- 1Institute of Molecular Biomedicine, Faculty of Medicine, Comenius University, Bratislava, Slovakia
- 2Department of Geriatric Medicine, Faculty of Medicine, Comenius University, Bratislava, Slovakia
- 3Institute of Pathophysiology, Faculty of Medicine, Comenius University, Bratislava, Slovakia
- 4Department of Molecular Biology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia
Despite ongoing vaccination COVID-19 is a global healthcare problem because of the lack of an effective targeted therapy. In severe COVID-19 manifesting as acute respiratory distress syndrome, uncontrolled innate immune system activation results in cytokine deregulation, damage-associated molecular patterns release upon tissue damage and high occurrence of thrombotic events. These pathomechanisms are linked to neutrophil function and dysfunction, particularly increased formation of neutrophil extracellular traps (NETs). While the association of NETs and severity of COVID-19 has been shown and proved, the causes of NETs formation are unclear. The aim of this review is to summarize potential inducers of NETs formation in severe COVID-19 and to discuss potential treatment options targeting NETs formation of removal.
Introduction
SARS-CoV-2 causes much more than just COVID-19. The world is still facing huge socio-economic problems that will likely persist much longer than the pandemic itself (1). Experts agree that a population-wide vaccination is the most effective weapon in the fight against SARS-CoV-2, but its application is not trivial in today’s world (2, 3). Due to the current state of misinformation, those who would not be vaccinated represent a significant portion of the population in many countries, although the situation is dynamic and changes with the number of vaccines that have been approved (2, 4–6). Unfortunately, if not enough people are vaccinated, the pandemic will not stop (7). If such a scenario occurs, the only remaining solution will be targeted and effective treatment of patients with severe COVID-19 (8). Several treatment strategies have already been proposed, but most of them do not decrease COVID-19 mortality, but at best reduce the time of hospitalization (9–11), with some even being ineffective and harmful (12). So far, the most successful approach seems to be immunosupressive therapy (11, 13), but to design the best treatment is only possible if pathogenesis of the disease is known in detail (14). And there are still gaps to fill.
Thrombosis in COVID-19
At the beginning of the pandemics, COVID-19 was almost exclusively viewed in the context of lung damage, and therefore artificial lung ventilation appeared to be a key therapeutic intervention (15). However, initial results from China, Italy, and the United States showed, that mortality of COVID-19 patients admitted to ICU that were in the need of mechanical ventilation was greater than 90% (16–18). Although the data were not so alarming in other countries later (17, 19), it was clear that the pathophysiology of COVID-19 required a more comprehensive view. A partial explanation was provided by a study published in the Lancet, where the authors showed that patients infected with SARS-CoV-2 show endothelial dysfunction due to endothelial inflammation, so-called endothelitis (20). The damaged endothelium facilitates coagulation and thrombus formation, whether in large vessels or in small arterioles and capillaries (21). This thrombosis and subsequent coagulopathy cannot, of course, be resolved by artificial lung ventilation and additional oxygenation (22). Thrombotic complications were indeed found to be one of the major issues in treating critically ill ICU patients with COVID-19 (23). It has become clear, that identifying the initiators and drivers of thrombosis is vital.
Neutrophil Extracellular Traps
DNA is found inside the nucleus and mitochondria of the cell and as the primary information-carrying molecule is protected by several membranes from external potentially damage-causing factors (24). The same membranes, however, protect the DNA also from release outside of the cell. Nevertheless, various types of cell death might lead to DNA release into the extracellular space (25). During inflammation, a significant source of this cell-free DNA (cfDNA) comes from a specific type of neutrophil response - the so-called NETosis, a process that results in the formation of neutrophil extracellular traps (NETs) (26). NETs are web-like structures composed of DNA-histone complexes decorated by antimicrobial proteins and enzymes such as myeloperoxidase (MPO), neutrophil elastase (NE), cathelicidin, calprotectin and many others (27). In fact, their composition varies and has been reported to be dependent on the stimulus that activates neutrophils and initiates NETs release (28, 29).
Induction of NETs Formation
Formation of NETs was initially discovered as a response of neutrophils to the presence of bacteria and immediately, their role in prevention of pathogen dissemination was recognized (26). Since then, the list of bacteria that can induce the formation of NETs has substantially grown (30–37). Neutrophils are also capable of sensing the size of the stimulus and can selectively form NETs in response to larger pathogens such as fungi and parasites (38–44). Interestingly, NETs formation was also found to be stimulated by viruses (Hantavirus, hRSV, HIV, influenza) but their role in antiviral defense in vivo remains unresolved (45–52). While NETs might potentially restrain virus particles and their individual components possess antiviral properties, NETs were not found to be induced during mild influenza infection and mice that are incapable of their formation do not display increased susceptibility to influenza virus (51, 52). On the other hand, NETs most likely mediate pathology of severe viral infections, where virus-induced tissue damage allows subsequent bacterial overgrowth that together with endogenous stimuli drives NETs release (53, 54). Pathogens are recognized by neutrophils through a variety of pattern recognition receptors (PRR’s) such as toll-like receptors (TLR’s) 2, 4, 7, 8 and 9, dectins 1 and 2 and can also induce NETs formation via activation of calcium signaling by calcium ionophores (55).
Sterile stimuli are also capable of NETs induction and even NETs themselves have been described to induce more NETs (56, 57). If excessive NETs formation damages endothelium or other tissue, neutrophils detect parts of free mitochondria that are released from dead cells as damage-associated molecular patterns (DAMPs) (58). More than 10 years ago, Carl J Hauser and colleagues found that despite billions of years of evolution, the immune system still recognizes mitochondria as bacteria (59). This may be important in the crush syndrome, in polytrauma, where patients end up in a septic shock-like condition even though they do not have any confirmed microbial infection (60). Individual mitochondrial DAMPs activate different receptors. Mitochondrial DNA contains unmethylated CpG islets that are ligands for the Toll-like receptor 9 (TLR9) (61–63). Formylated peptides and proteins of mitochondrial origin are recognized by formyl peptide receptors (FPR1-2) (64, 65) and saturated cardiolipin is able to activate TLR4 mediated signaling (66, 67). During viral pneumonia induced breakdown of pneumocytes, endothelocytes, pulmonary megakaryocytes or during the formation of NETs by neutrophils, free mitochondria are released (68, 69). These can subsequently activate the immune system either as intact organelles or as their individual mitochondrial DAMPs. Similar mechanism might be at play in severe COVID-19 infection.
Another endogenous stimulus such as activated platelets can induce NETs through the interaction of High mobility group box 1 (HMGB1) with the receptor for advanced glycation end products (RAGE) or TLR4 and P-selectin through binding to P-selectin glycoprotein ligand (70–72). NETs formation is also induced by the binding of anti-nuclear or anti-neutrophil antibodies and immobilized immune complexes to FcγRIIIb receptor (73–76), and even nanoparticles, cholesterol and monosodium urate crystals can stimulate NETs formation (77–82). Finally, phorbol 12-myristate 13-acetate (PMA) triggers NETs formation independently of any receptor via activation of protein kinase C (PKC) and production of reactive oxygen species (ROS) and is often used as positive control for NETs induction (30). All of the pathogenic, as well as non-infectious stimuli capable of NETs induction are listed in the Table 1.
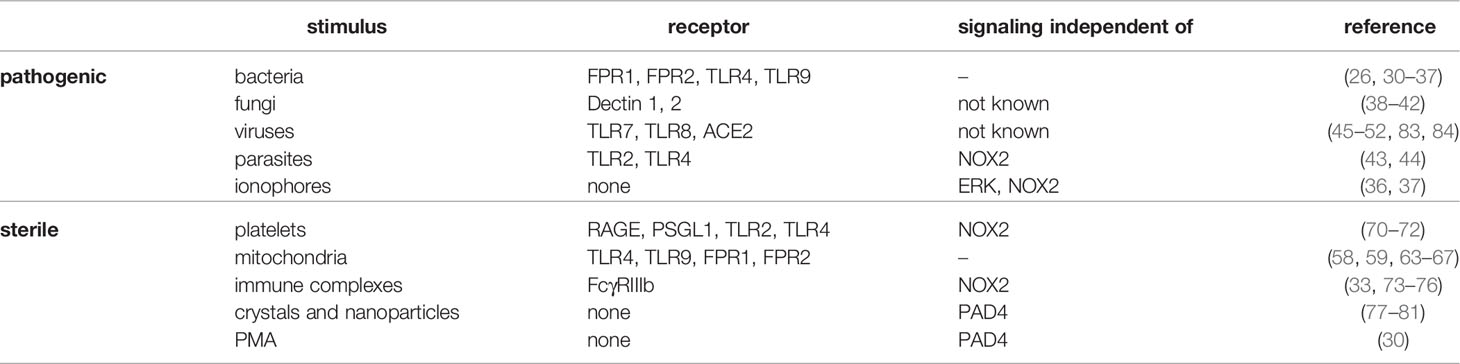
Table 1 Known pathogenic as well as sterile NETs inducers, corresponding receptors they interact with, along with a pathway the are independent of regarding NETs formation.
