
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 22 December 2022
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1089469
This article is part of the Research Topic Platelets and their Multi-faceted Roles in Health and Disease View all 7 articles
A correction has been applied to this article in:
Corrigendum: Platelet abnormalities in autoimmune thyroid diseases: A systematic review and meta-analysis
 Yu-tian Cao1,2
Yu-tian Cao1,2 Kai-yu Zhang2
Kai-yu Zhang2 Jing Sun1,2
Jing Sun1,2 Yan Lou1
Yan Lou1 Tian-su Lv2
Tian-su Lv2 Xinyi Yang1,2
Xinyi Yang1,2 Wen-hui Zhang1,2
Wen-hui Zhang1,2 Jiang-yi Yu1
Jiang-yi Yu1 Qi-biao Wu3
Qi-biao Wu3 Xi-qiao Zhou1*
Xi-qiao Zhou1*Background: Some degree of platelet index abnormality has been found clinically in the autoimmune thyroid disease (AITD), but the findings are not uniform.
Methods: The PubMed, Web of Science, Cochrane Library, and Embase databases were searched for relevant articles published up to August 16th, 2022, with no restrictions on the language of the articles. Reference lists of eligible articles were also searched. A random effect model was used to pool the standardized mean difference (SMD) and 95% confidence interval (95% CI) of platelet count (PLT), mean platelet volume (MPV), and platelet distribution width (PDW) between AITD patients and healthy controls, and subgroup analyses were performed.
Results: A total of 19 articles with 6173 people (3824 AITD patients and 2349 healthy people) were included in the meta-analysis. The results showed that PLT and MPV values were significantly increased in AITD patients when compared with healthy people (SMD: 0.164, 95% CI: 0.044 to 0.285; SMD: 0.256, 95% CI: 0.013 to 0.500), while no significant difference was found in PDW between the AITD group and the control group (SMD: 0.060, 95% CI: -0.164 to 0.284). Subgroup analysis according to disease type and thyroid function revealed that for PLT, this difference was only found in the Hashimoto’s thyroiditis (HT) and hypothyroid groups, but not in the Graves’ disease (GD) and hyperthyroid groups. For MPV, the results were the opposite of those for PLT: MPV was significantly higher in the GD, hyperthyroid, and euthyroid groups than in the control group, but not in the HT and hypothyroid groups. Sensitivity analysis showed that the stability of the pooled MPV was not good. No publication bias was found.
Conclusions: PLT and MPV are significantly elevated in patients with AITD, with PLT being more significantly elevated in HT and hypothyroidism, and MPV being more significantly increased in GD and hyperthyroidism. Appropriate clinical attention can be paid to the thyroid function of patients when abnormal platelet indices are found, and conversely, the consequences of abnormal platelet parameters such as elevated MPV lead to an increased occurrence of cardiovascular events, which should also be addressed in the AITD population.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022341823.
Autoimmune thyroid disease (AITD) is the most common autoimmune disease, mainly including Graves’ disease (GD) and Hashimoto’s thyroiditis (HT). GD is the most common cause of hyperthyroidism, which is mainly caused by thyrotropin receptor autoantibodies overstimulating thyroid-stimulating hormone receptors. Its clinical manifestations are mainly goiter and thyrotoxicosis, and some patients also have ocular abnormalities and localized dermopathy (1). HT is a disease characterized by thyroid specific autoantibodies (mainly including thyroid peroxidase antibody and thyroglobulin antibody), which mainly manifest as primary hypothyroidism caused by thyroid damage (2). The specific pathogenesis of AITD is not fully understood, but the interaction between genetic and environmental factors is key to the development of the disease, leading to dysfunction of lymphocytes and antigen-presenting cells, which disrupts immune homeostasis and causes thyroid autoimmunity (1, 2).
As a multifunctional cell, platelets have an important role in the regulation of immunity and inflammation, in addition to their hemostatic function (3). Platelets may be involved in the pathogenesis of AITD, and patients with AITD also have some degree of abnormal platelet parameters. Recent studies have found a higher platelet activation level in AITD (4); GD is associated with higher levels of activated lymphocytes and platelet-lymphocyte aggregates (5). In addition, it is not uncommon for AITD patients to be clinically complicated by immune thrombocytopenia (6), while reactive thrombocytosis has also been found in the HT population (7). Mean platelet volume (MPV), one of the indicators of platelet activation, meta-analysis has shown that there is a correlation between the increase MPV and the risk of thrombosis and cardiovascular events (8). Some studies found that MPV in AITD patients increased (9, 10), which increased the incidence and mortality of cardiovascular events, while others have concluded that MPV in the ATID group was not significantly different from that in the control group (11).
In view of these inconsistent findings, we conducted a meta-analysis to provide more comprehensive conclusions on changes in platelet parameters in AITD. As a simple and inexpensive laboratory indicator, studying the correlation between platelet parameters and AITD may provide broader clinical ideas for the prevention, diagnosis, and treatment of AITD and related platelet disorders.
This meta-analysis was conducted following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline (12) and the Preferred Reporting Items for a Systematic Review and Meta-analysis (PRISMA) guideline (Tables S1, S2) (13). The protocol for this meta-analysis was registered with PROSPERO (CRD42022341823).
We searched the PubMed, Web of Science, Cochrane Library, and Embase databases for relevant articles published up to August 16th, 2022. Our search strategy consisted of MeSH terms and entry terms with no restrictions on the language of the articles. We also scanned the reference lists of eligible articles for additional eligible articles that were not retrieved during the literature search. The details of the search strategy are presented in Table S3.
