- Department of Hematology and Medical Oncology, Indiana University School of Medicine, Indianapolis, IN, United States
Cytokines regulate both the innate and adaptive immune responses to cancer. Although antitumor activity has been seen for several cytokines in preclinical models, they have had limited success as single therapeutic agents in clinical trials of cancer immunotherapy. However, the possible combinations of cytokines with other immune therapeutics and the advancement in genetic engineering, synthetic biology and cellular and immune therapy has led to the revival of interest in cytokines as anticancer agents. This article will review several immunostimulatory cytokines with anticancer activity, focusing on the those that have been studied in treatment of lymphoma and highlighting recent advances of potential clinical relevance.
Introduction
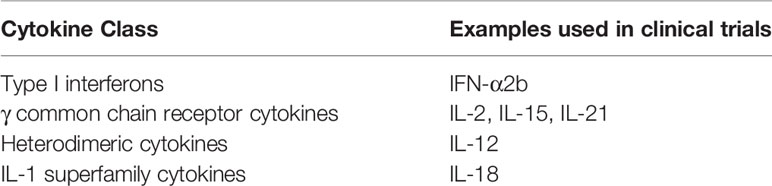
Cytokines are small glycoproteins and polypeptides that typically have a relatively short half-life and act in an autocrine and paracrine fashion. Cytokines mediate interactions between immune and nonimmune cells in the tumor microenvironment, which can either promote or inhibit the growth of cancer cells. Some cytokines, including interleukin-2 (IL-2), IL-12, IL-15, IL-18, IL-21, GM-CSF, CCL21 and type 1 interferons, have shown to have antitumor activity in preclinical studies (1, 2). Antitumor cytokine signaling could play a role in tumor antigen presentation, T-cell priming and activation, T cell infiltration and cancer cell death via stimulation of the adaptive and innate cell immunity (2, 3). Several cytokines, including IL-1B, IL-12, IL-18 and interferon (IFN)-γ, promote the differentiation of CD4 T cells into Th1 cells that can secrete cytokines, such as IL-2 and IFN-γ, that promote an antitumor response (4–7). Activation of natural killer (NK) cells by cytokines, including IL-2, IL-12, IL-15, IL-18, IFN-γ and CCL-5, can also augment antitumor immune responses. The cytokine antitumor activity seen in preclinical models led to the study of cytokines in clinical trials with GM-CSF, G-CSF, IL-2 and IFN-α being among the first studied (2). Cytokines have been explored in many solid and hematologic malignancies with the larger clinical trials mainly conducted in patients with melanoma, renal cell cancer, glioblastoma, breast cancer, lymphomas and leukemias (8). Despite the anticancer activity of numerous cytokines in preclinical models, only IL-2 and IFN-α showed sufficient clinical benefit as monotherapy in human clinical trials to warrant FDA approval. IL-2 is approved for treatment of advanced renal cell carcinoma (RCC) (9) and metastatic melanoma (10).IFN-α is approved for treatment of follicular lymphoma (11), hairy cell leukemia (12), AIDS-related Kaposi’s sarcoma (13), and melanoma (14). However, these cytokines have largely been supplanted in clinical practice by other immunotherapeutic targets, such as immune checkpoint inhibitors, with superior efficacy and more favorable toxicity profiles. Nevertheles, the possibility of combining cytokines with other immune therapies and the advancement in genetic engineering, synthetic biology and cellular and immune therapy have led to the revival of interest in cytokines as anticancer agents. This article will review several immunostimulatory cytokines with anticancer activity (Table 1), focusing on the those that have been studied in lymphoma (Table 2) and highlighting recent advances of potential clinical relevance.
Immunostimulatory Cytokines for Cancer Immunotherapy
Biology of IFN-α
Interferons are cytokines that are produced by malignant cells and dendritic cells. They are classified based on antigenic specificity into IFN-α, β and γ. IFN-α is a type 1 interferon and has been most extensively studied in anticancer therapy. The anticancer activity of IFN-α is likely due to its effect on multiple immune cells. It enhances proliferation and cytotoxicity of CD8 T cells. It also enhances cytotoxicity of NK cells and their expansion by stimulating IL-15 production. It plays a role in activation of the STING pathway post cytosolic DNA which plays a role in activation of Batf3+ dendritic cells, central to antigen presentation and hence to T cell effector functions. It may also upregulate the expression of MHC class I molecules on tumor cells. It also enhances the expression of PD-1 and PDL1 ligand on T-cell and neoplastic cells respectively, hence there is an interest in studying IFN-α in combination with immune checkpoint inhibitors (23). In preclinical models of lymphoma IFN-α was shown to have direct antitumor effects on neoplastic B cells by inducing apoptosis, inhibiting proliferation and cell cycle progression and promoting terminal differentiation in cancer cells (24, 25).
IFN-α-Based Immunotherapy for Cancer
Phase II trials involving IFN-α were conducted by the National Cancer Institute in non-Hodgkin lymphoma (NHL) patients. Conflicting results were seen in regards to the impact of IFN-α induction monotherapy and maintenance, and when combined with chemotherapy, on survival in NHL patients. IFN-α was used in treating low grade indolent NHLs where it showed some activity, however complete response (CR) and overall response rates were only 10% and 48% respectively (26–28). The introduction of rituximab in the late 1990s, led to better results when it was combined with interferon due to enhanced ADCC (28). IFN-α may also have a role in the treatment of myeloproliferative diseases (29). Several strategies to overcome the narrow therapeutic index of IFN-α include delivering IFN-α into tumor cells via immunocytokines and genetically engineered dendritic cells expressing vectors encoding IFN-α.
Biology of IL-2
IL-2 is one of the first cytokines to have been studied in anticancer treatment. IL-2 is a 4 alpha helix cytokine that has a major role in innate and adaptive immune responses. The IL-2 receptor has three subunits: IL-2Rα (CD25), IL-2Rβ (CD122), and the γ common chain (CD132). CD132 is also a component of the receptors for IL-4, IL-7, IL-9, IL-15, and IL-21. IL-2Rα binds IL-2 with low affinity and the IL-2Rβγc heterodimer binds IL-2 with intermediate affinity. The high affinity receptor for IL-2 is the IL-2Rαβγc heterotrimer (30). IL-2Rαβγc is constitutively expressed by CD4+ regulatory T cells (Tregs) and CD565bright NK cells and is transiently expressed on activated CD4+ and CD8+ T cells. The intermediate affinity IL-2Rβγc is constitutively expressed by CD56dim NK cells. IL-2 mediates antitumor immunity by promoting the proliferation and differentiation of activated CD8+ T cells into cytotoxic T lymphocytes (CTL) and by stimulating cytotoxicity and cytokine production of CTL and NK cells. However, IL-2 can also stimulate the proliferation and effector functions of Tregs, resulting in immunosuppressive effects that are not desirable in anticancer therapy (31–34).
IL-2-Based Immunotherapy Therapy for Lymphoma
Several studies established that continuous low dose of IL-2 results in expansion of NK cells while pulse intermediate dose IL-2 increases cytotoxic activity of NK cells (35–38). Synergistic activity against NHL was seen in mouse model when daily low dose IL-2 was administered with intermittent pulse intermediate dose IL2 and rituximab, likely due to enhanced ADCC mediated by NK cells. Although some early phase 1 studies showed promising results for the combination of rituximab and IL-2 (39, 40), other studies showed no significant clinical benefit (41, 42). Most of these studies used low dose IL-2, which could have preferentially expanded Tregs. The IL-2-based immunocytokines that showed positive results in preclinical lymphoma studies include L19-IL2 in NHL, HI-Leu 16-IL2 in lymphoma and HRS3scFv-IL12-Fc-IL12 in Hodgkin lymphoma. Diphteria toxin-IL-2 fused proteins showed promising results in a phase III trials of cutaneous T cell lymphoma (CTCL) patients (43). Currently, a phase II trial is ongoing for evaluating a similar diphtheria toxin-IL-2 fused protein with high bioavailability in patients with refractory/relapsed CTCL and peripheral T cell lymphomas (44).
