
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 30 March 2022
Sec. Viral Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.844023
This article is part of the Research Topic Immune Responses to HIV Infection: Basic, Clinical and Translational Research in East and Southeast Asia View all 43 articles
We aimed to analyze HIV-1 seroreversion caused by combination antiretroviral therapy (cART) and to explore antibody levels of anti-HIV-1 as an alternative biomarker of HIV-1 reservoir. We searched PubMed, Embase, the Cochrane Library, and Web of Science up to August 2021 for publications about the performance of HIV-1 serological assays or the association between antibody responses against HIV-1 and HIV-1 reservoirs. Potential sources of heterogeneity were explored by meta-regression analysis, including the year of publication, country, pretreatment viral load, sample size, the timing of treatment, time on cART, and principle or type of serological assay. Twenty-eight eligible studies with a total population of 1,883 were included in the meta-analysis. The pooled frequency of HIV-1 seronegativity is 38.0% (95% CI: 28.0%–49.0%) among children with vertical HIV-1 infection and cART initiation at the age of less than 6 months, while the percentage of HIV-1 seronegativity declined to 1.0% (95% CI: 0%–3.0%) when cART was initiated at the age of >6 months. For adult patients, 16.0% (95% CI: 9.0%–24.0%) of them were serologically negative when cART was initiated at acute/early infection of HIV-1, but the seronegative reaction was rarely detected when cART was started at chronic HIV-1 infection. Substantial heterogeneity was observed among the studies to estimate the frequency of HIV-1 seronegativity in the early-cART population (I2 ≥ 70%, p < 0.05 and all), while mild heterogeneity existed for the deferred-cART subjects. Moreover, anti-HIV-1 antibody response positively correlates with HIV-1 reservoir size with a pooled rho of 0.43 (95% CI: 0.28–0.55), suggesting that anti-HIV antibody level may be a feasible biomarker of HIV-1 reservoir size.
The WHO recommends early combination antiretroviral therapy (cART) for people living with HIV-1 infection regardless of CD4+ T-cell counts and sets the 90-90-90 goals by 2030 (1). By the end of 2020, 27.5 million people have received cART worldwide (2). Waning HIV-1 antibody response, even seroreversion, and failed seroconversion have been reported in the cART-treated population, particularly among children with a vertical HIV-1 infection or those with acute HIV-1 infection and early initiation of cART (3–13). Since HIV-1 detection is mainly dependent on serological tests, negative serostatus in cART-treated individuals would affect the diagnosis and management of HIV-1 infection. In fact, a large number of studies have reported different frequencies of HIV-1 seronegativity (4–6, 11–42), indicating the importance of a comprehensive analysis of cART-induced seronegativity.
Since the first-generation HIV-1 serological assays were approved by the U.S. Food and Drug Administration (FDA) in 1985 (43), more sensitive immunoassays from 2nd to 5th generation of HIV-1 tests, and user-friendly HIV-1 rapid tests have been developed and implemented in diagnosis and point-of-care testing of HIV-1 (44). To date, anti-HIV-1 antibody and p24 antigen detected by HIV-1 serological assays remain the most widely used biomarkers for clinical screening and diagnosis of HIV-1 infection. However, quite different frequencies of HIV-1 seronegativity and even contradictory results about HIV-1 serological testing have been reported among cART-treated populations with HIV-1 infection (4–6, 41). Thus, it is obviously necessary to conduct a systematic review and meta-regression analysis about the performance of HIV-1 serological assays among cART-treated HIV-infected population.
Furthermore, cART could affect HIV-1 neutralizing antibody (NAb) response and their ability to inhibit HIV-1 infection in vivo, but the dynamics of HIV-1 NAb have not yet been fully evaluated in particular in the era of cART. Unlike the rapid development of anti-HIV-1 binding antibodies, specific HIV-1 NAbs are usually induced months or years post HIV-1 infection with relatively low or moderate titers (45–47). Testing of HIV-1 NAb may be affected by antiretroviral agents in plasma of cART-treated patients (48–51), and discordant results have been reported (52–54).
Although lifelong administration of cART is successful in inhibiting HIV-1 replication and in controlling virus titers undetectable, the current antiretroviral regimens cannot eradicate HIV-1 due to the existence of the HIV-1 reservoir, which is still the main barrier of HIV-1 cure. It is important to monitor HIV-1 reservoir size for assessing the efficacy of cART and predicting viral rebound post cessation of cART (55). Various PCR-based or culture-based assays have been developed for measuring HIV-1 reservoir size but are limited in clinical application due to high cost and complexity (56). It has been reported that the intensity of anti-HIV-1 antibody response correlated well with the size of the HIV-1 reservoir since maturation and maintenance of antibody response depend on continuous stimulation of HIV-1 antigens (17, 19, 27, 28, 33, 39, 57–64). However, inconsistent results existed (21, 23, 32, 38). In this systematic review, we adapted a meta-analysis to analyze HIV-1 seroreversion caused by cART and to explore the possibility of anti-HIV-1 levels as an alternative biomarker for HIV-1 reservoir size.
PubMed, Embase, the Cochrane Library, and Web of Science up to August 2021 was searched for the studies about the performance of HIV-1 serological assays in cART-treated population by using the combination of terms including HIV/AIDS (“HIV” [MeSH]), antiretroviral therapy (anti-retroviral agents/therapeutic use [MeSH Terms]), performance characteristics (sensitivity and specificity [MeSH Terms]), and serologic test (serology [MeSH Terms]). Details of the search strategy for the aforementioned databases are illustrated in the Supplementary Materials. The reference lists of all the included studies as well as the relevant review articles were also screened to identify other related studies. In this study, the frequency of seronegativity, dynamics of HIV-1 Nab, and the association between anti-HIV titers and HIV-1 reservoir size among cART-treated patients were focused on. This meta-analysis was conducted following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Checklist S1, see Supplementary Materials) (65).
The included studies met the following criteria: 1) the study subjects were infected with HIV-1 and treated by cART; 2) the data were available for determining the frequency of seronegativity; and 3) serostatus was irrelevant to the influence of maternal antibodies in vertically infected children and the window period prior to seroconversion. The following reports were excluded: 1) if they were reviews, editorials, opinions, case reports, or animal studies; 2) if the number of cART-treated and cART-naive patients was not reported separately and could not be obtained from the authors; 3) if only overall estimates of the frequency of seronegativity were reported, but the timing of treatment is not available; 4) if no sufficient data for establishing the frequency of seronegativity in cART-treated children or adults, respectively.
Two authors (YL and HL) independently extracted the following information, i.e., the first author, year of publication, country, study subjects, sample size, pretreatment viral load (VL), median VL when the serological test was conducted, median months from HIV-1 diagnosis to cART initiation, median months on cART, principle or type of serological assays, and the corresponding frequency of seronegativity of each eligible study. Any disagreement between the two authors was resolved by discussing with the corresponding author ST to reach a consensus.
In order to decrease the effect of studies with extremely low frequency on the overall estimate, the data were transformed with the Freeman–Tukey double arcsine function before pooling the prevalence (66). The Wilson method (67) was used to calculate the 95% CIs around these estimates since the asymptotic method produces intervals that may extend below zero (68). Furthermore, both Cochran’s Q (reported as χ2 value and p-value) and the I2 statistic were applied to estimate the inter-study heterogeneity. A p < 0.05 from Cochrane’s chi-square (χ2) test or I2 statistic value >75% indicated substantial heterogeneity (69, 70). In our study, HIV-infected children and adults were separately analyzed because an infant’s immune response is different from that of an adult. In general, early HIV-1 infection refers to the initial 6-month time period after HIV-1 acquisition (71). Therefore, the cutoff value of 6 months was used in this study to group the early-cART and deferred-cART population. The study populations are then categorized into an early-cART group (≤6 months between HIV-1 diagnosis and cART initiation) and a deferred-cART group (>6 months) based on the timing of cART initiation. In consideration of different properties and testing principles of HIV-1 serological assays (44), the pooled frequency of seronegativity was estimated in the cART-treated population according to the test principles. Definition and determination of developed and developing countries are based on the criteria of World Economic Situation and Prospects 2022 published by the United Nations. When the frequency of seronegativity was evaluated by more than two serological assays with the same testing principles, the pooled estimates were adjusted for intra-study or within-study correlation (72). In situations with high inter-studies heterogeneity, a random-effects model was used; otherwise, a fixed-effect model was adapted (70). The potential sources of heterogeneity were further investigated through meta-regression analysis, including variables of the year of publication, sample size, country, pretreatment VL, the timing of treatment, months on cART, and principle or type of serological assay. Publication bias was assessed by funnel plot as well as Egger’s and Begg’s tests (73, 74). All the analyses were done in R software (version 3.5.2, R Foundation for Statistical Computing) by using the Package “meta.” Statistical tests were two-sided, and p < 0.05 was considered statistically significant.
Our searches returned a total of 2,321 records from 28 studies (4, 14–29, 31–40, 75). The median sample size is 41 (interquartile range (IQR): 16–101). A total of 1,883 subjects met the eligibility criteria and were included in the meta-analysis (Figure 1). Eleven studies (N = 565) were conducted in the United States, 5 (N = 376) in Thailand, 3 (N = 369) in South Africa, 2 (N = 75) in Italy, and one each in Zimbabwe (N = 129), Mali (N = 97), China (N = 73), Canada (N = 69), France (N = 44), Spain (N = 14), and the United Kingdom (N = 10). There were 20 surveys with a median sample size of 29 (IQR: 13–107) to evaluate the serostatus in cART-treated vertically infected children of ≥16 months old (4, 14–29, 31–33), while the frequency of seronegativity in the cART-treated adult population was reported in 8 investigations with a median sample size of 80 (IQR: 36–101) (34–40, 75).
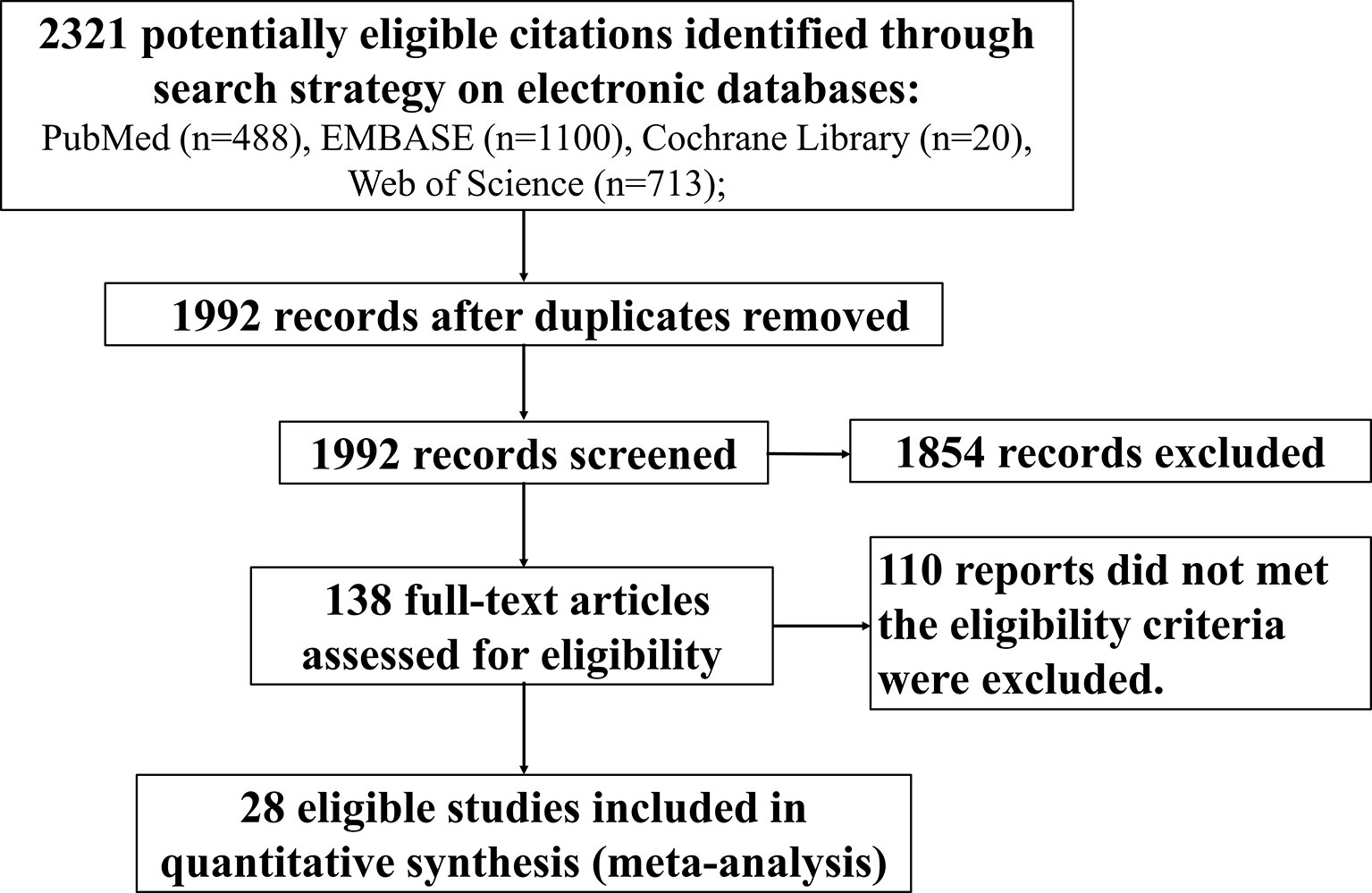
Figure 1 Flowchart depicting the systematic search conducted to identify eligible studies that reported frequency of HIV-1 seronegativity.
