- 1Department of Pediatrics, Division of Blood & Marrow Transplantation & Cellular Therapy, University of Minnesota, Minneapolis, MN, United States
- 2Io Therapeutics Inc., Santa Ana, CA, United States
Most allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients receive peripheral blood stem cell grafts resulting in a 30%–70% incidence of chronic graft-versus-host disease (cGVHD), a major cause of mortality and morbidity in long-term survivors. While systemic steroids remain the standard of care for first-line therapy, patients may require long-term administration, and those with steroid-resistant or refractory cGVHD have a worse prognosis. Although durable and deep responses with second-line therapies can be achieved in some patients, there remains an urgent need for new therapies. In this study, we evaluated the efficacy of IRX4204, a novel agonist that activates RXRs and is in clinical trials for cancer treatment to prevent and treat cGVHD in two complementary murine models. In a major histocompatibility complex mismatched, non-sclerodermatous multiorgan system model with bronchiolitis obliterans, IRX4204 prevented and reversed cGVHD including associated pulmonary dysfunction with restoration of germinal center T-follicular helper: T-follicular regulatory cell balance. In a minor histocompatibility antigen disparate sclerodermatous model, IRX4204 treatment significantly prevented and ameliorated skin cGVHD by reducing Th1 and Th17 differentiation due to anti-inflammatory properties. Together, these results indicate that IRX4204 is a promising therapeutic option to treat cGVHD with bronchiolitis obliterans or sclerodermatous manifestations.
Introduction
Chronic graft-versus-host disease (cGVHD) is a life-threatening complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT), which causes late non-relapse morbidity and mortality (1, 2). Although recent studies have advanced the understanding of GVHD pathophysiology, the first-line therapy remains corticosteroids, which can achieve a complete response in only 20%–50% of patients (3, 4). For patients that do not respond to steroid therapy, the mortality rate is high (5). Furthermore, allo-HSCT recipients undergoing broad immunosuppressant therapy are more prone to tumor relapse, infections, and drug-related toxicities. Therefore, novel targeted immunomodulatory strategies are highly warranted to improve clinical outcomes in allo-HSCT.
Chronic GVHD patients with either bronchiolitis obliterans (BO), an obstructive pulmonary disease, or scleroderma complications are poor responders to standard available therapies (6–9). Moreover, complete and durable responses in steroid refractory or dependent cGVHD are infrequent. Hence, multiple preclinical cGVHD models have been developed to represent aspects of the spectrum of cGVHD manifestations and further elucidate cGVHD pathogenesis in an attempt to develop therapeutic strategies (10–15). Since cGVHD with BO has a poor prognosis, a cGVHD BO model was developed as a platform to evaluate new therapies. Toward that end, B10.BR (H2k) recipients were conditioned with high-dose cyclophosphamide and total body irradiation prior to reconstitution with major histocompatibility complex (MHC)-disparate C57BL/6 (H2b) bone marrow and a low dose of T cells, recapitulating many of the clinical, functional, and pathological manifestations of cGVHD with BO (13, 16). Chronic GVHD pathogenesis with BO was dependent upon alloreactive donor CD4+ T cells that differentiated into T-follicular helper (Tfh) cells to activate germinal center (GC) B cells, resulting in pathogenic antibody production and disposition onto cGVHD target organs. Monocyte and macrophage recruitment into areas of lung injury results in stimulation of fibroblast release of profibrogenic molecules and fibrosis in target organs except the skin (17). In a minor histocompatibility antigen disparate scleroderma model, BALB/c (H2d) recipients underwent total body irradiation prior to reconstitution with B10.D2 (H2d) bone marrow and T cells resulting in fibrotic skin disease mediated by donor T helper 1 (Th1) and Th17 cells (18).
Retinoid X receptors (RXRs) are key members of the nuclear receptor (NR) superfamily due to their diverse roles in modulating various physiological processes (19). RXRs form homodimers (RXR-RXR) and heterodimers with other NRs, namely, retinoic acid receptors (RARs), thyroid hormone receptor, liver X receptors (LXRs), vitamin D receptor, farnesoid x receptor, nuclear receptor related 1 protein (Nurr1, Nr4a2), nerve growth factor IB (Nur77, Nr4a1), and peroxisome proliferator-associated receptors (PPARs) (20). Studies in mice have reported the direct role of RXR in controlling Th1 differentiation with loss of RXRα in CD4+ T cells leading to increased Th1 polarization and interferon gamma (IFN-γ) production (21). Since RXRα is also required to maintain the suppressive function of T regulatory cells (Tregs), RXR agonists could have dual benefits as a therapeutic strategy for controlling inflammatory disorders.
Rexinoids are RXR ligands that selectively bind and activate RXRs. Although rexinoids can have high therapeutic value in treating various metabolic disorders and cancers (22, 23), off-target responses due to RXR promiscuity with NRs other than RARα limited their clinical applications (24–26). For instance, in addition to RARα, Food and Drug Administration (FDA)-approved synthetic rexinoid bexarotene binds with liver-X-receptor (LXR) and PPAR (27–29). IRX4204 is a highly selective rexinoid with a potency of 100-fold more than bexarotene in activating RXRs. Specifically, IRX4204 does not transactivate RXR heterodimers of RAR, LXR, or PPAR (30), consistent with the selective biological function of IRX4204 and pointing to its use as a therapeutic agent. Recent studies have demonstrated the therapeutic efficacy of IRX4204 in controlling murine autoimmune disease and acute GVHD (aGVHD) (20, 31). Administration of IRX4204 in a murine model of multiple sclerosis attenuated the severity of the disease (20). We previously showed that IRX4204 treatment ameliorated aGVHD while retaining graft-versus-tumor (GVT) responses against leukemia and lymphoma cells (31). Since the pathophysiology of cGVHD is distinct from aGVHD, we sought to test the prophylactic and therapeutic efficacy of IRX4204 in two pathologically distinct cGVHD models. Herein, we demonstrate that IRX4204 administration prevents and treats both BO and sclerodermatous manifestations of cGVHD. Mechanistically, IRX4204 impairs pathogenic donor Tfh, Th1, and Th17 differentiation leading to protection from cGVHD.
Materials and Methods
Mice
Female C57BL/6 (B6;H2b) and BALB/c (H2d) mice were purchased from the National Cancer Institute. Female B10.BR (H2k) and B10.D2 (H2d) mice were purchased from The Jackson Laboratory. All mice ranged in age from 10 to 18 weeks. Mice were housed in a specific pathogen-free facility, and all studies were approved by the University of Minnesota’s Institutional Animal Care Committee.
BM Transplantation
For the BO model, B10.BR recipients were conditioned with cyclophosphamide on days −3 and −2 (120 mg/kg/day intraperitoneally). On day −1, recipients received total body irradiation (TBI) by X-ray [8.3 Gray (Gy) by X-ray)]. Recipients then received 10 × 106 B6 T-cell-depleted (TCD) bone marrow (BM) only or with 7–7.5 × 104 purified splenic T cells on day 0 (17, 32). In the scleroderma model, BALB/c mice were given lethal TBI (7 Gy by X-ray, day −1) and 107 B10.D2 TCD-BM only or with 1.8 × 106 CD4+ and 0.9 × 106 CD8 T cells (day 0) (32, 33). Mice were monitored daily for survival. Skin scores were assessed twice weekly (32).