The inclusion criteria were as follows (1): Patients: adult AITD patients (2); Control: adult in good health (3); Outcomes: platelet count (PLT), MPV, and platelet distribution width (PDW) (4); Study type: case control studies, cohort studies, and cross sectional studies (5); Studies included at least 20 patients to obtain good reliability.
The exclusion criteria were as follows (1): Animal experiments, review, conference abstracts, case reports and meta-analyses (2); Combined with severe primary systemic diseases such as cancer, acquired immune deficiency syndrome (3); Pregnant or lactating women, hematological diseases, and any other diseases could interfere with the platelet parameters (4); Subjects who had undergone thyroid surgery or iodine-131 therapy (5); Full-text or sufficient data could not be extracted.
Studies from the database were managed using EndNote X9 software to remove duplicate articles. The included articles based on inclusion and exclusion criteria were screened by T-SL and X-YY, who worked independently. The disagreement was discussed with another author (K-YZ) and subsequently resolved via consensus. Extracted research information includes (1): Background information: first author, publication year, country, study design, sample size, age, sex (2); Platelet parameters: PLT, MPV, and PDW. Values were presented as mean ± standard deviation (SD). When relevant data were missing, the corresponding author was contacted to obtain the information. If the same study contained multiple groups of available data, it would be divided into multiple independent individuals for data extraction. The process was performed independently by T-SL and X-YY. Any disagreements were resolved by discussion and consensus.
According to the recommendations of the Agency for Healthcare Research and Quality (AHRQ) for the quality evaluation criteria of observational studies, the Newcastle-Ottawa Scale (NOS) was used to evaluate cohort studies and case control studies (14–16), and the AHRQ methodology checklist was used to evaluate cross sectional studies (17). The NOS evaluates the quality of research from three aspects: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest for case control or cohort studies, respectively (Methods 1, 2). The quality of the study was assessed as follows: low quality = 0-3 stars; moderate quality = 4-6 stars; and high quality = 7-9 stars (18). The AHRQ methodology checklist includes 11 items: the definition of information source; inclusion and exclusion criteria; time period and continuity for identifying patients; blinding of personnel; assessments for quality assurance; confounding and missing data; and response rates and completeness of patients (Methods 3). If the answer is “unclear” or “no”, the item score is “0”. If the answer is “yes”, the score of the item is “1”. The quality of the study was assessed as follows: low quality = 0-3; moderate quality = 4-7; and high quality = 8-11 (17, 19). The quality of eligible articles was independently assessed by two investigators (T-SL and X-YY). Any disagreement was resolved by the third investigator (K-YZ).
The standardized mean difference (SMD) and 95% confidence interval (95% CI) of PLT, MPV, and PDW between AITD patients and healthy controls in each study were calculated and estimated. For studies using median, range and/or interquartile range, we used the methods proposed by Wan et al. (20) and Luo et al. (21) to estimate the sample mean and SD. Positive SMDs indicated higher platelet parameter values in AITD patients. If the changes in the outcomes were not reported. We used the conversion formulas recommended in the Cochrane Handbook Version 6.4 to calculate the changes in outcomes (22).
The I2 statistic was used to quantitatively evaluate the heterogeneity of studies. Studies with I² of 0-25%, 25-75%, and > 75% were considered to have low, moderate, and high heterogeneity, respectively. A random effect model was applied regardless of heterogeneity. Meta-regression analyses were performed to determine the source of heterogeneity. According to the disease type, thyroid function, quality assessment, region, and study type, we conducted subgroup analyses. The groups were as follows: GD, HT, and AITD; Hyperthyroid/Before treatment, Hypothyroid/Before treatment, Euthyroid/After treatment, and Unknown; High quality (NOS = 7-9 stars or AHRQ score = 8-11), Moderate quality (NOS = 4–6 stars or AHRQ score = 4-7), and Low quality (NOS = 0-3 stars or AHRQ score = 0-3); Turkey, China, and Others; Case control study and Cross sectional study.
Besides, sensitivity analysis was performed to evaluate the robustness of the results by removing one single study in each turn. If 10 or more studies were included, we would use the funnel plots and Egger’s test to investigate publication bias. If there was publication bias, the trim and fill method was conducted to rectify the funnel plot asymmetry.
All statistical analyses were done with Stata Version 15.0 (StataCorp) from September 6 to 10, 2022. The results were considered significant when a 2-tailed P < 0.05.
Through database searches, 759 records were found. After screening, 19 articles (5, 9, 11, 23–38) were finally selected for the meta-analysis. The specific screening process is shown in Figure 1, articles excluded after full text review are listed in Methods 4.
A total of 19 articles were included, 2 of which were from the same cross sectional study (26, 27). The characteristics of the 19 included articles are shown in Table S4. Among them, part of the data from 4 articles (23, 30, 36, 37) were presented as median (min-max) or median (25–75th percentile), which were transformed according to the methods mentioned in Data Synthesis and Data Analysis. A comparison table of the original data and the corresponding converted data is shown in Table S5. Based on the NOS and AHRQ methodology checklist, 17 articles (5, 11, 23–36, 38) were classified as high quality, and 2 articles (9, 37) as moderate quality. Detailed assessment scores are shown in Table S4.
