- 1Department of Immunology, The University of Texas Southwestern Medical Center, Dallas, TX, United States
- 2Department of Microbiology, Immunology & Genetics, The University of North Texas Health Sciences Center, Fort Worth, TX, United States
- 3Department of Microbiology, The University of Texas Southwestern Medical Center, Dallas, TX, United States
- 4Department of Pediatrics, The University of Texas Southwestern Medical Center, Dallas, TX, United States
The thymus, a primary lymphoid organ, produces the T cells of the immune system. Originating from the 3rd pharyngeal pouch during embryogenesis, this organ functions throughout life. Yet, thymopoiesis can be transiently or permanently damaged contingent on the types of systemic stresses encountered. The thymus also undergoes a functional decline during aging, resulting in a progressive reduction in naïve T cell output. This atrophy is evidenced by a deteriorating thymic microenvironment, including, but not limited, epithelial-to-mesenchymal transitions, fibrosis and adipogenesis. An exploration of cellular changes in the thymus at various stages of life, including mouse models of in-born errors of immunity and with single cell RNA sequencing, is revealing an expanding number of distinct cell types influencing thymus functions. The thymus microenvironment, established through interactions between immature and mature thymocytes with thymus epithelial cells (TEC), is well known. Less well appreciated are the contributions of neural crest cell-derived mesenchymal cells, endothelial cells, diverse hematopoietic cell populations, adipocytes, and fibroblasts in the thymic microenvironment. In the current review, we will explore the contributions of the many stromal cell types participating in the formation, expansion, and contraction of the thymus under normal and pathophysiological processes. Such information will better inform approaches for restoring thymus functionality, including thymus organoid technologies, beneficial when an individuals’ own tissue is congenitally, clinically, or accidentally rendered non-functional.
Introduction
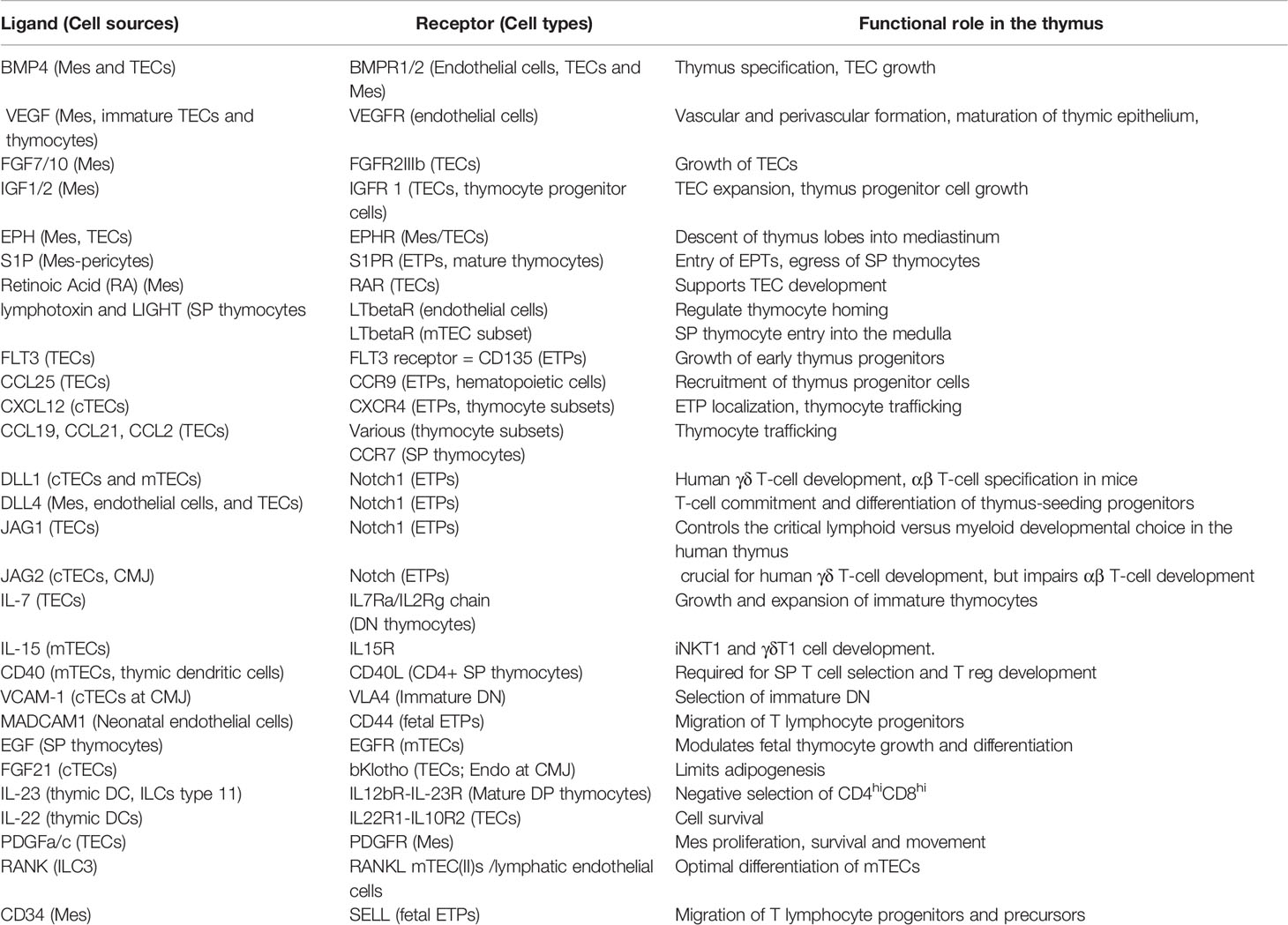
The thymus originates from the 3rd pharyngeal pouches (3rd PP), budding from one of the 5 temporary bilateral evaginations developing along the embryonic gut tube [reviewed in (1, 2)]. At this early stage, Paired Box 1 (PAX1), a member of the PAX family of transcription factors, is required for the patterning of these pharyngeal pouches. PAX1 is present in mesenchymal condensates surrounding the pouches, including the 3rd PP. Mutations in PAX1 cause thymus hypoplasia/aplasia, a phenotype more penetrant in humans than mice (3–6). The 3rd PP first patterns into the anterior-dorsal parathyroids at embryonic days 9.5-10 in the mouse, a time frame matching weeks 6-7 of gestation in humans. The ventrally positioned thymus anlage is specified at mouse embryonic days 10.5-11 (week 8 in human) (2). Both the parathyroids and thymus pattern as neural crest cell derived (NCC) mesenchymal cells (Mes) condense around a layer of columnar epithelial cells. Mes release Bone Morphogenic Protein 4 (BMP4), supporting the differentiation of some endothelial cells into thymus epithelial cells (TECs) (Table 1) (7). Vascular endothelial growth factor (VEGF), produced by Mes, and to some extent, by immature TECs and CD4-CD8- thymocytes, aids in tissue vascularization (8–11). A major change in the thymus occurs at e11.25 as immature TECs begin expressing the Forkhead Box N1 (FOXN1) transcription factor (1, 12, 13). FOXN1 positively regulates the expression of hundreds of genes, creating an environment suitable for T cell development (14). Among the up-regulated genes are those encoding chemokines, such as CCL25, which recruit early thymus progenitors (ETPs; also referred to as thymus progenitor cells) into the thymus tissue (15). The ETPs enter prior to tissue vascularization (16, 17). Thereafter, non-hematopoietic lineage cells, Mes, TECs and endothelial cells collectively cross-communicate with the hematopoietic cells to create a highly branched, three-dimensional (3D) epithelial meshwork (9, 18–21). This contrasts almost all other epithelial tissues in the body where cuboidal, squamous, or columnar features are retained (20, 22). As the thymus expands, Mes cells differentiate, forming the capsule, septae, perivascular cells (pericytes), vascular smooth muscle cells and fibroblasts. Pericytes envelope the endothelial vasculature and aid in nascent blood vessel formation (23). At early embryonic stages of thymopoiesis, T cell development proceeds in waves, with the earliest cell populations representing the γδ T cell lineage. Step-wise hematopoietic/non-hematopoietic cell-cell interactions promote thymus tissue expansion (19). These events are regulated by chemokine gradients, growth factor- and TNF- signaling pathways and Notch-notch ligand interactions, enabling immature TEC differentiation into two major subsets, cortical (cTECs) and medullary (mTECs) (Table 1). Cortical TECs secrete IL-7 and produce Delta-Ligand Like 4 (DLL4), supporting the expansion and differentiation of immature CD4-CD8- (DN) thymocytes (24–26). DLL4-Notch interactions induce expression of recombination activating genes (RAG1 and RAG2), initiating V(D)J recombination at the TCR beta locus (27). Those DN thymocytes successfully expressing the TCR beta protein signal via the pre-TCR complex, expand, and differentiate into CD4+CD8+ (DP) cells. At the DP stage, the TCR alpha locus undergoes RAG-mediated VJ recombination. DP cells successfully forming a cell surface αβ TCR complex then undergo a maturation/selection process. In this process, thymocytes expressing the appropriate TCR undergo positive and negative selection (28, 29). The selection is dictated by the capacity of the TCR to recognize self-peptide/MHC complexes expressed on the surface of either cTECs (mainly inducing positive selection) or mTECs (primarily inducing negative selection) [reviewed in (29–31)]. Positive selection establishes TCR self-restriction, which refers to those T cells with TCRs that have a weak avidity for self-peptide-self-MHC molecules. The selected thymocytes expand and differentiate into either CD4 or CD8 single positive (SP) cells. Countering positive selection, T cells expressing a TCR with too high an affinity for self-peptide/MHC are purged from the pool of immature thymocytes, a process termed negative selection. While negative selection can occur at the both DP and SP stages (29), a more robust clearance of potentially autoreactive T cells occurs when the SP cells enter the medullary region of the thymus (32). Therein, autoimmune regulator (AIRE) expressing mTECs purge autoreactive T cells [reviewed in (33, 34)]. The negative selection process also relies on thymus dendritic cells, which present peptide-MHC complexes stripped from the mTECs (35, 36). Finally, mTECs support the development of T regulatory cells (Tregs), a subset of CD4 SP cells that control the autoreactive potential of mature T cells due to their inherent ability to recognize self-peptide/MHC (30, 32, 37). Mature SP thymocytes surviving the selection gauntlet leave the thymus. This again involves chemokine gradients along with the release of sphingosine 1 phosphate (S1P) by pericytes at the corticomedullary junction (38, 39). S1P engages the S1P1 receptor on the mature SP thymocytes, facilitating their transit into the peripheral circulation. The hematopoietic cell seeding of the thymus and the developmental processes of positive and negative selection are maintained throughout life. However, the efficiency of these processes is dramatically curtailed during aging as adipogenesis, epithelial-to-mesenchymal transitions coupled with fibrosis disrupts the 3D structure of them meshwork and antagonizes thymopoiesis.