Formation of NETs is a double-edged sword (85). While being an extremely potent part of the antimicrobial defense, the emerging NETs must also be rapidly removed. Otherwise, the NETs activate other neutrophils and immunocompetent cells contributing to the inflammation that generates more NETs (55, 86). This creates a vicious cycle that is a key component in the pathogenesis of diseases as diverse as preeclampsia, sepsis or rheumatoid arthritis (71, 87, 88), and data suggests, that it is important for COVID-19 as well.
NETs Drive Thrombosis in COVID-19
The hypothesis that neutrophils and NETs are implicated in the formation of thrombi during severe SARS-CoV-2 infection has been proposed several times (89–94). Blood myeloperoxidase-DNA complex levels (i.e. NETs) were identified as a biomarker of an early response to SARS-CoV-2 infection, suggesting that circulating NETs are involved in COVID-19 pathology (95). Since then, several studies found that the production of NETs is increased in COVID-19 and their concentration is associated with severity of the disease and thrombosis (96–100), and NETs were found to be predominantly located in the lower respiratory tract of critically-ill patients (101). Skendros and his colleagues even proposed a mechanism of NETs induced thrombosis in COVID-19, where SARS-CoV-2 triggered complement activation leads to thrombin induced expression of tissue factor (TF) in neutrophils, which results in TF rich pro-coagulatory NETs (100). Increased NETs formation during SARS-CoV-2 infection has also been linked to ischemic stroke, underlying the importance of therapy focused on the inhibition of NETs formation (102). The fact that several studies and meta-analyses identified neutrophilia as one of the predictors of COVID-19 severity and an increased neutrophil to lymphocyte ratio has high predictive value if present at the beginning of the infection further underscores the role of neutrophils in early stages of COVID-19 pathology (103–107). In addition, the dysregulation of myeloid populations resulting in immature or dysfunctional neutrophils was found to be characteristic for developing severe, but not mild COVID-19 (108, 109). Lastly, genetic predisposition might also affect NETs mediated COVID-19 pathology. Genome-wide association study investigating genetic variants associated with circulating NETs levels in plasma revealed a variant in TMPRSS13 gene coding a type II transmembrane serine protease to be significantly associated with increased level of MPO-DNA complexes (110). Interestingly, the same protease TMPRSS13 was reported to enhance cellular uptake and replication of SARS-CoV-2, making it an interesting target for future investigation (111). Other study identified a variant on 3p21.31 region associated with increased respiratory failure risk in COVID-19 that enhances expression of leucine zipper transcription factor like 1 gene (LZTFL1). LZTFL1 regulates a viral response pathway and is associated with epithelial-mesenchymal transition and it is possible that this epithelial dysfunction is driven by neutrophil extracellular traps (112, 113).
Mechanisms of NETs Induction in COVID-19
Soon after it was found that SARS-CoV-2 infection results in the formation of neutrophil extracellular traps, the search for possible mechanisms of NETs induction in COVID-19 has begun. Arcanjo and his colleagues were the first to describe that both live, and heat-inactivated SARS-CoV-2 virus cultivated on and isolated from Vero cells could induce NETs formation at surprisingly low concentrations (83). Possible mechanism of SARS-CoV-2 induced NETs formation was later proposed by Veras and his colleagues. They reported that live, but not formaldehyde inactivated SARS-CoV-2 virus induces the formation of NETs and their induction is dependent on virus binding to neutrophil angiotensin converting enzyme (ACE2) receptor, again at interestingly low multiplicity of infection rate of 1 (84). Additionally, neutrophil elastase – a NETs component, is able to cleave S protein, resulting in an easier SARS-CoV-2 entry into the cell through ACE2, potentially increasing virus infectivity and its ability to stimulate immune response (114). Thus, as was already proposed, NETs formation might be induced by SARS-CoV-2 virus and at the same time increase its infectivity, making NETs and neutrophil elastase promising treatment targets (115). Whether these findings apply to a situation in vivo remains to be elucidated.
One possible factor linking endothelial dysfunction and deregulation of NETs formation with COVID-19 might be angiotensin 1-7, a product of ACE2, which functions as a key receptor for SARS-CoV-2 (116). Binding of the virus to this receptor leads to a reduction in the production of angiotensin 1-7 as a ligand of the Mas receptor (117, 118). The resulting imbalance between increasing angiotensin II and decreasing angiotensin 1-7 can stimulate endothelial dysfunction, an inflammatory response, induce NETs and thrombus formation (119–121). The consequences of these pathomechanisms are consistent with the histopathology of COVID-19 (122). Compared to influenza, lung necropsies in patients with COVID-19 showed similar diffuse alveolar damage but much more pronounced thrombosis with microangiopathy. Microthrombi were up to 9 times more frequent in the pulmonary circulation of COVID-19 when compared to influenza (122). Proposed mechanisms of NETs formation and induction of thrombosis in COVID-19 are illustrated in Figure 1.
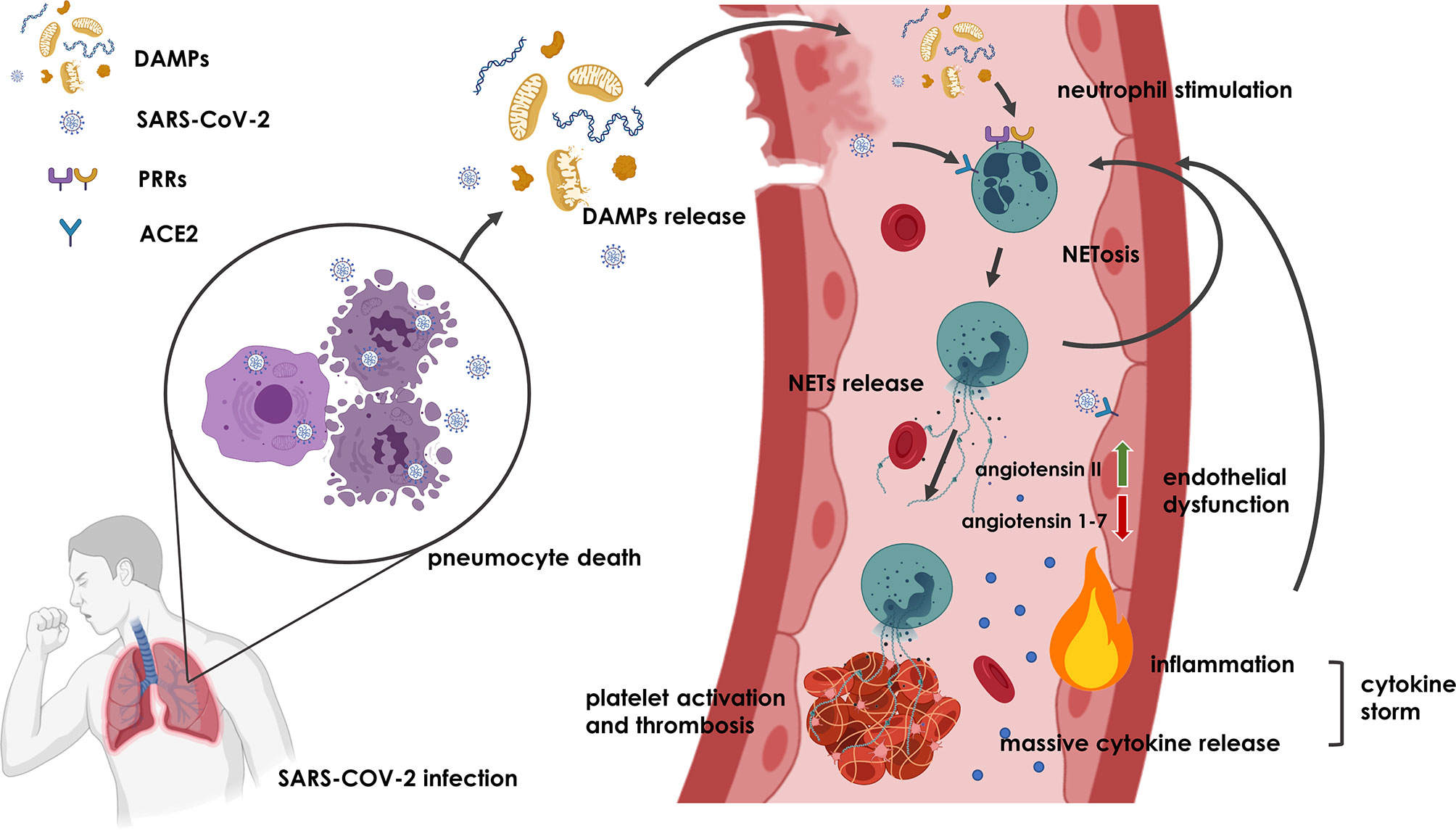
Figure 1 Potential mechanism underlying NETs formation and thrombosis induction in COVID-19. Upon SARS-CoV-2 infection, pneumocyte death and endothelial dysfunction result in the release of DAMPs and SARS-CoV-2 into extracellular space, where they bind to PRRs and ACE2 receptors and initiate activation of neutrophils and formation of NETs. NETs that are not removed from the circulation induce more NETs in a vicious circle and cause thrombosis and inflammation that might even lead to cytokine storm. Additionally, binding of SARS-CoV-2 on ACE2 receptor of endothelial cells may promote angiotensin II and angiotensin 1-7 imbalance leading to endothelial dysfunction and inflammation, which further contributes to NETs induction and thrombus formation. Figure was created with BioRender.com.
