
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 15 February 2022
Sec. Vaccines and Molecular Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.851028
This article is part of the Research Topic Irradiation Technologies for Vaccine Development View all 16 articles
 Eric R. James*
Eric R. James* Steve Matheny
Steve Matheny James Overby
James Overby B. Kim Lee Sim
B. Kim Lee Sim Abraham G. Eappen
Abraham G. Eappen Tao Li
Tao Li Ming Lin Li
Ming Lin Li Thomas L. Richie
Thomas L. Richie Sumana Chakravarty
Sumana Chakravarty Anusha Gunasekera
Anusha Gunasekera Tooba Murshedkar
Tooba Murshedkar Peter F. Billingsley
Peter F. Billingsley Stephen L. Hoffman
Stephen L. HoffmanIonizing radiation (UV, X-ray and ɣ) administered at an appropriate dose to pathogenic organisms can prevent replication while preserving metabolic activity. We have established the GMP process for attenuation by ionizing radiation of the Plasmodium falciparum (Pf) sporozoites (SPZ) in Sanaria® PfSPZ Vaccine, a protective vaccine against malaria. Mosquitoes raised and infected aseptically with Pf were transferred into infected mosquito transport containers (IMTC) and ɣ-irradiated using a 60Co source. PfSPZ were then extracted, purified, vialed, and cryopreserved. To establish the appropriate radiation conditions, the irradiation field inside the IMTCs was mapped using radiochromic film and alanine transfer dosimeters. Dosimeters were irradiated for times calculated to provide 120-170 Gy at the minimum dose location inside the IMTC and regression analysis was used to determine the time required to achieve a lower 95% confidence interval for 150 Gy. A formula incorporating the half-life of 60Co was then used to construct tables of irradiation times for each calendar day. From the mapping studies, formulae were derived to estimate the minimum and maximum doses of irradiation received inside the IMTC from a reference dosimeter mounted on the outside wall. For PfSPZ Vaccine manufacture a dose of 150 Gy was targeted for each irradiation event, a dose known to completely attenuate PfSPZ. The reference dosimeters were processed by the National Institute of Standards and Technology. There have been 587 irradiation events to produce PfSPZ Vaccine during 13 years which generated multiple lots released for pre-clinical studies and clinical trials. The estimated doses at the minimum dose location (mean 154.3 ± 1.77 Gy; range 150.0-159.3 Gy), and maximum dose location (mean 166.3 ± 3.65 Gy, range 155.7 to 175.3 Gy), in IMTCs were normally distributed. Overall dose uniformity was 1.078 ± 0.012. There was no siginifcant change in measured dose over 13 years. As of January 2022, 21 clinical trials of PfSPZ Vaccine have been conducted, with 1,740 volunteers aged 5 months to 61 years receiving 5,648 doses of PfSPZ Vaccine totalling >5.3 billion PfSPZ administered. There have been no breakthrough infections, confirming the consistency and robustness of the radiation attenuation process.
Radiation wavelengths shorter than ~124 nm that include far-UV, X-ray and ɣ, induce ionization effects that damage live cells principally through the generation of free radicals and their interaction with proteins, membranes and DNA. The dose of radiation can be selected to render cells or whole organisms metabolically active but incapable of replication. Used on eukaryotic pathogens, irradiation is an ideal method for developing live attenuated vaccines that are immunogenic and for which the ability to cause disease has been abrogated. Ionizing radiation of all three types has been used to attenuate parasitic protozoa and helminths (1–9)1.
The pioneering studies on attenuation of malaria sporozoites (SPZ) for assessing protective immunity were conducted with X-ray as the irradiation source (10). Subsequent studies used X-rays and ɣ irradiation, sourced either from 137Cs or 60Co (11–14). Sanaria®PfSPZ Vaccine is composed of SPZ, the infective stage of Plasmodium falciparum (Pf), that are irradiated in the mosquito using a 60Co source and subsequently extracted from the mosquito salivary glands, purified, formulated with cryoprotectant additives and cryopreserved (15, 16). In clinical trials, PfSPZ Vaccine induces >90% protection against controlled human malaria infection (CHMI) delivered by mosquito bite or by injection (17–19) and significant protection for at least two malaria transmission seasons against natural exposure to malaria in Africa (20). Attenuation by ɣ irradiation was adopted for the manufacture of PfSPZ Vaccine principally due to the ability to deliver a very accurate irradiation dose, shorter irradiation times than X-ray, ease of use, and a history of success in human trials (14) that used PfSPZ administered by the bite of Pf-infected, irradiated mosquitoes for immunizations.
We present here the process for development of a robust and reproducible method for the ɣ-irradiation of PfSPZ-infected mosquitoes delivered by a 60Co source, and the experience of using this method in the manufacture of PfSPZ Vaccine for clinical trials.
The irradiator, a JL Shepherd model 484-R-2, has three sources, an integral controller incorporating a timer and an air compressor with reservoir. The unit is fabricated principally from cast iron and lead, weighs approximately 6.5 metric tons, and houses the shielded irradiation chamber (Figure 1). The unit is calibrated annually by JL Shepherd (San Fernando, CA), and the controller and monitoring systems are calibrated independently every six months.