Neural Crest-Derived Mesenchymal Cell and Endothelial Cell Contributions to the Thymic Structure and Microenvironment
Neural crest cell (NCC)-derived mesenchymal cells (Mes) and endothelial cells are two key cell types essential for thymus formation, establishing the thymus vasculature. The vasculature serves as the entry site for T lymphocyte precursors or early thymus progenitors (ETPs), generated in fetal liver and bone marrow, to the thymic cortex. Endothelial cells form a cushion around the developing arterioles and veins, with the perivascular space comprising collagen fibers. Perivascular cells include pericytes and vascular smooth muscle cells (VSMCs) (40). The thymus anlage forms as Mes first localize around the 3rd PP (23). Mes release BMP4 and Sonic Hedgehog (SHH) in a spatially and temporally defined manner, initiating the patterning of the thymus and parathyroid regions, respectively (1, 7). Fibroblast growth factors (FGF7 and FGF10) and insulin growth factors (IGF1 and IGF2), secreted by Mes, are bound by the corresponding FGF- (FGFR2IIIb) and IGF- receptors (IGFR1) expressed on immature TECs, providing growth signals to the latter (41, 42). Multiple FGFs (FGF3, FGF8, FGF10, FGF15) are differentially expressed in a regionalized manner (43). Dysregulated activation of the FGF pathway leads to thymus hypoplasia, revealing the importance of temporal control of FGF expression levels (42, 43). A key role for Mes in thymus development has been shown using several distinct experimental approaches. First, extirpation of the cephalic neural folds in chick embryos (stage 9), a technique that depletes NCC-mesenchymal cells, results in thymus hypoplasia/aplasia (44). Second, mechanical removal of the Mes capsule from murine e12.5 thymuses causes a stunted expansion of the lobes when placed in culture (41, 45). Extraction of the capsule also limits tissue expansion when the thymus is grafted under the adult kidney capsule, despite adult kidney mesenchyme surrounding the tissue (41, 46–48). In the capsule stripped thymuses, T cell development is normal, as only a reduced cell number is noted relative to controls (41). Epidermal growth factor (EGF) addition can replace thymic mesenchyme to induce the lobulation of e13 embryonic thymuses, however this can occur in the absence of thymocytes (49, 50). Third, 22q11.2 deletion syndrome (often termed DiGeorge syndrome) causes congenital hypoplasia of the thymus (51, 52). The primary reason is a failure of Mes to support tissue expansion, established using murine models of the syndrome. Mesenchymal cells also induce MHC class II molecules on epithelial cells (49).
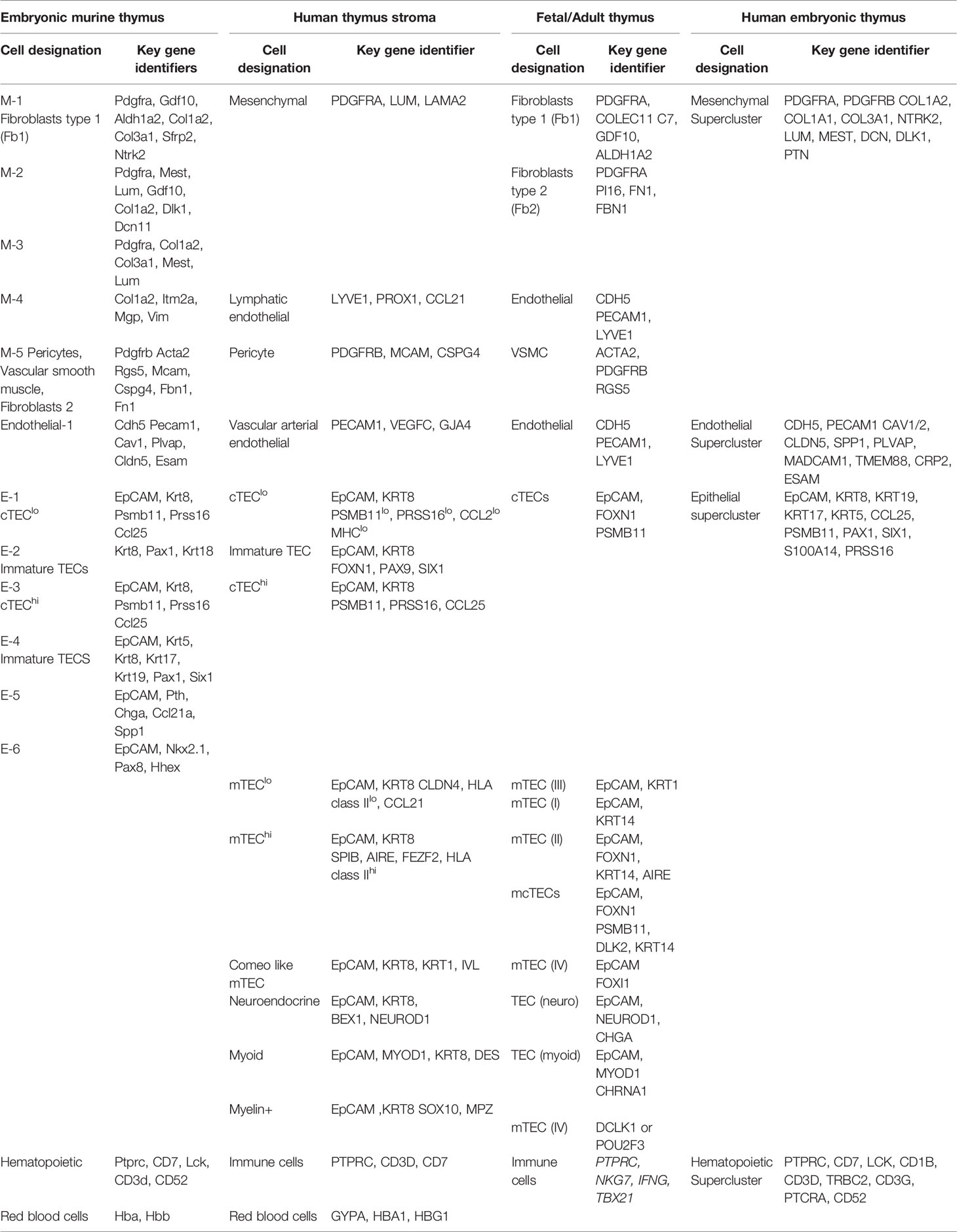
As the two thymus lobes expand, they pair and descend along the right and left subclavian arteries. The descent (between e11.0 and e12.5) involves erythropoietin-producing hepatocellular carcinoma (EPH) ligand-EPH receptor (EPHR) coupled Mes and TEC signaling (53). The Mes cells that differentiate into pericytes during embryogenesis are retained post-birth and into adulthood, determined by fate mapping neural crest cells with an embryonic specific Sox10-driven Cre expression (23, 54, 55). Such pericytes secrete sphingosine 1 phosphate (S1P) at the corticomedullary junction, which is needed to recruit thymus progenitor cells and enable the egress of the mature thymocytes into the circulation (38). Most studies have grouped the Mes populations collectively, principally based on the expression of Pdgfrα and/or fate mapping. More recent deep sequencing technologies, particularly single cell RNA sequencing is revealing a more complex heterogeneity among Mes cells (56, 57). Five Mes subtypes are evident in the developing embryonic thymus (Table 2), among these are pericytes, vascular smooth muscle cells and fibroblasts. Recent studies have revealed some heterogeneity between the capsular and medullary fibroblasts (58). Comparing human thymuses from fetal stages as well as postnatally by scRNA sequencing reveal 3 Mes subtypes; Fibroblast type 1 (PDGFRA, COLEC11, C7, GDF10), Fibroblast type 2 (PDGFRA, PI16, FN1, FBN1) and VSMCs (ACTA2) (Table 2) (57). Fibroblast types 1 and 2 (Fb1 and Fb2) are localized in peri lobular or interlobular positions, respectively. Fb1 cells express genes such as Aldehyde dehydrogenase 1A2 (ALDH1A2), which is needed for retinoic acid (RA) production, a morphogen that supports TEC development (47). Fb2 are often associated with large blood vessels lined with VSMCs. Fb2 express extracellular matrix protein and semaphorins that aid in vascular development (57). Notably, the fibroblast composition changes over time, with a Fibroblasts type 1 (Fb1) population prevalent in early developmental stages, while type 2 (Fb2) dominates in post-natal and adult thymus tissue (57). The antigens produced by these fibroblast subsets have a key role in central tolerance (58).
Endothelial cells are a second, critical non-hematopoietic cell type, needed for effective thymopoiesis. ScRNA sequencing data suggests the existence of one major endothelial cell type (56, 57, 59). As described in an earlier section, these cells form the vasculature/blood vessel network in the thymus. Of critical relevance to the specification of the embryonic thymus, some endothelial cells differentiate into TECs. The scRNA sequencing studies coupled with bioinformatics screens for paired ligand-receptor interactions between endothelial cells and thymocyte progenitors also reveals important contributions of MADCAM1-CD44, CFH-SELL, and CD34-SELL in thymopoiesis (Table 1). In adult tissues, endothelial cells regulate thymocyte homing via the lymphotoxin β receptor (LTβR) (60). The ligands for LTβR, lymphotoxin and LIGHT, are produced by mature T cells to activate endothelial-regulated homing functions. In adult thymus tissues following radiation-induced damage, the endothelial cells secrete BMP4 to support tissue regeneration (61). This is partly due to BMP4 enhancement of FOXN1 expression (61). It is likely that the embryonic endothelial cells also produce BMP4 in developing thymuses. In the post-natal thymus, the endothelial cells in the cortical region express high levels of claudin-5, which limits cell entry. This contrasts the endothelial cells in the cortical medullary junction (CMJ) and medulla, where many of the cells have lost claudin-5 expression, enabling T cell migration and egress following positive selection (62).
Epithelial Cell Control of Thymopoiesis
TECs are derived from endothelial cells around e10.5, beginning with the formation of immature bi-potent thymus epithelial cell (TEC) precursors (63, 64). These are defined by the expression of Cytokeratin 8, Cytokeratin 14, beta5t (encoded by Psmb11) and PLET1 (65). Immature TEC growth is sustained by various growth factors secreted by Mes, including FGFs and IGFs, as described above (Table 1). As the thymus and parathyroids coordinately expand, the immature TECs express high levels of E-cadherin, which facilitates the separation of the two tissues (53). The first identification of TEC progenitors revealed that mTECs and cTECs share a common origin (66, 67). Later complementary studies reported that embryonic TEPs expressing cortical markers can generate both cTECs and mTECs (68–70). The essential functional roles of TECs are mediated by FOXN1 (12, 71). FOXN1 is expressed in TECs following the initial specification of the thymus (10). The importance of FOXN1 in TECs was best revealed with spontaneously arising murine Foxn1 mutations along with the identification of humans who had autosomal recessive FOXN1 mutations, resulting in a Nude/SCID phenotype (12, 72–77). The Nude designation arises from alopecia universalis and nail dystrophy (75). In the skin, loss of FOXN1 prevents sufficient expression and deposition of keratins along the hair shaft, causing the hair follicle to curl and break instead of extruding through the epidermal layers (78). SCID arises because of an inability of the TECs to differentiate and expand without FOXN1, causing a block in T cell development at the early DN stage of thymopoiesis [reviewed in (13, 14, 79, 80)]. A key advance in understanding how FOXN1 controls TEC functions was the identification of its transcriptional targets by chromatin immunoprecipitation coupled with DNA sequencing. Using a FLAG-tagged Foxn1 BAC transgenic mouse line, a consensus nucleotide binding site of GACGC was identified (14). Among the ~500 or so direct targets of murine Foxn1 are chemokines and Notch ligands, Ccl25, Cxc112 and DLL4, respectively (14, 81). Other important gene targets include peptidases (e.g., Tasp1), proteases (e.g., Prss16), proteasome complex components (e.g., Psmb4, Psmb9, Psmb10, Psmb11, Psmb16, Psma4), peptide transporters required for MHC class I peptide presentation (e.g., Tap2), many keratins and Cd83 (14, 78, 81). Psmb11 encodes beta5t, a catalytic subunit of the thymus-specific proteasome (thymoproteome), generating peptides that are bound by MHC class I to support the positive selection of CD8+ T cells (81, 82). CD83 is required for the development of most CD4+ T cells (83). FOXN1 positively regulates itself, binding to the GACGC sequence present in its own promoter (78, 84, 85). In the skin, the Hoxc13 transcription factor positively regulates Foxn1 expression (78). Mutations in human HOXC13 diminish FOXN1 levels in the skin, causing ectodermal dysplasia, characterized by atrichia and nail dystrophy (86). Several Wnt glycoproteins also positively regulate Foxn1 expression (87). An analysis of conserved nucleotide sequences close to the Foxn1 target sequence reveals enrichment of TAp63 (one of two p63 gene transcription isoforms) and CREB binding sites. Notably, TAp63 participates in TEC homeostasis (88–90).
