- Department of Internal Medicine, Azienda Socio Sanitaria Territoriale (ASST) Ovest Milanese, Milan, Italy
Acting on the cytokine cascade is key to preventing disease progression and death in hospitalised patients with COVID-19. Among anti-cytokine therapies, interleukin (IL)-6 inhibitors have been the most used and studied since the beginning of the pandemic. Going through previous observational studies, subsequent randomised controlled trials, and meta-analyses, we focused on the baseline characteristics of the patients recruited, identifying the most favourable features in the light of positive or negative study outcomes; taking into account the biological significance and predictivity of IL-6 and other biomarkers according to specific thresholds, we ultimately attempted to delineate precise windows for therapeutic intervention. By stimulating scavenger macrophages and T-cell responsivity, IL-6 seems protective against viral replication during asymptomatic infection; still protective on early tissue damage by modulating the release of granzymes and lymphokines in mild-moderate disease; importantly pathogenic in severe disease by inducing the proinflammatory activation of immune and endothelial cells (through trans-signalling and trans-presentation); and again protective in critical disease by exerting homeostatic roles for tissue repair (through cis-signalling), while IL-1 still drives hyperinflammation. IL-6 inhibitors, particularly anti-IL-6R monoclonal antibodies (e.g., tocilizumab, sarilumab), are effective in severe disease, characterised by baseline IL-6 concentrations ranging from 35 to 90 ng/mL (reached in the circulation within 6 days of hospital admission), a ratio of partial pressure arterial oxygen (PaO2) and fraction of inspired oxygen (FiO2) between 100 and 200 mmHg, requirement of high-flow oxygen or non-invasive ventilation, C-reactive protein levels between 120 and 160 mg/L, ferritin levels between 800 and 1600 ng/mL, D-dimer levels between 750 and 3000 ng/mL, and lactate dehydrogenase levels between 350 and 500 U/L. Granulocyte-macrophage colony-stimulating factor inhibitors might have similar windows of opportunity but different age preferences compared to IL-6 inhibitors (over or under 70 years old, respectively). Janus kinase inhibitors (e.g., baricitinib) may also be effective in moderate disease, whereas IL-1 inhibitors (e.g., anakinra) may also be effective in critical disease. Correct use of biologics based on therapeutic windows is essential for successful outcomes and could inform future new trials with more appropriate recruiting criteria.
Introduction
As of October 1, 2021, the Coronavirus Disease 2019 (COVID-19) pandemic has caused over 200,000,000 cases and more than 4,500,000 deaths (1). Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is more often asymptomatic, on the other hand healthy carriers importantly contribute to the spread of the virus, and COVID-19 can manifest itself in different forms of severity, namely mild, moderate, severe and critical.
Mild forms affect patients with respiratory symptoms who are generally not hospitalised and do not require supplemental oxygen. Moderate forms affect patients with viral pneumonia who require low-flow supplemental oxygen (LFO, ≤ 5 liters per minute). Severe forms affect patients with bilateral interstitial pneumonia and acute respiratory distress syndrome (ARDS) requiring high-flow oxygen (HFO) or non-invasive ventilation (NIV). Critical forms affect patients admitted to intensive care unit (ICU) with severe ARDS, shock, and/or multiple organ failure, requiring invasive mechanical ventilation (IMV) with or without other organ support therapies, such as vasopressors, extracorporeal membrane oxygenation (ECMO), or dyalisis (2).
Severe and critical forms represent 14-15% and 2-5% of cases, respectively (3–6). Such life-threatening conditions are believed to result from a SARS-CoV-2-induced respiratory and systemic autoinflammatory disease, in which a dysregulated immune response, associated with exuberant cytokine release, would ultimately account for widespread vascular and tissue damage (7). Cytokines play a central role in the pathogenesis of COVID-19, emerging both as useful biomarkers in predicting disease evolution and as strategic targets for therapy. Discrete clusters of cytokines and chemokines are differentially expressed according to disease stage, with molecules involved in lymphoid priming being upregulated in moderate disease, and molecules involved in myeloid differentiation and migration being overexpressed in severe disease. A deep understanding of the qualitative, quantitative and temporal differences in cytokine pathways is therefore essential to appropriately choosing the right drug and the right timing for effective treatment (7, 8). While direct antiviral therapies may be useful in the early stages of the disease to inhibit virus replication, immunomodulatory and anti-cytokine therapies aimed at targeting harmful hyperinflammation would be crucial in the overt stages, to avoid critical deterioration and fatal outcomes. Since mortality rates can exceed 80% in critically-ill patients requiring IMV (9, 10), acting in severe progressive forms appears to be of the utmost importance in reducing lethality.
IL-6 in COVID-19 Inflammatory Cascade: Rationale for a Key Role in Severe Disease Progression
IL-6 levels in the circulation and bronchoalveolar lavage fluid of patients with COVID-19 progressively increase with disease severity (11, 12), reaching their peaks in critically-ill patients (13, 14). In the lungs of COVID-19 patients, IL-6 can be released by SARS-CoV-2 infected respiratory epithelial cells (15, 16), as well as by infiltrating CD14+CD16+ monocytes-macrophages and CD4+ T cells (17).
IL-6 can substantially contribute to the dysregulation of the immune response in COVID-19, basically by acting in two directions: on one side, it may cause a dysfunction of natural killer and cytotoxic CD8+ T cells associated with perforin and granzyme down-regulation (18), thereby dampening antiviral defenses; on the other side, it may inhibit the differentiation of regulatory T cells and elicit a T helper 17 (TH17)-like polarization of γ/δ and α/β CD4+ T cells, thus leading to uncontrolled hyperinflammation (7). At advanced stages, these mechanisms would result into a macrophage activation syndrome (MAS)-like condition, characterised by lymphocyte exhaustion and aberrant innate immune responses, vascular leakage, exudative-phase ARDS, coagulopathy, and multi-organ failure (7, 19). In severe COVID-19, in fact, increased levels of IL-6 have been found to be associated with higher viral load (20), lymphopenia and neutrophilia (11, 21), systemic inflammation (22), hypoxemia (23), and poor prognosis (22, 23). On the other hand, certain polymorphisms in the IL-6 receptor gene that attenuate IL-6 signalling have been shown to be protective against disease progression, resulting in a lower risk of hospitalisation for COVID-19 (24). Therapeutic blockade of IL-6 may therefore represent an effective strategy to prevent worsening of respiratory status and reduce overall mortality in these patients.
Inhibitors of IL-6 Signal in COVID-19: What to Hit and When?
IL-6 is a pleiotropic cytokine with multiple functions and a complex signalling involving two receptor subunits: IL-6Rα (IL-6R) and IL-6Rβ (130-kDa glycoprotein or gp130). Several signalling modalities have been identified, namely: cis-signalling (or classic mode), in which IL-6 binds to membrane IL-6R (e.g., on macrophages, hepatocytes, megacaryocytes, intestinal epithelial cells) and gp130; trans-signalling, in which a complex formed by IL-6 and soluble IL-6R (mainly cleaved by a disintegrin and metalloprotease-17 or ADAM-17) binds to membrane gp130 (e.g., in endothelial cells, neutrophils, monocytes, pneumocytes); and trans-presentation, in which IL-6 first binds to IL-6R on the membrane of one cell (e.g., dendritic cells) and then the complex binds to gp130 in another cell (e.g., CD4+ T cells) (25–27). Different modes result in different effects: cis-signalling mediates host protection against intracellular pathogens and tissue homeostasis, by upregulating opsonins in the liver and by inducing scavenger and regulatory functions in macrophages; trans-signalling drives proinflammatory activation of pneumocytes, adipose tissue-associated macrophages, gut-associated immune cells, neutrophils and endothelial cells; while trans-presentation promotes T-cell differentiation into pathogenic TH17 cells (25, 26) (Figure 1).
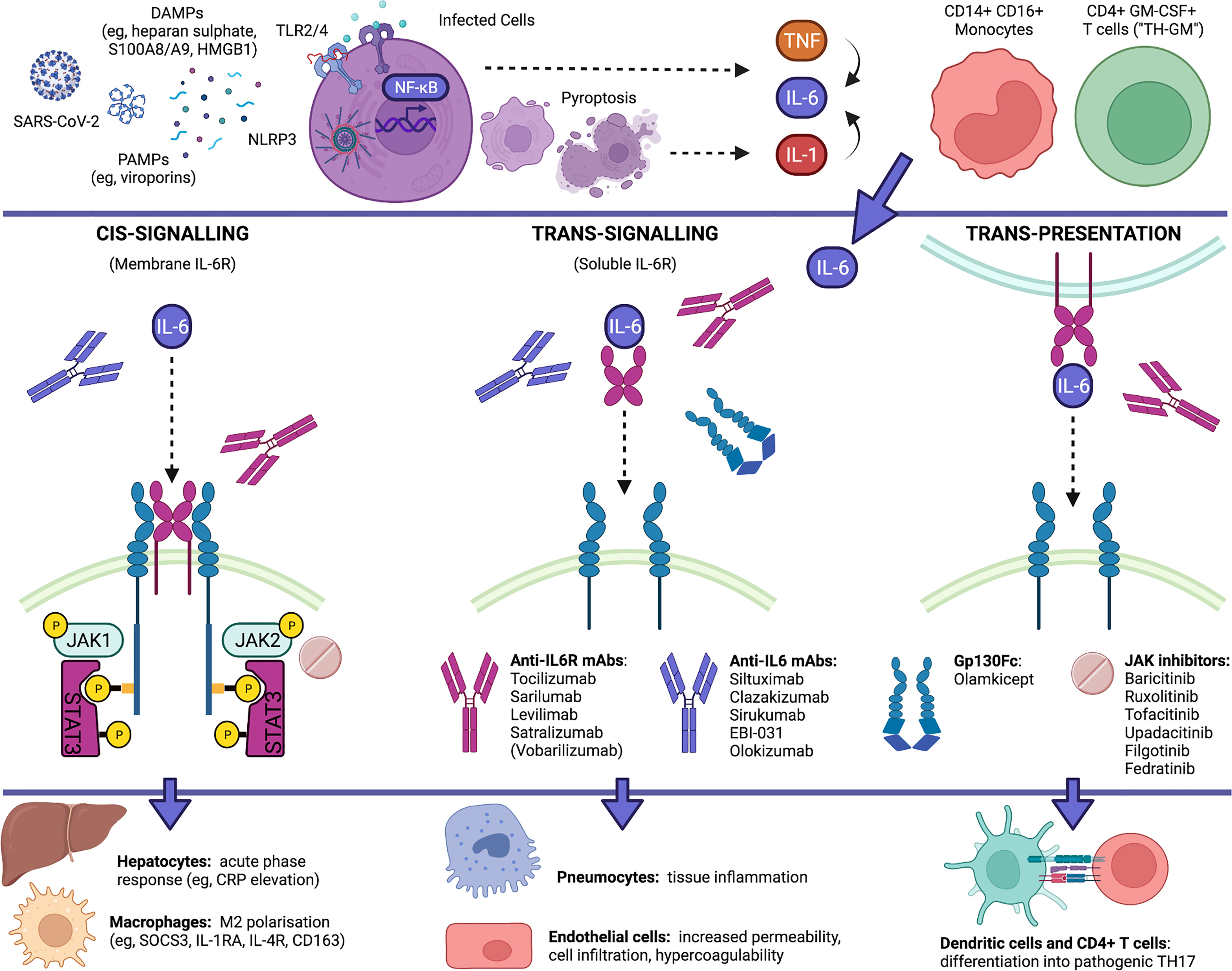
Figure 1 IL-6 pathways in COVID-19 and currently available blockers of IL-6 signalling. IL-6 is increasingly upregulated in COVID-19, being released by SARS-CoV-2 infected cells as well as by proinflammatory and infiltrating monocytes and T cells. IL-6 production is placed downstream of an autoinflammatory loop linked to pyroptosis and IL-1 production that is elicited by virus-associated PAMPs (e.g., viroporins) via the NLRP3 inflammasome and downstream of the activation of membrane TLRs by virus-induced DAMPs (e.g., heparan sulphate, alarmins S100A8/A9 and HMGB1), and occurs in parallel with the release of other cytokines (e.g., TNF, IL-10, IL-8, CCL2). IL-6 can signal through at least three distinct modalities. Anti-IL-6R antibodies, anti-IL-6 antibodies, gp130Fc and JAK inhibitors can differentially inhibit IL-6 signalling by acting at different sites. Whereas IL-6 trans-signalling and trans-presentation enhance the proinflammatory activation of pneumocytes, endothelial cells and T cells, IL-6 cis-signalling exerts homeostatic roles by eliciting the clearance of remnants through hepatic release of opsonins and the differentiation of scavenger macrophages. Created with Biorender.com.
IL-6 trans-signalling and trans-presentation are most likely pathogenic in severe progressive COVID-19: through trans-presentation, IL-6 may act, via signal transducer and activator of transcription (STAT) 3, to polarize granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing T cells (“TH-GM”), induced by IL-7, IL-23 and IL-1, into full-blown TH17 cells, thus amplifying neutrophil recruitment and activation (7); through trans-signalling, IL-6 may further contribute to upregulate chemokines (e.g., CCL2, IL-8), adhesion molecules (e.g., intercellular adhesion molecule-1, while internalizing vascular endothelial cadherin), their ligands (e.g., CD11b/CD18 integrin), and procoagulant factors (e.g., tissue factor-1, factor-III), thereby leading to increased endothelial permeability, CD14+CD16+ (intermediate/non-classical) monocyte and neutrophil migration from peripheral blood into inflamed tissues, formation of neutrophil extracellular traps (NETs), neutrophil-endothelial cell interactions, and hypercoagulability (7, 12, 17, 28–30). IL-6 trans-signalling and trans-presentation may therefore account for diffuse inflammation at various levels, including pulmonary, vascular, intestinal, and obesity-enhanced inflammation, up to the condition of shock, secondary to cytokine-mediated multi-organ dysfunction, vascular leakage and microthrombosis.