Pulmonary Function Tests
Pulmonary function tests (PFTs) were performed as previously described (16). Briefly, mice were anesthetized with Nembutal, intubated and ventilated using the Flexivent system (Scireq Montreal, QC). Pulmonary resistance, elastance, and compliance were reported using Flexivent software version 7. Chronic GVHD controls have increased pulmonary resistance and elastance along with decreased compliance as compared to BM-only controls in our BO cGVHD model (13, 16).
Flow Cytometry
For Tfh and GC B cells, single-cell suspensions of spleens were obtained and stained with fluorochrome-labeled anti-CD4 (RM4-5, BD), anti-CXCR5 (SPRCL5, Thermo Fisher Scientific, MA, USA), anti-PD-1 (J43, Thermo Fisher Scientific), anti-CD19 (eBio1D3, Thermo Fisher Scientific), anti-GL7 (GL-7, Thermo Fisher Scientific), and anti-Fas (J02, BD). Live/dead fixable viability dye (Thermo Fisher Scientific) was used for live/dead discrimination. GC B cells were defined as Fas and GL7 double-positive CD19 B cells. Tfh cells were defined as PD1 and CXCR5 high CD4+ Foxp3− T cells. Tfr cells were defined as PD1, and CXCR5 high CD4+ Foxp3+ T cells. Tregs were detected by staining cells for surface antigens, followed by fixation, permeabilization using a Foxp3/transcription factor staining buffer set (Thermo Fischer Scientific), and labeling with anti-Foxp3 (FJK-16s, Thermo Fisher Scientific). For intracellular cytokine staining experiment, lymph node and spleen cells were stimulated with cell stimulation cocktail (plus protein transport inhibitors) (Thermo Fisher Scientific) for 5 h at 37°C. After surface staining, cells were fixed, permeabilized, and stained with anti-interleukin-17 (anti-IL-17) (eBio17B7, Thermo Fisher Scientific) and anti-IFN-γ (XMG1.2, Thermo Fisher Scientific). BD LSRFortessa (BD Biosciences, CA, USA) was used to acquire cells, and analyses were performed using FlowJo software.
Immunofluorescence
For the GC detection, acetone-fixed 6-µm cryosections of spleens were stained with rhodamine-peanut agglutinin (Vector Laboratories, CA, USA). For CD4 and B-cell staining, sections were stained with CD4 FITC (RM4-5, Thermo Fisher Scientific) and B220 BV421 (RA3-6B2, BD). GCs are identified as PNA+ regions with B220+ and/or CD4+ areas surrounding them. Confocal images were acquired on an Olympus FluoView500 Confocal Laser Scanning Microscope at 200×, analyzed using FluoView3.2 software (Olympus), and quantified using a Voronoi tessellation methodology making use of EBImage (34).
NP-OVA Immunizations
B6 mice were immunized with 4-hydroxy-3-nitrophenylacetyl hapten (NP)-OVA (100 μg, Bioresearch Technologies, Novato, CA, USA), diluted in complete H37 Ra (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) in each flank (35). For IRX4204 prophylaxis, mice were given IRX4204 on days 0–7 daily (i.p.), then sacrificed 7 days post-immunization, and the inguinal lymph nodes were harvested and analyzed by flow cytometry. For therapeutic purposes, mice were treated with IRX4204 on days 8–14 daily (i.p.) 7 days post-immunization. On day 14, inguinal lymph nodes were harvested and analyzed by flow cytometry.
IRX4204
IRX4204 (Io Therapeutics, USA) was prepared in phosphate-buffered saline (PBS) [containing ~4% dimethyl sulfoxide (DMSO) and 1% Tween 80], once in a week and stored at 4°C (36). Chronic GVHD recipients were given vehicle or IRX4204 daily at a dose rate of 200 μg/mouse i.p as indicated.
Histopathology and Trichrome Staining
Tissue sections were embedded in optimal cutting temperature (OCT) compound, snap-frozen in liquid nitrogen, and stored at −80°C. Lungs were inflated by 75% OCT before harvest and freezing. Acetone-fixed 6-μm cryosections were hematoxylin and eosin stained and evaluated as described (37). For trichrome staining, 6-μm cryosections were fixed for overnight in Bouin’s solution and stained with the Masson’s trichrome staining kit (Sigma HT15) for detection of collagen deposition.
Statistical Analysis
GraphPad Prism 7 was used to conduct statistical analysis. One-way ANOVA with Bonferroni correction and Student’s t-test were used for statistical analysis as indicated. Error bars indicate mean ± standard error mean (significance: *p <.05; **p <.01; ***p <.001; ****p <.0001).
Results
IRX4204 Prevents and Reverses BO cGVHD
To evaluate the prophylactic efficacy of IRX4204 in cGVHD, we utilized a major MHC mismatched, murine multiorgan system model of cGVHD with BO. In this model, B10.BR recipients were preconditioned with cyclophosphamide and TBI followed by transplantation with donor B6 TCD BM alone or TCD-BM plus a low dose of T cells (13, 16). We previously reported that the loss of pulmonary function due to fibrotic change in the lung was detected as early as day 28 (13). Using a FlexiVent (SCIREQ) system, we performed PFTs on day 28 in cGVHD recipients treated with either vehicle or IRX4204. Recipients receiving IRX4204 daily from day 0 to 28 had significantly improved pulmonary function compared to those given vehicle (Figure 1A). These studies showed that cGVHD with BO was established by day 28 as demonstrated by PFTs and that IRX4204 given days 0–28 was sufficient to prevent cGVHD onset. Furthermore, mice given prophylactic IRX4204 showed improved PFTs by day 56 as compared to control recipients (Figure 1B).
