- 1Centre for Fish and Wildlife Health, Department of Pathobiology and Infectious Diseases, Vetsuisse Faculty, University of Bern, Bern, Switzerland
- 2Fish Immunology Group, CISA-INIA, Madrid, Spain
- 3Laboratory of Marine Biology and Ecology, Third Institute of Oceanography, Xiamen, China
There is growing awareness that a range of environmental chemicals target the immune system of fish and may compromise the resistance towards infectious pathogens. Existing concepts to assess chemical hazards to fish, however, do not consider immunotoxicity. Over recent years, the application of in vitro assays for ecotoxicological hazard assessment has gained momentum, what leads to the question whether in vitro assays using piscine immune cells might be suitable to evaluate immunotoxic potentials of environmental chemicals to fish. In vitro systems using primary immune cells or immune cells lines have been established from a wide array of fish species and basically from all immune tissues, and in principal these assays should be able to detect chemical impacts on diverse immune functions. In fact, in vitro assays were found to be a valuable tool in investigating the mechanisms and modes of action through which environmental agents interfere with immune cell functions. However, at the current state of knowledge the usefulness of these assays for immunotoxicity screening in the context of chemical hazard assessment appears questionable. This is mainly due to a lack of assay standardization, and an insufficient knowledge of assay performance with respect to false positive or false negative signals for the different toxicant groups and different immune functions. Also the predictivity of the in vitro immunotoxicity assays for the in vivo immunotoxic response of fishes is uncertain. In conclusion, the currently available database is too limited to support the routine application of piscine in vitro assays as screening tool for assessing immunotoxic potentials of environmental chemicals to fish.
Immunotoxicology Has Relevance for the Health of Fish Populations
In assessing the risk of environmental contaminants for fish, for a long time most consideration has been given to apical effects such as changes of survival, growth and reproduction (1, 2). This approach is based on the assumption that the apical effects are predictive for changes in population growth, although this assumption is partly questionable [e.g., (1, 3–5)]. More recently, a paradigm shift has taken place giving more emphasis on the role of sublethal effects for the ecological impact of toxicants [e.g., (2, 6–11)]. Particularly chemicals with specific modes of actions, for instance, pharmaceuticals, are unlikely to cause apical effects at environmental concentrations, still these concentrations may be high enough to modify physiological performance and life history traits of exposed organisms, what can have consequences for organism fitness and population growth.
To make sublethal effects assessment a valuable addition in evaluating the risks of environmental contaminants to fish populations, it is critical to not get lost in measuring an increasingly broad range of subtle parameters, but to focus on processes and traits that have potential toxicological and ecological inferences, and/or influence the vulnerability of species and populations towards chemical impacts (2, 8, 12, 13). In the present communication, we focus on the assessment of immunological effects of chemicals in fish. Immunity is directly linked to phenotypic fitness, survival probability and evolutionary selection (14–16). An immune system that is able to execute its basic functions, i.e. recognition and response, is essential for maintaining the integrity of the organism, and it prevents damage as it may arise from infectious pathogens or other environmental stressors including toxic chemicals (17). Environmental contaminants can interfere with the immune system of exposed organisms and this may result in immune dysfunction. Chemical effects on the immune system are usually not immediately lethal, although prolonged disturbances of immune homeostasis such as, e.g., chronic inflammation are known to be associated with increased morbidity and mortality (18–20). In addition, immune dysfunction is both a predisposing and enabling factor for pathogen-induced diseases and mortalities (4). Finally, since immune responses are costly, they may trade-off with other fitness-relevant life history traits (21), and through this mechanism, immune disturbances can indirectly impair reproduction and growth, reduce overwinter survival, or cause debilitation thereby increasing the risk of predation (12, 22–25).
Fish immunotoxicology aims to understand the impact of environmental contaminants on the health of fish and to assess the consequences for fish populations (26–30). There exists broad evidence that immunoactive chemicals are a relevant thread to fish populations. A wide array of environmental contaminants including legacy compounds, endocrine disrupting compounds (EDCs), pesticides, metals, and pharmaceuticals have been shown to impact the immune system of fishes [for reviews cf. (31–33)]. The International Council for the Exploration of the Sea (ICES) concluded that almost all known chemicals seem to impact the immune system of fishes (34). Also nanoparticles are known to interfere with fish immunity [for review see (35)]. Immune disturbances have been observed worldwide in fish populations living in contaminated habitats [e.g., (36–40)]. With respect to effluents from wastewater treatment works, which are major point sources of aquatic contamination, a number of studies reported immunotoxic effects for fish [e.g., (41, 42)]. Liney et al. (43) observed that the immunotoxic effects of wastewater effluents occurred at concentrations lower than those required to induce recognizable changes in the structure and function of the reproductive endocrine system. Similarly, Rehberger et al. (44) as well as Kernen et al. (45) reported that low concentrations of ethinylestradiol, a major environmental EDC, which disrupt reproductive functions of exposed fish, did also disrupt the immune functions. Collectively, the available findings indicate that chemically induced immunotoxicity is not an artifact of high dose laboratory experiments but that it is a relevant environmental hazard. Importantly, chemical effects on the fish immune system may cumulate with the impacts of other environmental stressors targeting the immune system, for instance, increasing water temperature or stress caused by habitat degradation (46, 47).
Given that immunotoxicity is a relevant ecotoxicological issue, the question is how to detect immunotoxic activities of environmental contaminants to fish? A range of methods and assays to investigate chemical impacts on the fish immune system is available [e.g., (26, 48, 49)], but currently there exist no generally accepted and/or standardized test procedures for assessing potential immunotoxic activities of chemicals to fish. Immunotoxicity appears to be a kind of “blind spot” in ecotoxicological hazard assessment. Hazard profiling of chemicals and other environmental agents relies on a diversity of methods, including computational techniques such as read-across or (Quantitative) Structure Activity Relationships (QSAR), on comparative approaches, i.e. using information from mammalian hazard testing for non-mammalian species, but also in vitro assays can play an important role. In fact, over recent years, in vitro assays have been increasingly applied for screening purposes in (eco)toxicological hazard assessment [e.g. (50–53)]. Immunotoxicity, however, was not or only marginally considered in these approaches, although a group of in vitro assays – in the sense of “Invitroomics” (54) - may well be able to test for the various immune targets potentially affected by toxic agents. The question to be addressed in this context is whether fish cell-based in vitro assays are indeed suitable and sufficient to evaluate the immunotoxicity of environmental chemicals. The aim of the present communication is to critically review the current state of knowledge on in vitro fish immunotoxicity testing. To this end, we will discuss what kind of in vitro assays are available using fish immune cells, for what toxicological purposes they have been applied, and what their strengths and weaknesses are.
Assessing the Immunotoxic Activity of Chemicals by In Vitro Approaches
Chemical impacts on immunity include both immunosuppression and immunostimulation (55, 56). The former can result in increased susceptibility to pathogen infections and pathogen-induced mortalities, activation of opportunistic microorganisms, or development of neoplasia (56); the latter can result in hypersensitivity reactions and autoimmune disorders – a response that has been frequently observed in man, but not yet reported for fish. Chemical-induced immunomodulation can have also indirect effects, for instance via resource trade-offs or endocrine-immune interactions, on other life history traits such as growth, reproduction and behaviour (57–59). Importantly, not each chemical-induced disturbance of an immune parameter will result in impaired immune functioning and competence. Only if the induced immune modulation is of sufficient strength and quality, an adverse effect will result (55, 60).
The immune system is, like the endocrine system, a highly complex system composed of a huge diversity of organs, cells, and mediator as well as effector molecules. The diffuse organization and the integrated functioning of the immune system complicates immunotoxicity assessment as it raises questions which targets within the system are impacted by the action of a chemical. A further complicating fact in immunotoxicity assessment is that the overall capacity of the immune system to maintain homeostasis and health as well as to defend the organism against external stressors depends not so much on an individual immune element but on the balance between individual components and the functioning at the systemic level. By means of networked self-control the immune system is able to buffer to some extent stressor-induced disturbances, and this makes it difficult to define thresholds of adversity or to extrapolate from the toxicant-induced modulation of a specific immune component to an overall impairment of immune functionality and capacity (60). In addition, toxic impacts often may not be visible in the resting immune system, but only when the immune system is activated, e.g., after a pathogen challenge (60, 61). Finally, immune responses may not be directly triggered by a toxicant, but as indirect response to other forms of toxicity. For instance, an inflammatory reaction may be a secondary response to a toxicant-induced tissue damage (62). Also toxicant effects on the microbiome can have consequences for immune system function [e.g., (63)].
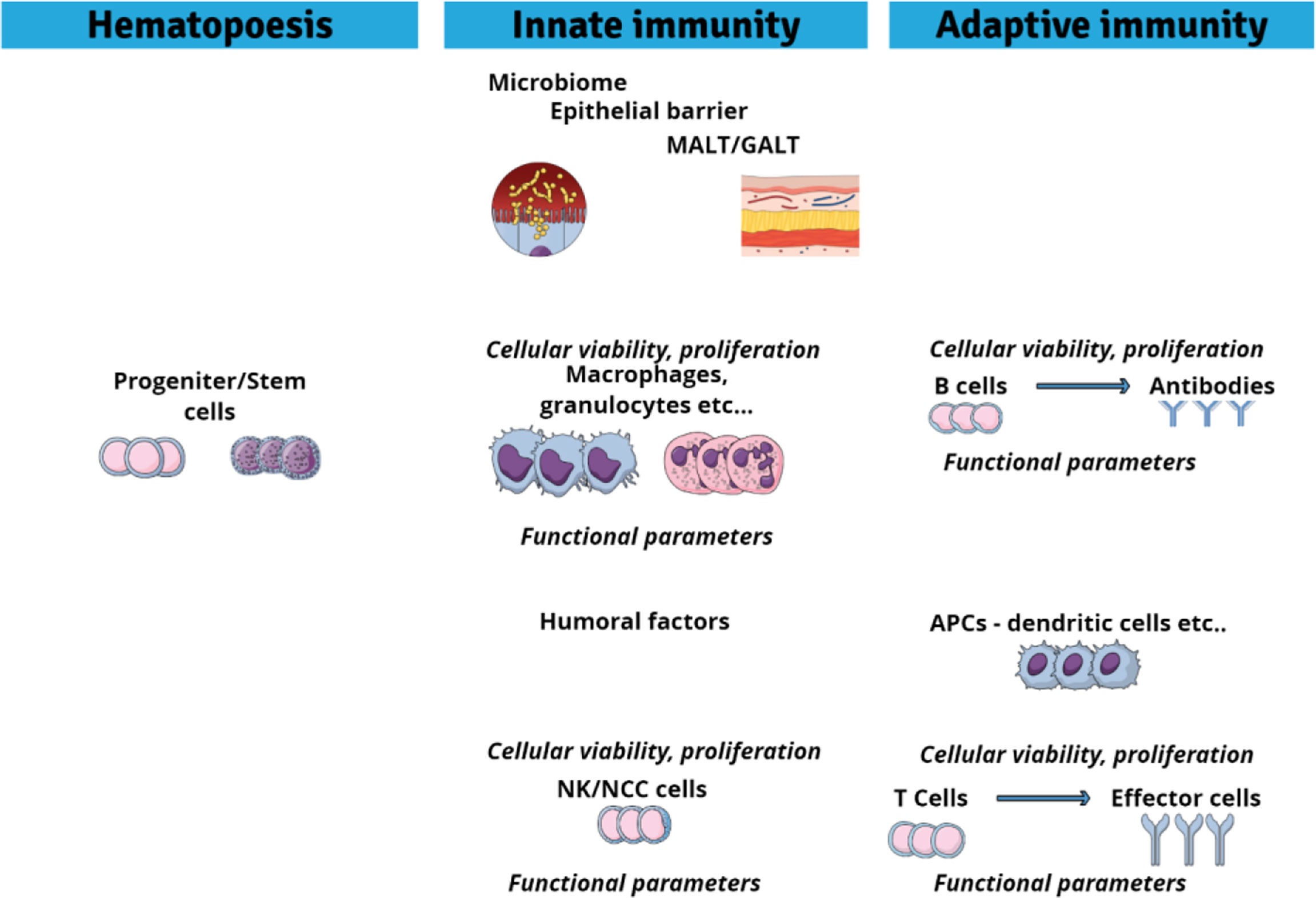
From what has been said above, it is evident that in vitro assays will be able to test only for certain forms of chemical impacts on the immune system (60, 64). In vitro assays cannot take into account for indirect effects such as neuro-immune-endocrine interactions, and they cannot reflect integrated responses resulting from the interactions of the individual immune components. However, in vitro assays can detect direct effects of chemicals on immune cell viability or proliferation. They can also distinguish if the chemical actions are selective for specific immune cell types. In addition, in vitro assays can test for the interference of chemicals with immune cell functioning, for instance, changes in signalling pathways, in the transcriptome, in oxidative burst or phagocytic activity, in antibody production, or in the production and release of soluble mediators such as cytokines. A challenge for in vitro immunotoxicity assessment, however, remains the fact that the immune system is composed of a diversity of cell types. Innate immunity involves, among others, granulocytes, neutrophils, macrophages, natural killer cells, or mast cells, while adaptive immunity involves B and T cells. In addition, macrophages and dendritic cells which act as antigen-presenting cells are linking the innate and adaptive arms of the immune system. Chemicals may target any of these immune cell types and their functions, and in vitro tests methodologies must be designed in a way to be able to assess the potential diversity in targets (65).
The use of in vitro assays for immunotoxicity testing is most advanced in human toxicology. Currently, immunotoxicity testing in human toxicology relies on animal tests, which include general immune endpoints in repeated dose studies and trigger-based tests on a case-by-case basis [e.g., (66–68)]. In the United States, the National Toxicology Program developed a tiered in vivo immunotoxicity testing strategy which includes tier I tests like the assessment of antigen-induced humoral immunity or cell-mediated immunity as well as general parameters like immune organ weight or histopathology (69). Tier II includes, among others, tests on hypersensitivity or on the cytotoxic T cell response. A tiered strategy for in vitro immunotoxicity assessment could start with an evaluation of myelotoxicity, which examines whether the toxicant leads to a decreased production of bone marrow-derived immune progenitor cells (64, 68, 70). For this purpose, often the humane/murine clonogenic CFU-GM (Colony Forming Unit – Granulocyte Macrophage) test is used (71). If a compound inhibits the proliferation of progenitor cells, it can be considered to be immunotoxic and a further evaluation is not needed. If a compound is not myelotoxic, the following tiers assess the impact of the chemical on the viability and function of differentiated immune cells, mainly on lymphocytes. Initially, the overt cytotoxicity of the chemical towards immune cells is determined, and then, using non-cytotoxic concentrations, the impacts on selected cell functions are tested, for instance, cytokine production or lymphocyte proliferation assays (64, 68, 70). The function tests include also genetically modified reporter gene cell systems like the “fluorescent cell chip” (72) or the IL-2 luciferase assay (73). Despite the fact that meanwhile a diversity of cell-based in vitro immunotoxicity assays is available and characterized, they are usually not yet included in the regulatory testing schemes of human toxicological risk assessment (60, 68).
Most success of in vitro immunotoxicity assays has been achieved in the area of chemical-induced immunostimulation such as skin sensitization. Here, in vitro assays are integrated in the assessment approach in the context of Adverse Outcome Pathways (AOP). The AOP framework intends to quantitatively link molecular initiating events of toxicity through a series of “key events” to adverse outcomes (74, 75). The AOP for skin sensitization starts with covalent interactions of the irritant with skin proteins as molecular initiating event, and then proceeds through a series of immune-related key events to end up in inflammation and allergic dermatitis. The immune key events which mechanistically link the MIE to the adverse outcome are induction of inflammatory cytokines, activation of dendritic cells, and activation and proliferation of T cells. For these immune responses, in vitro assays are available that provide quantitative concentration-response data to be integrated into the risk assessment of the skin sensitizing activity of chemicals (76).
One aspect that must not be neglected when using in vitro assays for immunotoxicity testing are technical issues. For instance, physicochemical characteristics of the test material or vehicle solvent may interfere with the in vitro systems. Also the influence of serum, as often used in cell cultures, on the bioavailability of the test chemicals, can be a confounding factor.
Finally, it is important to distinguish between in vitro and ex vivo assays. In the latter case, animals are exposed in vivo to the suspected immunotoxicant, and after the in vivo treatment, immune cells are isolated and tested for their functioning. Although the measurements on the isolated cells are done in vitro, the experiment still represents an in vivo study, because conditioning and treatment of the immune cells was done in the intact animal. The present communication will deal only with assays that do not involve treatments of animals, i.e. ex vivo assays are not considered.
Fish Immune Cells In Vitro: Cell Lines and Primary Cells
In vitro systems that have been used to study toxicant effects on fish immunity include mainly cell lines and primary cells, either in fresh suspensions or in primary culture. Therefore we will focus on these systems. Cells isolated from organs or tissues of an organism are primary cells; they typically are maintained for a few hours - usually as suspension – or for a few days – then either as two-dimensional monolayer culture or three-dimensional aggregate culture (77, 78). By convention, the primary cell system ends and a cell line arises at the time of the first subculture (54, 79). The cell lines proliferate in vitro and after a certain time period they are split up into subcultures, a process often referred to as passaging (78). Finite cell lines undergo only a limited number of passaging into subcultures, whereas continuous cell lines grow indefinitely (80, 81). In addition to primary cells and cell lines, also other in vitro systems like tissue explants could be used for immunotoxicity studies, but to date this interesting methodology has been rarely applied to immune organs (82).
