- 1Department of Allergy and Clinical Immunology, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 2School of Basic Medical Sciences, Guangzhou Medical University, Guangzhou, China
- 3International College of Education, Guangzhou Medical University, Guangzhou, China
- 4Department of Medicine, Firestone Institute for Respiratory Health, St. Joseph’s Healthcare and McMaster University, Hamilton, ON, Canada
Objective: This study aims to explore the potential of in situ airway differentiation of eosinophil progenitors (EoPs) and hematopoietic progenitor cells (HPCs) in sputum and peripheral blood from patients with non-asthmatic eosinophilic bronchitis (NAEB), eosinophilic asthma (EA), and healthy controls (HC).
Methods: Using flow cytometry, we enumerated sputum and blood HPCs and EoPs in patients with NAEB (n=15), EA (n=15), and HC (n=14) at baseline. Patients with NAEB and EA were then treated for 1 month with budesonide (200 μg, bid) or budesonide and formoterol (200/6 μg, bid), respectively. HPCs and EoPs in both compartments were re-evaluated.
Results: At baseline, NAEB and EA both had significantly greater numbers of sputum but not blood HPCs and EoPs (p<0.05) compared to HC. There were no differences between NAEB and EA. After 1 month of inhaled corticosteroid (ICS) treatment, NAEB patients showed a significant improvement in cough symptoms, but the attenuation of sputum HPC and EoP levels was not significant.
Conclusions: NAEB patients have increased airway levels of HPCs and EoPs. One-month treatment with ICS did not fully suppress the level of EoPs in NAEB. Controlling in situ airway differentiation of EoPs may control airway eosinophilia and provide long-term resolution of symptoms in NAEB.
Introduction
Non-asthmatic eosinophilic bronchitis (NAEB) is a common cause of chronic cough (1, 2). It is characterized by persistent troublesome cough and airway eosinophilia but lack of airway hyper-responsiveness (3). NAEB patients often respond well to inhaled corticosteroids but frequently relapse (4).
Previous studies have shown that NAEB is a T-helper 2 (Th2)-driven disease. In addition to airway eosinophilia, it was reported that, like asthmatics, NAEB patients have raised levels of inflammatory mediators and cytokines, such as histamine, cysteinyl-leukotrienes, interleukin (IL)-5, and eosinophilic cationic protein (5–7). Persistent sputum eosinophilia is a risk factor for relapse (8), suggesting that airway eosinophilia may be one major pathogenic mechanism in NAEB. Investigating the immunological processes that promote eosinophilic inflammation in the airways is important for the development of novel NAEB therapies.
Mature eosinophils differentiate from eosinophil-lineage committed progenitor cells (EoPs), which arise from bone-marrow-derived CD34+ hematopoietic progenitor cells (HPCs) (9). The differentiation and maturation of EoPs was originally thought to be restricted to the bone marrow (10, 11). However, increased numbers of HPCs and EoPs have been detected in the peripheral blood and tissue compartments from atopic subjects (12–15). It has now been proposed that in situ differentiation of EoPs can rapidly increase mucosal numbers of mature eosinophils during an inflammatory response, an element that may drive airway eosinophilia (16, 17). This suggests an important role of in situ eosinophil differentiation in the pathology of allergic diseases including asthma and allergic rhinitis.
We hypothesized that in situ differentiation of EoPs play a potential role in the pathogenesis of airway eosinophilia in NAEB. In the current study, we enumerated levels of HPCs and EoPs in induced sputum and peripheral blood from NAEB patients compared to eosinophilic asthmatics (EAs) and normal healthy controls (HCs). In addition, we repeated these measurements in NAEB and EA patients after 1 month of treatment with inhaled corticosteroids (ICS) or ICS plus long-acting beta-agonists (LABA), respectively.
Materials and Methods
Study Design and Participants
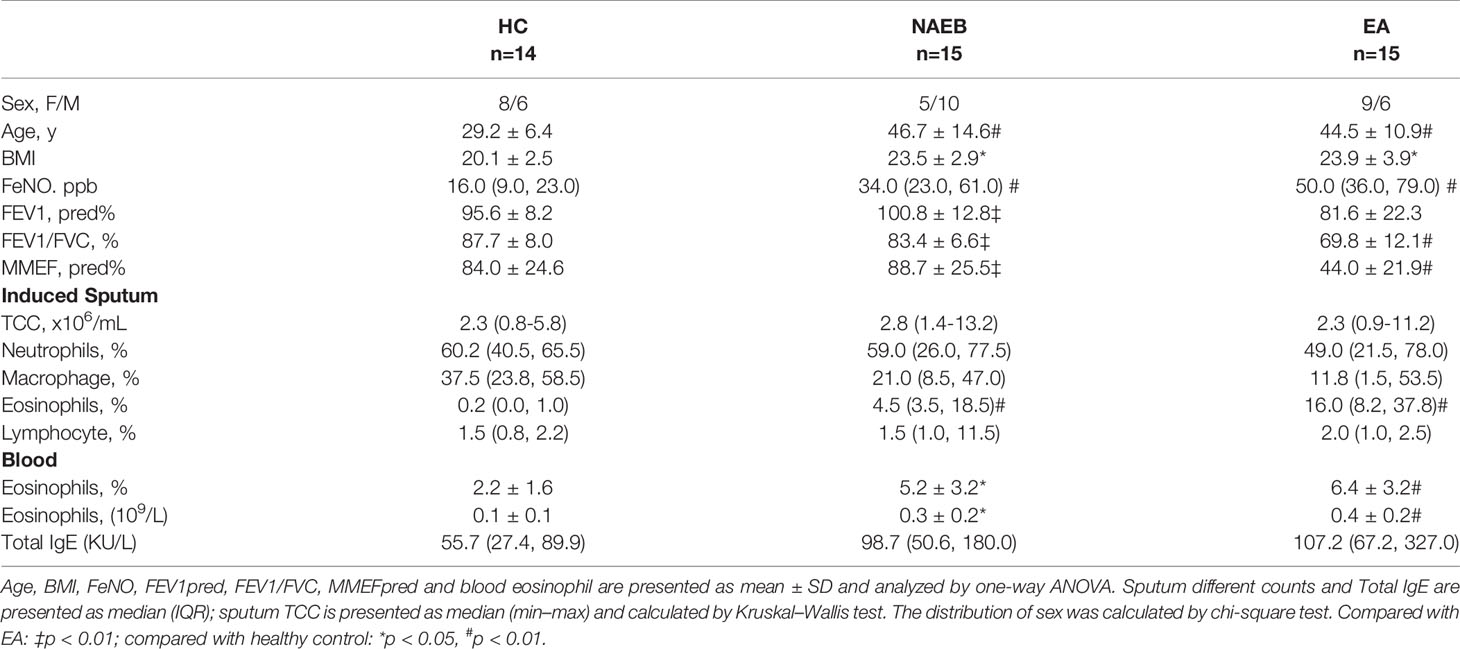
