- 1Department of Acupuncture, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Acupuncture, Guang’anmen Hospital, Chinese Academy of Traditional Chinese Medicine Ji’nan Hospital (Ji’nan Hospital of Traditional Chinese Medicine), Shandong, China
- 3Department of Traditional Chinese Medicine, Yuyuantan Community Health Center, Beijing, China
- 4Treatment Center of Traditional Chinese Medicine, Beijing Bo’ai Hospital, China Rehabilitation Research Center, Beijing, China
- 5Treatment Center of Traditional Chinese Medicine, School of Rehabilitation, Capital Medical University, Beijing, China
- 6Department of Acupuncture, Dongzhimen Hospital of Beijing University of Traditional Chinese Medicine, Beijing, China
Background: COVID-19 vaccines are required for individuals with myasthenia gravis (MG), as these patients are more likely to experience severe pneumonia, myasthenia crises, and higher mortality rate. However, direct data on the safety of COVID-19 vaccines in patients with MG are lacking, which results in hesitation in vaccination. This scoping was conducted to collect and summarize the existing evidence on this issue.
Methods: PubMed, Cochrane Library, and Web of Science were searched for studies using inclusion and exclusion criteria. Article titles, authors, study designs, demographics of patients, vaccination information, adverse events (AEs), significant findings, and conclusions of included studies were recorded and summarized.
Results: Twenty-nine studies conducted in 16 different countries in 2021 and 2022 were included. Study designs included case report, case series, cohort study, cross-sectional study, survey-based study, chart review, and systemic review. A total of 1347 patients were included. The vaccines used included BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, inactivated vaccines, and recombinant subunit vaccines. Fifteen case studies included 48 patients reported that 23 experienced new-onset, and five patients experienced flare of symptoms. Eleven other types of studies included 1299 patients reported that nine patients experienced new-onset, and 60 participants experienced flare of symptoms. Common AEs included local pain, fatigue, asthenia, cephalalgia, fever, and myalgia. Most patients responded well to treatment without severe sequelae. Evidence gaps include limited strength of study designs, type and dose of vaccines varied, inconsistent window of risk and exacerbation criteria, limited number of participants, and lack of efficacy evaluation.
Conclusion: COVID-19 vaccines may cause new-onset or worsening of MG in a small proportion of population. Large-scale, multicenter, prospective, and rigorous studies are required to verify their safety.
1 Introduction
It has been nearly three years since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory virus coronavirus 2 (SARS-CoV-2) (1–4). The world is promoting COVID-19 vaccination in order to reduce infections and combat this pandemic (5). More than 100 vaccine candidates have reached clinical trials (6, 7). Currently, there are mainly four types of COVID-19 vaccines in use: nucleic acid mRNA-based vaccines, viral vector vaccines, inactivated virus vaccines, and subunit vaccines (8, 9).
The relationship between COVID-19 infection and myasthenia gravis (MG) exacerbation has been observed in numerous studies. In COVID-19-infected patients with MG, severe pneumonia and myasthenia crises are more frequent, and their mortality rate is considerably higher (10, 11). Thirty-six of 91 patients who had both MG and COVID-19 infection were reported to experience flare of symptoms and required rescue therapy (36/91, 39.56%), and 22 died due to COVID-19 (22/91, 24.18%) (10). This suggests that it is necessary to help patients with MG find ways to resist COVID-19 as the pandemic continues around the world. Currently, vaccination is the best way to achieve this goal. Vaccines approved for use have shown significant protective effect and safety in the general population with low incidence of adverse events (AEs) (12). Nevertheless, it is important to examine the safety and tolerability of these vaccines in specific medical conditions, such as MG, where there is still a concern that exacerbation of symptoms may occur after vaccination due to the immune response activation (13). Some case studies have reported a possible association between new-onset or worsening MG and vaccination. The proposed mechanisms for vaccine-provoked autoimmune diseases include molecular mimicry between the spike protein of SARS-CoV-2 and host self-antigens and bystander activation (14–16). Direct data on the safety and tolerability of COVID-19 vaccines in patients with MG are lacking, which results in hesitation in vaccination (17).
To collect and analyze the existing evidence on the safety of COVID-19 vaccines in patients with MG, understand the current research situation, and determine future research priorities and trends, we decided to conduct this scoping review. This scoping review was written in accordance with the PRISMA Extension for Scoping Reviews (PRISMA-ScR) (18). The protocol was registered and accessible to the public on the Open Science Framework (OSF) with registration DOI https://doi.org/10.17605/OSF.IO/C2ZE3.
2 Materials and methods
2.1 Determining the review questions
Before starting to conduct this review, we defined our broad exploratory research question as, “What has been studied about the safety of COVID-19 vaccines in patients with MG?” More specific research questions included: What types of studies have been conducted? What types of vaccines have been used? What are the results of safety evaluation? What are the directions and priorities for future studies?
2.2 Literature search
Three databases, including PubMed, Cochrane Library, and Web of Science (WOS), were systematically searched to identify all studies with MeSH terms including “myasthenia gravis,” “COVID-19,” “SARS-CoV-2,” “vaccines,” “vaccination,” and “COVID-19 vaccines.” The supplementary file presents the detailed search strategy. All clinical studies that investigated the safety profile of COVID-19 vaccines in patients with MG were included, including case reports, case series, randomized controlled trials, cohort studies, and reviews. No restrictions were placed on languages (from database inception to October 2, 2022). We excluded repeatedly published articles, conference abstracts, and dissertations.
2.3 Literature selection
All articles retrieved from the three databases were imported into EndNote software. After removing duplicate records, the titles and abstracts were independently reviewed by two investigators, and those that matched the inclusion criteria were kept for additional review. Subsequently, the full texts were read to assess for eligibility. When the two investigators disagreed with whether or not to include an article, they should have a discussion before final decision. If there was no consensus, a third researcher assisted in determining to include the article or not.
2.4 Data extraction
The extracted data included: article title, author, journal, country of origin, year of publication, objectives, population and medical history, study designs, type and dose of vaccines, outcomes, AEs, significant findings, and conclusions. An Excel table was designed in advance for the data extraction. To ensure accuracy, two investigators collected the data independently and checked them in real-time.