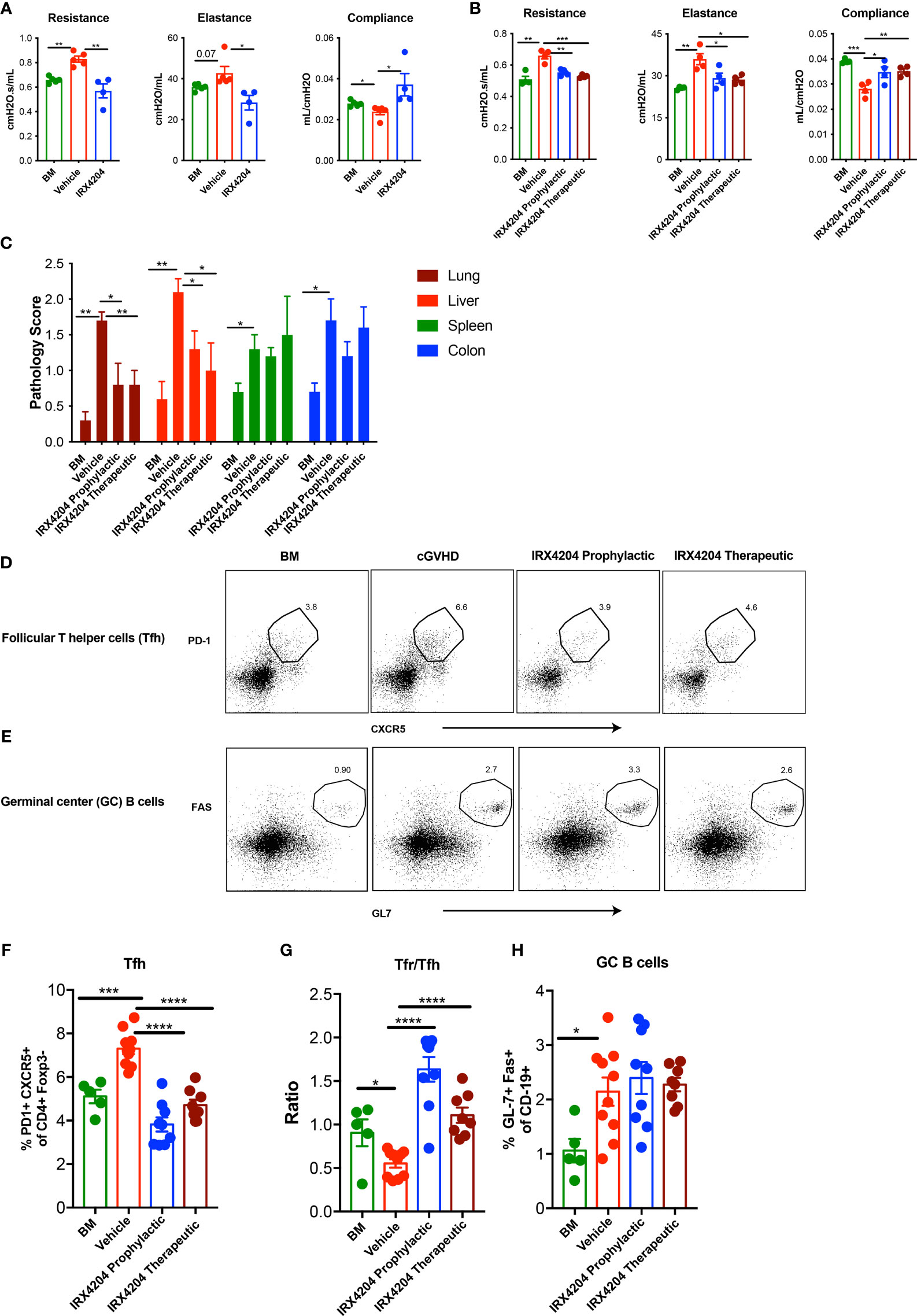
Figure 1 IRX4204 prevents and reverses established cGVHD-mediated BO. Conditioned B10.BR mice were transplanted with B6 donor BM ± T cells. A cohort of BM + T recipients were treated with IRX4204 either from days 0 to 28, or 28 to 56. Pulmonary tests including lung resistance, elastance, and compliance were performed on (A) day 28 and (B) day 56 post-transplantation n = 4 – 5/group. (C) Histopathology scores of hematoxylin and eosin–stained tissue sections from lung, liver, spleen, and colon on recipient on day 58. n = 5/group. Representative flow plots of (D) Tfh and (E) GC B cells. Frequency of Tfh (F), Ratio of Tfr/Tfh (G) and frequency of GC B cells (H) in recipient spleen on day 58. *p <.05; **p <.01; ***p <.001; ****p <.0001. Error bars represent standard error of the mean (SEM); n = 5 - 10 per group.
We next sought to determine whether IRX4204 could reverse the established cGVHD by treating cGVHD mice beginning on day 28. On day 56, cGVHD mice that were continuously treated from day 28 showed improved PFTs as compared to vehicle-treated cGVHD recipients and comparable to BM-only recipients (Figure 1B) Consistent with the reduced cGVHD clinical signs, pathology scores of lungs and liver were significantly lower in recipients of either prophylactic or therapeutic IRX4204 as compared to vehicle controls (Figure 1C). No significant changes were observed in the spleen and colon of mice that received IRX4204.
Increased Tfh and GC B cells can promote while Tfrs can inhibit BO cGVHD pathogenesis by controlling GC formation and allo- or auto-antibody production and deposition in cGVHD target tissues (17, 38). To determine whether IRX4204 treatment induces immune cell changes that could preclude or disrupt GC formation in cGVHD mice, we analyzed the frequency of Tfh, Tfrs, and GC B cells in day 58 post-BMT spleens of recipients treated daily with IRX4204 from day 0 to 28 or day 28 to 56. IRX4204-treated mice had a reduced frequency of pathogenic Tfh with an increased frequency of immunoregulatory Tfrs, resulting in elevated Tfr/Tfh ratios (Figures 1D, F, G). Cumulatively, these data demonstrate that IRX4204 treatment is effective at preventing and reversing cGVHD with BO as measured by PFTs, lung and liver pathology scores, and Tfr/Tfh imbalance associated with diminution of the GC immune responses.
IRX4204 Impairs GC Reactions
Although the GC B-cell frequency was not significantly different between the groups (Figures 1E, H) despite reduced Tfh and increased Tfr and Tfr/Tfh ratio, there was a statistical trend towards smaller GC size in recipients of IRX4204 prophylaxis (Figures 2A, B). Diminished GC size is consistent with the known cause–effect relationship between high GC numbers and size and cGVHD with BO (13, 17). Peri-bronchiolar collagen deposition is a characteristic feature of BO-cGVHD (13). We performed Masson’s trichrome staining to identify collagen in tissue section. Compared to vehicle controls, mice treated with IRX4204 either as prophylaxis or therapy had a decrease in the accumulation of collagen in the lungs around the bronchioles (Figure 2C).
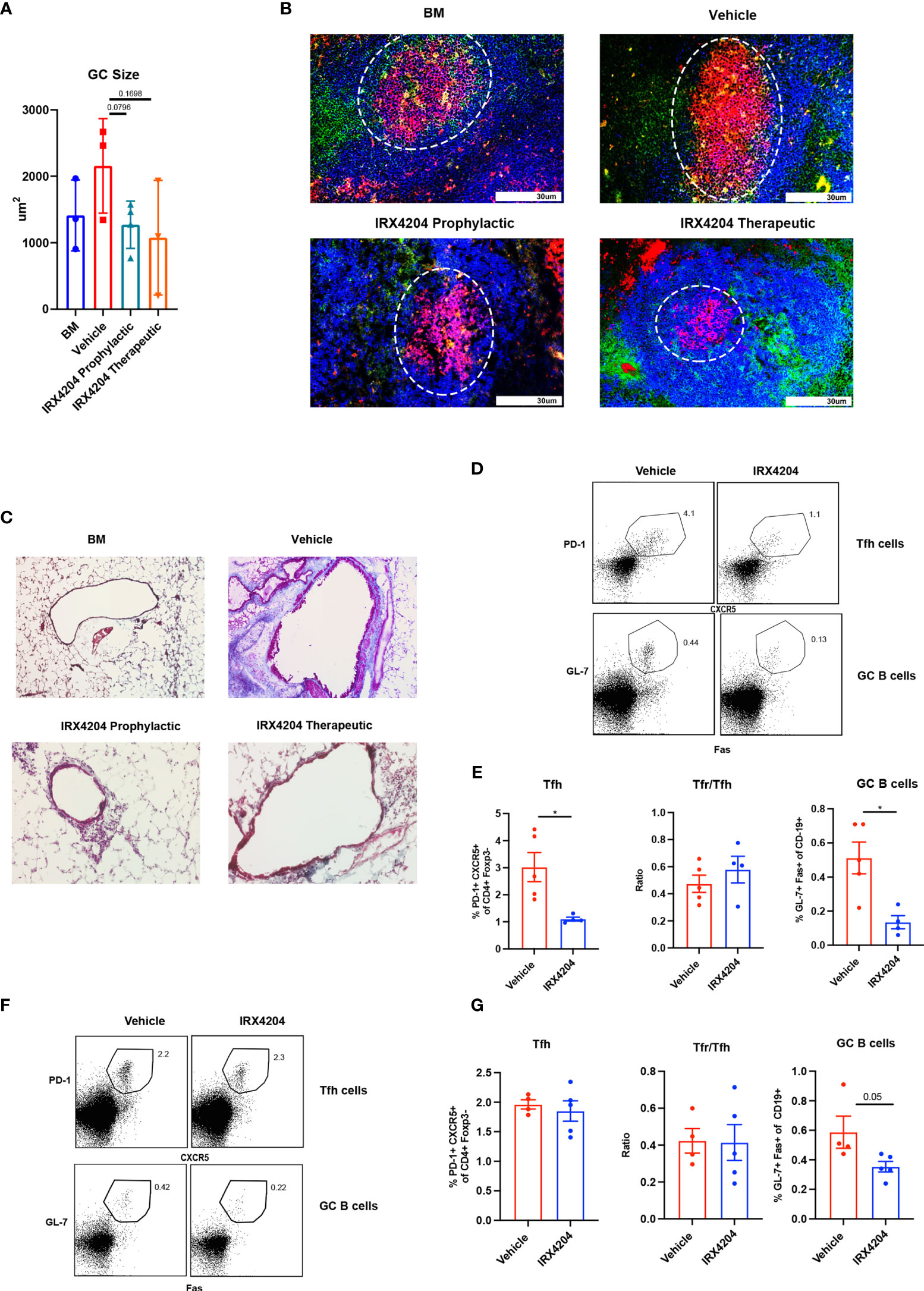
Figure 2 IRX4204 impairs GC B cell response in an immunization model but not in cGVHD. (A, B) Conditioned B10.BR mice were transplanted with B6 donor BM ± T cells. A cohort of BM + T recipients were treated with IRX4204 either from days 0 to 28, or days 28 to 56. n = 3 mice/group. (A) GC size and (B) representative splenic GC immunofluorescence images from BM only and BM plus T-cell mice on day 58 showing peanut agglutinin (PNA; Red). CD4 FITC (green) and B220 BV421 (blue). Germinal centers are highlighted in white circle. An Olympus FluoView500 confocal laser scanning microscope was used to acquire images at magnification 200×. (C) Representative images of Masson’s trichrome