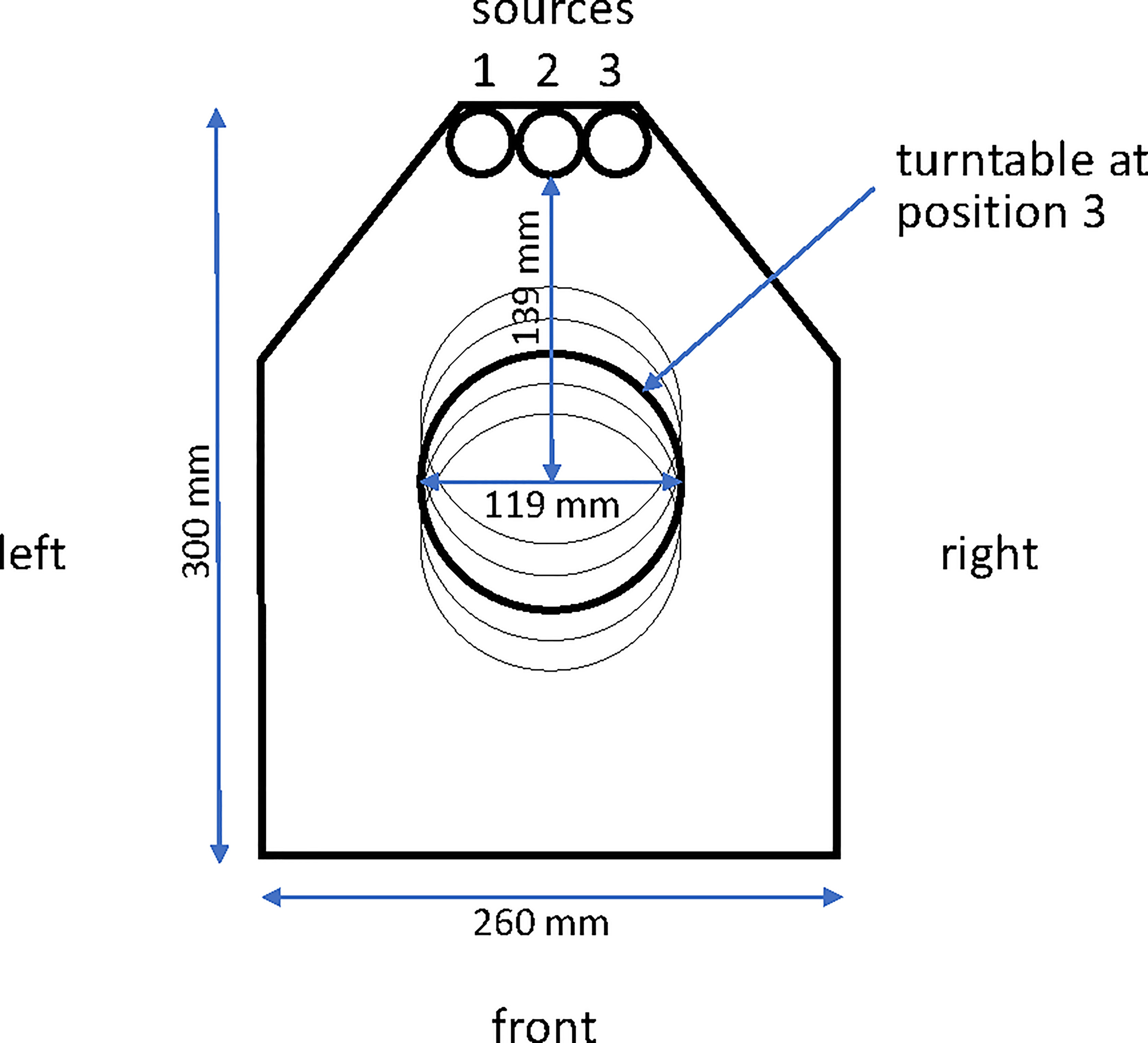
Figure 1 Plan view of the interior of the irradiator chamber. The three source tunnels (left #1, center #2 and right #3) are indicated at the apex of the drawing and the turntable is indicated at position 3 (of 5 potential positions) in the central axis of the chamber.
When installed in 2007, source tunnels 2 (center) and 3 (right) contained cobalt capsules with a total activity of 12,000 Ci. Nine years later, the 60Co had decayed through 1.9 half lives to 3,349 Ci so the 60Co capsule occupying source tunnel 2 was moved into source tunnel 1, and a new capsule with 8,400 Ci activity was added to source tunnel 2 to bring the total activity to 11,749 Ci. Sources are registered with the Nuclear Regulatory Commission and checked annually. In addition to the three source tunnels, the irradiator chamber contains a turntable that rotates at approximately 17 revolutions per minute and can be used at any of five positions at different distances from the irradiation sources. Turntable position 3 is used for IMTC mosquito irradiation (Figure 1).
Personnel qualified to operate the irradiator undergo FBI background checks, fingerprinting, and are issued a unique coded card for entry. Other security measures include a second coded door entry, a third door linked to an iris scanner, video surveillance at multiple locations and direct real-time video feed to the County Police Department (CPD) with an on-call Special Weapons and Tactics (SWAT) team.
Residual radiation around the irradiator both at rest and when active is equivalent to background, as indicated by routine dosimetry (processed quarterly) from multiple locations in the irradiator room. However, Sanaria provides operators with personal dosimeters that are maintained by a contract Radiation Safety Officer and processed by Landauer (Beltsville, MD). A survey meter (Technical Associates, Canoga Park, CA) connected to the irradiator controller broadcasts an alarm internally and to the CPD if radiation levels exceed threshold for safety or if the meter is disconnected or disabled. Additional monitoring and alarm systems are integral to the unit.
Aseptically-reared PfSPZ-infected Anopheles stephensi mosquitoes are transferred to Infected Mosquito Transport Containers (IMTC) for aseptic transport to the irradiator. The IMTC consists of a custom designed outer container (OC) fabricated from polycarbonate and composed of a cylinder with a screw-on base and a screw lid that incorporates a filter (Figure 2). The IMTC is assembled with the inner container (IC), a modified 1-pint cardboard cylinder, autoclaved, and mosquitoes are aspirated under aseptic conditions directly into the IC from the adult mosquito containers. Each IMTC is sealed inside a sterility maintenance bag (Steris, Erie, PA) which remains in place during irradiation. IMTCs were fabricated with the base able to fit within the circular wall of the irradiator chamber turntable and with a base thickness aimed to position the center of the vertical axis of the IC in the center of the irradiation field in the chamber.
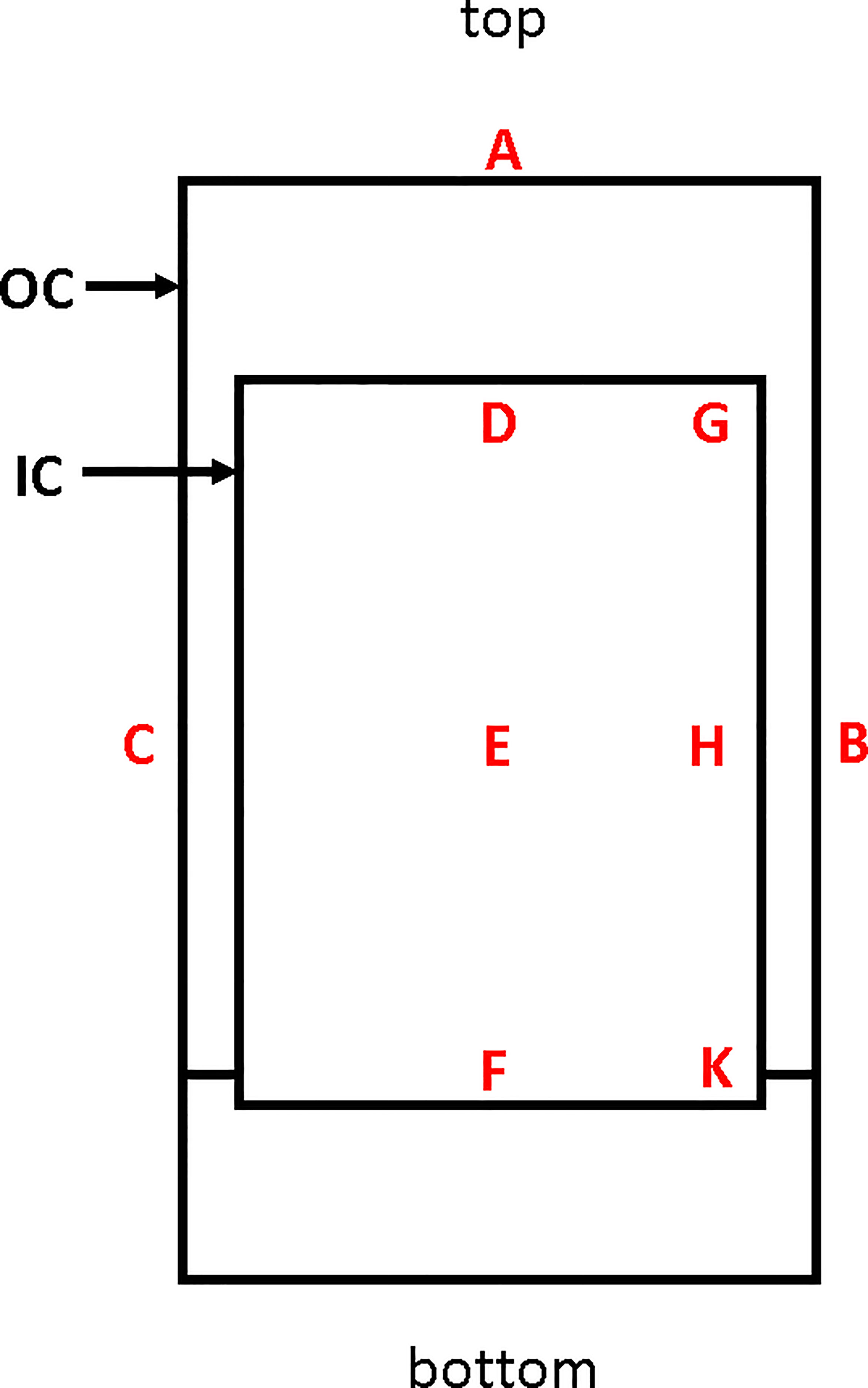
Figure 2 Sagittal sectional schematic diagram of an IMTC. The inner container (IC) is housed inside the outer container (OC), which is a custom machined 1 L polycarbonate container with screw top and screw base. All nine locations used for placement of alanine transfer dosimeters for mapping are indicated. The minimum dose of irradiation was recorded at position F up to the time when a new IC was incorporated when the minimum dose location changed to D. The maximum dose location is position H. The reference dosimeter used in PfSPZ Vaccine manufacturing runs is located at position B. The relationship between the dose received at B to the dose received at D (or previously, F) and between the dose at B and the dose at H are used to generate the formulae for estimating the doses at positions D/F and H during vaccine production.
