
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 25 January 2023
Sec. Immunological Tolerance and Regulation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1082950
This article is part of the Research TopicImmunomics: The Immune Regulatory Networks in the One Health PerspectiveView all 9 articles
 Hongwei Li1†
Hongwei Li1† Yiran Xu1,2†
Yiran Xu1,2† Wenhua Li1†
Wenhua Li1† Lingling Zhang1†
Lingling Zhang1† Xiaoli Zhang1
Xiaoli Zhang1 Bingbing Li1
Bingbing Li1 Yiwen Chen1
Yiwen Chen1 Xiaoyang Wang1,3
Xiaoyang Wang1,3 Changlian Zhu1,4*
Changlian Zhu1,4*The pathogenesis of autism spectrum disorder (ASD) is not well understood, especially in terms of immunity and inflammation, and there are currently no early diagnostic or treatment methods. In this study, we obtained six existing Gene Expression Omnibus transcriptome datasets from the blood of ASD patients. We performed functional enrichment analysis, PPI analysis, CIBERSORT algorithm, and Spearman correlation analysis, with a focus on expression profiling in hub genes and immune cells. We validated that monocytes and nonclassical monocytes were upregulated in the ASD group using peripheral blood (30 children with ASD and 30 age and sex-matched typically developing children) using flow cytometry. The receiver operating characteristic curves (PSMC4 and ALAS2) and analysis stratified by ASD severity (LIlRB1 and CD69) showed that they had predictive value using the “training” and verification groups. Three immune cell types – monocytes, M2 macrophages, and activated dendritic cells – had different degrees of correlation with 15 identified hub genes. In addition, we analyzed the miRNA-mRNA network and agents-gene interactions using miRNA databases (starBase and miRDB) and the DSigDB database. Two miRNAs (miR-342-3p and miR-1321) and 23 agents were linked with ASD. These findings suggest that dysregulation of the immune system may contribute to ASD development, especially dysregulation of monocytes and monocyte-derived cells. ASD-related hub genes may serve as potential predictors for ASD, and the potential ASD-related miRNAs and agents identified here may open up new strategies for the prevention and treatment of ASD.
Autism spectrum disorder (ASD) is a group of disorders characterized by impairment in social interaction and communication and the presence of restricted and repetitive behaviors and interests (1). It is often combined with intellectual disability (2), immune dysfunction (3), and inflammatory diseases of the gastrointestinal tract (4). ASD is a multifactorial process and is associated with an adverse maternal environment, children’s lifestyle, genetic factors, and immune, inflammatory, and psychosocial factors (5–8). Among these, immune and inflammatory factors have been shown to play an essential role in the development of autism (9–12). However, the pathogenesis caused by immune responses and inflammation remains unclear, and the regulatory mechanisms still require in-depth investigations.
Recent studies have shown that immune cells play a vital role in the occurrence and development of ASD. For example, abnormalities in the peripheral blood monocytes of the innate immune system are associated with behavioral changes in inflammatory subtype ASD (13), while NK cells from patients with high-functioning ASD show a high level of cell activation (14). Myeloid dendritic cells are increased in autistic children and are related to amygdala volume and repetitive behaviors (15). Microglia, as the resident macrophages in the central nervous system in persons with ASD, show elevated protein synthesis that causes autism-like synaptic and behavioral aberrations (16), and mediators from mast cells can activate microglia causing local inflammation and contributing to ASD symptoms (17). Moreover, regulatory B cells and T cells are decreased in children with ASD, and this plays a pivotal role in the evolution and severity of ASD (18). However, no study has analyzed the immune cell landscape in whole blood in persons with ASD and there is a need for a systematic approach to assessing the contribution of immune cells and key immune-related genes.
Non-coding RNAs with post-transcriptional regulatory functions for genes have been shown to be closely associated with ASD. For instance, the blood transcriptome in persons with ASD shows co-expression modules associated with ASD risk genes, including genes related to metabolism, immunity, neurodevelopment, and signal transduction (19). A recent study showed that micro-RNA (miRNA) is the key regulator of gene expression in neurodevelopmental transcriptional networks, and circulating miRNAs might therefore be potential predictors for ASD diagnosis and prognosis (20). In addition, many studies have focused on blood and saliva samples of ASD patients, and the results have shown that miRNAs might be of predictive significance in ASD because of their associations with inflammation and immunity (21–23). However, although miRNAs show great potential in treating cancer and other diseases (24), few studies on ASD have been reported. Also, the effects of known genes on ASD have not yet been fully elucidated, and more gene sets should be concentrated on, and potential interactions between genes might be further constructed and investigated such as miRNA-mRNA networks and protein-protein interaction (PPI) networks.
Bioinformatics analysis has attracted much attention with continuous breakthroughs in the discovery of novel genes and predictors. The purpose of this study was to collect and analyze the available ASD Gene Expression Omnibus (GEO) database to identify ASD-related immune cells and hub genes related to biological functions, gene networks, diagnosis, and treatment.
To collect all existing datasets that used children and adults with ASD’s peripheral blood for transcriptome studies, we searched the datasets from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) with the condition terms “Autism spectrum disorder,” “Autism,” “Autistic disorder,” “ASD,” the tissue term “blood,” and the organism term “Homo Sapiens.” The inclusion criteria were as follows: the datasets contained gene expression profiling by microarray or high-throughput RNA sequencing, the datasets included ASD and typically developing (TD) samples, and 20 or more samples were in the dataset. Thus, six eligible GEO Series (GSE) datasets were adopted, including GSE6575 (25), GSE18123 (26), GSE42133 (27), GSE111175 (28), GSE26415 (29), and GSE89594 (30). All transcriptomic datasets are listed in Supplementary Table 1. The GSE18123 dataset consisted of two RNA sequencing profiles from different GEO Platforms (GPL), so there were five datasets from children and two from adults. Among the five children’s datasets, four were used as “training” datasets, and one was used as the validation dataset. Figure 1 illustrates the workflow of this study. The R package “limma” (v3.50.3) was used to analyze each gene expression matrix with the threshold of p < 0.05 (31).
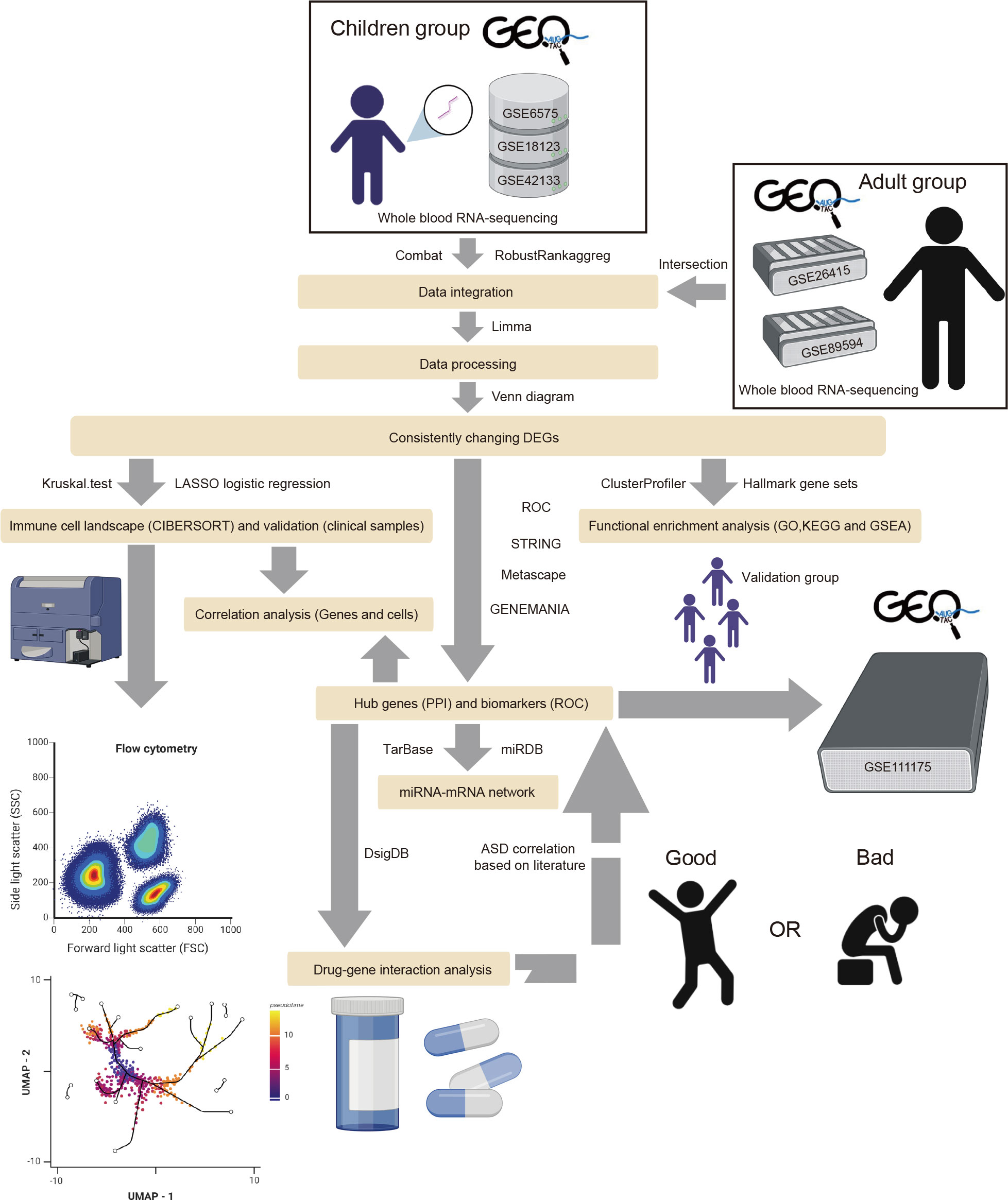
Figure 1 The flow diagram of the study. The adult group (GSE26415 and GSE89594) was only analyzed regarding GO, KEGG, GSEA, and immune cell infiltration.
The biological functions and corresponding pathways of the identified DEGs were determined through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses using the R package “clusterProfiler” (32) (v4.2.2) with a significance level of padjusted < 0.05. The online databases GENEMANIA (https://genemania.org/) (33) and Metascape (http://metascape.org/gp/index.html#/main/step1) (34) were used to predict gene function. Gene Set Enrichment Analysis (GSEA) was conducted in R software using the hallmarks gene set “h.all.v7.5.1.symbols.gm” (35). The results were visualized with the R packages “enrichplot” (v1.14.2) and “ggplot2” (v3.3.6).
The CIBERSORT analytical tool (https://cibersortx.stanford.edu/) was used to evaluate the abundance and differences of 22 types of immune cells between the ASD and TD group (36), including different kinds of B cells, dendritic cells, macrophages, mast cells, NK cells, T cells, eosinophils, monocytes, neutrophils, and plasma cells. The results were analyzed using the R packages “ggplot2” and “Kruskal.test”. For the training dataset, LASSO logistic regression was carried out to further select significantly different immune cells in combination with Kruskal.test. The Wilcoxon test and receiver operating characteristic (ROC) curves were calculated using R-based tools to assess the classifier performance for the LASSO regression model (v4.1.0).
A PPI network was constructed among the consistently changing DEGs using the STRING database (https://string-db.org) (37). The CytoHubba module in Cytoscape (v 3.7.1) was used to score the top 10 node genes using 12 different algorithms, namely MCC (Maximum Clique Centrality), DMNC (Density of Maximum Neighborhood Component), MNC (Maximum Neighborhood Component), Degree, EPC (Edge Percolated Component), BottleNeck, EcCentricity, Closeness, Radiality, Betweenness, Stress, and Clustering Coefficient. The hub genes generated from each algorithm were shown by the R package “UpSet” and validated using GSE111175.
