- 1Department of Translational Medical Sciences, University of Naples Federico II, Naples, Italy
- 2Medical Oncology, Department of Precision Medicine, University of Campania Luigi Vanvitelli, Naples, Italy
- 3Center for Basic and Clinical Immunology Research (CISI), University of Naples Federico II, Naples, Italy
- 4Moscati Hospital Pharmacy, Aversa, Italy
- 5World Allergy Organization (WAO) Center of Excellence, Naples, Italy
- 6Institute of Experimental Endocrinology and Oncology (IEOS), National Research Council, Naples, Italy
Immune checkpoint inhibitors (ICIs) block inhibitory molecules, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or its ligand, programmed cell death protein ligand 1 (PD-L1) and enhance antitumor T-cell activity. ICIs provide clinical benefits in a percentage of patients with advanced cancers, but they are usually associated with a remarkable spectrum of immune-related adverse events (irAEs) (e.g., rash, colitis, hepatitis, pneumonitis, endocrine, cardiac and musculoskeletal dysfunctions). Particularly patients on combination therapy (e.g., anti-CTLA-4 plus anti-PD-1/PD-L1) experience some form of irAEs. Different mechanisms have been postulated to explain these adverse events. Host factors such as genotype, gut microbiome and pre-existing autoimmune disorders may affect the risk of adverse events. Fatal ICI-related irAEs are due to myocarditis, colitis or pneumonitis. irAEs usually occur within the first months after ICI initiation but can develop as early as after the first dose to years after ICI initiation. Most irAEs resolve pharmacologically, but some appear to be persistent. Glucocorticoids represent the mainstay of management of irAEs, but other immunosuppressive drugs can be used to mitigate refractory irAEs. In the absence of specific trials, several guidelines, based on data from retrospective studies and expert consensus, have been published to guide the management of ICI-related irAEs.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the management of several advanced cancers (1, 2) and can result in durable responses in a percentage of patients (3–5). ICIs are monoclonal antibodies (mAbs) that block inhibitory molecules involved in regulation of immune system pathways, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (e.g., ipilimumab), programmed cell death protein 1 (PD-1) (e.g., nivolumab, pembrolizumab, cemiplimab), or its ligand programmed cell death protein ligand 1 (PD-L1) (e.g., atezolizumab, avelumab, durvalumab) in the tumor microenvironment, which leads to systemic immune cell activation (Figure 1) (6, 7). Immune checkpoints constitute mechanisms of central relevance in the regulation of immune response to avoid autoimmunity and limit tissue damage (8, 9). Immune checkpoints can be exploited by cancer cells as mechanisms of immunoevasion and immunoresistance (10).
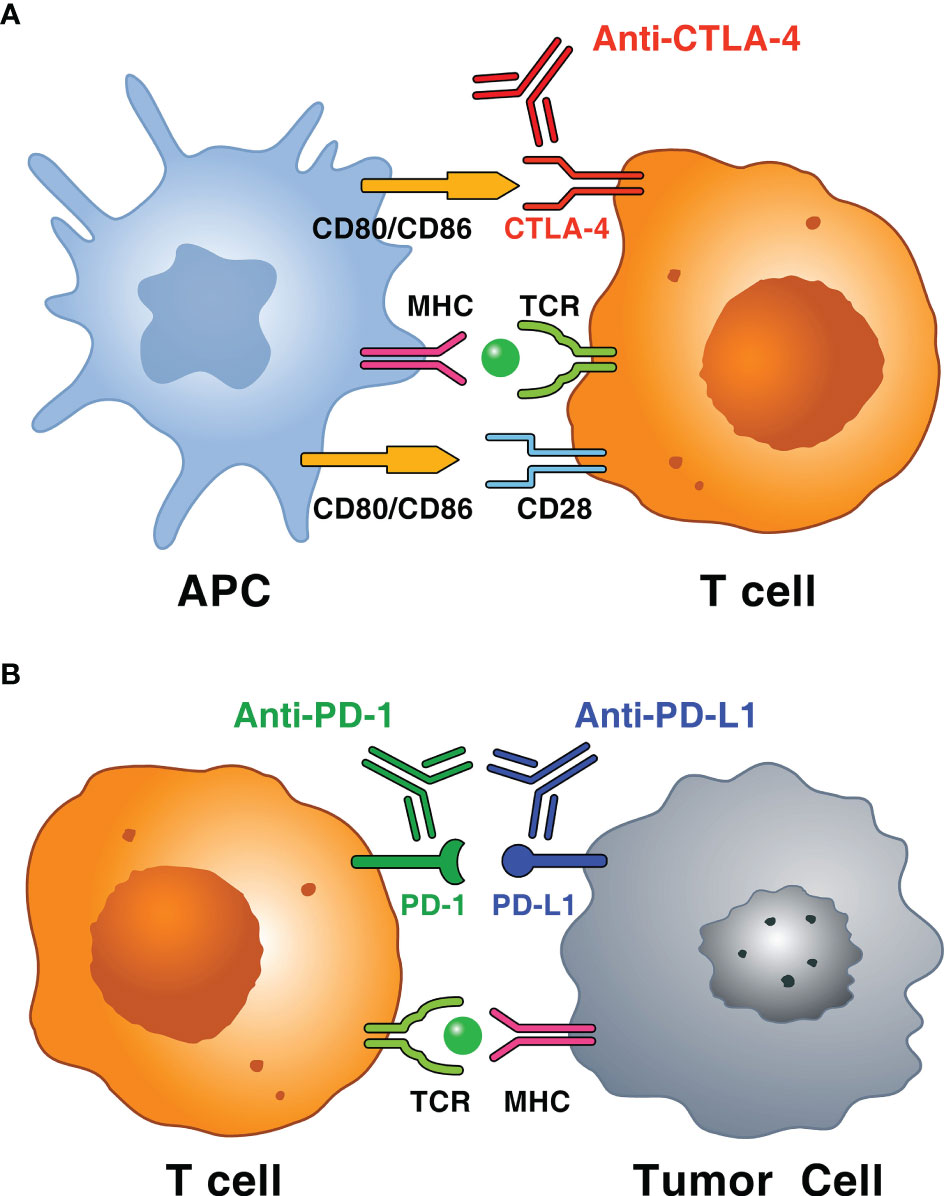
Figure 1 Schematic representation of immune mechanisms of immune checkpoints and immune checkpoint inhibitors (ICIs). (A) T cells, particularly CD4+ T cells in the lymph node, recognize tumor antigens in the context of MHC molecules or antigen-presenting cell (APC) and T cell receptor (TCR) on T cells. The interaction between CD80 (also known as B7-1) or CD86 (also known as B7-2) on APC and CD28 mediates T cell co-stimulation in conjunction with TCR signals. CTLA-4 on activated T cell interacts with both ligands (i.e., CD80 or CD86) with higher affinity and avidity than CD28 and, unlike CD28, sends an inhibitory signal to T cell. Monoclonal antibodies anti-CTLA-4 (i.e., ipilimumab) block this inhibitory pathway restoring T cell activity. (B) T cells, particularly cytotoxic CD8+ T cells, which recognize tumor antigens in the context of MHC class, result in the adaptive expression of PD-L1 on the surface of tumor cells. The interaction between PD-1 and PD-L1 negatively regulates the anti-tumor T cell response. This interaction is useful in preventing autoimmunity in physiological conditions, whereas cancer cells exploit this mechanism to escape from immune system upregulating PD-L1 expression. Anti-PD-1 (i.e., pembrolizumab, nivolumab and cemiplimab) and anti-PD-L1 mAbs (i.e., atezolizumab, avelumab and durvalumab) block this inhibitory pathway restoring T cell activity.
ICIs activate T cells and are often associated with a large spectrum of autoimmune responses, which are commonly referred to as “immune-related Adverse Events” (irAEs). The pleiotropic manifestations of irAEs can affect almost any organ (e.g., skin, colon, endocrine organs, joints, heart and lungs) and clinicians should be able to recognize and treat the heterogeneous manifestations of irAEs. Several comprehensive reviews have examined in detail the toxicity of ICIs affecting the skin (11–13), the gastrointestinal (14–16) and cardiovascular systems (17–24), the lung (25, 26), the endocrine organs (27–29), the joints (30, 31), the nervous (32) and the hematologic systems (33). In this review, we summarize the most recent observations and the complex pathophysiology and clinical characteristics of irAEs and their putative predictors and emerging therapies.
Incidence/Prevalence of IrAEs
Distinct immunological mechanisms underlie anti-CTLA-4 (ipilimumab) and anti-PD-1/PD-L1 checkpoint blockade (34). Therefore, it is not surprising that the incidence of any irAEs with these two groups of ICIs varies greatly. The pattern, incidence and severity of irAEs vary according to the type of ICI (anti-CTLA-4 or anti-PD-1/PD-L1) and the treatment schedule (monotherapy or combination therapy). It has been estimated that the incidence of irAEs in patients treated with anti-CTLA-4 mAb (ipilimumab) is higher than in those treated with anti-PD-1/PD-L1 mAbs (35). The highest incidence and the high-grade irAEs are usually associated with combination therapy of ipilimumab plus anti-PD-1/PD-L1 (36). In a large meta-analysis examining 16,485 patients, colitis and hypophysitis were more frequent with ipilimumab, while diabetes and pneumonitis were more frequent with anti-PD-1/PD-L1 (35). Colitis, hepatitis, pancreatitis, and ICI-associated diabetes are more likely to be high-grade (37).
Timing Of IrAEs
In melanoma patients treated with ipilimumab, the time of onset of skin-related irAEs is two to three weeks after ICI initiation, gastrointestinal and hepatic irAEs after six weeks, and endocrine irAEs after six to nine weeks (38, 39). Most high-grade irAEs resolve in two to five weeks with immunosuppression but some, such as arthritis, tend to persist (40). Endocrine irAEs (e.g., diabetes, thyroid dysfunctions) are usually irreversible and require prolonged hormone replacement therapy (39).
Fatal ICI-related irAEs tend to occur in the early phases of therapy and the incidence varies with the type of treatment. Fatalities are more common with combination therapy than with anti-PD-1/PD-L1 or anti-CTLA-4 (20). Fatality rates were approximately 39% for myocarditis and 5% for colitis (20).
There are some similarities between autoimmune manifestations of ICI-related irAEs and their spontaneous autoimmune counterparts but also several differences (41). For instance, ICI-induced diabetes can manifest with diabetic ketoacidosis, similar to T1D (42). The frequency of autoantibodies in ICI-induced diabetes is lower than in T1D (42, 43). ICI-induced hyperthyroidism is typically found at presentation and usually progresses to hypothyroidism (44, 45). ICI-induced colitis differs from inflammatory bowel disease (IBD) because is usually reversible (14).
Long-Term Adverse Effects of ICIs
ICIs have been successfully introduced in the treatment of various cancers only a few years ago. Therefore, there is limited experience on the long-term side effects of ICIs. Acute irAEs have thus far attracted major attention owing to their dramatic clinical presentation and need for urgent treatment. However, increasing evidences indicate that chronic irAEs are more prevalent than originally recognized (46, 47). Endocrinopathies (such as ICI-induced hypothyroidism and diabetes) and rheumatological toxicities (such as arthritis) are the most common chronic irAEs (48, 49). Endocrinopathies provide classical examples of irreversible damage of the relevant hormone-secreting cells. These syndromes are usually irreversible and require the use of lifelong exogenous hormone replacement therapy (45, 50). On the other hand, ICI-induced arthritis provides a classical example of smouldering inflammation in which ICIs trigger persistent subacute or chronic arthritis, closely mimicking that of rheumatoid arthritis (48, 51).
Several experimental studies have demonstrated that CTLA-4 and PD-1/PD-L1 axes are critical negative regulators of atherosclerosis (52–55). A recent retrospective study by Drobni et al. reported an association between ICIs with accelerated progression of atherosclerosis and cardiovascular events (56). They found increased atherosclerotic inflammatory activity 5 months after ICI therapy (57). Another retrospective study on 20 patients with melanoma found by positron emission tomography/computed tomography with 2-[18F] fluorodeoxyglucose (18F-FDG) that ICI therapy induced inflammatory activity in large arteries (57). The results of these two studies will certainly influence the approach to cardiovascular care for individuals receiving ICIs. Cardiac evaluation before initiation of ICI treatment should focus on long-term prevention rather than focusing only on early irAEs. Moreover, cancer trials should prospectively examine not only early but also late cardiovascular events (58).
Pathophysiology of IrAEs
Several immunopathogenic mechanisms (i.e., cellular autoimmunity, autoantibodies, complement activation, cytokines/chemokines release, genetics and alterations of the gut microbiome) have been suggested to be involved in the development of ICI-related irAEs (Figure 2).
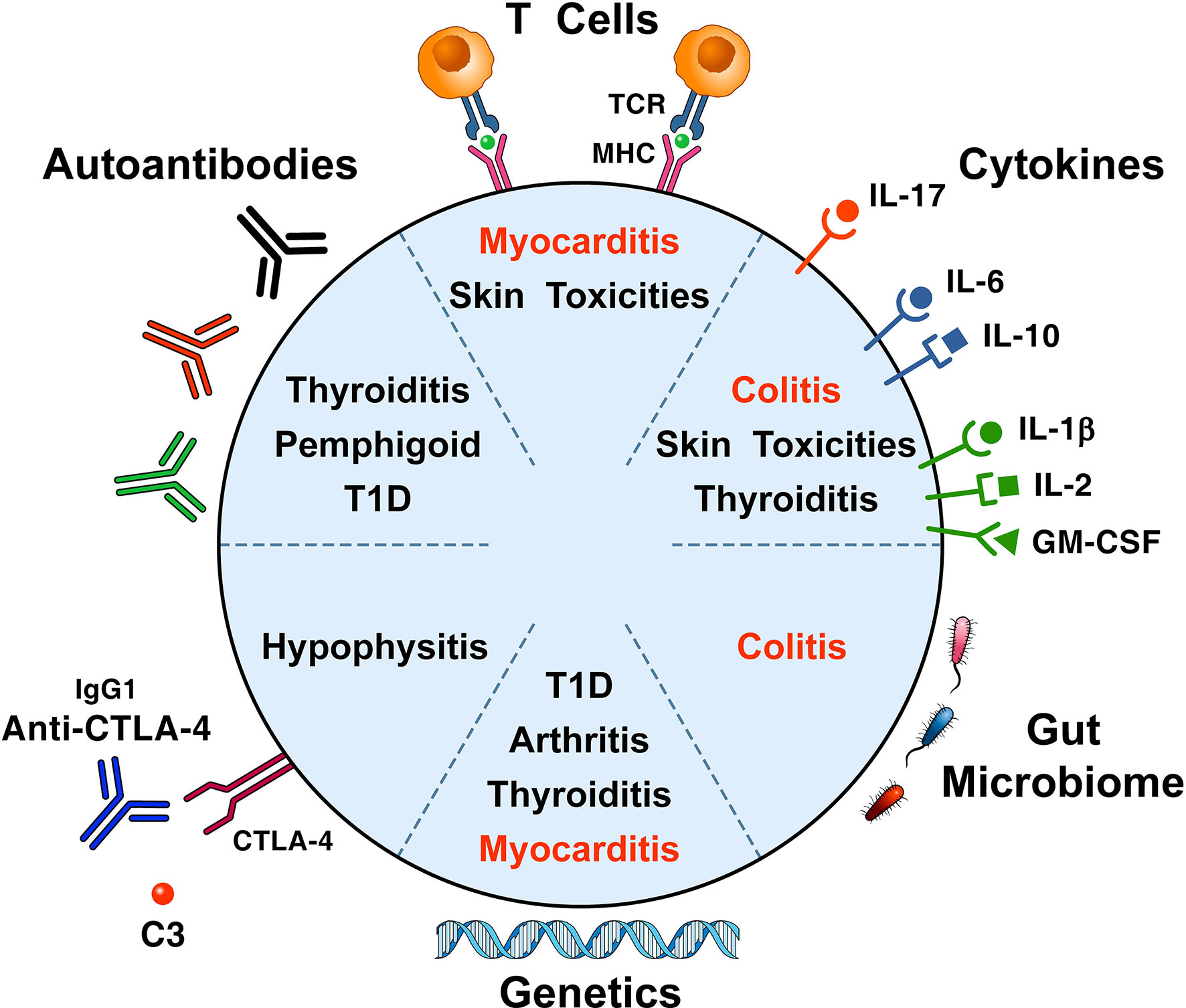
Figure 2 Proposed immunopathogenic mechanisms for the development of immune checkpoint-induced immune-related adverse events. A proposed mechanism postulates that self-antigens (e.g., heart and skeletal muscle antigens) activate T cell clones driving antitumor responses and organ-specific autoimmunity (24). Thyroid autoantibodies may be involved in patients who develop thyroid dysfunction (44, 59, 60), ICI-associated diabetes (42, 43), bullous pemphigoid (61), hypophysitis (62, 63), and myasthenia gravis (64). Cytokines/chemokines released from immune cells can cause immune-mediated tissue damage (12, 65, 66). The pivotal role of genetic factors in the development of ICI-associated irAEs, originally highlighted in mice (67, 68), has been confirmed in patients with arthritis (69), ICI-associated diabetes (42, 43, 70), and pruritus (71). There is growing evidence that gut microbiome may play a role in the development of experimental irAEs (72, 73) and of colitis in patients with melanoma (74, 75). irAEs contributing to most fatalities are presented in red.