The radiation dose received at any point in the irradiator chamber is inversely proportional to the distance from the sources. Thus the dose delivered inside the IMTC will vary both horizontally and vertically. By mapping the radiation field using radiochromic film, the maximum dose and minimum dose locations inside the IMTC can be identified (21). Two sets of radiochromic film mapping experiments were conducted, the first initially after the irradiator was installed and the second following the irradiator upgrade nine years later.
The first determination of the irradiation field was conducted inside the OC of the IMTC using GAFchromic HD-810 film [International Specialty Products (ISP), Wayne, NJ] rated for a dose range of 10-400 Gy. The film was trimmed to fit vertically into the OC, sandwiched between polystyrene plates and sealed in a black polyethylene pouch. Two different OCs were mapped using an irradiation time (provided by JL Shepherd) to target a dose on that particular day of 150 Gy in the center of the OC when the IMTC was placed on the turntable at position 3. The films were removed from their packaging at the National Institute of Standards and Technology (NIST, Gaithersburg, MD), and scanned into a Pharmacia-LKB 2222 UltroScan XL Laser Densitometer at 633 nm with a spot size of 100 μm. Measurements were made by stepping in both dimensions at a resolution of 0.6 mm. The data output was in arbitrary scanning laser densitometer (SLD) units related to optical absorbance. Average SLD values were determined at the film’s center, and the value used to normalize to the whole scan and to express the results in terms of percent increase or decrease relative to the dose at the center.
An additional set of radiochromic film assessments was made following the irradiator upgrade to confirm the distribution of the delivered radiation dose applied to the IC and confirm the minimum and maximum dose locations. Gafchromic Ashland Dose-Map™ film with an upper exposure bound of 50 Gy was sandwiched between Plexiglas sheets and sealed in black polypropylene. This smaller film package was supported inside the IC along the central vertical axis. An alanine transfer dosimeter was also placed on the outside of the OC of the IMTC at location B (Figure 2), and the IMTC exposed to a target dose of 50 Gy at the maximum dose location inside the IC.
To further characterize the distribution of radiation received by the IMTC and to determine the doses received at the minimum and maximum dose locations when targeting a received minimum dose of 150 Gy, the IC of the IMTC was also mapped using alanine transfer dosimeters (22–25). Alanine dosimeters (NIST High Dose Radiation Service, Gaithersburg, MD) were composed of Plexiglas vials each containing four alanine pellets. The dosimeters were positioned at the locations indicated in Figure 2. After irradiation, dosimeters were processed by NIST and the average dose received by the four pellets inside each Plexiglas vial was reported to the nearest whole Gy for that dosimeter.
Dosimeters located at the minimum dose location were irradiated on a specific date for times calculated to deliver doses ranging from 120 Gy to 170 Gy. Dosimeters were processed as above and the data for irradiation time and dose received by each dosimeter, were used in a regression analysis, including the upper and lower 95% confidence intervals, to determine the time required to deliver 150 Gy of radiation at the minimum dose location on that date. The value was also used to extrapolate back to the reference date when the irradiator was installed. This experiment was repeated after the irradiator source upgrade.
Two irradiation time tables were constructed spanning 1) the period from initial installation of the irradiator to the upgrade nine years later, and 2) all dates during the subsequent six years. For both timetables the baseline date was defined as the reference date from which to calculate the times to deliver the target minimum dose on all subsequent days according to the equation:
where:
t = time in minutes for the day of interest,
x = time in minutes at reference date,
y = number of days since reference date, and
T = ½ life of 60Co in days (1925.20 days).
The minimum and maximum doses of irradiation received by any mosquito inside the IMTC were estimated from the dose received by a dosimeter attached to the outside of the IMTC at location B (reference location) (Figure 2). To determine the formulae for estimating the dose received at the minimum dose location (location D or F) and the maximum dose location (location H) inside the IMTC from the dose received at the reference location, dosimeters were mounted on a cardboard scaffold at the three locations in the IC and at the external reference location.
Three sets of data were collected following installation of the irradiator, three more sets were collected when the irradiator was upgraded, and a final three sets were collected when the original IC was replaced by a new IC of slightly different dimensions. This last data set resulted in the minimum dose location changing from location F to location D (Figure 2).
In addition to dosimetry, the 6-day hepatocyte attenuation assay, a biological measure used to confirm attenuation, was performed using irradiated PfSPZ without cryopreservation (26); the result of this assay, along with the dosimetry data, is incorporated into the lot release certificate of analysis for PfSPZ Vaccine.
A manufacturing campaign for PfSPZ Vaccine consists of multiple sequential irradiation runs, generally up to 16. An alanine dosimeter is included at the reference location on the outside of each IMTC for every irradiation run. The irradiation time for any given date is indicated in the irradiation timetables. All dosimeters are processed by NIST. The data for the estimated doses delivered to the minimum dose location were calculated for all runs from the doses reported for the reference dosimeter.
