- 1Department of Traditional Chinese Medicine, Changhai Hospital, Naval Medical University, Shanghai, China
- 2School of Basic Medicine, Naval Medical University, Shanghai, China
- 3Clinical Cancer Institute, Center for Translational Medicine, Naval Medical University, Shanghai, China
Chimeric antigen receptor (CAR) engineering of natural killer (NK) cells is an attractive research field in tumor immunotherapy. While CAR is genetically engineered to express certain molecules, it retains the intrinsic ability to recognize tumor cells through its own receptors. Additionally, NK cells do not depend on T cell receptors for cytotoxic killing. CAR-NK cells exhibit some differences to CAR-T cells in terms of more precise killing, numerous cell sources, and increased effectiveness in solid tumors. However, some problems still exist with CAR-NK cell therapy, such as cytotoxicity, low transfection efficiency, and storage issues. Immune checkpoints inhibit immune cells from performing their normal killing function, and the clinical application of immune checkpoint inhibitors for cancer treatment has become a key therapeutic strategy. The application of CAR-T cells and immune checkpoint inhibitors is being evaluated in numerous ongoing basic research and clinical studies. Immune checkpoints may affect the function of CAR-NK cell therapy. In this review, we describe the combination of existing CAR-NK cell technology with immune checkpoint therapy and discuss the research of CAR-NK cell technology and future clinical treatments. We also summarize the progress of clinical trials of CAR-NK cells and immune checkpoint therapy.
1. Introduction
Innate immunity, also known as non-specific immunity, is a natural immune defense function that was gradually formed during the long-term development and evolution of the body. As an innate immune cell type, natural killer (NK) cells actively participate in the first line of defense against invasion by pathogenic microorganisms (1).
In the past few years, research into chimeric antigen receptor (CAR)-modified NK cell therapy has increased. CAR-modified NK cell therapy, similar to CAR-T cell therapy, involves the expression of synthetic receptors by genetically modified immune cells; these immune cells are redirected to tumor surface antigens for tumor clearance through the cytotoxicity of immune cells (2, 3). Researchers have used NK cells from different sources with various modular CAR designs against a variety of target antigens (4–6). CAR-T cell therapy and CAR-NK cell therapy have many advantages, but they also have common disadvantages. Such as Immune exhaustion caused by immune checkpoints may be one of the common problems to be solved in clinical treatment (7).
Immune checkpoint molecules are immunosuppressive molecules that are expressed on immune cells and regulate the degree of immune activation (8). Upon activation, immune checkpoint molecules maintain the immune system within normal levels, so that the immune system is not overactivated, preventing autoimmunity. In the tumor microenvironment, tumor cells express immune checkpoint inhibitory ligands, thereby stimulating the downstream signaling pathway of immune cells, leading to immune exhaustion and providing a more suitable environment for tumor cell survival (9). Immune checkpoint immunotherapy is currently used to regulate the activity of T cells and NK cells to kill tumor cells through a series of pathways such as co-inhibition or co-stimulation signals (8, 10, 11).
Previous studies have explored the combination of CAR-T cells with immunotherapy targeting programmed cell death protein 1/programmed cell death 1 ligand 1 (PD-1/PD-L1) (12). However, studies on the combination of CAR-NK cell with immune checkpoints therapy are limited (13). This review describes the current research on the combination of CAR-NK and immune checkpoint therapies with the aim of providing insights for clinical and basic research for cancer treatment.
2. NK cells
2.1. Human NK cells
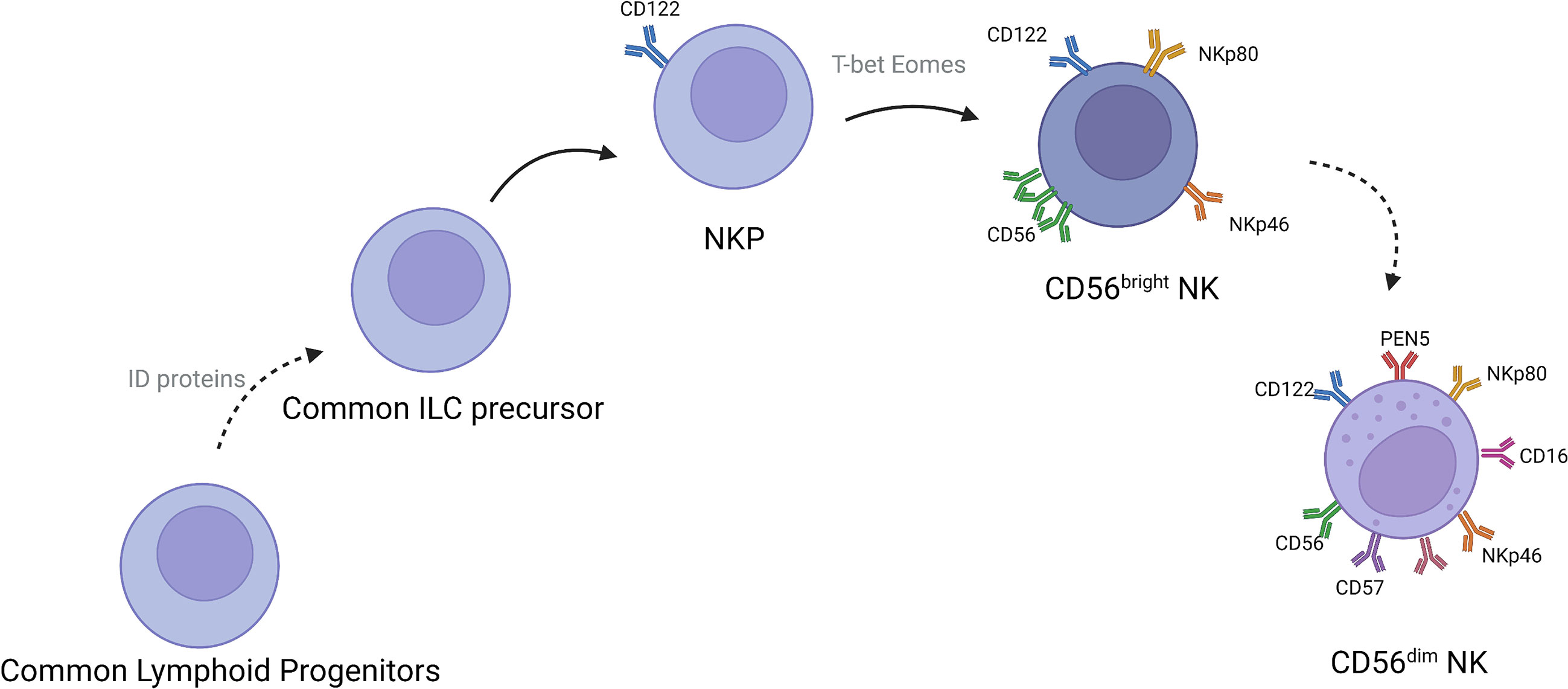
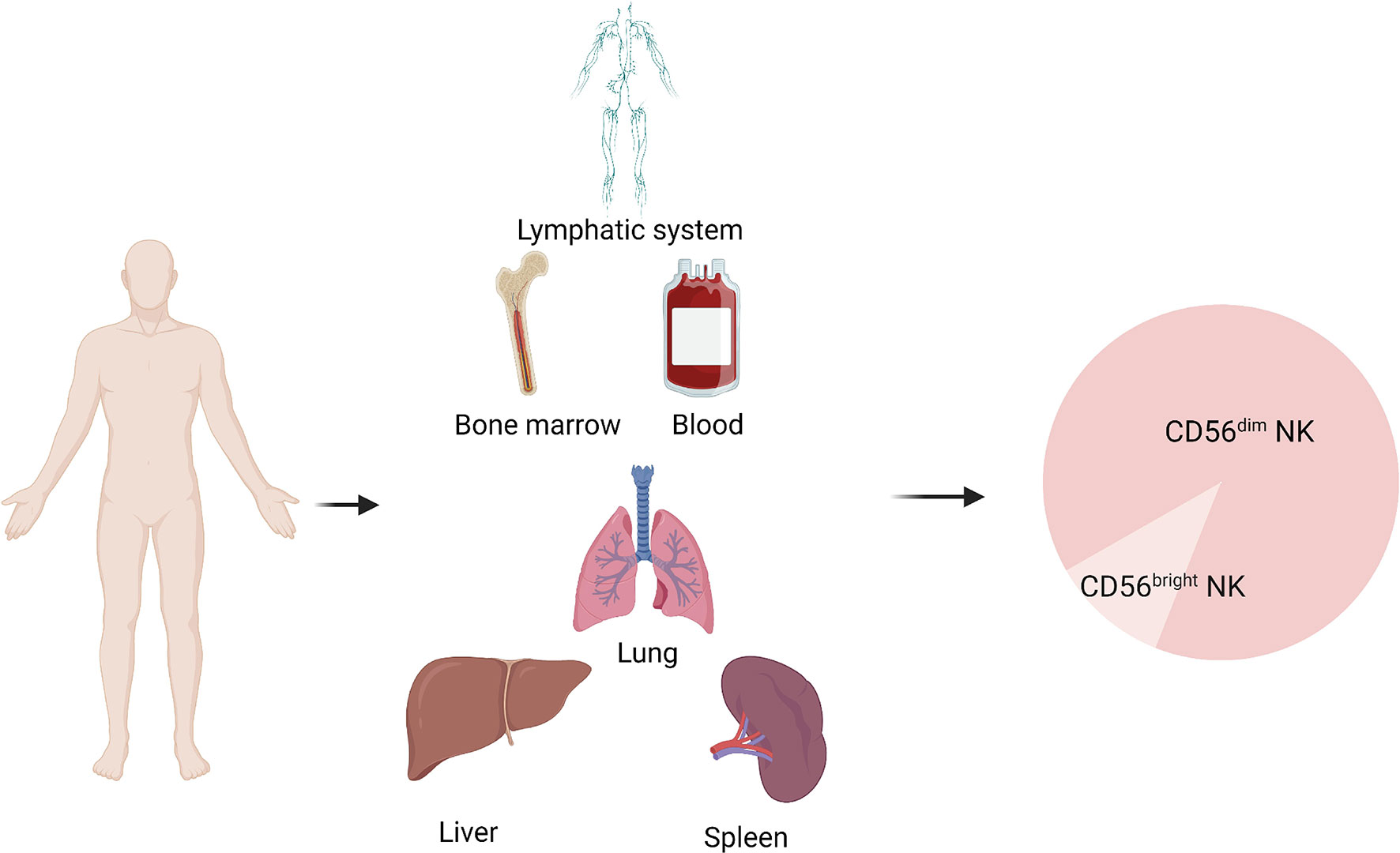
