
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 11 January 2023
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1103055
This article is part of the Research Topic Immunotherapy and Small Molecule Inhibitors as Combinational Cancer Therapeutics View all 19 articles
Introduction: The existence of many phase III randomized controlled trials (RCTs) of first-line treatment for unresectable hepatocellular carcinoma (HCC) puzzle doctors and patients in choosing the most effective treatment strategies. We aimed to assess the efficacy, safety, and cost-effectiveness of immunotherapy or targeted therapy as the first-line strategy for unresectable HCC.
Methods: The included clinical trials were retrieved from PubMed, Embase, the Cochrane library, and Web of Science databases, in which immunotherapy or targeted therapy was regarded as the first-line treatment for unresectable HCC, published in English between January 1, 2010, and September 20, 2022. We conducted a network meta-analysis (NMA) and cost-effectiveness analysis (CEA) from the Chinese payer’s perspective. Overall survival (OS), progression-free survival (PFS), the ranks of different treatments using P-score, and adverse events (AEs) were evaluated by NMA. Total costs, life-years (LYs), quality-adjusted life-years (QALYs), and incremental cost-benefit ratio (ICER) were estimated from 15-year Markov models developed by CEA.
Results: We identified 2,825 records, including 11,796 patients, from 15 RCTs. The NMA revealed that sintilimab plus a bevacizumab biosimilar (HR, 0.57; 95% CI, 0.43 to 0.75; P = 0.96) and camrelizumab plus rivoceranib (HR, 0.56; 95% CI, 0.41 to 0.66; P = 0.94) could lead to great improvements in OS and PFS compared with sorafenib-related survival. The CEA indicated that tislelizumab increased by 0.220 QALYs (0.312 LYs) and decreased by $1,938 compared with sorafenib, which yielded ICERs of -$8,809/QALY (-$2,612/LY). Sensitivity analysis showed that the model was stable.
Conclusion: Sintilimab plus a bevacizumab biosimilar and camrelizumab plus rivoceranib significantly prolonged OS and PFS, respectively. Further considering the pharmacoeconomics factors, tislelizumab is the most cost-effective first-line treatment strategy for unresectable HCC in China.
Primary liver cancer, including hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma and other rare types, was the sixth most commonly diagnosed cancer and the third leading cause of cancer death globally in 2020. Approximately 906,000 new cases of liver cancer were reported globally, out of which 830,000 had a fatal outcome (1). Based on previous data, it is estimated that primary liver cancer will be the fourth most commonly diagnosed cancer and the third leading cause of cancer death in 2022 in China, with 431,383 new cases and 302,327 deaths (2). Although there is a wide variety of treatment methods for primary liver cancer, their efficacy is still unsatisfactory due to the difficulty in early diagnosis, as most patients are diagnosed at the advanced stage of the illness. Currently, some therapeutic approaches include surgery, transarterial chemoembolization, hepatic arterial infusion chemotherapy, radiotherapy, targeted therapy, and immunotherapy (3). However, their efficacy of them is not so desirable. Therefore, it is necessary to recommend the most effective treatment for clinicians and patients to choose.
For patients struggling with unresectable HCC, cancer treatment strategies like targeted therapy and immunotherapy are proven to be effective. In the past decade, numerous immunotherapy drugs and targeted therapy drugs have been gradually tried to apply to the first-line treatment of unresectable HCC, such as brivanib, sunitinib, linifanib, sorafenib, sorafenib plus doxorubicin, sorafenib plus erlotinib, lenvatinib, nivolumab, donafenib, and atezolizumab plus bevacizumab (4–12). Currently, the first-line recommended drugs include sorafenib, lenvatinib, atezolizumab plus bevacizumab, durvalumab, and nivolumab (13). Some regimens have been reported in clinical trials for first-line treatment, like sintilimab plus a bevacizumab biosimilar, cabozantinib plus atezolizumab, tislelizumab, and camrelizumab, durvalumab, pembrolizumab plus lenvatinib (14–19). Additionally, some researchers conducted a comparison of the efficacy of several of them. Liu W et al. found that sintilimab plus a bevacizumab biosimilar was the most effective treatment (20). In addition, another paper demonstrated that atezolizumab plus bevacizumab and sintilimab plus a bevacizumab biosimilar were comparable in efficacy (21). These studies have also become data references for clinical medication.
