- Clinical Medical Research Center, Southwest Hospital, Army Military Medical University, Chongqing, China
Infection with SARS-CoV-2, the causative agent of the Coronavirus disease 2019 (COVID-19) pandemic, causes respiratory problems and multifaceted organ dysfunction. A crucial mechanism of COVID-19 immunopathy is the recruitment and activation of neutrophils at the infection site, which also predicts disease severity and poor outcomes. The release of neutrophil extracellular traps (NETs), occurring during a regulated form of neutrophil cell death known as NETosis, is a key effector function that mediates harmful effects caused by neutrophils. Abundant NETosis and NET generation have been observed in the neutrophils of many COVID-19 patients, leading to unfavorable coagulopathy and immunothrombosis. Moreover, excessive NETosis and NET generation are now more widely recognized as mediators of additional pathophysiological abnormalities following SARS-CoV-2 infection. In this minireview, we introduce subtypes of NET-producing neutrophils (e.g., low-density granulocytes) and explain the biological importance of NETs and the protein cargos of NETs in COVID-19. In addition, we discuss the mechanisms by which SARS-CoV-2 causes NETosis by upregulating viral processes (e.g., viral entry and replication) as well as host pro-NET mechanisms (e.g., proinflammatory mediator release, platelet activation, and autoantibody production). Furthermore, we provide an update of the main findings of NETosis and NETs in immunothrombosis and other COVID-19-related disorders, such as aberrant immunity, neurological disorders, and post COVID-19 syndromes including lung fibrosis, neurological disorder, tumor progression, and deteriorated chronic illness. Finally, we address potential prospective COVID-19 treatment strategies that target dysregulated NETosis and NET formation via inhibition of NETosis and promotion of NET degradation, respectively.
Introduction
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection (1). Pneumonia is a typical symptom of COVID-19 infection, while acute respiratory distress syndrome (ARDS) and multiple organ failure are common in severe COVID-19 patients (2). Immunopathological manifestations, including cytokine storms and impaired adaptive immunity, are the primary drivers behind COVID-19, with neutrophil infiltration being suggested as a significant cause. It has been observed that neutrophil extravasation occurs widely in pulmonary capillaries, myocardia, and the liver in post-mortem examinations of COVID-19 patients (3). Furthermore, SARS-CoV-2 infection has also been linked to increased neutrophil-to-lymphocyte ratios, which is associated with disease severity and clinical prognosis (4, 5). As a result, neutrophils and their effector mechanisms (e.g., degranulation, oxidative burst, and NETosis) are increasingly recognized as important mediators in the immunopathogenesis of COVID-19 (6).
NETosis is a special form of programmed cell death in neutrophils, which is characterized by the extrusion of DNA, histones, and antimicrobial proteins in a web-like structure known as neutrophil extracellular traps (NETs) (7). NETosis is induced by a range of microbial stimuli and proinflammatory mediators [e.g., interleukin (IL)-8 and IL-1β] (7). The increased generation of reactive oxygen species (ROS) is a crucial intracellular process that causes NETosis (8). ROS trigger proteases including protein arginine deiminase 4 (PAD 4), neutrophil elastase (NE), and Gasdermin D that catalyze the process of chromatin decondensation, nucleus disintegration, and cell rupture (7). Although NETs are important for preventing pathogen invasion, their excessive formation can result in a slew of negative consequences, such as autoimmune inflammation and tissue damage (9). When NETs are activated in the circulation, they can also induce hypercoagulability and thrombosis (10).
Previous studies have shown that NETosis and NET release are both elevated by circulatory or infiltrating neutrophils in COVID-19 patients (11, 12). Meanwhile, it has also been demonstrated that SARS-CoV-2 infection can directly induce NETosis and NET release in healthy neutrophils (13). Furthermore, NET production is regarded as a predictor of disease severity (11, 14) and clinical outcomes (15) in COVID-19. A key mechanism of NETosis and NET-related disease is the induction of immunothrombosis (16). However, NETosis and NETs are also increasingly recognized as mediators of other pathophysiological changes, such as immune dysfunction, neurological abnormities, and post COVID-19 disorders. Herein, we attempt to describe the biological significance of neutrophils and NETs in COVID-19 and the mechanisms involved in SARS-CoV-2-induced NETosis and NET release. We also discuss the roles of NETosis and NETs in immunothrombosis and other COVID-19-related disorders. In addition, we address future potential COVID-19 therapeutic options that target dysregulated NETosis and NET formation.
The Biology of Neutrophils and NETs in COVID-19
Maturation is an important aspect of neutrophil biology that significantly impacts NETosis and NET formation (17, 18). Mature neutrophils generally have a greater capacity to produce NETs in response to external stimuli (19, 20). However, a group of heterogeneous population of both mature and immature neutrophils, known as low-density granulocytes (LDGs), has recently been shown to be more prone to spontaneous NETosis and NET production in autoimmune disorders (21, 22). Importantly, LDG ratios are increased in individuals with COVID-19. The amounts of LDGs are further upregulated in individuals with severe COVID-19 where it is indicative for poor clinical prognoses (23–25). Furthermore, LDGs are prone to NETosis and NET formation, thereby contributing to COVID-19-related immunothrombosis and organ injury (26). Additional research has shown that a subset of immature (low CD10 expression, CD10low) LDGs display reduced capacity to produce NET and robust immunosuppressive capabilities that resemble myeloid-derived suppressor cells (27). In contrast, mature LDGs with higher CD10 and CD16 expression seem to be preferentially enhanced in COVID-19 and are more prone to NETosis and NET production (24, 27). For example, a unique LDG subset with intermediate expression of CD16 (CD16int) was clearly observed in COVID-19 patients. These LDGs spontaneously produce NETs and are favorably associated to lung inflammation and overall disease severity (23). Another study demonstrated the relationship between a cluster of CD33lowCD16+CD11b+ LDGs and increased detection of myeloperoxidase (MPO)-DNA, suggesting that this population plays a direct role in NETosis in COVID-19 (24). In addition, the presence of CD33lowCD16+CD11b+ LDGs is also associated with disease severity and prognosis.
NETs carry a wide range of protein cargos, which are either directly derived from the nucleus and granules or captured during the process of NET formation (28). These protein cargos are necessary for enabling NETs to exert their microbicidal effector function and cause pathological injury. In COVID-19, major NET protein cargos of NETs (i.e., NE, MPO, and histones) are significantly elevated. These protein cargos are associated with increased expression of proinflammatory mediators [e.g., IL-6, IL-8, and C-X-C motif receptor 2 (CXCR2)], multiple organ damage, increased risk of ventilation, and short-term mortality (29, 30). Other prothrombotic factors or proinflammatory damage-associated molecular pattern (DAMP) cargos are also enriched in NETs (27, 31). Intriguingly, Skendros et al. showed that COVID-19 neutrophils produce NETs carrying tissue factor (TF) (32). Moreover, platelet-rich plasma isolated from COVID-19 patients also stimulates the production of TF-containing NETs from healthy neutrophils, leading to thrombotic activity in human aortic endothelial cells in vitro (32). A separate study showed that the expression of high mobility group box 1 (HMGB1)–DNA complexes is upregulated in NETs released by COVID-19 patients, suggesting that circulating NETs contain higher levels of DAMPs (27). Taken together, these findings clearly illustrate the involvement of prothrombotic and proinflammatory cargos in NET-induced COVID-19 pathology.
Mechanisms of SARS-CoV-2-Induced NETosis and NET Formation
The establishment of intracellular infection is necessary for SARS-CoV-2 to directly induce NETosis and NET release in neutrophils. The first prerequisite is the successful neutrophil infiltration by the virus. Neutrophils with higher viral antigen loads promote more efficient NET production, while this process is hindered by inhibition of the classical entry mechanism involving angiotensin converting enzyme 2 (ACE2) and serine proteases (13). However, SARS-CoV-2 can also infect host cells through noncanonical receptors such as C-type lectin receptors (33, 34), which have recently been shown to mediate pattern recognition of dengue virus and NET formation (35). This suggests that C-type lectin receptors may also be involved in mediating NET release in COVID-19. In addition, NETosis and NET formation are also influenced by the viability and intracellular replication of SARS-CoV-2. When infected with viable rather than inactivated viruses, neutrophils are considerably more likely to undergo NETosis and produce NETs (13, 36). Furthermore, targeted pharmacological inhibition of viral replication reduces NET formation (13). Another mechanism behind SARS-COV-2-induced NETosis and NETs lies in the fact that SARS-CoV-2 infection causes neutrophils to increase the production of pro-NETosis mediators. Indeed, SARS-CoV-2 can increase oxidative burst in neutrophils and inhibit the antioxidant response, thereby aggravating immunopathologies in COVID-19, including NETosis and NETs (8, 37). For example, Arcanjo et al. confirmed that SARS-CoV-2 triggers NETosis in human neutrophils through increased ROS production (36). In another recent study, a self-sustained autocrine production loop of IL-8, another intrinsic and essential driver of NETosis, was discovered in pulmonary and peripheral blood neutrophils, which promotes NET formation and indicate the severity of COVID-19 (38).