To validate the most significant immune cells in children, the whole blood samples from 30 children with ASD and 30 age and sex-matched TD children were collected at the 3rd Affiliated Hospital of Zhengzhou University. All participants provided informed consent to participate in the study, which was approved by the Medical Ethics Committee of the 3rd Affiliated Hospital of Zhengzhou University (Ethical number 2020–56). All blood samples were processed within 8 h for flow cytometry with a mix of antibodies according to the manufacturer’s instructions. The antibodies purchased from BD Biosciences included CD45 (HI30, Cat# 564105) CD14 (M5E2, Cat# 561712), CD16 (3G8, Cat# 563692), and HLA-DR (G46-6, Cat# 560896). The data were analyzed using FlowJo software (TreeStar) and presented using the UMAP method.
The association of the hub genes with the infiltration levels of immune cells was explored and analyzed in R, and the correlation results were visualized using the “ggplot2” and “ggstatsplot” packages.
The GSE111175 dataset was used to assess the predictive effectiveness according to the validation group between the children with ASD and the children in the TD group using ROC curve analysis. The results were visualized with the “pROC”, “ggplot2”, and “ComplexHeatmap” packages.
The starBase (predicted program ≥ 2) (38) and miRDB (39) databases were used to predict the upstream miRNAs of hub mRNAs by taking intersections. Cytoscape (v 3.7.1) was used to visualize the mRNA-miRNA network.
The DSigDB database (http://tanlab.ucdenver.edu/DSigDB) was used to predict potential agents for hub genes related to ASD, with agents–hub genes ≥3 as the condition for the agents screen. The results were displayed using a Sankey diagram based on the “ggalluvial” (v0.12.3) package.
All data from the bioinformatics analyses were analyzed in R, and the data from routine blood tests were analyzed using SPSS software version 23 (IBM Corp). All data are presented as the mean ± standard deviation.
We conducted the analysis using the four children’s datasets and two adults’ datasets. For the children’s datasets, the “RRA” and “Batch correction” methods were used to get accurate DEGs from multiple children’s datasets. For the “Batch” dataset using the same platform, including GSE6575-GPL570 and GSE18123-GPL570, batch effects were removed using the ComBat function of the R package “SVA” (40). The 3D principal component analysis plots indicated that the processed data were more reliable after batch effect removal (Figures 2A, B). For “RRA” dataset using different platforms, including GSE18123-GPL6244 and GSE42133-GPL10558, the batch effect was corrected using the R package “RobustRankAggreg” (RRA) (41) (Figure 2C). As analyzed by “limma”, the final list of DEGs used for further analysis was generated as shown by the Venn diagram in Figure 2D. There were a total of 113 DEGs, of which 95 were consistently changing DEGs between these two datasets in the childhood group, including 57 co-upregulated and 38 co-downregulated genes (Supplementary Table 2 and Figure 2D). For the two adult datasets, DEGs were extracted as the intersection between GSE26415 and GSE89594 as shown in Supplementary Figure 1. A total of 25 consistently changing DEGs were screened between the GSE26415 and GSE89594 adult datasets, including 17 co-upregulated and 8 co-downregulated genes (Supplementary Table 3 and Supplementary Figure 1A).
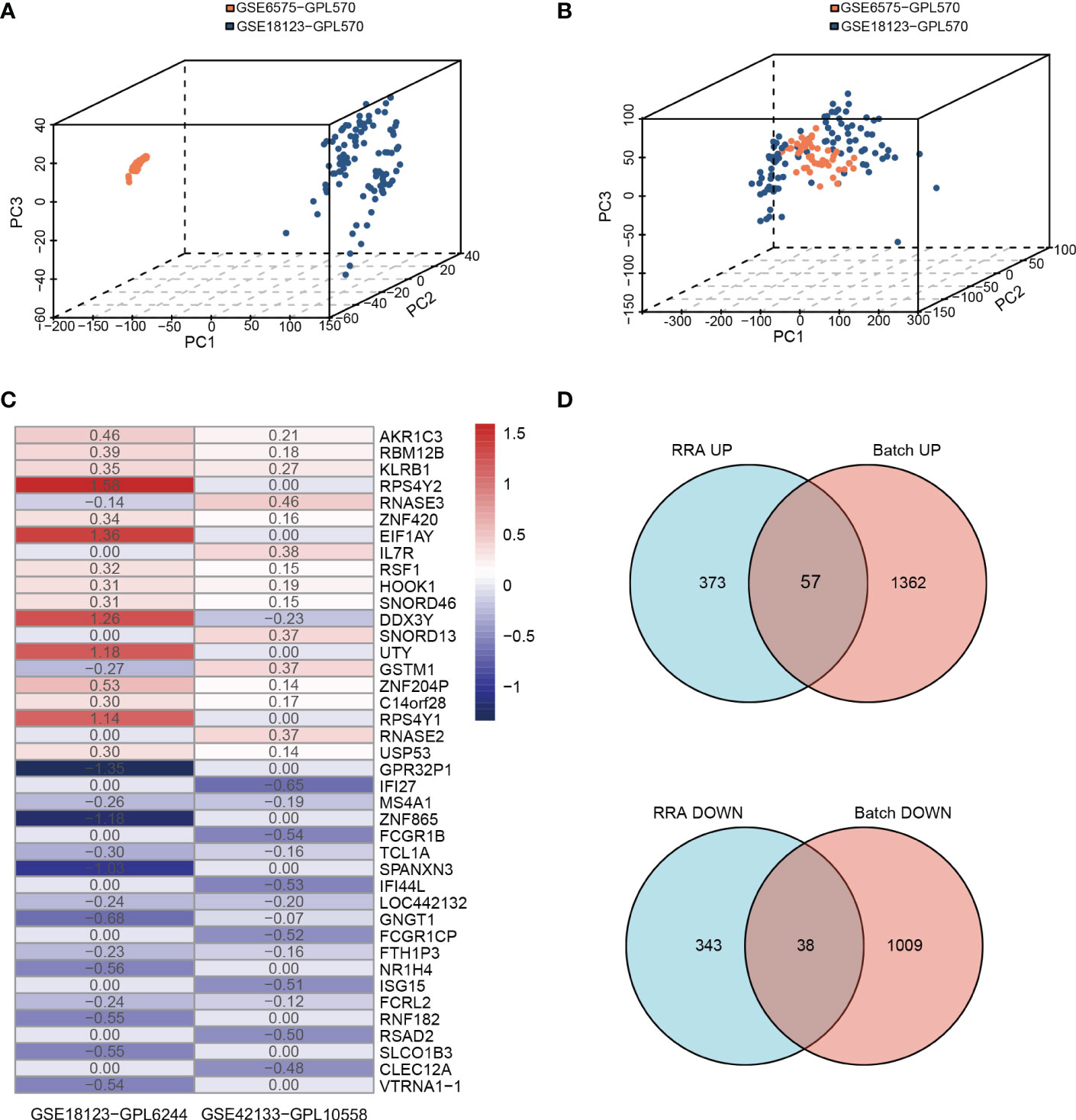
Figure 2 Identifying the DEGs from two methods in children’s databases. (A, B) Principle component analysis of the batch correction of GSE6575 and GSE18123: (A) before batch correction and (B) after batch correction. (C) The top 20 upregulated and 20 downregulated DEGs of the different platforms from two datasets determined by “RRA”. (D) Venn diagram showing the consistently changing DEGs screened by “RRA” and “Batch”.
To reveal the biological processes and signaling pathways behind the DEGs in children and adults, we performed a systematic analysis, including GO, KEGG, GSEA, and DisGeNET database prediction. For the childhood group, the 95 consistently changing DEGs were analyzed with Metascape and GSEA. In total, biological functions related to neuronal development (axon guidance, nervous system development, and Roundabout (ROBO) receptors), immunity (adaptive immune system, IFN-alpha/gamma response, complement, and IL2/Stat5 signaling), and cell development and metabolic processes (mTORC1 signaling, mitotic spindle formation, KRAS signaling, heme metabolism, and protein secretion) (Table 1; Figure 3A) were identified.
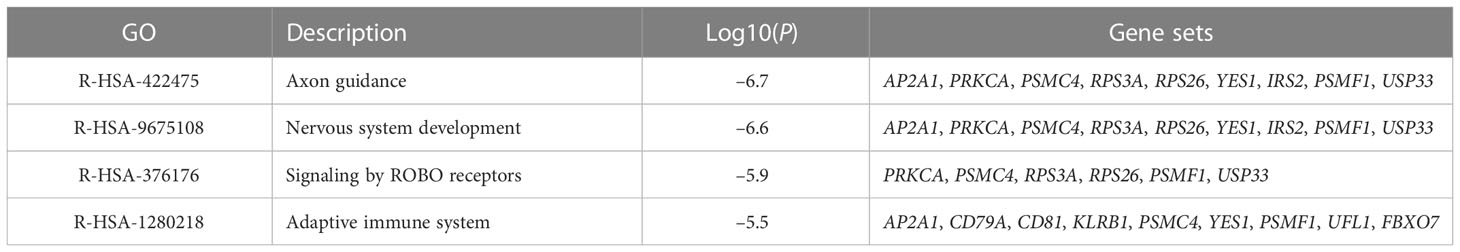
Table 1 The top four GO MCODE components identified in 95 consistently changing DEGs in the protein-protein interaction network.

Figure 3 Gene enrichment analysis based on DisGeNET and GSEA. Summary of the enrichment analysis in DisGeNET in the children (A) and adult (B) groups. The GSEA results showed the enriched activated and suppressed pathways in children (C) and adults (D).
Interestingly, the DisGeNET prediction suggested that the DEGs were mainly associated with erythrocyte-related parameters and disorders such as mean corpuscular hemoglobin (MCH), extramedullary hematopoiesis function, red cell distribution width (RDW), mean corpuscular hemoglobin concentration (MCHC), and anemia (Figure 3B; Supplementary Table 4). Indeed, these results were confirmed and validated when using peripheral blood samples collected from children with ASD. We observed significant differences between the ASD and TD groups for red blood cell count (RBC), hematocrit (HCT), MCH, coefficient of variation of RDW (RDW-CV), and MCHC (Table 2). Moreover, GO enrichment analysis of the DEGs suggested biological functions associated with erythrocyte differentiation (Supplementary Figure 2).