18 articles (26 available data) reported the difference in PLT between the AITD group and the control group. The results showed that PLT values were significantly increased in AITD patients when compared with healthy people (SMD: 0.164, 95% CI: 0.044 to 0.285, p = 0.008, Figure 2). For MPV, we pooled the effects of 9 articles (14 available data) and found that MPV values were significantly increased in AITD patients when compared with healthy people (SMD: 0.256, 95% CI: 0.013 to 0.500, p = 0.039, Figure 3). In the analysis of PDW (3 articles, 4 available data), no significant difference was found between the AITD group and the control group (SMD: 0.060, 95% CI, -0.164 to 0.284, p = 0.601, Figure 4).
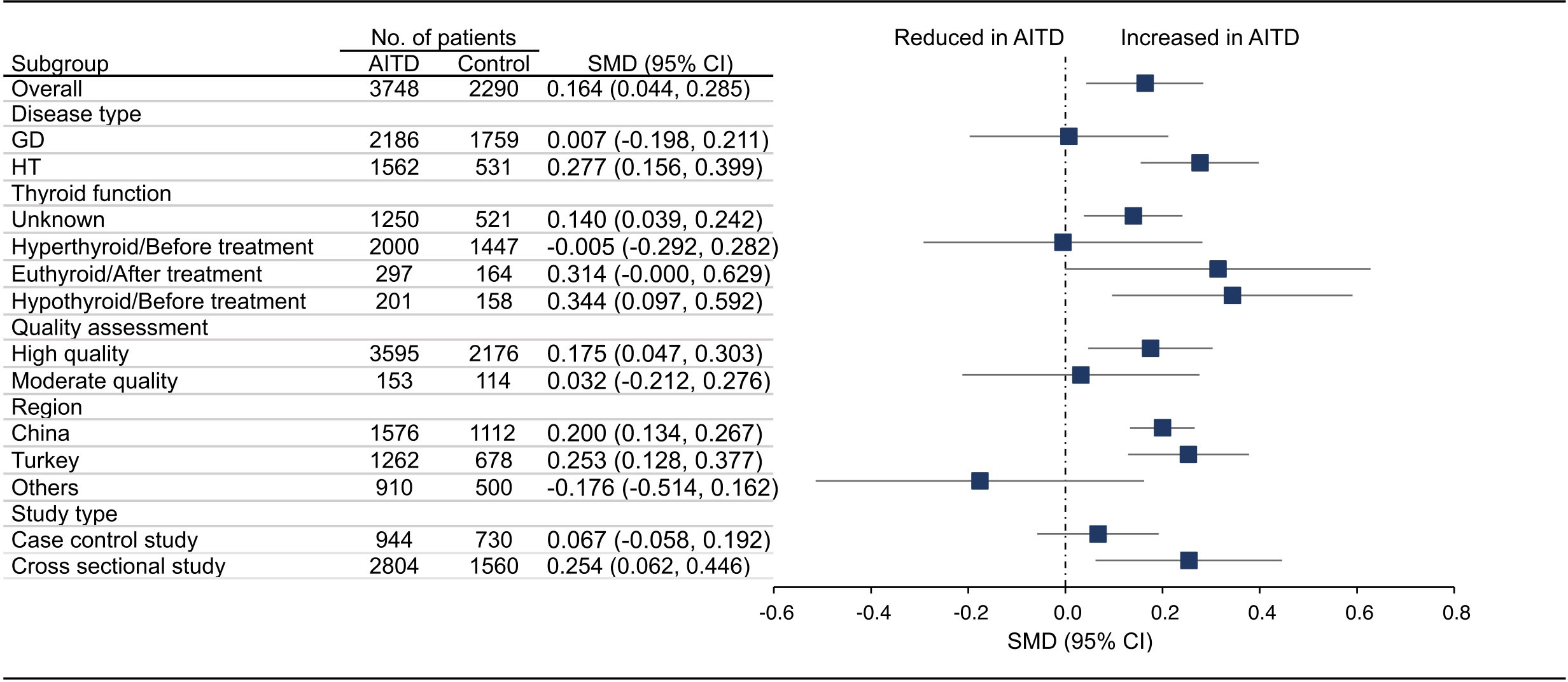
Figure 2 Standardized Mean Difference in PLT between the AITD Group and the Control Group. GD, Graves’ disease; HT, Hashimoto’s thyroiditis; AITD, autoimmune thyroid disease; PLT, platelet count; SMD, standardized mean difference; 95%CI, 95% confidence interval.