The purpose of these experiments was to determine the relative radiation dose delivered spatially, which is independent of a particular target dose or dose rate. Three pairs of radiochromic film images were recorded for the original study in the OC of the IMTC. After the first pair of images was obtained, the vertical positioning of the OC was adjusted upwards to improve the vertical gradient of received dose. An additional adjustment to the configuration of the IMTC was made after the second pair of images was obtained; results for the third pair are shown in Figure 3. Radiation exposure followed a gradient, with the highest dose received at the vertical midpoint on the side wall decreasing to the center of the OC and decreasing further both upwards and downwards along the central vertical axis. The lowest doses were recorded at the top center and bottom center of the OC.
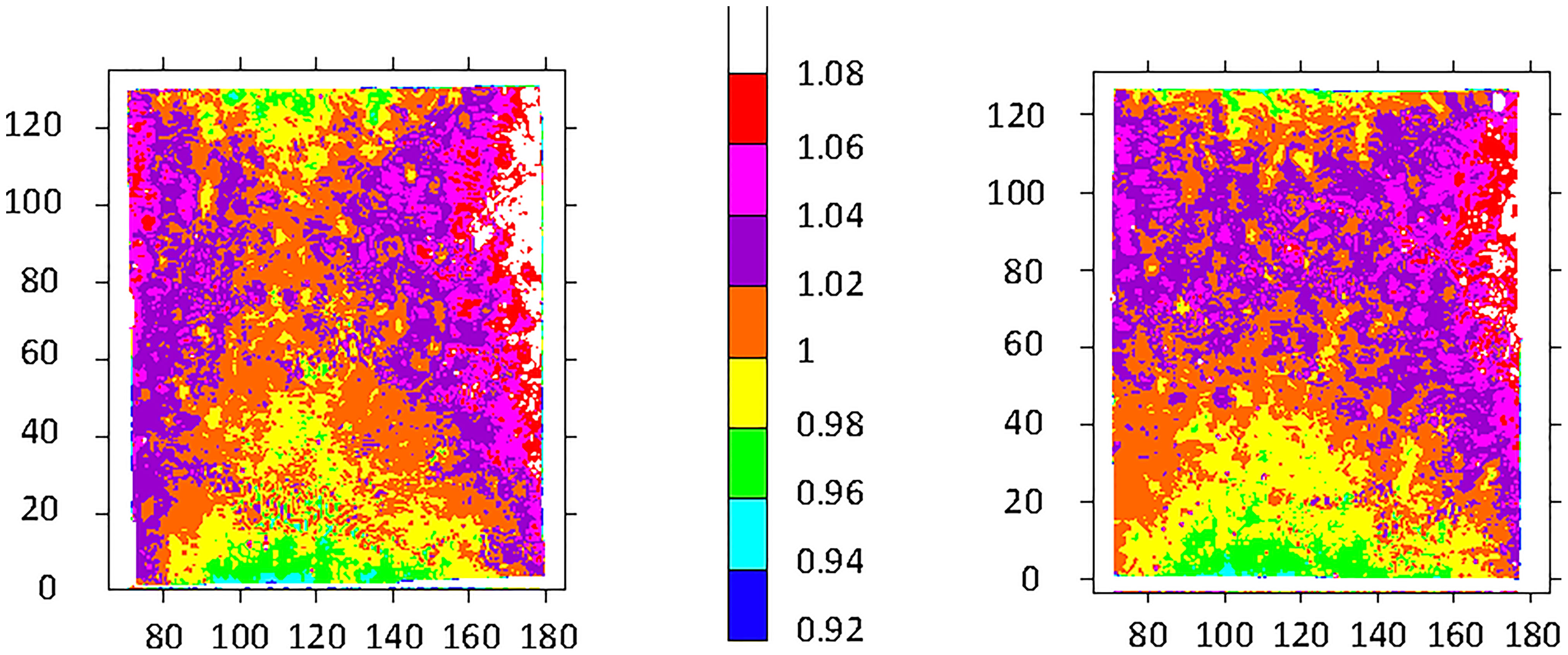
Figure 3 Test film results from two OCs exposed to 150 Gy. The target dose delivered was 150 Gy. The color scale represents proportional arbitrary scanning laser densitometry units normalized to 312 SLD units left, and to 320 SLD units right. The boundary between orange and yellow was assigned a value of 1. Horizontal axis: units in mm relative to the scanner base plate; vertical axis: distance in mm from the bottom of the film/container. See Figure 2 for the minimum dose locations (position F, bottom center of each film) and maximum dose locations (position H, at the side wall equator of each film).
Following upgrading of the irradiator, GAF chromic film was provided cut to fit the inside of the IC. For the three films the mean dose ± SD at the minimum dose location (position F) was 38.8 ± 0.64 Gy, and at the maximum dose location (position H) was 47.1 ± 0.25 Gy (Figure 4, Table 1). The dose uniformity, the ratio of highest dose to lowest dose, (dose at position H/dose at position F) was 1.216 ± 0.014 for this film using a target dose of 50 Gy.
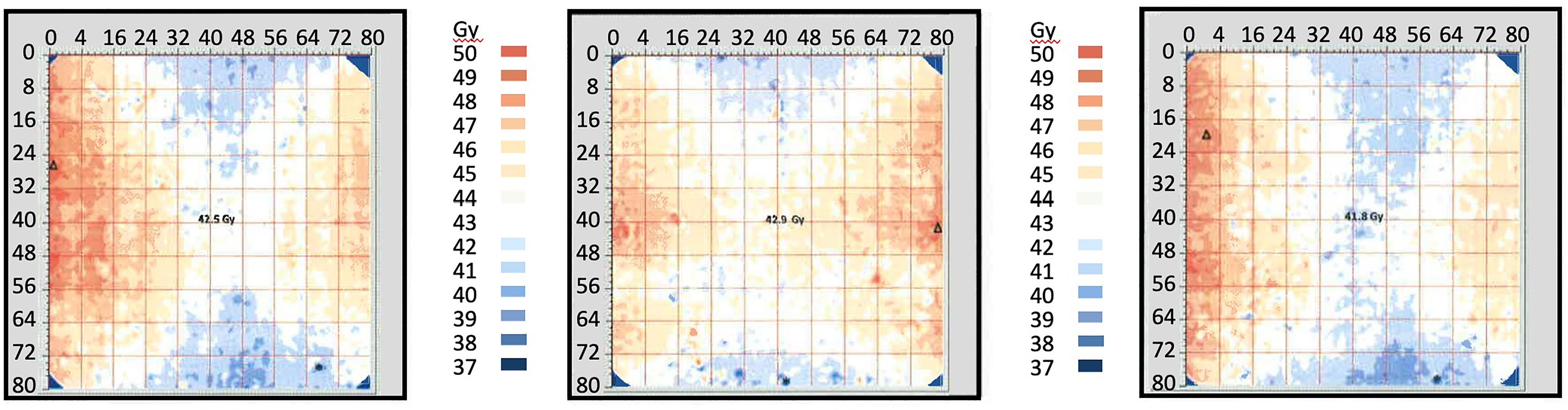
Figure 4 Radiochromic film mapping of the IC of the IMTCs: three runs. The image from run 1 is at left, from run 2 in the center and from run 3 is at right. The dose received at the center of the film (position E) was 42.9 Gy (run 1), 41.8 Gy (run 2) and 42.5 Gy (run 3). See Table 2 for dose levels recorded for the minimum dose locations (position F, bottom center of each film) and maximum dose locations (position H, at the side wall equator of each film).