staining. Collagen was identified as the area stained in blue. EVOS XL Imaging system was used to acquire images at magnification 200×. (D–G) WT mice were immunized with NP-OVA and treated with either vehicle or IRX4204 daily. (D, E) Flow plots of Tfh, GC B cells, and quantification of GC B cells, Tfh, and Tfr/Tfh ratio from inguinal draining lymph nodes 7 days post-immunization. IRX4204 or vehicle was given from day 0 to 7 (prophylactic). (F, G) Flow plots of Tfh, GC B cells, and quantification of GC B cells, Tfh, and Tfr/Tfh ratio from inguinal draining lymph nodes 14 days post-immunization. IRX4204 or vehicle was given from day 7 to 14 (therapeutic). *p < .05. Error bars represent standard error of the mean (SEM); n = 4/group.
To determine IRX4204 effects on GC reactions due to nominal antigen delivered in adjuvant in a non-cGVHD setting, wild-type mice were immunized with NP-OVA, emulsified in complete H37 Ra. One cohort of mice was treated daily with IRX4204 as prophylaxis for GC formation beginning on the day of immunization. A second cohort was treated with IRX4204 beginning after GC formation beginning on day 7 after immunization. After 7 days of IRX4204 administration, draining lymph nodes were harvested and the frequency of Tfh and GC B cells assayed. IRX4204 treatment as either prophylaxis (Figures 2D, E) or therapy (Figures 2F, G) significantly inhibited the induction of GC B cells compared to the vehicle treatment. However, we found that IRX4204 had the ability to reduce Tfh induction only when administered prophylactically (Figures 2D, E). Despite reduced Tfh in mice given IRX4204 prophylaxis, Tfr/Tfh ratios were unchanged. Not surprisingly, as Tfh were not reduced by IRX4204 given as a therapeutic agent, Tfr/Tfh ratio did differ between IRX4204 and vehicle (Figures 2F, G).
IRX4204 Is Effective as Prophylaxis and Therapy for Sclerodermatous cGVHD
Having demonstrated the potency of IRX4204 in attenuating cGVHD in the BO model that lacks skin manifestations, we next evaluated its efficacy in a classical model of sclerodermatous cGVHD. BALB/c recipients were lethally irradiated and transplanted with multiple minor mismatched B10.D2 donor BM ± T cells. In this model, cutaneous manifestations become apparent at approximately day 21 (39, 40). We tested the prophylactic and therapeutic efficacy of IRX4204 starting treatment from either day 0 or day 21 for a period of 50 days. We found that the administration of IRX4204 either as prophylaxis or therapy significantly improved cGVHD clinical and skin scores compared to vehicle controls (Figures 3A–C).
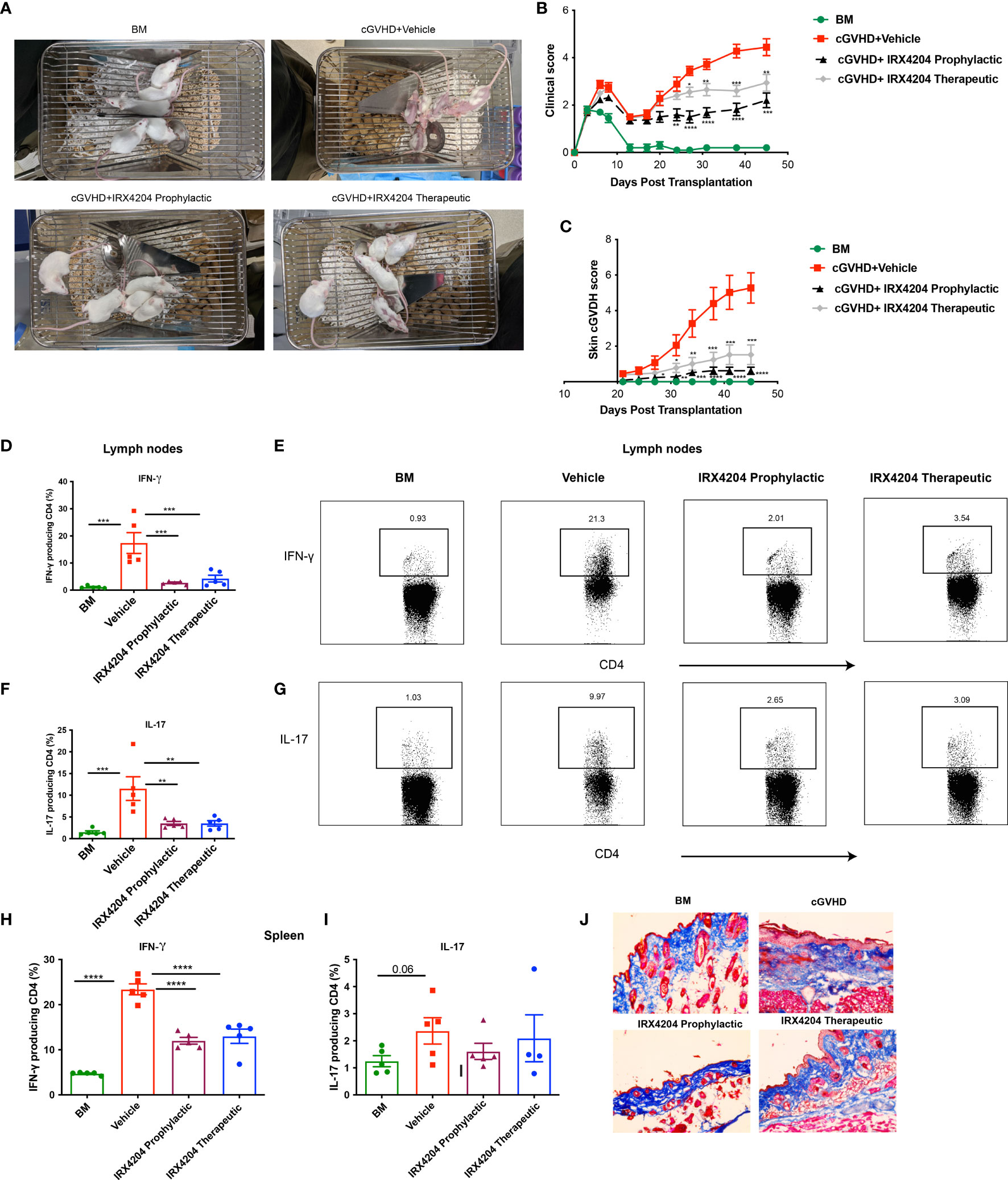
Figure 3 IRX4204 inhibits and treats sclerodermatous cGVHD. Lethally irradiated BALB/c mice were transplanted with B10.D2 BM only or with purified T cells (CD4, CD8: 1.8 x 106 and 0.9 x 106, respectively). Recipients were treated with IRX4204 either from day 0-21 (IRX4204 prophylactic) or day 21 (IRX4204 therapeutic). (A) Photographs of mice in BM, cGVHD + vehicle or IRX4204 treated cGVHD groups. (B) Clinical manifestations of cGVHD were assessed by giving scores to weight loss, activity, posture and fur condition. Healthy mice receive score 0. (C) Skin scores were assessed by measuring the area of skin with fur loss or sclerodermatous lesion. Intact skin was given a score of 0. (D–I) Peripheral lymph nodes (LNs) and spleens (SPL) were harvested on day 50 post-transplantation and stimulated with PMA and ionomycin in vitro. (D–G) The percentages and representative flow plots of (D–E) IFN-γ+ and (F, G) IL-17+ producing CD4+ T cells in LNs are shown. (H, I) The percentages of (H) IFN-γ+ and (I) IL-17+ producing CD4+ T cells in SPL are shown. (J) Trichrome staining of skin. Collagen was stained in blue. *p < .05; **p < .01; ***p < .001; ****p < .0001. Error bars represent standard error of the mean (SEM); n = 8 – 10 per group.
