- Department of Dermatology, University of Texas Southwestern Medical Center, Dallas, TX, United States
Lupus erythematosus is an autoimmune disease that may manifest in a variety of organs and tissues including the skin, kidney, brain, heart and lung. Many patients present with cutaneous lupus, where disease is often limited to the skin, but are at risk for developing systemic lupus. The objective of our present study is to perform a systematic review of studies that investigated patient cohorts and populations for the occurrence of cutaneous lupus progressing to systemic lupus. Inclusion criteria required that studies present longitudinal data of patients with limited cutaneous lupus erythematosus who were followed for development of systemic lupus erythematosus. Studies were excluded if patients had concurrent diagnosis of SLE, or if they failed to present longitudinal data. Medline and Embase were searched for English language studies using the Ovid platform. A total of 25 adult studies were identified, as well as 8 pediatric studies. The rate of cutaneous to systemic lupus progression ranged between 0% to 42% in the adult studies and 0% to 31% in the pediatric groups. The variability in these rates were due to differences in patient populations, study design, criteria used to diagnose systemic lupus, and follow-up time. Common risk factors associated with systemic lupus erythematosus development including having positive anti-nuclear antibodies, hematologic abnormalities, and higher number of lupus classification criteria at baseline. This study emphasizes the importance for providers to routinely monitor for systemic lupus in patients with cutaneous lupus.
Introduction
Cutaneous lupus erythematosus (CLE) is an autoimmune skin disease with a wide range of clinical presentations. Several subtypes exist including acute cutaneous lupus (ACLE), subacute cutaneous lupus (SCLE), and chronic cutaneous lupus (CCLE), with the most common CCLE subtype being discoid lupus erythematosus (DLE). As early as 1872, Moritz Kaposi identified a characteristic subset of patients with DLE and found that while they may present with limited cutaneous disease, some may progress to systemic involvement (1). Systemic involvement can range from mild in severity, affecting only a single organ system, to potentially severe systemic involvement, affecting multiple organ systems.
Since then, several classification criteria, including the American Rheumatism Association (ARA) criteria, American College of Rheumatology (ACR) criteria, Systemic Lupus International Collaborating Clinics (SLICC) criteria, and the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) criteria, have been developed to help clinicians monitor for the progression of CLE to systemic lupus erythematosus (SLE) (2–5). Clinically, the risk of patients with isolated CLE developing SLE is an area of interest to both the dermatologist and rheumatologist, and CLE patients. Current screening recommendations suggest monitoring patients for various lab abnormalities and clinical symptoms included in the lupus classification criteria sets, including the development of hematological abnormalities, autoantibodies including anti-nuclear antibodies (ANA) and double-stranded DNA (dsDNA) antibodies, and signs of joint, kidney or neurologic involvement (6). Current standard of care involves checking CLE patients for systemic disease on presentation as well as interval assessments for the development of SLE (6, 7).
The phenomenon of CLE developing to SLE has been studied in a variety of settings and populations, with the rate of progression ranging from zero to over thirty percent (8–10). Notably, methodologies amongst studies have often differed with respect to the studied population, definitional criteria of SLE, length of follow up, and study design. Prior reviews aimed at summarizing these studies have been limited to narrative reviews, narrow timeframe, or confined to a single subtype of CLE (11, 12). In order to better summarize these data, we performed a systematic reviews of all studies that have investigated patient cohorts and populations for the occurrence of CLE progressing to SLE. The information gleaned from this systematic review will help equip providers with counseling these patients about their prognosis and direct the management of these patients to track disease progression.
Methods
This systematic review was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (13). The objective was to identify studies of patients with skin limited cutaneous lupus and the rates of development of systemic lupus to better examine how studies evaluate and characterize this transition. The primary outcome of interest was the proportion of patients with CLE who developed SLE. Inclusion criteria were that studies identified cohorts of patients with CLE without SLE initially. Studies were excluded if patients had concurrent presentation of CLE and SLE, or did not present longitudinal data (either retrospective or prospective) for the development of SLE.