3 Results
3.1 Literature search results
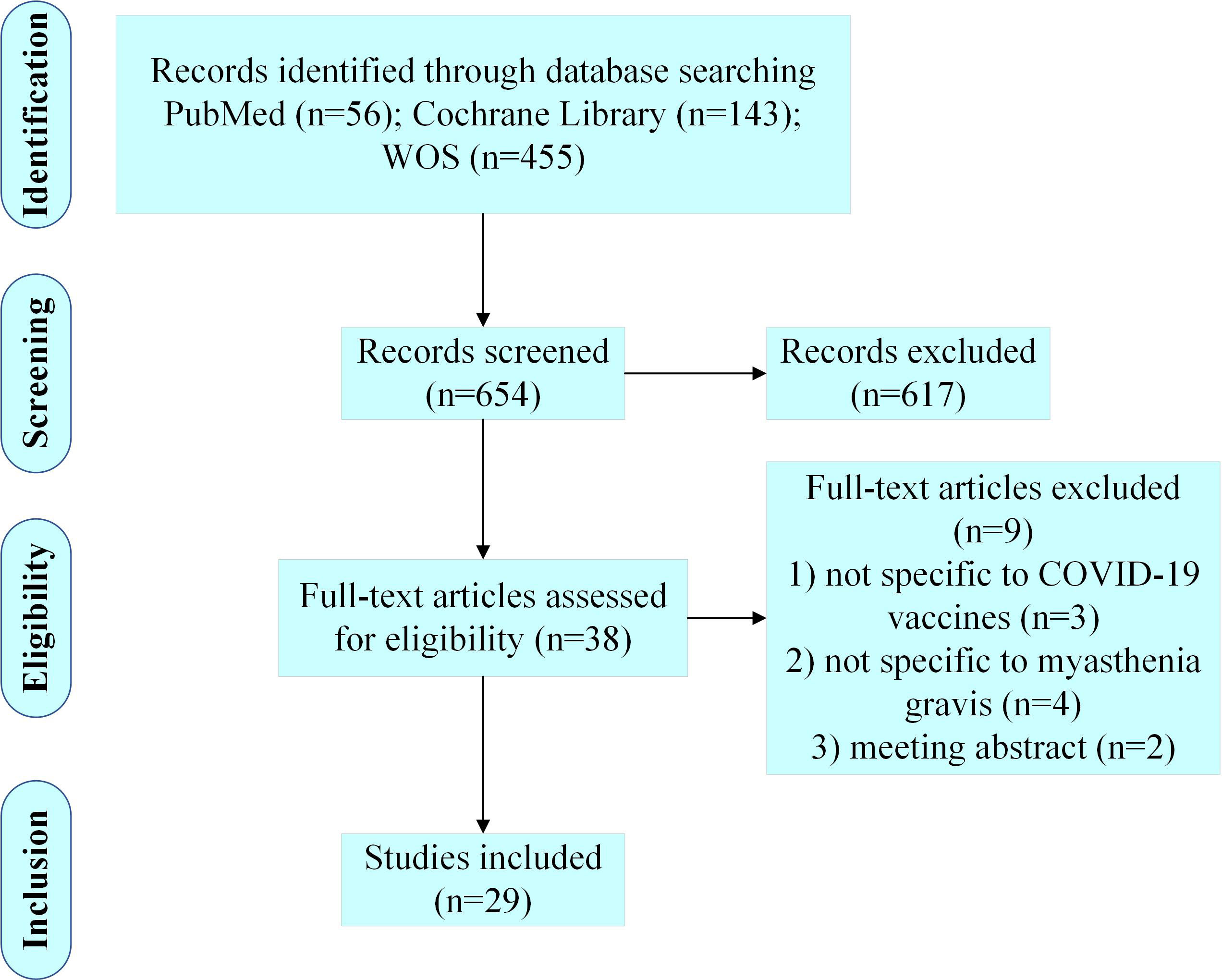
A total of 654 articles were retrieved from the three databases, and 29 articles were included in this review after comprehensive literature screening (Figure 1).
3.2 General characteristics of the included studies
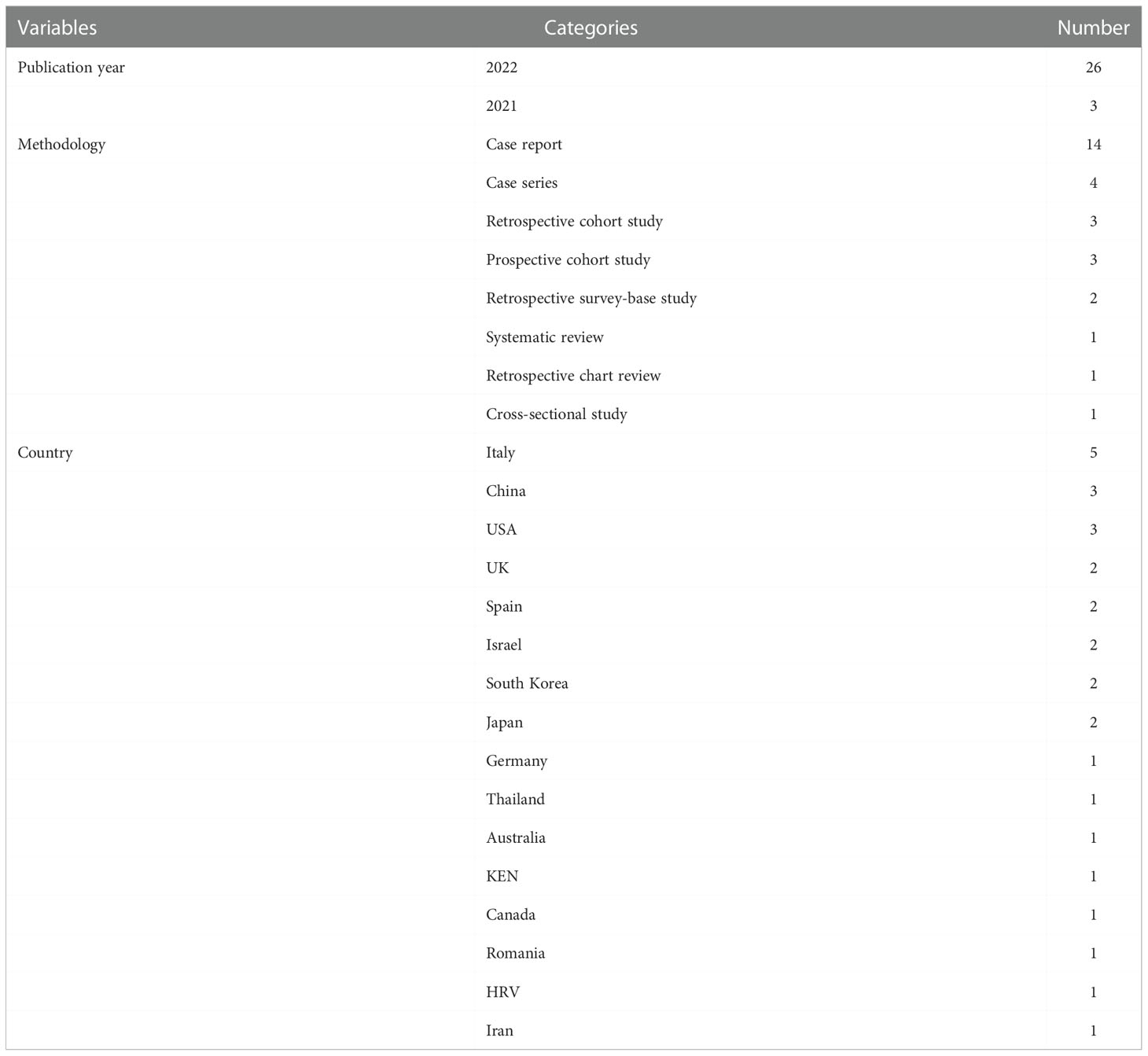
Table 1 displays general features of the 29 studies, including publishing year, methodology, and country of origin. Three of the included studies were published in 2021, and 26 in 2022. In terms of study design, case studies (18/29, 62.07%) were the most frequent, including 14 case reports and four case series. Other types of study designs included three retrospective cohort studies, three prospective cohort studies, two retrospective survey-based studies, one systematic review, one retrospective chart review, and one cross-sectional study. The 29 studies were conducted in 16 different countries, with Italy (5/29, 17.24%), China (3/29, 10.34%), and the United States (3/29, 10.34%) conducting the most relevant studies.
