
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 25 February 2022
Sec. Inflammation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.837588
This article is part of the Research Topic Insights in Inflammation: 2021 View all 18 articles
 Saerok Shim1†
Saerok Shim1† Siyoung Lee1†
Siyoung Lee1† Yasmin Hisham1†
Yasmin Hisham1† Sinae Kim1,2
Sinae Kim1,2 Tam T. Nguyen1,2
Tam T. Nguyen1,2 Afeisha S. Taitt1
Afeisha S. Taitt1 Jihyeong Hwang1
Jihyeong Hwang1 Hyunjhung Jhun3
Hyunjhung Jhun3 Ho-Young Park4
Ho-Young Park4 Youngmin Lee5
Youngmin Lee5 Su Cheong Yeom6
Su Cheong Yeom6 Sang-Yeob Kim7
Sang-Yeob Kim7 Yong-Gil Kim8*
Yong-Gil Kim8* Soohyun Kim1,2*
Soohyun Kim1,2*Cytokines are significantly associated with the homeostasis of immune responses in health and disease. Interleukin-32 (IL-32) is a cytokine originally discovered in natural killer cell transcript 4. IL-32 with different disorders has been described in terms of pathogenesis and the progression of diseases. Clinical studies have investigated IL-32 under various conditions, such as viral infection, autoimmune diseases, inflammatory diseases, certain types of cancer, vascular disease, and pulmonary diseases. The high expression of IL-32 was identified in different tissues with various diseases and found to have multiple transcripts of up to seven isoforms. However, the purification and biological activities of these isoforms have not been investigated yet. Therefore, in this study, we purified and compared the biological activity of recombinant IL-32 (rIL-32) isoforms. This is the first time for seven rIL-32 isoforms (α, β, δ, γ, ϵ, ζ, and θ) to be cloned and purified using an Escherichia coli expression system. Next, we evaluate the biological activities of these seven rIL-32 isoforms, which were used to treat different types of cells by assessing the levels of inflammatory cytokine production. The results revealed that rIL-32θ possessed the most dominant biological activity in both immune and non-immune cells.
Interleukin-32 (IL-32) cytokine was cloned in 1992 from natural killer cells and was formerly named natural killer cell transcript 4 (NK4). NK4 was renamed IL-32 in 2005 because it has a cytokine property (1, 2). It was found to induce several inflammatory cytokines, such as tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), macrophage inflammatory protein-2 (MIP2), interleukin-8 (IL-8), and interleukin-1 beta (IL-1β), and IL-32 acts like a proinflammatory cytokine (1–3).
Nevertheless, since its discovery, much knowledge remains to be determined. For the most part, its specific surface receptor has yet to be defined. Proteinase 3 (PR3) binds to IL-32 with very high affinity (4). PR3 is a serine proteinase produced from neutrophils as an enzyme, whereas it is also expressed on the membrane of monocytes. The possibility of IL-32 binding to integrins has been suggested (5), and this result was based on its amino acid composition containing an RGD motif, which ubiquitously presents in various genes. The IL-32 amino acid sequence has no known cytokine homolog; in addition, IL-32 was detected in most mammals except rodents (6).
A previous study reported that the IL-32 gene is composed of eight exons and presents within human chromosome 16p13.3 (1). According to its alternative splicing sites, more than seven transcripts have been suggested. However, seven isoforms with nine exons were described to be translated from its messenger RNA transcript (7). These isoforms are IL-32α, IL-32β, IL-32γ, IL-32δ, IL-32ε, IL-32ζ, and IL-32θ. As each isoform was discovered separately, the cell type, condition, and isoform function were varied. IL-32α, IL-32β, IL-32γ, and IL-32δ were mainly identified in IL-2-stimulated human NK cells; on the other hand, IL-32ε and IL-32ζ were found to be expressed in activated T cells (8). Lastly, IL-32θ was discovered from dendritic cells and Jurkat cells of human leukemia T cell line (9). These isoforms exhibited distinct effects in different conditions. Among the seven IL-32 isoforms, IL-32γ is the most-studied isoform, which also has the longest amino acid sequence.
IL-32 plays a vital modulator role in the pathogenesis of different diseases. Its involvement has been reported in various cancers, infections, and autoimmune and inflammatory disorders (6, 10, 11). Most autoimmune and inflammatory diseases associated with IL-32 are rheumatoid arthritis (RA), inflammatory bowel disease (IBD), psoriasis, chronic obstructive pulmonary disease (COPD), and asthma (3, 7, 10, 12–14). However, these clinical studies determined the levels of circulating IL-32 and then compared the patients to healthy controls. These studies fail to characterize the differences in IL-32 isoforms. However, the protein identification of each IL-32 isoform is subjected to a significant limitation because of the lack of a specific antibody to detect the IL-32 variant. Moreover, IL-32 secreted proteins are not easily purified since the structures of IL-32 isoforms are not thoroughly appraised.
At present, we were able to purify seven rIL-32 isoforms: with IL-32α, -β, -δ, and -γ purified in our previous study, whereas IL-32ϵ, -ζ, and -θ were puried for the first time in this study. Next, we assessed the biological activities of the seven rIL-32 isoforms in various cells by examining the production of inflammatory cytokines, such as IL-6, IL-8, TNFα, and MIP2. Seven rIL-32 isoforms show a different biological activity regarding different cell types.
All seven isoforms were cloned into pPROEX/HTa from Takara (Shiga, Japan) as previously described (8). IL-32α, -β, -γ, and -δ isoforms were cloned earlier, and the remaining three IL-32ϵ, -ζ, and -θ isoforms were constructed in this study for the first time using the closest isoform as the template, as shown in Figure 1. Briefly, pPROEX/HTa IL-32β plasmid was used to construct IL-32ζ and -θ. Then, IL-32θ was used to construct IL-32ϵ (Figure 1C). For the construction of a new isoform plasmid vector, we used overlap extension PCR with primers as indicated in Figure 1C. All PCR products were designed to have EcoRI and XbaI restriction enzyme sites in their 5′ and 3′ ends. Next, the PCR products were ligated into an expression vector using EcoRI and XbaI restriction enzymes (Takara) and confirmed by DNA sequencing analysis in Cosmogen (Seoul, Korea). These expression vectors were transformed into BL21-Codon Plus from Stratagene (San Diego, CA, USA) by heat shock method.
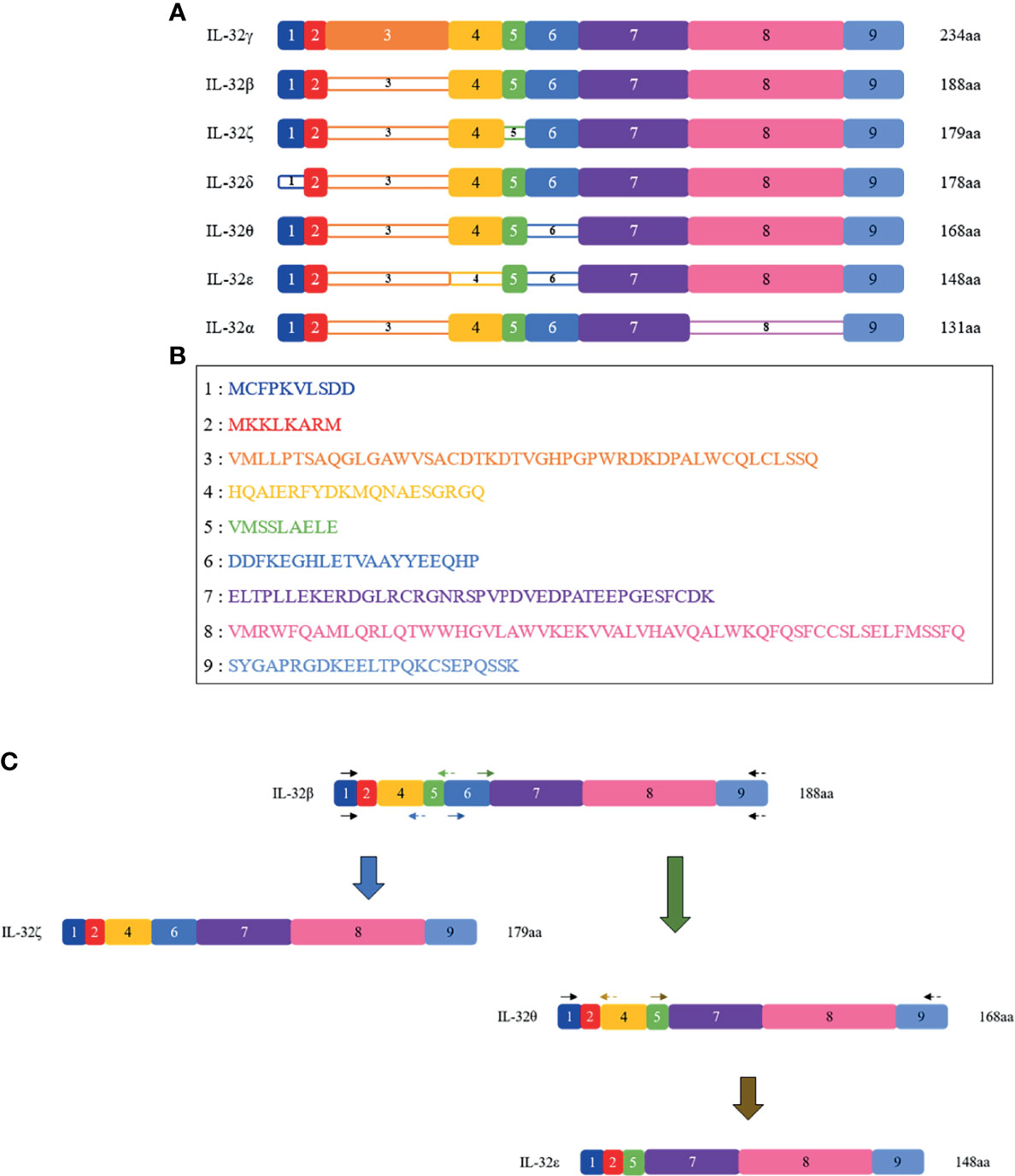
Figure 1 Schematic representations of the seven IL-32 isoforms. (A) Seven isoforms of IL-32 are shown with their present/absent domains. The name of each isoform is shown on the left, and their length in amino acid (aa) is on the right. The colored, numbered box represents exons from 1 to 9, numbered in line with the order of N-terminal; dense-colored boxes are indicated for the existing exons and decolored boxes for the absent exon. Starting from the longest isoform, IL-32γ isoform is represented on the top as it is the longest variant with 234 amino acid residues and the only one that contains all 9 exons, while on the bottom the shortest variant is present, which is IL-32α isoform with 131 amino acid residues. (B) Amino acid sequence of each exon. (C) Schematic PCR-based construction of IL-32ζ, IL-32θ, and IL-32ϵ; using IL-32β as a template, IL-32ζ and θ were built. Next, using IL-32θ as a template, IL-32ϵ was built. The black arrows represent outer primers and are the same in all constructs; the blue, green, and gold arrows represent the inner primers used to build IL-32ζ, IL-32θ, and IL-32ϵ, respectively. The forward primers are indicated as solid arrows, while the reverse primers are indicated as dashed arrows.