Piscine Immune Cell Lines
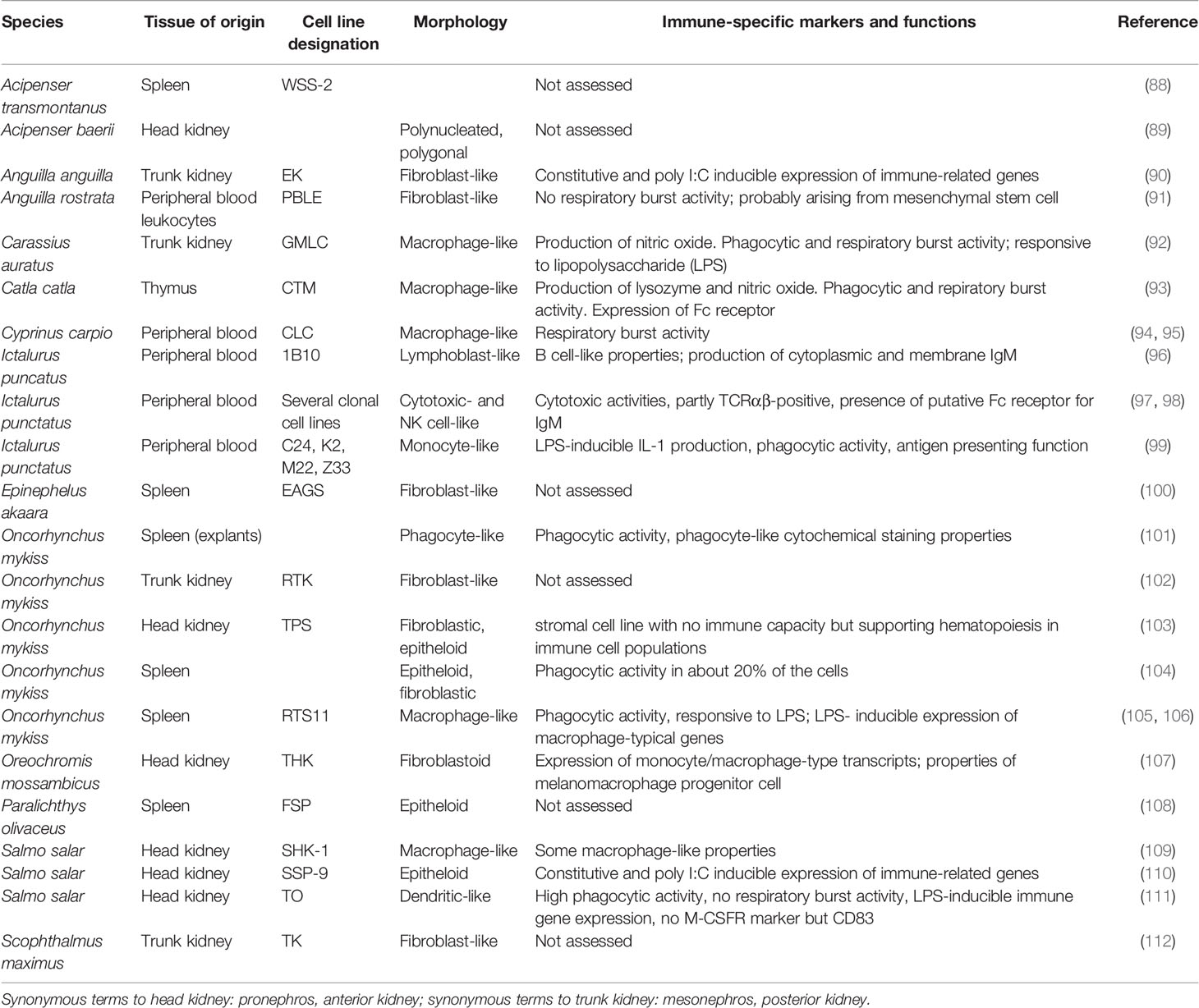
First introduced in the 1960s (83), the number of fish cell lines is continuously growing since then. While Fryer and Lannan (84) listed 152 fish cell lines, Lakra et al. (85) identified 283 cell lines, and these numbers keep increasing. Fish cell lines have been frequently used for the diagnosis of fish diseases as well as to study immunological responses [e.g., (54, 86, 87)]. Table 1 lists examples of cell lines derived from fish immune tissues including cell lines for which immunological functions have been demonstrated and cell lines for which no such characterization is available. The fact that a cell line originates from immune organs does not necessarily mean that it expresses immune functions. On the other hand, cell lines derived from non-immune tissues may display immune features such as lipopolysaccharide (LPS)- or cortisol-inducible immune gene expression (113, 114). Still, the use of cell lines derived from immune tissues and characterized for their immunological profile, like e.g. the macrophage line RTS11 (105, 106) appears to be preferable for immunological research and testing.
Genetically modified fish cell lines have been employed to study the function of immune genes and their role in resistance of fish to pathogens (115), but they have not yet been applied for immunotoxicity testing. Ecotoxicological screening batteries to characterize the toxicity profile of chemicals or environmental samples have occasionally included immune-related reporter systems (116), however, those systems were based on either yeast or mammalian cells, but not on fish cells. In addition, the measured endpoints like NFκB signalling were selected as indicators of cellular stress rather than as immunotoxicity endpoint.
While in vitro systems have been developed for phagocytic and lymphocytic cells of the fish immune system, little attention has been given to the antigen-presenting dendritic cells which bridge innate and adaptive immunity. An early attempt was made by Ganassin and Bols (117) who established long-term rainbow trout spleen cultures which produced cells displaying the morphology and motility typical of dendritic cells. Bassity and Clark (118), adapting mammalian protocols for the generation of dendritic cells, succeeded in culturing non-adherent cells, which were classified as dendritic cells because of their motility, tree-like morphology, phagocytotic abilities and the expression of dendritic cell markers. Also Pettersen et al. (111) succeeded in establishing a fish cell line with dendritic-like properties. However, all these systems have not yet been applied for immunotoxicity studies.
Primary Immune Cells of Fish
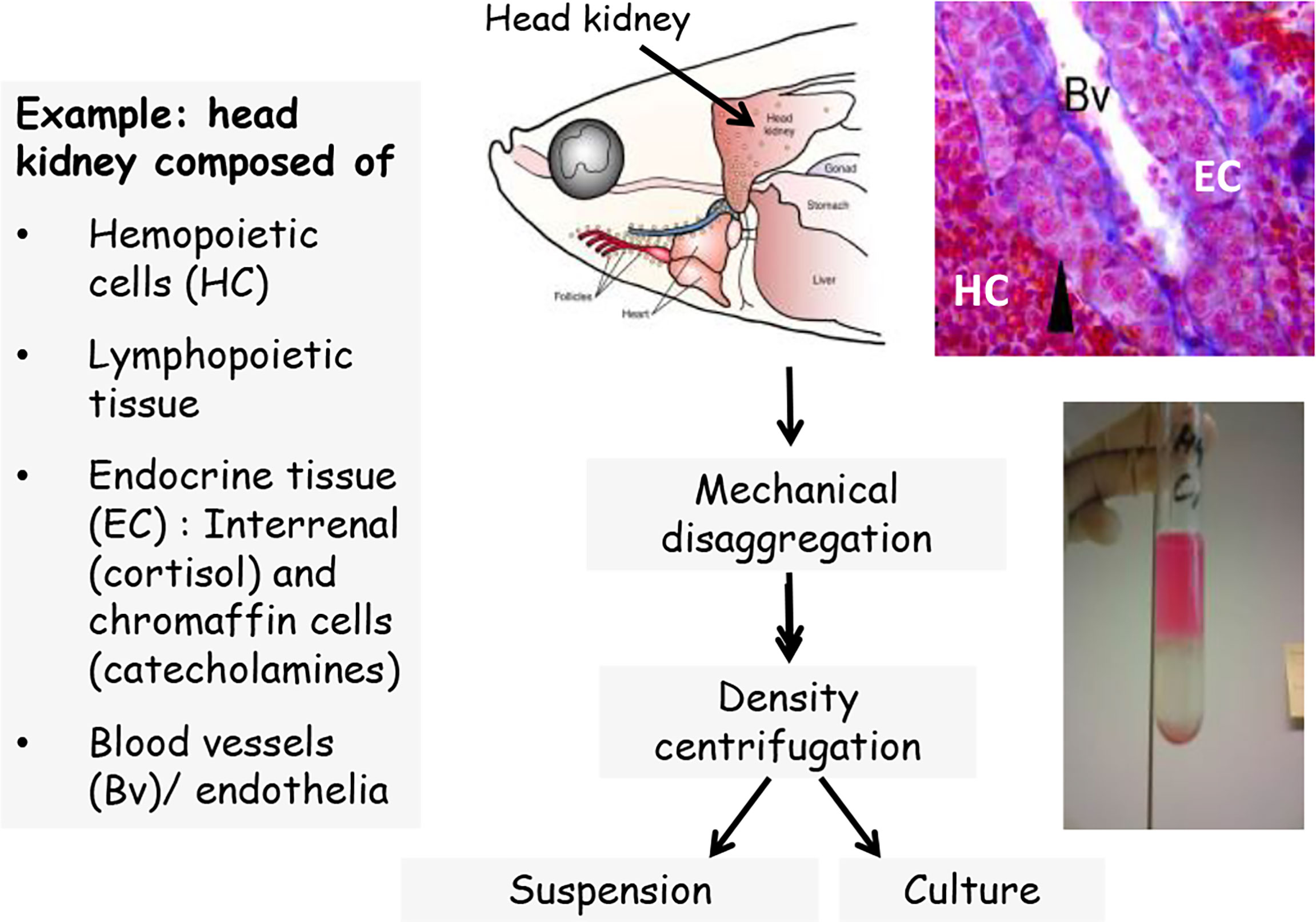
The leukocytes of fish include lymphocytes, polymorphonuclear granulocytes (e.g., neutrophils), mononuclear phagocytes (tissues macrophages and circulating monocytes), dendritic cells and natural killer cells (119, 120). The leukocytes differentiate from hematopoietic stem cells which give rise to the lymphoid and myeloid lineages (121). Methods for isolation of immune cells for in vitro studies are available for all immune organs of fish, including head kidney, trunk kidney, spleen, thymus, the lymphoid tissues in barrier organs like the gut as well as the blood and the peritoneal cavity [e.g. (122–129)]. The principal steps for isolating fish immune cells from lymphoid tissues involve the – usually mechanical – disaggregation of the organ, followed by density centrifugation and/or hypotonic lysis in order to separate the leukocytes from erythrocytes (Figure 1). Most density centrifugation methods used for immune cell isolation yield mixed leukocyte populations containing a variety of innate and adaptive immune cells. Subfractions enriched in specific immune cell types can be obtained through the choice of the density gradient used for the isolation step, or through separation steps during subsequent culture (79, 130, 131). For instance, innate and adaptive immune cells can be at least partly separated by culturing the cells overnight; then the phagocytes will attach to the culture plate while the lymphocytes will remain floating and can be washed away [e.g., (132, 133)]. Methods to characterize the composition of immune cell populations include cytochemical staining, immunostaining, flow cytometry or cell sorting [e.g., (61, 134–138)]. Importantly, the isolation method can influence the performance of the isolated cells (128).
After isolation and separation, the immune cells are maintained in vitro as suspension for a few hours, or they are cultured up to several days or weeks, either in suspension, as monolayer or as three-dimensional aggregate [e.g., (79, 127, 139–142)]. During culture, various factors like cell density, cell composition or medium composition influence the performance of the cells (128, 131, 142, 143). For instance, supplementation of the culture media with arginine or glutamine significantly enhanced the mitogenic response of naive T- and B-cells of channel catfish (144). Media factors can also trigger the further differentiation of cultured immune cells, for instance, the differentiation of head kidney leukocytes into specific macrophage sub-populations, (145, 146), or the polarization of macrophages into inflammatory M1 macrophages and anti-inflammatory M2 macrophages (147).
Application of Fish Cell-Based In Vitro Systems for Immunotoxicity Studies
Several reviews described basic principles of the piscine immune system, together with a discussion of the possible impacts of chemicals on fish immunity as well as the methodological approaches to test for immunotoxicity (26, 28–30, 32, 48). Here we focus on the use of in vitro assays in fish immunotoxicity assessment. As said above, a distinction must be made between ex vivo and in vitro approaches (32, 61, 79). In the first case, fishes are exposed in vivo, but afterwards the immune cells are isolated and their performance is studied in vitro. This approach reveals whether the in vivo exposure had consequences for the functional performance of the immune cells. The second approach is entirely in vitro. In the case of primary cells (see below), this means that the cells are isolated from control fish that have not been exposed to the toxicant. Still, in vivo factors like the nutritional status or the sex of the donor fish may influence the in vitro performance of the isolated immune cells [e.g., (148)]. Also the circadian time point of cell isolation may have an influence on the performance of the isolated cells (149).
A tool that might be of use for fish immunotoxicity assessment are fish embryos. Tests with embryonic life stages of fish are not considered as animal tests by law, and are increasingly used as alternatives to in vivo fish tests in the sense of the 3R (reduce, replace, refine) concept (150). Embryonic life stages of fishes have an at least partly functional immune system, for instance, in zebrafish, the innate immune system differentiates in the course of this developmental period (151, 152). Zebrafish embryos were extensively used as model organisms to study vertebrate hematopoietic development [e.g., (153)]. On that basis, zebrafish embryos may be well suitable as test system for assessing myelotoxic effects of chemicals in fish, however, this potential has been rarely used to date (154). In the present communication, we will not include fish embryos as immunotoxicity test systems, since we will strictly focus on in vitro methodologies.
Chemicals impact the immune system through direct interactions with immune cell survival, proliferation and functioning, and with the immune system communication. As a result, immunocompetence may get compromised (immunosuppression), leading to increased risk of infection and cancer. Alternatively, the toxic impact may cause immunostimulation, which can result in hypersensitivity, allergy or autoimmune reactions. Immunotoxicological research on fish focused to date almost exclusively on immunosuppressive effects, whereas immunostimulation, which is a frequent response of mammalian immunity to toxic exposure, either plays no role or has not been sufficiently studied in fish (32). Chemicals may also indirectly modulate the immune system, for instance, the costs incurred by the defense activities against the chemicals may trade-off with resource allocation to the maintenance and activation of the immune system. Further indirect effects of toxic chemicals on immunity can arise, for instance, when the chemicals cause cell damage and cell death in non-immune tissues, leading to the release of DAMPs (damage-associated molecular patterns) which then activate specific receptors on immune cells and trigger an immune responses (155).
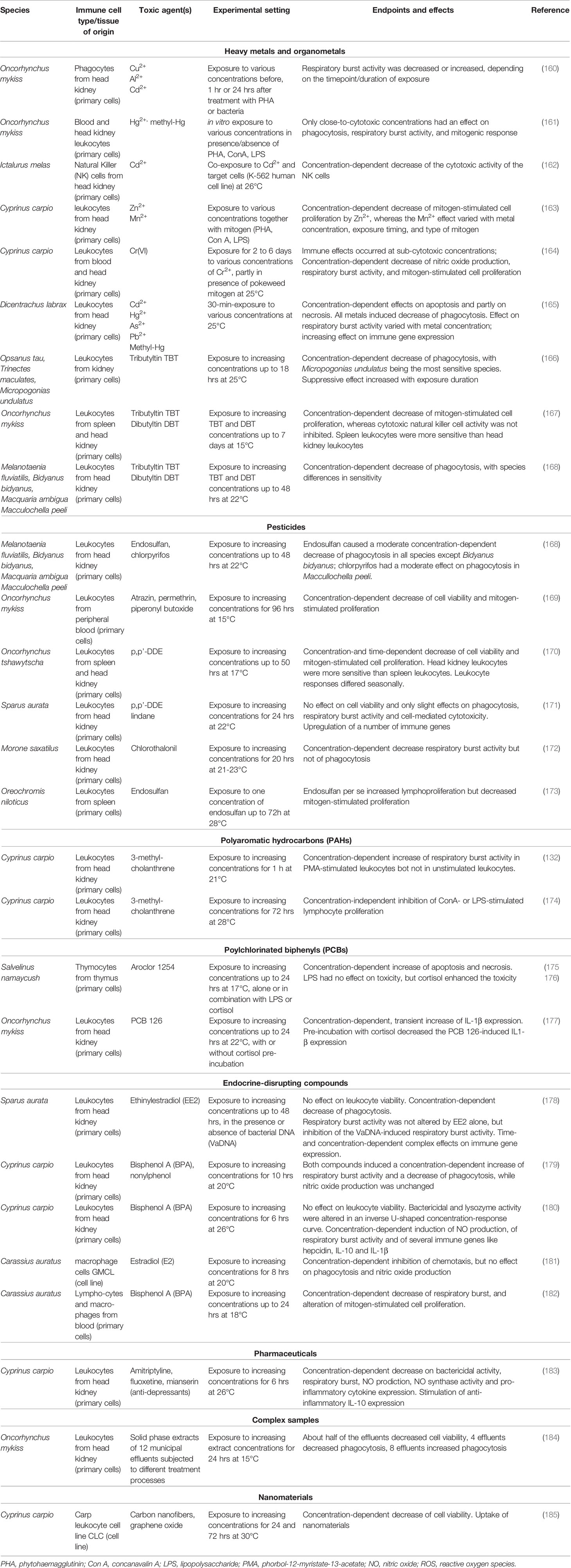
In vitro assays can detect direct chemical effects on cell viability, differentiation and proliferation as well as on cell functions (64, 68). The main potential immune cell targets are summarized in Figure 2. In the following we will discuss which in vitro cell systems and endpoints are available to assess immunotoxic activities of chemicals in fish. While a number of studies assessed immune endpoints, particularly immune gene expression, in non-immune cells of fish (90, 156–159), this review will focus on studies that employed immune cells. The vast majority of toxicological studies with fish immune cells were done using primary immune cells, while cell lines were rarely used (see also Table 2).