SARS-CoV-2 is significantly more effective at inducing NETosis in vivo than in cultured neutrophils (35). Furthermore, soluble substances in the plasma of COVID-19 patients also mediate NET formation in neutrophils from healthy people (39). These findings implicate indirect mechanism(s) underlying NETosis and NET formation induced by SARS-CoV-2. In fact, SARS-CoV-2 may stimulate the production of proinflammatory mediators or induce the release of DAMPs when contacting epithelial cells and other neighboring cells (e.g., macrophages) in the airway, resulting in a massive amplification of inflammatory and chemotactic responses. Proinflammatory mediators such as IL-8 and IL-1β are important NET-inducing mediators, and they are produced abundantly by SARS-CoV-2-infected epithelial cells and macrophages, increasing NETosis in tissues and intravascular neutrophils (40, 41). Another indirect route of SARS-CoV-2-induced NET production is platelet activation, which can enhance this process by interacting with neutrophils through toll-like receptor 4 (TLR4), platelet factor 4 (PF4), and extracellular vesicle-dependent processes (35, 42, 43). SARS-CoV-2 and its components (e.g., spike proteins and viral RNA) attach to platelets and increase their activation and aggregation in COVID-19, resulting in vascular injury and thrombosis, both of which are linked to NET formation (44, 45). For example, Wu et al. discovered NETs and overwhelming thromboses in the pulmonary tissues of individuals who had died from COVID-19 (43). The authors also discovered low viral load in the biopsied lungs from these individuals, as well as elevated PF4 expression, leading them to hypothesize that NET formation may be caused by activated platelets rather than SARS-CoV-2 itself. Anti-SARS-CoV-2 antibody or autoantibody overexpression is another typical indirect mechanism driving NETosis and NET formation. IgA2 antibodies were shown to be particularly high in severe SARS-CoV-2 infection, which correlates with circulating extracellular DNA. This, according to Staats et al., is indicative of NET development and predictive of catastrophic outcomes in COVID-19 patients (46). Other studies showed that autoantibodies against phospholipids and phospholipid-binding proteins (aPL antibodies) or PF4 are raised by SARS-CoV-2 infection (47) or vaccination (48). These autoantibodies then trigger NET release in neutrophils isolated from healthy individuals. COVID-19 also leads to the production of anti-NET autoantibodies, which are linked to increased circulating NETs and thromboinflammation in patients. Notably, autoantibodies have been shown to prevent NETs from being degraded by healthy control serum, allowing them to persist in individuals with COVID-19 (49).
NETosis and NETs in Coagulopathy and Immunothrombosis in COVID-19
Immune cells like neutrophils interact directly with platelets and plasma coagulation factors, causing coagulopathy and thrombosis in COVID-19 patients, a condition known as immunothrombosis (39, 50). In COVID-19 patients, abnormal coagulation is common, and in most cases, disseminated intravascular coagulation (DIC) was observed in individuals with COVID-19 during hospitalization before they eventually died (51). Pathological findings also indicated that neutrophil-produced NETs are major elements of micro- and macro-vascular thrombi (52, 53). In addition, the collaboration of numerous components in NETs, including platelets, endothelial cells, coagulation factors, and inorganic polyphosphate, is required for NET-induced intravascular coagulation (32, 54). In COVID-19 and ex-COVID-19 cases, the major components of NETs, such as genomic DNA and citrullinated histone H3, have been suggested as coagulation inducers. For example, when cell free-DNA from human neutrophils spikes into plasma, it triggers thrombin generation by binding to factor XII (FXII), which is enhanced by diminished anticoagulant factors and poor fibrinolysis (55, 56). Complements also stimulate tissue factor production and interact with the platelet/NETs/thrombin axis, making them important players in COVID-19 immunothrombosis (32, 57). Furthermore, histones boost thrombin synthesis in a dose-dependent manner, in a process that relies on activatable platelets as well as platelet activation indirectly, but not by platelet tissue factor (58). Other components, such as neutrophil elastase and cathepsin G via protease-activated receptors, and neutrophil elastase can also degrade tissue factor pathway inhibitors to promote coagulation (59). NETs and histones may also stimulate platelets to be procoagulant by upregulating the expression of P-selectin (54).
Within COVID-19 patients, NETs can cause microvascular thrombosis, tissue damage, and organ failure (60, 61). NETs are released into the intravascular circulation, bind to vessel walls, capture platelets and microvesicles, and subsequently obstruct blood flow. Endothelial in situ microvascular thrombosis, which may be induced by NETs, is one cause of thrombosis in COVID-19 (62). Type I interferons (63) and the NOD-like receptor protein 3 (NLRP3) inflammasome (64) are two examples of proinflammatory pathways that can be triggered by NET formation. In addition, the cytotoxic action of the enzymes and histones that are released from NETs can promote endothelial cell death and endothelial dysfunction (65, 66), which is considered a critical pathogenic mechanism of organ injury in COVID-19 patients (67, 68). Moreover, NET production promotes lung epithelial cell death and intravascular thrombus formation, thereby crucial for inducing acute lung injury in COVID-19 (69, 70). Indeed, due to the presence of abundant pulmonary vascular neutrophils and dynamically released platelets, NET-induced immunothrombosis is more likely to occur, resulting in occlusion of the microvasculature and ischemic injury (16, 71). Autopsies of people who have died from COVID-19 have also revealed occlusive thrombi inside the pulmonary vasculature (72). These autopsies have also shown that incidences of alveolar capillary microthrombi in COVID-19 patients are significantly higher than in influenza patients (72). NETs may also contribute to renal failure and liver injury in COVID-19 patients due to their pro-thrombotic activity. Numerous microthrombi in the hepatic sinusoids, as well as ischemic-type hepatic necrosis, are found in the postmortem livers of COVID-19 patients (73, 74). Furthermore, inflammatory microvascular thrombi, which contain NET components and platelets, have been observed in the lungs, kidneys, and hearts of COVID-19 patients (75).
NETosis and NETs in the Pathology of COVID-19 Beyond Immunothrombosis
NETosis and NETs are increasingly recognized as causes of vascular injury that induce immunothrombosis, as summarized in the preceding section. In addition, autoantigens, proinflammatory mediators, proteases, and cytotoxic enzymes are exposed during NETosis, leading to aberrant immunity such as cytokine storms, autoimmune disorders, and immunosuppression. NETosis and NETs may also have a role in the development of post COVID-19 syndromes, including lung fibrosis, neurological disorders, tumor growth, and worsening of concomitant diseases.
Cytokine Storm
In sepsis (76) and sterile inflammatory conditions (28, 77), NETs and other by-products of NETosis have been shown to act as direct inflammation amplifiers. Hyperinflammation (also known as a “cytokine storm”) is typical in COVID-19 and works in tandem with immunothrombosis to promote ARDS and extensive organ failure (78, 79). According to Ouwendijk et al., NET-specific MPO–DNA complexes in patient plasma corelate with SARS-CoV-2 viral load and plasma inflammatory markers [e.g., C-reactive protein (CRP) and IL-6], implying a NET-associated cytokine storm in COVID-19 (80). In a more recent study, Torres-Ruiz and colleagues directly simulated monocyte-derived macrophages with NETs and NET protein cargos extracted from COVID-19 patients, revealing a significant increase in major proinflammatory mediators such as IL-6, IL-8, IL-17A, tumor necrosis factor α (TNF-α), and granulocyte macrophage colony-stimulating factor (GM-CSF) (27). NETs are also thought to trigger IL-1β secretion in monocytes/macrophages, resulting in a signaling loop that augments the resolution of inflammation after SARS-CoV-2 infection (41). Furthermore, SARS-CoV-2 drives NETosis and NET formation to allow for the release of free DNA and by-products (e.g., elastases and histones). This may trigger surrounding macrophages and endothelial cells to secrete excessive proinflammatory cytokines and chemokines, which, in turn, enhance NET formation and form a positive feedback of cytokine storms in COVID-19 (81, 82).