Cellular Autoimmunity
The importance of T cells in the mechanisms of ICI-associated irAEs is supported by genetic loss-of-function studies in mice (67, 68). Autoreactive T cells can be activated by shared antigens between tumor and peripheral tissues. Shared T cell clones in the tumor, heart, and skeletal muscle were found in melanoma patients, who died from fatal myocarditis and myositis after treatment with anti-CTLA-4/PD-1 mAbs (76). The exact mechanism of cardiac toxicity remains unknown, but it has been suggested that shared antigens may drive both antitumor responses and organ-specific autoimmunity (24). Shared T cell antigens were found in the skin and tumor of lung cancer patients who developed skin toxicities (77). Similarly, vitiligo is common in melanoma patients treated with ICIs (78). Recently, Lozano and collaborators demonstrated that in melanoma patients treated with anti-PD-1 or anti-PD-1 and anti-CTLA-4 combination, two pretreatment factors in peripheral blood - activated CD4 memory T cell abundance and TCR diversity - were associated with severe irAEs development (79).
CTLA-4 is a modulator of Tregs (80) and these cells act as gatekeepers for the prevention of autoimmunity. The role of Tregs in ICI-induced irAEs deserves additional studies (81). It has been suggested that tissue-resident memory T cells (Trm) in tumor microenvironment can play a role in irAEs (82). Single-cell analysis of ICI-associated colitis patient samples found expansion of CTLA-4+ Treg cells and differentiation of CD8 Trm cells to cytotoxic effector cells (83).
Humoral Immunity
Anti-thyroglobulin (TG) and/or anti-thyroid peroxidase (TPO) autoantibodies are found in 13–70% of patients who develop ICI-related thyroid dysfunction (44, 59, 60, 84). Thyroid autoantibodies increase the risk of ICI-induced thyroid dysfunction (85–87) and β-cell autoantibodies are found in approximately 50% of patients with ICI-induced diabetes (42, 43, 88). Autoantibodies anti-BP180 can be found in the majority of patients with anti-PD-L1-associated bullous pemphigoid (61).
Human pituitary cells express CTLA-4 at both mRNA and protein levels and in an animal model the injection of anti-CTLA-4 antibodies induced lymphocytic infiltration and complement activation of the pituitary gland (62). Anti-pituitary antibodies were detected only in patients with ipilimumab-associated hypophysitis but not in those without hypophysitis (62). Autoantibodies against guanine nucleotide-binding protein G subunit alpha (GNAL) and integral membrane protein 2B (ITM2B) have been found associated with ICI-induced hypophysitis (63). These findings implicate both autoantibodies and T cell-mediated processes in ICI-associated pituitary destruction (89). Overexpression of pituitary CTLA-4 was reported in a patient with severe ipilimumab-associated hypophysitis (90). It has been suggested that hypophysitis is caused by complement activation from endogenous autoantibodies and/or exogenous IgG1 anti-CTLA-4 (ipilimumab) (62). IgG1, used in ipilimumab, activates the classic complement pathway explaining the elevated frequency of pituitary gland damage compared with its occurrence in patients treated with anti-PD-1/anti-PD-L-1 IgG4 antibodies (91, 92).
Anti-acetylcholine receptor antibodies can be found in approximately 50% of patients with ICI-induced myasthenia gravis (64). Patients with ICI-induced arthritis are commonly rheumatoid factor (RF) and cyclic citrullinated peptide (CCP) negative (69). Unfortunately, in the majority of these studies the presence of autoantibodies prior to ICI initiation has not been evaluated.
Cytokines and Chemokines
Cytokine release syndrome (CRS) is a systemic inflammatory disorder characterized by a massive release of cytokines (93). It can present with a variety of symptoms ranging from mild (e.g., fever, fatigue, nausea, rash) to life threating, sometime fatal. A recent analysis of WHO global pharmacovigilance database, found that ICI-related CRS can occur in ICI-treated patients (94). On similar ground, cytokines and chemokines are involved in different irAEs. Increased baseline IL-17 concentrations were temporally associated with subsequent development of ICI-associated colitis (65). Increased IL-6 and IL-10 concentrations were found in patients with skin irAEs (12). Increased concentrations of IL-1β, IL-2, and GM-CSF at baseline have been associated with ICI-related thyroid dysfunction (66). High concentrations of T cell chemotactic chemokines (i.e., CXCL9 and CXCL10) are associated with irAEs (95). A recent study in patients with melanoma treated with combined immune checkpoint blockade (CICB) targeting CTLA-4 and PD-1 who developed ≥ grade 3 colitis demonstrates an intestinal overexpression of IL1β and TNF compared to normal tissue (96).
Genetic Factors
Genetic factors influence the development and progression of several autoimmune disorders. The importance of genetic factors in the development of ICI-associated irAEs was originally highlighted by genetic loss-of-function studies in mice (67, 68). Thus, the possibility exists that genetic susceptibility may play a role in the pathogenesis of irAEs. Experimental studies have demonstrated that CTLA-4 and PD-1 deletion or inhibition can cause autoimmune myocarditis with lymphocytic infiltration of cytotoxic T-cells (67, 97–99). The majority (≅ 52%) of patients with ICI-related arthritis possess the RA-associated HLA-DR susceptibility allele (69). The majority of patients with ICI-related diabetes had at least one HLA-DR risk allele (70). HLA-DR4 predominance has been reported in patients with ICI-induced diabetes (42, 43). HLA-DRB1*04:05 has been associated with ICI-induced arthritis (69) and HLA-DRB1*11:01 with pruritis (71). A multicenter study found that a polygenic risk score (PRS) for thyroid disorders is associated with developing thyroid irAEs in patients with non-small cell lung cancer (NSCLC) treated with anti-PD-1 or anti-PD-1 and anti-CTLA-4 combination (84). In this study, thyroid irAEs were associated with better response to ICIs. Moreover, in a phase 3 randomized controlled trial (RCT), it was found that PRS for dermatological autoimmune diseases were associated with increased risk for skin irAEs and longer overall survival in bladder cancer patients treated with atezolizumab (100).
Microbiome
The multiple interactions among tumor microenvironment, microbiome, host factors, and response to ICI, and the development of ICI-associated irAEs are largely unknown (101). There is evidence that the gut microbiome might play a role in tumor response (102–104). For instance, gut microbiome modulates response to anti-PD-1 in melanoma patients (105) and epithelial tumors (103). Mice repleted with B. fragilis less likely developed irAEs after exposure to anti-CTLA-4 inhibitors (72). Moreover, microbiota-derived peptides from Bacteroides induced autoimmune myocarditis (73).
Melanoma patients treated with ipilimumab and with baseline gut microbiome enriched for Faecalibacterium and other Firmicutes had longer progression-free survival and overall survival (74). In a prospective study of melanoma treated with ipilimumab, patients with abundant Bacteroidetes phylum less likely developed ICI-induced colitis (75). A recent study found that gut microbiota signatures are associated with irAEs to CICB targeting CTLA-4 and PD-1 in melanoma patients and in experimental models (96). In this study, the rate of any grade of irAEs was high (93.5%) and 49% of patients experienced severe (≥ grade 3) irAEs. The alpha diversity of the gut microbiome in patients who did or did not develop severe irAEs was similar. However, Bacteroides intestinalis (B. intestinalis) were more abundant in patients with ≥ grade 3 irAEs versus those who did not. In melanoma patients who developed colitis there was an overexpression of mucosal IL1β and IL-17, but not TNF. These fascinating results were corroborated by results in experimental models in which mice, gavaged with different strains of B. intestinalis following gut sterilization with antibiotics, showed overexpression of Il1b. Moreover, fecal microbiota transplant (FMT) in antibiotic-treated animals using fecal material from human donors harboring high endogenous levels of B. intestinalis induced ileal overexpression of Il1b after administration of CICB. Collectively, these human and experimental studies highlight a contribution of commensal microbiota to intestinal damage associated with CICB.
Putative Biomarkers of Immune-Related Adverse Events
Several studies have or are evaluating the possibility of identifying biomarkers of irAEs associated with different types of ICIs. Table 1 lists genetic, clinical, immune, microbial and tumor biomarkers that have been linked to irAEs. In particular, some studies have identified an association between HLA and irAEs. The association of baseline antibodies (85–87, 109, 110), baseline cytokine levels (65, 66, 111, 112), and immune cell changes (79, 83, 113–116) suggests that humoral and cellular immunity play a role in some specific irAEs. In particular, activated CD4 memory T cell abundance and TCR diversity in peripheral blood are associated with severe irAEs development in patients with melanoma (79). The emerging results from parallel human and experimental models highlight a contribution of specific gut microbiota to the development of intestinal irAEs (74, 75, 96). It should be emphasized that the small size of these studies requires validation in larger cohorts of patients with different types of cancer.
Clinical Manifestations of ICI Associated Autoimmunity
The results of a meta-analysis of 35 trials demonstrated the extreme heterogeneity of manifestations and severity of autoimmune complications of ICIs (35). Figure 3 illustrates that irAEs can affect nearly every organ in association with ICIs.
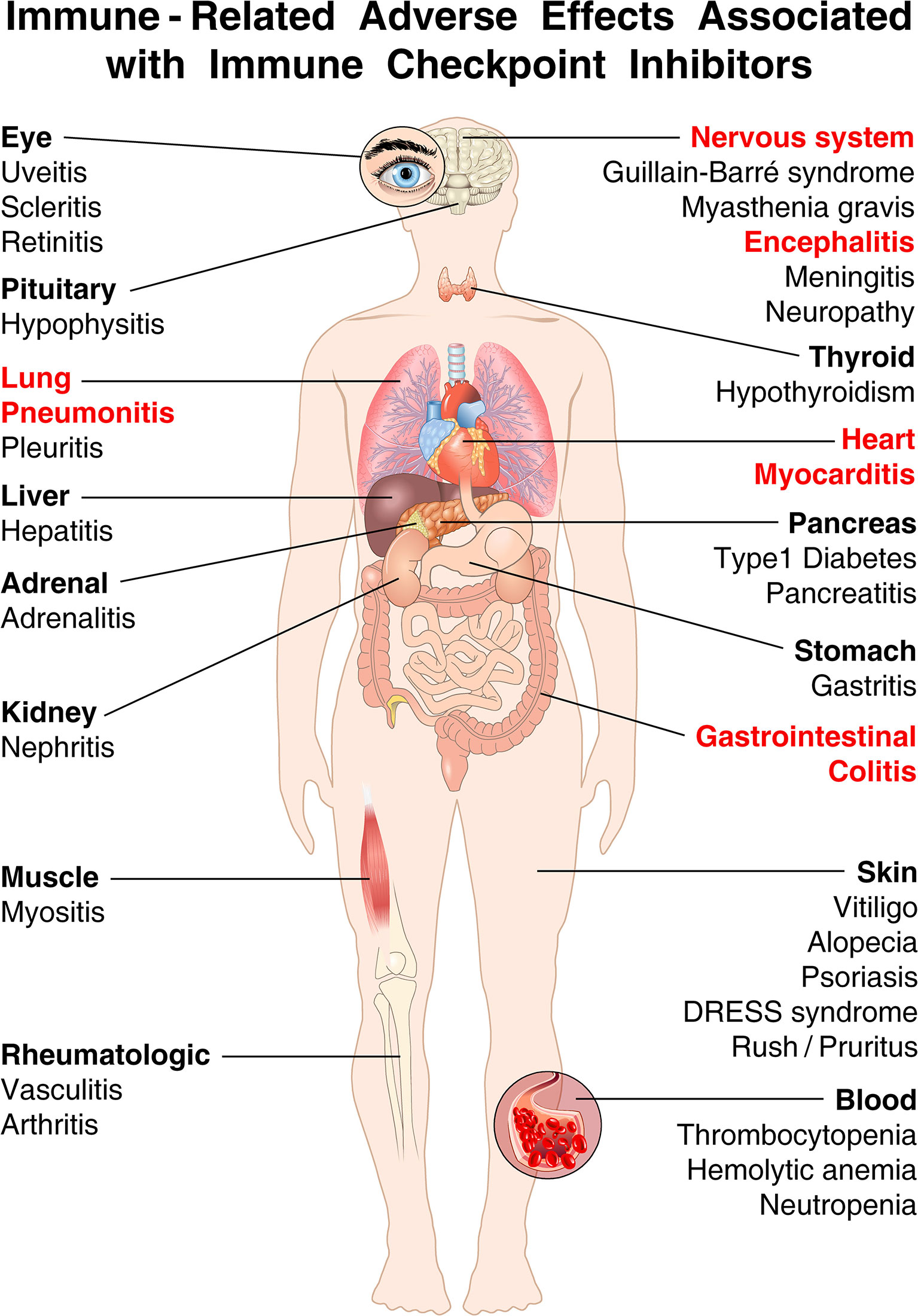
Figure 3 The spectrum of organs affected by irAEs associated with immune checkpoint inhibitors (ICIs) is very broad. Shown are the most common immune-related adverse events (irAEs) that clinicians can encounter in cancer patients treated with ICIs. irAEs contributing to most fatalities are highlighted in red [modified from (19)]. ICI-associated diabetes is almost exclusively seen in patients treated with anti-PD-1/PD-L1 antibodies, and rarely with ipilimumab monotherapy (42, 118). By contrast, hypophysitis occurs more often in patients receiving ipilimumab (119). Colitis occurs more commonly with ipilimumab and with combined immune checkpoint blockade than with anti-PD-1/PD-L1 alone (112, 120). Endocrinopathies (such as hypothyroidism, hypophysitis, and adrenal insufficiency) and rheumatological disorders have the highest incidence of development into subacute/chronic toxicity (49). Endocrine toxicities, unlike many other irAEs, are not managed using high-dose glucocorticoids, which have no effect on either initial severity and resolution (121, 122). Although acute myocarditis was the first cardiovascular irAEs associated with ICIs (123), an important unanswered question relates to the long-term cardiovascular sequelae of ICIs (49, 56, 57).
Skin Rash
Skin manifestations during ICI therapy show the highest incidence of irAEs and appear higher in patients with melanoma than in patients with other malignancies (124). ICI-related skin manifestations include maculopapular, eczematous, psoriasiform, lichenoid, and bullous eruptions, frequently associated with pruritus (125). Vitiligo associated with anti-PD-1 therapy differs from canonical vitiligo because of a patchy distribution, occurring in sun-exposed areas, and lacking the Köebner phenomenon (126). Bullous skin disease tends to occur in patients treated with anti-PD-1/PD-L1 rather than ipilimumab (127). Rare cases of severe Stevens-Johnson syndrome/toxic epidermal necrolysis have been reported (128).
