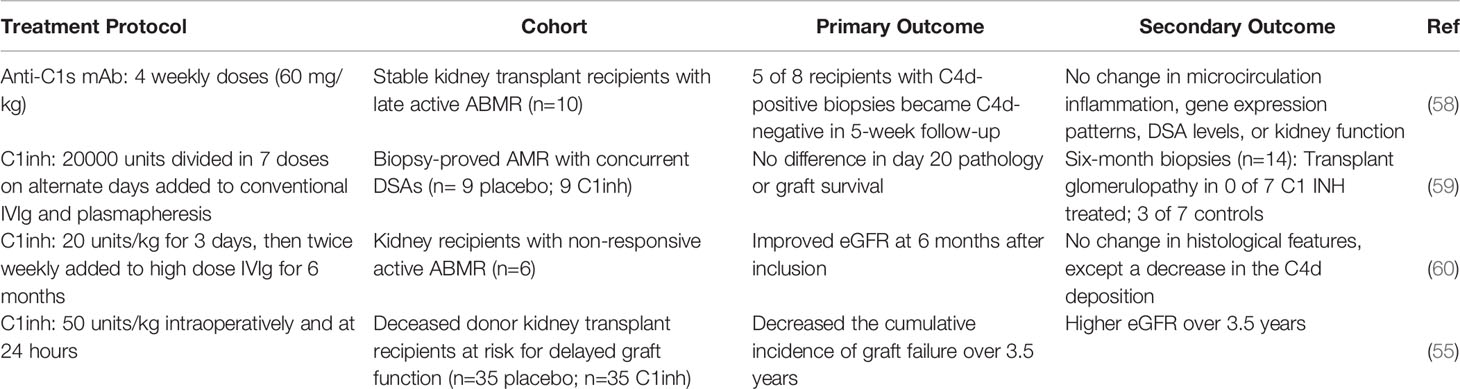
Based on current knowledge, it is plausible that C1q production by resident macrophages promotes non-inflammatory clearance of injured tissue from transplanted organs. In contrast, the complete C1 complex initiates the classical cascade of complement. This interpretation would support strategies to preserve or enhance C1q production while inhibiting the activity of C1r and C1s. Two such therapeutic interventions have been tested in transplant recipients. One is C1inh (55, 56) and the other is a monoclonal antibody to C1s (57, 58). Both biologics have been tested in patients experiencing antibody mediated rejection (Table 2). In general, this approach has decreased complement activation temporarily, but has little or no effect on long-term outcomes (58–60). Conversely, several experimental studies indicate that C1inh can be effective in decreasing injury due to ischemia-reperfusion (61–64). One extended clinical study included 70 recipients of deceased donor kidney transplants at risk for delayed graft function. C1inh (n=35) versus placebo (n=35) administered intraoperatively and at 24 hours resulted in decreased cumulative incidence of graft failure and higher eGFR over 3.5 years (55, 56). These clinical studies have been designed to prevent initiation of the complement cascade by C1, and the effects on the PRR function of C1q were not examined. With increasing application of ex vivo perfusion of organs before transplantation, enhancing or preserving C1q PRR functions should be considered. This could be accomplished by eliminating CCR2+ C1qlo resident macrophages, while preserving CCR2-C1qhi resident macrophages (31). Alternatively or additionally, biologically modified C1q could be added to the perfusate (65). Various mutant C1q molecules have been described that retain PRR function but lack binding sites for C1r or C1s (66). C1q is 460 kDa and can diffuse across endothelial barriers into the interstitial spaces. As a result, perfusates containing C1q would supplement C1q in the interstitium of organs. We propose that increasing C1q function locally would increase local and systemic clearance of apoptotic and necrotic cells from transplants and modulate sensitization (23, 30, 39). While abundant evidence indicates that C1q modulates the immune responses to autoantigens, little data is available regarding alloimmune responses. The one study of cardiac allografts in C1q deficient mice only investigated the effects of C1q deficiency in the recipient (12). Although absence of C1q in the recipient resulted in increased donor specific alloantibody titers, the recent data demonstrating resident macrophages are potent sources of C1q suggests that testing the effects of C1q deficiency in the donor would be informative especially in models where warm and cold ischemic times are extended to reflect the ischemic times incurred by clinical organ transplants retrieved from deceased donors (50, 67, 68).