Among the 20 selected studies that focus on cART-treated children (Table 1), the frequency of seronegativity was reported in 11 studies in which early-treated children were included with a median age of 2.2 months (IQR: 1.7–2.7, N = 583) when cART started (4, 14–16, 20–22, 26, 29, 32, 33) and in 4 studies that deferred-treated children were included with a median age of 55.7 months (IQR: 37.4–86.4, N = 587) (18, 23–25). In addition, 5 studies included both early-treated and deferred-treated children and separately reported the frequency of seronegativity in the two groups (17, 19, 27, 28, 31). Of the 8 studies that investigated the serostatus in cART-treated adults (Table 2), 4 (34, 36, 38, 75) and 3 (35, 39, 40) studies recruited early-cART (N = 366) and deferred-cART-treated patients (N = 275), respectively. Only one study covered both early-cART (N = 9) and deferred-cART (N = 10) subjects but analyzed the frequency of seronegativity separately (37).
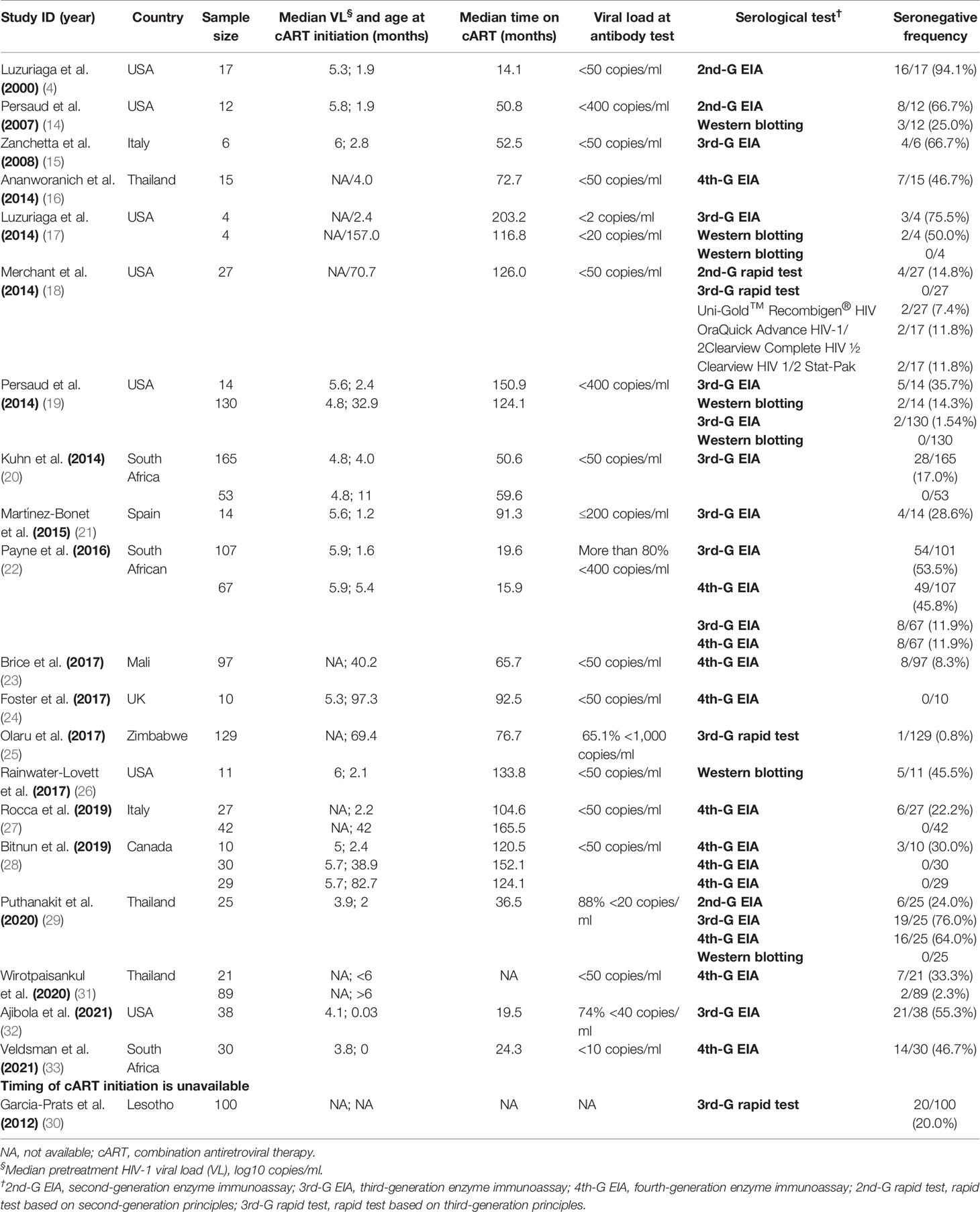
Table 1 Estimated frequency of HIV-1 seronegativity at or after 16 months of age in cART-treated vertically HIV-1-infected children.
The overall pooled frequency of HIV-1 seronegativity was 38.0% (95% CI: 28.0%–49.0%) among early-treated children, while the frequency declined to 1.0% (0%–3.0%) for the deferred-treated children (Table 3). Moreover, the frequency of HIV-1 seronegativity detected by various serological assays was significantly different. For the children who initiated cART at the age of <6 months, the pooled frequency of seronegativity was 63.0% (16.0%–99.0%) for the 2nd-G enzyme immunoassay (EIA) or 2nd-G rapid tests, 41.0% (19.0%–64.0%) for the 3rd-G EIA, 45.0% (12.0%–81.0%) for the 3rd-G rapid tests, 36.0% (23.0%–51.0%) for the 4th-G EIA, and 19.0% (1.0%–47.0%) for Western blotting analysis (Figure 2A). However, a relatively low frequency of seronegativity was observed in the children when cART started at the age of >6 months. The frequency of seronegativity was 15.0% (3.0%–31.0%) for the 2nd-G EIA or 2nd-G rapid tests, 1.0% (0%–3.0%) for the 3rd-G EIA, 1.0% (0%–13.0%) for the 3rd-G rapid tests, 1.0% (0%–4.0%) for the 4th-G EIA, and 0.0% for Western blotting testing (Figure 2B).
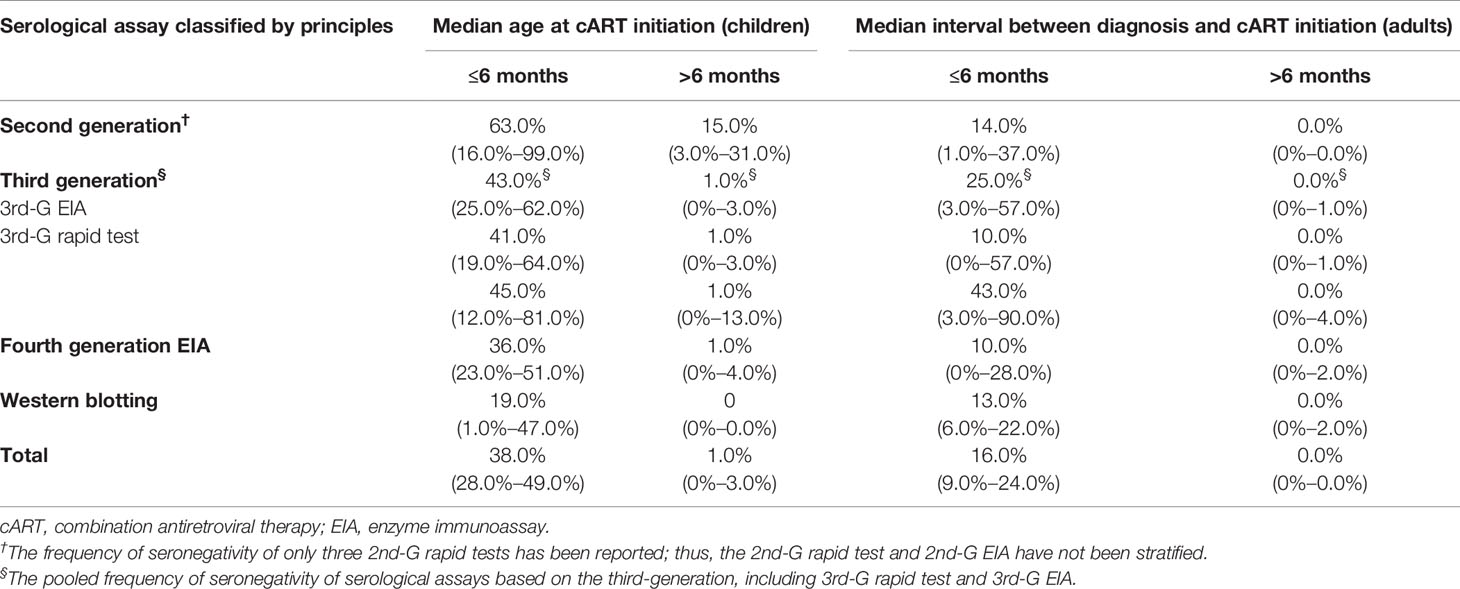
Table 3 The pooled frequency of seronegativity (95% CI) for HIV-1 serological assays in cART-treated population stratified by detection principles and timing of antiretroviral therapy started.
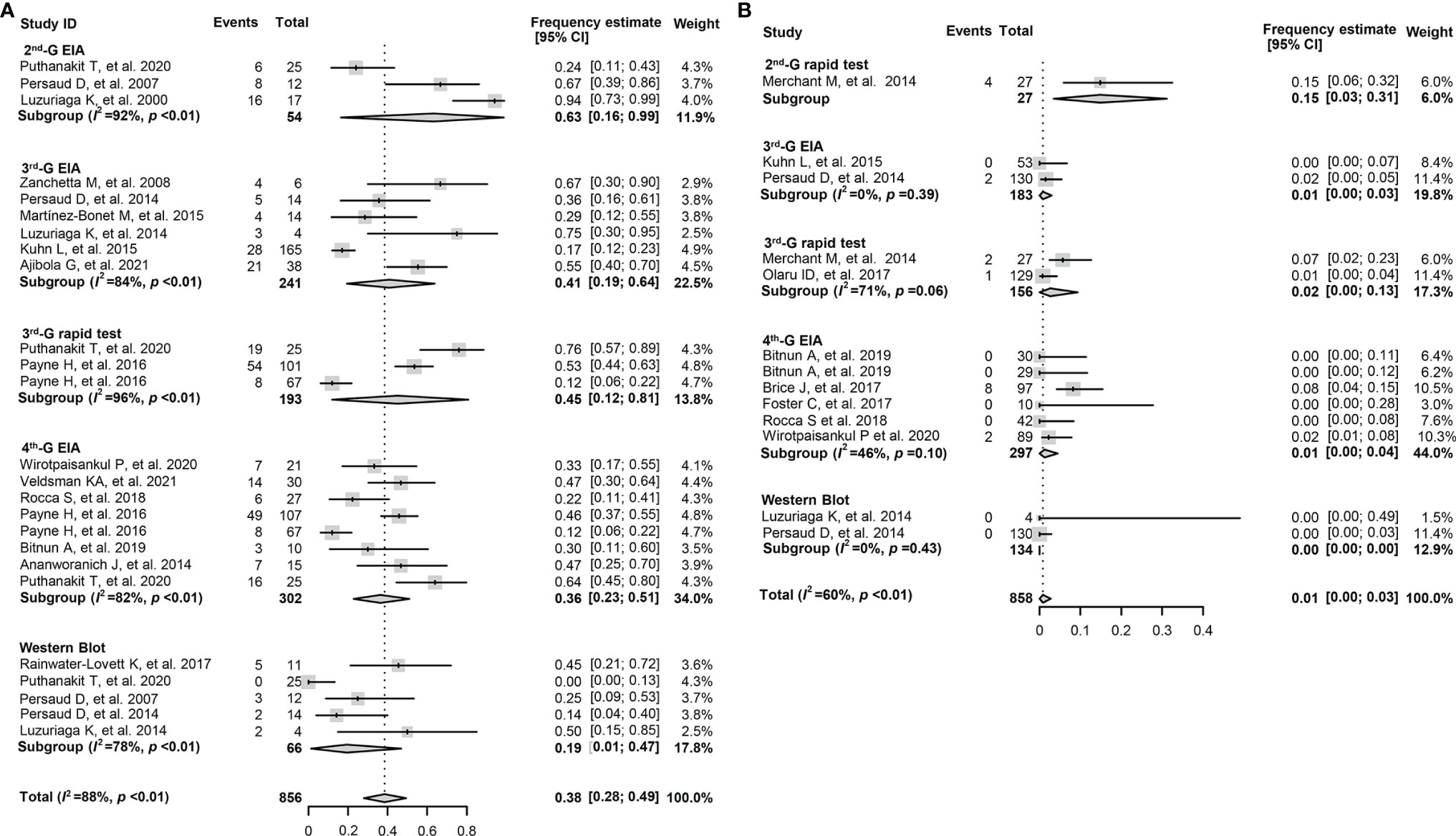
Figure 2 Forest plots of the pooled frequency of HIV-1 seronegativity in HIV-infected children according to the principles of HIV-1 serological assays. (A) The pooled frequency of HIV-1 seronegativity in vertically HIV-infected children of ≥16 months old and cART started at the age of <6 months. (B) The pooled frequency of HIV-1 seronegativity in vertically HIV-infected children of ≥16 months old and cART started at the age of >6 months. 2nd-G EIA, second-generation enzyme immunoassay; 3rd-G EIA, third-generation enzyme immunoassay; 4th-G EIA, fourth-generation enzyme immunoassay; 2nd-G rapid test, rapid test based on second-generation principles; 3rd-G rapid test, rapid test based on third-generation principles; cART, combination antiretroviral therapy.