3.3 Demographic characteristics of study participants
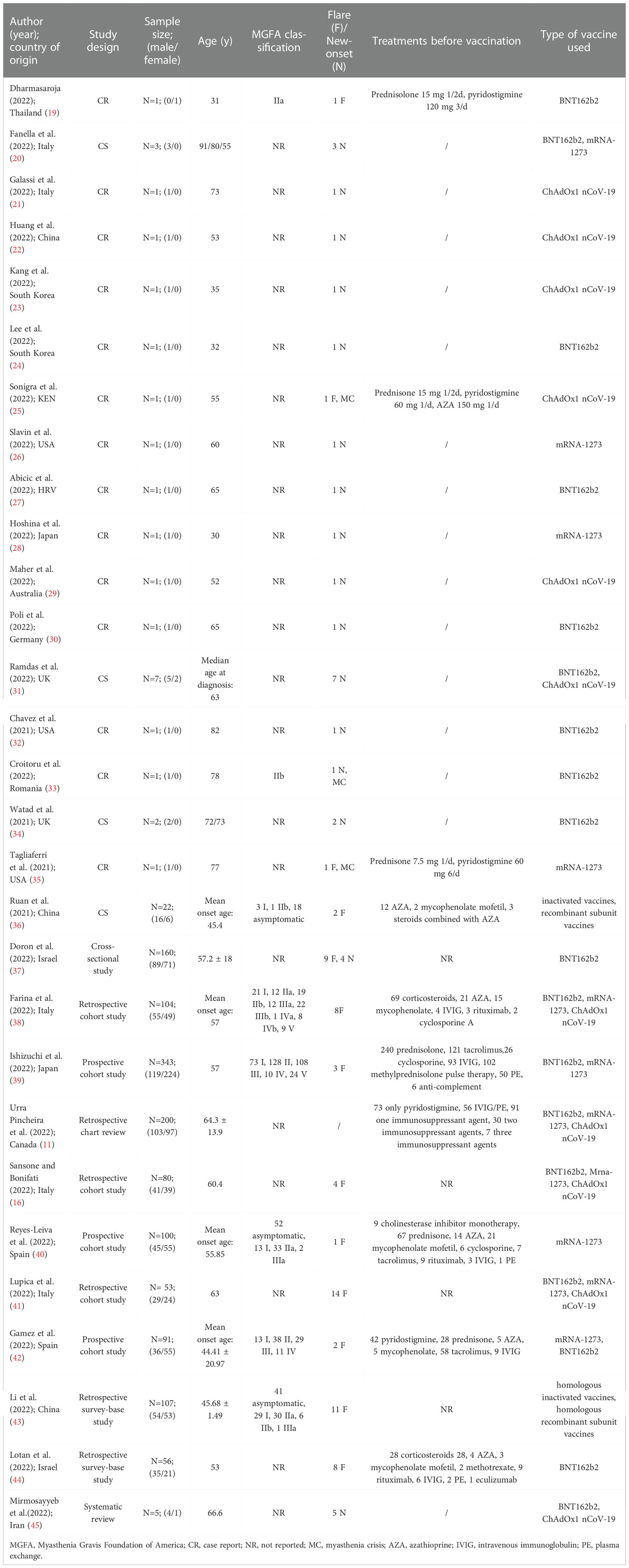
Table 2 shows the detailed demographic and clinical features of the participants. In the 29 studies, 1347 participants in total were included, including 649 males (649/1347, 48.18%) and 698 females (698/1347, 51.82%). Four studies reported the mean onset age of the patients, and one study reported the median age at MG diagnosis. In the other 24 studies, the age of the patients ranged from 31 to 91 years. Only seven studies involving 769 patients reported the specific Myasthenia Gravis Foundation of America (MGFA) classification of participants. Among the 769 patients, 111 were asymptomatic, 152 were in grade I, 269 were in grade II, 174 were in grade III, 30 were in grade IV, and 33 were in grade V. Ten studies reported the detailed treatment for the patients before vaccination. The vaccines used in all included studies included BNT162b2 (Comirnaty, developed by BioNTech and Pfizer), mRNA-1273 (Spikevax, previously called Moderna, developed by Moderna Biotech), ChAdOx1 nCoV-19 (Vaxzevria, developed by AstraZeneca), inactivated vaccines, and recombinant subunit vaccines.
3.4 Summary of case studies
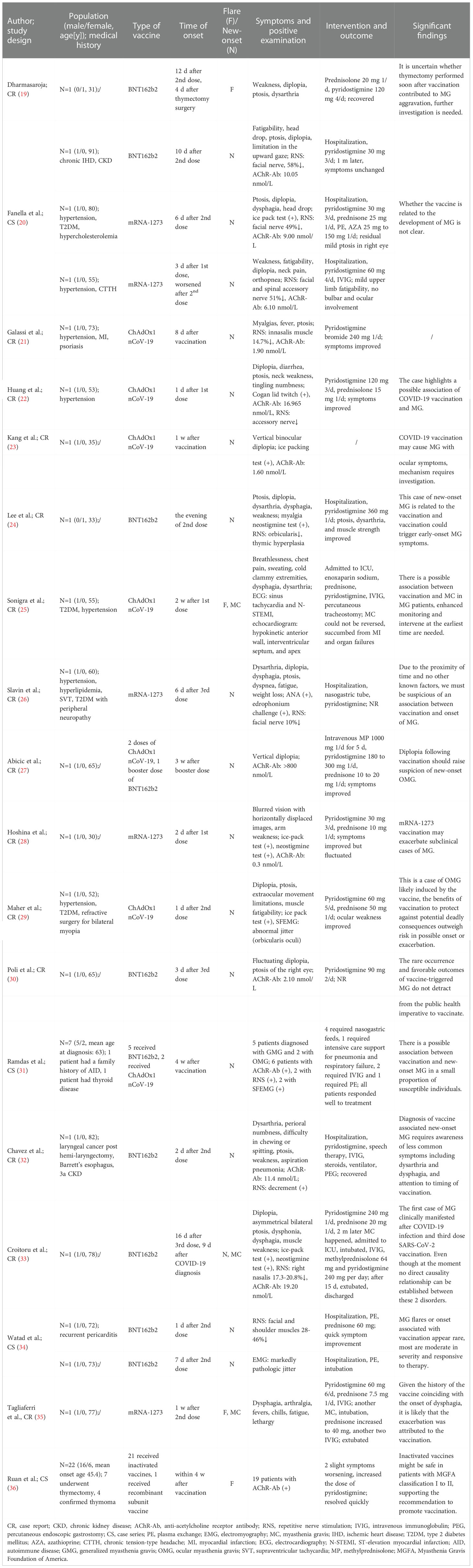
Table 3 presents detailed information of the included 18 case studies (19–36) involving 48 patients. Twenty-eight of the 48 participants experienced the onset or flare of MG, and three of them experienced myasthenia crisis. Twenty-three participants had new occurrences of MG: 12 (12/23, 52.17%) after receiving BNT162b2, six (6/23, 26.09%) after ChAdOx1 nCoV-19, four (4/23, 17.39%) after mRNA-1273, and one (1/23, 4.35%) after two doses of ChAdOx1 nCoV-19 and one booster dose of BNT162b2. Five patients had flares of MG symptoms after vaccination: one (1/5, 20.00%) after mRNA-1273, one (1/5, 20.00%) after BNT162b2, one (1/5, 20.00%) after ChAdOx1 nCoV-19, one (1/5, 20.00%) after the inactivated vaccine, and one (1/5, 20.00%) after the recombinant subunit vaccine. Four patients reported explicit onset or flare of symptoms after the first dose, nine after the second, and four after the third dose. The exact time of onset or flare ranged from the evening of vaccination to four weeks after each dose. Common MG symptoms after vaccination included dysarthria, perioral numbness, fatigability, dysphagia, dysphonia, diplopia, and ptosis. Fifteen studies reported that the patients responded well to treatment, and two studies did not report the results of the patients after the intervention. One study reported the death of a patient due to myocardial infarction, organ failure, and myasthenia crisis after receiving one dose of ChAdOx1 nCoV-19 (25). The results of case studies showed that there is a possible association between COVID-19 vaccination and worsening or new-onset MG. However, further studies and data are needed to support this finding.