Targeting NETs Formation
Whether a neutrophil decides to form a NET depends on the context, i.e. also on the size, number and structural properties of the potential inducers (123). Understanding NETosis on a molecular level is extremely important, as the knowledge of signaling pathways involved in NETs induction will enable for selective inhibition of NETs formation, rather than just unspecific attenuation of inflammation. As was mentioned above, both pathogenic and sterile stimuli activate neutrophils through binding of various membrane and intracellular receptors and via MEK–extracellular-signal-regulated kinase (ERK) and protein kinase C (PKC) induce the production of ROS. ROS then activate MPO, which triggers oxidative activation of NE required for the degradation of actin cytoskeleton and subsequent histone processing upon NE nuclear translocation (41, 124, 125). Histone citrullination by protein-arginine deiminase type 4 (PAD4) further enhances chromatin decondensation and after mixing with cytoplasmatic components and permeabilization of the plasma membrane, NET is released into the extracellular space (30, 126–128).
To date, several compounds that target components of this pathway have been suggested as a potential intervention in COVID-19, most notable of them being Chloramidine, an inhibitor of PAD4 and NE inhibitor Sivelestat (ONO-5046), that has already been approved for the treatment of ARDS in Japan (92). While Sivelestat improves pulmonary function and oxygen saturation in ARDS patients, meta-analysis of completed clinical trials did not show improvement in survival of patients with ARDS (129). Currently, new generation of NE inhibitors (Lonodelestat, Alvelestat, CHF6333 and Elafin) have entered clinical trials, albeit neither NE nor PAD4 inhibitors are currently tested in clinical trials investigating COVID-19. Other, less specific drugs that could inhibit neutrophil recruitment or indirectly attenuate NETs formation such as Colchicine, Disulfiram, Anakinra, N-Acetyl Cysteine, Azithromycin, Aspirin, Cyclosporine A and Metformin are being clinically evaluated in COVID-19 but only two will inspect the effect of intervention on NETs formation (92). One retrospective study will examine the effect of Anakinra and the other examined the effect of disulfiram, but no results are currently available (NCT04594356, NCT04594343). Finally, hydroxychloroquine that interferes with NETs formation through inhibition of TLR9 has been proposed as a therapeutical intervention for COVID-19, although it has already been shown that it does not improve clinical outcome and mortality of patients with COVID-19 (130, 131).
Recently, mtDNA has been identified as an activator of cyclic GMP-AMP synthase (cGAS)-Stimulator of interferon genes (STING) signaling that drives aberrant type I interferon (type I IFN) response in COVID-19 (132). Moreover, pharmacological inhibition of STING improved disease outcome in a murine model of SARS-CoV-2 induced lung inflammation. Since type I IFN is also known to be an inducer of NETs formation, therapeutical targeting of DAMPs that are released from dead pneumocytes after SARS-CoV-2 infection should also be considered (133, 134). In fact, it has already been proposed, that cell-free mitochondria constitute a potential treatment target, since inhibition of their recognition by neutrophils could result in decreased neutrophil reactivity and NETs formation (135).
Another possible therapeutic strategy is to focus on the removal of NETs. NETs clearance is important for preventing sterile inflammation and thrombosis and is carried out by monocytes and macrophages, but also depends on the plasma nuclease activity (136). Because of histones, antimicrobial peptides and other proteins that bind DNA with high affinity, NETs may be partially resistant to deoxyribonuclease (137, 138). Additionally, anti-NET antibodies found in the plasma of COVID-19 patients likely also stabilize NETs and impair their clearance (139). Nevertheless, exogenous administration of recombinant deoxyribonuclease 1 has already been shown to decrease the concentration of plasma levels of cell free DNA and NETs in vitro and may be used as a potential therapeutic intervention (140). There are currently 8 registered clinical trials evaluating NETs in COVID-19 patients (NCT04409925, NCT04541979, NCT05139901, NCT04359654, NCT04402970, NCT04817332, NCT04594356, NCT04594343). Of those, NCT04594356, NCT04594343 were mentioned above and will investigate the effect of Anakinra and Disulfiram, and NCT04817332 evaluates the effect of protease inhibitor Brensocatib, that is expected to reduce NE activity. The remaining five are investigating the effect of recombinant human DNase 1 (rhDNase 1) on NET quantity, with NCT04402970 having already published results (141). In this study, treatment with rhDNase 1 was associated with decreased DNA-MPO complexes (i.e. NETs) in lungs as well as improved oxygenation. This study was however limited by its small sample size of 30 patients, and while a small decrease in mortality was observed upon rhDNase 1 treatment, it was not statistically significant and a more extensive trial would be warranted. All of the currently available as well as proposed treatments targeting NETs are listed in the Table 2.
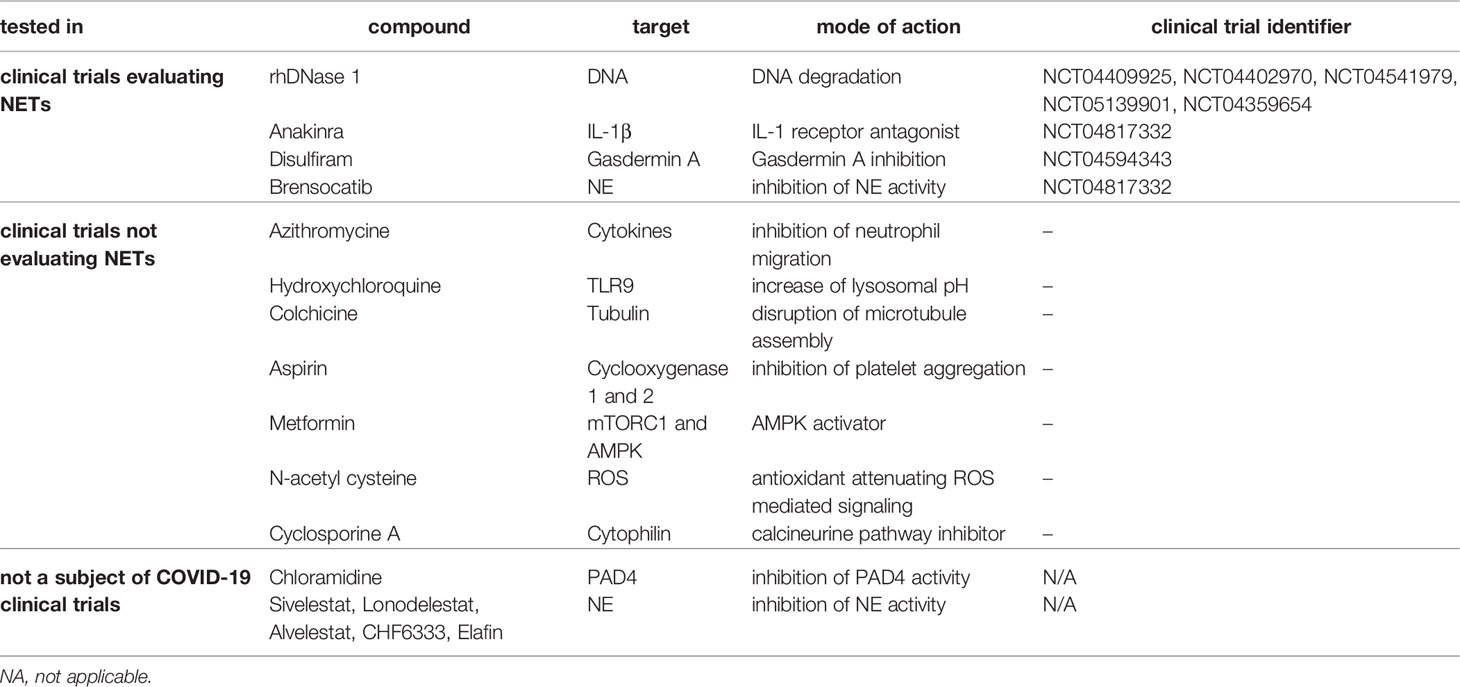
Table 2 Compounds that degrade or inhibit the formation of NETs and their corresponding targets with proposed mechanism of action in relation to clinical trials with COVID-19 patients.