Autoimmune Diseases
NET release enables self-antigen exposure and autoantibody production, thereby increasing the autoinflammatory response (83). In COVID-19, SARS-CoV-2 infection may also induce autoimmune symptoms through mechanisms involving NET production (83, 84). First, SARS-CoV-2-induced NET production may be a key source of autoantigens. Virus-affected protein autoantigens have also been used to study autoimmune sequelae in COVID-19 in a series of multi-omic investigations (85–87). The authors in these studies proposed a pathway for virus-induced protein modifications that generate autoantigens, thereby suggesting NET-associated protein alterations (e.g., citrullinated histones) as autoantigens in COVID-19 (88). Furthermore, Wang et al. reported the discovery of unknown autoantigens related to nucleic acid and nucleocytoplasmic transport in COVID-19. As NETs carry substantial amounts of nuclear components, their role in this process is plausible (87). Second, anti-NET antibodies and other autoantibodies are elevated in COVID-19. For example, Zuo et al. tested anti-NET antibodies in 328 hospitalized patients with COVID-19 and found anti-NET IgG and IgM levels to be higher in patients than in healthy controls, which is also linked to poor clinical outcomes (49). In addition, Torres-Ruiz et al. found that patients with COVID-19 who have higher anti-NET antibodies are more likely to be detected with positive autoantibodies [e.g., antinuclear antibodies (ANA) and anti-neutrophil cytoplasmic antibodies (ANCA)], suggesting that COVID-19 NETs may act as potential inducers for autoimmune responses (27).
Immunosuppression
COVID-19 patients have weakened adaptive immunity as well as a high level of inflammation (89). Although there is no clear evidence for NET-associated immunosuppression in COVID-19, it is worth noting that tumor-associated NETosis and NETs promote an immunosuppressive environment in which anti-tumor immunity is compromised (90, 91). NETs have also been shown to enhance macrophage pyroptosis in sepsis (92) and to be associated with endothelial apoptosis in COVID-19 patients (93), thereby further facilitating an immunosuppressive microenvironment. Furthermore, persistent immunosuppression may result in bacterial co-infection or secondary infection, which has emerged as a new threat for COVID-19 patients (94). For example, Kreitmann et al. found that early bacterial coinfections were more prevalent in COVID-19 patients than those infected with other viruses (95). In another study, de Buhr et al. presume that NETs and NET-degraded fragments may cause bacterial coinfections in COVID-19 patients (96). The authors’ hypothesis is based on their previous ex-COVID-19 studies, which showed that host intrinsic nucleases that degrade NETs and cell-free DNA may facilitate infection with certain bacteria (e.g., Actinobacillus pleuropneumoniae) that do not synthesize intrinsic nicotinamide adenine dinucleotides (NAD) de novo but instead rely on degraded NET products to obtain extrinsic NAD (97). In another ex-COVID-19 study, NETs in the cerebrospinal fluid of patients with pneumococcal meningitis were demonstrated to interfere with bacterial clearance (98). As a result, these findings may provide novel implications for the possibility of NET-induced immunosuppression in COVID-19 in the context of co-existing bacterial infection.
Post-COVID-19 Syndrome
Patients infected by SARS-CoV-2 have also shown chronic sequelae for weeks or even months, a condition termed as “post COVID-19 syndrome” or “long COVID” (99, 100). Following initial onset of COVID-19, an estimated 50% or more of COVID-19 survivors may develop multi-organ problems (e.g., pulmonary dysfunction and neurologic impairment) or have worsening concomitant chronic illness (100–102). Notably, a recent study found that circulating markers of NETs returned to comparable levels with healthy controls after 4 months of infection, implying that NETs are persistent and may be involved in post COVID-19 syndrome pathology (30, 103, 104).
Pulmonary fibrosis is a common post COVID-19 respiratory conditions due to epithelial–mesenchymal transition (EMT) after SARS-CoV-2 infection and inflammation (105). Pandolfi et al. showed that NETs in the bronchoalveolar lavage fluid of severe COVID-19 patients cause EMT in lung epithelial cells (106). The authors also found that SARS-CoV-2 infection increases NETosis in co-cultured lung epithelial cells, macrophages, and neutrophils, as evidenced by increased SMA (a mesenchymal marker) expression and decreased E-cadherin (an epithelial marker) expression. Other studies reported that NETs and NET-associated proteins favor the expression of inflammatory and fibrotic genes (107), or increase the number and activity of fibroblasts (108) in ex-COVID-19 fibrosis induced by chemical or infectious stimuli. These data may indirectly implicate NETs as important players in the pathogenesis of pulmonary fibrosis post COVID-19.
Another common post-COVID-19 condition is persistent neurologic impairment (109, 110), which is characterized by cerebrovascular abnormalities and symptoms such as fatigue, myalgia, and cognitive impairment (111). Direct SARS-CoV-2 infection, persistent neuro-inflammation by central or peripheral mediators, and cerebrovascular abnormalities, all of which are linked to the excessive production of NETs, are among the mechanisms responsible for neurologic impairment following COVID-19 (102, 110). For example, Lou et al. highlighted the impact of SARS-CoV-2 infection on cerebrovascular disorders, citing increased NET formation and related immunothrombosis, inflammation, and antiphospholipid antibody production as major impacts (112). In addition, Pramitasuri et al. found NET-induced vasculopathy (e.g., procoagulant activity and endothelial dysfunction) and neuroinflammation (e.g., extruded NET components mediated neuronal damage, NETs-IL-1 loop, and IL-17 cascades) in ischemic stroke following COVID-19 (113). These findings could point to a potential link between NETs and COVID-19-related long-term neurological diseases.
COVID-19 also has a long-term influence on tumor progression (114). Patients with tumors have been shown to be more vulnerable to SARS-CoV-2 infection and subsequent development of severe COVID-19 (115). In addition, Saini et al. suggested that patients who have recovered from COVID-19 may have an increased risk of developing cancer or of cancer progression and metastasis (116). Moreover, NETs have been shown to change the tumor microenvironment (117), awaken cancer cells (118), and enhance tumor progression and metastasis (119). As a result, the persistent presence of NETs may mediate the long-term effects of tumor induction or worsening in COVID-19. This hypothesis, however, will need to be proven in future studies using more direct approaches.
COVID-19 may aggravate pre-existing chronic illnesses such as hypertension (120), obesity (121), and diabetes (122), in addition to having a direct effect on the host. Given the circulatory and infiltrating nature of neutrophils, the broad occurrence of NETosis and NETs in COVID-19 may facilitate the worsening of various chronic disorders. For example, Thierry et al. found that NETs and their by-products (e.g., elastase) may induce hypertension, thrombosis, and vasculitis in COVID-19 (82). Furthermore, hypertensive patients infected by SARS-CoV-2 have greater neutrophil counts than COVID-19 patients without hypertension, implying a potential relationship between NETs and COVID-19-related hypertension (120). Moreover, plasmatic NET by-products (e.g., MPO–DNA complexes) were found to be higher in extremely obese or diabetic patients (82, 123). The authors of these studies also speculated about the tangled association between NETs, chronic obesity, and diabetes, and how these could lead to long-term pathologies, such as delayed wound healing, increased cardiovascular disease, and thromboembolic events following COVID-19.