The overall pooled frequency of HIV-1 seronegativity was 16.0% (9.0%–24.0%) in the early cART-treated adult population. In contrast, only a few serologically negative cases were reported when cART started >6 months after HIV-1 diagnosis (Table 3). Furthermore, for the early cART-treated adult patients, the pooled frequency of seronegativity was 14.0% (1.0%–37.0%) for the 2nd-G EIA or 2nd-G rapid tests, 10.0% (0%–57.0%) for the 3rd-G EIA, 43.0% (3.0%–90.0%) for the 3rd-G rapid tests, 10.0% (0%–28.0%) for the 4th-G EIA, and 13.0% (6.0%–22.0%) for Western blotting testing (Figure 3A). No seronegative cases were reported in the deferred cART-treated group regardless of the serological assays used (Figure 3B). Due to the higher frequency of seronegativity detected by HIV-1 rapid tests, we repeated the meta-analysis by excluding the data from the 2nd and 3rd-generation rapid tests and observed similar differences of seronegativity frequency detected by different serological assays as those aforementioned (Table 4). Of note, the 2nd-generation EIA appeared to yield higher seronegativity frequency than other assays in both early-treated children (63.0%, 95% CI: 16.0%–99.0%) and adult patient (16.0%, 95% CI: 0%–49.0%, Table 4).
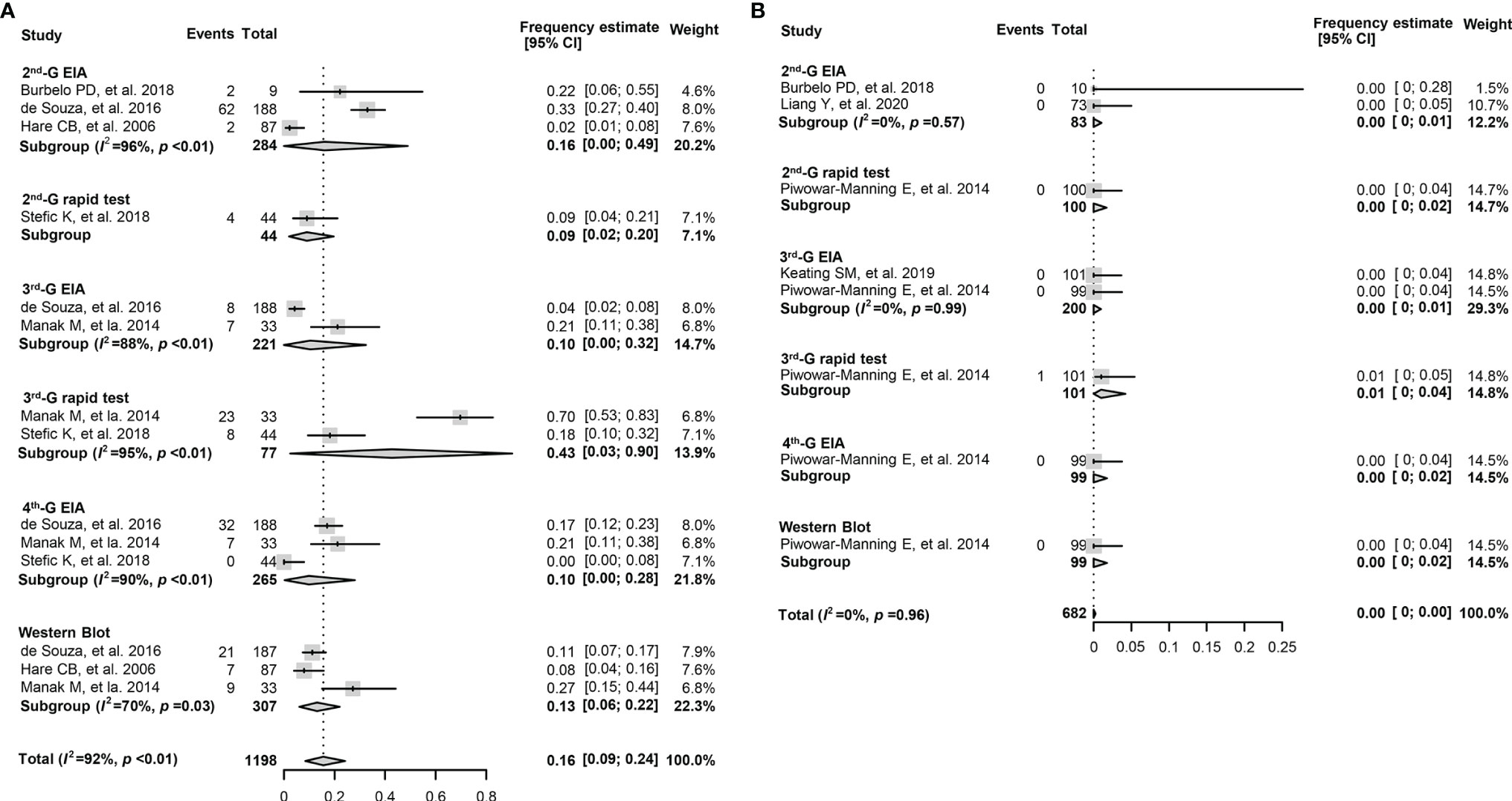
Figure 3 Forest plots of the pooled frequency of HIV-1 seronegativity in HIV-infected adults according to the principles of HIV serological assays. (A) The pooled frequency of HIV-1 seronegativity in early-treated (<6 months post diagnosis) adult patients. (B) The pooled frequency of HIV-1 seronegativity in deferred-treated (>6 months post diagnosis) adult patients. 2nd-G EIA, second-generation enzyme immunoassay; 3rd-G EIA, third-generation enzyme immunoassay; 4th-G EIA, fourth-generation enzyme immunoassay; 2nd-G rapid test, rapid test based on second-generation principles; 3rd-G rapid test, rapid test based on third-generation principles.
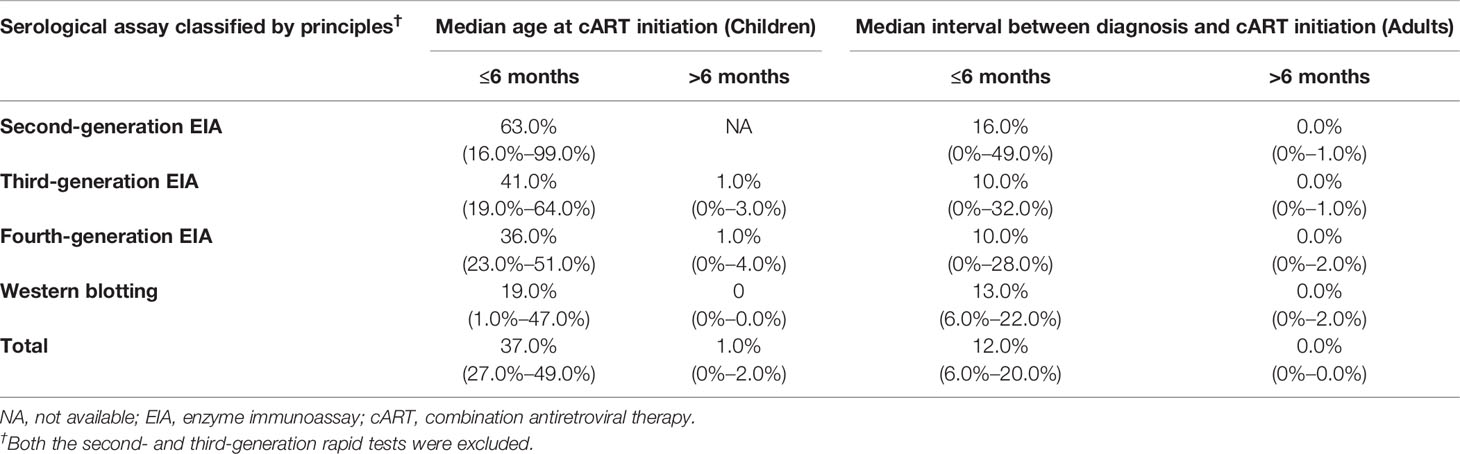
Table 4 The pooled frequency of seronegativity (95% CI) for HIV-1 serological EIA assays in cART-treated population stratified by detection principles and timing of antiretroviral therapy started.
Substantial heterogeneity was observed across the subgroups in the children with early cART (I2 ranges from 78% to 92%, p < 0.01) and early cART-treated adults (I2 ranges from 70% to 96%, p < 0.05). Therefore, we explored the potential sources of heterogeneity through multivariate meta-regression analysis. For cART-treated children, after other potential confounders were adjusted, only the timing of cART initiation remained significant, while deferred treatment was significantly associated with a lower frequency of seronegativity (β = −0.590, p < 0.001, Table 5). The multivariate analysis was not conducted in the cART-treated adult population since there were only 8 studies included. It is believed that meta-regression analysis is generally appropriate for the analysis of more than 10 studies. Taken together, the timing of anti-retroviral treatment including the age for the cART-treated children played a critical role in determining the serostatus of HIV-infected subjects (Figure 4). The frequency of HIV-1 seronegativity was inversely correlated with the time interval from HIV-1 diagnosis or infection to cART initiation for HIV-infected adults.

Table 5 Multivariate meta-regression for the frequency of HIV-1 seronegativity in cART-treated children.
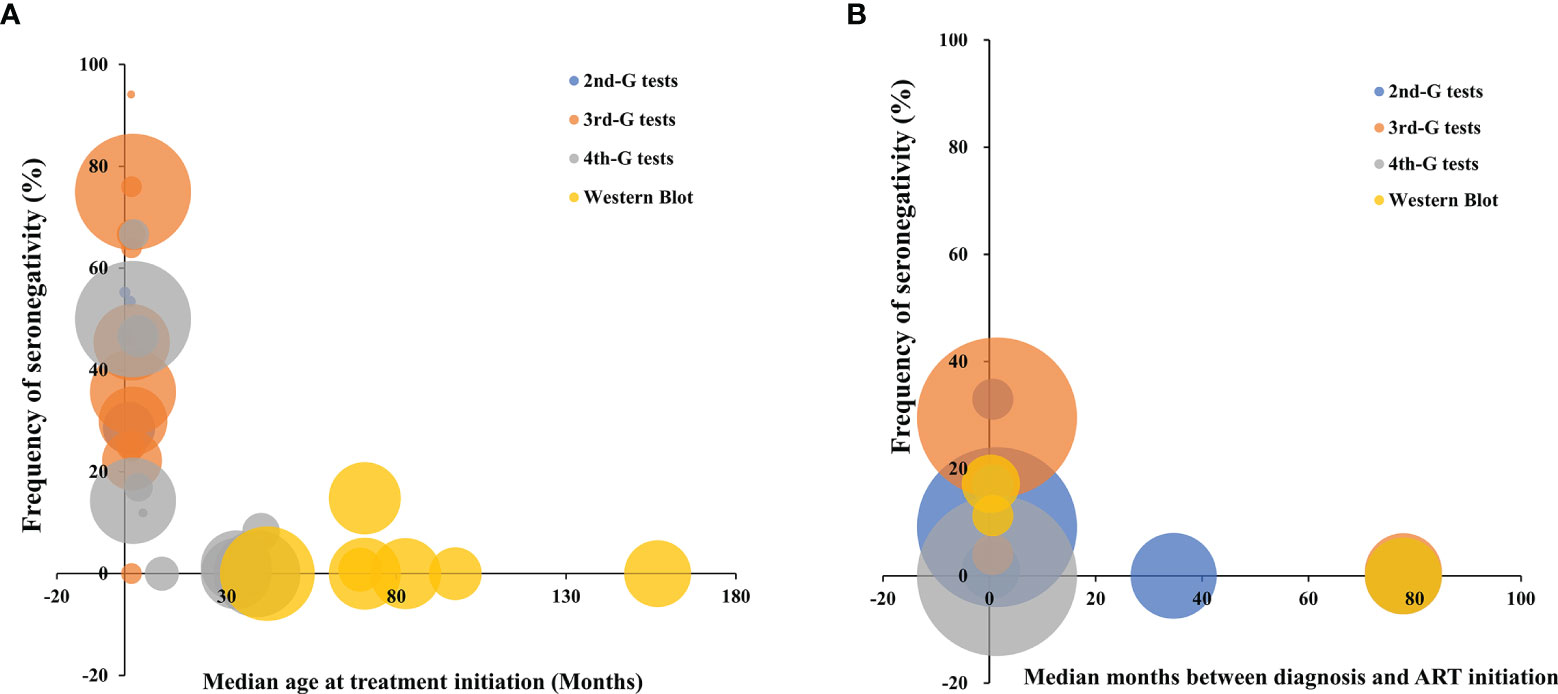
Figure 4 The distribution of frequency of HIV-1 seronegativity in cART-treated vertically HIV-infected children (A) and adult patients (B) according to the time of cART initiation and the principles of HIV-1 serological assays. Bubble size is proportional to the duration of cART. 2nd-G test, HIV-1 serological test based on second-generation principle; 3rd-G test, HIV-1 serological test based on third-generation principle; 4th-G test, HIV-1 serological test based on fourth-generation principle; cART, combination antiretroviral therapy.
Potential publication bias was assessed by funnel plot and Egger’s and Begg’s tests (Figure 5). Overall, the funnel plots were inverted symmetry, and no evidence of significant publication bias was obtained for the surveys that recruited early-treated children (Figure 5A, Egger’s test, p = 0.164; Begg’s test, p = 0.482), deferred-treated children (Figure 5B, Egger’s test, p = 0.421; Begg’s test, p = 0.111), early-treated adults (Figure 5C, Egger’s test, p = 0.571; Begg’s test, p = 0.144), or deferred-treated adults (Figure 5D, Egger’s test, p = 0.336; Begg’s test, p = 0.113).