Sclerodermatous cGVHD is mediated by donor CD4+ T cells producing IFN-γ and IL-17 (18). To explore the cellular mechanisms associated with cGVHD protection in IRX4204-treated recipients, we examined the frequency of IFN-γ+ and IL-17+ producing CD4+ T cells in recipient peripheral lymph nodes and spleens. On day 50, compared to control, we found that IRX4204 treatment, both prophylactic and therapeutic, significantly reduced the frequency of CD4+ T cells producing IFN-γ and IL-17 in peripheral lymph nodes (Figures 3D–G). In addition, the frequency of splenic CD4+ IFN-γ+ but not IL-17 producing T cells was significantly reduced in the spleens of mice given IRX4204 as prophylaxis or therapy (Figures 3H, I). To evaluate whether IRX4204 reduces collagen deposition on skin, serially sectioned skin tissues were stained with Masson’s trichrome stain. Compared to vehicle controls, mice treated with IRX4204 either as prophylaxis or therapy had a decrease in the accumulation of collagen in the skin (Figure 3J). Taken together, IRX4204 alleviated sclerodermatous cGVHD associated with reduced Th1 and Th17 differentiation in secondary lymphoid organs.
Discussion
Identification of novel immunomodulatory therapies to prevent and treat cGVHD remains the unmet clinical need in allo-HSCT. In the current study, we demonstrated that a highly potent rexinoid, IRX4204, prevented and treated cGVHD by impairing pathogenic donor T-cell responses in two preclinical models with distinct pathophysiology. Therefore, targeting the RXR pathway with IRX4204 is a promising therapeutic strategy to reduce cGVHD. Furthermore, IRX4204 impairs GC B-cell generation in mice immunized with NP-OVA.
In the MHC mismatched BO model, IRX4204 treatment prevented and reversed established cGVHD as reflected by improved PFTs. Although we found that IRX4204 was effective in reducing the cGVHD-mediated lung and liver pathology, there was no effect on spleen and colon pathology. The lack of effect may be due to contrasting mechanisms of cGVHD in different organs. We previously demonstrated that Tfh, a subset of CD4+ T cells, plays a key role in promoting BO cGVHD pathogenesis by supporting GC B-cell affinity maturation and differentiation to produce pathogenic antibodies (17). An earlier study reported that RXR activation has been shown to negatively affect Tfh differentiation in a chronic model of inflammation (41). A more recent study reported that RARα signaling enhances Tfh differentiation in an airway inflammation mouse model (42), perhaps through inhibiting IL2Rα upregulation on T cells, as IL2R signaling inhibits Tfh differentiation by repressing BCL6. In the current study, we reasoned that IRX4204 would modulate Tfh differentiation in cGVHD mice by directly impairing donor T-cell responses. Indeed, we found that IRX4204 treatment reduced the frequency of pathogenic Tfh cells, consistent with the known direct suppressive effect on the proliferation of murine and human T cells in vitro (31) and in vivo in IRX4204-treated aGVHD mice (31).
Chronic GVHD patients have a reduced frequency of Tregs (43), which leads to the lack of functional tolerance and subsequent immune dysregulation. Restoring immune tolerance through either expansion of Tregs in vivo or adoptive Treg transfer has been shown to be an effective strategy to reduce cGVHD (44–46). Here, we show the increased frequency of Tfrs when recipient mice were treated with IRX4204 compared with vehicle-treated recipients. IRX4204 is a potent activator of Nr4a2, which has been shown to promote Treg generation and stability (47). Consistent with the Nr4a2 activity, IRX4204 fostered in vitro induced Treg (20, 31) and pTreg generation in aGVHD mice (31).
Studies have shown that Tfrs impair the production of pathogenic antibodies by negatively regulating Tfh and GC B-cells responses (38, 48). Therefore, it is likely that both increased Tfr frequency and Tfr/Tfh ratios in IRX4204-treated mice contribute to the alleviated cGVHD. Furthermore, this cGVHD/BO model depends upon Tfh production of IL-21. IL-21 is counterregulatory to pTreg production, as we reported in an aGVHD model (49), and Treg function (50, 51). Taken together, increased Tfr in IRX4204-treated mice may be due to Nr4a2 pathway activation in donor T cells resulting in suppression of Tfh IL-21 production, fostering Treg suppressor function and the generation of Tregs and Tfrs over Tfhs. By employing the NP-OVA immunization model, we observed that prophylactic IRX4204 reduced the frequency of Tfh and GC B cells, although unexpectedly, we did not observe any significant differences in GC B cells between IRX4204 and vehicle-treated mice in cGVHD/BO mice. However, compared to vehicle-treated mice, IRX4204-prophylactically treated mice had a statistical trend toward smaller GC size.
Scleroderma is a serious and severe fibrosing disorder that occurs in the majority of cGVHD patients affecting the skin, subcutaneous tissue, and fascia (52). In an MHC-matched, multiple minor antigen mismatched scleroderma mouse model (32, 53), IRX4204 treatment as either a prophylactic or therapeutic significantly reduced sclerodermatous cGVHD with improved clinical outcomes. A previous study showed that in a scleroderma cGVHD model, transplantation of either donor IL-17−/− or IFN-γ−/− T cells significantly ameliorated the disease (18). In the current study, we observed a reduced frequency of Th1 and Th17 cells in IRX4204-treated recipients, consistent with previous studies showing that IRX4204 impaired Th1 and Th17 differentiation in aGVHD and experimental autoimmune encephalomyelitis, respectively (20, 31). RA signaling is required to maintain the stability of Th1 cells (54). We and others demonstrated that heightened RA synthesis during allo-HSCT exacerbated aGVHD lethality by enhancing Th1 differentiation of donor T cells (55–57). IRX4204 may directly suppress Th1 differentiation via Nr4a2 activation, as Nr4a2 has been shown to repress Th1 lineage commitment (47) or as a result of inadequate RA signaling in donor T cells.