English language literature was searched using the MEDLINE and Embase databases. Databases were searched from inception until the date of the search using the Ovid platform. Databases were searched for articles with keywords, titles, abstracts including cutaneous lupus or its subtypes (i.e. discoid lupus, lupus panniculitus, lupus profundus, bullous lupus, subacute cutaneous lupus, lupus tumidus) and systemic lupus. Two separate reviewers (P.C. and A.W.) independently appraised all studies meeting inclusion and exclusion criteria. Disagreements were discussed and consensus reached involving a third reviewer (B.F.C.) whenever appropriate. Full text articles were then screened for inclusion in the present study and reference lists of primary studies were searched for additional studies meeting inclusion criteria.
Results
After removing duplicates in the OVID platform, a total of 2,842 titles and abstracts were screened for articles potentially meeting inclusion criteria. Of these, 85 full-text articles were selected for in-depth review with a total of 33 articles relevant articles identified meeting our inclusion criteria. This included 25 articles of adult CLE patients, and 8 pediatric CLE studies, which will be summarized in the following sections. A complete PRISMA flow chart is included in Supplementary Figure 1 (13).
Adult CLE
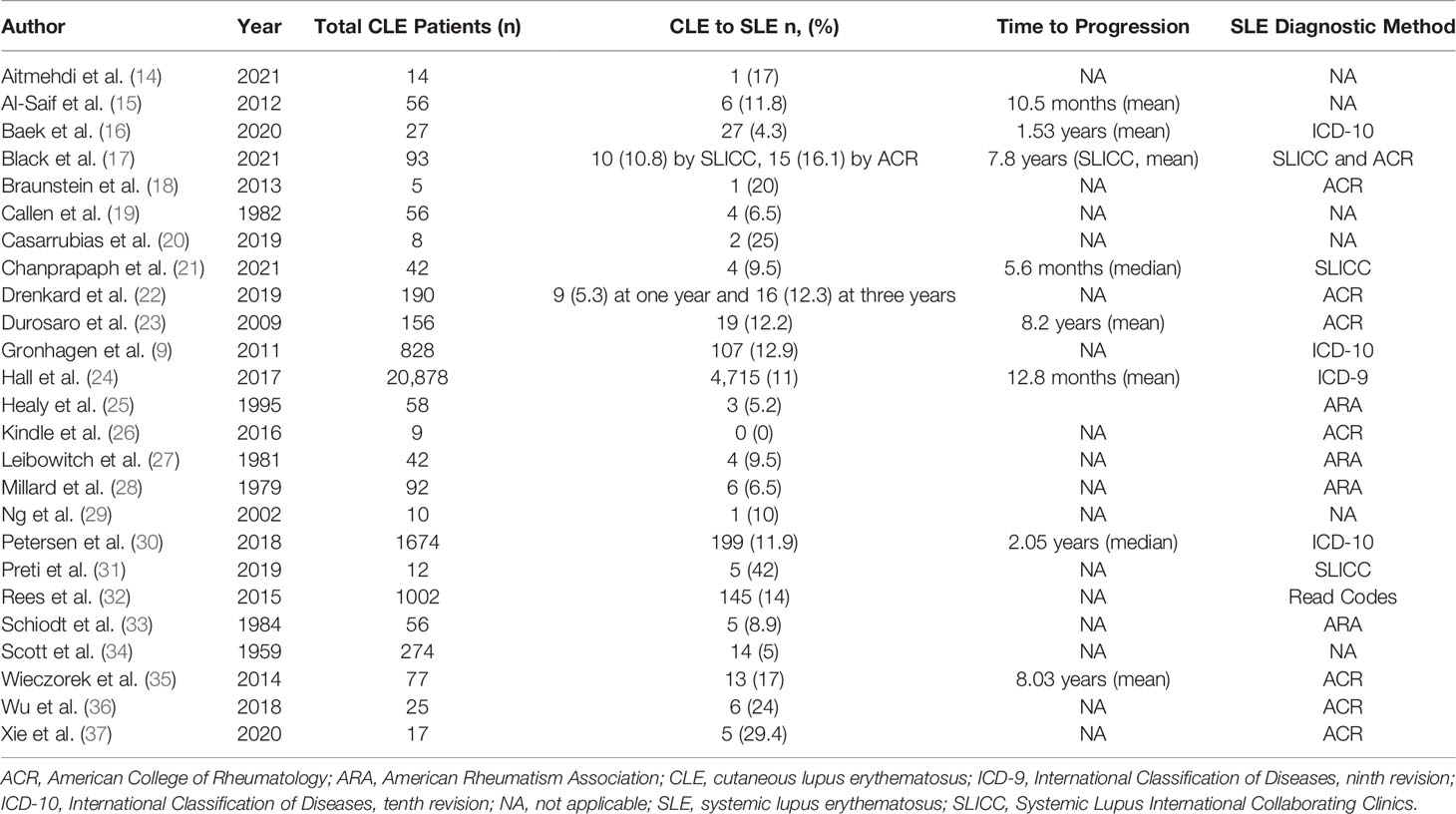
Studies looking at adult CLE patients reported a broad range of CLE to SLE progression. The rate of CLE to SLE progression ranged from 0 to 42 percent of CLE patients developing SLE (Table 1). The number of patients with CLE only and therefore eligible to progress varied widely amongst studies, ranging from small cohorts of only 5 patients to large, database studies of over 20,000 patients (18, 24, 30). DLE was the most commonly studied CLE subtype amongst all studies examined (20/25). SCLE was the second most commonly represented subtype (10/25). Notably, one study found that patients with SCLE had higher rates of progression than those with DLE (9). Most studies analyzed CLE patients from multiple subtypes. While several studies did report on various CLE subtypes other than DLE (e.g. lupus erythematosus panniculitis, lupus erythematosus tumidus), this accounted for a relatively small proportion of the overall data studied.