Seven recombinant rIL-32 (α, -β, -γ, -δ, -ϵ, -ζ, and -θ) proteins were expressed in E. coli with 4-h isopropyl β-D-1-thiogalactopyranoside induction at 37°C. rIL-32β, -γ, and -θ were purified with Ni-NTA agarose from Qiagen (Hilden, Germany), and the others were purified with TALON® Magnetic Bead (Takara) using his6-tag at the N-terminus of rIL-32 isoform proteins. Among the affinity-purified proteins, rIL-32β, -γ, and -ϵ were subjected to a high-performance liquid chromatography column from Grace (Stockbridge, GA), and rIL-32α, -δ, -ζ, and -θ were subjected to an anion exchange column (HiTrap Q FF, 1 ml) from GE Healthcare (Chicago, IL, USA). After that, we checked their concentration by silver staining, Bradford assay, and BCA assay. Next, to check the bands of purified rIL-32 isoform proteins, we did western blotting with mouse anti-his6-tag mAb from R&D system (Minneapolis, MN, USA). The rIL-32 proteins were tested with a LAL chromogenic endotoxin quantitation kit from Thermo Fisher (Waltham, MA, USA). The endotoxin level was below 0.5 EU per 1 μg of rIL-32 protein, which is approximately 0.05 ng in 1 μg of rIL-32.
The expression levels of IL-32 in normal tissues were identified using GTEx Portal (https://www.gtexportal.org/home/).
THP-1 and Raw 264.7, A459 cell lines were obtained from ATCC (Manassas, VA, USA). The THP-1 monocytes and Raw 264.7 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 μg/ml penicillin, and 100 μg/ml streptomycin. A549 was cultured in Ham’s F12K medium containing the same reagents. Mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium containing the same reagents. All cell culture media were from Welgene Biotech (Taipei, Taiwan). The culture condition was as follows: under humidified 5% CO2 at 37°C. THP-1 (2.5 × 104/well), Raw 264.7 (5.0 × 104/well), and A549 (2.5 × 104/well) were seeded in a 96-well plate of 100 μl volume. THP-1 and Raw 264.7 were treated with different concentrations of purified rIL-32 isoforms (11.1, 33.3, and 100 ng/ml) in 100-μl-volume media, and the control was treated with the media alone. A549 and MEFs were treated with different concentrations of purified rIL-32 isoforms (100, 200, and 1,000 ng/ml) in 100-μl-volume media, and the control was treated with media alone. After 18 h of stimulation, the supernatants of THP-1 and Raw 264.7 were assessed for human IL-8 and mouse TNFα measurements, respectively. The A549 and MEF supernatants were assessed for human and mouse IL-6 measurements, respectively. All tested cytokines were determined by ELISA kits (R&D system).
To verify that rIL-32 isoforms induced various cytokines in primary cells, we prepared splenocytes, bone marrow cells, and lung cells from C57BL/6 from Orient Bio (Seoul, Korea). All animal procedures were reviewed and approved by the Konkuk University Institutional Animal Care Committee. A C57BL/6 mouse was dissected, and the spleen, bone, and lung were isolated. We mashed the spleens and collected bone marrow cells from bones. These were centrifugated, washed with Dulbecco’s phosphate-buffered saline, and suspended in RPMI1640 medium supplemented with 10% FBS, 100 μg/ml penicillin, and 100 μg/ml streptomycin. In the case of lung cells, these were chopped, centrifugated, treated with collagenase V, and suspended in RPMI 1640 medium supplemented with 10% FBS, 100 μg/ml penicillin, and 100 μg/ml streptomycin. The MEF cells were prepared as follows: the fetus was isolated at 13.5 days of pregnancy. The fetus was chopped and digested with trypsin and DNase 1 and then suspended and cultured in DMEM medium supplemented with 10% FBS, 100 μg/ml penicillin, and 100 μg/ml streptomycin. The isolated primary mouse cells were seeded as follows: splenocytes (4.0 × 106/well), bone marrow cells (1.0 × 106/well), lung cells (2.5 × 104/well), and MEFs (2.5 × 104/well). After having been stimulated for 18 h with rIL-32 isoforms, mouse TNFα, mouse IL-6, and mouse MIP2 were assessed by using the ELISA set (R&D system).
All data were analyzed by Graph Pad Prism v.9 to perform one-way or two-way ANOVA, followed by Tukey’s post-hoc analysis. P-values <0.05 were considered statistically significant and were indicated in the figure legends.
Seven IL-32 isoforms were constructed and cloned as shown in Figure 1A. IL-32 was divided into 9 small domains, and domain 8 is the longest. The amino acid sequence of each domain was illustrated with different colors, as shown in Figure 1B, corresponding to the color of the domain in Figure 1A. Each isoform of complete open reading frame in pPROEX/HTa E. coli had its expression vector confirmed by DNA sequencing. Multi-step (his6-tag purification and ion-exchange chromatography or high-performance liquid chromatography) purification was employed to obtain seven pure isoforms of rIL-32 protein. Figure 2A shows the 10% SDS-PAGE analysis of rIL-32 isoforms, with the dominant bands of each isoform corresponding to its theoretical molecular size as follows: IL-32α: 19.8 kDa, IL-32β: 25.5 kDa, IL-32γ: 31.5 kDa, IL-32δ: 24.2 kDa, IL-32ϵ: 20.7 kDa, IL-32ζ: 24.4 kDa, and IL-32θ: 23.0 kDa, plus 5.4 kDa of N-terminus his6-tag. All seven rIL-32 isoforms were migrated slowly; therefore, the molecular weight in silver staining was slightly higher than the actual molecular weight of each rIL-32 isoform. In addition to this, some rIL-32 isoforms appeared as a dimer and multiple bands. To confirm whether these bands were purified rIL-32 from E. coli or not, we did a western blot analysis using mouse anti-his6 tag mAb. As shown in Figure 2B, all protein bands in silver staining were found to be bound with mouse anti-his6 tag mAb to confirm the purity of the final seven rIL-32 isoform proteins.