Although RA signaling has been shown to exacerbate aGVHD (55–57), Nishimura and colleagues (18) demonstrated that the in vivo administration of synthetic retinoid attenuated scleroderma cGVHD by reducing the differentiation of Th1 and Th17. Since IRX4204 preferentially activates RXR homodimers, the endogenous RA signaling pathway that is heightened in GVHD may be impaired in cGVHD mice due to the competitive binding of RXRs to the agonist or other receptors, reducing binding with RARs. Whereas the RXR agonist tributyltin inhibited Th17 differentiation that mechanistically may be the consequence of LXR-RXR pathway activation (58), IRX4204 inhibition of Th17 differentiation in vitro and in vivo (20) does not require LXR activation, suggesting that RXR homodimers or other RXR binding partners are involved in controlling Th17 differentiation. Notably, IL-17 is known to support GC formation, ectopic lymphoid follicles, and antibody class switching in mouse B cells (59–61). In cGVHD/BO model, the in vivo administration of neutralizing anti-IL-17 antibody or small molecule RORγt inhibitors given as a therapeutic markedly alleviated cGVHD (62).
Taken together, our results suggest that targeting the RXR pathway with IRX4204 represents a novel immunomodulatory strategy to prevent or treat cGVHD. IRX4204 is currently in phase I and II clinical trials (NCT0154007 and NCT02991651) to treat cancer. Having shown the beneficial effects of IRX4204 in two distinct cGVHD preclinical models, our studies support consideration for clinical testing of IRX4204 in patients who do not respond to FDA-approved drugs for steroid refractory cGVHD.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by University of Minnesota.
Author Contributions
GT designed and performed research, provided, and analyzed the data, and wrote the paper. MZ, FM, RF, JD, SR, MR and EA performed experiments, analyzed the data and edited the manuscript. APM performed histopathological analysis. MS provided IRX4204 and edited the manuscript. BB designed, organized, and supervised research and edited the paper. All authors contributed to the article and approved the submitted version.
Conflict of Interest
BB receives remuneration as an advisor to Kamon Pharmaceuticals, Five Prime Therapeutics, Regeneron Pharmaceuticals, Magenta Therapeutics, and BlueRock Therapeuetics; research support from Fate Therapeutics, RXi Pharmaceuticals, Alpine Immune Sciences, Abbvie, the Leukemia and Lymphoma Society, the Children’s Cancer Research Fund, and the KidsFirst Fund and is a cofounder of Tmunity. Author MS is shareholder, officer, and director of the company Io Therapeutics, Inc., which is developing IRX4204 for commercialization.The reviewer DW has declared a past co-authorship with one of the authors BB at the time of review.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Jamie Panthera for excellent animal husbandry. This work was supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases P01 AI056299, R01 AI034495, and T32 AI007313 (FM), and National Heart, Lung, and Blood Institute R01 HL56067 (BB). GT was supported by the Canadian Institutes of Health Research (CIHR) fellowship.
References
1. Socie G, Ritz J. Current Issues in Chronic Graft-Versus-Host Disease. Blood (2014) 124(3):374–84. doi: 10.1182/blood-2014-01-514752
2. Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-Versus-Host Disease and Therapeutic Targets. N Engl J Med (2017) 377(26):2565–79. doi: 10.1056/NEJMra1703472
3. Wolff D, Gerbitz A, Ayuk F, Kiani A, Hildebrandt GC, Vogelsang GB, et al. Consensus Conference on Clinical Practice in Chronic Graft-Versus-Host Disease (GVHD): First-Line and Topical Treatment of Chronic GVHD. Biol Blood Marrow Transplant (2010) 16(12):1611–28. doi: 10.1016/j.bbmt.2010.06.015
4. Westin JR, Saliba RM, De Lima M, Alousi A, Hosing C, Qazilbash MH, et al. Steroid-Refractory Acute GVHD: Predictors and Outcomes. Adv Hematol (2011) 2011:601953. doi: 10.1155/2011/601953
5. Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and Second-Line Systemic Treatment of Acute Graft-Versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant (2012) 18(8):1150–63. doi: 10.1016/j.bbmt.2012.04.005
6. Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis Obliterans in Chronic Graft-Versus-Host Disease: Analysis of Risk Factors and Treatment Outcomes. Biol Blood Marrow Transplant (2003) 9(10):657–66. doi: 10.1016/S1083-8791(03)00242-8
7. Inamoto Y, Storer BE, Petersdorf EW, Nelson JL, Lee SJ, Carpenter PA, et al. Incidence, Risk Factors, and Outcomes of Sclerosis in Patients With Chronic Graft-Versus-Host Disease. Blood (2013) 121(25):5098–103. doi: 10.1182/blood-2012-10-464198
8. Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis Obliterans Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation-an Increasingly Recognized Manifestation of Chronic Graft-Versus-Host Disease. Biol Blood Marrow Transplant (2010) 16(1 Suppl):S106–14. doi: 10.1016/j.bbmt.2009.11.002
9. Kitko CL, White ES, Baird K. Fibrotic and Sclerotic Manifestations of Chronic Graft-Versus-Host Disease. Biol Blood Marrow Transplant (2012) 18(1 Suppl):S46–52. doi: 10.1016/j.bbmt.2011.10.021
10. Zhang Y, McCormick LL, Desai SR, Wu C, Gilliam AC. Murine Sclerodermatous Graft-Versus-Host Disease, a Model for Human Scleroderma: Cutaneous Cytokines, Chemokines, and Immune Cell Activation. J Immunol (2002) 168(6):3088–98. doi: 10.4049/jimmunol.168.6.3088
11. Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, et al. Donor CD4+ T and B Cells in Transplants Induce Chronic Graft-Versus-Host Disease With Autoimmune Manifestations. Blood (2006) 107(7):2993–3001. doi: 10.1182/blood-2005-09-3623
12. Chu YW, Gress RE. Murine Models of Chronic Graft-Versus-Host Disease: Insights and Unresolved Issues. Biol Blood Marrow Transplant (2008) 14(4):365–78. doi: 10.1016/j.bbmt.2007.12.002
13. Srinivasan M, Flynn R, Price A, Ranger A, Browning JL, Taylor PA, et al. Donor B-Cell Alloantibody Deposition and Germinal Center Formation Are Required for the Development of Murine Chronic GVHD and Bronchiolitis Obliterans. Blood (2012) 119(6):1570–80. doi: 10.1182/blood-2011-07-364414
14. MacDonald KP, Hill GR, Blazar BR. Chronic Graft-Versus-Host Disease: Biological Insights From Preclinical and Clinical Studies. Blood (2017) 129(1):13–21. doi: 10.1182/blood-2016-06-686618
15. Deng R, Hurtz C, Song Q, Yue C, Xiao G, Yu H, et al. Extrafollicular CD4(+) T-B Interactions are Sufficient for Inducing Autoimmune-Like Chronic Graft-Versus-Host Disease. Nat Commun (2017) 8(1):978. doi: 10.1038/s41467-017-00880-2
16. Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A New Murine Model for Bronchiolitis Obliterans Post-Bone Marrow Transplant. Am J Respir Crit Care Med (2007) 176(7):713–23. doi: 10.1164/rccm.200702-335OC
17. Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, et al. Increased T Follicular Helper Cells and Germinal Center B Cells Are Required for cGVHD and Bronchiolitis Obliterans. Blood (2014) 123(25):3988–98. doi: 10.1182/blood-2014-03-562231
18. Nishimori H, Maeda Y, Teshima T, Sugiyama H, Kobayashi K, Yamasuji Y, et al. Synthetic Retinoid Am80 Ameliorates Chronic Graft-Versus-Host Disease by Down-Regulating Th1 and Th17. Blood (2012) 119(1):285–95. doi: 10.1182/blood-2011-01-332478
19. Mangelsdorf DJ, Evans RM. The RXR Heterodimers and Orphan Receptors. Cell (1995) 83(6):841–50. doi: 10.1016/0092-8674(95)90200-7
20. Chandraratna RA, Noelle RJ, Nowak EC. Treatment With Retinoid X Receptor Agonist IRX4204 Ameliorates Experimental Autoimmune Encephalomyelitis. Am J Transl Res (2016) 8(2):1016–26.