NETosis and NET-based Therapeutic Approaches to COVID-19
With the growing recognition of the roles of NETosis and NETs in COVID-19, a few treatment approaches have recently been reported that inhibit NETosis and NET release, or promote NET degradation (Table 1). The first approach involves interfering with SARS-CoV-2 to stimulate neutrophils or antagonize the function of other extracellular pro-NETosis factors. For example, antigen–antibody complexes can stimulate FcγRIIA receptors on neutrophils to induce NETs in COVID-19 via spleen tyrosine kinase (SYK) phosphorylation and downstream signaling (124). R406 is the active ingredient of the SYK inhibitor, fostamatinib, which suppresses NETosis in neutrophils from healthy donors when activated by COVID-19 patient plasma (124). Furthermore, a synthetic glycopolymer agonist of sialic acid-binding immunoglobulin-type lectins (Siglec)-9, a checkpoint receptor in neutrophils, might reduce NETosis triggered by viral PAMPs (e.g., R848) or COVID-19 patient plasma (126). In COVID-19, the complement system is also overactive, and complement enriched NETs or the by-product of complement activation, known as anaphylatoxins, are linked to clinical symptoms of COVID-19 (32). Carboxypeptidase B2 (CPB2) is a natural plasma enzyme that degrades C3a and C5a anaphylatoxins (142). Zhang et al. found that recombinant CPB2 inhibits NET production in neutrophils isolated from COVID-19 patients and reduces the damage to cultured vascular endothelial cells (125). Other research has also revealed that antibodies against cytokines like IL-8, IL-6, and IL-1β not only reduce the resolution of inflammation but also block their pro-NETosis function (70, 133, 143). The second strategy targets the intracellular mechanisms that drive NETosis in response to SARS-CoV-2 infection. For example, vitamin C has been tested in phase 2 clinical trials aimed at reducing COVID-19-associated mortality by reducing excessive activation of the inflammatory response (135). Of note, vitamin C is an antioxidant that significantly attenuates PMA-induced NETosis in healthy neutrophils by scavenging ROS (144). Therefore, vitamin C may also inhibit NETosis and NET production in COVID-19. Proteases are important mediators in the NETosis process. Antiproteases that inhibit histone deacetylase (e.g., Belinostat), block NE activity (Alvelestat), or inhibit Gasdermin D (Disulfiram) have been shown to inhibit NETosis. These antiproteases have undergone preclinical (136) or clinical testing (11, 60) to demonstrate their efficacy in COVID-19. PAD4 is another crucial pro-NET enzyme that is upregulated in COVID-19 and is responsible for NET release (138, 145). Therefore, the use of PAD4 inhibitors, such as Cl-amidine, YW-56, and GSK484, may prevent both the prothrombotic and proinflammatory effects of SARS-CoV-2 by reducing NET production (138). For example, Cl-amidine has been shown to suppress NET release in both SARS-CoV-2-infected healthy neutrophils and blood neutrophils from COVID-19 patients (13). Cl-amidine also prevents apoptosis of lung epithelial cells due to NET release, suggesting that PAD4 inhibitors could be used to prevent immunothrombosis and lung injury in COVID-19 (13, 138). The third strategy involves mediating NET degradation. In fact, COVID-19 has been found to have a deficiency in NET clearance, resulting in pulmonary thrombo-inflammation (55). DNase-1 and DNase-1L3 are important catalyzers involved in the dissolution of NETs and the prevention of thromboembolic events caused by NETs (141). In this regard, DNases are currently being tested in clinical trials for the treatment of COVID-19 (55, 139). In another study using proteomic profiling, Fisher et al. showed that recombinant DNase treatment lowers NET production to promote recovery in COVID-19 patients (146). More research into the mechanisms underlying NET formation in COVID-19 may pave the way for other novel therapeutic approaches (e.g., extracellular histone neutralizers or NET inhibitors) (140).
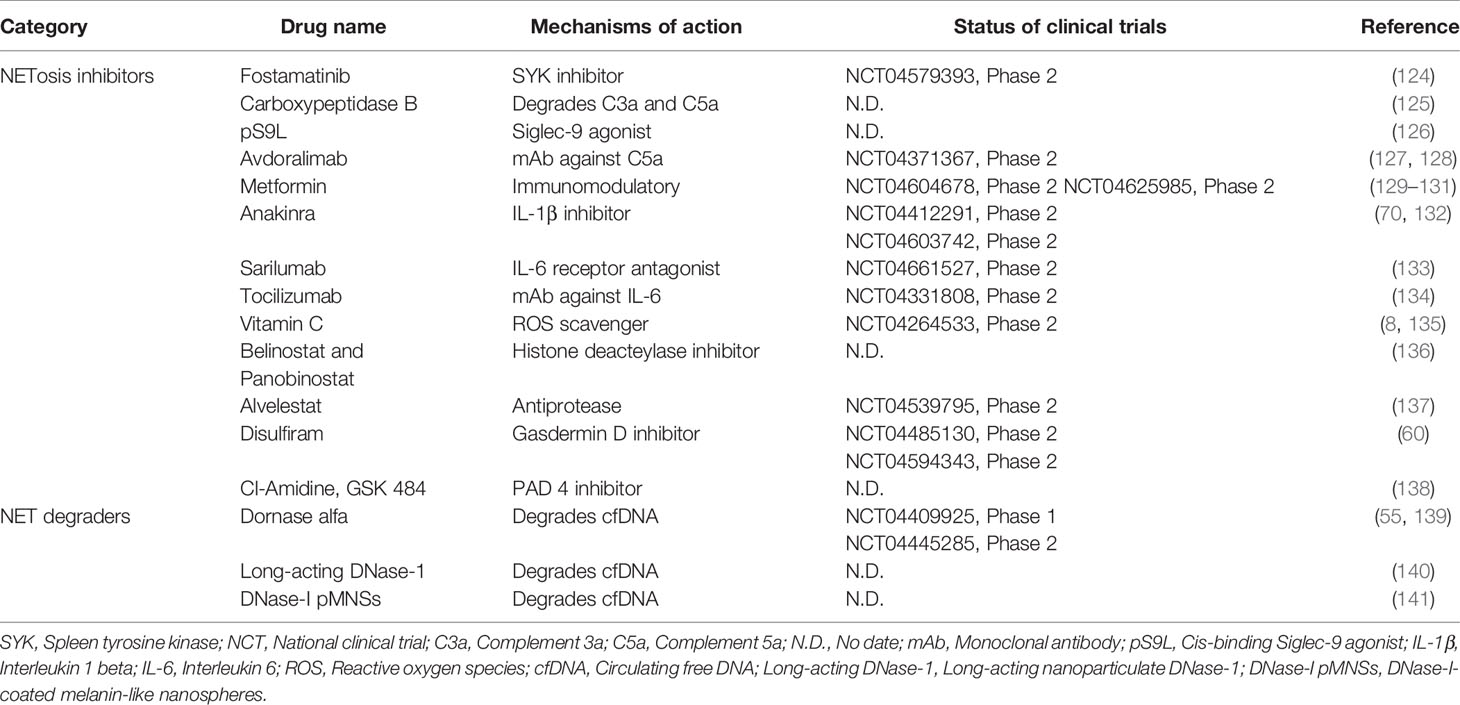
Table 1 Anti-COVID-19 drugs in preclinical or clinical development via NETosis and NETs targeting mechanisms.
Conclusions
SARS-CoV-2 infection triggers NETosis and NET formation in both circulatory and infiltrating neutrophils, resulting in pulmonary injury, extensive inflammation, and the formation of a typical COVID-19 thrombus. The characteristic COVID-19 NET-induced thrombus contributes to microvascular obstruction and organ damage. However, new evidence reveals the role of NETs in pathological processes other than thrombosis, such as chronic aberrant immunity and long-term COVID-19. Given the ongoing COVID-19 pandemic, more people may develop both acute and chronic symptoms following SARS-CoV-2 infection. In this regard, urgent interventions are required to suppress the triggering factors involved in these processes or otherwise speeding up the degradation machinery, both of which may help to reduce NETosis and avoid the negative consequences of NET formation. In addition, precise management will be necessary to avoid disruption of protective neutrophil activities and NET formation. Furthermore, because DNase treatment may enhance the release of breakdown products from NETs (e.g., histones and proteases), it is important to avoid unwanted negative effects when developing novel anti-COVID-19 therapies that target NETs (96).