In summary, while the function of C1q as part of the C1 complex that initiates the classical complement cascade has been extensively examined in the transplant field especially in the context of antibody-mediated rejection, greater appreciation of the anti-inflammatory functions of C1q could open novel approaches to limiting graft injury.
Author Contributions
All authors participated in reviewing the literature, writing and editing the manuscript. RK and HN performed experiments that led to the literature search and hypothesis. All authors approved the submitted version.
Funding
All authors are supported by grants NIH R01 AI165513 and PO1 AI087586 from the NIAID.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, et al. Capillary Deposition of C4d Complement Fragment and Early Renal Graft Loss. Kidney Int (1993) 43(6):1333–8. doi: 10.1038/ki.1993.187
2. Lederer SR, Schneeberger H, Albert E, Johnson JP, Gruber R, Land W, et al. Early Renal Graft Dysfunction. The Role of Preformed Antibodies to DR-Typed Lymphoblastoid Cell Lines. Transplantation (1996) 61(2):313–9. doi: 10.1097/00007890-199601270-00025
3. Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff ‘09 Meeting Report: Antibody Mediated Graft Deterioration and Implementation of Banff Working Groups. Am J Transplant (2010) 10(3):464–71. doi: 10.1111/j.1600-6143.2009.02987.x
4. Tambur AR, Herrera ND, Haarberg KM, Cusick MF, Gordon RA, Leventhal JR, et al. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transplant (2015) 15(9):2421–30. doi: 10.1111/ajt.13295
5. Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, et al. Detection of C3d-Binding Donor-Specific Anti-HLA Antibodies at Diagnosis of Humoral Rejection Predicts Renal Graft Loss. J Am Soc Nephrol (2015) 26(2):457–67. doi: 10.1681/ASN.2013101144
6. Bartel G, Wahrmann M, Schwaiger E, Kikic Z, Winzer C, Horl WH, et al. Solid Phase Detection of C4d-Fixing HLA Antibodies to Predict Rejection in High Immunological Risk Kidney Transplant Recipients. Transpl Int (2013) 26(2):121–30. doi: 10.1111/tri.12000
7. Tatapudi VS, Montgomery RA. Therapeutic Modulation of the Complement System in Kidney Transplantation: Clinical Indications and Emerging Drug Leads. Front Immunol (2019) 10:2306. doi: 10.3389/fimmu.2019.02306
8. Lachmann P. Complement Before Molecular Biology. Mol Immunol (2006) 43(6):496–508. doi: 10.1016/j.molimm.2005.04.005
9. Stegert M, Bock M, Trendelenburg M. Clinical Presentation of Human C1q Deficiency: How Much of a Lupus? Mol Immunol (2015) 67(1):3–11. doi: 10.1016/j.molimm.2015.03.007
10. van Schaarenburg RA, Schejbel L, Truedsson L, Topaloglu R, Al-Mayouf SM, Riordan A, et al. Marked Variability in Clinical Presentation and Outcome of Patients With C1q Immunodeficiency. J Autoimmun (2015) 62:39–44. doi: 10.1016/j.jaut.2015.06.002
11. Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, et al. Homozygous C1q Deficiency Causes Glomerulonephritis Associated With Multiple Apoptotic Bodies. Nat Genet (1998) 19(1):56–9. doi: 10.1038/ng0598-56
12. Csencsits K, Burrell BE, Lu G, Eichwald EJ, Stahl GL, Bishop DK. The Classical Complement Pathway in Transplantation: Unanticipated Protective Effects of C1q and Role in Inductive Antibody Therapy. Am J Transplant (2008) 8(8):1622–30. doi: 10.1111/j.1600-6143.2008.02295.x
13. Lepow IH, Naff GB, Todd EW, Pensky J, Hinz CF. Chromatographic Resolution of the First Component of Human Complement Into Three Activities. J Exp Med (1963) 117:983–1008. doi: 10.1084/jem.117.6.983
14. Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. Complement Is Activated by IgG Hexamers Assembled at the Cell Surface. Science (2014) 343(6176):1260–3. doi: 10.1126/science.1248943
15. Zwarthoff SA, Widmer K, Kuipers A, Strasser J, Ruyken M, Aerts PC, et al. C1q Binding to Surface-Bound IgG is Stabilized by C1r2s2 Proteases. Proc Natl Acad Sci USA (2021) 118(26):e2102787118. doi: 10.1073/pnas.2102787118
16. Endo Y, Takahashi M, Fujita T. Lectin Complement System and Pattern Recognition. Immunobiology (2006) 211(4):283–93. doi: 10.1016/j.imbio.2006.01.003
17. Matsushita M, Matsushita A, Endo Y, Nakata M, Kojima N, Mizuochi T, et al. Origin of the Classical Complement Pathway: Lamprey Orthologue of Mammalian C1q Acts as a Lectin. Proc Natl Acad Sci USA (2004) 101(27):10127–31. doi: 10.1073/pnas.0402180101
18. Gaboriaud C, Frachet P, Thielens NM, Arlaud GJ. The Human C1q Globular Domain: Structure and Recognition of Non-Immune Self Ligands. Front Immunol (2011) 2:92. doi: 10.3389/fimmu.2011.00092
19. Korb LC, Ahearn JM. C1q Binds Directly and Specifically to Surface Blebs of Apoptotic Human Keratinocytes: Complement Deficiency and Systemic Lupus Erythematosus Revisited. J Immunol (1997) 158(10):4525–8.