In Vitro Assays Related to Hematopoietic Progenitor Cells
Chemicals can cause a suppression in the production of progenitor cells that will differentiate into leukocytes, erythrocytes or thrombocytes. In mammals, this effect takes place in the bone marrow and is designated as bone marrow suppression or myelotoxicity. For the in vitro evaluation of myelotoxic activities of chemicals, bone marrow culture systems have been established which enable to assess proliferation and differentiation of pluripotent hematopoietic stem cells or of progenitors of specific blood cell lineages (186). An example is provided by the Colony-Forming Unit Granulocyte-Macrophage (CFU-GM) assay, which quantifies the number of surviving bone marrow progenitors as a function of chemical concentration (64, 186). Assessment of myelotoxicity is used as first step in a tiered in vitro immunotoxicity testing; if a chemical is found to be myelotoxic, this means that the organisms will no longer be able to produce immune cells and sustain a functional immune system. Therefore a further testing is no longer necessary (68).
In fish, the main hematopoietic organs include head kidney, spleen, and trunk kidney, their relative importance varying across species (121). Primary culture systems that support the proliferation and differentiation of piscine hematopoietic stem cells have been established from spleen and kidney (79, 117, 187–189). Although these systems hold promise for immunotoxicological studies, they have not been used yet for this purpose.
In Vitro Assays Related to Epithelial Barrier Immunity
Mucosal epithelia establish a barrier between the environment and the internal milieu of the organism. Regulation of immunity at the barrier epithelia is critical to preserving host integrity. It is here where the immune cells and the mucosa-associated lymphoid tissues (MALT) are initially exposed to external factors including toxicants. Mucosal epithelia also harbour microbial communities, the microbiome, which influence mucosal homeostasis and immune function. Immune responses of epithelial barriers involve an intricate network of molecular pathways, epithelial cells, intraepithelial immune cells as well as the microbiome (190–192).
Toxicants can impair the epithelial barrier immunity of fish (27, 193–195). The challenge in using in vitro systems for studying toxic effects on mucosal immunity is that they have to reproduce the integration and interaction of immune processes as they occur the in vivo setting. Most in vitro models lack organ architecture, thereby reducing the possibilities for cell-cell interactions and interactions between the different components driving the immune response. Several approaches were developed over recent years to overcome these difficulties. Typically, cell lines such as the rainbow trout RTgutGC line are cultured on semipermeable supports in order to establish tight, polarized epithelia (196–198). The realism of these systems can be improved by combining different cell lines, by modifying the culture environment or by including microbial communities (199, 200). These in vitro systems have been successfully used to study the impact of toxicants on mucosal biotransformation and oxidative stress pathways (157, 158). In addition, they are responsive to immune stimuli such as lipopolysaccharide, and are able to upregulate pro-inflammatory cytokines (159). A logical next step could be, analogous to what is done with in vitro models of mammalian mucosal barriers [e.g. (201)], to include immunocompetent cells into the epithelial layers. Overall, three-dimensional in vitro systems, particularly when incorporating immune cells, hold promise as tools for studying xenobiotic effects on mucosal immunity of fish.
Phagocyte-Based In Vitro Assays
The blood cells of teleost fish consist of erythrocytes (> 90%), thrombocytes and leukocytes (120). Fish thrombocytes may exert leukocyte-like functions, but this is discussed controversially (120, 202). Since they have not been used for in vitro immunotoxicity assays, we will not further consider them here.
Phagocytic cells of the innate arm of the teleostean immune system arise from myeloid stem cells and include monocytes/macrophages and granulocytes (eosinophilic, basophilic, neutrophil) (26, 28, 30, 120, 203). Granulocytes and monocytes/macrophages migrate to pathogens, and incorporate and kill them. They are present in the peripheral blood from where they can invade (injured) tissues. Monocytes, after migrating out of the blood into tissues, differentiate into macrophages. There exist also resident phagocytic cell populations, for instance, microglial cells in the brain or Kupffer cells in the liver, which form the so-called reticuloendothelial system (RES) (28). The majority of in vitro fish immunotoxicity studies used cells isolated from the head kidney and the peripheral blood (129). Importantly, the isolated cell populations may show significant functional differences, depending on their cellular composition, their tissue of origin, the physiology of the donor fish, and/or the culture microenvironment (120). Also cell lines with properties of phagocytic cell types are available, for instance, the goldfish macrophage cell line GMLC (181), or the monocyte-macrophage cell line RTS11 from rainbow trout (105), however, innate immune cell lines have been rarely applied in immunotoxicity studies. The most frequently used in vitro system to investigate chemical impacts on innate immune functions of fish are freshly isolated or cultured phagocytes. As pointed out by Fournier et al. (204) and Bols et al. (79), phagocytes have properties especially useful in the context of ecotoxicology. Phagocytosis is conserved in all animals what allows cross-species comparisons. Often phagocytes can be collected by non-lethal techniques. In the intact animal, exposure of phagocytes to xenobiotics is assured because phagocyte populations are found at all potential site of xenobiotic entry (gills, gut, skin). Because of their capability for pino- and phagocytosis, phagocytes can take up not only dissolved but also particulate foreign materials and protein-bound chemicals. Finally, phagocytes are able to metabolize xenobiotics (135, 205).
The fish phagocyte populations used for in vitro studies are often of mixed composition, i.e. they contain hematopietic precursor cells, monocytes/macrophages, and various types of granulocytes. For instance, Ribas et al. (206) reported that a phagocyte population isolated from the head kidney of the freshwater fish, Hoplias malabaricus, was composed of 71% hematopoietic precursor cells, 19% macrophages and 9% monocytes. The endpoints most frequently measured in in vitro toxicity studies with fish immune cells include chemical effects on cell viability, phagocytotic activity, respiratory burst activity and immune gene expression (32, 79, 129).
Cytotoxicity is commonly understood in the sense that the cell is killed by the chemical agent. If the chemical perturbs the metabolic or structural integrity of the cell, this can cause cell death. Toxicants can also trigger physiological cell death, i.e., apoptosis, as it has been shown, for instance, for organochlorine contaminants and polyaromatic hydrocarbons (PAHs) (175, 207). Methods to determine cytotoxicity are principally based on (i) the assessment of cell membrane integrity, e.g., by determining the exclusion of dyes such as trypan blue, (ii) the retainment of intracellular components such as cytoplasmic enzymes, or (iii) the measurement of the cellular metabolic activity (78, 208). In numerous in vitro fish immunotoxicological studies, such methods have been applied to evaluate at which concentration the test chemical causes the death of the immune cells [e.g., (161, 165, 172, 209, 210), see also Table 2]. It is important to distinguish between the cytotoxic and concentration of a test chemical and the concentration at which it causes specific immune functional effects, e.g., altered phagocytotic activity. The cytotoxic concentration of a test chemical activates numerous non-specific defense and lethality mechanisms. This concentration represents no specific immunotoxic activity, but general baseline cytotoxicity (208, 211, 212). To test for specific immunotoxic effects in vitro, it is essential to apply non-cytotoxic concentrations in order to avoid false positive responses caused by the interference of the cytotoxic concentrations with cell viability (213, 214).
Phagocytosis is a non-specific immune function which refers to the cellular uptake and intracellular processing of pathogens, foreign particles, cellular debris and macromolecules. Among immune cells, particularly cells of the innate arm such as monocytes, macrophages and granulocytes possess phagocytic capabilities, but in fish also B cells and thrombocytes display phagocytic activities (202, 215–217). The analysis of chemical-induced suppression of phagocytosis is of toxicological relevance as it can disturb the clearance of pathogens, the processing and presentation of antigens as well as cytokine secretion and immune system communication, what may result in a compromised immunocompetence of the organism. Methodologically, phagocytotic activity is determined by measuring the ingestion of (inactivated) bacteria or plastic beads. Often these materials are fluorescently labelled so that their uptake can be monitored by flow cytometry, in fluorescent plate readers, or by fluorescence microscopy (129, 218–221). Assay conditions such as incubation time or particle concentration, the analytical method as well as whether the phagocytes are activated or resting influence the results of phagocytosis assays (184, 222). Also the use of appropriate controls to distinguish between particles adhered to the surface of the cells or ingested by the cells is mandatory (214, 217). Importantly, toxicant effects on phagocytosis may be detectable only after activation of the cells by exposure to pathogenic signals such as bacterial lipopolysaccharide (LPS); the activation can lead to enhanced phagocytosis, enhanced production of reactive oxygen intermediates and nitric oxide, and enhanced secretion of pro-inflammatory cytokines (IL-1 TNFa, IL-6, IL-12, IL-1 [e.g., (141, 214, 223, 224)]. The results of the phagocytosis assay are presented as percentage of phagocytosing cells in the cell population, or as number of particles engulfed per cell (26, 61, 184, 225). This assay provides a simple assessment of an important (innate) immune mechanism, and consequently, it has been frequently used for in vitro immunotoxicity studies with innate immune cells of fish. Diverse groups of chemicals as well as natural toxins and micro/nanoparticles have been shown to modulate the phagocytic activity of the cells. Generally a trend for suppression of phagocytosis has been observed in in vitro immunotoxicity studies with innate immune cells of fish; only rarely a stimulation has been reported (226). Remarkably, the in vitro assays appear to reflect species as well as sex differences in the phagocytic response to toxicants (168, 227).
Respiratory or oxidative burst by phagocytes involves the production of reactive oxygen species (ROS) as well as nitrogen radicals (NO). The ROS reaction is catalysed by a NADPH oxidase complex (228, 229). The radicals produced by the respiratory burst serve to kill invading microorganisms, either extracellularly, or intracellularly after phagocytosis, and thus respiratory burst has an important role in the immune response towards pathogens (230). In studies with fish leukocytes, frequently used methods to measure the respiratory burst activity are the reduction of the dye nitroblue tetrazolium, and the luminal-enhanced chemoluminescence (27, 61, 79, 231). Various stimulants have been used to induce phagocyte respiratory burst, including pathogen-derived PAMPs, or agents such as concavalin A, zymosan phorbol myristate acetate (PMA), with the choice of the stimulating agent being of relevance for the response of the cells against pathogens and toxicants (27, 61, 129, 232–234). Analysis of the respiratory burst activity has been frequently applied in immunotoxicity studies with isolated fish phagocytes (see Table 2). While for phagocytosis activity, generally suppressive effects of toxicants have been reported, for respiratory burst activity also chemical-induced activation was observed, particularly for estrogenic endocrine disruptors, but the effects vary largely with exposure conditions and whether the immune cells are resting or stimulated cells (cf. Table 2). For instance, in vitro exposure to Ni2+ did not affect the respiratory burst activity of activated peritoneal macrophages of rainbow trout, but it affected the basal ROS production of these cells (235). Also biological factors such as species differences influence the results of the in vitro studies (27, 236) as well as the purity of the test agent (237). Toxicants can also potentiate the induction of phagocyte ROS production by stimulants such as PMA, as it has been shown by Reynaud et al. (132) for the PAH, 3-methylcholanthrene (3MC). This potentiating effect could involve 3MC biotransformation – as phagocytes possess the capacity for xenobiotic metabolism (135) - and/or modulation of intracellular Ca levels. This example may illustrate the complexity of mechanisms through which chemicals can modulate phagocyte functions.
The examination of immune gene expression is increasingly used for in vitro studies on toxic effects on fish phagocytes, both with primary cells and with cell lines. Methodologically, RT-PCR methods for the measurement of individual genes and global transcriptomic analyses have been applied. As with phagocytosis and respiratory burst, transcriptomic responses of fish phagocytes are largely influenced by biological factors and experimental/technical settings. Mainly pro-inflammatory cytokines such as TNF-α, IL-1β, or IL-6 were studied, but also anti-inflammatory cytokines such as IL-10 (see Table 2). The transcriptomic analyses revealed the regulation of key immune signalling pathways such as NFκB, ERK1/2, Toll-like receptor, or B cell receptor by the toxicants, often together with cellular stress-related pathways like Jak-STAT (173, 180, 183, 238). Gene expression approaches were also applied for mechanistic studies, for instance, to unravel the role of the estrogen receptors (ERs) or the peroxisome proliferator-activated receptor,PPARγ, in mediating chemical effects on immune functions, or to study the interaction between toxicants and hormones of the stress axis in modulating immune gene expression (177, 180, 210, 239, 240).
Further endpoints that have been used, although relatively rarely, for in vitro immunotoxicity studies with piscine phagocytes include the assessment of phagocyte chemotaxis and bactericidal assays. In order to engulf and incorporate pathogens, phagocytes have the ability of chemotaxis, and this property appears to be sensitive to the action of toxicants (181, 241). Ni2+, for instance, altered the migration of rainbow trout macrophages in vitro (235). The bactericidal or bacterial killing assay measures the capacity of isolated phagocytes to kill bacteria. It has been applied, for instance, to examine the immunosuppressive effect of pentachlorophenol on phagocytes of Fundulus heteroclitus (242).
Lymphocyte-Based In Vitro Assays
The lymphoid lineage of leukocytes includes B-lymphocytes, natural killer (NK) cells and T lymphocytes, the latter being composed of cytotoxic T cells, T helper cells and regulatory T cells (26, 28, 30, 243–245). The T cells of teleost fish display gene expression patterns that resemble the T cell subpopulations known from mammals, namely cytotoxic (CD8), helper (CD4) and regulatory (Treg) T cells (203, 246). Also teleost B cells are composed of diverse subsets, and express different heavy Ig chain classes, including IgM, Ig T/Z, and IgD (247, 248). NK cells, like the cytotoxic T cells, initiate the killing of altered, tumorous and infected cells, but different to cytotoxic T cells, they do not depend on specific antigen presentation for recognizing infected cells. When NK cells were first described in fish, they were designated as non-specific cytotoxic cells (NCC). While the NK cells belong to the innate arm of the immune system, the cytotoxic T cells belong to the adaptive arm. Evolutionary-wise, there appears to be a homology between teleost lymphocytes and mammalian innate-like lymphocytes (249).
To assess immunotoxic effects on fish lymphocytes in vitro, typically no purified or enriched lymphocyte cultures are used, but mixed leukocyte cultures, which are then treated under conditions that elicit B or T cell-specific responses. An example is provided by the widely used lymphoproliferation or lymphocyte blastogenesis assay. When lymphocytes are challenged with a pathogen, they undergo proliferation. The immunocompetence of a fish will be compromised if a toxic agent suppresses the functional ability of lymphocytes to proliferate. To assess whether a chemical can inhibit lymphocyte proliferation, leukocytes isolated from blood or from lymphoid organs are exposed during in vitro culture to mitogens, and the magnitude of cell proliferation is then measured as endpoint. Commonly used mitogens for T cells include the plant lectins, phytohaemagglutinin (PHA) and concanavalin A, while for B cells, lipopolysaccharide (LPS) from the wall of gram-negative bacteria is used, and pokeweed mitogen serves as mixed mitogen for B and T cells (79). Methods to measure the induced cell proliferation include the DNA-incorporation of radiolabeld thymidine or thymidine analogues, the determination of the cells undergoing mitosis (mitotic index), or colorimetric methods (61, 250–252). Also flow cytometry is a frequently used method (253), also because it provides the option to determine the proliferation of specific lymphocyte subpopulations (254). The proliferative response obtained in in vitro blastogenesis assays varies with a number of factors including the mitogen type and concentration, the length of incubation time or the composition of the culture medium (79). The lymphoproliferation assay has been widely applied in the immunotoxicity assessment of diverse compounds including metals, PAHs, endocrine disruptors or natural toxins [(163, 172, 174, 182, 255–259), see also Table 2].
As an indicator of the functionality of the antibody producing B cells, the plaque forming cell (PFC) assay can be used (260). Although the plaque formation is determined in vitro, the assay is, strictly speaking, an ex vivo assay, since the fish has to be injected with the antigen – mostly sheep red blood cells - in vivo. The toxicant exposure is usually also done in vivo. Only occasionally the assay is performed fully in vitro (261), but to the best our knowledge, this approach has not been used for toxicity studies. Since it is not an entirely in vitro test, the PFC assay will not be discussed here.
In vitro approaches were also used to study toxic impacts of chemicals on thymocyte precursor cells. Sweet et al. (175) found that exposure of isolated thymic cells of Lake trout (Salvelinus namaycush) to various organochlorine contaminants (Aroclor 1254, hexachlorocyclohexane isomers) displayed high levels of apoptosis. Subsequent studies confirmed these findings and showed that the presence of cortisol enhanced the chemical toxicity (176).
NK cells and cytotoxic T cells are responsible for the cell-mediated cytotoxicity (CMC). For various in vitro preparations of fish immune cells, an allogeneic and xenogeneic activity has been demonstrated. These preparations contained cells expressing markers of NK cells and/or cytotoxic T cells (cf. 203). For NK cells, methods for their isolation and primary culture have been developed (79, 262). While a number of studies used ex vivo approaches to investigate toxic effects on fish NK cells [e.g., (263)], toxic effects on NK cells have been very rarely studied using in vitro systems. An example is the work of Viola et al. (162) who showed that Cd had an inhibitory effect on the ability of catfish NK cells to kill foreign cells of a human cell line.
Perspectives in Using In Vitro Systems for Immunotoxicity Assessment With Fishes
The growing evidence that diverse environmental contaminants including pharmaceuticals, endocrine disruptors, polyaromatic hydrocarbons, organochlorines, or plastic materials can interfere with immune functioning of fish argues for a consideration of immunotoxicity in the ecotoxicological hazard assessment. However, as recently pointed out by Johnson et al. (2) adding more and more tests to the existing battery of the Organisation of Economic Cooperation and Development (OECD) battery of ecotoxicity tests is not realistic, for both practical and ethical reasons. Here, mode of action- and mechanism-oriented screening and toxicity profiling strategies increasingly rely on in vitro assays as rapid, non-animal and cost-effective tools, as exemplified by the US EPA ToxCast Program for monosubstancess [e.g., (213, 264)] or by bioanalytical approaches for complex environmental samples [e.g., (52, 265)]. Such approaches could well integrate in vitro assays for immunotoxicity screening. In fact, the ToxCast Programme includes already a number of human cell-based assays that express genes or proteins of the innate and adaptive immune responses, although functional immune endpoints are not yet included (266). Here, we aimed to evaluate the availability and potential of fish-based in vitro assays to screen for immunotoxic activities of environmental agents to fish.