Following the European Society of Medical Oncology (ESMO) congress, the latest progress of first-line treatment regimens for HCC was updated in 2022, some of which have yielded breakthrough achievements. In the RATIONALE-301 trial, tislelizumab, compared with sorafenib, showed a non-inferiority efficacy in prolonging median overall survival (mOS) [15.9 vs 13.1 months; Hazard ratio (HR), 0.85; 95% confidence interval (CI), 0.712 to 1.019; P = 0.0398] (16). In the SHR-1210-III-310 trial, camrelizumab plus rivoceranib significantly prolonged mOS (22.1 vs 15.2 months; R, 0.62; 95% CI, 0.49 to 0.80; P < 0.0001) and mPFS (5.6 vs 3.7 months; HR, 0.52; 95% CI, 0.41 to 0.65; P < 0.0001) compared with sorafenib (17). In addition, lenvatinib plus pembrolizumab prolonged mOS (21.2 vs 19.0 months; HR, 0.840; 95% CI, 0.708 to 0.997; P = 0.0227) and mPFS (8.2 vs 8.1 months; HR, 0.834; 95% CI, 0.712 to 0.978) compared with lenvatinib alone but did not reach the significance threshold in the LEAP-002 study (19).
Considering that the previous research did not include these regimens comprehensively to compare efficacy indirectly, we conducted this network meta-analysis. However, due to the high morbidity and mortality of primary liver cancer in the Chinese population and most patients diagnosed as an advanced stage at the initial visit, the country and society are facing a huge burden of medical and health care. What’s more, a growing number of novel drugs with expensive cost showed satisfactory efficacy. Therefore, we also performed a cost-effectiveness analysis to evaluate which regimen can balance clinical benefits and medical cost better from Chinese payers’ perspectives.
We performed this work according to the PRISMA statement, including a PRISMA NMA checklist (Supplementary Table 1).
We searched PubMed, Embase, the Cochrane library, and Web of Science for English-language publications from January 1, 2010, to September 20, 2022, with the search terms “nivolumab”, “pembrolizumab”, “atezolizumab”, “camrelizumab”, “durvalumab”, “tislelizumab”, “PD-1”, “PD-L1”, “immunotherapy”, “sorafenib”, “sunitinib”, “linifanib”, “lenvatinib”, “donafenib”, “bevacizumab”, “targeted therapy”, “molecular targeted therapy”, “unresectable hepatocellular carcinoma”, and “clinical trial” (Supplementary Table 2). We also retrieved abstracts from the conferences of the European Society of Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO). The chosen literature for the conduction of this study should abide by the following inclusion criteria: (1) phase III RCTs for unresectable hepatocellular carcinoma; (2) included immunotherapy or targeted therapy treatment arm instead of locoregional therapy as the first-line treatment; (3) the endpoint included OS and PFS. The exclusion criteria are as follows: (1) a number of patients in the experimental group <10; (2) detailed reports of adverse effects were not available; (3) not published in English. Two independent reviewers (K.L. and Y.W.Z.) screened these studies to exclude repeated articles and those articles not meet the inclusion criteria and extracted relevant data. In the case of disagreement between the two reviewers, we invited a third independent reviewer (H.Z.) for evaluation. We selected the most updated report when there are several reports from the same clinical trial. When we find potentially included abstracts, we first determine whether they meet the inclusion criteria based on their content. In addition, we will contact researchers or the marketing department of the corresponding medical company to obtain detailed data to make the final decision. The bias risk assessment for these clinical trials was performed according to the Cochrane Collaboration guideline (22).
The HRs and its 95% CI for OS and PFS of various treatment schemes were obtained using the R software (version 4.1.1, available: http://www.rproject.org) along with the package of “netmeta”. Since there is little data to evaluate the heterogeneity between clinical trials, we established a fixed-effect model. In addition, we compared indirectly the safety of different regimens and calculated the odds ratio (OR) and its 95% CI of all grade adverse events (AEs) and ≥ 3-grade AEs. Finally, we used P-score to rank the efficacy and safety of each regimen.
We performed this work according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist (Supplementary Table 3), while this analysis did not involve human subjects or animal study.
The patients have received the first-line treatment of various regimens. In the case of progressed disease (PD) or intolerable AEs, patients received regorafenib recommended by the National Comprehensive Cancer Network (NCCN®) guidelines and the Chinese Society of Clinical Oncology (CSCO) guidelines as subsequent therapy (13, 23, 24). On the other hand, the remaining patients received the best supportive care (BSC) until death, and those who reached death received terminal care. The specific use of different drugs is detailed in Supplementary Table 4. According to these RCTs and the published articles, we assumed that patients were 65 kg in weight, and 164 cm in height with a body surface area of 1.72 m2 (25, 26) (Table 1).
To evaluate the cost-effectiveness of the different first-line regimens of patients with unresectable HCC, we constructed a Markov model with a length of 6 weeks and three health states (PFS, progressive disease (PD), and death) (Supplementary Figure 1) using TreeAge Pro 2020 (TreeAge Software, Williamstown, MA, https://www.treeage.com) and the the time horizon was 15 years. The costs and effects were discounted at a rate of 3% per year (34). The outputs we measured included the total cost, life-years (LYs), quality-adjusted LYs (QALYs), and incremental cost-effectiveness ratios (ICERs). The willingness-to-pay (WTP) was $37,653 per QALY in China (25).