Fifteen patients with NAEB were recruited in the First Affiliated Hospital of Guangzhou Medical University between June 2016 and May 2017. Fifteen patients with EA and 14 HCs were included as disease and health controls. Pulmonary function, fractional exhaled nitric oxide, sputum differential counts, complete blood counts (CBC), serum IgE, symptom scores, and questionnaires including Asthma Control Test (ACT), Leicester Cough Questionnaires (LCQ), and Visual Analogue Scale (VAS) were recorded. Levels of sputum and blood HPCs and EoPs were enumerated by flow cytometry in all participants. NAEB patients were prescribed budesonide (200 μg, bid), and EA patients were prescribed budesonide/formoterol (200/6 μg, bid) for 1 month. Following this, FeNO, sputum differential count, CBC, and flow cytometric assessments of progenitor cells were re-evaluated in both patient groups.
NAEB was diagnosed according to the Chinese cough guidelines (18). The subjects had (1) persistent cough for more than 8 weeks; (2) a normal chest radiograph; (3) sputum eosinophilia (sputum Eos% ≥ 2.5%); and (4) normal spirometry and normal methacholine airway responsiveness. The subjects with EA were diagnosed according to the GINA 2015 criteria (19). All patients with EA had characteristic symptoms (such as wheezing, shortness of breath, chest tightness, or cough); increased sputum eosinophilia (sputum Eos% ≥ 3%), and >12% forced expiratory volume in 1 s (FEV1) reversibility after short-acting bronchodilator or a positive methacholine provocative test. All patients with NAEB or EA were not currently on any steroid therapy. The healthy controls (n=14) had normal spirometry, negative methacholine provocative test, and no history of respiratory disease, allergies, or systemic disease. All subjects were non-smokers. Subjects were excluded if they had experienced a respiratory infection in the past 4 weeks or had a history of bronchiectasis, chronic obstructive pulmonary disease, or other chronic pulmonary diseases.
Sputum Induction and Cell Isolation
Sputum was induced by inhalation of an aerosol of hypertonic saline as previously described (20). Sputum samples were processed by selecting the mucus plugs, mixing with 4 parts 0.1% dithiothreitol, then filtered through a 48-mm nylon mesh and centrifuged at 3,000 rpm for 10 min at 4°C. The cell pellet was re-suspended in phosphate-buffered saline (PBS). The sputum supernatants were stored at −80°C. The cell smear was prepared and stained with hematoxylin–eosin stain. The differential cell counts of sputum samples were obtained by counting 400 non-squamous cells. The remaining cells were subjected to immunofluorescence staining and enumeration by flow cytometry.
PBMC Isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from venous blood by Ficoll density gradient centrifugation. In brief, whole blood was diluted 1:1 with PBS and layered onto Ficoll-PAQUE Plus (GE Healthcare, Marlborough, MA, USA), centrifuged at 1,200g and 4°C for 25 min with the brake off. The buffy coat was collected and washed twice (500g for 5 min) with PBS. Cell count and viability were quantified in a Neubauer’s chamber with 0.4% Trypan Blue Solution.
Flow Cytometry
Freshly isolated blood-derived mononuclear cells and sputum-extracted cells were immediately incubated with fluorescence-labeled antibodies to define cell subpopulations. Antibodies (BD Biosciences) used for flow cytometry were FITC-CD45, PE-Cy5-CD34, and PE-CD125. Cells were analyzed by FACSVerse analytical flow cytometry (BD Company, San Diego, CA, USA). The percentage of HPCs (FSCmediumSSClowCD45dullCD34+) and EoPs (FSCmediumSSClowCD45dullCD34+CD125+) were determined using FlowJo software (BD Biosciences). The absolute numbers of HPCs or EoPs were calculated by multiplying percentage of HPCs or EoPs within R2 (singlets with medium FSC and low SSC) in flow cytometry with the absolute number of lymphocytes in blood routine test or sputum cell counts. The gating strategy is shown in Supplementary Figure S1.