Author Contributions
XL conceived the review article, reviewed the literature, and made substantial revisions before submission. YZ reviewed the literature, contributed to the writing of the manuscript, and prepared the table. XC reviewed the literature and contributed to the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation (81902015 and 81801964).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cao X. COVID-19: Immunopathology and Its Implications for Therapy. Nat Rev Immunol (2020) 20:269–70. doi: 10.1038/s41577-020-0308-3
2. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 Pathophysiology: A Review. Clin Immunol (2020) 215:108427. doi: 10.1016/j.clim.2020.108427
3. Bian XW. Autopsy of COVID-19 Patients in China. Natl Sci Rev (2020) 7:1414–8. doi: 10.1093/nsr/nwaa123
4. Cai J, Li H, Zhang C, Chen Z, Liu H, Lei F, et al. The Neutrophil-To-Lymphocyte Ratio Determines Clinical Efficacy of Corticosteroid Therapy in Patients With COVID-19. Cell Metab (2021) 33:258–69. doi: 10.1016/j.cmet.2021.01.002
5. Wang H, Zhang Y, Mo P, Liu J, Wang H, Wang F, et al. Neutrophil to CD4+ Lymphocyte Ratio as a Potential Biomarker in Predicting Virus Negative Conversion Time in COVID-19. Int Immunopharmacol (2020) 85:106683. doi: 10.1016/j.intimp.2020.106683
6. Cavalcante-Silva L, Carvalho D, Lima EA, Galvao J, Da SJ, Sales-Neto JM, et al. Neutrophils and COVID-19: The Road So Far. Int Immunopharmacol (2021) 90:107233. doi: 10.1016/j.intimp.2020.107233
7. Thiam HR, Wong SL, Wagner DD, Waterman CM. Cellular Mechanisms of NETosis. Annu Rev Cell Dev Biol (2020) 36:191–218. doi: 10.1146/annurev-cellbio-020520-111016
8. Schonrich G, Raftery MJ, Samstag Y. Devilishly Radical NETwork in COVID-19: Oxidative Stress, Neutrophil Extracellular Traps (NETs), and T Cell Suppression. Adv Biol Regul (2020) 77:100741. doi: 10.1016/j.jbior.2020.100741
9. Gupta S, Kaplan MJ. The Role of Neutrophils and NETosis in Autoimmune and Renal Diseases. Nat Rev Nephrol (2016) 12:402–13. doi: 10.1038/nrneph.2016.71
10. Sorensen OE, Borregaard N. Neutrophil Extracellular Traps - the Dark Side of Neutrophils. J Clin Invest (2016) 126:1612–20. doi: 10.1172/JCI84538
11. Janiuk K, Jabłońska E, Garley M. Significance of NETs Formation in COVID-19. Cells Basel (2021) 10:151. doi: 10.3390/cells10010151
12. Narasaraju T, Tang BM, Herrmann M, Muller S, Chow VTK, Radic M. Neutrophilia and NETopathy as Key Pathologic Drivers of Progressive Lung Impairment in Patients With COVID-19. Front Pharmacol (2020) 11:870. doi: 10.3389/fphar.2020.00870
13. Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2-Triggered Neutrophil Extracellular Traps Mediate COVID-19 Pathology. J Exp Med (2020) 217:e20201129. doi: 10.1084/jem.20201129
14. Didangelos A. COVID-19 Hyperinflammation: What About Neutrophils? Msphere (2020) 5:e00367-20. doi: 10.1128/mSphere.00367-20
15. Huckriede J, Anderberg SB, Morales A, de Vries F, Hultstrom M, Bergqvist A, et al. Evolution of NETosis Markers and DAMPs Have Prognostic Value in Critically Ill COVID-19 Patients. Sci Rep (2021) 11:15701. doi: 10.1038/s41598-021-95209-x
16. Kvietys PR, Fakhoury H, Kadan S, Yaqinuddin A, Al-Mutairy E, Al-Kattan K. COVID-19: Lung-Centric Immunothrombosis. Front Cell Infect Microbiol (2021) 11:679878. doi: 10.3389/fcimb.2021.679878
17. Lukasova E, Koristek Z, Klabusay M, Ondrej V, Grigoryev S, Bacikova A, et al. Granulocyte Maturation Determines Ability to Release Chromatin NETs and Loss of DNA Damage Response; These Properties Are Absent in Immature AML Granulocytes. Biochim Biophys Acta (2013) 1833:767–79. doi: 10.1016/j.bbamcr.2012.12.012
18. Kwiecien I, Rutkowska E, Kulik K, Klos K, Plewka K, Raniszewska A, et al. Neutrophil Maturation, Reactivity and Granularity Research Parameters to Characterize and Differentiate Convalescent Patients From Active SARS-CoV-2 Infection. Cells Basel (2021) 10:2332. doi: 10.3390/cells10092332
19. Mackey J, Coffelt SB, Carlin LM. Neutrophil Maturity in Cancer. Front Immunol (2019) 10:1912. doi: 10.3389/fimmu.2019.01912
20. Snoderly HT, Boone BA, Bennewitz MF. Neutrophil Extracellular Traps in Breast Cancer and Beyond: Current Perspectives on NET Stimuli, Thrombosis and Metastasis, and Clinical Utility for Diagnosis and Treatment. Breast Cancer Res (2019) 21:145. doi: 10.1186/s13058-019-1237-6
21. Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil Extracellular Traps Enriched in Oxidized Mitochondrial DNA Are Interferogenic and Contribute to Lupus-Like Disease. Nat Med (2016) 22:146–53. doi: 10.1038/nm.4027
22. Hassani M, Hellebrekers P, Chen N, van Aalst C, Bongers S, Hietbrink F, et al. On the Origin of Low-Density Neutrophils. J Leukoc Biol (2020) 107:809–18. doi: 10.1002/JLB.5HR0120-459R
23. Morrissey SM, Geller AE, Hu X, Tieri D, Ding C, Klaes CK, et al. A Specific Low-Density Neutrophil Population Correlates With Hypercoagulation and Disease Severity in Hospitalized COVID-19 Patients. JCI Insight (2021) 6:e148435. doi: 10.1172/jci.insight.148435
24. Cabrera LE, Pekkarinen PT, Alander M, Nowlan KHA, Nguyen NA, Jokiranta S, et al. Characterization of Low-Density Granulocytes in COVID-19. PloS Pathog (2021) 17:e1009721. doi: 10.1371/journal.ppat.1009721
25. Torres-Ruiz J, Pérez-Fragoso A, Maravillas-Montero JL, Llorente L, Mejía-Domínguez NR, Páez-Franco JC, et al. Redefining COVID-19 Severity and Prognosis: The Role of Clinical and Immunobiotypes. Front Immunol (2021) 12:689966. doi: 10.3389/fimmu.2021.689966
26. Obermayer A, Jakob L, Haslbauer JD, Matter MS, Tzankov A, Stoiber W. Neutrophil Extracellular Traps in Fatal COVID-19-Associated Lung Injury. Dis Markers (2021) 2021:1–10. doi: 10.1155/2021/5566826
27. Torres-Ruiz J, Absalón-Aguilar A, Nuñez-Aguirre M, Pérez-Fragoso A, Carrillo-Vázquez DA, Maravillas-Montero JL, et al. Neutrophil Extracellular Traps Contribute to COVID-19 Hyperinflammation and Humoral Autoimmunity. Cells Basel (2021) 10:2545. doi: 10.3390/cells10102545
28. Papayannopoulos V. Neutrophil Extracellular Traps in Immunity and Disease. Nat Rev Immunol (2018) 18:134–47. doi: 10.1038/nri.2017.105
29. Gueant JL, Gueant-Rodriguez RM, Fromonot J, Oussalah A, Louis H, Chery C, et al. Elastase and Exacerbation of Neutrophil Innate Immunity Are Involved in Multi-Visceral Manifestations of COVID-19. Allergy (2021) 76:1846–58. doi: 10.1111/all.14746
30. Ng H, Havervall S, Rosell A, Aguilera K, Parv K, von Meijenfeldt FA, et al. Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19. Arterioscler Thromb Vasc Biol (2021) 41:988–94. doi: 10.1161/ATVBAHA.120.315267
31. Cicco S, Cicco G, Racanelli V, Vacca A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediat Inflamm (2020) 2020:1–25. doi: 10.1155/2020/7527953
32. Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and Tissue Factor-Enriched Neutrophil Extracellular Traps Are Key Drivers in COVID-19 Immunothrombosis. J Clin Invest (2020) 130:6151–7. doi: 10.1172/JCI141374
33. Lempp FA, Soriaga LB, Montiel-Ruiz M, Benigni F, Noack J, Park YJ, et al. Lectins Enhance SARS-CoV-2 Infection and Influence Neutralizing Antibodies. Nature (2021) 598:342–7. doi: 10.1038/s41586-021-03925-1
34. Lu Q, Liu J, Zhao S, Gomez CM, Laurent-Rolle M, Dong J, et al. SARS-CoV-2 Exacerbates Proinflammatory Responses in Myeloid Cells Through C-Type Lectin Receptors and Tweety Family Member 2. Immunity (2021) 54:1304–19. doi: 10.1016/j.immuni.2021.05.006
35. Sung PS, Hsieh SL. C-Type Lectins and Extracellular Vesicles in Virus-Induced NETosis. J BioMed Sci (2021) 28:46. doi: 10.1186/s12929-021-00741-7