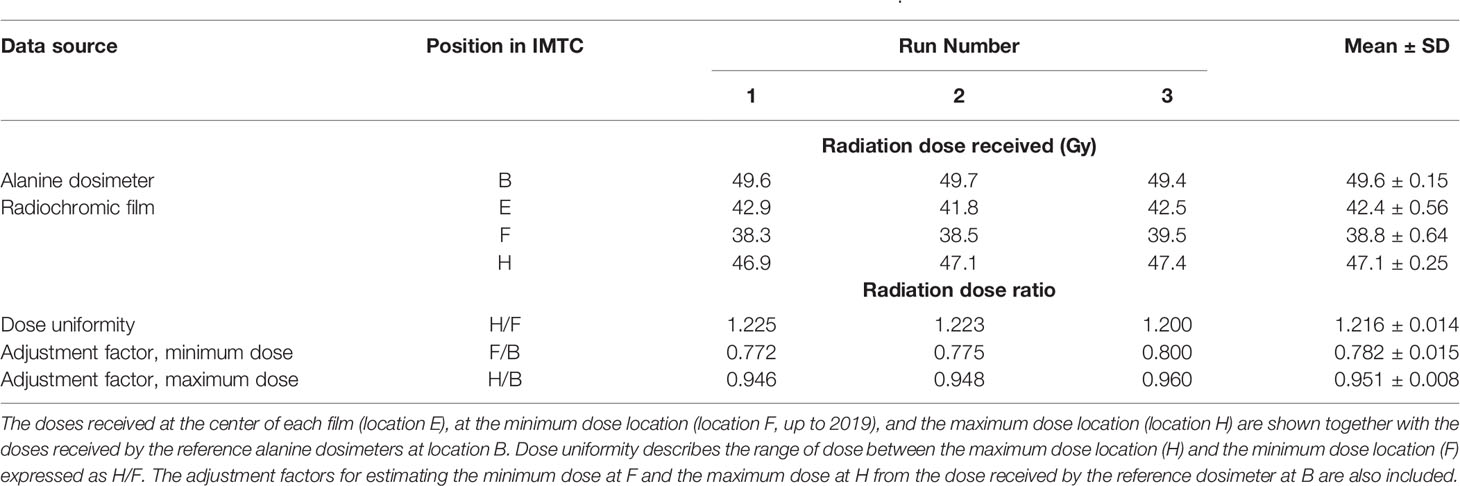
Table 1 Radiation doses recorded in the ICs of the IMTCs in the radiochromic film study in Figure 4.
Experiments were conducted using alanine transfer dosimeters with a dose calculated to deliver 150 Gy to the minimum dose location. The most recent set of these experiments was performed after the changeover to the new IC in the IMTC. These studies used dosimeters placed at the six locations inside the IC (Figure 2) to define the minimum and maximum dose locations, and two dosimeters on the exterior of the IMTC at positions A and B (Figure 2). In this study, dose uniformity was tighter at 1.09 ± 0.004 (Table 2) than seen with GAF chromic film. This alanine dosimeter mapping study also established that the minimum dose was received at location D (154 ± 1.0 Gy) rather than location F (155 ± 1.0 Gy). This change in minimum dose location was a consequence of the changeover to the new IC of the IMTC which was, as indicated above, slightly taller (by 8.7 mm). The position of the base of the IC is fixed, so that the additional height of the IC moved the top of the IC and the minimum dose location higher up the vertical axis of the unit into a lower isodose band. The ratios between the minimum dose and the reference dose (0.834 ± 0.008) and the maximum dose and the reference dose (0.908 ± 0.005) were also established for estimating the minimum and maximum doses delivered during PfSPZ Vaccine production runs. For example, if the dosimeter at the reference location received a dose of 182 Gy, then 151.8 Gy and 165.3 Gy would be received at the minimum and maximum dose locations, respectively, and the dose uniformity (ratio of highest dose to lowest dose), would be 1.089.
Dosimeters placed at the minimum dose location in the IC of the IMTC were irradiated for different times to deliver doses between 120 and 170 Gy. Regression analysis of irradiation time vs. dose was used to determine the time to deliver a dose of 150 Gy with lower 95% confidence interval (Figures 5A, B). This dose was defined as the target minimum dose and the time to deliver this dose extrapolated from the regression analysis as the time on that date to deliver the target dose of irradiation. This time was then extended to the reference date and that value incorporated as the reference time (x in Equation 1) for constructing the calendar of irradiation timetables.
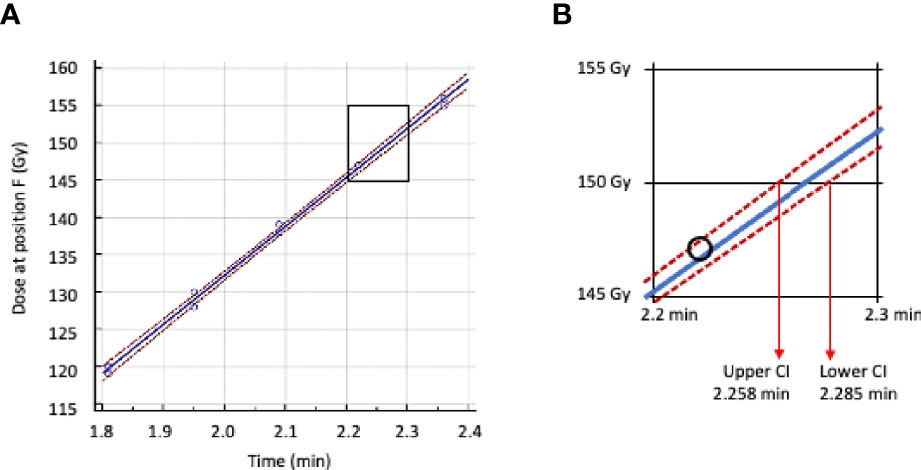
Figure 5 Regression analysis of irradiation time vs dose received at location F (minimum dose location) inside the IMTC. (A) Regression line plot in blue, 95% confidence intervals in red. (B) Area in A between 145 Gy and 155 Gy enlarged with the lower and upper 95% confidence intervals. The time adopted for calculating the target dose of 150 Gy is 2.285 min.
The estimated irradiation doses at the minimum dose location (locations F or D) in the IC were calculated using the formulae obtained by dosimetry. Three different sets of conversion factors were used following dosimetry calibration of the irradiator for 1) the period when two sources were active in the irradiator (first 9 years), 2) after the irradiator upgrade when all three sources were active (next 4 years), and 3) after introduction of the new IC (all subsequent times). For all irradiation runs the PfSPZ-infected mosquitoes were irradiated at ambient temperature inside the irradiator chamber which was typically 23°C, a temperature optimum for PfSPZ (26).