Challenges and Future Directions for IL-2-Based Cancer Immunotherapy
High dose IL-2 is approved by the FDA for treatment of metastatic RCC and metastatic melanoma. However due to the various challenges with high dose IL-2 monotherapy including its substantial toxicity and modest efficacy, it is infrequently used and has largely been replaced by other immunotherapeutic agents (45). Low dose IL-2 could preferentially expand Tregs over the activation of NK and CD8 T cells (30). The high doses of IL-2 required for immune stimulation of NK and CD8 T cells is associated with serious side effects such as hypotension, organ failure, cytopenias and vascular leak syndrome (46). To improve the pharmacokinetics and pharmacodynamics of IL-2 and reduce its systemic toxicity several strategies have been pursued. Immunocytokines, in which IL-2 is linked to an antibody that targets tumor associated-antigens, have been efficacious in preclinical models and are currently being used in IL-2 based therapy trials (45). IL-2-based immunocytokines have been tested in combination with other cytokines which enhance the activation of NK cells (47). Combining IL-2 immunocytokines with other immunocytokines, chemotherapeutics, and immunotherapeutic agents such as immune checkpoint inhibitors will likely be implemented in future IL-2-based immunotherapies (45, 48). The administration of intratumoral IL-2 may decrease systemic toxicity (45, 48). Adjusting the design of manufactured IL-2/IL-2 immunocytokines to preferentially stimulate NK and CD8 T cells over the expansion of Tregs is one of approaches to optimize IL-2-based therapies. The structure of IL-2 cytokine/immunocytokines have been engineered to have higher affinity for the IL-2Rβγc heterodimer and diminished binding to the IL-2Rα on Tregs. IL-2 has also been successfully combined with an IL-2 antibody that masks the IL-2Rα binding site of IL-2, thus abolishing binding of engineered IL-2 to Tregs. Pegylation of IL-2-based immunocytokines can increase their half life and also block IL-2 from binding to IL-2Rα (49–51). Novel IL-2 fusion proteins have been designed to enhance the activity and proliferation of NK cells and have shown promising results in preclinical models. The OCMP-mutIL-2 fusion protein binds with high affinity to NKG2D on NK cells and contains mutations that confer preferential binding to IL-2Rβγc rather than IL2Rα (52, 53). Adjusting the structure of the IL-2-based therapies can also alleviate the risk of toxicities such as vascular leak syndrome.
Biology of IL-12
IL-12 is a heterodimeric cytokine that is secreted by antigen presenting cells (APCs), including dendritic cells and cells of monocyte/macrophage lineage, in response to pathogen-associated molecular patterns, damage-associated molecular patterns, cytokine stimulation and direct immune cell–cell contact (1, 54). IL-12 binds with high affinity to the IL-12Rβ1/IL-12Rβ2 heterodimer that is constitutively expressed by NK cells and after activation by T cells and B cells. Binding of IL-12 to its receptor leads to recruitment and activation of JAK2 and TYK2 with subsequent activation of STAT4 (54). IL-12 has been used in cancer immunotherapy based on its anti-tumor activity in preclinical models mediated by various effects on the adaptive and the innate immune system and bridging them together (54). IL-12 is critical for the production of IFN-γ by NK and T cells (55). IL-12 promotes the differentiation of naïve CD4 Th0 cells into Th1 cells (56) and the CD8 T cells into CTL. It also augments the cytolytic activity and the growth of activated T cells and NK cells (57). IL-12 enhances NK mediated ADCC (58), B cell survival and IgG production. IL-12 also has antiangiogenic effects by enhancing the production of monokine induced by IFN-γ (MIG; CXCL9) and IFN-γ-induced protein 10 (IP-10;CXCL10) (59). IL-12 also exerts antitumor effects through regulation of peritumoral extracellular matrix, tumor stroma, and enhancing the processing and expression of MHC class I molecules (54).
IL-12-Based Immunotherapy for Lymphoma
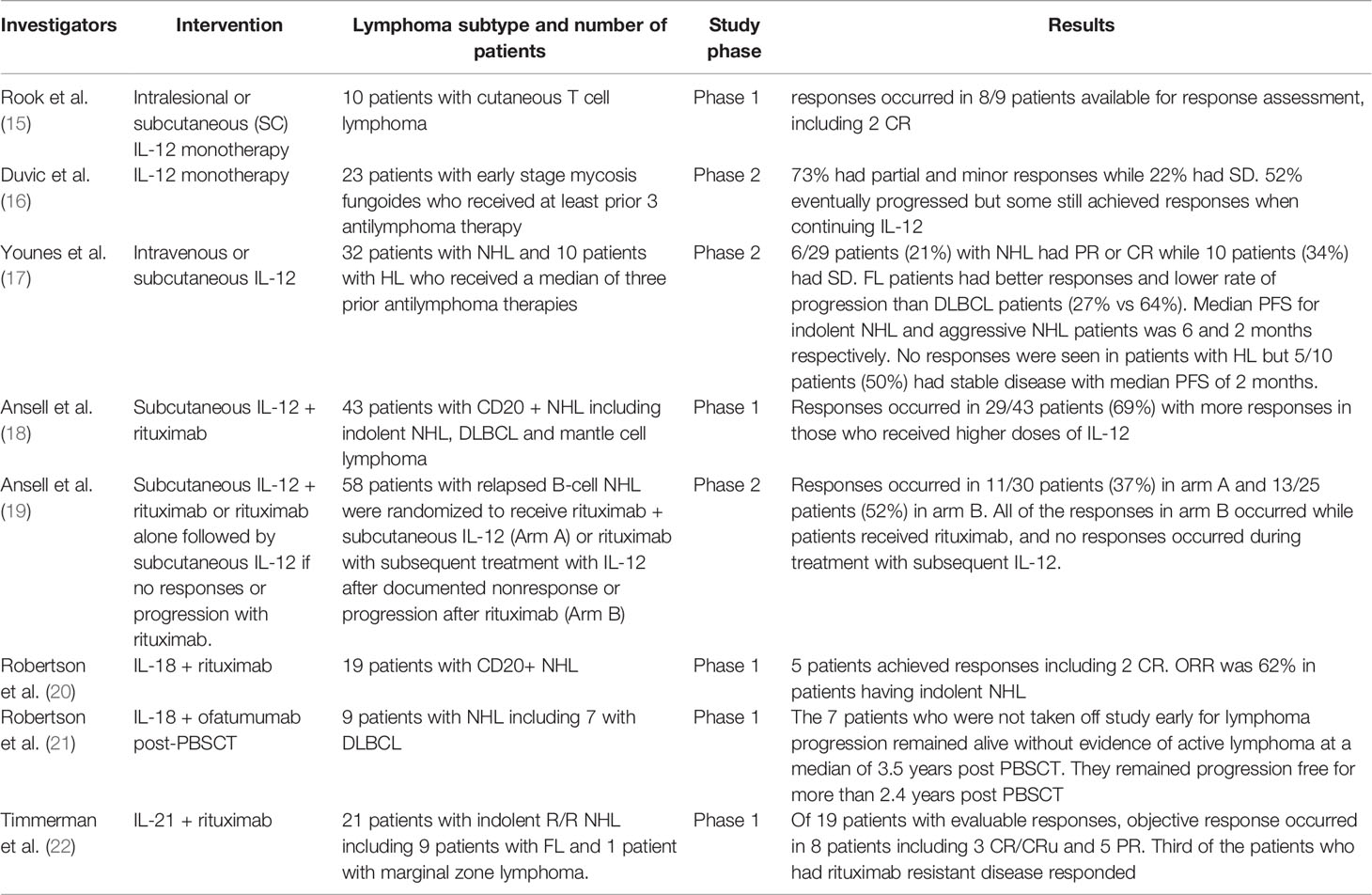
The decrease in function of Th1 cells and underproduction of IFN-γ in the tumor environment of CTCL, makes IL-12 an attractive therapeutic option for this lymphoma subtype, especially in as much as cutaneous lesions could be easily accessed (54, 60). In a study of 10 patients with CTCL who received biweekly intralesional or subcutaneous (SC) injections of IL-12 monotherapy, 9 patients were available for response assessment at safety analysis time, responses occurred in 8/9 patients including 2 CR. Side effects were short lived (15). SC IL-12 monotherapy was also evaluated in a phase II trial of 23 patients with early stage mycosis fungoides whom received at least 3 prior antilymphoma therapies, 10/23 patients received IL-12 injections for at least 6 months and continued for 2 years. 73% had partial and minor responses while 22% had stable disease. However, 52% eventually progressed but some still achieved responses when continuing IL-12. 5 patients stopped therapy due to side effects and 1 patient died with hemolytic anemia that was likely attributed to the drug (16). A recent phase 2 study (NCT02542124) combining IL-12 with low dose total skin electron beam therapy has been initiated with the rationale that IL-12 may also decrease radiation toxicity.