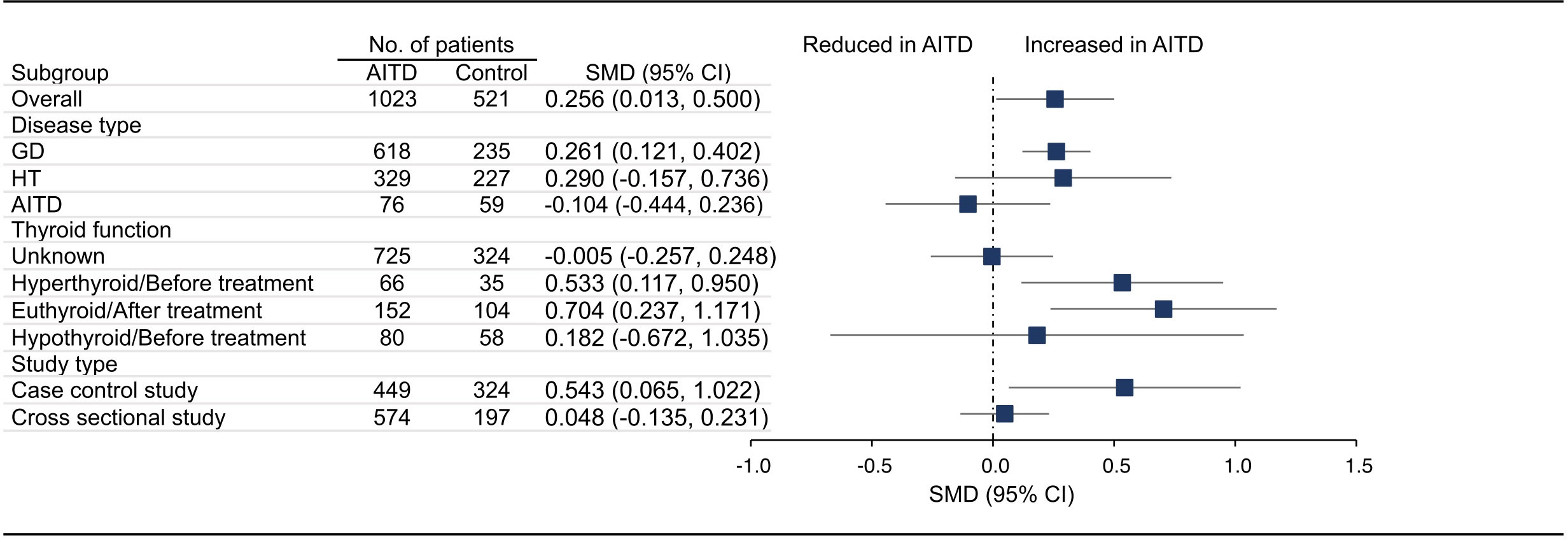
Figure 3 Standardized Mean Difference in MPV between the AITD Group and the Control Group. GD, Graves’ disease; HT, Hashimoto’s thyroiditis; AITD, autoimmune thyroid disease; MPV, mean platelet volume; SMD, standardized mean difference; 95%CI, 95% confidence interval.
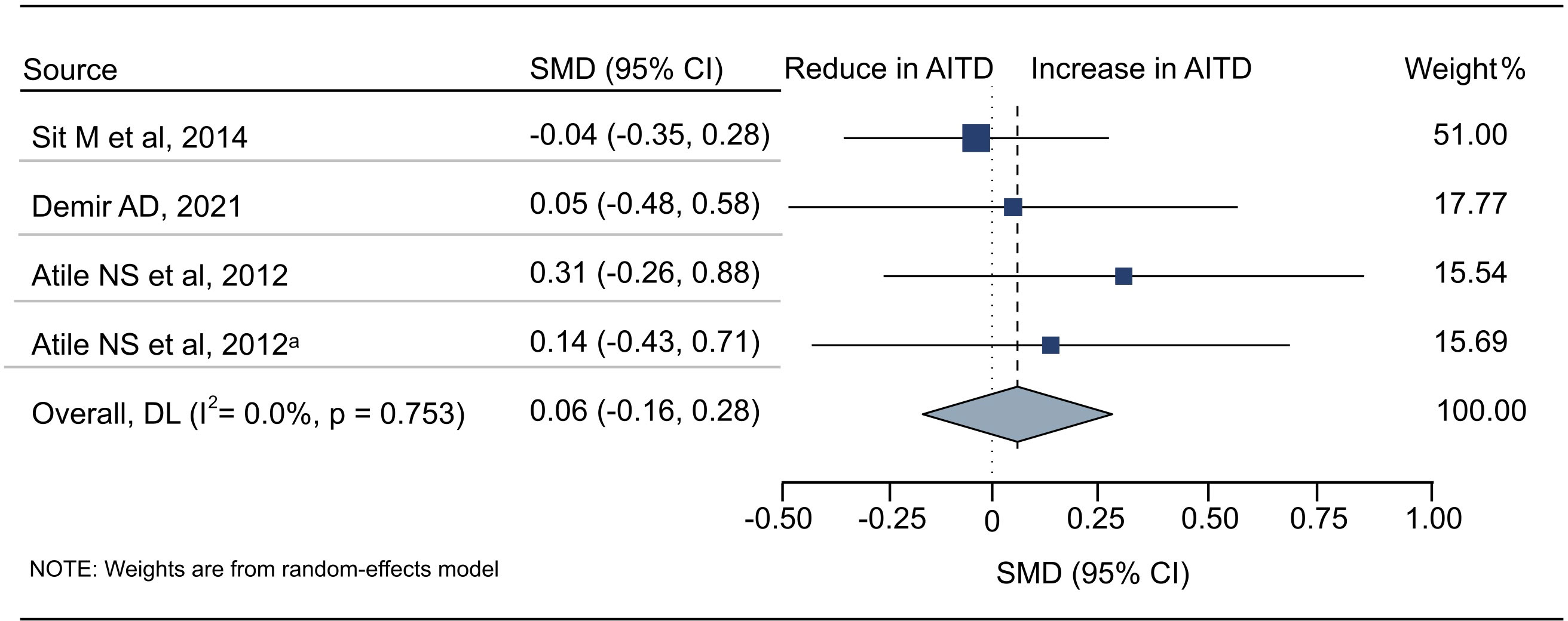
Figure 4 Standardized Mean Difference in PDW between the AITD Group and the Control Group. AITD, autoimmune thyroid disease; PDW, platelet distribution width; SMD, standardized mean difference; 95%CI, 95% confidence interval. a: the second available data from the same study.