Whereas the soluble decoy receptor sgp130Fc (e.g., olamkicept) selectively blocks trans-signalling, anti-IL-6 monoclonal antibodies (e.g., siltuximab, clazakizumab, olokizumab) can block cis-signalling and trans-signalling, and anti-IL-6R monoclonal antibodies (e.g., tocilizumab, sarilumab) can block all three mechanisms, including trans-presentation (25). By acting on soluble IL-6R, monoclonal antibodies might also affect the signalling of IL-27p28/IL-30, possibly interfering with the induction of CXCL10 (31), another chemokine that is strongly upregulated in COVID-19 (7). Since tocilizumab, but not siltuximab, has been shown from meta-analyses to be effective in patients hospitalised with COVID-19 (32), blocking trans-presentation and IL-6-induced TH17 polarization would be particularly crucial to prevent severe to critical disease progression.
Conversely, IL-6 cis-signalling mainly drives hepatic synthesis and secretion of acute-phase reactants (e.g., CRP elevation in the blood), and exerts negative feedback mechanisms on proinflammatory cytokines, by suppressing their production (e.g., TNF) (33), stimulating their decoy receptors (e.g., sTNFRp55, IL-1RA) (34), or inducing negative regulators of their intracellular pathways (e.g., suppressor of cytokine signalling 3, or SOCS3, inhibiting IL-6, IL-7 and IL-23 signals; and IL-4R, inhibiting TH17 maturation) (7, 25, 26, 35). In the circulation of patients with COVID-19, IL-6 reaches its peak at advanced stages, on average after the second week of disease, and, notably, this increase is accompanied by peak concentrations of IL-10 and CRP (11, 13). This suggests that in critical COVID-19, strong elevation of IL-6 (e.g., > 100-120 pg/mL) and CRP levels (e.g., > 160-200 mg/L) could reflect augmented IL-6 cis-signalling in the attempt to exert homeostatic roles, while IL-10 further strengthens the predominance of SOCS3 over STAT3 signal (36). Therefore, inhibiting membrane IL-6R at this stage would not be useful (and might even worsen inflammation), as indicated by poor outcomes with tocilizumab in critically-ill patients. Furthermore, since IL-6 cis-signalling elicits host defence against pathogens and promotes the growth and survival of hepatocytes, megacaryocytes and intestinal epithelial cells (25, 26), membrane IL-6R blockade might promote adverse events and complications, including serious infections (e.g., ventilator-associated pneumonia) (37, 38), increased transaminases, thrombocytopenia and intestinal perforation, particularly in patients who are critically-ill with COVID-19, as they are intubated, immunosuppressed, and inflamed or hypoperfused at multiple levels.
In early stages of COVID-19, in which IL-6 may play protective roles, IL-6R blockade would not be useful either. Many viruses, in fact, including SARS-CoV-2, are able to induce SOCS3 as a virulence factor, which inhibits STAT1/IFN and gp130/JAK2/STAT3 signals to elude antiviral responses and virus clearance, thus allowing viral replication (39–41); collaterally, STAT3 down-modulation results in a compensatory induction of IL-6 mediated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (41), thereby already mimicking the effects of anti-IL-6R agents. Moreover, viral pneumonia in mild-moderate COVID-19 might be substantially mediated by cytokines and cellular pathways pathogenically upstream of IL-6, such as IL-33, IL-9 or IFNγ, which can be released by virus-damaged respiratory epithelial cells, type-2 innate lymphoid cells and γ/δ T cells (7); indeed, at this stage, IL-6 may negatively modulate T-cell release of IFNγ and granzymes (18, 42).
In fact, tocilizumab was observed to reduce IL-17 and TNF (i.e., cytokines associated with severe disease), yet it provoked a transitory increase in IFNγ (probably associated with moderate disease), and a strong increase in IL-6 and IL-10 levels (highly associated with critical disease) (43).
Taken together, benefits from therapeutic blockade of IL-6 can be obtained in COVID-19 by disrupting proinflammatory IL-6 trans-presentation and trans-signalling, which would be predominant and pathogenic in severe disease; anti-IL-6 treatment should therefore be given to patients with severe and rapidly progressive COVID-19, within the second week of symptom onset (or within the first week of hospitalisation), that is before the increased IL-6 and CRP levels further rise uncontrollably. By contrast, IL-6 inhibition no longer appears to be useful in critically-ill patients, in whom IL-6 cis-signalling predominates and would now exert anti-inflammatory and homeostatic roles. IL-6 inhibition does not seem to be useful even in early stages of COVID-19, as IL-6 signalling would be protective, indeed already blocked by the virus, and mild-moderate disease would be primarily driven by viral replication and additional upstream cytokines (Figure 2).
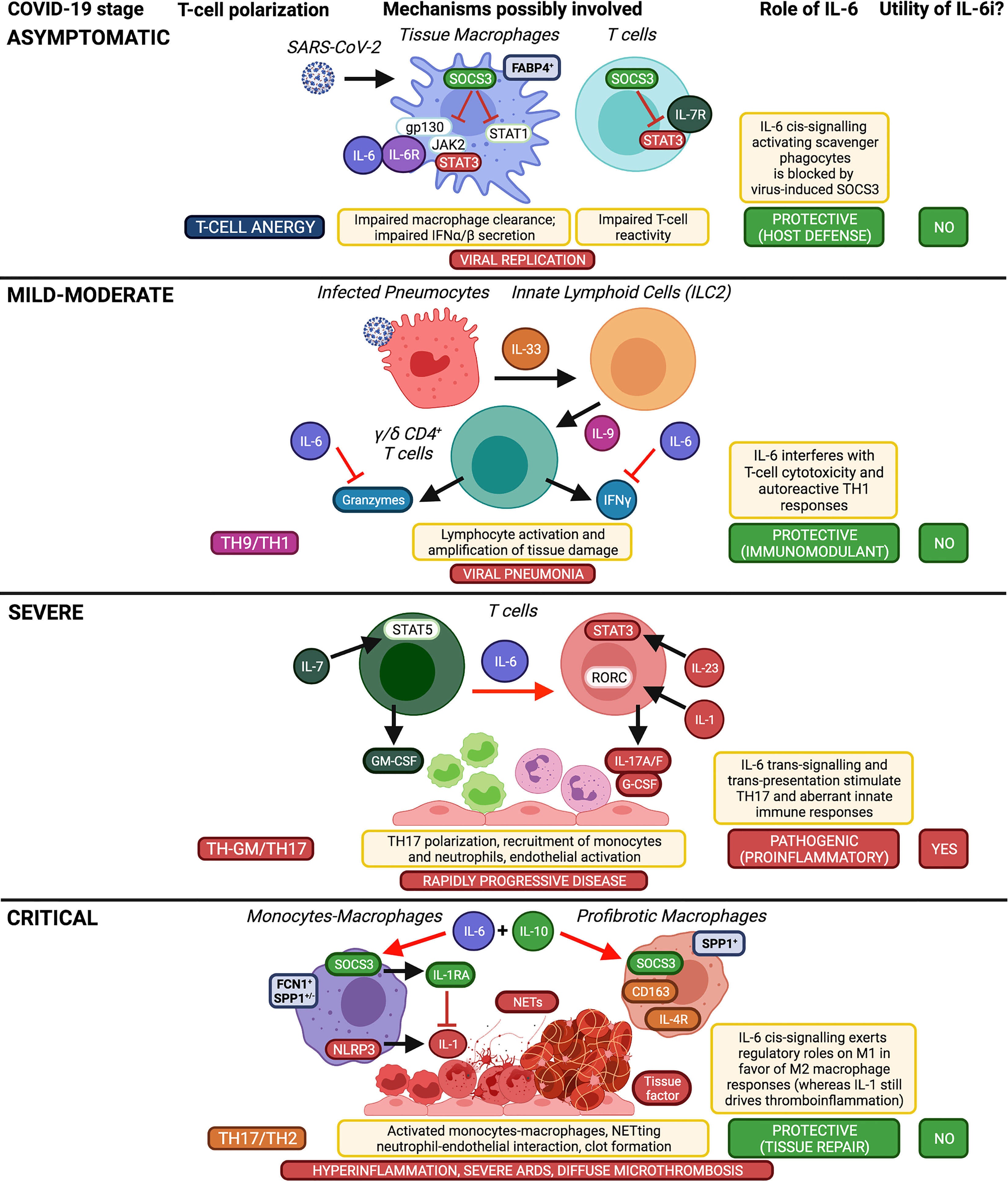
Figure 2 Implications of IL-6 in COVID-19 pathogenesis and therapeutic opportunity of using IL-6 inhibitors according to disease stage. Taking into account the well-established functions of IL-6 on immune cells (i.e. pro-M2, anti-Treg, anti-cytotoxic, anti-TH1, pro-TH17), here we schematically summarize the differential roles of IL-6 in the inflammatory cascade of COVID-19 according to disease stages and supposed T-cell polarization, which affect the usefulness or otherwise of IL-6 inhibitors (IL-6i). Created with Biorender.com.
Tocilizumab in Early Observational Studies: What Have We Learned From the Italian Experience?
Tocilizumab is an anti-IL-6R monoclonal antibody that is approved, and widely available in both intravenous and subcutaneous formulations, for certain acute and chronic inflammatory disorders that share many clinical and pathogenic aspects with severe COVID-19, such as autoinflammatory febrile diseases (e.g., Still’s disease), autoimmune diseases that are complicated by interstitial lung disease (e.g., rheumatoid arthritis), vasculitis (e.g., giant cell arteritis), and cytokine storm syndromes (e.g., MAS complicating Still’s disease, chimeric antigen receptor T-cell therapy-induced cytokine release syndrome or CAR-T cell-induced CRS). In these contexts, tocilizumab can efficiently act on fever, fatigue, pain, anxiety and depression, anemia, acute-phase response, deterioration of lung function, and vascular manifestations (44).
Italy was among the first countries after China to face the COVID-19 outbreak. It was soon realised that patients hospitalised with COVID-19 who worsened and died had developed a hyperinflammatory reaction largely responsible for severe ARDS and multi-organ failure. Tocilizumab and other IL-6 inhibitors (i.e., sarilumab, siltuximab) were then tried as compassionate off-label use in an effort to relieve harmful inflammation.
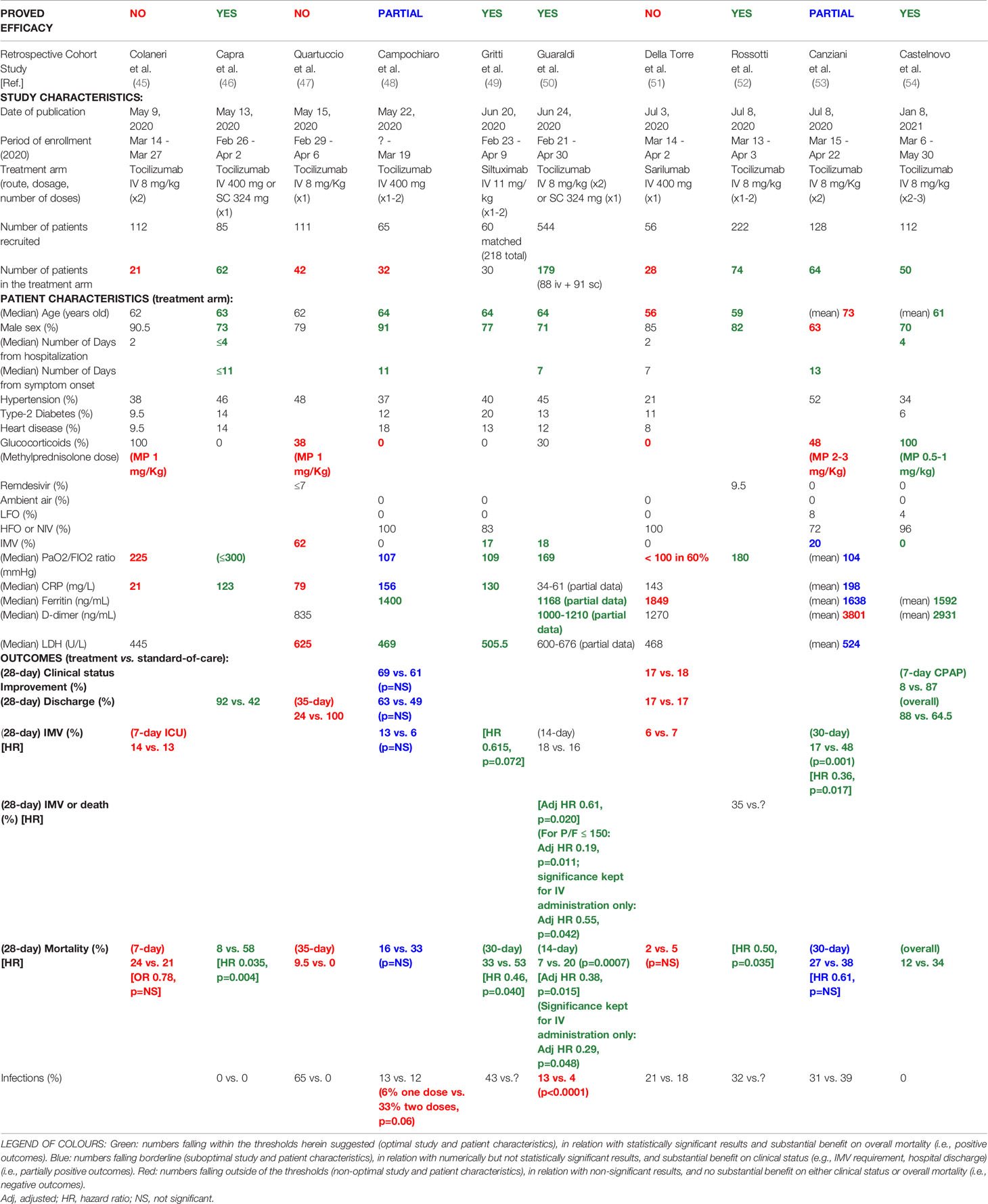
From the literature, we identified 10 retrospective cohort studies, published between May 9, 2020, and January 8, 2021, that enrolled patients hospitalised with COVID-19 during the “first wave” of the outbreak in Italy (overall between the end of February and the end of May, 2020), who underwent treatment with IL-6 inhibitors versus standard of care only (45–54). Studies were somewhat heterogenous in the number of patients recruited or included in the treatment arm, as well as in treatment protocols (including route of administration, dosage, number and timing of infusions), co-treatments, and disease severity (PaO2/FIO2 ratio and magnitude of systemic inflammation) (Table 1).