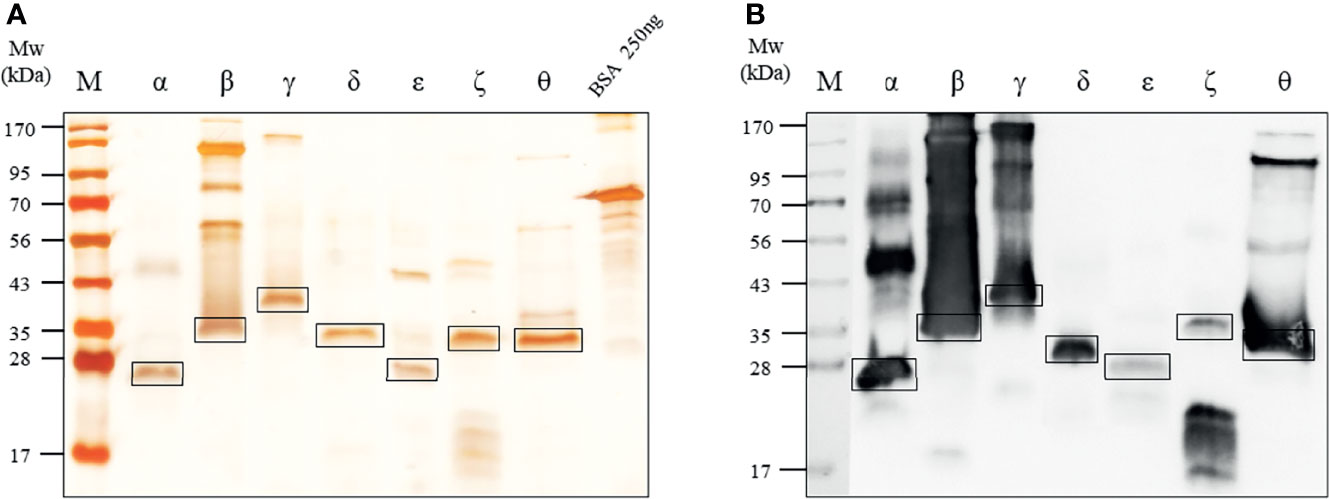
Figure 2 Expression of seven rIL-32 isoform proteins. (A) 10% SDS-PAGE analysis followed by silver staining for the seven purified rIL-32 isoforms of rIL-32 after a multi-step purification procedure compared with a known concentration of bovine serum albumin. The molecular weight (kDa) and rIL-32 isoforms are indicated at the top of their respective lanes in the following arrangement: α, β, γ, δ, ϵ, ζ, and θ. kDa; kilodalton. (B) Western blot analysis for seven rIL-32 proteins were loaded and probed with mouse anti-his6 tag mAb.
It has been reported that the expression of IL-32 cytokine is increased in a variety of inflammatory autoimmune diseases and certain infections and cancers. We evaluated the expression of IL-32 in normal tissues using GTEx portal. Additionally, IL-32 is expressed in many cell types, including immune and non-immune cells, exhibiting different activities, which may be due to differences in cell types and/or stimulus and different isoform expressions related to cell types. However, a comparison of the activity of IL-32 isoforms has not been elucidated. IL-32 expression in normal tissues revealed that the highest expression of IL-32 was found in the spleen, followed by Epstein–Barr virus-transformed lymphocytes and then lung tissues (Figure 3). Therefore, we compared the activity of the seven IL-32 isoforms in several cell types, including immune cells, lung cells, and fibroblasts. THP-1 (human-derived monocytes) and Raw 264.7 (mouse-derived monocytes/macrophages) cell lines were used to evaluate the biological activity of rIL-32 isoforms. Primary mouse bone marrow and splenocytes were also isolated to evaluate the biological activity of rIL-32 isoforms. rIL-32 promotes the differentiation of monocytes into macrophage-like cells, inducing proinflammatory cytokines such as TNFα, IL-6, and IL-8 (15). Therefore, we treated the selected cell types with the seven rIL-32 isoforms and measured the cytokine productions to evaluate the biological activity of each rIL-32 isoform and determine the dominant isoform in each cell type.

Figure 3 Expression of IL-32 in normal tissue samples. IL-32 gene expression analysis on normal tissue using GTEx portal; values of expression are shown in transcript per million and are calculated from a gene model with the isoforms collapsed to a single gene. The box plots are shown as median and 25th and 75th percentiles; the outliers are defined as above or below 1.5 times the interquartile range and are shown as points. The highest IL-32 expression was found in the spleen, followed by Epstein–Barr virus-transformed lymphocytes, and lung, whereas the lowest was observed in all tissue types of the brain.
The biological activity of the seven purified rIL-32 isoforms was assessed in immune cells. First, THP-1 and Raw 264.7 were stimulated with the seven rIL-32 isoforms, and a cell culture supernatant was used to assess the levels of IL-8 and TNFα production, respectively (Figure 4). Moreover, mouse isolated primary bone marrow and splenocytes were stimulated with the seven rIL-32 isoforms. Next, IL-6 from bone marrow as well as IL-6, TNFα, and MIP2 from splenocytes were assessed (Figure 5). The production of the measured cytokines was significantly increased by three rIL-32 isoforms, which were rIL-32θ, -γ, and -β isoforms, in a dose-dependent manner. These results were consistent in immune cell lines (THP-1 and Raw 264.7) and primary mouse immune cells (splenocytes and bone marrow cells). At the same time, the remaining four (rIL-32-α, -δ, -ϵ, and -ζ) isoforms have a weak or no activity in cytokine production.
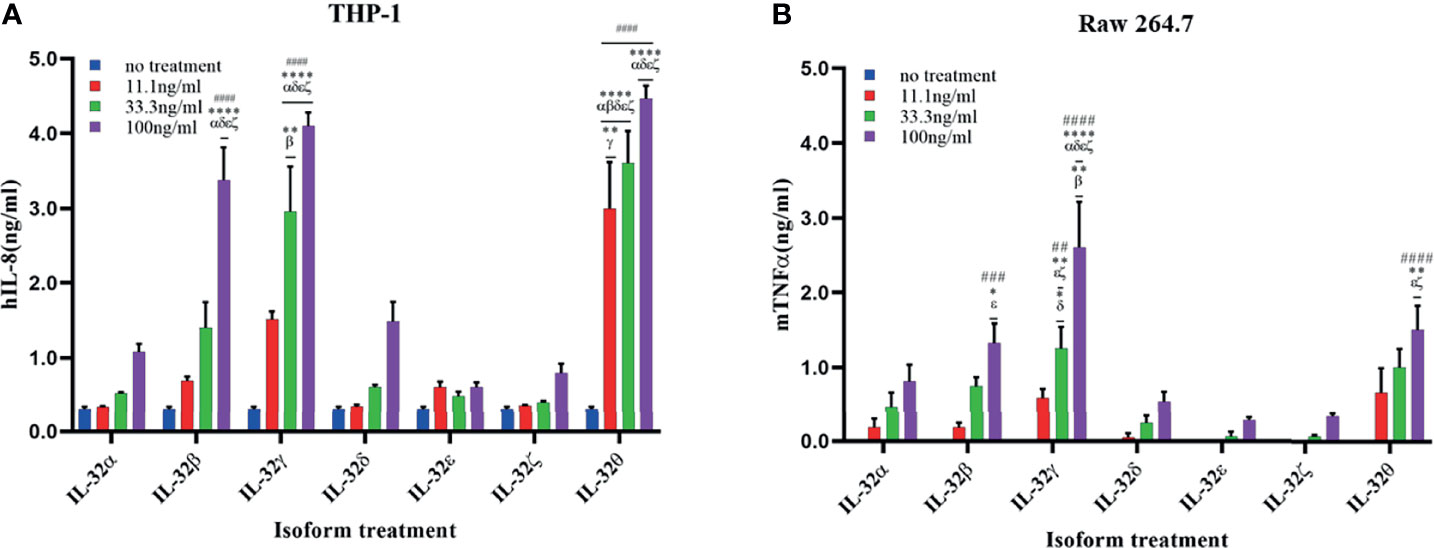
Figure 4 Biological activities of seven rIL-32 isoforms in immune cell lines. rIL-32 isoforms with different concentrations were treated in cells for 18 h. The levels of IL-8 and TNFα were measured in the supernatant of THP-1 (A) and Raw 264.7 (B), respectively, by using ELISA. The bar graph represents the level of cytokines, mean ± SEM. Statistical testing was performed using two-way ANOVA followed by Tukey’s post-hoc analysis. ##p < 0.01, ###p < 0.001, ####p <0.0001 as compared to no treatment control within the same isoform treatment. *p < 0.05, **p < 0.01, ****p < 0.0001 as compared to other displayed isoform symbols treated with the same concentration.