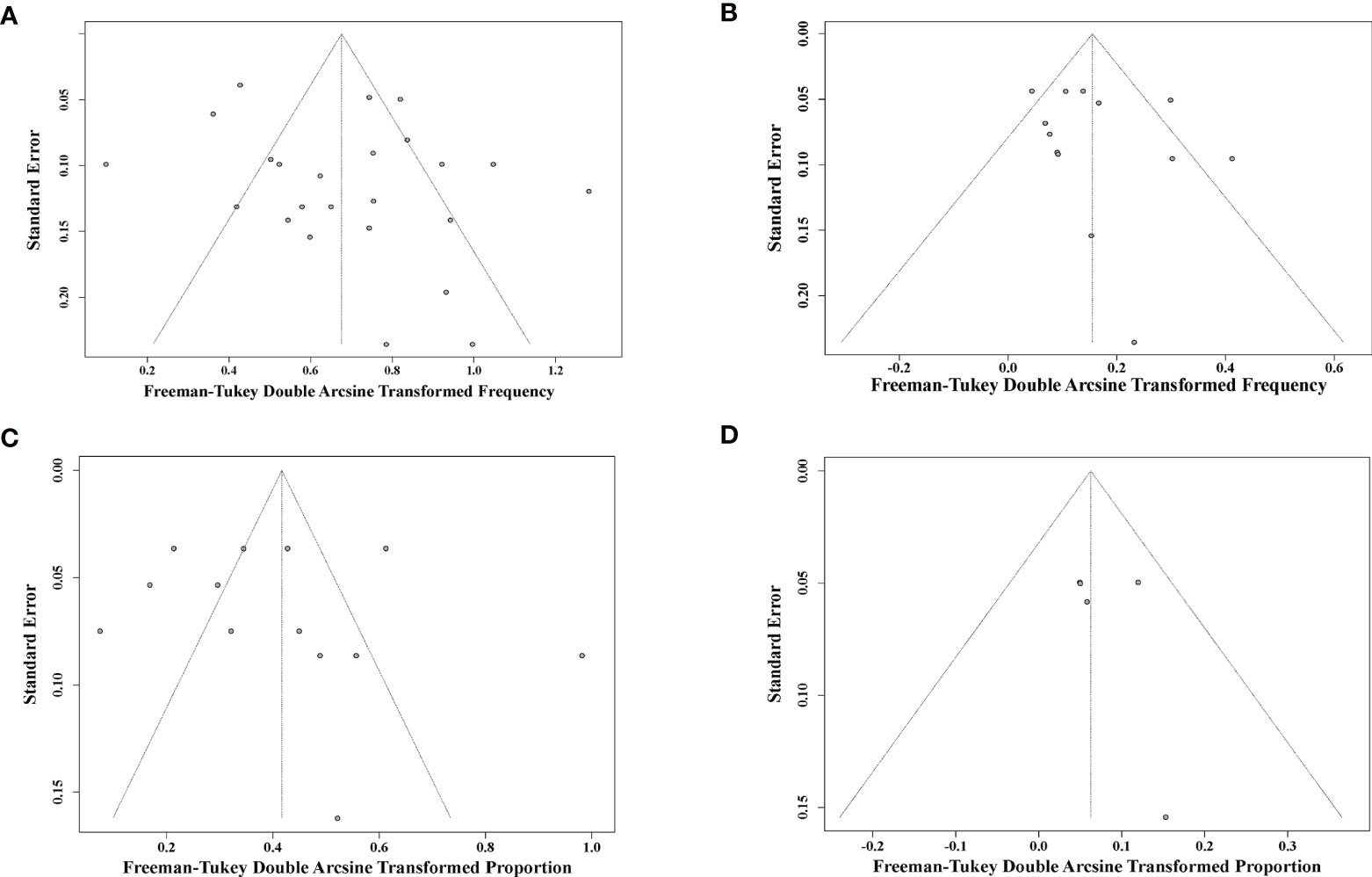
Figure 5 Funnel plot shows publication bias of the identified surveys for early-cART children (A), deferred-cART children (B), early-cART adults (C), and deferred-cART adults (D). cART, combination antiretroviral therapy.
The publications about the association between anti-HIV-1 antibody response and HIV-1 reservoir size were identified through PubMed searches up to August 2021 using the following terms: “HIV persistence,” “latent reservoir,” “HIV reservoirs,” “cell associated HIV DNA,” “viral reservoirs,” “provirus burden,” “HIV serostatus,” and “HIV antibodies.” A total of 18 reports (17, 19, 21, 23, 27, 28, 32, 33, 38, 39, 57–64) were selected, and the median sample size of these studies was 48 (IQR: 22–98, N = 1,525). Out of 9 studies conducted in the children with vertical HIV-1 infection (17, 19, 21, 23, 27, 28, 32, 33, 62) with a median sample size of 30 (IQR: 23–83, N = 489), 6 (66.7%, 6/9) studies reported a significant association between anti-HIV titers and the size of HIV-1 reservoir (Table 6) (17, 19, 27, 28, 33, 62). Eight of the other 9 studies conducted in adult patients (median sample size = 51, IQR: 18–109, N = 1,106) also reported a significant association between HIV-1-specific antibody response and the reservoir size (Table 7) (39, 57–61, 63, 64). Of note, the majority of the studies to investigate the association between HIV-1 antibody response and HIV-1 reservoir size are in the HIV-infected subjects with viremia suppression (<50 copies/ml, Tables 6 and 7).
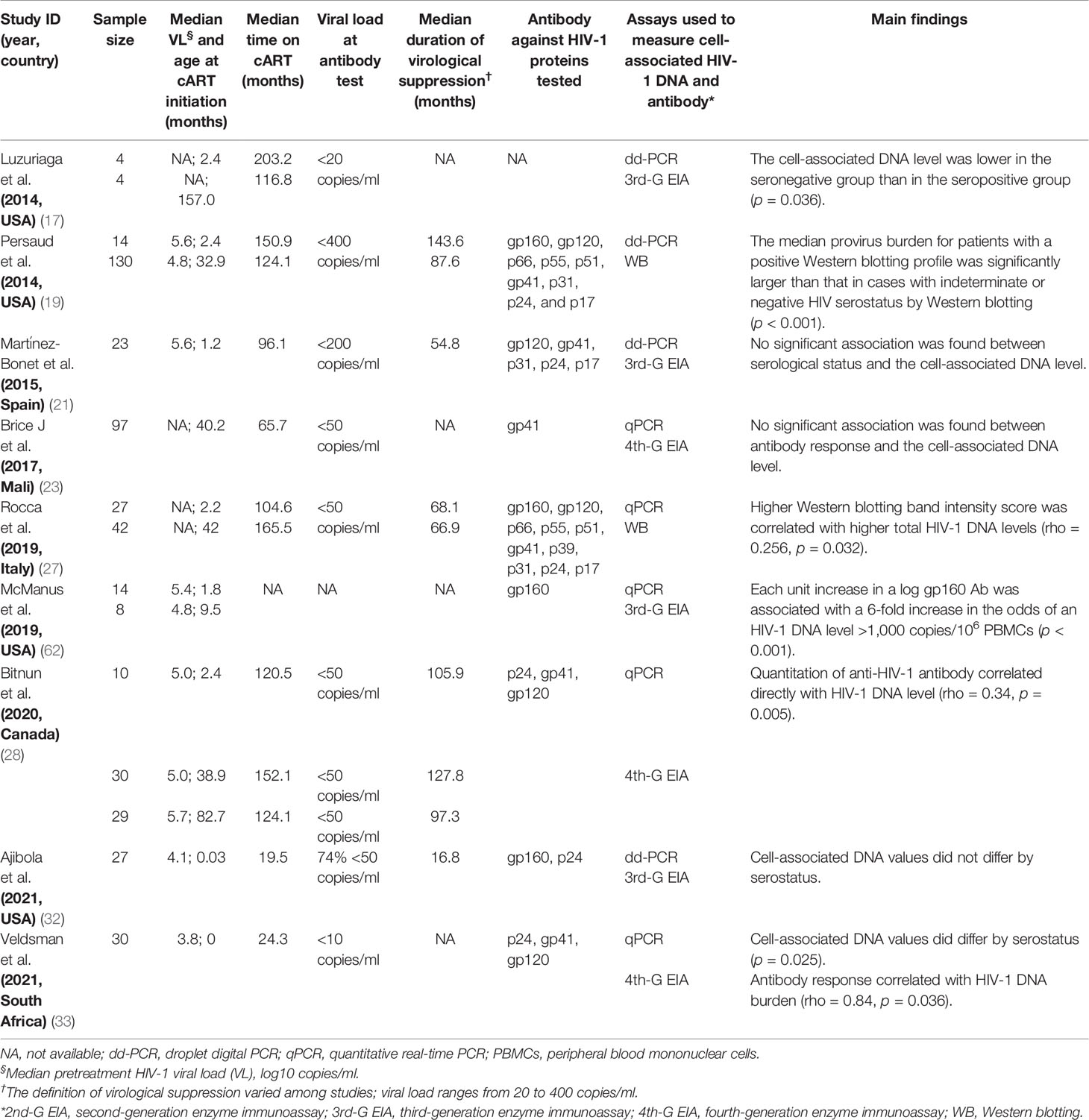
Table 6 Estimated statistical association between cell-associated HIV-1 DNA burden and quantitative or qualitative anti-HIV antibody in children with vertically HIV-1 infection.
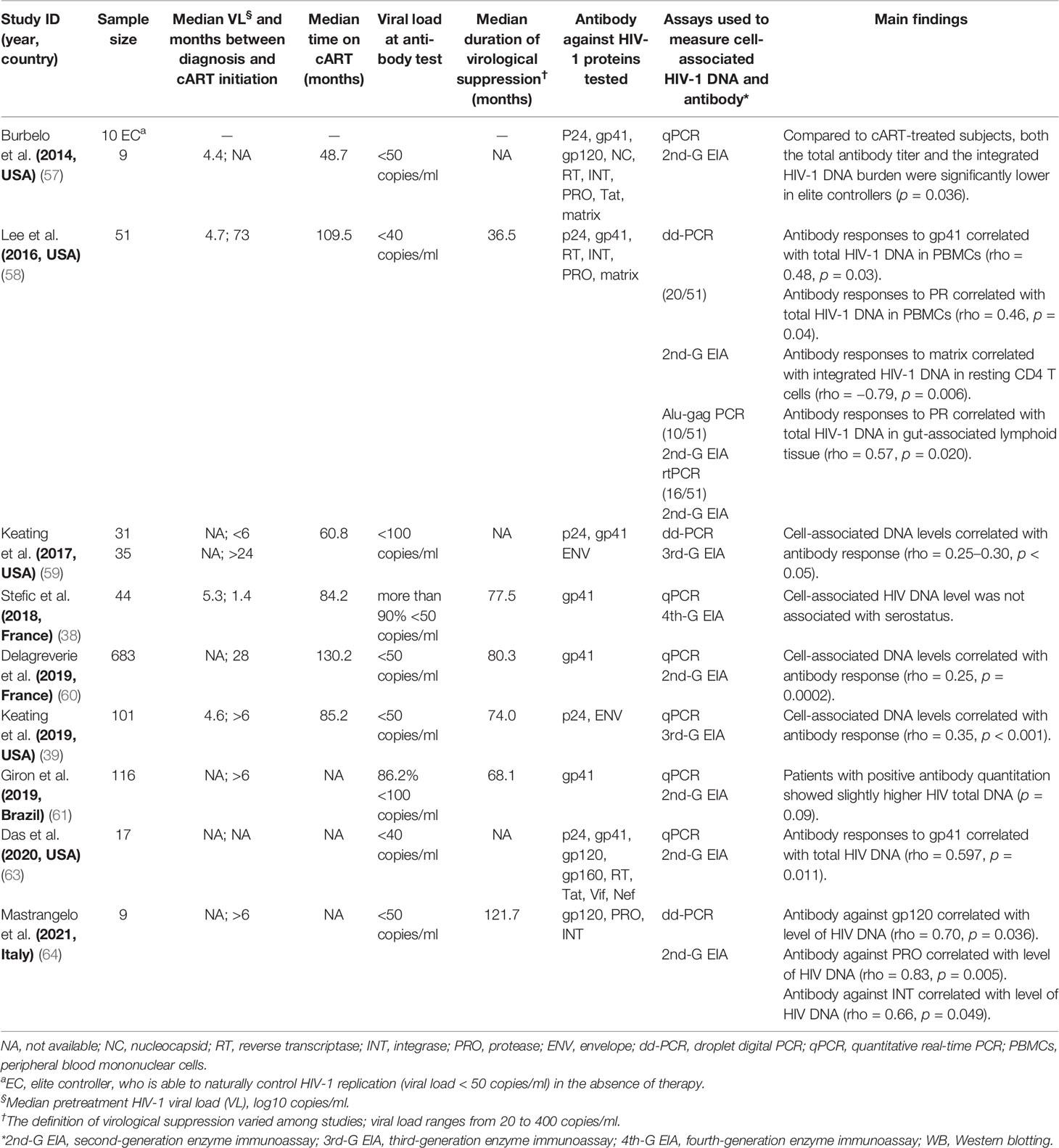
Table 7 Estimated statistical association between cell-associated HIV-1 DNA burden and quantitative or qualitative anti-HIV antibody among adult cases.
Furthermore, several studies reported more cell-associated DNA copies in the HIV-1 seropositive population than the seronegative group (17, 33, 57, 61), and high levels of anti-HIV-1 binding antibodies positively correlated with HIV-1 DNA burden in peripheral blood mononuclear cells (PBMCs) with a Spearman’s correlation coefficient (rho) of 0.25~0.84 (p < 0.05) (27, 28, 33, 39, 58–60, 63, 64). However, inconsistent results were reported in other studies (21, 23, 32, 38). Stefic et al. reported no significant association between HIV-1 serostatus tested by HIV-1 rapid test and cell-associated HIV-1 DNA level in early-treated adults (38).