Statistical Analysis
Statistical analysis was performed by using SPSS Version23.0 (SPSS Inc., Chicago, IL, USA). Age, body mass index (BMI), FEV1pred, MMEFpred, FEV1/forced vital capacity (FEV1/FVC), and blood eosinophil were presented as mean ± SD and analyzed by one-way ANOVA followed by the Tukey post-test for multiple comparisons. Sputum differential counts, FeNO, serum total IgE, HPCs, and EoPs levels were presented as median (IQR) and analyzed by Kruskal–Wallis test and Dunn’s post-hoc analyses for multiple comparisons. Comparisons of LCQ, VAS, ACT, FeNO, sputum and blood eosinophils, HPCs, and EoPs before and after treatment were calculated using a paired t-test or Wilcoxon matched-pairs sign rank test. Correlations among clinical parameters were computed using a Spearman test. Differences were considered to be statistically significant when p<0.05.
Results
Demographics and Clinical Characteristics at Baseline
Baseline characteristics of participants in the study are shown in Table 1. NAEB and EA patients were significantly older and had a higher BMI than HC controls (all p<0.01). Similar to EA, NAEB showed higher levels of FeNO, sputum eosinophils, and blood eosinophils compared with HC (p<0.05). No differences of FeNO, sputum, and blood eosinophils were found between NAEB and EA.
Airway HPCs and EoPs Are Increased in Patients With NAEB
PBMCs were successful obtained from all subjects for flow cytometry detection. Sputum samples for flow cytometry were obtained from 14 patients with NAEB, 11 patients with EA, and 9 subjects with HC.
In the airway, sputum HPCs levels in NAEB [770 (8,219) cells/ml] and EA [742 (1,322) cells/ml] were significantly higher than in the HC group [135 (343) cells/ml] (both p<0.05, Figure 1A). In addition, sputum EoPs levels were significantly higher in patients with NAEB [91 (219) cells/ml] and EA [69 (199) cells/ml] compared to the HC group [17 (26) cells/ml] (both p<0.05, Figure 1B). In contrast, there were no significant differences in sputum HPCs or EoPs levels between NAEB and EA. Overall, there was a strong correlation between sputum HPCs and EoPs levels (r=0.778, p<0.001, Supplementary Figure S2) and a moderate association between sputum EoPs and eosinophils levels (r=0.378, p<0.05, Supplementary Figure S2).
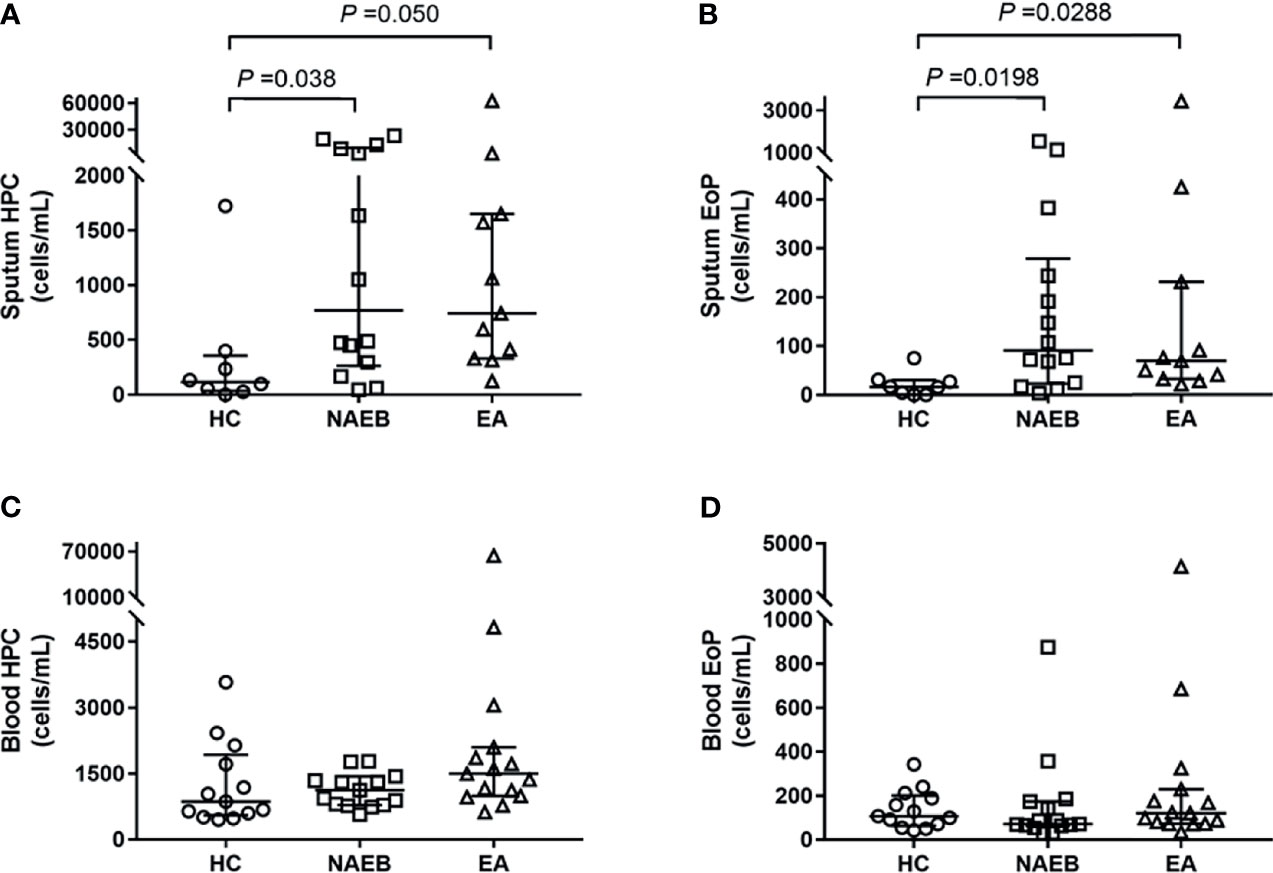
Figure 1 Absolute numbers of hematopoietic progenitor cells (HPC) and eosinophilic progenitor cells (EoPs) in three groups at baseline. Absolute numbers of sputum HPC cells (A) and EoP cells (B), and the peripheral blood HPC cells (C) and EoP cells (D). Absolute cell numbers enumerated by flow cytometry are presented as cells/ml, and data are presented as median (IQR) values. HC, healthy control group; NAEB, non-asthmatic eosinophilic bronchitis group; EA, eosinophilic asthmatics group.