NK cells are mainly present in lymph nodes, bone marrow, peripheral blood, lungs, spleen, and liver (14). These cells develop from CD34+ hematopoietic progenitor cells. After developing into lymphoid progenitor cells, the cells gradually downregulate CD34 and upregulate CD56 and then develop into NK cells (Figure 1). CD56-expressing cells are divided into CD56bright and CD56dim subsets, defined on the basis of density of CD56 expression. More than 90% of NK cells subtype in the body is CD56dim NK cells (15–17), which play an important roles in tumor immunotherapy (Figure 2). CD56bright NK cells are immature NK cells and either function as progenitor cells of CD56dim NK cells or as effector cells. Compare to CD56dim NK cells, CD56bright NK cells exert less cytotoxic effects and secrete certain cytokines, growth factors, and chemokines to play immunomodulatory roles. CD56dim NK cells exhibit weak cytokine secretion activity, but these cells show natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity, with more lethality compared with CD56bright cells (18–21). Their main target cells are tumor cells, virus-infected cells, and parasites, and these cells initiate and participate in the adaptive immune response. They also show good therapeutic effects in rheumatoid arthritis (22, 23).
2.2. NK cells target and kill tumor cells
Tumor development is caused by abnormal cell proliferation. Tumor progression involves metastasis from the primary site to other sites and invasion of vital organs and organ failure, resulting in patient death (24, 25). The body has various strategies to prevent tumor development through checks by the immune system (26). Therefore, the mechanisms by which NK cells find and kill tumor cells require elucidation to potentially identify new strategies to improve outcome of cancer patients.
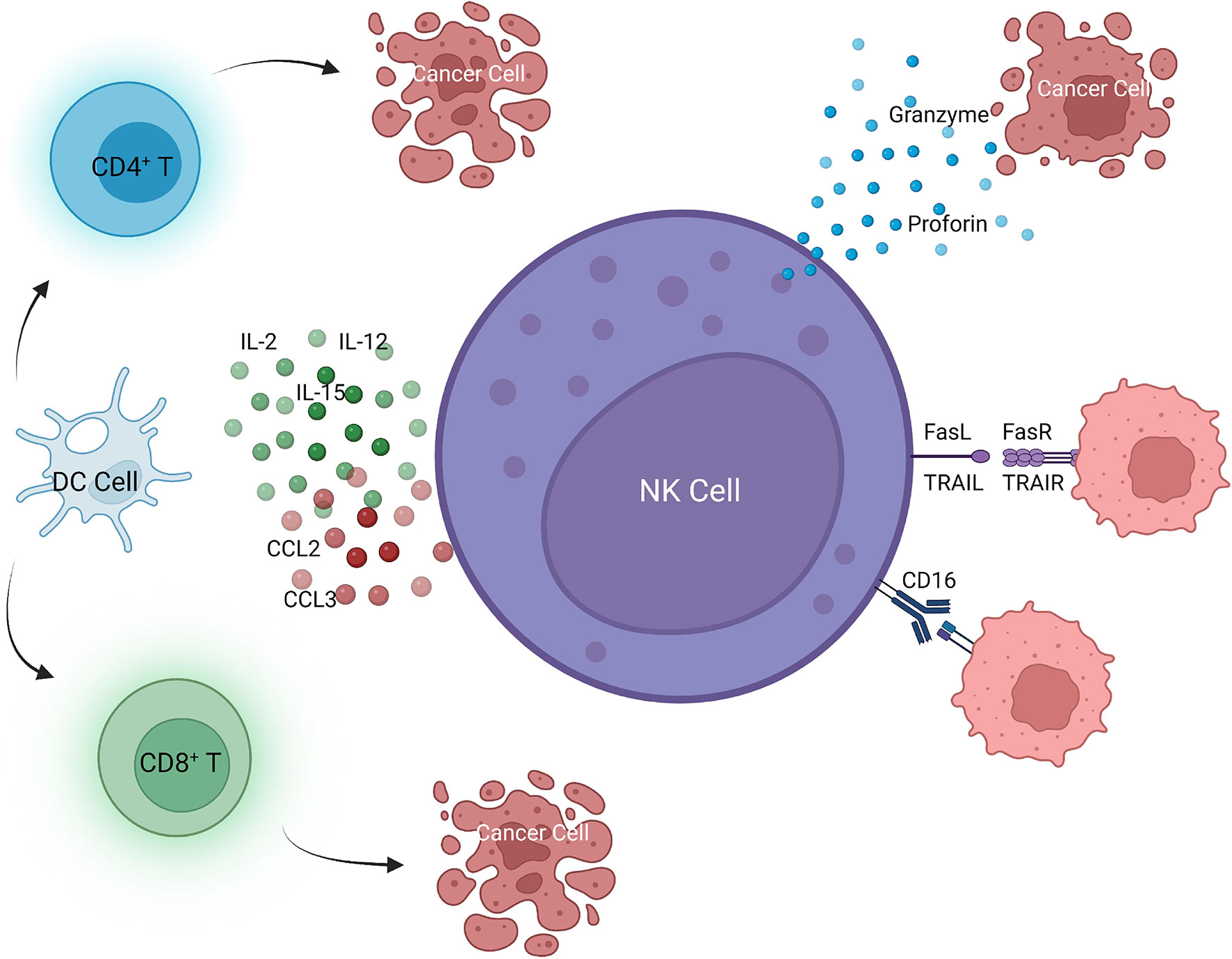
Tumor cells have reduced expression of major histocompatibility complex class I (MHC-I) early during tumor growth to avoid surveillance by the immune system. However, tumor cells with low MHC-I expression could stimulate NK cells, allowing NK cells to detect and kill tumor cells at an early stage (27, 28). NK cells eliminate tumor cells through four pathways. One is killing targeted tumor cells by releasing perforin and granzyme-containing cytoplasmic granules, leading to apoptosis of tumor cells. Granzymes are released into the intracellular space in a calcium-dependent manner (29, 30). Perforin in cytoplasmic granules induces cell membrane perforation, allowing granzyme to enter tumor cells, which leads to cell death receptor–mediated apoptosis (31). Second, NK cells can secret tumor necrosis factor (TNF) superfamily members, such as FasL and TNF-related apoptosis-inducing ligand (TRAIL), which can bind to their receptors to induce apoptosis of target cells (32, 33). The third pathway is to induce tumor cell apoptosis by limiting tumor angiogenesis and enhancing adaptive immunity by releasing effector molecules with anti-cancer properties, such as interferon-γ (IFN-γ) (29, 34). Stimulating cytokines, such as interleukin(IL)-2, IL-12, IL-15, and IL-18 and cytokines leading to IFN production, enhance the anti-tumor effect of NK cells (29, 35). NK cells also produce chemokines to recruit macrophages, dendritic cells, and T cells to cooperate in suppressing tumor growth (36, 37). Fourth, Fc receptor (CD16) is expressed and mediates the antibody-dependent cell-mediated cytotoxicity (ADCC) effect (38) (Figure 3).
2.3. Immune escape of tumor cells
Although NK cells play an important role in immune surveillance, tumor cells also could escape immune surveillance by NK cells through various mechanisms (39). Tumor cells can down regulate ligands recognized by NK cell receptors through metalloproteinase mediated cleavage and other mechanisms, leading to immune escape (40, 41). Second, during tumor development, tumor cells and factors in the tumor microenvironment release a variety of immunosuppressive factors to escape immune surveillance by NK cells (42). Third, NK cells are inhibited by immunosuppressive cells after immune escape of tumor cells (43).
3. Combined applications of CAR-NK cells and immune checkpoint inhibitors