Increased concentration of NETs components and cfDNA were negatively associated with clinical outcomes, indicating that NETs formation should be potentially evaluated not only as a novel target for therapeutic interventions, but could also be used as a clinical biomarker (142). A case study by Zuo and colleagues found remnants of NETs such as cfDNA, citrullinated histone H3, myeloperoxidase and its complexes in patient sera were associated with higher risk of thrombosis, in spite of previous prophylactic anticoagulation (143). While this phenomenon should be further explored, these results suggest that standard anticoagulation treatment may not be sufficient and targeting NETs formation and promoting their degradation should be prioritized.
Today, it still remains unclear what induces the formation of NETs during SARS-CoV-2 infection, why geriatric and not immunosuppressed patients are at higher risk of death from COVID-19, and how to best intervene to avoid the negative consequences of increased NETs production. Since the early outbreak in Wuhan, old age was found to be a major risk factor for mortality of COVID-19 patients (144). While it has been hypothesized, that increased risk of thrombotic complications is attributed to individuals with specific genetic conditions that favor the release of NETs and are therefore predisposed for abnormal coagulation (145), so far, no studies have stratified COVID-19 patients ex ante based on NETs formation. Whether elderly people and those with underlying health problems such as diabetes or asthma are at the highest risk of developing severe COVID-19 because of altered neutrophil function and NETs formation remains to be determined.
Conclusion
NETs research is in an exciting phase. While the evidence for the procoagulatory properties of NETs and their involvement in the COVID-19 pathology is growing stronger, insight into the mechanisms initiating their formation is still lacking. To develop targeted therapies focused on NETs inhibition is only possible if the factors that are involved in their induction are elucidated, and that requires extensive preclinical studies followed by clinical trials. This work presents current knowledge on the stimuli that might activate neutrophils and induce the formation of NETs during SARS-CoV-2 infection and highlights possible treatment options for COVID-19, but also for several other pathologies with shared pathogenesis involving NETs formation. Many unknowns need to be resolved, but understanding the complexities of NETs formation in vivo would be beneficial beyond the current pandemic.
Author Contributions
MP, MD, and PC conceptualized and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors are supported by Slovak research and development agency (grant number PP-COVID-20-0016).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The Socio-Economic Implications of the Coronavirus Pandemic (COVID-19): A Review. Int J Surg (2020) 78:185–93. doi: 10.1016/j.ijsu.2020.04.018
2. Xantus GZ, Burke D, Kanizsai P. How to Best Handle Vaccine Decliners: Scientific Facts and Psychological Approach. Postgrad Med J (2021) 9:postgradmedj-2021-139835. doi: 10.1136/postgradmedj-2021-139835
3. Corey L, Mascola JR, Fauci AS, Collins FS. A Strategic Approach to COVID-19 Vaccine R&D. Science (2020) 368(6494):948–50. doi: 10.1126/science.abc5312
4. Golob JL, Lugogo N, Lauring AS, Lok AS. SARS-CoV-2 Vaccines: A Triumph of Science and Collaboration. JCI Insight (2021) 6(9):e149187. doi: 10.1172/jci.insight.149187
5. Johnson NF, Velásquez N, Restrepo NJ, Leahy R, Gabriel N, El Oud S, et al. The Online Competition Between Pro- and Anti-Vaccination Views. Nature (2020) 582(7811):230–3. doi: 10.1038/s41586-020-2281-1
6. Megget K. Even Covid-19 Can’t Kill the Anti-Vaccination Movement. Bmj (2020) 369:m2184. doi: 10.1136/bmj.m2184
7. Lim W, Zhang P. Herd Immunity and a Vaccination Game: An Experimental Study. PloS One (2020) 15(5):e0232652. doi: 10.1371/journal.pone.0232652
8. Pedersen SF, Ho YC. SARS-CoV-2: A Storm Is Raging. J Clin Invest (2020) 130(5):2202–5. doi: 10.1172/JCI137647
9. Galan LEB, Santos NMD, Asato MS, Araújo JV, de Lima Moreira A, Araújo AMM, et al. Phase 2 Randomized Study on Chloroquine, Hydroxychloroquine or Ivermectin in Hospitalized Patients With Severe Manifestations of SARS-CoV-2 Infection. Pathog Glob Health (2021) 115(4):235–42. doi: 10.1080/20477724.2021.1890887
10. Rezagholizadeh A, Khiali S, Sarbakhsh P, Entezari-Maleki T. Remdesivir for Treatment of COVID-19; An Updated Systematic Review and Meta-Analysis. Eur J Pharmacol (2021) 897:173926. doi: 10.1016/j.ejphar.2021.173926
11. Kim MS, An MH, Kim WJ, Hwang TH. Comparative Efficacy and Safety of Pharmacological Interventions for the Treatment of COVID-19: A Systematic Review and Network Meta-Analysis. PloS Med (2020) 17(12):e1003501. doi: 10.1371/journal.pmed.1003501
12. Ghazy RM, Almaghraby A, Shaaban R, Kamal A, Beshir H, Moursi A, et al. A Systematic Review and Meta-Analysis on Chloroquine and Hydroxychloroquine as Monotherapy or Combined With Azithromycin in COVID-19 Treatment. Sci Rep (2020) 10(1):22139. doi: 10.1038/s41598-020-77748-x
13. Nugroho CW, Suryantoro SD, Yuliasih Y, Rosyid AN, Asmarawati TP, Andrianto L, et al. Optimal Use of Tocilizumab for Severe and Critical COVID-19: A Systematic Review and Meta-Analysis. F1000Res (2021) 10:73. doi: 10.12688/f1000research.45046.1
14. Manjili RH, Zarei M, Habibi M, Manjili MH. COVID-19 as an Acute Inflammatory Disease. J Immunol (2020) 205(1):12–9. doi: 10.4049/jimmunol.2000413
15. Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q, et al. Intubation and Ventilation Amid the COVID-19 Outbreak: Wuhan’s Experience. Anesthesiology (2020) 132(6):1317–32. doi: 10.1097/ALN.0000000000003296
16. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. Jama (2020) 323(16):1574–81. doi: 10.1001/jama.2020.5394
17. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. Jama (2020) 323(20):2052–9. doi: 10.1001/jama.2020.6775
18. Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical Course and Outcomes of 344 Intensive Care Patients With COVID-19. Am J Respir Crit Care Med (2020) 201(11):1430–4. doi: 10.1164/rccm.202003-0736LE
19. Auld SC, Caridi-Scheible M, Blum JM, Robichaux C, Kraft C, Jacob JT, et al. ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019. Crit Care Med (2020) 48(9):e799–804. doi: 10.1097/CCM.0000000000004457
20. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet (2020) 395(10234):1417–8. doi: 10.1016/S0140-6736(20)30937-5
21. Mosleh W, Chen K, Pfau SE, Vashist A. Endotheliitis and Endothelial Dysfunction in Patients With COVID-19: Its Role in Thrombosis and Adverse Outcomes. J Clin Med (2020) 9(6):1862. doi: 10.3390/jcm9061862
22. Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, et al. Endotheliopathy in COVID-19-Associated Coagulopathy: Evidence From a Single-Centre, Cross-Sectional Study. Lancet Haematol (2020) 7(8):e575–82. doi: 10.1016/S2352-3026(20)30216-7
23. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of Thrombotic Complications in Critically Ill ICU Patients With COVID-19. Thromb Res (2020) 191:145–7. doi: 10.1016/j.thromres.2020.04.013
24. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, Structures, and Functions of Circulating DNA in Oncology. Cancer Metastasis Rev (2016) 35(3):347–76. doi: 10.1007/s10555-016-9629-x
25. Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and Death of Circulating Cell-Free DNA. Cancer Biol Ther (2019) 20(8):1057–67. doi: 10.1080/15384047.2019.1598759
26. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil Extracellular Traps Kill Bacteria. Science (2004) 303(5663):1532–5. doi: 10.1126/science.1092385
27. Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense Against Candida Albicans. PloS Pathog (2009) 5(10):e1000639. doi: 10.1371/journal.ppat.1000639
28. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci Transl Med (2013) 5(178):178ra40. doi: 10.1126/scitranslmed.3005580
29. Mitsios A, Arampatzioglou A, Arelaki S, Mitroulis I, Ritis K. NETopathies? Unraveling the Dark Side of Old Diseases Through Neutrophils. Front Immunol (2016) 7:678. doi: 10.3389/fimmu.2016.00678
30. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel Cell Death Program Leads to Neutrophil Extracellular Traps. J Cell Biol (2007) 176(2):231–41. doi: 10.1083/jcb.200606027
31. Hamaguchi S, Seki M, Yamamoto N, Hirose T, Matsumoto N, Irisawa T, et al. Case of Invasive Nontypable Haemophilus Influenzae Respiratory Tract Infection With a Large Quantity of Neutrophil Extracellular Traps in Sputum. J Inflammation Res (2012) 5:137–40. doi: 10.2147/JIR.S39497
32. Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee WY, Sanz MJ, et al. Molecular Mechanisms of NET Formation and Degradation Revealed by Intravital Imaging in the Liver Vasculature. Nat Commun (2015) 6:6673. doi: 10.1038/ncomms7673
33. Van Avondt K, van der Linden M, Naccache PH, Egan DA, Meyaard L. Signal Inhibitory Receptor on Leukocytes-1 Limits the Formation of Neutrophil Extracellular Traps, But Preserves Intracellular Bacterial Killing. J Immunol (2016) 196(9):3686–94. doi: 10.4049/jimmunol.1501650
34. Hoppenbrouwers T, Autar ASA, Sultan AR, Abraham TE, van Cappellen WA, Houtsmuller AB, et al. In Vitro Induction of NETosis: Comprehensive Live Imaging Comparison and Systematic Review. PloS One (2017) 12(5):e0176472. doi: 10.1371/journal.pone.0176472
35. Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 Is Essential for Antibacterial Innate Immunity Mediated by Neutrophil Extracellular Traps. J Exp Med (2010) 207(9):1853–62. doi: 10.1084/jem.20100239
36. Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, Thompson PR, et al. Diverse Stimuli Engage Different Neutrophil Extracellular Trap Pathways. Elife (2017) 6:e24437. doi: 10.7554/eLife.24437
37. Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 Channel and Mitochondrial ROS Mediate NADPH Oxidase-Independent NETosis Induced by Calcium Influx. Proc Natl Acad Sci USA (2015) 112(9):2817–22. doi: 10.1073/pnas.1414055112
38. Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, et al. Restoration of NET Formation by Gene Therapy in CGD Controls Aspergillosis. Blood (2009) 114(13):2619–22. doi: 10.1182/blood-2009-05-221606
39. Rohm M, Grimm MJ, D’Auria AC, Almyroudis NG, Segal BH, Urban CF. NADPH Oxidase Promotes Neutrophil Extracellular Trap Formation in Pulmonary Aspergillosis. Infect Immun (2014) 82(5):1766–77. doi: 10.1128/IAI.00096-14
40. Loures FV, Röhm M, Lee CK, Santos E, Wang JP, Specht CA, et al. Recognition of Aspergillus Fumigatus Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps. PloS Pathog (2015) 11(2):e1004643. doi: 10.1371/journal.ppat.1004643
41. Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al. Myeloperoxidase Is Required for Neutrophil Extracellular Trap Formation: Implications for Innate Immunity. Blood (2011) 117(3):953–9. doi: 10.1182/blood-2010-06-290171
42. Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil Extracellular Traps Capture and Kill Candida Albicans Yeast and Hyphal Forms. Cell Microbiol (2006) 8(4):668–76. doi: 10.1111/j.1462-5822.2005.00659.x
43. Gabriel C, McMaster WR, Girard D, Descoteaux A. Leishmania Donovani Promastigotes Evade the Antimicrobial Activity of Neutrophil Extracellular Traps. J Immunol (2010) 185(7):4319–27. doi: 10.4049/jimmunol.1000893
44. Sousa-Rocha D, Thomaz-Tobias M, Diniz LF, Souza PS, Pinge-Filho P, Toledo KA. Trypanosoma Cruzi and Its Soluble Antigens Induce NET Release by Stimulating Toll-Like Receptors. PloS One (2015) 10(10):e0139569. doi: 10.1371/journal.pone.0139569
45. Raftery MJ, Lalwani P, Krautkrämer E, Peters T, Scharffetter-Kochanek K, Krüger R, et al. β2 Integrin Mediates Hantavirus-Induced Release of Neutrophil Extracellular Traps. J Exp Med (2014) 211(7):1485–97. doi: 10.1084/jem.20131092
46. Souza PSS, Barbosa LV, Diniz LFA, da Silva GS, Lopes BRP, Souza PMR, et al. Neutrophil Extracellular Traps Possess Anti-Human Respiratory Syncytial Virus Activity: Possible Interaction With the Viral F Protein. Virus Res (2018) 251:68–77. doi: 10.1016/j.virusres.2018.04.001
47. Cortjens B, de Boer OJ, de Jong R, Antonis AF, Sabogal Piñeros YS, Lutter R, et al. Neutrophil Extracellular Traps Cause Airway Obstruction During Respiratory Syncytial Virus Disease. J Pathol (2016) 238(3):401–11. doi: 10.1002/path.4660
48. Funchal GA, Jaeger N, Czepielewski RS, Machado MS, Muraro SP, Stein RT, et al. Respiratory Syncytial Virus Fusion Protein Promotes TLR-4-Dependent Neutrophil Extracellular Trap Formation by Human Neutrophils. PloS One (2015) 10(4):e0124082. doi: 10.1371/journal.pone.0124082
49. Barr FD, Ochsenbauer C, Wira CR, Rodriguez-Garcia M. Neutrophil Extracellular Traps Prevent HIV Infection in the Female Genital Tract. Mucosal Immunol (2018) 11(5):1420–8. doi: 10.1038/s41385-018-0045-0
50. Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil Extracellular Traps Mediate a Host Defense Response to Human Immunodeficiency Virus-1. Cell Host Microbe (2012) 12(1):109–16. doi: 10.1016/j.chom.2012.05.015
51. Hemmers S, Teijaro JR, Arandjelovic S, Mowen KA. PAD4-Mediated Neutrophil Extracellular Trap Formation Is Not Required for Immunity Against Influenza Infection. PloS One (2011) 6(7):e22043. doi: 10.1371/journal.pone.0022043
52. Ellis TN, Kuehn MJ. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiol Mol Biol Rev (2010) 74(1):81–94. doi: 10.1128/MMBR.00031-09
53. Pillai PS, Molony RD, Martinod K, Dong H, Pang IK, Tal MC, et al. Mx1 Reveals Innate Pathways to Antiviral Resistance and Lethal Influenza Disease. Science (2016) 352(6284):463–6. doi: 10.1126/science.aaf3926
54. Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive Neutrophils and Neutrophil Extracellular Traps Contribute to Acute Lung Injury of Influenza Pneumonitis. Am J Pathol (2011) 179(1):199–210. doi: 10.1016/j.ajpath.2011.03.013