In the blood, there was no significant difference in HPCs or EoPs levels in NAEB [HPCs, 1,123 (650)/ml; EoPs, 71 (110)/ml] compared with the HC [HPCs, 952 (1,246)/ml; EoPs, 118 (117)/ml] or EA group [HPCs, 1,498 (1,109)/ml; EoPs, 121 (156)] (Figures 1C, D). Neither blood HPCs levels nor EoPs levels correlated with blood eosinophil, sputum HPCs, or EoPs levels (Supplementary Figure S1).
HPCs and EoPs Levels Following Treatment With ICS for NAEB or ICS/LABA for EA
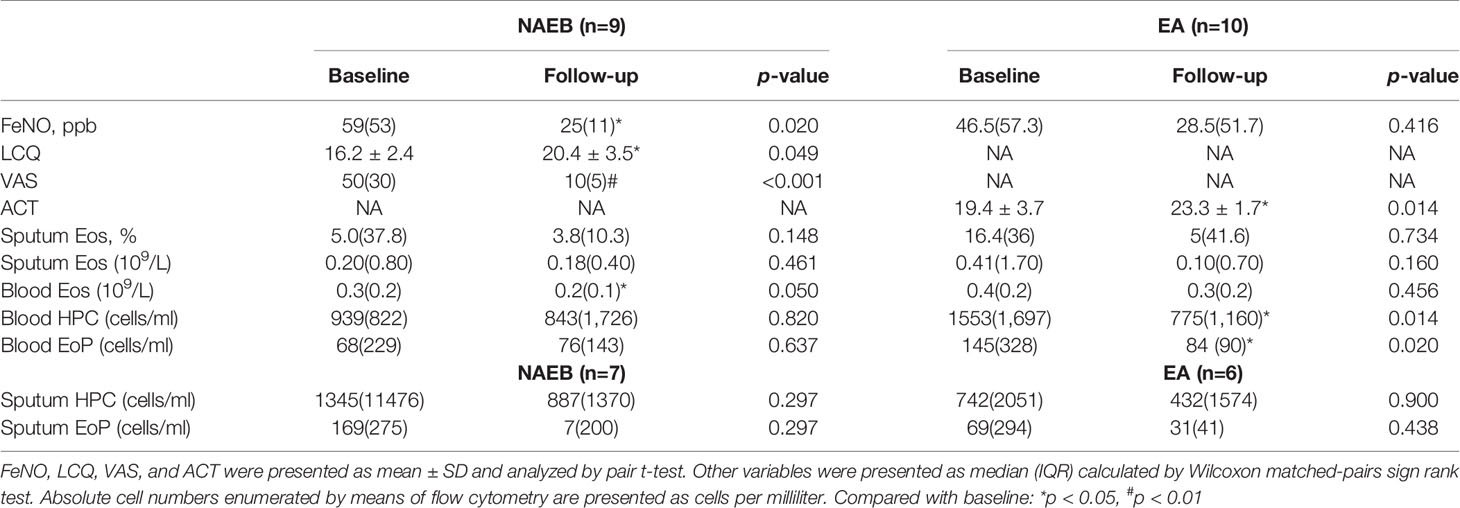
After 1-month ICS treatment, patients with NAEB presented an improvement in cough symptoms as reflected by a significant improvement of LCQ [16.2 ± 2.4 vs. 20.4 ± 3.5] and VAS [50 (30) vs. 10 (5)] (all p<0.05). One patient reported complete resolution of symptoms. In addition, the NAEB group displayed decreased levels of FeNO [59 (53) vs. 25 (11)] and blood eosinophils [0.3 (0.2) vs. 0.2 (0.1)] (all p<0.05). There was reduction in the level of sputum HPCs, EoPs, and eosinophils [HPCs, 1,345(11,476) vs. 887(1,370); EoPs, 169(275) vs. 7(200), cells/ml; Eos, 5.0(37.8) vs. 3.8(10.3), %], although this change did not achieve significance (Figure 2 and Table 2).