Studies used several different metrics to define SLE. Most studies (7/25) used the 1982 ACR SLE criteria (18, 22, 23, 26, 35–37). Four studies pre-dated the development of the 1982 ACR criteria and used ARA criteria (25, 27, 28, 33). Two studies used the 2012 SLICC classification criteria (21, 31). None have employed the 2019 EULAR/ACR criteria. One study used more than one classification criteria set to compare rates of CLE to SLE progression. From a cohort of 93 patients with CLE, our group reported 10.8% developing SLE under the SLICC criteria and 16.1% under the ACR criteria, highlighting potential differences between criteria sets (17). Five adult studies used diagnostic codes for large data sets (9, 16, 24, 30, 32). Six studies did not specify a defined criteria set/methodology (14, 15, 19, 20, 29, 34).
The length of follow up was variable among studies. For instance, 11 out of 25 studies only reported a range of years from which records were reviewed instead of average follow-up time (9, 15, 20–23, 27, 30–33). Some studies chose to report a range of years from which records were obtained and a minimum length of follow up of 6 months (16, 17, 37). Other studies chose to report median or mean length of time to follow up, ranging from a median of 40 to 48 months or a mean of 16.7 months to 5.75 years (14, 19, 26, 29). In addition, some studies reported variable rates that were dependent on length of follow up. For instance, Gronhagen et al. reported that when follow up data for one year was analyzed, 9.7% of CLE patients developed SLE; when sufficient follow up data was available for 3 years, this shifted to 16.7% (9).
Heterogeneous data on risk factors for CLE to SLE progression and time to progression were available from a minority of studies. From the adult studies, the most common patient and clinical risk factors associated with SLE development included positive ANA (5/25), hematologic abnormalities (2/25), and number of classification criteria met at baseline (2/25) (15, 17, 21, 25, 28, 35). Studies often differed on significant risk factors. Al-Saif et al. reported that CLE patients who progressed to SLE had more sunlight exposure, were ANA positive, and had a positive dsDNA antibody. They also found that progression of disease was significantly correlated with an earlier age of onset (p=0.044). Our group identified baseline risk factors for disease progression under the SLICC criteria including positive ANA (p=0.02), SLICC immunologic criteria (p=0.002), and SLICC total criteria (p=0.007) (17). Other studies identified baseline risk factors including non-scarring alopecia and high initial ANA titer ≥1:320 (21), hematologic abnormalities and positive ANA (28), and mucocutaneous criteria, positive ANA, total number of ACR criteria, and generalized DLE (35). Time to progression was reported inconsistently among studies and ranged anywhere from a mean of 5.6 months to a median of 8.2 years for adult cohorts (21, 23). One study reported significantly different median time to progression for subtypes of CLE including 3.04 years for DLE, 1.65 years for SCLE, and 1.04 years for localized CLE (p=0.018) (30).