In Vitro Assays With Fish Immune Cells for Mechanistic Studies
In vitro systems are well suitable to investigate the mechanisms through which toxicants interfere with immune cell functions. This is illustrated, for instance, by studies on the interference of PAHs with Ca and cAMP signalling and biotransformation processes of fish immune cells (132, 135, 267), the influence of endocrine disruptors and pesticides on signal transduction pathways (180, 183, 240, 268), the interference of toxicants with cell differentiation processes (259), or by studies that examine whether immunotoxic effects are caused by a direct action of a chemical on the immune cells or whether they may be caused by indirect effects (269). Also toxicokinetic aspects such as determinants for binding and uptake of toxic agents by immune cells can well be examined using in vitro systems (222, 227). In addition, in vitro systems offer the possibility to compare immunotoxic processes at the cellular level under equivalent conditions across species (79, 168, 236). Likewise, in vitro systems provide the option to study cell type-specific responses to toxic agents, e.g., to compare phagocytes versus lymphocytes, to compare leukocytes originating from different immune organs, or to compare immune cells versus stromal cells (141, 182, 270). Finally, the role of the physiological condition of the donor fish in the immune cell response can be detected. For instance, Ottinger and Kaattari (271) showed that the sensitivity of rainbow trout leukocytes to aflatoxin varied with the season, i.e. cells prepared from fish during January to June were significantly less sensitive than those from July to December.
In Vitro Assays With Fish Immunce Cells to Screen for Immunotoxic Activities of Environmental Agents
The relatively few in vitro toxicity studies with fish immune cells are dispersed between toxicant classes, toxic modes of action, immune cell types, immune endpoints, fish species and assay conditions. For instance, for the group of arylhydrocarbon receptor (AhR)-binding PCBs, which are well known immunotoxicants impacting the health and disease status of wild fish populations [cf. (272)], there is fairly good number of ex vivo studies available, however, to the best of our knowledge only six studies have investigated the PCB effects on fish immune cells in vitro. Quabius et al. (177) exposed primary cultures of rainbow trout anterior kidney cells to the AhR-binding PCB 126, and observed a transient, but significant induction of the cytokine IL-1β. The induction was potentiated by the presence of LPS. Zhang et al. (273, 274) exposed isolated Carassius auratus lymphocytes to PCBs and found that the EC50 for apoptosis was higher for higher chlorine substitution, and for coplanar than for non-planar structure. Vazzana et al. (275) treated isolated lymphocytes of sharpsnout sea bream, Diplodus puntazzo, with Aroclor 1254, a PCB mixture, and observed an enhanced respiratory burst activity of the cells. Sweet et al. (175) and Miller et al. (176) exposed thymocytes of Lake trout to Aroclor 1254 and found that this resulted in a significant increase of thymocyte apoptosis. Such a data set, while valuable, is too fragmented and limited to come up with an at least partly conclusive immunotoxicity profile of AhR-binding PCBs in fish, or recommendations for the most appropriate assay for PCB immunotoxicity screening.
Further factors that currently limit the utility of piscine in vitro assays for immunotoxicity screening include the lack of assay standardization (79) as well as the still limited repertoire of in vitro immunoassays. Currently there exist no fish correlates for important screening assays as they are frequently used in human immunotoxicology, for instance, myelotoxicity assays, the NK killing assay, fluorescent cell chip assays or assays assessing multiple immune endpoints [cf. (60, 65, 68)].
A critical question in the use of in pisicine vitro assays for immunotoxicity screening is whether they correctly classify test agents as potentially immune-active or –inactive, i.e. if they produce false positives or false negatives. Rehberger et al. (214) tested five immunotoxic chemicals and two non-immunotoxic chemicals at sub-cytotoxic concentrations using an in vitro assay with head kidney leukocytes of rainbow trout. The five immunotoxicants were correctly classified as immunotoxicants, although the pharmaceutical diclofenac elicited a rather weak response (pointing to the need of using a test battery, since depending on the mode of immunotoxic action, different in vitro assays will show different sensitivities). However, the two non-immunotoxic chemicals, displaying a narcotic mode of action – butanol and ethylene glycol – also induced an immune response. This false positive result may be explained by an overlap of the cellular immune response with the cellular stress response. The two cellular responses share a number of receptors and signalling pathways (276), many of them being located or associated with the cell membrane. Chemicals with narcotic mode of action interfere with the cell membrane organization and fluidity, and this activates stress pathways (277) which then may converge with immune-related receptors and signalling pathways (214). If the hypothesis of an interference between the cellular stress and immune responses is correct, this would represent a principal obstacle in using cell-based assays for in vitro immunotoxicity screening.
In Vitro Assays With Fish Immunce Cells to Predict In Vivo Immunotoxicity in Fish
The discussion on false positives and negative results leads to the question how (qualitatively) predictive the in vitro results are for the in vivo immunotoxic action of environmental agents. Again, the available fragmentary database makes it difficult to provide a conclusive answer. For instance, the in vitro findings on the apoptotic effects of PCBs on fish thymocytes do well agree with the established suppressive effect of PCBs on the fish thymus in vivo (cf. 272). In contrast, the in vitro observations that PCBs induce the respiratory burst activity of leukocytes (273–275) is not in line with reports that in vivo PCB exposure of fish results in the suppression of the respiratory burst activity (263, 278). Rehberger et al. (32) performed a meta-analysis of published in vitro and in vivo immunotoxicological studies with fishes and found some correlations but also numerous misfits. The authors emphasized that the robustness of the correlations across the different studies was weak due to the low number of data points. Also when analysing only those publications that included a direct in vivo-in vitro comparison within the same study, for instance, the study of Cabas et al. (178) who compared the immunological effects of an estrogen-active endocrine disruptor in gilthead seabream (Sparus aurata) in vivo and in the isolated head kidney leukocytes in vitro, a conclusive answer was not possible because the number of studies was too low.
In human toxicology, the regulatory assessment of immunotoxicity still exclusively relies on in vivo tests, although the use of in vitro approaches for the prediction of direct immunosuppressive effect is increasingly discussed (68, 186, 279). A decision tree approach has been suggested for the in vitro assessment of chemical-induced immunosuppression which combines different cell systems and endpoints in a tiered manner (60, 68). Another approach is the incorporation of in vitro assays to test key events in the context of Adverse Outcome Pathways (AOPs), as it has been applied for skin sensitisation safety assessment (76). The currently limited database available for fish in vitro immunotoxicity assays would not support the identification of the most appropriate assays for such a tiered testing strategy. Also, AOPs that could integrate in vitro measurements of immune endpoints, comparable to the skin sensitisation AOP in humans, do currently not exist for fish. This does not mean that the application of in vitro testing for immunotoxicity assessment in fish is principally not possible, but it simply means that the existing database is too limited and it would need substantial and systematic research efforts to fill in the existing knowledge gaps. The recent years have seen substantial progress in the utilization of in vitro methodologies for ecotoxicological hazard assessment (280–282), but for the field of immunotoxicology we are not there yet.
Author Contributions
HS wrote a first draft of the manuscript, which was then edited and further developed by KR, CB, and BJ. All authors have read and agreed to the published version of the manuscript.
Funding
HS was supported by the grants 310030E-164266, 31003A_153427, and 31003A_130640 from the Swiss National Science Foundation SNSF. CB was funded by Swiss National Science Foundation (SNSF) Post Doc Mobility Fellowship number P400PB_183824. BJ was supported by the National Natural Science Foundation of China under the number 4197721.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Calow P, Forbes VE. Does Ecotoxicology Inform Ecological Risk Assessment? Environ Sci Technol (2003) 37:146A–51A. doi: 10.1021/es0324003
2. Johnson AC, Jin X, Nakada N, Sumpter JP. Learning From the Past and Considering the Future of Chemicals in the Environment. Science (2020) 367:384–7. doi: 10.1126/science.aay6637
3. Roex EWM, van Gestel AP, van Wezel AP, van Straalen NM. Ratios Between Acute Aquatic Toxicity and Effects on Population Growth Rates in Relation to Toxicant Mode of Action. Environ Toxicol Chem (2001) 19:685–93. doi: 10.1002/etc.5620190321
5. Stark JD. How Closely do Acute Lethal Concentration Estimates Predict Effects of Toxicants on Populations? Integr Environ Assess Manag (2005) 1:109–13. doi: 10.1897/IEAM_2004-002r.1
6. Relyea R, Hoverman J. Assessing the Ecology in Ecotoxicology: A Review and Synthesis in Freshwater Systems. Ecol Lett (2006) 9:1157–71. doi: 10.1111/j.1461-0248.2006.00966.x
7. Baldwin DH, Spromberg JA, Collier TK, Scholz NL. A Fish of Many Scales: Extrapolating Sublethal Pesticide Exposures to the Productivity of Wild Salmon Populations. Ecol Appl (2009) 19:2004–15. doi: 10.1890/08-1891.1
8. Segner H. Moving Beyond a Descriptive Aquatic Toxicology: The Value of Biological Process and Trait Information. Aquat Toxicol (2011) 105:50–5. doi: 10.1016/j.aquatox.2011.06.016
9. Van den Brink PJ, Alexander A, Desrosiers M, Goe3dkoep W, Goethals P, Liess M, et al. Traits-Based Approaches in Bioassessment and Ecological Risk Assessment: Strengths, Weaknesss, Opportunities and Threats. Integr Environ Assess Manag (2011) 7:198–208. doi: 10.1002/ieam.109
10. Hamilton PB, Cowx IG, Oleksiak MF, Griffiths AM, Grahn M, Stevens JR, et al. Population-Level Consequences for Wild Fish Exposed to Sub-Lethal Concentrations of Chemicals a Critical Review. Fish Fish (2016) 17:545–66. doi: 10.1111/faf.12125
11. Murphy CA, Nisbet RM, Antczak P, Garcia-Reyero N, Gergs A, Lika K, et al. Incorporating Suborganismal Processes Into Dynamic Energy Budget Models for Ecological Risk Assessment. Integr Environ Assess Manag (2018) 14:615–24. doi: 10.1002/ieam.4063
12. Van Straalen NM. Biodiversity of Ecotoxicological Responses in Animals. Netherlands J Zool (1994) 43:112–29. doi: 10.1163/156854294X00097
13. Todgham AE, Stilman JH. Physiological Responses to Shifts in Multiple Environmental Stressors: Relevance in a Changing World. Integr Comp Biol (2013) 53:539–44. doi: 10.1093/icb/ict086
14. Lazarro BP, Little TJ. Immunity in a Variable World. Philos Trans R Soc B (2009) 364:15–26. doi: 10.1098/rstb.2008.0141
15. Schulenburg H, Kurtz J, Moret Y, Siva-Jothy MTR. Introduction. Ecological Immunology. Philos Trans R Soc B (2009) 364:3–14. doi: 10.1098/rstb.2008.0249
16. Graham AL, Shuker DM, Pollitt LC, Auld SK JR, Wilson AJ, Little TJ. Fitness Consequences of Immune Responses: Strengthening the Empirical Framework for Ecoimmunology. Funct Ecol (2011) 25:5–17. doi: 10.1111/j.1365-2435.2010.01777.x
17. Matzinger P. The Danger Model: A Renewed Sense of Self. Science (2002) 296:301–5. doi: 10.1126/science.1071059
18. Feist SW, Longshaw M. Histopathology of Fish Parasite Infections – Importance for Populations. J Fish Biol (2008) 73:2143–60. doi: 10.1111/j.1095-8649.2008.02060.x
19. Bonaccio M, di Castelnuovo A, Pounis G, de Curtis A, Costanzo S, Persichillo M, et al. A Score of Low-Grade Inflammation and Risk of Mortality: Prospective Findings From the Moli-Sani Study. Haematologica (2016) 101:1434–41. doi: 10.3324/haematol.2016.144055
20. Dai YJ, Cao XF, Zhang DD, Li XF, liu WB, Jiang GZ. Chronic Inflammation Is a Key to Inducing Injury in Blunt Snout Bream (Megalobrama Amblycephala) Fed With a High Fat Diet. Dev Comp Immunol (2019) 97:28–37. doi: 10.1016/j.dci.2019.03.009
21. Sheldon BC, Verhulst S. Ecological Immunology: Costly Parasite Defences and Trade-Offs in Evolutionary Ecology. Trends Ecol Evol (1996) 11:317–21. doi: 10.1016/0169-5347(96)10039-2
22. Pacioni C, Eden P, Reiss A, Ellis T, Knowles G, Wayne AF. Disease Hazard Identification and Assessment Associated With Wildlife Population Declines. Ecol Manag Restor (2015) 16:142–52. doi: 10.1111/emr.12155
23. Ferguson LV, Kortet R, Sinclair BJ. Eco-Immunology in the Cold: The Role of Immunity in Shaping the Overwinter Survival of Ectotherms. J Exp Biol (2018) 221:jeb163873. doi: 10.1242/jeb.163873
24. Wernicke von Siebenthal E, Rehberger K, Bailey C, Ros A, Herzog EL, Segner H. Trade-Offs Under Water: Physiological Plasticity of Rainbow Trout (Oncorhynchus mykiss) Confronted by Multiple Stressors. Fishes (2018) 3:49. doi: 10.3390/fishes3040049
25. Song H, Xu D, Tian L, Chen R, Wang L, Tan P, et al. Overwinter Mortality in Yellow Drum (Nibea albiflora): Insights From Growth and Immune Response to Cold and Starvation Stress. Fish Shellfish Immunol (2019) 92:341–7. doi: 10.1016/j.fsi.2019.06.030
26. Anderson DP, Zeeman MG. Immunotoxicology in Fish. In: Rand GM, author. Fundamentals of Aquatic Toxicology. Washington: Taylor & Francis (1995). p. 371–92.
27. Bols NC, Brubacher JL, Ganassin RC, Lee LEJ. Ecotoxicology and Innate Immunity in Fish. Dev Comp Immunol (2001) 25:853–73. doi: 10.1016/S0145-305X(01)00040-4
28. Rice CD. Fish Immunotoxicology. In: Target Organ Toxicity in Marine and Freshwater Teleosts. Abingdon, UK: Taylor & Francis (2001). p. 96–138.
29. Burnett KG. Impacts of Environmental Toxicants and Natural Variables on the Immune System of Fishes. In: Mommsen TP, Moon TW, authors. Biochemistry and Molecular Biology of Fishes, vol. 6. Amsterdam, NL: Elsevier (2005). p. 231–53.
30. Carlson E, Zelikoff J. The Immune System of Fish. In: The Toxicology of Fishes. Boca Raton FL: CRC Press (2008). p. 489–529.
31. Poulsen AH, Escher BI. Chemically-Induced Immunosuppression and Disease Susceptibility in Marine Wildlife: A Literature Review. The University of Queensland, Australia: National Centre for Envrionmental Toxicology (Entox (2012).
32. Rehberger K, Werner I, Hitzfeld B, Segner H, Baumann L. 20 Years of Fish Immunotoxicology – What We Know and Where We Are. Crit Rev Toxicol (2017) 47:509–35. doi: 10.1080/10408444.2017.1288024
33. Kataoka C, Kashiwada S. Ecologcial Risks Due to Immunotoxicologcial Effects on Aquatic Organsisms. Int J Mol Sci (2021) 22:8305. doi: 10.3390/ijms22158305
34. International Council for the Exploration of the Sea ICES. Effects of Contaminants on the Immune System in Fish and Shellfish. Report (2005). Available at: www.ices.dk7sites/pub/Publication%20Reports/Advice/2005/may/Immune%20System.pdf.
35. Rastgar S, Ardeshir RA, Segner H, Tyler CR, Peijnenburg WJGM, Wang Y, et al. Immunotoxic Effects of Metal-Based Nanoparticles in Bivalves and Fish.