The first study of IL-12 monotherapy in NHL and Hodgkin lymphoma (HL) patients was reported by Younes et al. (17) in which 32 patients with NHL and 10 patients with HL who received a median of three prior antilymphoma therapies were enrolled. NHL patients had diffuse large B-cell lymphoma (DLBCL) and grade 1-2 follicular lymphoma (FL). 11 patients received 250 ng/kg of intravenous (IV) IL-12 daily for 5 days every 3 weeks (preceded by an initial test dose of 250 ng/kg) and 31 patients received twice-weekly IL-12 SC injections at 500 ng/kg (in case of toxicity, the dosage was reduced to 300 ng/kg). 39 patients (93%) were present for response assessment. 6/29 patients (21%) with NHL had partial remission (PR) or CR while 10 patients (34%) had stable disease. FL patients had better responses and lower rate of progression than DLBCL patients (27% vs 64%). Median progression free survival (PFS) for indolent NHL and aggressive NHL patients was 6 and 2 months respectively. Overall responses in those who received IV and SC IL-12 were 40% and 7% respectively. All responders had low burden disease. IL-12 did not affect the CD4 T cell count but the mean CD8 T cell count was significantly increased. Levels of VEGF and bFGF (reflecting angiogenesis) were either decreased or stable after IL-12 injections in most patients who responded, and were stable or increased in most patients who did not respond or progressed. No responses were seen in patients with HL but 5/10 patients (50%) had stable disease with median PFS of 2 months. All HL patients received SC IL-12 and it is possible this could have influenced the results. IL-12 was tolerable in most patients with 95% developing fevers that mostly responding to acetaminophen. Fatigue, malaise and arthralgia were commonly reported and were more common in those who received IV IL-12. 9 patients required dose reductions including 3 patients who developed grade 3 hepatotoxicity and all received SC IL-12. All hepatotoxicities were grade 1 and 2. Treatment was prematurely stopped in 4 patients (17). In a phase 1 study of a group with heterogenous types of lymphomas, IL-12 was given SC twice weekly at doses 500 and 300 ng/kg or lower along with rituximab with the aim to enhance NK mediated ADCC. PR and CR were seen in 69% with more responses in those who received higher doses of IL-12 (18). However, in the phase 2 study, comparable response rates were seen with rituximab monotherapy and combination of rituximab and IL-12 (19). A phase II study has been initiated to evaluate the combination of IL-12 with salvage R-ICE or R-DHAP in patients with relapsed/refractory aggressive B-cell NHL with the rationale that IL-12 may enhance responses but also decrease chemotherapy toxicity (NCT02544724).
The safety of using IV IL-12 after autologous peripheral blood stem cell transplantation (PBSCT) was established in a phase 1 trial by Robertson et al. in which 12 patients including 10 with hematologic malignancies (8 NHL, 2 HL, 2 plasma cell myeloma) with very high risk of relapse received three different initial doses of IV IL-12. Time to progression ranged from 10.5 to 50.8 months with 3/8 of the NHL patients and 1/2 of HL patients, did not progress during the observation period (median of 32.4 months after initiating IL-12 therapy) (61). An obstacle to optimal IL-12-based immunotherapy after PBSCT is the profound, acquired STAT4 deficiency that can occur in this setting (62).
Challenges and Future Directions for IL-12-Based Cancer Immunotherapy
Significant systemic toxicities have been reported in studies with IL-12 that are mainly due to increased production of IFN-γ, TNF, IP-10 and MIG. Reported adverse events and toxicities in clinical trials include fatigue, fever, arthralgia, headaches and malaise (63–67). Erythropoietin analogues and G-CSF were successful in reducing anemia and bone marrow suppression respectively (54). It seems that systemic toxicity may be reduced if IL-12 is delivered locally into the tumor bed and is being implemented in current IL-12 clinical trials (54).
The anticancer effect of IL-12 in preclinical models was enhanced by combining it with chemotherapeutics, other cytokines, antibodies, antiangiogenic agents, radiotherapy, adoptive therapy, and tumor vaccines. For example, in a preclinical Burkitt lymphoma model, the anticancer effect of IL-12 was potentiated by the addition of vasostatin. Chemotherapeutic agents could augment the anticancer activity by increasing the release and presentation of tumor antigens. However, some chemotherapeutic agents could suppress the immune response by inhibiting immune effector cells (62).
Despite the anticancer activity seen in preclinical models, IL-12 monotherapy did not yield the same results in human clinical trials. One possible explanation for this discrepancy is the negative feedback and immunoregulatory effect that could result from increased IL-10 and TIM3 production that could lead to an increase in IFN-γ levels and increase PDL1 expression on cancer cells (54). Also, the human tumor environment may be more heterogenous than preclinical models with more immunosuppressive effect and tumor escape mechanisms.
Delivering IL-12 regionally or locally into the tumor environment may overcome its narrow therapeutic index by enhancing efficacy and decreasing systemic toxicity. With the advancement in genetic engineering techniques and adoptive cell therapies, IL-12 is regaining attention (68). In recent cancer experiments and early studies, IL-12 has been genetically delivered via engineered viruses or nonviral vectors such as IL-12 expressing plasmids (69–71). IL-12 plasmids are being studied in CTCL (54). Adoptive immunotherapy with the utilization of genetically modified IL-12 expressing lymphocytes have also been implemented (72). The idea of delivering CAR T cells transduced with inducible IL-12 is also being considered (73). IL-12 has also been combined with cancer vaccines including dendritic cell-tumor cell fusion vaccines (74). IL-12-based Immunocytokines in which IL-12 is attached to monoclonal antibodies that target specific tumor antigens may also help decrease systemic toxicity and improve efficacy of IL-12.
Biology of IL-15
IL-15 is a four alpha helix cytokine (75). The receptor for IL-15 is a heterotrimer that includes IL-15Rα (CD215), which is specific to IL-15, CD122 (β subunit common to IL-2 and IL-15) and the γc chain (CD132) (76). IL-15 is produced by APCs (macrophages, monocytes, and dendritic cells) as well as several other cell types. After IL-15 protein is translated from mRNA, it binds with high affinity to intracellular IL-15Rα and the IL-15/IL-15α complex is transported to the cell surface of the producing cells. The IL-15-IL-15Rα complex is transpresented to the IL2/IL-15Rβγc heterodimer expressed on NK and CD8 T cells (75, 77–79). The main immunostimulatory effects of IL-15 that may lead to its antitumor activity include enhancing the proliferation and survival of T cells, proliferation and differentiation of NK cells, and differentiation of CTL. IL-15 can cause prolonged expansion and activation of both NK cells and CD8 T cells (78, 80, 81). Despite sharing some biologic effects with IL-2, the use of IL-15 in anticancer therapy has potential advantages compared to IL-2. Potential advantages of IL-15 are that it causes less expansion of Tregs and less activation-induced death of effector T cells compared to IL-2. IL-15 also does not appear to cause major capillary leakage that can be a serious toxicity of IL-2 (82, 83).
Challenges and Future Directions for IL-15-Based Cancer Immunotherapy
Despite the potent antitumor activity of IL-15 seen in some preclinical models, clinical results have not been as impressive, with issues that include a short half-life, relatively modest bioactivity in vivo, and reliance on transpresentation. Several approaches have been taken to overcome these challenges. Constructing superagonist complexes by combining IL-15 with various forms of IL-15Rα led to a superior antitumor activity than IL-15 monotherapy. Administration of a superagonist is thought to mimic transpresentation of IL-15 by IL-15Rα to effector cells expressing the IL-2/IL-15βγc heterodimer and thus enhancing IL-15 bioactivity (77, 79). Novel IL-15-based therapies have combined it in preclinical studies with other immunotherapeutic agents, such as monoclonal antibodies (rituximab, alemtuzumab), CD40 agents, cancer vaccines, and other cytokines (IL-12, IL-18, and IL-21) (82, 84). When these preclinical model approaches translated to human clinical trials, tumor responses have been disappointing, perhaps due to the immunosuppressive tumor microenvironment. Thus, combining IL-15-based therapies with immune checkpoint inhibitors that target the immunosuppressive environment is rational (82). IL-15-based therapy with dendritic vaccines and oncolytic viruses expressing IL-15 or IL-15- IL-15Rα are also being investigated in preclinical studies (82, 85). Toxicities and adverse events that were observed with recombinant IL-15 include fever, hypotension, appetite changes, weight loss, diarrhea, rash, transient grade 3–4 neutropenia and anemia. These adverse events were less severe when IL-15 was given SC and intermittently. A concern with the use of IL-15 arises from its potent stimulatory effect on NK cells and CD8 T cells, which could lead to autoimmune toxicities (78). Future studies are needed to determine the optimal administration route and dosage for IL-15- based therapy to minimize toxicity and enhance its bioactivity. Most of the ongoing/recruiting early phase clinical trials of IL-15 based therapy in lymphoma patients are using IL-15 super agonists and in combination with other anticancer therapeutics due to the challenges with IL-15 monotherapy mentioned before. Phase 1 trial has been initiated to evaluate the safety and efficacy of the IL-15 superagonist ALT -803 in combination with rituximab in patients with relapsed/refractory indolent NHL who received at least 1 prior rituximab treatment (NCT02384954).