55. Papayannopoulos V. Neutrophil Extracellular Traps in Immunity and Disease. Nat Rev Immunol (2018) 18(2):134–47. doi: 10.1038/nri.2017.105
56. Cui BB, Tan CY, Schorn C, Tang HH, Liu Y, Zhao Y. Neutrophil Extracellular Traps in Sterile Inflammation: The Story After Dying? Autoimmunity (2012) 45(8):593–6. doi: 10.3109/08916934.2012.719952
57. Herster F, Bittner Z, Archer NK, Dickhöfer S, Eisel D, Eigenbrod T, et al. Neutrophil Extracellular Trap-Associated RNA and LL37 Enable Self-Amplifying Inflammation in Psoriasis. Nat Commun (2020) 11(1):105. doi: 10.1038/s41467-019-13756-4
58. Itagaki K, Kaczmarek E, Lee YT, Tang IT, Isal B, Adibnia Y, et al. Mitochondrial DNA Released by Trauma Induces Neutrophil Extracellular Traps. PloS One (2015) 10(3):e0120549. doi: 10.1371/journal.pone.0120549
59. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury. Nature (2010) 464(7285):104–7. doi: 10.1038/nature08780
60. Thurairajah K, Briggs GD, Balogh ZJ. The Source of Cell-Free Mitochondrial DNA in Trauma and Potential Therapeutic Strategies. Eur J Trauma Emerg Surg (2018) 44(3):325–34. doi: 10.1007/s00068-018-0954-3
61. Zhang JZ, Liu Z, Liu J, Ren JX, Sun TS. Mitochondrial DNA Induces Inflammation and Increases TLR9/NF-κb Expression in Lung Tissue. Int J Mol Med (2014) 33(4):817–24. doi: 10.3892/ijmm.2014.1650
62. Bao W, Xia H, Liang Y, Ye Y, Lu Y, Xu X, et al. Toll-Like Receptor 9 Can be Activated by Endogenous Mitochondrial DNA to Induce Podocyte Apoptosis. Sci Rep (2016) 6:22579. doi: 10.1038/srep22579
63. Fang C, Wei X, Wei Y. Mitochondrial DNA in the Regulation of Innate Immune Responses. Protein Cell (2016) 7(1):11–6. doi: 10.1007/s13238-015-0222-9
64. Kaczmarek E, Hauser CJ, Kwon WY, Riça I, Chen L, Sandler N, et al. A Subset of Five Human Mitochondrial Formyl Peptides Mimics Bacterial Peptides and Functionally Deactivates Human Neutrophils. J Trauma Acute Care Surg (2018) 85(5):936–43. doi: 10.1097/TA.0000000000001971
65. Weiß E, Kretschmer D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol (2018) 39(10):815–29. doi: 10.1016/j.it.2018.08.005
66. Coats SR, Hashim A, Paramonov NA, To TT, Curtis MA, Darveau RP. Cardiolipins Act as a Selective Barrier to Toll-Like Receptor 4 Activation in the Intestine. Appl Environ Microbiol (2016) 82(14):4264–78. doi: 10.1128/AEM.00463-16
67. Pizzuto M, Lonez C, Baroja-Mazo A, Martínez-Banaclocha H, Tourlomousis P, Gangloff M, et al. Saturation of Acyl Chains Converts Cardiolipin From an Antagonist to an Activator of Toll-Like Receptor-4. Cell Mol Life Sci (2019) 76(18):3667–78. doi: 10.1007/s00018-019-03113-5
68. Herold S, Becker C, Ridge KM, Budinger GR. Influenza Virus-Induced Lung Injury: Pathogenesis and Implications for Treatment. Eur Respir J (2015) 45(5):1463–78. doi: 10.1183/09031936.00186214
69. Darden DB, Hawkins RB, Larson SD, Iovine NM, Prough DS, Efron PA. The Clinical Presentation and Immunology of Viral Pneumonia and Implications for Management of Coronavirus Disease 2019. Crit Care Explor (2020) 2(4):e0109. doi: 10.1097/CCE.0000000000000109
70. Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P-Selectin Promotes Neutrophil Extracellular Trap Formation in Mice. Blood (2015) 126(2):242–6. doi: 10.1182/blood-2015-01-624023
71. Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Septic Blood. Nat Med (2007) 13(4):463–9. doi: 10.1038/nm1565
72. Tadie JM, Bae HB, Jiang S, Park DW, Bell CP, Yang H, et al. HMGB1 Promotes Neutrophil Extracellular Trap Formation Through Interactions With Toll-Like Receptor 4. Am J Physiol Lung Cell Mol Physiol (2013) 304(5):L342–9. doi: 10.1152/ajplung.00151.2012
73. Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting Neutrophils Are Major Inducers of Type I IFN Production in Pediatric Systemic Lupus Erythematosus. Sci Transl Med (2011) 3(73):73ra20. doi: 10.1126/scitranslmed.3001201
74. Van Avondt K, Fritsch-Stork R, Derksen RH, Meyaard L. Ligation of Signal Inhibitory Receptor on Leukocytes-1 Suppresses the Release of Neutrophil Extracellular Traps in Systemic Lupus Erythematosus. PloS One (2013) 8(10):e78459. doi: 10.1371/journal.pone.0078459
75. Behnen M, Leschczyk C, Moller S, Batel T, Klinger M, Solbach W, et al. Immobilized Immune Complexes Induce Neutrophil Extracellular Trap Release by Human Neutrophil Granulocytes via FcgammaRIIIB and Mac-1. J Immunol (2014) 193(4):1954–65. doi: 10.4049/jimmunol.1400478
76. Alemán OR, Mora N, Cortes-Vieyra R, Uribe-Querol E, Rosales C. Differential Use of Human Neutrophil Fcγ Receptors for Inducing Neutrophil Extracellular Trap Formation. J Immunol Res (2016) 2016:2908034. doi: 10.1155/2016/2908034
77. Muñoz LE, Bilyy R, Biermann MH, Kienhöfer D, Maueröder C, Hahn J, et al. Nanoparticles Size-Dependently Initiate Self-Limiting NETosis-Driven Inflammation. Proc Natl Acad Sci USA (2016) 113(40):E5856–65. doi: 10.1073/pnas.1602230113
78. Desai J, Foresto-Neto O, Honarpisheh M, Steiger S, Nakazawa D, Popper B, et al. Particles of Different Sizes and Shapes Induce Neutrophil Necroptosis Followed by the Release of Neutrophil Extracellular Trap-Like Chromatin. Sci Rep (2017) 7(1):15003. doi: 10.1038/s41598-017-15106-0
79. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil Extracellular Traps License Macrophages for Cytokine Production in Atherosclerosis. Science (2015) 349(6245):316–20. doi: 10.1126/science.aaa8064
80. Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated Neutrophil Extracellular Traps Limit Inflammation by Degrading Cytokines and Chemokines. Nat Med (2014) 20(5):511–7. doi: 10.1038/nm.3547
81. Desai J, Kumar SV, Mulay SR, Konrad L, Romoli S, Schauer C, et al. PMA and Crystal-Induced Neutrophil Extracellular Trap Formation Involves RIPK1-RIPK3-MLKL Signaling. Eur J Immunol (2016) 46(1):223–9. doi: 10.1002/eji.201545605
82. Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, et al. Neutrophil Extracellular Trap Formation Is Associated With IL-1beta and Autophagy-Related Signaling in Gout. PloS One (2011) 6(12):e29318. doi: 10.1371/journal.pone.0029318
83. Arcanjo A, Logullo J, Menezes CCB, de Souza Carvalho Giangiarulo TC, Dos Reis MC, de Castro GMM, et al. The Emerging Role of Neutrophil Extracellular Traps in Severe Acute Respiratory Syndrome Coronavirus 2 (COVID-19). Sci Rep (2020) 10(1):19630. doi: 10.1038/s41598-020-76781-0
84. Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2-Triggered Neutrophil Extracellular Traps Mediate COVID-19 Pathology. J Exp Med (2020) 217(12):e20201129. doi: 10.1084/jem.20201129
85. Kaplan MJ, Radic M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J Immunol (2012) 189(6):2689–95. doi: 10.4049/jimmunol.1201719
86. McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and Neutrophil Extracellular Traps Collaborate to Promote Intravascular Coagulation During Sepsis in Mice. Blood (2017) 129(10):1357–67. doi: 10.1182/blood-2016-09-741298