20. Paidassi H, Tacnet-Delorme P, Garlatti V, Darnault C, Ghebrehiwet B, Gaboriaud C, et al. C1q Binds Phosphatidylserine and Likely Acts as a Multiligand-Bridging Molecule in Apoptotic Cell Recognition. J Immunol (2008) 180(4):2329–38. doi: 10.4049/jimmunol.180.4.2329
21. Ye JJ, Bian X, Lim J, Medzhitov R. Adiponectin and Related C1q/TNF-Related Proteins Bind Selectively to Anionic Phospholipids and Sphingolipids. Proc Natl Acad Sci USA (2020) 117(29):17381–8. doi: 10.1073/pnas.1922270117
22. Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, et al. A Hierarchical Role for Classical Pathway Complement Proteins in the Clearance of Apoptotic Cells In Vivo. J Exp Med (2000) 192(3):359–66. doi: 10.1084/jem.192.3.359
23. Prabagar MG, Do Y, Ryu S, Park JY, Choi HJ, Choi WS, et al. SIGN-R1, a C-Type Lectin, Enhances Apoptotic Cell Clearance Through the Complement Deposition Pathway by Interacting With C1q in the Spleen. Cell Death Differ (2013) 20(4):535–45. doi: 10.1038/cdd.2012.160
24. Bohlson SS, O’Conner SD, Hulsebus HJ, Ho MM, Fraser DA. Complement, C1q, and C1q-Related Molecules Regulate Macrophage Polarization. Front Immunol (2014) 5:402. doi: 10.3389/fimmu.2014.00402
25. Ling GS, Crawford G, Buang N, Bartok I, Tian K, Thielens NM, et al. C1q Restrains Autoimmunity and Viral Infection by Regulating CD8(+) T Cell Metabolism. Science (2018) 360(6388):558–63. doi: 10.1126/science.aao4555
26. Arlaud GJ, Gaboriaud C, Garnier G, Circolo A, Thielens NM, Budayova-Spano M, et al. Structure, Function and Molecular Genetics of Human and Murine C1r. Immunobiology (2002) 205(4-5):365–82. doi: 10.1078/0171-2985-00139
27. Chen G, Tan CS, Teh BK, Lu J. Molecular Mechanisms for Synchronized Transcription of Three Complement C1q Subunit Genes in Dendritic Cells and Macrophages. J Biol Chem (2011) 286(40):34941–50. doi: 10.1074/jbc.M111.286427
28. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-Resident Macrophages Self-Maintain Locally Throughout Adult Life With Minimal Contribution From Circulating Monocytes. Immunity (2013) 38(4):792–804. doi: 10.1016/j.immuni.2013.04.004
29. Malone AF, Wu H, Fronick C, Fulton R, Gaut JP, Humphreys BD. Harnessing Expressed Single Nucleotide Variation and Single Cell RNA Sequencing To Define Immune Cell Chimerism in the Rejecting Kidney Transplant. J Am Soc Nephrol (2020) 31(9):1977–86. doi: 10.1681/ASN.2020030326
30. Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, et al. An Abundant Tissue Macrophage Population in the Adult Murine Heart With a Distinct Alternatively-Activated Macrophage Profile. PLoS One (2012) 7(5):e36814. doi: 10.1371/journal.pone.0036814
31. Kopecky BJ, Dun H, Amrute JM, Lin CY, Bredemeyer AL, Terada Y, et al. Donor Macrophages Modulate Rejection After Heart Transplantation. bioRxiv (2021). doi: 10.1101/2021.09.17.459296
32. Dangi A, Natesh NR, Husain I, Ji Z, Barisoni L, Kwun J, et al. Single Cell Transcriptomics of Mouse Kidney Transplants Reveals a Myeloid Cell Pathway for Transplant Rejection. JCI Insight (2020) 5(20):e141321. doi: 10.1172/jci.insight.141321
33. Kusumoto H, Hirosawa S, Salier JP, Hagen FS, Kurachi K. Human Genes for Complement Components C1r and C1s in a Close Tail-to-Tail Arrangement. Proc Natl Acad Sci USA (1988) 85(19):7307–11. doi: 10.1073/pnas.85.19.7307