Biology of IL-18
IL-18 is an immunostimulatory cytokine that regulates both innate and adaptive immune responses. IL-18, a member of the IL-1 superfamily of cytokines, is produced by several cell types, including macrophages, dendritic cells, and epithelial cells. IL-18 binds to a receptor complex composed of at least two subunits, IL-18Rα (IL-1Rrp1) and IL-18Rβ (AcPL). Preclinical models and phase 1 trials established efficacy and biological activity of IL-18 against solid tumors and lymphoma (20, 86, 87). IL-18 could promote antitumor responses by stimulating production of IFN-γ, activating NK cells and monocytes/macrophages and enhancing their ADCC. Increased production of IFN-γ by IL-18 leads to increased production of CXCL9 (MIG) and CXCL10 (IP-10) that exert an antiangiogenic effect. IL-18 could also enhance the differentiation of Th1 cells and facilitate the priming of effector cells including helper NK cells into tumor site (20). Despite the biological activity of IL-18 seen in early studies, IL-18 monotherapy had limited efficacy in cancer patients (86–88). Thus, several approaches are being implemented in studies to enhance the efficacy of IL-18 such as combining it with other therapeutics.
IL-18-Based Immunotherapy for Lymphoma
IL-18 showed in vitro and in vivo synergistic activity when combined with the CD20 monoclonal antibody rituximab in a preclinical study (89). This led to a phase 1 study in which 19 patients with CD20+ NHL received rituximab and escalating doses of human recombinant IL-18. Eleven patients had rituximab-refractory disease. 5 patients achieved responses including 2 CR. The response rate was 62% for the 8 patients with indolent lymphoma who received doses of IL-18 that were associated with optimal biologic responses as defined by ancillary biomarker studies. Overall response rates were similar in patients with rituximab-refractory and rituximab-sensitive disease. The combination was deemed to be safe with a similar safety profile to IL-18 monotherapy. The objective responses are likely attributable to enhanced ADCC mediated by NK cells and monocyte/macrophages against rituximab-sensitized lymphoma cells (20). Although rituximab has been combined in clinical studies with other cytokines such as IL-2, IL-12 and GM-CSF, IL-18 has the advantage of enhancing the ADCC mediated by both NK cells and macrophages/monocytes. IL-2 and IL-12 enhance preferentially NK cell-mediated ADCC and GM-CSF preferentially enhances ADCC mediated by macrophages/monocytes (20). Ofatumumab is monoclonal antibody that binds to a different epitope of CD20 than that recognized by rituximab. Preclinical studies showed that IL-18 plus ofatumumab was more effective than IL-18 plus rituximab in a lymphoma xenograft model. IL-18-based immunotherapy might circumvent the acquired STAT4 deficiency that impairs IL-12-based treatment after PBSCT. A phase 1 study showed that the combination of ofatumumab with IL-18 in 9 patients with NHL (7 DLBCL) post high dose chemotherapy and PBSCT is feasible and tolerable with no dose limiting toxicities. Although efficacy could not be determined from this phase 1 study, the seven patients who were not taken off study early for lymphoma progression remained alive without evidence of active lymphoma at a median of 3.5 years post PBSCT. All of these patients have remained progression free for more than 2.4 years post PBSCT (21). Other approaches include utilizing IL-18 secreting CAR T cells and IL-18-based adoptive transfer (such as IL-12/IL-15/IL18 preactivated NK cells) (90, 91) with ongoing clinical trials as promising results were seen in early clinical trials of AML patients (92, 93).
Challenges and Future Directions for IL-18-Based Cancer Immunotherapy
IL-18 has a very favorable toxicity profile but has had limited efficacy as a monotherapy. IL-18 binding protein (IL-18BP) is a secreted antagonist that binds to IL-18 with high affinity and neutralizes its biological activity. IFN-γ induced by IL-18 may exert a negative feedback loop by increasing the expression of IL-18BP (91). Zhou et al. developed a form of IL-18 that is resistant to inhibition by IL-18BP and showed a potent antitumor responses in mice (94). IFN-γ induced by IL-18 could also stimulate upregulation of PD-L1 on tumors cells, providing a rationale for combining IL-18 and PD-1 checkpoint inhibitors in cancer immunotherapy. Other approaches to enhance the antitumor activity of IL-18 include combining it with chemotherapy and other immune therapeutics (monoclonal antibodies,other cytokines, cancer vaccines) (91). IL-18 secreting CAR T cells and IL-18-IL2 fusion proteins are under investigation.
Complicating the development of IL-18-based cancer immunotherapy are preclinical studies showing the IL-18 can promote tumor invasiveness and progression in some experiments. It is possible that IL-18 levels may have an impact on tumor regression/growth as administration of low dose IL-18 in murine melanoma model promoted metastasis due to suppressed mature NK cell number, while high doses of IL-18 that resulted in IL-18 serum levels > 1 ng/mL inhibited tumor growth without a decrease in mature NK cell number (95). The lowest doses of hIL-18 given to lymphoma patients resulted in IL-18 plasma levels of > 10 ng/mL and biomarker studies have shown in vivo activation rather than suppression of NK cells during IL-18-based immunotherapy (20, 87). Further studies are needed to determine the precise conditions under which IL-18 promotes or inhibits tumor growth versus regression. IL-18BP-based therapy may be a potential therapeutic modality for clinical scenarios where IL-18 can promote tumor growth.
Biology of IL-21
IL-21 is a 4 helix bundle cytokine that binds to the IL-21R (CD360)/γc (CD132) heterodimeric receptor complex. IL-21 is mainly secreted by T follicular helper cells, Th17 cells and NK T cells. IL-21 stimulates innate and adaptive immune responses and showed antitumor activity in preclinical models. IL-21 enhances the proliferation of CD4 T cells and the proliferation and cytotoxic activity of CD8 T cells. IL-21 suppressed Treg cells in vitro and together with IL-16, IL-21 can enhance the differentiation of CD4 T cells into TH17 cells. IL-21 enhances maturation and cytotoxic activity of NK cells. ADCC activity of NK cells can be enhanced by IL-21 reflected by increased CD16 expression. At low doses, IL-21 enhances proliferation of NK cells while at high doses and in the presence of IL-2 and IL-15, IL-21 decreases the proliferation of NK cells (96). The effect of IL-21 on B-cells is variable and is influenced by the type and differentiation stage of the B lymphocyte which will be discussed in the next section. Despite the antitumor activity of IL-21 in preclinical models of solid and hematologic malignancies, this was not met with the same success when taken into human clinical trials. Current approaches to enhance the antitumor activity of IL-21 include combining it with other cytokines and monoclonal antibodies and co-administration with adoptively-transferred cells and tumor cell vaccines (96).
IL-21 Based Immunotherapy for Lymphoma
The effect of IL-21 on B-cells is influenced by the stage differentiation of the B-lymphocyte. IL-21 can either enhance the proliferation or apoptosis of the B cells. Given that IL-21 has an indirect antitumor activity by enhancing the cytotoxic activity and proliferation of CD8 T cells and NK cells and that it can promote apoptosis of the B-cell at certain stages by upregulation of pro-apoptotic proteins such as Bim and downregulation of anti-apoptotic proteins, there has been an interest in studying IL-21 in the treatment of lymphoma. IL-21 led to complete regression of DLBCL in xenograft models and extended their survival by promoting apoptosis of B-cells (96, 97). Glebert et al. showed that IL-21 had antiproliferative effect on 2 mantle cell lymphoma (MCL) cell lines with increased pro-apoptotic proteins and decreased anti-apoptotic protein (98). IL-21 combined with rituximab was evaluated in a phase 1 clinical trial involving 21 patients with indolent R/R NHL including 9 patients with FL and 1 patient with marginal zone lymphoma. Of 19 patients with evaluable responses, objective response occurred in 8 patients including 3 CR/CRu and 5 PR. A third of the patients who had rituximab-resistant disease responded (22). Contrarily, clinical studies showed that IL-21 can promote the proliferation of Hodgkin lymphoma (99), EBV transformed lymphomas (100) and ALK-positive anaplastic large cell lymphoma (101) suggesting that blocking the effect of IL-21 may be a potential therapeutic approaches in these lymphoma subtypes.