36. Luebke RW, Hodson PV, Faisal M, Ross PS, Grasman KA, Zelikoff JT. Aquatic Pollution-Induced Immunotoxicity in Wildlife Species. Fundam Appl Toxicol (1997) 37:1–15. doi: 10.1006/faat.1997.2310
37. Arkoosh MR, Collier TK. Ecological Risk Assessment Paradigm for Salmon: Analyzing Immune Function to Evaluate Risk. Hum Ecol Risk Assess (2002) 8:265–72. doi: 10.1080/20028091056908
38. Iwanowicz LR, Blazer VS, Hill NP, McCormick SD, DeVault DS, Ottinger CA. Histologic, Immunologic and Endocrine Biomarkers Indicate Contaminant Effects in Fishes of the Ashtabula River. Ecotoxicology (2012) 21:165–82. doi: 10.1007/s10646-011-0776-0
39. Whitehead A. Interactions Between Oil-Spill Pollutants and Natural Stressors can Compound Ecotoxicological Effects. Integr Comp Biol (2013) 53:635–47. doi: 10.1093/icb/ict080
40. Gao Y, Xu H, Li L, Niu C. Immune Defense Parameters of Wild Fish as Sensitive Biomarkers for Ecological Risk Assessment in Shallow Sea Ecosystems: A Case Study With Wild Mullet (Liza haematocheila) in Liaodong Bay. Ecotoxicol Environ Saf (2020) 194:11033. doi: 10.1016/j.ecoenv.2020.110337
41. Hoeger B, van den Hevel MR, Hitzfeld BC, Dietrich DR. Effects of Treated Sewage Effluent on Immune Function in Rainbow Trout, Oncorhynchus mykiss. Aquat Toxicol (2004) 70:345–55. doi: 10.1016/j.aquatox.2004.10.010
42. Hebert N, Gagné F, Cejka P, Cyr D, Marcogliese DJ, Blaise C. The Effects of Primary-Treated Municipal Effluent on the Immune System of Rainbow Trout (Oncorhynchus mykiss): Exposure Duration and Contribution of Suspended Particles. Comp Biochem Physiol (2008) 148C:258–64. doi: 10.1016/j.cbpc.2008.06.007
43. Liney KE, Hagger JA, Tyler CR, Depledge MH, Galloway TS, Jobling S. Health Effects in Fish of Long-Term Exposure to Effluents From Wastewater Treatment Works. Environ Health Perspect (2006) 114(suppl 1):81–9. doi: 10.1289/ehp.8058
44. Rehberger K, Wernicke von Siebenthal E, Bailey C, Bregy P, Fasel M, Herzog EL, et al. Long-Term Exposure to Low 17α-Ethinylestradiol (EE2) Concentrations Disrupts Both the Reproductive and the Immune System of Juvenile Rainbow Trout, Oncorhynchus mykiss. Environ Int (2020) 142:105836. doi: 10.1016/j.envint.2020.105836
45. Kernen L, Phan A, Bo J, Herzog EL, Huynh J, Segner H, et al. Estrogens as Immunotoxicants: 17α-Ethinylestradiol Exposure Retards Thymus Development in Zebrafish (Danio Rerio). Aquat Toxicol (2022) 242:106025. doi: 10.1016/j.aquatox.2021.106025
46. Jacobson KC. Cumulative Effects of Natural and Anthropogenic Stress on Immune Function and Disease Resistance in Juvenile Chinook Salmon. J Aquat Anim Health (2003) 15:1–12. doi: 10.1577/1548-8667(2003)015<0001:CEONAA>2.0.CO;2
47. Petitjean Q. Direct and Indirect Effects of Multiple Environmental Stressors on Fish Health in Human-Altered Rivers. Sci Total Environ (2020) 742:140657. doi: 10.1016/j.scitotenv.2020.140657
48. Segner H, Wenger M, Möller AM, Koellner B, Casanova-Nakayama A. Immunotoxic Effects of Environmental Toxicants in Fish – How to Assess Them? Environ Sci Pollut Res (2012) 19:2465–78. doi: 10.1007/s11356-012-0978-x
49. Ye RR, Peterson DR, Seemann F, Kitamura SI, Lee JS, Lau TCK, et al. Immune Competence Assessment in the Marine Medaka (Oryzias melastigma) – A Holistic Approach for Immunotoxicology. Environ Sci Pollut Res (2017) 24:27687–701. doi: 10.1007/s11356-016-7208-x
50. Castaño A, Bols NC, Braunbeck T, Dierickx P, Halder M, Isomaa B, et al. The Use of Fish Cells in Ecotoxicology. The Report and Recommendations of ECVAM Workshop. Altern Lab Anim (2003) 31:317–51. doi: 10.1177/026119290303100314
51. Judson RS, Houck KA, kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, et al. In Vitro Screening of Environmental Chemicals for Targeted Testing Prioritization: The ToxCast Project. Envrion Health Perspect (2010) 118:485–92. doi: 10.1289/ehp.0901392
52. Escher BI, Allinson M, Altenburger R, Bain PA, Balaquer P, Busch W, et al. Benchmarking Organic Micropollutants in Wastewater, Recycled Water and Drinking Water With In Vitro Bioassays. Environ Sci Technol (2014) 48:1940–56. doi: 10.1021/es403899t
53. Barrick A, Chatel A, Bruneau M, Mouneyrac C. The Role of High-Throughput Screening in Ecotoxicology and Engineered Nanomaterials. Environ Toxicol Chem (2017) 36:1704–14. doi: 10.1002/etc.3811
54. Bols NC, Pham PH, Dayeh VR, Lee LEJ. Invitromatics, Invitrome and Invitromics: Introduction of Three New Terms Fro In Vitro Biology and Illustration of Their Use With the Fish Cell Lines of Rainbow Trout. In Vitro Cell Dev Biol Anim (2017) 53:383–405. doi: 10.1007/s11626-017-0142-5
55. Kimber I, Dearman RJ. Immune Responses: Adverse Versus Non-Adverse Effects. Toxicol Pathol (2002) 30:54–8. doi: 10.1080/01926230252824707
56. Selgrade MK. Immunotoxicity – The Risk Is Real. Toxicol Sci (2007) 100:328–32. doi: 10.1093/toxsci/kfm244
57. Segner H, Verburg-van Kemenade BML, Chadzinska M. The Immunomodulatory Function of the Hypothalamus-Pituitary-Gonad Axis: Proximate Mechanism for Reproduction-Immune Trade-Offs? Dev Comp Immunol (2017) 66:43–60. doi: 10.1016/j.dci.2016.07.004
58. Corsini E, Ruffo F, Racchi M. Steroid Hormones, Endocrine Disrupting Compounds and Immunotoxicology. Curr Opin Toxicol (2018) 10:69–73. doi: 10.1016/j.cotox.2018.01.006
59. Manley K, Han W, Zelin G, Lawrence DA. Crosstalk Between the Immune, Endocrine, and Nervous Systems in Immunotoxicology. Curr Opin Toxicol (2018) 10:37–45. doi: 10.1016/j.cotox.2017.12.003
60. Hartung T, Corsini E. Immunotoxicology: Challenges in the 21st Century and In Vitro Opportunities. ALTEX (2013) 30:411–26. doi: 10.14573/altex.2013.4.411
61. Koellner B, Wasserrab B, Kotterba G, Fischer U. Evaluation of Immune Functions of Rainbow Trout (Oncorhynchus mykiss)—How Can Environmental Influences Be Detected? Toxicol Lett (2002) 131:83–95. doi: 10.1016/S0378-4274(02)00044-9
62. Luster MI, Rosenthal GJ. Chemical Agents and the Immune Response. Envrion Health Perspect (1993) 100:219–26. doi: 10.1289/ehp.93100219
63. Redfern LK, Jayasundara N, Singleton DR, Di Giulio RT, Carlson J, Summer SJ, et al. The Role of Gut Microbial Community and Metabolomic Shifts in Adaptive Resistance of Atlantic Killifish (Fundulus heteroclitus) to Polycyclic Aromatic Hydrocarbons. Sci Total Environ (2021) 776:145955. doi: 10.1016/j.scitotenv.2021.145955
64. Lankveld DPK, van Loveren H, Baken KA, Vandebriel RJ. In Vitro Testing for Direct Immunotoxicity: State of the Art. Methods Mol Biol (2010) 598:401–23. doi: 10.1007/978-1-60761-401-2_26
65. Aiba S, Kimura Y. In Vitro Test Methods to Evaluate the Effects of Chemicals on Innate and Adaptive Immune Responses. Curr Opin Toxicol (2017) 5:6–12. doi: 10.1016/j.cotox.2017.06.010
66. Boverhof DR, Ladics G, Luebke B, Botham J, Corsini E, Evans E. Approaches and Considerations for the Assessment of Immunotoxicity for Environmental Chemicals: A Workshop Summary. Regul Toxicol Pharmacol (2014) 68:96–107. doi: 10.1016/j.yrtph.2013.11.012
67. Luster MI. A Historical Perspective of Immunotoxicology. J Immunotoxicol (2014) 11:197–202. doi: 10.3109/1547691X.2013.837121
68. Corsini E, Roggen EL. Overview of In Vitro Assessment of Immunotoxicity. Curr Opin Toxicol (2017) 5:13–8. doi: 10.1016/j.cotox.2017.06.016
69. Blank JA, Luster MI, Langone JJ, Wilson SD. Immunotoxicology – Regulatory and Risk Assessment Concepts. Int J Toxicol (2000) 19:95–106. doi: 10.1080/109158100224908
70. Gennari A, Ban M, Braun A, Casati S, Corsini E, Dastych J, et al. The Use of In Vitro Systems for Evaluating Immunotoxicity: The Report and Recommendations of an ECVAM Workshop. J Immunotoxicol (2005) 2:61–83. doi: 10.1080/15476910590965832
71. Pessina A, Malerba I, Gribaldo L. Hematotoxicity Testing by Cell Clonogenic Assay in Drug Development and Preclinical Trials. Curr Pharm Des (2005) 11:1055–65. doi: 10.2174/1381612053381648
72. Ringerike T, Ulleras U, Völker R, Verlaan B, Eikeset A, Trazska D, et al. Detection of Immunotoxicity Using T-Cell Based Cytokine Reporter Cell Lines (Cell Chip). Toxicology (2005) 206:257–72. doi: 10.1016/j.tox.2004.08.014
73. Kimura Y, Yasumo R, Watanabe M, Kobayashi M, Iwaki T, Fujimura C, et al. An International Validation Study of the IL-2 Luc Assays for Evaluating Potential Immunotoxic Effects of Chemicals on TZ Cells and a Proposal for Reference Data for Immunotoxic Chemicals. Toxicol In Vitro (2020) 66:104832. doi: 10.1016/j.tiv.2020.104832
74. Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. Adverse Outcome Pathways: A Conceptual Framework to Support Ecotoxicology Research and Risk Assessment. Environ Toxicol Chem (2011) 29:730–41. doi: 10.1002/etc.34
75. Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, et al. Increasing Scientific Confidence in Adverse Outcome Pathways: Application of Tailored Bradford-Hill Considerations for Evaluating Weight of Evidence. Regul Toxicol Pharmacol (2015) 72:514–37. doi: 10.1016/j.yrtph.2015.04.004
76. Reisinger K, Hoffmann S, Alepee N, Ashikaga T, Barroso J, Elcombe C, et al. Systematic Evaluation of Non-Animal Test Methods for Skin Sensitisation Safety Assessment. Toxicol In Vitro (2015) 29:259–70. doi: 10.1016/j.tiv.2014.10.018
77. Segner H. Fish Cell Lines as a Tool in Aquatic Toxicology. In: Braunbeck T, Hinton DE, Streit B, authors. Fish Ecotoxicology. Basel: Birkhäuser (1998). p. 1–38.
78. Bols NC, Dayeh VR, Lee LEJ, Schirmer K. Use of Fish Cell Lines in the Toxicology and Ecotoxicology of Fish. In: Mommsen TP, Moon TW, authors. Biochemistry and Molecular Biology of Fishes. Vol. 6 Toxicology. New York: Elsevier, Amserdam (2005). p. 43–84.
79. Bols NC, DeWitte-Orr SS, Brubacher JL, Dixon B, Ganassin RC. Cell Culture Approaches in Aquatic Immunotoxicology. In: Mothersill C, Austin B, authors. In Vitro Methods in Aquatic Toxicology. Chichester: Springer Praxis (2003). p. 399–420.
80. Bols NC, Lee LEJ. Cell Lines: Availability, Propagation and Isolation. In: Hochachka PW, Mommsent TP, authors. Biochemistry and Molecular Biology of Fishes, vol. 3. Amsterdam: Elsevier (1994). p. 145–59.
81. Ostrander GK, Blair JB, Stark BA, Marley GM, Bales WD, Veltri RW, et al. Long-Term Primary Culture Epithelia Cells From Rainbow Trout (Oncorhynchus mykiss) Liver. In Vitro Cell Dev Biol Anim (1995) 31:367–78. doi: 10.1007/BF02634286
82. LeClair EE. The Last Half Century of Fish Explants and Organ Culture. Zebrafish (2021) 18:1–19. doi: 10.1089/zeb.2020.1935
83. Wolf K, Quimby MC. Established Eurythermic Lines of Fish Cells In Vitro. Science (1962) 135:1065–6. doi: 10.1126/science.135.3508.1065
84. Fryer JL, Lannan CN. Three Decades of Fish Cell Culture: A Current Listing of Cell Lines Derived From Fishes. J Tiss Cult Methods (1994) 16:87–94. doi: 10.1007/BF01404816
85. Lakra WS, Swaminathan TR, Joy KP. Development, Characterization, Conservation and Storage of Fish Cell Lines: A Review. Fish Physiol Biochem (2011) 37:1–20. doi: 10.1007/s10695-010-9411-x
86. Villena AJ. Applications and Needs of Fish and Shellfish Cell Culture for Disease Control in Aquaculture. Rev Fish Biol Fish (2003) 13:111–40. doi: 10.1023/A:1026304212673
87. Goswami M, Yaswanth BS, Trudeau V, Lakra WS. Role and Relevance of Fish Cell Lines in Advanced In Vitro Research. Mol Biol Rep (2022). doi: 10.1007/s11033-021-06997-4
88. Hedrick RP, McDowell TS, Rosemark R, Aronstein D, Lannan CN. Two Cell Lines From White Sturgeon. Trans Am Fish Soc (1991) 120:528–34. doi: 10.1577/1548-8659(1991)120<0528:TCLFWS>2.3.CO;2
89. Ciba P, Schicktanz S, Anders E, Siegl E, Stielow A, Klink E, et al. Long-Term Culture of a Cell Population From Siberian Sturgeon (Acipenser baerii) Head Kidney. Fish Physiol Biochem (2008) 34:367–72. doi: 10.1007/s10695-007-9196-8
90. Chen B, Zheng Z, Yang J, Chi H, Huang H, Gong H. Development and Characterization of a New Cell Line Derivd From European Eel Anguilla anguilla Kidney. Biol Open (2019) 8:bio037507. doi: 10.1242/bio.037507
91. DeWitte-Orr SJ, Lepic K, Bryson SP, Walsh SK, Lee LEJ, Bols NC. Development of a Continuous Cell Line, PBLE, From an American Eel Peripheral Blood Leukocyte Preparation. In Vitro Cell Dev Biol Anim (2006) 42:263–72. doi: 10.1290/0604023.1
92. Wang R, Neumann NF, Shen Q, Belosevic M. Establishment and Characterization of Macrophage Cell Line From the Goldfish. Fish Shellfish Immunol (1995) 5:329–46. doi: 10.1006/fsim.1995.0032
93. Chaudhary DK, Sood N, Rathore G, Pradhan PK, Punia P, Agarwal NK. Establishment and Characterization of Macrophage Cell Line From Thymus of Catla catla. Aquacult Res (2014) 45:299–311. doi: 10.1111/j.1365-2109.2012.03227.x
94. Faisal M, Ahne W. A Cell Line (CLC) of Adherent Peripheral Blood Mononuclear Leucocytes of Normal Common Carp Cyprinus carpio. Dev Comp Immunol (1990) 14:255–60. doi: 10.1016/0145-305X(90)90097-X
95. Weyts FAA, Rombout JHWM, Verburg-Kemenade BML. A Common Carp (Cyprinus Carpio) Leukocyte Cell Line Shares Morphological and Functional Characteristiscs With Macrophages. Fish Shellfish Immunol (1997) 7:123–32. doi: 10.1006/fsim/1996.0069
96. Miller NW, Rycyzyn MA, Wilson MR, Warr GW, Naftel JP, Clem WL. Development and Characterization of Channel Catfish Long Term B Cell Lines. J Immunol (1994) 152:2180–9.
97. Stuge TB, Wilson MR, Zhou H, Barker KS, Bengten E, Chinchar G, et al. Development and Analysis of Various Clonal Alloantigen-Dependent Cytotoxic Cell Lines From Channel Catfish. J Immunol (2000) 164:2971–7. doi: 10.4049/jimmunol.164.6.2971
98. Shen L, Stuge TB, Zhou H, Khayat M, Barker KS, Quiniou SMA, et al. Channel Catfish Cytotoxic Cells: A Mini—Review. Dev Comp Immunol (2002) 26:141–9. doi: 10.1016/S0145-305X(01)00056-8
99. Vallejo AN, Elsaesser CF, Miller NW, Clem LW. Spontaneous Development of Functionally Active Long-Term Monocyte-Like Cell Lines From Channel Catfish. In Vitro Cell Dev Biol (1991) 27A:279–86. doi: 10.1007/BF02630904
100. Huang X, Huang Y, Sun J, Han X, Qin Q. Characterization of Two Grouper Epinephales Akaara Cell Lines: Application to Studies of Singapor Grouper Iridovirus (SGIV) Propagation and Virus-Host Interaction. Aquaculture (2009) 292:172–9. doi: 10.1016/j.aquaculture.2009.04.019
101. Moritomo T, Anderson DP, Schill WB. Establishment of a Cell Line With Reticulo-Endothelial Characteristics From a Rainbow Trout Spleen Explant. Fish Pathol (1990) 25:165–72. doi: 10.3147/jsfp.25.165
102. Andral R, Hurard C, Elziere-Papyanni P, Vivares CP. Establishment and Characterization of a Rainbow Trout Kidney Cell Line, RTK. In: Perkins FO, Cheng TC, authors. Pathology in Marine Science. San Diego: Academic Press (1990). p. 33–42.