Most patients have mild skin reactions, while severe (grade 3-4) toxicities are found in a low proportion of patients (5.8% for the ipilimumab + nivolumab combination, even lower values for anti-PD-1 monotherapy), significantly lower than the toxicities of other sites (such as hepatic or gastrointestinal) (129). The management of skin toxicities needs specific treatment for the type and severity of the condition: topical glucocorticoids are effective in treating low-grade skin reactions, whereas high-grade events must be treated with systemic glucocorticoids.
Diarrhea and Colitis
Diarrhea is the most frequent manifestation of gastrointestinal toxicity from ICI and is one of the main causes of emergency department visits for patients treated with ICI (130). Colitis occurs more commonly with ipilimumab and with CICB than with anti-PD-1/PD-L1 mAbs alone (120, 131). No differences have been described in the incidence of diarrhea/colitis in different types of malignancy (132). The presence of ulcerative lesions appears to be related to a greater probability of glucocorticoid resistance and greater severity of diarrhea (133). Lesions primarily affect the distal colon, but lesions can occur in more proximal tracts. Colitis-associated autoantibodies are seldom present (134). Prophylactic budesonide is not effective against ipilimumab-induced colitis (135). Infliximab induces a shorter time to symptom resolution than glucocorticoids (136). Vedolizumab (an antibody directed against α4-β7 integrin), has also been used as glucocorticoid sparing agent (137). There is some evidence that early treatment with either infliximab or vedolizumab reduces symptom duration and glucocorticoid administration (138). Several clinical trials are evaluating the safety and efficacy of infliximab (NCT05034536, NCT03293784, NCT04407247) or certolizumab (NCT03293784) in clinical response of ICI-induced colitis.
Recent clinical and experimental models indicate that higher abundance of B. intestinalis is associated with high grade-colitis in melanoma patients treated with CICB (96). The gut microbiome appears to mediate intestinal toxicity via IL-1β and treatment of mice with an anti-IL-1R antagonist (anakinra) reduced intestinal inflammation. A clinical trial is evaluating the safety and efficacy of anakinra on irAEs and cytokine profiles in patients with different cancers (NCT04576429).
Hepatitis and Pancreatitis
Immune-mediated liver injury caused by ICIs can present with fatigue, fever, nausea, and jaundice. The severity of hepatic toxicity can be classified in relation to the increase in liver enzymes (AST and ALT) and bilirubinemia. The incidence of irAEs varies between 3% to 9% for anti-CTLA-4, and 0.7% to 1.8% for anti-PD-1/PD-L1 (139). CICB (ipilimumab + nivolumab) is associated with an incidence of any-grade hepatotoxicity of 29% and high-grade hepatotoxicity of 17% (39). Approximately 50% of the patients with ICI-associated liver injury have antinuclear antibodies and 19% have anti-smooth muscle antibodies (140). Pancreatitis can also occur in response to ICIs (141).
Thyroiditis
Thyroid dysfunction is the most common endocrinologic irAEs due to ICIs (27, 29, 45). The median time of onset is approximately 6 weeks after the start of immunotherapy. ICI-associated thyroid dysfunction is more common after anti-PD-1/PD-L1 antibodies or combination therapy than with ipilimumab alone (41, 142, 143). Patients receiving ICIs should undergo regular thyroid testing (i.e., TSH and T4). Patients usually experience a first transient phase of hyperthyroidism, followed by euthyroidism or hypothyroidism (45, 144). The presence of thyroid autoantibodies (anti-TPO and anti-TG) before ICI treatment increases the risk of thyroid dysfunction in patients treated with nivolumab or pembrolizumab (85–87, 109). The mechanism responsible for ICI-induced thyroid dysfunction is unclear. It has been hypothesized that polymorphic variants of the PDCD1 in some individuals might predispose them to an increased risk of thyroid dysfunction (145). It is also unknown whether thyroid autoantibodies are the cause of thyroid dysfunction or the result of an immunological response to thyroid antigens released during ICI-related thyroiditis (146). ICI therapy can be continued with close follow-up and monitoring of TSH and T4 in the context of thyroiditis. In the presence of more severe irAEs, ICI should be held until symptoms resolve. Glucocorticoids do not improve the clinical course of thyroid dysfunction (121).
Hypophysitis
Hypophysitis is a specific complication of ipilimumab treatment and rarely occurs in anti-PD-1/PD-L1-treated individuals (62, 91, 92). Most patients present with headache and/or fatigue (147, 148). Magnetic resonance imaging (MRI) of the brain highlights an expansion of the pituitary gland and/or infundibulum (147–149). Enlargement on MRI precedes the clinical diagnosis of ipilimumab-related hypophysitis (147). The pituitary gland decreases in size over 4-12 weeks leading to atrophy (147, 150, 151). Importantly, a normal MRI does not completely rule out hypophysitis, and therapy should be based on clinical symptoms and pituitary hormone levels (152). Glucocorticoids do not improve the degree or duration of hypophysitis (149). Hormone deficiencies are managed with the corresponding hormone replacement (149).
Diabetes
Although not very common (42), PD-1 pathway blockade can cause autoimmune ICI-induced diabetes mellitus, which usually (≅ 70%) presents as diabetic ketoacidosis (29, 50, 153–156). ICI-associated diabetes rarely occurs in patients treated with ipilimumab (42, 118). In these individuals, C-peptide levels are very low and approximately 50% of patients have T1D associated antibodies (anti-glutamic acid decarboxylase) (43). Human leukocyte antigen risk alleles (HLA-DR4, -DQ8, -DR3, and DR2) can be associated with high frequency of spontaneous T1D (157). In ICI-induced diabetes, there is a predominance of HLA-DR4, which is present in approximately 70% of patients. Other HLA alleles associated with high risk of spontaneous T1D are not overrepresented in ICI-associated diabetes (42, 43). ICI-induced diabetes tends to be permanent (50) and attempts with glucocorticoids administration showed no recovery (158).
Pneumonitis
Early clinical trials and meta-analyses suggested an incidence of ICI-associated pneumonitis of 3-5% (159–163); recent studies examining real-world populations suggest this could be as high as 13-19% (164–166). While the incidence of all-grade pneumonitis appears to be higher in the real-world population as opposed to clinical trials, the percentage of ≥ grade 3 pneumonitis appears to be relatively consistent across both populations (163–165, 167).
Immune-related pneumonia represents one of the main causes of death during treatment with anti-PD-1/PD-L1 alone and the fourth cause during combined treatment with ipilimumab plus anti-PD-1/PD-L1 (14% of total cases) after colitis, myocarditis and hepatitis (20). Anti-CTLA-4 treatment causes a lower incidence of immune-related pneumonia compared to treatment with anti-PD-1/PD-L1 alone (168). Immune-related pneumonia are usually associated with CICB (169). One-third of these patients are asymptomatic, whereas the others present with dyspnea and/or cough (159). Radiographic findings on chest computed tomography (CT) do not highlight specific characteristics (159, 170). Previous thoracic radiotherapy and previous lung disorders are predictors of pneumonitis associated with anti-PD-1 (171, 172). The differential diagnosis of these patients should be made after ruling out other causes of similar lung involvement. This issue is particularly relevant during the current outbreak of COVID-19 (173). Guidelines on the management of ICI-related pneumonitis have been published from ESMO (174) and ASCO/NCCN (175–177). A clinical trial is evaluating the safety and efficacy of infliximab versus intravenous immunoglobulin therapy (IVIG) in treating glucocorticoid-refractory pneumonitis (NCT04438382).
Arthritis
Arthritis is not very common (≅ 4%) in patients with ICIs (178). A systematic review encompassing 372 patients found that the time of onset of arthritis ranged from 1 day to 53 months (median time: 4 months) (51); 49% had polyarthritis, 17% oligoarthritis, 10% arthralgia, and 21% polymyalgia rheumatica (178–181). More than half of patients had a “rheumatoid arthritis-like” presentation (51). RF and anti-citrullinated peptide antibodies (ACPA) are present in ≅19% of patients (51, 180, 182). Treatment of irAEs should be guided by severity (183). Most patients can be managed with non-steroidal anti-inflammatory drugs or intra-articular glucocorticoid injections. More severe patients, especially those who received CICB, require systemic glucocorticoids (182). Arthritis often persists even after stopping ICIs and may require prolonged immunosuppression with biological disease-modifying anti-rheumatic drugs (DMARDs) (40). Diagnostic and management algorithms for rheumatoid irAEs have been recently proposed (183).
Myositis
ICI-related myositis can manifest in the form of acute or subacute myalgia or muscle weakness (183, 184). When concomitant myocarditis and myasthenia gravis-like symptoms (e.g., ptosis and oculomotor weakness) occur, fatality rates are relatively high (185). Muscle biopsy shows inflammation and myonecrosis (184). Myositis-associated antibodies (anti-TIF1-y, SRP, Ro52; PL-7, PL-12, or SRP) can be detected in ICI-associated myositis (186). Anti-striated muscle antibodies can be found even without clinical evidence of myasthenia gravis (187, 188). Glucocorticoids are the first-line therapy for ICI-associated myositis. Initial dosing can range from 0.5 mg/Kg prednisone daily up to 2,000 mg IV methylprednisolone (183). IVIG and plasmapheresis have been used in refractory cases (183, 184, 186).
Myocarditis
The true incidence of ICI-associated myocarditis remains uncertain. Early ICI-based cancer trials did not prospectively screen for myocarditis (189). Moreover, because the diagnosis of myocarditis can be difficult, cases could easily be missed. Recent reports suggest that the incidence of ICI-associated myocarditis is 0.27% to 1.14% (76, 190). Myocarditis is an infrequent, but often lethal complication of ICI therapy (76, 191). Elevated troponin level and abnormal electrocardiogram were found in the majority of these patients with ICI-associated myocarditis. Interestingly, half of these patients showed preserved ejection fraction (190). The clinical manifestation of ICI-associated myocarditis is variable. Fulminant cases characterized by early-onset have been described (19, 123, 192). In these cases, cardiac arrhythmias are common (23, 190). The association of skeletal myositis and myasthenia gravis following ICI therapy should orientate for myocarditis (185, 193). “Smouldering” cases of myocarditis have been also reported (194).
Diagnosis of ICI-associated myocarditis is challenging and includes a combination of biomarker tests (troponin), cardiac MRI, late gadolinium enhancement, and possibly biopsy (T cell infiltrate) (195). Major adverse cardiac events (MACE) can occur also in patients with preserved ejection fraction. A troponin T level ≥ 1.5 ng/mL was associated with a marked increase in MACE during follow-up (190). The precise mechanisms by which ICIs cause cardiotoxicity remain undefined. Existing data support T cell-mediated immunity as a major component in pathogenesis, but many fundamental questions remain (24). Early and aggressive treatment with high doses of glucocorticoids is critical (132, 190, 196). Treatment of ICI-associated myocarditis includes ICI discontinuation, supportive management, and glucocorticoids (175). Prednisone (0.5 to 2.0 mg/kg), followed by 4–6 week taper upon symptoms improvement, is recommended (24, 175, 197). Despite this treatment, mortality remains substantial, and individual case reports demonstrate successful treatment with alemtuzumab (anti-CD52 mAb) (198), or abatacept (a fusion protein composed of the extracellular domain of CTLA-4 and the Fc region of human IgG1) (1). Prospective clinical trials are needed to compare the safety and efficacy of different immunosuppressive therapies in ICI-associated myocarditis. Recent experimental data point to the hypothesis that anti-PD-1 therapy induces a smouldering disruption of cardiac immunity towards an inflammatory phenotype, with manifest consequences on cardiac function in the presence of a second hit, in the form of systemic stress induced by presence of a tumor (199). This also indicates the possibility that the inflammatory phenotype raises the risk for the development of myocarditis upon exposure to additional, yet unknown risk factors (200). Furthermore, treatment with anti-PD-1 may first produce only a latent inflammatory involvement associated to dysregulated cardiac metabolism that may progress to overt myocarditis in a subset of patients (200, 201).
Neurological
Neurological complications of ICIs (headache, myasthenia gravis, peripheral neuropathy, meningitis, and encephalitis) are uncommon (≅ 1%) (32, 77, 184, 202, 203). Myasthenia gravis (77) and encephalitis are more common with anti-PD-1 antibodies, whereas Guillain Barré and meningitis are more common with ipilimumab. MG associated with myositis and myocarditis has a poor prognosis (64, 203). Approximately 50% of MG patients have anti-acetylcholine receptor antibodies (64). Glucocorticoids and, in some patients, IVIG, are the mainstay of therapy (64).
Hematologic
In contrast to other anticancer therapies, hematological irAEs in patients treated with ICIs are uncommon. Neutropenia, autoimmune hemolytic anemia, and immune thrombocytopenia can occur rarely (≅ 5%) (204, 205). A positive direct antiglobulin test is present in the majority (≅ 60%) of patients with ICI-related autoimmune hemolytic anemia (206). Glucocorticoids are the first line of therapy; IVIG or rituximab can be considered in difficult cases. Neutropenic patients can be treated with G-CSF (204, 206).
Renal
Renal dysfunction is rare with ipilimumab and with anti-PD-1/PD-L1 therapies occurring in <1% of patients (207). The incidence is higher with combination of ipilimumab plus anti-PD-1/PD-L1 reaching approximately 4% (208, 209). Renal dysfunction is usually due to acute interstitial nephritis (210) or, more rarely, to glomerulonephritis (211).
Ocular
irAEs of the eye are rare and occur in <1% of patients treated with ICIs (212, 213). Uveitis can be a complication of ICI treatment (214). Few cases of Vogt-Koyanagi-Harada disease have been described in melanoma patients, which hinted a possible cross-reactivity between T lymphocytes targeting melanoma cells and the melanocytes of the eye (215). In the cases of ICI-associated sicca syndrome, oral manifestations are more common than the ocular ones (216). Anti-Ro (SS-A) and anti-La (SS-B) are usually negative (216).
Pre-Existing Autoimmune Disease and ICIs
Cancer patients with underlying autoimmune disease were initially excluded from ICI RCT (162, 217–219). Therefore, the prevalence and incidence of exacerbations of pre-existing autoimmune disorders was not immediately appreciated. Patients with autoimmune disease, however, represent 20 to 50 million people in the United States alone, and one study reported that approximately 13% of lung cancer patients had a concurrent diagnosis of autoimmune disease (220).
There is now evidence that irAEs are more frequent and occur faster in cancer patients with several autoimmune diseases (psoriasis, rheumatoid arthritis, IBD, systemic lupus erythematosus, vasculitis) (106–108). These findings suggest that a close monitoring is mandatory during early phases of treatment. The majority of autoimmune flares and irAEs can be managed without ICI discontinuation, but fatalities can occur (221). Conflicting results have been reported in studies concerning the risk of autoimmune flares or irAEs in patients with active versus inactive autoimmune disease (106, 108, 221, 222). Patients with underlying autoimmune disorders should be carefully managed by multidisciplinary teams.
IrAEs and Efficacy of ICIs
In one large, retrospective study of ipilimumab, the treatment outcomes were similar in patients with and without irAEs (223). Subsequent studies reported that melanoma patients who develop vitiligo or endocrine complications have better tumor response and improved survival (122, 175, 224–226). The results of randomized trials have shown that patients who discontinue ICIs due to toxicity respond better than patients without irAEs (227). Patients that developed thyroiditis after PD-1 or PD-L1 blockade had longer overall survival compared to the thyroid irAE negative group (60, 225). Prospective studies are needed to verify whether different irAEs are associated with improved tumor response to ICIs.