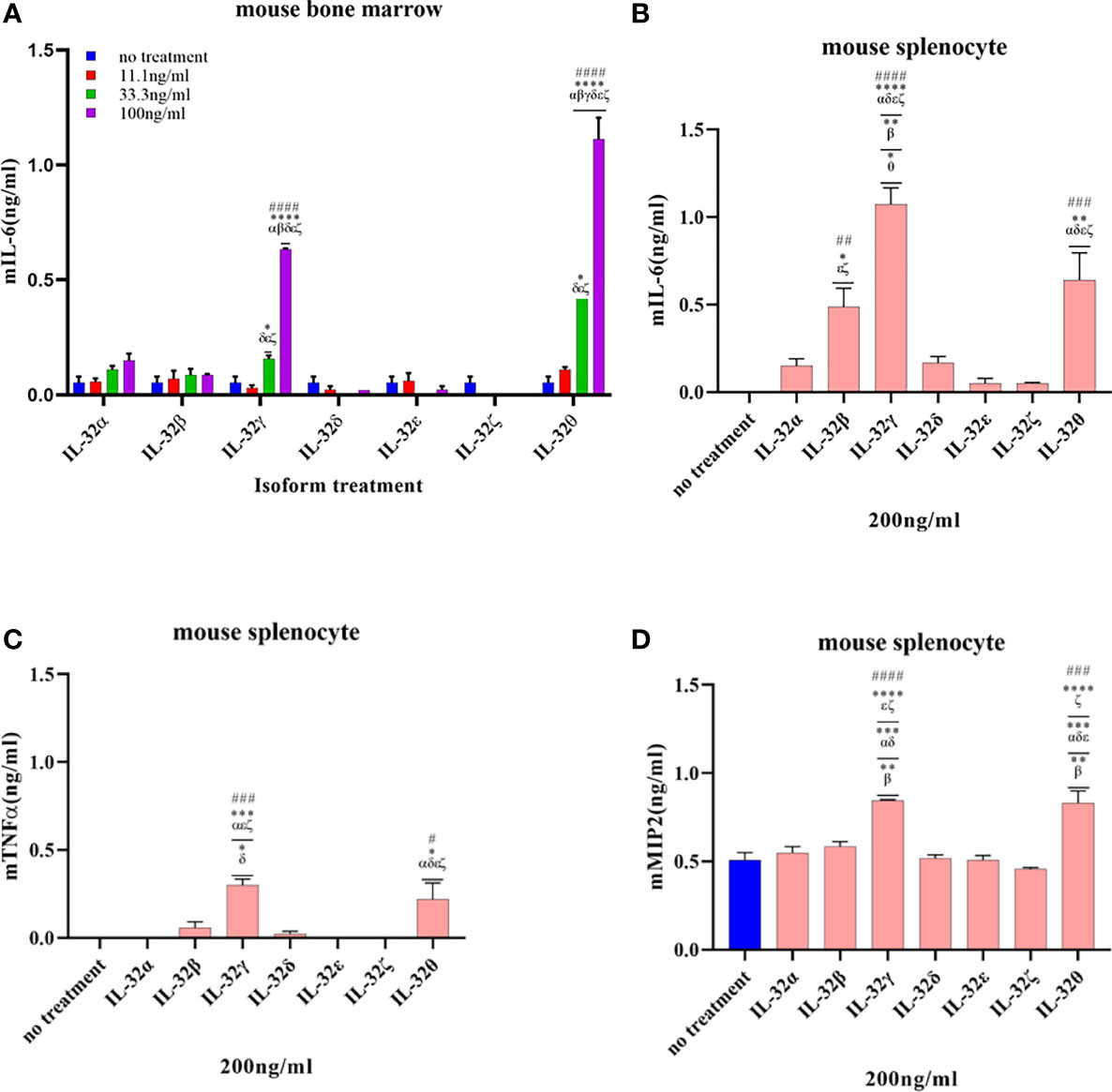
Figure 5 Biological activities of seven rIL-32 isoforms in the primary mouse immune cells. rIL-32 isoforms with different concentrations were treated for 18 h in mouse bone marrow. The level of IL-6 was measured by ELISA (A), and 200 ng/ml of each isoform was treated for 18 h in mouse splenocytes. The levels of IL-6, TNFα, and MIP2 were measured by ELISA [(B–D), respectively]. The bar graph represents the level of cytokines, mean ± SEM. Statistical testing was performed using two-way ANOVA (A) and one-way ANOVA (B–D), followed by Tukey’s post-hoc analysis. #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 as compared to the no-treatment control within the same isoform treatment. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as compared to other displayed isoform symbols treated with the same concentration.
In the case of THP-1 cells that were stimulated with different concentrations of rIL-32θ, these showed ±4 folds of IL-8 production compared to non-stimulated cells, and all three concentrations (11.1, 33.3, and 100 ng/ml) were significantly increasing the IL-8 levels, followed by rIL-32γ with concentrations of 33.3 and 100 ng/ml and then rIL-32β with a higher concentration only 100 ng/ml, which thus significantly induced IL-8 production. Interestingly, only rIL-32θ induced a significant amount of IL-8 production at a low concentration. Thus, rIL-32θ was considered the most potent rIL-32 isoform in this cell line. On the other hand, both rIL-32α and rIL-32δ showed ±1 ng/ml production of IL-8 at their highest concentration of 100 ng/ml. rIL-32ϵ and rIL-32ζ did not induce IL-8 production (Figure 4A).
The results from Raw 264.7 cells were similar to the THP-1 results, with one difference in the dominant isoform, which was rIL-32γ showing ±2.5 folds of TNFα production instead compared to non-stimulated cells at the concentrations of 33.3 and 100 ng/ml. However, rIL-32θ and rIL-32β induced significant TNFα production at 100 ng/ml (Figure 4B).
Next, the seven rIL-32 isoforms were treated in primary mouse bone marrow cells and splenocytes. The effect of each rIL-32 isoform on the production of cytokines is shown in Figure 5. In bone marrow cells, all rIL-32 isoforms at a low concentration (11.1 ng/ml) did not induce IL-6 production. Only two isoforms (rIL-32γ and rIL-32θ) induced the production of IL-6 at 33.3 and 100 ng/ml. However, the levels of IL-6 were significantly higher at 100 ng/ml of rIL-32θ, followed by rIL-32γ isoform (Figure 5A). Concurrently, rIL-32γ and rIL-32θ imply significantly higher IL-6, TNFα, and MIP2 in the primary mouse splenocytes as shown in Figures 5B–D, respectively.
Biological activity was assessed in human A549 lung cells and primary mouse lung cells. Both cells were treated with rIL-32 seven isoforms of different concentrations for 18 h; then, the levels of IL-6 were assessed (Figure 6). In both lung cells, rIL-32θ showed a highly significant production of IL-6 than the other six isoforms in a concentration-dependent manner. However, unlike immune cells, rIL-32γ showed weak or no biological activities in A549 (Figure 6A) and mouse isolated lung cells (Figure 6B), respectively. Moreover, rIL-32δ, -β, and -α demonstrated a significant biological activity at high concentrations, in terms of IL-6 production, only on A549 cells. The remaining isoforms, rIL-32ϵ and rIL-32ζ, still have a very weak activity on A549 cells.
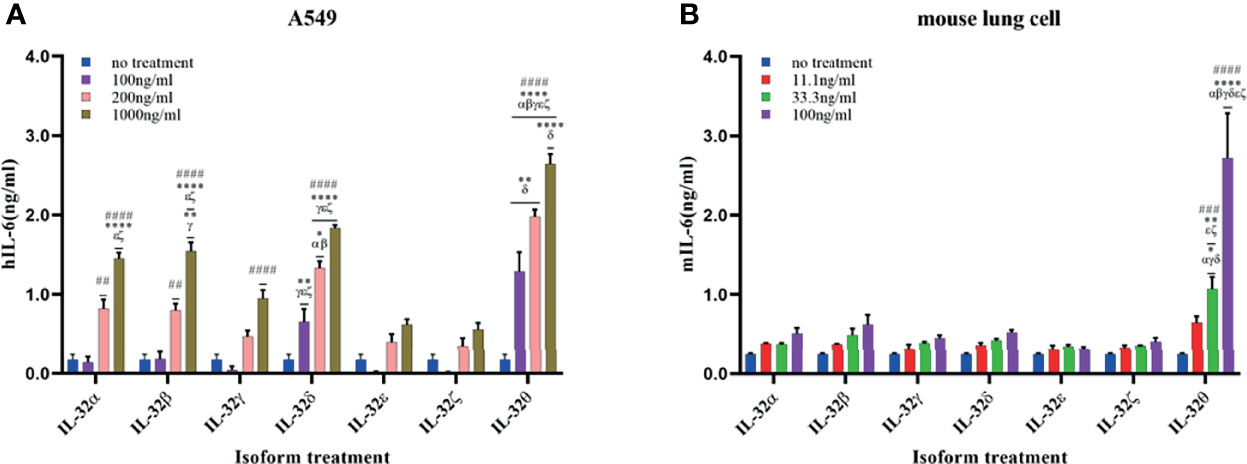
Figure 6 Biological activities of seven rIL-32 isoforms in lung cells. rIL-32 isoforms with different concentrations were treated for 18 h in human A549 lung cells (A) and primary mouse lung cells (B). IL-6 production was measured by ELISA. The bar graph represents the level of cytokines, mean ± SEM. Statistical testing was performed using two-way ANOVA, followed by Tukey’s post-hoc analysis. ##p < 0.01, ###p < 0.001, ####p < 0.0001 as compared to no-treatment control within the same isoform treatment. *p < 0.05, **p < 0.01, ****p < 0.0001 as compared to other displayed isoform symbols treated with the same concentration.