Meta-regression analyses were performed according to covariates including disease type, thyroid function, quality assessment, study type, and region. The results showed that these covariates were not possible sources of heterogeneity (Table S6). For PDW, since the number of included studies was less than 10, no meta-regression analysis was conducted.
Furthermore, we conducted subgroup analyses according to the disease type, thyroid function, quality assessment, region, and study type. As shown in Figure 2, HT group had significantly higher PLT than healthy people (SMD: 0.277, 95% CI: 0.156 to 0.399), while the difference in GD group was not statistically significant (SMD: 0.007, 95% CI: -0.198 to 0.211). For thyroid function, PLT in thyroid function unknown group (SMD: 0.140, 95% CI: 0.039 to 0.242) and hypothyroid/before treatment group (SMD: 0.344, 95% CI: 0.097 to 0.592) was significantly higher than that of the control group, while there was no significant difference in hyperthyroid/before treatment group (SMD: -0.005, 95% CI: -0.292 to 0.282) and euthyroid/after treatment group (SMD: 0.314, 95% CI: -0.000 to 0.629). For studies of different quality scores, regions, and study types, the PLT in high quality group (SMD: 0.175, 95% CI: 0.047 to 0.303), China (SMD: 0.200, 95% CI: 0.134 to 0.267) and Turkey group (SMD: 0.253, 95% CI: 0.128 to 0.377), and cross sectional group (SMD: 0.254, 95% CI: 0.062 to 0.446) was significantly higher than that in the control group, while the difference in moderate quality group (SMD: 0.032, 95% CI: -0.212 to 0.276), other countries group (SMD: -0.176, 95% CI: -0.514 to 0.162), and case control group (SMD: 0.067, 95% CI: -0.058 to 0.192) was not significant (Figures S1A–E).
For MPV, we conducted subgroup analyses according to disease type, thyroid function, and study type. As shown in Figure 3, MPV in GD group (SMD: 0.261, 95% CI: 0.121 to 0.402), hyperthyroid/before treatment group (SMD: 0.533, 95% CI: 0.117 to 0.950), euthyroid/after treatment group (SMD: 0.704, 95% CI: 0.237 to 1.171), and case control group (SMD: 0.543, 95% CI: 0.065 to 1.022) was significantly higher than that of the control group, while there was no significant difference in HT group (SMD: 0.290, 95% CI: -0.157 to 0.736), AITD group (SMD: -0.104, 95% CI: -0.444 to 0.236), thyroid function unknown group (SMD: -0.005, 95% CI: -0.257 to 0.248), hypothyroid/before treatment group (SMD: 0.182, 95% CI: -0.672 to 1.035) and cross sectional group (SMD: 0.048, 95% CI: -0.135 to 0.231, Figures S2A–E). Due to insufficient data, we did not conduct subgroup analysis on MPV based on quality assessment and region. There were too few studies on PDW, and no subgroup analysis was conducted.
Sensitivity analysis showed that no single study significantly influenced the difference in PLT between AITD patients and healthy people. However, the stability of the pooled MPV was not good. After more than half of the studies were eliminated separately, the pooled results of the remaining studies were not statistically significant (95% CI exceeded 0, Table S7). In addition, funnel plots were roughly symmetrical (Figure S3), and Egger’s test further showed that there was no publication bias among included studies in PLT and MPV (Table S8, Figure S4). For PDW, we did not conduct publication bias analysis because fewer than 10 studies were included.
In this meta-analysis, we found that PLT and MPV were significantly higher in the AITD population. After further grouping according to disease type and thyroid function, PLT in the HT group, thyroid function unknown group, and hypothyroid/before treatment group was significantly higher than that of the control group; MPV in the GD group, euthyroid/after treatment group, and hyperthyroid/before treatment group was significantly higher than that in the control group.
Regarding changes in PLT in AITD, this difference was found only in the HT and hypothyroid groups, but not in the GD and hyperthyroid groups. Such a result does not seem to be a coincidence, as HT patients tend to develop hypothyroidism later in life while GD is the main cause of hyperthyroidism. Beyan et al. first reported the case of reactive thrombocytosis caused by HT. A 31-year-old man with HT stopped taking levothyroxine on his own and found that PLT increased to 715×109/L during routine follow-up, and the examination showed that the patient was in a subclinical hypothyroidism state. When he started thyroid hormone replacement therapy again, PLT gradually decreased to normal (7). In addition to this case report, Bilge et al. found a significantly higher PLT in the HT group than the control group (31). Besides, several other studies, although lacking statistical differences, also suggested a slightly higher PLT in the HT group compared to controls (24, 26). HT is the major cause of hypothyroidism, and studies have found that thyroid hormone levels are also associated with PLT. One trial found an increase in PLT in hypothyroid patients two weeks after stopping thyroid hormone supplementation (39); another study showed a significant negative correlation between PLT and thyroid hormone in hypothyroid patients (10).
