- 1Peking University People’s Hospital, Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Beijing, China
- 2Peking-Tsinghua Center for Life Sciences, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China
- 3Research Unit of Key Technique for Diagnosis and Treatments of Hematologic Malignancies, Chinese Academy of Medical Sciences, Beijing, China
For allogeneic hematopoietic stem cell transplantation (allo-HSCT) recipients, preemptive interferon-α (IFN-α) therapy is considered as a useful method to eliminate the minimal residual disease (MRD). Our purpose is to assess the long-term efficacy of preemptive IFN-α therapy in acute myeloid leukemia (AML) patients following allo-HSCT based on two registry studies (#NCT02185261 and #NCT02027064). We would present the final data and unpublished results of long-term clinical outcomes with extended follow-up. We adopted polymerase chain reaction (PCR) and multiparameter flow cytometry (MFC) to monitor MRD, and a positive result of bone marrow specimen examined by either of them would be identified as the MRD-positive status. Subcutaneous injections of recombinant human IFN-α-2b were performed for 6 cycles, and prolonged IFN-α therapy could be permitted at the request of patients. The median cycles were 3.5 (range, 0.5–30.5) cycles. A total of 9 patients suffered from grade ≥3 toxicities (i.e., infectious: n = 6; hematologic: n = 3). The 6-year cumulative incidences of relapse and non-relapse mortality following IFN-α therapy were 13.0% (95% confidence interval [CI], 5.4–20.6%) and 3.9% (95%CI, 0.0–17.6%), respectively. The probability of disease-free survival at 6 years following IFN-α therapy was 83.1% (95%CI, 75.2–91.9%). The probability of overall survival at 6 years following IFN-α therapy was 88.3% (95%CI, 81.4–95.8%). The cumulative incidences of total chronic graft-versus-host disease (cGVHD) and severe cGVHD at 6 years following IFN-α therapy were 66.2% (95%CI, 55.5–77.0%) and 10.4% (95%CI, 3.6–17.2%), respectively. Multivariable analysis showed that an alternative donor was associated with a lower risk of relapse and the better disease-free survival. Thus, preemptive IFN-α therapy could clear MRD persistently, prevent relapse truly, and improve long-term survival in AML patients following allo-HSCT.
Introduction
In acute myeloid leukemia (AML) patients following allogeneic hematopoietic stem cell transplantation (allo-HSCT), relapse is the most important cause for transplant failure (1, 2). Patients who still suffer from the disease while cannot be detected by morphological analysis can be identified by the minimal residual disease (MRD) monitoring (3). Polymerase chain reaction (PCR) assays based on detecting genetic abnormalities associated with leukemia and multiparameter flow cytometry (MFC) based on detecting leukemia-associated immunophenotypes (LAIPs) can be employed to monitor MRD. Many studies provided evidence that MRD monitoring could predict forthcoming relapse after allo-HSCT (3–6).
Impending relapse could be reversed by prompt therapies at the early stage with relatively low-level disease. Thus, patients who have MRD receiving preemptive interventions are reasonable. Unlike maintenance or phylactic treatments, MRD-directed preemptive treatments can help risk stratification and spare some patients in remission from further therapy.
Chemotherapy in combination with donor lymphocyte infusion (Chemo-DLI) has emerged as a major preemptive intervention, and it can persistently clear MRD, prevent relapse, and improve survival (7–10). However, some patients fail to receive DLI because the second donation is unavailable (e.g., the unrelated donor, or the related donor refuse to donate lymphocytes). DLI can induce severe graft-versus-host disease (GVHD) (11). In addition, it is reported that 20–40% patients would suffer from aplasia following DLI (12) which may be related to the extent of residual host hematopoiesis (13). Another potential preemptive intervention, hypomethylating agents (HMAs) treatment, may also be useful for AML patients following allo-HSCT (14, 15). Whereas, several studies reported that the long-term efficacy of HMAs treatment was unsatisfactory, despite it could delay the hematologic relapse (16–19).
The fact that interferon-α (IFN-α) impacts on AML through immune activation (20, 21) has rekindled the interest in the utility of IFN-α in AML patients following allo-HSCT as an immunotherapeutic option (22–26). In addition, it is convenient to perform IFN-α therapy on an outpatient basis. For allo-HSCT recipients, several studies indicated the safety of IFN-α therapy was acceptable (27–30), and our two prospective registry studies (NCT02185261 and NCT02027064) observed that preemptive IFN-α therapy could clear the MRD effectively (31, 32). However, the follow-ups of these studies were relatively short. It is still unknown that whether IFN-α therapy can decrease relapse truly or it can only delay the hematologic relapse. Thus, we should further identify the long-term clinical outcomes of preemptive IFN-α therapy for AML patients receiving allo-HSCT.
Thus, we included AML patients who were enrolled in NCT02185261 and NCT02027064 and aimed to assess the long-term efficacy of preemptive IFN-α therapy in AML patients following allo-HSCT. We would present the final data and unpublished results of long-term clinical outcomes with extended follow-up.
Method
Patients
We have reported the short-term results of two registry studies (i.e., #NCT02185261 and #NCT02027064) which were designed to assess the safety and efficacy of preemptive IFN-α therapy (31, 32). Detailed criteria had been reported and summarized in Supplementary Methods. In brief, AML patients who achieved engraftment and regained MRD positive after allo-HSCT could be enrolled. Considering the potential synergistic effect between Chemo-DLI and IFN-α therapy, the patients receiving both therapies were excluded in this extension study [NCT02185261: n = 15; NCT02027064: n = 9; Chemo-DLI group: n = 15, which had been reported by Mo et al. (33)]. Aiming at further evaluating the long-term efficacy of IFN-α therapy, patients who had MRD and received preemptive Chemo-DLI during the same period were also enrolled as controls (Figure 1) because the long-term efficacy of Chemo-DLI had been confirmed (7, 34). The endpoint analysis of the last follow-up was conducted on July 1, 2021. All participants or guardians gave written informed consent in accordance with the Declaration of the Helsinki, and approval was given by the Peking University People’s Hospital Institutional Review Board.
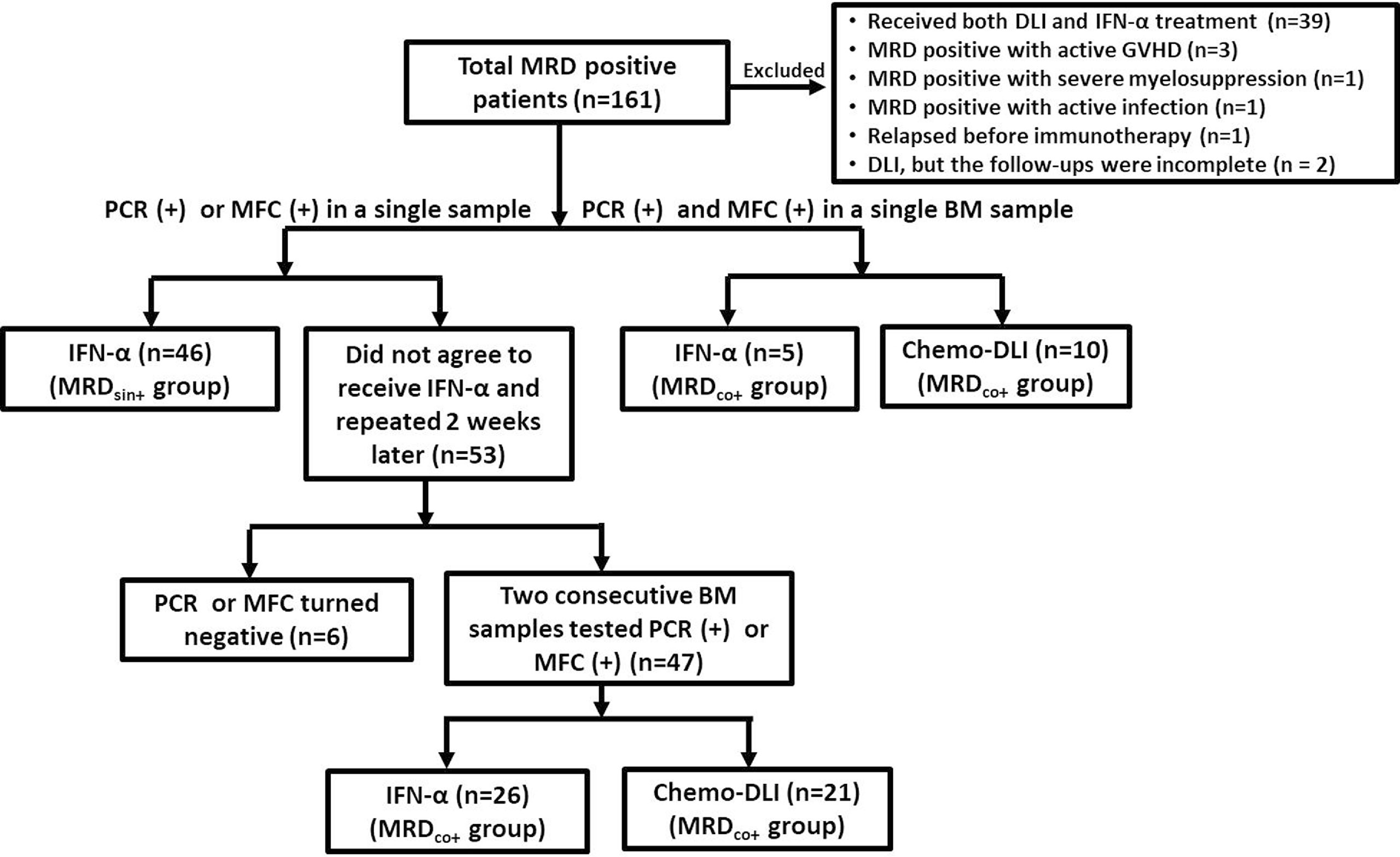
Figure 1 Diagram of enrolled patients. Among the 53 patients with MRDsin+ who did not agree to receive any interventions, 22 of them reduced immunosuppression after the MRDsin+ diagnosis, but only 2 of them showed MRD clearings when the tests were repeated 2 weeks after the first positive results were obtained. Four patients showed MRD negative without any interventions. (MRDsin+ group, n=46; MRDco+ group: IFN-α n = 31, Chemo-DLI n = 31).