36. Arcanjo A, Logullo J, Menezes CCB, de Souza Carvalho Giangiarulo TC, Dos Reis MC, de Castro GMM, et al. The Emerging Role of Neutrophil Extracellular Traps in Severe Acute Respiratory Syndrome Coronavirus 2 (COVID-19). Sci Rep-Uk (2020) 10:19630. doi: 10.1038/s41598-020-76781-0
37. Laforge M, Elbim C, Frere C, Hemadi M, Massaad C, Nuss P, et al. Tissue Damage From Neutrophil-Induced Oxidative Stress in COVID-19. Nat Rev Immunol (2020) 20:515–6. doi: 10.1038/s41577-020-0407-1
38. Kaiser R, Leunig A, Pekayvaz K, Popp O, Joppich M, Polewka V, et al. Self-Sustaining IL-8 Loops Drive a Prothrombotic Neutrophil Phenotype in Severe COVID-19. JCI Insight (2021) 6:e150862. doi: 10.1172/jci.insight.150862
39. Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood (2020) 136:1169–79. doi: 10.1182/blood.2020007008
40. Park JH, Lee HK. Re-Analysis of Single Cell Transcriptome Reveals That the NR3C1-CXCL8-Neutrophil Axis Determines the Severity of COVID-19. Front Immunol (2020) 11:2145. doi: 10.3389/fimmu.2020.02145
41. Yaqinuddin A, Kashir J. Novel Therapeutic Targets for SARS-CoV-2-Induced Acute Lung Injury: Targeting a Potential IL-1β/Neutrophil Extracellular Traps Feedback Loop. Med Hypotheses (2020) 143:109906. doi: 10.1016/j.mehy.2020.109906
42. Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat Med (2007) 13:463–9. doi: 10.1038/nm1565
43. Wu M, Chen Y, Xia H, Wang C, Tan CY, Cai X, et al. Transcriptional and Proteomic Insights Into the Host Response in Fatal COVID-19 Cases. Proc Natl Acad Sci USA (2020) 117:28336–43. doi: 10.1073/pnas.2018030117
44. Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, et al. SARS-CoV-2 Binds Platelet ACE2 to Enhance Thrombosis in COVID-19. J Hematol Oncol (2020) 13:120. doi: 10.1186/s13045-020-00954-7
45. Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, Khalki L, et al. Platelets can Associate With SARS-Cov-2 RNA and Are Hyperactivated in COVID-19. Circ Res (2020) 127:1404–18. doi: 10.1161/CIRCRESAHA.120.317703
46. Staats L, Pfeiffer H, Knopf J, Lindemann A, Furst J, Kremer AE, et al. IgA2 Antibodies Against SARS-CoV-2 Correlate With NET Formation and Fatal Outcome in Severely Diseased COVID-19 Patients. Cells Basel (2020) 9:2676. doi: 10.3390/cells9122676
47. Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, et al. Prothrombotic Autoantibodies in Serum From Patients Hospitalized With COVID-19. Sci Transl Med (2020) 12:eabd3876. doi: 10.1126/scitranslmed.abd3876
48. Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, et al. Insights in ChAdOx1 Ncov-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia. Blood (2021) 138:2256–68. doi: 10.1182/blood.2021013231
49. Zuo Y, Yalavarthi S, Navaz SA, Hoy CK, Harbaugh A, Gockman K, et al. Autoantibodies Stabilize Neutrophil Extracellular Traps in COVID-19. JCI Insight (2021) 6:e150111. doi: 10.1172/jci.insight.150111
50. Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, et al. Platelet Gene Expression and Function in Patients With COVID-19. Blood (2020) 136:1317–29. doi: 10.1182/blood.2020007214
51. Arachchillage D, Laffan M. Abnormal Coagulation Parameters Are Associated With Poor Prognosis in Patients With Novel Coronavirus Pneumonia. J Thromb Haemost (2020) 18:1233–4. doi: 10.1111/jth.14820
52. Dolhnikoff M, Duarte-Neto AN, de Almeida MR, Da SL, de Oliveira EP, Saldiva P, et al. Pathological Evidence of Pulmonary Thrombotic Phenomena in Severe COVID-19. J Thromb Haemost (2020) 18:1517–9. doi: 10.1111/jth.14844
53. Sonzogni A, Previtali G, Seghezzi M, Grazia AM, Gianatti A, Licini L, et al. Liver Histopathology in Severe COVID 19 Respiratory Failure Is Suggestive of Vascular Alterations. Liver Int (2020) 40:2110–6. doi: 10.1111/liv.14601
54. McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and Neutrophil Extracellular Traps Collaborate to Promote Intravascular Coagulation During Sepsis in Mice. Blood (2017) 129:1357–67. doi: 10.1182/blood-2016-09-741298
55. Englert H, Rangaswamy C, Deppermann C, Sperhake J, Krisp C, Schreier D, et al. Defective NET Clearance Contributes to Sustained FXII Activation in COVID-19-Associated Pulmonary Thrombo-Inflammation. Ebiomedicine (2021) 67:103382. doi: 10.1016/j.ebiom.2021.103382
56. Noubouossie DF, Reeves BN, Strahl BD, Key NS. Neutrophils: Back in the Thrombosis Spotlight. Blood (2019) 133:2186–97. doi: 10.1182/blood-2018-10-862243
57. Bonaventura A, Vecchie A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial Dysfunction and Immunothrombosis as Key Pathogenic Mechanisms in COVID-19. Nat Rev Immunol (2021) 21:319–29. doi: 10.1038/s41577-021-00536-9
58. Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular Histones Promote Thrombin Generation Through Platelet-Dependent Mechanisms: Involvement of Platelet TLR2 and TLR4. Blood (2011) 118:1952–61. doi: 10.1182/blood-2011-03-343061
59. Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal Coupling of Coagulation and Innate Immunity via Neutrophil Serine Proteases. Nat Med (2010) 16:887–96. doi: 10.1038/nm.2184
60. Ackermann M, Anders H, Bilyy R, Bowlin GL, Daniel C, De Lorenzo R, et al. Patients With COVID-19: In the Dark-NETs of Neutrophils. Cell Death Differ (2021) 28:3125–39. doi: 10.1038/s41418-021-00805-z
61. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil Extracellular Traps in COVID-19. JCI Insight (2020) 5:e138999. doi: 10.1172/jci.insight.138999
62. Poor HD. Pulmonary Thrombosis and Thromboembolism in COVID-19. Chest (2021) 160:1471–80. doi: 10.1016/j.chest.2021.06.016
63. Rosa BA, Ahmed M, Singh DK, Choreno-Parra JA, Cole J, Jimenez-Alvarez LA, et al. IFN Signaling and Neutrophil Degranulation Transcriptional Signatures Are Induced During SARS-CoV-2 Infection. Commun Biol (2021) 4:290. doi: 10.1038/s42003-021-01829-4
64. Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil Extracellular Trap-Associated Protein Activation of the NLRP3 Inflammasome Is Enhanced in Lupus Macrophages. J Immunol (2013) 190:1217–26. doi: 10.4049/jimmunol.1202388
65. Pieterse E, Rother N, Garsen M, Hofstra JM, Satchell SC, Hoffmann M, et al. Neutrophil Extracellular Traps Drive Endothelial-To-Mesenchymal Transition. Arterioscler Thromb Vasc Biol (2017) 37:1371–9. doi: 10.1161/ATVBAHA.117.309002
66. Becker K, Beythien G, de Buhr N, Stanelle-Bertram S, Tuku B, Kouassi NM, et al. Vasculitis and Neutrophil Extracellular Traps in Lungs of Golden Syrian Hamsters With SARS-CoV-2. Front Immunol (2021) 12:640842. doi: 10.3389/fimmu.2021.640842
67. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med (2020) 180:934–43. doi: 10.1001/jamainternmed.2020.0994
68. Dupont A, Rauch A, Staessens S, Moussa M, Rosa M, Corseaux D, et al. Vascular Endothelial Damage in the Pathogenesis of Organ Injury in Severe COVID-19. Arteriosc Thromb Vasc Biol (2021) 41:1760–73. doi: 10.1161/ATVBAHA.120.315595
69. Szturmowicz M, Demkow U. Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int J Mol Sci (2021) 22:8854. doi: 10.3390/ijms22168854
70. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps. J Exp Med (2020) 217:e20200652. doi: 10.1084/jem.20200652
71. Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, et al. The Lung Is a Site of Platelet Biogenesis and a Reservoir for Haematopoietic Progenitors. Nature (2017) 544:105–9. doi: 10.1038/nature21706
72. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med (2020) 383:120–8. doi: 10.1056/NEJMoa2015432