34. Reboul A, Prandini MH, Bensa JC, Colomb MG. Characterization of C1q, C1s and C-1 Inh Synthesized by Stimulated Human Monocytes In Vitro. FEBS Lett (1985) 190(1):65–8. doi: 10.1016/0014-5793(85)80428-2
35. Loos M, Storz R, Muller W, Lemmel EM. Immunofluorescence Studies on the Subcomponents of the First Component of Complement (C1): Detection of C1q and C1s in Different Cells of Biopsy Material and on Human as Well as on Guinea Pig Peritoneal Macrophages. Immunobiology (1981) 158(3):213–24. doi: 10.1016/S0171-2985(81)80071-X
36. Xavier S, Sahu RK, Bontha SV, Mass V, Taylor RP, Megyesi J, et al. Complement C1r Serine Protease Contributes to Kidney Fibrosis. Am J Physiol Renal Physiol (2019) 317(5):F1293–304. doi: 10.1152/ajprenal.00357.2019
37. Prada AE, Zahedi K, Davis AE 3rd. Regulation of C1 Inhibitor Synthesis. Immunobiology (1998) 199(2):377–88. doi: 10.1016/S0171-2985(98)80042-9
38. Poon PH, Schumaker VN, Phillips ML, Strang CJ. Conformation and Restricted Segmental Flexibility of C1, the First Component of Human Complement. J Mol Biol (1983) 168(3):563–77. doi: 10.1016/S0022-2836(83)80302-7
39. Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM. Tissue-Resident Macrophages Are Locally Programmed for Silent Clearance of Apoptotic Cells. Immunity (2017) 47(5):913–27.e6. doi: 10.1016/j.immuni.2017.10.006
40. Conway BR, O’Sullivan ED, Cairns C, O’Sullivan J, Simpson DJ, Salzano A, et al. Kidney Single-Cell Atlas Reveals Myeloid Heterogeneity in Progression and Regression of Kidney Disease. J Am Soc Nephrol (2020) 31(12):2833–54. doi: 10.1681/ASN.2020060806
41. Puranik AS, Leaf IA, Jensen MA, Hedayat AF, Saad A, Kim KW, et al. Kidney-Resident Macrophages Promote a Proangiogenic Environment in the Normal and Chronically Ischemic Mouse Kidney. Sci Rep (2018) 8(1):13948. doi: 10.1038/s41598-018-31887-4
42. Nagano H, Nadeau KC, Takada M, Kusaka M, Tilney NL. Sequential Cellular and Molecular Kinetics in Acutely Rejecting Renal Allografts in Rats. Transplantation (1997) 63(8):1101–8. doi: 10.1097/00007890-199704270-00009
43. Naesens M, Li L, Ying L, Sansanwal P, Sigdel TK, Hsieh SC, et al. Expression of Complement Components Differs Between Kidney Allografts From Living and Deceased Donors. J Am Soc Nephrol (2009) 20(8):1839–51. doi: 10.1681/ASN.2008111145
44. Moosig F, Damm F, Knorr-Spahr A, Ritgen M, Zeuner RA, Kneba M, et al. Reduced Expression of C1q-mRNA in Monocytes From Patients With Systemic Lupus Erythematosus. Clin Exp Immunol (2006) 146(3):409–16. doi: 10.1111/j.1365-2249.2006.03225.x
45. Mueller FB, Yang H, Lubetzky M, Verma A, Lee JR, Dadhania DM, et al. Landscape of Innate Immune System Transcriptome and Acute T Cell-Mediated Rejection of Human Kidney Allografts. JCI Insight (2019) 4(13):e128014. doi: 10.1172/jci.insight.128014
46. Vonbrunn E, Ries T, Sollner S, Muller-Deile J, Buttner-Herold M, Amann K, et al. Multiplex Gene Analysis Reveals T-Cell and Antibody-Mediated Rejection-Specific Upregulation of Complement in Renal Transplants. Sci Rep (2021) 11(1):15464. doi: 10.1038/s41598-021-94954-3