Pediatric CLE
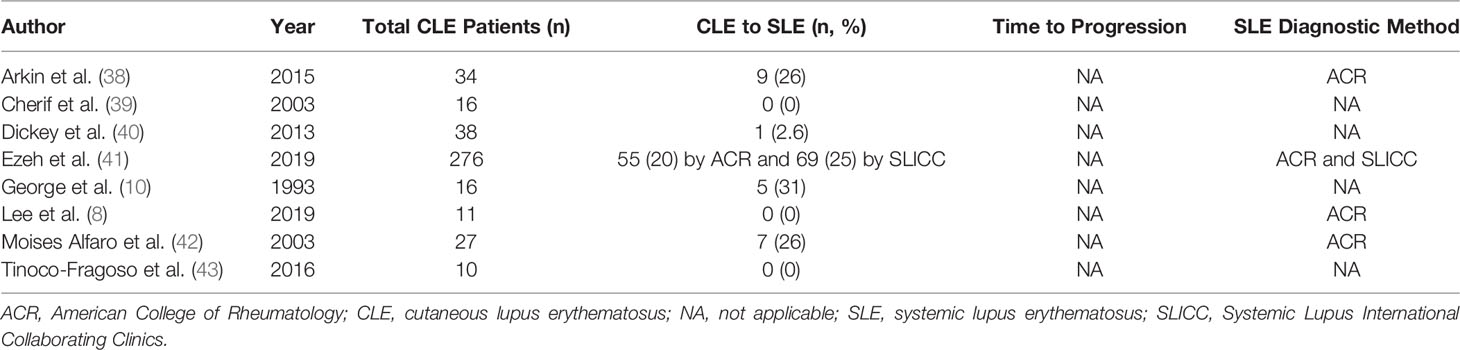
Eight studies looking at CLE to SLE progression amongst pediatric cohorts were found. Similar to the adult cohort studies, there was also a broad range of progression rates among pediatric populations, ranging from 0 to 31 percent of patients developing SLE (Table 2). However, the cohort size of patients with CLE and therefore eligible to progress to SLE was notably smaller than that of adult cohort studies, ranging from 10 to 276 total patients (41, 43). Similar to adult studies, DLE was the most commonly analyzed subtype representing over 60% of pediatric studies. Two studies examined a mixed cohort of multiple subtypes (8, 40). One small cohort study was dedicated to lupus erythematosus profundus (43).
In terms of criteria sets for SLE diagnosis, pediatric studies most commonly used the ACR criteria to define SLE progression (3/8 studies) (8, 38, 42). Ezeh et al. reported rates of progression for both ACR (20%) and SLICC (25%) criteria in the same cohort of patients (41). The remainder of pediatric studies did not specify a specific classification or diagnostic criteria used to determine the progression of CLE to SLE in their patient cohorts (10, 39, 40, 43). Like adult studies, follow-up length for pediatric cohorts was variably reported, with studies reporting a median follow up time ranging between 1 and 11 years (8, 10).
Only three studies commented on risk factors for progression. Risk factors included: higher age at diagnosis of DLE and positive autoantibodies, positive serologies and higher-titer ANA, and positive family history for rheumatic disease (p<0.05) (38, 41, 42). Only one study, Arkin et al., reported data on time to progression and noted that pediatric patients were at greatest risk for CLE to SLE progression within the first year after CLE diagnosis (38). However, they note that their study was limited to a follow-up duration of 5 years.
Discussion
This systematic review encompassed a broad range of studies, reporting on both adult and pediatric CLE groups. In adults, all but one study showed a proportion of CLE patients ultimately developing SLE. While a minority of CLE patients will go on to develop SLE, this proportion is sizeable enough to highlight the need for CLE patients to have ongoing monitoring for the development of SLE. Interestingly, data was somewhat more bimodal in the pediatric studies, with several studies reporting that no CLE patients progressing to SLE, but other studies reporting higher risk of 20%-30%. This discrepancy in reported risks may reflect study level characteristics or varying patient populations. The relatively limited number of pediatric studies highlights the need for more data to better characterize the risk of developing SLE within the pediatric population.