Multiple studies have linked cancer and the immune system (44–46). Similar to organ transplantation, studies have shown that the immune system can recognize and respond to tumors. Therefore, research has focused on developing anti-tumor strategies by activating the immune system (47, 48). Tumor immunotherapy, including adoptive cell therapy and immune checkpoint therapy, are likely to revolutionize the treatment of malignant tumors (49). NK cell immunotherapy mainly includes adoptive NK cell therapy and NK cell–based ADCC functional antibody therapy. Adoptive NK cell therapy could exploit the intrinsic anti-tumor potentials of NK cells (50). Besides, NK cells can also be modified by gene editing (49, 51–53). However, these NK cell immunotherapies also have some limitations, such as life-threatening toxicity, insignificant efficacy in solid tumors, and poor durability [55]. Therefore, we propose combining CAR-immune cell therapy with other anti-tumor therapies to help improve the anti-tumor effect, inhibit toxicity, and improve the prognosis of patients (7, 12, 54–56).
3.1. CAR-NK cells
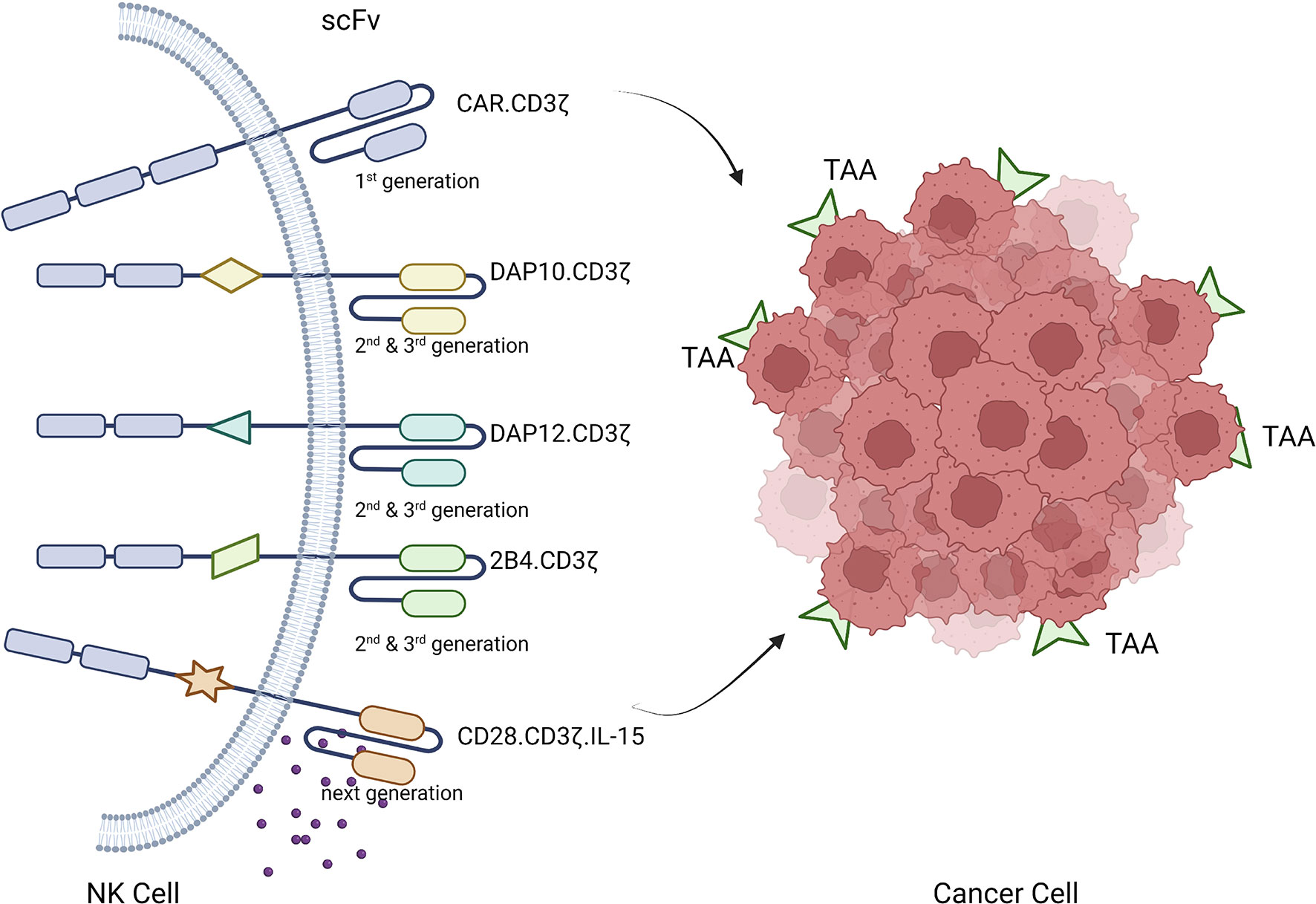
CAR-based gene modification of immune cells to express synthetic receptors redirects immune cells to tumor surface antigens for tumor clearance through immune cell cytotoxicity (57, 58). CAR molecules expressed on NK cells contain an extracellular domain, a transmembrane region, and one or more intracellular signaling domains (56, 59). The extracellular domain includes a signal peptide and an antigen-recognizing single-chain antibody fragment, which mainly recognizes tumor associated antigens (TAAs) on tumor cells. A hinge region connects this structure to the transmembrane region, which is also connected intracellularly to the intracellular domain containing the activation signal. The hinge assembly connects the ectodomain to the transmembrane domain. The transmembrane domain, a hydrophobic α-helix, crosses the membrane between the spacer and domain at end of the signal peptide. The inner domain (signaling domain) is relatively complex and is a functional component of CAR-immune cells that controls their activation, proliferation, and survival (60–62). Successful CAR design is achieved by a combination of careful design and functional testing. The inner domain of the CAR transmits costimulatory signals to immune cells in response to antigen recognition by the outer domain, enabling them to initiate cytotoxic functions (63). Similar to CAR-T cell therapy, several generations of CAR-NK cells have been developed. First-generation CAR-NK cells, similar to CAR-T cells, contain only CD3ζ signals. The CAR construct has been fine-tuned to induce a more potent anti-tumor response, increase antigen affinity, and prolong in vivo persistence using multiple genetic engineering technologies. Various costimulatory elements have been studied, such as second- and third-generation CAR-NK cells, which carry one or two additional costimulatory signals, respectively. Costimulatory molecules are derived from the immunoglobulin superfamily (CD28 and inducible costimulator), TNF receptor superfamily (4-1BB, OX40, CD40, and CD27), SLAM-related receptor family (2B4), and other domains including CD40L and Toll-like receptor (3, 56, 64–66). Compared with early CAR-NK constructs, which are mainly based on the costimulatory domain involved in T cell activation, NK cell–specific signal adapters become valuable due to their increasingly powerful functions (67, 68). In a preclinical study investigating CD19-directed CAR-NK cells, the addition of DAP10, a physiological adapter of NKG2D, resulted in enhanced anti-tumor potency compared with constructs using CD3ζ signals alone (4, 68, 69). Other studies have reported that the addition of DAP12 to prostate stem cell antigen–targeted CAR constructs and 2B4 to mesodermin-targeted CAR-NK cells amplifies anti-tumor effects (70, 71).