It was noteworthy that most of the studies with positive results had recruited at least 50 patients in the treatment arm (46, 50, 52, 54), whereas those showing negative or non-significant results included fewer patients (45, 47, 48, 51), thus suggesting that results might have been influenced by the small size of the cohorts. Most of the patients were men (≥ 70%), aged between 59 and 64 years old; however, some studies with negative or partial results included more women (53) and/or patients that were on average older than 70 (53) or younger than 59 (51). No obvious difference in the overall distribution of comorbidities (e.g., hypertension, diabetes, heart disease) was noticed between studies with positive and negative results.
According to the data available, patients were generally enrolled for treatment within the second week of symptom onset and within 4 days of hospitalisation. Tocilizumab was given mainly intravenously, at various doses (400-800 mg), in single or multiple infusions (1 to 3 infusions, at intervals of 12-72 hours). It was noted that two infusions could increase the rate of secondary infections and bacteremia compared to a single infusion (48, 50), while the effects of subcutaneous administration on mortality and composite outcomes (i.e., IMV + death) lost statistical significance in the subgroup analysis (50).
Concerning concomitant therapies, standard of care originally included drugs (e.g., hydroxychloroquine, azithromycin, lopinavir/ritonavir) subsequently proved ineffective against COVID-19 (55, 56), while remdesivir was not available or administered to a negligible number of patients (47, 52). Since the RECOVERY data on the efficacy of intermediate-to-low doses of dexamethasone and the benefit of combining tocilizumab with dexamethasone on overall survival were published later (57, 58), standard of care and treatment protocols did not yet include the use of glucocorticoids. In some cases, steroids were given to a minority of patients (<50%) (47, 50, 53), generally at high doses (i.e., methylprednisolone 1-2 mg/Kg per day) as supportive therapy (45, 53). In one study, in which all tocilizumab patients were co-treated with lower doses of steroids (i.e., methylprednisolone 0.5-1 mg/Kg per day, tapered and suspended within 7-10 days), tocilizumab showed good results (54).
Most importantly, patients who successfully responded to IL-6 inhibitors had severe disease, but neither critical nor moderate disease. Studies in which most of the patients required IMV showed negative results (47), whereas treatment was effective in case series without or with low numbers of intubated patients (49, 50, 54). On the other hand, due to the overwhelming of ICU during the first months of the outbreak, patients that underwent IMV were probably less than they should have been (48, 51). Parameters to be considered more reliable of disease severity could instead be the pressure of arterial oxygen to fractional inspired oxygen concentration (PaO2/FIO2) ratio and possibly some inflammatory markers. In fact, patients from studies reporting significantly reduced mortality with IL-6 inhibitors had baseline median PaO2/FIO2 ratios between 110 and 200 mmHg (i.e., severe disease) (46, 49, 50, 52); studies with median PaO2/FIO2 ratios between 100 and 110 mmHg (i.e., severe-to-critical disease) showed numerically but not statistically significant differences in mortality (48, 53) and in some cases reported a significant reduction in IMV requirement (53); whereas studies with median PaO2/FIO2 ratios < 100 (i.e., critical disease) or > 200 mmHg (i.e., moderate disease) failed to show substantial differences in mortality, IMV requirement or hospital discharge (45, 47, 51).
Looking at inflammatory markers, studies with positive results reported median CRP values around 120-130 mg/L (46, 49), whereas studies with only partial results or negative results reported values ≥ 160 mg/L (48, 53) or ≤ 80 mg/L (45, 47). Moreover, patient series with ferritin levels < 1600 ng/mL and D-dimer levels < 3000 ng/mL (50, 54) showed better outcomes compared to those with higher values (51, 53) (Table 1).
Univariate and multivariate analyses confirmed many of these observations. Compared to patients who did not respond to IL-6 inhibitors, patients who improved after treatment showed significant differences in baseline median values of: age (59-63 versus 74 years old) (48, 59), male percentage (100 versus 70%) (48), PaO2/FIO2 ratio (112-137 versus 81-86 mmHg) (48, 51), CRP (128 versus 186 mg/L) (48), IL-6 (52-58 versus 99-120 pg/ml) (51, 59), and neutrophil-to-lymphocyte ratio (3.5 versus 8) (59). Similar numerical differences in age, PaO2/FIO2 ratio, IL-6 and neutrophil-to-lymphocyte ratio were actually observed between ICU patients requiring IMV and non-ICU patients requiring NIV/HFO (47, 59), further indicating that IL-6 blockade responders belong to this second category of patients.
At multivariate regression analysis, independent predictors of clinical improvement and survival upon treatment were: age ≤ 70-75 years old (48, 59, 60), PaO2/FIO2 ratio ≥ 100 (48, 51, 60), IL-6 values < 91 pg/mL (59), administration of tocilizumab within 6 days (61), and no need of IMV (60). Conversely, independent predictors of ICU admission and mortality were: comorbidities (in particular kidney impairment) (53), IMV at baseline (9, 53), and D-dimer levels > 3500 ng/ml (61). Both tocilizumab and glucocorticoid use were predictors of reduced mortality at 14 days, in the case of tocilizumab specifically in patients not requiring IMV (62).
After treatment, good responders showed control values of ferritin returning < 1400 ng/ml, D-dimer < 3000 ng/ml and lactate dehydrogenase (LDH) < 400 U/L, while non-responders had ferritin > 1800 ng/ml, D-dimer > 5000 ng/ml and LDH > 500 U/L (63). Lower levels of IL-6 24 hours after the infusion were found predictive of clinical improvement at day 7 (62). Irrespective of the main outcome, IL-6 inhibitors led to a fast decrease and normalisation of CRP levels, and a rapid resolution of fever and fatigue (45, 50, 51, 54, 63).
More Conclusive Evidence: Results From Ramdomised Controlled Trials
From the early stages of the pandemic, IL-6 has been a well-documented biomarker of disease severity and poor prognosis, and considered among the most promising targets in the treatment of the COVID-19 cytokine storm (19). About half of the clinical trials being conducted worldwide testing monoclonal antibodies for the management of severe COVID-19 have been performed with IL-6 inhibitors, and in particular with tocilizumab (64).
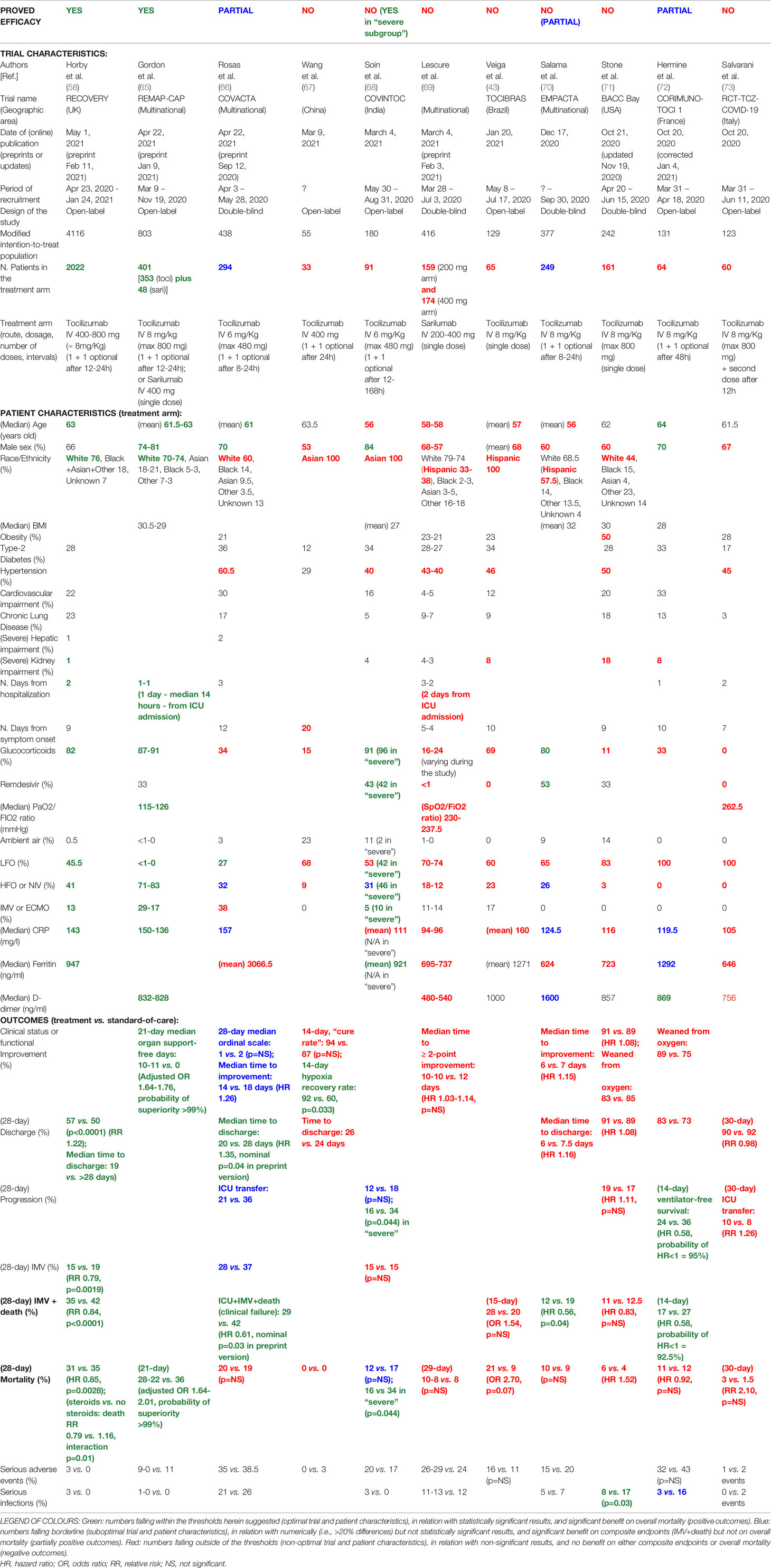
As of May 1, 2021, 11 RCTs with tocilizumab (10 RCTs) and/or sarilumab (2 RCTs) were published in peer-reviewed journals (43, 58, 65–73). Many of the observations made in the above reported Italian observational studies were confirmed in the RCTs. Studies were heterogenous in sample size, patient composition (e.g., age, sex, ethnicity), treatment protocol (e.g., dosage, number and timing of infusions), concomitant therapies (e.g., glucocorticoids, antivirals), and disease severity (e.g., respiratory status, oxygen requirement, inflammatory markers) (Table 2).
Many studies with negative results recruited fewer than 100 patients into the treatment arm (43, 67, 68, 73), while the best outcomes were reported in the two largest trials (i.e., RECOVERY and REMAP-CAP) (58, 65), again suggesting that some inconclusive results may have suffered from low statistical power. Studies with worse results also included patients younger (< 59 years old) (43, 68–70) than other studies with better results (61-64 years old) (58, 65, 66, 72). Excluding RECOVERY, studies with overall negative outcomes included fewer men (< 70%) (43, 67, 69–71, 73) compared to other studies with positive or partially positive outcomes (≥ 70%) (65, 66, 72). Whereas successful trials included high numbers of Caucasian White patients (≥ 70%) (58, 65), worse results were obtained in trials with fewer White patients and relatively more Hispanic White and/or Black Afroamerican patients (43, 69–71), or with Asian patients (67, 68). Studies with poor outcomes also included numerous patients with comorbidities (e.g., obesity, hypertension, kidney impairment) (43, 68, 69, 71).
Treatment was given intravenously, at various doses (tocilizumab 400-800 mg or 6-8 mg/Kg, in 1-2 infusions, at variable intervals ranging from 8 hours to 7 days; or sarilumab 200-400 mg in a single infusion), and was mostly started within the first 3 days of hospitalisation and/or within 2 weeks from symptom onset. Importantly, in severe-to-critical patients, treatment was successful if given within 24 hours from ICU admission (65), but not later (69).
In successful trials, standard of care included glucocorticoids, which were then administered to the great majority of the patients (> 80%) (58, 65), whereas substantially fewer patients were co-treated with steroids in studies with negative results (43, 67, 69, 71, 73). Antivirals, and in particular remdesivir, were given in variable proportion, without obvious implications in the overall outcome.
Most remarkably, best responses on disease progression and mortality were obtained in trials enrolling a substantial proportion of patients requiring HFO or NIV at baseline (> 40%, ie. severe disease), a limited amount of patients requiring LFO (< 50%, ie. moderate disease), and only a minority of patients requiring IMV (< 30%, ie. critical disease) (58, 65). Even in COVINTOC trial (68), an Indian RCT reporting overall negative or partial results, positive outcomes were observed when analysing specifically the “severe subgroup”, in which these proportions were respected. As observed for earlier Italian studies (although the data here are largely lacking), a baseline median PaO2/FIO2 (or SaO2/FIO2) ratio > 110 mmHg was reported in a successful trial (65), whereas values > 200 mmHg were reported in negative trials (69, 73).