Studies used a variety of different metrics to define SLE. Larger population studies used diagnostic codes to identify patients with SLE. While this may be less rigorous on a patient level basis, it does allow for examining a significantly broader segment of the population and provide greater context of this phenomenon. For smaller studies, specific SLE classification criteria, including the ARA, ACR, and SLICC criteria, were employed for each patient and their disease course. Studies that examined multiple diagnostic criteria both supported the risk of transition to SLE. The similarly reported rates within studies that employed multiple SLE diagnostic criteria suggests that this distinction may not account greatly for the discrepancies in progression rates between studies. For example, Ezeh et al. reported on both SLICC and ACR criteria, yielding 20% progression under ACR criteria and 25% under SLICC criteria (41). Conversely, Black et al. reported 10.8% development from CLE to SLE using SLICC criteria and 16.1% with ACR criteria (17). The small variation in rates were thought to be, in part due to application of photosensitivity as a diagnostic criteria in ACR but not SLICC.
A variety of risk factors have been proposed to influence the risk of development of SLE, which was more commonly studied in adult CLE patients than pediatric CLE patients. Disease severity, CLE subtype, autoantibodies (anti-dsDNA and anti-Smith), arthritis, and high titers of ANAs have been reported to be more commonly found in CLE patients progressing to SLE than those who have not (11, 44). In our review of prior studies, the most common risk factor reported was a positive ANA (15, 17, 21, 28, 35, 41). Other common risk factors included hematologic abnormalities, age at CLE onset, lupus specific antibodies like dsDNA, and mucocutaneous criteria (15, 21, 25, 28, 35, 38, 41). Disparities in risk factor reporting can be attributed to differences in study design, population, and methods of reporting SLE diagnosis. Future larger-scale studies with uniform SLE diagnosis reporting are needed to further confirm risk factors that portend higher chance for systemic progression in CLE patients. In addition, most CLE patients who ultimately progressed to SLE in the studies examined by this review rarely met criteria that would signify involvement of major organ systems (e.g. renal, neuro), highlighting the overall mild severity of systemic involvement seen in CLE patients who progress to SLE (17, 21, 35).
It has been hypothesized that antimalarial treatment with may slow or prevent the progression of systemic disease (45). To address this hypothesis, there is an ongoing multi-center randomized controlled trial looking at whether hydroxychloroquine can halt progression of lupus in at-risk individuals such as those with CLE (46). Given that lupus medications may slow development to SLE, the rate of progression may be higher in untreated CLE individuals. While none of the reported studies looked at effects of therapies on progression, we hypothesize that because most patients in these studies were under treatment, reported rates of progression from CLE to SLE may be conservative.
In conclusion, this study summarized findings from adult and pediatric CLE patient groups showing ranges of progression to SLE. Prior studies showing up to 42% of CLE patients progressing to SLE highlight the importance for monitoring CLE patients for the development of systemic disease clinically at routine intervals. We recommend that providers perform complete review of systems to identify any new systemic symptoms such as small joint pains, and thorough skin exams to check for worsening skin disease and presence of oral ulcers lasting more than two weeks. Laboratory tests including ANAs and complete blood counts can be also ordered, with positive ANA titers being followed up with additional autoantibody tests including dsDNA and extractable nuclear antibody tests (6). Importantly, larger multi-center studies using standard and uniform reporting of SLE diagnosis and heterogeneous populations are necessary to better estimate rates of and identify risk factors for development of SLE in CLE patients.
Author Contributions
PC, AW, and BC contributed to conception and design of the study. PC and AW contributed to the acquisition and analysis of the data. PC and AW drafted the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
BC is an investigator for Daavlin Corporation and Biogen Incorporated and Pfizer Incorporated. He is a consultant for Bristol Meyers Squibb, EMD Serono, Horizon Therapeutics, and Biogen Incorporated.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.866319/full#supplementary-material
Supplementary Figure 1 | PRISMA Flow Diagram for literature search. Diagram shows searching and selection strategy at each stage of search.
References
1. Smith CD, Cyr M. The History of Lupus Erythematosus: From Hippocrates to Osler. Rheum Dis Clin North Am (1988) 14(1):1–14. doi: 10.1016/S0889-857X(21)00942-X
2. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol (2019) 71(9):1400–12. doi: 10.1002/art.40930
3. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum (1982) 25(11):1271–7. doi: 10.1002/art.1780251101
4. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and Validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum (2012) 64(8):2677–86. doi: 10.1002/art.34473
5. Cohen AS. Preliminary Criteria for the Classification of Systemic Lupus Erythematosus. Bull Rheum Dis (1971) 21:643–8.