Fibroblasts are cells that are mainly accountable for maintaining the extracellular matrix and are found within many tissues and organs such as the skin and lungs. Therefore, we measured the production of IL-6 in MEF cells treated with different concentrations of the seven isoforms to assess their biological activity (Figure 7). Like the immune and lung cells, rIL-32θ showed the highest production of IL-6. Nevertheless, a significant induction was found only with the high concentration of isoform at 1,000 ng/ml. Moreover, IL-32δ and IL-32β also showed a significant production of IL-6 following rIL-32θ at the high concentration of 1,000 ng/ml. rIL-32α and rIL-32γ showed a slightly non-significant production of IL-6, whereas rIL-32ϵ and rIL-32ζ isoforms did not induce cytokine production.
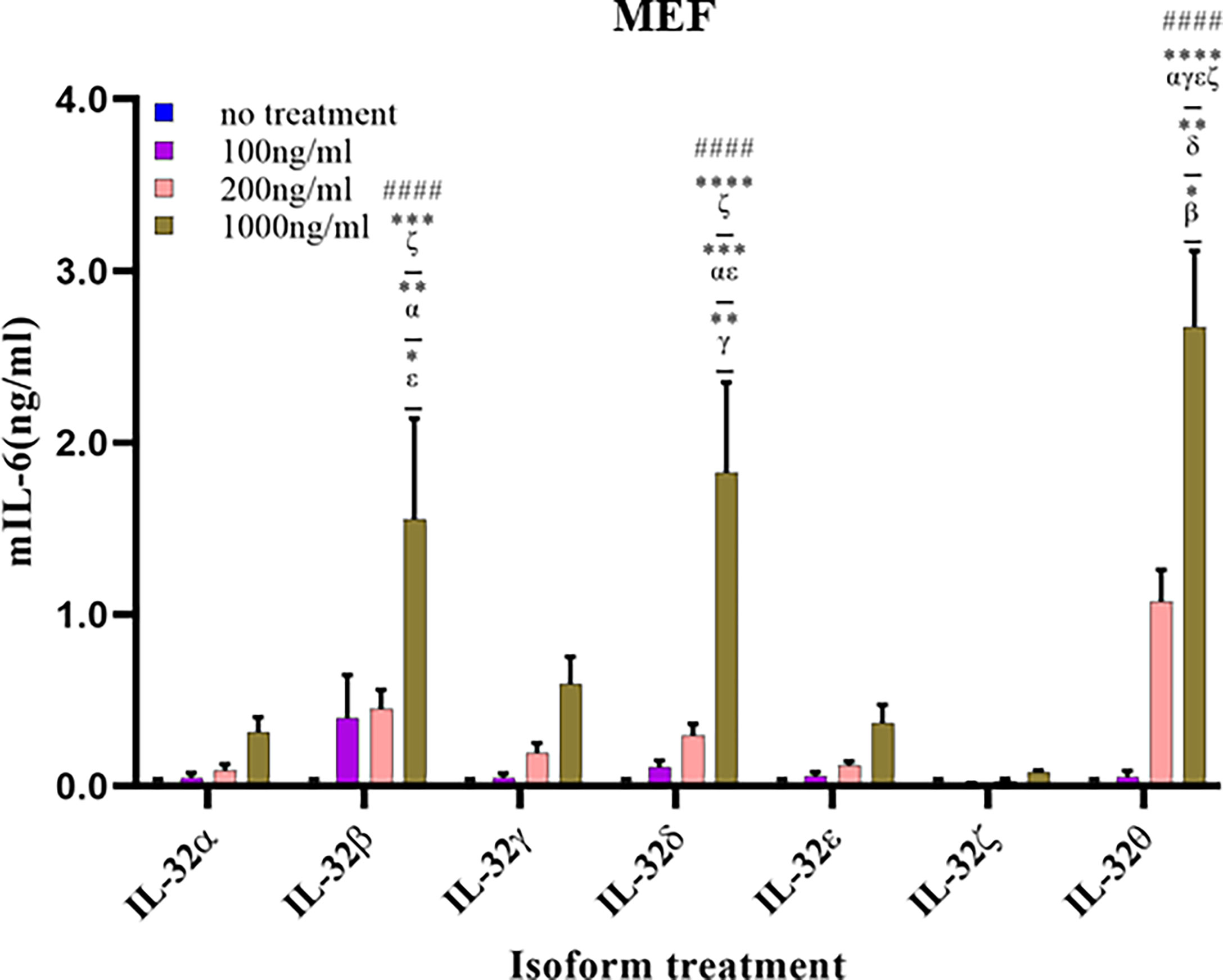
Figure 7 Biological activities of seven rIL-32 isoforms in mouse embryonic fibroblast. rIL-32 isoforms with different concentrations were treated for 18 h. The bar graph represents the level of IL-6 production, mean ± SEM, measured by ELISA. Statistical testing was performed using two-way ANOVA, followed by Tukey’s post-hoc analysis. ####p < 0.0001 as compared to no-treatment control within the same isoform treatment. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as compared to other displayed isoform symbols treated with the same concentration.
IL-32 is a novel multifunctional cytokine involved in various cell functions, differentiation, pro- or anti-inflammatory cytokines stimulation, and apoptosis (15–22). This cytokine promotes the induction of crucial inflammatory cytokines such as IL-1β, TNFα, IL-6, IL-8, and MIP2 (1, 2, 15, 23). Its expression engages numerous pathogenesis disorders, including inflammatory, autoimmune diseases, cancers, and infections (6, 24–26). IL-32 is found to come up with different splice variants (7, 27). However, there are limitations on IL-32 isoform characterization and correlation to define biological processes or disease conditions.
In this study, we were able to purify seven rIL-32 isoforms and evaluate their biological activity in different cell types, which may shed light on the specific activities of these seven IL-32 isoforms. Among them, four rIL-32 isoforms (IL-32α, -β, -γ, and -δ) were previously purified (8). Moreover, the remaining three rIL-32 isoforms (IL-32ϵ, -ζ, and -θ) were successfully constructed and purified for the first time in this study.
The expression of IL-32 in normal tissue revealed a high expression among various cell types, e.g., lung cells, fibroblasts, and immune cells, including monocytes and bone marrow (Figure 3). IL-32 is highly associated as well with disease conditions relating to these cell types, like rheumatoid arthritis, COPD, asthma, atopic dermatitis (AD), and certain cancers (6, 10, 12, 24, 28, 29). Therefore, we investigated the differences in the biological activity of the seven IL-32 isoforms within immune cells, lung cells, and fibroblasts. This study illustrated the need for a fundamental activity study regarding each IL-32 isoform.
So far, the expression of IL-32 has been correlated with numerous autoimmune diseases, among them RA and IBD that were the most-studied conditions in this regard. In the case of RA and compared to both healthy controls and patients with osteoarthritis, IL-32 expression was higher in RA patients (30); moreover, the synovial biopsies of RA patients exhibit a reduction of IL-32 upon anti-TNFα treatment. This interchange between IL-32 and TNFα suggests an intensification of inflammatory processes in RA (18). Regarding IBD, IL-32 has been suggested to have a role in the pathogenesis of IBD as it promotes the production of TNFα, IL-6, and IL-1β cytokines (31). To a lesser extent, patients with autoimmune diseases, including psoriasis, granulomatosis with polyangiitis, myasthenia graves, and type 2 diabetes, have also demonstrated a higher serum level of IL-32 than healthy controls (32–34). This difference was linked to disorder severity, suggesting its usefulness in being an inflammatory marker and outcome predictor.
More recently, IL-32 is also involved in type 1 diabetes; its mRNA levels in beta-cells were higher than in those in control subjects (35). These results are in line with the outcome of Jhun et al., who found that IL-32, specifically the gamma isoform, hastens streptozotocin-induced type 1 diabetes (36). In addition to autoimmune diseases, IL-32 is involved in respiratory inflammation conditions, such as COPD and asthma (12–14, 29, 37, 38). Its expression in lung tissue is enhanced in COPD patients and was associated with the obstruction degree of airflow in vivo (12). Besides this, IL-32 was found to play a role in gastric inflammation and cancer (39, 40), altogether signifying the execution of IL-32 in several inflammatory conditions with different patterns that could be explained by the existence of different isoforms that play different roles. Nevertheless, many of these studies fail to convey the IL-32 isoforms concerning disease conditions.