47. Cernoch M, Hruba P, Kollar M, Mrazova P, Stranavova L, Lodererova A, et al. Intrarenal Complement System Transcripts in Chronic Antibody-Mediated Rejection and Recurrent IgA Nephropathy in Kidney Transplantation. Front Immunol (2018) 9:2310. doi: 10.3389/fimmu.2018.02310
48. Yue S, Zhou H, Wang X, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. Prolonged Ischemia Triggers Necrotic Depletion of Tissue-Resident Macrophages To Facilitate Inflammatory Immune Activation in Liver Ischemia Reperfusion Injury. J Immunol (2017) 198(9):3588–95. doi: 10.4049/jimmunol.1601428
49. Dieude M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, et al. The 20S Proteasome Core, Active Within Apoptotic Exosome-Like Vesicles, Induces Autoantibody Production and Accelerates Rejection. Sci Transl Med (2015) 7(318):318ra200. doi: 10.1126/scitranslmed.aac9816
50. Lee JY, Arumugarajah S, Lian D, Maehara N, Haig AR, Suri RS, et al. Recombinant Apoptosis Inhibitor of Macrophage Protein Reduces Delayed Graft Function in a Murine Model of Kidney Transplantation. PLoS One (2021) 16(4):e0249838. doi: 10.1371/journal.pone.0249838
51. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc Natl Acad Sci USA (2016) 113(8):E968–77. doi: 10.1073/pnas.1521230113
52. Zeng F, Chen Z, Chen R, Shufesky WJ, Bandyopadhyay M, Camirand G, et al. Graft-Derived Extracellular Vesicles Transported Across Subcapsular Sinus Macrophages Elicit B Cell Alloimmunity After Transplantation. Sci Transl Med (2021) 13(585):eabb0122. doi: 10.1126/scitranslmed.abb0122
53. Cardinal H, Dieude M, Hebert MJ. The Emerging Importance of Non-HLA Autoantibodies in Kidney Transplant Complications. J Am Soc Nephrol (2017) 28(2):400–6. doi: 10.1681/ASN.2016070756
54. Clotet-Freixas S, Kotlyar M, McEvoy CM, Pastrello C, Rodriguez-Ramirez S, Farkona S, et al. Increased Autoantibodies Against Ro/SS-A, CENP-B, and La/SS-B in Patients With Kidney Allograft Antibody-Mediated Rejection. Transplant Direct (2021) 7(10):e768. doi: 10.1097/TXD.0000000000001215
55. Huang E, Vo A, Choi J, Ammerman N, Lim K, Sethi S, et al. Three-Year Outcomes of a Randomized, Double-Blind, Placebo-Controlled Study Assessing Safety and Efficacy of C1 Esterase Inhibitor for Prevention of Delayed Graft Function in Deceased Donor Kidney Transplant Recipients. Clin J Am Soc Nephrol (2020) 15(1):109–16. doi: 10.2215/CJN.04840419
56. Jordan SC, Choi J, Aubert O, Haas M, Loupy A, Huang E, et al. A Phase I/II, Double-Blind, Placebo-Controlled Study Assessing Safety and Efficacy of C1 Esterase Inhibitor for Prevention of Delayed Graft Function in Deceased Donor Kidney Transplant Recipients. Am J Transplant (2018) 18(12):2955–64. doi: 10.1111/ajt.14767
57. Colonna L, Parry GC, Panicker S, Elkon KB. Uncoupling Complement C1s Activation From C1q Binding in Apoptotic Cell Phagocytosis and Immunosuppressive Capacity. Clin Immunol (2016) 163:84–90. doi: 10.1016/j.clim.2015.12.017
58. Eskandary F, Jilma B, Muhlbacher J, Wahrmann M, Regele H, Kozakowski N, et al. Anti-C1s Monoclonal Antibody BIVV009 in Late Antibody-Mediated Kidney Allograft Rejection-Results From a First-in-Patient Phase 1 Trial. Am J Transplant (2018) 18(4):916–26. doi: 10.1111/ajt.14528