We collected data from the OS and PFS curves from included RCTs using GetData Graph Digitizer (version 2.26; http://www.getdata-graph-digitizer.com/index.php), whereas the Weibull distribution was chosen as the best-fitting parameter model. This selection was made among well-known models such as log-logistic, Gompertz, Weibull, exponential, and log-normal distribution according to Akaike’s information criterion (AIC) and Bayesian information criterion (BIC) (Supplementary Figure 2 and Table 5) (36). As for sorafenib, we reconstructed the survival curves according to the survival data reported by each clinical trial. We got two parameters, shape (γ) and scale (λ), using R software (25). We used previously published utilities of 0.76 and 0.68 as the mean health utility value for the PFS and the PD state, respectively (27). We also consider the disutility values of grade 3/4 AEs in our analysis (28).
Direct medical costs were taken solely into account from the Chinese payers’ perspective, including costs of drugs, AEs management (grade 3 or higher AEs with an incidence rate higher than 5%) (27, 29–34), BSC, terminal care, follow-up, and monitoring (30, 35). The drug price and part of the cost of AEs management are from Xiangya Hospital of Central South University. The remaining costs were derived from previously published literature (Table 1). All costs are exchanged into US dollars at the rate of $1 = ¥6.8917.
We performed one-way sensitivity analysis and probabilistic sensitivity analysis to evaluate the uncertainty of the model results. One-way sensitivity analysis was conducted within a variance of 20% from their baseline values according to varied values of a certain parameter. We also performed a probabilistic sensitivity analysis to assess the probability of effectiveness of the treatment regimens through 10000 Monte Carlo repetitions.
The current NMA was conducted upon 15 phase III RCTs in which 2,825 records were screened and 11,796 patients were enrolled (Supplementary Figure 3). The model diagram of the NMA is shown in Supplementary Figure 4. These trials involved regimens brivanib (N = 577), donafenib (N = 328), durvalumab (N = 389), lenvatinib (N = 877), linifanib (N = 514), nivolumab (N = 371), sunitinib (N = 530), tislelizumab (N = 342), atezolizumab plus bevacizumab (N = 336), cabozantinib plus atezolizumab (N = 432), camrelizumab plus rivoceranib (N = 272), durvalumab plus tremelimumab (N = 393), pembrolizumab plus lenvatinib (N = 395), sintilimab plus a bevacizumab biosimilar (N = 381), sorafenib plus doxorubicin (N = 180), sorafenib plus erlotinib (N = 358), and sorafenib (N = 4,925) (Supplementary Table 6). The risk of bias is shown in Supplementary Table 7. From the indirect comparisons of the NMA, sintilimab plus a bevacizumab biosimilar (HR, 0.57; 95% CI, 0.43 to 0.75 or 1.75; 1.33 to 2.32; P-score = 0.96) and camrelizumab plus rivoceranib (HR, 0.56; 95% CI, 0.41 to 0.66 or 1.92; 1.53 to 2.42; P-score = 0.94) could lead to great improvements in OS and PFS compared with the sorafenib-related survival (Figures 1 and 2). The HRs for OS and PFS of active treatment compared with the sorafenib treatment are shown in Figures 1 and 2. The forest plot revealed that tislelizumab had a lower likelihood of all-grade (OR, 0.14; 95% CI, 0.07 to 0.25; P-score = 0.05) and grade 3 or higher AEs (0.25; 0.18 to 0.35; P-score = 0.05) than those of sorafenib, respectively (Supplementary Figure 5).
A Markov model was composed of 11,403 participants with a 15-year time horizon, in which the durvalumab plus tremelimumab group was excluded. The total costs and QALYs (LYs) of brivanib, sunitinib, linifanib, sorafenib plus doxorubicin, sorafenib plus erlotinib, lenvatinib, cabozantinib plus atezolizumab, durvalumab, tislelizumab, nivolumab, donafenib, pembrolizumab plus lenvatinib, camrelizumab plus rivoceranib, atezolizumab plus bevacizumab, sintilimab plus a bevacizumab biosimilar, and sorafenib were $64,723 and 0.374 (0.524), $26,922 and 0.945 (1.379), $59,860 and 1.379 (1.681), $38,052 and 1.186 (1.717), $35,787 and 1.297 (1.888), $28,080 and 1.367 (1.942), $56,396 and 1.410 (1.994), $33,972 and 1.498 (2.128), $26,808 and 1.509 (2.149), $32,703 and 1.515 (2.148), $31,063 and 1.535 (2.180), $44,731 and 1.594 (2.260), $40,307 and 1.795 (2.603), $73,457 and 1.870 (2.646), $56,259 and 2.076 (2.950), and $28,746 and 1.289 (1.837), which yielded ICERs of -$39,319 (-$27,401), $5,302 ($3,983), -$250,919 (-$199,449), -$83,838 (-$77,550), $138,059 ($880,125), -$3,680 (-$6,343), $228,512 ($176,115), $25,005 ($17,959), -$8,809 (-$2,612), $17,509 ($12,724), $9,419 ($6,755), $52,410 ($37,790), $22,848 ($15,093), $76,955 ($55,267), and $34,959 ($24,675) per QALY (LY) gained than sorafenib, respectively (Table 2). Our results demonstrate that tislelizumab or lenvatinib versus sorafenib as first-line systematic treatment were dominant. Further pairwise comparative analysis, treatment with tislelizumab produced an additional 0.142 QALYs (0.207 LYs) and a cost reduction of $1,272 compared with lenvatinib, resulting in an ICER of -$8,958/QALY (-6,145/LY). A comparison of ICER’s pairwise treatment strategies is shown in Supplementary Table 8. In summary, tislelizumab was cost-effective as the first-line strategy for unresectable HCC in China.