Lately, with increasing inconsistent reports regarding the role of IL-32, there is a large agreement that these different functions may relate to the different IL-32 isoforms. As mentioned earlier, most of the previous studies assessed the level of IL-32 with lack of specific isoform consideration. However, limited studies have demonstrated a few properties of some isoforms—for example, IL-32α has shown pro- and anti-inflammatory properties as it induces pro-inflammatory cytokine expression, thus suppressing its inflammatory role in the spinal cord. Besides this, the ability of IL-32α to promote the differentiation of osteoclast has been reported (17).
IL-32β also has both pro- and anti-inflammatory properties; it induces cytokine production of both IL-10 and TNFα in phorbol-12-myristate-13-acetate-stimulated cells, K562, and THP-1, respectively (1, 41, 42). In addition, this isoform also improves the adhesion ability of inflammatory cells to activate endothelial cells along with the consequent induction of proinflammatory cytokines. Therefore, it is involved in vascular inflammation propagation and the modulation of lipid accumulation (43, 44).
The longest isoform, IL-32γ, exhibits mainly a pro-inflammatory property and accordingly induces pro-inflammatory cytokine expression. Moreover, IL-32γ promotes the migration of activated T cells via chemokine (C-C motif) ligand 5 (CCL5) production in dendritic cells (DCs), stimulates the maturation and activation of DCs, and therefore increases the production of IL-12 and IL-6 (45, 46). In ankylosing spondylitis joint, IL-32γ plays an enhancement role in the differentiation of osteoblast (47). In RA patients, the level of IL-32γ was found to be upregulated significantly in both CD14+ monocytes and synovial membrane (16, 48). Therefore, it has been suggested that this isoform activates osteoclasts and, subsequently, tissue resorption. Furthermore, IL-32γ has shown a potent antiviral activity versus several viruses, specifically influenza A virus, vesicular stomatitis virus, herpes simplex virus 2, and human immunodeficiency virus (49–52).
IL-32δ is another isoform that generally demonstrates a proinflammatory property; it inhibits the production of IL-10. This inhibition occurs through the modulation of IL-32β; thus, this observation reveals that IL-32 is controlled by its isoforms (53). On the contrary, IL-32θ has mainly anti-inflammatory effects and has an inhibitory role on monocyte differentiation (41, 54). In patients with acute myeloid leukemia, IL-32θ regulates the production of TNFα negatively (55). In addition, IL-32θ negatively regulates CCL5 expression, an inflammatory chemokine secreted in several conditions such as viral infection and cancer, at both mRNA and protein levels. This data suggests the intracellular modulator role of IL-32θ under inflammation (56). Additionally, the isoform of IL-32θ has been found to suppress epithelial–mesenchymal transition, resulting in inhibition of invasion and migration of colon cancer cells under in vitro and in vivo assessments (57). Lastly, for IL-32ϵ, its transcript was elevated in the IBD mucosa, thus suggesting a protective activity (58). However, the present study showed that the IL-32θ isoform has the most prominent activity among the seven IL-32 isoforms.
There has been a widespread acceptance that IL-32γ is the most biologically active isoform (6, 8, 19, 26), as recent results suggest such interpretation. This conclusion is probably attributable to IL-32γ as it is the most-studied isoform. Here our data is in line with the interpretation regarding IL-32γ, specifically within the immune cells along with IL-32θ isoform. In more detail, we observed a higher activity of IL-32θ isoform in human-derived monocytes, THP-1, followed by the IL-32γ isoform (Figure 4A). However, in mouse-derived cells, IL-32γ exhibits maximum activity. Remarkably, IL-32β activity was directly following IL-32γ and IL-32θ among the tested immune cells (Figures 4 and 5). It is noteworthy that IL-32 switches between its isoforms under certain conditions were reported to reduce the inflammation as a safety control. This shift of transcripts has been indicated between IL-32γ and IL-32β isoforms (19). A similar shifting may be the case with IL-32θ to reduce its potent activity. More investigation is needed to confirm this suggestion and thus specify the key exon/domain/peptide signal responsible for the splicing change in both cases as well as examine the possibility of dimerization of IL-32β to reduce its section.
So far, few studies have been conducted on IL-32θ compared to the IL-32γ isoform. Interestingly, IL-32θ was the most active isoform in most cell types except in mouse Raw 264.7 and splenocyte (Figures 4B and 5B). These in vitro results using a human-derived cell line (A549) showed four active isoforms with a difference in activity as reflected by the different levels of IL-6 production; these are IL-32θ, -δ, -β, and -α, in descending order. In comparison, ex vivo results using mouse-derived cells demonstrated a significant IL-6 production only with the IL-32θ isoform. In addition, IL-6 production in MEF showed that IL-32θ has the highest significant activity, followed by IL-32δ and IL-32β with comparable results. As mentioned above, IL-32θ has been suggested to play an intracellular modulatory role in breast cancer cells (56, 59). On the other hand, a study conducted on asthma patients showed a lower IL-32γ compared to healthy controls (37) as well as rIL-32γ that showed a negative regulatory effect in an asthma mouse model (14). However, this report did not consider the behavior of the IL-32θ isoform.
Inflammatory skin conditions have revealed the changes in levels of IL-32 with restricted and conflicting data regarding isoforms. A study comparing patients with asthma, psoriasis, and AD to healthy subjects found that IL-32 was higher in asthmatic and AD patients’ serum (60). They suggest that the release of IL-32 is mainly from apoptotic cells in both conditions, which is also in line with their in vitro results. Therefore, they declared the usefulness of using IL-32 serum levels in diagnosis to examine patients with AD or asthma. In addition, they mentioned the possibility of targeting IL-32 as a therapeutic purpose. There are some contradicting reports of Al-Shobaili et al., Meyer et al., and Lee et al. on the one hand, whereas Al-Shobaili et al. and Meyer et al. have found that the levels of IL-32 are increasing in psoriasis and AD, respectively (22, 61). Lee et al. reported that IL-32 exhibits a suppressor role for AD (60). Thus far, additional studies are needed to explain the role of these isoforms in different stages and different stimuli and their impact on each other.
In summary, we purified seven rIL-32 isoforms using the E. coli expression system and evaluated their biological activities using various cell types. Along with rIL-32γ, rIL-32θ revealed similar or higher activities in all tested cells. However, the behavior of IL-32 isoforms could be different at baseline and other conditions, as it may be influenced by many factors, such as different stimuli, health/disease conditions, cell type, and genetic background. Moreover, rIL-32ϵ and rIL-32ζ both showed little or no activity in the tested cells. Nevertheless, our results indicated the necessity to illuminate each rIL-32 isoform. Therefore, both mRNA and protein levels, in the forthcoming studies, should be considered. Furthermore, specific monoclonal antibodies that recognize each isoform are needed to accomplish this need, such as in the case of IL-32γ (62).
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
All animal procedures were reviewed and approved by the Konkuk University Institutional Animal Care Committee.
SS and SL designed the study, analyzed the data, and performed the experiments. SS, SL, SK, TTN, AT, and JH, performed the experiments. HJ, YL, SCY, and Y-GK analyzed the data. Funding acquisition was carried out by HJ, H-YP, S-YK, Y-GK, and SK. SS and YH examined the data. AT edited the manuscript. SK designed the study, supervised the project, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2022. This work was supported by the National Research Foundation of Korea (NRF-2021R1F1A1057397). This research was supported by the Main Research Program (E0210602-02) of the Korea Food Research Institute (KFRI), funded by the Ministry of Science and ICT. S-YK and Y-GK were supported by NRF-2021M3A9G1026605. SL was supported by NRF-2019R1I1A1A01057699.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AD, atopic dermatitis; CCL, chemokine (C-C motif) ligand; COPD, chronic obstructive pulmonary disease; DC, dendritic cells; E. coli, Escherichia coli; IBD, inflammatory bowel disease; IL, interleukin; MIP2, macrophage inflammatory protein-2; MEFs, mouse embryonic fibroblasts; NK4, natural killer cell transcript 4; PR3, proteinase 3; rIL-32, recombinant interleukin-32; RA, rheumatoid arthritis; TNFα, tumor necrosis factor-α.
1. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: A Cytokine and Inducer of Tnfalpha. Immunity (2005) 22(1):131–42. doi: 10.1016/j.immuni.2004.12.003
2. Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, et al. IL-32 Synergizes With Nucleotide Oligomerization Domain (NOD) 1 and NOD2 Ligands for IL-1beta and IL-6 Production Through a Caspase 1-Dependent Mechanism. Proc Natl Acad Sci USA (2005) 102p(45):16309–14. doi: 10.1073/pnas.0508237102
3. Nam SY, Jeong HJ, Kim HM. Kaempferol Impedes IL-32-Induced Monocyte-Macrophage Differentiation. Chem Biol Interact (2017) 274:107–15. doi: 10.1016/j.cbi.2017.07.010
4. Novick D, Rubinstein M, Azam T, Rabinkov A, Dinarello CA, Kim SH. Proteinase 3 Is an IL-32 Binding Protein. Proc Natl Acad Sci USA (2006) 103(9):3316–21. doi: 10.1073/pnas.0511206103
5. Heinhuis B, Netea MG, Dinarello CA, Joosten LA. Interleukin-32: A Predominantly Intracellular Proinflammatory Mediator That Controls Cell Activation and Cell Death. Cytokine (2012) 60(2):321–7. doi: 10.1016/j.cyto.2012.07.010
6. Kim S. Interleukin-32 in Inflammatory Autoimmune Diseases. Immune Netw (2014) 14(3):123–7. doi: 10.4110/in.2014.14.3.123
7. Sohn DH, Nguyen TT, Kim S, Shim S, Lee S, Lee Y, et al. Structural Characteristics of Seven IL-32 Variants. Immune Netw (2019) 19(2):e8. doi: 10.4110/in.2019.19.e8
8. Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, et al. Identification of the Most Active Interleukin-32 Isoform. Immunology (2009) 126(4):535–42. doi: 10.1111/j.1365-2567.2008.02917.x
9. Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Ham SY, et al. Interaction Network Mapping Among IL-32 Isoforms. Biochimie (2014) 101:248–51. doi: 10.1016/j.biochi.2014.01.013
10. Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH, Park MH. Interleukin 32, Inflammation and Cancer. Pharmacol Ther (2017) 174:127–37. doi: 10.1016/j.pharmthera.2017.02.025
11. Briukhovetska D, Dorr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in Cancer: From Biology to Therapy. Nat Rev Cancer (2021) 21(8):481–99. doi: 10.1038/s41568-021-00363-z
12. Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, et al. IL-32, a Novel Proinflammatory Cytokine in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med (2008) 178(9):894–901. doi: 10.1164/rccm.200804-646OC
13. Rong B, Fu T, Rong C, Liu W, Li K, Liu H. Correlation Between Serum IL-32 Concentration and Clinical Parameters of Stable COPD: A Retrospective Clinical Analysis. Sci Rep (2020) 10(1):12092. doi: 10.1038/s41598-020-69000-3
14. Bang BR, Kwon HS, Kim SH, Yoon SY, Choi JD, Hong GH, et al. Interleukin-32gamma Suppresses Allergic Airway Inflammation in Mouse Models of Asthma. Am J Respir Cell Mol Biol (2014) 50(6):1021–30. doi: 10.1165/rcmb.2013-0234OC
15. Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, et al. Interleukin-32 Induces the Differentiation of Monocytes Into Macrophage-Like Cells. Proc Natl Acad Sci USA (2008) 105(9):3515–20. doi: 10.1073/pnas.0712381105
16. Kim YG, Lee CK, Oh JS, Kim SH, Kim KA, Yoo B. Effect of Interleukin-32gamma on Differentiation of Osteoclasts From CD14+ Monocytes. Arthritis Rheum (2010) 62(2):515–23. doi: 10.1002/art.27197
17. Mabilleau G, Sabokbar A. Interleukin-32 Promotes Osteoclast Differentiation But Not Osteoclast Activation. PloS One (2009) 4(1):e4173. doi: 10.1371/journal.pone.0004173
18. Heinhuis B, Koenders MI, van Riel PL, van de Loo FA, Dinarello CA, Netea MG, et al. Tumour Necrosis Factor Alpha-Driven IL-32 Expression in Rheumatoid Arthritis Synovial Tissue Amplifies an Inflammatory Cascade. Ann Rheum Dis (2011) 70(4):660–7. doi: 10.1136/ard.2010.139196
19. Heinhuis B, Koenders MI, van de Loo FA, Netea MG, van den Berg WB, Joosten LA. Inflammation-Dependent Secretion and Splicing of IL-32{Gamma} in Rheumatoid Arthritis. Proc Natl Acad Sci USA (2011) 108(12):4962–7. doi: 10.1073/pnas.1016005108
20. Kang JW, Choi SC, Cho MC, Kim HJ, Kim JH, Lim JS, et al. A Proinflammatory Cytokine Interleukin-32beta Promotes the Production of an Anti-Inflammatory Cytokine Interleukin-10. Immunology (2009) 128(1 Suppl):e532–40. doi: 10.1111/j.1365-2567.2008.03025.x
21. Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, et al. Involvement of IL-32 in Activation-Induced Cell Death in T Cells. Int Immunol (2006) 18(2):233–40. doi: 10.1093/intimm/dxh339
22. Meyer N, Zimmermann M, Burgler S, Bassin C, Woehrl S, Moritz K, et al. IL-32 Is Expressed by Human Primary Keratinocytes and Modulates Keratinocyte Apoptosis in Atopic Dermatitis. J Allergy Clin Immunol (2010) 125(4):858–865 e10. doi: 10.1016/j.jaci.2010.01.016
23. Saetta M, Baraldo S, Corbino L, Turato G, Braccioni F, Rea F, et al. CD8+Ve Cells in the Lungs of Smokers With Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med (1999) 160(2):711–7. doi: 10.1164/ajrccm.160.2.9812020
24. Yan H, He D, Huang X, Zhang E, Chen Q, Xu R, et al. Role of Interleukin-32 in Cancer Biology. Oncol Lett (2018) 16(1):41–7. doi: 10.3892/ol.2018.8649
25. Dinarello CA, Kim SH. IL-32, a Novel Cytokine With a Possible Role in Disease. Ann Rheum Dis (2006) 65(Suppl 3):iii61–4. doi: 10.1136/ard.2006.058511
26. Zhou Y, Zhu Y. Important Role of the IL-32 Inflammatory Network in the Host Response Against Viral Infection. Viruses (2015) 7(6):3116–29. doi: 10.3390/v7062762
27. Heinhuis B, Plantinga TS, Semango G, Kusters B, Netea MG, Dinarello CA, et al. Alternatively Spliced Isoforms of IL-32 Differentially Influence Cell Death Pathways in Cancer Cell Lines. Carcinogenesis (2016) 37(2):197–205. doi: 10.1093/carcin/bgv172
28. Sloot YJE, Smit JW, Joosten LAB, Netea-Maier RT. Insights Into the Role of IL-32 in Cancer. Semin Immunol (2018) 38:24–32. doi: 10.1016/j.smim.2018.03.004
29. Xin T, Chen M, Duan L, Xu Y, Gao P. Interleukin-32: Its Role in Asthma and Potential as a Therapeutic Agent. Respir Res (2018) 19(1):124. doi: 10.1186/s12931-018-0832-x
30. Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, et al. IL-32, a Proinflammatory Cytokine in Rheumatoid Arthritis. Proc Natl Acad Sci USA (2006) 103(9):3298–303. doi: 10.1073/pnas.0511233103
31. Shioya M, Nishida A, Yagi Y, Ogawa A, Tsujikawa T, Kim-Mitsuyama S, et al. Epithelial Overexpression of Interleukin-32alpha in Inflammatory Bowel Disease. Clin Exp Immunol (2007) 149(3):480–6. doi: 10.1111/j.1365-2249.2007.03439.x
32. Bae S, Kim YG, Choi J, Hong J, Lee S, Kang T, et al. Elevated Interleukin-32 Expression in Granulomatosis With Polyangiitis. Rheumatol (Oxf) (2012) 51(11):1979–88. doi: 10.1093/rheumatology/kes163
33. Na SJ, So SH, Lee KO, Choi YC. Elevated Serum Level of Interleukin-32alpha in the Patients With Myasthenia Gravis. J Neurol (2011) 258(10):1865–70. doi: 10.1007/s00415-011-6036-7
34. Fadaei R, Bagheri N, Heidarian E, Nouri A, Hesari Z, Moradi N, et al. Serum Levels of IL-32 in Patients With Type 2 Diabetes Mellitus and its Relationship With TNF-Alpha and IL-6. Cytokine (2020) 125:154832. doi: 10.1016/j.cyto.2019.154832