3.5 Summary of other studies
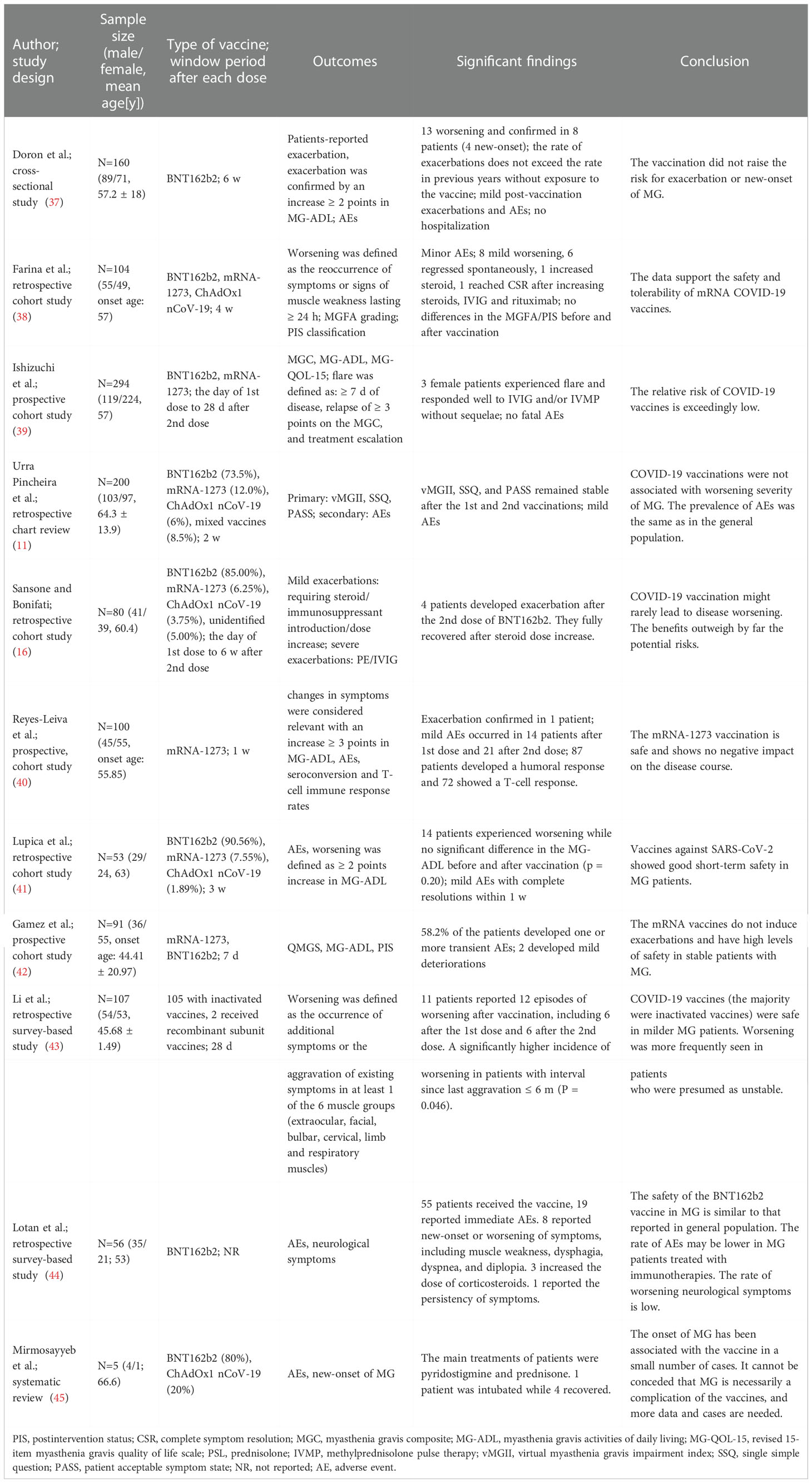
Table 4 presents detailed information of the included 11 articles other than case studies, involving 1299 patients. The criteria for defining exacerbation or onset of MG varied among the studies. Common criteria included changes in scales, such as myasthenia gravis activities of daily living scale (MG-ADL) or quantitative myasthenia gravis scale (QMG), symptom changes and duration, and treatment escalation. The window of risk ranged from seven days to six weeks after each vaccine dose. Ten studies reported that 60 participants (60/1299, 4.62%) experienced exacerbations, and nine had new-onset MG (9/1299, 0.69%). One retrospective chart review reported no symptom flares in 200 patients with MG after vaccination (11). Common AEs reported in these studies included local pain, fatigue, asthenia, cephalalgia, fever, and myalgia. The following are brief introductions of the included studies:
Doron et al. (37) conducted a cross-sectional study including 160 participants in an Israeli hospital to examine the safety of BNT162b2 vaccines. A questionnaire was sent to document whether they had an exacerbation of symptoms (confirmed by a minimum two-point increase on the MG-ADL), AEs, duration of exacerbations, and treatment change within 6 weeks after each dose. One hundred and fifty participants received the vaccines and 13 patients (13/150, 8.70%), including nine previously diagnosed MG and four new-onset MG, reported worsening myasthenia symptoms, and exacerbation was confirmed in eight patients (8/150, 5.30%). The flares lasted for less than three weeks in six patients, over three months in six patients, and one had an undeterminable duration. No hospitalization was necessary for any of these exacerbations, and three patients with previously diagnosed MG had increased prednisone dosages. In the 2021 vaccination period, seven patients reported an exacerbation, compared to six patients in the control period in 2020 (P = 0.880). The data showed that the exposure to vaccines did not lead to a significant increase in the rate of MG exacerbations. According to the electronic medical records of this hospital, there were 14 cases of newly diagnosed MG that visited the hospital in 2021, 19 in 2020, 27 in 2019, and 15 in 2018. The notion that the incidence of MG and vaccination are positively correlated is not supported by these data. Only mild AEs, such as flu-like symptoms, local pain, or isolated fatigue with a short duration, were reported. The findings support repeated vaccination in patients with MG.