Management of IrAEs
No trials are evaluating the efficacy of different irAEs treatments. Therefore, management of irAEs are based on retrospective studies and expert consensus (11, 29, 31, 45, 174, 175, 228, 229). Low-grade irAEs usually do not need ICI discontinuation and immunosuppressive treatment. Higher grade irAEs may require both therapeutic strategies. Table 2 schematically summarizes the anti-inflammatory and immunosuppressive drugs routinely used to treat ICI-related irAEs.
Glucocorticoids are powerful anti-inflammatory and immunosuppressive drugs commonly employed as first-line treatment in patients with ICI-induced irAEs. The efficacy of glucocorticoids varies tremendously in the treatment of different types of irAEs. For instance, these compounds are effective in most cases but do not reverse hypophysitis, and high doses may worsen outcomes (122). By contrast, initial high-dose glucocorticoids were superior to intermediate and low-dose steroid in the treatment of ICI-associated myocarditis (242). Another important issue is the timing of initiation of glucocorticoid administration. In the above retrospective, observational study, early (≤ 24 hours) administration of glucocorticoids can also vary in different irAEs. For example, arthritis is also unique since inflammation often persists even after stopping ICIs and may require prolonged glucocorticoid treatment (40) and/or biological DMARDs (243).
Glucocorticoids are associated with potential multiple side effects and impact on anti-tumor response. Low doses of glucocorticoids used to treat irAEs do not affect the response rates and/or the survival of ICI-treated patients (223, 224, 230). A meta-analysis found that glucocorticoids do not negatively affect survival (230). However, glucocorticoids before or early during ICI treatment may negatively affect outcomes (231–234). Baseline treatment of lung cancer patients with anti-PD-1 and high doses (> 10 mg/day) of prednisone negatively affects outcomes compared to those treated with low dose glucocorticoids (231, 235). The side effects of glucocorticoids depend on the daily dose, the cumulative dose administered and possibly the type of underlying disease (244). Therefore, awareness of specific glucocorticoid-induced side effects is required.
irAEs refractory to glucocorticoids may require the administration of a mAb targeting TNF-α (i.e., infliximab) to treat certain irAEs such as colitis (16, 136, 237, 245) and pneumonitis (25, 175, 246). Preclinical studies suggest that prophylactic TNF-α inhibition eliminated ICI-induced colitis without affecting anti-tumor response (241). There is conflicting evidence on the effect of short-term TNF-α inhibition with infliximab to treat ICI-induced irAEs on overall survival in cancer patients (237–240). A comprehensive review has examined in detail the role of different cytokines in the pathophysiology of irAEs. In addition, the authors provided an in-depth analysis of strategies to uncouple the cytokine response that participates in ICI-associated irAEs (247).
Potential Therapies and Prevention of IrAEs
The increasing use of ICIs in a growing number of solid and hematologic cancers requires us to offer the best-targeted therapies of irAEs. Table 3 summarizes the potential therapies of irAEs.
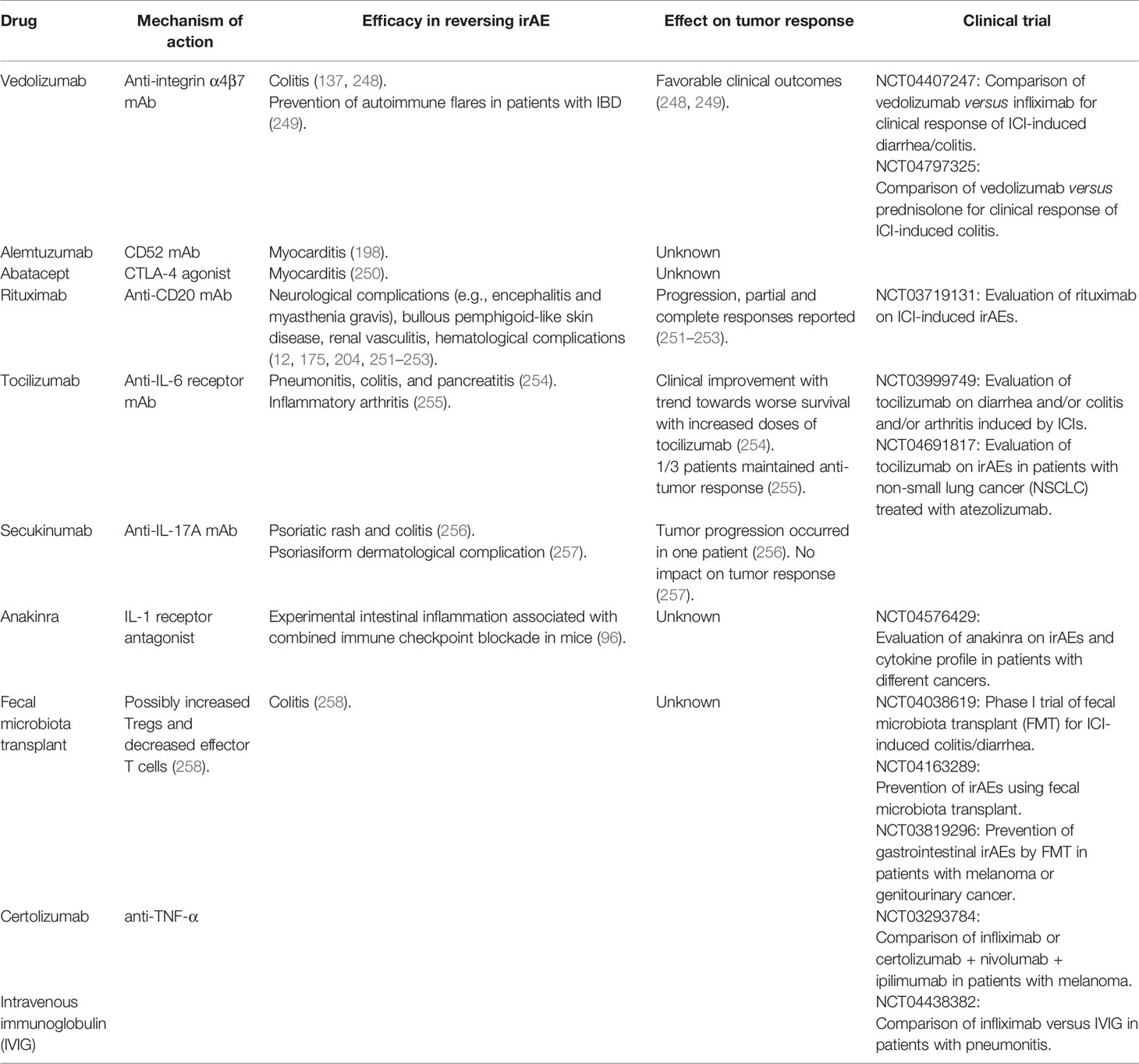
Table 3 Emerging and potential future therapies for immune adverse events associated with immune checkpoint inhibitors.
Understanding the pathophysiology of ICI-associated myocarditis and developing effective treatments is of great importance. The quest for novel therapies for glucocorticoid-resistant ICI myocarditis is a clinical unmet need. If symptoms and laboratory findings in ICI myocarditis do not regress upon high-dose glucocorticoids, other immunosuppressant agents [e.g. mycophenolate mofetil, methotrexate, calcineurin inhibitors, intravenous immunoglobulin (IVIG), anti-thymocyte globulin, rituximab and infliximab] may be considered for treatment of ICIs cardiotoxicity, but data are still controversial (201, 259, 260).
Alemtuzumab (anti-CD52 mAb) and abatacept, a protein consisting of the human CTLA-4 extracellular domain fused to the Fc portion of IgG, acting as a CTLA-4 agonist, have been employed for the treatment of single cases of glucocorticoid-refractory myocarditis (198, 250). Concerns with abatacept are possible infections and tumor progression. Abatacept has also been used to treat ICI-induced MG (261). Alemtuzumab causes T cell depletion and its impact on tumor growth remains unknown. Overall, the evidence available at present is insufficient to support any of the anecdotal, albeit reasonable, strategies outlined above and more evidence-based guidance in this critical care is urgently needed. While blocking TNF-α in heart failure (HF) has been proven contraindicated in symptomatic (NYHA III and IV) patients (262, 263), anti-TNF-α may be a promising approach to prevent the early stages of cardiotoxicity from anti-PD-1 immunotherapy (200).
Vedolizumab is a specific anti-integrin α4β7 antibody, used for the treatment of IBD (264–266). Preclinical studies have reported that vedolizumab induces remission in ICI-induced glucocorticoid-refractory colitis with good safety profiles (137, 248). Early treatment with vedolizumab is a potential treatment of ICI-associated colitis (138). Two clinical trials are evaluating the safety and efficacy of vedolizumab in the treatment of ICI-induced colitis (NCT04407247, NCT04797325).
Rituximab, a mAb targeting CD20, has been used in glucocorticoid-refractory encephalitis and myasthenia gravis (251, 252), bullous pemphigoid-like skin disease (12), renal vasculitis (253), and hematological complications (204).
Tocilizumab, a mAb anti-IL-6 receptor, has been used to treat ICI-associated arthritis (255) and glucocorticoid-refractory irAEs (254). The safety and efficacy of tocilizumab on ICI-associated irAEs are under evaluation in two clinical trials (NCT03999749, NCT04691817).
IL-17 blockade has been used in few patients with colon cancer and melanoma (256, 257). A recent study found that there is an upregulation of intestinal IL-1β in melanoma patients treated with CICB who developed high-grade colitis (96). In two mouse models, CICB was associated with intestinal inflammation characterized by upregulation of Il1b, but not Tnfa or Il6. Interestingly, mice concurrently treated with CICB and anakinra (anti-IL-1R) showed less intestinal inflammation. These parallel studies in humans and mice suggest that severe intestinal inflammation associated with CICB could be prevented by an IL-1R antagonist. A clinical trial is evaluating the safety and efficacy of anakinra on ICI-induced irAEs (NCT04576429).
Gut microbiome can influence efficacy of PD-1-based immunotherapy (103, 105). A clinical trial is prospectively analyzing the intestinal microbiome as predictor of ICI-associated irAEs (NCT04107311). Fecal microbiota transplant (FMT), which transfers an entire microbiome from a healthy donor to a recipient, is a therapeutic tool with several potential applications but numerous caveats (267). FMT may be a potential mechanism for treating ICI-induced colitis (258). FMT can be performed via an oral capsule containing fecal extracts (268–270), colonoscopy-guided transfer (269, 271) or enema (272, 273). Microbiome composition varies widely among healthy donors and can affects success rates (274, 275). FMT involves the risk for transmission of infectious agents via FMT (276). Regulatory authorities have released specific guidelines to offer FMT with safety at the time of COVID-19 pandemic (277). Two patients with refractory ICI-associated colitis were successfully treated with FMT (258). Clinical and experimental studies are needed to evaluate the efficacy of this approach as well as to provide further mechanistic insights. In summary, there is some evidence that microbiome manipulation could potentially impact cancer course and perhaps ICI-associated irAEs. Several clinical trials are evaluating the safety and efficacy of FMT on the prevention/treatment of intestinal irAEs (NCT04038619, NCT04163289, NCT03819296).
Conclusions and Perspectives
Emerging real-world data suggests the incidence of ICI-associated irAEs may be higher than previously found in clinical trials. This trend has the potential to increase further as the use of canonical ICIs (anti-CTLA-4 and anti-PD-1/PD-L1), alone or in combination, is increasing exponentially and nearly 50% of patients treated will experience some form of irAEs (278). Furthermore, the combination of first and/or second generation of ICIs (e.g., anti-TIGIT) or with anti-angiogenic agents (279) could open a new scenario of irAEs associated with novel forms of cancer immunotherapy.
Great efforts have been devoted to the identification of genetic, humoral and cellular biomarkers predictive of irAEs associated with ICIs. Although these putative biomarkers have not been incorporated into clinical practice, they have highlighted some novel aspects of irAE pathogenesis. For instance, recent human and experimental studies have highlighted the contribution of specific gut microbiota to intestinal damage caused by CICB in melanoma patients (96). This study also suggests that specific peripheral blood signatures are associated with a risk of developing toxicity after CICB. Of course, additional studies of larger cohorts of ICI-treated patients with different cancers will be needed to validate these findings.
Management of irAEs is essentially based on retrospective studies and expert consensus (11, 29, 31, 45, 174, 175, 228, 229). Glucocorticoids, commonly employed as first-line treatment of irAEs, do not affect certain toxicities, and high doses may negatively affect outcomes (122, 231–234). Awareness of glucocorticoid-induced side effects is required. A wide spectrum of emerging therapeutic options, including mAbs (anti-TNF-α, anti-IL-6, anti-CD20, anti-IL-1R) (12, 96, 241, 251, 252, 254) and FMT (96, 258) are under clinical and experimental investigations. There is currently no clinical evidence that irAEs can be pharmacologically prevented. However, there is experimental evidence that prophylactic administration anti-TNF-α (241) or anti-IL-1R (96) is associated with less intestinal inflammation caused by CICB in different tumor models.
Table 4 presents some of the outstanding pathophysiological and therapeutic questions that should be addressed to better understand the complexity of different irAEs. Due to the clinical complexity surrounding autoimmune disease development and management, Clinical Immunologists should be involved in the care of cancer patients before, during, and after checkpoint blockade. Perhaps, multidisciplinary irAE management teams, facilitating prompt diagnosis and treatment should be enlisted. Further research is needed to improve early diagnosis, understand immunological and genetic mechanisms, and develop management algorithms for these disorders.

Table 4 Outstanding pathophysiological and therapeutic questions relevant to immune-related adverse events (irAEs) associated with immune checkpoint inhibitors.
Author Contributions
RP, GC, FC, CT, and GV wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by grants from the CISI-Lab Project (University of Naples Federico II) and TIMING Project and Campania Bioscience (Regione Campania).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the medical artist Fabrizio Fiorbianco for preparing figures and the administrative staff (Dr. Roberto Bifulco, Dr. Anna Ferraro and Dr. Maria Cristina Fucci), without whom it would not be possible to work as a team. The authors apologize to the many researchers who have contributed importantly to the field and whose work was not cited due to space constraints.
Abbreviations
ACPA, anti-citrullinated peptide antibodies; B. intestinalis, Bacteroides intestinalis; CT, chest computed tomography; CICB, combined immune checkpoint blockade; CCP, cyclic citrullinated peptide; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; DMARDs, disease modifying anti-rheumatic drugs; FMT, fecal microbiota transplant; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; GNAL, guanine nucleotide-binding protein G subunit alpha; HLA, human leukocyte antigen; ICIs, immune checkpoint inhibitors; irAEs, immune-related adverse events; IBD, inflammatory bowel disease; ITM2B, integral membrane protein 2B; IVIG), intravenous immunoglobulin therapy; MRI, magnetic resonance imaging; MACE, major adverse cardiac events; mAbs, monoclonal antibodies; MCP-1, monocyte chemoattractant protein-1; NCI-CTCAE, national cancer institute common terminology criteria for adverse events; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death protein ligand 1; PRS, polygenic risk score; RCT, randomized controlled trial; RF, rheumatoid factor; SS-A, Sjogren’s syndrome antibodies anti-Ro; SS-B, Sjogren’s syndrome antibodies anti-La; TG, thyroglobulin; TPO, thyroid peroxidase; Trm, tissue-resident memory T; 18F-FDG, tomography/computed tomography with 2-[18F] fluorodeoxyglucose; TNF-α, tumor necrosis factor-α; T1D, type 1 diabetes.
