
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 27 May 2022
Sec. Autoimmune and Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.859926
This article is part of the Research TopicChronic Autoimmune Arthritis, Infections and VaccinesView all 18 articles
Efficient protection against coronavirus disease 2019 (COVID-19) has been achieved by immunization with mRNA-based vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, efficient immune responses against this novel virus by vaccination are accompanied by a wide variety of side effects. Indeed, flares or new-onset of autoimmune disorders have been reported soon after the COVID-19 vaccination. Although pro-inflammatory cytokine responses play pathogenic roles in the development of autoimmunity, cytokines charactering COVID-19 vaccination-related autoimmune responses have been poorly understood. Given that mRNA derived from COVID-19 vaccine is a potent inducer for pro-inflammatory cytokine responses, these cytokines might mediate autoimmune responses after COVID-19 vaccination. Here we report a case with new-onset rheumatoid arthritis (RA) following COVID-19 vaccination. Serum concentrations not only of arthrogenic cytokines, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), but also of type I interferon (IFN) were elevated at the active phase in this case. Induction of remission by methotrexate and tocilizumab was accompanied by a marked reduction in serum concentrations of type I IFN, IL-6, and TNF-α. These results suggest that production of type I IFN, IL-6, and TNF-α induced by COVID-19 vaccination might be involved in this case with new-onset RA.
Coronavirus disease 2019 (COVID-19) sometimes causes autoimmunity (1). Molecular mimicry has been considered to be involved in the development of autoimmunity due to cross-reactivity of a COVID-19 spike protein to human antigens (1). Given that BNT162b2 (BioNTech-Pfizer) is a mRNA-based vaccine expressing the spike protein, it is possible that BNT162b2 vaccination can be a trigger for the development of autoimmunity (2–4). In fact, new-onset or flares of autoimmune disorders including rheumatoid arthritis (RA), adult-onset Still’s disease, inflammatory bowel diseases, and anti-neutrophil cytoplasmic antibody-associated vasculitis have been observed soon after the COVID-19 vaccination (5–9). Immunopathogenesis underlying COVID-19 vaccination-related autoimmunity has not been fully understood. In this regard, sensing of double-stranded and single-stranded RNA derived from the mRNA-based vaccine by toll-like receptors (TLRs), retinoic acid inducible gene-I (RIG-I), and melanoma differentiation-associated gene 5 (MDA5) can be potent triggers for production of type I interferons (IFN-I) and pro-inflammatory mediators (2–4). Therefore, it is likely that excessive production of IFN-I and pro-inflammatory cytokines is involved in the development of autoimmune responses associated with COVID-19 vaccination. However, cytokines causing autoimmunity have not been identified in such cases. Here, we report a case with new-onset RA following COVID-19 vaccination. In this case, serum and synovial fluid concentrations of interleukin (IL)-6, tumor necrosis factor-α (TNF-α), and IFN-I were elevated, suggesting that pro-inflammatory cytokine responses triggered by COVID-19 vaccination might be involved in the development of de novo RA.
A 53-year-old healthy Japanese man received BNT162b2 vaccination twice. His grandmother had RA. Successful immunization was verified by a marked elevation of anti-COVID-19-specific Ab titer (510 U/mL, normal range <15). Four weeks after the final vaccination, his left knee joint became swollen and painful. Soon after, he noticed bilateral omalgia and morning stiffness. Blood examination revealed marked leukocytosis (white blood cell, WBC count; 14,600/μL, normal range; 4,000~9,000) and elevated levels of C-reactive protein (CRP 8.45 mg/dL, normal range; 0~0.60). WBC counts and CRP levels were normal before the second vaccination (Figure 1A). His serum levels of anti-cyclic citrullinated peptide antibody and rheumatoid factor were 1,200 U/mL (normal range; 0~4.4) and 51 U/mL (normal range; 0~15), respectively. Magnetic resonance imaging showed diffuse knee effusion. Based on these typical symptoms and serological analyses, he was diagnosed as RA.
Initial treatment with methotrexate (MTX, 3 mg/day) with an escalating dose schedule (1 mg/2 weeks) and prednisolone (5 mg/day) failed to induce remission. PSL was switch to subcutaneous injection of etanercept (50 mg/week), which was again unsuccessful for the induction of remission. MTX (6 mg/day) in combination with subcutaneous injection of tocilizumab (162 mg/week) normalized serum concentration of CRP and WBC count (Figure 1A). Around one month after the injection of tocilizumab, induction of remission was achieved and bilateral omalgia, swollen of the left knee, and morning stiffness disappeared at this time point. He was treated with MTX and tocilizumab (162 mg/every two weeks) and maintained complete remission for more than ten months without adverse events.