Farina et al. (38) conducted a retrospective cohort study to investigate the safety of COVID-19 vaccines in 104 patients with MG from two centers in Italy. Ninety-eight (98/104, 94.20%) participants received at least two doses of vaccines four weeks before the survey, and 63 of them (63/98, 64.20%) received the booster dose. The vaccines with frequent use included BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19. Worsening was defined as the reoccurrence of symptoms or signs of muscle weakness lasting for at least 24 hours. Minor AEs such as local pain and fever were reported. Mild MG worsening after vaccination was observed in eight cases (8/104, 7.70%), mostly after BNT162b2 (7/8, 87.50%). The frequency of worsening among anti-muscle-specific tyrosine kinase antibody-positive MG (MuSK-MG) cases (3/9, 33.30%) was significantly higher than that among anti-acetylcholine receptor antibody-positive MG (AChR-MG) cases (4/83, 4.80%) and seronegative cases (1/12, 8.30%). Six of the eight patients experienced spontaneous regression of symptoms (6/8, 75.00%). After receiving the second dose of mRNA-1275, one seronegative patient who had diplopia, limb weakness, and bulbar deficiency required an increase in steroids dose. One MuSK-MG patient experienced complete resolution of symptoms after addition of intravenous immunoglobulin (IVIG) and rituximab. Generally, the findings favored of the safety and tolerability of the COVID-19 vaccines in patients with MG.
Ishizuchi et al. (39) conducted a prospective cohort study involving 343 patients with MG in Japan. A total of 294 patients (294/343, 85.70%) received COVID-19 vaccines, BNT162b2 in 254, and mRNA-1273 in 40. No fatal AEs were reported. Three female patients with early onset MG (3/294, 1.02%), two who received BNT162b2 and one who received mRNA-1273, experienced flare, two with the generalized form, and one with the ocular form. The timing of onset varied from two days after the first dose to 14 days after the second dose. The patients reported a mean increased scores of the myasthenia gravis composite (MGC) from three to 12, MG-ADL from two to nine, and revised 15-item myasthenia gravis quality of life scale (MG-QOL-15) from four to 15, indicating that worsening of symptoms severely impaired quality of life. The patients responded well to IVIG and/or methylprednisolone pulse therapy (IVMP) without further sequelae. The data indicated that the COVID-19 vaccination had an exceedingly low risk. However, postponing vaccination may be safer for patients with severe symptoms, such as bulbar involvement or myasthenia crisis. It is important to keep a close eye on symptom changes, especially in the first week following vaccination.
Urra Pincheira et al. (11) conducted a retrospective chart review in Canada involving 200 patients who received two vaccine doses. In total, 147 patients received BNT162b2 (147/200, 73.50%), 24 received mRNA-1273 (24/200, 12.00%), and 12 received ChAdOx1 nCoV-19 (12/200, 6.00%). Seventeen patients received mixed vaccines (17/200, 8.50%). There were no significant differences in the virtual myasthenia gravis impairment index (vMGII), patient acceptable symptom state (PASS), and single simple question (SSQ) before and after vaccination. Nearly 90.00% of the patients were PASS yes at the end of the study, with a mean SSQ value of 82.00% and mean vMGII of seven, indicating a well-controlled population. None of the patients required hospitalization. About 60.00% of individuals after the first dose and 56.00% after the second dose, respectively, reported AEs, such as injection site pain and fatigue.
Sansone and Bonifati (16) conducted a retrospective cohort study in Italy involving 80 patients who received two doses of COVID-19 vaccines. 68 patients received BNT162b2 (68/80, 85.00%), five received mRNA-1273 (5/80, 6.25%), three received ChAdOx1 nCoV-19 (3/80, 3.75%), and four received an unidentified type of vaccine (4/80, 5.00%). Sixty-nine patients (69/80, 86.25%) did not show any exacerbation within the window period of risk. It was difficult to identify an exacerbation in seven patients (7/80, 8.75%) during the window period because of plasma exchange (PE) or IVIG treatment on a regular basis. Four patients (4/80, 5.00%) had MG exacerbation after receiving the second dose of BNT162b2, two patients required steroid/immunosuppressant introduction or dose increase with rapid control of the symptoms, and two required PE/IVIG. These data suggest that COVID-19 vaccines rarely result in worsening MG. As some studies have shown that the combination of COVID-19 and MG might exacerbate either of the two, the researchers concluded that the benefits of vaccination outweigh the potential risks.
Reyes-Leiva et al. (40) performed a prospective cohort study that included 100 participants in Spain to determine the safety profile of mRNA-1273 vaccines in patients with MG. All participants received two mRNA-1273 vaccine doses. The researchers collected the MG-ADL scores seven days before and seven days after each dose. Symptom changes were considered to be relevant to the vaccination if the MG-ADL increased three points or more. Eight (8/100, 8.00%) and ten (10/100, 10.00%) patients reported a significant increase in MG-ADL after the first and second doses, respectively. In these 18 patients, after the first dose, the mean MG-ADL increase was 3.25 points, and after the second dose, it was 3.90 points. This worsening was self-limiting in 17 participants, and they were not considered to have an exacerbation. The remaining patient with generalized myasthenia gravis (GMG) experienced a four-month exacerbation after the second dose. The MG-ADL score increased by three points, and the patient required treatment modification. Fourteen (14/100, 14.00%) participants reported mild AEs after the first dose, and 21 (21/100, 21.00%) after the second dose. No association was found between the rates of AEs and immunosuppressive treatment. The results of this study verified the safety of vaccination.
Lupica et al. (41) performed a retrospective cohort study involving 53 patients with MG who received two COVID-19 vaccine doses in Italy. Exacerbation was confirmed if the MG-ADL score increased two points or more before and after vaccination. Forty-eight patients received BNT162b2 (48/53, 90.57%), four received mRNA-1273 (4/53, 7.54%), and one received ChAdOx1 nCoV-19 (1/53, 1.89%). The MG-ADL score increased in 14 patients (14/53, 28.30%) after vaccination, which was more common in females compared than in males (P = 0.048). However, there was no substantial difference in the MG-ADL score prior to and following vaccination in all patients. (P = 0.20). Two patients (2/53, 3.77%), seven patients (7/53, 13.21%), and 15 patients (15/53, 28.31%) reported AEs after the first, second, or both doses, respectively. Common AEs included local pain, asthenia, cephalalgia, fever, and myalgia. The results of this study support the use of COVID-19 vaccines in patients with MG.
Gamez et al. (42) conducted a prospective cohort study involving 91 patients in Spain to investigate the safety of mRNA vaccines in patients with well-controlled MG. Thirty-eight patients (38/91, 41.80%) developed at least one transient adverse event. No significant association was found in the rates of AEs, or steroid use or dose change. Two patients (2/91, 2.20%) experienced changes in functional status, and none of the patients experienced myasthenia crisis. The first patient experienced diplopia (QMG from nine to 11 and MG-ADL from three to five) lasting four days, and no treatment modification was required. The second patient experienced lower limb fatigue (QMG from four to seven and MG-ADL from one to six) lasting two weeks, and the prednisone dose was increased. The data from this study support the safety of COVID-19 vaccines in patients with well-controlled MG.