103. Diago ML, Lopez-Fierro MP, Razquin B, Villena A. Establishment and Characterization of a Poronephric Stromal Cell Line (TPS) From Rainbow Trout, Oncorhynchus mykiss. Fish Shellfish Immunol (1995) 5:441–57. doi: 10.1006/fsim.1995.0042
104. Flano E, Lopez-Fierro P, Alvarez F, Razquin B, Villena A. Splenic Cultures From Rainbow Trout, Onocrhynchus mykiss: Establishment and Characterization. Fish Shellfish Immunol (1998) 8:589–606. doi: 10.1006/fsim.1998.0165
105. Ganassin RC, Bols NC. Development of a Monocyte-Macrophage-Like Cell Line, RTS11, From Rainbow Trout Spleen. Fish Shellfish Immunol (1998) 8:457–76. doi: 10.1006/fsim.1998.0153
106. Brubacher JL, Secombes CJ, Zou J, Bols NC. Constitutive and LPS-Induced Gene Expression in a Macrophgae-Like Cell Line From the Rainbow Trout (Oncorhynchus mykiss). Dev Comp Immunol (2000) 24:565–74. doi: 10.1016/S0145-305X(00)00019-7
107. Wen CM. Development and Characterization of a Cell Line From Tilapia Head Kidney With Melanomacrophage Characteristics. Fish Shellfish Immunol (2016) 49:442–9. doi: 10.1016/j.fsi.2016.01.013
108. Kang MS, Oh MJ, Kim YJ, Kawai K, Jung SJ. Establishment and Characterization of Two New Cell Lines Derived From Flounder, Paralichthys olivaceus (Temmicnk & Schlegel). J Fish Dis (2003) 26:657–65. doi: 10.1046/j.1365-2761.2003.00499.x
109. Dannevig BH, Brudeseth BE, Gjoen T, Rode M, Wergeland HI, Evensen O, et al. Characterization of a Long-Term Cell Line (SHK-1) Developed From the Head Kidney of Atlantic Salmon (Salmo salar L.). Fish Shellfish Immunol (1997) 7:213–26. doi: 10.1006/fsim.1996.0076
110. Rodriguez Saint Jean S, Gonzalez C, Monras M, Romero A, Ballesteros N, Enriquez R, et al. Establishment and Characterization of a New Cell Line (SSP-9) Derived From Atlantic Salmon Salmo salar That Expressses Type I Ifn. J Fish Biol (2014) 85:1526–45. doi: 10.1111/jfb.12503
111. Pettersen EF, Ingerslev HC, Stavang V, Egenberg M, Wergeland HI. A Highly Phagocytic Cell Line TO From Atlantic Salmon Is CD83 Positive and M-CSFR Negative, Indicating a Dendritic-Like Cell Line. Fish Shellfish Immunol (2008) 25:809–19. doi: 10.1016/j.fsi.2008.08.014
112. Wang N, Wang XL, Sha ZX, Tian YS, Chen SL. Development and Characterization of a New Marine Fish Cell Line From Turbot (Scophthalmus maximus). Fish Physiol Biochem (2010) 36:1227–34. doi: 10.1007/s10695-010-9402-y
113. Castro R, Zou J, Secombes CJ, Martin SAM. Cortisol Modulates the Induction of Inflammatory Gene Expression in a Rainbow Trout Macrophage Cell Line. Fish Shellfish Immunol (2011) 30:215–23. doi: 10.1016/j.fsi.2010.10.010
114. De Bruijn I, Belmonte R, Anderson VL, Saraiva M, Wang T, van West P, et al. Immune Gene Expression in Trout Cell Lines Infected With the Fish Pathogenic Oomycete Saprolegnia parasitica. Dev Comp Immunol (2012) 38:4–54. doi: 10.1016/j.dci.2012.03.018
115. Collet B, Collins C, Lester K. Engineered Cell Lines for Fish Health Research. Dev Comp Immunol (2018) 80:34–40. doi: 10.1016/j.dci.2017.01.013
116. Neale PA, Altenburger R, Aït-Aïssa S, Brion F, Busch W, de Aragão Umbuzeiro G. Development of a Bioanalytical Test Battery for Water Quality Monitoring: Fingerprinting Identified Micropollutants and Their Contribution to Effects in Surface Water. Water Res (2017) 123:734–50. doi: 10.1016/j.watres.2017.07.016
117. Ganassin RC, Bols NC. Development of Long-Term Rainbow Trout Spleen Cultures That Are Haemopoietic and Produce Dendritic Cells. Fish Shellfish Immunol (1996) 6:17–34. doi: 10.1006/fsim.1996.0003
118. Bassity E, Clark TG. Functional Identification of Dendritic Cells in the Teleost Model, Rainbow Trout (Oncorhynchus mykiss). PloS One (2012) 7:e33196. doi: 10.1371/journal.pone.0033196
119. Ellis AE. The Leucocytes of Fish: A Review. J Fish Biol (1977) 11:453–91. doi: 10.1111/j.1095-8649.1977.tb04140.x
120. Zhu W, Su J. Immune Functions of Phagocytic Blood Cells in Teleost. Rev Aquacult (2021). doi: 10.1111/raq.12616
121. Kondera E. Haematopoiesis and Haematopietic Organs in Fish. Sci Ann Pol Soc Anim Prod (2019) 15:9–16. doi: 10.5604/01.3001.0013.4535
122. Braun-Nesje R, Bertheussen K, Kaplan G, Seljelid R. Salmonid Macrophages: Separation, In Vitro Culture and Characterization. J Fish Dis (1981) 15:295–304. doi: 10.1111/j.1365-2761.1981.tb01118.x
123. Bayne CJ. Pronephric Leukocytes of Cyprinus carpio: Isolation, Separation and Characterization. Vet Immunol Immunopathol (1986) 12:141–51. doi: 10.1016/0165-2427(86)90118-2
124. Zelikoff JT, Enane NA, Bowser D, Squibb KS, Frenkel K. Development of Fish Peritoneal Macrophages as a Model for Higher Vertebrates in Immunotoxicological Studies. I. Characterization of Trout Macrophage Morphological, Functional and Biochemical Properties. Fundam Appl Toxicol (1991) 16:576–89. doi: 10.1016/0272-0590(91)90097-N
125. Garduno RA, Kay WW. Isolation and Culture of Head Kidney Macrophages. In: Hochachka PW, Mommsen TP, authors. Biochemistry and Molecular Biology of Fishes, vol. 3. Amsterdam: Elsevier (1994). p. 328–39.
126. Afonso A, Ellis AE, Silva MT. The Leucocyte Population of the Unstimulated Peritoneal Cavity of Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol (1997) 7:335–48. doi: 10.1006/fsim.1997.0089
127. Sorensen KK, Sveinbjornsson B, Dalmo RA, Smedsrod B, Bertheussen K. Isolation, Cultivation and Characteriztaion of Head Kidney Macrophages From Atlantic Cod, Gadus morhua L. J Fish Dis (1997) 20:93–107. doi: 10.1046/j.1365-2761.1997.d01-112.x
128. Samai HC, Rioult D, Bado-Nilles A, Delahaut L, Jubreaux J, Geffard A, et al. Procedures for Leukocyte Isolation From Lymphoid Tissues and Consequences on Immune Endpoints Used to Evaluate Fish Immune Status: A Case Study on Roach (Rutilus rutilus). Fish Shellfish Immunol (2018) 74:190–204. doi: 10.1016/j.fsi.2017.12.040
129. Lulijwa R, Alfaro AC, Merien F, Meyer J, Young T. Advances in Salmonid Fish Immunology: A Review of Methods and Techniques for Lymphoid Tissue and Peripheral Blood Leucocyte Isolation and Application. Fish Shellfish Immunol (2019) 95:44–80. doi: 10.1016/j.fsi.2019.10.006
130. Congleton JL, Greenlee AR, Ristow SS. Isolation of Leucocytes From the Anterior Kidney and Spleen of Rainbow Trout in a Self-Generating Density Gradient. J Fish Biol (1990) 36:575–85. doi: 10.1111/j.1095-8649.1990.tb03558.x
131. Verburg-van Kemenade BML, Weyts FAA, Debets R, Flik G. Carp Macrophages and Neutrophilic Granulocytes Secrete an Interleukin-1-Like Factor. Dev Comp Immunol (1995) 19:59–70. doi: 10.1016/0145-305X(94)00047-J
132. Reynaud S, Duchiron C, Deschaux P. 3-Methylcholanthrene Increased Phorbol-3-Myristate 13-Acetate-Induced Respiratory Burst Activity and Intracellular Calcium Levels in Common Carp (Cyprinus carpio L.) Macrophages. Toxicol Appl Pharmacol (2001) 175:1–9. doi: 10.1006/taap.2001.9217
133. MacKenzie S, Iliev D, Liarte C, Koskinen H, Planas JV, Goetz FW, et al. Transcriptional Analysis of LPS-Stimulated Activation of Trout (Oncorhynchus mykiss) Monocyte/Macrophage Cells in Primary Culture Treated With Cortisol. Mol Immunol (2006) 43:1340–8. doi: 10.1016/j.molimm.2005.09.005
134. DeLuca D, Wilson M, Warr GW. Lymphocyte Heterogeneity in the Trout, Salmo gairdneri, Defined With Monoclonal Antibodies to IgM. Eur J Immunol (1983) 13:546–51. doi: 10.1002/eji.1830130706
135. Nakayama A, Riesen I, Koellner B, Eppler E, Segner H. Surface Marker-Defined Head Kidney Granulocytes and B-Lymphocytes of Rainbow Trout Express Benzo[a]Pyrene-Inducible Cytochrome P4501A Protein. Toxicol Sci (2008) 103:86–96. doi: 10.1093/toxsci/kfn024
136. Korytar T, Thi HD, Takizawa F, Koellner B. A Multicolour Flow Cytoimetry Identifying Defined Leukocyte Subsets or Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol (2013) 35:2017–9. doi: 10.1016/j.fsi.2013.09.025
137. Castro R, Bromage E, Abos B, Pignatelli J, Gonzalez Granja A, Luque A, et al. CCR7 Is Mainly Expressed in Teleost Gills, Where It Defines an IgD+IgM- B Lymphocyte Subset. J Immunol (2014) 192:1257–66. doi: 10.4049/jimmunol.1302471
138. Haugland GT, Ronneseth A, Wergeland HL. Flow Cytometry Analyses of Phagocytic and Respiratory Burst Activities and Cytochemical Characterization of Leucocytes Isolated From Wrasse (Labrus bergylta A.). Fish Shellfish Immunol (2014) 39:51–60. doi: 10.1016/j.fsi.2014.04.023
139. Miller NW, McKinney EC. In Vitro Culture of Fish Leukocytes. In: Hochachka PW, Mommsen TP, authors. Biochemistry and Molecular Biology of Fishes, vol. 3. Amsterdam: Elsevier (1994). p. 341–53.
140. Neumann NF, Barreda DR, Belosevic M. Generation and Functional Analysis of Distinct Macrophage Sub-Populations From Goldfish (Carassius auratus L.) Kidney Leukocyte Cultures. Fish Shellfish Immunol (2000) 10:1–20. doi: 10.1006/fsim.1999.0221
141. Fierro-Castro C, Barrioluengo L, Lopez-Fierro P, Razquin BE, Carracedo B, Villena AJ. Fish Cell Cultures as In Vitro Models of Pro-Inflammatory Responses Elicited by Immunostimulants. Fish Shellfish Immunol (2012) 33:389–400. doi: 10.1016/j.fsi.2012.05.019
142. Qiu W, Liu S, Chen J, Hu L, Wu M, Yang M. The Primary Culture of Carp (Cyprinus carpio) Macrophages and the Verification of Its Phagocytic Activity. In Vitro Cell Dev Biol Anim (2016) 52:10–9. doi: 10.1007/s11626-015-9942-7
143. Zou J, Holland J, Pleguezuelos O, Cunningham C, Secombes CJ. Factors Influencing the Expression of Interleukin-1β in Cultured Rainbow Trout (Oncorhynchus mykiss) Leucocytes. Dev Comp Immunol (2000) 24:575–82. doi: 10.1016/S0145-305X(99)00085-3
144. Pohlenz C, Buentello A, Mwangi W, Gatlin DM. Arginine and Glutamine Supplementation to Culture Media Improves the Performance of Various Channel Catfish Immune Cells. Fish Shellfish Immunol (2012) 32:762–8. doi: 10.1016/j.fsi.2012.01.029
145. Belosevic M, Hannington PC, Barreda DR. Development of Goldfish Macrophages In Vitro. Fish Shellfish Immunol (2007) 20:152–71. doi: 10.1016/j.fsi.2004.10.010
146. Katzenback BA, Belosevic M. Colony-Stimulating Factor-1 Receptor Protein Expression Is a Specific Marker for Goldfish (Carassius auratus L.) Macrophage Progenitors and Their Differentiated Cell Types. Fish Shellfish Immunol (2012) 32:434–45. doi: 10.1016/j.fsi.2011.12.003
147. Wentzel AS, Janssen JJE, de Boer VCJ, van Veen WG, Forlenza M, Wiegertjes GF. Fish Macrophages Show Distinct Metabolic Signatures Upon Polarization. Front Immunol (2020) 11:152. doi: 10.3389/fimmu.2020.00152
148. Ottinger CA, Smith CR, Blazer VS. In Vitro Immune Function in Laboratory-Reared Age-0 Smallmouth Bass (Micropterus dolomieu) Relative to Diet. Fish Shellfish Immunol (2019) 95:1–10. doi: 10.1016/j.fsi.2019.10.005
149. Zeeman M. Modulation of the Immune Response in Fish. Vet Immunol Immunopathol (1986) 12:235–41. doi: 10.1016/0165-2427(86)90127-3
150. Embry MR, Belanger SE, Braunbeck TA, Galay-Burgos M, Halder M, Hinton DE, et al. The Fish Embryo Toxicity Test as an Animal Alternative Method in Hazard and Risk Assessment and Scientific Research. Aquat Toxicol (2010) 97:79–87. doi: 10.1016/j.aquatox.2009.12.008
151. Willett CE, Cortes A, Zuasti A, Zapata AG. Early Hematopoiesis and Developing Lymphoid Organs in the Zebrafish. Dev Dyn (1999) 214:323–36. doi: 10.1002/(SICI)1097-0177(199904)214:4<323::AID-AJA5>3.0.CO;2-3
152. Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and Maturation of the Immune System in Zebrafish, Danio rerio: A Gene Expression Profiling, in Situ Hybridization and Immunological Study. Dev Comp Immunol (2004) 28:9–28. doi: 10.1016/S0145-305X(03)00103-4
153. Avagyan S, Zon LI. Fish to Learn: Insights Into Blood Development and Blood Disorders From Zebrafish Hematopoiesis. Hum Gene Ther (2016) 27:287–94. doi: 10.1089/hum.2016.024
154. Pang S, Gao Y, Li A, Yao X, Qu G, Hu L, et al. Tetrabromobisphenol A Perturbs Erythropoiesis and Impairs Blood Circulation in Zebrafish Embryos. Environ Sci Technol (2020) 54:12998–3007. doi: 10.1021/acs.est.0c02934
155. Amadori M. The Innate Immune Response to Noninfectious Stressors: Human and Animal Models. Amsterdam: Academic Press (2016).