Concerning acute-phase reactants, in line with previous Italian cohort studies, trials with positive outcomes reported baseline median CRP values overall ranging between 120 and 155 mg/L. In particular: good efficacy (i.e., significantly reduced progression and mortality) was reported in trials with CRP values between 130 and 150 mg/L (58, 65); partial efficacy (i.e., significantly reduced progression including ICU transfer and IMV rate, but not overall mortality) was observed in trials with CRP values between 120 and 125 mg/L (70, 72) or around 155 mg/L (66); and no efficacy was obtained in trials with CRP values ≤ 115 mg/L (69, 71, 73) or ≥ 160 mg/L (43). Furthermore, whereas the two major successful trials largely included patients with only modestly increased levels of ferritin and D-dimer (58, 65), trials that reported poor outcomes mostly included patients with lower (69, 71, 73) or higher (43) values of ferritin (< 800 or > 1200-1400 ng/ml) and D-dimer (< 700 or > 1000-1500 ng/ml), and trials that reported partial efficacy showed dichotomous trends (70, 72).
No significant increase in serious adverse events associated with IL-6 inhibitors was noted. Indeed, in some trials, serious infections were numerically lower in the treatment arm (66, 71, 72), and significantly lower in one study (71) (Table 2). Results from the MARIPOSA study suggest a more favourable profile for tocilizumab at a dose of 8 mg/Kg versus 4 mg/Kg in terms of lower mortality and fewer serious adverse events (74).
Confirmations From Meta-Analyses and Current Guidelines
Early meta-analyses of the first published RCTs did not show significant differences in mortality with anti-IL-6R agents, although they showed significantly lower rates of ICU transfer, IMV, and composite outcome of IMV or death, along with a lower rate of serious infections (22, 75, 76). This could have been due to the small size and composite primary endpoints of early trials, probably underpowered to detect differences in mortality, and to the high heterogeneity among the studies. However, subsequent meta-analyses of more recently completed RCTs including RECOVERY, or even unpublished data through searches of electronic databases and contact with experts, ultimately concluded that, in patients hospitalised with COVID-19, treatment with IL-6 antagonists, and in particular tocilizumab (with much higher certainty than sarilumab, but not siltuximab), results into a significant reduction in 28-day all-cause mortality (2, 32, 58), in line with previous meta-analyses of observational cohort studies (22, 77, 78). Specifically, high probability of reduced risk of mortality and clinically meaningful mortality benefit was observed in patients receiving concomitant glucocorticoids and non-invasive ventilation or high-flow oxygen, with no need of IMV or cardiovascular support (32, 79). A significant interaction was seen in the subgroup analysis in regard to concomitant steroid therapy (2), while other meta-analyses confirmed the independent strong benefit of steroid therapy, particularly low-dose dexametasone (not high-dose methylprednisolone or hydrocortisone), on survival of severe and critical patients (80). Lower odds of mortality with tocilizumab were observed in patients with CRP values between 75 and 150 mg/L compared to patients with lower or higher levels; yet, a statistical significance was not reached (32).
According to the NIH COVID-19 treatment guidelines (81), largely based on the findings and recruiting criteria of RECOVERY and REMAP-CAP trials, tocilizumab (at a single intravenous dose of 8 mg/Kg actual body weight up to 800 mg) or sarilumab (in case tocilizumab is not available, at a single intravenous dose of 400 mg) are recommended, in combination with dexamethasone (6 mg per day, intravenously, for up to 10 days), for recently hospitalised patients (within 3 days from hospital admission and/or within 24 hours from ICU admission), with a rapid respiratory decompensation requiring increasing amounts of oxygen (in particular, HFO or NIV, or soon after starting IMV), and substantially increased markers of inflammation (e.g., CRP ≥ 75 mg/L).
Therapeutic Windows: To Cure the Right Patient at the Right Time We Need Clear Cut-Offs
A correct stratification of the patients is crucial for achieving satisfactory results with tocilizumab in the treatment of COVID-19, in clinical trials as in clinical practise. Although only large individual patient data meta-analyses would best indicate which patients are more likely to benefit from IL-6R blockade, from this review of the data we aimed to identify, for some important respiratory and inflammatory parameters, the minimum and maximum thresholds between which to build up a therapeutic window.
Relatively simple numerical cut-offs would actually reflect the complexity and heterogeneity of disease biology, helping to recognize the stage and progression of the disease, the prevailing pathogenic aspects, the predominant inflammatory patterns, and the actual significance of IL-6 in a given patient.
IL-6 levels are significantly correlated with worsening oxygen exchange in the lungs of COVID-19 patients and are major predictors of disease progression and mortality (23). Plasma levels of IL-6 < 7-10 pg/ml are found in healthy subjects (and presumably asymptomatic carriers); < 20 pg/ml (median 17 pg/ml) in low-risk patients (i.e., mild-moderate disease); > 35 pg/ml (around 2/3-fold higher median values) in patients at risk of progression (i.e., moderate-to-severe disease); > 55 pg/ml in patients with a complicated course (i.e., severe disease); and > 80 pg/ml (median values 86-91 pg/ml) in patients developing severe respiratory failure requiring ventilatory support and at high risk of in-hospital death (i.e., severe-to-critical and critical disease) (82–87). These thresholds are in line with results of univariate and multivariate analysis of the Italian observational studies exposed above (51, 59). Interestingly, daily monitoring of IL-6 levels showed that, in severe progressive forms, IL-6 increases up to 85-90 pg/ml at 6-7 days of hospitalisation, and reaching these values precedes a further increase to peak concentrations above 120-300 pg/ml on days 9-10 (88). Acting within 6 days of hospitalisation in severe patients, identified by means of baseline IL-6 values between 35 and 90 pg/ml, may therefore represent the right time to interrupt the inflammatory cascade and prevent lethal cytokine storm.
A good response to IL-6R blockade is expected when IL-6 elevation is accompanied at baseline by an increase in CRP values. Higher levels of both IL-6 and CRP are, in fact, associated with disease severity in COVID-19 (89). Indeed, cytokine blockade was not effective in COVID-19-unrelated ARDS (showing higher IL-6 levels but much lower CRP values as compared to severe COVID-19), while it is effective in CAR T-cell induced CRS (showing high concentrations of both IL-6 and CRP) (90). On the other hand, tocilizumab does not reduce serum IL-6 concentrations, which are instead variably increased upon treatment, but it does efficiently reduce CRP levels, which reflect IL-6 bioactivity. CRP levels < 80-100 mg/L (median 77 mg/L) are found in patients at low risk of progression (i.e., mild-moderate disease), whereas CRP levels > 180-200 mg/L (median 194 mg/L) are detected in patients with high rates of mortality (i.e., critical disease) (86, 87). From the observational studies and RCTs reported above, it emerges that patients with CRP values between 120 and 160 mg/L are actually those with a severe progressive disease who are most likely to respond successfully to IL-6 inhibitors.
In regard to serum ferritin levels, observations from the aforementioned retrospective cohort studies and RCTs seem to confirm other reports (91, 92), according to which low-risk patients can be identified for values < 400-800 ng/ml (i.e., mild-moderate disease); patients at high risk of progression for values between 800 and 1400-1600 ng/ml (i.e., severe progressive disease); and patients at high risk of death for values > 1600-2000 ng/ml (i.e., critical disease). Severe hyperferritinemia (> 1600-2000 ng/ml) is a criterion for MAS (93, 94), and probably indicates an overt state of MAS-like hyperinflammation in COVID-19 that specifically occurs in critically-ill patients (19). Due to the postulated regulatory role of IL-6 at this stage, tocilizumab would be no longer effective in these cases; conversely, activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome, which is typical of hyperferritinemic syndromes, would be crucial also in critical COVID-19 (7), and therapies targeting IL-1, such as anakinra, could still be effective (95). In addition to being associated with intense macrophage activation and release of IL-1, high ferritin levels are also associated with severe hepatocellular damage and LDH elevation, as well as with development of diffuse microthrombosis and coagulopathy with severely increased D-dimer concentrations (19). In fact, high D-dimer levels were found to predict mortality and poor response to tocilizumab in COVID-19 patients (61, 96, 97); indeed, treatment with tocilizumab could even lead to a further increase in D-dimer, despite the decrease in fibrinogen (52, 63). Altogether, COVID-19 patients characterised by serum ferritin levels > 1600-2000 ng/mL, LDH > 500-550 U/L, and D-dimer > 3000-5000 ng/mL, are most likely hyperinflamed and critically-ill patients who do not respond to IL-6R blockade (but may still respond to IL-1 blockade). It remains to be assessed whether, in addition to these absolute thresholds, the ratio of CRP to ferritin (or D-dimer) may inform the advisability of using tocilizumab or anakinra (or full-dose anticoagulants). Most recently, the soluble urokinase plasminogen activator receptor (suPAR) has been proposed as an alternative and more sensitive biomarker for early identification of those COVID-19 patients at risk of progression who release high amounts of alarmins S100A8/A9 and IL-1α and particularly respond to anakinra, thus allowing the extension of the use of anakinra even to more moderate forms of disease (98).
Concerning respiratory parameters and oxygen requirements, as exposed in the previous paragraphs, RCTs and meta-analyses have concluded that the best responders to IL-6 inhibitors, particularly tocilizumab, are hypoxic and severely progressing patients requiring high-flow oxygen or non-invasive ventilation, while from retrospective cohort studies it can be suggested that this subgroup basically corresponds to patients with values of PaO2/FIO2 ratio between 100 and 200 mmHg. In this subset of patients, median values of CRP, ferritin, D-dimer, LDH and neutrophil-to-lymphocyte ratio in blood actually fall within the optimal ranges proposed here (47, 59, 95). By contrast, critically-ill patients (showing values of PaO2/FIO2 ratio <100 mmHg and requiring IMV) and mild-moderate patients (showing values of PaO2/FIO2 ratio > 200-300 mmHg and requiring low-flow or no oxygen) generally show poor responses to IL-6 inhibitors. Nevertheless, results from a large RCT evaluating sarilumab recently posted as a preprint (99) seem to suggest that IL-6 inhibitors can reduce the risk of death by up to half even in ICU patients undergoing IMV, in case these are co-treated with steroids and still show relatively low median values of IL-6 and CRP, substantially falling within our proposed response ranges.
A schematic summary of the suggested items and cut-offs to consider in COVID-19 to discern good and bad responders to tocilizumab (and other agents) is provided in Figure 3.
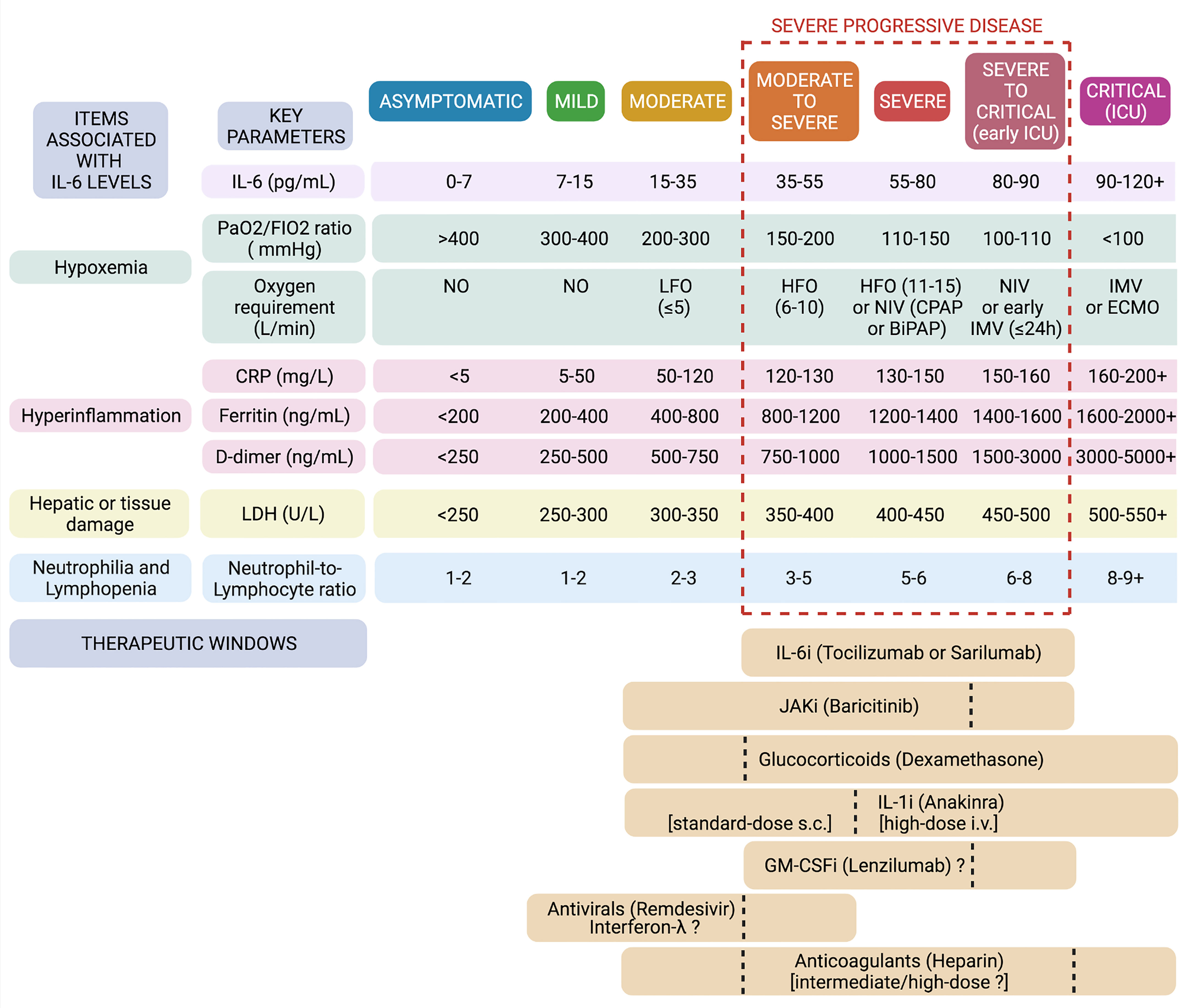
Figure 3 Defining therapeutic windows for IL-6 inhibitors and other biologics in COVID-19. In the light of baseline characteristics of previous successful and unsuccessful trials, univariate and multivariate analyses, meta-analyses, and studies on the predictivity of IL-6 and other biomarkers, we set thresholds and ranges for some major respiratory and laboratory parameters in the attempt to frame specific windows of opportunity for tocilizumab and other agents in the immunotherapy of COVID-19. Created with Biorender.com.