6. O’Brien JC, Chong BF. Not Just Skin Deep: Systemic Disease Involvement in Patients With Cutaneous Lupus. J Invest Dermatol Symp Proc (2017) 18(2):S69–74. doi: 10.1016/j.jisp.2016.09.001
7. Lu Q, Long H, Chow S, Hidayat S, Danarti R, Listiawan Y, et al. Guideline for the Diagnosis, Treatment and Long-Term Management of Cutaneous Lupus Erythematosus. J Autoimmun (2021) 123:102707. doi: 10.1016/j.jaut.2021.102707
8. Lee SK, Baek J, Roh JY, Kim HJ. Clinical Characteristics of Pediatric Cutaneous Lupus Erythematosus: Experience From a Tertiary Referral Center in Korea. Lupus (2019) 28(7):888–92. doi: 10.1177/0961203319851568
9. Gronhagen CM, Fored CM, Granath F, Nyberg F. Cutaneous Lupus Erythematosus and the Association With Systemic Lupus Erythematosus: A Population-Based Cohort of 1088 Patients in Sweden. Br J Dermatol (2011) 164(6):1335–41. doi: 10.1111/j.1365-2133.2011.10272.x
10. George PM, Tunnessen WW Jr. Childhood Discoid Lupus Erythematosus. Arch Dermatol (1993) 129(5):613–7. doi: 10.1001/archderm.129.5.613
11. Chong BF, Song J, Olsen NJ. Determining Risk Factors for Developing Systemic Lupus Erythematosus in Patients With Discoid Lupus Erythematosus. Br J Dermatol (2012) 166(1):29–35. doi: 10.1111/j.1365-2133.2011.10610.x
12. Zhou W, Wu H, Zhao M, Lu Q. New Insights Into the Progression From Cutaneous Lupus to Systemic Lupus Erythematosus. Expert Rev Clin Immunol (2020) 16(8):829–37. doi: 10.1080/1744666x.2020.1805316
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (Clin Res Ed) (2021) 372:n71. doi: 10.1136/bmj.n71
14. Aitmehdi R, Arnaud L, Frances C, Senet P, Monfort JB, de Risi-Pugliese T, et al. Long-Term Efficacy and Safety Outcomes of Lenalidomide for Cutaneous Lupus Erythematosus: A Multicenter Retrospective Observational Study of 40 Patients. J Am Acad Dermatol (2021) 84(4):1171–4. doi: 10.1016/j.jaad.2020.11.014
15. Al-Saif FM, Al-Balbeesi AO, Al-Samary AI, Al-Rashid SB, Halwani M, Al-Mekhadab E, et al. Discoid Lupus Erythematosus in a Saudi Population: Clinical and Histopathological Study. J Saudi Soc Dermatol Dermatologic Surg (2012) 16(1):9–12. doi: 10.1016/j.jssdds.2011.09.003
16. Baek YS, Park SH, Baek J, Roh JY, Kim HJ. Cutaneous Lupus Erythematosus and Its Association With Systemic Lupus Erythematosus: A Nationwide Population-Based Cohort Study in Korea. J Dermatol (2020) 47(2):163–5. doi: 10.1111/1346-8138.15162
17. Black SM, Walocko F, Li X, Chong BF. Development of Systemic Lupus in Patients With Cutaneous Lupus Using the 2012 Systemic Lupus International Collaborating Clinics (Slicc) Classification Criteria for Systemic Lupus Erythematosus. J Am Acad Dermatol (2021) 85(1):200–2. doi: 10.1016/j.jaad.2020.12.061
18. Braunstein I, Goodman NG, Rosenbach M, Okawa J, Shah A, Krathen M, et al. Lenalidomide Therapy in Treatment-Refractory Cutaneous Lupus Erythematosus: Histologic and Circulating Leukocyte Profile and Potential Risk of a Systemic Lupus Flare. J Am Acad Dermatol (2012) 66(4):571–82. doi: 10.1016/j.jaad.2011.01.015
19. Callen JP. Chronic Cutaneous Lupus Erythematosus. Clinical, Laboratory, Therapeutic, and Prognostic Examination of 62 Patients. Arch Dermatol (1982) 118(6):412–6. doi: 10.1001/archderm.1982.01650180046015
20. Casarrubias AC, Flores SM. Lupus Panniculits: Experience From a Third-Level Hospital. J Dermatol Nurses’ Assoc Conference: 24th World Congress Dermatol Milan Italy (2020) 12(2).