21. Du X, Tabeta K, Mann N, Crozat K, Mudd S, Beutler B. An Essential Role for Rxr Alpha in the Development of Th2 Responses. Eur J Immunol (2005) 35(12):3414–23. doi: 10.1002/eji.200535366
22. Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR Modulation in Cancer and Metabolic Disease. Nat Rev Drug Discovery (2007) 6(10):793–810. doi: 10.1038/nrd2397
23. Ren G, Kim T, Kim HS, Young ME, Muccio DD, Atigadda VR, et al. A Small Molecule, UAB126, Reverses Diet-Induced Obesity and Its Associated Metabolic Disorders. Diabetes (2020) 69(9):2003–16. doi: 10.2337/db19-1001
24. Miller VA, Benedetti FM, Rigas JR, Verret AL, Pfister DG, Straus D, et al. Initial Clinical Trial of a Selective Retinoid X Receptor Ligand, LGD1069. J Clin Oncol (1997) 15(2):790–5. doi: 10.1200/JCO.1997.15.2.790
25. Lenhard JM, Lancaster ME, Paulik MA, Weiel JE, Binz JG, Sundseth SS, et al. The RXR Agonist LG100268 Causes Hepatomegaly, Improves Glycaemic Control and Decreases Cardiovascular Risk and Cachexia in Diabetic Mice Suffering From Pancreatic Beta-Cell Dysfunction. Diabetologia (1999) 42(5):545–54. doi: 10.1007/s001250051193
26. Pinaire JA, Reifel-Miller A. Therapeutic Potential of Retinoid X Receptor Modulators for the Treatment of the Metabolic Syndrome. PPAR Res (2007) 2007:94156. doi: 10.1155/2007/94156
27. Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, et al. Design and Synthesis of Potent Retinoid X Receptor Selective Ligands That Induce Apoptosis in Leukemia Cells. J Med Chem (1995) 38(16):3146–55. doi: 10.1021/jm00016a018
28. Lalloyer F, Pedersen TA, Gross B, Lestavel S, Yous S, Vallez E, et al. Rexinoid Bexarotene Modulates Triglyceride But Not Cholesterol Metabolism via Gene-Specific Permissivity of the RXR/LXR Heterodimer in the Liver. Arterioscler Thromb Vasc Biol (2009) 29(10):1488–95. doi: 10.1161/ATVBAHA.109.189506
29. Dickey AS, Sanchez DN, Arreola M, Sampat KR, Fan W, Arbez N, et al. PPARdelta Activation by Bexarotene Promotes Neuroprotection by Restoring Bioenergetic and Quality Control Homeostasis. Sci Transl Med (2017) 9(419). doi: 10.1126/scitranslmed.aal2332
30. Wang J, Bi W, Zhao W, Varghese M, Koch RJ, Walker RH, et al. Selective Brain Penetrable Nurr1 Transactivator for Treating Parkinson’s Disease. Oncotarget (2016) 7(7):7469–79. doi: 10.18632/oncotarget.7191
31. Thangavelu G, Wang C, Loschi M, Saha A, Osborn M, Furlan SN, et al. Repurposing a Novel Anti-Cancer RXR Agonist to Attenuate Acute GVHD and Maintain Graft-Versus-Leukemia Responses. Blood (2020) 137:1090–103. doi: 10.1182/blood.2020005628
32. Du J, Paz K, Flynn R, Vulic A, Robinson TM, Lineburg KE, et al. Pirfenidone Ameliorates Murine Chronic GVHD Through Inhibition of Macrophage Infiltration and TGF-Beta Production. Blood (2017) 129(18):2570–80. doi: 10.1182/blood-2017-01-758854
33. Radojcic V, Pletneva MA, Yen HR, Ivcevic S, Panoskaltsis-Mortari A, Gilliam AC, et al. STAT3 Signaling in CD4+ T Cells Is Critical for the Pathogenesis of Chronic Sclerodermatous Graft-Versus-Host Disease in a Murine Model. J Immunol (2010) 184(2):764–74. doi: 10.4049/jimmunol.0903006
34. Pau G, Fuchs F, Sklyar O, Boutros M, Huber W. EBImage–an R Package for Image Processing With Applications to Cellular Phenotypes. Bioinformatics (2010) 26(7):979–81. doi: 10.1093/bioinformatics/btq046
35. Sage PT, Sharpe AH. In Vitro Assay to Sensitively Measure T(FR) Suppressive Capacity and T(FH) Stimulation of B Cell Responses. Methods Mol Biol (2015) 1291:151–60. doi: 10.1007/978-1-4939-2498-1_13
36. Vuligonda V, Thacher SM, Chandraratna RA. Enantioselective Syntheses of Potent Retinoid X Receptor Ligands: Differential Biological Activities of Individual Antipodes. J Med Chem (2001) 44(14):2298–303. doi: 10.1021/jm0100584
37. Blazar BR, Taylor PA, McElmurry R, Tian L, Panoskaltsis-Mortari A, Lam S, et al. Engraftment of Severe Combined Immune Deficient Mice Receiving Allogeneic Bone Marrow via In Utero or Postnatal Transfer. Blood (1998) 92(10):3949–59. doi: 10.1182/blood.V92.10.3949.422k26_3949_3959
38. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ Follicular Regulatory T Cells Control the Germinal Center Response. Nat Med (2011) 17(8):975–82. doi: 10.1038/nm.2425
39. Claman HN, Jaffee BD, Huff JC, Clark RA. Chronic Graft-Versus-Host Disease as a Model for Scleroderma. II. Mast Cell Depletion With Deposition of Immunoglobulins in the Skin and Fibrosis. Cell Immunol (1985) 94(1):73–84. doi: 10.1016/0008-8749(85)90086-3
40. McCormick LL, Zhang Y, Tootell E, Gilliam AC. Anti-TGF-Beta Treatment Prevents Skin and Lung Fibrosis in Murine Sclerodermatous Graft-Versus-Host Disease: A Model for Human Scleroderma. J Immunol (1999) 163(10):5693–9.