Concentrations of IFN-α, IFN-β, IL-6, and TNF-α were measured using enzyme-linked immunosorbent assay kits from R&D systems. Serum samples were obtained at the active and remitted phases whereas joint fluid samples were taken at the active phase.
One major question arising from this case is whether BNT162b2 vaccination promoted the development of RA. Pro-inflammatory cytokines such as IL-6 and TNF-α underlie the immuno-pathogenesis of RA whereas the IFN-I responses are not so prominent in RA (10). mRNA derived from BNT162b2 can cause strong IFN-I responses through sensing by TLRs, RIG-I, and MDA5 (2–4). Therefore, it is possible that not only IL-6 and TNF-α but also IFN-I is involved in the development of RA in this case. As expected, serum concentrations of TNF-α and IL-6 were markedly reduced after treatment with MTX and tocilizumab (Figure 1B). Interestingly, serum concentrations of IFN-α and IFN-β were also high before the treatment and declined at the remission phase (Figure 1B). In addition, synovial fluid obtained from the left knee before the treatment contained high levels of IFN-I as well as IL-6. Thus, this new-onset RA after BNT162b2 vaccination was characterized by IFN-I as well as IL-6 and TNF-α.
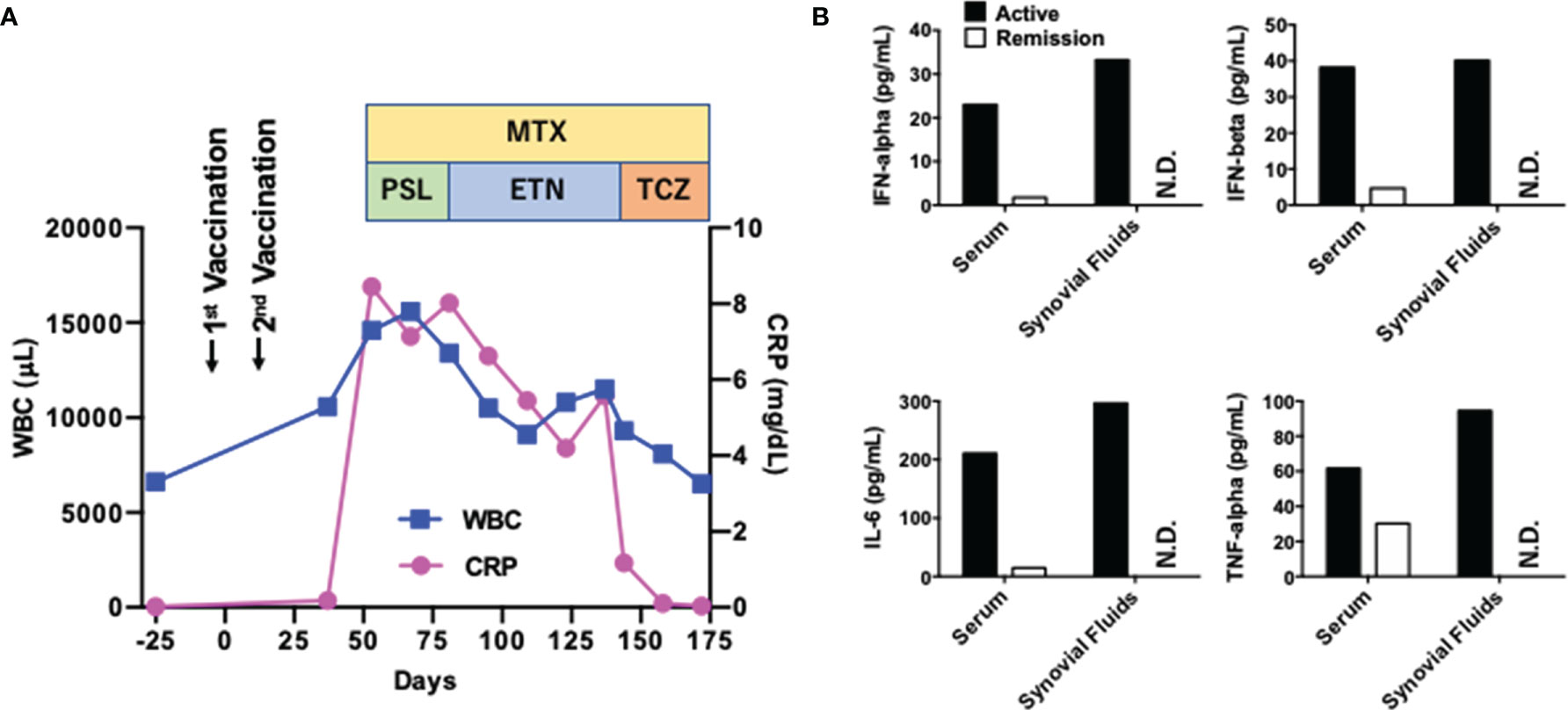
Figure 1 Clinical course of a patient with rheumatoid arthritis exhibiting systemic pro-inflammatory cytokine responses. (A) Clinical course. The first vaccination day was defined as Day 0. WBC, white blood cell; CRP, C-reactive protein; MTX, methotrexate; PSL, prednisolone; ETN, etanercept; TCZ, tocilizumab. (B) Serum concentrations of cytokines at the active (Day 53) and remitted (Day 172) phases. Serum concentrations of IL-6, TNF-α, IFN-α, and IFN-β at the active and remitted phases are shown. Synovial fluids were obtained at the active phase alone. N.D., not done.