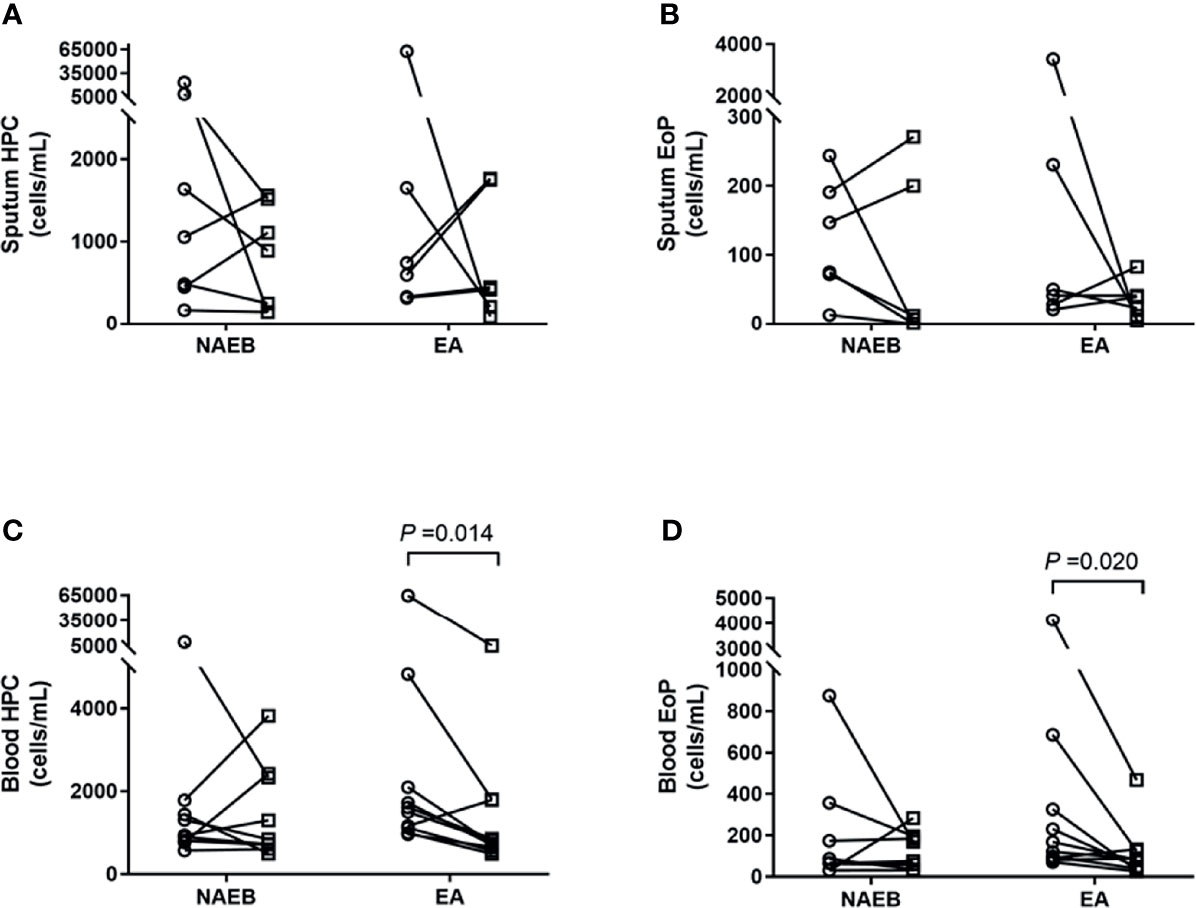
Figure 2 Enumeration of hematopoietic progenitor cells (HPC) and eosinophilic progenitor cells (EoPs) in sputum and peripheral blood after 1 month of ICS treatment in NAEB and ICS/LABA treatment in EA. The changes in sputum HPC counts (A) and EoP counts (B), and blood HPC counts (C) and EoP counts (D) before (open circle) and after (open square) therapy. NAEB, Non-asthmatic eosinophilic bronchitis group. EA, eosinophilic asthmatics group.
In patients with EA, an improvement in symptoms was observed as reflected by an increasing ACT score (19.4 ± 3.7 vs. 23.3 ± 1.7) (p<0.05). A significant reduction was found in blood HPCs and EoPs levels [HPCs, 1,553(1,697) vs. 775(1,160); EoPs, 145(328) vs. 84 (90), cells/ml] (all p<0.05) but not mature eosinophils. In contrast, no differences were found in FeNO levels or sputum eosinophils, HPCs, or EoPs after treatment in these patients (Figure 2 and Table 2). For the follow-up time point, when data from EA and NAEB patients were combined, the sputum EoPs level did not correlate with sputum HPCs nor eosinophils levels (Supplementary Figure S2B).
Discussion
The current study has demonstrated, for the first time, that NAEB patients have significantly increased levels of EoPs and HPC in the airways when compared to healthy controls. One-month ICS therapy improved symptoms in NAEB patients; however, the reduction in inflammatory indices including airway EoPs, HPCs, and mature eosinophils was not significant. The results suggest that in situ airway differentiation of EoPs may be one of the possible pathways mediating the airway eosinophilia in NAEB. In addition, longer treatment strategies may be required to investigate whether normalization of lung inflammatory cells may further improve symptoms of NAEB and chances of future relapse.
Immune cells that contribute to airway eosinophilia in NAEB are not clearly defined. Brightling et al. (6) previously reported that the proportion of BALF IL-4+CD4+ T cells and the number of IL-4+ and IL-5+ cells in bronchial submucosa are significantly higher in NAEB compared to HC controls, suggesting that the Th2 cell may drive airway eosinophilia in these patients. In addition to the traffic of mature eosinophils from the periphery, in situ eosinophilopoiesis has been proposed as an additional process that may drive airway eosinophilia in allergic asthma and rhinitis. It can rapidly increase mucosal levels of mature eosinophils during an inflammatory response. Studies have shown that the increase in numbers of sputum EoPs precedes the development of airway eosinophilia after allergen inhalational challenge in asthmatics (21). Cameron et al. (16) found that local IL-5-dependent differentiation of EoPs was observed when nasal biopsies were cultured ex vivo with IL-5 or ragweed allergen resulting in a reduction in CD34 immunopositive/IL-5Rα mRNA+ cells and a concurrent increase in the number of MBP immunoreactive cells, likely mature eosinophils. Our data showed increased levels of sputum HPCs, EoPs in NAEB, and a moderate association between sputum EoPs and sputum eosinophilia indicating, that in situ differentiation may mediate local increases in mature eosinophil levels in NAEB. Consistent with this hypothesis, NAEB patients had increased concentrations of IL-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF), which could induce HPCs and EoPs differentiation into eosinophils within the airways (6, 22, 23). In addition, recent studies have suggested that HPCs and EoPs may act as pro-inflammatory effector cells following activation by epithelial cell-derived alarmin cytokines (24). Upon stimulation with thymic stomal lymphopoietin (TSLP) and/or interleukin-33 (IL-33), HPCs shown to express TSLPR and ST2 (25) release high levels of IL-5, IL-13, and GM-CSF (26). Whether NAEB has higher levels of alarmin cytokines in the airways and whether sputum HPC and EoP express greater amounts of type 2 cytokines compared to healthy controls remains to be investigated.