As immature TECs expand, they develop into two major subsets, designated as cortical or medullary TECs based on their location within the thymus. Cortical TECs are defined by the expression of Cathepsin L, TSPP and PSMB11 [reviewed in (91)]. These cells support the recruitment of early thymus progenitors from the blood via selected chemokines (CCL21, CCL25) as well as the progression of thymocytes from the DN to DP stages of thymopoiesis (CCL19, CCL25, CXCL12). CCL9, CCL21, CCL25, CCL12, positively regulated by FOXN1, provide directional cues for developing thymocytes (39, 92). DLL4 levels, which are much higher on cTECs than mTECs, signal immature thymocytes via Notch to progress from the DN to DP subset (25, 93). The conditional targeting of DLL4 in TECs leads to a severe failure of T cell development at the DN1 stage of early DN thymocyte progression. Likewise, the elimination of the receptor (Notch 1) for DLL4 on hematopoietic progenitor cells prevents T cell development (94). Cortical TECs also secrete IL7, a cytokine that stimulates DN thymocyte growth via the IL7Ra/IL2Rg receptor (24, 26).
The medullary regions of the thymus form during embryogenesis as small pockets that expand and coalesce into the larger clusters present post-natally. These mTECs differentiate/proliferate following interactions with mature SP thymocytes. In the embryonic period, Notch signaling is needed for the formation of mTECs, with RANK-mediated signaling more important for the mTECs post-natally (95, 96). The differentiation involves CD4 SP thymocyte expression of Notch and/or RANK in combination with CD40L and EGFR (95–101). Once formed, mTECs are defined by the expression of Cathepsin S, CD40, CCR7 ligand (CCL19/CCL21) and AIRE [reviewed in (91)]. Medullary TECs mediate negative selection of SP T cells along with the positive selection of T regulatory cell subsets (102, 103) [reviewed in (30, 32)]. One of the mTEC subsets produces the chemokines CCL19 and CCL25 to attract positively selected, CCR7 expressing thymocytes into the medullary region (104). Mice lacking the lymphotoxin beta receptor fail to develop these mTECs (105). As the SP thymocytes traffic through the medullary region, they interact with a second subset of mTECs, defined by the expression of the autoimmune regulator (AIRE) gene. AIRE enforces the expression of “tissue-restricted” proteins that eliminate autoreactive T cells (33, 106). AIRE facilitates this by releasing RNA polymerase II from promoter regions where this enzyme is stalled, a mechanism normally used to prevents widespread gene expression (107). Mesenchymal epithelial transition factor (c-Met) is expressed by TECs along with early T progenitors. The specific targeting of c-Met in TECs results in age-dependent progressive reduction in TECs number coupled with lower regulatory T cells (108). Similarly, the targeted loss of Shh in TECs reduces both cTEC and mTEC numbers (109). While most studies have detailed the development of cTEC and mTEC from embryonic TEC progenitors, thymus epithelial progenitors have also been identified in adult tissues (110, 111). These adult progenitor populations can give rise to both cortical and medullary TEC subsets (110, 111).
While initial characterizations of TECs relied on cell surface protein expression and fate mapping to define subsets, scRNA sequencing is revealing many additional epithelial and TEC subsets. For example, an analysis of embryonic day 13-13.5 fetal thymuses, when 80% of the cells are stromal (Mes, TEC, endothelial), reveal 6 distinct epithelial subsets (Table 2). Multiple other studies have revealed different TEC populations at different developmental stages (112–116). Among these are 2 subsets of immature TECs along with cTEClo and cTEChi subsets, with the lo and hi referring to MHC class II levels. At e13.-13.5 stage, no mTECs are evident. A 5th epithelial subset expresses parathyroid hormone (PTH), suggesting either the presence of some parathyroid TECs or the existence of a bi-functional TEC. The 6th epithelial subset (E-6) expresses Nkx2.1, which may be a thyroid or parathyroid precursor cell type. ScRNA sequencing of human thymuses obtained at gestational weeks 19 and 23 (e14.5-e16.5 in murine embryos) and postnatal samples from 6-day old newborn (e20) and an infant (10 months) reveals 3 major epithelial groups broken down into 9 subclusters (57). Two clusters are categorized as cTECs based on the characteristic genes (PSMB11, PRSS16, CCL25). One cluster is the cTEClo, which is rapidly proliferating based on Ki67+ expression. The second cluster is the cTEChi. mTECs are also evident in the embryonic tissues at this developmental stage, and split into 3 distinct subgroups partly defined by the levels of HLA class II. These are mTEClo (CLDN4 and lower levels of HLA class II), mTEChi (SPIB, AIRE, FEZF2, higher levels of HLA class II), and corneocyte-like mTECs (KRT1, IVL) (56). Cells in the mTEClo cluster express high levels of the chemokine CCL21, similar to the CCL21-expressing post-natal mTEClo population described in mice (105). Another epithelial cluster, marked by FOXN1, PAX9, and SIX1, represents immature TECs, and is evident in early developmental stages and remains in postnatal and adult periods. PAX9, and SIX1 are not present in cTECs or mTECs, implicating this cluster as a potential progenitor cell not yet committed to a specific lineage. The additional epithelial clusters identified in human thymuses include neuroendocrine (BEX1, NEUROD1), muscle-like myoid (MYOD1, DES), and myelin+ epithelial cell markers (SOX10, MPZ) (56, 57). A mixed medullary/cortical TEC subset (mcTECs), marked by expression of DLK2, is present in late fetal and post-natal human thymuses. Finally, a rare population of tuft-like mTECs is present in human and mouse thymuses, although the genes DCLK1 and POU2F3 used to define this population are not specific to TECs in humans (57).
The various TEC subsets constitute the basic thymic microenvironment. They provide cytokines, chemokines, multiple molecular signals, and cell-to-cell interactions to control thymocyte proliferation, differentiation, and selection. This process leads thymocytes from immature to mature, and in turn, TECs themselves also get maturation from bi-potent epithelial progenitors to functional cTECs and mTECs. The added complexity of many different subsets suggests distinct roles for each in the formation and regeneration of the thymus at different stages.
The Changing Cellular Landscape in an Aging Thymus
Post-adolescence, the thymus begins a slow and continuous atrophy (117, 118). Tissue changes include TEC loses, increasing epithelial-to-mesenchymal transitions (EMT) coupled with their differentiation into fibroblasts, adipogenesis, loss of cortical-medullary boundaries, and increases in perivascular spaces (118, 119). Although recruitment of thymus progenitors from the bone marrow is not critically impacted, alternations in T cell development, defects in T cell selection, a contracted TCR repertoire diversity, and reduced egress of naive SP T cells are apparent with aging (120). TEC loses by both EMT and cell death, coupled with the tapered expression of FOXN1 contribute to diminished thymopoiesis (91, 121, 122). In fact, enforced expression of Foxn1 in an aged thymus can restore thymus size and functionality, comparable to that seen with a young thymus (123–125). As the thymus ages, TEC production of IL-7 and FGF21 tapers. FGF21 losses increase intra-thymic adipogenesis and decrease peri-thymic brown adipose tissue (126). The obligate co-receptor for FGF21 is beta-Klotho (KLB), present on the endothelial cells at the corticomedullary junction (126). Reduced signaling via FGF21/KLB comprises endothelial functions. However, KLB-deficient mice have a pronounced thymus hypoplasia not intrinsic to TECs or bone marrow cells, revealing a systemic effect (127). Notably, the KLB-deficient mice have high levels of vitamin D, and thymus hypoplasia is rectified by nutritional restriction of Vitamin D (127).
By mid-age, ectopic adipocytes make up 50% of the thymus (119). Although the adipocytes come from a variety of tissues, mesenchymal transitions via EMT are one proposed source (126). The adipocytes fill the perivascular space and impede entry of thymus progenitor cells (128, 129). Intra-thymically distributed adipocytes also release cytokines, steroids, and hormones that negatively impact thymopoiesis (130–133). Among these are leukemia inhibitory factor (LIF), oncostatin M and IL-6. In mouse models of aging, caloric restriction or systemic administration of Ghrelin improves T cell output by reducing adipogenesis and delaying age-dependent thymic involution (134, 135). EMT further generates an expanding number of fibroblasts within the thymus, increasing fibroblast/TEC ratios (136). Whether these fibroblasts directly contribute to reduced thymus functions, as seen with pulmonary fibrosis, remains to be established (137).
Thymus Regeneration Technologies for the Young and the Old
The thymus is extremely stress sensitive, with transient cell losses reaching 90% evident following infections, glucocorticoid treatments, chemotherapy, and radiation exposure (138, 139). Dependent on the severity of the stress and/or the age of the thymus, the damage can be transient (if the damage is only in hematopoietic lineage cells) or permanent (the damage mostly happens in non-hematopoietic lineage cells). Because of this, many clinical interventions are being considered to improve/rejuvenate thymus functions. Among these are cytokines and growth factors that support the non-hematopoietic lineage stromal cell populations and/or developing thymocytes. For example, TEC differentiation/expansion can be improved with IL-22, Keratin growth factor (KGF), EGF, BMP4, RANKL and 2 microRNAs, miR-205 or miR-29a (61, 97, 99, 140–144). Group 3 innate lymphoid (ILC3s) cells in the thymus are one important cell population that facilitate thymus recovery following stress (145). In response to whole body radiation exposure, CD103+ thymus dendritic cells release IL-23, which signals the ILC3s to secrete IL-22. The IL-22 receptor (IL-22Ra/IL-10Rb) is principally expressed on cTECs and mTECs, with ligand mediated signaling supporting TEC survival and functionality (141). BMP4, produced by endothelial cells, similarly enhances TEC functions, including an upregulation of FOXN1 along with its downstream targets (61). Additional cytokines, ligands and chemokines that improve T cell development include IL-7, Fms-Like Tyrosine Kinase 3 Ligand (FLT3L), DLL4 and the chemokines CXCL12, CCL19, CCL21, and CCL25 (91, 146–150). In a clinical setting, enhanced thymopoiesis is accomplished by administration of FLT3L, and this is done for patients receiving a bone marrow transplant (151–153). FLT3L increases progenitor cell uptake into the thymus (152). The potential use of the many of the other proteins that support thymopoiesis for clinical treatments requires further validation as many will impact cell populations outside the thymus. Two possible solutions to overcome the pleotropic effects of these various cytokines/chemokines or ligands include direct intrathymic injections, or administration of encapsulated nanoparticles with selectively for the thymus (154, 155). For example, intrathymic injections of Foxn1, consisting of an COOH-terminal TAT transduction domain linked to full-length Foxn1, transiently improves TEC numbers and thymopoiesis in mouse models of hematopoietic stem cell transplantation (155). However, recombinant Foxn1 protein injections into a thymus also result in the protein entering hematopoietic cells, and this can have potentially harmful outcomes (156). Therefore, direct intrathymic injections of post-natally derived TECs or with Foxn1-reprogrammed fibroblasts are alternate strategies with good rejuvenation effects (157, 158). Furthermore, direct intrathymic injections of encapsulated cytokines and/or growth factors and even reprogrammed cells into the thymus may be of therapeutic value.