For the initial 9-year period the formula for estimating the dose received at location F was x0.8973 and for estimating the maximum dose at location H, the formula used was x0.9471. For the 4 years after the irradiator upgrade the conversion factor used for the dose at location F was x0.845, and for the maximum dose location H, was x0.9141. The current conversion factor used to estimate the minimum dose at location D from the dose received at location B for the period of 2019-present shown in Table 2 is x0.834, and to convert the dose at location B to the estimated dose at the maximum dose location, location H, the conversion factor is x0.908.
The estimated minimum dose received in each of 587 irradiation events during GMP manufacturing of PfSPZ Vaccine was very consistent (Figure 6), ranging from 150.0 Gy to 159.3 Gy, with a mean estimated minimum dose of 154.3 ± 1.77 Gy. The mean estimated maximum dose delivered in all irradiation runs was 166.3 ± 3.65 Gy, (range 155.7 Gy to 175.3 Gy), well below the highest acceptable maximum dose of 190 Gy. The overall dose uniformity was 1.078 ± 0.012.
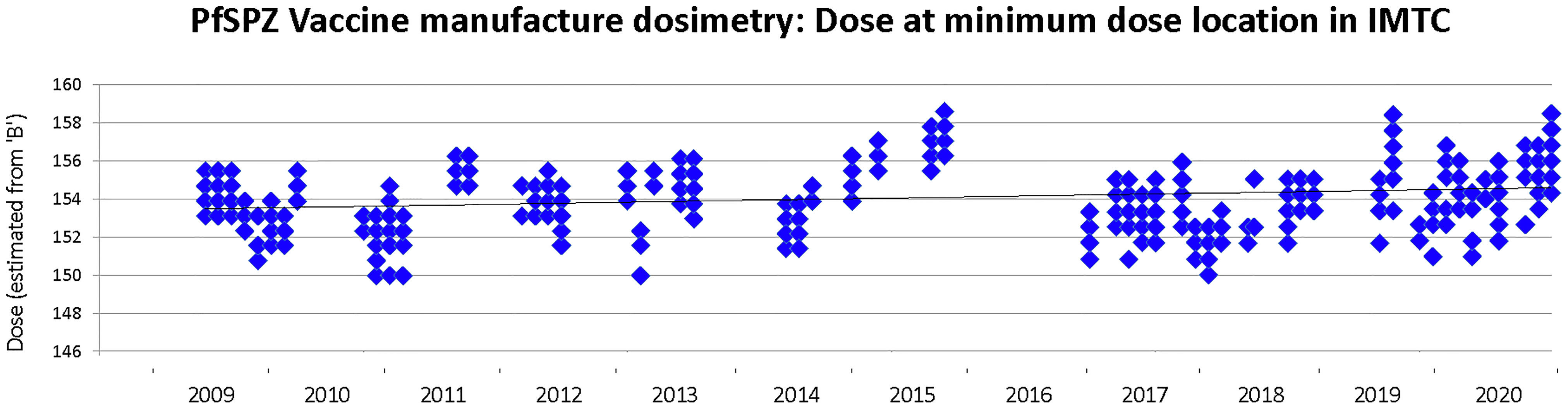
Figure 6 Minimum dose of irradiation received by any mosquito during PfSPZ Vaccine production campaigns between 2009-2020. The target minimum dose is 150 Gy and the minimum acceptable dose is 142.5 Gy. Not all production campaigns were converted into lots released for clinical use, and none of the clinical released lots included PfSPZ irradiated below 150 Gy.
The viability of PfSPZ in production lots is assessed using a sporozoite membrane integrity assay (SMIA), and is routinely conducted on vaccine bulk product prior to fill-finish. The SMIA results for PfSPZ Vaccine (radiation-attenuated) and PfSPZ Challenge (non-irradiated) have been published previously on several occasions: for example, the viability of PfSPZ after irradiation and prior to cryopreservation was reported as 97% (17) and for non-irradiated PfSPZ prior to cryopreservation the viability has been reported as 98.2% (27).
In the 21 clinical trials conducted to date using radiation-attenuated PfSPZ, 1,740 volunteers aged 5 months to 61 years have received 5,648 doses of PfSPZ Vaccine, meaning that >5.3 x 109 irradiated PfSPZ have been administered to human subjects. There have been no breakthrough infections. The 100% infectious dose (ID100) for non-irradiated PfSPZ (Sanaria® PfSPZ Challenge (NF54)) administered by direct venous inoculation (DVI) is 3.2 x 103 PfSPZ (27), which has been confirmed in 79 of 79 malaria-naive subjects receiving their first CHMI given by injection (28–31). The highest dose of PfSPZ Vaccine administered has been 2.7 x 106 PfSPZ, which represents 840x the ID100 [(32, 33), Sirima et al, submitted for publication]. Overall, the equivalent of more than 1.6 x 106 ID100s have been administered without a breakthrough.
We describe here the studies supporting the GMP radiation-attenuation methodology used in the manufacture of PfSPZ Vaccine, a radiation-attenuated, purified and cryopreserved, metabolically-active, non-replicating, whole sporozoite vaccine against malaria which has demonstrated unparalelled efficacy, safety and tolerability. In the 21 clinical trials conducted to date, three trials have shown 100% protection against homologous controlled human malaria infection (CHMI) (17, 19, 20).
Some of the initial concerns considered during development of a radiation-attenuated malaria vaccine were that attenuation by irradiation would be difficult to manage, that the parasites would have the potential to cause infections because of inadequate attenuation, or would cause an inferior and non-protective immune response due to over attenuation. None of these three scenarios has occurred.
Although the precise mechanisms whereby irradiation prevents replication is not understood, it is likely that damage to DNA results in multiple redundant defects providing a high level of assurance that no individual parasite would be capable of replication. Parasite genes that do appear to be downregulated following irradiation include those for DNA repair (34). Thus, with over 5.3 x 109 PfSPZ irradiated and subsequently administered to humans, not one has broken through. As the data presented here demonstrate, the manufacturing process for PfSPZ Vaccine maintains a tight control over the attenuation process.
PfSPZ administered directly by the bite of mosquitoes subjected to 150 Gy did not lead to breakthrough infections whereas PfSPZ exposed to a dose 120 Gy PfSPZ were not fully attenuated and breakthrough infections occurred (11, 12). In the in vitro 6-day hepatocyte attenuation assay, liver stage parasites develop from PfSPZ subjected to 120 Gy, but not after an irradiation dose of 142.5 Gy (data to be published separately). For the rodent malaria parasite, P. yoelii (Py), which has a lower tolerance to irradiation than Pf, the minimum predicted dose to achieve full attenuation in mice is 92.4 Gy (35). At a radiation dose of 100 Gy, PySPZ were fully attenuated; injection of 1x105 irradiated PySPZ to each of 41 mice failed to lead to infection, compared to a dose of just 2.78 non-irradiated PySPZ that infected 50% of the mice (33). The minimum attenuating dose for P. berghei (Pb) also appears to be 100 Gy (10), although most studies of immunity stimulated by PbSPZ utilize 120-150 Gy (36, 37).