The results of one-way sensitivity showed that HRs of OS of the tislelizumab versus sorafenib when comparing tislelizumab and sorafenib, followed by the cost of sorafenib, regorafenib, and tislelizumab. When comparing lenvatinib and sorafenib, HRs of OS of the lenvatinib versus sorafenib, followed by the costs of lenvatinib, sorafenib and regorafenib (Figure 3). In the probabilistic sensitivity analysis, the acceptability curve showed that for different WTP values, the most cost-effective solutions are also different (Figure 4). For example, tislelizumab is a better choice when it is less than the WTP threshold of $37,653/QALY in China. When the WTP value is between $37,653/QALY and $80,000/QALY, camrelizumab plus rivoceranib is the better choice. When WTP is greater than $80,000/QALY, sintilimab plus a bevacizumab biosimilar is a better choice (Figure 4). The scatter plot showed that the probability of tislelizumab, lenvatinib, donafenib, nivolumab, camrelizumab plus rivoceranib, durvalumab, sintilimab plus a bevacizumab biosimilar, pembrolizumab plus lenvatinib, sorafenib plus erlotinib, sunitinib, atezolizumab plus bevacizumab, brivanib, linifanib, sorafenib plus doxorubicin, cabozantinib plus atezolizumab therapies being cost-effective were 96.7%, 86.3%, 84.5%, 82.4%, 75.4%, 73.6%, 60.0%, 24.0%, 0.3%, 1.6%, 0.9%, 0%, 0%, 0%, and 0% compared with sorafenib at a WTP threshold of $37,653/QALY, respectively (Supplementary Figure 6).
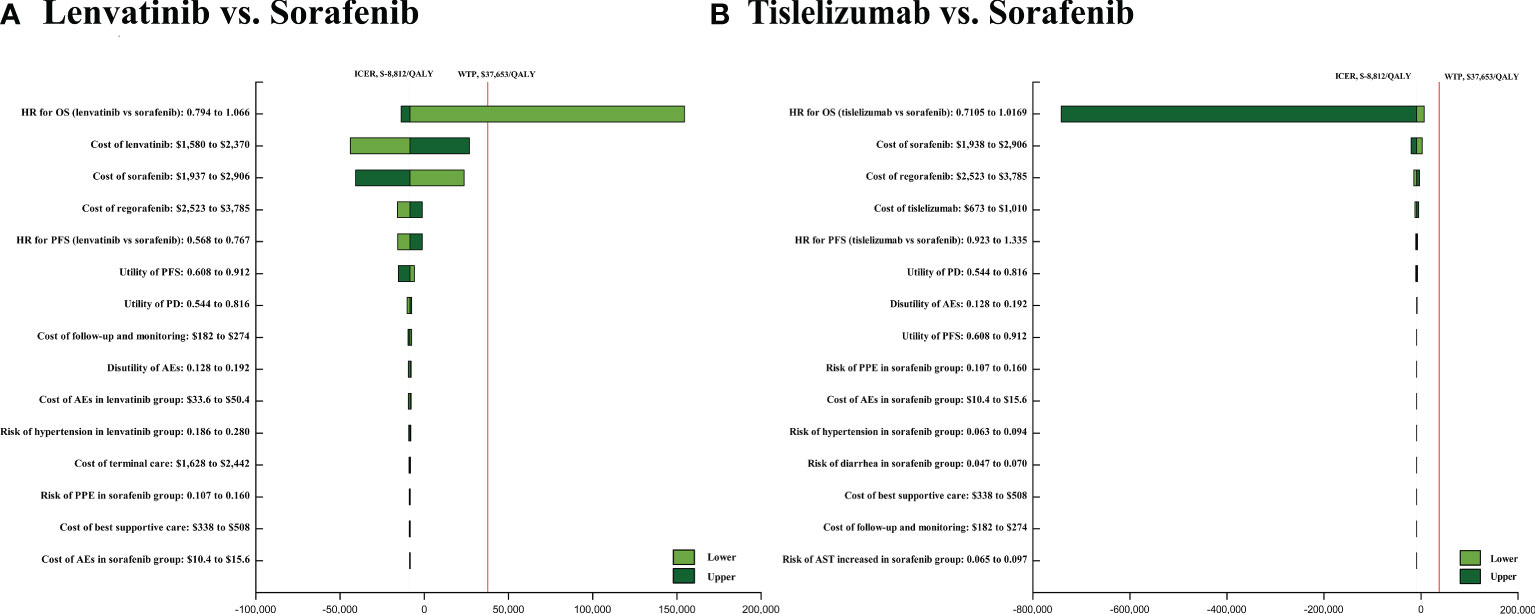
Figure 3 The one-way sensitivity analyses of lenvatinib vs sorafenib (A), tislelizumab vs. sorafenib (B). PFS, progression-free survival; PD, disease progressed; AEs, adverse events.