The structural design of the first three generations of CARs depended on the immune cell receptor domain, which has some limitations in cellular immunotherapy. Most current CAR constructs rely on the CD3ζ chain signaling domain, and strong activation signals are important to induce effective anti-tumor responses but they may also lead to rapid exhaustion of effector cells. Thus, a combination of costimulatory domains can be used to calibrate the desired immune cell response. Compared with 4-1BB-based CARs, CD28-based CARs have faster effector features and induce higher expression of IFN-γ, granzyme B, and TNF-α. However, this strong costimulatory signal also leads to activation-induced cell death. Conversely, 4-1BB-CD3ζ signaling preferentially induces memory-related genes and sustains anti-tumor activity. The reason may be that the 4-1BB domain ameliorates NK cell depletion induced by the CD28 domain (68, 70, 72, 73).
Fourth-generation constructs, termed armed CARs, are more effective and incorporate molecular payloads that confer additional features and functions to CAR-modified immune cells that are not present in any physiological immune cell receptor. This approach enables engineering of the CAR structure. Current clinical trials of CAR-NK cells are investigating second- and third-generation CAR-NK constructs that eliminate all circulating adoptive NK cells by inducing IL-15 expression to enhance caspase 9 activity to prevent adverse toxicity (13, 74, 75).
3.2. Advantages of CAR-NK cells
CAR-NK cells have the potential to be applied in other medical fields. These cells may be safer than CAR-T cells because cytokines secreted by activated NK cells are safer and usually suppress the proinflammatory cytokines such as TNF-α, IL-1, and IL-6 released by CAR-T cells. Additionally, CAR-NK cells reduce the risk of GVHD because they are not restricted to MHC (51, 76). CAR-NK cells may also have various cytotoxic effects as they recognize and kill targets through engineered killing capabilities and natural cytotoxic receptors (77). Interestingly, Clinical trials CAR-NK cells can recognize and kill the residual tumor cells after long-term treatment. because CAR-NK cells contain CAR-dependent and CAR-independent target recognition and killing capabilities, the incidence of tumor escape in CAR-NK therapy is less (59, 78, 79). In addition, mature NK cells have a short lifespan in blood, which reduces the risk of cellular memory responses and cellular defects resulting from targeted/non-tumor effects (80). What is more, a large number of NK cell lines are available for CAR modification. Because of the low risks of alloreactivity and graft-versus-host disease (GVHD), allogeneic CAR-NK cells can be obtained from various sources including PB, UCB, iPSCs, hESCs, and NK-92 cells (13, 76, 81). Finally, the cost of CAR-NK cells is lower than that of CAR-T cells and thus CAR-NK may have greater market potential. The gradually improved technology makes it possible to store, thaw and reinfuse these cells, and can also carry out genetic engineering or genetic editing technology when needed (78) (Figure 4).
3.3. Disadvantages of CAR-NK cells
Despite the many advantages of NK cells, the application of CAR-NK cells has several challenges. Expansion of NK cells in vitro is the first limitation for CAR-NK cell immunotherapy. The number of NK cells obtained from a single donor is insufficient for treatment, which makes expansion and activation of NK cells critical (64, 82). Second, because the location of CAR binding epitope and its distance from CAR-NK cell surface affect its ability to bind antigen and activate CAR-NK cells, the current CAR used in NK cells has a structure that causes a first magnetic resistance, reducing the ability of these cells to bind antigens (83). Additionally, the production process of usually requires 2–3 weeks to culture NK cells and produce cytokines (IL-2 alone or in combination with IL-15 or an anti-CD3 monoclonal antibody) (13, 84). NK cells do not survive in the absence of cytokines. Therefore, exogenous cytokines must be provided to allow infused NK cells to survive and proliferate in vivo (64, 85). The source of NK cells is also an issue. Autologous NK cells need to be frozen and thawed. However, this reduces their anti-tumor effect and survival rate (86). Additionally, exogenous cytokines may have adverse effects such as systemic toxicity (70, 78, 87).