References
1. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov (2018) 8:1069–86. doi: 10.1158/2159-8290.CD-18-0367
2. Sharma P, Allison JP. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies With Curative Potential. Cell (2015) 161:205–14. doi: 10.1016/j.cell.2015.03.030
3. Lebbe C, Weber JS, Maio M, Neyns B, Harmankaya K, Hamid O, et al. Survival Follow-Up and Ipilimumab Retreatment of Patients With Advanced Melanoma Who Received Ipilimumab in Prior Phase II Studies. Ann Oncol (2014) 25:2277–84. doi: 10.1093/annonc/mdu441
4. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377:1345–56. doi: 10.1056/Nejmoa1709684
5. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol (2019) 5:1411–20. doi: 10.1001/jamaoncol.2019.2187
6. Ribas A, Wolchok JD. Cancer Immunotherapy Using Checkpoint Blockade. Science (2018) 359:1350–5. doi: 10.1126/science.aar4060
7. Fritz JM, Lenardo MJ. Development of Immune Checkpoint Therapy for Cancer. J Exp Med (2019) 216:1244–54. doi: 10.1084/jem.20182395
8. Topalian SL, Sharpe AH. Balance and Imbalance in the Immune System: Life on the Edge. Immunity (2014) 41:682–4. doi: 10.1016/j.immuni.2014.11.005
9. Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, et al. CTLA-4 Suppresses the Pathogenicity of Self Antigen-Specific T Cells by Cell-Intrinsic and Cell-Extrinsic Mechanisms. Nat Immunol (2010) 11:129–35. doi: 10.1038/ni.1835ni.1835
10. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders or Major Toxicity With Ipilimumab. Ann Oncol (2017) 28:368–76. doi: 10.1093/annonc/mdw443
11. Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J Cutan Med Surg (2021) 25:59–76. doi: 10.1177/1203475420943260
12. Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA, Freites-Martinez A, et al. Treatment Outcomes of Immune-Related Cutaneous Adverse Events. J Clin Oncol (2019) 37:2746–58. doi: 10.1200/JCO.18.02141
13. Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am J Clin Dermatol (2018) 19:345–61. doi: 10.1007/s40257-017-0336-3
14. Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L, et al. Enterocolitis Due to Immune Checkpoint Inhibitors: A Systematic Review. Gut (2018) 67:2056–67. doi: 10.1136/gutjnl-2018-316948
15. Siakavellas SI, Bamias G. Checkpoint Inhibitor Colitis: A New Model of Inflammatory Bowel Disease? Curr Opin Gastroenterol (2018) 34:377–83. doi: 10.1097/MOG.0000000000000482
16. Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, et al. Immune-Checkpoint Inhibitor-Induced Diarrhea and Colitis in Patients With Advanced Malignancies: Retrospective Review at MD Anderson. J Immunother Cancer (2018) 6:37. doi: 10.1186/s40425-018-0346-6
17. Herrmann J. Adverse Cardiac Effects of Cancer Therapies: Cardiotoxicity and Arrhythmia. Nat Rev Cardiol (2020) 17:474–502. doi: 10.1038/s41569-020-0348-1
18. Neilan TG, Rothenberg ML, Amiri-Kordestani L, Sullivan RJ, Steingart RM, Gregory W, et al. Myocarditis Associated With Immune Checkpoint Inhibitors: An Expert Consensus on Data Gaps and a Call to Action. Oncologist (2018) 23:874–8. doi: 10.1634/theoncologist.2018-0157
19. Varricchi G, Galdiero MR, Marone G, Criscuolo G, Triassi M, Bonaduce D, et al. Cardiotoxicity of Immune Checkpoint Inhibitors. ESMO Open (2017) 2:e000247. doi: 10.1136/esmoopen-2017-000247
20. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
21. Varricchi G, Galdiero MR, Tocchetti CG. Cardiac Toxicity of Immune Checkpoint Inhibitors: Cardio-Oncology Meets Immunology. Circulation (2017) 136:1989–92. doi: 10.1161/CIRCULATIONAHA.117.029626
22. Tocchetti CG, Galdiero MR, Varricchi G. Cardiac Toxicity in Patients Treated With Immune Checkpoint Inhibitors: It Is Now Time for Cardio-Immuno-Oncology. J Am Coll Cardiol (2018) 71:1765–7. doi: 10.1016/j.jacc.2018.02.038
23. Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation (2017) 136:2085–7. doi: 10.1161/CIRCULATIONAHA.117.030571
24. Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, Kitsis RN. Immune Checkpoint Inhibitor-Associated Myocarditis: Manifestations and Mechanisms. J Clin Invest (2021) 131:e145186. doi: 10.1172/JCI145186145186
25. Reuss JE, Suresh K, Naidoo J. Checkpoint Inhibitor Pneumonitis: Mechanisms, Characteristics, Management Strategies, and Beyond. Curr Oncol Rep (2020) 22:56. doi: 10.1007/s11912-020-00920-z
26. Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of Pneumonitis and Pneumonia Associated With Immune Checkpoint Inhibitors for Solid Tumors: A Systematic Review and Meta-Analysis. Front Immunol (2019) 10:108. doi: 10.3389/fimmu.2019.00108
27. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer Immunotherapy - Immune Checkpoint Blockade and Associated Endocrinopathies. Nat Rev Endocrinol (2017) 13:195–207. doi: 10.1038/nrendo.2016.205
28. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr Rev (2019) 40:17–65. doi: 10.1210/er.2018-00006
29. Ferrari SM, Fallahi P, Elia G, Ragusa F, Ruffilli I, Patrizio A, et al. Autoimmune Endocrine Dysfunctions Associated With Cancer Immunotherapies. Int J Mol Sci (2019) 20:2560. doi: 10.3390/ijms20102560
30. Calabrese LH, Calabrese C, Cappelli LC. Rheumatic Immune-Related Adverse Events From Cancer Immunotherapy. Nat Rev Rheumatol (2018) 14:569–79. doi: 10.1038/s41584-018-0074-9
31. Kostine M, Finckh A, Bingham CO, Visser K, Leipe J, Schulze-Koops H, et al. EULAR Points to Consider for the Diagnosis and Management of Rheumatic Immune-Related Adverse Events Due to Cancer Immunotherapy With Checkpoint Inhibitors. Ann Rheum Dis (2021) 80:36–48. doi: 10.1136/annrheumdis-2020-217139
32. Spain L, Tippu Z, Larkin JM, Carr A, Turajlic S. How We Treat Neurological Toxicity From Immune Checkpoint Inhibitors. ESMO Open (2019) 4:e000540. doi: 10.1136/esmoopen-2019-000540
33. Michot JM, Lazarovici J, Tieu A, Champiat S, Voisin AL, Ebbo M, et al. Haematological Immune-Related Adverse Events With Immune Checkpoint Inhibitors, How to Manage? Eur J Cancer (2019) 122:72–90. doi: 10.1016/j.ejca.2019.07.014
34. Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell (2017) 170:1120–1133 e1117. doi: 10.1016/j.cell.2017.07.024
35. Arnaud-Coffin P, Maillet D, Gan HK, Stelmes JJ, You B, Dalle S, et al. A Systematic Review of Adverse Events in Randomized Trials Assessing Immune Checkpoint Inhibitors. Int J Cancer (2019) 145:639–48. doi: 10.1002/ijc.32132
36. Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, et al. The Safety and Tolerability of Combined Immune Checkpoint Inhibitors (Anti-PD-1/PD-L1 Plus Anti-CTLA-4): A Systematic Review and Meta-Analysis. BMC Cancer (2019) 19:559. doi: 10.1186/s12885-019-5785-z
37. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. JAMA Oncol (2019) 5:1008–19. doi: 10.1001/jamaoncol.2019.0393
38. Weber JS, Kahler KC, Hauschild A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. J Clin Oncol (2012) 30:2691–7. doi: 10.1200/JCO.2012.41.6750
39. Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. J Clin Oncol (2017) 35:3815–22. doi: 10.1200/JCO.2016.72.1167
40. Braaten TJ, Brahmer JR, Forde PM, Le D, Lipson EJ, Naidoo J, et al. Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis Persists After Immunotherapy Cessation. Ann Rheum Dis (2020) 79:332–8. doi: 10.1136/annrheumdis-2019-216109
41. Chan KK, Bass AR. Autoimmune Complications of Immunotherapy: Pathophysiology and Management. BMJ (2020) 369:m736. doi: 10.1136/bmj.m736
42. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes (2018) 67:1471–80. doi: 10.2337/dbi18-0002dbi18-0002
43. de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, van der Auwera BJ, et al. Immune Checkpoint Inhibitors and Type 1 Diabetes Mellitus: A Case Report and Systematic Review. Eur J Endocrinol (2019) 181:363–74. doi: 10.1530/EJE-19-0291EJE-19-0291
44. de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, et al. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. J Clin Endocrinol Metab (2016) 101:4431–9. doi: 10.1210/jc.2016-2300
45. Elia G, Ferrari SM, Galdiero MR, Ragusa F, Paparo SR, Ruffilli I, et al. New Insight in Endocrine-Related Adverse Events Associated to Immune Checkpoint Blockade. Best Pract Res Clin Endocrinol Metab (2020) 34:101370. doi: 10.1016/j.beem.2019.101370
46. Owen CN, Bai X, Quah T, Lo SN, Allayous C, Callaghan S, et al. Delayed Immune-Related Adverse Events With Anti-PD-1-Based Immunotherapy in Melanoma. Ann Oncol (2021) 32:917–25. doi: 10.1016/j.annonc.2021.03.204
47. Patrinely JR Jr., Johnson R, Lawless AR, Bhave P, Sawyers A, Dimitrova M, et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-Risk Resected Melanoma. JAMA Oncol (2021) 7:744–8. doi: 10.1001/jamaoncol.2021.0051
48. Cappelli LC, Bingham CO 3rd. Spectrum and Impact of Checkpoint Inhibitor-Induced irAEs. Nat Rev Rheumatol (2021) 17:69–70. doi: 10.1038/s41584-020-00546-2
49. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-Checkpoint Inhibitors: Long-Term Implications of Toxicity. Nat Rev Clin Oncol (2022) 1–14. doi: 10.1038/s41571-022-00600-w
50. de Filette J, Andreescu CE, Cools F, Bravenboer B, Velkeniers B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated With Immune Checkpoint Inhibitors. Horm Metab Res (2019) 51:145–56. doi: 10.1055/a-0843-3366
51. Ghosh N, Tiongson MD, Stewart C, Chan KK, Jivanelli B, Cappelli L, et al. Checkpoint Inhibitor-Associated Arthritis: A Systematic Review of Case Reports and Case Series. J Clin Rheumatol (2020) 27:e317-22. doi: 10.1097/RHU.0000000000001370
52. Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, et al. Single-Cell Immune Landscape of Human Atherosclerotic Plaques. Nat Med (2019) 25:1576–88. doi: 10.1038/s41591-019-0590-4
53. Strauss L, Mahmoud MAA, Weaver JD, Tijaro-Ovalle NM, Christofides A, Wang Q, et al. Targeted Deletion of PD-1 in Myeloid Cells Induces Antitumor Immunity. Sci Immunol (2020) 5:eaay1863. doi: 10.1126/sciimmunol.aay18635/43/eaay1863
54. Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic Immune Responses Are Regulated by the PD-1/PD-L Pathway in Mice. J Clin Invest (2007) 117:2974–82. doi: 10.1172/JCI31344
55. Poels K, van Leent MMT, Boutros C, Tissot H, Roy S, Meerwaldt AE, et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell-Driven Plaque Inflammation in Atherosclerosis. JACC CardioOncol (2020) 2:599–610. doi: 10.1016/j.jaccao.2020.08.007
56. Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation (2020) 142:2299–311. doi: 10.1161/CIRCULATIONAHA.120.049981
57. Calabretta R, Hoeller C, Pichler V, Mitterhauser M, Karanikas G, Haug A, et al. Immune Checkpoint Inhibitor Therapy Induces Inflammatory Activity in Large Arteries. Circulation (2020) 142:2396–8. doi: 10.1161/CIRCULATIONAHA.120.048708
58. Minasian L, Dimond E, Davis M, Adhikari B, Fagerstrom R, Fabian C, et al. The Evolving Design of NIH-Funded Cardio-Oncology Studies to Address Cancer Treatment-Related Cardiovascular Toxicity. JACC CardioOncol (2019) 1:105–13. doi: 10.1016/j.jaccao.2019.08.007
59. Mazarico I, Capel I, Gimenez-Palop O, Albert L, Berges I, Luchtenberg F, et al. Low Frequency of Positive Antithyroid Antibodies is Observed in Patients With Thyroid Dysfunction Related to Immune Check Point Inhibitors. J Endocrinol Invest (2019) 42:1443–50. doi: 10.1007/s40618-019-01058-x
60. Yamauchi I, Yasoda A, Matsumoto S, Sakamori Y, Kim YH, Nomura M, et al. Incidence, Features, and Prognosis of Immune-Related Adverse Events Involving the Thyroid Gland Induced by Nivolumab. PloS One (2019) 14:e0216954. doi: 10.1371/journal.pone.0216954
61. Siegel J, Totonchy M, Damsky W, Berk-Krauss J, Castiglione F Jr., Sznol M, et al. Bullous Disorders Associated With Anti-PD-1 and Anti-PD-L1 Therapy: A Retrospective Analysis Evaluating the Clinical and Histopathologic Features, Frequency, and Impact on Cancer Therapy. J Am Acad Dermatol (2018) 79:1081–8. doi: 10.1016/j.jaad.2018.07.008
62. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci Transl Med (2014) 6:230ra245. doi: 10.1126/scitranslmed.3008002
63. Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H, et al. Autoimmune Antibodies Correlate With Immune Checkpoint Therapy-Induced Toxicities. Proc Natl Acad Sci USA (2019) 116:22246–51. doi: 10.1073/pnas.1908079116
64. Johansen A, Christensen SJ, Scheie D, Hojgaard JLS, Kondziella D. Neuromuscular Adverse Events Associated With Anti-PD-1 Monoclonal Antibodies: Systematic Review. Neurology (2019) 92:663–74. doi: 10.1212/WNL.0000000000007235
65. Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, et al. Baseline Circulating IL-17 Predicts Toxicity While TGF-Beta1 and IL-10 are Prognostic of Relapse in Ipilimumab Neoadjuvant Therapy of Melanoma. J Immunother Cancer (2015) 3:39. doi: 10.1186/s40425-015-0081-181
66. Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, et al. Predictive and Sensitive Biomarkers for Thyroid Dysfunctions During Treatment With Immune-Checkpoint Inhibitors. Cancer Sci (2020) 111:1468–77. doi: 10.1111/cas.14363
67. Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 Leads to Massive Lymphoproliferation and Fatal Multiorgan Tissue Destruction, Revealing a Critical Negative Regulatory Role of CTLA-4. Immunity (1995) 3:541–7. doi: 10.1016/1074-7613(95)90125-6
68. Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science (2001) 291:319–22. doi: 10.1126/science.291.5502.319
69. Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA. Association of HLA-DRB1 Shared Epitope Alleles and Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis. Rheumatology (Oxford) (2019) 58:476–80. doi: 10.1093/rheumatology/key358
70. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune Checkpoint Inhibitor-Induced Type 1 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Med (2019) 36:1075–81. doi: 10.1111/dme.14050
71. Hasan Ali O, Berner F, Bomze D, Fassler M, Diem S, Cozzio A, et al. Human Leukocyte Antigen Variation is Associated With Adverse Events of Checkpoint Inhibitors. Eur J Cancer (2019) 107:8–14. doi: 10.1016/j.ejca.2018.11.009
72. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science (2015) 350:1079–84. doi: 10.1126/science.aad1329
73. Gil-Cruz C, Perez-Shibayama C, De Martin A, Ronchi F, van der Borght K, Niederer R, et al. Microbiota-Derived Peptide Mimics Drive Lethal Inflammatory Cardiomyopathy. Science (2019) 366:881–6. doi: 10.1126/science.aav3487
74. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated With Ipilimumab. Ann Oncol (2017) 28:1368–79. doi: 10.1093/annonc/mdx108
75. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal Microbiome Analyses Identify Melanoma Patients at Risk for Checkpoint-Blockade-Induced Colitis. Nat Commun (2016) 7:10391. doi: 10.1038/ncomms10391
76. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant Myocarditis With Combination Immune Checkpoint Blockade. N Engl J Med (2016) 375:1749–55. doi: 10.1056/NEJMoa1609214
77. Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:1043–7. doi: 10.1001/jamaoncol.2019.0402
78. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-Related Adverse Events With Immune Checkpoint Blockade: A Comprehensive Review. Eur J Cancer (2016) 54:139–48. doi: 10.1016/j.ejca.2015.11.016
79. Lozano AX, Chaudhuri AA, Nene A, Bacchiocchi A, Earland N, Vesely MD, et al. T Cell Characteristics Associated With Toxicity to Immune Checkpoint Blockade in Patients With Melanoma. Nat Med (2022) 28:353–62. doi: 10.1038/s41591-021-01623-z
80. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 Control Over Foxp3+ Regulatory T Cell Function. Science (2008) 322:271–5. doi: 10.1126/science.1160062
81. Liu Y, Zheng P. How Does an Anti-CTLA-4 Antibody Promote Cancer Immunity? Trends Immunol (2018) 39:953–6. doi: 10.1016/j.it.2018.10.009
82. Blanc C, Hans S, Tran T, Granier C, Saldman A, Anson M, et al. Targeting Resident Memory T Cells for Cancer Immunotherapy. Front Immunol (2018) 9:1722. doi: 10.3389/fimmu.2018.01722
83. Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell (2020) 182:655–71.e622. doi: 10.1016/j.cell.2020.06.001
84. Luo J, Martucci VL, Quandt Z, Groha S, Murray MH, Lovly CM, et al. Immunotherapy-Mediated Thyroid Dysfunction: Genetic Risk and Impact on Outcomes With PD-1 Blockade in Non-Small Cell Lung Cancer. Clin Cancer Res (2021) 27:5131–40. doi: 10.1158/1078-0432.CCR-21-0921
85. Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, et al. Predictive Factors of Nivolumab-Induced Hypothyroidism in Patients With Non-Small Cell Lung Cancer. In Vivo (2017) 31:1035–9. doi: 10.21873/invivo.11166
86. Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, et al. Association of Antithyroglobulin Antibodies With the Development of Thyroid Dysfunction Induced by Nivolumab. Cancer Sci (2018) 109:3583–90. doi: 10.1111/cas.13800
87. Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol (2019) 5:376–83. doi: 10.1001/jamaoncol.2018.5860
88. Bingley PJ. Clinical Applications of Diabetes Antibody Testing. J Clin Endocrinol Metab (2010) 95:25–33. doi: 10.1210/jc.2009-1365
89. Lin HH, Gutenberg A, Chen TY, Tsai NM, Lee CJ, Cheng YC, et al. In Situ Activation of Pituitary-Infiltrating T Lymphocytes in Autoimmune Hypophysitis. Sci Rep (2017) 7:43492. doi: 10.1038/srep43492
90. Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, et al. Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights Into Pathogenesis From an Autopsy Series. Am J Pathol (2016) 186:3225–35. doi: 10.1016/j.ajpath.2016.08.020
91. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and Activity of Anti-PD-L1 Antibody in Patients With Advanced Cancer. N Engl J Med (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
92. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-Programmed-Death-Receptor-1 Treatment With Pembrolizumab in Ipilimumab-Refractory Advanced Melanoma: A Randomised Dose-Comparison Cohort of a Phase 1 Trial. Lancet (2014) 384:1109–17. doi: 10.1016/S0140-6736(14)60958-2
93. Godel P, Shimabukuro-Vornhagen A, von Bergwelt-Baildon M. Understanding Cytokine Release Syndrome. Intensive Care Med (2018) 44:371–3. doi: 10.1007/s00134-017-4943-5
94. Ceschi A, Noseda R, Palin K, Verhamme K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front Pharmacol (2020) 11:557. doi: 10.3389/fphar.2020.00557
95. Khan S, Khan SA, Luo X, Fattah FJ, Saltarski J, Gloria-McCutchen Y, et al. Immune Dysregulation in Cancer Patients Developing Immune-Related Adverse Events. Br J Cancer (2019) 120:63–8. doi: 10.1038/s41416-018-0155-1
96. Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut Microbiota Signatures are Associated With Toxicity to Combined CTLA-4 and PD-1 Blockade. Nat Med (2021) 27:1432–41. doi: 10.1038/s41591-021-01406-6
97. Lucas JA, Menke J, Rabacal WA, Schoen FJ, Sharpe AH, Kelley VR. Programmed Death Ligand 1 Regulates a Critical Checkpoint for Autoimmune Myocarditis and Pneumonitis in MRL Mice. J Immunol (2008) 181:2513–21. doi: 10.4049/jimmunol.181.4.2513
98. Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies Against Cardiac Troponin I are Responsible for Dilated Cardiomyopathy in PD-1-Deficient Mice. Nat Med (2003) 9:1477–83. doi: 10.1038/nm955nm955
99. Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 Protects Against Inflammation and Myocyte Damage in T Cell-Mediated Myocarditis. J Immunol (2012) 188:4876–84. doi: 10.4049/jimmunol.1200389
100. Khan Z, Di Nucci F, Kwan A, Hammer C, Mariathasan S, Rouilly V, et al. Polygenic Risk for Skin Autoimmunity Impacts Immune Checkpoint Blockade in Bladder Cancer. Proc Natl Acad Sci USA (2020) 117:12288–94. doi: 10.1073/pnas.1922867117
101. Anderson R, Theron AJ, Rapoport BL. Immunopathogenesis of Immune Checkpoint Inhibitor-Related Adverse Events: Roles of the Intestinal Microbiome and Th17 Cells. Front Immunol (2019) 10:2254. doi: 10.3389/fimmu.2019.02254
102. Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science (2015) 350:1084–9. doi: 10.1126/science.aac4255science.aac4255[pii
103. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy Against Epithelial Tumors. Science (2018) 359:91–7. doi: 10.1126/science.aan3706
104. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The Commensal Microbiome is Associated With Anti-PD-1 Efficacy in Metastatic Melanoma Patients. Science (2018) 359:104–8. doi: 10.1126/science.aao3290
105. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut Microbiome Modulates Response to Anti-PD-1 Immunotherapy in Melanoma Patients. Science (2018) 359:97–103. doi: 10.1126/science.aan4236
106. Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, et al. Autoimmune Diseases and Immune-Checkpoint Inhibitors for Cancer Therapy: Review of the Literature and Personalized Risk-Based Prevention Strategy. Ann Oncol (2020) 31:724–44. doi: 10.1016/j.annonc.2020.03.285
107. Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, et al. Safety and Efficacy of Anti-Programmed Death 1 Antibodies in Patients With Cancer and Pre-Existing Autoimmune or Inflammatory Disease. Eur J Cancer (2018) 91:21–9. doi: 10.1016/j.ejca.2017.12.008
108. Kehl KL, Yang S, Awad MM, Palmer N, Kohane IS, Schrag D. Pre-Existing Autoimmune Disease and the Risk of Immune-Related Adverse Events Among Patients Receiving Checkpoint Inhibitors for Cancer. Cancer Immunol Immunother (2019) 68:917–26. doi: 10.1007/s00262-019-02321-z
109. Kobayashi T, Iwama S, Yasuda Y, Okada N, Tsunekawa T, Onoue T, et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J Endocr Soc (2018) 2:241–51. doi: 10.1210/js.2017-00432
110. Gowen MF, Giles KM, Simpson D, Tchack J, Zhou H, Moran U, et al. Baseline Antibody Profiles Predict Toxicity in Melanoma Patients Treated With Immune Checkpoint Inhibitors. J Transl Med (2018) 16:82. doi: 10.1186/s12967-018-1452-4
111. Valpione S, Pasquali S, Campana LG, Piccin L, Mocellin S, Pigozzo J, et al. Sex and Interleukin-6 are Prognostic Factors for Autoimmune Toxicity Following Treatment With Anti-CTLA4 Blockade. J Transl Med (2018) 16:94. doi: 10.1186/s12967-018-1467-x
112. Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin Cancer Res (2019) 25:1557–63. doi: 10.1158/1078-0432.CCR-18-2795
113. Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, et al. Early B Cell Changes Predict Autoimmunity Following Combination Immune Checkpoint Blockade. J Clin Invest (2018) 128:715–20. doi: 10.1172/JCI9679896798[pii
114. Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated With Immune-Checkpoint Inhibitors. Oncologist (2019) 24:1128–36. doi: 10.1634/theoncologist.2018-
115. Giommoni E, Giorgione R, Paderi A, Pellegrini E, Gambale E, Marini A, et al. Eosinophil Count as Predictive Biomarker of Immune-Related Adverse Events (irAEs) in Immune Checkpoint Inhibitors (ICIs) Therapies in Oncological Patients. Immuno (2021) 1:253. doi: 10.3390/immuno1030017
116. Schindler K, Harmankaya K, Kuk D, Mangana J, Michielin O, HoellerReinhard Dummer C, et al. Abstract: Correlation of Absolute and Relative Eosinophil Counts With Immune-Related Adverse Events in Melanoma Patients Treated With Ipilimumab. J Clin Oncol (2014) 32:9096. doi: 10.1200/jco.2014.32.15_suppl.9096
117. Sakata Y, Kawamura K, Ichikado K, Shingu N, Yasuda Y, Eguchi Y, et al. The Association Between Tumor Burden and Severe Immune-Related Adverse Events in non-Small Cell Lung Cancer Patients Responding to Immune-Checkpoint Inhibitor Treatment. Lung Cancer (2019) 130:159–61. doi: 10.1016/j.lungcan.2019.02.011
118. Wright JJ, Salem JE, Johnson DB, Lebrun-Vignes B, Stamatouli A, Thomas JW, et al. Increased Reporting of Immune Checkpoint Inhibitor-Associated Diabetes. Diabetes Care (2018) 41:e150–1. doi: 10.2337/dc18-1465dc18-1465
119. Faje A, Reynolds K, Zubiri L, Lawrence D, Cohen JV, Sullivan RJ, et al. Hypophysitis Secondary to Nivolumab and Pembrolizumab is a Clinical Entity Distinct From Ipilimumab-Associated Hypophysitis. Eur J Endocrinol (2019) 181:211–9. doi: 10.1530/EJE-19-0238EJE-19-0238
120. Dougan M, Pietropaolo M. Time to Dissect the Autoimmune Etiology of Cancer Antibody Immunotherapy. J Clin Invest (2020) 130:51–61. doi: 10.1172/JCI131194131194
121. Ma C, Hodi FS, Giobbie-Hurder A, Wang X, Zhou J, Zhang A, et al. The Impact of High-Dose Glucocorticoids on the Outcome of Immune-Checkpoint Inhibitor-Related Thyroid Disorders. Cancer Immunol Res (2019) 7:1214–20. doi: 10.1158/2326-6066.CIR-18-0613
122. Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-Dose Glucocorticoids for the Treatment of Ipilimumab-Induced Hypophysitis is Associated With Reduced Survival in Patients With Melanoma. Cancer (2018) 124:3706–14. doi: 10.1002/cncr.31629
123. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased Reporting of Fatal Immune Checkpoint Inhibitor-Associated Myocarditis. Lancet (2018) 391:933. doi: 10.1016/S0140-6736(18)30533-6
124. Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grunwald V, et al. Diagnosis, Monitoring and Management of Immune-Related Adverse Drug Reactions of Anti-PD-1 Antibody Therapy. Cancer Treat Rev (2016) 45:7–18. doi: 10.1016/j.ctrv.2016.02.003
125. Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory Eruptions Associated With Immune Checkpoint Inhibitor Therapy: A Single-Institution Retrospective Analysis With Stratification of Reactions by Toxicity and Implications for Management. J Am Acad Dermatol (2019) 80:990–7. doi: 10.1016/j.jaad.2018.10.062
126. Larsabal M, Marti A, Jacquemin C, Rambert J, Thiolat D, Dousset L, et al. Vitiligo-Like Lesions Occurring in Patients Receiving Anti-Programmed Cell Death-1 Therapies are Clinically and Biologically Distinct From Vitiligo. J Am Acad Dermatol (2017) 76:863–70. doi: 10.1016/j.jaad.2016.10.044
127. Aggarwal P. Disproportionality Analysis of Bullous Pemphigoid Adverse Events With PD-1 Inhibitors in the FDA Adverse Event Reporting System. Expert Opin Drug Saf (2019) 18:623–33. doi: 10.1080/14740338.2019.1619693
128. Lacouture M, Sibaud V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am J Clin Dermatol (2018) 19:31–9. doi: 10.1007/s40257-018-0384-3
129. Soldatos TG, Dimitrakopoulou-Strauss A, Larribere L, Hassel JC, Sachpekidis C. Retrospective Side Effect Profiling of the Metastatic Melanoma Combination Therapy Ipilimumab-Nivolumab Using Adverse Event Data. Diagnostics (Basel) (2018) 8:76. doi: 10.3390/diagnostics8040076
130. El Majzoub I, Qdaisat A, Thein KZ, Win MA, Han MM, Jacobson K, et al. Adverse Effects of Immune Checkpoint Therapy in Cancer Patients Visiting the Emergency Department of a Comprehensive Cancer Center. Ann Emerg Med (2019) 73:79–87. doi: 10.1016/j.annemergmed.2018.04.019
131. Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in Patients With Cancer After Antibody Blockade of Cytotoxic T-Lymphocyte-Associated Antigen 4. J Clin Oncol (2006) 24:2283–9. doi: 10.1200/JCO.2005.04.5716
132. Wang DY, Ye F, Zhao S, Johnson DB. Incidence of Immune Checkpoint Inhibitor-Related Colitis in Solid Tumor Patients: A Systematic Review and Meta-Analysis. Oncoimmunology (2017) 6:e1344805. doi: 10.1080/2162402X.2017.1344805
133. Wang Y, Abu-Sbeih H, Mao E, Ali N, Qiao W, Trinh VA, et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm Bowel Dis (2018) 24:1695–705. doi: 10.1093/ibd/izy1044989161
134. Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, et al. Cancer Immunotherapy With Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J Crohns Colitis (2016) 10:395–401. doi: 10.1093/ecco-jcc/jjv227
135. Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study Comparing the Tolerability and Efficacy of Ipilimumab Administered With or Without Prophylactic Budesonide in Patients With Unresectable Stage III or IV Melanoma. Clin Cancer Res (2009) 15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024
136. Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL Jr., Abdel-Wahab N, et al. Infliximab Associated With Faster Symptom Resolution Compared With Corticosteroids Alone for the Management of Immune-Related Enterocolitis. J Immunother Cancer (2018) 6:103. doi: 10.1186/s40425-018-0412-0
137. Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, et al. Vedolizumab Treatment for Immune Checkpoint Inhibitor-Induced Enterocolitis. Cancer Immunol Immunother (2017) 66:581–92. doi: 10.1007/s00262-017-1962-6
138. Abu-Sbeih H, Ali FS, Wang X, Mallepally N, Chen E, Altan M, et al. Early Introduction of Selective Immunosuppressive Therapy Associated With Favorable Clinical Outcomes in Patients With Immune Checkpoint Inhibitor-Induced Colitis. J Immunother Cancer (2019) 7:93. doi: 10.1186/s40425-019-0577-1
139. Suzman DL, Pelosof L, Rosenberg A, Avigan MI. Hepatotoxicity of Immune Checkpoint Inhibitors: An Evolving Picture of Risk Associated With a Vital Class of Immunotherapy Agents. Liver Int (2018) 38:976–87. doi: 10.1111/liv.13746
140. De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of Liver Injury Induced by Cancer Immunotherapy Using Immune Checkpoint Inhibitors. J Hepatol (2018) 68:1181–90. doi: 10.1016/j.jhep.2018.01.033
141. Abu-Sbeih H, Tang T, Lu Y, Thirumurthi S, Altan M, Jazaeri AA, et al. Clinical Characteristics and Outcomes of Immune Checkpoint Inhibitor-Induced Pancreatic Injury. J Immunother Cancer (2019) 7:31. doi: 10.1186/s40425-019-0502-7
142. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety Profiles of Anti-CTLA-4 and Anti-PD-1 Antibodies Alone and in Combination. Nat Rev Clin Oncol (2016) 13:473–86. doi: 10.1038/nrclinonc.2016.58
143. Varricchi G, Loffredo S, Marone G, Modestino L, Fallahi P, Ferrari SM, et al. The Immune Landscape of Thyroid Cancer in the Context of Immune Checkpoint Inhibition. Int J Mol Sci (2019) 20:3934. doi: 10.3390/ijms20163934ijms20163934
144. Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, et al. Immune-Related Thyroiditis With Immune Checkpoint Inhibitors. Thyroid (2018) 28:1243–51. doi: 10.1089/thy.2018.0116
145. Orlov S, Salari F, Kashat L, Walfish PG. Induction of Painless Thyroiditis in Patients Receiving Programmed Death 1 Receptor Immunotherapy for Metastatic Malignancies. J Clin Endocrinol Metab (2015) 100:1738–41. doi: 10.1210/jc.2014-4560
146. Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, et al. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J Clin Endocrinol Metab (2017) 102:2770–80. doi: 10.1210/jc.2017-004483805504
147. Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, et al. Ipilimumab-Induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients With Metastatic Melanoma. J Clin Endocrinol Metab (2014) 99:4078–85. doi: 10.1210/jc.2014-2306
148. Albarel F, Gaudy C, Castinetti F, Carre T, Morange I, Conte-Devolx B, et al. Long-Term Follow-Up of Ipilimumab-Induced Hypophysitis, a Common Adverse Event of the Anti-CTLA-4 Antibody in Melanoma. Eur J Endocrinol (2015) 172:195–204. doi: 10.1530/EJE-14-0845
149. Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, et al. Systemic High-Dose Corticosteroid Treatment Does Not Improve the Outcome of Ipilimumab-Related Hypophysitis: A Retrospective Cohort Study. Clin Cancer Res (2015) 21:749–55. doi: 10.1158/1078-0432.CCR-14-2353
150. Carpenter KJ, Murtagh RD, Lilienfeld H, Weber J, Murtagh FR. Ipilimumab-Induced Hypophysitis: MR Imaging Findings. AJNR Am J Neuroradiol (2009) 30:1751–3. doi: 10.3174/ajnr.A1623
151. Juszczak A, Gupta A, Karavitaki N, Middleton MR, Grossman AB. Ipilimumab: A Novel Immunomodulating Therapy Causing Autoimmune Hypophysitis: A Case Report and Review. Eur J Endocrinol (2012) 167:1–5. doi: 10.1530/EJE-12-0167
152. Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The Price of Tumor Control: An Analysis of Rare Side Effects of Anti-CTLA-4 Therapy in Metastatic Melanoma From the Ipilimumab Network. PloS One (2013) 8:e53745. doi: 10.1371/journal.pone.0053745
153. Kotwal A, Haddox C, Block M, Kudva YC. Immune Checkpoint Inhibitors: An Emerging Cause of Insulin-Dependent Diabetes. BMJ Open Diabetes Res Care (2019) 7:e000591. doi: 10.1136/bmjdrc-2018-000591
154. Gaudy C, Clevy C, Monestier S, Dubois N, Preau Y, Mallet S, et al. Anti-PD1 Pembrolizumab Can Induce Exceptional Fulminant Type 1 Diabetes. Diabetes Care (2015) 38:e182–183. doi: 10.2337/dc15-1331dc15-1331
155. Mellati M, Eaton KD, Brooks-Worrell BM, Hagopian WA, Martins R, Palmer JP, et al. Anti-PD-1 and Anti-PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care (2015) 38:e137–138. doi: 10.2337/dc15-0889dc15-0889
156. Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, et al. Precipitation of Autoimmune Diabetes With Anti-PD-1 Immunotherapy. Diabetes Care (2015) 38:e55–57. doi: 10.2337/dc14-234938/4/e55
157. James EA, Mallone R, Kent SC, DiLorenzo TP. T-Cell Epitopes and Neo-Epitopes in Type 1 Diabetes: A Comprehensive Update and Reappraisal. Diabetes (2020) 69:1311–35. doi: 10.2337/dbi19-002269/7/1311
158. Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, et al. Autoimmune Diabetes Induced by PD-1 Inhibitor-Retrospective Analysis and Pathogenesis: A Case Report and Literature Review. Cancer Immunol Immunother (2017) 66:1399–410. doi: 10.1007/s00262-017-2033-8
159. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol (2017) 35:709–17. doi: 10.1200/JCO.2016.68.2005
160. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
161. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
162. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
163. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-Analysis. JAMA Oncol (2016) 2:1607–16. doi: 10.1001/jamaoncol.2016.24532544610
164. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic Impact and Risk Factors of Immune-Related Pneumonitis in Patients With Non-Small-Cell Lung Cancer Who Received Programmed Death 1 Inhibitors. Clin Lung Cancer (2019) 20:442–450 e444. doi: 10.1016/j.cllc.2019.07.006
165. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol (2018) 13:1930–9. doi: 10.1016/j.jtho.2018.08.2035
166. Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, Incidence, and Risk Factors of Immune Checkpoint Inhibitor-Related Pneumonitis in Patients With non-Small Cell Lung Cancer. Lung Cancer (2018) 125:150–6. doi: 10.1016/j.lungcan.2018.09.015
167. Khunger M, Jain P, Rakshit S, Pasupuleti V, Hernandez AV, Stevenson J, et al. Safety and Efficacy of PD-1/PD-L1 Inhibitors in Treatment-Naive and Chemotherapy-Refractory Patients With Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Clin Lung Cancer (2018) 19:e335–48. doi: 10.1016/j.cllc.2018.01.002
168. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and Class-Specific Patterns of Immune-Related Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Ann Oncol (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
169. Wu J, Hong D, Zhang X, Lu X, Miao J. PD-1 Inhibitors Increase the Incidence and Risk of Pneumonitis in Cancer Patients in a Dose-Independent Manner: A Meta-Analysis. Sci Rep (2017) 7:44173. doi: 10.1038/srep44173
170. Larsen BT, Chae JM, Dixit AS, Hartman TE, Peikert T, Roden AC. Clinical and Histopathologic Features of Immune Checkpoint Inhibitor-Related Pneumonitis. Am J Surg Pathol (2019) 43:1331–40. doi: 10.1097/PAS.0000000000001298
171. Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, et al. Risk Factors for Pneumonitis in Patients Treated With Anti-Programmed Death-1 Therapy: A Case-Control Study. Cancer Med (2018) 7:4115–20. doi: 10.1002/cam4.1579
172. Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y, et al. Pre-Existing Pulmonary Fibrosis is a Risk Factor for Anti-PD-1-Related Pneumonitis in Patients With non-Small Cell Lung Cancer: A Retrospective Analysis. Lung Cancer (2018) 125:212–7. doi: 10.1016/j.lungcan.2018.10.001
173. Rossi E, Schinzari G, Tortora G. Pneumonitis From Immune Checkpoint Inhibitors and COVID-19: Current Concern in Cancer Treatment. J Immunother Cancer (2020) 8:e000952. doi: 10.1136/jitc-2020-000952
174. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of Toxicities From Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2017) 28:iv119–42. doi: 10.1093/annonc/mdx2253958159
175. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2018) 36:1714–68. doi: 10.1200/JCO.2017.77.6385
176. Gomatou G, Tzilas V, Kotteas E, Syrigos K, Bouros D. Immune Checkpoint Inhibitor-Related Pneumonitis. Respiration (2020) 99:932–42. doi: 10.1159/000509941
177. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J Natl Compr Canc Netw (2019) 17:255–89. doi: 10.6004/jnccn.2019.0013
178. Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, et al. Rheumatic Disorders Associated With Immune Checkpoint Inhibitors in Patients With Cancer-Clinical Aspects and Relationship With Tumour Response: A Single-Centre Prospective Cohort Study. Ann Rheum Dis (2018) 77:393–8. doi: 10.1136/annrheumdis-2017-212257
179. Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res (Hoboken) (2017) 69:1751–63. doi: 10.1002/acr.23177
180. Smith MH, Bass AR. Arthritis After Cancer Immunotherapy: Symptom Duration and Treatment Response. Arthritis Care Res (Hoboken) (2019) 71:362–6. doi: 10.1002/acr.23467
181. Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L. Polymyalgia Rheumatica-Like Syndrome From Checkpoint Inhibitor Therapy: Case Series and Systematic Review of the Literature. RMD Open (2019) 5:e000906. doi: 10.1136/rmdopen-2019-000906
182. Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, Naidoo J, et al. Clinical Presentation of Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis Differs by Immunotherapy Regimen. Semin Arthritis Rheum (2018) 48:553–7. doi: 10.1016/j.semarthrit.2018.02.011
183. Cappelli LC, Bingham CO 3rd. Expert Perspective: Immune Checkpoint Inhibitors and Rheumatologic Complications. Arthritis Rheumatol (2021) 73:553–65. doi: 10.1002/art.41587
184. Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Aure K, et al. Immune Checkpoint Inhibitor-Related Myositis and Myocarditis in Patients With Cancer. Neurology (2018) 91:e985–94. doi: 10.1212/WNL.0000000000006124
185. Anquetil C, Salem JE, Lebrun-Vignes B, Johnson DB, Mammen AL, Stenzel W, et al. Immune Checkpoint Inhibitor-Associated Myositis: Expanding the Spectrum of Cardiac Complications of the Immunotherapy Revolution. Circulation (2018) 138:743–5. doi: 10.1161/CIRCULATIONAHA.118.035898
186. Moreira A, Loquai C, Pfohler C, Kahler KC, Knauss S, Heppt MV, et al. Myositis and Neuromuscular Side-Effects Induced by Immune Checkpoint Inhibitors. Eur J Cancer (2019) 106:12–23. doi: 10.1016/j.ejca.2018.09.033
187. Liewluck T, Kao JC, Mauermann ML. PD-1 Inhibitor-Associated Myopathies: Emerging Immune-Mediated Myopathies. J Immunother (2018) 41:208–11. doi: 10.1097/CJI.0000000000000196
188. Shah M, Tayar JH, Abdel-Wahab N, Suarez-Almazor ME. Myositis as an Adverse Event of Immune Checkpoint Blockade for Cancer Therapy. Semin Arthritis Rheum (2019) 48:736–40. doi: 10.1016/j.semarthrit.2018.05.006
189. Groarke JD, Cheng S, Moslehi J. Cancer-Drug Discovery and Cardiovascular Surveillance. N Engl J Med (2013) 369:1779–81. doi: 10.1056/NEJMp1313140
190. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
191. Hu JR, Florido R, Lipson EJ, Naidoo J, Ardehali R, Tocchetti CG, et al. Cardiovascular Toxicities Associated With Immune Checkpoint Inhibitors. Cardiovasc Res (2019) 115:854–68. doi: 10.1093/cvr/cvz0265304411[pii
192. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular Toxicities Associated With Immune Checkpoint Inhibitors: An Observational, Retrospective, Pharmacovigilance Study. Lancet Oncol (2018) 19:1579–89. doi: 10.1016/S1470-2045(18)30608-9
193. Allenbach Y, Anquetil C, Manouchehri A, Benveniste O, Lambotte O, Lebrun-Vignes B, et al. Immune Checkpoint Inhibitor-Induced Myositis, the Earliest and Most Lethal Complication Among Rheumatic and Musculoskeletal Toxicities. Autoimmun Rev (2020) 19:102586. doi: 10.1016/j.autrev.2020.102586
194. Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, et al. Smoldering Myocarditis Following Immune Checkpoint Blockade. J Immunother Cancer (2017) 5:91. doi: 10.1186/s40425-017-0296-4
195. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation (2019) 140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497
196. Mir H, Alhussein M, Alrashidi S, Alzayer H, Alshatti A, Valettas N, et al. Cardiac Complications Associated With Checkpoint Inhibition: A Systematic Review of the Literature in an Important Emerging Area. Can J Cardiol (2018) 34:1059–68. doi: 10.1016/j.cjca.2018.03.012
197. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing Toxicities Associated With Immune Checkpoint Inhibitors: Consensus Recommendations From the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer (2017) 5:95. doi: 10.1186/s40425-017-0300-z
198. Esfahani K, Buhlaiga N, Thebault P, Lapointe R, Johnson NA, Miller WH Jr. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N Engl J Med (2019) 380:2375–6. doi: 10.1056/NEJMc1903064
199. Thackeray JT, Pietzsch S, Stapel B, Ricke-Hoch M, Lee CW, Bankstahl JP, et al. Insulin Supplementation Attenuates Cancer-Induced Cardiomyopathy and Slows Tumor Disease Progression. JCI Insight (2017) 2e93098. doi: 10.1172/jci.insight.9309893098
200. Michel L, Helfrich I, Hendgen-Cotta UB, Mincu RI, Korste S, Mrotzek SM, et al. Targeting Early Stages of Cardiotoxicity From Anti-PD1 Immune Checkpoint Inhibitor Therapy. Eur Heart J (2022) 43:316–29. doi: 10.1093/eurheartj/ehab4306352226
201. Varricchi G, Galdiero MR, Tocchetti CG. Novel Actors on the Stage of Cardiac Dysfunction Induced by Anti-PD1 Oncological Treatments. Eur Heart J (2021) 43:330–2. doi: 10.1093/eurheartj/ehab5846359545[pii
202. Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological Adverse Events Associated With Immune Checkpoint Inhibitors: Review of the Literature. Eur J Cancer (2017) 73:1–8. doi: 10.1016/j.ejca.2016.12.001
203. Johnson DB, Manouchehri A, Haugh AM, Quach HT, Balko JM, Lebrun-Vignes B, et al. Neurologic Toxicity Associated With Immune Checkpoint Inhibitors: A Pharmacovigilance Study. J Immunother Cancer (2019) 7:134. doi: 10.1186/s40425-019-0617-x
204. Delanoy N, Michot JM, Comont T, Kramkimel N, Lazarovici J, Dupont R, et al. Haematological Immune-Related Adverse Events Induced by Anti-PD-1 or Anti-PD-L1 Immunotherapy: A Descriptive Observational Study. Lancet Haematol (2019) 6:e48–57. doi: 10.1016/S2352-3026(18)30175-3
205. Kramer R, Zaremba A, Moreira A, Ugurel S, Johnson DB, Hassel JC, et al. Hematological Immune Related Adverse Events After Treatment With Immune Checkpoint Inhibitors. Eur J Cancer (2021) 147:170–81. doi: 10.1016/j.ejca.2021.01.013
206. Leaf RK, Ferreri C, Rangachari D, Mier J, Witteles W, Ansstas G, et al. Clinical and Laboratory Features of Autoimmune Hemolytic Anemia Associated With Immune Checkpoint Inhibitors. Am J Hematol (2019) 94:563–74. doi: 10.1002/ajh.25448
207. Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, Gastrointestinal, Hepatic, Endocrine, and Renal Side-Effects of Anti-PD-1 Therapy. Eur J Cancer (2016) 60:190–209. doi: 10.1016/j.ejca.2016.02.025
208. Murakami N, Motwani S, Riella LV. Renal Complications of Immune Checkpoint Blockade. Curr Probl Cancer (2017) 41:100–10. doi: 10.1016/j.currproblcancer.2016.12.004
209. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse Effects of Immune-Checkpoint Inhibitors: Epidemiology, Management and Surveillance. Nat Rev Clin Oncol (2019) 16:563–80. doi: 10.1038/s41571-019-0218-0
210. Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR, et al. Clinicopathological Features of Acute Kidney Injury Associated With Immune Checkpoint Inhibitors. Kidney Int (2016) 90:638–47. doi: 10.1016/j.kint.2016.04.008
211. Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, et al. Nephrotoxicity of Immune Checkpoint Inhibitors Beyond Tubulointerstitial Nephritis: Single-Center Experience. J Immunother Cancer (2019) 7:2. doi: 10.1186/s40425-018-0478-8