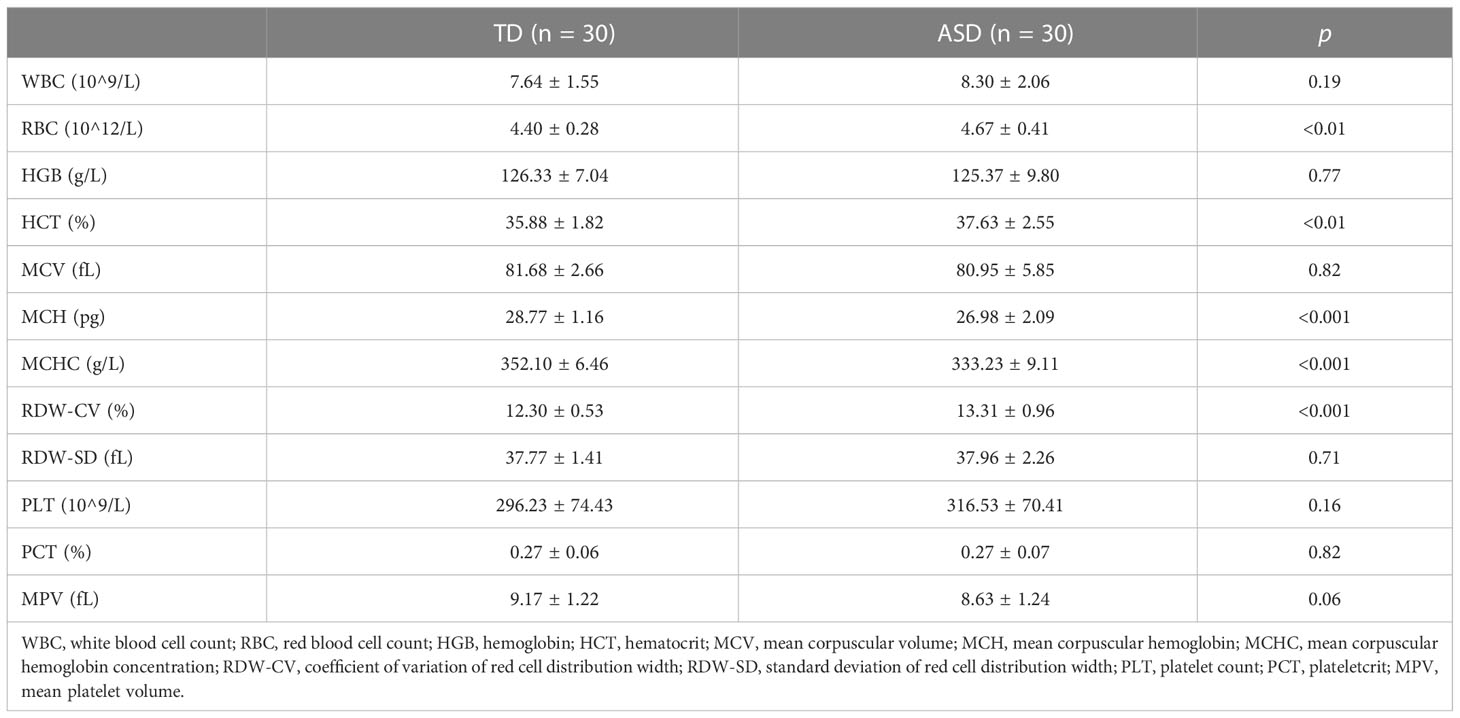
Table 2 The comparison of routine blood tests between TD and ASD groups in the clinical children’s samples.
It is noteworthy that when analyzing the functional enrichment of DEGs using the “RRA” and “Batch” method separately, we found that the B cell receptor (BCR) signaling pathway was significantly enriched in both (Supplementary Figures 3A, B). Similarly, when analyzing all 113 DEGs instead of the consistently changing 95 DEGs between the two methods using the Metascape database, we also found the BCR signaling pathway to be a significant pathway (Supplementary Figure 3C). In addition, several DEGs, including PIR-B (LILRB1), IgA, CD81, and VAV, were found to be involved in the BCR signaling pathway (data not shown).
For the adult group, Metascape analysis of the 25 consistently changing DEGs suggested that deubiquitination, regulation of hormone levels, cell morphogenesis involved in neuron differentiation, and positive regulation of catabolic process were the most enriched terms (Supplementary Figure 1B). GENEMANIA analysis showed that the TGF-β/SMAD signaling pathway was a significantly altered signaling pathway (Supplementary Figure 1C), and some metabolic disorders and immune disease-related processes were significantly enriched according to the DisGeNET analysis, such as hyperinsulinism, scleroderma, juvenile arthritis, inflammation, and celiac disease (Figure 3C). In addition, GSEA analysis showed that oxidative phosphorylation, MYC targets v1, E2F targets, and protein secretion were suppressed, while heme metabolism and KRAS signaling DN were activated, all of which were the opposite of what was seen in the childhood group. It is also noteworthy that estrogen response was among the biological processes enriched in the adult ASD datasets compared to children (Figure 3D).
Overall, the biological process analysis using DEGs showed that immune function, neuronal development, and metabolic disorders were common in both children and adults with ASD. However, there were obvious differences between the two groups. For example, erythrocyte differentiation was involved in children with ASD, while estrogen response affected the course of adults with ASD.
The analysis above clearly showed that immune function is among the most significantly affected biological functions in both children and adults with ASD. In addition, immune responses and inflammation have been suggested to play a crucial role in childhood ASD progression, so we conducted a comprehensive analysis of the immune cell profiles in the selected datasets. The “Batch” dataset for children (101 ASD children and 45 TD children) and the GSE26415 dataset for adults (21 ASD adults and 21 TD adults) were used because of the high quality of their sequencing data and the availability of the CIBERSORT algorithm.
Using the CIBERSORT algorithm, 22 immune cell types were identified in each sample from each group (Supplementary Figure 4). Among these, naive B cells, monocytes, neutrophils, resting NK cells, naive CD4+ T cells, and CD8+ T cells were the main immune cells in children, while monocytes, neutrophils, memory-activated CD4+ T cells, and CD8+ T cells were similarly enriched in adults. In addition, there were significant differences between the ASD and TD groups regarding resting/activated dendritic cells, M0/M2 macrophages, and monocytes in children and M0 macrophages, resting mast cells, and resting/activated NK cells in adults (Figure 4A).
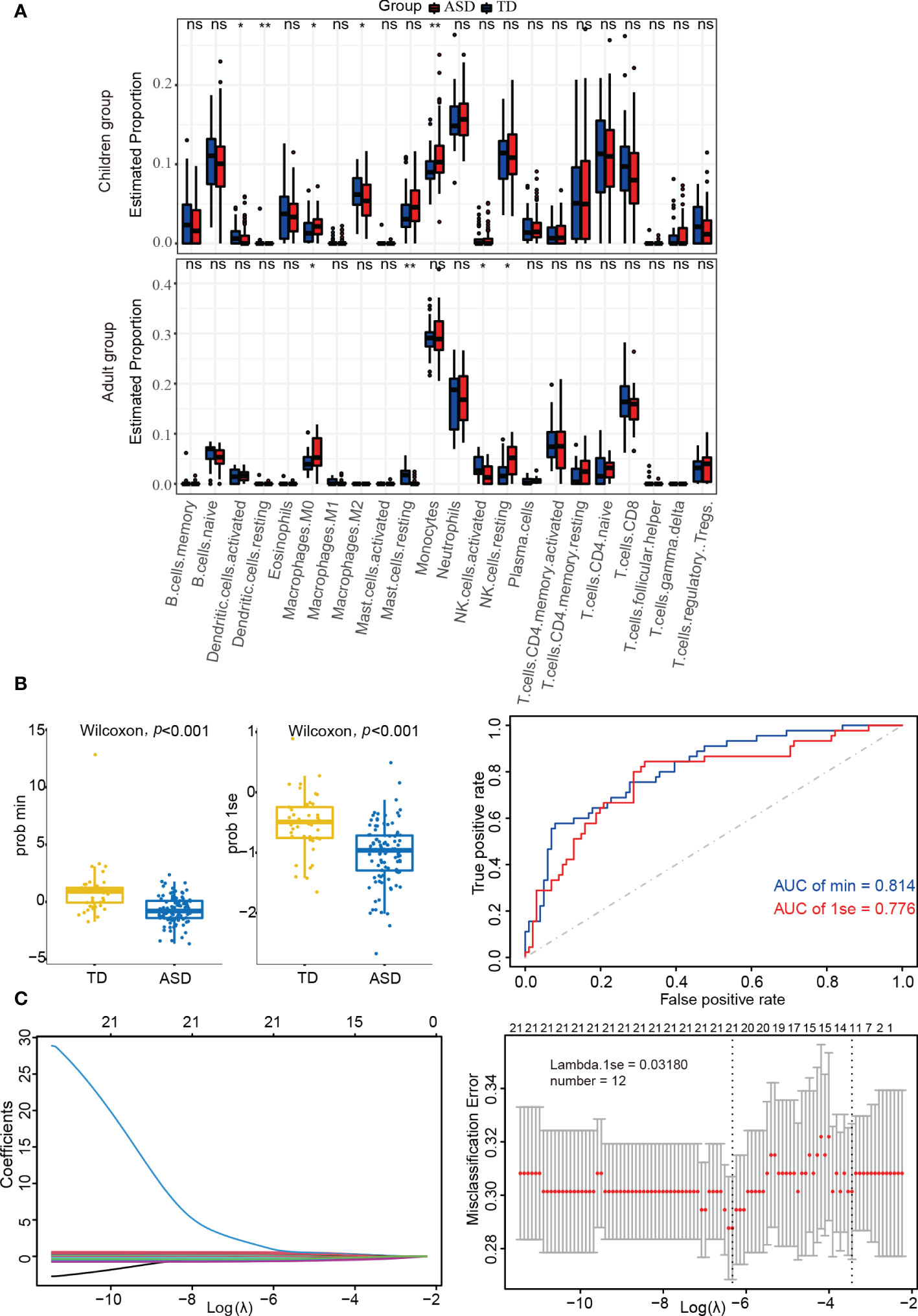
Figure 4 Identifying the significantly different infiltrations of immune cells. (A) Kruskal test analysis in children and adults. (B) LASSO regression analysis in children’s datasets. (C) Box-plots and ROC curves for assessing classifier performance in the LASSO regression model. ns: not significant; *p < 0.05; **p < 0.01.
LASSO regression analysis can more accurately identify significant differences in immune cells. As shown in Supplementary Table 5 and Figure 4B, the LASSO regression model was found to have good classifier performance according to Wilcoxon’s test and area under the ROC curve (AUC) analysis. When further analyzing the data in children with ASD, 12 types of immune cells were extracted (Figure 4C). Among these, four cell types (monocytes, M2 macrophages, and resting/activated dendritic cells) from two algorithms (Kruskal.test and LASSO logistic regression) were identified.
It has been suggested that monocytes play a major role in ASD development in children (42). To validate the results obtained from the integration analysis above, monocyte phenotypes were further identified and confirmed using peripheral blood samples. We collected a total of 60 peripheral whole blood samples, including 30 ASD and 30 age and sex-matched TD children, and performed flow cytometry analysis (Figure 5A). In the UMAP analysis, each of the monocyte subtype populations, including intermediate monocytes, classical monocytes, and nonclassical monocytes (ncMos), were shown as distinct cell clusters (Figure 5B). The flow cytometry analysis showed that among all analyzed monocyte subtypes, there were significant differences in total monocytes (p < 0.05) and ncMos (p < 0.001) (Figure 5C).

Figure 5 The expression of the monocyte phenotype. (A) Monocytes were typed using flow cytometry. (B) UMAP for monocytes and monocyte subtypes in children with ASD and TDs. (C) The percentage of monocytes and subtypes in dot plots. *p < 0.05; ***p < 0.001. Mo, monocyte; iMo, intermediate monocyte; cMo, classical monocyte; ncMo, nonclassical monocyte; ns, not significant.
Hub genes usually refer to genes with a connectivity degree greater than 10 in the genetic interaction network, and these play important roles in biological systems. Here, we used the “Batch” and “RRA” datasets as the training dataset and GSE111175 as the validation dataset for children with ASD. Notably, due to the integration and consistency of the data in the “Batch” and “RRA” datasets, we used the expression data from the “Batch” dataset to represent the expression levels of the hub genes in the training dataset.
The PPI network used to identify the hub genes was obtained when 95 consistently changing DEGs in children with ASD were analyzed by STRING (Supplementary Figure 5). Using the CytoHubba function, there were a total of 15 hub genes (Figure 6A), including AHSP, ALAS2, SELENBP1, AP2A1, BCL2L1, CD3G, MAP1LC3A, CD69, DCAF12, EPB42, GMPR, IGF2R, LILRB1, PSMC4, and SLC4A1, and the expression levels of the hub genes were determined in the training (Figure 6B) and validation (Figure 6C) datasets in children with ASD and TD. Clearly, the validation dataset confirmed only ALAS2, SELENBP1, CD3G, MAP1LC3A, CD69, DCAF12, IGF2R, PSMC4, and SLC4A1 as significantly differently expressed hub genes between children with ASD and TD and not the genes AHSP, AP2A1, BCL2L1, EPB42, GMPR, and LILRB.