87. Gupta A, Hasler P, Gebhardt S, Holzgreve W, Hahn S. Occurrence of Neutrophil Extracellular DNA Traps (NETs) in Pre-Eclampsia: A Link With Elevated Levels of Cell-Free DNA? Ann N Y Acad Sci (2006) 1075:118–22. doi: 10.1196/annals.1368.015
88. Jorch SK, Kubes P. An Emerging Role for Neutrophil Extracellular Traps in Noninfectious Disease. Nat Med (2017) 23(3):279–87. doi: 10.1038/nm.4294
89. Yaqinuddin A, Kashir J. Novel Therapeutic Targets for SARS-CoV-2-Induced Acute Lung Injury: Targeting a Potential IL-1β/Neutrophil Extracellular Traps Feedback Loop. Med Hypotheses (2020) 143:109906. doi: 10.1016/j.mehy.2020.109906
90. Thierry AR. Does the Newly Observed Inflammatory Syndrome in Children Demonstrate a Link Between Uncontrolled Neutrophil Extracellular Traps Formation and COVID-19? Pediatr Res United States (2020) 89(4):716–7. doi: 10.1038/s41390-020-0996-1
91. Cicco S, Cicco G, Racanelli V, Vacca A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediators Inflammation (2020) 2020:7527953. doi: 10.1155/2020/7527953
92. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps. J Exp Med (2020) 217(6):e20200652. doi: 10.1084/jem.20200652
93. Golonka RM, Saha P, Yeoh BS, Chattopadhyay S, Gewirtz AT, Joe B, et al. Harnessing Innate Immunity to Eliminate SARS-CoV-2 and Ameliorate COVID-19 Disease. Physiol Genomics (2020) 52(5):217–21. doi: 10.1152/physiolgenomics.00033.2020
94. Thierry AR, Roch B. SARS-CoV2 may Evade Innate Immune Response, Causing Uncontrolled Neutrophil Extracellular Traps Formation and Multi-Organ Failure. Clin Sci (Lond) (2020) 134(12):1295–300. doi: 10.1042/CS20200531
95. Guéant JL, Fromonot J, Guéant-Rodriguez RM, Lacolley P, Guieu R, Regnault V. Blood Myeloperoxidase-DNA, a Biomarker of Early Response to SARS-CoV-2 Infection? Allergy (2021) 76(3):892–6. doi: 10.1111/all.14533
96. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil Extracellular Traps (NETs) as Markers of Disease Severity in COVID-19. medRxiv (2020) 14:2020.04.09.20059626. doi: 10.1101/2020.04.09.20059626
97. Petito E, Falcinelli E, Paliani U, Cesari E, Vaudo G, Sebastiano M, et al. Association of Neutrophil Activation, More Than Platelet Activation, With Thrombotic Complications in Coronavirus Disease 2019. J Infect Dis (2021) 223(6):933–44. doi: 10.1093/infdis/jiaa756
98. Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood (2020) 136(10):1169–79. doi: 10.1182/blood.2020007008
99. Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, et al. Neutrophil Extracellular Traps Infiltrate the Lung Airway, Interstitial, and Vascular Compartments in Severe COVID-19. J Exp Med (2020) 217(12):e20201012. doi: 10.1084/jem.20201012
100. Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and Tissue Factor-Enriched Neutrophil Extracellular Traps Are Key Drivers in COVID-19 Immunothrombosis. J Clin Invest (2020) 130(11):6151–7. doi: 10.1172/JCI141374
101. Ouwendijk WJD, Raadsen MP, van Kampen JJA, Verdijk RM, von der Thusen JH, Guo L, et al. Neutrophil Extracellular Traps Persist at High Levels in the Lower Respiratory Tract of Critically Ill COVID-19 Patients. J Infect Dis (2021) 223(9):1512–21. doi: 10.1093/infdis/jiab050
102. Pramitasuri TI, Laksmidewi A, Putra IBK, Dalimartha FA. Neutrophil Extracellular Traps in Coronavirus Disease-19-Associated Ischemic Stroke: A Novel Avenue in Neuroscience. Exp Neurobiol (2021) 30(1):1–12. doi: 10.5607/en20048
103. Guan J, Wei X, Qin S, Liu X, Jiang Y, Chen Y, et al. Continuous Tracking of COVID-19 Patients’ Immune Status. Int Immunopharmacol (2020) 89(Pt A):107034. doi: 10.1016/j.intimp.2020.107034
104. Webb BJ, Peltan ID, Jensen P, Hoda D, Hunter B, Silver A, et al. Clinical Criteria for COVID-19-Associated Hyperinflammatory Syndrome: A Cohort Study. Lancet Rheumatol (2020) 2(12):e754–63. doi: 10.1016/S2665-9913(20)30343-X
105. Singh K, Mittal S, Gollapudi S, Butzmann A, Kumar J, Ohgami RS. A Meta-Analysis of SARS-CoV-2 Patients Identifies the Combinatorial Significance of D-Dimer, C-Reactive Protein, Lymphocyte, and Neutrophil Values as a Predictor of Disease Severity. Int J Lab Hematol (2021) 43(2):324–8. doi: 10.1111/ijlh.13354
106. Lagunas-Rangel FA. Neutrophil-To-Lymphocyte Ratio and Lymphocyte-to-C-Reactive Protein Ratio in Patients With Severe Coronavirus Disease 2019 (COVID-19): A Meta-Analysis. J Med Virol (2020) 92(10):1733–4. doi: 10.1002/jmv.25819
107. Lagunas-Rangel FA, Chávez-Valencia V. Laboratory Findings That Predict a Poor Prognosis in COVID-19 Patients With Diabetes: A Meta-Analysis. Endocrinol Diabetes Nutr (Engl Ed) (2021) 68(7):520–2. doi: 10.1016/j.endien.2021.11.002
108. Parackova Z, Zentsova I, Bloomfield M, Vrabcova P, Smetanova J, Klocperk A, et al. Disharmonic Inflammatory Signatures in COVID-19: Augmented Neutrophils’ But Impaired Monocytes’ and Dendritic Cells’ Responsiveness. Cells (2020) 9(10):2206. doi: 10.3390/cells9102206
109. Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell (2020) 182(6):1419–40.e23. doi: 10.1016/j.cell.2020.08.001
110. Donkel SJ, Portilla Fernández E, Ahmad S, Rivadeneira F, van Rooij FJA, Ikram MA, et al. Common and Rare Variants Genetic Association Analysis of Circulating Neutrophil Extracellular Traps. Front Immunol (2021) 12:615527. doi: 10.3389/fimmu.2021.615527
111. Kishimoto M, Uemura K, Sanaki T, Sato A, Hall WW, Kariwa H, et al. TMPRSS11D and TMPRSS13 Activate the SARS-CoV-2 Spike Protein. Viruses (2021) 13(3):384. doi: 10.3390/v13030384
112. Downes DJ, Cross AR, Hua P, Roberts N, Schwessinger R, Cutler AJ, et al. Identification of LZTFL1 as a Candidate Effector Gene at a COVID-19 Risk Locus. Nat Genet (2021) 53(11):1606–15. doi: 10.1038/s41588-021-00955-3
113. Pandolfi L, Bozzini S, Frangipane V, Percivalle E, De Luigi A, Violatto MB, et al. Neutrophil Extracellular Traps Induce the Epithelial-Mesenchymal Transition: Implications in Post-COVID-19 Fibrosis. Front Immunol (2021) 12:663303. doi: 10.3389/fimmu.2021.663303
114. Watanabe R, Matsuyama S, Shirato K, Maejima M, Fukushi S, Morikawa S, et al. Entry From the Cell Surface of Severe Acute Respiratory Syndrome Coronavirus With Cleaved S Protein as Revealed by Pseudotype Virus Bearing Cleaved S Protein. J Virol (2008) 82(23):11985–91. doi: 10.1128/JVI.01412-08
115. Thierry AR. Anti-Protease Treatments Targeting Plasmin(ogen) and Neutrophil Elastase May Be Beneficial in Fighting COVID-19. Physiol Rev (2020) 100:1597–8. doi: 10.1152/physrev.00019.2020
116. Li Y, Zhou W, Yang L, You R. Physiological and Pathological Regulation of ACE2, the SARS-CoV-2 Receptor. Pharmacol Res (2020) 157:104833. doi: 10.1016/j.phrs.2020.104833
117. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A Crucial Role of Angiotensin Converting Enzyme 2 (ACE2) in SARS Coronavirus-Induced Lung Injury. Nat Med (2005) 11(8):875–9. doi: 10.1038/nm1267
118. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The Pivotal Link Between ACE2 Deficiency and SARS-CoV-2 Infection. Eur J Intern Med (2020) 76:14–20. doi: 10.1016/j.ejim.2020.04.037