21. Chanprapaph K, Tankunakorn J, Suchonwanit P, Rutnin S. Dermatologic Manifestations, Histologic Features and Disease Progression Among Cutaneous Lupus Erythematosus Subtypes: A Prospective Observational Study in Asians. Dermatol Ther (2021) 11(1):131–47. doi: 10.1007/s13555-020-00471-y
22. Drenkard C, Shenvi N, Easley K, Lim SS. The Georgia Lupus Registry: A Population-Based Estimate of the Incidence of Sle in Patients With Chronic Cutaneous Lupus. Lupus (2010) 1):10. doi: 10.1177/09612033100190010101
23. Durosaro O, Davis MDP, Reed KB, Rohlinger AL. Incidence of Cutaneous Lupus Erythematosus, 1965-2005 a Population-Based Study. Arch Dermatol (2009) 145(3):249–53. doi: 10.1001/archdermatol.2009.21
24. Hall SA, Allen JK, Payas N, Merola JF, Franchimont N, Dilley AB. Temporal Relationship of Cutaneous Lupus Erythematosus and Systemic Lupus Erythematosus: A Large, Retrospective Cohort Study. Lupus Sci Med (2017) 4(Supplement 1):A186–A7. doi: 10.1136/lupus-2017-000215.405
25. Healy E, Kieran E, Rogers S. Cutaneous Lupus Erythematosus - a Study of Clinical and Laboratory Prognostic Factors in 65 Patients. Irish J Med Sci (1995) 164(2):113–5. doi: 10.1007/BF02973274
26. Kindle SA, Wetter DA, Davis MDP, Pittelkow MR, Sciallis GF. Lenalidomide Treatment of Cutaneous Lupus Erythematosus: The Mayo Clinic Experience. Int J Dermatol (2016) 55:e431-9. doi: 10.1111/ijd.13226
27. Leibowitch M, Droz D, Noel LH, Avril MF, Leibowitch J. Clq Deposits at the Dermoepidermal Junction: A Marker Discriminating for Discoid and Systemic Lupus Erythematosus. J Clin Immunol (1981) 1(2):119–24. doi: 10.1007/BF00915389
28. Millard LG, Rowell NR. Abnormal Laboratory Test Results and Their Relationship to Prognosis in Discoid Lupus Erythematosus. A Long-Term Follow-Up Study of 92 Patients. Arch Dermatol (1979) 115(9):1055–8. doi: 10.1001/archderm.115.9.1055
29. Ng PPL, Tan SH, Tan T. Lupus Erythematosus Panniculitis: A Clinicopathologic Study. Int J Dermatol (2002) 41(8):488–90. doi: 10.1046/j.1365-4362.2002.01510.x
30. Petersen MP, Moller S, Bygum A, Voss A, Bliddal M. Epidemiology of Cutaneous Lupus Erythematosus and the Associated Risk of Systemic Lupus Erythematosus: A Nationwide Cohort Study in Denmark. Lupus (2018) 27(9):1424–30. doi: 10.1177/0961203318777103
31. Preti C, Bendjuia G, Manzano RE, Schroh R, Mascaro JM, Feinsilber D. Lupus Erythematosus Tumidus: A Clinical and Epidemiological Study. J Dermatol Nurses’ Assoc Conference: 24th World Congress Dermatol Milan Italy (2020) 12(2).