Transplant Regimens
The principal myeloablative preconditioning regimen was cytosine arabinoside (Ara-C), busulfan (3.2 mg/kg/day, day -8, day -7, and day -6), cyclophosphamide (1.8 g/m2/day, day -5 and day -4), and simustine (250 mg/m2, day -3). Ara-C was administered at 4 g/m2/day (day -10 and day -9) to the human leukocyte antigen (HLA)-haploidentical donor (HID) group, at 2 g/m2/day (day -10 and day -9) to the HLA-unrelated donor (URD) group, and at 2 g/m2/day (day -9) to the HLA-matched sibling donor (MSD) group. In addition, HID and URD groups received rabbit anti-thymocyte globulin (thymoglobulin, 2.5 mg/kg/d, day -5, day -4, day -3, and day -2; Sanofi, France) to prevent GVHD. In addition, all the patients received cyclosporine A (CSA), mycophenolate mofetil (MMF), and short-term methotrexate (MTX) as GVHD prophylaxis (Supplementary Methods) (2, 35–40).
The Protocols of Preemptive IFN-α Therapy and Chemo-DLI
MRD Monitoring After Allo-HSCT
MRD monitoring based on LAIPs and Wilms’ tumor gene 1 (WT1) in AML patients in the study NCT02185261 [patients with t(15,17), inv (16), t(9,22), t(8,21), or t(16,16) mutations were excluded] and based on RUNX1-RUNX1T1 transcripts in AML patients with t(8,21) in the study NCT02027064 (detailed information were summarized in Supplementary Methods) (31, 32, 41, 42). MRD was monitored at 1, 2, 3, 4.5, 6, 9, and 12 months after allo-HSCT and at 6-month intervals thereafter. We adopted both PCR and MFC to monitor MRD because multiple methods were recommended to ensure the sensitivity and specificity of MRD monitoring (3, 14, 43), and a positive result of bone marrow (BM) specimen examined by either of them would be identified as the MRD-positive status.
MRDsin+ status was defined as cases in which a single BM sample tested positive by PCR or MFC. MRDco+ status included: 1). cases in which 2 consecutive BM samples tested positive by PCR or MFC within a 2-week interval; or 2). those tested positive by both PCR and MFC in a single BM sample (Figure 1).
The Protocols of Preemptive IFN-α Therapy, Chemo-DLI, and GVHD Treatment After Preemptive Immunotherapy
CSA was used as the immunosuppressant and tapered according to the time of MRD occurring. Patients in early-onset MRD (EMRD) group used IFN-α with CSA, and CSA was gradually tapered and then ceased if the patients did not experience new onset GVHD at 100 days after allo-HSCT. For the patients in late-onset MRD (LMRD) group, if they had stopped CSA, they received IFN-α without immunosuppressants. If CSA trough blood concentration was less than 100 ng/ml, LMRD patients stopped CSA before they received IFN-α therapy. Otherwise, LMRD patients received IFN-α and gradually tapered CSA and then ceased if the patients did not experience new onset GVHD (Figure 2). Whether patients discontinued immunosuppressants before IFN-α therapy was described in Table 1.
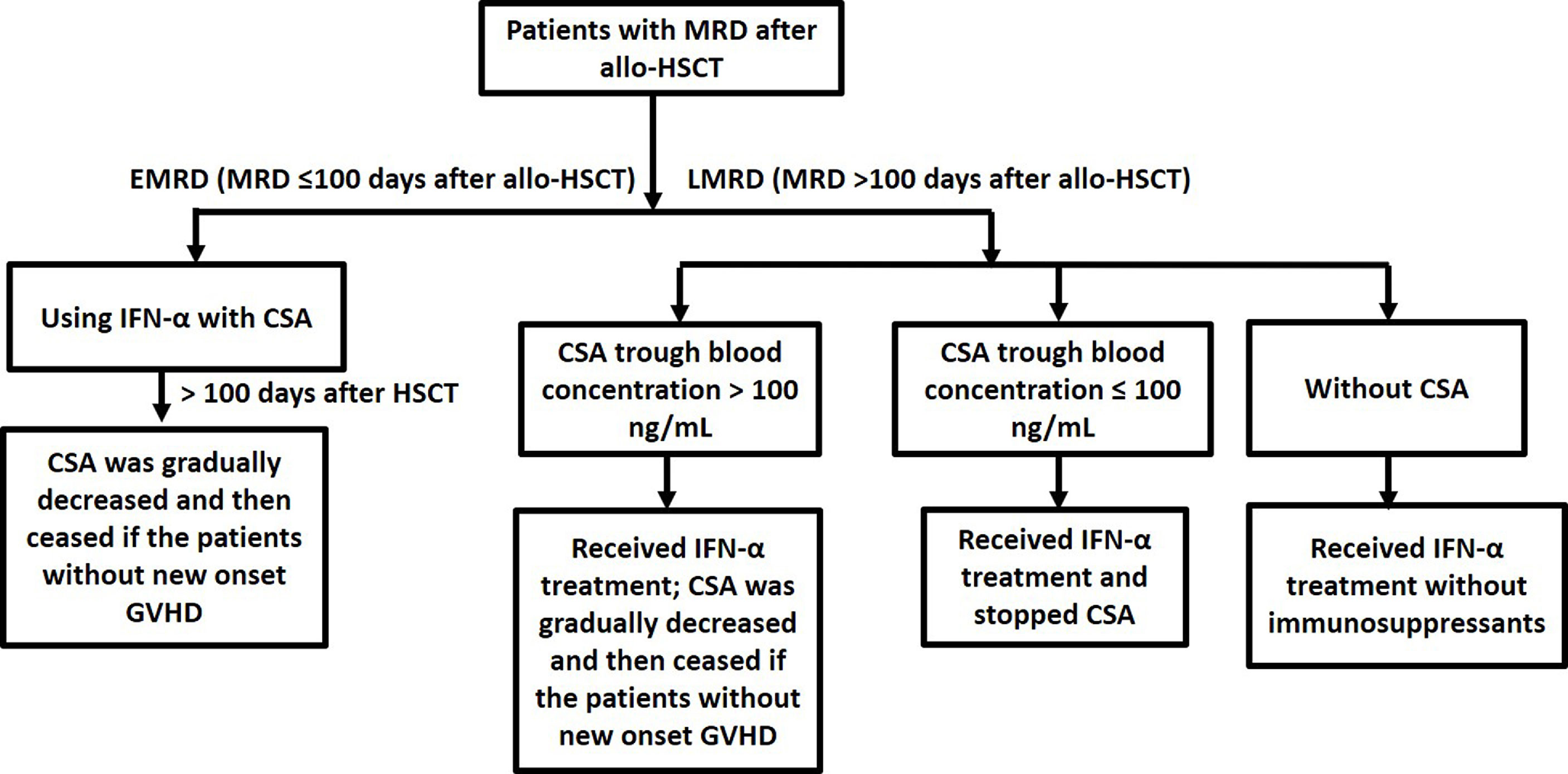
Figure 2 Using cyclosporine A in MRD-positive patients who received preemptive IFN-α therapy after allo-HSCT.
Patients with MRDsin+ were recommended to receive IFN-α therapy. For the patients who did not agree to receive IFN-α therapy (n = 53), the tests were repeated 2 weeks after positive results for PCR or MFC results were obtained. Reducing immunosuppressant use was accepted and not considered as preemptive intervention in the present study (n = 22), but only 2 patients achieved MRD negative after that. Forty-seven patients showed 2 consecutive positive BM samples (i.e., MRDco+) (Figure 1). Patients with MRDco+ should receive preemptive intervention.
Detailed information of IFN-α therapy is summarized in Supplementary Methods. Recombinant human IFN-α-2b injections (Anferon; Tianjin Hualida Biotechnology Co., Ltd., Tianjin, China) were administered subcutaneously for 6 cycles (twice or thrice weekly in every 4 weeks cycle). For patients older than 16 years, IFN-α injections were given at dosages of 3 million units, and for those younger than 16 years, they were given at 3 million units per square meter (capped by 3 million units). Prolonged IFN-α therapy could be permitted at the request of patients.