Similar to CAR-T cells, NK cells lack effective gene transfer strategies (88). Both viral and non-viral vectors have been used to genetically engineer CAR-NK cells (89). While the transduction efficiency of retroviral vectors is high, these vectors may cause insertional mutations, carcinogenesis, and other adverse effects (90). However, while lentiviral vectors have a low incidence of insertional mutations, their transfection efficiency in peripheral blood NK cells is as low as 20% (91). mRNA transfection is also considered to be a safe and practical transfection method for CAR-NK cells. In a xenograft tumor model, receptor expression exceeded 80% at 24h after electroporation of mRNA, and NK cells transfected with mRNA exerted marked cytotoxicity (92). Studies have recently shown that mRNA transfection avoids targeted non-tumor toxicity, a major limiting factor in the clinical application of CAR-modified cell immunotherapy (92, 93). However, the anti-tumor effect of CAR-NK cells transfected with mRNA by electroporation is temporary because the expression of CARs does not exceed 3 days.
4. CAR-NK cell and immune checkpoint therapies
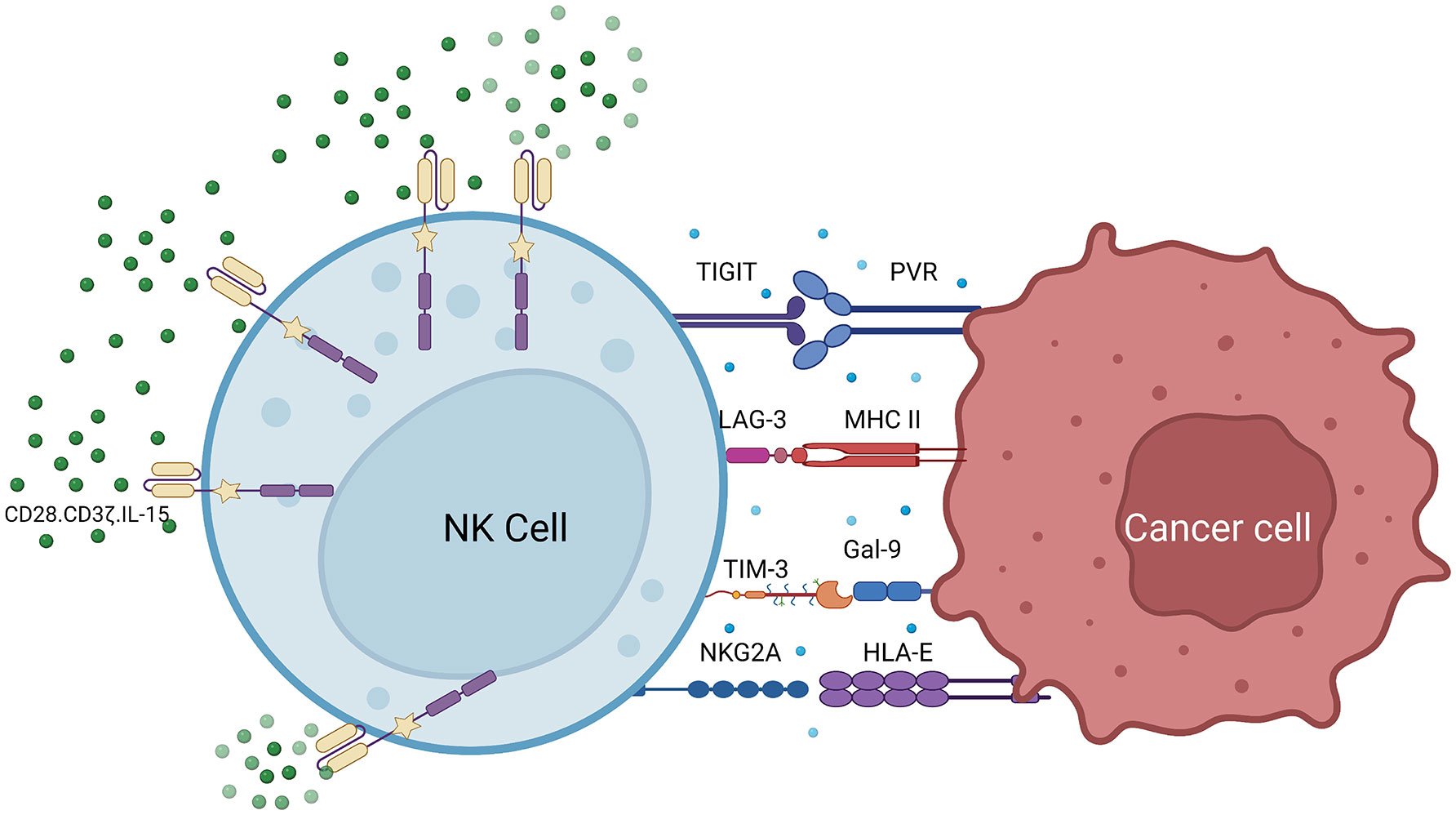
CAR-NK cells have better targeting ability compared with other immune cells. In addition to recognizing tumor surface antigens through CARs, CAR-NK cells recognize tumor cells through various receptors such as natural cytotoxic receptors NKp46, NKp44, NKp30, NKG2D, and DNAM-1 (CD226) (13, 60, 94, 95). Despite the success of adoptive NK cell therapy, immune cell depletion remains a therapeutic barrier. To develop the next generation of CAR-NK cells, the negative regulator of NK cells may be a potential new design direction (96–98). T cell immune checkpoints affect CAR-T cell therapy. For example, PD-1 is an immune checkpoint receptor expressed on the T cell surface. It binds to PD-L1 expressed by target cells and sends an inactivating signal to T cells, thereby inhibiting the immune activity of T cells (12, 99, 100). However, many cancer cells express high levels of PD-L1, leading to cancer cell survival after T cell engagement. This can be overcome by targeted therapy. PD-1/PD-L1 axis inhibitors have been proven to achieve good clinical effects (101). However, PD-1 expression on NK cells is very low. Additionally, there are immune checkpoint receptors on NK cells that may regulate the functions of CAR-NK cells, such as T cell immunoreceptor with Ig and ITIM domains (TIGIT), NKG2A, lymphocyte activation gene-3 (LAG-3), and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) (Figure 5) (95–97).
4.1. TIGIT
TIGIT, also known as WUCAM, Vstm3, and VSIG9, is a member of the poliovirus receptor (PVR)/Nectin family and immunoglobulin (Ig) superfamily (11, 102, 103). TIGIT is an inhibitory receptor (104) that inhibits T and NK cell activation (102). TIGIT is a transmembrane glycoprotein composed of three domains, the extracellular Ig variable domain, type I transmembrane domain, and short intracellular domain, and has an immune receptor tyrosine-based inhibition motif (ITIM) and immunoglobulin tyrosine tail-like phosphorylation motif (104, 105).
Previous studies have shown that TIGIT is expressed in all types of human NK cells (106). Poliovirus receptor (PVR, CD155), a physical ligand of TIGIT, which can bind TIGIT and activate immunosuppressive signal through the cytoplasmic ITIM domain of TIGIT (98). CD155 also plays an important role in both NK- and T cell-mediated immunity in humans and mice and is expressed on T cells, B cells, macrophages, and dendritic cells (105, 107, 108). CD155 is frequently overexpressed in human malignant tumors. As an immunomodulatory molecule, CD226 is a costimulatory molecule of T cells and NK cells, while TIGIT and CD96 are co inhibitory molecules, which can competitively bind CD155 (109). However, TIGIT has the highest affinity for CD155, and CD226 has the lowest affinity for CD155, as evidenced by direct radioligand binding analysis and competition experiments (110, 111). Therefore, the balance between the three competitively binding CD155 may play an important role in maintaining NK cells immune functions (112).