119. Fraga-Silva RA, Pinheiro SV, Gonçalves AC, Alenina N, Bader M, Santos RA. The Antithrombotic Effect of Angiotensin-(1-7) Involves Mas-Mediated NO Release From Platelets. Mol Med (2008) 14(1-2):28–35. doi: 10.2119/2007-00073.Fraga-Silva
120. da Silveira KD, Coelho FM, Vieira AT, Sachs D, Barroso LC, Costa VV, et al. Anti-Inflammatory Effects of the Activation of the Angiotensin-(1-7) Receptor, MAS, in Experimental Models of Arthritis. J Immunol (2010) 185(9):5569–76. doi: 10.4049/jimmunol.1000314
121. Romero A, San Hipólito-Luengo Á, Villalobos LA, Vallejo S, Valencia I, Michalska P, et al. The Angiotensin-(1-7)/Mas Receptor Axis Protects From Endothelial Cell Senescence via Klotho and Nrf2 Activation. Aging Cell (2019) 18(3):e12913. doi: 10.1111/acel.12913
122. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med (2020) 383(2):120–8. doi: 10.1056/NEJMoa2015432
123. Manfredi AA, Ramirez GA, Rovere-Querini P, Maugeri N. The Neutrophil’s Choice: Phagocytose vs Make Neutrophil Extracellular Traps. Front Immunol (2018) 9:288. doi: 10.3389/fimmu.2018.00288
124. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil Elastase and Myeloperoxidase Regulate the Formation of Neutrophil Extracellular Traps. J Cell Biol (2010) 191(3):677–91. doi: 10.1083/jcb.201006052
125. Porto BN, Stein RT. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front Immunol (2016) 7:311. doi: 10.3389/fimmu.2016.00311
126. Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET Formation. Front Immunol (2012) 3:360. doi: 10.3389/fimmu.2012.00360
127. Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone Hypercitrullination Mediates Chromatin Decondensation and Neutrophil Extracellular Trap Formation. J Cell Biol (2009) 184(2):205–13. doi: 10.1083/jcb.200806072
128. Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, et al. Human PAD4 Regulates Histone Arginine Methylation Levels via Demethylimination. Science (2004) 306(5694):279–83. doi: 10.1126/science.1101400
129. Tagami T, Tosa R, Omura M, Fukushima H, Kaneko T, Endo T, et al. Effect of a Selective Neutrophil Elastase Inhibitor on Mortality and Ventilator-Free Days in Patients With Increased Extravascular Lung Water: A Post Hoc Analysis of the PiCCO Pulmonary Edema Study. J Intensive Care (2014) 2(1):67. doi: 10.1186/s40560-014-0067-y
130. Gasmi A, Peana M, Noor S, Lysiuk R, Menzel A, Gasmi Benahmed A, et al. Chloroquine and Hydroxychloroquine in the Treatment of COVID-19: The Never-Ending Story. Appl Microbiol Biotechnol (2021) 105(4):1333–43. doi: 10.1007/s00253-021-11094-4
131. Zhang S, Zhang Q, Wang F, Guo X, Liu T, Zhao Y, et al. Hydroxychloroquine Inhibiting Neutrophil Extracellular Trap Formation Alleviates Hepatic Ischemia/Reperfusion Injury by Blocking TLR9 in Mice. Clin Immunol (2020) 216:108461. doi: 10.1016/j.clim.2020.108461
132. Di Domizio J, Gulen MF, Saidoune F, Thacker VV, Yatim A, Sharma K, et al. The cGAS-STING Pathway Drives Type I IFN Immunopathology in COVID-19. Nature (2022). doi: 10.1038/s41586-022-04421-w
133. Moreira-Teixeira L, Stimpson PJ, Stavropoulos E, Hadebe S, Chakravarty P, Ioannou M, et al. Type I IFN Exacerbates Disease in Tuberculosis-Susceptible Mice by Inducing Neutrophil-Mediated Lung Inflammation and NETosis. Nat Commun (2020) 11(1):5566. doi: 10.1038/s41467-020-19412-6
134. Grunwell JR, Stephenson ST, Mohammad AF, Jones K, Mason C, Opolka C, et al. Differential Type I Interferon Response and Primary Airway Neutrophil Extracellular Trap Release in Children With Acute Respiratory Distress Syndrome. Sci Rep (2020) 10(1):19049. doi: 10.1038/s41598-020-76122-1
135. Vorobjeva NV, Sud’ina GF, Chernyak BV. Mitochondria Are Potential Targets for the Development of New Drugs Against Neutrophilic Inflammation in Severe Pneumonia Including COVID-19. Front Pharmacol (2021) 12:609508. doi: 10.3389/fphar.2021.609508
136. Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, et al. Host DNases Prevent Vascular Occlusion by Neutrophil Extracellular Traps. Science (2017) 358(6367):1202–6. doi: 10.1126/science.aam8897
137. Neumann A, Völlger L, Berends ET, Molhoek EM, Stapels DA, Midon M, et al. Novel Role of the Antimicrobial Peptide LL-37 in the Protection of Neutrophil Extracellular Traps Against Degradation by Bacterial Nucleases. J Innate Immun (2014) 6(6):860–8. doi: 10.1159/000363699
138. Masuda S, Nonokawa M, Futamata E, Nishibata Y, Iwasaki S, Tsuji T, et al. Formation and Disordered Degradation of Neutrophil Extracellular Traps in Necrotizing Lesions of Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis. Am J Pathol (2019) 189(4):839–46. doi: 10.1016/j.ajpath.2019.01.007
139. Zuo Y, Yalavarthi S, Navaz SA, Hoy CK, Harbaugh A, Gockman K, et al. Autoantibodies Stabilize Neutrophil Extracellular Traps in COVID-19. JCI Insight (2021) 6(15):e150111. doi: 10.1172/jci.insight.150111
140. Lee YY, Park HH, Park W, Kim H, Jang JG, Hong KS, et al. Long-Acting Nanoparticulate DNase-1 for Effective Suppression of SARS-CoV-2-Mediated Neutrophil Activities and Cytokine Storm. Biomaterials (2021) 267:120389. doi: 10.1016/j.biomaterials.2020.120389
141. Holliday ZM, Earhart AP, Alnijoumi MM, Krvavac A, Allen LH, Schrum AG. Non-Randomized Trial of Dornase Alfa for Acute Respiratory Distress Syndrome Secondary to Covid-19. Front Immunol (2021) 12:714833. doi: 10.3389/fimmu.2021.714833
142. Ng H, Havervall S, Rosell A, Aguilera K, Parv K, von Meijenfeldt FA, et al. Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19. Arterioscler Thromb Vasc Biol (2020) 41(2):Atvbaha120315267. doi: 10.1161/ATVBAHA.120.315267
143. Zuo Y, Zuo M, Yalavarthi S, Gockman K, Madison JA, Shi H, et al. Neutrophil Extracellular Traps and Thrombosis in COVID-19. J Thromb Thromb (2021) 51(2):446–53. doi: 10.1007/s11239-020-02324-z
144. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
145. Ding J, Hostallero DE, El Khili MR, Fonseca GJ, Milette S, Noorah N, et al. A Network-Informed Analysis of SARS-CoV-2 and Hemophagocytic Lymphohistiocytosis Genes’ Interactions Points to Neutrophil Extracellular Traps as Mediators of Thrombosis in COVID-19. PloS Comput Biol (2021) 17(3):e1008810. doi: 10.1371/journal.pcbi.1008810
Keywords: COVID – 19, thrombosis, neutrophil, extracellular traps (ETs), DAMPs
Citation: Pastorek M, Dúbrava M and Celec P (2022) On the Origin of Neutrophil Extracellular Traps in COVID-19. Front. Immunol. 13:821007. doi: 10.3389/fimmu.2022.821007
Received: 23 November 2021; Accepted: 17 February 2022;
Published: 11 March 2022.
Edited by:
Julia Kzhyshkowska, Heidelberg University, GermanyReviewed by:
Alain R. Thierry, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceKonstantinos Kambas, Hellenic Pasteur Institut, Greece
Copyright © 2022 Pastorek, Dúbrava and Celec. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Celec, cGV0ZXIuY2VsZWNAaW1ibS5zaw==
 Michal Pastorek
Michal Pastorek Martin Dúbrava2
Martin Dúbrava2 Peter Celec
Peter Celec