In line with previous studies (17, 21), we found that asthmatic patients showed elevated levels of airway HPCs and EoPs. However, no differences in blood EoPs were observed. This might be related to the severity of the asthmatic patients enrolled, as blood and sputum EoPs in severe asthma are significantly higher than in mild asthma (17). In the current study, the asthmatic patients were mild and steroid naive. Our previous data (17) found a 10-fold greater number of sputum EoPs in prednisone-dependent severe asthmatics compared to mild asthmatics, while these cells were comparable in the PB of both subject groups, suggesting that an enhanced eosinophilopoietic environment exits in the airways of severe asthmatics with persistent eosinophilia.
In contrast to the EA group, neither blood HPCs nor blood EoPs in the NAEB group were different when compared to HC controls. We speculated that there may be systemic component involving mobilization of progenitor cells from bone marrow in EA compared to NAEB where the inflammatory responses appear to be localized to the airways. Eotaxin/CCR3 and SDF-1/CXCR4 axes are reported to play a role promoting progenitors release from the bone marrow in EA and might be less prominent or involved in progenitor cell trafficking to the airways in NAEB (27, 28). This is supported by our previous findings where we found no difference in serum levels of eotaxin or IL-5 in NAEB compared to HC, suggesting that NAEB displayed only mild systemic inflammation (21).
After 1 month of treatment with ICS in NAEB, or ICS/LABA in EA, we found an inconsistent relationship between the improvement of symptoms and relief of inflammation. Despite a significant reduction in blood eosinophils, sputum levels of HPCs, EoPs, and mature eosinophil only showed a trend of not a significant decline in NAEB. In addition, a moderate correlation was found between sputum EoPs and eosinophils in these patients; this might fit out with the hypothesis that that local airway levels of EoP may contribute to the airway eosinophilia. Despite that the attenuation of sputum HPC and EoP levels in EA patients after ICS treatment was not significant, ICS treatment may still have a suppression role on local airway eosinophil differentiation to mature cells. The reasons are as follows: first, the weaker correlation between sputum EOS and HPC or EoP might provide indirect evidence that ICS treatment suppresses differentiation of EoPs into mature eosinophils (Supplementary Figure S2B). Second, Kim et al. reported that an increase in CD34+ mononuclear cells occurs in steroid-treated nasal polyps (29). A possible mechanism that they proposed is that inhibition of the differentiation of mature cells from progenitors may cause more residing CD34+ progenitor cells in the local tissue. Since we have previously reported a higher rate of recurring episodes after 1 month of ICS treatment compared to 3 months treatment, during a 1-year follow-up in NAEB (30), our current data suggest that a longer treatment strategy may be required to fully investigate whether reduction in local eosinophilopoietic processes may reduce airway eosinophilia and further improve the management of NAEB.
Some limitations of the study should be noted. First, we measured the total numbers of HPCs and EoPs, and the numbers of activated or cytokine-producing HPCs and EoPs were not determined. Second, our current study is an initial observational, small group study that is hypothesis generating, which provides indirect evidence of the existence of in situ airway differentiation in NAEB as reflected by the increased sputum EoPs in NAEB and its moderate correlation with sputum eosinophils. The significance of in situ airway differentiation of EoPs leading to airway eosinophilia in NAEB needs to be further investigated.
In summary, this study demonstrated that increases in HPCs and EoPs in NAEB are predominantly found in the airways and that these cells may contribute to the airway eosinophilia. One-month ICS treatment improved the symptoms in NAEB but did not fully suppress the airway inflammatory responses.
Data Availability Statement
The datasets generated for this study can be obtained from the corresponding author upon reasonable request. Requests to access the datasets should be directed to Y2hlbl9yY2hAMTYzLmNvbQ==.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethic Committee of the First Affiliated Hospital of Guangzhou Medical University. The IRB number of the ethic approval is 2016-52. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RS, PB, and RC planned the study and designed the experiments. JXL, WQL, SZ, LF, SXZ, DSHS, DS, and WL cared for the patients and provided clinical information. CZ, RX, and BL performed the experiments. CZ, RX, and BL performed the statistical analysis and wrote the manuscript. JL, KL, NZ, RS, PB, and RC contributed to made critical revision of the manuscript. RS, PB, and RC supervised the work. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81870079), State Key Laboratory of Respiratory Disease (SKLRD-QN-201702), Guangzhou Science and Technology Project/Nanshan Medical Foundation (202102010349), Incubation Project for Innovation Team of GMU (Grant 2017-159), and Incubation Program of National Science Found for Distinguished Young Scholars (GMU2020-207).