TIGIT interference has been shown to restore NK cell function and inhibit tumor growth in ovarian and breast cancer (113, 114). Notably, 58% sequence homology is found between human and mouse TIGIT; while the ITIM sequence in the cytoplasmic tail of TIGIT is the same in mice and humans (11, 107, 115). Similar to the inhibitory effect of human TIGIT, murine TIGIT also inhibits the cytotoxicity of mouse NK cells. Because of the cross-species specificity of the protein, human and mouse TIGIT have different binding properties. Human TIGIT binds more ligands to play an inhibitory role in immunity (104, 116).
4.2. NKG2A
NKG2A, an immunosuppressive receptor, is an attractive target for immunocheckpoint therapy. Approximately half of human peripheral blood NK cells express NKG2A (117). It is mainly expressed in CD56bright NK cells and gradually decreases during NK cell maturation (118). NKG2A is a one-way type II integral membrane glycoprotein containing cytoplasmic, transmembrane, and extracellular lectin-like domains. NKG2A has two types of ITIM, which are mainly involved in immunosuppressive regulation (119–121).
The NKG2 protein is a C-type lectin that dimerizes with CD94 on the cell surface (122, 123). The non-classical MHC class I molecule human leukocyte antigen-E (HLA-E) is the main ligand of NKG2A/CD94, and its expression is approximately 25 times lower than that of classical MHC class I molecules. It is expressed in most normal tissues, and the interaction between NKG2A and HLA-E inhibits NK and T cell activation (97, 124, 125). Binding of NKG2A/CD94 receptors to peptide-presenting HLA-E leads to phosphorylation of ITIMs in NKG2A. Phosphorylated ITIMs are responsible for the recruitment and activation of intracellular phosphatases SHP-1 and SHP-2, thereby inhibiting the activation signals generated by activating receptors in NK cells (119, 126). HLA-E expression is generally increased in tumor cells (125), which provides NKG2A with more opportunities to inhibit NK cell activation. Similar to other immune checkpoint molecules, NKG2A is used by tumor cells for immune evasion. Therefore, disruption of the interaction between NKG2A and its ligands may enhance the anti-tumor immune response (127, 128). Previous studies have shown that blocking of inhibitory checkpoints in NK cells may also be effective for some metastatic carcinomas (129, 130). NKG2A is also involved in the pathological processes of immune-mediated diseases such as autoimmune diseases, inflammatory diseases, parasitic infections, and transplant rejection. These findings suggest that NKG2A is a novel therapeutic target for various immune-mediated diseases (131, 132).
4.3. LAG-3
Lymphocyte activation gene-3 (LAG-3), also known as CD223, is a 503 amino acid protein encoded by the LAG3 gene. LAG-3 is an immune checkpoint receptor protein localized in the cell membrane (133). The extracellular portion of the molecule consists of four immunoglobulin-like domains (D1–D4), which shows rigidity between D1 and D2 as well as D3 and D4 and relative flexibility between D1 and D2 as well as D3 and D4 (134). The human LAG3 gene is located on chromosome 12 (12p13), a similar location as the gene for CD4 (12p13.31). While LAG-3 and CD4 share only approximately 20% identical at the protein level, they have a highly homologous protein structure (135, 136). The cytoplasmic tail of LAG-3 has three key elements: a serine phosphorylation site, a KIEELE motif, and a glutamic proline dipeptide repeat. The KIEELE motif is highly conserved and may be involved in the transduction of the downstream inhibitory signal of LAG-3 because LAG-3 protein lacking this structure cannot exert an inhibitory effect on T cells (137). LAG-3 is selectively transcribed in activated T and NK cells. LAG-3 is mainly expressed in activated T, NK, B, and plasma cell dendritic cells. LAG-3 is expressed on NK cells, invariant NK T cells, Treg cells, and CD4+ and CD8+ subsets of T lymphocytes activated by antigens (138–141).
The role of LAG-3 in regulating NK cell functions is unclear, but similar to CD4, LAG-3 binds to major histocompatibility complex II (MHC-II) molecules. Compared with CD4, LAG-3 has a higher affinity for MHC-II (approximately 100 times) because LAG-3 enhances the interaction with MHC-II, and this interaction occurs through a ring structure composed of 30 amino acids in its D1 domain (138, 140, 142, 143). LAG-3 selectively binds to the stable antigen peptide–MHC-II molecular complex (pMHC-II). Therefore, LAG-3 preferentially inhibits the activation of CD4+ T cells with stable pMHC-II (143, 144). In NK cells, an increase in LAG-3 protein expression correlates with time post-infection and white pulp localization. One study suggested that upregulation of NK cells by LAG-3 causes the surrounding MHC-II+ cells to send inhibitory feedback signals, thereby terminating inhibition of T cells by NK cells (145). NK cells from LAG-3-deficient mice are defective in killing specific cancer cells (146). Considering of the effect of LAG-3 on the NK cell effector function, targeting LAG-3 may be useful in immunotherapy (147).
4.4. TIM-3
TIM-3 protein is a type I membrane protein also known as hepatitis A virus cell receptor 2 (HAVCR2), which is a negative regulator of anti-tumor immunity (148). It is a member of the TIM family that contain eight members, TIM1–TIM8. Among the protein family, TIM1, TIM3, and TIM4 are expressed in humans (149). TIM-3 was discovered in 2002 (150). TIM-3 includes three regions: the extracellular, transmembrane, and intracellular regions. The extracellular region consists of the N-terminal extracellular immunoglobulin variable (IgV) domain with an FG-CC loop and N-linked glycan, the mucin domain containing O-linked glycosylation sites, and the stalk domain containing N-linked glycan. The intracellular region consists of a cytoplasmic tail with five tyrosine residues (151, 152). TIM-3 is expressed on terminally differentiated CD4+ T cell subsets, such as Th1, Th17, and Tregs cells, and type 1 CD8+ T cells, but not on Th2 cells. It is also expressed on B cells, macrophages, dendritic cells, natural killer cells, mast cells, and monocytes (153, 154).
TIM-3 has been shown to inhibit tumor growth in various preclinical cancer models. The IgV domain contains binding sites for its ligands. Phosphatidylserine, carcinoembryonic antigen-associated cell adhesion molecule, and high mobility group protein 1 bind to the FG-CC loop, while Gal9 binds to N-linked glycans (151, 155). TIM-3 binds to its ligand galectin-9 to induce Th1 cell depletion (148, 156). The interaction of TIM-3 with its ligands also causes peripheral immune tolerance, and blocking TIM-3 eliminates the development of Th1 cell tolerance (148, 157). Although TIM-3 is a marker of T cell exhaustion (153), its expression is not associated with NK cell dysfunction in healthy donors. TIM-3 is also expressed in NK cells, the cytolytic activity of TIM-3+ NK cells from healthy humans is higher than that of TIM-3- NK cells, and the TIM-3+ NK cells can kill K562 cells by releasing IFN-γ (158–160).