212. Antoun J, Titah C, Cochereau I. Ocular and Orbital Side-Effects of Checkpoint Inhibitors: A Review Article. Curr Opin Oncol (2016) 28:288–94. doi: 10.1097/CCO.0000000000000296
213. Zhou L, Wei X. Ocular Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors in Lung Cancer. Front Immunol (2021) 12:701951. doi: 10.3389/fimmu.2021.701951
214. Bitton K, Michot JM, Barreau E, Lambotte O, Haigh O, Marabelle A, et al. Prevalence and Clinical Patterns of Ocular Complications Associated With Anti-PD-1/PD-L1 Anticancer Immunotherapy. Am J Ophthalmol (2019) 202:109–17. doi: 10.1016/j.ajo.2019.02.012
215. Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee JM, et al. Ocular Adverse Events Following Use of Immune Checkpoint Inhibitors for Metastatic Malignancies. Ocul Immunol Inflamm (2020) 28:854–9. doi: 10.1080/09273948.2019.1583347
216. Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, et al. Sicca Syndrome Associated With Immune Checkpoint Inhibitor Therapy. Oncologist (2019) 24:1259–69. doi: 10.1634/theoncologist.2018-0823
217. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab Versus Ipilimumab in Advanced Melanoma. N Engl J Med (2015) 372:2521–32. doi: 10.1056/NEJMoa1503093
218. Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
219. Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and Ipilimumab Versus Ipilimumab in Untreated Melanoma. N Engl J Med (2015) 372:2006–17. doi: 10.1056/NEJMoa1414428
220. Khan SA, Pruitt SL, Xuan L, Gerber DE. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol (2016) 2:1507–8. doi: 10.1001/jamaoncol.2016.2238
221. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med (2018) 168:121–30. doi: 10.7326/M17-20732667695
222. Tison A, Quere G, Misery L, Funck-Brentano E, Danlos FX, Routier E, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol (2019) 71:2100–11. doi: 10.1002/art.41068
223. Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol (2015) 33:3193–8. doi: 10.1200/JCO.2015.60.8448
224. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol (2017) 35:785–92. doi: 10.1200/JCO.2015.66.1389
225. Kotwal A, Kottschade L, Ryder M. PD-L1 Inhibitor-Induced Thyroiditis Is Associated With Better Overall Survival in Cancer Patients. Thyroid (2020) 30:177–84. doi: 10.1089/thy.2019.0250
226. Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol (2016) 152:45–51. doi: 10.1001/jamadermatol.2015.2707
227. Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373:1270–1. doi: 10.1056/NEJMc1509660
228. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation (2020) 141:e69–92. doi: 10.1161/CIR.0000000000000745
229. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail (2020) 13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405
230. Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, et al. Association of Steroids Use With Survival in Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers (Basel) (2020) 12:546. doi: 10.3390/cancers12030546
231. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol (2018) 36:2872–8. doi: 10.1200/JCO.2018.79.0006
232. Scott SC, Pennell NA. Early Use of Systemic Corticosteroids in Patients With Advanced NSCLC Treated With Nivolumab. J Thorac Oncol (2018) 13:1771–5. doi: 10.1016/j.jtho.2018.06.004
233. Fuca G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, et al. Modulation of Peripheral Blood Immune Cells by Early Use of Steroids and its Association With Clinical Outcomes in Patients With Metastatic non-Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. ESMO Open (2019) 4:e000457. doi: 10.1136/esmoopen-2018-000457
234. Chasset F, Pages C, Biard L, Roux J, Sidina I, Madelaine I, et al. Single-Center Study Under a French Temporary Authorization for Use (TAU) Protocol for Ipilimumab in Metastatic Melanoma: Negative Impact of Baseline Corticosteroids. Eur J Dermatol (2015) 25:36–44. doi: 10.1684/ejd.2014.2471
235. Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol (2019) 37:1927–34. doi: 10.1200/JCO.19.00189
236. Draghi A, Borch TH, Radic HD, Chamberlain CA, Gokuldass A, Svane IM, et al. Differential Effects of Corticosteroids and Anti-TNF on Tumor-Specific Immune Responses: Implications for the Management of irAEs. Int J Cancer (2019) 145:1408–13. doi: 10.1002/ijc.32080
237. Verheijden RJ, May AM, Blank CU, Aarts MJB, van den Berkmortel F, van den Eertwegh AJM, et al. Association of Anti-TNF With Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res (2020) 26:2268–74. doi: 10.1158/1078-0432.CCR-19-3322
238. Lesage C, Longvert C, Prey S, Maanaoui S, Dreno B, Machet L, et al. Incidence and Clinical Impact of Anti-TNFalpha Treatment of Severe Immune Checkpoint Inhibitor-Induced Colitis in Advanced Melanoma: The Mecolit Survey. J Immunother (2019) 42:175–9. doi: 10.1097/CJI.0000000000000268
239. Chen AY, Wolchok JD, Bass AR. TNF in the Era of Immune Checkpoint Inhibitors: Friend or Foe? Nat Rev Rheumatol (2021) 17:213–23. doi: 10.1038/s41584-021-00584-4
240. Suijkerbuijk KPM, Verheijden RJ. TNF Inhibition for Immune Checkpoint Inhibitor-Induced irAEs: The Jury is Still Out. Nat Rev Rheumatol (2021) 17:505. doi: 10.1038/s41584-021-00640-z
241. Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, et al. Prophylactic TNF Blockade Uncouples Efficacy and Toxicity in Dual CTLA-4 and PD-1 Immunotherapy. Nature (2019) 569:428–32. doi: 10.1038/s41586-019-1162-y
242. Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-Associated Myocarditis. Circulation (2020) 141:2031–4. doi: 10.1161/CIRCULATIONAHA.119.044703
243. Galdiero MR, Morelli E, Triggianese P, Carucci L, Punziano A, Pinto A, et al. First Report of De Novo Nivolumab-Induced Oligoarthritis in a Young Man With Relapsing Classic Hodgkin Lymphoma. J Clin Rheumatol (2020) 27:S877–8. doi: 10.1097/RHU.0000000000001459
244. Shimba A, Ikuta K. Control of Immunity by Glucocorticoids in Health and Disease. Semin Immunopathol (2020) 42:669–80. doi: 10.1007/s00281-020-00827-8
245. Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M. Concurrent Therapy With Immune Checkpoint Inhibitors and TNFalpha Blockade in Patients With Gastrointestinal Immune-Related Adverse Events. J Immunother Cancer (2019) 7:226. doi: 10.1186/s40425-019-0711-0
246. Kalisz KR, Ramaiya NH, Laukamp KR, Gupta A. Immune Checkpoint Inhibitor Therapy-Related Pneumonitis: Patterns and Management. Radiographics (2019) 39:1923–37. doi: 10.1148/rg.2019190036
247. Kang JH, Bluestone JA, Young A. Predicting and Preventing Immune Checkpoint Inhibitor Toxicity: Targeting Cytokines. Trends Immunol (2021) 42:293–311. doi: 10.1016/j.it.2021.02.006
248. Abu-Sbeih H, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z, et al. Outcomes of Vedolizumab Therapy in Patients With Immune Checkpoint Inhibitor-Induced Colitis: A Multi-Center Study. J Immunother Cancer (2018) 6:142. doi: 10.1186/s40425-018-0461-4
249. Frohne CC, Llano EM, Perkovic A, Cohen RD, Luke JJ. Complete Response of Metastatic Melanoma in a Patient With Crohn’s Disease Simultaneously Receiving Anti-Alpha4beta7 and Anti-PD1 Antibodies. J Immunother Cancer (2019) 7:1. doi: 10.1186/s40425-018-0484-x
250. Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N Engl J Med (2019) 380:2377–9. doi: 10.1056/NEJMc1901677
251. Ito M, Fujiwara S, Fujimoto D, Mori R, Yoshimura H, Hata A, et al. Rituximab for Nivolumab Plus Ipilimumab-Induced Encephalitis in a Small-Cell Lung Cancer Patient. Ann Oncol (2017) 28:2318–9. doi: 10.1093/annonc/mdx252
252. Crusz SM, Radunovic A, Shepherd S, Shah S, Newey V, Phillips M, et al. Rituximab in the Treatment of Pembrolizumab-Induced Myasthenia Gravis. Eur J Cancer (2018) 102:49–51. doi: 10.1016/j.ejca.2018.07.125
253. Mamlouk O, Lin JS, Abdelrahim M, Tchakarov AS, Glass WF, Selamet U, et al. Checkpoint Inhibitor-Related Renal Vasculitis and Use of Rituximab. J Immunother Cancer (2020) 8:e000750. doi: 10.1136/jitc-2020-000750
254. Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the Management of Immune Mediated Adverse Events Secondary to PD-1 Blockade. J Oncol Pharm Pract (2019) 25:551–7. doi: 10.1177/1078155217745144
255. Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, et al. Successful Treatment of Arthritis Induced by Checkpoint Inhibitors With Tocilizumab: A Case Series. Ann Rheum Dis (2017) 76:2061–4. doi: 10.1136/annrheumdis-2017-211560
256. Esfahani K, Miller WH Jr. Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N Engl J Med (2017) 376:1989–91. doi: 10.1056/NEJMc1703047
257. Johnson D, Patel AB, Uemura MI, Trinh VA, Jackson N, Zobniw CM, et al. IL17A Blockade Successfully Treated Psoriasiform Dermatologic Toxicity From Immunotherapy. Cancer Immunol Res (2019) 7:860–5. doi: 10.1158/2326-6066.CIR-18-0682
258. Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal Microbiota Transplantation for Refractory Immune Checkpoint Inhibitor-Associated Colitis. Nat Med (2018) 24:1804–8. doi: 10.1038/s41591-018-0238-9
259. Zhang RS, Padegimas A, Murphy KM, Evans PT, Peters CJ, Domenico CM, et al. Treatment of Corticosteroid Refractory Immune Checkpoint Inhibitor Myocarditis With Infliximab: A Case Series. Cardiooncology (2021) 7:13. doi: 10.1186/s40959-021-00095-x
260. Cautela J, Zeriouh S, Gaubert M, Bonello L, Laine M, Peyrol M, et al. Intensified Immunosuppressive Therapy in Patients With Immune Checkpoint Inhibitor-Induced Myocarditis. J Immunother Cancer (2020) 8:e001887. doi: 10.1136/jitc-2020-001887
261. Kumar B, Ballas Z. Adverse Events Associated With Immune Checkpoint Blockade. N Engl J Med (2018) 378:1164. doi: 10.1056/NEJMc1801663
262. Mann DL. Innate Immunity and the Failing Heart: The Cytokine Hypothesis Revisited. Circ Res (2015) 116:1254–68. doi: 10.1161/CIRCRESAHA.116.302317
263. Frantz S, Falcao-Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, et al. The Innate Immune System in Chronic Cardiomyopathy: A European Society of Cardiology (ESC) Scientific Statement From the Working Group on Myocardial Function of the ESC. Eur J Heart Fail (2018) 20:445–59. doi: 10.1002/ejhf.1138
264. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med (2013) 369:699–710. doi: 10.1056/NEJMoa1215734
265. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med (2013) 369:711–21. doi: 10.1056/NEJMoa1215739
266. Sands BE, Peyrin-Biroulet L, Loftus EV Jr., Danese S, Colombel JF, Toruner M, et al. Vedolizumab Versus Adalimumab for Moderate-To-Severe Ulcerative Colitis. N Engl J Med (2019) 381:1215–26. doi: 10.1056/NEJMoa1905725
267. Varricchi G, Poto R, Ianiro G, Punziano A, Marone G, Gasbarrini A, et al. Gut Microbiome and Common Variable Immunodeficiency: Few Certainties and Many Outstanding Questions. Front Immunol (2021) 12:712915. doi: 10.3389/fimmu.2021.712915
268. Hirsch BE, Saraiya N, Poeth K, Schwartz RM, Epstein ME, Honig G. Effectiveness of Fecal-Derived Microbiota Transfer Using Orally Administered Capsules for Recurrent Clostridium Difficile Infection. BMC Infect Dis (2015) 15:191. doi: 10.1186/s12879-015-0930-z
269. Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium Difficile Infection: A Randomized Clinical Trial. JAMA (2017) 318:1985–93. doi: 10.1001/jama.2017.17077
270. Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium Difficile Infection. JAMA (2014) 312:1772–8. doi: 10.1001/jama.2014.13875
271. Allegretti JR, Korzenik JR, Hamilton MJ. Fecal Microbiota Transplantation via Colonoscopy for Recurrent C. Difficile Infection. J Vis Exp (2014) 52154. doi: 10.3791/52154
272. Silverman MS, Davis I, Pillai DR. Success of Self-Administered Home Fecal Transplantation for Chronic Clostridium Difficile Infection. Clin Gastroenterol Hepatol (2010) 8:471–3. doi: 10.1016/j.cgh.2010.01.007
273. Tariq R, Pardi DS, Bartlett MG, Khanna S. Low Cure Rates in Controlled Trials of Fecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection: A Systematic Review and Meta-Analysis. Clin Infect Dis (2019) 68:1351–8. doi: 10.1093/cid/ciy7215078604
274. de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, et al. Donor Metabolic Characteristics Drive Effects of Faecal Microbiota Transplantation on Recipient Insulin Sensitivity, Energy Expenditure and Intestinal Transit Time. Gut (2020) 69:502–12. doi: 10.1136/gutjnl-2019-318320
275. Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol (2019) 9:2. doi: 10.3389/fcimb.2019.00002
276. Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic Review: The Global Incidence of Faecal Microbiota Transplantation-Related Adverse Events From 2000 to 2020. Aliment Pharmacol Ther (2021) 53:33–42. doi: 10.1111/apt.16148
277. Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, et al. Reorganisation of Faecal Microbiota Transplant Services During the COVID-19 Pandemic. Gut (2020) 69:1555–63. doi: 10.1136/gutjnl-2020-321829
278. Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving Towards Personalized Treatments of Immune-Related Adverse Events. Nat Rev Clin Oncol (2020) 17:504–15. doi: 10.1038/s41571-020-0352-8
Keywords: cancer, cytotoxic T lymphocyte-associated protein (CTLA-4), immunotherapy, immune checkpoint inhibitor (ICI), immune-related adverse event (irAE), programmed cell death protein -1 (PD-1), PD-L1
Citation: Poto R, Troiani T, Criscuolo G, Marone G, Ciardiello F, Tocchetti CG and Varricchi G (2022) Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events. Front. Immunol. 13:804597. doi: 10.3389/fimmu.2022.804597
Received: 29 October 2021; Accepted: 07 March 2022;
Published: 30 March 2022.
Edited by:
Fabio Malavasi, University of Turin, ItalyReviewed by:
Arabella Young, University of California, San Francisco, United StatesYinghong Wang, University of Texas MD Anderson Cancer Center, United States
Copyright © 2022 Poto, Troiani, Criscuolo, Marone, Ciardiello, Tocchetti and Varricchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilda Varricchi, Z2lsZGFuZXRAZ21haWwuY29t
†ORCID: Remo Poto, orcid.org/0000-0002-4723-0167
Teresa Troiani, orcid.org/0000-0002-0646-4901
Gjada Criscuolo, orcid.org/0000-0002-5928-1869
Giancarlo Marone, orcid.org/0000-0003-0233-7830
Fortunato Ciardiello, orcid.org/0000-0002-3369-4841
Carlo Gabriele Tocchetti, orcid.org/0000-0001-5983-688X
Gilda Varricchi, orcid.org/0000-0002-9285-4657
 Remo Poto
Remo Poto Teresa Troiani
Teresa Troiani Gjada Criscuolo1,3†
Gjada Criscuolo1,3† Giancarlo Marone
Giancarlo Marone Fortunato Ciardiello
Fortunato Ciardiello Carlo Gabriele Tocchetti
Carlo Gabriele Tocchetti Gilda Varricchi
Gilda Varricchi