At present, the morbidity and mortality of primary liver cancer, especially HCC, are very high in the world, and most patients are in the advanced stage. Among all regions of the world, the highest incidence of HCC has been observed in Asia, and China has recorded many cases (37). Furthermore, Chinese patients with unresectable HCC face substantial financial pressure due to medical expenses. From 2012 to 2014, the expenditures of an HCC patient were ¥55,529 ($8,057) in 13 Chinese provinces on average, which included ¥4,592 ($666) of non-medical and the rest ¥50,937 ($7,391) of medical expenditures (38). Due to the poor prognosis and medical care burden of unresectable HCC, numerous clinical trials are conducted to explore an effective treatment option (16, 17, 19, 37). For the moment, there are a large quantity of novel drugs for unresectable HCC to choose, such as brivanib, sunitinib, linifanib, sorafenib plus doxorubicin, sorafenib plus erlotinib, lenvatinib, cabozantinib plus atezolizumab, durvalumab, tislelizumab, nivolumab, donafenib, pembrolizumab plus lenvatinib, camrelizumab plus rivoceranib, atezolizumab plus bevacizumab, sintilimab plus a bevacizumab biosimilar, and sorafenib. Although these therapeutic strategies are effective for treating unresectable HCC, the high cost of these drugs places a heavy burden on social health resources and patients. In addition, there is a lack of head-to-head clinical trials of 16 treatment strategies to show which one is better, and fewer studies to consider its overall cost-effectiveness. Therefore, we performed the first well-rounded network meta-analysis and cost-effectiveness analysis of first-line systemic regiments for unresectable HCC to facilitate treatment strategies for patients and clinicians.
Additionally, there is a lack of head-to-head clinical trials to determine which one was the best choice. Even more, only a few studies discussed the overall cost-effectiveness of each strategy separately. Therefore, this was the first well-rounded NMA and cost-effectiveness analysis evaluating the first-line systematic regimens for unresectable HCC to facilitate treatment strategies for both patients and clinicians.
The last ten years have shown that many novel drugs have been approved to treat irresectable liver cancer. Sorafenib was the first approved molecularly-targeted drug and was regarded as a standard in improving the prognosis of patients struggling with unresectable HCC. In this study, we also uniformly selected sorafenib as a control. However, our findings differed slightly from those of published research (21). Network meta-analysis results revealed that sintilimab plus a bevacizumab biosimilar showed the best effectiveness in prolonging OS, followed by camrelizumab plus rivoceranib and atezolizumab plus bevacizumab. On the other hand, camrelizumab plus rivoceranib ranked the first in prolonging PFS, followed by pembrolizumab plus lenvatinib and sintilimab plus a bevacizumab biosimilar. The anti-angiogenesis effect of immunotherapy combined with targeted therapy is obvious, which highlights the characteristics and advantages of immunotherapy, and gradually changes the existing clinical standard and treatment mode of unresectable HCC. However, all grade and grade 3 or higher AEs in camrelizumab plus rivoceranib group is much higher than other groups. The grade 3 or higher AEs in camrelizumab plus rivoceranib group included hypertension, hepatic insufficiency, palmar-plantar erythro-dysesthesia, and so on, most of which are related to TKI (17). Meanwhile, all grade and grade 3 or higher AEs in the immunotherapy alone group are much lower than other group, such as durvalumab, tislelizumab, and nivolumab. The latter indicated the possibility of immunotherapy being a new trend in curing patients with unresectable HCC. In conclusion, NMA provides strong evidence for the efficacy of immunotherapy in combination with targeted therapy as the first-line treatment for unresectable HCC. Our estimates of survival suggest that the efficacy of immunotherapy plus targeted therapy is better than others. Although immunotherapy remains the most favorable safety, the increased efficacy of combination therapy comes at the cost of a high risk of toxicity, which often results in permanent discontinuation. Therefore, doctors and patients need to consider both efficacy and safety when selecting treatment options according to the patient’s condition in clinical practice.