Although mRNA-based COVID-19 vaccines are very effective for the protection against SARS-CoV-2 infection, the induction of efficient immune responses is concomitant with a wide variety of side effects (2–4). New-onset or exacerbation of autoimmune disorders has been reported in patients soon after COVID-19 vaccination (5–9). Given that pro-inflammatory cytokine responses play crucial roles in the development of autoimmune disorders, it is likely that COVID-19 vaccination can be a trigger for production of pro-inflammatory cytokines. Different from conventional vaccines, mRNA-based COVID-19 vaccines do not contain adjuvants with potent immuno-stimulatory functions (2–4). In this regards, recognition of mRNAs derived from COVID-19 vaccine by TLRs, RIG-I, and MDA5 may induce pro-inflammatory cytokines, including IFN-I (2–4). However, molecular mechanisms accounting for the development of autoimmunity following COVID-19 vaccination have not been fully understood. Here we show a case of de novo RA following COVID-19 vaccination and characterized by systemic pro-inflammatory cytokine responses including IFN-I, IL-6, and TNF-α. Although new-onset or exacerbation of autoimmune disorders have been reported in patients soon after COVID-19 vaccination (5–9), little information was available regarding pro-inflammatory cytokine responses in such cases.
Although IL-6 and TNF-α are well-established arthrogenic cytokines, roles of IFN-I have been poorly defined. The IFN-I signature is less conspicuous in RA than in the other IFN-I-dependent autoimmunity such as systemic lupus erythematosus (11–13). Thus, this case was atypical in that IFN-I responses as well as prototypical pro-inflammatory cytokine responses were parallel to disease activities. In this regard, we speculate that BNT162b2 vaccination could be a trigger for RA development. This notion is supported by the fact that mRNA derived from BNT162b2 is a potent inducer of IFN-I responses through activation of TLRs, RIG-I, and MDA5 (2, 3). Consistent with high concentrations of IFN-I in the synovial fluid of this case, Lande et al. confirmed the presence of IFN-I in the synovial fluid of patients with RA (14). Taken together, this case suggests that initial IFN-I responses induced by BNT162b2 vaccination might trigger arthrogenic cytokine responses accounting for the development of typical RA via induction of IL-6 and TNF-α. This idea is fully supported by the fact that IFN-I can function as upstream cytokines with the ability to induce production of prototypical inflammatory cytokines such as IL-6 and TNF-α (15). However, we cannot exclude the possibility that the timing of RA development with regard to vaccination was coincidental. Moreover, this patient was successfully treated by the blockade of IL-6, which suggests predominant roles of this cytokine. Future studies performing large-scale epidemiological analyses in new-onset or flare RA patients following COVID-19 vaccination are required to establish its link. It would be intriguing to examine whether IFN-I as well as arthrogenic cytokine (IL-6 and TNF-α) responses are elevated in COVID-19 vaccination-associated RA.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Kindai University Faculty of Medicine. The patients/participants provided their written informed consent to participate in this study.
TW and KM wrote the manuscript draft and measured concentrations of cytokines. TW, KM, AH, TY, KK, and MK revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported in part by a Grant-in-Aid for Scientific Research (22K07996) from Japan Society for the Promotion of Science, Yakult Bioscience Foundation, Smoking Research Foundation and Takeda Science Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Ms. Yukiko Ueno for her secretarial assistance.