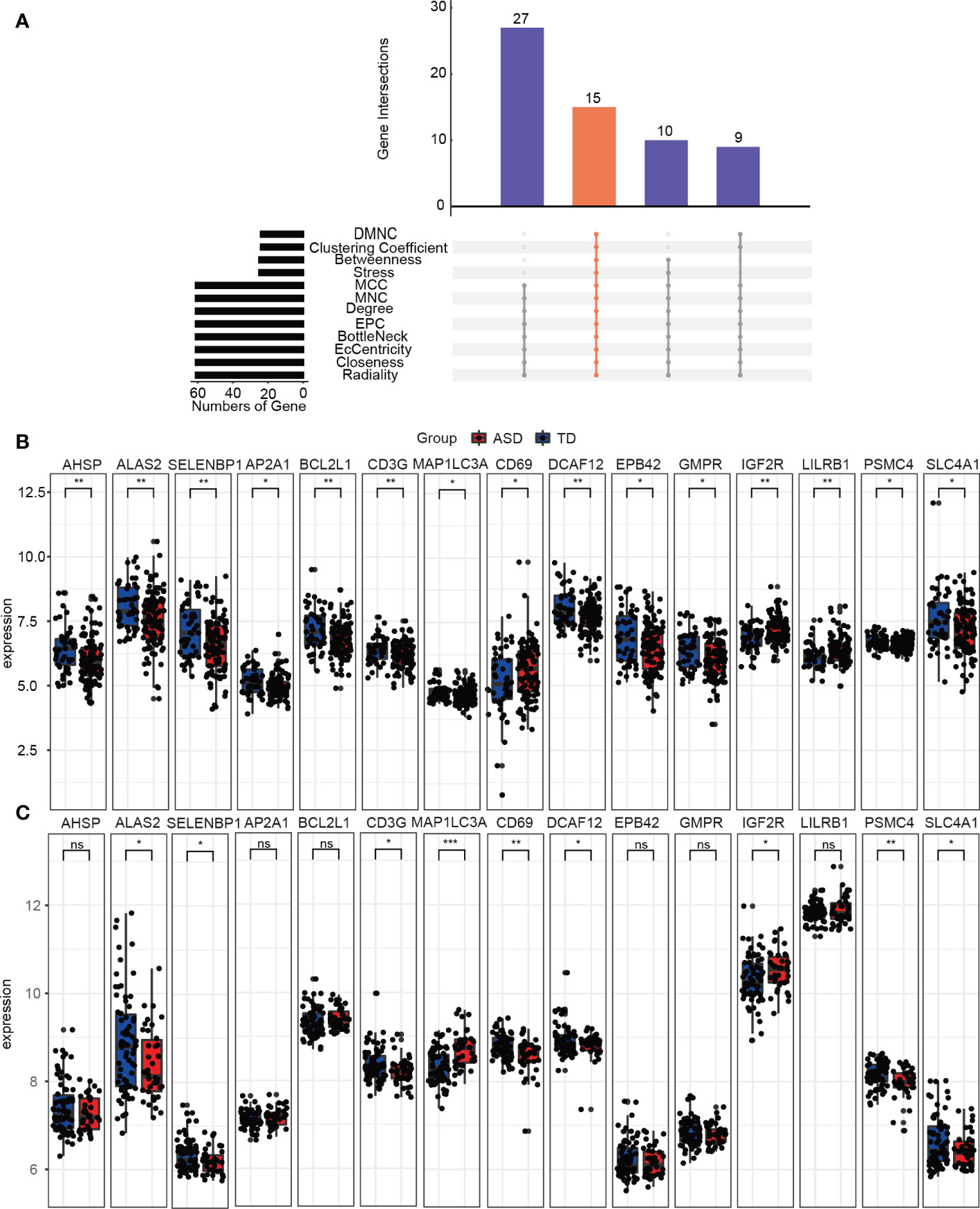
Figure 6 Identifying the hub genes. (A) The 12 algorithms used to screen for hub genes in the R package “UpSet”. Expression levels of 15 hub genes in the (B) training and (C) validation groups. ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
Furthermore, as shown in Figure 7A, ALAS2, CD3G, DCAF12, IGF2R, PSMC4, SELENBP1, and SLC4A1 were significantly and consistently altered hub genes in the training and validation datasets, which suggested that these genes were critical to ASD and might be used as predictors for ASD in children. An AUC more than 0.60 was considered to be relatively good predictive accuracy. The AUC values of PSMC4 and ALAS2 were 0.633 (95% CI 0.534–0.732) and 0.651 (95% CI 0.559–0.744) in the training group and 0.677 (95% CI 0.574–0.779) and 0.625 (95% CI 0.517–0.733) in the validation group, respectively. In the logistic regression model the combined AUC values of PSMC4 and ALAS2 reached 0.668 (95% CI 0.575–0.762) and 0.729 (95% CI 0.630–0.829), respectively, in both datasets (Table 3; Figure 7B). In addition, we also undertook diagnostic performance analyses of the remaining five possible marker genes, including single and multiple gene combination diagnostic analyses. However, we did not find any additional predictors in the two datasets when considering higher diagnostic efficiency and lower cost. Therefore, PSMC4 and ALAS2 might act as predictors with moderate strength (43).
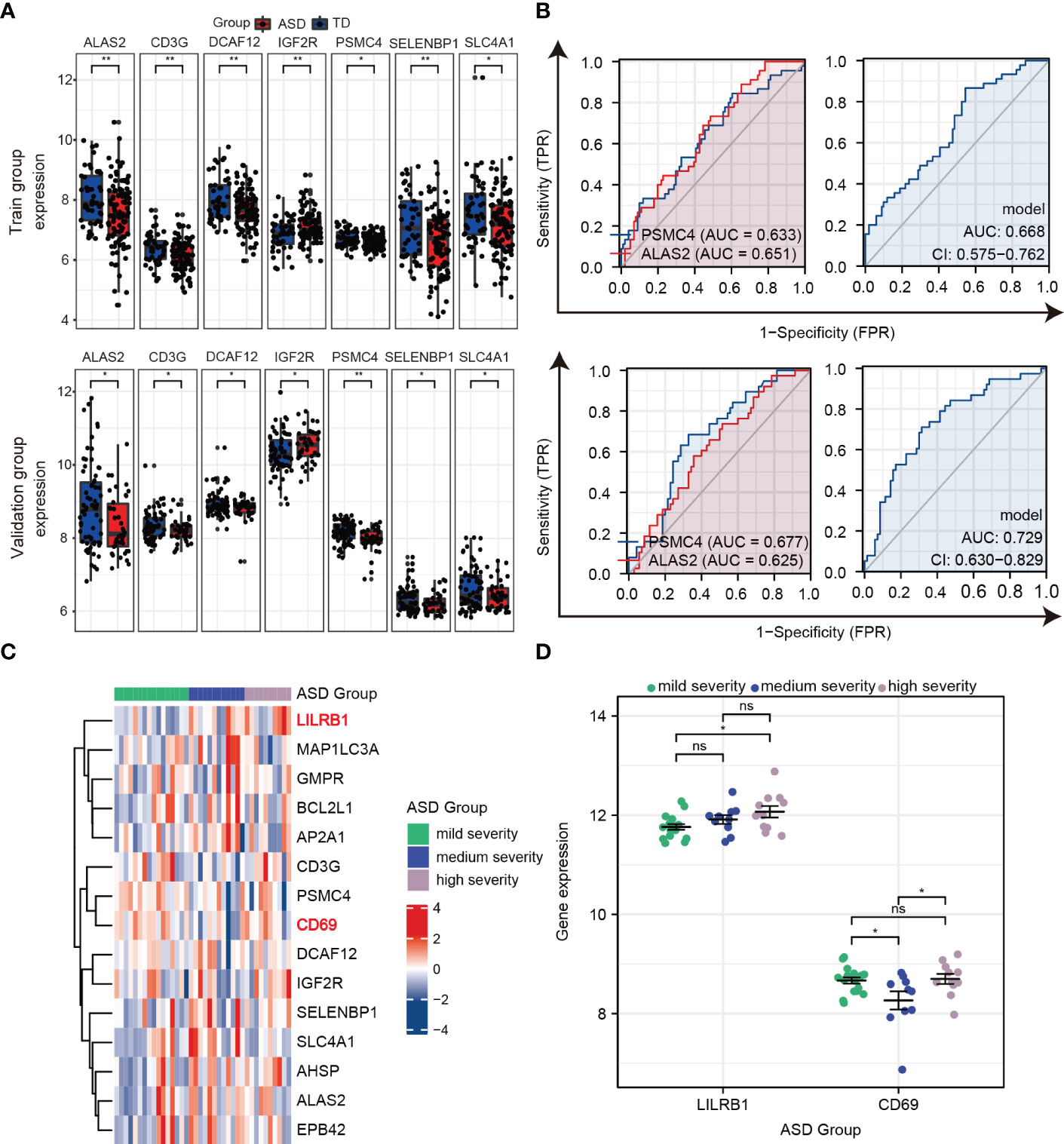
Figure 7 Verifying the predictors from hub genes in the training and validation groups. (A) Seven hub genes with consistent changes in expression levels in the training group (top) and validation group (bottom). (B) ROC curve and logistic regression model to assess the predictive accuracy of PSMC4 and ALAS2 in the training group (top) and validation group (bottom). (C) Heat maps of the expression of 15 hub genes in the three groups according to ASD severity. (D) Prognostic values for LILRB1 and CD69 in dot plots. ns, not significant; *p < 0.05; **p < 0.01.
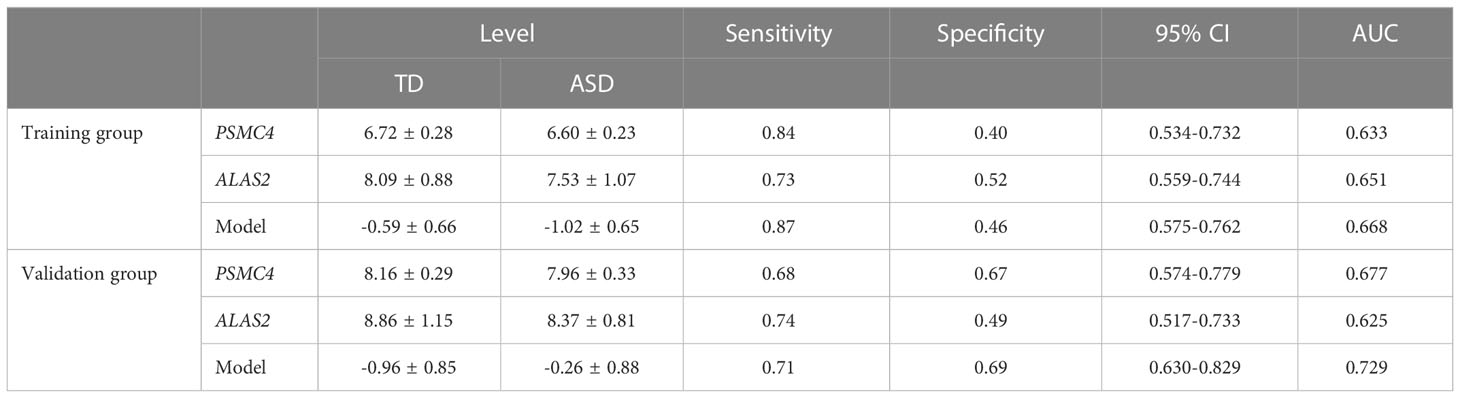
Table 3 Receiver operative characteristic curves of PSMC4, ALAS2, and Model (PSMC4+ALAS2) in the training and validation group.