It is a cost-based innovative treatment strategy that we need to take into consideration in China, an economic powerhouse. The baseline results of the cost-effectiveness analysis indicated that tislelizumab and lenvatinib increased by 0.220 and 0.078 QALYs and decreased by $1,938 and $666 compared with sorafenib, respectively. What’s more, tislelizumab increased by 0.142 QALYs and decreased by $1,272 compared with lenvatinib. The treatment of and sintilimab plus a bevacizumab biosimilar, atezolizumab plus bevacizumab, and camrelizumab plus rivoceranib are related to better efficacy in all first-line strategy, producing 2.076, 1.870, and 1.795 QALYs respectively, which are accordance with the results of NMA. However, considering the medical expenditure of all treatment strategies, tislelizumab is the dominant cost-effective strategies as the first-line treatment for unresectable HCC. The main reasons were: first, considering the affordability of Chinese patients, the price of tislelizumab negotiated with the government is the lowest among all first-line treatment. Second, the costs of dealing with AEs were the least due to the low incidence of grade 3 or higher AEs. Third, patients receiving BSC, who are intolerant to standard treatment strategies for unresectable HCC, accounted for a relatively small percentage.
Our cost-effectiveness analysis is sensitive to the relative efficacy of the first-line treatment for unresectable HCC. The analysis suggested that the economic outcome of lenvatinib became more favorable in patients with lower HR of OS compared with sorafenib and worse in patients with higher HR. However, regardless of whether the HR of OS was higher or lower in tislelizumab group compared with sorafenib, the economic outcome was favorable. This finding is similar to previously published researches, in which the HR of OS is the most influential factor (28, 30, 39–42). Changes in WTP values also affect economic outcomes. Tislelizumab was the most cost-effective treatment option at a WTP threshold of $37,653 per QALY, whereas camrelizumab plus rivoceranib was a preferable option at a WTP threshold of $37,653 to $80,000 per QALY and sintilimab plus a bevacizumab biosimilar was an affordable option at a WTP threshold of higher than $80,000 per QALY. Due to China’s vast territory and abundant resources, GDP per capita varies greatly. We also calculated WTP values for different regions of China. For example, The WTP values of Beijing, Shanghai, Jiangsu, Guangdong, Hubei, Neimenggu, Anhui, Hunan, Jiangxi, Guizhou, Guangxi, Heilongjiang and Gansu were $80,053/QALY, $75,656/QALY, $59,768/QALY, $42,964/QALY, $37,698/QALY, $37,132/QALY, $30,646/QALY, $30,167/QALY, $28,513/QALY, $22,114/QALY, $21,740/QALY, $20,329/QALY and $17,804/QALY, respectively (43). Surprisingly, tislelizumab is the best strategy of choice in underdeveloped and relatively developed regions of China. In most developed regions, camrelizumab plus rivoceranib is regarded as the best choice. Only in Beijing, sintilimab plus a bevacizumab biosimilar is an optional choice. Therefore, the different economic factors of different regions should be taken into account when approving novel medicines for clinical use. Currently, the drugs used in the first-line treatment of liver cancer have certain clinical benefits and may be used on a large scale, but their economic toxicity still exists. Economic toxicity can bankrupt patients with high treatment costs, cause cancer patients to stop treatment, and even lead to poor patient outcomes in China (34). In the CSCO guidelines version 2022, sorafenib, lenvatinib, donafenib, sintilimab plus a bevacizumab biosimilar, and camrelizumab plus rivoceranib are recommended first-line strategies, with a wide range of costs (23). Thus, our results can be used to find a reasonable balance between the price of new drugs and their clinical efficacy, inform national regulatory agencies when making healthcare decisions, and make a significant contribution to adequately address economic toxicity.
There are some limitations in our study. Firstly, when using the network meta-analysis method to compare first-line treatment regimens indirectly, we assumed that the included studies did not differ in patient characteristics and summarized the chemotherapy groups, and selected a fixed-effect model. However, it is a difference that we cannot eliminate. For instance, the ORIENT-32 trial recruited participants from the Chinese population and the IMbrave150 trial and LEAP-002 trial recruited globally. Secondly, considering that there were multiple survival curves of sorafenib, we pooled and reconstructed the survival curves according to the original survival data of the sorafenib group in each clinical trial. Thirdly, we inferred the long-term survival benefit in terms of the short-term survival data of each experiment, which will change with the change of long-term follow-up. This is an inevitable limitation in our model. Therefore, it is necessary to verify and evaluate the concordance of these health outcomes in a model with real-world data. In addition, we only considered the occurrence of more than 5% of grade 3 or higher adverse events, which may underestimate the cost of adverse events. Nevertheless, the cost and disutility of AEs were not the significant factors influencing the results
Briefly, sintilimab plus a bevacizumab biosimilar and camrelizumab plus rivoceranib showed the best efficacy in prolonging OS and PFS compared with sorafenib, respectively. What’s more, we found that tislelizumab is the most cost-effective first-line treatment strategy for unresectable HCC in China at the WTP of $37,653 QALY. In economically developed areas of China, camrelizumab plus rivoceranib is also a recommended cost-effective treatment strategy. The results could help clinicians select the most appropriate drugs for their patients and set reimbursement policies.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors, it does not require the approval of the independent ethics committee.