Going Beyond IL-6
In managing COVID-19, growing interest is turning to Janus kinase (JAK) inhibitors. Among these, baricitinib was originally identified using artificial intelligence as a suitable candidate for COVID-19 treatment, due to its ability to bind with high affinity to some key regulators of clathrin-mediated endocytosis, namely AP2-associated protein kinase-1 (AAK1) and possibly cyclic G-associated kinase (GAK), thus inhibiting the entry of SARS-CoV-2 into cells and the intracellular assembly of virus particles (100). Such antiviral effects were more recently demonstrated in vitro using 3D cultures of primary human liver cells, in which baricitinib was able to reduce SARS-CoV-2 infectivity and viral load by directly inhibiting numb-associated kinases and impeding IFNα2-mediated induction of the SARS-CoV-2 receptor angiotensin-converting enzyme 2 (ACE2) (101). Plasma concentrations of baricitinib at therapeutic doses would be sufficient to exert antiviral effects in vivo (100).
Moreover, and perhaps most importantly, JAK inhibitors exert multidirectional anti-inflammatory effects by simultaneously inhibiting intracellular signals induced by several cytokines. Acting on JAK1 would broadly interfere with the signalling downstream of cytokine receptors belonging to the gp130 family (e.g., IL-6, IL-27), IFN family (e.g., IFNγ), γ-chain family (e.g., IL-4, IL-9, IL-7) and others (e.g., thymic stromal lymphopoietin, IL-13, G-CSF); acting on JAK2 would specifically interfere with GM-CSF, IL-23, IL-12 and IL-6 signals; while acting on JAK3 would more potently interfere with the γ-chain receptor family (102).
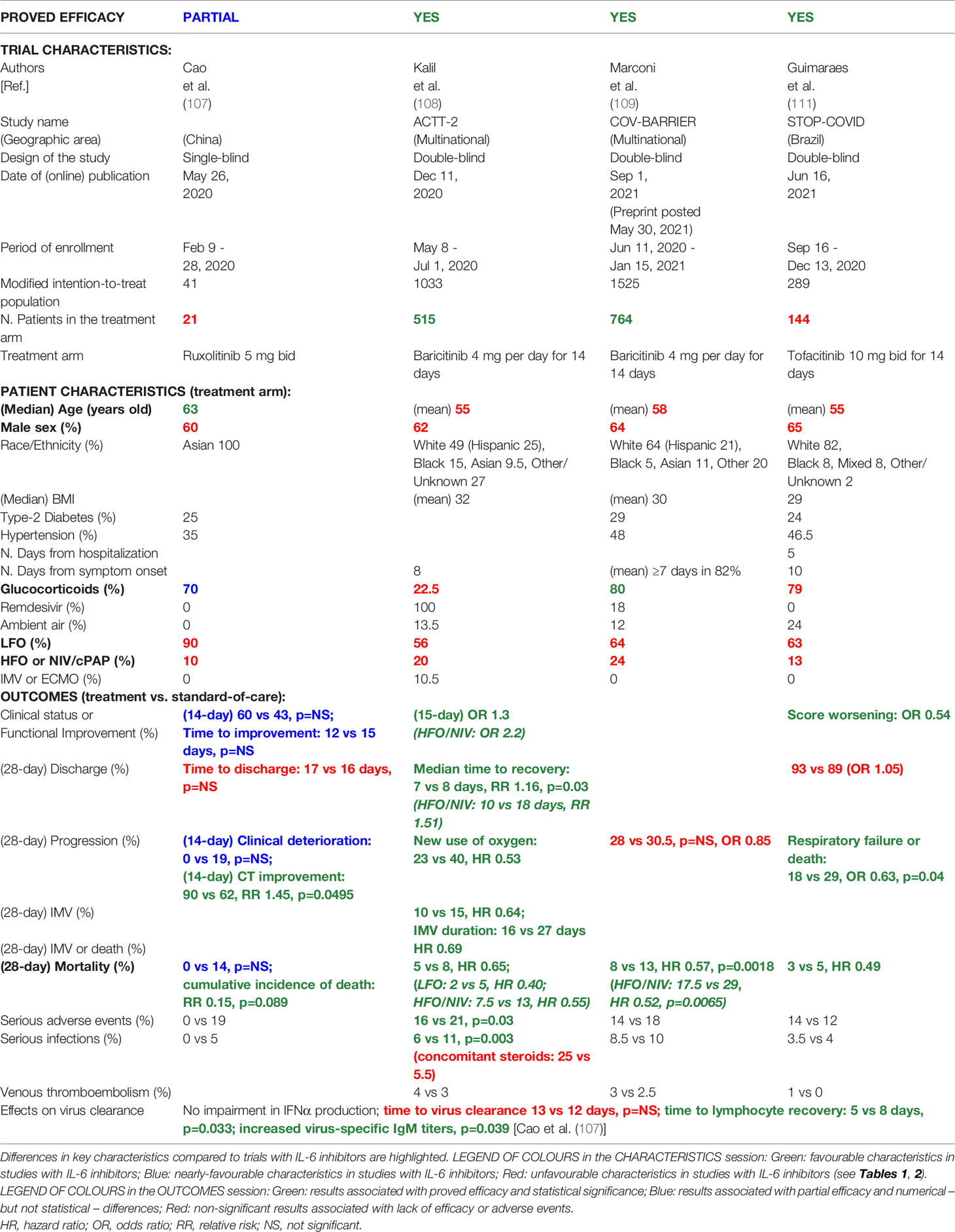
Different JAK inhibitors have been proposed for the treatment of COVID-19, namely: JAK1/JAK2/TYK2 inhibitors (e.g., baricitinib, ruxolitinib) (103–109), selective JAK2 inhibitors (e.g., fedratinib) (110), and - more recently - JAK1/JAK3 inhibitors (e.g., tofacitinib) (111). Probably due to their ability to block various inflammatory pathways including not only IL-6/STAT3 and TH17 (advanced stages), but also TH9 and TH1/TH-GM pathways (upstream stages) (Figure 2) (7, 104–106), in addition to their presumed direct effects on viral infection and replication (early stages) (100, 101), JAK inhibitors appeared from both observational studies (103, 104, 106) and RCTs (107–109, 111) to be effective not only in severe forms of COVID-19, but also in patients with moderate disease (Figure 3), the latters representing the vast majority of patients in these studies (Table 3). Furthermore, baricitinib may work even in the absence of concomitant steroids, and in series involving more women and younger patients (103, 104, 108, 109). Nevertheless, as with tocilizumab, the most significant results with baricitinib (on time-to-recovery or all-cause mortality, according to ACTT-2 and COV-BARRIER trials, respectively) were obtained in patients with severe disease requiring at baseline HFO or NIV (81, 108, 109) (Table 3).
IL-1 inhibitors, in particular the recombinant IL-1α/β receptor antagonist anakinra, have been shown in observational studies to reduce mortality in both severe and critical disease (112, 113) (Figure 3). Whereas both IL-6 and IL-1 inhibitors would have a significant impact on mortality in patients showing a PaO2/FIO2 ratio ≥ 100 mmHg, only IL-1 inhibitors would remain effective in patients with a PaO2/FIO2 ratio < 100 mmHg (95). In fact, as previously mentioned, critical COVID-19 assumes the connotations of a hyperferritinemic syndrome or an autoinflammatory febrile disease, in which an aberrant activation of the NLRP3 inflammasome, in this case resulting from the conspicuous presence of monocytes-macrophages and viral products (e.g., viroporins), provokes intense production of IL-1α and IL-1β, which ultimately drive hyperinflammation and severe ARDS (7, 27), whereas IL-6 would now play regulatory roles. Two phase-3 RCTs evaluating the efficacy of IL-1 inhibitors in patients hospitalised with COVID-19 have recently been published, confirming significant beneficial effects on clinical status and survival for anakinra (98), but not for the anti-IL-1β monoclonal antibody canakinumab (114), thereby suggesting a central contribution of the alarmin IL-1α to acute lung inflammation and COVID-19 pathogenesis (7).
Limited data are available from RCTs in regard to therapies targeting other cytokines involved in severe COVID-19. Among GM-CSF inhibitors, otilimab (115) conferred a benefit on survival and respiratory status in a subgroup of patients aged ≥ 70 years old; lenzilumab (116) improved ventilator-free survival in hypoxic patients not receiving IMV; while for mavrilimumab (117) results were inconclusive, reporting numerical but not statistical differences on mortality, possibly due to the small size and the low power of the study. It also remains to adequately investigate the effects of inhibiting IL-17, by means of anti-IL-17A/F therapies (e.g., bimekizumab), in severe forms, or the alarmin cytokine IL-33, by means of anti-ST2 agents (e.g., astegolimab), at least in early and moderate stages, or in post-COVID fibrosis (7).
Regarding glucocorticoids, treatment with low-to-intermediate doses of steroids, particularly dexamethasone, has been shown to be associated with reduced mortality in hypoxic patients who require either IMV or supplemental oxygen, but not in those who do not require oxygen support (57, 80). Although the main RCT in this regard (57) did not distinguish between high-flow and low-flow oxygen requirements, other studies have reported non-significant effects of steroids in non-severe patients requiring only low-flow oxygen (118, 119) (Figure 3). In case of further disease progression despite glucocorticoid treatment, the addition of tocilizumab led to significantly better outcomes (120, 121), consistent with the synergistic effects of tocilizumab and dexametasone observed in the RCTs and meta-analyses discussed above (2, 58), and possibly explained by the synergism of IL-6R signal blockade combined with steroid-induced down-regulation of IL-6 (or other cytokines).
Since patients with COVID-19 at high risk of progression are characterised by early interferonopathy and viral replication (122), recombinant type-I (IFNβ) and especially type-III (IFNλ) interferons, as well as endosomal TLR agonists inducing IFNs, have been proposed in patients with mild-moderate disease and high viral load (7, 123–125), similarly to what was suggested in regard to antivirals (remdesivir) (126). Nevertheless, it has been argued that antivirals might help even in more advanced stages of the disease in combination with anti-inflammatory drugs (81, 126) (Figure 3).
COVID-19 is also characterised by acquired thrombophilia, and hypercoagulability might concur with hyperinflammation as a cause of death. Increased rates of venous thromboembolism have been observed in COVID-19 patients and autopsy studies demonstrated microvascular thrombosis in about 80% of cases (127). Furthermore, heparan sulfate may mediate virus interaction with host membrane receptors, thus promoting virus entry and inflammatory cell activation (7, 128). Heparin has been included in the treatment of COVID-19 with the double rationale of impeding clot formation and competing with membrane heparan sulfate. On the other side, hospitalisation could contribute to the observed risk of venous thromboembolism and the actual impact of pulmonary embolism and/or microvascular thrombosis on severe respiratory failure and mortality in COVID-19 is still controversial (127). Intensified anticoagulant strategies can halve the risk of venous thromboembolism but can also double the risk of bleeding (76), especially in critically-ill patients, possibly due to tissue hypoperfusion, concomitant supportive therapy with high-dose hydrocortisone, liver failure and coagulopathy. The question of whether to prefer anticoagulant therapy or prophylaxis is therefore the subject of intense debate. Plasma concentrations of D-dimer might be used to guide anticoagulation dose in hospitalised patients (97). Presumably, D-dimer levels < 750-1000 ng/mL (moderate disease) may suggest a low-dose prophylaxis; levels between 1000 and 3000 ng/mL (severe disease) may suggest intermediate- or high-dose therapeutic regimens; while levels > 3000-5000 ng/mL (critical disease) may warn of the risk of bleeding with intensified strategies (Figure 3). In fact, a recent meta-analysis seems to show that a full-dose anticoagulation may reduce mortality in severe, yet non-critical, patients (76).
Further data from ongoing or recently completed clinical trials are eagerly awaited (Table 4). In this regard, it will be particularly interesting to assess the effect of combining IL-6 inhibitors with antivirals (e.g., tocilizumab + remdesivir, tocilizumab + favipiravir), anticoagulants (e.g., tocilizumab + full-dose heparin), IL-1 inhibitors (e.g., tocilizumab + anakinra, tocilizumab + colchicine), or other immunotherapies (e.g., tocilizumab + pembrolizumab). Ongoing head-to-head comparative studies (e.g., tocilizumab ± remdesivir versus baricitinib ± remdesivir) are of great interest as well.