As mentioned in the introduction, platelets are also important players in immunity and inflammation. Activated platelets synthesize and release pro-thrombotic and pro-inflammatory factors from the granule system. Many platelet-derived factors contribute to the formation of inflammatory response, the most important of which is P-selectin, which binds to the P-selectin glycoprotein ligand-1 on endothelial or immune cells, thereby enabling platelets to bind to the inflamed endothelium, recruit circulating leukocytes, promote platelet leukocyte aggregation, and initiate an inflammatory response at the injured site (3, 40). As HT is an autoimmune thyroiditis and is characterized pathologically by an intra-thyroidal lymphocytic infiltrate, platelets are likely to be involved in its pathogenesis and cause changes in platelet parameters. Thrombopoietin produced by the liver plays an important role in megakaryocyte proliferation, differentiation, and platelet formation (41). It was found that inflammatory factors can stimulate the production of thrombopoietin, which also means that PLT may increase significantly in inflammatory states (42). In addition, elevated levels of tumor necrosis factor-α were found to lead to platelet hyperreactivity in the model of aging mouse (43). On the one hand, it may induce transcriptional reprogramming of megakaryocytes, leading to an increase in PLT (43). On the other hand, it stimulates platelet activation and adhesion, forming more platelet-leukocyte aggregates that further promote the progression of immune inflammation, forming a vicious circle. Although a study has shown a significantly higher PLT in GD (29), our results did not find a significant difference in PLT between the GD or hyperthyroid population and the healthy population. Such results are curious, as GD is also an immune disease, so why does it not show elevated PLT like HT? We speculate that it may be related to the following factors: Elevated thyroid hormone levels in GD patients enhance the phagocytosis of the reticuloendothelial system and shorten the platelet lifespan (44), which is superimposed on the increase in platelets due to immunoinflammatory factors, resulting in insignificant changes.
For MPV changes in AITD, the results were the opposite of those in PLT. The MPV was significantly higher in the GD, hyperthyroid/before treatment, and euthyroid/after treatment groups than in the control group, while it was not statistically significant in the HT and hypothyroid groups. One explanation is that the active reticuloendothelial phagocyte system in patients with GD leads to a decrease in platelets (44), while the newborn platelets are larger in size (45). However, it is noteworthy that MPV was also statistically significant in the euthyroid group, which contradicts the conventional perception that abnormal thyroid hormone levels lead to elevated MPV. MPV was found to be significantly higher in euthyroid patients with AITD than in controls (9, 10), and MPV was positively correlated with thyroid peroxidase antibody (46). These studies suggest that the mechanism of MPV abnormalities in AITD patients may be immune and inflammatory rather than hormonal disorders. Of course, this also needs to be verified in more clinical studies. The meta-analysis showed that increased MPV was strongly associated with cardiovascular events (8). MPV as one of the indicators of platelet activation, AITD patients have significantly higher levels of platelet activation (4), and thyroid dysfunction is strongly associated with thrombosis, increasing the morbidity and mortality of cardiovascular events (47–49). Therefore, appropriate clinical attention can be paid to changes in MPV in AITD patients to assess their risk factors for cardiovascular events and intervene in a timely manner. Notably, the multifaceted nature of platelets gives them a place in human health and in a considerable number of diseases, and abnormal platelet indices are not limited to AITD. In recent years, there have been an increasing number of studies related to this issue, and to our knowledge, this is the first meta-analysis to assess differences in platelet parameters between AITD and healthy populations. We have drawn some conclusions from the data analysis and hope that this will also make a small contribution to research in the field of platelets.
Even so, there were some limitations to our work. First, most of the studies were conducted in China and Turkey, and there were differences in the platelet analyzers used, which can lead to bias and made it difficult to generalize these findings to a broader scope. Second, partial summary results had great heterogeneity. Although subgroup analysis explained some of the sources of heterogeneity, there were still some that they couldn’t explain. Third, sensitivity analysis showed that the stability of the pooled MPV was not good, which limits the interpretability of our result. Finally, although we considered the effect of disease type, thyroid function, quality assessment, region, and study type on platelets, we were unable to extract sufficient data for other confounding factors, such as gender and age, for further analysis.
In conclusion, this meta-analysis showed that PLT and MPV were significantly elevated in AITD patients when compared with healthy people. Subgroup analysis based on disease type and thyroid function revealed that this elevation was more significant for PLT in the HT and hypothyroid populations and for MPV in the GD and hyperthyroid populations. The clinical findings of platelet abnormalities can appropriately focus on the patient’s thyroid function, and conversely, the consequences of platelet abnormalities should be prevented in the AITD population. However, these findings must be considered in the context of above limitations, and more clinical studies are needed to validate them.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YC and XZ conceived and designed the study. KZ, TL and XY contributed to data collection. YL, TL, WZ and JS conducted the data analysis and interpretation. YC and KZ drafted the initial manuscript. XZ, JY and QW revised the manuscript. All authors contributed to the article and approved the submitted version.
The study was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The funding source was used for article processing charges. Beyond that, the funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1089469/full#supplementary-material
AITD, Autoimmune thyroid disease; GD, Graves’ disease; HT, Hashimoto’s thyroiditis; MPV, Mean platelet volume; MOOSE, Meta-analysis of Observational Studies in Epidemiology; PRISMA, Preferred Reporting Items for a Systematic Review and Meta-analysis; PLT, Platelet count; PDW, Platelet distribution width; SD, Standard deviation; AHRQ, Agency for Healthcare Research and Quality; NOS, Newcastle-Ottawa Scale; SMD, Standardized mean difference; 95% CI, 95% confidence interval.
1. Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, et al. Graves’ disease. Nat Rev Dis Primers (2020) 6:52. doi: 10.1038/s41572-020-0184-y
2. Ralli M, Angeletti D, Fiore M, D’Aguanno V, Lambiase A, Artico M, et al. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev (2020) 19:102649. doi: 10.1016/j.autrev.2020.102649
3. Dib PRB, Quirino-Teixeira AC, Merij LB, Pinheiro MBM, Rozini SV, Andrade FB, et al. Innate immune receptors in platelets and platelet-leukocyte interactions. J Leukocyte Biol (2020) 108:1157–82. doi: 10.1002/Jlb.4mr0620-701r
4. Tomczynska M, Salata I, Bijak M, Saluk-Bijak J. The potential contribution and role of a blood platelets in autoimmune thyroid diseases. J Cell Mol Med (2018) 22:6386–90. doi: 10.1111/jcmm.13862
5. Kuznik BI, Vitkovsky YA, Gvozdeva OV, Solpov AV, Magen E. Lymphocyte-platelet crosstalk in graves’ disease. Am J Med Sci (2014) 347:206–10. doi: 10.1097/MAJ.0b013e3182831726
6. Ito S, Fujiwara S, Murahashi R, Nakashima H, Matsuoka S, Ikeda T, et al. Clinical association between thyroid disease and immune thrombocytopenia. Ann Hematol (2021) 100:345–52. doi: 10.1007/s00277-020-04343-5
7. Beyan C, Kaptan K. Reactive thrombocytosis accompanying subclinical hypothyroidism due to hashimoto’s thyroiditis. Blood Coagul Fibrin (2013) 24:649–51. doi: 10.1097/MBC.0b013e32836069f5
8. Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost (2010) 8:148–56. doi: 10.1111/j.1538-7836.2009.03584.x
9. Carlioglu A, Timur O, Durmaz SA, Ayhan ME. Mean platelet volume in euthyroid patients with hashimoto’s thyroiditis. Blood Coagul Fibrinolysis (2015) 26:282–4. doi: 10.1097/mbc.0000000000000236
10. Gur EB, Karadeniz M, Inceefe H, Tatar S, Turan GA, Genc M, et al. Thyroid antibodies in euthyroid and subclinical hypothyroidic pregnant women with autoimmune hypothyroidism: effects on hematological parameters and postpartum hemorrhage. Ginekol Pol (2015) 86:666–71. doi: 10.17772/gp/57810
11. Arpaci D, Gurol G, Ergenc H, Yazar H, Tocoglu AG, Ciftci IH, et al. A controversial new approach to address hematological parameters in hashimoto’s thyroiditis. Clin Lab (2016) 62:1225–31. doi: 10.7754/Clin.Lab.2015.150927
12. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
13. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
14. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
15. Wells GA, Shea BO, Connell D, Peterson J, Welch V, Losos M, et al. NewCastle - Ottawa quality assessment scale – cohort studies . Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed September 1, 2022).
16. Wells GA, Shea BO, Connell D, Peterson J, Welch V, Losos M, et al. NewCastle - Ottawa quallty assessment scale – case control studies . Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed September 1, 2022).
17. Rostom A, Dube C, Cranney A, Saloojee N, Sy R, Garritty C, et al. Rockville (MD): Agency for healthcare research and quality (US) (2004). Available at: http://www.ncbi.nlm.nih.gov/books/NBK35156 (Accessed September 1, 2022). Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms.
18. Wells GA, Shea BO, Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses . Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed September 1, 2022).
19. Hu J, Dong Y, Chen X, Liu Y, Ma D, Liu X, et al. Prevalence of suicide attempts among Chinese adolescents: A meta-analysis of cross-sectional studies. Compr Psychiatry (2015) 61:78–89. doi: 10.1016/j.comppsych.2015.05.001
20. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14:135. doi: 10.1186/1471-2288-14-135
21. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res (2018) 27:1785–805. doi: 10.1177/0962280216669183
22. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022) . Available at: https://www.training.cochrane.org/handbook (Accessed September 1, 2022).
23. He P, Yang H, Lai Q, Kuang Y, Huang Z, Liang X, et al. The diagnostic value of blood cell-derived indexes in subacute thyroiditis patients with thyrotoxicosis: a retrospective study. Ann Transl Med (2022) 10:322. doi: 10.21037/atm-22-719
24. Demir AD. Relationship of the platelet distribution width/platelet count ratio with thyroid antibody levels in patients with hashimoto’s thyroiditis. J Int Med Res (2021) 49:3000605211043241. doi: 10.1177/03000605211043241
25. Szydelko J, Litwinczuk M, Szydelko M, Matyjaszek-Matuszek B. Neutrophil-to-Lymphocyte, monocyte-to-Lymphocyte and platelet-to-Lymphocyte ratios in relation to clinical parameters and smoking status in patients with graves’ orbitopathy-novel insight into old tests. J Clin Med (2020) 9. doi: 10.3390/jcm9103111
26. Onalan E, Donder E. Neutrophil and platelet to lymphocyte ratio in patients with hypothyroid hashimoto’s thyroiditis. Acta BioMed (2020) 91:310–14. doi: 10.23750/abm.v91i2.8592
27. Onalan E, Aslan M. Could neutrophil to lymphocyte ratio be a marker in hashimoto’s thyroiditis? J Pak Med Assoc (2020) 70:1381–83. doi: 10.5455/JPMA.32518
28. Dasgupta R, Atri A, Jebasingh F, Hepzhibah J, Christudoss P, Asha H, et al. Platelet-lymphocyte ratio as a novel surrogate marker to differentiate thyrotoxic patients with graves disease from subacute thyroiditis: a cross-sectional study from south India. Endocr Pract (2020) 26:939–44. doi: 10.4158/ep-2020-0086
29. Turan E. Evaluation of neutrophil-to-lymphocyte ratio and hematologic parameters in patients with graves’ disease. Bratisl Med J (2019) 120:476–79. doi: 10.4149/bll_2019_076
30. Taşkaldiran I, Omma T, Önder ÇE, Firat SN, Koç G, Kiliç MK, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, and platelet-tolymphocyte ratio in different etiological causes of thyrotoxicosis. Turk J Med Sci (2019) 49:1687–92. doi: 10.3906/sag-1901-116
31. Bilge M, Yesilova A, Adas M, Helvaci A. Neutrophil- and platelet- to lymphocyte ratio in patients with euthyroid hashimoto’s thyroiditis. Exp Clin Endocrinol Diabetes (2019) 127:545–49. doi: 10.1055/a-0723-3441
32. Gu X, Wu S, Xu J, Hong Y, Yang L, Lin Y. Implication of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with thyrotoxicosis: significance in differential diagnosis. Chin J Endocrinol Metab (2017) 33:491–96.