Conclusion
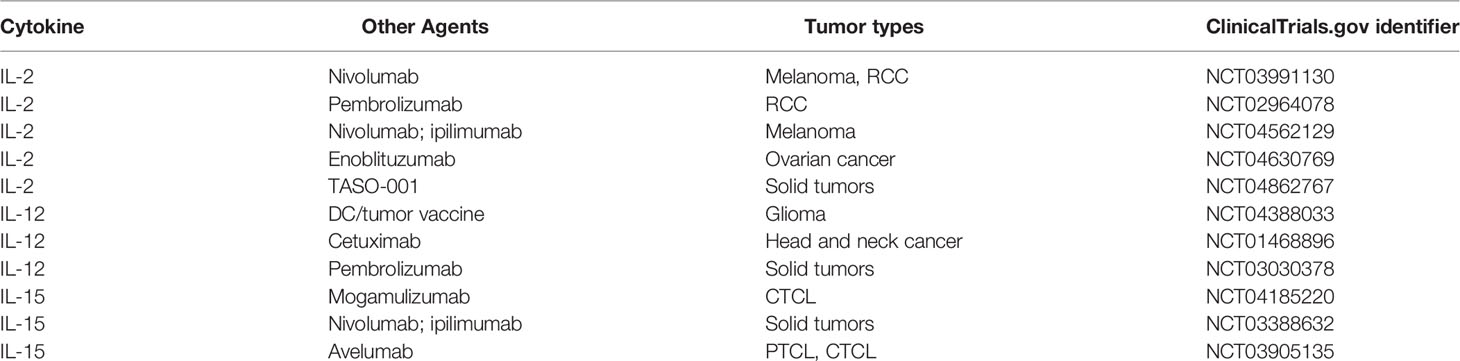
Despite promising antitumor activity of several cytokines in preclinical models, cytokines as monotherapy have had limited success when tested in clinical trials. Indeed, only IFN-α and IL-2 are currently approved for treatment of cancer. However, advances in genetic engineering, synthetic biology, and cellular and immune therapy have led to a renewal of interest in cytokines for cancer immunotherapy. Combinations of cytokines with monoclonal antibodies to tumor antigens, checkpoint inhibitors, vaccines, and cell-based therapies are being investigated in clinical trials (Table 3). Moreover, autologous and/or allogeneic T cell products have been designed to express immunostimulatory cytokines in addition to CAR that direct them to tumor-associated antigens (102–104). A phase I clinical trial of ST-067, a recombinant human IL-18 engineered to abolish binding and neutralization of its activity by IL-18BP, has been initiated. Innovative approaches to cytokine-based cancer immunotherapy are being actively pursued and, it is to be hoped, will improve outcomes for patients with lymphoma and other malignancies.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
The research of MJR is supported by gift from Eugene and Nancy Bate.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, et al. Cytokines in Clinical Cancer Immunotherapy. Br J Cancer (2019) 120(1):6–15. doi: 10.1038/s41416-018-0328-y
2. Qiu Y, Su M, Liu L, Tang Y, Pan Y, Sun J. Clinical Application of Cytokines in Cancer Immunotherapy. Drug Des Devel Ther (2021) 15:2269–87. doi: 10.2147/DDDT.S308578
3. Chen DS, Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
4. Mantovani A, Barajon I, Garlanda C. Il-1 and Il-1 Regulatory Pathways in Cancer Progression and Therapy. Immunol Rev (2018) 281(1):57–61. doi: 10.1111/imr.12614
5. Micallef MJ, Tanimoto T, Kohno K, Ikeda M, Kurimoto M. Interleukin 18 Induces the Sequential Activation of Natural Killer Cells and Cytotoxic T Lymphocytes to Protect Syngeneic Mice From Transplantation With Meth a Sarcoma. Cancer Res (1997) 57(20):4557–63.
6. Tugues S, Burkhard S, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, et al. New Insights Into Il-12-Mediated Tumor Suppression. Cell Death Differ (2015) 22(2):237–46. doi: 10.1038/cdd.2014.134
7. Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol (2018) 9:847. doi: 10.3389/fimmu.2018.00847
8. Kirkwood JM, Ernstoff M. Melanoma: Therapeutic Options With Recombinant Interferons. Semin Oncol (1985) 12(4 Suppl 5):7–12.
9. Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of Treatment of 255 Patients With Metastatic Renal Cell Carcinoma Who Received High-Dose Recombinant Interleukin-2 Therapy. J Clin Oncol (1995) 13(3):688–96. doi: 10.1200/JCO.1995.13.3.688
10. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-Dose Recombinant Interleukin 2 Therapy for Patients With Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. J Clin Oncol (1999) 17(7):2105. doi: 10.1200/JCO.1999.17.7.2105
11. Solal-Celigny P, Lepage E, Brousse N, Reyes F, Haioun C, Leporrier M, et al. Recombinant Interferon Alfa-2b Combined With a Regimen Containing Doxorubicin in Patients With Advanced Follicular Lymphoma. Groupe D'etude Des Lymphomes De L'adulte. N Engl J Med (1993) 329(22):1608–14. doi: 10.1056/NEJM199311253292203
12. Golomb HM, Jacobs A, Fefer A, Ozer H, Thompson J, Portlock C, et al. Alpha-2 Interferon Therapy of Hairy-Cell Leukemia: A Multicenter Study of 64 Patients. J Clin Oncol (1986) 4(6):900–5. doi: 10.1200/JCO.1986.4.6.900
13. Groopman JE, Gottlieb MS, Goodman J, Mitsuyasu RT, Conant MA, Prince H, et al. Recombinant Alpha-2 Interferon Therapy for Kaposi's Sarcoma Associated With the Acquired Immunodeficiency Syndrome. Ann Intern Med (1984) 100(5):671–6. doi: 10.7326/0003-4819-100-5-671
14. Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon Alfa-2b Adjuvant Therapy of High-Risk Resected Cutaneous Melanoma: The Eastern Cooperative Oncology Group Trial Est 1684. J Clin Oncol (1996) 14(1):7–17. doi: 10.1200/JCO.1996.14.1.7
15. Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, Sherman ML, et al. Interleukin-12 Therapy of Cutaneous T-Cell Lymphoma Induces Lesion Regression and Cytotoxic T-Cell Responses. Blood (1999) 94(3):902–8. doi: 10.1182/blood.V94.3.902.415k23_902_908
16. Duvic M, Sherman ML, Wood GS, Kuzel TM, Olsen E, Foss F, et al. A Phase Ii Open-Label Study of Recombinant Human Interleukin-12 in Patients With Stage Ia, Ib, or Iia Mycosis Fungoides. J Am Acad Dermatol (2006) 55(5):807–13. doi: 10.1016/j.jaad.2006.06.038
17. Younes A, Pro B, Robertson MJ, Flinn IW, Romaguera JE, Hagemeister F, et al. Phase Ii Clinical Trial of Interleukin-12 in Patients With Relapsed and Refractory Non-Hodgkin’s Lymphoma and Hodgkin’s Disease. Clin Cancer Res (2004) 10(16):5432–8. doi: 10.1158/1078-0432.CCR-04-0540
18. Ansell SM, Witzig TE, Kurtin PJ, Sloan JA, Jelinek DF, Howell KG, et al. Phase 1 Study of Interleukin-12 in Combination With Rituximab in Patients With B-Cell Non-Hodgkin Lymphoma. Blood (2002) 99(1):67–74. doi: 10.1182/blood.v99.1.67
19. Ansell SM, Geyer SM, Maurer MJ, Kurtin PJ, Micallef IN, Stella P, et al. Randomized Phase Ii Study of Interleukin-12 in Combination With Rituximab in Previously Treated Non-Hodgkin's Lymphoma Patients. Clin Cancer Res (2006) 12(20 Pt 1):6056–63. doi: 10.1158/1078-0432.CCR-06-1245
20. Robertson MJ, Kline J, Struemper H, Koch KM, Bauman JW, Gardner OS, et al. A Dose-Escalation Study of Recombinant Human Interleukin-18 in Combination With Rituximab in Patients With Non-Hodgkin's Lymphoma. J Immunother (2013) 36(6):331. doi: 10.1097/CJI.0b013e31829d7e2e
21. Robertson MJ, Stamatkin CW, Pelloso D, Weisenbach J, Prasad NK, Safa AR. A Dose-Escalation Study of Recombinant Human Interleukin-18 in Combination With Ofatumumab After Autologous Peripheral Blood Stem Cell Transplantation for Lymphoma. J Immunother (2018) 41(3):151–7. doi: 10.1097/CJI.0000000000000220
22. Timmerman JM, Byrd JC, Andorsky DJ, Yamada RE, Kramer J, Muthusamy N, et al. A Phase I Dose-Finding Trial of Recombinant Interleukin-21 and Rituximab in Relapsed and Refractory Low Grade B-Cell Lymphoproliferative Disorders. Clin Cancer Res (2012) 18(20):5752–60. doi: 10.1158/1078-0432.CCR-12-0456
23. Budhwani M, Mazzieri R, Dolcetti R. Plasticity of Type I Interferon-Mediated Responses in Cancer Therapy: From Anti-Tumor Immunity to Resistance. Front Oncol (2018) 8:322. doi: 10.3389/fonc.2018.00322
24. Adolf G, Haas O, Fischer P, Swetly P. Spontaneous Production of A-and B-Interferon in Human Lymphoblastoid and Lymphoma Cell Lines. Arch Virol (1982) 72(3):169–78. doi: 10.1007/BF01348962
25. Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. Ifn-Alpha Directly Promotes Programmed Cell Death-1 Transcription and Limits the Duration of T Cell-Mediated Immunity. J Immunol (2011) 186(5):2772–9. doi: 10.4049/jimmunol.1003208
26. Hansen RM, Borden EC. Current Status of Interferons in the Treatment of Cancer. Oncology (Williston Park) (1992) 6(11):19–24; discussion 6, 9.