To further estimate the sensitivity of using these hub genes as indicators for ASD severity, we extracted the Autism Diagnostic Observation Schedule Social Affect (ADOS-SA) deficit scores that were positively correlated with ASD severity from the GSE111175 dataset. The children with ASD were divided into the following three groups according to severity: mild (5 to 11), medium (12 to 15), and high (16 to 21). The heatmap showed the expression of 15 hub genes in the three groups (Figure 7C), and we used scatter plots to show gene expression patterns among the three groups to make it more intuitively understandable. In particular, LIlRB1 could distinguish between high and mild severity ASD (p < 0.05), and CD69 had relatively lower expression in the medium severity group compared to the mild and high severity groups (p < 0.05) (Figure 7D). These results indicate that LIlRB1 and CD69 can be used as indicators for ASD severity in children.
To investigate the correlations between hub genes and immune cell profiles in children with ASD, we performed Spearman correlation analysis using the “Batch” dataset. First, we explored the correlation between immune cells in children with ASD and TD, and we found that monocytes were positively associated with M0/M1 macrophages but negatively related to M2 macrophages in children with ASD, whereas M0 macrophages were negatively related to M2 macrophages in TD children (Figure 8A). Second, the correlation between 15 hub genes and 4 significantly differentially expressed immune cells (Figure 8B) showed that activated dendritic cells were positively correlated with AHSP, LILRB1, and CD69 (r > 0.20, p < 0.05) but negatively correlated with PSMC4 (r < –0.20, p < 0.05). In addition, M2 macrophages showed a positive correlation with AP2A1, MAP1LC3A, EPB42, BCL2L1, GMPR, SLC4A1, and AHSP (r > 0.20, p < 0.05) and a negative correlation with CD3G (r < –0.20, p < 0.05) in children with ASD (Figure 8C). Lastly, the graphs of the linear correlation indicated that monocytes were significantly and positively related to LIlRB1 (r > 0.40, p < 0.001) (Figure 8D).
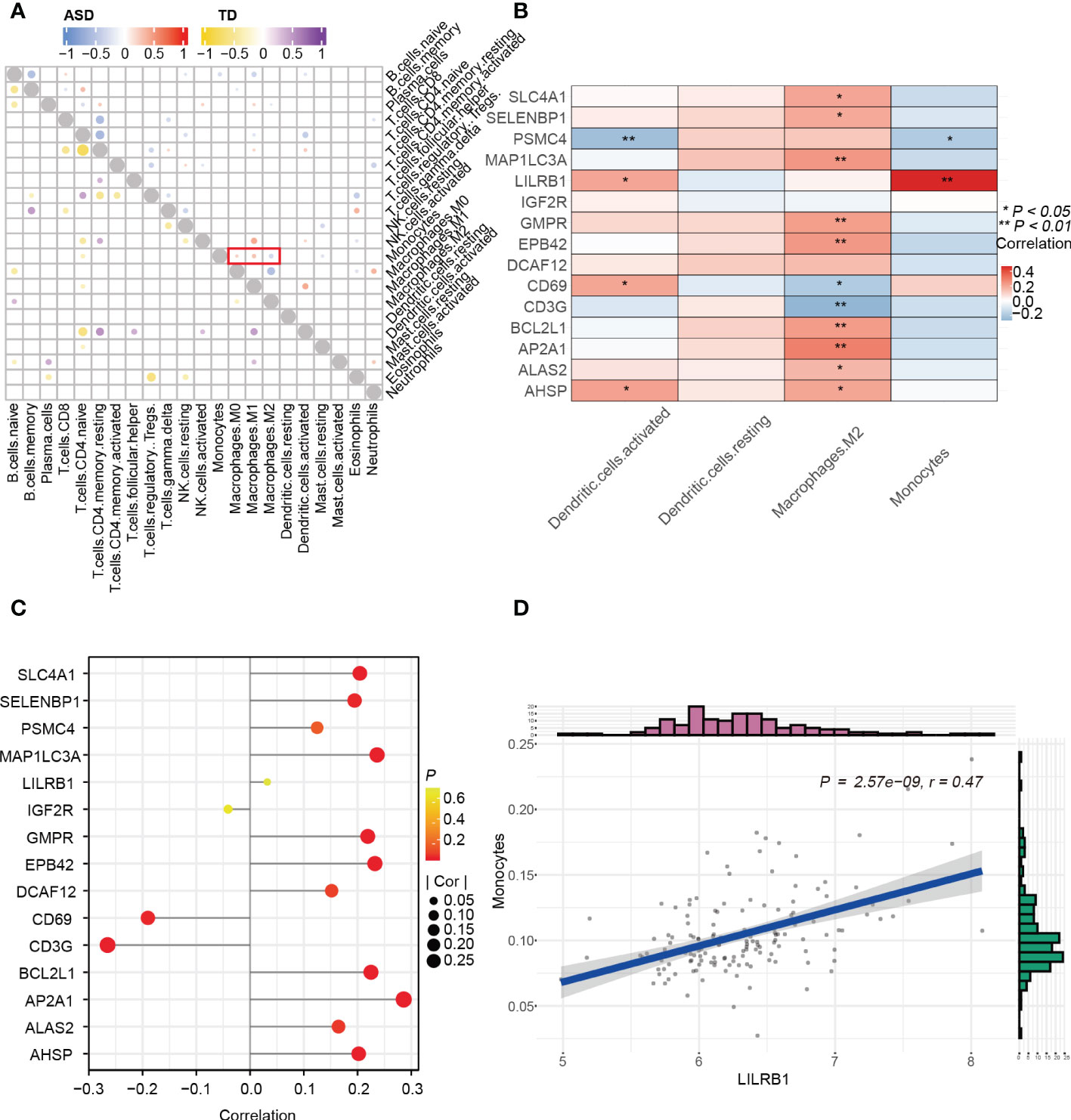
Figure 8 Correlation between immune cells and genes. (A) Correlation between 15 hub genes and 4 significantly differential immune cells. (B) Lollipop diagram of the correlation between 15 hub genes and M2 Macrophages with |r| > 0.20, p < 0.05. (C) Correlation among immune cells in the child ASD and TD groups. (D) Scatter plot of significantly related LIlRB1 and monocytes with r > 0.40 and p < 0.001. *p < 0.05; **p < 0.01.
To gain further insight into the regulatory mechanisms and to identify potential agents affecting ASD in children, we next tried to identify the miRNAs and agents that influence identified 15 hub genes.
A total of 118 candidate miRNAs were predicted to target the 7 identified hub genes. There were 10 miRNAs targeting AP2A1, 18 miRNAs targeting BCL2L1, 33 miRNAs targeting CD69, 37 miRNAs targeting DCAF2, 5 miRNAs targeting GMPR, 33 miRNAs targeting IGF2R, and 2 miRNAs targeting LIlRB1 (Figure 9A). We then summarized those miRNAs that regulated more than one hub gene in Table 4. Of these, hsa-miR-342-3p and hsa-miR-1321 interacted most intensively with robust hub genes (target hub genes = 3).
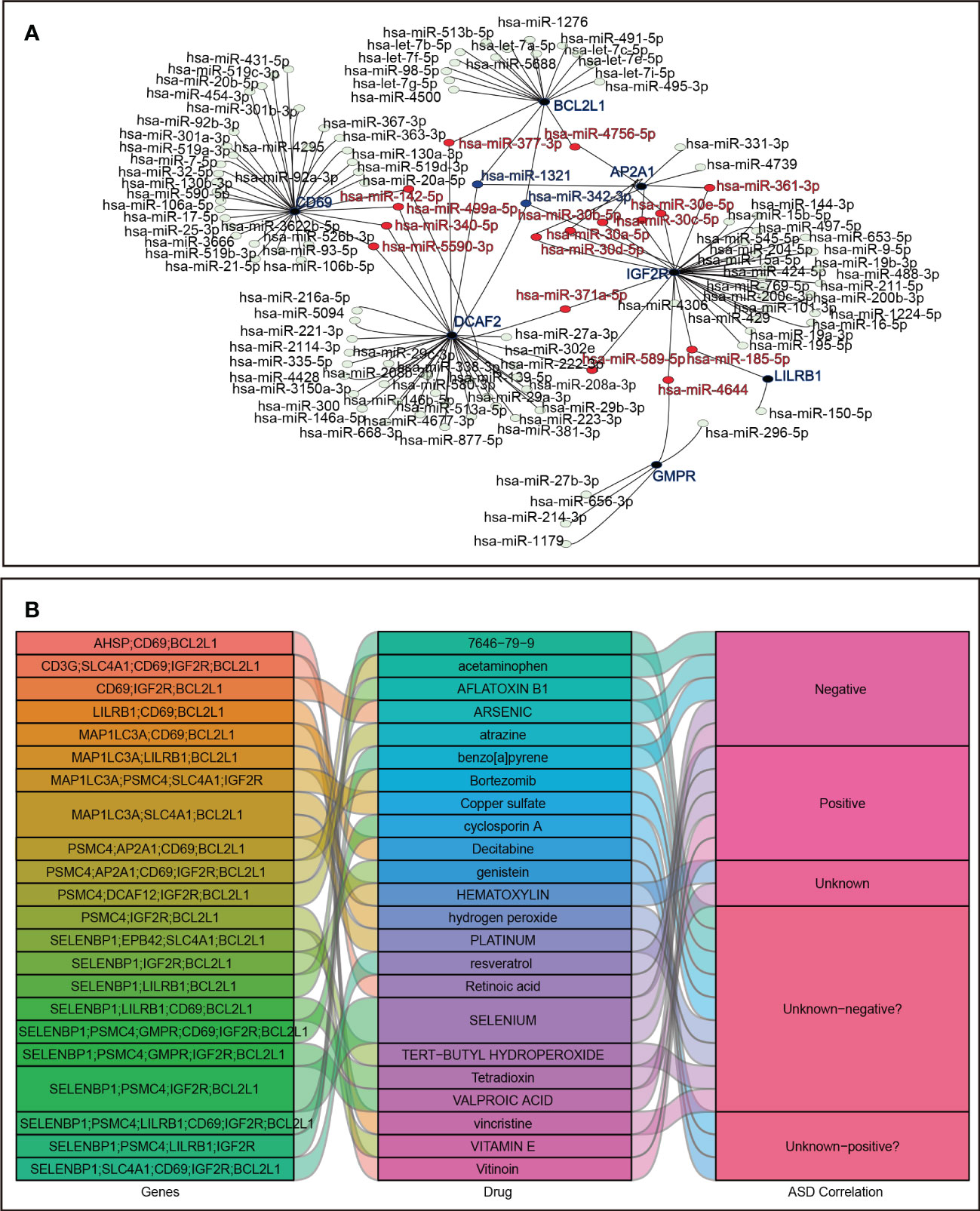
Figure 9 Prediction in miRNA-mRNA and agents-genes. (A) miRNA-mRNA network. (B) Sankey diagram for agents–hub genes.
In addition, using the DSigDB database, the 23 agents affecting ASD were shown in a Sankey diagram with agents–hub genes ≥ 3, and the agents-disease associations were also displayed, including positive, negative, and unknown correlations with ASD status (Figure 9B).
This study identified seven ASD-related hub genes (PSMC4, CD3G, IGF2R, DCAF12, SELENBP1, ALAS2, SLC4A1) and found that PSMC4 and ALAS2 had good diagnostic efficacy. In addition, LIlRB1 and CD69 had the ability to diagnose mild, medium, and high severity ASD. Among these, CD3G encodes the CD3-gamma polypeptide, which plays an important role in autoimmunity. It has been reported that CD3γ-deficient patients present with Treg phenotypic and functional defects (44); however, no study has been reported on the relationship between CD3G and ASD. Here, we found that the expression of CD3G was lower in children with ASD, which shows that there were Treg cell abnormalities in these children (45). Both Treg cells and M2 macrophages can suppress overly aggressive immune responses, and thus loss of Treg cell function may require more M2 macrophages to restore immunosuppressive function, which could be the reason that CD3G had a negative correlation with M2 macrophages.