Because it was unclear that whether IFN-α therapy could play a role in patients in more advanced stage (e.g., MRDco+ or high-level MRD) when these two registry studies started while the efficacy of Chemo-DLI had been already identified (7, 8), it was the first option for them to receive preemptive Chemo-DLI. Patients who were unable to be treated with Chemo-DLI due to provider or patient refusal were enrolled in these two studies and given IFN-α therapy (Figure 1 and Supplementary Methods) (7, 44).
Definition and Assessment
Disease risk index (DRI) was evaluated according to the criteria of Armand et al. (45). The diagnosis of GVHD was made according to international criteria (46, 47). High-level MRD status included: 1). WT1 transcript levels ≥1.0%, 2). RUNX1-RUNX1T1 transcripts <3.5 log reduction from diagnosis, or 3). LAIPs positivity in ≥1.0% of cells with LAIPs in post-HSCT BM samples; the other status was defined as low-level MRD. The definitions of LMRD, EMRD, relapse and, non-relapse mortality (NRM) were shown in Supplementary Methods (31, 32).
Statistical Analysis
In the study of #NCT02185261 and #NCT02027064, the primary endpoint was relapse, and the secondary endpoints were cGVHD, NRM, disease-free survival (DFS), and overall survival (OS). χ2 and Fisher’s exact tests for categorical data and Mann–Whitney U-test for continuous variable were performed to compare the characteristics of patients between groups. The Kaplan–Meier estimator was utilized to calculate the probabilities of OS and DFS. OS was measured until all-cause mortality, and DFS was measured until relapse or death. Patients without an event were censored at final follow-up. The cumulative incidence function was adopted to calculate the incidence of cGVHD, relapse, and NRM (48). Univariable and multivariable Cox regression analysis are described in Supplementary Methods. Two-sided P-values were adopted. Statistical analysis was performed by R software 4.0.0 (https://www.r-project.org) and SPSS 23 (SPSS Inc./IBM, Armonk, NY, USA).
Results
Long-Term Clinical Outcomes of Preemptive IFN-α Therapy
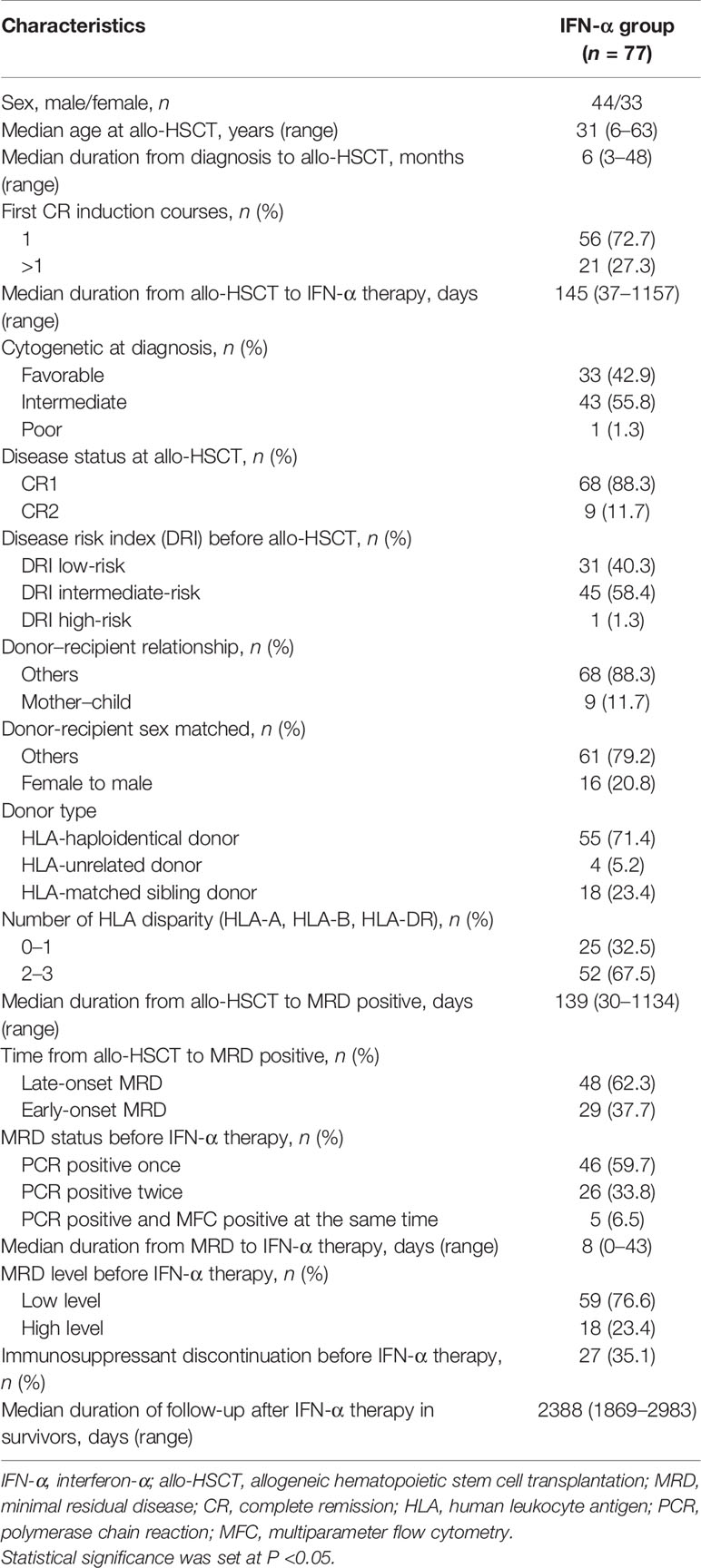
The characteristics of 77 AML patients after preemptive IFN-α therapy following allo-HSCT are summarized in Table 1 and Figure 3. The median age of patients receiving IFN-α was 31 (range, 6–63) years, and 5 children (≤14 years) were included. Thirty-three (42.9%) patients had favorable cytogenetic at diagnosis. Thirty-one and 46 patients were included in MRDco+ and MRDsin+ group, respectively, and the comparisons of their characteristics were summarized in Supplementary Table 1. The median time of follow-up in survivors was 2,388 (range, 1,869–2,983) days. The median cycles of IFN-α therapy were 3.5 (range, 0.5–30.5) cycles, and 27 patients received more than 6 cycles. Nine patients suffered from grade ≥3 toxicities (i.e., infectious: n = 6; hematologic: n = 3). MRD evolution after IFN-α therapy had been described in detail (31, 32). Characteristics of aGVHD following IFN-α therapy are shown in Supplementary Table 2. In this extension study, we focused on the long-term clinical outcomes of patients in these two registry studies, and the short-term clinical outcomes which had been reported were not repeated.
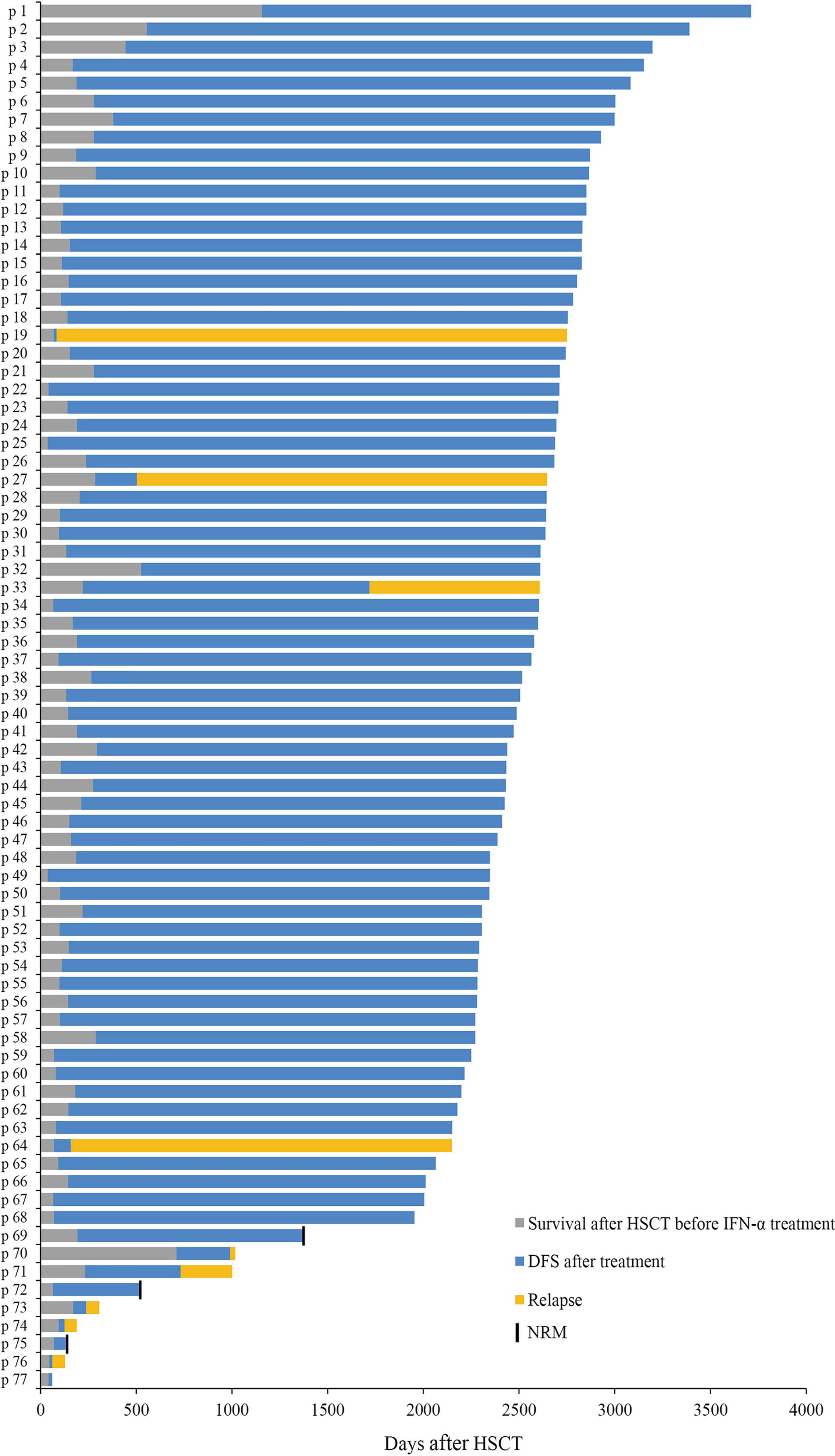
Figure 3 Response. Swimmer plot displaying all patients who received preemptive IFN-α therapy after allo-HSCT.