Spearman’s correlation coefficient between HIV-1-specific antibody titers and HIV-1 DNA burden was reported in 9 studies, although the heterogeneity was significant (I2 = 74%, p < 0.01) (27, 28, 33, 39, 58–60, 63, 64). After adjusting intra-study and within-study correlations (Figure 6), we pooled the results together using random-effects meta-analyses and found a pooled rho of 0.43 (95% CI: 0.28–0.55) for all the studies analyzed. Further stratified analysis indicated that the pooled rho was 0.53 (95% CI: 0.09–0.80, I2 = 89%, p < 0.01) for children and 0.33 (95% CI: 0.21–0.43, I2 = 33%, p = 0.19) for adults (Figure 6A). In order to explore the source of heterogeneity, we conducted a sensitivity analysis and excluded two studies that were considered to be heterogeneous (33, 64). After the two heterogeneous studies were excluded, no notable heterogeneity was found in the studies included (p = 0.58). The overall pooled rho was 0.30 (0.14–0.45) and 0.28 (0.21–0.36) for children and adults, respectively (Figure 6B). Of note, Veldsman et al. reported a very strong correlation (rho = 0.84) between anti-HIV titers and reservoir size in children who received cART upon birth (33) probably due to the inhibition of both HIV-1 seeding from the reservoir and anti-HIV-1 antibody response during the very early cART (71, 76).
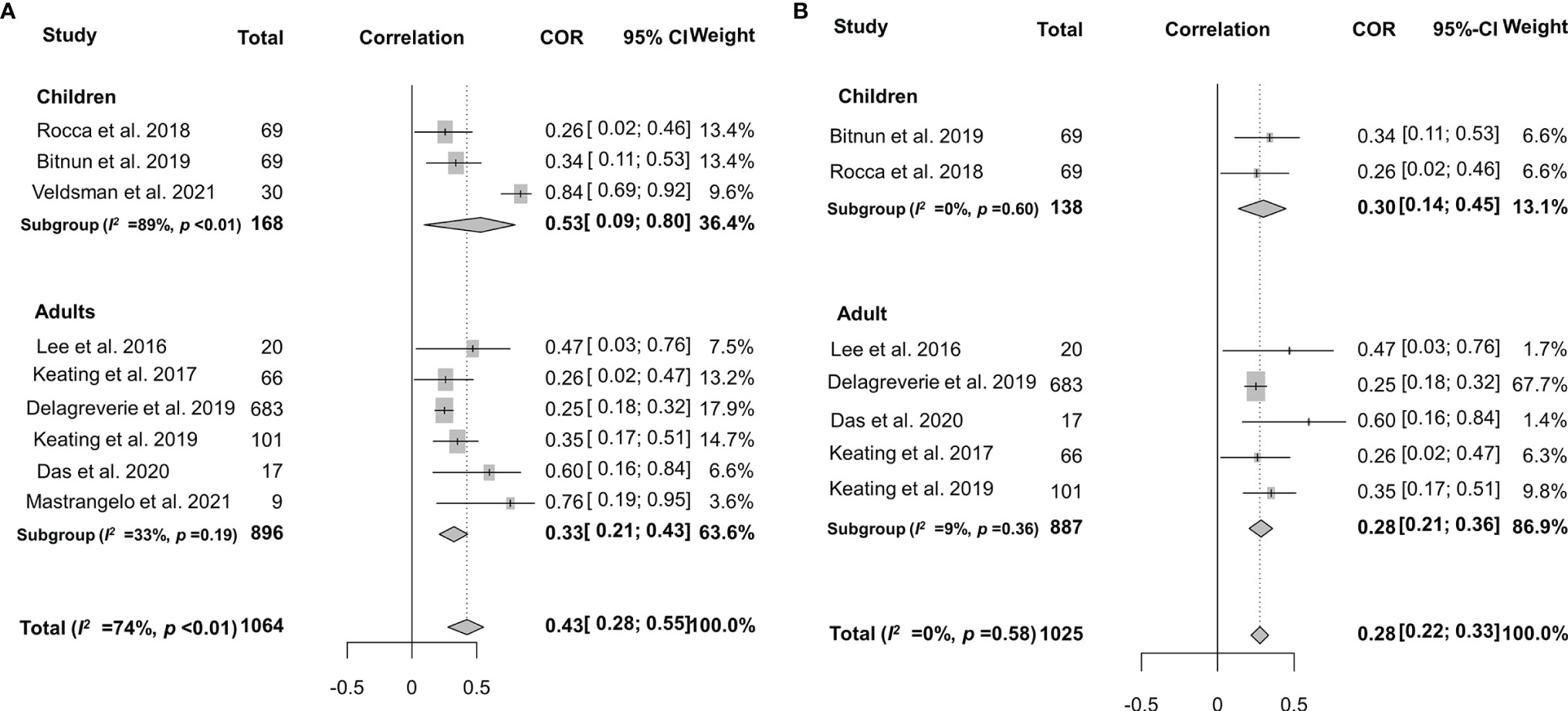
Figure 6 Forest plots of the pooled Spearman’s correlation coefficient (rho) with the corresponding 95% CIs for the correlation between HIV-1-specific antibody titer and HIV-1 DNA burden in patients for all the eligible studies (A) or the studies without significant heterogeneity (B).
As mentioned above, the frequency of seronegativity was significantly affected by the serological assays in particular HIV-1 rapid tests. Therefore, false-negative serostatus may underestimate the real association. To determine the potential impact of HIV-1 serological assays, we analyzed the correlation between anti-HIV antibody levels and the size of the latent reservoir under a stratified analysis according to the serological test principles (Figure 7A). We found that the 2nd-generation tests appeared to yield a slightly stronger correlation (rho = 0.37) than others (rho 0.26–0.34, Figure 7B). Potential publication bias was also assessed by funnel plot and Egger’s and Begg’s tests. The funnel plots were inverted symmetry, and no evidence of significant publication bias was obtained for the surveys (Figure 8, Egger’s test, p = 0.068; Begg’s test, p = 0.025).
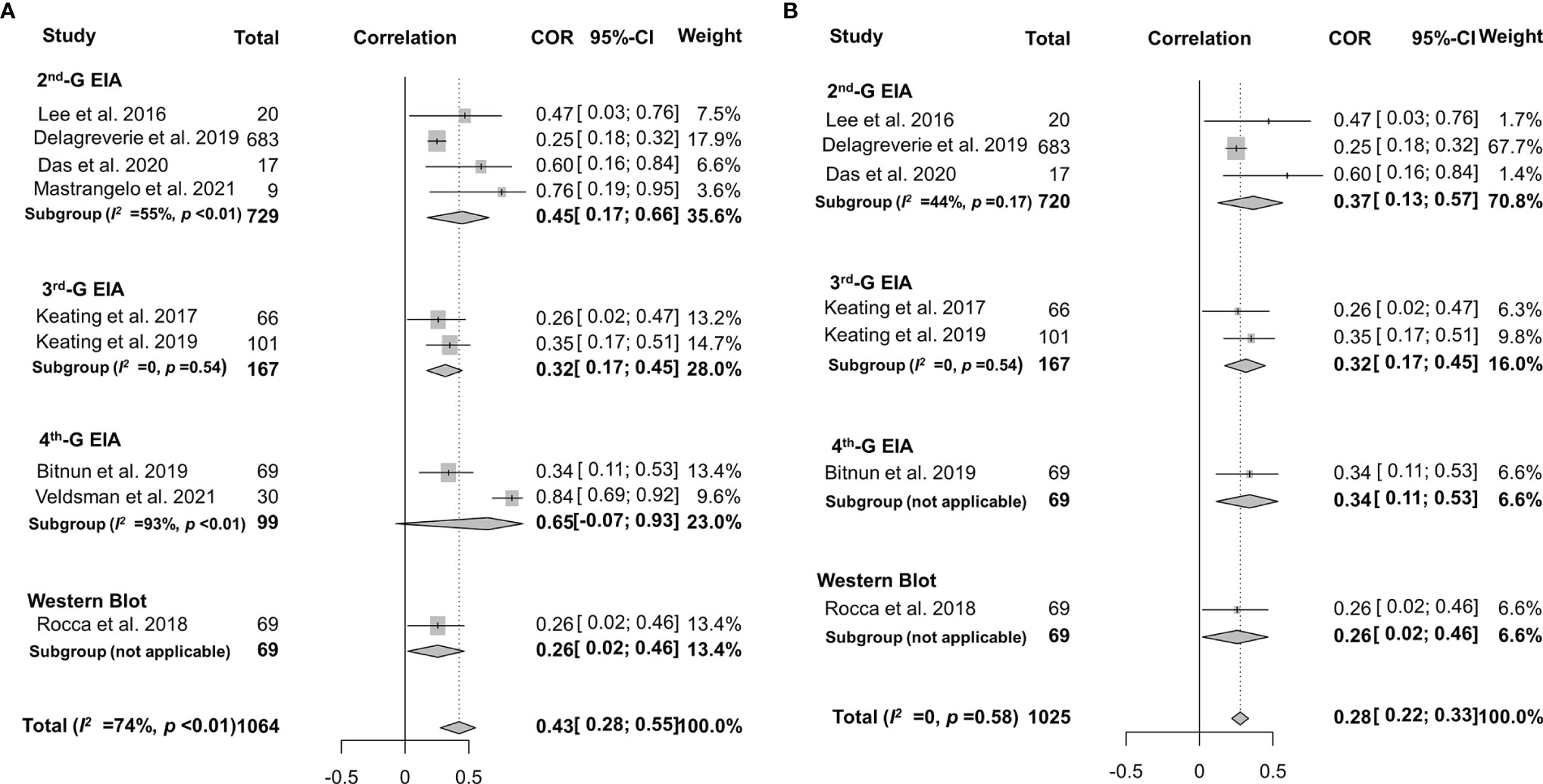
Figure 7 Forest plots of the pooled Spearman’s correlation coefficient (rho) with the corresponding 95% CIs for the correlation between HIV-1-specific antibody titer and HIV-1 DNA burden among HIV-infected population according to the serological test principles for all the eligible studies (A) or the studies without significant heterogeneity (B).
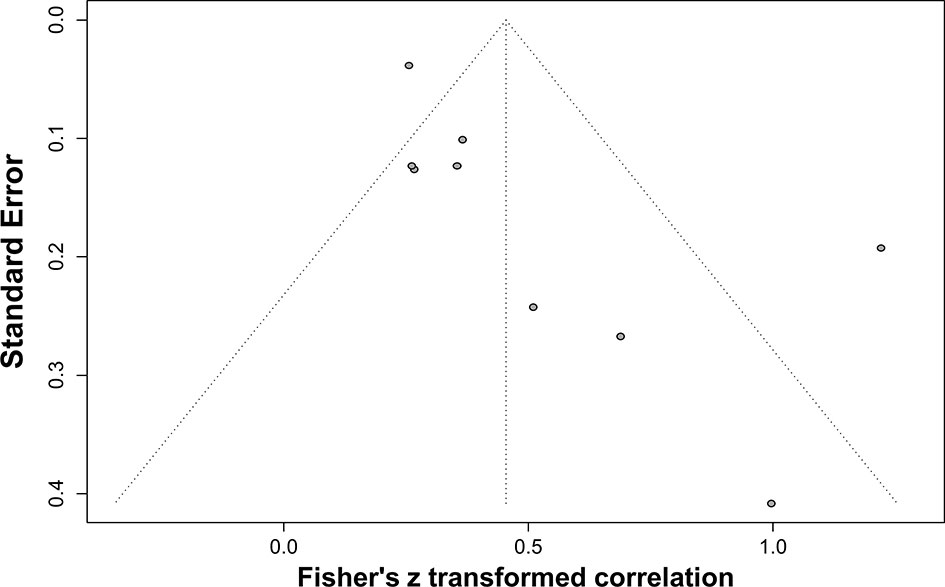
Figure 8 Funnel plot shows publication bias of the identified surveys to investigate the correlation between HIV-1-specific antibody titer and HIV-1 DNA burden.
Except for the window period of HIV-1 infection (77) or progression to AIDS (43, 78), HIV-1 seronegativity among cART-naïve patients is extremely rare (79). HIV-1 seronegativity caused by cART is a new issue that remains to be elucidated. In our systematic review, we uncover 38% and 16% of HIV-1 seronegativity in early-treated children and adults, respectively (Table 3). In contrast, only about 1% of deferred-treated children and adults were HIV-1 seronegative (Table 3). Furthermore, the 2nd generation of HIV-1 serological assays and rapid tests are prone to yield negative results for cART-treated patients (Table 4). To our best knowledge, this is the first systematic review to summarize the frequency of HIV-1 seronegativity in cART-treated patients. Negative HIV-1 serostatus caused by antiretroviral treatment may be due to the failure of seroconversion for the subjects who are acutely infected with HIV-1, or seroreversion caused by the efficient inhibition of HIV-1 replication, but does not mean the cure or eradication of HIV-1 infection since interruption of cART will result in virus rebound and rapid increase of anti-HIV antibody levels (75, 80–82). Since early cART would restrict HIV-1-specific antibody response and even result in seronegativity, the results of HIV-1 serological testing must be interpreted with caution in the era of cART.
Two key factors that may affect the frequency of HIV-1 seronegativity are the serological assays used to detect HIV-1 infection and the time to initiate cART. It is clear that the 2nd-generation EIA and rapid tests are more likely to result in seronegativity due to their relatively low sensitivity and specificity. The major difference between the 2nd- and 3rd-generation EIA is the detection of IgG antibodies against HIV-1 only for the 2nd-G EIA and both IgG and IgM antibodies for the 3rd-G EIA. O’Connell et al. found that the low sensitivity of HIV-1 rapid tests is due to its low efficiency of detecting antibodies against HIV-1 gp41, which is a major antibody produced in HIV-infected subjects (5). Therefore, the difference in seronegativity frequency detected by different generations of serological tests is because of the testing principles and the antigens used in the tests (36, 44). The difference in seronegativity reported in the studies may not be caused by sampling bias since the different performance of the 2nd-, 3rd-, and 4th-generation EIA and Western blotting analysis was reported when testing the same samples from an early-treated adult population (36). Compared to that in the 2nd-generation EIA, the frequency of HIV-1 seronegativity in the cART-treated children reduced by 22.0%, 27.0%, and 44.0% in the 3rd-G EIA, 4th-G EIA, and Western blotting, respectively (Table 4). For adult patients, the frequency of HIV-1 seronegativity reduced by 6.0%, 6.0%, and 3.0% in 3rd-G EIA, 4th-G EIA, and Western blotting, respectively (Table 4). These results clearly confirmed the better performance and reliability of the 4th-G EIA and Western blotting tests in detecting anti-HIV antibodies even in the era of cART. HIV-1 4th-G EIA can simultaneously detect IgM and IgG antibodies against HIV-1/-2 and group O virus as well as HIV-1 p24 antigen and shows superior sensitivity (83). It should thus be implemented as a routine assay when possible.