LILRB1 and CD69 are essential genes in immune regulation through T cells and play roles in the development of autoimmune responses (46–49). The most intriguing result in our study was that LILRB1 was tightly related to ASD. LILRB1 is expressed mainly in myeloid lineage cells like monocytes and dendritic cells, and because LILRB1 expression increases with progressive ASD severity the circulating monocyte numbers increase progressively with advancing disease, which is similar to previous reports (50). These results suggest that LIlRB1 plays a crucial role in ASD processes, and in-depth explorations will provide us with a better understanding of the mechanisms of autism. CD69, the early T cell activation marker, can promote the proliferation and activation of T cells, and dendritic cells act as professional antigen-presenting cells to initiate T cell-mediated immune responses, which could explain why CD69 was positively related with activated dendritic cells in our study.
SELENBP1, as its name suggests, combines with selenium and participates in various intracellular selenium transport mechanisms (51). Hence, deficiency of SELENBP1 causes deficiency of selenium, leading to certain neurologic diseases such as recent-onset schizophrenia (52) and autism (53, 54). Consistent with other studies, we also observed that SELENBP1 was lower in ASD children than in TD children, which indicates that low selenium levels is a risk indicator for autism. This finding provides further evidence for the addition of selenium supplements to ASD treatments.
IGF2R, the receptor that binds to insulin-like growth factor 2 (IGF2), is important for fetal growth and development (55). IGF2 via IGF2R reverses the abnormal activity of the AMPK-mTOR-S6K pathway and rescues active translation at synapses in autism-like phenotypes in mice (56), and bidirectional regulation of IGF2 in autoimmune diseases has been suggested to be related to the differential activation of IGF1R and IGF2R, which regulates both the anti- and pro-inflammatory effects of macrophages (57). Thus IGF2R plays a key role in neuronal development, and its increased expression in cases of ASD implies that there are different degrees and types of activation of macrophages in ASD. As we expected, M0 macrophages levels were increased in ASD patients, which indicates that there are abnormalities in immunologic homeostasis. In addition, the decreased numbers of M2 macrophages and increased numbers of monocytes suggest that children with ASD are in a relatively pro-inflammatory state.
DCAF12 and PSMC4 are both involved in the degradation of ubiquitinated proteins (58, 59), but there have been no studies showing a relation between these two genes and autism. Here we observed that DCAF12 and PSMC4 in the ASD group had reduced expression compared to TD group. This suggests that there might be a problem with the degradation of ubiquitinated proteins in children with autism. Therefore, we further explored whether there might be another pathway for protein degradation, such as autophagy. Interestingly, MAP1LC3A, also known as microtubule-associated protein light chain 3 (LC3), is lipidated to form LC3-II and is generally considered to be a good indicator of macroautophagy (60). We found that MAP1LC3A was a DEG in both the training and validation groups. Although the trends were opposite in these two groups, we cannot exclude that autophagy takes part in the development of autism. In addition to macroautophagy’s role in protein degradation, local protein is also cleared by dendritic cells. We found that PSMC4 was negatively correlated with activated dendritic cells, which suggests that more activated dendritic cells might prevent the accumulation of proteins due to the reduced degradation caused by decreased PSMC4.
In the DEG analysis, erythrocyte differentiation, erythrocyte-related disorders, and heme metabolism were enriched in children with ASD, and our clinical data from routine blood tests validated the significant changes in erythrocyte-related parameters (RBC, HCT, MCH, RDW-CV, and MCHC) in ASD patients. Erythrocyte parameters can reflect iron status (61), and thus the results presented above imply that abnormalities in iron metabolism affect the course of ASD, as reported in previous studies (62, 63). ALAS2 (64, 65), SLC4A1 (66), AHSP (67), EPB42 (68), and GMPR (69) were identified as ASD hub genes, and these are associated with erythrocytes, especially with regard to changes in hemoglobin, which are closely related to iron metabolism. A review article summarized the evidence showing that iron content regulates macrophage polarization (70). Our study found that SLC4A1 had a positive correlation with M2 macrophages, which suggests that decreased SLC4A1 affects erythrocyte formation and may cause an imbalance in heme-iron metabolism in macrophages and may further result in a decrease in the production of anti-inflammatory M2 macrophages. In summary, our data provide genetic and cellular evidence supporting the correlation between iron metabolism abnormalities and ASD, and further studies are needed to validate these findings. Compared to children, hormone levels and early/late estrogen response were enriched in adults with ASD. Although recent research reported that prenatal estrogens contribute to the risk of autism (71), there is no study on the link between adults with ASD and estrogen. A previous study showed that estrogen is indispensable for immune robustness and for neural functions (72), so abnormal activation of estrogen may contribute to immune and neurological abnormalities in adults with ASD.
In the GSEA analysis in adults with ASD, the MYC targets v1, E2F targets, protein secretion, heme metabolism, and KRAS signaling DN showed opposite trends in inhibition or activation compared to children with ASD. These age-related differences in ASD may indicate distinct disease characteristics between children and adults, and this suggests the need for different approaches to raising awareness and adjusting treatment for ASD patients in different age groups.
In terms of the immune cell landscape, decreased activated NK cells and increased resting NK cells suggest that reduced NK cell number and activity may result in immune disorders in adults with ASD. In children with ASD, we postulate that ncMos are abnormal. A recent article showed that ncMos regulate autoimmune and inflammatory diseases (73) and can directly regulate the adaptive immune response by regulating the activity of specific subpopulations of other immune cells. Hence, the significantly elevated ncMo levels indicate that ASD is a class of immune-mediated disorders, especially disorders in the adaptive immune system. Also, identifying ncMos that exhibit specific pathogenic roles is important for the development of targeted autism therapies.
We predicted that BCL2L1, DCAF2, and IGF2R are the target genes of miR-342-3p and that BCL2L1, DCAF2, and AP2A1 are the target genes of miR-1321, which suggested miRNAs’ therapeutic value in treating ASD. Previous studies showed that miR-342-3p controls macrophage survival by targeting BCL2L1 (74) and that miR-342 is up-regulated in lymphoblastoid cell lines in autism cases (75). In addition, hsa-miR-1321 has been suggested to play a role in the development of cancer (76, 77). However, there are no data reporting these miRNA-gene regulatory axes in peripheral blood samples from children with ASD. Because the identified hub genes are tightly linked to the initiation and progression of ASD, the targeted therapies using miR-342-3p and miR-1321 might be a promising novel treatment modality for autism.
We further found identified agents-gene axes using the DSigDB database. The existing studies have shown different types of agents affecting ASD, including those that improve ASD (resveratrol, vitinoin, vitamin E, and retinoic acid) (78–82) and those that exacerbate ASD (valproic acid, arsenic, benzo[a]pyrene, and acetaminophen) (83–86). Moreover, there are ASD-related agents with unknown risks, including reagents with neurotoxicity and immunotoxicity (platinum, bortezomib, tert-butyl hydroperoxide, vincristine, atrazine, 7646-79-9, hydrogen peroxide, aflatoxin B1, and copper sulfate) (87–95) or agents providing neuroprotection and immune regulation (genistein, cyclosporin A, and decitabine) (96–98) and agents that require exploration in terms of immune and neurological function (hematoxylin and tetradioxin). Interestingly, selenium has a bidirectional modulatory effect on autism, and supplementation with selenium can improve autism-like behaviors in animal models (99), while prenatal exposure to high levels of selenium may affect childhood neurodevelopment and induce ASD (100). Overall, these agents play distinct roles in the ASD process, which suggests that agents should be tailored to the biological functions of the hub genes to be targeted.
There are some limitations in our study. First, the datasets were from different GEO datasets, and there was not enough corresponding clinical information for assessment and prediction. Second, we focused on ASD in children due to the lack of more readily available adult datasets and samples, and this hindered us from exploring difference between children and adults in a comprehensive and objective manner. Third, we analyzed ASD in children and adults as groups with no details regarding specific ages, which may mask the greater and/or earlier age-related changes. Fourth, we used bioinformatics methods to screen hub genes and miRNA, and experimental verification of such interactions is lacking. Fifth, the clinical sample size was small, which may not support rigorous statistical analysis.
In summary, our study identifies four potential blood predictors (PSMC4, ALAS2, LIlRB1, and CD69), four dysregulated immune cell types (monocytes, M2 macrophages, and resting and activated dendritic cells), two miRNAs (miR-342-3p and miR-1321) and 23 potential agents affecting ASD. The gene expression profiles are age-related in ASD, and we show for the first time that ncMos are upregulated in children with ASD. This study enriches our knowledge of the molecular mechanisms of ASD and promotes the development of early diagnosis and treatment strategies for childhood ASD.
Publicly available datasets were analyzed in this study. This data can be found here: GEO database (http://www.ncbi.nlm.nih.gov/geo) GSE6575, GSE18123, GSE42133, GSE111175, GSE26415, and GSE89594.
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of the 3rd Affiliated Hospital of Zhengzhou University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
HL analyzed the data and wrote the manuscript. YX designed the study and analyzed the data. WL provided the clinical data of ASD patients. LZ conducted the validation experiments. XZ, BL, YC, and XW ensured the accuracy and integrity of the results. CZ devised and supervised the study. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (U21A20347 and 82203969), the Henan Key Laboratory of Population Defects Prevention (ZD202103), the Department of Science and Technology of Henan Province, China (212102310221, 222102310161), the Henan Province Medical Research Project (LHGJ20190349), the Swedish Research Council (2018–02267), Swedish Governmental grants to scientists working in health care (ALFGBG-965197), the Brain Foundation (FO2022-0120), the Adlerbert Research Foundation (2021–496, 2022–582), and Stiftelsen Edit Jacobsons Donationsfond (2021–102).