cGVHD
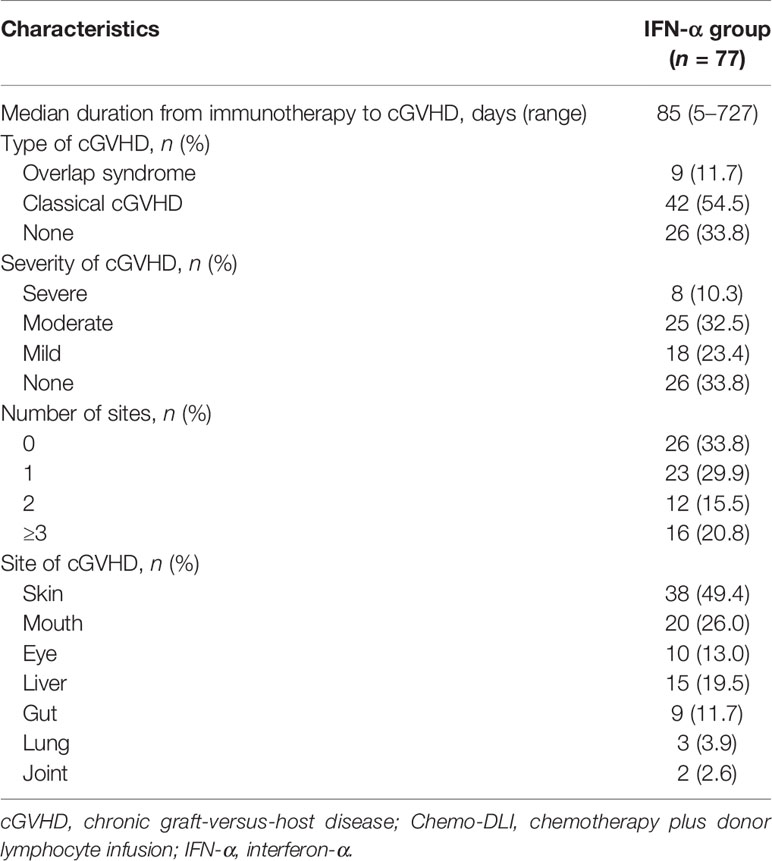
The cGVHD characteristics are summarized in Table 2. Fifty-one patients experienced cGVHD after IFN-α therapy, 29 had been reported previously while 22 were identified during the extended follow-up. The median duration from IFN-α therapy to cGVHD was 85 (range, 5–727) days. The 6-year cumulative incidences of total cGVHD and severe cGVHD following IFN-α therapy were 66.2% (95% confidence interval [CI], 55.5–77.0%) and 10.4% (95%CI, 3.6–17.2%), respectively.
Relapse
Ten patients showed relapse following preemptive IFN-α therapy, 7 had been reported previously while 3 were identified during the extended follow-up. The median duration from IFN-α therapy to relapse was 79 (range, 15–1,499) days. The 6-year cumulative incidence of relapse (CIR) following preemptive IFN-α therapy was 13.0% (95%CI, 5.4–20.6%), which was comparable between MRDsin+ and MRDco+ groups (8.7% vs. 19.4%, P = 0.173, Supplementary Figure 1A), and was lower in the low-level group compared with high-level group (8.5% vs. 27.8%, P = 0.024, Figure 4A). The 6-year CIR was 7.7% (95%CI, 0.0–18.1%) and 15.7% (95%CI, 5.7–25.7%), respectively, for those detected MRD within and beyond 100 days after allo-HSCT (P = 0.315, Supplementary Figure 2A), which was 11.5% (95%CI, 2.7–20.3%) and 16.0% (95%CI, 1.3–30.7%), respectively, for those detected MRD within and beyond 6 months after allo-HSCT (P = 0.669, Supplementary Figure 2B).
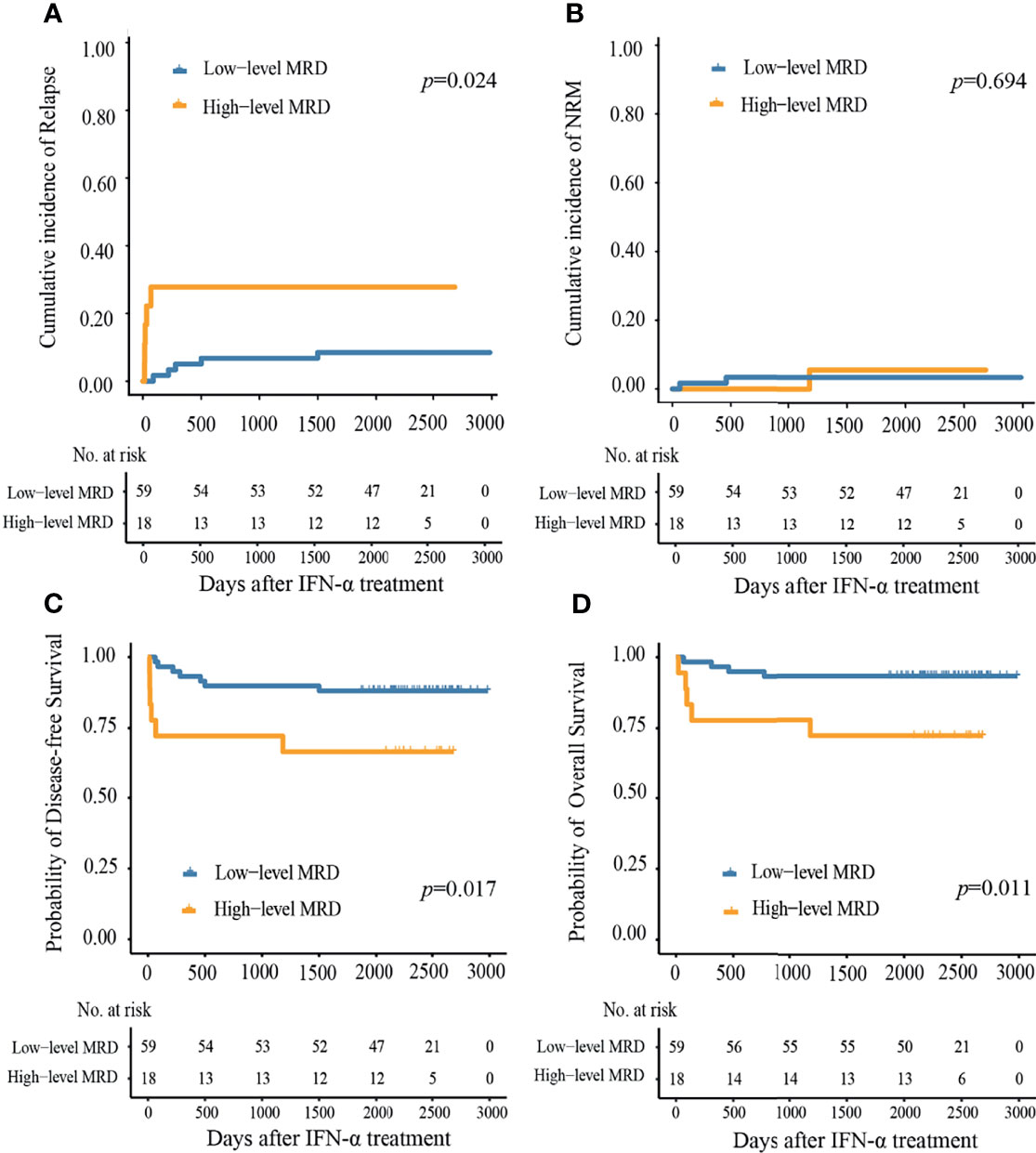
Figure 4 Cumulative incidence of (A) relapse, (B) non-relapse mortality, (C) disease-free survival, and (D) overall survival at 6 years after preemptive IFN-α therapy in the low- and high-level MRD groups.