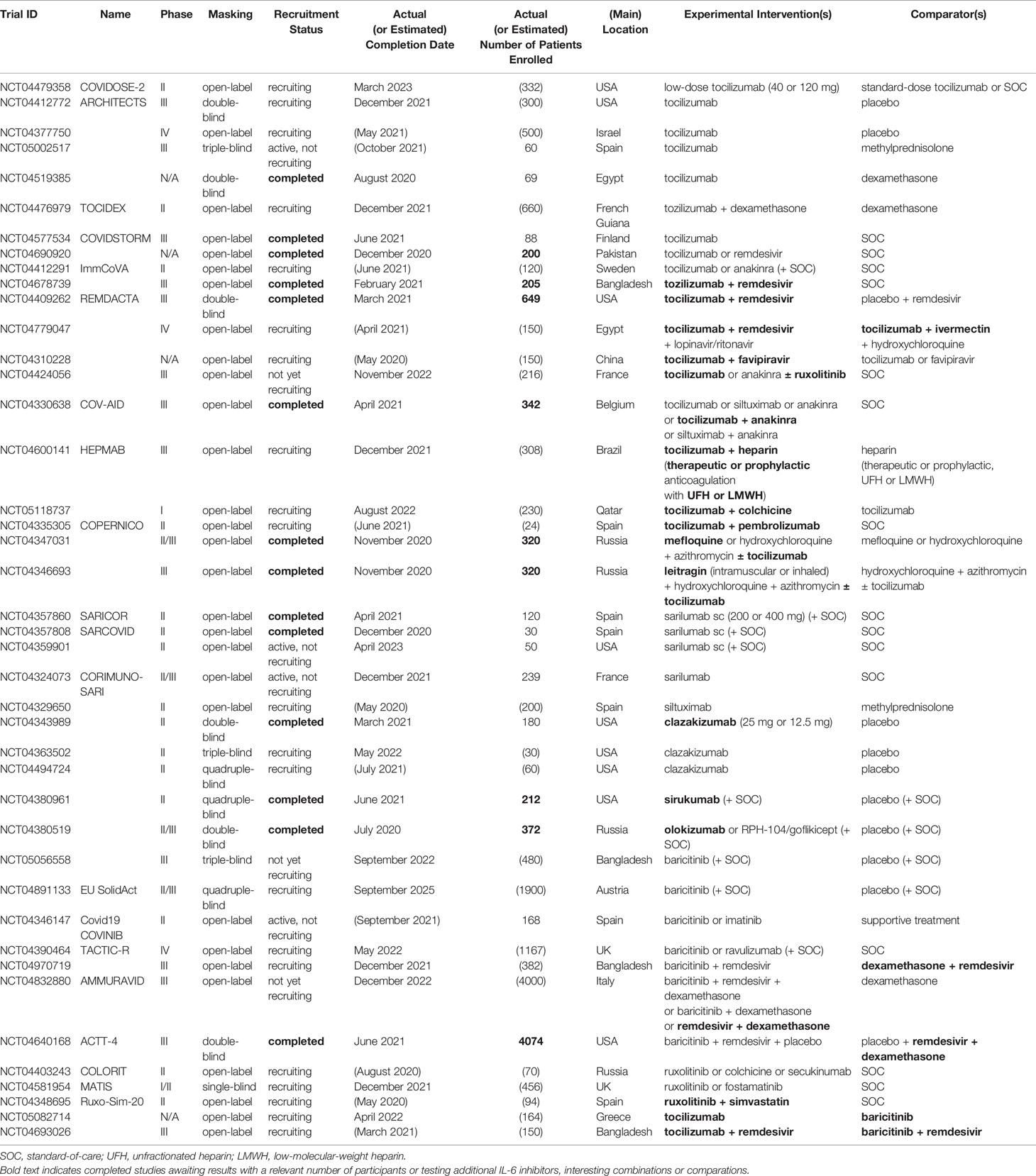
Table 4 Randomized controlled clinical trials with agents targeting the IL-6 signalling in COVID-19 that are ongoing or awaiting results (as reported in Clinicaltrials.gov; last accessed December 6, 2021).
Discussion and Conclusions
Severe COVID-19 is a hyperinflammatory and life-threatening pulmonary and systemic disease, so prompt intervention on the cytokine cascade can importantly prevent clinical deterioration and mortality.
The beneficial effects of tocilizumab and other biologics in the management of COVID-19 have long been debated owing to large discrepancies in study results, possibly due to differences in sample size (i.e., studies often underpowered to detect significant differences in mortality), patient series composition (i.e., disease severity, magnitude of systemic inflammation, age, comorbidities, and perhaps sex and ethnicity), and treatment protocols (i.e., dosage, timing and co-treatments, particularly with steroids).
Here, we attempted to dissect the heterogeneity of these studies as well as the complexity of the inflammatory cascade in COVID-19, the differential roles of IL-6 in relation to disease stage and severity, and the biological significance of clinical parameters and laboratory markers, in order to identify and summarize the baseline characteristics of patients who should best respond to treatment with IL-6 inhibitors or other biologics, thus ultimately defining precise therapeutic windows.
Therapeutic approaches for COVID-19 based on disease stage have been suggested by subgroup analyses of clinical trials and in part summarized in previous editorials (129). Our review extends therapeutic options to anti-cytokine monoclonal antibodies and decoy receptors, such as IL-6, GM-CSF, and IL-1 inhibitors, in addition to JAK inhibitors, antivirals and glucocorticoids, with the ultimate aim of highlighting their differential windows of opportunity. Importantly, our estimation of disease severity is based not only on respiratory status, here assessed by means of the PaO2/FIO2 ratio and oxygen requirement, but also on key circulating biomarkers, namely IL-6, CRP, ferritin, D-dimer and LDH levels. Furthermore, here we have distinguished a “severe-to-critical” stage corresponding to early ICU patients, including those undergoing IMV within the past 24 hours (i.e., ordinal score between 6 and 7 on the NIAID 8-point ordinal scale for assessing clinical status), for whom – so far - there is evidence of benefit for IL-6 inhibitors, but not for JAK inhibitors.
In summary, due to the pathogenic role of IL-6 signal (trans-signalling and trans-presentation) specifically in the severe stage of the disease, IL-6 inhibitors, and particularly anti-IL-6R monoclonal antibodies (e.g., tocilizumab, sarilumab), appear to be effective in patients with severe COVID-19, mostly characterised by baseline IL-6 levels between 35 and 90 ng/mL (typically reached within 6 days of hospitalisation), PaO2/FIO2 ratios between 100 and 200 mmHg, requirement of HFO or NIV, CRP levels between 120 and 160 mg/L, ferritin levels between 800 and 1600 ng/mL, D-dimer levels between 750 and 3000 ng/mL, and LDH levels between 350 and 500 U/L. Patients aged between 59 and 64 years old, males, and non-Hispanic White, might respond better than others to IL-6 inhibitors.
Because GM-CSF also plays an important role in severe disease, GM-CSF inhibitors (e.g., lenzilumab, otilimab, mavrilimumab) may have a therapeutic window similar to that of IL-6 inhibitors, perhaps with a difference in age preference (i.e., better outcomes obtained for patients aged ≤ 70 years old with tocilizumab, ≥ 70 years old with otilimab).
Since JAK1 and JAK2 transmit both IL-6 and GM-CSF signals, as well as the signal of various cytokines placed aside or upstream in the inflammatory cascade (e.g., IL-9, IFNγ, IL-7, IL-23, G-CSF), JAK inhibitors, and particularly JAK1/JAK2 inhibitors (e.g., baricitinib, which might also directly interfere with viral replication), appear to be effective not only in severe forms but also in moderate COVID-19 (thus including younger patients and more women), and even in the absence of concomitant steroids.
Whereas IL-6 acquires homeostatic roles in critical stages (cis-signalling), IL-1α and IL-1β are instead proinflammatory and pathogenic in both severe and critical disease, being increasingly released with severe hyperferritinemia and hyperactivation of monocytes-macrophages; IL-1 inhibitors, in fact, and particularly the decoy IL-1 receptor binding to both IL-1 isoforms (e.g., anakinra), appear to be effective not only in moderate-to-severe forms but also in critical COVID-19.
It is hoped that this narrative overview of the current literature may offer useful insights into the proper use of biologics in COVID-19, in future trials as in the real world.
Author Contributions
GZ conceived the study, searched the literature, wrote the manuscript, and prepared the figures and tables. AT, LC, and AL contributed to search the literature and reviewed the manuscript. NM, PF, IS, and AM critically edited the manuscript. All authors have read and approved the final version submitted.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACE2, angiotensin-converting enzyme 2; ARDS, acute respiratory distress syndrome; CAR-T, chimeric antigen receptor T-cell therapy; CCL, C-C motif chemokine ligand; CD, cluster of differentiation; cPAP, continuous positive airway pressure; CXCL, C-X-C motif chemokine ligand; COVID-19, Coronavirus Disease 2019; CRP, C-reactive protein; CRS, cytokine release syndrome; DAMPs, damage-associated molecular patterns; ECMO, extracorporeal membrane oxygenation; FABP4, fatty acid-binding protein 4; Fc, fragment crystallisable; FCN1, ficolin-1; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; gp130, 130-kDa glycoprotein; HFO, high-flow oxygen; i, inhibitors; HMGB1, high mobility group box-1; ICU, intensive care unit; IFN, interferon; IL, interleukin; IMV, invasive mechanical ventilation; JAK, Janus kinase; LDH, lactate dehydrogenase; LFO, low-flow oxygen; M1 or M2, type-I or type-II (classically or alternatively) activated macrophages; mAbs, monoclonal antibodies; MAS, macrophage activation syndrome; NETs, neutrophil extracellular traps; NIAID, National Institute of Allergy and Infectious Diseases; NIH, National Institutes of Health; NIV, non-invasive ventilation; NLRP3, NLR family pyrin domain containing 3; PAMPs, pathogen-associated molecular patterns; PaO2/FIO2 ratio, pressure of arterial oxygen to fractional inspired oxygen concentration; -R, receptor; -RA, receptor antagonist; RCT, randomised controlled trial; RORC, retinoic acid orphan receptor C; s-, soluble; SaO2, peripheral arterial oxygen saturation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOCS, suppressor of cytokine signalling; SPP1, secreted phosphoprotein 1; STAT, signal transducer and activator of transcription; suPAR, soluble urokinase plasminogen activator receptor; TH, T helper; TH-GM, GM-CSF-producing T helper cells; TLR, Toll-like receptor; TNF, tumor necrosis factor; Treg, regulatory T cells.
References
1. Available at: https://www.google.com/search?q=covid-19+cases.
2. Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Graña C, Schmucker C, et al. Interleukin-6 Blocking Agents for Treating COVID-19: A Living Systematic Review. Cochrane Database Syst Rev (2021) 3(3):CD013881. doi: 10.1002/14651858.CD013881
3. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med (2020) 382(18):1708–20. doi: 10.1056/NEJMoa2002032
4. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA (2020) 324(8):782–93. doi: 10.1001/jama.2020.12839
5. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA (2020) 323(13):1239–42. doi: 10.1001/jama.2020.2648
6. Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep (2020) 69(24):759–65. doi: 10.15585/mmwr.mm6924e2
7. Zizzo G, Cohen PL. Imperfect Storm: Is Interleukin-33 the Achilles Heel of COVID-19? Lancet Rheumatol (2020) 2(12):e779–90. doi: 10.1016/S2665-9913(20)30340-4
8. Buszko M, Nita-Lazar A, Park JH, Schwartzberg PL, Verthelyi D, Young HA, et al. Lessons Learned: New Insights on the Role of Cytokines in COVID-19. Nat Immunol (2021) 22(4):404–11. doi: 10.1038/s41590-021-00901-9
9. Morena V, Milazzo L, Oreni L, Bestetti G, Fossali T, Bassoli C, et al. Off-Label Use of Tocilizumab for the Treatment of SARS-CoV-2 Pneumonia in Milan, Italy. Eur J Intern Med (2020) 76:36–42. doi: 10.1016/j.ejim.2020.05.011
10. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA (2020) 323(20):2052–9. doi: 10.1001/jama.2020.6775
11. Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K, et al. Immunopathological Characteristics of Coronavirus Disease 2019 Cases in Guangzhou, China. Immunology (2020) 160(3):261–8. doi: 10.1111/imm.13223
12. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients With COVID-19. Nat Med (2020) 26(6):842–4. doi: 10.1038/s41591-020-0901-9
13. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling Serum Cytokines in COVID-19 Patients Reveals IL-6 and IL-10 Are Disease Severity Predictors. Emerg Microbes Infect (2020) 9(1):1123–30. doi: 10.1080/22221751.2020.1770129
14. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
15. Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, et al. Comparative Replication and Immune Activation Profiles of SARS-CoV-2 and SARS-CoV in Human Lungs: An Ex Vivo Study With Implications for the Pathogenesis of COVID-19. Clin Infect Dis (2020) 71:1400–9. doi: 10.1093/cid/ciaa410
16. Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell (2020) 181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026
17. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T Cells and Inflammatory Monocytes Incite Inflammatory Storm in Severe COVID-19 Patients. Natl Sci Rev (2020) 7:998–1002. doi: 10.1093/nsr/nwaa041
18. Cifaldi L, Prencipe G, Caiello I, Bracaglia C, Locatelli F, De Benedetti F, et al. Inhibition of Natural Killer Cell Cytotoxicity by Interleukin-6: Implications for the Pathogenesis of Macrophage Activation Syndrome. Arthritis Rheumatol (2015) 67:3037–46. doi: 10.1002/art.39295
19. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of Cytokines Including Interleukin-6 in COVID-19 Induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev (2020) 19:102537. doi: 10.1016/j.autrev.2020.102537
20. Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin Infect Dis (2020) 71(8):1937–42. doi: 10.1093/cid/ciaa449
21. Wang F, Qu M, Zhou X, Zhao K, Lai C, Tang Q, et al. The Timeline and Risk Factors of Clinical Progression of COVID-19 in Shenzhen, China. J Transl Med (2020) 18(1):270. doi: 10.1186/s12967-020-02423-8
22. Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, et al. Efficacy and Safety of Tocilizumab in COVID-19 Patients: A Living Systematic Review and Meta-Analysis. Clin Microbiol Infect (2021) 27(2):215–27. doi: 10.1016/j.cmi.2020.10.036
23. Santa Cruz A, Mendes-Frias A, Oliveira AI, Dias L, Matos AR, Carvalho A, et al. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front Immunol (2021) 12:613422. doi: 10.3389/fimmu.2021.613422
24. Bovijn J, Lindgren CM, Holmes MV. Genetic Variants Mimicking Therapeutic Inhibition of IL-6 Receptor Signaling and Risk of COVID-19. Lancet Rheumatol (2020) 2(11):e658–9. doi: 10.1016/S2665-9913(20)30345-3
25. Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: Designing Specific Therapeutics for a Complex Cytokine. Nat Rev Drug Discovery (2018) 17(6):395–412. doi: 10.1038/nrd.2018.45
26. Narazaki M, Kishimoto T. The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int J Mol Sci (2018) 19(11):3528. doi: 10.3390/ijms19113528
27. Nasonov E, Samsonov M. The Role of Interleukin 6 Inhibitors in Therapy of Severe COVID-19. BioMed Pharmacother (2020) 131:110698. doi: 10.1016/j.biopha.2020.110698
28. Mazzone A, Castelnovo L, Tamburello A, Gatti A, Brando B, Faggioli P, et al. Monocytes Could Be a Bridge From Inflammation to Thrombosis on COVID-19 Injury: A Case Report. Thromb Update (2020) 1:100007. doi: 10.1016/j.tru.2020.100007
29. Gatti A, Radrizzani D, Viganò P, Mazzone A, Brando B. Decrease of Non-Classical and Intermediate Monocyte Subsets in Severe Acute SARS-CoV-2 Infection. Cytometry A. (2020) 97(9):887–90. doi: 10.1002/cyto.a.24188
30. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological Findings of COVID-19 Associated With Acute Respiratory Distress Syndrome. Lancet Respir Med (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
31. Petes C, Mariani MK, Yang Y, Grandvaux N, Gee K. Interleukin (IL)-6 Inhibits IL-27- and IL-30-Mediated Inflammatory Responses in Human Monocytes. Front Immunol (2018) 9:256. doi: 10.3389/fimmu.2018.00256
32. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-Analysis. JAMA (2021) 326(6):499–518. doi: 10.1001/jama.2021.11330
33. Aderka D, Le JM, Vilcek J. IL-6 Inhibits Lipopolysaccharide-Induced Tumor Necrosis Factor Production in Cultured Human Monocytes, U937 Cells, and in Mice. J Immunol (1989) 143(11):3517–23.
34. Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an Anti-Inflammatory Cytokine: Induction of Circulating IL-1 Receptor Antagonist and Soluble Tumor Necrosis Factor Receptor P55. Blood (1994) 83(1):113–8. doi: 10.1182/blood.V83.1.113.bloodjournal831113
35. Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and Resistance to Regulation by IL-4 During Th17 Maturation. J Immunol (2011) 187(9):4440–50. doi: 10.4049/jimmunol.1002860
36. Niemand C, Nimmesgern A, Haan S, Fischer P, Schaper F, Rossaint R, et al. Activation of STAT3 by IL-6 and IL-10 in Primary Human Macrophages Is Differentially Modulated by Suppressor of Cytokine Signaling 3. J Immunol (2003) 170(6):3263–72. doi: 10.4049/jimmunol.170.6.3263
37. Kimmig LM, Wu D, Gold M, Pettit NN, Pitrak D, Mueller J, et al. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Front Med (Lausanne) (2020) 7:583897. doi: 10.3389/fmed.2020.583897
38. Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19. Clin Infect Dis (2021) 73(2):e445–54. doi: 10.1093/cid/ciaa954
39. Johnson HM, Lewin AS, Ahmed CM. SOCS, Intrinsic Virulence Factors, and Treatment of COVID-19. Front Immunol (2020) 11:582102. doi: 10.3389/fimmu.2020.582102
40. Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, et al. SOCS3 Regulates the Plasticity of Gp130 Signaling. Nat Immunol (2003) 4(6):546–50. doi: 10.1038/ni932
41. Liu S, Yan R, Chen B, Pan Q, Chen Y, Hong J, et al. Influenza Virus-Induced Robust Expression of SOCS3 Contributes to Excessive Production of IL-6. Front Immunol (2019) 10:1843. doi: 10.3389/fimmu.2019.01843
42. Diehl S, Rincón M. The Two Faces of IL-6 on Th1/Th2 Differentiation. Mol Immunol (2002) 39(9):531–6. doi: 10.1016/s0161-5890(02)00210-9
43. Veiga VC, Prats JAGG, Farias DLC, Rosa RG, Dourado LK, Zampieri FG, et al. Coalition Covid-19 Brazil VI Investigators. Effect of Tocilizumab on Clinical Outcomes at 15 Days in Patients With Severe or Critical Coronavirus Disease 2019: Randomised Controlled Trial. BMJ (2021) 372:n84. doi: 10.1136/bmj.n84
44. Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 Biology Into Effective Treatments. Nat Rev Rheumatol (2020) 16(6):335–45. doi: 10.1038/s41584-020-0419-z
45. Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. The Covid Irccs San Matteo Pavia Task Force. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results From SMAtteo COvid19 REgistry (SMACORE). Microorganisms (2020) 8(5):695. doi: 10.3390/microorganisms8050695
46. Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of Low Dose Tocilizumab on Mortality Rate in Patients With COVID-19 Related Pneumonia. Eur J Intern Med (2020) 76:31–5. doi: 10.1016/j.ejim.2020.05.009
47. Quartuccio L, Sonaglia A, McGonagle D, Fabris M, Peghin M, Pecori D, et al. Profiling COVID-19 Pneumonia Progressing Into the Cytokine Storm Syndrome: Results From a Single Italian Centre Study on Tocilizumab Versus Standard of Care. J Clin Virol (2020) 129:104444. doi: 10.1016/j.jcv.2020.104444
48. Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and Safety of Tocilizumab in Severe COVID-19 Patients: A Single-Centre Retrospective Cohort Study. Eur J Intern Med (2020) 76:43–9. doi: 10.1016/j.ejim.2020.05.021
49. Gritti G, Raimondi F, Ripamonti D, Riva I, Landi F, Alborghetti L, et al. IL-6 Signalling Pathway Inactivation With Siltuximab in Patients With COVID-19 Respiratory Failure: An Observational Cohort Study. medRxiv (2020) 04.01.20048561. doi: 10.1101/2020.04.01.20048561
50. Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in Patients With Severe COVID-19: A Retrospective Cohort Study. Lancet Rheumatol (2020) 2(8):e474–84. doi: 10.1016/S2665-9913(20)30173-9
51. Della-Torre E, Campochiaro C, Cavalli G, De Luca G, Napolitano A, La Marca S, et al. Interleukin-6 Blockade With Sarilumab in Severe COVID-19 Pneumonia With Systemic Hyperinflammation: An Open-Label Cohort Study. Ann Rheum Dis (2020) 79(10):1277–85. doi: 10.1136/annrheumdis-2020-218122
52. Rossotti R, Travi G, Ughi N, Corradin M, Baiguera C, Fumagalli R, et al. Safety and Efficacy of Anti-Il6-Receptor Tocilizumab Use in Severe and Critical Patients Affected by Coronavirus Disease 2019: A Comparative Analysis. J Infect (2020) 81(4):e11–7. doi: 10.1016/j.jinf.2020.07.008
53. Canziani LM, Trovati S, Brunetta E, Testa A, De Santis M, Bombardieri E, et al. Humanitas and Gavazzeni / Castelli COVID-19 Task Forces. Interleukin-6 Receptor Blocking With Intravenous Tocilizumab in COVID-19 Severe Acute Respiratory Distress Syndrome: A Retrospective Case-Control Survival Analysis of 128 Patients. J Autoimmun (2020) 114:102511. doi: 10.1016/j.jaut.2020.102511
54. Castelnovo L, Tamburello A, Lurati A, Zaccara E, Marrazza MG, Olivetti M, et al. Anti-IL6 Treatment of Serious COVID-19 Disease: A Monocentric Retrospective Experience. Med (Baltimore) (2021) 100(1):e23582. doi: 10.1097/MD.0000000000023582
55. Ghazy RM, Almaghraby A, Shaaban R, Kamal A, Beshir H, Moursi A, et al. A Systematic Review and Meta-Analysis on Chloroquine and Hydroxychloroquine as Monotherapy or Combined With Azithromycin in COVID-19 Treatment. Sci Rep (2020) 10(1):22139. doi: 10.1038/s41598-020-77748-x
56. Echarte-Morales J, Minguito-Carazo C, Del Castillo-García S, Borrego-Rodríguez J, Rodríguez-Santamarta M, Sánchez-Muñoz E, et al. Effect of Hydroxychloroquine, Azithromycin and Lopinavir/Ritonavir on the QT Corrected Interval in Patients With COVID-19. J Electrocardiol (2021) 64:30–5. doi: 10.1016/j.jelectrocard.2020.11.012
57. Collaborative Group RECOVERY, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients With Covid-19. N Engl J Med (2021) 384(8):693–704. doi: 10.1056/NEJMoa2021436
58. RECOVERY Collaborative Group. Tocilizumab in Patients Admitted to Hospital With COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet (2021) 397(10285):1637–45. doi: 10.1016/S0140-6736(21)00676-0
59. Gremese E, Cingolani A, Bosello SL, Alivernini S, Tolusso B, Perniola S, et al. Sarilumab Use in Severe SARS-CoV-2 Pneumonia. EClinicalMedicine (2020) 27:100553. doi: 10.1016/j.eclinm.2020.100553
60. Perrone F, Piccirillo MC, Ascierto PA, Salvarani C, Parrella R, Marata AM, et al. Tocilizumab for Patients With COVID-19 Pneumonia. The Single-Arm TOCIVID-19 Prospective Trial. J Transl Med (2020) 18(1):405. doi: 10.1186/s12967-020-02573-9
61. Sciascia S, Aprà F, Baffa A, Baldovino S, Boaro D, Boero R, et al. Pilot Prospective Open, Single-Arm Multicentre Study on Off-Label Use of Tocilizumab in Patients With Severe COVID-19. Clin Exp Rheumatol (2020) 38(3):529–32.
62. Pomponio G, Ferrarini A, Bonifazi M, Moretti M, Salvi A, Giacometti A, et al. Tocilizumab in COVID-19 Interstitial Pneumonia. J Intern Med (2021) 289(5):738–46. doi: 10.1111/joim.13231
63. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the Treatment of Severe COVID-19 Pneumonia With Hyperinflammatory Syndrome and Acute Respiratory Failure: A Single Center Study of 100 Patients in Brescia, Italy. Autoimmun Rev (2020) 19(7):102568. doi: 10.1016/j.autrev.2020.102568
64. Patel S, Saxena B, Mehta P. Recent Updates in the Clinical Trials of Therapeutic Monoclonal Antibodies Targeting Cytokine Storm for the Management of COVID-19. Heliyon (2021) 7(2):e06158. doi: 10.1016/j.heliyon.2021.e06158
65. Investigators REMAP-CAP, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients With Covid-19. N Engl J Med (2021) 384(16):1491–502. doi: 10.1056/NEJMoa2100433
66. Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in Hospitalized Patients With Severe Covid-19 Pneumonia. N Engl J Med (2021) 384(16):1503–16. doi: 10.1056/NEJMoa2028700
67. Wang D, Fu B, Peng Z, Yang D, Han M, Li M, et al. Tocilizumab in Patients With Moderate or Severe COVID-19: A Randomized, Controlled, Open-Label, Multicenter Trial. Front Med (2021) 15(3):486–94. doi: 10.1007/s11684-020-0824-3
68. Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, et al. Tocilizumab Plus Standard Care Versus Standard Care in Patients in India With Moderate to Severe COVID-19-Associated Cytokine Release Syndrome (COVINTOC): An Open-Label, Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Respir Med (2021) 9(5):511–21. doi: 10.1016/S2213-2600(21)00081-3
69. Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, et al. Sarilumab in Patients Admitted to Hospital With Severe or Critical COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir Med (2021) 9(5):522–32. doi: 10.1016/S2213-2600(21)00099-0
70. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized With Covid-19 Pneumonia. N Engl J Med (2021) 384(1):20–30. doi: 10.1056/NEJMoa2030340
71. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of Tocilizumab in Patients Hospitalized With Covid-19. N Engl J Med (2020) 383(24):2333–44. doi: 10.1056/NEJMoa2028836
72. Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern Med (2021) 181(1):32–40. doi: 10.1001/jamainternmed.2020.6820
73. Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern Med (2021) 181(1):24–31. doi: 10.1001/jamainternmed.2020.6615
74. Kumar PN, Hernández-Sánchez J, Nagel S, Feng Y, Cai F, Rabin J, et al. Safety and Efficacy of Tocilizumab 4 or 8 mg/kg in Hospitalized Patients With Moderate to Severe Coronavirus Disease 2019 Pneumonia: A Randomized Clinical Trial. Open Forum Infect Dis (2021) 9(1):ofab608. doi: 10.1093/ofid/ofab608
75. Lin WT, Hung SH, Lai CC, Wang CY, Chen CH. The Effect of Tocilizumab on COVID-19 Patient Mortality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int Immunopharmacol (2021) 96:107602. doi: 10.1016/j.intimp.2021.107602
76. Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug Treatments for Covid-19: Living Systematic Review and Network Meta-Analysis. BMJ (2020) 370:m2980. doi: 10.1136/bmj.m2980. Update in: BMJ
77. Boregowda U, Perisetti A, Nanjappa A, Gajendran M, Kutti G Sridharan, Goyal H. Addition of Tocilizumab to the Standard of Care Reduces Mortality in Severe COVID-19: A Systematic Review and Meta-Analysis. Front Med (Lausanne) (2020) 7:586221. doi: 10.3389/fmed.2020.586221
78. Han Q, Guo M, Zheng Y, Zhang Y, De Y, Xu C, et al. Current Evidence of Interleukin-6 Signaling Inhibitors in Patients With COVID-19: A Systematic Review and Meta-Analysis. Front Pharmacol (2020) 11:615972. doi: 10.3389/fphar.2020.615972
79. Albuquerque AM, Tramujas L, Sewanan LR, Brophy JM. Tocilizumab in COVID-19 – A Bayesian Reanalysis of RECOVERY. medRxiv preprint (2021). doi: 10.1101/2021.06.15.21258966
80. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-Analysis. JAMA (2020) 324(13):1330–41. doi: 10.1001/jama.2020.17023
81. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/ (Accessed September 30, 2021).