Li et al. (43) performed a retrospective survey-based study involving 107 patients to investigate the safety of inactivated COVID-19 vaccines in China. Worsening was defined as the appearance of new symptoms or aggravation of pre-existing symptoms in at least one of the extraocular, facial, cervical, bulbar, limb, or respiratory muscles within 28 days after each dose. One hundred and seven patients received at least one dose (105 received inactivated vaccines and two received homologous recombinant subunit vaccines). Eleven participants (11/107, 10.28%), including six after the first dose and six after the second dose, experienced 12 bouts of deterioration in total after vaccination. Only one female patient required an increase in prednisone dose, as well as the addition of tacrolimus, and she recovered two months later. In patients who were deemed unstable (with an interval since last aggravation less than six months), the exacerbation rate was noticeably higher (P = 0.046). The only independent factor correlated to exacerbation, according to logistic regression, was the interval since last worsening less than six months (P = 0.01). The data from this study showed that inactivated vaccines are safe for milder patients. Patients who were thought to be potentially unstable experienced worsening more frequently. After vaccination, patients presumed to be stable also showed mild worsening.
Lotan et al. (44) conducted a survey-based study in Israel documenting immediate AEs and the new-onset or worsening of neurological symptoms in patients with MG to investigate the safety and tolerability of the BNT162b2 vaccines. Fifty-five patients received vaccines, 51 received two doses, and four received one dose. Nineteen patients (19/55, 34.55%) reported immediate AEs, such as pain at the injection site, fatigue, headache, and dizziness following vaccination, four after the first dose, six after the second dose, and nine after both doses. AEs were more commonly seen in patients aged < 55 years. Eight patients (8/55, 14.55%) experienced occurrence or exacerbation of neurological symptoms, such as muscle weakness, dysphagia, dyspnea, dysarthria, ptosis, and diplopia, four after the first dose, two after the second dose, and two after both doses. Three patients experienced symptom onset or worsening within a few hours after the vaccine, four after one to four days, and one after one week. One of the eight patients had to increase the dose of corticosteroids. Six patients reported symptom relief within a month, while two experienced persistent symptoms for more than a month. The researchers concluded that the safety profile of BNT162b2 vaccine in patients with MG is comparable to that in the general population. Patients receiving immunotherapy may experience fewer AEs.
Mirmosayyeb et al. (45) conducted a case report-based systematic review to investigate whether MG is a complication of COVID-19 vaccines. Four studies that reported five new-onset MG cases were included. Their study found that the onset of MG was associated with vaccination in a few cases. However, due to the lack of sufficient data, more studies and cases are required before reaching a conclusion.
4 Discussion
4.1 Summary of findings
Our scoping review identified 29 studies to examine the existing evidence on the safety of COVID-19 vaccines in patients with MG. The article title, author, journal, country of origin, year of publication, purpose of the study, population and medical history, methodology, type and dose of vaccines, outcomes, AEs, significant findings, and conclusions were recorded. The 29 studies were conducted in 16 different countries and included 1347 participants. Case studies included 48 participants and 28 of them experienced new-onset or worsening of MG after vaccination, suggesting that there may be an association between the onset or flare of MG and COVID-19 vaccines. Ten observational studies and one systemic review reported 60 exacerbations (60/1299, 4.62%) and nine new-onsets (9/1299, 0.69%) of MG. Common AEs included local pain, fatigue, asthenia, cephalalgia, fever, and myalgia. The data suggest that only a minority of patients who received the vaccines reported worsening as well as mild AEs, supporting the safety of COVID-19 vaccines in the MG population. However, most studies still called for more prospective, multicenter, large-scale, and rigorously conducted studies to provide strong evidence and verify their safety.
4.2 Gaps in the evidence and research priorities
4.2.1 Limited strength of study designs
There were too many case studies and retrospective studies in all of the included articles. There could be a significant publication bias as negative findings are usually not published in an article. Thus, case studies can provide relatively limited evidence to support the safety of COVID-19 vaccines in patients with MG. However, the accumulation of cases demonstrates that potential risks exist between vaccination and MG onset or flare. Case studies can also provide meaningful lessons and references to clinicians in understanding the clinical features of MG after vaccination, window of risk, treatment, and prognosis. Recall errors are common in retrospective studies and may cause bias in the results of the studies, leading to limited strength of evidence. Therefore, more prospective and rigorously-designed studies are required in the future.
4.2.2 Type and dose of vaccines varied
Currently, most studies have evaluated the safety of mRNA vaccines because of their broad availability. The safety of different types of vaccines deserves further detailed research because people in different countries and regions can only access certain types of vaccines. For example, inactivated vaccines are mainly used in China, but their safety in patients with MG is under-evaluated. The participants in the 29 studies received different doses of vaccines, ranging from one to three doses. At present, many countries are promoting intensive vaccination, and the potential risks of different doses or boost vaccination need to be observed in depth and comprehensively evaluated in the future.
4.2.3 Inconsistent window of risk and exacerbation criteria
The window of risk observed after vaccination in different studies was different. Some studies chose six weeks as the risk period because this period was considered a vaccine-related effect in studies in the context of Guillain-Barre syndrome, and influenza vaccination (46), and MG exacerbation after influenza vaccination (47). The criteria for MG exacerbation after vaccination also differed. Some studies required clinicians to make objective judgments according to changes in scales, while some studies had relatively subjective criteria for aggravation. The standardized and unified window of risk and aggravation criteria are the basis for accurately evaluating the relationship between vaccination and new-onset or flare of MG. If the observed window of risk is exceeded or the specified standard is not met, caution should be exercised before concluding that there is a relationship between MG flares or onset and vaccination.
4.2.4 Limited number of participants
The number of patients included in all studies was small, which limits the reliability of the conclusions. To obtain highly reliable conclusions, observational studies require a large sample size. Due to the heterogeneity of MG, whether the safety of vaccines for different MG populations is consistent deserves further study. If patients with different severities, antibody types, immunosuppressive treatment schemes, and different levels of stability have different risks or AEs after vaccination, more large-sample, in-depth research and analysis should be conducted in these fields.
4.2.5 Lack of efficacy evaluation
Only a few studies have investigated the protective effect of COVID-19 vaccines in patients with MG when evaluating the safety. As the pandemic seems to have a severe impact on patients with MG, in addition to the risks and AEs of vaccination, attention should be paid to evaluating the effectiveness of vaccines against SARS-CoV-2 infection. The benefits and risks should be compared objectively to provide more reasonable instructions to patients with MG in clinical practice.
4.3 Strength and limitations
This scoping review comprehensively searched articles about the safety of COVID-19 vaccines in patients with MG in three databases using standard literature retrieval methods, and summarized the research results on this topic. This study had several limitations. First, since the main purpose of this review was to collect and organize all the evidence, the quality of the included studies was not evaluated. Second, no specific conclusions could be drawn to answer more detailed questions because no analysis was conducted on the data of the included studies. Third, as the main purpose of this scoping review was to provide a comprehensive summary of evidence relating to safety, we refrained from assessing the effectiveness of vaccines against SARS-CoV-2. Finally, the researchers conducting this review were all clinicians. If basic researchers were involved, they might have a more in-depth view of this review.