For manufacturing PfSPZ Vaccine, we chose a target minimum dose of 150 Gy with an acceptable minimum dose of 142.5 Gy (5% below the target dose) and a maximum dose of 190 Gy. If over-attenuated, the PfSPZ could potentially lose their ability to stimulate a protective immune response in the liver. For the rodent parasite PySPZ, doubling the attenuating dose (i.e. to 200 Gy) does not lead to a diminution of protection. However, we wanted to provide a tighter range for the PfSPZ so selected, in the absence of any available data, a dose of 190 Gy as the upper threshold for acceptance. In practice, as indicated here, the highest level of irradiation for the PfSPZ has been considerably lower with a range maximum of 175.3 Gy, which is well below the acceptable maximum dose. Overall, the mean estimated minimum dose was 154.3 Gy and the mean estimated maximum dose was 166.3 Gy for vaccine lots.
Irradiation using 60Co is a physical process. When incorporating the defined half life decay of the sources (Equation 1) it should be possible to deliver a precise dose of radiation and for there to be no meaningful variability between runs on the same day or between runs on different days. However, the irradiator has moving parts with some inertia or variability – these include the rotation of the turntable in the irradiator chamber, the position of the reference dosimeter relative to the sources when they are activated during a run, and the speed with which the source rods are elevated by air pressure from the compressor. There are uncertainties in the alanine processing methodology to which NIST assigns a value of ±1.8%. Together some of these factors may account for a portion of the overall ±3.1% (150-159.3 Gy) variability seen between runs (Figure 6).
60Co irradiation has proven to be a robust, repeatable and reliable process, but has drawbacks because of attendant security issues which significantly increase the cost, including running personnel background checks, training and certification, and security of controlled access and monitoring. These are fixed costs that would be diluted by scaling up production, however, because of the half life decay, the sources deplete over time and have to be replenished. Our irradiator has undergone one upgrading event at considerable cost. In the future it is likely that 60Co irradiation will be replaced by X-ray irradiation, and Sanaria has begun to explore the process of making this transition.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conceptualization: EJ and SH. Methodology: EJ, SM, JO, BS, AE, TL, ML, PB, TR, SC, AG, and TM. Funding acquisition: EJ and SH. Project administration: EJ and SH. Supervision: EJ and SH. Writing – original draft: EJ. Writing – review & editing: EJ, SH, PB, and TR. All authors contributed to the article and approved the submitted version.
The work was supported by components of several Small Business Research Innovation awards to SH from the National Institute of Allergy and Infectious Disease of NIH, principally 43AI058499 and 44AI058499.
All authors are employed by Sanaria Inc.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the NIAID/NIH SBIR program for support and Drs Stephen Sletzer, Marc Desrosiers, Lonnie Cumberland and Ileana Pazos of the NIST High Dose Dosimetry Service for providing advice and guidance. We thank Greg Smith ABHP, Sanaria’s Radiation Safety Officer, RSO Inc, Laurel, MD, and also Mark A Smith PhD, CHP of Ionaktis LLC, Charlotte, NC, contract Radiation Physicist for data and document reviews.
1. Jarrett WF, Jennings FW, McIntyre WI, Mulligan W, Urquhart GM. Irradiated Helminth Larvae in Vaccination. Proc R Soc Med (1958) 51:743–4. doi: 10.1177/003591575805100912
2. Sharma RL, Bhat TK, Dhar DN. Control of Sheep Lungworm in India. Parasitol Today (1988) 4:33–6. doi: 10.1016/0169-4758(88)90061-0
3. Miller TA. Effect of Route of Administration of Vaccine and Challenge on the Immunogenic Efficiency of Double Vaccination With Irradiated Ancylostoma caninum Larvae. J Parasitol (1965) 51:200–6. doi: 10.2307/3276081
4. Tromba FG. Immunization of Pigs Against Experimental Ascaris suum Infection by Feeding Ultraviolet-Attenuated Eggs. J Parasitol (1978) 64:651–6. doi: 10.2307/3279954
5. Taylor MG, James ER, Nelson GS, Bickle Q, Dunne DW, Webbe G. Immunisation of Sheep Against Schistosoma mattheei Using Either Irradiated Cercariae or Irradiated Schistosomula. J Helminthol (1976) 50:1–9. doi: 10.1017/s0022149x00028753
6. Hsü SY, Hsü HF, Burmeister LF. Schistosoma mansoni: Vaccination of Mice With Highly X-Irradiated Cercariae. Exp Parasitol (1981) 52:91–104. doi: 10.1016/0014-4894(81)90065-5
7. Bushara HO, Hussein MF, Saad AM, Taylor MG, Dargie JD, Marshall TF, et al. Immunization of Calves Against Schistosoma bovis Using Irradiated Cercariae or Schistosomula of S. bovis. Parasitol (1978) 77:303–11. doi: 10.1017/s0031182000050265
8. Ruppel A, Shi YE, Moloney NA. Schistosoma mansoni and S. japonicum: Comparison of Levels of Ultraviolet Irradiation for Vaccination of Mice With Cercariae. Parasitol (1990) 101:23–6. doi: 10.1017/s0031182000079701
9. Sadun EH. Immunization in Schistosomiasis by Previous Exposure to Homologous and Heterologous Cercariae by Inoculation of Preparations From Schistosomes and by Exposure to Irradiated Cercariae. Ann NY Acad Sci (1963) 113:418. doi: 10.1111/j.1749-6632.1963.tb40680.x
10. Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective Immunity Produced by the Injection of X-Irradiated Sporozoites of Plasmodium berghei. Nature (1967) 216:160–2. doi: 10.1038/216160a0
11. Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of Man Against Sporozoite-Induced falciparum Malaria. Am J Med Sci (1973) 266:169–77. doi: 10.1097/00000441-197309000-00002
12. Rieckmann KH, Beaudoin RL, Cassells JS, Sell KW. Use of Attenuated Sporozoites in the Immunization of Human Volunteers Against falciparum Malaria. Bull WHO (1979) 57(Suppl 1):261–5.