Furthermore, the time to initiate cART also significantly affects seronegativity among cART-treated patients. Early initiation of cART, in particular in children <6 months old or in adults with acute HIV-1 infection, will increase the probability of seronegativity. This is probably because early antiretroviral treatment inhibits the development of chronic HIV-1 infection, prevents maturation of specific immune responses against HIV-1, or restricts the size of HIV-1 reservoir (4, 7, 8, 12, 84–88). An excellent example is that early cART may help HIV-infected children to be cured or to achieve a functional cure to maintain HIV-1 free for a long time even in the absence of antiretroviral therapy (89–91). Moreover, it was reported that a higher frequency of HIV-1 seronegativity correlated well with a longer time of virological suppression (25, 38, 75). Time on virological suppression does not equal to, but theoretically should be proportional to, time on treatment. Taken together, early cART, long-term treatment, and efficient virus suppression may affect the immune response against HIV-1. At present, the best strategy to control HIV-1 epidemics is still early diagnosis, early initiation of antiretroviral therapy, and efficient viral suppression, which in turn may result in more seronegative cases. It is necessary to have a guideline to deal with the seronegative issue in the era of cART. HIV-1 serological assays should be evaluated in the serum samples from cART-treated subjects in order to further refine their design and improve their performance.
It is still unclear how antiretroviral therapy affects antibody response. Antibody generation and affinity maturation occur within germinal centers in lymph nodes where B cells mutate and are selected by T cells based on their ability to bind to an antigen (92). Moreover, cART can interfere with this process in multiple ways including reducing the production of HIV-1 antigens, which may be detrimental to antibody responses, but also halting or potentially reversing T-cell exhaustion and preventing CD4+ T cell decline, which may improve antibody responses. The obvious difference between the early and deferred-treated populations in antibody response may be due to the sufficient antigen harboring in the follicular dendritic cells to continuously stimulate antibody production in HIV-1 chronically infected subjects. Thus, a decline in the amount of circulating HIV-1 antigens after cART treatment would have little impact on the maintenance of antibody levels (93, 94). Also, it is well known that long-lived germinal centers in chronic HIV-1 infection can induce long-term affinity maturation of broadly neutralizing antibodies in response to viral escape mutants (95). However, different from the decreased HIV-1-specific binding antibody after antiretroviral treatment, there is no consensus on the role of antiretroviral therapy in the induction and maturation of anti-HIV-1 neutralizing antibodies (11, 48, 50–52, 54, 96–98) (Table 8). This discrepancy may also be partially caused by different cART regimens used (52), or different HIV-1 strains or inhibitory thresholds adopted in neutralization assay (11, 48).
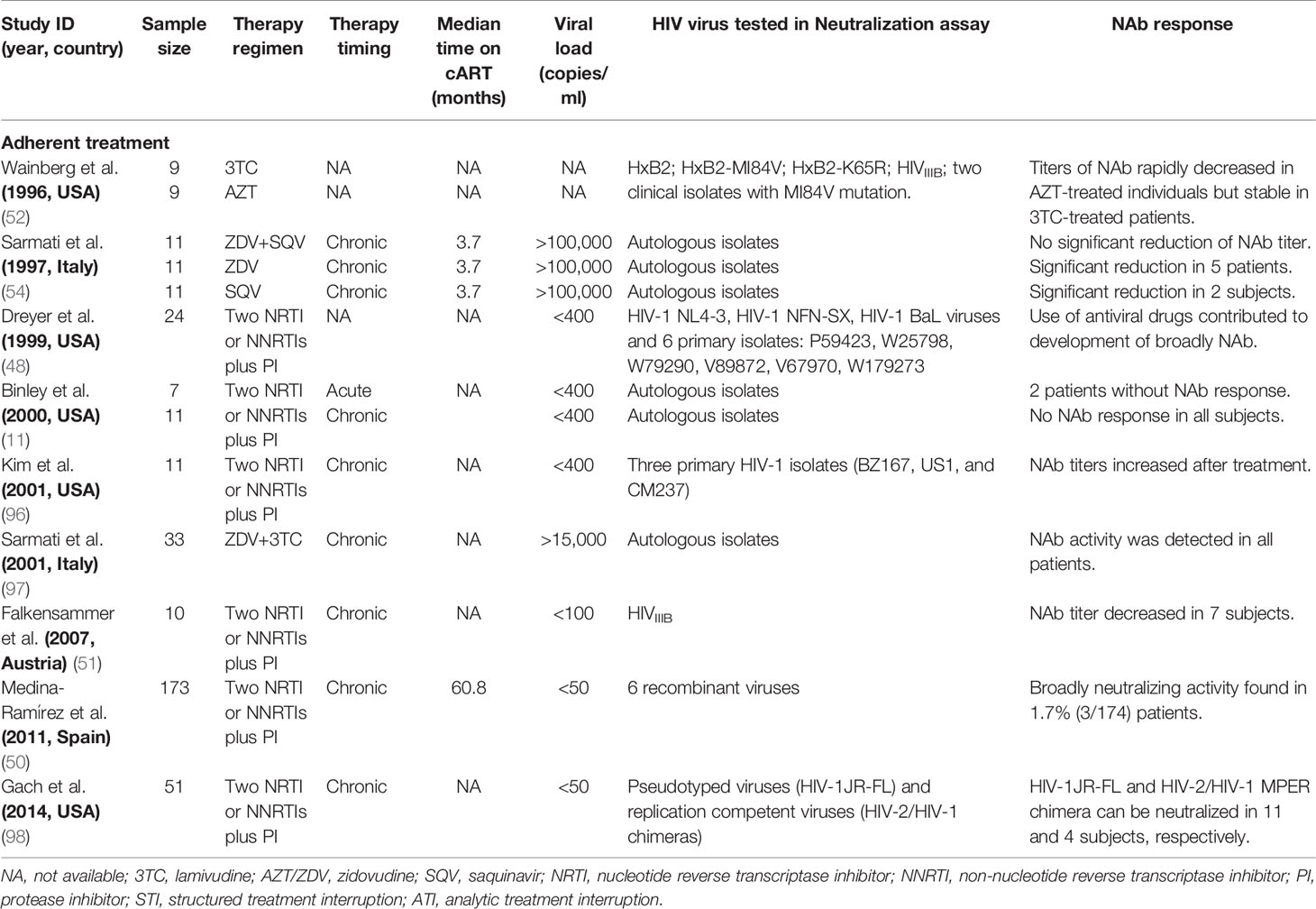
Table 8 Effect of antiviral therapy on HIV-1-specific neutralizing antibodies (NAb) in adult patients.
Furthermore, Zaongo et al. have recently reviewed the role of cART and HIV-1 VL in influencing HIV-1-specific antibody response (99). The HIV-1-specific antibody is usually detected 14 days after infection in adults (100); however, HIV-infected infants develop autogenous antibodies approximately at the age of 4 months (101). Therefore, the window period for cART to impede the autogenous antibody response against HIV-1 is much longer in infants than in adults. These findings may explain the higher frequency of HIV-1 seronegativity in early-treated children (38.0%) than in early-treated adults (16.0%).
The correlation between HIV-1 VL and antibody response is another debated issue. Unfortunately, HIV-1 VLs vary among HIV-infected subjects and are affected by HIV-1 subtypes (102, 103). In this meta-analysis, only 3 (10.7%, 3/28) studies (21, 35, 38) provided information on HIV-1 subtypes as a whole, but not for every HIV-infected individual in these studies, and it is impossible to analyze the correlation between HIV-1 subtypes and seronegativity. However, when pretreatment HIV-1 RNA levels were included in the meta-regression model, the results showed that pretreatment HIV-1 VL did not significantly affect the frequency of seronegativity (Table 5). Furthermore, it is more likely that higher VLs result in both larger reservoirs and more strong antibody responses; however, small HIV-1 reservoirs and a low level of anti-HIV antibodies were observed in cART-treated patients who are under virological suppression, especially for early-treated populations (19, 84, 104). Therefore, based on their synchronous response to cART, it is reasonable to assume that anti-HIV antibody levels may be a suitable proxy for the size of the latent reservoir. Our meta-analysis results indicated that the levels of anti-HIV-1 antibody response were positively associated with the size of HIV-1 reservoir and supported that anti-HIV titers might be a feasible biomarker of HIV-1 reservoir burden and may be used to assess the efficiency of cART in particular in the resource-limiting settings, although further studies are needed (99).
The major limitation of our analysis is the missing data for some important factors in the majority of the studies, including HIV-1 subtypes, pretreatment CD4+ cell counts, and ethnicity (84, 99). Because only 10.7% (3/28) and 11.1% (2/18) studies reported the HIV-1 subtype and ethnicity/race of study subjects, we did not analyze the effect of viral subtypes and ethnicity in cART-induced seronegativity or HIV-1 reservoir. The independent effect of these factors in antibody response or HIV-1 reservoir size should be further assessed.
In conclusion, seronegativity in the HIV-infected and cART-treated populations is an important issue particularly when cART is initiated during acute/early HIV-1 infection. In the era of cART, it is definitely necessary to develop an appropriate algorithm for HIV-1 diagnosis and management of cART-treated individuals. Additionally, more studies are needed for further validation of the HIV-1-specific antibody as a feasible biomarker of the HIV-1 reservoir.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
ST and YL conceived the study. YL and HL collected, analyzed, and interpreted the data. YL made the tables and figures and wrote the manuscript. ED and ST revised the manuscript. All authors reviewed, revised, and approved the final report.
This work was supported by the National Major Science and Technology Project (grant number 2018ZX10732-401-003-003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.844023/full#supplementary-material
1. WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. Geneva: World Health Organization (2017).
2. WHO. HIV/AIDS. World Health Organization (2021). Available at: https://www.who.int/news-room/facts-in-pictures/detail/hiv-aids
3. Luzuriaga K, Bryson Y, Krogstad P, Robinson J, Stechenberg B, Lamson M, et al. Combination Treatment With Zidovudine, Didanosine, and Nevirapine in Infants With Human Immunodeficiency Virus Type 1 Infection. N Engl J Med (1997) 336:1343–9. doi: 10.1056/NEJM199705083361902
4. Luzuriaga K, McManus M, Catalina M, Mayack S, Sharkey M, Stevenson M, et al. Early Therapy of Vertical Human Immunodeficiency Virus Type 1 (HIV-1) Infection: Control of Viral Replication and Absence of Persistent HIV-1-Specific Immune Responses. J Virol (2000) 74:6984–91. doi: 10.1128/JVI.74.15.6984-6991.2000
5. O’Connell RJ, Merritt TM, Malia JA, VanCott TC, Dolan MJ, Zahwa H, et al. Performance of the OraQuick Rapid Antibody Test for Diagnosis of Human Immunodeficiency Virus Type 1 Infection in Patients With Various Levels of Exposure to Highly Active Antiretroviral Therapy. J Clin Microbiol (2003) 41:2153–5. doi: 10.1128/JCM.41.5.2153-2155.2003
6. O’Connell RJ, Agan BK, Anderson SA, Malia JA, Michael NL. Sensitivity of the Multispot HIV-1/HIV-2 Rapid Test Using Samples From Human Immunodeficiency Virus Type 1-Positive Individuals With Various Levels of Exposure to Highly Active Antiretroviral Therapy. J Clin Microbiol (2006) 44:1831–3. doi: 10.1128/JCM.44.5.1831-1833.2006
7. Markowitz M, Vesanen M, Tenner-Racz K, Cao Y, Binley JM, Talal A, et al. The Effect of Commencing Combination Antiretroviral Therapy Soon After Human Immunodeficiency Virus Type 1 Infection on Viral Replication and Antiviral Immune Responses. J Infect Dis (1999) 179:527–37. doi: 10.1086/314628
8. Adalid-Peralta L, Grangeot-Keros L, Rudent A, Ngo-Giang-Huong N, Krzysiek R, Goujard C, et al. Impact of Highly Active Antiretroviral Therapy on the Maturation of Anti-HIV-1 Antibodies During Primary HIV-1 Infection. HIV Med (2006) 7:514–9. doi: 10.1111/j.1468-1293.2006.00406.x
9. Jurriaans S, Sankatsing SU, Prins JM, Schuitemaker H, Lange J, van der Kuyl AC, et al. HIV-1 Seroreversion in an HIV-1-Seropositive Patient Treated During Acute Infection With Highly Active Antiretroviral Therapy and Mycophenolate Mofetil. AIDS (London England) (2004) 18:1607–8. doi: 10.1097/01.aids.0000131367.05823.ce
10. Kassutto S, Johnston MN, Rosenberg ES. Incomplete HIV Type 1 Antibody Evolution and Seroreversion in Acutely Infected Individuals Treated With Early Antiretroviral Therapy. Clin Infect Dis (2005) 40:868–73. doi: 10.1086/428127
11. Binley JM, Trkola A, Ketas T, Schiller D, Clas B, Little S, et al. The Effect of Highly Active Antiretroviral Therapy on Binding and Neutralizing Antibody Responses to Human Immunodeficiency Virus Type 1 Infection. J Infect Dis (2000) 182:945–9. doi: 10.1086/315774
12. Manak MM, Jagodzinski LL, Shutt A, Malia JA, Leos M, Ouellette J, et al. Decreased Seroreactivity in Individuals Initiating Antiretroviral Therapy During Acute HIV Infection. J Clin Microbiol (2019) 57. doi: 10.1128/JCM.00757-19
13. Curlin ME, Gvetadze R, Leelawiwat W, Martin M, Rose C, Niska RW, et al. Analysis of False-Negative Human Immunodeficiency Virus Rapid Tests Performed on Oral Fluid in 3 International Clinical Research Studies. 64:1663–9. doi: 10.1093/cid/cix228
14. Persaud D, Ray SC, Kajdas J, Ahonkhai A, Siberry GK, Ferguson K, et al. Slow Human Immunodeficiency Virus Type 1 Evolution in Viral Reservoirs in Infants Treated With Effective Antiretroviral Therapy. AIDS Res Hum Retroviruses (2007) 23:381–90. doi: 10.1089/aid.2006.0175
15. Zanchetta M, Anselmi A, Vendrame D, Rampon O, Giaquinto C, Mazza A, et al. Early Therapy in HIV-1-Infected Children: Effect on HIV-1 Dynamics and HIV-1-Specific Immune Response. Antivir Ther (2008) 13:47–55.