For individuals with in-born errors of immunity, the clinical treatment options for restoring thymopoiesis are not straightforward. First, an assessment of whether hematopoietic or stromal cell populations are causal to thymus hypoplasia is needed. If genetic mutations are inherent to the hematopoietic cells, bone marrow transplants are an effective clinical treatment (159). However, individuals wherein the non-hematopoietic stromal/epithelial cells of the thymus are impacted, allogeneic thymus tissue transplants remain the only FDA approved therapy (Enzyvant, Inc.) (160–162). For such tissue transplants, the donor thymus is depleted of most hematopoietic cells, leaving a residual mixture of pericytes, endothelial cells, TECs, and some mature CD4+ T cells (160). These transplant strategies work for those with a severe T cell lymphopenia. Among the patients who will benefit from such transplants include those with 22q11.2 deletion syndrome (DiGeorge), autosomal recessive FOXN1 mutations, PAX1 mutations, CHARGE (Coloboma, heart defects, anal atresia, growth retardation, genital abnormalities, ear abnormalities) syndrome, or those who had diabetic embryopathies (6, 80, 160). However, the allogeneic transplant approach is not suitable for individuals who retain some thymus functionality, since they will have sufficient peripheral T cell numbers to cause transplant rejection. Among these are patients undergoing chemo ablative therapies. An equally important group to consider are those who had partial or complete thymectomies, often done during restorative cardiothoracic surgeries since the thymus affects surgical access to the heart (163–165). In individuals who were thymectomized at a young age, low naïve peripheral T cell numbers and a reduced TCR repertoire is evident later in life (166, 167). Noteworthy, complete thymectomies are also done for patients with thymomas or the autoimmune disorder Myasthenia gravis (168). Sadly, radiation-mediated thymus ablation was a common standard-of-care treatment for infants during the years 1910-1960 (169, 170). The prevailing medical notion at the time was that a large thymus (which is actually normal) was causing lung compression and/or asthma, leading to sudden infant death syndrome and status thymicolymphaticus (171). Regardless of reasons for why a thymus is “removed”, there are currently no effective strategies for restoring thymopoiesis. Putative solutions emerge from recent advances in thymus regeneration technologies, supported with two lines of evidence. First, it is clear thymus transplants work for patients with a thymus aplasia, as well as with the Nude/SCID mice, where the transplanted tissue supports T cell development (161, 162, 172, 173). This indicates that artificial thymic organoid (ATO) technologies may become an effective clinical approach for tissue regeneration. Second, organoid technologies are rapidly advancing for many types of tissues, although regenerating an effective thymus remains a significant challenge (91).
A quintessential breakthrough for ATOs was the initial development of stromal cell lines (OP9) expressing the Notch ligands Delta-like ligand 1 (DLL1) or DLL4, with both supporting T cell development to the SP stage by engaging Notch on immature thymocytes (174, 175). DLL4 functions better than DLL1 due to the lack of a proline rich domain at the Module at the N-terminus of Notch Ligand (MNNL) (176). This is consistent with the non-redundant role for DLL4 in vivo. An important modification to the OP9 monolayer system was the use a unique murine stromal cell line that re-establishes a 3-d epithelial meshwork upon co-culture with hematopoietic stem and progenitor cells (HSPC) (177). HSPCs can be from either cord- or peripheral- blood or bone marrow (177). While the stromal cell lines were first developed to express human DLL1 (MS5-hDLL1), human DLL4 is now used (177–179). Grown in serum-free culture conditions to eliminate variations caused by serum, human T cell differentiation proceeds to the SP stage in the ATOs (177–179). While the ATO technology is a definite improvement, the organoids are not designed for transplant purposes. Limitations include the low overall numbers of T cells that are generated (1 to 3 x 106 cells), an abnormal CD4/CD8 ratio < 1 and the use of murine cell lines. ATOs can, however, reveal whether a patient with probable in-born error of immunity would need a bone marrow or thymus transplant. For example, patients with mutations in genes affecting stromal cell subsets (mesenchymal, TECs, endothelial) will retain HSPCs capable of thymus development (178, 179). Those with mutations affecting either hematopoietic cell differentiation and or T cell functions (IL2Rg chain, RAG, Artemis, T cell components) will not support T cell development, confirming that a patient will likely need a bone marrow transplants. Notably, the current ATOs now use DLL4 expressing stromal cells (MS5-hDLL4) cultured with patient derived CD34+ cells (178, 179).
A third strategic advance for improving thymus organoid technologies is the addition of decellularized thymus scaffolds. Comprising the extracellular matrix from thymuses, these support TEC recolonization and bone marrow cell reconstitution. The reconstituted scaffolds are grafted under the kidney capsule of nude/SCID mice TEC and support T cell development (180–183). Disadvantages of such scaffold approaches include the need for primary human thymus tissues, complex experimental manipulation, and low cell yields. However, recent research has revealed techniques to expand TECs and interstitial cells from human thymuses in large numbers, potentially suitable for clinical approaches (184). Another strategy to generate many functional TECs is to use embryonic fibroblasts, which are reprogrammed with Foxn1 over-expression systems, and are termed inducible TECs. These cells can be reaggregated to generate an ectopic de novo thymus under the kidney capsule in the mouse models (48). Host T cell progenitors seed the de novo thymus-like organ generated by the transplant, and normal thymocyte distributions are observed after 4 weeks. Additionally, typical thymus microstructures are evident in the de novo thymus engrafted tissue (48). Combining the various techniques holds promise for personalized thymus regeneration approaches.
Discussion
The key to effective thymus regeneration requires knowledge of how particular cell subsets contribute to thymopoiesis (Tables 1, 2). Thus, ad-mixing various cell subsets from different tissues, isolated at distinct development stages, will likely be ineffective. A multi-omics profiling of key stromal cell populations; endothelium, epithelium and fibroblasts, performed with 12 distinct mouse organs including the thymus, reveals global transcriptome patterns that are quite divergent when comparing similar cell types obtained from different tissues (185). In fact, the 3 major stromal populations are more related transcriptionally when obtained from the same tissue source, such as the thymus. This suggests that individual tissue environments create transcriptional similarities among the stromal cell populations (185). Such a finding argues that selection of the “correct” stromal tissue from a “thymus” programmed environment is essential for developing effective thymus organoids. Notably, thymus organogenesis involves strategic cellular interactions among different cell clusters using selected receptor-ligand pairs (186). By integrating scRNA sequencing with known receptor-ligand pair interactions, the connectivity among TECs, mesenchymal cells, and endothelial cells is emerging (59). Combing this information with the HLA haplotype of the cells needed for the host and considerations about the patient, thymectomized versus one with an in-born error of immunity, will better inform thymus regenerative technologies. The 4 key cell types to consider for thymus regeneration are mesenchymal cells, endothelial cells, endothelial-derived TECs and hematopoietic stem cells with adequate thymopoietic potential. The last two decades of thymus research suggest that personalized thymus regeneration techniques incorporating these 4 cell types are nearing realization.
Author Contributions
PB, D-MS, and NvO wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Our work was supported, in part, by grants from the National Institutes of Health. National Institutes of Health grant R01AI114523 (NvO) National Institutes of Health grant R21AI144140 (NvO).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely appreciate insights into human thymus hypoplasia’s from our discussions with Drs. Christian Wysocki (UT Southwestern Medical Center), Maria Teresa de la Morena (Seattle Children’s and the University of Washington) and Mary Louise Markert (Duke University Medical Center), clinicians who care for patients with diverse in-born errors of immunity.
References
1. Gordon J, Manley NR. Mechanisms of Thymus Organogenesis and Morphogenesis. Development (2011) 138(18):3865–78. doi: 10.1242/dev.059998
2. Farley AM, Morris LX, Vroegindeweij E, Depreter ML, Vaidya H, Stenhouse FH, et al. Dynamics of Thymus Organogenesis and Colonization in Early Human Development. Development (2013) 140(9):2015–26. doi: 10.1242/dev.087320
3. Paganini I, Sestini R, Capone GL, Putignano AL, Contini E, Giotti I, et al. A Novel PAX1 Null Homozygous Mutation in Autosomal Recessive Otofaciocervical Syndrome Associated With Severe Combined Immunodeficiency. Clin Genet (2017) 92(6):664–8. doi: 10.1111/cge.13085
4. Su DM, Manley NR. Hoxa3 and Pax1 Transcription Factors Regulate the Ability of Fetal Thymic Epithelial Cells to Promote Thymocyte Development. J Immunol (2000) 164(11):5753–60. doi: 10.4049/jimmunol.164.11.5753
5. Wallin J, Eibel H, Neubuser A, Wilting J, Koseki H, Balling R. Pax1 Is Expressed During Development of the Thymus Epithelium and Is Required for Normal T-Cell Maturation. Development (1996) 122(1):23–30. doi: 10.1242/dev.122.1.23
6. Yamazaki Y, Urrutia R, Franco LM, Giliani S, Zhang K, Alazami AM, et al. PAX1 Is Essential for Development and Function of the Human Thymus. Sci Immunol (2020) 5(44):eaax1036. doi: 10.1126/sciimmunol.aax1036
7. Gordon J, Patel SR, Mishina Y, Manley NR. Evidence for an Early Role for BMP4 Signaling in Thymus and Parathyroid Morphogenesis. Dev Biol (2010) 339(1):141–54. doi: 10.1016/j.ydbio.2009.12.026
8. Mori K, Itoi M, Tsukamoto N, Amagai T. Foxn1 Is Essential for Vascularization of the Murine Thymus Anlage. Cell Immunol (2010) 260(2):66–9. doi: 10.1016/j.cellimm.2009.09.007
9. Cuddihy AR, Ge S, Zhu J, Jang J, Chidgey A, Thurston G, et al. VEGF-Mediated Cross-Talk Within the Neonatal Murine Thymus. Blood (2009) 113(12):2723–31. doi: 10.1182/blood-2008-06-162040
10. Bryson JL, Griffith AV, Hughes B, Saito F, Takahama Y, Richie ER, et al. Cell-Autonomous Defects in Thymic Epithelial Cells Disrupt Endothelial-Perivascular Cell Interactions in the Mouse Thymus. PloS One (2013) 8(6):e65196–e. doi: 10.1371/journal.pone.0065196
11. Muller SM, Terszowski G, Blum C, Haller C, Anquez V, Kuschert S, et al. Gene Targeting of VEGF-A in Thymus Epithelium Disrupts Thymus Blood Vessel Architecture. Proc Natl Acad Sci USA (2005) 102(30):10587–92. doi: 10.1073/pnas.0502752102
12. Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T. New Member of the Winged-Helix Protein Family Disrupted in Mouse and Rat Nude Mutations. Nature (1994) 372(6501):103–7. doi: 10.1038/372103a0
13. Vaidya HJ, Briones Leon A, Blackburn CC. FOXN1 in Thymus Organogenesis and Development. Eur J Immunol (2016) 46(8):1826–37. doi: 10.1002/eji.201545814
14. Žuklys S, Handel A, Zhanybekova S, Govani F, Keller M, Maio S, et al. Foxn1 Regulates Key Target Genes Essential for T Cell Development in Postnatal Thymic Epithelial Cells. Nat Immunol (2016) 17(10):1206–15. doi: 10.1073/pnas.1301799110
15. Wurbel M-A, Philippe J-M, Nguyen C, Victorero G, Freeman T, Wooding P, et al. The Chemokine TECK Is Expressed by Thymic and Intestinal Epithelial Cells and Attracts Double- and Single-Positive Thymocytes Expressing the TECK Receptor CCR9. Eur J Immunol (2000) 30(1):262–71. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0
16. Calderon L, Boehm T. Three Chemokine Receptors Cooperatively Regulate Homing of Hematopoietic Progenitors to the Embryonic Mouse Thymus. Proc Natl Acad Sci USA (2011) 108(18):7517–22. doi: 10.1073/pnas.1016428108
17. Liu C, Ueno T, Kuse S, Saito F, Nitta T, Piali L, et al. The Role of CCL21 in Recruitment of T-Precursor Cells to Fetal Thymi. Blood (2005) 105(1):31–9. doi: 10.1182/blood-2004-04-1369
18. van Ewijk W, Brekelmans PJ, Jacobs R, Wisse E. Lymphoid Microenvironments in the Thymus and Lymph Node. Scanning Microsc (1988) 2(4):2129–40.