Conflict of Interest
RS reports grants and personal fees from Genentech Inc, grants and personal fees from Astra Zeneca, grants and personal fees from Teva Pharmaceuticals, personal fees from GSK, all outside the scope of this work; PO’B has grants in aid and speaker’s fees from AstraZeneca, grants in aid from Bayer, Genentech, Merck, and Novartis, and has received speaker’s fees from GSK, Chiesi and Covis, all outside the scope of this work. RC reports grants and speaker’s fees from AstraZeneca GSK, Sanofi and Novartis, all outside the scope of this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.737968/full#supplementary-material
References
1. Brightling CE, Ward R, Goh KL, Wardlaw AJ, Pavord ID. Eosinophilic Bronchitis Is an Important Cause of Chronic Cough. Am J Resp Crit Care (1999) 160:406–10. doi: 10.1164/ajrccm.160.2.9810100
2. Lai K, Chen R, Lin J, Huang K, Shen H, Kong L, et al. A Prospective, Multicenter Survey on Causes of Chronic Cough in China. Chest (2013) 143:613–20. doi: 10.1378/chest.12-0441
3. Gibson PG, Dolovich J, Denburg J, Ramsdale EH, Hargreave FE. Chronic Cough: Eosinophilic Bronchitis Without Asthma. Lancet (London England) (1989) 1:1346–8. doi: 10.1016/s0140-6736(89)92801-8
4. Gibson PG, Hargreave FE, Girgis-Gabardo A, Morris M, Denburg JA, Dolovich J. Chronic Cough With Eosinophilic Bronchitis: Examination for Variable Airflow Obstruction and Response to Corticosteroid. Clin Exp Allergy (1995) 25:127–32. doi: 10.1111/j.1365-2222.1995.tb01017.x
5. Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of Airway Immunopathology of Eosinophilic Bronchitis and Asthma. Thorax (2003) 58(6):528–32. doi: 10.1136/thorax.58.6.528
6. Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 Cytokine Expression in Bronchoalveolar Lavage Fluid T Lymphocytes and Bronchial Submucosa Is a Feature of Asthma and Eosinophilic Bronchitis. J Allergy Clin Immunol (2002) 110:899–905. doi: 10.1067/mai.2002.129698
7. Brightling CE, Ward R, Woltmann G, Bradding P, Sheller JR, Dworski R, et al. Induced Sputum Inflammatory Mediator Concentrations in Eosinophilic Bronchitis and Asthma. Am J Resp Crit Care (2000) 162:878–82. doi: 10.1164/ajrccm.162.3.9909064
8. Lai K, Liu B, Xu D, Han L, Lin L, Xi Y, et al. Will Nonasthmatic Eosinophilic Bronchitis Develop Into Chronic Airway Obstruction?: A Prospective, Observational Study. Chest (2015) 148:887–94. doi: 10.1378/chest.14-2351
9. Sehmi R, Denburg JA. Differentiation of Human Eosinophils. Role in Allergic Inflammation. Chem Immunol (2000) 76:29–44. doi: 10.1159/000058788
10. Sanderson CJ. Interleukin-5, Eosinophils, and Disease. BLOOD (1992) 79:3101–9. doi: 10.1182/blood.V79.12.3101.bloodjournal79123101
11. Clutterbuck EJ, Hirst EM, Sanderson CJ. Human Interleukin-5 (IL-5) Regulates the Production of Eosinophils in Human Bone Marrow Cultures: Comparison and Interaction With IL-1, IL-3, IL-6, and GMCSF. Blood (1989) 73:1504–12. doi: 10.1182/blood.V73.6.1504.bloodjournal7361504
12. Gibson PG, Dolovich J, Girgis-Gabardo A, Morris MM, Anderson M, Hargreave FE, et al. The Inflammatory Response in Asthma Exacerbation: Changes in Circulating Eosinophils, Basophils and Their Progenitors. Clin Exp Allergy (1990) 20:661–8. doi: 10.1111/j.1365-2222.1990.tb02705.x
13. Gibson PG, Manning PJ, O’Byrne PM, Girgis-Gabardo A, Dolovich J, Denburg JA, et al. Allergen-Induced Asthmatic Responses. Relationship Between Increases in Airway Responsiveness and Increases in Circulating Eosinophils, Basophils, and Their Progenitors. Am Rev Respir Dis (1991) 143:331–5. doi: 10.1164/ajrccm/143.2.331
14. Sehmi R, Howie K, Sutherland DR, Schragge W, O’Byrne PM, Denburg JA. Increased Levels of CD34+ Hemopoietic Progenitor Cells in Atopic Subjects. Am J Resp Cell Mol (1996) 15:645–55. doi: 10.1165/ajrcmb.15.5.8918371
15. Dorman SC, Babirad I, Post J, Watson RM, Foley R, Jones GL, et al. Progenitor Egress From the Bone Marrow After Allergen Challenge: Role of Stromal Cell-Derived Factor 1alpha and Eotaxin. J Allergy Clin Immunol (2005) 115:501–7. doi: 10.1016/j.jaci.2004.11.017
16. Cameron L, Christodoulopoulos P, Lavigne F, Nakamura Y, Eidelman D, McEuen A, et al. Evidence for Local Eosinophil Differentiation Within Allergic Nasal Mucosa: Inhibition With Soluble IL-5 Receptor. J Immunol (Baltimore Md.: 1950) (2000) 164:1538–45. doi: 10.4049/jimmunol.164.3.1538
17. Sehmi R, Smith SG, Kjarsgaard M, Radford K, Boulet L, Lemiere C, et al. Role of Local Eosinophilopoietic Processes in the Development of Airway Eosinophilia in Prednisone-Dependent Severe Asthma. Clin Exp Allergy (2016) 46:793–802. doi: 10.1111/cea.12695
18. The Asthma Workgroup of Chinese Society of Respiratory Diseases (CSRD), Chinese Medical Association. The Chinese National Guidelines on Diagnosis and Management of Cough (December 2010). Chin Med J-PEKING (2011) 124:3207–19. doi: 10.3760/cma.j.issn.0366-6999.2011.20.002