41. Leber A, Abedi V, Hontecillas R, Viladomiu M, Hoops S, Ciupe S, et al. Bistability Analyses of CD4+ T Follicular Helper and Regulatory Cells During Helicobacter Pylori Infection. J Theor Biol (2016) 398:74–84. doi: 10.1016/j.jtbi.2016.02.036
42. Scholz J, Kuhrau J, Heinrich F, Heinz GA, Hutloff A, Worm M, et al. Vitamin A Controls the Allergic Response Through T Follicular Helper Cell as Well as Plasmablast Differentiation. Allergy (2021) 76(4):1109–22. doi: 10.1111/all.14581
43. Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced Frequency of FOXP3+ CD4+CD25+ Regulatory T Cells in Patients With Chronic Graft-Versus-Host Disease. Blood (2005) 106(8):2903–11. doi: 10.1182/blood-2005-03-1257
44. Matsuoka K, Koreth J, Kim HT, Bascug G, McDonough S, Kawano Y, et al. Low-Dose Interleukin-2 Therapy Restores Regulatory T Cell Homeostasis in Patients With Chronic Graft-Versus-Host Disease. Sci Transl Med (2013) 5(179):179ra43. doi: 10.1126/scitranslmed.3005265
45. McDonald-Hyman C, Flynn R, Panoskaltsis-Mortari A, Peterson N, MacDonald KP, Hill GR, et al. Therapeutic Regulatory T-Cell Adoptive Transfer Ameliorates Established Murine Chronic GVHD in a CXCR5-Dependent Manner. Blood (2016) 128(7):1013–7. doi: 10.1182/blood-2016-05-715896
46. Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, et al. Interleukin-2 and Regulatory T Cells in Graft-Versus-Host Disease. N Engl J Med (2011) 365(22):2055–66. doi: 10.1056/NEJMoa1108188
47. Sekiya T, Kashiwagi I, Inoue N, Morita R, Hori S, Waldmann H, et al. The Nuclear Orphan Receptor Nr4a2 Induces Foxp3 and Regulates Differentiation of CD4+ T Cells. Nat Commun (2011) 2:269. doi: 10.1038/ncomms1272
48. Sage PT, Sharpe AH. T Follicular Regulatory Cells in the Regulation of B Cell Responses. Trends Immunol (2015) 36(7):410–8. doi: 10.1016/j.it.2015.05.005
49. Hippen KL, Bucher C, Schirm DK, Bearl AM, Brender T, Mink KA, et al. Blocking IL-21 Signaling Ameliorates Xenogeneic GVHD Induced by Human Lymphocytes. Blood (2012) 119(2):619–28. doi: 10.1182/blood-2011-07-368027
50. Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, et al. IL-21 Counteracts the Regulatory T Cell-Mediated Suppression of Human CD4+ T Lymphocytes. J Immunol (2007) 178(2):732–9. doi: 10.4049/jimmunol.178.2.732
51. Clough LE, Wang CJ, Schmidt EM, Booth G, Hou TZ, Ryan GA, et al. Release From Regulatory T Cell-Mediated Suppression During the Onset of Tissue-Specific Autoimmunity Is Associated With Elevated IL-21. J Immunol (2008) 180(8):5393–401. doi: 10.4049/jimmunol.180.8.5393
52. Lee SJ, Flowers ME. Recognizing and Managing Chronic Graft-Versus-Host Disease. Hematol Am Soc Hematol Educ Program (2008) 2008:134–41. doi: 10.1182/asheducation-2008.1.134
53. Thangavelu G, Du J, Paz KG, Loschi M, Zaiken MC, Flynn R, et al. Inhibition of Inositol Kinase B Controls Acute and Chronic Graft-Versus-Host Disease. Blood (2020) 135(1):28–40. doi: 10.1182/blood.2019000032
54. Brown CC, Esterhazy D, Sarde A, London M, Pullabhatla V, Osma-Garcia I, et al. Retinoic Acid is Essential for Th1 Cell Lineage Stability and Prevents Transition to a Th17 Cell Program. Immunity (2015) 42(3):499–511. doi: 10.1016/j.immuni.2015.02.003
55. Chen X, Dodge J, Komorowski R, Drobyski WR. A Critical Role for the Retinoic Acid Signaling Pathway in the Pathophysiology of Gastrointestinal Graft-Versus-Host Disease. Blood (2013) 121(19):3970–80. doi: 10.1182/blood-2012-08-445130
56. Aoyama K, Saha A, Tolar J, Riddle MJ, Veenstra RG, Taylor PA, et al. Inhibiting Retinoic Acid Signaling Ameliorates Graft-Versus-Host Disease by Modifying T-Cell Differentiation and Intestinal Migration. Blood (2013) 122(12):2125–34. doi: 10.1182/blood-2012-11-470252
57. Thangavelu G, Lee YC, Loschi M, Schaechter KM, Feser CJ, Koehn BH, et al. Dendritic Cell Expression of Retinal Aldehyde Dehydrogenase-2 Controls Graft-Versus-Host Disease Lethality. J Immunol (2019) 202(9):2795–805. doi: 10.4049/jimmunol.1800899
58. Takeuchi H, Yokota-Nakatsuma A, Ohoka Y, Kagechika H, Kato C, Song SY, et al. Retinoid X Receptor Agonists Modulate Foxp3(+) Regulatory T Cell and Th17 Cell Differentiation With Differential Dependence on Retinoic Acid Receptor Activation. J Immunol (2013) 191(7):3725–33. doi: 10.4049/jimmunol.1300032
59. Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, et al. Th17 Cells Induce Ectopic Lymphoid Follicles in Central Nervous System Tissue Inflammation. Immunity (2011) 35(6):986–96. doi: 10.1016/j.immuni.2011.10.015
60. Blaho VA, Buczynski MW, Dennis EA, Brown CR. Cyclooxygenase-1 Orchestrates Germinal Center Formation and Antibody Class-Switch via Regulation of IL-17. J Immunol (2009) 183(9):5644–53. doi: 10.4049/jimmunol.0901499
61. Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, et al. Interleukin 17-Producing T Helper Cells and Interleukin 17 Orchestrate Autoreactive Germinal Center Development in Autoimmune BXD2 Mice. Nat Immunol (2008) 9(2):166–75. doi: 10.1038/ni1552
Keywords: RXR, TFH, TfR, IL-17, germinal center B cells
Citation: Thangavelu G, Zaiken MC, Mohamed FA, Flynn R, Du J, Rhee SY, Riddle MJ, Aguilar EG, Panoskaltsis-Mortari A, Sanders ME and Blazar BR (2022) Targeting the Retinoid X Receptor Pathway Prevents and Ameliorates Murine Chronic Graft-Versus-Host Disease. Front. Immunol. 13:765319. doi: 10.3389/fimmu.2022.765319
Received: 26 August 2021; Accepted: 26 January 2022;
Published: 11 March 2022.
Edited by:
Antoine Toubert, Université Paris Diderot, FranceReviewed by:
Cynthia Giver, Emory University, United StatesDietlinde Wolf, University of Miami, United States
Copyright © 2022 Thangavelu, Zaiken, Mohamed, Flynn, Du, Rhee, Riddle, Aguilar, Panoskaltsis-Mortari, Sanders and Blazar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Govindarajan Thangavelu, Z292aW5kYXJhamFuLnRoYW5nYXZlbHVAZ21haWwuY29t
 Govindarajan Thangavelu
Govindarajan Thangavelu Michael C. Zaiken
Michael C. Zaiken Fathima A. Mohamed
Fathima A. Mohamed Ryan Flynn
Ryan Flynn Jing Du1
Jing Du1 Angela Panoskaltsis-Mortari
Angela Panoskaltsis-Mortari Martin E. Sanders
Martin E. Sanders Bruce R. Blazar
Bruce R. Blazar