35. Kallionpaa H, Somani J, Tuomela S, Ullah U, de Albuquerque R, Lonnberg T, et al. Early Detection of Peripheral Blood Cell Signature in Children Developing Beta-Cell Autoimmunity at a Young Age. Diabetes (2019) 68(10):2024–34. doi: 10.2337/db19-0287
36. Jhun H, Choi J, Hong J, Lee S, Kwak A, Kim E, et al. IL-32gamma Overexpression Accelerates Streptozotocin (STZ)-Induced Type 1 Diabetes. Cytokine (2014) 69(1):1–5. doi: 10.1016/j.cyto.2014.05.002
37. Kwon JW, Chang HS, Heo JS, Bae DJ, Lee JU, Jung CA, et al. Characteristics of Asthmatics With Detectable IL-32gamma in Induced Sputum. Respir Med (2017) 129:85–90. doi: 10.1016/j.rmed.2017.06.005
38. Rong Y, Xiang XD, Li YM, Peng ZY, Li JX. IL-32 was Involved in Cigarette Smoke-Induced Pulmonary Inflammation in COPD. Clin Respir J (2015) 9(4):430–5. doi: 10.1111/crj.12157
39. Sakitani K, Hirata Y, Hayakawa Y, Serizawa T, Nakata W, Takahashi R, et al. Role of Interleukin-32 in Helicobacter Pylori-Induced Gastric Inflammation. Infect Immun (2012) 80(11):3795–803. doi: 10.1128/IAI.00637-12
40. Khawar MB, Abbasi MH, Sheikh N. IL-32: A Novel Pluripotent Inflammatory Interleukin, Towards Gastric Inflammation, Gastric Cancer, and Chronic Rhino Sinusitis. Mediat Inflamm (2016) 2016:8413768. doi: 10.1155/2016/8413768
41. Heinhuis B, Koenders MI, van den Berg WB, Netea MG, Dinarello CA, Joosten LA. Interleukin 32 (IL-32) Contains a Typical Alpha-Helix Bundle Structure That Resembles Focal Adhesion Targeting Region of Focal Adhesion Kinase-1. J Biol Chem (2012) 287(8):5733–43. doi: 10.1074/jbc.M111.288290
42. Kang JW, Park YS, Kim MS, Lee DH, Bak Y, Ham SY, et al. Interleukin (IL)-32beta-Mediated CCAAT/Enhancer-Binding Protein Alpha (C/Ebpalpha) Phosphorylation by Protein Kinase Cdelta (Pkcdelta) Abrogates the Inhibitory Effect of C/Ebpalpha on IL-10 Production. J Biol Chem (2013) 288(33):23650–8. doi: 10.1074/jbc.M113.465575
43. Kobayashi H, Huang J, Ye F, Shyr Y, Blackwell TS, Lin PC. Interleukin-32beta Propagates Vascular Inflammation and Exacerbates Sepsis in a Mouse Model. PloS One (2010) 5(3):e9458. doi: 10.1371/journal.pone.0009458
44. Lee DH, Hong JE, Yun HM, Hwang CJ, Park JH, Han SB, et al. Interleukin-32beta Ameliorates Metabolic Disorder and Liver Damage in Mice Fed High-Fat Diet. Obes (Silver Spring) (2015) 23(3):615–22. doi: 10.1002/oby.21001
45. Son MH, Jung MY, Choi S, Cho D, Kim TS. IL-32gamma Induces Chemotaxis of Activated T Cells via Dendritic Cell-Derived CCL5. Biochem Biophys Res Commun (2014) 450(1):30–5. doi: 10.1016/j.bbrc.2014.05.052
46. Jung MY, Son MH, Kim SH, Cho D, Kim TS. IL-32gamma Induces the Maturation of Dendritic Cells With Th1- and Th17-Polarizing Ability Through Enhanced IL-12 and IL-6 Production. J Immunol (2011) 186(12):6848–59. doi: 10.4049/jimmunol.1003996
47. Lee EJ, Lee EJ, Chung YH, Song DH, Hong S, Lee CK, et al. High Level of Interleukin-32 Gamma in the Joint of Ankylosing Spondylitis Is Associated With Osteoblast Differentiation. Arthritis Res Ther (2015) 17:350. doi: 10.1186/s13075-015-0870-4
48. Moon YM, Yoon BY, Her YM, Oh HJ, Lee JS, Kim KW, et al. IL-32 and IL-17 Interact and Have the Potential to Aggravate Osteoclastogenesis in Rheumatoid Arthritis. Arthritis Res Ther (2012) 14(6):R246. doi: 10.1186/ar4089
49. Li W, Sun W, Liu L, Yang F, Li Y, Chen Y, et al. IL-32: A Host Proinflammatory Factor Against Influenza Viral Replication Is Upregulated by Aberrant Epigenetic Modifications During Influenza a Virus Infection. J Immunol (2010) 185(9):5056–65. doi: 10.4049/jimmunol.0902667
50. Nold MF, Nold-Petry CA, Pott GB, Zepp JA, Saavedra MT, Kim SH, et al. Endogenous IL-32 Controls Cytokine and HIV-1 Production. J Immunol (2008) 181(1):557–65. doi: 10.4049/jimmunol.181.1.557
51. Rasool ST, Tang H, Wu J, Li W, Mukhtar MM, Zhang J, et al. Increased Level of IL-32 During Human Immunodeficiency Virus Infection Suppresses HIV Replication. Immunol Lett (2008) 117(2):161–7. doi: 10.1016/j.imlet.2008.01.007
52. Zepp JA, Nold-Petry CA, Dinarello CA, Nold MF. Protection From RNA and DNA Viruses by IL-32. J Immunol (2011) 186(7):4110–8. doi: 10.4049/jimmunol.1000081
53. Nold-Petry CA, Nold MF, Zepp JA, Kim SH, Voelkel NF, Dinarello CA. IL-32-Dependent Effects of IL-1beta on Endothelial Cell Functions. Proc Natl Acad Sci USA (2009) 106(10):3883–8. doi: 10.1073/pnas.0813334106
54. Kim MS, Kang JW, Park YS, Lee DH, Bak Y, Kwon T, et al. IL-32theta Inhibits Monocytic Differentiation of Leukemia Cells by Attenuating Expression of Transcription Factor PU.1. Oncotarget (2015) 6(6):4394–405. doi: 10.18632/oncotarget.3013
55. Kim MS, Kang JW, Jeon JS, Kim JK, Kim JW, Hong J, et al. IL-32theta Gene Expression in Acute Myeloid Leukemia Suppresses TNF-Alpha Production. Oncotarget (2015) 6(38):40747–61. doi: 10.18632/oncotarget.5688
56. Bak Y, Kang JW, Kim MS, Park YS, Kwon T, Kim S, et al. IL-32theta Downregulates CCL5 Expression Through its Interaction With Pkcdelta and STAT3. Cell Signal (2014) 26(12):3007–15. doi: 10.1016/j.cellsig.2014.09.015
57. Bak Y, Kwon T, Bak IS, Hong J, Yu DY, Yoon DY. IL-32theta Inhibits Stemness and Epithelial-Mesenchymal Transition of Cancer Stem Cells via the STAT3 Pathway in Colon Cancer. Oncotarget (2016) 7(6):7307–17. doi: 10.18632/oncotarget.7007
58. Imaeda H, Andoh A, Aomatsu T, Osaki R, Bamba S, Inatomi O, et al. A New Isoform of Interleukin-32 Suppresses IL-8 MRNA Expression in the Intestinal Epithelial Cell Line HT-29. Mol Med Rep (2011) 4(3):483–7. doi: 10.3892/mmr.2011.442
59. Khawar MB, Mukhtar M, Abbasi MH, Sheikh N. IL-32theta: A Recently Identified Anti-Inflammatory Variant of IL-32 and Its Preventive Role in Various Disorders and Tumor Suppressor Activity. Am J Transl Res (2017) 9(11):4726–37.
60. Lee YS, Han SB, Ham HJ, Park JH, Lee JS, Hwang DY, et al. IL-32gamma Suppressed Atopic Dermatitis Through Inhibition of MiR-205 Expression via Inactivation of Nuclear Factor-Kappa B. J Allergy Clin Immunol (2020) 146(1):156–68. doi: 10.1016/j.jaci.2019.12.905
61. Al-Shobaili HA, Farhan J, Zafar U, Rasheed Z. Functional Role of Human Interleukin-32 and Nuclear Transcription Factor-Kb in Patients With Psoriasis and Psoriatic Arthritis. Int J Health Sci (Qassim) (2018) 12(3):29–34.
Keywords: interleukin-32, recombinant protein, isoforms, IL-32θ, inflammatory cytokine
Citation: Shim S, Lee S, Hisham Y, Kim S, Nguyen TT, Taitt AS, Hwang J, Jhun H, Park H-Y, Lee Y, Yeom SC, Kim S-Y, Kim Y-G and Kim S (2022) Comparison of the Seven Interleukin-32 Isoforms’ Biological Activities: IL-32θ Possesses the Most Dominant Biological Activity. Front. Immunol. 13:837588. doi: 10.3389/fimmu.2022.837588
Received: 16 December 2021; Accepted: 17 January 2022;
Published: 25 February 2022.
Edited by:
Pierre Miossec, Université Claude Bernard Lyon 1, FranceReviewed by:
Kwang Dong Kim, Gyeongsang National University, South KoreaCopyright © 2022 Shim, Lee, Hisham, Kim, Nguyen, Taitt, Hwang, Jhun, Park, Lee, Yeom, Kim, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Gil Kim, YmVzdG1kMjAwMEBhbWMuc2VvdWwua3I=; Soohyun Kim, c29vaHl1bkBrb25rdWsuYWMua3I=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.