82. Zhang J, Hao Y, Ou W, Ming F, Liang G, Qian Y, et al. Serum Interleukin-6 Is an Indicator for Severity in 901 Patients With SARS-CoV-2 Infection: A Cohort Study. J Transl Med (2020) 18(1):406. doi: 10.1186/s12967-020-02571-x
83. Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: A Systematic Review and Meta-Analysis. Rev Med Virol (2020) 30(6):1–9. doi: 10.1002/rmv.2141
84. Aziz M, Fatima R, Assaly R. Elevated Interleukin-6 and Severe COVID-19: A Meta-Analysis. J Med Virol (2020) 92(11):2283–5. doi: 10.1002/jmv.25948
85. Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the Controversy: Interleukin-6 and the COVID-19 Cytokine Storm Syndrome. Eur Respir J (2020) 56(4):2003006. doi: 10.1183/13993003.03006-2020
86. Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, et al. Elevated Levels of IL-6 and CRP Predict the Need for Mechanical Ventilation in COVID-19. J Allergy Clin Immunol (2020) 146(1):128–136.e4. doi: 10.1016/j.jaci.2020.05.008
87. Laguna-Goya R, Utrero-Rico A, Talayero P, Lasa-Lazaro M, Ramirez-Fernandez A, Naranjo L, et al. IL-6-Based Mortality Risk Model for Hospitalized Patients With COVID-19. J Allergy Clin Immunol (2020) 146(4):799–807.e9. doi: 10.1016/j.jaci.2020.07.009
88. Tian H, Sui Y, Tian S, Zou X, Xu Z, He H, et al. Case Report: Clinical Treatment of the First Critical Patient With Coronavirus Disease (COVID-19) in Liaocheng, Shandong Province. Front Med (Lausanne) (2020) 7:249. doi: 10.3389/fmed.2020.00249
89. Caricchio R, Gallucci M, Dass C, Zhang X, Gallucci S, Fleece D, et al. Preliminary Predictive Criteria for COVID-19 Cytokine Storm. Ann Rheum Dis (2021) 80(1):88–95. doi: 10.1136/annrheumdis-2020-218323
90. Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison With Other Inflammatory Syndromes. Lancet Respir Med (2020) 8(12):1233–44. doi: 10.1016/S2213-2600(20)30404-5
91. Gómez-Pastora J, Weigand M, Kim J, Wu X, Strayer J, Palmer AF, et al. Hyperferritinemia in Critically Ill COVID-19 Patients - Is Ferritin the Product of Inflammation or a Pathogenic Mediator? Clin Chim Acta (2020) 509:249–51. doi: 10.1016/j.cca.2020.06.033
92. Kappert K, Jahić A, Tauber R. Assessment of Serum Ferritin as a Biomarker in COVID-19: Bystander or Participant? Insights by Comparison With Other Infectious and non-Infectious Diseases. Biomarkers (2020) 25(8):616–25. doi: 10.1080/1354750X.2020.1797880
93. Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and Validation of the HScore, a Score for the Diagnosis of Reactive Hemophagocytic Syndrome. Arthritis Rheumatol (2014) 66(9):2613–20. doi: 10.1002/art.38690
94. Davì S, Minoia F, Pistorio A, Horne A, Consolaro A, Rosina S, et al. Performance of Current Guidelines for Diagnosis of Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol (2014) 66(10):2871–80. doi: 10.1002/art.38769
95. Della-Torre E, Lanzillotta M, Campochiaro C, Cavalli G, De Luca G, Tomelleri A, et al. Respiratory Impairment Predicts Response to IL-1 and IL-6 Blockade in COVID-19 Patients With Severe Pneumonia and Hyper-Inflammation. Front Immunol (2021) 12:675678. doi: 10.3389/fimmu.2021.675678
96. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients With COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
97. Conte G, Cei M, Evangelista I, Colombo A, Vitale J, Mazzone A, et al. The Meaning of D-Dimer Value in Covid-19. Clin Appl Thromb Hemost (2021) 27:10760296211017668. doi: 10.1177/10760296211017668
98. Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al. Early Treatment of COVID-19 With Anakinra Guided by Soluble Urokinase Plasminogen Receptor Plasma Levels: A Double-Blind, Randomized Controlled Phase 3 Trial. Nat Med (2021) 27(10):1752–60. doi: 10.1038/s41591-021-01499-z
99. Sivapalasingam S, Lederer DJ, Bhore R, Hajizadeh N, Criner G, Hosain R, et al. A Randomized Placebo-Controlled Trial of Sarilumab in Hospitalized Patients With Covid-19. medRxiv preprint (2021). doi: 10.1101/2021.05.13.21256973
100. Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: Combining Antiviral and Anti-Inflammatory Treatments. Lancet Infect Dis (2020) 20(4):400–2. doi: 10.1016/S1473-3099(20)30132-8
101. Stebbing J, Sánchez Nievas G, Falcone M, Youhanna S, Richardson P, Ottaviani S, et al. JAK Inhibition Reduces SARS-CoV-2 Liver Infectivity and Modulates Inflammatory Responses to Reduce Morbidity and Mortality. Sci Adv (2021) 7(1):eabe4724. doi: 10.1126/sciadv.abe4724
102. Hammarén HM, Virtanen AT, Raivola J, Silvennoinen O. The Regulation of JAKs in Cytokine Signaling and its Breakdown in Disease. Cytokine (2019) 118:48–63. doi: 10.1016/j.cyto.2018.03.041
103. Cantini F, Niccoli L, Nannini C, Matarrese D, Natale MED, Lotti P, et al. Beneficial Impact of Baricitinib in COVID-19 Moderate Pneumonia; Multicentre Study. J Infect (2020) 81(4):647–79. doi: 10.1016/j.jinf.2020.06.052
104. Bronte V, Ugel S, Tinazzi E, Vella A, De Sanctis F, Canè S, et al. Baricitinib Restrains the Immune Dysregulation in Patients With Severe COVID-19. J Clin Invest (2020) 130(12):6409–16. doi: 10.1172/JCI141772
105. Petrone L, Petruccioli E, Alonzi T, Vanini V, Cuzzi G, Najafi Fard S, et al. In-Vitro Evaluation of the Immunomodulatory Effects of Baricitinib: Implication for COVID-19 Therapy. J Infect (2021) 82(4):58–66. doi: 10.1016/j.jinf.2021.02.023
106. Vannucchi AM, Sordi B, Morettini A, Nozzoli C, Poggesi L, Pieralli F, et al. Compassionate Use of JAK1/2 Inhibitor Ruxolitinib for Severe COVID-19: A Prospective Observational Study. Leukemia (2021) 35(4):1121–33. doi: 10.1038/s41375-020-01018-y
107. Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in Treatment of Severe Coronavirus Disease 2019 (COVID-19): A Multicenter, Single-Blind, Randomized Controlled Trial. J Allergy Clin Immunol (2020) 146(1):137–146.e3. doi: 10.1016/j.jaci.2020.05.019
108. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib Plus Remdesivir for Hospitalized Adults With Covid-19. N Engl J Med (2021) 384(9):795–807. doi: 10.1056/NEJMoa2031994
109. Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and Safety of Baricitinib for the Treatment of Hospitalised Adults With COVID-19 (COV-BARRIER): A Randomised, Double-Blind, Parallel-Group, Placebo-Controlled Phase 3 Trial. Lancet Respir Med (2021) 9:1407–18. doi: 10.1016/S2213-2600(21)00331-3
110. Wu D, Yang XO. TH17 Responses in Cytokine Storm of COVID-19: An Emerging Target of JAK2 Inhibitor Fedratinib. J Microbiol Immunol Infect (2020) 53(3):368–70. doi: 10.1016/j.jmii.2020.03.005
111. Guimarães PO, Quirk D, Furtado RH, Maia LN, Saraiva JF, Antunes MO, et al. Tofacitinib in Patients Hospitalized With Covid-19 Pneumonia. N Engl J Med (2021) 385(5):406–15. doi: 10.1056/NEJMoa2101643
112. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 Blockade With High-Dose Anakinra in Patients With COVID-19, Acute Respiratory Distress Syndrome, and Hyperinflammation: A Retrospective Cohort Study. Lancet Rheumatol (2020) 2(6):e325–31. doi: 10.1016/S2665-9913(20)30127-2
113. Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for Severe Forms of COVID-19: A Cohort Study. Lancet Rheumatol (2020) 2(7):e393–400. doi: 10.1016/S2665-9913(20)30164-8
114. Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, et al. Effect of Canakinumab vs Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized With Severe COVID-19: A Randomized Clinical Trial. JAMA (2021) 326(3):230–9. doi: 10.1001/jama.2021.9508
115. Patel J, Beishuizen A, Ruiz XB, Boughanmi H, Cahn A, Criner GJ, et al. A Randomized Trial of Otilimab in Severe COVID-19 Pneumonia (OSCAR). medRxiv (2021) 04.14.21255475. doi: 10.1101/2021.04.14.21255475
116. Temesgen Z, Burger CD, Baker J, Polk C, Libertin CR, Kelley CF, et al. Lenzilumab in Hospitalised Patients With COVID-19 Pneumonia (LIVE-AIR): A Phase 3, Randomised, Placebo-Controlled Trial. Lancet Respir Med (2021) 1:S2213-2600(21)00494-X. doi: 10.1016/S2213-2600(21)00494-X
117. Cremer PC, Abbate A, Hudock K, McWilliams C, Mehta J, Chang SY, et al. Mavrilimumab in Patients With Severe COVID-19 Pneumonia and Systemic Hyperinflammation (MASH-COVID): An Investigator Initiated, Multicentre, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Rheumatol (2021) 3(6):e410–8. doi: 10.1016/S2665-9913(21)00070-9
118. Rodríguez-Baño J, Pachón J, Carratalà J, Ryan P, Jarrín I, Yllescas M, et al. Treatment With Tocilizumab or Corticosteroids for COVID-19 Patients With Hyperinflammatory State: A Multicentre Cohort Study (SAM-COVID-19). Clin Microbiol Infect (2021) 27(2):244–52. doi: 10.1016/j.cmi.2020.08.010
119. Yuan M, Xu X, Xia D, Tao Z, Yin W, Tan W, et al. Effects of Corticosteroid Treatment for Non-Severe COVID-19 Pneumonia: A Propensity Score-Based Analysis. Shock (2020) 54(5):638–43. doi: 10.1097/SHK.0000000000001574
120. Campins L, Boixeda R, Perez-Cordon L, Aranega R, Lopera C, Force L. Early Tocilizumab Treatment Could Improve Survival Among COVID-19 Patients. Clin Exp Rheumatol (2020) 38(3):578.
121. Ramiro S, Mostard RLM, Magro-Checa C, van Dongen CMP, Dormans T, Buijs J, et al. Historically Controlled Comparison of Glucocorticoids With or Without Tocilizumab Versus Supportive Care Only in Patients With COVID-19-Associated Cytokine Storm Syndrome: Results of the CHIC Study. Ann Rheum Dis (2020) 79(9):1143–51. doi: 10.1136/annrheumdis-2020-218479
122. Trouillet-Assant S, Viel S, Gaymard A, Pons S, Richard JC, Perret M, et al. Type I IFN Immunoprofiling in COVID-19 Patients. J Allergy Clin Immunol (2020) 146(1):206–8. doi: 10.1016/j.jaci.2020.04.029
123. Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, et al. Triple Combination of Interferon Beta-1b, Lopinavir-Ritonavir, and Ribavirin in the Treatment of Patients Admitted to Hospital With COVID-19: An Open-Label, Randomised, Phase 2 Trial. Lancet (2020) 395(10238):1695–704. doi: 10.1016/S0140-6736(20)31042-4
124. Feld JJ, Kandel C, Biondi MJ, Kozak RA, Zahoor MA, Lemieux C, et al. Peginterferon Lambda for the Treatment of Outpatients With COVID-19: A Phase 2, Placebo-Controlled Randomised Trial. Lancet Respir Med (2021) 9(5):498–510. doi: 10.1016/S2213-2600(20)30566-X
125. Dyavar SR, Singh R, Emani R, Pawar GP, Chaudhari VD, Podany AT, et al. Role of Toll-Like Receptor 7/8 Pathways in Regulation of Interferon Response and Inflammatory Mediators During SARS-CoV2 Infection and Potential Therapeutic Options. BioMed Pharmacother (2021) 141:111794. doi: 10.1016/j.biopha.2021.111794
126. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med (2020) 383(19):1813–26. doi: 10.1056/NEJMoa2007764
127. Chan N, Eikelboom J. Hypercoagulability and Thrombosis in COVID-19: A Modifiable Cause for Mortality? Eur Heart J (2021) 42(33):3143–5. doi: 10.1093/eurheartj/ehab417
128. Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell (2020) 183(4):1043–1057.e15. doi: 10.1016/j.cell.2020.09.033
Keywords: COVID-19, cytokines, IL-6, therapy, tocilizumab, sarilumab, baricitinib, anakinra
Citation: Zizzo G, Tamburello A, Castelnovo L, Laria A, Mumoli N, Faggioli PM, Stefani I and Mazzone A (2022) Immunotherapy of COVID-19: Inside and Beyond IL-6 Signalling. Front. Immunol. 13:795315. doi: 10.3389/fimmu.2022.795315
Received: 14 October 2021; Accepted: 25 January 2022;
Published: 22 February 2022.
Edited by:
Alexei A. Grom, Cincinnati Children’s Research Foundation, United StatesReviewed by:
Shetty Ravi Dyavar, Adicet Bio, Inc, United StatesDelia Goletti, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), Italy
Copyright © 2022 Zizzo, Tamburello, Castelnovo, Laria, Mumoli, Faggioli, Stefani and Mazzone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaetano Zizzo, Z2FldGFuby56aXp6b0Bhc3N0LW92ZXN0bWkuaXQ=
 Gaetano Zizzo
Gaetano Zizzo Antonio Tamburello
Antonio Tamburello Laura Castelnovo
Laura Castelnovo Paola Maria Faggioli
Paola Maria Faggioli