19. Global Strategy for Asthma Management and Prevention (2015). Available at: http://guide.medlive.cn/guideline/12751.
20. Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, et al. Indices of Airway Inflammation in Induced Sputum: Reproducibility and Validity of Cell and Fluid-Phase Measurements. Am J Respir Crit Care Med (1996) 154(2 Pt 1):308–17. doi: 10.1164/ajrccm.154.2.8756799
21. Dorman SC, Efthimiadis A, Babirad I, Watson RM, Denburg JA, Hargreave FE, et al. Sputum CD34+IL-5Ralpha+ Cells Increase After Allergen: Evidence for In Situ Eosinophilopoiesis. Am J Resp Crit Care (2004) 169:573–7. doi: 10.1164/rccm.200307-1004OC
22. Zhan C, Xu R, Liu J, Zhang S, Luo W, Chen R, et al. Increased Sputum IL-17a Level in Non-Asthmatic Eosinophilic Bronchitis. Lung (2018) 196:699–705. doi: 10.1007/s00408-018-0166-y
23. Zhang R, Luo W, Liang Z, Tan Y, Chen R, Lu W, et al. Eotaxin and IL-4 Levels Are Increased in Induced Sputum and Correlate With Sputum Eosinophils in Patients With Nonasthmatic Eosinophilic Bronchitis. Medicine (2017) 96:e6492. doi: 10.1097/MD.0000000000006492
24. Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ Hemopoietic Progenitor Cells Are Potent Effectors of Allergic Inflammation. J Allergy Clin Immunol (2009) 123:472–8. doi: 10.1016/j.jaci.2008.10.022
25. Hui CCK, Rusta-Sallehy S, Asher I, Heroux D, Denburg JA. The Effects of Thymic Stromal Lymphopoietin and IL-3 on Human Eosinophil-Basophil Lineage Commitment: Relevance to Atopic Sensitization. Immunity Inflamm Dis (2014) 2:44–55. doi: 10.1002/iid3.20
26. Smith SG, Gugilla A, Mukherjee M, Merim K, Irshad A, Tang W, et al. Thymic Stromal Lymphopoietin and IL-33 Modulate Migration of Hematopoietic Progenitor Cells in Patients With Allergic Asthma. J Allergy Clin Immunol (2015) 135:1594–602. doi: 10.1016/j.jaci.2014.12.1918
27. Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic Stem Cells Are Uniquely Selective in Their Migratory Response to Chemokines. J Exp Med (2002) 195:1145–54. doi: 10.1084/jem.20011284
28. Lévesque J, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 Chemotactic Interaction During Hematopoietic Stem Cell Mobilization Induced by GCSF or Cyclophosphamide. J Clin Invest (2003) 111:187–96. doi: 10.1172/JCI15994
29. Kim YK, Uno M, Hamilos DL, Beck L, Bochner B, Schleimer R, et al. Immunolocalization of CD34 in Nasal Polyposis. Effect of Topical Corticosteroids. Am J Resp Cell Mol (1999) 20:388–97. doi: 10.1165/ajrcmb.20.3.3060
Keywords: non-asthmatic eosinophilic bronchitis, hematopoietic progenitor cells, eosinophil progenitors, airway inflammation, eosinophil
Citation: Zhan C, Xu R, Li B, Liu J, Liang W, Zhang S, Fang L, Zhong S, de Silva SDSH, Sivapalan D, Luo W, Li J, Lai K, Zhong N, Sehmi R, O’Byrne PM and Chen R (2022) Eosinophil Progenitors in Patients With Non-Asthmatic Eosinophilic Bronchitis, Eosinophilic Asthma, and Normal Controls. Front. Immunol. 13:737968. doi: 10.3389/fimmu.2022.737968
Received: 08 July 2021; Accepted: 03 March 2022;
Published: 31 March 2022.
Edited by:
Wendy W. J. Unger, Erasmus MC-Sophia Children’s Hospital, NetherlandsReviewed by:
Selma Van Staveren, Erasmus University Medical Center, NetherlandsLili Chen, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2022 Zhan, Xu, Li, Liu, Liang, Zhang, Fang, Zhong, de Silva, Sivapalan, Luo, Li, Lai, Zhong, Sehmi, O’Byrne and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruchong Chen, Y2hlbl9yY2hAMTYzLmNvbQ==; Roma Sehmi, c2VobWlyQG1jbWFzdGVyLmNh; Paul M. O’Byrne, b2J5cm5lcEBtY21hc3Rlci5jYQ==
†These authors have contributed equally to this work and share first authorship
‡Lead contact
 Chen Zhan
Chen Zhan Rong Xu1†
Rong Xu1† Bizhou Li
Bizhou Li Jiaxing Liu
Jiaxing Liu Wanqin Liang
Wanqin Liang Shuxin Zhong
Shuxin Zhong Wei Luo
Wei Luo Jing Li
Jing Li Kefang Lai
Kefang Lai Nanshan Zhong
Nanshan Zhong Roma Sehmi
Roma Sehmi Paul M. O’Byrne
Paul M. O’Byrne Ruchong Chen
Ruchong Chen