KL, YZ, and HZ designed the experiment. YZ, and KL performed the experiments. YZ and KL analyzed the data. HZ contributed analysis tools and funding. YZ, KL, and HZ wrote the manuscript. KL and YZ contributed equally. All authors contributed to the article and approved the submitted version.
This work was supported by the Clinical Research Project of Xiangya Hospital (grant number, 2016L06 to HZ).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1103055/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and united states, 2022: profiles, trends, and determinants. Chin Med J (Engl) (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
3. Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang M, et al. Immunotherapy for hepatocellular carcinoma: Current status and future prospects. Front Immunol (2021) 12:765101. doi: 10.3389/fimmu.2021.765101
4. Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol (2013) 31:3517–24. doi: 10.1200/JCO.2012.48.4410
5. Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol (2013) 31:4067–75. doi: 10.1200/JCO.2012.45.8372
6. Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol (2015) 33:172–9. doi: 10.1200/JCO.2013.54.3298
7. Abou-Alfa GK, Shi Q, Knox JJ, Kaubisch A, Niedzwiecki D, Posey J, et al. Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: Phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol (2019) 5:1582–8. doi: 10.1001/jamaoncol.2019.2792
8. Zhu AX, Rosmorduc O, Evans TR, Ross PJ, Santoro A, Carrilho FJ, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol (2015) 33:559–66. doi: 10.1200/JCO.2013.53.7746
9. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
10. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol (2022) 23:77–90. doi: 10.1016/S1470-2045(21)00604-5
11. Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: A randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol (2021) 39:3002–11. doi: 10.1200/JCO.21.00163
12. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76:862–73. doi: 10.1016/j.jhep.2021.11.030
13. National comprehensive cancer network clinical practice guidelines in oncology (NCCN guidelines): Hepatobiliary cancers, version 2.2022 . Available at: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (Accessed July 15, 2022).
14. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
15. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23:995–1008. doi: 10.1016/S1470-2045(22)00326-6
16. Qin S, Kudo M, Meyer T, Finn RS, Vogel A, Bai Y, et al. LBA36 final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol (2022) 33:S1402–S3. doi: 10.1016/j.annonc.2022.08.033
17. Qin S, Chan LS, Gu S, Bai Y, Ren Z, Lin X, et al. LBA35 camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol (2022) 33:S1401–S2. doi: 10.1016/j.annonc.2022.08.032
18. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol (2022) 40:379–. doi: 10.1200/JCO.2022.40.4_suppl.379
19. Finn RS, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, et al. LBA34 primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol (2022) 33:S1401. doi: 10.1016/j.annonc.2022.08.031
20. Liu W, Quan B, Lu S, Tang B, Li M, Chen R, et al. First-line systemic treatment strategies for unresectable hepatocellular carcinoma: A systematic review and network meta-analysis of randomized clinical trials. Front Oncol (2021) 11:771045. doi: 10.3389/fonc.2021.771045
21. Fulgenzi CAM, D'Alessio A, Airoldi C, Scotti L, Demirtas CO, Gennari A, et al. Comparative efficacy of novel combination strategies for unresectable hepatocellular carcinoma: A network metanalysis of phase III trials. Eur J Cancer. (2022) 174:57–67. doi: 10.1016/j.ejca.2022.06.058
22. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
23. Available at: http://www.csco.org.cn/cn/index.aspx (Accessed 2022).
24. Available at: http://www.nhc.gov.cn/ (Accessed 2022).
25. Zhu Y, Liu K, Wang K, Peng L. Vascular endothelial growth factor receptor inhibitors in Chinese patients with advanced radioactive iodine-refractory differentiated thyroid cancer: A network meta-analysis and cost-effectiveness analysis. Front Endocrinol (Lausanne). (2022) 13:909333. doi: 10.3389/fendo.2022.909333
26. Zhu Y, Liu K, Ding D, Wang K, Liu X, Tan X. Chemo-immunotherapy regimes for recurrent or metastatic nasopharyngeal carcinoma: A network meta-analysis and cost-effectiveness analysis. Front Pharmacol (2022) 13:858207. doi: 10.3389/fphar.2022.858207
27. Liao W, Huang J, Hutton D, Zhu G, Wu Q, Wen F, et al. Cost-effectiveness analysis of cabozantinib as second-line therapy in advanced hepatocellular carcinoma. Liver Int (2019) 39:2408–16. doi: 10.1111/liv.14257
28. Li L, Yang S, Chen Y, Tian L, He Y, Wu B, et al. Immune checkpoint inhibitors plus an anti-VEGF antibody as the first-line treatment for unresectable hepatocellular carcinoma: A network meta-analysis and cost-effectiveness analysis. Front Pharmacol (2022) 13:891008. doi: 10.3389/fphar.2022.891008
29. Cai H, Zhang L, Li N, Zheng B, Liu M. Lenvatinib versus sorafenib for unresectable hepatocellular carcinoma: a cost-effectiveness analysis. J Comp Eff Res (2020) 9:553–62. doi: 10.2217/cer-2020-0041
30. Zhao M, Pan X, Yin Y, Hu H, Wei J, Bai Z, et al. Cost-effectiveness analysis of five systemic treatments for unresectable hepatocellular carcinoma in China: An economic evaluation based on network meta-analysis. Front Public Health (2022) 10:869960. doi: 10.3389/fpubh.2022.869960
31. Meng R, Zhang X, Zhou T, Luo M, Qiu Y. Cost-effectiveness analysis of donafenib versus lenvatinib for first-line treatment of unresectable or metastatic hepatocellular carcinoma. Expert Rev Pharmacoecon Outcomes Res (2022) 22:1079–86. doi: 10.1080/14737167.2022.2079498
32. Zhou T, Cao Y, Wang X, Yang L, Wang Z, Ma A, et al. Economic evaluation of sintilimab plus bevacizumab versus sorafenib as a first-line treatment for unresectable hepatocellular carcinoma. Adv Ther (2022) 39:2165–77. doi: 10.1007/s12325-022-02079-4
33. Li M, Lin S, Wilson L, Huang P, Wang H, Lai S, et al. Cost-effectiveness analysis of hepatic arterial infusion of FOLFOX combined sorafenib for advanced hepatocellular carcinoma with portal vein invasion. Front Oncol (2021) 11:562135. doi: 10.3389/fonc.2021.562135
34. Zhu Y, Liu K, Ding D, Zhou Y, Peng L. Pembrolizumab plus chemotherapy as first-line treatment for advanced esophageal cancer: A cost-effectiveness analysis. Adv Ther (2022) 39:2614–29. doi: 10.1007/s12325-022-02101-9
35. Guan H, Wang C, Zhao Z, Han S. Cost-effectiveness of donafenib as first-line treatment of unresectable hepatocellular carcinoma in China. Adv Ther (2022) 39:3334–46. doi: 10.1007/s12325-022-02185-3
36. Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making. (2013) 33:743–54. doi: 10.1177/0272989X12472398
37. Sayiner M, Golabi P, Younossi ZM. Disease burden of hepatocellular carcinoma: A global perspective. Dig Dis Sci (2019) 64:910–7. doi: 10.1007/s10620-019-05537-2
38. Zou H, Li M, Lei Q, Luo Z, Xue Y, Yao D, et al. Economic burden and quality of life of hepatocellular carcinoma in greater China: A systematic review. Front Public Health (2022) 10:801981. doi: 10.3389/fpubh.2022.801981
39. Chiang CL, Chan SK, Lee SF, Wong IO, Choi HC. Cost-effectiveness of pembrolizumab as a second-line therapy for hepatocellular carcinoma. JAMA Netw Open (2021) 4:e2033761. doi: 10.1001/jamanetworkopen.2020.33761
40. Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open (2021) 4:e210037. doi: 10.1001/jamanetworkopen.2021.0037
41. Chiang CL, Chan SK, Lee SF, Choi HC. First-line atezolizumab plus bevacizumab versus sorafenib in hepatocellular carcinoma: A cost-effectiveness analysis. Cancers (Basel). (2021) 13:931. doi: 10.3390/cancers13050931
42. Hou Y, Wu B. Atezolizumab plus bevacizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma: a cost-effectiveness analysis. Cancer Commun (Lond). (2020) 40:743–5. doi: 10.1002/cac2.12110
43. Wangyi. Available at: https://www.163.com/dy/article/HEBAKHK80553CGLS.html (Accessed September, 2022).
Keywords: unresectable hepatocellular carcinoma, immunotherapy, targeted therapy, network meta-analysis, cost-effectiveness analysis
Citation: Liu K, Zhu Y and Zhu H (2023) Immunotherapy or targeted therapy as the first-line strategies for unresectable hepatocellular carcinoma: A network meta-analysis and cost-effectiveness analysis. Front. Immunol. 13:1103055. doi: 10.3389/fimmu.2022.1103055
Received: 19 November 2022; Accepted: 27 December 2022;
Published: 11 January 2023.
Edited by:
Mohammad Azhar Aziz, Aligarh Muslim University, IndiaReviewed by:
Mohammad A. AL-Mterin, University of Nizwa, OmanCopyright © 2023 Liu, Zhu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Zhu, emh1aG9uZzA3MTlAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.