16. Ananworanich J, Puthanakit T, Suntarattiwong P, Chokephaibulkit K, Kerr SJ, Fromentin R, et al. Reduced Markers of HIV Persistence and Restricted HIV-Specific Immune Responses After Early Antiretroviral Therapy in Children. AIDS (London England) (2014) 28:1015–20. doi: 10.1097/QAD.0000000000000178
17. Luzuriaga K, Tabak B, Garber M, Chen YH, Ziemniak C, McManus MM, et al. HIV Type 1 (HIV-1) Proviral Reservoirs Decay Continuously Under Sustained Virologic Control in HIV-1-Infected Children Who Received Early Treatment. J Infect Dis (2014) 210:1529–38. doi: 10.1093/infdis/jiu297
18. Merchant M, Wright M, Kabat W, Yogev R. Long-Term Highly Suppressed HIV-Infected Children and Adolescents With Negative Rapid HIV Tests Due to Significant Antibody Loss. J Clin Virol (2014) 59:172–6. doi: 10.1016/j.jcv.2013.11.012
19. Persaud D, Patel K, Karalius B, Rainwater-Lovett K, Ziemniak C, Ellis A, et al. Influence of Age at Virologic Control on Peripheral Blood Human Immunodeficiency Virus Reservoir Size and Serostatus in Perinatally Infected Adolescents. JAMA Pediatr (2014) 168:1138–46. doi: 10.1001/jamapediatrics.2014.1560
20. Kuhn L, Schramm DB, Shiau S, Strehlau R, Pinillos F, Technau K, et al. Young Age at Start of Antiretroviral Therapy and Negative HIV Antibody Results in HIV-Infected Children When Suppressed. AIDS (London England) (2015) 29:1053–60. doi: 10.1097/QAD.0000000000000677
21. Martínez-Bonet M, Puertas MC, Fortuny C, Ouchi D, Mellado MJ, Rojo P, et al. Establishment and Replenishment of the Viral Reservoir in Perinatally HIV-1-Infected Children Initiating Very Early Antiretroviral Therapy.Clin Infect Dis (2015) 61:1169–78. doi: 10.1093/cid/civ456
22. Payne H, Mkhize N, Otwombe K, Lewis J, Panchia R, Callard R, et al. Reactivity of Routine HIV Antibody Tests in Children Who Initiated Antiretroviral Therapy in Early Infancy as Part of the Children With HIV Early Antiretroviral Therapy (CHER) Trial: A Retrospective Analysis. Lancet Infect Dis (2015) 15:803–9. doi: 10.1016/S1473-3099(15)00087-0
23. Brice J, Sylla M, Sayon S, Telly F, Bocar-Fofana D, Murphy R, et al. Qualitative and Quantitative HIV Antibodies and Viral Reservoir Size Characterization in Vertically Infected Children With Virological Suppression. J Antimicrobial Chemother (2017) 72:1147–51 doi: 10.1093/jac/dkw537.
24. Foster C, Pace M, Kaye S, Hopkins E, Jones M, Robinson N, et al. Early Antiretroviral Therapy Reduces HIV DNA Following Perinatal HIV Infection. AIDS (London England) (2017) 31:1847–51. doi: 10.1097/QAD.0000000000001565
25. Olaru ID, McHugh G, Dakshina S, Majonga E, Dauya E, Bandason T, et al. False-Negative HIV Tests Using Oral Fluid Tests in Children Taking Antiretroviral Therapy From Harare, Zimbabwe. J Int AIDS Soc (2017) 20:21751. doi: 10.7448/IAS.20.7.21751
26. Rainwater-Lovett K, Ziemniak C, Watson D, Luzuriaga K, Siberry G, Petru A, et al. Paucity of Intact Non-Induced Provirus With Early, Long-Term Antiretroviral Therapy of Perinatal HIV Infection. PloS One (2017) 12:e0170548. doi: 10.1371/journal.pone.0170548
27. Rocca S, Zangari P, Cotugno N, De Rossi A, Ferns B, Petricone D, et al. Human Immunodeficiency Virus (HIV)-Antibody Repertoire Estimates Reservoir Size and Time of Antiretroviral Therapy Initiation in Virally Suppressed Perinatally HIV-Infected Children. J Pediatr Infect Dis Soc (2019) 8:433–8. doi: 10.1093/jpids/piy080
28. Bitnun A, Ransy DG, Brophy J, Kakkar F, Hawkes M, Samson L, et al. Clinical Correlates of Human Immunodeficiency Virus-1 (HIV-1) DNA and Inducible HIV-1 RNA Reservoirs in Peripheral Blood in Children With Perinatally Acquired HIV-1 Infection With Sustained Virologic Suppression for at Least 5 Years. Clin Infect Dis (2020) 70:859–66. doi: 10.1093/cid/ciz251
29. Puthanakit T, Ananworanich J, Akapirat S, Pattanachaiwit S, Ubolyam S, Assawadarachai V, et al. Pattern and Frequency of Seroreactivity to Routinely Used Serologic Tests in Early-Treated Infants With HIV. J Acquir Immune Defic Syndr (2020) 83:260–6. doi: 10.1097/QAI.0000000000002254
30. Garcia-Prats AJ, Draper HR, Sanders JE, Agrawal AK, Mohapi EQ, Schutze GE. False-Negative Post-18-Month Confirmatory HIV Tests in HIV DNA PCR-Positive Children: A Retrospective Analysis. AIDS (London England) (2012) 26:1927–34. doi: 10.1097/QAD.0b013e32835705bf
31. Wirotpaisankul P, Lapphra K, Maleesatharn A, Rungmaitree S, Wittawatmongkol O, Phongsamart W, et al. HIV Seronegativity in Children, Adolescents and Young Adults Living With Perinatally Acquired HIV: A Cross-Sectional Study in Thailand. J Int AIDS Soc (2020) 23:e25614. doi: 10.1002/jia2.25614
32. Ajibola G, Garcia-Broncano P, Maswabi K, Bennett K, Hughes MD, Moyo S, et al. Viral Reservoir in Early-Treated HIV-Infected Children and Markers for Sustained Viral Suppression. Clin Infect Dis (2021) 73:e997–e1003. doi: 10.1093/cid/ciab143
33. Veldsman KA, Laughton B, Janse van Rensburg A, Zuidewind P, Dobbels E, Barnabas S, et al. Viral Suppression is Associated With HIV-Antibody Level and HIV-1 DNA Detectability in Early Treated Children at 2 Years of Age. AIDS (London England) (2021) 35:1247–52. doi: 10.1097/QAD.0000000000002861
34. Manak MM, Peel S, Malia J, Shutt A, Akapirat S, Pankam T, et al. Loss of HIV Serological Markers Following Early Treatment of Acute HIV Infection. Topics Antiviral Med (2014) 22:187–8.
35. Piwowar-Manning E, Fogel JM, Laeyendecker O, Wolf S, Cummings V, Marzinke MA, et al. Failure to Identify HIV-Infected Individuals in a Clinical Trial Using a Single HIV Rapid Test for Screening. HIV Clin Trials (2014) 15:62–8. doi: 10.1310/hct1502-62
36. de Souza MS, Pinyakorn S, Akapirat S, Pattanachaiwit S, Fletcher JL, Chomchey N, et al. Initiation of Antiretroviral Therapy During Acute HIV-1 Infection Leads to a High Rate of Nonreactive HIV Serology. Clin Infect Dis (2016) 63:555–61. doi: 10.1093/cid/ciw365
37. Burbelo PD, Price RW, Hagberg L, Hatano H, Spudich S, Deeks SG, et al. Anti-Human Immunodeficiency Virus Antibodies in the Cerebrospinal Fluid: Evidence of Early Treatment Impact on Central Nervous System Reservoir? J Infect Dis (2018) 217:1024–32. doi: 10.1093/infdis/jix662
38. Stefic K, Novelli S, Mahjoub N, Seng R, Molina JM, Cheneau C, et al. Nonreactive Human Immunodeficiency Virus Type 1 Rapid Tests After Sustained Viral Suppression Following Antiretroviral Therapy Initiation During Primary Infection. J Infect Dis (2018) 217:1793–7. doi: 10.1093/infdis/jiy120
39. Keating SM, Jones RB, Lalama CM, Bosch RJ, McMahon D, Hampton D, et al. Brief Report: HIV Antibodies Decline During Antiretroviral Therapy But Remain Correlated With HIV DNA and HIV-Specific T-Cell Responses. J Acquir Immune Defic Syndr (2019) 81:594–9. doi: 10.1097/QAI.0000000000002080
40. Liang Y, Li L, Shui J, Hu F, Wang H, Xia Y, et al. Reduction of Anti-HIV Antibody Responses in Subjects Receiving Antiretroviral Therapy During Chronic HIV-1 Infection. J Clin Virol (2020) 128:104414. doi: 10.1016/j.jcv.2020.104414
41. Delaney KP, Branson BM, Uniyal A, Phillips S, Candal D, Owen SM, et al. Evaluation of the Performance Characteristics of 6 Rapid HIV Antibody Tests. Clin Infect Dis (2011) 52:257–63. doi: 10.1093/cid/ciq068
42. Fogel JM, Piwowar-Manning E, Debevec B, Walsky T, Schlusser K, Laeyendecker O, et al. Brief Report: Impact of Early Antiretroviral Therapy on the Performance of HIV Rapid Tests and HIV Incidence Assays. J Acquir Immune Defic Syndr (2017) 75:426–30. doi: 10.1097/QAI.0000000000001421
43. Spivak AM, Sydnor ER, Blankson JN, Gallant JE. Seronegative HIV-1 Infection: A Review of the Literature. AIDS (London England) (2010) 24:1407–14. doi: 10.1097/QAD.0b013e32833ac65c
44. Alexander TS. Human Immunodeficiency Virus Diagnostic Testing: 30 Years of Evolution. Clin Vaccine Immunol (2016) 23:249–53. doi: 10.1128/CVI.00053-16
45. Overbaugh J, Morris L. The Antibody Response Against HIV-1. Cold Spring Harb Perspect Med (2012) 2:a007039. doi: 10.1101/cshperspect.a007039
46. Abela IA, Kadelka C, Trkola A. Correlates of Broadly Neutralizing Antibody Development. Curr Opin HIV AIDS (2019) 14:279–85. doi: 10.1097/COH.0000000000000552
47. Tomaras GD, Haynes BF. HIV-1-Specific Antibody Responses During Acute and Chronic HIV-1 Infection. Curr Opin HIV AIDS (2009) 4:373–9. doi: 10.1097/COH.0b013e32832f00c0
48. Dreyer K, Kallas EG, Planelles V, Montefiori D, McDermott MP, Hasan MS, et al. Primary Isolate Neutralization by HIV Type 1-Infected Patient Sera in the Era of Highly Active Antiretroviral Therapy. AIDS Res Hum Retroviruses (1999) 15:1563–71. doi: 10.1089/088922299309856
49. Montefiori DC, Hill TS, Vo HT, Walker BD, Rosenberg ES. Neutralizing Antibodies Associated With Viremia Control in a Subset of Individuals After Treatment of Acute Human Immunodeficiency Virus Type 1 Infection. J Virol (2001) 75:10200–7. doi: 10.1128/JVI.75.21.10200-10207.2001
50. Medina-Ramírez M, Sánchez-Merino V, Sánchez-Palomino S, Merino-Mansilla A, Ferreira CB, Pérez I, et al. Broadly Cross-Neutralizing Antibodies in HIV-1 Patients With Undetectable Viremia. J Virol (2011) 85:5804–13. doi: 10.1128/JVI.02482-10
51. Falkensammer B, Freissmuth D, Hübner L, Speth C, Dierich MP, Stoiber H. Changes in HIV-Specific Antibody Responses and Neutralization Titers in Patients Under ART. Front Biosci (2007) 12:2148–58. doi: 10.2741/2218
52. Wainberg MA, Drosopoulos WC, Salomon H, Hsu M, Borkow G, Parniak M, et al. Enhanced Fidelity of 3TC-Selected Mutant HIV-1 Reverse Transcriptase. Science (1996) 271:1282–5. doi: 10.1126/science.271.5253.1282
53. Schmidtmayerová H, Lackovicová M, Stanková M, Brůcková M, Surový I, Ujhelyi E, et al. Virus Neutralizing Antibodies at Different Stages of the HIV Disease: Increased Levels After Azidothymidine Treatment. Acta Virol (1992) 36:157–65.