The study benefited from GEO database, which provides large numbers of datasets. We also thank all participants for making a significant contribution to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1082950/full#supplementary-material
1. Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: classification, diagnosis and therapy. Pharmacol Ther (2018) 190:91–104. doi: 10.1016/j.pharmthera.2018.05.007
2. McDonnell CG, Boan AD, Bradley CC, Seay KD, Charles JM, Carpenter LA. Child maltreatment in autism spectrum disorder and intellectual disability: Results from a population-based sample. J Child Psychol Psychiatry (2019) 60(5):576–84. doi: 10.1111/jcpp.12993
3. Hughes HK, Mills Ko E, Rose D, Ashwood P. Immune dysfunction and autoimmunity as pathological mechanisms in autism spectrum disorders. Front Cell Neurosci (2018) 12:405. doi: 10.3389/fncel.2018.00405
4. Li Q, Han Y, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci (2017) 120:120. doi: 10.3389/fncel.2017.00120
5. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet (2018) 392(10146):508–20. doi: 10.1016/S0140-6736(18)31129-2
6. Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis primers (2020) 6(1):1–23. doi: 10.1038/s41572-019-0138-4
7. Manoli DS, State MW. Autism spectrum disorder genetics and the search for pathological mechanisms. Am J Psychiatry (2021) 178(1):30–8. doi: 10.1176/appi.ajp.2020.20111608
8. Gyawali S, Patra BN. Autism spectrum disorder: Trends in research exploring etiopathogenesis. Psychiatry Clin Neurosci (2019) 73(8):466–75. doi: 10.1111/pcn.12860
9. Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology (2017) 42(1):284–98. doi: 10.1038/npp.2016.158
10. Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci (2015) 16(8):469–86. doi: 10.1038/nrn3978
11. Abruzzo P, Matté A, Bolotta A, Federti E, Ghezzo A, Guarnieri T, et al. Plasma peroxiredoxin changes and inflammatory cytokines support the involvement of neuro-inflammation and oxidative stress in autism spectrum disorder. J Trans Med (2019) 17(1):1–12. doi: 10.1186/s12967-019-2076-z
12. Shmarina GV, Ershova ES, Simashkova NV, Nikitina SG, Chudakova JM, Veiko NN, et al. Oxidized cell-free DNA as a stress-signaling factor activating the chronic inflammatory process in patients with autism spectrum disorders. J Neuroinflam (2020) 17(1):1–13. doi: 10.1186/s12974-020-01881-7
13. Jyonouchi H, Geng L, Davidow AL. Cytokine profiles by peripheral blood monocytes are associated with changes in behavioral symptoms following immune insults in a subset of ASD subjects: an inflammatory subtype? J Neuroinflamm (2014) 11(1):1–13. doi: 10.1186/s12974-014-0187-2
14. Bennabi M, Tarantino N, Gaman A, Scheid I, Krishnamoorthy R, Debré P, et al. Persistence of dysfunctional natural killer cells in adults with high-functioning autism spectrum disorders: stigma/consequence of unresolved early infectious events? Mol Autism (2019) 10(1):1–13. doi: 10.1186/s13229-019-0269-1
15. Breece E, Paciotti B, Nordahl CW, Ozonoff S, Van de Water JA, Rogers SJ, et al. Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Brain Behavior Immun (2013) 31:69–75. doi: 10.1016/j.bbi.2012.10.006
16. Xu Z-X, Kim GH, Tan J-W, Riso AE, Sun Y, Xu EY, et al. Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat Commun (2020) 11(1):1–17. doi: 10.1038/s41467-020-15530-3
17. Theoharides TC, Kavalioti M, Tsilioni I. Mast cells, stress, fear and autism spectrum disorder. Int J Mol Sci (2019) 20(15):3611. doi: 10.3390/ijms20153611
18. De Giacomo A, Gargano CD, Simone M, Petruzzelli MG, Pedaci C, Giambersio D, et al. B and T immunoregulation: A new insight of b regulatory lymphocytes in autism spectrum disorder. Front Neurosci (2021) 15:732611. doi: 10.3389/fnins.2021.732611
19. Gao H, Zhong J, Huang Q, Wu X, Mo X, Lu L, et al. Integrated systems analysis explores dysfunctional molecular modules and regulatory factors in children with autism spectrum disorder. J Mol Neurosci (2021) 71(2):358–68. doi: 10.1007/s12031-020-01658-w
20. Salloum-Asfar S, Satheesh NJ, Abdulla SA. Circulating miRNAs, small but promising biomarkers for autism spectrum disorder. Front Mol Neurosci (2019) 12:253. doi: 10.3389/fnmol.2019.00253
21. Atwan H, Assarehzadegan M-A, Shekarabi M, Jazayeri SM, Barfi S, Shoormasti RS, et al. Assessment of miR-181b-5p, miR-23a-3p, BCL-2, and IL-6 in peripheral blood mononuclear cells of autistic patients; likelihood of reliable biomarkers. Iranian J Allergy Asthma Immunol (2020) 19(1):74–83. doi: 10.18502/ijaai.v19i1.2420
22. Jyonouchi H, Geng L, Streck DL, Dermody JJ, Toruner GA. MicroRNA expression changes in association with changes in interleukin-1ss/interleukin10 ratios produced by monocytes in autism spectrum disorders: their association with neuropsychiatric symptoms and comorbid conditions (observational study). J Neuroinflamm (2017) 14(1):1–14. doi: 10.1186/s12974-017-1003-6
23. Vaccaro T, Sorrentino JM, Salvador S, Veit T, Souza DO, De Almeida RF. Alterations in the microRNA of the blood of autism spectrum disorder patients: effects on epigenetic regulation and potential biomarkers. Behav Sci (2018) 8(8):75. doi: 10.3390/bs8080075
24. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov (2017) 16(3):203–22. doi: 10.1038/nrd.2016.246
25. Gregg JP, Lit L, Baron CA, Hertz-Picciotto I, Walker W, Davis RA, et al. Gene expression changes in children with autism. Genomics (2008) 91(1):22–9. doi: 10.1016/j.ygeno.2007.09.003
26. Kong SW, Collins CD, Shimizu-Motohashi Y, Holm IA, Campbell MG, Lee I-H, et al. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PloS One (2012) 7(12):e49475. doi: 10.1371/journal.pone.0049475
27. Pramparo T, Pierce K, Lombardo MV, Barnes CC, Marinero S, Ahrens-Barbeau C, et al. Prediction of autism by translation and immune/inflammation coexpressed genes in toddlers from pediatric community practices. JAMA Psychiatry (2015) 72(4):386–94. doi: 10.1001/jamapsychiatry.2014.3008
28. Gazestani VH, Pramparo T, Nalabolu S, Kellman BP, Murray S, Lopez L, et al. A perturbed gene network containing PI3K–AKT, RAS–ERK and WNT–β-catenin pathways in leukocytes is linked to ASD genetics and symptom severity. Nat Neurosci (2019) 22(10):1624–34. doi: 10.1038/s41593-019-0489-x
29. Kuwano Y, Kamio Y, Kawai T, Katsuura S, Inada N, Takaki A, et al. Autism-associated gene expression in peripheral leucocytes commonly observed between subjects with autism and healthy women having autistic children. PloS One (2011) 6(9):e24723. doi: 10.1371/journal.pone.0024723
30. Kimura R, Swarup V, Tomiwa K, Gandal MJ, Parikshak NN, Funabiki Y, et al. Integrative network analysis reveals biological pathways associated with williams syndrome. J Child Psychol Psychiatry (2019) 60(5):585–98. doi: 10.1111/jcpp.12999
31. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res (2015) 43(7):e47–e. doi: 10.1093/nar/gkv007
32. Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an r package for comparing biological themes among gene clusters. Omics: J Integr Biol (2012) 16(5):284–7. doi: 10.1089/omi.2011.0118
33. Franz M, Rodriguez H, Lopes C, Zuberi K, Montojo J, Bader GD, et al. GeneMANIA update 2018. Nucleic Acids Res (2018) 46(W1):W60–4. doi: 10.1093/nar/gky311
34. Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun (2019) 10(1):1–10. doi: 10.1038/s41467-019-09234-6
35. Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database hallmark gene set collection. Cell systems (2015) 1(6):417–25. doi: 10.1016/j.cels.2015.12.004
36. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods (2015) 12(5):453–7. doi: 10.1038/nmeth.3337
37. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res (2015) 43(D1):D447–52. doi: 10.1093/nar/gku1003
38. Li J-H, Liu S, Zhou H, Qu L-H, Yang J-H. starBase v2. 0: decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-seq data. Nucleic Acids Res (2014) 42(D1):D92–D7. doi: 10.1093/nar/gkt1248
39. Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res (2020) 48(D1):D127–31. doi: 10.1093/nar/gkz757
40. Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics (2012) 28(6):882–3. doi: 10.1093/bioinformatics/bts034
41. Kolde R, Laur S, Adler P, Vilo J. Robust rank aggregation for gene list integration and meta-analysis. Bioinformatics (2012) 28(4):573–80. doi: 10.1093/bioinformatics/btr709
42. Nadeem A, Ahmad SF, Attia SM, Bakheet SA, Al-Harbi NO, Al-Ayadhi LY. Activation of IL-17 receptor leads to increased oxidative inflammation in peripheral monocytes of autistic children. Brain Behavior Immun (2018) 67:335–44. doi: 10.1016/j.bbi.2017.09.010
43. Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin practice (2006) 12(2):132–9. doi: 10.1111/j.1365-2753.2005.00598.x
44. Rowe JH, Delmonte OM, Keles S, Stadinski BD, Dobbs AK, Henderson LA, et al. Patients with CD3G mutations reveal a role for human CD3γ in treg diversity and suppressive function. Blood J Am Soc Hematol (2018) 131(21):2335–44. doi: 10.1182/blood-2018-02-835561
45. Mostafa GA, Al Shehab A, Fouad NR. Frequency of CD4+ CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. J Child neurol (2010) 25(3):328–35. doi: 10.1177/0883073809339393
46. Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells (1994) 12(5):456–65. doi: 10.1002/stem.5530120502
47. Gorabi AM, Hajighasemi S, Kiaie N, Hayat SMG, Jamialahmadi T, Johnston TP, et al. The pivotal role of CD69 in autoimmunity. J Autoimmun (2020) 111:102453. doi: 10.1016/j.jaut.2020.102453
48. Iglesias M, Augustin JJ, Alvarez P, Santiuste I, Postigo J, Merino J, et al. Selective impairment of TH17-differentiation and protection against autoimmune arthritis after overexpression of BCL2A1 in t lymphocytes. PloS One (2016) 11(7):e0159714. doi: 10.1371/journal.pone.0159714
49. Gustafson CE, Qi Q, Hutter-Saunders J, Gupta S, Jadhav R, Newell E, et al. Immune checkpoint function of CD85j in CD8 T cell differentiation and aging. Front Immunol (2017) 8:692. doi: 10.3389/fimmu.2017.00692
50. Chen H-R, Chen C-W, Mandhani N, Short-Miller JC, Smucker MR, Sun Y-Y, et al. Monocytic infiltrates contribute to autistic-like behaviors in a two-hit model of neurodevelopmental defects. J Neurosci (2020) 40(49):9386–400. doi: 10.1523/JNEUROSCI.1171-20.2020
51. Kanazawa T, Chana G, Glatt SJ, Mizuno H, Masliah E, Yoneda H, et al. The utility of SELENBP1 gene expression as a biomarker for major psychotic disorders: replication in schizophrenia and extension to bipolar disorder with psychosis. Am J Med Genet Part B: Neuropsychiatr Genet (2008) 147(6):686–9. doi: 10.1002/ajmg.b.30664
52. Chau EJ, Mostaid MS, Cropley V, McGorry P, Pantelis C, Bousman CA, et al. Downregulation of plasma SELENBP1 protein in patients with recent-onset schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry (2018) 85:1–6. doi: 10.1016/j.pnpbp.2018.03.010
53. Mehta SQ, Behl S, Day PL, Delgado AM, Larson NB, Stromback LR, et al. Evaluation of zn, Cu, and Se levels in the north American autism spectrum disorder population. Front Mol Neurosci (2021) 14:665686. doi: 10.3389/fnmol.2021.665686
54. Tschinkel PFS, Bjørklund G, Conón LZZ, Chirumbolo S, Nascimento VA. Plasma concentrations of the trace elements copper, zinc and selenium in Brazilian children with autism spectrum disorder. Biomed Pharmacother (2018) 106:605–9. doi: 10.1016/j.biopha.2018.06.174
55. Brown J, Jones EY, Forbes BE. Keeping IGF-II under control: Lessons from the IGF-II–IGF2R crystal structure. Trends Biochem Sci (2009) 34(12):612–9. doi: 10.1016/j.tibs.2009.07.003
56. Steinmetz AB, Stern SA, Kohtz AS, Descalzi G, Alberini CM. Insulin-like growth factor II targets the mTOR pathway to reverse autism-like phenotypes in mice. J Neurosci (2018) 38(4):1015–29. doi: 10.1523/JNEUROSCI.2010-17.2017
57. Wang X, Lin L, Lan B, Wang Y, Du L, Chen X, et al. IGF2R-initiated proton rechanneling dictates an anti-inflammatory property in macrophages. Sci Adv (2020) 6(48):eabb7389. doi: 10.1126/sciadv.abb7389
58. Lidak T, Baloghova N, Korinek V, Sedlacek R, Balounova J, Kasparek P, et al. CRL4-DCAF12 ubiquitin ligase controls MOV10 RNA helicase during spermatogenesis and T cell activation. Int J Mol Sci (2021) 22(10):5394. doi: 10.3390/ijms22105394
59. Zavodszky E, Peak-Chew S-Y, Juszkiewicz S, Narvaez AJ, Hegde RS. Identification of a quality-control factor that monitors failures during proteasome assembly. Science (2021) 373(6558):998–1004. doi: 10.1126/science.abc6500
60. Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, et al. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature (2009) 461(7264):654–8. doi: 10.1038/nature08455
61. Torsvik IK, Markestad T, Ueland PM, Nilsen RM, Midttun Ø, Bjørke Monsen A-L. Evaluating iron status and the risk of anemia in young infants using erythrocyte parameters. Pediatr Res (2013) 73(2):214–20. doi: 10.1038/pr.2012.162
62. Gunes S, Ekinci O, Celik T. Iron deficiency parameters in autism spectrum disorder: clinical correlates and associated factors. Ital J pediatrics (2017) 43(1):1–6. doi: 10.1186/s13052-017-0407-3
63. De Giacomo A, Medicamento S, Pedaci C, Giambersio D, Giannico OV, Petruzzelli MG, et al. Peripheral iron levels in autism spectrum disorders vs. other neurodevelopmental disorders: Preliminary data. Int J Environ Res Public Health (2022) 19(7):4006. doi: 10.3390/ijerph19074006
64. Harigae H, Nakajima O, Suwabe N, Yokoyama H, Furuyama K, Sasaki T, et al. Aberrant iron accumulation and oxidized status of erythroid-specific δ-aminolevulinate synthase (ALAS2)–deficient definitive erythroblasts. Blood J Am Soc Hematol (2003) 101(3):1188–93. doi: 10.1182/blood-2002-01-0309
65. Li Y, Liu S, Sun H, Yang Y, Qi H, Ding N, et al. Mir-218 inhibits erythroid differentiation and alters iron metabolism by targeting alas2 in k562 cells. Int J Mol Sci (2015) 16(12):28156–68. doi: 10.3390/ijms161226088
66. Gallagher PG. Disorders of red cell volume regulation. Curr Opin hematol (2013) 20(3):201–7. doi: 10.1097/MOH.0b013e32835f6870
67. Dickson CF, Rich AM, D'Avigdor WM, Collins DA, Lowry JA, Mollan TL, et al. α-hemoglobin-stabilizing protein (AHSP) perturbs the proximal heme pocket of oxy-α-hemoglobin and weakens the iron-oxygen bond. J Biol Chem (2013) 288(27):19986–20001. doi: 10.1074/jbc.M112.437509
68. Theodosia A Kalfa AHB, Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, et al. EPB42-related hereditary spherocytosis. Seattle: Seattle (WA: University of Washington (2014). Anne Amemiya.