NRM
Supplementary Table 3 showed the causes of NRM. Three patients died of NRM after preemptive IFN-α therapy, 2 had been reported previously while 1 was identified during the extended follow-up. The median duration from preemptive IFN-α therapy to NRM was 460 (range, 52–1181) days. The 6-year cumulative incidence of NRM following IFN-α therapy was 3.9% (95%CI, 0.0–17.6%), which was 4.3 and 3.2%, respectively, in the MRDsin+ group and MRDco+ group (Supplementary Figure 1B), and was 5.6 and 3.4%, respectively, in the high- and low-level groups (Figure 4B).
DFS
At 6 years following IFN-α therapy, the probability of DFS was 83.1% (95%CI, 75.2–91.9%). They were comparable between MRDsin+ group and MRDco+ group (87.0% vs. 77.4%, P = 0.270, Supplementary Figure 1C), and were worse in high-level MRD group compared to those of low-level MRD group (66.7% vs. 88.1%, P = 0.017, Figure 4C).
OS
At 6 years following IFN-α therapy, the probability of OS was 88.3% (95%CI, 81.4–95.8%). They were comparable between MRDsin+ and MRDco+ groups (91.3% vs. 83.9%, P = 0.300, Supplementary Figure 1D), and were worse in high-level MRD group compared to those of low-level MRD group (72.2% vs. 93.2%, P = 0.011, Figure 4D).
Multivariable Analysis
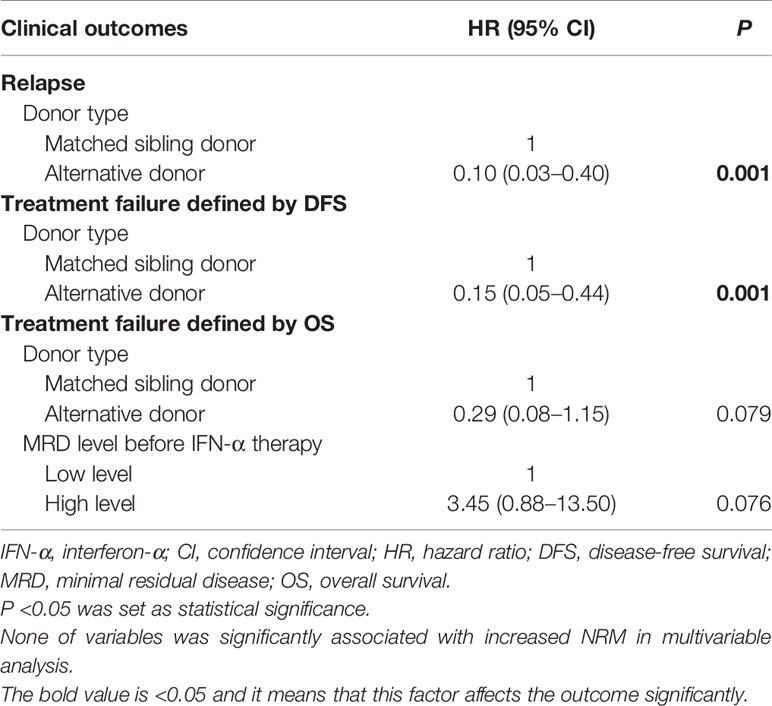
In patients receiving preemptive IFN-α therapy, an alternative donor was associated with a lower risk of relapse and the better DFS. An alternative donor and a low-level MRD before IFN-α therapy were associated with the better OS, of borderline statistical significance (Table 3 and Supplementary Table 4).
Long-Term Clinical Outcomes of Preemptive Chemo-DLI
Thirty-one patients received preemptive Chemo-DLI during the same period (Supplementary Table 5). The median time of follow-up in survivors was 2,696 (range, 2,190–3,072) days. The median courses of DLI were 1 (range, 1–6) courses, and 4 of them received more than 1 course. The cGVHD occurred in 15 patients following Chemo-DLI, the median duration from Chemo-DLI to cGVHD was 59 (range, 33–404) days. The cumulative incidences of total and severe cGVHD at 6 years following preemptive Chemo-DLI were 48.4% (95%CI, 30.1–66.7%) and 22.6% (95%CI, 7.5–37.7%), respectively.
After preemptive Chemo-DLI, relapse occurred in 10 patients and the median duration from DLI to relapse was 59 (range, 21–207) days. NRM occurred in 6 patients following preemptive Chemo-DLI (Supplementary Table 3), and the median duration from DLI to NRM was 97 (range, 20–362) days. The 6-year cumulative incidence of NRM and relapse following Chemo-DLI was 19.4% (95%CI, 5.1–33.7%) and 32.3% (95%CI, 15.4–49.2%), respectively. The 6-year probability of DFS after Chemo-DLI was 48.4% (95%CI, 33.6–69.6%), and the 6-year probability of OS after Chemo-DLI was 64.5% (95%CI, 49.7–83.8%).
In patients with high-level MRD, the 6-year cumulative incidence of relapse and DFS following IFN-α were comparable to that of Chemo-DLI group (relapse, 27.8% vs. 45.5%, P = 0.430; DFS, 66.7% vs. 41.7%, P = 0.190). In patients with MRDco+, the 6-year cumulative incidence of relapse following IFN-α group was comparable with Chemo-DLI group (19.5% vs. 35.6%, P = 0.174), and the 6-year probability of DFS of IFN-α group was better than that of Chemo-DLI group (77.4% vs. 48.4%, P = 0.017) (Supplementary Figure 3).
In the cohort including patients receiving preemptive IFN-α therapy and Chemo-DLI, multivariable analysis showed that MRD status and intervention methods (i.e., MRDco+ receiving Chemo-DLI vs. MRDco+ receiving IFN-α therapy vs. MRDsin+ receiving IFN-α therapy) were associated with clinical outcomes, and the MRDsin+ receiving IFN-α therapy group had a lower relapse risk, a lower risk of NRM and the better survival (Supplementary Tables 6, 7).
Clinical Outcomes of MRD-Positive Patients Without IFN-α Therapy During the Same Period
During the same period, 11 patients with MRD failed to receive preemptive IFN-α therapy because of following reasons: active GVHD (n = 3), severe myelosuppression (n = 1), active infection: (n = 1), and MRD turned negative without interventions (n = 6) (Supplementary Table 8 and Figure 1). The median time of follow-up in survivors was 2,054 (range, 1,591–2,454) days. Seven of them experienced relapse. The 6-year cumulative incidence of CIR and NRM following MRD positive was 63.6% (95%CI, 32.7–94.5%) and 0.0%, respectively. The 6-year probability of DFS following MRD positive was 36.4% (95%CI, 16.6–79.5%) and 6-year probability of OS following MRD positive was 54.5% (95%CI, 31.8–93.6%) (Supplementary Figure 4).
Discussion
Numerous studies have suggested that IFN-α could play a role in inducing anti-leukemic responses in vivo (20); however, only single case reports or studies with small sample sizes have supported that IFN-α could be a treatment choice for AML (22, 49–51). Thus, the clinical utility of IFN-α in AML patients has not been established (20). In this extension study, the 6-year rates of relapse, NRM, DFS, and OS following preemptive IFN-α therapy were 13.0, 3.9, 83.1, and 88.3%, respectively. To our knowledge, this extension study is the first to confirm the long-term efficacy of preemptive IFN-α therapy in AML patients following allo-HSCT. In addition, this study confirmed the persistent anti-leukemic responses induced by IFN-α therapy in AML patients.
We previously reported that over 70% of MRD patients achieved a negative status following IFN-α therapy (31, 32). In this extension study, we observed that the long-term efficacy of preemptive IFN-α therapy was satisfactory. Although several studies have reported that maintenance IFN-α therapy could not prevent relapse in AML patients who received chemotherapy (20, 52, 53), Jiang et al. (21) recently reported that maintenance IFN-α therapy could prevent relapse in favorable-risk AML after consolidation chemotherapy. For patients receiving allo-HSCT, the most important mechanism for clearing leukemia cells is the graft-versus-leukemia effect. IFN-α therapy showed immunomodulatory effects in MRD patients following allo-HSCT (54), and the anti-leukemic activity of IFN-α might be through immune activation (20, 25). In addition, IFN-α might preferably be chosen by leukemia patients with a low tumor burden (20). Therefore, IFN-α therapy would be more beneficial for MRD patients following allo-HSCT.
RUNX1-RUNX1T1 tested by RQ-PCR in NCT02027064 and LAIPs tested by MFC in NCT02185261 were used as markers for MRD. RUNX1-RUNX1T1, which is one of the recurrent genetic abnormalities, is proved to be a stable and effective MRD marker (55, 56). In addition, we observed that the relapse rate was nearly one-third, even in patients with low-level RUNX1-RUNX1T1 after allo-HSCT (Qin et al., data unpublished), if no preemptive interventions were administered. Zhao et al. reported that the sensitivity and specificity for LAIPs to predict relapse were 25.9 and 98.8% (57, 58), respectively. Moreover, the relapse rate of patients who showed LAIPs positive after allo-HSCT was reported to be 82.4% (43). However, although the sensitivity of MFC is relatively low, it is still recommended as an accepted MRD marker by the European LeukemiaNet (ELN) consensus (59).