Cytokine stimulation increases the expression of TIM-3 on CD56dim and CD56bright NK cell subsets (161). TIM-3 expression on peripheral blood NK cells is increased in many cancer patients compared with healthy individuals. The expression of TIM-3 on NK cells increased with the development of disease stage. It has been reported that the survival rate of lung adenocarcinoma patients decreases with the increase of TIM-3+ NK cell percentage (155, 161, 162). Moreover, an study in esophageal cancer reported that tumor-infiltrating TIM-3+ NK cells showed a reduction in IFN-γ production and degranulation capacity compared with TIM-3+ counterparts (163). It has been reported that TIM-3 blockade can enhance the function of immune cells in multiple myeloma and melanoma (164, 165). Therefore, TIM-3 is expressed on fully functionally mature and/or activated NK cells and may function as an inhibitory receptor to inhibit NK cell functions similar to killer cell inhibitory receptor (KIR) and NKG2A.
5. Combination of CAR-NK cells therapy and immune checkpoint therapies for various tumors
Despite the remarkable success of adoptive NK cell therapy, immune cell depletion remains a barrier for therapeutic efficacy (166). To develop the next generation of CAR-NK cells, the identification of negative regulators of NK cell immune functions is required. Some checkpoint receptors, such as PD-1, LAG-3, TIM-3, TIGIT, and killer cell lectin-like receptor subfamily G member 1 (KLRG1), are upregulated in exhausted NK cells (96, 98). NKG2A is one of the most prominent inhibitory NK cell receptors, and its gene deletion is associated with increased NK cell cytotoxicity against tumors (117). Blocking TIGIT prevents NK cell depletion (102, 105, 107). Additionally, cytokine-inducible Src homology 2-containing (CIS) protein, which is an important cytokine checkpoint upstream of IL-15 signaling, is induced by the addition of cytokines, achieving enhanced metabolic fitness and effector functions in CAR-NK cells (70, 167). Other studies have revealed the positive effects of PD-1/PD-L1 and CTLA-4 blockade on NK cells (168).
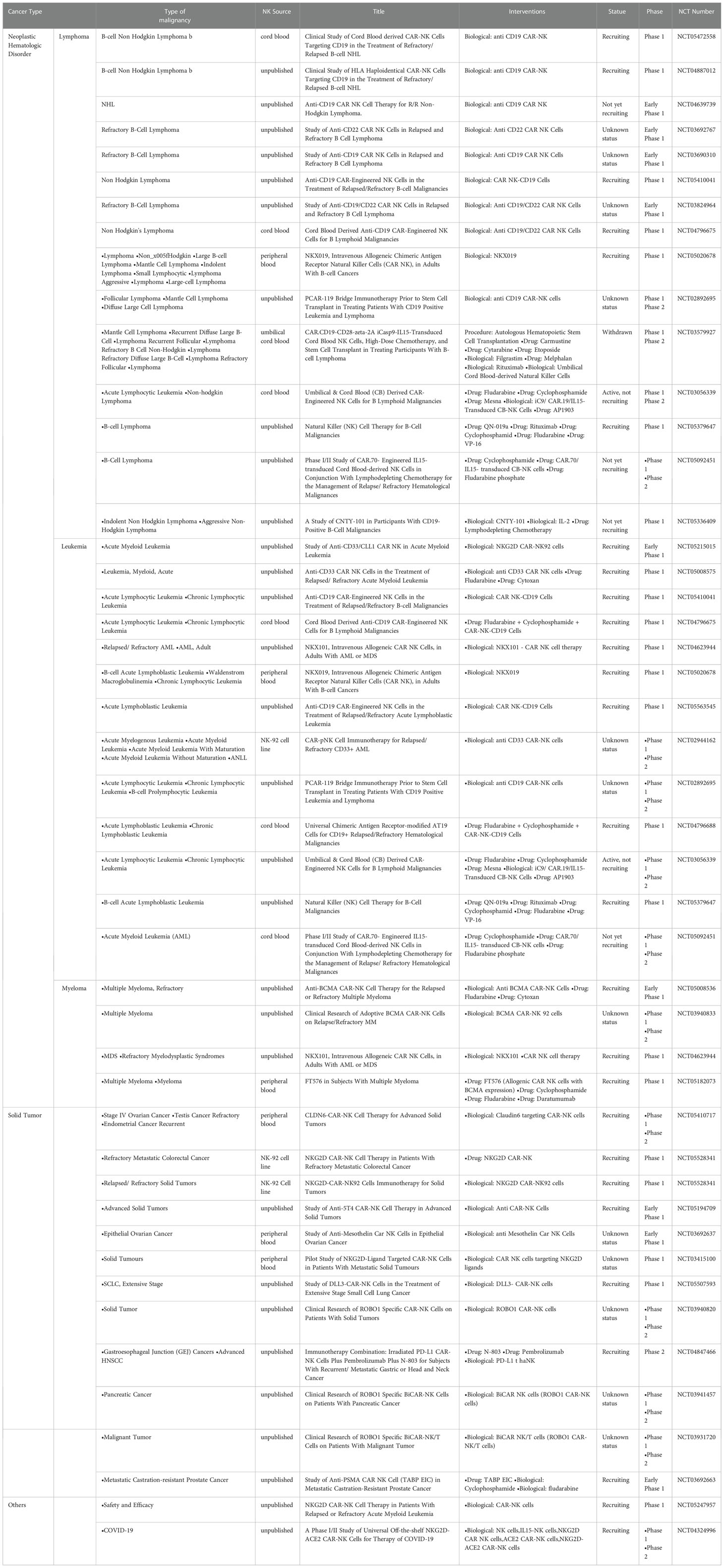
CAR-NK therapy combined with immune checkpoint therapy showed better therapeutic effects compared with single therapy in clinical treatment (13). CAR-NK cell therapy has shown preliminary clinical significance. In addition to being effective against hematological and lymphoid tumors, NK cells have been used as an important treatment strategy for solid tumors (169, 170). For example, a phase I/IIa trial of CAR-NK cell therapy in 11 patients with relapsed/refractory non-Hodgkin’s lymphoma (NHL) or chronic lymphocytic leukemia (CLL) was recently reported (NCT00505245). Of the 11 patients in the trial, 8 patients (73%) were treated, and 7 patients had a complete response to the treatment with no evidence of disease at a median follow-up of 13.8 months. Most patients had a significant response within 30 days after receiving cell infusion, showing a progressive response, and the durability of the treatment was confirmed up to 1 year after infusion.
The safety of CAR-NK cell infusion has been shown by the absence of serious adverse events during patient treatment and follow-up (clinicaltrials.gov, accessed on January 1, 2020). While some patients in remission experienced disease relapse or required additional anti-cancer therapy, cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), or GVHD of any grade has not been reported. Engineered hiPSC-derived allogeneic NK cells are expected to be a safe and effective off-the-shelf cell therapy drug (171). The function of transgenic NK cells is significantly enhanced and they have a significant killing ability for hematological and solid tumors. As of October 2022, 39 clinical trials of CAR-NK therapy have been registered, mainly in the United States, China, and European countries (clinicaltrials.gov). Most targets of CAR-NK therapy are in hematological tumors, but also in solid malignancies such as pancreatic, ovarian, and prostate cancers. Most clinical trials use allogeneic NK cells, mainly from healthy donors, or NK cell lines such as NK92 (Table 1).