32. Rees F, Doherty M, Grainge M, Lanyon P, Davenport G, Zhang W. How Often Does Cutaneous Lupus Evolve Into Systemic Lupus? A Uk Cohort Study. Ann Rheum Dis (2015) 2:1090. doi: 10.1136/annrheumdis-2015-eular.1052
33. Schiodt M. Oral Discoid Lupus Erythematosus. Ii. Skin Lesions and Systemic Lupus Erythematosus in Sixty-Six Patients With 6-Year Follow-Up. Oral Surg Oral Med Oral Pathol (1984) 57(2):177–80. doi: 10.1016/0030-4220(84)90208-1
34. Scott A, Rees EG. The Relationship of Systemic Lupus Erythematosus and Discoid Lupus Erythematosus; a Clinical and Hematological Study. AMA Arch Derm (1959) 79(4):422–35. doi: 10.1001/archderm.1959.01560160040005
35. Wieczorek IT, Propert KJ, Okawa J, Werth VP. Systemic Symptoms in the Progression of Cutaneous to Systemic Lupus Erythematosus. JAMA Dermatol (2014) 150(3):291–6. doi: 10.1001/jamadermatol.2013.9026
36. Wu MY, Wang CH, Ng CY, Kuo TT, Chang YC, Yang CH, et al. Periorbital Erythema and Swelling as a Presenting Sign of Lupus Erythematosus in Tertiary Referral Centers and Literature Review. Lupus (2018) 27(11):1828–37. doi: 10.1177/0961203318792358
37. Xie Y, Liu B, Wu Z. Increased Interleukin-9 Levels in Skin Lesions From Cutaneous Lupus Erythematosus Patients May Predict the Progression to Systemic Lupus Erythematosus. J Dermatol Sci (2021) 101(1):78–80. doi: 10.1016/j.jdermsci.2020.10.016
38. Arkin LM, Ansell L, Rademaker A, Curran ML, Miller ML, Wagner A, et al. The Natural History of Pediatric-Onset Discoid Lupus Erythematosus. J Am Acad Dermatol (2015) 72(4):628–33. doi: 10.1016/j.jaad.2014.12.028
39. Cherif F, Mebazaa A, Mokni M, El Euch D, Azaiz MI, Dhahri ABO. Childhood Discoid Lupus Erythematosus: A Tunisian Retrospective Study of 16 Cases. Pediatr Dermatol (2003) 20(4):295–8. doi: 10.1046/j.1525-1470.2003.20402.x
40. Dickey BZ, Holland KE, Drolet BA, Galbraith SS, Lyon VB, Siegel DH, et al. Demographic and Clinical Characteristics of Cutaneous Lupus Erythematosus at a Paediatric Dermatology Referral Centre. Br J Dermatol (2013) 169(2):428–33. doi: 10.1111/bjd.12383
41. Ezeh N, Buhr K, Nguyen C, Al Ahmed O, Ardoin S, Barton V, et al. Baseline Clinical and Serological Findings in Pediatric-Onset Discoid Lupus Erythematosus: Analysis of a Multicenter Retrospective Cohort Study. Arthritis Rheumatol (2019) 71(Supplement 10):5091–4. doi: 10.1002/art.41108
42. Moises-Alfaro C, Berron-Perez R, Carrasco-Daza D, Gutierrez-Castrellon P, Ruiz-Maldonado R. Discoid Lupus Erythematosus in Children: Clinical, Histopathologic, and Follow-Up Features in 27 Cases. Pediatr Dermatol (2003) 20(2):103–7. doi: 10.1046/j.1525-1470.2003.20201.x
43. Tinoco-Fragoso F, Bernal-Lopez LE, Lammoglia-Ordiales L. Lupus Erythematosus Profundus in Children, a Case Series. J Am Acad Dermatol (2016) 1:AB214.
44. Zhu JL, Black SM, Chong BF. Role of Biomarkers in the Diagnosis and Prognosis of Patients With Cutaneous Lupus Erythematosus. Ann Trans Med (2021) 9(5):429. doi: 10.21037/atm-20-5232
45. James JA, Kim-Howard XR, Bruner BF, Jonsson MK, McClain MT, Arbuckle MR, et al. Hydroxychloroquine Sulfate Treatment Is Associated With Later Onset of Systemic Lupus Erythematosus. Lupus (2007) 16(6):401–9. doi: 10.1177/0961203307078579
Keywords: cutaneous lupus erythematosus (CLE), systemic lupus erythematosus, systematic review, autoimmunity, progression
Citation: Curtiss P, Walker AM and Chong BF (2022) A Systematic Review of the Progression of Cutaneous Lupus to Systemic Lupus Erythematosus. Front. Immunol. 13:866319. doi: 10.3389/fimmu.2022.866319
Received: 31 January 2022; Accepted: 21 February 2022;
Published: 11 March 2022.
Edited by:
V. Michael Holers, University of Colorado Denver, United StatesReviewed by:
Carlo Chizzolini, Université de Genève, SwitzerlandCopyright © 2022 Curtiss, Walker and Chong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin F. Chong, YmVuLmNob25nQHV0c291dGh3ZXN0ZXJuLmVkdQ==
 Paul Curtiss
Paul Curtiss Benjamin F. Chong
Benjamin F. Chong