59. Montgomery RA, Orandi BJ, Racusen L, Jackson AM, Garonzik-Wang JM, Shah T, et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody-Mediated Rejection Following Kidney Transplantation: Results of a Randomized Double-Blind Placebo-Controlled Pilot Study. Am J Transplant (2016) 16(12):3468–78. doi: 10.1111/ajt.13871
60. Viglietti D, Gosset C, Loupy A, Deville L, Verine J, Zeevi A, et al. C1 Inhibitor in Acute Antibody-Mediated Rejection Nonresponsive to Conventional Therapy in Kidney Transplant Recipients: A Pilot Study. Am J Transplant (2016) 16(5):1596–603. doi: 10.1111/ajt.13663
61. Danobeitia JS, Zens TJ, Chlebeck PJ, Zitur LJ, Reyes JA, Eerhart MJ, et al. Targeted Donor Complement Blockade After Brain Death Prevents Delayed Graft Function in a Nonhuman Primate Model of Kidney Transplantation. Am J Transplant (2020) 20(6):1513–26. doi: 10.1111/ajt.15777
62. Delpech PO, Thuillier R, SaintYves T, Danion J, Le Pape S, van Amersfoort ES, et al. Inhibition of Complement Improves Graft Outcome in a Pig Model of Kidney Autotransplantation. J Transl Med (2016) 14(1):277. doi: 10.1186/s12967-016-1013-7
63. Eerhart MJ, Reyes JA, Blanton CL, Danobeitia JS, Chlebeck PJ, Zitur LJ, et al. Complement Blockade in Recipients Prevents Delayed Graft Function and Delays Antibody-Mediated Rejection in a Nonhuman Primate Model of Kidney Transplantation. Transplantation (2022) 106(1):60–71. doi: 10.1097/TP.0000000000003754
64. Poppelaars F, Jager NM, Kotimaa J, Leuvenink HGD, Daha MR, van Kooten C, et al. C1-Inhibitor Treatment Decreases Renal Injury in an Established Brain-Dead Rat Model. Transplantation (2018) 102(1):79–87. doi: 10.1097/TP.0000000000001895
65. Franzin R, Stasi A, Fiorentino M, Simone S, Oberbauer R, Castellano G, et al. Renal Delivery of Pharmacologic Agents During Machine Perfusion to Prevent Ischaemia-Reperfusion Injury: From Murine Model to Clinical Trials. Front Immunol (2021) 12:673562. doi: 10.3389/fimmu.2021.673562
66. Espericueta V, Manughian-Peter AO, Bally I, Thielens NM, Fraser DA. Recombinant C1q Variants Modulate Macrophage Responses But do Not Activate the Classical Complement Pathway. Mol Immunol (2020) 117:65–72. doi: 10.1016/j.molimm.2019.10.008
67. Heylen L, Pirenne J, Samuel U, Tieken I, Naesens M, Sprangers B, et al. The Impact of Anastomosis Time During Kidney Transplantation on Graft Loss: A Eurotransplant Cohort Study. Am J Transplant (2017) 17(3):724–32. doi: 10.1111/ajt.14031
Keywords: complement, C1q, tissue resident macrophages, pattern recognition receptor, B cells, donor specific antibodies, antibody-mediated rejection
Citation: Khedraki R, Noguchi H and Baldwin WM III (2022) Balancing the View of C1q in Transplantation: Consideration of the Beneficial and Detrimental Aspects. Front. Immunol. 13:873479. doi: 10.3389/fimmu.2022.873479
Received: 10 February 2022; Accepted: 07 March 2022;
Published: 24 March 2022.
Edited by:
Marilia Cascalho, University of Michigan, United StatesReviewed by:
Georg Böhmig, Medical University of Vienna, AustriaCopyright © 2022 Khedraki, Noguchi and Baldwin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William M. Baldwin III, YmFsZHdpd0BjY2Yub3Jn
 Raneem Khedraki1,2
Raneem Khedraki1,2 William M. Baldwin III
William M. Baldwin III