19. van Ewijk W, Hollander G, Terhorst C, Wang B. Stepwise Development of Thymic Microenvironments In Vivo Is Regulated by Thymocyte Subsets. Development (2000) 127(8):1583–91. doi: 10.1242/dev.127.8.1583
20. van Ewijk W, Wang B, Hollander G, Kawamoto H, Spanopoulou E, Itoi M, et al. Thymic Microenvironments, 3-D Versus 2-D? Semin Immunol (1999) 11(1):57–64. doi: 10.1006/smim.1998.0158
21. Guo J, Rahman M, Cheng L, Zhang S, Tvinnereim A, Su DM. Morphogenesis and Maintenance of the 3D Thymic Medulla and Prevention of Nude Skin Phenotype Require FoxN1 in Pre- and Post-Natal K14 Epithelium. J Mol Med (Berl) (2011) 89(3):263–77. doi: 10.1007/s00109-010-0700-8
22. Clevers H. Modeling Development and Disease With Organoids. Cell (2016) 165(7):1586–97. doi: 10.1016/j.cell.2016.05.082
23. Müller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, et al. Neural Crest Origin of Perivascular Mesenchyme in the Adult Thymus. J Immunol (2008) 180(8):5344–51. doi: 10.4049/jimmunol.180.8.5344
24. Alves NL, Richard-Le Goff O, Huntington ND, Sousa AP, Ribeiro VS, Bordack A, et al. Characterization of the Thymic IL-7 Niche In Vivo. Proc Natl Acad Sci USA (2009) 106(5):1512–7. doi: 10.1073/pnas.0809559106
25. Hozumi K, Mailhos C, Negishi N, Hirano K, Yahata T, Ando K, et al. Delta-Like 4 Is Indispensable in Thymic Environment Specific for T Cell Development. J Exp Med (2008) 205(11):2507–13. doi: 10.1084/jem.20080134
26. Mazzucchelli RI, Warming S, Lawrence SM, Ishii M, Abshari M, Washington AV, et al. Visualization and Identification of IL-7 Producing Cells in Reporter Mice. PloS One (2009) 4(11):e7637. doi: 10.1371/journal.pone.0007637
27. Chi X, Li Y, Qiu X. V(D)J Recombination, Somatic Hypermutation and Class Switch Recombination of Immunoglobulins: Mechanism and Regulation. Immunology (2020) 160(3):233–47. doi: 10.1111/imm.13176
28. Saito T, Watanabe N. Positive and Negative Thymocyte Selection. Crit Rev Immunol (1998) 18(4):359–70. doi: 10.1615/CritRevImmunol.v18.i4.40
29. Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and Negative Selection of the T Cell Repertoire: What Thymocytes See (and Don't See). Nat Rev Immunol (2014) 14(6):377–91. doi: 10.1038/nri3667
30. Taniguchi RT, Anderson MS. The Role of Aire in Clonal Selection. Immunol Cell Biol (2011) 89(1):40–4. doi: 10.1038/icb.2010.132
31. Morimoto R, Swann J, Nusser A, Trancoso I, Schorpp M, Boehm T. Evolution of Thymopoietic Microenvironments. Open Biol (2021) 11(2):200383. doi: 10.1098/rsob.200383
32. Cheng M, Anderson MS. Thymic Tolerance as a Key Brake on Autoimmunity. Nat Immunol (2018) 19(7):659–64. doi: 10.1038/s41590-018-0128-9
33. Mathis D, Benoist C. Aire. Annu Rev Immunol (2009) 27(1):287–312. doi: 10.1146/annurev.immunol.25.022106.141532
35. Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J Immunol (2017) 198(6):2223–31. doi: 10.4049/jimmunol.1601629
36. Hasegawa H, Matsumoto T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front Immunol (2018) 9:350. doi: 10.3389/fimmu.2018.00350
37. Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, et al. The Thymic Medulla Is Required for Foxp3+ Regulatory But Not Conventional CD4+ Thymocyte Development. J Exp Med (2013) 210(4):675–81. doi: 10.1084/jem.20122070
38. Zachariah MA, Cyster JG. Neural Crest-Derived Pericytes Promote Egress of Mature Thymocytes at the Corticomedullary Junction. Science (2010) 328(5982):1129–35. doi: 10.1126/science.1188222
39. Hu Z, Lancaster JN, Ehrlich LIR. The Contribution of Chemokines and Migration to the Induction of Central Tolerance in the Thymus. Front Immunol (2015) 6:398. doi: 10.3389/fimmu.2015.00398
40. Cordier AC, Haumont SM. Development of Thymus, Parathyroids, and Ultimo-Branchial Bodies in NMRI and Nude Mice. Am J Anat (1980) 157(3):227–63. doi: 10.1002/aja.1001570303
41. Jenkinson WE, Rossi SW, Parnell SM, Jenkinson EJ, Anderson G. PDGFRalpha-Expressing Mesenchyme Regulates Thymus Growth and the Availability of Intrathymic Niches. Blood (2007) 109(3):954–60. doi: 10.1182/blood-2006-05-023143
42. Revest J-M, Suniara RK, Kerr K, Owen JJT, Dickson C. Development of the Thymus Requires Signaling Through the Fibroblast Growth Factor Receptor R2-IIIb. J Immunol (2001) 167(4):1954–61. doi: 10.4049/jimmunol.167.4.1954
43. Gardiner JR, Jackson AL, Gordon J, Lickert H, Manley NR, Basson AM. Localised Inhibition of FGF Signalling in the Third Pharyngeal Pouch Is Required for Normal Thymus and Parathyroid Organogenesis. Development (2012) 139(18):3456–66. doi: 10.1242/dev.079400
44. Bockman DE, Kirby ML. Neural Crest Function in Thymus Development. Immunol Ser (1989) 45:451–67.
45. Auerbach R. Morphogenetic Interactions in the Development of the Mouse Thymus Gland. Dev Biol (1960) 2(3):271–84. doi: 10.1016/0012-1606(60)90009-9
46. Jenkinson WE, Jenkinson EJ, Anderson G. Differential Requirement for Mesenchyme in the Proliferation and Maturation of Thymic Epithelial Progenitors. J Exp Med (2003) 198(2):325–32. doi: 10.1084/jem.20022135
47. Sitnik KM, Kotarsky K, White AJ, Jenkinson WE, Anderson G, Agace WW. Mesenchymal Cells Regulate Retinoic Acid Receptor-Dependent Cortical Thymic Epithelial Cell Homeostasis. J Immunol (2012) 188(10):4801–9. doi: 10.4049/jimmunol.1200358
48. Bredenkamp N, Ulyanchenko S, O'Neill KE, Manley NR, Vaidya HJ, Blackburn CC. An Organized and Functional Thymus Generated From FOXN1-Reprogrammed Fibroblasts. Nat Cell Biol (2014) 16(9):902–8. doi: 10.1038/ncb3023
49. Itoi M, Tsukamoto N, Yoshida H, Amagai T. Mesenchymal Cells Are Required for Functional Development of Thymic Epithelial Cells. Int Immunol (2007) 19(8):953–64. doi: 10.1093/intimm/dxm060
50. Shinohara T, Honjo T. Epidermal Growth Factor can Replace Thymic Mesenchyme in Induction of Embryonic Thymus Morphogenesis In Vitro. Eur J Immunol (1996) 26(4):747–52. doi: 10.1002/eji.1830260404
51. Du Q, de la Morena MT, van Oers NSC. The Genetics and Epigenetics of 22q11.2 Deletion Syndrome. 2 Deletion Syndr Front Genet (2019) 10:1365. doi: 10.3389/fgene.2019.01365
52. DiGeorge A. Congenital Absence of the Thymus and Its Immunological Consequences: Concurrence With Congenital Hypoparathyroidism. Birth Defects Orig ArtSerIV (1968) 1:116–21.
53. Foster KE, Gordon J, Cardenas K, Veiga-Fernandes H, Makinen T, Grigorieva E, et al. EphB-Ephrin-B2 Interactions Are Required for Thymus Migration During Organogenesis. Proc Natl Acad Sci USA (2010) 107(30):13414–9. doi: 10.1073/pnas.1003747107
54. Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, et al. Contribution of Neural Crest-Derived Cells in the Embryonic and Adult Thymus. J Immunol (2008) 180(5):3183–9. doi: 10.4049/jimmunol.180.5.3183
55. Sitnik KM, Wendland K, Weishaupt H, Uronen-Hansson H, White AJ, Anderson G, et al. Context-Dependent Development of Lymphoid Stroma From Adult CD34(+) Adventitial Progenitors. Cell Rep (2016) 14(10):2375–88. doi: 10.1016/j.celrep.2016.02.033
56. Bautista JL, Cramer NT, Miller CN, Chavez J, Berrios DI, Byrnes LE, et al. Single-Cell Transcriptional Profiling of Human Thymic Stroma Uncovers Novel Cellular Heterogeneity in the Thymic Medulla. Nat Commun (2021) 12(1):1096. doi: 10.1038/s41467-021-21346-6
57. Park J-E, Botting RA, Conde CD, Popescu D-M, Lavaert M, Kunz DJ, et al. A Cell Atlas of Human Thymic Development Defines T Cell Repertoire Formation. Science (2020) 367(6480):eaay3224. doi: 10.1126/science.aay3224
58. Nitta T, Tsutsumi M, Nitta S, Muro R, Suzuki EC, Nakano K, et al. Fibroblasts as a Source of Self-Antigens for Central Immune Tolerance. Nat Immunol (2020) 21(10):1172–80. doi: 10.1038/s41590-020-0756-8
59. Zeng Y, Liu C, Gong Y, Bai Z, Hou S, He J, et al. Single-Cell RNA Sequencing Resolves Spatiotemporal Development of Pre-Thymic Lymphoid Progenitors and Thymus Organogenesis in Human Embryos. Immunity (2019) 51(5):930–48.e6. doi: 10.1016/j.immuni.2019.09.008
60. Shi Y, Wu W, Chai Q, Li Q, Hou Y, Xia H, et al. Ltβr Controls Thymic Portal Endothelial Cells for Haematopoietic Progenitor Cell Homing and T-Cell Regeneration. Nat Commun (2016) 7(1):12369. doi: 10.1038/ncomms12369
61. Wertheimer T, Velardi E, Tsai J, Cooper K, Xiao S, Kloss CC, et al. Production of BMP4 by Endothelial Cells Is Crucial for Endogenous Thymic Regeneration. Sci Immunol (2018) 3(19):eaal2736. doi: 10.1126/sciimmunol.aal2736
62. Nagatake T, Zhao YC, Ito T, Itoh M, Kometani K, Furuse M, et al. Selective Expression of Claudin-5 in Thymic Endothelial Cells Regulates the Blood-Thymus Barrier and T-Cell Export. Int Immunol (2021) 33(3):171–82. doi: 10.1093/intimm/dxaa069
63. Bleul CC, Corbeaux T, Reuter A, Fisch P, Mönting JS, Boehm T. Formation of a Functional Thymus Initiated by a Postnatal Epithelial Progenitor Cell. Nature (2006) 441(7096):992–6. doi: 10.1038/nature04850
64. Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ. Clonal Analysis Reveals a Common Progenitor for Thymic Cortical and Medullary Epithelium. Nature (2006) 441(7096):988–91. doi: 10.1038/nature04813
65. Ulyanchenko S, O’Neill Kathy E, Medley T, Farley Alison M, Vaidya Harsh J, Cook Alistair M, et al. Identification of a Bipotent Epithelial Progenitor Population in the Adult Thymus. Cell Rep (2016) 14(12):2819–32. doi: 10.1016/j.celrep.2016.02.080
66. Bennett AR, Farley A, Blair NF, Gordon J, Sharp L, Blackburn CC. Identification and Characterization of Thymic Epithelial Progenitor Cells. Immunity (2002) 16(6):803–14. doi: 10.1016/S1074-7613(02)00321-7
67. Gill J, Malin M, Hollander GA, Boyd R. Generation of a Complete Thymic Microenvironment by MTS24(+) Thymic Epithelial Cells. Nat Immunol (2002) 3(7):635–42. doi: 10.1038/ni812