5 Conclusion
This scoping review provides an overview of the evidence that is currently available about the safety of COVID-19 vaccines in patients with MG. Case studies have demonstrated that COVID-19 vaccines may cause new-onset or worsening of MG in a small proportion of the population. Some retrospective and prospective observational studies have reported only a few AEs or worsening of MG, supporting the safety of COVID-19 vaccines in patients with MG. Large-scale, multicenter, prospective, and rigorous studies are needed to verify their safety.
Author contributions
WZ, JN, and LM screened the literature. WC, YT, and QS extracted data of all the studies. SP wrote the draft of the review. SL, YY, and RF revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Innovation Fund of China Academy of Chinese Medical Sciences (CI2021A01309).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1103020/full#supplementary-material
References
1. Yi J, Miao J, Zuo Q, Owusu F, Dong Q, Lin P, et al. COVID-19 pandemic: A multidisciplinary perspective on the pathogenesis of a novel coronavirus from infection, immunity and pathological responses. Front Immunol (2022) 13:978619. doi: 10.3389/fimmu.2022.978619
2. Baratella E, Ruaro B, Marrocchio C, Starvaggi N, Salton F, Giudici F, et al. Interstitial lung disease at high resolution ct after SARS-CoV-2-Related acute respiratory distress syndrome according to pulmonary segmental anatomy. J Clin Med (2021) 10(17):3985. doi: 10.3390/jcm10173985
3. Gao L, Zheng C, Shi Q, Xiao K, Wang L, Liu Z, et al. Evolving trend change during the COVID-19 pandemic. Front Public Health (2022) 10:957265. doi: 10.3389/fpubh.2022.957265
4. Pelosi P, Tonelli R, Torregiani C, Baratella E, Confalonieri M, Battaglini D, et al. Different methods to improve the monitoring of noninvasive respiratory support of patients with severe Pneumonia/Ards due to COVID-19: An update. J Clin Med (2022) 11(6):1704. doi: 10.3390/jcm11061704
5. Akande OW, Akande TM. COVID-19 pandemic: A global health burden. Niger Postgrad Med J (2020) 27(3):147–55. doi: 10.4103/npmj.npmj_157_20
6. Rueda-Fernández M, Melguizo-Rodríguez L, Costela-Ruiz VJ, González-Acedo A, Ramos-Torrecillas J, Illescas-Montes R. The current status of COVID-19 vaccines. a scoping review. Drug Discov Today (2022) 27(11):103336. doi: 10.1016/j.drudis.2022.08.004
7. Wang K, Wang L, Li M, Xie B, He L, Wang M, et al. Real-word effectiveness of global COVID-19 vaccines against SARS-CoV-2 variants: A systematic review and meta-analysis. Front Med (Lausanne) (2022) 9:820544. doi: 10.3389/fmed.2022.820544
8. Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the pfizer-biontech and Oxford-astrazeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. Bmj (2021) 373:n1088. doi: 10.1136/bmj.n1088
9. Zhou Z, Zhu Y, Chu M. Role of COVID-19 vaccines in SARS-CoV-2 variants. Front Immunol (2022) 13:898192. doi: 10.3389/fimmu.2022.898192
10. Muppidi S, Guptill JT, Jacob S, Li Y, Farrugia ME, Guidon AC, et al. COVID-19-Associated risks and effects in myasthenia gravis (CARE-MG). Lancet Neurol (2020) 19(12):970–1. doi: 10.1016/s1474-4422(20)30413-0
11. Urra Pincheira A, Alnajjar S, Katzberg H, Barnett C, Daniyal L, Rohan R, et al. Retrospective study on the safety of COVID-19 vaccination in myasthenia gravis. Muscle Nerve (2022) 66(5):558–61. doi: 10.1002/mus.27657
12. Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, immunogenicity and safety of COVID-19 vaccines: A systematic review and meta-analysis. Front Immunol (2021) 12:714170. doi: 10.3389/fimmu.2021.714170
13. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med (2021) 385(12):1078–90. doi: 10.1056/NEJMoa2110475
14. Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: What is the evidence? LANCET (2003) 362(9396):1659–66. doi: 10.1016/s0140-6736(03)14802-7
15. Lagoumintzis G, Chasapis CT, Alexandris N, Kouretas D, Tzartos S, Eliopoulos E, et al. Nicotinic cholinergic system and COVID-19: In silico identification of interactions between α7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-V and SARS-CoV-2 spike glycoproteins. Food Chem Toxicol (2021) 149:112009. doi: 10.1016/j.fct.2021.112009
16. Sansone G, Bonifati DM. Vaccines and myasthenia gravis: A comprehensive review and retrospective study of SARS-CoV-2 vaccination in a Large cohort of myasthenic patients. J Neurol (2022) 269(8):3965–81. doi: 10.1007/s00415-022-11140-9
17. Zhou Q, Zhou R, Yang H, Yang H. To be or not to be vaccinated: That is a question in myasthenia gravis. Front Immunol (2021) 12:733418. doi: 10.3389/fimmu.2021.733418
18. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. Prisma extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med (2018) 169(7):467–73. doi: 10.7326/m18-0850
19. Dharmasaroja P. Early flare-ups of myasthenia gravis after thoracoscopic thymectomy in a patient recently receiving BNT162b2 mRNA COVID-19 vaccination. Cureus (2022) 14(1):e21571. doi: 10.7759/cureus.21571
20. Fanella G, Baiata C, Candeloro E, Toscano G, Colnaghi S, Mauri M, et al. New-onset myasthenia gravis after mRNA SARS-CoV-2 vaccination: A case series. Neurological Sci Off J Ital Neurological Soc Ital Soc Clin Neurophysiol (2022) 43(10):5799–802. doi: 10.1007/s10072-022-06284-5
21. Galassi G, Rispoli V, Iori E, Ariatti A, Marchioni A. Coincidental onset of ocular myasthenia gravis following ChAdOx1 n-CoV-19 vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). ISRAEL Med Assoc J (2022) 24(1):9–10.