54. Sarmati L, Nicastri E, el-Sawaf G, Ventura L, Salanitro A, Ercoli L, et al. Increase in Neutralizing Antibody Titer Against Sequential Autologous HIV-1 Isolates After 16 Weeks Saquinavir (Invirase) Treatment. J Med Virol (1997) 53:313–8. doi: 10.1002/(SICI)1096-9071(199712)53:4<313::AID-JMV1>3.0.CO;2-A
55. Abdel-Mohsen M, Richman D, Siliciano RF, Nussenzweig MC, Howell BJ, Martinez-Picado J, et al. Recommendations for Measuring HIV Reservoir Size in Cure-Directed Clinical Trials. Nat Med (2020) 26:1339–50. doi: 10.1038/s41591-020-1022-1
56. Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 Cure: Measuring the Latent Reservoir. Trends Microbiol (2015) 23:192–203. doi: 10.1016/j.tim.2015.01.013
57. Burbelo PD, Bayat A, Rhodes CS, Hoh R, Martin JN, Fromentin R, et al. HIV Antibody Characterization as a Method to Quantify Reservoir Size During Curative Interventions. J Infect Dis (2014) 209:1613–7. doi: 10.1093/infdis/jit667
58. Lee SA, Bacchetti P, Chomont N, Fromentin R, Lewin SR, O’Doherty U, et al. Anti-HIV Antibody Responses and the HIV Reservoir Size During Antiretroviral Therapy. PloS One (2016) 11:e0160192. doi: 10.1371/journal.pone.0160192
59. Keating SM, Pilcher CD, Jain V, Lebedeva M, Hampton D, Abdel-Mohsen M, et al. HIV Antibody Level as a Marker of HIV Persistence and Low-Level Viral Replication. J Infect Dis (2017) 216:72–81. doi: 10.1093/infdis/jix225
60. Delagreverie HM, Grude M, Lambert-Niclot S, Nere ML, Jadand C, Leport C, et al. Anti-Gp41 Antibody Levels Reflect HIV Viral Suppression and Cellular Reservoir in Long-Term Antiretroviral-Treated Trial Participants. J Antimicrobial Chemother (2019) 74:1389–94. doi: 10.1093/jac/dkz004
61. Giron LB, Tenore SB, Janini LMR, Sucupira MCA, Diaz RS. Laboratory Surrogate Markers of Residual HIV Replication Among Distinct Groups of Individuals Under Antiretroviral Therapy. PloS One (2019) 14:e0217502. doi: 10.1371/journal.pone.0217502
62. McManus M, Henderson J, Gautam A, Brody R, Weiss ER, Persaud D, et al. Quantitative Human Immunodeficiency Virus (HIV)-1 Antibodies Correlate With Plasma HIV-1 RNA and Cell-Associated DNA Levels in Children on Antiretroviral Therapy. Clin Infect Dis (2019) 68:1725–32 doi: 10.1093/cid/ciab143.
63. Das J, Devadhasan A, Linde C, Broge T, Sassic J, Mangano M, et al. Mining for Humoral Correlates of HIV Control and Latent Reservoir Size. PloS Pathog (2020) 16:e1008868. doi: 10.1371/journal.ppat.1008868
64. Mastrangelo A, Burbelo PD, Galli L, Poli A, Alteri C, Scutari R, et al. Anti-HIV Antibodies Are Representative of the Latent Reservoir But Do Not Correlate With Viral Control in People With Long-Lasting Virological Suppression Undergoing Analytical Treatment Interruption (APACHE Study). J Antimicrobial Chemother (2021) 76:1646–8. doi: 10.1093/jac/dkab060
65. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
66. Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Math Statist (1950) 21:607–11. doi: 10.1214/aoms/1177729756
67. Wilson EB. Probable Inference, the Law of Succession, and Statistical Inference. J Am Stat Assoc (1927) 22:209–12. doi: 10.1080/01621459.1927.10502953
68. Newcombe RG. Two-Sided Confidence Intervals for the Single Proportion: Comparison of Seven Methods. Stat Med (1998) 17:857–72. doi: 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E
69. Higgins JP, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
70. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. Bmj (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
71. Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, et al. Antiretroviral Therapy Initiated Within 6 Months of HIV Infection Is Associated With Lower T-Cell Activation and Smaller HIV Reservoir Size. J Infect Dis (2013) 208:1202–11. doi: 10.1093/infdis/jit311
72. van Houwelingen HC, Arends LR, Stijnen T. Advanced Methods in Meta-Analysis: Multivariate Approach and Meta-Regression. Stat Med (2002) 21:589–624. doi: 10.1002/sim.1040
73. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50:1088–101. doi: 10.2307/2533446
74. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
75. Hare CB, Pappalardo BL, Busch MP, Karlsson AC, Phelps BH, Alexander SS, et al. Seroreversion in Subjects Receiving Antiretroviral Therapy During Acute/Early HIV Infection. Clin Infect Dis (2006) 42:700–8. doi: 10.1086/500215
76. Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R, et al. Impact of Multi-Targeted Antiretroviral Treatment on Gut T Cell Depletion and HIV Reservoir Seeding During Acute HIV Infection. PloS One (2012) 7:e33948. doi: 10.1371/journal.pone.0033948
77. Gallerano D, Cabauatan CR, Sibanda EN, Valenta R. HIV-Specific Antibody Responses in HIV-Infected Patients: From a Monoclonal to a Polyclonal View. Int Arch Allergy Immunol (2015) 167:223–41. doi: 10.1159/000438484
78. Soriano V, Dronda F, Gonzalez-Lopez A, Chaves F, Bravo R, Gutierrez M, et al. HIV-1 Causing AIDS and Death in a Seronegative Individual. Vox Sang (1994) 67:410–1. doi: 10.1111/j.1423-0410.1994.tb01286.x
79. Roy MJ, Damato JJ, Burke DS. Absence of True Seroreversion of HIV-1 Antibody in Seroreactive Individuals. Jama (1993) 269:2876–9. doi: 10.1001/jama.1993.03500220062027
80. Hainaut M, Peltier CA, Goetghebuer T, van der Linden D, Marissens D, Zissis G, et al. Seroreversion in Children Infected With HIV Type 1 Who Are Treated in the First Months of Life Is Not a Rare Event. Clin Infect Dis (2005) 41:1820–1. doi: 10.1086/498313
81. Killian MS, Norris PJ, Rawal BD, Lebedeva M, Hecht FM, Levy JA, et al. The Effects of Early Antiretroviral Therapy and Its Discontinuation on the HIV-Specific Antibody Response. AIDS Res Hum Retroviruses (2006) 22:640–7. doi: 10.1089/aid.2006.22.640
82. Stephenson KE, Neubauer GH, Bricault CA, Shields J, Bayne M, Reimer U, et al. Antibody Responses After Analytic Treatment Interruption in Human Immunodeficiency Virus-1-Infected Individuals on Early Initiated Antiretroviral Therapy. Open Forum Infect Dis (2016) 3:ofw100. doi: 10.1093/ofid/ofw100
83. Tan WS, Chow EP, Fairley CK, Chen MY, Bradshaw CS, Read TR. Sensitivity of HIV Rapid Tests Compared With Fourth-Generation Enzyme Immunoassays or HIV RNA Tests. AIDS (London England) (2016) 30:1951–60. doi: 10.1097/QAD.0000000000001134
84. Bachmann N, von Siebenthal C, Vongrad V, Turk T, Neumann K, Beerenwinkel N, et al. Determinants of HIV-1 Reservoir Size and Long-Term Dynamics During Suppressive ART. Nat Commun (2019) 10:3193. doi: 10.1038/s41467-019-10884-9
85. Hocqueloux L, Avettand-Fènoël V, Jacquot S, Prazuck T, Legac E, Mélard A, et al. Long-Term Antiretroviral Therapy Initiated During Primary HIV-1 Infection Is Key to Achieving Both Low HIV Reservoirs and Normal T Cell Counts. J Antimicrobial Chemother (2013) 68:1169–78. doi: 10.1093/jac/dks533
86. Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, et al. Early Antiretroviral Therapy Reduces AIDS Progression/Death in Individuals With Acute Opportunistic Infections: A Multicenter Randomized Strategy Trial. PloS One (2009) 4:e5575. doi: 10.1371/journal.pone.0005575
87. Chiappini E, Galli L, Tovo PA, Gabiano C, Gattinara GC, Guarino A, et al. Virologic, Immunologic, and Clinical Benefits From Early Combined Antiretroviral Therapy in Infants With Perinatal HIV-1 Infection. AIDS (London England) (2006) 20:207–15. doi: 10.1097/01.aids.0000200529.64113.3e
88. Ananworanich J, Chomont N, Eller LA, Kroon E, Tovanabutra S, Bose M, et al. HIV DNA Set Point Is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine (2016) 11:68–72. doi: 10.1016/j.ebiom.2016.07.024
89. Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr., Chun TW, et al. Absence of Detectable HIV-1 Viremia After Treatment Cessation in an Infant. N Engl J Med (2013) 369:1828–35. doi: 10.1056/NEJMoa1302976
90. Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, et al. Viremic Relapse After HIV-1 Remission in a Perinatally Infected Child. N Engl J Med (2015) 372:786–8. doi: 10.1056/NEJMc1413931
91. Frange P, Faye A, Avettand-Fenoël V, Bellaton E, Descamps D, Angin M, et al. HIV-1 Virological Remission Lasting More Than 12 Years After Interruption of Early Antiretroviral Therapy in a Perinatally Infected Teenager Enrolled in the French ANRS EPF-CO10 Paediatric Cohort: A Case Report. The Lancet. HIV (2016) 3:e49–54. doi: 10.1016/S2352-3018(15)00232-5
92. Doria-Rose NA, Joyce MG. Strategies to Guide the Antibody Affinity Maturation Process. Curr Opin Virol (2015) 11:137–47. doi: 10.1016/j.coviro.2015.04.002
93. Amor A, Toro C, Jimenez V, Simon A, Ramos B, Soriano V. Seroreversion of HIV Antibodies in Patients With Prolonged Suppression of Viraemia Under HAART. AIDS (London England) (2006) 20:1460–2. doi: 10.1097/01.aids.0000233584.10209.43
94. Cornelissen M, Jurriaans S, Prins JM, Bakker M, van der Kuyl AC. Absence of Seroreversion in 80 HAART-Treated HIV-1 Seropositive Patients With at Least Five-Years Undetectable Plasma HIV-1 Viral Load. AIDS Res Ther (2006) 3:3. doi: 10.1186/1742-6405-3-3
95. Wendel BS, Fu Y, Hahn B, He C, Hernandez SM, Qu M, et al. Rapid HIV Progression Is Associated With Extensive Ongoing Somatic Hypermutation. J Immunol (Baltimore Md: 1950) (2020) 205:587–94. doi: 10.4049/jimmunol.1901161
96. Kim JH, Mascola JR, Ratto-Kim S, VanCott TC, Loomis-Price L, Cox JH, et al. Selective Increases in HIV-Specific Neutralizing Antibody and Partial Reconstitution of Cellular Immune Responses During Prolonged, Successful Drug Therapy of HIV Infection. AIDS Res Hum Retroviruses (2001) 17:1021–34. doi: 10.1089/088922201300343708
97. Sarmati L, d’Ettorre G, Nicastri E, Ercoli L, Uccella I, Massetti P, et al. Neutralizing Antibodies Against Autologous Human Immunodeficiency Virus Type 1 Isolates in Patients With Increasing CD4 Cell Counts Despite Incomplete Virus Suppression During Antiretroviral Treatment. Clin Diagn Lab Immunol (2001) 8:822–4. doi: 10.1128/CDLI.8.4.822-824.2001
98. Gach JS, Achenbach CJ, Chromikova V, Berzins B, Lambert N, Landucci G, et al. HIV-1 Specific Antibody Titers and Neutralization Among Chronically Infected Patients on Long-Term Suppressive Antiretroviral Therapy (ART): A Cross-Sectional Study. PloS One (2014) 9:e85371. doi: 10.1371/journal.pone.0085371
99. Zaongo SD, Sun F, Chen Y. Are HIV-1-Specific Antibody Levels Potentially Useful Laboratory Markers to Estimate HIV Reservoir Size? A Rev Front Immunol (2021) 12:786341. doi: 10.3389/fimmu.2021.786341
100. Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. Initial B-Cell Responses to Transmitted Human Immunodeficiency Virus Type 1: Virion-Binding Immunoglobulin M (IgM) and IgG Antibodies Followed by Plasma Anti-Gp41 Antibodies With Ineffective Control of Initial Viremia. J Virol (2008) 82:12449–63. doi: 10.1128/JVI.01708-08
101. Pollack H, Zhan MX, Ilmet-Moore T, Ajuang-Simbiri K, Krasinski K, Borkowsky W. Ontogeny of Anti-Human Immunodeficiency Virus (HIV) Antibody Production in HIV-1-Infected Infants. Proc Natl Acad Sci USA (1993) 90:2340–4. doi: 10.1073/pnas.90.6.2340
102. Morrison CS, Demers K, Kwok C, Bulime S, Rinaldi A, Munjoma M, et al. Plasma and Cervical Viral Loads Among Ugandan and Zimbabwean Women During Acute and Early HIV-1 Infection. AIDS (London England) (2010) 24:573–82. doi: 10.1097/QAD.0b013e32833433df
103. Novitsky V, Ndung’u T, Wang R, Bussmann H, Chonco F, Makhema J, et al. Extended High Viremics: A Substantial Fraction of Individuals Maintain High Plasma Viral RNA Levels After Acute HIV-1 Subtype C Infection. AIDS (London England) (2011) 25:1515–22. doi: 10.1097/QAD.0b013e3283471eb2
104. Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-Treatment HIV-1 Controllers With a Long-Term Virological Remission After the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PloS Pathog (2013) 9:e1003211 doi: 10.1371/journal.ppat.1003211.
Keywords: antiretroviral therapy, HIV-1 serostatus, HIV-1 reservoir, meta-analysis, anti-HIV-1 antibody
Citation: Liang Y, Lin H, Dzakah EE and Tang S (2022) Influence of Combination Antiretroviral Therapy on HIV-1 Serological Responses and Their Implications: A Systematic Review and Meta-Analysis. Front. Immunol. 13:844023. doi: 10.3389/fimmu.2022.844023
Received: 27 December 2021; Accepted: 04 March 2022;
Published: 30 March 2022.
Edited by:
Weiming Tang, University of North Carolina at Chapel Hill, United StatesReviewed by:
Rajat Desikan, Certara UK Limited, United KingdomCopyright © 2022 Liang, Lin, Dzakah and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shixing Tang, dGFtZ3NoaXhpbmdAc211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.