68. Baik S, Jenkinson EJ, Lane PJ, Anderson G, Jenkinson WE. Generation of Both Cortical and Aire(+) Medullary Thymic Epithelial Compartments From CD205(+) Progenitors. Eur J Immunol (2013) 43(3):589–94. doi: 10.1002/eji.201243209
69. Ohigashi I, Zuklys S, Sakata M, Mayer CE, Zhanybekova S, Murata S, et al. Aire-Expressing Thymic Medullary Epithelial Cells Originate From Beta5t-Expressing Progenitor Cells. Proc Natl Acad Sci USA (2013) 110(24):9885–90. doi: 10.1073/pnas.1301799110
70. Ribeiro AR, Rodrigues PM, Meireles C, Di Santo JP, Alves NL. Thymocyte Selection Regulates the Homeostasis of IL-7-Expressing Thymic Cortical Epithelial Cells In Vivo. J Immunol (2013) 191(3):1200–9. doi: 10.4049/jimmunol.1203042
71. Nehls M, Kyewski B, Messerle M, Waldschütz R, Schüddekopf K, Smith AJH, et al. Two Genetically Separable Steps in the Differentiation of Thymic Epithelium. Science (1996) 272(5263):886–9. doi: 10.1126/science.272.5263.886
72. Schlake T, Schorpp M, Nehls M, Boehm T. The Nude Gene Encodes a Sequence-Specific DNA Binding Protein With Homologs in Organisms That Lack an Anticipatory Immune System. Proc Natl Acad Sci (1997) 94(8):3842–7. doi: 10.1073/pnas.94.8.3842
73. Bosticardo M, Yamazaki Y, Cowan J, Giardino G, Corsino C, Scalia G, et al. Heterozygous FOXN1 Variants Cause Low TRECs and Severe T Cell Lymphopenia, Revealing a Crucial Role of FOXN1 in Supporting Early Thymopoiesis. Am J Hum Genet (2019) 105(3):549–61. doi: 10.1016/j.ajhg.2019.07.014
74. Giardino G, Sharapova SO, Ciznar P, Dhalla F, Maragliano L, Radha Rama Devi A, et al. Expanding the Nude SCID/CID Phenotype Associated With FOXN1 Homozygous, Compound Heterozygous, or Heterozygous Mutations. J Clin Immunol (2021) 41(4):756–68. doi: 10.1007/s10875-021-00967-y
75. Pignata C, Fiore M, Guzzetta V, Castaldo A, Sebastio G, Porta F, et al. Congenital Alopecia and Nail Dystrophy Associated With Severe Functional T-Cell Immunodeficiency in Two Sibs. Am J Med Genet (1996) 65(2):167–70. doi: 10.1002/(SICI)1096-8628(19961016)65:2<167::AID-AJMG17>3.0.CO;2-O
76. Vigliano I, Gorrese M, Fusco A, Vitiello L, Amorosi S, Panico L, et al. FOXN1 Mutation Abrogates Prenatal T-Cell Development in Humans. J Med Genet (2011) 48(6):413–6. doi: 10.1136/jmg.2011.089532
77. Du Q, Huynh LK, Coskun F, Molina E, King MA, Raj P, et al. FOXN1 Compound Heterozygous Mutations Cause Selective Thymic Hypoplasia in Humans. J Clin Invest (2019) 129(11):4724–38. doi: 10.1172/JCI127565
78. Potter CS, Pruett ND, Kern MJ, Baybo MA, Godwin AR, Potter KA, et al. The Nude Mutant Gene Foxn1 Is a HOXC13 Regulatory Target During Hair Follicle and Nail Differentiation. J Invest Dermatol (2011) 131(4):828–37. doi: 10.1038/jid.2010.391
79. Romano R, Palamaro L, Fusco A, Giardino G, Gallo V, Del Vecchio L, et al. FOXN1: A Master Regulator Gene of Thymic Epithelial Development Program. Front Immunol (2013) 4:187. doi: 10.3389/fimmu.2013.00187
80. Bhalla P, Wysocki CA, van Oers NSC. Molecular Insights Into the Causes of Human Thymic Hypoplasia With Animal Models. Front Immunol (2020) 11:830. doi: 10.3389/fimmu.2020.00830
81. Uddin MM, Ohigashi I, Motosugi R, Nakayama T, Sakata M, Hamazaki J, et al. Foxn1-Beta5t Transcriptional Axis Controls CD8(+) T-Cell Production in the Thymus. Nat Commun (2017) 8:14419. doi: 10.1038/ncomms14419
82. Murata S, Sasaki K, Kishimoto T, S-i N, Hayashi H, Takahama Y, et al. Regulation of CD8+ T Cell Development by Thymus-Specific Proteasomes. Science (2007) 316(5829):1349–53. doi: 10.1126/science.1141915
83. Fujimoto Y, Tu L, Miller AS, Bock C, Fujimoto M, Doyle C, et al. CD83 Expression Influences CD4+ T Cell Development in the Thymus. Cell (2002) 108(6):755–67. doi: 10.1016/S0092-8674(02)00673-6
84. Larsen BM, Cowan JE, Wang Y, Tanaka Y, Zhao Y, Voisin B, et al. Identification of an Intronic Regulatory Element Necessary for Tissue-Specific Expression of Foxn1 in Thymic Epithelial Cells. J Immunol (2019) 203(3):686–95. doi: 10.4049/jimmunol.1801540
85. Handel AE, Holländer GA. Comment on “Identification of an Intronic Regulatory Element Necessary for Tissue-Specific Expression of Foxn1 in Thymic Epithelial Cells”. J Immunol (2019) 203(9):2355–. doi: 10.4049/jimmunol.1900948
86. Ali RH, Habib R, Ud-Din N, Khan MN, Ansar M, Ahmad W. Novel Mutations in the Gene HOXC13 Underlying Pure Hair and Nail Ectodermal Dysplasia in Consanguineous Families. Br J Dermatol (2013) 169(2):478–80. doi: 10.1111/bjd.12302
87. Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, et al. Wnt Glycoproteins Regulate the Expression of FoxN1, the Gene Defective in Nude Mice. Nat Immunol (2002) 3(11):1102–8. doi: 10.1038/ni850
88. Burnley P, Rahman M, Wang H, Zhang Z, Sun X, Zhuge Q, et al. Role of the P63-FoxN1 Regulatory Axis in Thymic Epithelial Cell Homeostasis During Aging. Cell Death Dis (2013) 4(11):e933. doi: 10.1038/cddis.2013.460
89. Candi E, Rufini A, Terrinoni A, Giamboi-Miraglia A, Lena AM, Mantovani R, et al. Δnp63 Regulates Thymic Development Through Enhanced Expression of FgfR2 and Jag2. Proc Natl Acad Sci (2007) 104(29):11999–2004. doi: 10.1073/pnas.0703458104
90. Romano R-A, Smalley K, Magraw C, Serna VA, Kurita T, Raghavan S, et al. Δnp63 Knockout Mice Reveal Its Indispensable Role as a Master Regulator of Epithelial Development and Differentiation. Dev (Cambr Engl) (2012) 139(4):772–82. doi: 10.1242/dev.071191
91. Alawam AS, Anderson G, Lucas B. Generation and Regeneration of Thymic Epithelial Cells. Front Immunol (2020) 11:858. doi: 10.3389/fimmu.2020.00858
92. Jenkinson WE, Rossi SW, Parnell SM, Agace WW, Takahama Y, Jenkinson EJ, et al. Chemokine Receptor Expression Defines Heterogeneity in the Earliest Thymic Migrants. Eur J Immunol (2007) 37(8):2090–6. doi: 10.1002/eji.200737212
93. Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, et al. Delta-Like 4 Is the Essential, Nonredundant Ligand for Notch1 During Thymic T Cell Lineage Commitment. J Exp Med (2008) 205(11):2515–23. doi: 10.1084/jem.20080829
94. Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, et al. Deficient T Cell Fate Specification in Mice With an Induced Inactivation of Notch1. Immunity (1999) 10(5):547–58. doi: 10.1016/S1074-7613(00)80054-0
95. Liu D, Kousa AI, O'Neill KE, Rouse P, Popis M, Farley AM, et al. Canonical Notch Signaling Controls the Early Thymic Epithelial Progenitor Cell State and Emergence of the Medullary Epithelial Lineage in Fetal Thymus Development. Development (2020) 147(12):e178982. doi: 10.1242/dev.178582
96. Li J, Gordon J, Chen ELY, Xiao S, Wu L, Zúñiga-Pflücker JC, et al. NOTCH1 Signaling Establishes the Medullary Thymic Epithelial Cell Progenitor Pool During Mouse Fetal Development. Development (2020) 147(12):e178998. doi: 10.1242/dev.178988
97. Satoh R, Kakugawa K, Yasuda T, Yoshida H, Sibilia M, Katsura Y, et al. Requirement of Stat3 Signaling in the Postnatal Development of Thymic Medullary Epithelial Cells. PloS Genet (2016) 12(1):e1005776. doi: 10.1371/journal.pgen.1005776
98. Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, et al. The Tumor Necrosis Factor Family Receptors RANK and CD40 Cooperatively Establish the Thymic Medullary Microenvironment and Self-Tolerance. Immunity (2008) 29(3):423–37. doi: 10.1016/j.immuni.2008.06.015
99. Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, et al. The Cytokine RANKL Produced by Positively Selected Thymocytes Fosters Medullary Thymic Epithelial Cells That Express Autoimmune Regulator. Immunity (2008) 29(3):438–50. doi: 10.1016/j.immuni.2008.06.018
100. Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, et al. Rank Signaling Links the Development of Invariant Gammadelta T Cell Progenitors and Aire(+) Medullary Epithelium. Immunity (2012) 36(3):427–37. doi: 10.1016/j.immuni.2012.01.016
101. White AJ, Nakamura K, Jenkinson WE, Saini M, Sinclair C, Seddon B, et al. Lymphotoxin Signals From Positively Selected Thymocytes Regulate the Terminal Differentiation of Medullary Thymic Epithelial Cells. J Immunol (2010) 185(8):4769–76. doi: 10.4049/jimmunol.1002151
102. Alexandropoulos K, Danzl NM. Thymic Epithelial Cells: Antigen Presenting Cells That Regulate T Cell Repertoire and Tolerance Development. Immunol Res (2012) 54(1-3):177–90. doi: 10.1007/s12026-012-8301-y
103. Klein L, Robey EA, Hsieh CS. Central CD4(+) T Cell Tolerance: Deletion Versus Regulatory T Cell Differentiation. Nat Rev Immunol (2019) 19(1):7–18. doi: 10.1038/s41577-018-0083-6
104. Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7-Mediated Migration of Developing Thymocytes to the Medulla Is Essential for Negative Selection to Tissue-Restricted Antigens. Proc Natl Acad Sci (2009) 106(40):17129–33. doi: 10.1073/pnas.0906956106
105. Lkhagvasuren E, Sakata M, Ohigashi I, Takahama Y. Lymphotoxin β Receptor Regulates the Development of CCL21-Expressing Subset of Postnatal Medullary Thymic Epithelial Cells. J Immunol (2013) 190(10):5110–7. doi: 10.4049/jimmunol.1203203
106. Passos GA, Speck-Hernandez CA, Assis AF, Mendes-da-Cruz DA. Update on Aire and Thymic Negative Selection. Immunology (2018) 153(1):10–20. doi: 10.1111/imm.12831
107. Giraud M, Yoshida H, Abramson J, Rahl PB, Young RA, Mathis D, et al. Aire Unleashes Stalled RNA Polymerase to Induce Ectopic Gene Expression in Thymic Epithelial Cells. Proc Natl Acad Sci (2012) 109(2):535–40. doi: 10.1073/pnas.1119351109
108. Su M, Hu R, Song Y, Liu Y, Lai L. Targeted Deletion of C-Met in Thymic Epithelial Cells Leads to an Autoimmune Phenotype. Immunol Cell Biol (2018) 96(2):229–35. doi: 10.1111/imcb.1026