73. Zhao CL, Rapkiewicz A, Maghsoodi-Deerwester M, Gupta M, Cao W, Palaia T, et al. Pathological Findings in the Postmortem Liver of Patients With Coronavirus Disease 2019 (COVID-19). Hum Pathol (2021) 109:59–68. doi: 10.1016/j.humpath.2020.11.015
74. Li D, Ding X, Xie M, Tian D, Xia L. COVID-19-Associated Liver Injury: From Bedside to Bench. J Gastroenterol (2021) 56:218–30. doi: 10.1007/s00535-021-01760-9
75. Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation (2020) 142:1176–89. doi: 10.1161/CIRCULATIONAHA.120.048488
76. Denning NL, Aziz M, Gurien SD, Wang P. DAMPs and NETs in Sepsis. Front Immunol (2019) 10:2536. doi: 10.3389/fimmu.2019.02536
77. Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-Associated Molecular Pattern-Activated Neutrophil Extracellular Trap Exacerbates Sterile Inflammatory Liver Injury. Hepatology (2015) 62:600–14. doi: 10.1002/hep.27841
78. Borges L, Pithon-Curi TC, Curi R, Hatanaka E. COVID-19 and Neutrophils: The Relationship Between Hyperinflammation and Neutrophil Extracellular Traps. Mediators Inflamm (2020) 2020:8829674. doi: 10.1155/2020/8829674
79. Soy M, Keser G, Atagunduz P, Tabak F, Atagunduz I, Kayhan S. Cytokine Storm in COVID-19: Pathogenesis and Overview of Anti-Inflammatory Agents Used in Treatment. Clin Rheumatol (2020) 39:2085–94. doi: 10.1007/s10067-020-05190-5
80. Ouwendijk W, Raadsen MP, van Kampen J, Verdijk RM, von der Thusen JH, Guo L, et al. High Levels of Neutrophil Extracellular Traps Persist in the Lower Respiratory Tract of Critically Ill Patients With Coronavirus Disease 2019. J Infect Dis (2021) 223:1512–21. doi: 10.1093/infdis/jiab050
81. Tomar B, Anders HJ, Desai J, Mulay SR. Neutrophils and Neutrophil Extracellular Traps Drive Necroinflammation in COVID-19. Cells Basel (2020) 9:1383. doi: 10.3390/cells9061383
82. Thierry AR, Roch B. Neutrophil Extracellular Traps and By-Products Play a Key Role in COVID-19: Pathogenesis, Risk Factors, and Therapy. J Clin Med (2020) 9:2942. doi: 10.3390/jcm9092942
83. Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y. The SARS-CoV-2 as an Instrumental Trigger of Autoimmunity. Autoimmun Rev (2021) 20:102792. doi: 10.1016/j.autrev.2021.102792
84. Liu Y, Sawalha AH, Lu Q. COVID-19 and Autoimmune Diseases. Curr Opin Rheumatol (2021) 33:155–62. doi: 10.1097/BOR.0000000000000776
85. Wang JY, Roehrl MW, Roehrl VB, Roehrl MH. A Master Autoantigen-Ome Links Alternative Splicing, Female Predilection, and COVID-19 to Autoimmune Diseases. bioRxiv (2021) 2021.07.30.454526. doi: 10.1101/2021.07.30.454526
86. Wang JY, Zhang W, Roehrl MW, Roehrl VB, Roehrl MH. An Autoantigen Profile From Jurkat T-Lymphoblasts Provides a Molecular Guide for Investigating Autoimmune Sequelae of COVID-19. bioRxiv (2021) 2021.07.05.451199. doi: 10.1101/2021.07.05.451199
87. Wang JY, Zhang W, Roehrl VB, Roehrl MW, Roehrl MH. An Autoantigen-Ome From HS-Sultan B-Lymphoblasts Offers a Molecular Map for Investigating Autoimmune Sequelae of COVID-19. bioRxiv (2021) 2021.04.05.438500. doi: 10.1101/2021.04.05.438500
88. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci Transl Med (2013) 5:140r–78r. doi: 10.1126/scitranslmed.3005580
89. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA (2020) 324:782–93. doi: 10.1001/jama.2020.12839
90. Teijeira A, Garasa S, Ochoa MC, Villalba M, Olivera I, Cirella A, et al. IL8, Neutrophils, and NETs in a Collusion Against Cancer Immunity and Immunotherapy. Clin Cancer Res (2021) 27:2383–93. doi: 10.1158/1078-0432.CCR-20-1319
91. Zhang Y, Chandra V, Riquelme SE, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17-Induced Neutrophil Extracellular Traps Mediate Resistance to Checkpoint Blockade in Pancreatic Cancer. J Exp Med (2020) 217:e20190354. doi: 10.1084/jem.20190354
92. Chen L, Zhao Y, Lai D, Zhang P, Yang Y, Li Y, et al. Neutrophil Extracellular Traps Promote Macrophage Pyroptosis in Sepsis. Cell Death Dis (2018) 9:597. doi: 10.1038/s41419-018-0538-5
93. Hussman JP. Cellular and Molecular Pathways of COVID-19 and Potential Points of Therapeutic Intervention. Front Pharmacol (2020) 11:1169. doi: 10.3389/fphar.2020.01169
94. Vaillancourt M, Jorth P. The Unrecognized Threat of Secondary Bacterial Infections With COVID-19. Mbio (2020) 11:e01806-20. doi: 10.1128/mBio.01806-20
95. Kreitmann L, Monard C, Dauwalder O, Simon M, Argaud L. Early Bacterial Co-Infection in ARDS Related to COVID-19. Intensive Care Med (2020) 46:1787–9. doi: 10.1007/s00134-020-06165-5
96. de Buhr N, von Kockritz-Blickwede M. The Balance of Neutrophil Extracellular Trap Formation and Nuclease Degradation: An Unknown Role of Bacterial Coinfections in COVID-19 Patients? Mbio (2021) 12:e03304-20. doi: 10.1128/mBio.03304-20
97. de Buhr N, Bonilla MC, Pfeiffer J, Akhdar S, Schwennen C, Kahl BC, et al. Degraded Neutrophil Extracellular Traps Promote the Growth of Actinobacillus Pleuropneumoniae. Cell Death Dis (2019) 10:657. doi: 10.1038/s41419-019-1895-4
98. Mohanty T, Fisher J, Bakochi A, Neumann A, Cardoso J, Karlsson C, et al. Neutrophil Extracellular Traps in the Central Nervous System Hinder Bacterial Clearance During Pneumococcal Meningitis. Nat Commun (2019) 10:1667. doi: 10.1038/s41467-019-09040-0
99. Mahase E. Covid-19: What Do We Know About “Long Covid”? BMJ (2020) 370:m2815. doi: 10.1136/bmj.m2815
100. Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-Term and Long-Term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open (2021) 4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568
101. Sapkota HR, Nune A. Long COVID From Rheumatology Perspective - a Narrative Review. Clin Rheumatol (2021) 41:337–48. doi: 10.1007/s10067-021-06001-1
102. Crook H, Raza S, Nowell J, Young M, Edison P. Long Covid-Mechanisms, Risk Factors, and Management. BMJ (2021) 374:n1648. doi: 10.1136/bmj.n1648
103. Sawadogo SA, Dighero-Kemp B, Ouedraogo DD, Hensley L, Sakande J. How NETosis Could Drive “Post-COVID-19 Syndrome” Among Survivors. Immunol Lett (2020) 228:35–7. doi: 10.1016/j.imlet.2020.09.005
104. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-Month Consequences of COVID-19 in Patients Discharged From Hospital: A Cohort Study. Lancet (2021) 397:220–32. doi: 10.1016/S0140-6736(20)32656-8
105. Giacomelli C, Piccarducci R, Marchetti L, Romei C, Martini C. Pulmonary Fibrosis From Molecular Mechanisms to Therapeutic Interventions: Lessons From Post-COVID-19 Patients. Biochem Pharmacol (2021) 193:114812. doi: 10.1016/j.bcp.2021.114812
106. Pandolfi L, Bozzini S, Frangipane V, Percivalle E, De Luigi A, Violatto MB, et al. Neutrophil Extracellular Traps Induce the Epithelial-Mesenchymal Transition: Implications in Post-COVID-19 Fibrosis. Front Immunol (2021) 12:663303. doi: 10.3389/fimmu.2021.663303
107. Suzuki M, Ikari J, Anazawa R, Tanaka N, Katsumata Y, Shimada A, et al. PAD4 Deficiency Improves Bleomycin-Induced Neutrophil Extracellular Traps and Fibrosis in Mouse Lung. Am J Respir Cell Mol Biol (2020) 63:806–18. doi: 10.1165/rcmb.2019-0433OC
108. Negreros M, Flores-Suarez LF. A Proposed Role of Neutrophil Extracellular Traps and Their Interplay With Fibroblasts in ANCA-Associated Vasculitis Lung Fibrosis. Autoimmun Rev (2021) 20:102781. doi: 10.1016/j.autrev.2021.102781
109. The LN. Long COVID: Understanding the Neurological Effects. Lancet Neurol (2021) 20:247. doi: 10.1016/S1474-4422(21)00059-4
110. Jarrahi A, Ahluwalia M, Khodadadi H, Da SLSE, Kolhe R, Hess DC, et al. Neurological Consequences of COVID-19: What Have We Learned and Where Do We Go From Here? J Neuroinflamm (2020) 17:286. doi: 10.1186/s12974-020-01957-4