69. Van Hout CV, Tachmazidou I, Backman JD, Hoffman JD, Liu D, Pandey AK, et al. Exome sequencing and characterization of 49,960 individuals in the UK biobank. Nature (2020) 586(7831):749–56. doi: 10.1038/s41586-020-2853-0
70. Mu Q, Chen L, Gao X, Shen S, Sheng W, Min J, et al. The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci Bulletin (2021) 66(17):1806–16. doi: 10.1016/j.scib.2021.02.010
71. Baron-Cohen S, Tsompanidis A, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah M, et al. Foetal oestrogens and autism. Mol Psychiatry (2020) 25(11):2970–8. doi: 10.1038/s41380-019-0454-9
72. Patel S, Homaei A, Raju AB, Meher BR. Estrogen: the necessary evil for human health, and ways to tame it. Biomed Pharmacother (2018) 102:403–11. doi: 10.1016/j.biopha.2018.03.078
73. Narasimhan PB, Marcovecchio P, Hamers AA, Hedrick CC. Nonclassical monocytes in health and disease. Annu Rev Immunol (2019) 37:439–56. doi: 10.1146/annurev-immunol-042617-053119
74. Czimmerer Z, Varga T, Kiss M, Vázquez CO, Doan-Xuan QM, Rückerl D, et al. The IL-4/STAT6 signaling axis establishes a conserved microRNA signature in human and mouse macrophages regulating cell survival via miR-342-3p. Genome Med (2016) 8(1):1–22. doi: 10.1186/s13073-016-0315-y
75. Sarachana T, Zhou R, Chen G, Manji HK, Hu VW. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med (2010) 2(4):1–18. doi: 10.1186/gm144
76. Luo M, Zhang L, Yang H, Luo K, Qing C. Long non−coding RNA NEAT1 promotes ovarian cancer cell invasion and migration by interacting with miR−1321 and regulating tight junction protein 3 expression. Mol Med Rep (2020) 22(4):3429–39. doi: 10.3892/mmr.2020.11428
77. Chakraborty S, Nath D. A study on microRNAs targeting the genes overexpressed in lung cancer and their codon usage patterns. Mol Biotechnol (2022) 64(10):1095–1119. doi: 10.1007/s12033-022-00491-3
78. Malaguarnera M, Khan H, Cauli O. Resveratrol in autism spectrum disorders: Behavioral and molecular effects. Antioxidants (2020) 9(3):188. doi: 10.3390/antiox9030188
79. Santos-Terra J, Deckmann I, Carello-Collar G, Nunes GD-F, Bauer-Negrini G, Schwingel GB, et al. Resveratrol prevents cytoarchitectural and interneuronal alterations in the valproic acid rat model of autism. Int J Mol Sci (2022) 23(8):4075. doi: 10.3390/ijms23084075
80. Riebold M, Mankuta D, Lerer E, Israel S, Zhong S, Nemanov L, et al. All-trans retinoic acid upregulates reduced CD38 transcription in lymphoblastoid cell lines from autism spectrum disorder. Mol Med (2011) 17(7):799–806. doi: 10.2119/molmed.2011.00080
81. Pangrazzi L, Balasco L, Bozzi Y. Natural antioxidants: a novel therapeutic approach to autism spectrum disorders? Antioxidants (2020) 9(12):1186. doi: 10.3390/antiox9121186
82. Yang L, Xia Z, Feng J, Zhang M, Miao P, Nie Y, et al. Retinoic acid supplementation rescues the social deficits in Fmr1 knockout mice. Front Genet (2022) 13:928393. doi: 10.3389/fgene.2022.928393
83. Taleb A, Lin W, Xu X, Zhang G, Zhou Q-G, Naveed M, et al. Emerging mechanisms of valproic acid-induced neurotoxic events in autism and its implications for pharmacological treatment. Biomed Pharmacother (2021) 137:111322. doi: 10.1016/j.biopha.2021.111322
84. Skogheim TS, Weyde KVF, Engel SM, Aase H, Surén P, Øie MG, et al. Metal and essential element concentrations during pregnancy and associations with autism spectrum disorder and attention-deficit/hyperactivity disorder in children. Environ Int (2021) 152:106468. doi: 10.1016/j.envint.2021.106468
85. Santos JX, Rasga C, Marques AR, Martiniano H, Asif M, Vilela J, et al. A role for gene-environment interactions in autism spectrum disorder is supported by variants in genes regulating the effects of exposure to xenobiotics. Front Neurosci (2022) 16:862315. doi: 10.3389/fnins.2022.862315
86. Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, et al. Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry (2020) 77(2):180–9. doi: 10.1001/jamapsychiatry.2019.3259
87. Fumagalli G, Monza L, Cavaletti G, Rigolio R, Meregalli C. Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Front Immunol (2021) 11:626687. doi: 10.3389/fimmu.2020.626687
88. Yan W, Wu Z, Zhang Y, Hong D, Dong X, Liu L, et al. The molecular and cellular insight into the toxicology of bortezomib-induced peripheral neuropathy. Biomed Pharmacother (2021) 142:112068. doi: 10.1016/j.biopha.2021.112068
89. Adams JD, Klaidman LK, Huang Y-M, Cheng JJ, Wang Z-J, Nguyen M, et al. The neuropathology of intracerebroventriculart-butylhydroperoxide. Mol Chem neuropathol (1994) 22(2):123–42. doi: 10.1007/BF03160100
90. Khan TM, Benaich N, Malone CF, Bernardos RL, Russell AR, Downes GB, et al. Vincristine and bortezomib cause axon outgrowth and behavioral defects in larval zebrafish. J Peripheral Nervous System (2012) 17(1):76–89. doi: 10.1111/j.1529-8027.2012.00371.x
91. Shan W, Hu W, Wen Y, Ding X, Ma X, Yan W, et al. Evaluation of atrazine neurodevelopment toxicity in vitro-application of hESC-based neural differentiation model. Reprod Toxicol (2021) 103:149–58. doi: 10.1016/j.reprotox.2021.06.009
92. Akinrinde A, Adebiyi O. Neuroprotection by luteolin and gallic acid against cobalt chloride-induced behavioural, morphological and neurochemical alterations in wistar rats. Neurotoxicology (2019) 74:252–63. doi: 10.1016/j.neuro.2019.07.005
93. Bouayed J, Soulimani R. Evidence that hydrogen peroxide, a component of oxidative stress, induces high-anxiety-related behaviour in mice. Behav Brain Res (2019) 359:292–7. doi: 10.1016/j.bbr.2018.11.009
94. De Santis B, Raggi ME, Moretti G, Facchiano F, Mezzelani A, Villa L, et al. Study on the association among mycotoxins and other variables in children with autism. Toxins (2017) 9(7):203. doi: 10.3390/toxins9070203
95. Kaur S, Raj K, Gupta Y, Singh S. Allicin ameliorates aluminium-and copper-induced cognitive dysfunction in wistar rats: Relevance to neuro-inflammation, neurotransmitters and aβ (1–42) analysis. JBIC J Biol Inorganic Chem (2021) 26(4):495–510. doi: 10.1007/s00775-021-01866-8
96. Rosenfeld CS. Effects of phytoestrogens on the developing brain, gut microbiota, and risk for neurobehavioral disorders. Front Nutr (2019) 6:142. doi: 10.3389/fnut.2019.00142
97. Liddicoat AM, Lavelle EC. Modulation of innate immunity by cyclosporine a. Biochem Pharmacol (2019) 163:472–80. doi: 10.1016/j.bcp.2019.03.022
98. Zambuzi FA, Cardoso-Silva PM, Castro RC, Fontanari C, FdS E, Frantz FG. Decitabine promotes modulation in phenotype and function of monocytes and macrophages that drive immune response regulation. Cells (2021) 10(4):868. doi: 10.3390/cells10040868
99. Wu H, Zhao G, Liu S, Zhang Q, Wang P, Cao Y, et al. Supplementation with selenium attenuates autism-like behaviors and improves oxidative stress, inflammation and related gene expression in an autism disease model. J Nutr Biochem (2022) 107:109034. doi: 10.1016/j.jnutbio.2022.109034
Keywords: autism spectrum disorder, whole blood, immune cells landscape, predictor, bioinformatics analysis
Citation: Li H, Xu Y, Li W, Zhang L, Zhang X, Li B, Chen Y, Wang X and Zhu C (2023) Novel insights into the immune cell landscape and gene signatures in autism spectrum disorder by bioinformatics and clinical analysis. Front. Immunol. 13:1082950. doi: 10.3389/fimmu.2022.1082950
Received: 28 October 2022; Accepted: 28 December 2022;
Published: 25 January 2023.
Edited by:
Michele Costanzo, University of Naples Federico II, ItalyReviewed by:
Paul Yao, Hainan Women and Children’s Medical Center, ChinaCopyright © 2023 Li, Xu, Li, Zhang, Zhang, Li, Chen, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changlian Zhu, Y2hhbmdsaWFuLnpodUBuZXVyby5ndS5zZTs=; emh1Y0B6enUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.