We previously reported that WT1-positive patients were more likely to experience relapse compared to persistent WT1-negative patients after allo-HSCT (7, 28, 41), and other institutes reported similar results as well (34, 60–62). In particular, Zhao et al. (43) reported that the relapse rate in WT1-positive patients was 60.7%, and the sensitivity and specificity for WT1 to predict relapse were 68.5 and 90.6%, respectively. However, some studies excluded WT1 from MRD markers for AML and doubted its specificity and sensitivity (59). Thus, we combined WT1 with LAIPs to further improve the sensitivity and specificity (3, 43). Moreover, several studies reported that combined WT1 and LAIPs could predict relapse and direct preemptive interventions effectively (7, 8, 28, 43, 63). Thus, the methods for MRD monitoring were reliable in the present study.
Nevertheless, because WT1 is not a leukemia specific marker, patients receiving WT1-directed IFN-α therapy may be at risk of overtreatment. We observed that few cases of severe toxicity occurred during the treatment of IFN-α, which may have minimized the influence of the relatively low specificity of WT1 monitoring. In addition, the risk of post-transplant relapse could also be reduced by maintenance of IFN-α therapy after allo-HSCT (25, 64). New molecular markers with higher sensitivity and specificity could further improve the efficacy of preemptive IFN-α therapy in AML patients.
MRDco+ was suggested to be a more advanced stage for AML than MRDsin+, and tapering immunosuppressants alone could not clear MRD effectively. Zhao et al. (43) reported that MRDco+ patients had a higher rate of relapse (WT1+ twice: 72.0%; MFC+ twice: 100.0%; MFC+ and WT1+: 92.3%) than MRDsin+ patients (WT1+ once: 60.7%; MFC+ once: 82.4%). In addition, of the 53 MRDsin+ patients for whom repeated tests were conducted 2 weeks after positive results were obtained, 47 showed two consecutive positive BM samples (MRDco+), although 20 of them had tapered the immunosuppressants in the present study. Thus, the 6-year CIR of MRDsin+ patients receiving preemptive IFN-α therapy was only 8.7%, suggesting that this strategy contributed towards controlling the disease promptly.
Preemptive Chemo-DLI was preferred in patients in MRDco+ patients when these two registry studies started, and those who were unable to receive DLI were enrolled to receive preemptive IFN-α therapy. However, the clinical outcomes of the MRDsin+ and MRDco+ groups were comparable in patients after preemptive IFN-α therapy. In addition, MRDco+ patients who received IFN-α therapy achieved better DFS than those who received Chemo-DLI. Thus, although some patients in more advanced stages choosing Chemo-DLI may induce unavoidable bias, it might not influence the favorable outcomes of preemptive IFN-α therapy.
In this extension study, the 6-year rates for CIR, NRM, DFS, and OS following Chemo-DLI were 32.3, 19.4, 48.4, and 64.5%, respectively. Although this was similar to our previous results (8), Chemo-DLI did not appear to be superior to IFN-α therapy. This might be because nearly 60% of the patients received preemptive IFN-α therapy due to MRDsin+. Most of them could clear MRD and promptly stop the evolution from MRDsin+ to MRDco+, which would screen patients who were more sensitive to immunotherapy. We observed that more patients with high-level MRD were enrolled in the Chemo-DLI group, which may be because some of them disagreed to receive IFN-α therapy. Although high-level MRD patients receiving preemptive IFN-α therapy showed outcomes similar to those receiving Chemo-DLI, it could not be concluded that IFN-α therapy was superior to Chemo-DLI in AML patients with MRD because this was not a randomized controlled trial (RCT). NPM1 is recognized as a molecular marker for MRD assessment. In the present study, four patients had NPM1 mutation at diagnosis. One of them achieved NPM1 positive when he was categorized as MRD positive (i.e., LAIPs positive). However, the other 3 patients did not monitor NPM1 regularly after allo-HSCT because our institute did not make NPM1 monitoring mandatory for AML patients receiving HSCT in 2014–2015. NPM1 has been added to the panel of markers for MRD monitoring now (59), and future studies can further assess the efficacy of preemptive IFN-α therapy based on NPM1 monitoring.
This study has some limitations. This was not a randomized controlled trial and selection bias was a pertinent issue. MRD patients who failed to receive preemptive IFN-α therapy during the same period had several complications (e.g., active infection or GVHD), which may have contributed to their shorter survival. Future RCTs should evaluate the efficacy of preemptive IFN-α therapy in AML patients in greater detail. Considering that MRD monitoring methods are relatively complicated and not popularized to every hospital, and Magenau et al. (25) also reported that early administration of type-1 IFN could limit relapse after allo-HSCT without increasing toxicity or rates of severe aGVHD. Thus, patients who could not monitor MRD regularly after allo-HSCT received IFN-α maintenance therapy is also reasonable. Moreover, the efficacy of preemptive IFN-α therapy and IFN-α maintenance therapy for relapse prophylaxis could also be compared by RCTs in the future.
In summary, our study illustrated that for AML patients with MRD following allo-HSCT, preemptive IFN-α therapy could clear MRD persistently, prevent relapse, and lead to improvements in long-term survival. This strategy was adopted to provide appropriate and timely therapy to suitable patients. It is convenient to perform IFN-α therapy without severe toxicity on an outpatient basis. Therefore, preemptive IFN-α therapy can be popularized easily, and it could help improve relapse prophylaxis strategies in AML patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking University People’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
X-DM and X.-J.H. designed the study. M-ZS, X-HZ, L-PX, YW, C-HY, HC, Y-HC, WH, F-RW, J-ZW, X-SZ, Y-ZQ, Y-JC, and K-Y L conducted data collection. M-ZS, X-DM, and X-JH conducted data analysis and drafted manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Program of the National Natural Science Foundation of China (grant number 82170208), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant number 81621001), the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant number 2019-I2M-5-034), the Key Program of the National Natural Science Foundation of China (grant number 81930004), and the Fundamental Research Funds for the Central Universities.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors appreciate Dr. Dao-Xing Deng for his help in data collection, and thank Shan-Mei Liao, Yue Cai, and Hui-Xin Liu for their assistances in data analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.757002/full#supplementary-material
References
1. Yan CH, Xu LP, Wang FR, Chen H, Han W, Wang Y, et al. Causes of Mortality After Haploidentical Hematopoietic Stem Cell Transplantation and the Comparison With HLA-Identical Sibling Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2016) 51(3):391–7. doi: 10.1038/bmt.2015.306
2. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Disease Risk Comorbidity Index for Patients Receiving Haploidentical Allogeneic Hematopoietic Transplantation. Engineering (2021) 7(2):162–9. doi: 10.1016/j.eng.2020.12.005
3. Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML After Hematopoietic Stem Cell Transplantation: Methods of Monitoring and Preventive Strategies. A Review From the ALWP of the EBMT. Bone Marrow Transplant (2016) 51(11):1431–8. doi: 10.1038/bmt.2016.167
4. Campana D, Leung W. Clinical Significance of Minimal Residual Disease in Patients With Acute Leukaemia Undergoing Haematopoietic Stem Cell Transplantation. Br J Haematol (2013) 162(2):147–61. doi: 10.1111/bjh.12358
5. Mo XD, Lv M, Huang XJ. Preventing Relapse After Haematopoietic Stem Cell Transplantation for Acute Leukaemia: The Role of Post-Transplantation Minimal Residual Disease (MRD) Monitoring and MRD-Directed Intervention. Br J Haematol (2017) 179(2):184–97. doi: 10.1111/bjh.14778
6. Wang Y, Chen H, Chen J, Han M, Hu J, Jiong H, et al. The Consensus on the Monitoring, Treatment, and Prevention of Leukemia Relapse After Allogeneic Hematopoietic Stem Cell Transplantation in China. Cancer Lett (2018) 438:63–75. doi: 10.1016/j.canlet.2018.08.030
7. Yan C, Liu D, Liu K, Xu L, Liu Y, Chen H, et al. Risk Stratification-Directed Donor Lymphocyte Infusion Could Reduce Relapse of Standard-Risk Acute Leukemia Patients After Allogeneic Hematopoietic Stem Cell Transplantation. Blood (2012) 119(14):3256–62. doi: 10.1182/blood-2011-09-380386
8. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Salvage Chemotherapy Followed by Granulocyte Colony-Stimulating Factor-Primed Donor Leukocyte Infusion With Graft-vs.-Host Disease Control for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation: Prognostic Factors and Clinical Outcomes. Eur J Haematol (2016) 96(3):297–308. doi: 10.1111/ejh.12591
9. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Comparison of Outcomes After Donor Lymphocyte Infusion With or Without Prior Chemotherapy for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Ann Hematol (2017) 96(5):829–38. doi: 10.1007/s00277-017-2960-7