22. Huang BD, Hsueh HW, Yang SH, Lin CW. New-onset myasthenia gravis after ChAdOx1 nCoV-19 vaccine inoculation. J Neuroophthalmol (2022). doi: 10.1097/wno.0000000000001548
23. Kang MC, Park K-A, Min J-H, Oh SY. Myasthenia gravis with ocular symptoms following a ChAdOx1 nCoV-19 vaccination: A case report. Am J Ophthalmol Case Rep (2022) 27:101620–. doi: 10.1016/j.ajoc.2022.101620
24. Lee MA, Lee C, Park JH, Lee JH. Early-onset myasthenia gravis following COVID-19 vaccination. J Korean Med Sci (2022) 37(10):e50. doi: 10.3346/jkms.2022.37.e50
25. Sonigra KJ, Sarna K, Vaghela VP, Guthua S. An interesting case of fatal myasthenic crisis probably induced by the COVID-19 vaccine. Cureus (2022) 14(3):e23251–e. doi: 10.7759/cureus.23251
26. Slavin E, Fitzig J, Neubert C, Garcia-Lopez F, Cuevas-Trisan R. New-onset myasthenia gravis confirmed by electrodiagnostic studies after a third dose of SARS-CoV-2 mRNA-1273 vaccine: A case report. Am J Phys Med Rehabil (2022) 101(12):e176–9. doi: 10.1097/phm.0000000000002076
27. Abicic A, Sitas B, Adamec I, Bilic E, Habek M. New-onset ocular myasthenia gravis after booster dose of COVID-19 vaccine. Cureus (2022) 14(7):e27213–e. doi: 10.7759/cureus.27213
28. Hoshina Y, Sowers C, Baker V. Myasthenia gravis presenting after administration of the mRNA-1273 vaccine. Eur J Case Rep Internal Med (2022) 9(7):003439–. doi: 10.12890/2022_003439
29. Maher DI, Hogarty D, Ben Artsi E. Acute onset ocular myasthenia gravis after vaccination with the Oxford-astrazeneca COVID-19 vaccine. Orbit (Amsterdam Netherlands) (2022) 1–5. doi: 10.1080/01676830.2022.2062777
30. Poli K, Poli S, Ziemann U. Multiple autoimmune syndromes including acute disseminated encephalomyelitis, myasthenia gravis, and thyroiditis following messenger ribonucleic acid-based COVID-19 vaccination: A case report. Front Neurol (2022) 13:913515. doi: 10.3389/fneur.2022.913515
31. Ramdas S, Hum RM, Price A, Paul A, Bland J, Burke G, et al. SARS-CoV-2 vaccination and new-onset myasthenia gravis: A report of 7 cases and review of the literature. Neuromuscular Disord NMD (2022) 32(10):785–9. doi: 10.1016/j.nmd.2022.09.001
32. Chavez A, Pougnier C. A case of COVID-19 vaccine associated new diagnosis myasthenia gravis. J Prim Care Community Health (2021) 12:21501327211051933–. doi: 10.1177/21501327211051933
33. Croitoru CG, Cuciureanu DI, Prutianu I, Cianga P. Autoimmune myasthenia gravis after COVID-19 in a triple vaccinated patient. Arch Clin cases (2022) 9(3):104–7. doi: 10.22551/2022.36.0903.10212
34. Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following Mrna/DNA SARS-CoV-2 vaccination. Vaccines (2021) 9(5):435. doi: 10.3390/vaccines9050435
35. Tagliaferri AR, Narvaneni S, MdH A, Grist W. A case of COVID-19 vaccine causing a myasthenia gravis crisis. Cureus (2021) 13(6):e15581–e. doi: 10.7759/cureus.15581
36. Ruan Z, Tang Y, Li C, Sun C, Zhu Y, Li Z, et al. COVID-19 vaccination in patients with myasthenia gravis: A single-center case series. Vaccines (2021) 9(10):1112. doi: 10.3390/vaccines9101112
37. Doron A, Piura Y, Vigiser I, Kolb H, Regev K, Nesher N, et al. BNT162b2 mRNA COVID-19 vaccine three-dose safety and risk of COVID-19 in patients with myasthenia gravis during the alpha, delta, and omicron waves. J Neurol (2022) 269:6193–201. doi: 10.1007/s00415-022-11303-8
38. Farina A, Falso S, Cornacchini S, Spagni G, Monte G, Mariottini A, et al. Safety and tolerability of SARS-CoV-2 vaccination in patients with myasthenia gravis: A multicenter experience. Eur J Neurol (2022) 29(8):2505–10. doi: 10.1111/ene.15348
39. Ishizuchi K, Takizawa T, Sekiguchi K, Motegi H, Oyama M, Nakahara J, et al. Flare of myasthenia gravis induced by COVID-19 vaccines. J Neurol Sci (2022) 436:120225. doi: 10.1016/j.jns.2022.120225
40. Reyes-Leiva D, Lopez-Contreras J, Moga E, Pla-Junca F, Lynton-Pons E, Rojas-Garcia R, et al. Immune response and safety of SARS-CoV-2 mRNA-1273 vaccine in patients with myasthenia gravis. Neurol Neuroimmunol Neuroinflamm (2022) 9(4):e200002. doi: 10.1212/nxi.0000000000200002
41. Lupica A, Di Stefano V, Iacono S, Pignolo A, Quartana M, Gagliardo A, et al. Impact of COVID-19 in AChR myasthenia gravis and the safety of vaccines: Data from an Italian cohort. Neurol Int (2022) 14(2):406–16. doi: 10.3390/neurolint14020033
42. Gamez J, Gamez A, Carmona F. Safety of mrna COVID-19 vaccines in patients with well-controlled myasthenia gravis. Muscle Nerve (2022) 66(5):612–7. doi: 10.1002/mus.27703
43. Li HY, Shao LY, Song M, Hu SM, Yue YX, Li HF. Safety of inactivated SARS-CoV-2 vaccines in myasthenia gravis: A survey-based study. Front Immunol (2022) 13:923017. doi: 10.3389/fimmu.2022.923017
44. Lotan I, Hellmann MA, Friedman Y, Stiebel-Kalish H, Steiner I, Wilf-Yarkoni A. Early safety and tolerability profile of the BNT162b2 COVID-19 vaccine in myasthenia gravis. NEUROMUSCULAR Disord (2022) 32(3):230–5. doi: 10.1016/j.nmd.2022.01.013
45. Mirmosayyeb O, Moases Ghaffary E, Mazdak M, Bagheri Z, Bagherieh S, Shaygannejad V. Is myasthenia gravis a real complication of the COVID-19 vaccine? a case report-based systematic review. Can J Infect Dis Med Microbiol = J canadien Des maladies infectieuses la microbiologie medicale (2022) 2022:5009450–. doi: 10.1155/2022/5009450
46. Souayah N, Nasar A, Suri MF, Qureshi AI. Guillain-Barre syndrome after vaccination in United States a report from the CDC/FDA vaccine adverse event reporting system. Vaccine (2007) 25(29):5253–5. doi: 10.1016/j.vaccine.2007.03.053
Keywords: myasthenia gravis, COVID-19, SARS-CoV-2, vaccines, safety
Citation: Peng S, Tian Y, Meng L, Fang R, Chang W, Yang Y, Li S, Shen Q, Ni J and Zhu W (2022) The safety of COVID-19 vaccines in patients with myasthenia gravis: A scoping review. Front. Immunol. 13:1103020. doi: 10.3389/fimmu.2022.1103020
Received: 19 November 2022; Accepted: 06 December 2022;
Published: 22 December 2022.
Edited by:
Barbara Ruaro, University of Trieste, ItalyReviewed by:
Chiara Bozzi, University of Trieste, ItalyAlessia Giovanna Andrisano, Azienda Ospedaliero Universitaria Ospedali Riuniti Di Trieste, Italy
Copyright © 2022 Peng, Tian, Meng, Fang, Chang, Yang, Li, Shen, Ni and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenzeng Zhu, zhuwenzeng@gamyy.cn
 Siyang Peng
Siyang Peng Yukun Tian1
Yukun Tian1