109. Saldaña JI, Solanki A, Lau C-I, Sahni H, Ross S, Furmanski AL, et al. Sonic Hedgehog Regulates Thymic Epithelial Cell Differentiation. J Autoimmun (2016) 68:86–97. doi: 10.1016/j.jaut.2015.12.004
110. Meireles C, Ribeiro AR, Pinto RD, Leitao C, Rodrigues PM, Alves NL. Thymic Crosstalk Restrains the Pool of Cortical Thymic Epithelial Cells With Progenitor Properties. Eur J Immunol (2017) 47(6):958–69. doi: 10.1002/eji.201746922
111. Wong K, Lister NL, Barsanti M, Lim JM, Hammett MV, Khong DM, et al. Multilineage Potential and Self-Renewal Define an Epithelial Progenitor Cell Population in the Adult Thymus. Cell Rep (2014) 8(4):1198–209. doi: 10.1016/j.celrep.2014.07.029
112. Baran-Gale J, Morgan MD, Maio S, Dhalla F, Calvo-Asensio I, Deadman ME, et al. Ageing Compromises Mouse Thymus Function and Remodels Epithelial Cell Differentiation. Elife (2020) 9:e56221. doi: 10.7554/eLife.56221
113. Bornstein C, Nevo S, Giladi A, Kadouri N, Pouzolles M, Gerbe F, et al. Single-Cell Mapping of the Thymic Stroma Identifies IL-25-Producing Tuft Epithelial Cells. Nature (2018) 559(7715):622–6. doi: 10.1038/s41586-018-0346-1
114. Cowan JE, Malin J, Zhao Y, Seedhom MO, Harly C, Ohigashi I, et al. Myc Controls a Distinct Transcriptional Program in Fetal Thymic Epithelial Cells That Determines Thymus Growth. Nat Commun (2019) 10(1):5498. doi: 10.1038/s41467-019-13465-y
115. Miller CN, Proekt I, von Moltke J, Wells KL, Rajpurkar AR, Wang H, et al. Thymic Tuft Cells Promote an IL-4-Enriched Medulla and Shape Thymocyte Development. Nature (2018) 559(7715):627–31. doi: 10.1038/s41586-018-0345-2
116. Wells KL, Miller CN, Gschwind AR, Wei W, Phipps JD, Anderson MS, et al. Combined Transient Ablation and Single-Cell RNA-Sequencing Reveals the Development of Medullary Thymic Epithelial Cells. Elife (2020) 9:e60188. doi: 10.7554/eLife.60188
117. Palmer D. The Effect of Age on Thymic Function. Front Immunol (2013) 4:316. doi: 10.3389/fimmu.2013.00316
118. Dixit VD. Thymic Fatness and Approaches to Enhance Thymopoietic Fitness in Aging. Curr Opin Immunol (2010) 22(4):521–8. doi: 10.1016/j.coi.2010.06.010
119. Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP. Analysis of the Human Thymic Perivascular Space During Aging. J Clin Invest (1999) 104(8):1031–9. doi: 10.1172/JCI7558
120. Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su DM. The Aged Thymus Shows Normal Recruitment of Lymphohematopoietic Progenitors But Has Defects in Thymic Epithelial Cells. Int Immunol (2007) 19(10):1201–11. doi: 10.1093/intimm/dxm095
121. Ortman CL, Dittmar KA, Witte PL, Le PT. Molecular Characterization of the Mouse Involuted Thymus: Aberrations in Expression of Transcription Regulators in Thymocyte and Epithelial Compartments. Int Immunol (2002) 14(7):813–22. doi: 10.1093/intimm/dxf042
122. Sun L, Guo J, Brown R, Amagai T, Zhao Y, Su D-M. Declining Expression of a Single Epithelial Cell-Autonomous Gene Accelerates Age-Related Thymic Involution. Aging Cell (2010) 9(3):347–57. doi: 10.1111/j.1474-9726.2010.00559.x
123. Bredenkamp N, Nowell CS, Blackburn CC. Regeneration of the Aged Thymus by a Single Transcription Factor. Development (2014) 141(8):1627–37. doi: 10.1242/dev.103614
124. Chen L, Xiao S, Manley NR. Foxn1 Is Required to Maintain the Postnatal Thymic Microenvironment in a Dosage-Sensitive Manner. Blood (2009) 113(3):567–74. doi: 10.1182/blood-2008-05-156265
125. Zook EC, Krishack PA, Zhang S, Zeleznik-Le NJ, Firulli AB, Witte PL, et al. Overexpression of Foxn1 Attenuates Age-Associated Thymic Involution and Prevents the Expansion of Peripheral CD4 Memory T Cells. Blood (2011) 118(22):5723–31. doi: 10.1182/blood-2011-03-342097
126. Youm Y-H, Horvath TL, Mangelsdorf DJ, Kliewer SA, Dixit VD. Prolongevity Hormone FGF21 Protects Against Immune Senescence by Delaying Age-Related Thymic Involution. Proc Natl Acad Sci USA (2016) 113(4):1026–31. doi: 10.1073/pnas.1514511113
127. Xing Y, Smith MJ, Goetz CA, McElmurry RT, Parker SL, Min D, et al. Thymic Epithelial Cell Support of Thymopoiesis Does Not Require Klotho. J Immunol (2018) 201(11):3320–8. doi: 10.4049/jimmunol.1800670
128. Cavallotti C, D'Andrea V, Tonnarini G, Cavallotti C, Bruzzone P. Age-Related Changes in the Human Thymus Studied With Scanning Electron Microscopy. Microsc Res Tech (2008) 71(8):573–8. doi: 10.1002/jemt.20588
129. Mori K, Itoi M, Tsukamoto N, Kubo H, Amagai T. The Perivascular Space as a Path of Hematopoietic Progenitor Cells and Mature T Cells Between the Blood Circulation and the Thymic Parenchyma. Int Immunol (2007) 19(6):745–53. doi: 10.1093/intimm/dxm041
130. Yang H, Youm YH, Sun Y, Rim JS, Galbán CJ, Vandanmagsar B, et al. Axin Expression In Thymic Stromal Cells Contributes to an Age-Related Increase in Thymic Adiposity and is Associated With Reduced Thymopoiesis Independently of Ghrelin Signaling. J Leukoc Biol (2009) 85(6):928–38. doi: 10.1189/jlb.1008621
131. Youm Y-H, Yang H, Sun Y, Smith RG, Manley NR, Vandanmagsar B, et al. Deficient Ghrelin Receptor-Mediated Signaling Compromises Thymic Stromal Cell Microenvironment by Accelerating Thymic Adiposity *. J Biol Chem (2009) 284(11):7068–77. doi: 10.1074/jbc.M808302200
132. Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC, et al. Leukemia Inhibitory Factor, Oncostatin M, IL-6, and Stem Cell Factor mRNA Expression in Human Thymus Increases With Age and Is Associated With Thymic Atrophy. J Immunol (2000) 164(4):2180–7. doi: 10.4049/jimmunol.164.4.2180
133. Sempowski GD, Rhein ME, Scearce RM, Haynes BF. Leukemia Inhibitory Factor Is a Mediator of Escherichia Coli Lipopolysaccharide-Induced Acute Thymic Atrophy. Eur J Immunol (2002) 32(11):3066–70. doi: 10.1002/1521-4141(200211)32:11<3066::AID-IMMU3066>3.0.CO;2-J
134. Yang H, Youm Y-H, Dixit VD. Inhibition of Thymic Adipogenesis by Caloric Restriction Is Coupled With Reduction in Age-Related Thymic Involution. J Immunol (2009) 183(5):3040–52. doi: 10.4049/jimmunol.0900562
135. Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm Y-H, Smith RG, et al. Ghrelin Promotes Thymopoiesis During Aging. J Clin Invest (2007) 117(10):2778–90. doi: 10.1172/JCI30248
136. Nitta T, Ota A, Iguchi T, Muro R, Takayanagi H. The Fibroblast: An Emerging Key Player in Thymic T Cell Selection. Immunol Rev (2021) 302(1):68–85. doi: 10.1111/imr.12985
137. Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. N Engl J Med (2020) 383(10):958–68. doi: 10.1056/NEJMra2005230
138. Kinsella S, Dudakov JA. When the Damage Is Done: Injury and Repair in Thymus Function. Front Immunol (2020) 11:1745. doi: 10.3389/fimmu.2020.01745
139. Gruver AL, Sempowski GD. Cytokines, Leptin, and Stress-Induced Thymic Atrophy. J Leukoc Biol (2008) 84(4):915–23. doi: 10.1189/jlb.0108025
140. Rossi SW, Kim M-Y, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, et al. RANK Signals From CD4+3– Inducer Cells Regulate Development of Aire-Expressing Epithelial Cells in the Thymic Medulla. J Exp Med (2007) 204(6):1267–72. doi: 10.1084/jem.20062497
141. Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, et al. Interleukin-22 Drives Endogenous Thymic Regeneration in Mice. Science (2012) 336(6077):91–5. doi: 10.1126/science.1218004
142. Rossi SW, Jeker LT, Ueno T, Kuse S, Keller MP, Zuklys S, et al. Keratinocyte Growth Factor (KGF) Enhances Postnatal T-Cell Development via Enhancements in Proliferation and Function of Thymic Epithelial Cells. Blood (2007) 109(9):3803–11. doi: 10.1182/blood-2006-10-049767
143. Hoover AR, Dozmorov I, MacLeod J, Du Q, de la Morena MT, Forbess J, et al. MicroRNA-205 Maintains T Cell Development Following Stress by Regulating Forkhead Box N1 and Selected Chemokines. J Biol Chem (2016) 291(44):23237–47. doi: 10.1074/jbc.M116.744508
144. Papadopoulou AS, Dooley J, Linterman MA, Pierson W, Ucar O, Kyewski B, et al. The Thymic Epithelial microRNA Network Elevates the Threshold for Infection-Associated Thymic Involution via miR-29a Mediated Suppression of the IFN-[Alpha] Receptor. Nat Immunol (2012) 13(2):181–7. doi: 10.1038/ni.2193
145. Romera-Hernandez M, Aparicio-Domingo P, Cupedo T. Damage Control: Rorγt+ Innate Lymphoid Cells in Tissue Regeneration. Curr Opin Immunol (2013) 25(2):156–60. doi: 10.1016/j.coi.2013.01.007
146. Shitara S, Hara T, Liang B, Wagatsuma K, Zuklys S, Holländer GA, et al. IL-7 Produced by Thymic Epithelial Cells Plays a Major Role in the Development of Thymocytes and Tcrγδ+ Intraepithelial Lymphocytes. J Immunol (2013) 190(12):6173–9. doi: 10.4049/jimmunol.1202573
147. Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, et al. Interleukin 7 Receptor-Deficient Mice Lack Gammadelta T Cells. Proc Natl Acad Sci (1996) 93(14):7172–7. doi: 10.1073/pnas.93.14.7172
148. Fry TJ, Sinha M, Milliron M, Chu Y-W, Kapoor V, Gress RE, et al. Flt3 Ligand Enhances Thymic-Dependent and Thymic-Independent Immune Reconstitution. Blood (2004) 104(9):2794–800. doi: 10.1182/blood-2003-11-3789
149. Trotman-Grant AC, Mohtashami M, De Sousa Casal J, Martinez EC, Lee D, Teichman S, et al. DL4-μbeads Induce T Cell Lineage Differentiation From Stem Cells in a Stromal Cell-Free System. Nat Commun (2021) 12(1):5023. doi: 10.1038/s41467-021-25245-8
150. Abramson J, Anderson G. Thymic Epithelial Cells. Annu Rev Immunol (2017) 35(1):85–118. doi: 10.1146/annurev-immunol-051116-052320
151. De Felice L, Di Pucchio T, Breccia M, Agostini F, Mascolo MG, Guglielmi C, et al. Flt3L Enhances the Early Stem Cell Compartment After Ex Vivo Amplification of Umbilical Cord Blood CD34+ Cells. Bone Marrow Transpl (1998) 22 Suppl 1:S66–7.