10. Schmid C, Kuball J, Bug G. Defining the Role of Donor Lymphocyte Infusion in High-Risk Hematologic Malignancies. J Clin Oncol (2021) 39(5):397–418. doi: 10.1200/jco.20.01719
11. Orti G, Barba P, Fox L, Salamero O, Bosch F, Valcarcel D. Donor Lymphocyte Infusions in AML and MDS: Enhancing the Graft-Versus-Leukemia Effect. Exp Hematol (2017) 48:1–11. doi: 10.1016/j.exphem.2016.12.004
12. Keil F, Haas OA, Fritsch G, Kalhs P, Lechner K, Mannhalter C, et al. Donor Leukocyte Infusion for Leukemic Relapse After Allogeneic Marrow Transplantation: Lack of Residual Donor Hematopoiesis Predicts Aplasia. Blood (1997) 89(9):3113–7. doi: 10.1182/blood.V89.9.3113
13. Bar M, Sandmaier BM, Inamoto Y, Bruno B, Hari P, Chauncey T, et al. Donor Lymphocyte Infusion for Relapsed Hematological Malignancies After Allogeneic Hematopoietic Cell Transplantation: Prognostic Relevance of the Initial CD3+ T Cell Dose. Biol Blood Marrow Transplant (2013) 19(6):949–57. doi: 10.1016/j.bbmt.2013.03.001
14. Yafour N, Beckerich F, Bulabois CE, Chevallier P, Daguindau E, Dumesnil C, et al. How to Prevent Relapse After Allogeneic Hematopoietic Stem Cell Transplantation in Patients With Acute Leukemia and Myelodysplastic Syndrome. Curr Res Transl Med (2017) 65(2):65–9. doi: 10.1016/j.retram.2017.06.001
15. Schroeder T, Rautenberg C, Haas R, Germing U, Kobbe G. Hypomethylating Agents for Treatment and Prevention of Relapse After Allogeneic Blood Stem Cell Transplantation. Int J Hematol (2018) 107(2):138–50. doi: 10.1007/s12185-017-2364-4
16. Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for Treatment of Imminent Relapse in MDS or AML Patients After Allogeneic HSCT: Results of the RELAZA Trial. Leukemia (2012) 26(3):381–9. doi: 10.1038/leu.2011.234
17. Drozd-Sokołowska J, Gil L, Waszczuk-Gajda A, Mądry K, Piekarska A, Dutka M, et al. Azacitidine Use After Allogeneic Stem Cell Transplantation-Results From the Polish Adult Leukemia Group. Transplant Proc (2016) 48(5):1802–5. doi: 10.1016/j.transproceed.2016.01.078
18. Sockel K, Wermke M, Radke J, Kiani A, Schaich M, Bornhauser M, et al. Minimal Residual Disease-Directed Preemptive Treatment With Azacitidine in Patients With NPM1-Mutant Acute Myeloid Leukemia and Molecular Relapse. Haematologica (2011) 96(10):1568–70. doi: 10.3324/haematol.2011.044388
19. Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable Residual Disease-Guided Treatment With Azacitidine to Prevent Haematological Relapse in Patients With Myelodysplastic Syndrome and Acute Myeloid Leukaemia (RELAZA2): An Open-Label, Multicentre, Phase 2 Trial. Lancet Oncol (2018) 19(12):1668–79. doi: 10.1016/s1470-2045(18)30580-1
20. Anguille S, Lion E, Willemen Y, Van Tendeloo VF, Berneman ZN, Smits EL. Interferon-Alpha in Acute Myeloid Leukemia: An Old Drug Revisited. Leukemia (2011) 25(5):739–48. doi: 10.1038/leu.2010.324
21. Jiang H, Liu XH, Kong J, Wang J, Jia JS, Lu SY, et al. Interferon-α as Maintenance Therapy can Significantly Reduce Relapse in Patients With Favorable-Risk Acute Myeloid Leukemia. Leuk Lymphoma (2021) 62(12):2949–56. doi: 10.1080/10428194.2021.1948027
22. Gesundheit B, Shapira MY, Resnick IB, Amar A, Kristt D, Dray L, et al. Successful Cell-Mediated Cytokine-Activated Immunotherapy for Relapsed Acute Myeloid Leukemia After Hematopoietic Stem Cell Transplantation. Am J Hematol (2009) 84(3):188–90. doi: 10.1002/ajh.21346
23. Mo X, Zhao X, Xu L, Liu D, Zhang X, Chen H, et al. Interferon Alpha: The Salvage Therapy for Patients With Unsatisfactory Response to Minimal Residual Disease-Directed Modified Donor Lymphocyte Infusion. Chin Med J (Engl) (2014) 127(14):2583–7. doi: 10.3760/cma.j.issn.0366-6999.20132908
24. Tang X, Song YH, Sun A, Zhu X, Ruan C, Wu D. Successful Treatment of Relapsed Acute Myeloid Leukemia Without Chemotherapy. J Clin Oncol (2016) 34(13):e117–9. doi: 10.1200/jco.2012.48.0442
25. Magenau JM, Peltier DC, Riwes M, Pawarode A, Parkin B, Braun TM, et al. Type-1 Interferon to Prevent Leukemia Relapse After Allogeneic Transplantation. Blood Adv (2021) 5(23):5047–56. doi: 10.1182/bloodadvances.2021004908
26. Henden AS, Varelias A, Leach J, Sturgeon E, Avery J, Kelly J, et al. Pegylated Interferon-2alpha Invokes Graft-Versus-Leukemia Effects in Patients Relapsing After Allogeneic Stem Cell Transplantation. Blood Adv (2019) 3(20):3013–9. doi: 10.1182/bloodadvances.2019000453
27. Mo X, Zhang X, Xu L, Wang Y, Yan C, Chen H, et al. Minimal Residual Disease-Directed Immunotherapy for High-Risk Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Front Med (2019) 13(3):354–64. doi: 10.1007/s11684-018-0665-5
28. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Interferon-Alpha: A Potentially Effective Treatment for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2015) 21(11):1939–47. doi: 10.1016/j.bbmt.2015.06.014
29. Liu S, Luo X, Zhang X, Xu L, Wang Y, Yan C, et al. Preemptive Interferon-α Treatment Could Protect Against Relapse and Improve Long-Term Survival of ALL Patients After Allo-HSCT. Sci Rep (2020) 10(1):20148. doi: 10.1038/s41598-020-77186-9
30. Henden AS, Varelias A, Leach J, Sturgeon E, Avery J, Kelly J, et al. Pegylated Interferon-2α Invokes Graft-Versus-Leukemia Effects in Patients Relapsing After Allogeneic Stem Cell Transplantation. Blood Adv (2019) 3(20):3013–9. doi: 10.1182/bloodadvances.2019000453
31. Mo XD, Wang Y, Zhang XH, Xu LP, Yan CH, Chen H, et al. Interferon-Alpha Is Effective for Treatment of Minimal Residual Disease in Patients With T (8) Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Prospective Registry Study. Oncologist (2018) 23(11):1349–57. doi: 10.1634/theoncologist.2017-0692
32. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. IFN-α Is Effective for Treatment of Minimal Residual Disease in Patients With Acute Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Registry Study. Biol Blood Marrow Transplant (2017) 23(8):1303–10. doi: 10.1016/j.bbmt.2017.04.023
33. Mo X, Zhang X, Xu L, Wang Y, Yan C, Chen H, et al. Interferon-Alpha Salvage Treatment Is Effective for Patients With Acute Leukemia/Myelodysplastic Syndrome With Unsatisfactory Response to Minimal Residual Disease-Directed Donor Lymphocyte Infusion After Allogeneic Hematopoietic Stem Cell Transplantation. Front Med (2019) 13(2):238–49. doi: 10.1007/s11684-017-0599-3
34. Dominietto A, Pozzi S, Miglino M, Albarracin F, Piaggio G, Bertolotti F, et al. Donor Lymphocyte Infusions for the Treatment of Minimal Residual Disease in Acute Leukemia. Blood (2007) 109(11):5063–4. doi: 10.1182/blood-2007-02-072470
35. Huang XJ, Xu LP, Liu KY, Liu DH, Wang Y, Chen H, et al. Partially Matched Related Donor Transplantation can Achieve Outcomes Comparable With Unrelated Donor Transplantation for Patients With Hematologic Malignancies. Clin Cancer Res (2009) 15(14):4777–83. doi: 10.1158/1078-0432.CCR-09-0691
36. Wang Y, Liu Q-F, Xu L-P, Liu K-Y, Zhang X-H, Ma X, et al. Haploidentical vs Identical-Sibling Transplant for AML in Remission: A Multicenter, Prospective Study. Blood (2015) 125(25):3956–62. doi: 10.1182/blood-2015-02-627786
37. Wang Y, Liu QF, Lin R, Yang T, Xu YJ, Mo XD, et al. Optimizing Antithymocyte Globulin Dosing in Haploidentical Hematopoietic Cell Transplantation: Long-Term Follow-Up of a Multicenter, Randomized Controlled Trial. Sci Bull (2021) 66(24):2498–505. doi: 10.1016/j.scib.2021.06.002
38. Mo XD, Zhang YY, Zhang XH, Xu LP, Wang Y, Yan CH, et al. The Role of Collateral Related Donors in Haploidentical Hematopoietic Stem Cell Transplantation. Sci Bull (2018) 63(2095-9273):1376. doi: 10.1016/j.scib.2018.08.008
39. Liu SN, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Prognostic Factors and Long-Term Follow-Up of Basiliximab for Steroid-Refractory Acute Graft-Versus-Host Disease: Updated Experience From a Large-Scale Study. Am J Hematol (2020) 95(8):927–36. doi: 10.1002/ajh.25839
40. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The Consensus From The Chinese Society of Hematology on Indications, Conditioning Regimens and Donor Selection for Allogeneic Hematopoietic Stem Cell Transplantation: 2021 Update. J Hematol Oncol (2021) 14(1):145. doi: 10.1186/s13045-021-01159-2
41. Zhao XS, Jin S, Zhu HH, Xu LP, Liu DH, Chen H, et al. Wilms’ Tumor Gene 1 Expression: An Independent Acute Leukemia Prognostic Indicator Following Allogeneic Hematopoietic SCT. Bone Marrow Transplant (2012) 47(4):499–507. doi: 10.1038/bmt.2011.121
42. Qin YZ, Wang Y, Xu LP, Zhang XH, Chen H, Han W, et al. The Dynamics of RUNX1-RUNX1T1 Transcript Levels After Allogeneic Hematopoietic Stem Cell Transplantation Predict Relapse in Patients With T (8) Acute Myeloid Leukemia. J Hematol Oncol (2017) 10(1):44. doi: 10.1186/s13045-017-0414-2
43. Zhao XS, Yan CH, Liu DH, Xu LP, Liu YR, Liu KY, et al. Combined Use of WT1 and Flow Cytometry Monitoring can Promote Sensitivity of Predicting Relapse After Allogeneic HSCT Without Affecting Specificity. Ann Hematol (2013) 92(8):1111–9. doi: 10.1007/s00277-013-1733-1
44. Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, et al. Diagnosis and Management of Acute Graft-Versus-Host Disease. Br J Haematol (2012) 158(1):30–45. doi: 10.1111/j.1365-2141.2012.09129.x
45. Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, et al. A Disease Risk Index for Patients Undergoing Allogeneic Stem Cell Transplantation. Blood (2012) 120(4):905–13. doi: 10.1182/blood-2012-03-418202
46. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant (1995) 15(6):825–8.
47. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant (2005) 11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004
48. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of Failure Probabilities in the Presence of Competing Risks: New Representations of Old Estimators. Stat Med (1999) 18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o
49. Shaffer L, Giralt S, Champlin R, Chan KW. Treatment of Leukemia Relapse After Bone Marrow Transplantation With Interferon-Alpha and Interleukin 2. Bone Marrow Transplant (1995) 15(2):317–9.
50. Singhal S, Treleaven J, Mehta J, Powles R. Sensitivity of Secondary Acute Myeloid Leukemia Relapsing After Allogeneic Bone Marrow Transplantation to Immunotherapy With Interferon-Alpha 2b. Bone Marrow Transplant (1997) 19(11):1151–3. doi: 10.1038/sj.bmt.1700793
51. Giralt S, O'Brien S, Talpaz M, Van BK, Chan KW, Rondón G, et al. Interferon-Alpha and Interleukin-2 as Treatment for Leukemia Relapse After Allogeneic Bone Marrow Transplantation. Cytokines Mol Ther (1995) 1(2):115–22.
52. Ankerst J, Fäldt R, Nilsson PG, Flodgren P, Sjögren HO. Complete Remission in a Patient With Acute Myelogenous Leukemia Treated With Leukocyte α-Interferon and Cimetidine. Cancer Immunol Immunother (1984) 17(1):69–71. doi: 10.1007/BF00205501
53. Palva IP, Almqvist A, Elonen E, Hanninen A, Jouppila J, Jarventie G, et al. Value of Maintenance Therapy With Chemotherapy or Interferon During Remission of Acute Myeloid Leukaemia. Eur J Haematol (1991) 47(3):229–33. doi: 10.1111/j.1600-0609.1991.tb01560.x
54. Lin XJ, Dai HP, Wang AJ, Chen F, Ma X, Sun AN, et al. Effects of Preemptive Interferon-Alpha Monotherapy in Acute Leukemia Patients With Relapse Tendency After Allogeneic Hematopoietic Stem Cell Transplantation: A Case-Control Study. Ann Hematol (2018) 97(11):2195–204. doi: 10.1007/s00277-018-3429-z
55. Wang Y, Wu DP, Liu QF, Qin YZ, Wang JB, Xu LP, et al. In Adults With T (8)AML, Posttransplant RUNX1/RUNX1T1-Based MRD Monitoring, Rather Than C-KIT Mutations, Allows Further Risk Stratification. Blood (2014) 124(12):1880–6. doi: 10.1182/blood-2014-03-563403
56. Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H, et al. MRD-Directed Risk Stratification Treatment may Improve Outcomes of T (8) AML in the First Complete Remission: Results From the AML05 Multicenter Trial. Blood (2013) 121(20):4056–62. doi: 10.1182/blood-2012-11-468348
57. Ravandi F, Jorgensen J, Borthakur G, Jabbour E, Kadia T, Pierce S, et al. Persistence of Minimal Residual Disease Assessed by Multiparameter Flow Cytometry Is Highly Prognostic in Younger Patients With Acute Myeloid Leukemia. Cancer (2017) 123(3):426–35. doi: 10.1002/cncr.30361
58. Ravandi F, Walter RB, Freeman SD. Evaluating Measurable Residual Disease in Acute Myeloid Leukemia. Blood Adv (2018) 2(11):1356–66. doi: 10.1182/bloodadvances.2018016378
59. Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J, et al. Minimal/measurable Residual Disease in AML: A Consensus Document From the European LeukemiaNet MRD Working Party. Blood (2018) 131(12):1275–91. doi: 10.1182/blood-2017-09-801498
60. Nomdedeu JF, Esquirol A, Carricondo M, Pratcorona M, Hoyos M, Garrido A, et al. Bone Marrow WT1 Levels in Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplasia: Clinically Relevant Time Points and 100 Copies Threshold Value. Biol Blood Marrow Transplant (2018) 24(1):55–63. doi: 10.1016/j.bbmt.2017.09.001
61. Rautenberg C, Pechtel S, Hildebrandt B, Betz B, Dienst A, Nachtkamp K, et al. Wilms' Tumor 1 Gene Expression Using a Standardized European LeukemiaNet-Certified Assay Compared to Other Methods for Detection of Minimal Residual Disease in Myelodysplastic Syndrome and Acute Myelogenous Leukemia After Allogeneic Blood Stem Cell Transplantation. Biol Blood Marrow Transplant (2018) 24(11):2337–43. doi: 10.1016/j.bbmt.2018.05.011
62. Rautenberg C, Lauseker M, Kaivers J, Jager P, Fischermanns C, Pechtel S, et al. Prognostic Impact of Pretransplant Measurable Residual Disease Assessed by Peripheral Blood WT1-mRNA Expression in Patients With AML and MDS. Eur J Haematol (2021) 107(2):283–92. doi: 10.1111/ejh.13664
63. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. IFN-Alpha Is Effective for Treatment of Minimal Residual Disease in Patients With Acute Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Registry Study. Biol Blood Marrow Transplant (2017) 23(8):1303–10. doi: 10.1016/j.bbmt.2017.04.023
Keywords: acute myeloid leukemia, hematopoietic stem cell transplantation, interferon-α, minimal residual disease, preemptive
Citation: Shen M-Z, Zhang X-H, Xu L-P, Wang Y, Yan C-H, Chen H, Chen Y-H, Han W, Wang F-R, Wang J-Z, Zhao X-S, Qin Y-Z, Chang Y-J, Liu K-Y, Huang X-J and Mo X-D (2022) Preemptive Interferon-α Therapy Could Protect Against Relapse and Improve Survival of Acute Myeloid Leukemia Patients After Allogeneic Hematopoietic Stem Cell Transplantation: Long-Term Results of Two Registry Studies. Front. Immunol. 13:757002. doi: 10.3389/fimmu.2022.757002
Received: 11 August 2021; Accepted: 10 January 2022;
Published: 28 January 2022.
Edited by:
Alok Srivastava, Christian Medical College & Hospital, IndiaReviewed by:
John Magenau, University of Michigan, United StatesNavin Khattry, Tata Memorial Hospital, India
Copyright © 2022 Shen, Zhang, Xu, Wang, Yan, Chen, Chen, Han, Wang, Wang, Zhao, Qin, Chang, Liu, Huang and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Dong Mo, bXhkNDUzQDE2My5jb20=
 Meng-Zhu Shen
Meng-Zhu Shen Xiao-Hui Zhang
Xiao-Hui Zhang Lan-Ping Xu
Lan-Ping Xu Yu Wang
Yu Wang Chen-Hua Yan1
Chen-Hua Yan1 Ya-Zhen Qin
Ya-Zhen Qin Xiao-Jun Huang
Xiao-Jun Huang Xiao-Dong Mo
Xiao-Dong Mo