13. Edelman R, Hoffman SL, Davis JR, Beier M, Sztein MB, Losonsky G, et al. Long-Term Persistence of Sterile Immunity in a Volunteer Immunized With X-Irradiated Plasmodium falciparum Sporozoites. J Infect Dis (1993) 168:1066–70. doi: 10.1093/infdis/168.4.1066
14. Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of Humans Against Malaria by Immunization With Radiation-Attenuated Plasmodium falciparum Sporozoites. J Infect Dis (2002) 185:1155–64. doi: 10.1086/339409
15. Hoffman SL, Billingsley PF, James ER, Richman A, Loyevsky M, Li T, et al. Development of a Metabolically Active, non-Replicating Sporozoite Vaccine to Prevent Plasmodium falciparum Malaria. Hum Vaccin (2010) 6:97–106. doi: 10.1126/science.1211548
16. Richie TL, Billingsley PF, Sim BKL, James ER, Chakravarty S, Epstein JE, et al. Progress With Plasmodium falciparum Sporozoite (PfSPZ)-Based Malaria Vaccines. Vaccine (2015) 33:7452–61. doi: 10.1016/j.vaccine.2015.09.096
17. Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. VRC 312 Study Team. Protection Against Malaria by Intravenous Immunization With a Nonreplicating Sporozoite Vaccine. Science (2013) 341(6152):1359–65. doi: 10.1126/science.1241800
18. Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, et al. Protection Against Plasmodium falciparum Malaria by PfSPZ Vaccine. JCI Insight (2017) 2:e89154. doi: 10.1172/jci.insight.89154
19. Jongo SA, Church LWP, Mtoro AT, Schindler T, Chakravarty S, Ruben AJ, et al. Increase of Dose Associated With Decrease in Protection Against Controlled Human Malaria Infection by PfSPZ Vaccine in Tanzanian Adults. Clin Infect Dis (2020) 71:2849–57. doi: 10.1093/cid/ciz1152
20. Sissoko MS, Healy SA, Katile A, Zaidi I, Hu Z, Kamate B, et al. Safety and Efficacy of a Three-Dose Regimen of Plasmodium falciparum Sporozoite Vaccine in Adults During an Intense Malaria Transmission Season in Mali: A Randomised, Controlled Phase 1 Trial. Lancet Infect Dis (2021) :S1473-3099(21)00332-7. doi: 10.1016/S1473-3099(21)00332-7
21. Devic S. Radiochromic Film Dosimetry: Past, Present, and Future. Phys Med (2011) 27:122–34. doi: 10.1016/j.ejmp.2010.10.001
22. McLaughlin WL, Desrosiers MF. Dosimetry Systems for Radiation Processing. Radiat Phys Chem (1995) 46:1163–74. doi: 10.1016/0969-806X(95)00349-3
23. Burns DT, Allisy-Roberts PJ, Desrosiers MF, Sharpe PHG, Pimpinella M, Lourenço V, et al. Supplementary Comparison CCRI(I)-S2 of Standards for Absorbed Dose to Water in 60Co Ɣ Radiation at Radiation Processing Dose Levels. Metrologia (2011) 48(Tech. Suppl):06009, 1–18.
24. Nagy VYU, Desrosiers MF. A Complex Time Dependence of the EPR Signal of Irradiated L-α -Alanine. Appl Radiat Isot (1996) 47:789–93. doi: 10.1016/0969-8043(96)00053-X
25. Humphreys JC, Puhl JM, Seltzer SM, McLaughlin WL, Desrosiers MF, Bensen DL, et al. Radiation Processing Dosimetry Calibration Services and Measurement Assurance Program. Gaithersburg, MD: NIST Special Publication (1998) 250–44. doi: 10.6028/NIST.SP.250-44
26. Siu NF, Sedegah M, Hoffman SL. In Vitro Survival and Retention of Infectivity of Plasmodium yoelii Sporozoites Over Extended Periods of Time. J Parasitol (1994) 80(5):826–9. doi: 10.2307/3283266
27. Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, et al. Live Attenuated Malaria Vaccine Designed to Protect Through Hepatic CD8⁺ T Cell Immunity. Science (2011) 334(6055):475–80. doi: 10.1126/science.1211548
28. Mordmüller B, Supan C, Sim BKL, Gómez-Pérez GP, Ospina Salazar CL, Held J, et al. Direct Venous Inoculation of Plasmodium falciparum Sporozoites for Controlled Human Malaria Infection: A Dose-Finding Trial in Two Centres. Malar J (2015) 14:117. doi: 10.1186/s12936-015-0628-0
29. Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile Protection Against Human Malaria by Chemoattenuated PfSPZ Vaccine. Nature (2017) 542(7642):445–9. doi: 10.1038/nature21060
30. Gómez-Pérez GP, Legarda A, Muñoz J, Sim BKL, Ballester MR, Dobaño C, et al. Controlled Human Malaria Infection by Intramuscular and Direct Venous Inoculation of Cryopreserved Plasmodium falciparum Sporozoites in Malaria-Naive Volunteers: Effect of Injection Volume and Dose on Infectivity Rates. Malar J (2015) 14:306. doi: 10.1186/s12936-015-0817-x
31. Murphy SC, Deye GA, Sim BKL, Galbiati S, Kennedy JK, Cohen KW, et al. PfSPZ-CVac Efficacy Against Malaria Increases From 0% to 75% When Administered in the Absence of Erythrocyte Stage Parasitemia: A Randomized, Placebo-Controlled Trial With Controlled Human Malaria Infection. PloS Pathog (2021) 17(5):e1009594. doi: 10.1371/journal.ppat.1009594
34. Hoffman BU, Chattopadhyay R. Plasmodium falciparum: Effects of Radiation on Levels of Gene Transcripts in Sporozoites. Exp Prasiitol (2008) 118(2):247–52. doi: 10.1016/j.exppara.2007.08.014
35. Chattopadhyay R, Conteh S, Li M, James ER, Epstein JE, Hoffman SL. The Effects of Radiation on the Safety and Protective Efficacy of an Attenuated Plasmodium yoelii Sporozoite Malaria Vaccine. Vaccine (2009) 27(27):3675–80. doi: 10.1016/j.vaccine.2008.11.073
36. Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Williams J, Krzych U. Protracted Protection to Plasmodium berghei Malaria is Linked to Functionally and Phenotypically Heterogeneous Liver Memory CD8+ T Cells. J Immunol (2003) 171:2024–34. doi: 10.4049/jimmunol.171.4.2024
37. Parmar R, Patel H, Yadav N, Parikh R, Patel K, Mohankrishnan A, et al. Infectious Sporozoites of Plasmodium berghei Effectively Activate Liver CD8 α+ Dendritic Cells. Front Immunol (2018) 9:192:192. doi: 10.3389/fimmu.2018.00192
32. Lyke KE, Singer A, Berry AA, Reyes S, Chakravarty S, James ER, et al. Multidose Priming and Delayed Boosting Improve Plasmodium falciparum Sporozoite Vaccine Efficacy Against Heterologous P. falciparum Controlled Human Malaria Infection. Clin Infect Dis (2021) 73(7):e2424–35. doi: 10.1093/cid/ciaa1294
Keywords: radiation, attenuation, malaria, sporozoite, vaccine
Citation: James ER, Matheny S, Overby J, Sim BKL, Eappen AG, Li T, Li ML, Richie TL, Chakravarty S, Gunasekera A, Murshedkar T, Billingsley PF and Hoffman SL (2022) A First for Human Vaccinology: GMP Compliant Radiation Attenuation of Plasmodium falciparum Sporozoites for Production of a Vaccine Against Malaria. Front. Immunol. 13:851028. doi: 10.3389/fimmu.2022.851028
Received: 08 January 2022; Accepted: 25 January 2022;
Published: 15 February 2022.
Edited by:
Viskam Wijewardana, International Atomic Energy Agency, AustriaReviewed by:
Takafumi Tsuboi, Ehime University, JapanCopyright © 2022 James, Matheny, Overby, Sim, Eappen, Li, Li, Richie, Chakravarty, Gunasekera, Murshedkar, Billingsley and Hoffman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric R. James, ZWphbWVzQHNhbmFyaWEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.