33. Aktas G, Sit M, Dikbas O, Erkol H, Altinordu R, Erkus E, et al. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of hashimoto’s thyroiditis. Rev Assoc Med Bras (2017) 63:1065–68. doi: 10.1590/1806-9282.63.12.1065
34. Savas E, Sahin AZ, Aksoy SN, Tascan A, Sayiner ZA, Ozkaya M. Serum levels of inflammatory markers in patients with thyroid dysfunction and their association with autoimmunity status. Int J Clin Exp Med (2016) 9:4485–90.
35. Keskin H, Kaya Y, Cadirci K, Kucur C, Ziypak E, Simsek E, et al. Elevated neutrophil-lymphocyte ratio in patients with euthyroid chronic autoimmune thyreotidis. Endocr Regul (2016) 50:148–53. doi: 10.1515/enr-2016-0017
36. Sit M, Kargi E, Aktas G, Dikbas O, Alcelik A, Savli H. Mean platelet volume should be a useful indicator in diagnosis of hashimoto’s thyroiditis. Acta Med Mediterr (2014) 30:1263–66.
37. Aktas G, Sit M, Dikbas O, Tekce BK, Savli H, Tekce H, et al. Could red cell distribution width be a marker in hashimoto’s thyroiditis? Exp Clin Endocrinol Diabetes (2014) 122:572–4. doi: 10.1055/s-0034-1383564
38. Atile NS, Bilir BE, Bilir B, Guldiken S. Mean platelet volume levels in patients with overt hypothyroidism before and after levothyroxine treatment. Acta Endocrinol-Buch (2012) 8:607–13. doi: 10.4183/aeb.2012.607
39. van Doormaal JJ, van der Meer J, Oosten HR, Halie MR, Doorenbos H. Hypothyroidism leads to more small-sized platelets in circulation. Thromb Haemost (1987) 58:964–65. doi: 10.1055/s-0038-1646037
40. Deppermann C, Kubes P. Start a fire, kill the bug: The role of platelets in inflammation and infection. Innate Immun-London (2018) 24:335–48. doi: 10.1177/1753425918789255
41. Machlus KR, Italiano JE Jr. The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol (2013) 201:785–96. doi: 10.1083/jcb.201304054
42. Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest (2005) 115:3339–47. doi: 10.1172/Jci26674
43. Davizon-Castillo P, McMahon B, Aguila S, Bark D, Ashworth K, Allawzi A, et al. TNF-α-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood (2019) 134:727–40. doi: 10.1182/blood.2019000200
44. Kurata Y, Nishioeda Y, Tsubakio T, Kitani T. Thrombocytopenia in graves’ disease: effect of T3 on platelet kinetics. Acta haematologica (1980) 63:185–90. doi: 10.1159/000207396
45. Holinstat M. Normal platelet function. Cancer Metastasis Rev (2017) 36:195–98. doi: 10.1007/s10555-017-9677-x
46. Erem C, Ersoz HO, Karti SS, Ukinc K, Hacihasanoglu A, Deger O, et al. Blood coagulation and fibrinolysis in patients with hyperthyroidism. J Endocrinol Invest (2002) 25:345–50. doi: 10.1007/bf03344016
47. Sohn SY, Lee E, Lee MK, Lee JH. The association of overt and subclinical hyperthyroidism with the risk of cardiovascular events and cardiovascular mortality: Meta-analysis and systematic review of cohort studies. Endocrinol Metab (Seoul) (2020) 35:786–800. doi: 10.3803/EnM.2020.728
48. Kim HJ, Kang T, Kang MJ, Ahn HS, Sohn SY. Incidence and mortality of myocardial infarction and stroke in patients with hyperthyroidism: A nationwide cohort study in Korea. Thyroid (2020) 30:955–65. doi: 10.1089/thy.2019.0543
Keywords: autoimmune thyroid disease, Grave’s disease, Hashimoto autoimmune thyroiditis, platelet count, mean platelet volume, systematic review, meta - analysis
Citation: Cao Y-t, Zhang K-y, Sun J, Lou Y, Lv T-s, Yang X, Zhang W-h, Yu J-y, Wu Q-b and Zhou X-q (2022) Platelet abnormalities in autoimmune thyroid diseases: A systematic review and meta-analysis. Front. Immunol. 13:1089469. doi: 10.3389/fimmu.2022.1089469
Received: 04 November 2022; Accepted: 06 December 2022;
Published: 22 December 2022.
Edited by:
Gianni Guidetti, University of Pavia, ItalyReviewed by:
Natasa Stanisavljevic, University Medical Center, SerbiaCopyright © 2022 Cao, Zhang, Sun, Lou, Lv, Yang, Zhang, Yu, Wu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi-qiao Zhou, emhvdXhpcWlhb0BuanVjbS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.