152. Williams KM, Moore AR, Lucas PJ, Wang J, Bare CV, Gress RE. FLT3 Ligand Regulates Thymic Precursor Cells and Hematopoietic Stem Cells Through Interactions With CXCR4 and the Marrow Niche. Exp Hematol (2017) 52:40–9. doi: 10.1016/j.exphem.2017.05.005
153. Anandasabapathy N, Breton G, Hurley A, Caskey M, Trumpfheller C, Sarma P, et al. Efficacy and Safety of CDX-301, Recombinant Human Flt3L, at Expanding Dendritic Cells and Hematopoietic Stem Cells in Healthy Human Volunteers. Bone Marrow Transpl (2015) 50(7):924–30. doi: 10.1038/bmt.2015.74
154. Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective Organ Targeting (SORT) Nanoparticles for Tissue-Specific mRNA Delivery and CRISPR–Cas Gene Editing. Nat Nanotechnol (2020) 15(4):313–20. doi: 10.1038/s41565-020-0669-6
155. Song Y, Su M, Zhu J, Di W, Liu Y, Hu R, et al. FOXN1 Recombinant Protein Enhances T-Cell Regeneration After Hematopoietic Stem Cell Transplantation in Mice. Eur J Immunol (2016) 46(6):1518–28. doi: 10.1002/eji.201546196
156. Ruan L, Zhang Z, Mu L, Burnley P, Wang L, Coder B, et al. Biological Significance of FoxN1 Gain-of-Function Mutations During T and B Lymphopoiesis in Juvenile Mice. Cell Death Dis (2014) 5(10):e1457. doi: 10.1038/cddis.2014.432
157. Kim M-J, Miller CM, Shadrach JL, Wagers AJ, Serwold T. Young, Proliferative Thymic Epithelial Cells Engraft and Function in Aging Thymuses. J Immunol (2015) 194(10):4784–95. doi: 10.4049/jimmunol.1403158
158. Oh J, Wang W, Thomas R, Su D-M. Thymic Rejuvenation via FOXN1-Reprogrammed Embryonic Fibroblasts (FREFs) to Counteract Age-Related Inflammation. JCI Insight (2020) 5(18):e140313. doi: 10.1172/jci.insight.140313
159. Lankester AC, Albert MH, Booth C, Gennery AR, Güngör T, Hönig M, et al. EBMT/ESID Inborn Errors Working Party Guidelines for Hematopoietic Stem Cell Transplantation for Inborn Errors of Immunity. Bone Marrow Transplant (2021) 56(9):2052–62. doi: 10.1038/s41409-021-01378-8
160. Kreins AY, Bonfanti P, Davies EG. Current and Future Therapeutic Approaches for Thymic Stromal Cell Defects. Front Immunol (2021) 12:835. doi: 10.3389/fimmu.2021.655354
161. Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, et al. Review of 54 Patients With Complete DiGeorge Anomaly Enrolled in Protocols for Thymus Transplantation: Outcome of 44 Consecutive Transplants. Blood (2007) 109(10):4539–47. doi: 10.1182/blood-2006-10-048652
162. Markert ML, Marques JG, Neven B, Devlin BH, McCarthy EA, Chinn IK, et al. First Use of Thymus Transplantation Therapy for FOXN1 Deficiency (Nude/SCID): A Report of 2 Cases. Blood (2011) 117(2):688–96. doi: 10.1182/blood-2010-06-292490
163. Halnon NJ, Jamieson B, Plunkett M, Kitchen CMR, Pham T, Krogstad P. Thymic Function and Impaired Maintenance of Peripheral T Cell Populations in Children With Congenital Heart Disease and Surgical Thymectomy. Pediatr Res (2005) 57(1):42–8. doi: 10.1203/01.PDR.0000147735.19342.DE
164. Sauce D, Larsen M, Fastenackels S, Duperrier A, Keller M, Grubeck-Loebenstein B, et al. Evidence of Premature Immune Aging in Patients Thymectomized During Early Childhood. J Clin Invest (2009) 119(10):3070–8. doi: 10.1172/JCI39269
165. Roosen J, Oosterlinck W, Meyns B. Routine Thymectomy in Congenital Cardiac Surgery Changes Adaptive Immunity Without Clinical Relevance. Interact Cardiovasc Thorac Surg (2014) 20(1):101–6. doi: 10.1093/icvts/ivu343
166. Gudmundsdottir J, Söderling J, Berggren H, Óskarsdóttir S, Neovius M, Stephansson O, et al. Long-Term Clinical Effects of Early Thymectomy: Associations With Autoimmune Diseases, Cancer, Infections, and Atopic Diseases. J Allergy Clin Immunol (2018) 141(6):2294–7.e8. doi: 10.1016/j.jaci.2018.01.037
167. Cavalcanti NV, Palmeira P, Jatene MB, de Barros Dorna M, Carneiro-Sampaio M. Early Thymectomy Is Associated With Long-Term Impairment of the Immune System: A Systematic Review. Front Immunol (2021) 12:5043. doi: 10.3389/fimmu.2021.774780
168. Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo H-C, Marx A, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med (2016) 375(6):511–22. doi: 10.1056/NEJMoa1602489
169. Dally A. Status Lymphaticus: Sudden Death in Children From "Visitation of God" to Cot Death. Med Hist (1997) 41(1):70–85. doi: 10.1017/S0025727300062049
170. Adams MJ, Shore RE, Dozier A, Lipshultz SE, Schwartz RG, Constine LS, et al. Thyroid Cancer Risk 40+ Years After Irradiation for an Enlarged Thymus: An Update of the Hempelmann Cohort. Radiat Res (2010) 174(6):753–62. doi: 10.1667/RR2181.1
171. Symmers D. The Cause Of Sudden Death In Status Lymphaticus. Am J Dis Children (1917) 14(6):463–9. doi: 10.1001/archpedi.1917.01910120068004
172. Chinn IK, Milner JD, Scheinberg P, Douek DC, Markert ML. Thymus Transplantation Restores the Repertoires of Forkhead Box Protein 3 (FoxP3)+ and FoxP3- T Cells in Complete DiGeorge Anomaly. Clin Exp Immunol (2013) 173(1):140–9. doi: 10.1111/cei.12088
173. Davies EG. Immunodeficiency in DiGeorge Syndrome and Options for Treating Cases With Complete Athymia. Front Immunol (2013) 4:322. doi: 10.3389/fimmu.2013.00322
174. Schmitt TM, Zúñiga-Pflücker JC. Induction of T Cell Development From Hematopoietic Progenitor Cells by Delta-Like-1 In Vitro. Immunity (2002) 17(6):749–56. doi: 10.1016/S1074-7613(02)00474-0
175. Balciunaite G, Ceredig R, Fehling H-J, Zúñiga-Pflücker J-C, Rolink AG. The Role of Notch and IL-7 Signaling in Early Thymocyte Proliferation and Differentiation. Eur J Immunol (2005) 35(4):1292–300. doi: 10.1002/eji.200425822
176. Hirano K-I, Suganami A, Tamura Y, Yagita H, Habu S, Kitagawa M, et al. Delta-Like 1 and Delta-Like 4 Differently Require Their Extracellular Domains for Triggering Notch Signaling in Mice. eLife (2020) 9:e50979. doi: 10.7554/eLife.50979
177. Seet CS, He C, Bethune MT, Li S, Chick B, Gschweng EH, et al. Generation of Mature T Cells From Human Hematopoietic Stem and Progenitor Cells in Artificial Thymic Organoids. Nat Methods (2017) 14(5):521–30. doi: 10.1038/nmeth.4237
178. Bosticardo M, Pala F, Calzoni E, Ottavia M, Delmonte OM, Dobbs K, et al. Artificial Thymic Organoids Represent a Reliable Tool to Study T-Cell Differentiation in Patients With Severe T-Cell Lymphopenia. Blood Adv (2020) 4(12):2611–6. doi: 10.1182/bloodadvances.2020001730
179. Bifsha P, Leiding JW, Pai S-Y, Colamartino ABL, Hartog N, Church JA, et al. Diagnostic Assay to Assist Clinical Decisions for Unclassified Severe Combined Immune Deficiency. Blood Adv (2020) 4(12):2606–10. doi: 10.1182/bloodadvances.2020001736
180. Poznansky MC, Evans RH, Foxall RB, Olszak IT, Piascik AH, Hartman KE, et al. Efficient Generation of Human T Cells From a Tissue-Engineered Thymic Organoid. Nat Biotechnol (2000) 18(7):729–34. doi: 10.1038/77288
181. Chung B, Montel-Hagen A, Ge S, Blumberg G, Kim K, Klein S, et al. Engineering the Human Thymic Microenvironment to Support Thymopoiesis In Vivo. Stem Cells (2014) 32(9):2386–96. doi: 10.1002/stem.1731
182. Fan Y, Tajima A, Goh SK, Geng X, Gualtierotti G, Grupillo M, et al. Bioengineering Thymus Organoids to Restore Thymic Function and Induce Donor-Specific Immune Tolerance to Allografts. Mol Ther (2015) 23(7):1262–77. doi: 10.1038/mt.2015.77
183. Tajima A, Pradhan I, Trucco M, Fan Y. Restoration of Thymus Function With Bioengineered Thymus Organoids. Curr Stem Cell Rep (2016) 2(2):128–39. doi: 10.1007/s40778-016-0040-x
184. Campinoti S, Gjinovci A, Ragazzini R, Zanieri L, Ariza-McNaughton L, Catucci M, et al. Reconstitution of a Functional Human Thymus by Postnatal Stromal Progenitor Cells and Natural Whole-Organ Scaffolds. Nat Commun (2020) 11(1):6372. doi: 10.1038/s41467-020-20082-7
185. Krausgruber T, Fortelny N, Fife-Gernedl V, Senekowitsch M, Schuster LC, Lercher A, et al. Structural Cells Are Key Regulators of Organ-Specific Immune Responses. Nature (2020) 583(7815):296–302. doi: 10.1038/s41586-020-2424-4
Keywords: thymus, FOXN1, thymus epithelial cells, mesenchymal cells, endothelial cells, T cell development, thymus regeneration, thymus organoid technologies
Citation: Bhalla P, Su D-M and van Oers NSC (2022) Thymus Functionality Needs More Than a Few TECs. Front. Immunol. 13:864777. doi: 10.3389/fimmu.2022.864777
Received: 28 January 2022; Accepted: 03 May 2022;
Published: 10 June 2022.
Edited by:
Loretta Tuosto, Sapienza University of Rome, ItalyReviewed by:
Donald Palmer, Royal Veterinary College (RVC), United KingdomNuno L. Alves, Universidade do Porto, Portugal
Copyright © 2022 Bhalla, Su and van Oers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolai S. C. van Oers, bmljb2xhaS52YW5vZXJzQHV0c291dGh3ZXN0ZXJuLmVkdQ==
 Pratibha Bhalla
Pratibha Bhalla Dong-Ming Su
Dong-Ming Su Nicolai S. C. van Oers
Nicolai S. C. van Oers