156. Estevez RA, Contreras Mostazo MG, Rodriguez E, Espinoza JC, Kuznar J, Jonsson ZO, et al. Inducers of Salmon Innate Immunity: An In Vitro and In Vivo Approach. Fish Shellfish Immunol (2018) 72:247–58. doi: 10.1016/j.fsi.2017.10.058
157. Langan LM, Arossa S, Owen SF, Jha AN. Assessing the Impact of Benzo(a)Pyrene With the In Vitro Fish Gut Model: An Integrated Approach for Eco-Genotoxicologcial Studies. Mutat Res Genet Toxicol Environ Mutagen (2018) 826:53–64. doi: 10.1016/j.mrgentox.2017.12.009
158. Langan LM, Owen SF, Trznadel M, Dodd NJ, Jackson SK, Purcell WM, et al. Spheroid Size Does Not Impact Metabolism of the β-Blocker Propanolol in 3D Intestinal Fish Model. Front Pharmacol (2018) 9:947. doi: 10.3389/fphar.2018.00947
159. Schug H, Yue Y, Krese R, Fischer S, Kortner TM, Schirmer K. Time- and Concentration-Dependent Expression of Immune and Barrier Genes in the RTgutGC Fish Intestinal Model Following Immune Stimulation. Fish Shellfish Immunol (2019) 88:308–17. doi: 10.1016/j.fsi.2019.02.036
160. Elsasser MS, Roberson BS, Hetrick FM. Effects of Metals on the Chemiluminiscent Response of Rainbow Trout (Salmo Gairdneri) Phagocytes. Vet Immunol Immunopharmacol (1986) 12:243–50. doi: 10.1016/0165-2427(86)90128-5
161. Voccia I, Krzystyniak K, Dunier M, Flipo D, Fournier M. In Vitro Mercury-Related Cytotoxicity and Functional Impairment Oft He Immune Cells of Rainbow Trout (Oncorhynchus mykiss). Aquat Toxicol (1994) 29:37–48. doi: 10.1016/0166-445X(94)90046-9
162. Viola A, Pregnolato G, Albergoni V. Effect of In Vitro Cadmium Exposure on Natural Killer (NK) Cells of Catfish, Ictalurus melas. Fish Shellfish Immunol (1996) 6:167–72. doi: 10.1006/fsim.1996.0017
163. Ghanmi Z, Rouabhia M, Othmane O, Deschaux P. Effects of Metal Ions on Cyprinid Fish Immune Response: In Vitro Effects of Zn2+ and Mn2+ on the Mitogenic Response of Carp Pronephros Lymphocytes. Ecotoxicol Environ Saf (1989) 17:183–9. doi: 10.1016/0147-6513(89)90037-7
164. Steinhagen D, Helmus T, Maurer S, Michael RD, Leibold W, Scharsack JP, et al. Effect of Hexavalent Carcinogenic Chromium on Carp Cyprinus carpio Immune Cells. Dis Aquat Organ (2004) 62:155–61. doi: 10.3354/dao062155
165. Morcillo P, Cordero H, Meseguer J, Esteban MA, Cuesta A. In Vitro Immunotoxicologcial Effects of Heavy Metals on European Sea Bass (Dicentrarchus labrax L.) Head Kidney Leucocytes. Fish Shellfish Immunol (2015) 47:245–54. doi: 10.1016/j.fsi.2015.09.011
166. Wishkovsky A, Mathews AS, Weeks BA. Effect of Tributyltin on the Chemiluminiscent Response of Phagocytes From Three Species of Estuarine Fish. Arch Environ Contam Toxicol (1989) 18:826–31. doi: 10.1007/BF01160296
167. O’Halloran K, Ahokas JT, Wright PFA. Response of Fish Immune Cells to In Vitro Organotin Exposures. Aquat Toxicol (1998) 40:141–56. doi: 10.1016/S0166-445X(97)00054-4
168. Harford AJ, O’Halloran K, Wright PFA. The Effects of In Vitro Pesticide Exposures on the Phagocytic Function of Four Native Australian Freshwater Fish. Aquat Toxicol (2005) 75:330–42. doi: 10.1016/j.aquatox.2005.09.005
169. Shelley LK, Ross PS, Kennedy CJ. Immunotoxic and Cytotoxic Effects of Atrazine, Permethrine and Piperonyl Butoxide to Rainbow Trout Following In Vitro Exposure. Fish Shellfish Immunol (2012) 33:455–8. doi: 10.1016/j.fsi.2012.05.020
170. Misumi I, Vella AT, Leong JAC, Nakanishi T, Schreck CB. P,P’-DDE Depresses the Immune Competence of Chinook Salmon (Oncorhynchus tshawytscha) Leukocytes. Fish Shellfish Immunol (2005) 19:97–114. doi: 10.1016/j.fsi.2004.11.005
171. Cuesta A, Meseguer J, Esteban MA. Effects of the Organochlorines P,P'-DDE and Lindane on Gilthead Seabream Leucocyte Immune Parameters and Gene Expression. Fish Shellfish Immunol (2008) 25:682–8. doi: 10.1016/j.fsi.2008.02.006
172. Baier-Anderson C, Anderson RS. Evaluation of the Immunotoxicity of Chlorothalonil to Striped Bass Phagocytes Following In Vitro Exposure. Environ Toxicol Chem (1998) 17:1546–51. doi: 10.1002/etc.5620170815
173. Tellez-Banuelos MC, Ortiz-Lazareno PC, Santerre A, Casas-Solis J, Bravo-Cuellar A, Zaitseva G. Effects of Low Concentrations of Endosulfan on Proliferation, ERK1/2 Pathway, Apoptosis and Senescence in Nile Tilapia (Oreochromis niloticus) Splenocytes. Fish Shellfish Immunol (2011) 31:1291–6. doi: 10.1016/j.fsi.2011.10.003
174. Reynaud S, Durchiron C, Deschaux P. 3-Methylcholanthrene Inhibits Lymphocyte Proliferation and Increases Intracellular Calcium Levels in Common Carp (Cyprinus carpio). Aquat Toxicol (2003) 63:319–31. doi: 10.1016/S0166-445X(02)00188-1
175. Sweet LI, Passino-Reader DR, Meier PG, Omann GM. Thymocyte Viability, Apoptosis and Necrosis: In Vitro Effects of Organochlorine Contaminants. Fish Shellfish Immunol (1998) 8:77–90. doi: 10.1006/fsim.1997.0126
176. Miller GG, Sweet LI, Adams JV, Omann GM, Passino-Reader DR, Meier PG. In Vitro Toxicity and Interactions of Environmental Contaminants (Aroclor 1254 and Mercury) and Immunomodulatory Agents (Lipopolysaccharide and Cortisol) on Thymocytes From Lake Trout (Salvelinus Namaycush). Fish Shellfish Immunol (2002) 13:11–26. doi: 10.1006/fsim.2001.0381
177. Quabius ES, Krupp G, Secombes CJ. Polychlorinated Biphenyl 126 Affects Expression of Genes Involved in Stress-Immune Interaction in Primary Cultures of Rainbow Trout Anterior Kidney Cells. Environ Toxicol Chem (2005) 24:3053–60. doi: 10.1897/05-110R.1
178. Cabas I, Liarte S, García-Alcázar A, Meseguer J, Mulero V, García-Ayala A. 17beta-Ethynylestradiol Alters the Immune Response of the Teleost Gilthead Seabream (Sparus aurata L.) Both In Vivo and In Vitro. Dev Comp Immunol (2012) 36:547–56. doi: 10.1016/j.dci.2011.09.011
179. Gushiken Y, Watanuki H, Sakai M. In Vitro Effect of Carp Phagocytic Cells by Bisphenol A and Nonylphenol. Fish Sci (2002) 68:178–83. doi: 10.1046/j.1444-2906.2002.00405.x
180. Yang M, Qiu W, Chen B, Chen J, Liu S, Wu M, et al. The In Vitro Immune Modulatory Effect of Bisphenol A on Fish Macrophages via Estrogen Receptor α and Nuclear Factor κB Signalling. Environ Sci Technol (2015) 49:1888–95. doi: 10.1021/es505163v
181. Wang R, Belosevic M. The In Vitro Effects of Estradiol and Cortisol on the Function of a Long-Term Goldfish Macrophage Cell Line. Dev Comp Immunol (1995) 19:327–36. doi: 10.1016/0145-305X(95)00018-O
182. Yin D, Hu S, Gu Y, Wei L, Liu S, Zhang A. Immunotoxicity of Bisphenol A to Carassius auratus Lymphocytes and Macrophages Following In Vitro Exposure. J Environ Sci (2007) 19:232–7. doi: 10.1016/S1001-0742(07)60038-2
183. Qiu W, Wu M, Liu S, Chen B, Pan C, Yang M, et al. Suppressive Immunoregulatory Effects of Three Antidepressants via Inhibition of the Nuclear Factor-κb Activation Assessed Using Primary Macrophages of Carp (Cypinus carpio). Toxicol Appl Pharmacol (2017) 322:1–8. doi: 10.1016/j.taap.2017.03.002
184. Gagné F, Fortier M, Fournier M, Smyth SA. In Vitro Immunotoxicity of Untreated and Treated Urban Wastewaters Using Various Treatment Processes to Rainbow Trout Leukocytes. J Environ Sci (2013) 25:1400–7. doi: 10.1016/S1001-0742(12)60202-2
185. Kalman J, Merino C, Fernandez-Cruz ML, Navas JM. Usefulness of Fish Cell Lines for the Initial Characterization of Toxicity and Cellular Fate of Graphene-Related Materials (Carbon Nanofibers and Graphen Oxide). Chemosphere (2019) 218:347–58. doi: 10.1016/j.chemosphere.2018.11.130
186. Galbiati V, Mitjans M, Corsini E. Present and Future of In Vitro Immunotoxicology in Drug Development. J Immunotoxicol (2010) 7:255–67. doi: 10.3109/1547691X.2010.509848
187. Ganassin RC, Bols NC. Growth of Rainbow Trout Hemopoietic Cells in Methylcellulose and Methods for Monitoring Their Proliferative Response in This Matrix. Methods Cell Sci (2000) 22:147–52. doi: 10.1023/A:1009835814441
188. Moritomo T, Kihara R, Nakayama S, Itoue T, Imagawa T, Watanabe T. Culture of Carp Hematopoietic Cells. Fish Pathol (1996) 31:59–63. doi: 10.3147/jsfp.31.59
189. Katakura F, Yamaguchi T, Yoshida M, Moritomo T, Naganishi T. Demonstration of T Cell and Macrophage Progenitors in Carp (Cyprinus carpio) Kidney Hematopoietic Tissues. Development of Clonal Assay System for Carp Hematopoietic Cells. Dev Comp Immunol (2010) 34:685–9. doi: 10.1016/j.dci.2010.01.015
190. Rombout JH, Yang G, Kiron V. Adaptive Immune Responses at Mucosal Surfaces of Teleost Fish. Fish Shellfish Immunol (2014) 40:634–43. doi: 10.1016/j.fsi.2014.08.020
191. Salinas I. The Mucosal Immune System of Teleost Fish. Biology (2015) 4:525–39. doi: 10.3390/biology4030525
193. Guardiola FADoiguardi M, Parisi MG, Trapani MR, Meseguer J, Cuesta A, Cammarat M, et al. Evaluation of Waterborne Exposure to Heavy Metals in Innate Immune Defences Present on Skin Mucus of Gilthead Seabream (Sparus aurata). Fish Shellfish Immunol (2015) 45:112–23. doi: 10.1016/j.fsi.2015.02.010
194. Rashidian G, Lazado CC, Mahboub HH, Mohammadi-Aloucheh R, Prokic MD, Nada HS, et al. Chemcially and Green-Synthesized ZnO Nanoparticles Alter Key Immunological Molecules in Common Carp (Cyprinus Carpio) Skin Mucus. Int J Mol Sci (2021) 22:3270. doi: 10.3390/ijms22063270
195. Zhang Y, Zhang P, Shang X, Lu Y, Li Y. Exposure of Lead on Intestinal Structural Integrity and the Diversity of Gut Microbiota of Common Carp. Comp Biochem Physiol (2021) 239C:108877. doi: 10.1016/j.cbpc.2020.108877
196. Geppert M, Sigg L, Schirmer K. A Novel Two-Compartment Barrier Model for Investigating Nanoparticle Transport in Fish Intestinal Epithelial Cells. Environ Sci Nano (2016) 3:388–95. doi: 10.1039/C5EN00226E
197. Minghetti M, Drieschner C, Bramaz N, Schug H, Schirmer K. A Fish Intestinal Epithelial Barrier Model Established From the Rainbow Trout (Oncorhynchus mykiss) Cell Line, RTgutGC. Cell Biol Toxicol (2017) 33:539–55. doi: 10.1007/s10565-017-9385-x
198. Drieschner C, Minghetti M, Wu S, Renaud P, Schirmer K. Ultrathin Alumina Membranes as Scaffold for Epithelial Culture From the Intestine of Rainbow Trout. ACS Appl Mater Interfaces (2017) 9:9496–505. doi: 10.1021/acsami.7b00705
199. Drieschner C, Könemann S, Renaud P, Schirmer K. Fish-Gut-on-a-Chip: Development of a Microfluidic Bioreactor to Study the Role of Fish Intestine In Vitro. Lab Chip (2019) 19:3268–76. doi: 10.1039/C9LC00415G
200. Kazlauskaite R, Cheaib B, Heys C, Ijaz UZ, Connelly S, Sloan W, et al. SalmoSim: The Development of a Three-Compartment In Vitro Simulator of the Atlantic Salmon GI Tract and Associated Microbial Communities. Microbiome (2021) 9:179. doi: 10.1186/s40168-021-01134-6
201. Leonard F, Collnot EM, Lehr CM. A Three-Dimensional Coculture of Enterocytes, Monocytes and Dendritic Cells to Model Inflamed Intestinal Mucosa In Vitro. Mol Pharmaceut (2010) 7:2103–19. doi: 10.1021/mp1000795
202. Koellner B, Fischer U, Rombout JHWM, Taverne-Thiele JJ, Hansen JD. Potential Involvement of Rainbow Trout Thrombocytes in Immune Functions: A Study Using a Panel of Monoclonal Antibodies and RT-PCR. Dev Comp Immunol (2004) 28:1049–62. doi: 10.1016/j.dci.2004.03.005
203. Fischer U, Koppang EO, Nakanishi T. Teleost T and NK Cell Immunity. Fish Shellfish Immunol (2013) 35:197–206. doi: 10.1016/j.fsi.2013.04.018
204. Fournier M, Cyr D, Blakley B, Boermans H, Brousseau P. Phagocytosis as a Biomarker of Immunotoxicity in Wildlife Species Exposed to Environmental Xenobiotics. Am Zool (2000) 40:412–20. doi: 10.1093/icb/40.3.412
205. Möller AM, Hermsen C, Floehr T, Lamoree MH, Segner. Tissue-Specific Metabolism of Benzo(a)Pyrene in Rainbow Trout (Oncorhynchus mykiss) – A Comparison Between Liver and Immune Organs. Drug Metab Dispos (2014) 42:111–8. doi: 10.1124/dmd.113.053777
206. Ribas JL, da Silva CA, de Andrade L, Galvan GL, Cestari MM, Trindade ES, et al. Effects of Antiinflammatory Drugs in Primary Kidney Cell Culture of a Freshwater Fish. Fish Shellfish Immunol (2014) 40:296–303. doi: 10.1016/j.fsi.2014.07.009
207. Reynaud S, Duchiron C, Deschaux P. 3-Methylcholanthren Induces Lymphocyte and Phagocyte Apoptosis in Common Carp In Vitro. Aquat Toxicol (2004) 66:307–18. doi: 10.1016/j.aquatox.2003.10.003
208. Segner H, Braunbeck T. Endpoints for In Vitro Toxicity Testing With Fish Cells. In: Mothersill C, Austin B, authors. In Vitro Methods in Aquatic Toxicology. Chichester: Springer Praxis (2003). p. 77–141.
209. Sieroslawaska A, Rymuszka A. Assessment of the Cytotoxic Impact of Cyanotoxin Beta-N-Methylamino-L-Alanine on a Fish Immune Cell Line. Aquat Toxicol (2019) 212:214–21. doi: 10.1016/j.aquatox.2019.05.012
210. Rastgar S, Ardeshir RA, Zabihi E, Mohavedinia A, Salati AP. Immunotoxicity of Estrogen and Nonylphenol on Apoptosis and Expression of ERs in Goldfish Macrophage: Opening New Avenue for Discovering the Role of Experimental Model Systems and Sexes. Aquat Toxicol (2019) 209:159–67. doi: 10.1016/j.aquatox.2019.02.007
211. Clemedson C, Andersson M, Aoki Y, Barile FA, Calleja MA, Castaño A, et al. MEIC Evaluation of Acute Systemic Toxicity. Part IV. In Vitro Results From 67 Toxicity Assays Used to Test Reference Chemicals 31-50 and a Comparative Cytotoxicity Analysis. Altern Lab Anim (1998) 26:131–83. doi: 10.1177/026119299802601s03
212. Escher BI, Glauch L, König M, Mayer P, Schlichting R. Baseline Toxicity and Volatility Cutoff in Reporter Gene Assays Used for High Throughput Screening. Chem Res Toxicol (2019) 32:1646–55. doi: 10.1021/acs.chemrestox.9b00182
213. Judson R, Houck K, Martin M, Richard AM, Knudsen TB, Shah I, et al. Analysis of the Effects of Cell Stress and Cytotoxicity on In Vitro Assay Activity Across a Diverse Chemical and Assay Space. Toxicol Sci (2016) 152:323–39. doi: 10.1093/toxsci/kfw092
214. Rehberger K, Escher BI, Scheidegger A, Werner I, Segner H. Evaluation of an In Vitro Assay to Screen for the Immunotoxic Potential of Chemicals to Fish. Sci Rep (2021) 11:3167. doi: 10.1038/s41598-021-82711-5
215. Li J, Barreda DR, Zhang Y, Boshra H, Gelman AE, Lapatra S, et al. B Lymphocytes From Early Vertebrates Have Potent Phagocytic and Microbicidal Abilities. Nat Immunol (2006) 7:1116–24. doi: 10.1038/ni1389
216. Nagasawa T, Nakayasu C, Rieger AM, Barreda DR, Somamoto T, Nakao M. Phagocytosis by Thrombocytes Is a Conserved Innate Immune Mechanisms in Lower Vertebrates. Front Immunol (2014) 5:445. doi: 10.3389/fimmu.2014.00445
217. Zwollo P, Quddos F, Bagdassarian C, Seeley ME, Hale RC, Abderhalden L. Polstyrene Microplastics Reduce Abundance of Developing B Cells in Rainbow Trout (Oncorhynchus Mykiss) Primary Cultures. Fish Shellfish Immunol (2021) 114:102–11. doi: 10.1016/j.fsi.2021.04.014
218. Seeley KR, Gillespie PD, Weeks BA. A Simple Technique for the Rapid Spectrophotometric Determination of Phagocytosis by Fish Macrophages. Mar Environ Res (1990) 30:37–41. doi: 10.1016/0141-1136(90)90009-D
219. Esteban MA, Mulero V, Munoz J, Meseguer J. Methodological Aspects of Assessing Phagocytosis of Vibrio anguillarum by Leucocytes of Gilthead Seabream (Sparus Aurata L.) by Flow Cytometry and Electron Microscopy. Cell Tiss Res (1998) 293:133–41. doi: 10.1007/s004410051105
220. Harford AJ, O’Halloran K, Wright PFA. Flow Cytometric Analysis and Optimisation for Measuring Phagocytosis in Three Australian Freshwater Fish. Fish Shellfish Immunol (2006) 20:562–73. doi: 10.1016/j.fsi.2005.07.005
221. Park Y, Abisshira-Garcia IS, Thalmann S, Wiegertjees GF, Barreda DR, Olsvik PA, et al. Imaging Flow Cytometric Protocols for Examining Phagocytosis of Microplastics and Bioparticles by Immune Cells of Aquatic Animals. Front Immunol (2020) 11:203. doi: 10.3389/fimmu.2020.00203