27. Ziegler-Heitbrock HW, Thiel E. Recombinant Ifn-Alpha in Lymphomas. J Invest Dermatol (1990) 95(6 Suppl):213S–5S. doi: 10.1111/1523-1747.ep12875789
28. Zhang L, Tai Y-T, Ho MZG, Qiu L, Anderson KC. Interferon-Alpha-Based Immunotherapies in the Treatment of B Cell-Derived Hematologic Neoplasms in Today’s Treat-To-Target Era. Exp Hematol Oncol (2017) 6(1):1–9. doi: 10.1186/s40164-017-0081-6
29. Gu W, Yang R, Xiao Z, Zhang L. Clinical Outcomes of Interferon Therapy for Polycythemia Vera and Essential Thrombocythemia: A Systematic Review and Meta-Analysis. Int J Hematol (2021) 114(3):342–54. doi: 10.1007/s12185-021-03171-1
30. Mitra S, Leonard WJ. Biology of Il-2 and Its Therapeutic Modulation: Mechanisms and Strategies. J Leukoc Biol (2018) 103(4):643–55. doi: 10.1002/JLB.2RI0717-278R
31. Boyman O, Sprent J. The Role of Interleukin-2 During Homeostasis and Activation of the Immune System. Nat Rev Immunol (2012) 12(3):180–90. doi: 10.1038/nri3156
32. Spolski R, Li P, Leonard WJ. Biology and Regulation of Il-2: From Molecular Mechanisms to Human Therapy. Nat Rev Immunol (2018) 18(10):648–59. doi: 10.1038/s41577-018-0046-y
33. Waldmann TA. The Biology of Interleukin-2 and Interleukin-15: Implications for Cancer Therapy and Vaccine Design. Nat Rev Immunol (2006) 6(8):595–601. doi: 10.1038/nri1901
34. Carnemolla B, Borsi L, Balza E, Castellani P, Meazza R, Berndt A, et al. Enhancement of the Antitumor Properties of Interleukin-2 by Its Targeted Delivery to the Tumor Blood Vessel Extracellular Matrix. Blood (2002) 99(5):1659–65. doi: 10.1182/blood.v99.5.1659
35. Caligiuri MA, Murray C, Soiffer R, Klumpp T, Seiden M, Cochran K, et al. Extended Continuous Infusion Low-Dose Recombinant Interleukin-2 in Advanced Cancer: Prolonged Immunomodulation Without Significant Toxicity. J Clin Oncol (1991) 9(12):2110–9. doi: 10.1200/JCO.1991.9.12.2110
36. Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional Consequences of Interleukin 2 Receptor Expression on Resting Human Lymphocytes. Identification of a Novel Natural Killer Cell Subset With High Affinity Receptors. J Exp Med (1990) 171(5):1509–26. doi: 10.1084/jem.171.5.1509
37. Meropol NJ, Barresi GM, Fehniger TA, Hitt J, Franklin M, Caligiuri MA. Evaluation of Natural Killer Cell Expansion and Activation in Vivo With Daily Subcutaneous Low-Dose Interleukin-2 Plus Periodic Intermediate-Dose Pulsing. Cancer Immunol Immunother (1998) 46(6):318–26. doi: 10.1007/s002620050493
38. Caligiuri MA, Murray C, Robertson MJ, Wang E, Cochran K, Cameron C, et al. Selective Modulation of Human Natural Killer Cells in Vivo After Prolonged Infusion of Low Dose Recombinant Interleukin 2. J Clin Invest (1993) 91(1):123–32. doi: 10.1172/JCI116161
39. Eisenbeis CF, Grainger A, Fischer B, Baiocchi RA, Carrodeguas L, Roychowdhury S, et al. Combination Immunotherapy of B-Cell Non-Hodgkin’s Lymphoma With Rituximab and Interleukin-2: A Preclinical and Phase I Study. Clin Cancer Res (2004) 10(18):6101–10. doi: 10.1158/1078-0432.CCR-04-0525
40. Gluck WL, Hurst D, Yuen A, Levine AM, Dayton MA, Gockerman JP, et al. Phase I Studies of Interleukin (Il)-2 and Rituximab in B-Cell Non-Hodgkin’s Lymphoma: Il-2 Mediated Natural Killer Cell Expansion Correlations With Clinical Response. Clin Cancer Res (2004) 10(7):2253–64. doi: 10.1158/1078-0432.CCR-1087-3
41. Khan KD, Emmanouilides C, Benson DM Jr., Hurst D, Garcia P, Michelson G, et al. A Phase 2 Study of Rituximab in Combination With Recombinant Interleukin-2 for Rituximab-Refractory Indolent Non-Hodgkin's Lymphoma. Clin Cancer Res (2006) 12(23):7046–53. doi: 10.1158/1078-0432.CCR-06-1571
42. Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, et al. Allogeneic Natural Killer Cells for Refractory Lymphoma. Cancer Immunol Immunother (2010) 59(11):1739–44. doi: 10.1007/s00262-010-0896-z
43. Prince HM, Duvic M, Martin A, Sterry W, Assaf C, Sun Y, et al. Phase Iii Placebo-Controlled Trial of Denileukin Diftitox for Patients With Cutaneous T-Cell Lymphoma. J Clin Oncol (2010) 28(11):1870–7. doi: 10.1200/JCO.2009.26.2386
44. Maruyama D, Ando K, Yamamoto K, Kiyohara E, Terui Y, Fukuhara N, et al. Phase 2 Study of E7777, a Diphtheria Toxin Fragment-Interleukin-2 Fusion Protein, in Japanese Patients With Relapsed or Refractory Peripheral and Cutaneous T-Cell Lymphoma. Blood (2019) 134:4032. doi: 10.1182/blood-2019-121723
45. Mortara L, Balza E, Bruno A, Poggi A, Orecchia P, Carnemolla B. Anti-Cancer Therapies Employing Il-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-Tumor Efficacy. Front Immunol (2018) 9:2905. doi: 10.3389/fimmu.2018.02905
46. Pachella LA, Madsen LT, Dains J. The Toxicity and Benefit of Various Dosing Strategies for Interleukin-2 in Metastatic Melanoma and Renal Cell Carcinoma. J Adv Pract Oncol (2015) 6(3):212. doi: 10.6004/jadpro.2015.6.3.3
47. Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural Killer Cell–Mediated Eradication of Neuroblastoma Metastases to Bone Marrow by Targeted Interleukin-2 Therapy. Blood (1998) 91(5):1706–15. doi: 10.1182/blood.V91.5.1706
48. Pretto F, Elia G, Castioni N, Neri D. Preclinical Evaluation of Il2-Based Immunocytokines Supports Their Use in Combination With Dacarbazine, Paclitaxel and Tnf-Based Immunotherapy. Cancer Immunol Immunother (2014) 63(9):901–10. doi: 10.1007/s00262-014-1562-7
49. Bentebibel S-E, Hurwitz ME, Bernatchez C, Haymaker C, Hudgens CW, Kluger HM, et al. A First-In-Human Study and Biomarker Analysis of Nktr-214, a Novel Il2rβγ-Biased Cytokine, in Patients With Advanced or Metastatic Solid Tumors. Cancer Discov (2019) 9(6):711–21. doi: 10.1158/2159-8290.CD-18-1495
50. Diab A, Hurwitz ME, Cho DC, Papadimitrakopoulou V, Curti BD, Tykodi SS, et al. Nktr-214 (Cd122-Biased Agonist) Plus Nivolumab in Patients With Advanced Solid Tumors: Preliminary Phase 1/2 Results of Pivot. J Clin Oncol (2018) 36(15_suppl):3006. doi: 10.1200/JCO.2018.36.15_suppl.3006
51. Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, et al. Cergutuzumab Amunaleukin (Cea-Il2v), a Cea-Targeted Il-2 Variant-Based Immunocytokine for Combination Cancer Immunotherapy: Overcoming Limitations of Aldesleukin and Conventional Il-2-Based Immunocytokines. Oncoimmunology (2017) 6(3):e1277306. doi: 10.1080/2162402X.2016.1277306
52. Ghasemi R, Lazear E, Wang X, Arefanian S, Zheleznyak A, Carreno BM, et al. Selective Targeting of Il-2 to Nkg2d Bearing Cells for Improved Immunotherapy. Nat Commun (2016) 7(1):12878. doi: 10.1038/ncomms12878
53. Kang TH, Mao CP, He L, Tsai YC, Liu K, La V, et al. Tumor-Targeted Delivery of Il-2 by Nkg2d Leads to Accumulation of Antigen-Specific Cd8+ T Cells in the Tumor Loci and Enhanced Anti-Tumor Effects. PloS One (2012) 7(4):e35141. doi: 10.1371/journal.pone.0035141
54. Lasek W, Zagozdzon R, Jakobisiak M. Interleukin 12: Still a Promising Candidate for Tumor Immunotherapy? Cancer Immunol Immunother (2014) 63(5):419–35. doi: 10.1007/s00262-014-1523-1
55. Stern AS, Podlaski FJ, Hulmes JD, Pan YC, Quinn PM, Wolitzky AG, et al. Purification to Homogeneity and Partial Characterization of Cytotoxic Lymphocyte Maturation Factor From Human B-Lymphoblastoid Cells. Proc Natl Acad Sci USA (1990) 87(17):6808–12. doi: 10.1073/pnas.87.17.6808
56. Trinchieri G, Wysocka M, D'Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, et al. Natural Killer Cell Stimulatory Factor (Nksf) or Interleukin-12 Is a Key Regulator of Immune Response and Inflammation. Prog Growth Factor Res (1992) 4(4):355–68. doi: 10.1016/0955-2235(92)90016-B
57. Zeh 3H, Hurd S, Storkus W, Lotze M. Interleukin-12 Promotes the Proliferation and Cytolytic Maturation of Immune Effectors: Implications for the Immunotherapy of Cancer. J Immunother Emphasis Tumor Immunol (1993) 14(2):155–61. doi: 10.1097/00002371-199308000-00012
58. Parihar R, Dierksheide J, Hu Y, Carson WE. Il-12 Enhances the Natural Killer Cell Cytokine Response to Ab-Coated Tumor Cells. J Clin Invest (2002) 110(7):983–92. doi: 10.1172/JCI15950
59. Angiolillo AL, Sgadari C, Tosato G. A Role for the Interferon-Inducible Protein 10 in Inhibition of Angiogenesis by Interleukin-12. Ann N Y Acad Sci (1996) 795(1):158–67. doi: 10.1111/j.1749-6632.1996.tb52664.x
60. Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, et al. Immunopathogenesis and Therapy of Cutaneous T Cell Lymphoma. J Clin Invest (2005) 115(4):798–812. doi: 10.1172/JCI24826
61. Robertson MJ, Pelloso D, Abonour R, Hromas RA, Nelson RP Jr., Wood L, et al. Interleukin 12 Immunotherapy After Autologous Stem Cell Transplantation for Hematological Malignancies. Clin Cancer Res (2002) 8(11):3383–93.
62. Robertson MJ, Chang H-C, Pelloso D, Kaplan MH. Impaired Interferon-Γ Production as a Consequence of Stat4 Deficiency After Autologous Hematopoietic Stem Cell Transplantation for Lymphoma. Blood (2005) 106(3):963–70. doi: 10.1182/blood-2005-01-0201
63. Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, et al. Phase I Evaluation of Intravenous Recombinant Human Interleukin 12 in Patients With Advanced Malignancies. Clin Cancer Res (1997) 3(3):409–17.
64. Bajetta E, Del Vecchio M, Mortarini R, Nadeau R, Rakhit A, Rimassa L, et al. Pilot Study of Subcutaneous Recombinant Human Interleukin 12 in Metastatic Melanoma. Clin Cancer Res (1998) 4(1):75–85.
65. Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. Effects of Single-Dose Interleukin-12 Exposure on Interleukin-12–Associated Toxicity and Interferon-Γ Production. Blood (1997) 90(7):2541–8.
66. Robertson MJ, Cameron C, Atkins MB, Gordon MS, Lotze MT, Sherman ML, et al. Immunological Effects of Interleukin 12 Administered by Bolus Intravenous Injection to Patients With Cancer. Clin Cancer Res (1999) 5(1):9–16.
67. Portielje JE, Kruit WH, Schuler M, Beck J, Lamers CH, Stoter G, et al. Phase I Study of Subcutaneously Administered Recombinant Human Interleukin 12 in Patients With Advanced Renal Cell Cancer. Clin Cancer Res (1999) 5(12):3983–9.
68. Waldmann TA. Cytokines in Cancer Immunotherapy. Cold Spring Harb Perspect Biol (2018) 10(12):a028472. doi: 10.1101/cshperspect.a028472
69. Amer MH. Gene Therapy for Cancer: Present Status and Future Perspective. Mol Cell Ther (2014) 2(1):27. doi: 10.1186/2052-8426-2-27
70. Teo PY, Cheng W, Hedrick JL, Yang YY. Co-Delivery of Drugs and Plasmid DNA for Cancer Therapy. Adv Drug Deliv Rev (2016) 98:41–63. doi: 10.1016/j.addr.2015.10.014
71. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-Viral Vectors for Gene-Based Therapy. Nat Rev Genet (2014) 15(8):541–55. doi: 10.1038/nrg3763
72. Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, et al. Tumor-Infiltrating Lymphocytes Genetically Engineered With an Inducible Gene Encoding Interleukin-12 for the Immunotherapy of Metastatic Melanoma. Clin Cancer Res (2015) 21(10):2278–88. doi: 10.1158/1078-0432.CCR-14-2085
73. Chmielewski M, Abken H. Car T Cells Transform to Trucks: Chimeric Antigen Receptor–Redirected T Cells Engineered to Deliver Inducible Il-12 Modulate the Tumour Stroma to Combat Cancer. Cancer Immunol Immunother (2012) 61(8):1269–77. doi: 10.1007/s00262-012-1202-z
74. Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, et al. Vaccination of Glioma Patients With Fusions of Dendritic and Glioma Cells and Recombinant Human Interleukin 12. J Immunother (2004) 27(6):452–9. doi: 10.1097/00002371-200411000-00005
75. Steel JC, Waldmann TA, Morris JC. Interleukin-15 Biology and Its Therapeutic Implications in Cancer. Trends Pharmacol Sci (2012) 33(1):35–41. doi: 10.1016/j.tips.2011.09.004
76. Budagian V, Bulanova E, Paus R, Bulfone-Paus S. Il-15/Il-15 Receptor Biology: A Guided Tour Through an Expanding Universe. Cytokine Growth Factor Rev (2006) 17(4):259–80. doi: 10.1016/j.cytogfr.2006.05.001
77. Isvoranu G, Marinescu B, Surcel M, Ursaciuc C, Manda G. Immunotherapy in Cancer-In Vivo Study of the Anti-Tumor Activity of the Il-15/Il-15r Alfa Combination in an Experimental Model of Melanoma. Farmacia (2015) 63:631–6.