111. Williams S, Wynford-Thomas R, Robertson NP. Long-COVID: Neurological Manifestations and Management. J Neurol (2021) 268:4915–7. doi: 10.1007/s00415-021-10847-5
112. Lou M, Yuan D, Liao S, Tong L, Li J. Potential Mechanisms of Cerebrovascular Diseases in COVID-19 Patients. J Neurovirol (2021) 27:35–51. doi: 10.1007/s13365-021-00948-2
113. Pramitasuri TI, Laksmidewi A, Putra I, Dalimartha FA. Neutrophil Extracellular Traps in Coronavirus Disease-19-Associated Ischemic Stroke: A Novel Avenue in Neuroscience. Exp Neurobiol (2021) 30:1–12. doi: 10.5607/en20048
114. Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer Cell (2020) 38:629–46. doi: 10.1016/j.ccell.2020.09.018
115. Pathania AS, Prathipati P, Abdul BA, Chava S, Katta SS, Gupta SC, et al. COVID-19 and Cancer Comorbidity: Therapeutic Opportunities and Challenges. Theranostics (2021) 11:731–53. doi: 10.7150/thno.51471
116. Saini G, Aneja R. Cancer as a Prospective Sequela of Long COVID-19. Bioessays (2021) 43:e2000331. doi: 10.1002/bies.202000331
117. Huang H, Zhang H, Onuma AE, Tsung A. Neutrophil Elastase and Neutrophil Extracellular Traps in the Tumor Microenvironment. Adv Exp Med Biol (2020) 1263:13–23. doi: 10.1007/978-3-030-44518-8_2
118. Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil Extracellular Traps Produced During Inflammation Awaken Dormant Cancer Cells in Mice. Science (2018) 361:eaao4227. doi: 10.1126/science.aao4227
119. Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, et al. DNA of Neutrophil Extracellular Traps Promotes Cancer Metastasis via CCDC25. Nature (2020) 583:133–8. doi: 10.1038/s41586-020-2394-6
120. Xia F, Zhang M, Cui B, An W, Chen M, Yang P, et al. COVID-19 Patients With Hypertension Are at Potential Risk of Worsened Organ Injury. Sci Rep (2021) 11:3779. doi: 10.1038/s41598-021-83295-w
121. Clemmensen C, Petersen MB, Sorensen T. Will the COVID-19 Pandemic Worsen the Obesity Epidemic? Nat Rev Endocrinol (2020) 16:469–70. doi: 10.1038/s41574-020-0387-z
122. Khunti K, Davies MJ, Kosiborod MN, Nauck MA. Long COVID - Metabolic Risk Factors and Novel Therapeutic Management. Nat Rev Endocrinol (2021) 17:379–80. doi: 10.1038/s41574-021-00495-0
123. D’Abbondanza M, Martorelli EE, Ricci MA, De Vuono S, Migliola EN, Godino C, et al. Increased Plasmatic NETs by-Products in Patients in Severe Obesity. Sci Rep (2019) 9:14678. doi: 10.1038/s41598-019-51220-x
124. Strich JR, Ramos-Benitez MJ, Randazzo D, Stein SR, Babyak A, Davey RT, et al. Fostamatinib Inhibits Neutrophils Extracellular Traps Induced by COVID-19 Patient Plasma: A Potential Therapeutic. J Infect Diseases (2021) 223:981–4. doi: 10.1093/infdis/jiaa789
125. Zhang Y, Han K, Du C, Li R, Liu J, Zeng H, et al. Carboxypeptidase B Blocks Ex Vivo Activation of the Anaphylatoxin-Neutrophil Extracellular Trap Axis in Neutrophils From COVID-19 Patients. Crit Care (2021) 25:51. doi: 10.1186/s13054-021-03482-z
126. Delaveris CS, Wilk AJ, Riley NM, Stark JC, Yang SS, Rogers AJ, et al. Synthetic Siglec-9 Agonists Inhibit Neutrophil Activation Associated With COVID-19. ACS Cent Sci (2021) 7:650–7. doi: 10.1021/acscentsci.0c01669
127. Java A, Apicelli AJ, Liszewski MK, Coler-Reilly A, Atkinson JP, Kim AHJ, et al. The Complement System in COVID-19: Friend and Foe? JCI Insight (2020) 5:e140711. doi: 10.1172/jci.insight.140711
128. Jodele S, Kohl J. Tackling COVID-19 Infection Through Complement-Targeted Immunotherapy. Br J Pharmacol (2021) 178:2832–48. doi: 10.1111/bph.15187
129. Chen X, Guo H, Qiu L, Zhang C, Deng Q, Leng Q. Immunomodulatory and Antiviral Activity of Metformin and Its Potential Implications in Treating Coronavirus Disease 2019 and Lung Injury. Front Immunol (2020) 11:2056. doi: 10.3389/fimmu.2020.02056
130. Ibrahim S, Lowe JR, Bramante CT, Shah S, Klatt NR, Sherwood N, et al. Metformin and Covid-19: Focused Review of Mechanisms and Current Literature Suggesting Benefit. Front Endocrinol (2021) 12:587801. doi: 10.3389/fendo.2021.587801
131. Dalan R. Metformin, Neutrophils and COVID-19 Infection. Diabetes Res Clin Pr (2020) 164:108230. doi: 10.1016/j.diabres.2020.108230
132. Gustine JN, Jones D. Immunopathology of Hyperinflammation in COVID-19. Am J Pathol (2021) 191:4–17. doi: 10.1016/j.ajpath.2020.08.009
133. Khiali S, Rezagholizadeh A, Entezari-Maleki T. A Comprehensive Review on Sarilumab in COVID-19. Expert Opin Biol Th (2021) 21:615–26. doi: 10.1080/14712598.2021.1847269
134. Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern Med (2021) 181:32–40. doi: 10.1001/jamainternmed.2020.6820
135. Bae M, Kim H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System Against COVID-19. Molecules (2020) 25:5346. doi: 10.3390/molecules25225346
136. Hamam HJ, Palaniyar N. Post-Translational Modifications in NETosis and NETs-Mediated Diseases. Biomolecules (2019) 9:369. doi: 10.3390/biom9080369
137. Thierry AR. Anti-Protease Treatments Targeting Plasmin(Ogen) and Neutrophil Elastase May Be Beneficial in Fighting COVID-19. Physiol Rev (2020) 100:1597–8. doi: 10.1152/physrev.00019.2020
138. Elliott W, Guda MR, Asuthkar S, Teluguakula N, Prasad DVR, Tsung AJ, et al. PAD Inhibitors as a Potential Treatment for SARS-CoV-2 Immunothrombosis. Biomedicines (2021) 9:1867. doi: 10.3390/biomedicines9121867
139. Weber AG, Chau AS, Egeblad M, Barnes BJ, Janowitz T. Nebulized in-Line Endotracheal Dornase Alfa and Albuterol Administered to Mechanically Ventilated COVID-19 Patients: A Case Series. Mol Med (2020) 26:91. doi: 10.1186/s10020-020-00215-w
140. Lee YY, Park HH, Park W, Kim H, Jang JG, Hong KS, et al. Long-Acting Nanoparticulate DNase-1 for Effective Suppression of SARS-CoV-2-Mediated Neutrophil Activities and Cytokine Storm. Biomaterials (2021) 267:120389. doi: 10.1016/j.biomaterials.2020.120389
141. Park HH, Park W, Lee YY, Kim H, Seo HS, Choi DW, et al. Bioinspired DNase-I-Coated Melanin-Like Nanospheres for Modulation of Infection-Associated NETosis Dysregulation. Adv Sci (Weinh) (2020) 7:2001940. doi: 10.1002/advs.202001940
142. Morser J, Shao Z, Nishimura T, Zhou Q, Zhao L, Higgins J, et al. Carboxypeptidase B2 and N Play Different Roles in Regulation of Activated Complements C3a and C5a in Mice. J Thromb Haemost (2018) 16:991–1002. doi: 10.1111/jth.13964
143. Yang L, Liu L, Zhang R, Hong J, Wang Y, Wang J, et al. IL-8 Mediates a Positive Loop Connecting Increased Neutrophil Extracellular Traps (NETs) and Colorectal Cancer Liver Metastasis. J Cancer (2020) 11:4384–96. doi: 10.7150/jca.44215
144. Mohammed BM, Fisher BJ, Kraskauskas D, Farkas D, Brophy DF, Fowler AR, et al. Vitamin C: A Novel Regulator of Neutrophil Extracellular Trap Formation. Nutrients (2013) 5:3131–51. doi: 10.3390/nu5083131
145. Arisan ED, Uysal-Onganer P, Lange S. Putative Roles for Peptidylarginine Deiminases in COVID-19. Int J Mol Sci (2020) 21:4662. doi: 10.3390/ijms21134662
Keywords: NETosis, NETs, COVID-19, immunothrombosis, post COVID-19 syndrome, immunopathology
Citation: Zhu Y, Chen X and Liu X (2022) NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond. Front. Immunol. 13:838011. doi: 10.3389/fimmu.2022.838011
Received: 17 December 2021; Accepted: 08 February 2022;
Published: 02 March 2022.
Edited by:
Bart Tummers, St. Jude Children’s Research Hospital, United StatesReviewed by:
José Jiram Torres-Ruiz, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoCopyright © 2022 Zhu, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Liu, bGl1eDA3MDRAdG1tdS5lZHUuY24=
†These authors have contributed equally to this work
 Yuanfeng Zhu
Yuanfeng Zhu Xiaoli Chen
Xiaoli Chen Xin Liu
Xin Liu