222. Yue Y, Li X, Sigg L, Suter MJF, Pillai S, Behra R, et al. Interaction of Silver Nanoparticles With Algae and Fish Cells : A Side by Side Comparison. J Nanobiotechnology (2017) 15:16. doi: 10.1186/s12951-017-0254-9
223. Solem ST, Jorgensen JB, Robertson B. Stimulation of Respiratory Burst and Phagocytotic Activity in Atlantic Salmon (Salmo salar L.) Macrophages by Lipopolysaccharide. Fish Shellfish Immunol (1995) 5:475–91. doi: 10.1016/S1050-4648(95)80049-2
224. Sjelelid R, Dalmo R. The Immunomodulatory Effect of LPS, Laminaran and Sulpahated Laminaran (1,3, D-Glucan)on Atlantic Salmon, Salmo salar L., Macrophages In Vitro. J Fish Dis (1995) 18:175–86. doi: 10.1111/j.1365-2761.1995.tb00275.x
225. Gagné F, Bruneau A, Turcotte P, Gagnon C, Lacaze E. An Investigation of the Immunotoxicity of Oil Sands Processed Water and Leachates in Trout Leukocytes. Ecotoxicol Environ Saf (2017) 141:43–51. doi: 10.1016/j.ecoenv.2017.03.012
226. Watanuki H, Gushiken Y, Sakai M. In Vitro Modulation of Common Carp (Cyprinus Carpio) Phagocytic Cells by Di-N-Butyl Phthalate and Di-2-Ethylhexal Phthalate. Aquat Toxicol (2003) 63:119–26. doi: 10.1016/S0166-445X(02)00172-8
227. Hayashi Y, Miclaus T, Murugadoss S, Takamiya M, Scavenius C, Kjaer-Sorensen K, et al. Female Versus Male Biological Identities of Nanoparticles Determine the Interaction With Immune Cells in Fish. Environ Sci Nano (2017) 4:895. doi: 10.1039/C7EN00071E
228. Secombes CJ, Cross AR, Sharp GJE, Garcia R. NADPH Oxidase-Like Activity in Rainbow Trout Oncorhynchus mykiss (Walbaum) Macrophages. Dev Comp Immunol (1992) 16:405–13. doi: 10.1016/0145-305X(92)90042-B
229. Grayfer L, Hodginson JW, Belosevic M. Antimicrobial Responses of Teleost Phagopcytes and Innate Immune Evasion Strategies of Intracellular Bacteria. Dev Comp Immunol (2014) 43:223–42. doi: 10.1016/j.dci.2013.08.003
230. Neumann NF, Stafford JL, Barreda D, Ainsworth AJ, Belosevic M. Antimicrobial Mechanisms of Fish Phagocytes and Their Role in Host Defense. Dev Comp Immunol (2001) 25:807–25. doi: 10.1016/S0145-305X(01)00037-4
231. Vera-Jimenez NI, Pietettri D, Wiegertjes GF, Nielsen ME. Comparative Study of β-Glucan Induced Respiratory Burts Measured by Nitroblue Tetratzolium Assay and Real Time Luninol-Enhanced Chemiluminsecence Assay in Common Carp (Cyprinus carpio L.). Fish Shellfish Immunol (2013) 34:1216–22. doi: 10.1016/j.fsi.2013.02.004
232. Novoa B, Figueras A, Ashton I, Secombes CJ. In Vitro Studies on Teh Regulation of Rainbow Trout (Oncorhynchus mykiss) Macrophage Respiratory Burst Activity. Dev Comp Immunol (1996) 20:207–16. doi: 10.1016/0145-305X(96)00011-0
233. Holen E, Lie KK, Araujo P, Olsvik PA. Pathogen Recognition and Mechansims in Atlantic Cod (Gadus morhua) Head Kidney Cells: Bacteria (LPS) and Virus (Poly I:C) Signals Through Different Pathways and Affect Distinct Genes. Fish Shellfish Immunol (2012) 33:267–76. doi: 10.1016/j.fsi.2012.05.013
234. Pietretti D, Vera-Jimenez NI, Hoole D, Wiegertjes GF. Oxidative Burst and Nitric Oxide Responses in Carp Macrophages Induced by Zymosan, MacroGard, and Selective Dectin-1 Agonists Suggest Recognition by Multiple Pattern Recognition Receptors. Fish Shellfish Immunol (2013) 35:847–57. doi: 10.1016/j.fsi.2013.06.022
235. Bowser DH, Frenkel K, Zelikoff JT. Effects of In Vitro Nickel Exposure on the Macrophage-Mediated Immune Functions of Rainbow Trout (Oncorhynchus mykiss). Bull Environ Contam Toxicol (1994) 52:367–73. doi: 10.1007/BF00197823
236. Moritomo T, Serata K, Teshirogi K, Aikawa H, Inoue Y, Itou T, et al. Flow Cytometric Analysis of the Neutrophil Respiratory Burst of Ayu, Plecoglossus altivelis: Comparison With Other Fresh Water Fish. Fish Shellfish Immunol (2003) 15:29–83. doi: 10.1016/S1050-4648(02)00136-5
237. Roszell LE, Anderson RS. In Vitro Immunomodulation of Pentachlorophenol in Phagocytes From an Estuarine Teleost, Fundulus heteroclitus, as Measured by Chemiluminiscence Activityl. Arch Environ Contam Toxicol (1993) 25:492–6. doi: 10.1007/BF00214338
238. Liu S, Pan C, Tang Y, Chen F, Yang M, Wang KJ. Identification of Novel Long-Coding RNAs Involved in Bisphenol A Induced Immunotoxicity in Fish Primary Macrophages. Fish Shellfish Immunol (2020) 100:152–60. doi: 10.1016/j.fsi.2020.03.006
239. Iwanowicz LR, Stafford JL, Patino R, Bentgen E, Miller NW, Blazer VS. Channel Catfish (Ictalurus Punctatus) Leukocytes Express Estrogen Receptor Isoforms Erα and Erβ and Are Functionally Modulated by Estrogens. Fish Shellfish Immunol (2014) 40:109–19. doi: 10.1016/j.fsi.2014.06.021
240. Qiu W, Yang M, Liu J, Luo S, Wong M, Zheng C. Bisphenol-S-Induced Chronic Inflammatory Stress in Liver via Peroxisome Proliferator-Activated Receptor γ Using Fish In Vivo and In Vitro Models. Environ Pollut (2019) 246:963–71. doi: 10.1016/j.envpol.2018.11.039
241. Aaltonen TM, Jokinen EI, Lappivaara J, Markkula SE, Salo HM, Lepännen H, et al. Effects of Primary and Secondary-Treated Bleached Kraft Mill Effluents on the Immune System and Physiological Parameters of Roach. Aquat Toxicol (2000) 51:55–67. doi: 10.1016/S0166-445X(00)00101-6
242. Roszell LE, Anderson RS. Hydrogen Peroxide Production and Bactericidal Activity in Pronephros Phagocyte Sub-Populations From Fundulus heteroclitus Following Exposure to Pentachlorophenol. Mar Environ Res (1997) 43:1–9. doi: 10.1016/0141-1136(95)00024-0
243. Castro R, Tafalla C. Overview of Fish Immunity. In: Beck P, Peatman E, authors. Mucosal Health in Aquaculture. Amsterdam: Elsevier (2015). p. 3–54.
244. Smith NC, Rise ML, Christian SL. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish and Lobe-Finned Fish. Front Immunol (2019) 10:2292. doi: 10.3389/fimmu.2019.02292
245. Yamagauchi T, Takizawa F, Furihata M, Soto-Lampe V, Dijkstra JM, Fischer U. Teleost Cytotoxic T Cells. Fish Shellfish Immunol (2019) 95:422–39. doi: 10.1016/j.fsi.2019.10.041
246. Nakanishi T, Shibasaki Y, Matsuura Y. T Cells in Fish. Biology (2015) 4:640–63. doi: 10.3390/biology4040640
247. Zwollo P. Dissecting Teleost B Cell Differentiation Using Transcription Factors. Dev Comp Immunol (2011) 35:898–905. doi: 10.1016/j.dci.2011.01.009
248. Perdiguero P, Morel E, Tafalla C. Diversity of Rainbow Trout Blood B Cells as Revealed by Single Cell RNA Sequencing. Biology (2021) 10:511. doi: 10.3390/biology10060511
249. Scapigliatti G, Fausto AM, Pichietti S. Fish Lymphocytes: An Evolutionary Equivalent of Mammalian Innate-Like Lymphocytes? Front Immunol (2018) 9:971. doi: 10.3389/fimmu.2018.00971
250. Miller NW, Clem LW. A Culture System for Mitogen-Induced Proliferation of Channel Catfish (Ictalurus punctatus) Peripheral Blood Lymphocytes. J Tiss Cult Methods (1988) 11:69–73. doi: 10.1007/BF01404135
251. Reitan LJ, Thuvander A. In Vitro Stimulation of Salmonid Leukocytes With Mitogens and With Aeromonas Salmonicida. Fish Shellfish Immunol (1991) 1:297–307. doi: 10.1016/S1050-4648(05)80067-1
252. Gauthier DT, Cartwright DD, Densmore CL, Blazer VS, Ottinger CA. Measurement of In Vitro Leucocyte Mitogenesis in Fish: ELISA Based Detection of the Thymidine Analogue 5’-Bromo-2’deoxyuridine. Fish Shellfish Immunol (2003) 14:279–88. doi: 10.1006/fsim.2002.0436
253. Scharsack JP, Steinhagen D, Leibold W, Rabe U, Korting W, Schuberth HJ. Flow Cytometric Analysis of Mitogen-Induced Activation of Rainbow Trout (Oncorhynchus mykiss) Peripheral Blood Leucocytes. J Vet Med (2001) B47:331–9. doi: 10.1046/j.1439-0450.2001.00454.x
254. Milston RH, Vella AT, Crippen TL, Fitzpatrick MS, Leong JAC, Schreck CB. In Vitro Detection of Functional Humoral Immunocompetence in Juvenile Chinook Salmon (Oncorhynchus Tshawytscha) Using Flow Cytometry. Fish Shellfish Immunol (2003) 15:145–58. doi: 10.1016/S1050-4648(02)00151-1
255. Faisal M, Huggett RJ. Effects of Polycyclic Aromatic Hydrocarbons on the Lymphocyte Mitogenic Responses in Spot, Leiostomus Xanthurus. Mar Environ Res (1993) 35:121–4. doi: 10.1016/0141-1136(93)90024-T
256. Carlson EA, Li Y, Zelikoff JT. Exposure of Japanes Medaka (Oryzias Latipes) to Benzo(a)Pyrene Suppresses Immune Function and Host Resistance Against Bacterial Challenge. Aquat Toxicol (2002) 56:289–301. doi: 10.1016/S0166-445X(01)00223-5
257. Reynaud S, Deschaux P. The Effects of 3-Methylcholanthrene on Lymphocyte Proliferation in the Common Carp (Cyprinus carpio). Toxicology (2005) 211:156–64. doi: 10.1016/j.tox.2005.02.015
258. Rymuszka A, Adaszek Ł. Pro- and Anti-Inflammatory Cytokine Expression in Carp Blood and Head Kidney Leukocytes Exposed to Cyanotoxin Stress – An In Vitro Study. Fish Shellfish Immunol (2007) 22:289–92. doi: 10.1016/j.fsi.2006.06.002
259. Martins K, Applegate B, Hagedorn B, Kennish J, Zwollo P. Di(2-Ethyhexyl) Phthalate Inhibits B Cell Proliferation and Reduces the Abundance of IgM-Secreting Cells in Cultured Immune Tissues of the Rainbow Trout. Fish Shellfish Immunol (2015) 44:332–41. doi: 10.1016/j.fsi.2015.02.037
260. Smith DA, Schurig GG, Smith SA, Holladay SD. The Hemolytic Plaque-Forming Cell Assay in Tilapia (Oreochromis niloticus) Exposed to Benzo(a)Pyrene: Enhanced or Depressed Plaque Formation Depends on Dosing Schedule. Toxicol Methods (1999) 9:57–79. doi: 10.1080/105172399242726
261. El-Gendy KS, Aly NM, El-Sebae AH. Effects of Edifenphos and Glyphosate on the Immune Response and Protein Biosynthesis of Bolti Fish (Tilapia nilotica). J Environ Sci Health B (1998) 33:135–49. doi: 10.1080/03601239809373135
262. Ristow S, Evans DL, Jaso-Friedmann L. Analyzing Nonspecific Cytotoxic Cells in Fish. Methods Cell Biol (2000) 121:347–57. doi: 10.1385/1-59259-044-6:347
263. Rice CD, Schlenk D. Immune Function and Cytochrome P4501 Activity After Acute Exposure to 3,3’,4,4’,5-Pentachlorobiphenyl (PCB 126) in Channel Catfish. J Aquat Anim Health (1995) 7:195–204. doi: 10.1577/1548-8667(1995)007<0195:IFACPA>2.3.CO;2
264. Richard AM, Judson RS, Houck KA, Grulke CM, Volaqrqth P, ThillainadarajahI, et al. ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem Res Toxicol (2016) 29:1225–51. doi: 10.1021/acs.chemrestox.6b00135
265. Hamers T, Legler J, Blaha L, Hylland K, Marigomez I, Schipper CA, et al. Expert Opinion on Toxicity Profiling – Report From a NORMAN Expert Group Meeting. Integr Environ Assess Manag (2013) 9:185–91. doi: 10.1002/ieam.1395
266. Naidenko OV, Andrews DQ, Temkin AM, Stoiber T, Uche UI, Evans S, et al. Investigating Molecular Mechanisms of Immunotoxicity and the Utility of ToxCast for Immunotoxicity Screening of Chemicals Added to Food. Int J Environ Res Public Health (2021) 18:3332. doi: 10.3390/ijerph18073332
267. Duchiron C, Reynaud S, Deschaux P. Lindane-Induced Macrophage Activating Factor (MAF) Production by Peripheral Blood Leukocytes (PBLs) of Rainbow Trout (Oncorhynchus mykiss): Involvement of Intracellular cAMP Mobilization. Aquat Toxicol (2002) 56:81–91. doi: 10.1016/S0166-445X(01)00193-X
268. Diaz-Resendiz KJG, Bernal-Ortega JA, Covantes-Rosales CE, Ortiz-Lazareno PC, Toledo-Ibarra GA, Ventura Ramon GH, et al. In Vitro Effect of Diazoxon, a Metabolite of Diazinon, on Proliferation, Sognal Transduction, and Death Induction in Mononuclear Cells of Nile Tilapia Fish (Oreochromis niloticus). Fish Shellfish Immunol (2020) 105:8–15. doi: 10.1016/j.fsi.2020.07.001
269. Giron-Perez MI, Zaitseva G, Casas-Solis J, Santerre A. Effects of Diazinon and Diazoxon on the Lymphoproliferation Rate of Splenocytes From Male Nile Tilapia (Oreochromis niloticus): The Immunosuppressive Effect Could Involve an Increase of Acetylcholine Levels. Fish Shellfish Immunol (2008) 25:517–21. doi: 10.1016/j.fsi.2008.07.002
270. Betoulle S, Duchiron C, Deschaux P. Lindane Differently Modulates Intracellular Calcium Levels in Two Populations of Rainbow Trout (Oncorhynchus mykiss) Immune Cells : Head Kidney Phagocytes and Peripheral Leukocytes. Toxicology (2000) 145:203–15. doi: 10.1016/S0300-483X(99)00226-7
271. Ottinger CA, Kaattari SL. Sensitivity of Rainbow Trout Leukocytes to Aflatoxin B1. Fish Shellfish Immunol (1998) 8:515–30. doi: 10.1006/fsim.1998.0154
272. Segner H, Bailey C, Tafalla C, Bo J. Immunotoxicity of Xenobiotics in Fish: A Role for the Aryl Hydrocarbon Receptor (AhR)? Int J Mol Sci (2021) 22:9460. doi: 10.3390/ijms22179460
273. Zhang H, Zhang J, Zhu Y. In Vitro Investigations for the QSAR Mechanism of Lymphocytes Apoptosis Induced by Substituted Aromatic Toxicants. Fish Shellfish Immunol (2008) 25:710–7. doi: 10.1016/j.fsi.2008.02.008
274. Zhang J, Zhang H, Ni W. Oxidative Stress and Apoptosis of Carassius auratus Lymphocytes Induced by Nonplanar (PCB 153) and Coplanar (PCB 126) Polychlorinated Biphenyl Congeners In Vitro. J Environ Sci (2009) 21:1284–9. doi: 10.1016/S1001-0742(08)62416-X
275. Vazzana M, Reas G, Cammarata M, Arizza V, Ferrantelli V, Parrinello N. Aroclor 1254 Vinhibits the Chemiluminescence Response of Peritoneal Cavity Cells From Sharpsnout Sea Bream (Diplodus puntazzo). Fish Shellfish Immunol (2014) 39:498–502. doi: 10.1016/j.fsi.2014.05.030
276. Muralidharan S, Mandrekar P. Cellular Stress Response and the Innate Immune Signalling: Integrating Pathways in Host Defense and Inflammation. J Leukoc Biol (2013) 94:1167–84. doi: 10.1189/jlb.0313153
277. Los DA, Murata N. Membrane Fluidity and Its Roles in the Perception of Environmental Signals. Biochim Biophys Acta (2004) 1666:142–57. doi: 10.1016/j.bbamem.2004.08.002
278. Regala RP, Rice CD, Schwedtke CE, Dorciak IR. The Effects of Tributyltin (TBT) and 3,3’,4,4’,5-Pentachlorbiphenyl (PCB-126) on Antibody Response and Phagocyte Oxidative Burst Activity in Channel Catfish. Arch Environ Contam Toxicol (2001) 40:386–91. doi: 10.1007/s002440010187
279. Settivari RS, Krieger SM, Hindle S, Gehen SC, Hollnagel HM, Boverhof DR. Current Status of Alternative Methods for Assessing Immunotoxicity: A Chemcial Industry Perspctive. Curr Opin Toxicol (2017) 5:19–27. doi: 10.1016/j.cotox.2017.06.015
280. Schirmer K. Proposal to Improve Vertebrate Cell Cultures to Establish Them as Substitutes for the Regulatory Testing of Chemicals and Effluents Using Fish. Toxicology (2006) 224:163–83. doi: 10.1016/j.tox.2006.04.042
281. Norberg-King TJ, Embry MR, Belanger SE, Braunbeck T, Butler JD, Dorn PB, et al. An International Perspective on the Tools and Concepts for Effluent Toxicity Assessments in the Context of Animal Alternatives. Environ Toxicol Chem (2018) 37:2745–57. doi: 10.1002/etc.4259
Keywords: fish, immunotoxicity, in vitro, toxicity screening, ecotoxicological hazard assessment, comparative immunity, fish immune cells
Citation: Segner H, Rehberger K, Bailey C and Bo J (2022) Assessing Fish Immunotoxicity by Means of In Vitro Assays: Are We There Yet? Front. Immunol. 13:835767. doi: 10.3389/fimmu.2022.835767
Received: 14 December 2021; Accepted: 01 February 2022;
Published: 28 February 2022.
Edited by:
Nguyen T. K. Vo, Wilfrid Laurier University, CanadaReviewed by:
Vivian Dayeh, University of Waterloo, CanadaLeon Grayfer, George Washington University, United States
Copyright © 2022 Segner, Rehberger, Bailey and Bo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helmut Segner, aGVsbXV0LnNlZ25lckB2ZXRzdWlzc2UudW5pYmUuY2g=
 Helmut Segner
Helmut Segner Kristina Rehberger1
Kristina Rehberger1 Christyn Bailey
Christyn Bailey