1. Ehrenfeld M, Tincani A, Andreoli L, Cattalini M, Greenbaum A, Kanduc D, et al. Covid-19 and Autoimmunity. Autoimmun Rev (2020) 19(8):102597. doi: 10.1016/j.autrev.2020.102597
2. Teijaro JR, Farber DL. COVID-19 Vaccines: Modes of Immune Activation and Future Challenges. Nat Rev Immunol (2021) 21(4):195–7. doi: 10.1038/s41577-021-00526-x
3. Sprent J, King C. COVID-19 Vaccine Side Effects: The Positives About Feeling Bad. Sci Immunol (2021) 6(60). doi: 10.1126/sciimmunol.abj9256
4. Sahin U, Kariko K, Tureci O. mRNA-Based Therapeutics–Developing a New Class of Drugs. Nat Rev Drug Discovery (2014) 13(10):759–80. doi: 10.1038/nrd4278
5. Hakroush S, Tampe B. Case Report: ANCA-Associated Vasculitis Presenting With Rhabdomyolysis and Pauci-Immune Crescentic Glomerulonephritis After Pfizer-BioNTech COVID-19 mRNA Vaccination. Front Immunol (2021) 12:762006. doi: 10.3389/fimmu.2021.762006
6. Leone F, Cerasuolo PG, Bosello SL, Verardi L, Fiori E, Cocciolillo F, et al. Adult-Onset Still's Disease Following COVID-19 Vaccination. Lancet Rheumatol (2021) 3(10):e678–80. doi: 10.1016/S2665-9913(21)00218-6
7. Weaver KN, Zhang X, Dai X, Watkins R, Adler J, Dubinsky MC, et al. Impact of SARS-CoV-2 Vaccination on Inflammatory Bowel Disease Activity and Development of Vaccine-Related Adverse Events: Results From PREVENT-COVID. Inflamm Bowel Dis (2021). doi: 10.1093/ibd/izab302
8. Sangha M, Roitman I, Sultan K, Swaminath A. SARS-CoV-2 Immunization in Patients With Inflammatory Bowel Disease May Result in Disease Flares. Am J Gastroenterol (2021) 116(12):2480–1. doi: 10.14309/ajg.0000000000001416
9. Terracina KA, Tan FK. Flare of Rheumatoid Arthritis After COVID-19 Vaccination. Lancet Rheumatol (2021) 3(7):e469–70. doi: 10.1016/S2665-9913(21)00108-9
10. McInnes IB, Schett G. Cytokines in the Pathogenesis of Rheumatoid Arthritis. Nat Rev Immunol (2007) 7(6):429–42. doi: 10.1038/nri2094
11. Higgs BW, Liu Z, White B, Zhu W, White WI, Morehouse C, et al. Patients With Systemic Lupus Erythematosus, Myositis, Rheumatoid Arthritis and Scleroderma Share Activation of a Common Type I Interferon Pathway. Ann Rheum Dis (2011) 70(11):2029–36. doi: 10.1136/ard.2011.150326
12. Watanabe T, Minaga K, Kamata K, Kudo M, Strober W. Mechanistic Insights Into Autoimmune Pancreatitis and IgG4-Related Disease. Trends Immunol (2018) 39(11):874–89. doi: 10.1016/j.it.2018.09.005
13. Chasset F, Dayer JM, Chizzolini C. Type I Interferons in Systemic Autoimmune Diseases: Distinguishing Between Afferent and Efferent Functions for Precision Medicine and Individualized Treatment. Front Pharmacol (2021) 12:633821. doi: 10.3389/fphar.2021.633821
14. Lande R, Giacomini E, Serafini B, Rosicarelli B, Sebastiani GD, Minisola G, et al. Characterization and Recruitment of Plasmacytoid Dendritic Cells in Synovial Fluid and Tissue of Patients With Chronic Inflammatory Arthritis. J Immunol (2004) 173(4):2815–24. doi: 10.4049/jimmunol.173.4.2815
Keywords: rheumatoid arthritis, COVID-19 vaccination, type I IFN, IL-6, TNF-alpha
Citation: Watanabe T, Minaga K, Hara A, Yoshikawa T, Kamata K and Kudo M (2022) Case Report: New-Onset Rheumatoid Arthritis Following COVID-19 Vaccination. Front. Immunol. 13:859926. doi: 10.3389/fimmu.2022.859926
Received: 22 January 2022; Accepted: 28 April 2022;
Published: 27 May 2022.
Edited by:
Michele Maria Luchetti Gentiloni, Marche Polytechnic University, ItalyReviewed by:
Rita Consolini, University of Pisa, ItalyCopyright © 2022 Watanabe, Minaga, Hara, Yoshikawa, Kamata and Kudo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomohiro Watanabe, dG9tb2hpcm9AbWVkLmtpbmRhaS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.