78. Robinson TO, Schluns KS. The Potential and Promise of Il-15 in Immuno-Oncogenic Therapies. Immunol Lett (2017) 190:159–68. doi: 10.1016/j.imlet.2017.08.010
79. Stoklasek TA, Schluns KS, Lefrançois L. Combined Il-15/Il-15rα Immunotherapy Maximizes Il-15 Activity in Vivo. J Immunol (2006) 177(9):6072–80. doi: 10.4049/jimmunol.177.9.6072
80. Waldmann TA, Miljkovic MD, Conlon KC. Interleukin-15 (Dys)Regulation of Lymphoid Homeostasis: Implications for Therapy of Autoimmunity and Cancer. J Exp Med (2020) 217(1):e20191062. doi: 10.1084/jem.20191062
81. Waldmann TA. Interleukin-15 in the Treatment of Cancer. Expert Rev Clin Immunol (2014) 10(12):1689–701. doi: 10.1586/1744666X.2014.973856
82. Isvoranu G, Surcel M, Munteanu AN, Bratu OG, Ionita-Radu F, Neagu MT, et al. Therapeutic Potential of Interleukin-15 in Cancer (Review). Exp Ther Med (2021) 22(1):675. doi: 10.3892/etm.2021.10107
83. Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, et al. Safety (Toxicity), Pharmacokinetics, Immunogenicity, and Impact on Elements of the Normal Immune System of Recombinant Human Il-15 in Rhesus Macaques. Blood (2011) 117(18):4787–95. doi: 10.1182/blood-2010-10-311456
84. Waldmann TA, Dubois S, Miljkovic MD, Conlon KC. Il-15 in the Combination Immunotherapy of Cancer. Front Immunol (2020) 11:868. doi: 10.3389/fimmu.2020.00868
85. Stephenson K, Barra N, Davies E, Ashkar A, Lichty B. Expressing Human Interleukin-15 From Oncolytic Vesicular Stomatitis Virus Improves Survival in a Murine Metastatic Colon Adenocarcinoma Model Through the Enhancement of Anti-Tumor Immunity. Cancer Gene Ther (2012) 19(4):238–46. doi: 10.1038/cgt.2011.81
86. Robertson MJ, Mier JW, Logan T, Atkins M, Koon H, Koch KM, et al. Clinical and Biological Effects of Recombinant Human Interleukin-18 Administered by Intravenous Infusion to Patients With Advanced Cancer. Clin Cancer Res (2006) 12(14 Pt 1):4265–73. doi: 10.1158/1078-0432.CCR-06-0121
87. Robertson MJ, Kirkwood JM, Logan TF, Koch KM, Kathman S, Kirby LC, et al. A Dose-Escalation Study of Recombinant Human Interleukin-18 Using Two Different Schedules of Administration in Patients With Cancer. Clin Cancer Res (2008) 14(11):3462–9. doi: 10.1158/1078-0432.CCR-07-4740
88. Tarhini AA, Millward M, Mainwaring P, Kefford R, Logan T, Pavlick A, et al. A Phase 2, Randomized Study of Sb-485232, Rhil-18, in Patients With Previously Untreated Metastatic Melanoma. Cancer (2009) 115(4):859–68. doi: 10.1002/cncr.24100
89. Srivastava S, Pelloso D, Feng H, Voiles L, Lewis D, Haskova Z, et al. Effects of Interleukin-18 on Natural Killer Cells: Costimulation of Activation Through Fc Receptors for Immunoglobulin. Cancer Immunol Immunother (2013) 62(6):1073–82. doi: 10.1007/s00262-013-1403-0
90. Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained Effector Function of Il-12/15/18–Preactivated Nk Cells Against Established Tumors. J Exp Med (2012) 209(13):2351–65. doi: 10.1084/jem.20120944
91. Fabbi M, Carbotti G, Ferrini S. Context-Dependent Role of Il-18 in Cancer Biology and Counter-Regulation by Il-18bp. J Leukoc Biol (2015) 97(4):665–75. doi: 10.1189/jlb.5RU0714-360RR
92. Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful Adoptive Transfer and in Vivo Expansion of Human Haploidentical Nk Cells in Patients With Cancer. Blood (2005) 105(8):3051–7. doi: 10.1182/blood-2004-07-2974
93. Cooley S, He F, Bachanova V, Vercellotti GM, DeFor TE, Curtsinger JM, et al. First-In-Human Trial of Rhil-15 and Haploidentical Natural Killer Cell Therapy for Advanced Acute Myeloid Leukemia. Blood Adv (2019) 3(13):1970–80. doi: 10.1182/bloodadvances.2018028332
94. Zhou T, Damsky W, Weizman O-E, McGeary MK, Hartmann KP, Rosen CE, et al. Il-18bp Is a Secreted Immune Checkpoint and Barrier to Il-18 Immunotherapy. Nature (2020) 583(7817):609–14. doi: 10.1038/s41586-020-2422-6
95. Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, et al. Il-18 Induces Pd-1–Dependent Immunosuppression in Cancer. Cancer Res (2011) 71(16):5393–9. doi: 10.1158/0008-5472.CAN-11-0993
96. Bhatt S, Sarosiek KA, Lossos IS. Interleukin 21–Its Potential Role in the Therapy of B-Cell Lymphomas. Leuk Lymphoma (2017) 58(1):17–29. doi: 10.1080/10428194.2016.1201568
97. Sarosiek KA, Malumbres R, Nechushtan H, Gentles AJ, Avisar E, Lossos IS. Novel Il-21 Signaling Pathway Up-Regulates C-Myc and Induces Apoptosis of Diffuse Large B-Cell Lymphomas. Blood (2010) 115(3):570–80. doi: 10.1182/blood-2009-08-239996
98. Gelebart P, Zak Z, Anand M, Dien-Bard J, Amin H, Lai R. Interleukin-21 Effectively Induces Apoptosis in Mantle Cell Lymphoma Through a Stat1-Dependent Mechanism. Leukemia (2009) 23(10):1836–46. doi: 10.1038/leu.2009.100
99. Scheeren FA, Diehl SA, Smit LA, Beaumont T, Naspetti M, Bende RJ, et al. Il-21 Is Expressed in Hodgkin Lymphoma and Activates Stat5: Evidence That Activated Stat5 Is Required for Hodgkin Lymphomagenesis. Blood (2008) 111(9):4706–15. doi: 10.1182/blood-2007-08-105643
100. Wu L, Ehlin-Henriksson B, Zhu H, Ernberg I, Klein G. Ebv Counteracts Il-21-Induced Apoptosis in an Ebv-Positive Diffuse Large B-Cell Lymphoma Cell Line. Int J Cancer (2013) 133(3):766–70. doi: 10.1002/ijc.28067
101. Bard JD, Gelebart P, Anand M, Zak Z, Hegazy SA, Amin HM, et al. Il-21 Contributes to Jak3/Stat3 Activation and Promotes Cell Growth in Alk-Positive Anaplastic Large Cell Lymphoma. Am J Pathol (2009) 175(2):825–34. doi: 10.2353/ajpath.2009.080982
102. Martinez Bedoya D, Dutoit V, Migliorini D. Allogeneic Car T Cells: An Alternative to Overcome Challenges of Car T Cell Therapy in Glioblastoma. Front Immunol (2021) 12:640082. doi: 10.3389/fimmu.2021.640082
103. Rafiq S, Hackett CS, Brentjens RJ. Engineering Strategies to Overcome the Current Roadblocks in Car T Cell Therapy. Nat Rev Clin Oncol (2020) 17(3):147–67. doi: 10.1038/s41571-019-0297-y
Keywords: cytokines, immunotherapy, interleukins, cancer, lymphoma
Citation: Atallah-Yunes SA and Robertson MJ (2022) Cytokine Based Immunotherapy for Cancer and Lymphoma: Biology, Challenges and Future Perspectives. Front. Immunol. 13:872010. doi: 10.3389/fimmu.2022.872010
Received: 09 February 2022; Accepted: 22 March 2022;
Published: 20 April 2022.
Edited by:
Jun Wang, Dalhousie University, CanadaReviewed by:
Kaiyuan Ni, Massachusetts Institute of Technology, United StatesFrancisco Borrego, Biocruces Bizkaia Health Research Institute, Spain
Copyright © 2022 Atallah-Yunes and Robertson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suheil Albert Atallah-Yunes, c2F0YWxsYUBpdS5lZHU=
 Suheil Albert Atallah-Yunes
Suheil Albert Atallah-Yunes Michael J. Robertson
Michael J. Robertson