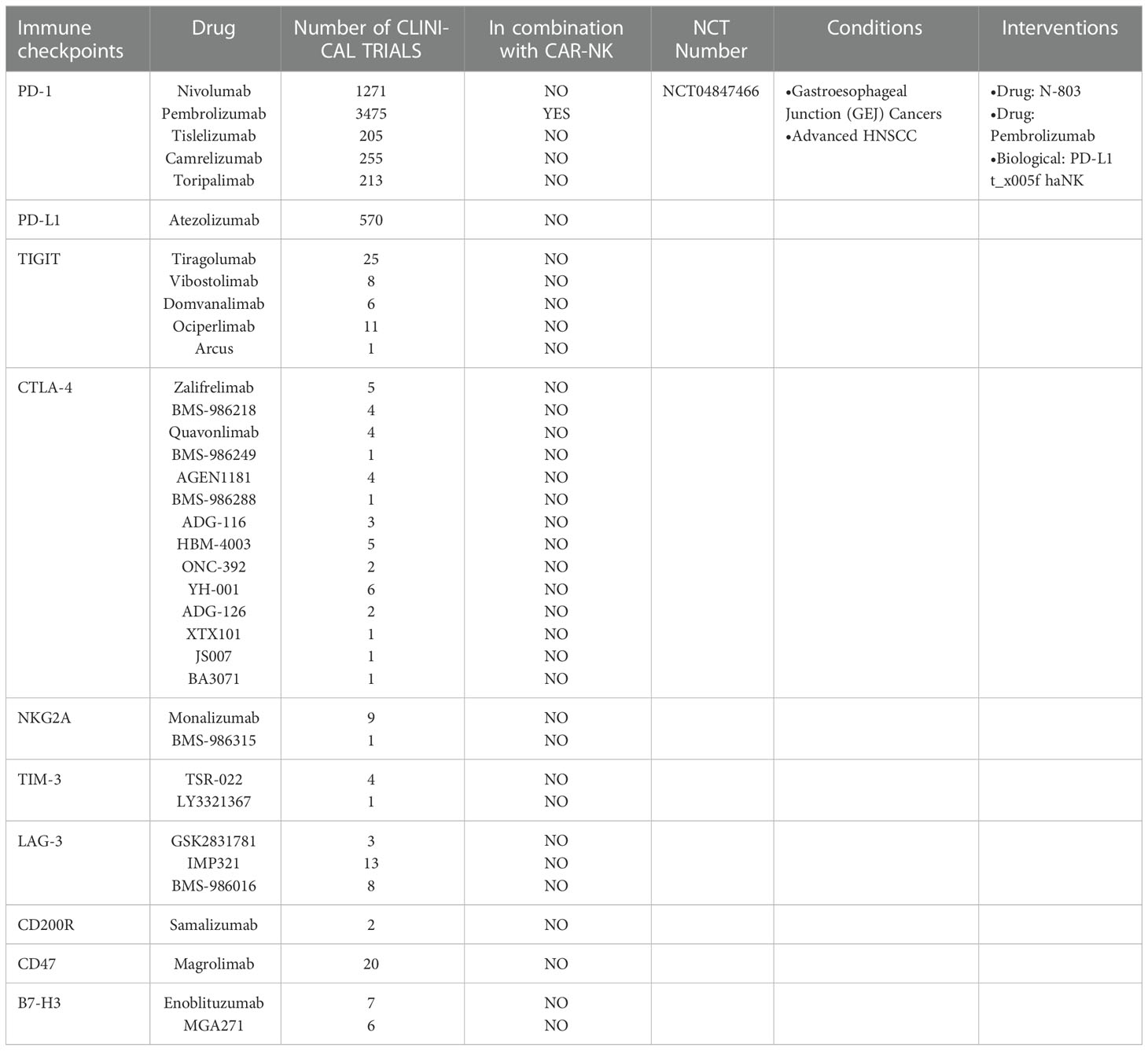
A cytotoxic T-lymphocyte-associated protein 4 (CTLA4) inhibitor, ipilimumab, has been used to treat patients with advanced gastric cancer in a phase II clinical study (NCT01585987). Tremelimumab was evaluated in a phase II trial as a second-line treatment for patients with metastatic gastric adenocarcinoma. Pembrolizumab, a PD1 inhibitor, was approved by the FDA as a third-line treatment for patients with PD-L1-positive advanced gastric cancer. Nivolumab is a Food and Drug Administration (FDA)-approved drug as a third-line treatment for patients with advanced gastric cancer. Tebotelimab, another PD-1 inhibitor, blocks PD-1 and LAG-3 checkpoint molecules independently or synergistically. Additionally, durvalumab was used in a phase I B/II clinical trial in patients with advanced gastroesophageal cancer. In a phase III trial (NCT02625623), avelumab was used as third-line therapy. The TIGIT inhibitor tiragolumab is in a phase III trial and ociperlimab is in a phase II trial. Relatlimab, a LAG-3 inhibitor, has also entered clinical trials (Table 2).
The combination of CAR-T cells and nivolumab (anti-PD-1 antibody) has been used in the treatment of relapsed or refractory classical Hodgkin’s lymphoma (CHL) (63). The PD-1/PD-L1 axis inhibits the cytotoxicity of CAR-T cells, thereby protecting tumor cells from being killed (56), which poses a challenge for CAR-T cell therapy. Therefore, the combination of CAR-NK cells therapy and immunocheckpoint therapy may become a new potential direction. CAR-NK will be developed into a safe, effective, and “off-the-shelf” cancer immunotherapy. In addition, immune checkpoint and CAR target can be designed together to optimize NK cell activation and cytotoxicity to overcome tumor suppression and escape.
6. Conclusion
In this review, we discussed CAR-NK therapy, preparation of CAR-NK cells, clinical progress, and the advantages and disadvantages of CAR-NK cells. Although CAR-NK cells have unique advantages, some challenges still exist, including the long time and high cost of CAR-NK cell preparation, biological toxicity, limited storage and transportation. The efficacy of CAR-NK cells in the treatment of solid tumors is limited. Regulatory challenges remain in terms of safety and clinical efficacy. We also propose the combination of CAR-NK cell and immune checkpoint therapies for future clinical applications. Ongoing research may resolve the challenges of CAR-NK cells and immune checkpoint therapies. Overcoming these issues will help provide new breakthroughs in the treatment of tumors by CAR modifications.
Author contributions
KY and YZ were responsible for preparation of manuscript. GS, XZ, and MS were responsible for data collection. JC, KY, XL, and LW were responsible for supervision, reviewing,editing and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by the National Natural Science Foundation of China (No. 82202912, 81774244 and 81303112), the Natural Science Foundation of Shanghai (12ZR1437400), and Shanghai Sailing Project from the Science and Technology Commission of Shanghai Municipality (22YF1458800).
Acknowledgments
We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moffett A, Shreeve N. Local immune recognition of trophoblast in early human pregnancy: Controversies and questions. Nat Rev Immunol (2022). doi: 10.1038/s41577-022-00777-2
2. Siegler EL, Zhu Y, Wang P, Yang L. Off-the-Shelf CAR-NK cells for cancer immunotherapy. Cell Stem Cell (2018) 23:160–1. doi: 10.1016/j.stem.2018.07.007
3. MacKay M, Afshinnekoo E, Rub J, Hassan C, Khunte M, Baskaran N, et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat Biotechnol (2020) 38:233–44. doi: 10.1038/s41587-019-0329-2
4. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med (2020) 382:545–53. doi: 10.1056/NEJMoa1910607
5. Zheng L, Ren L, Kouhi A, Khawli LA, Hu P, Kaslow HR, et al. A humanized lym-1 CAR with novel DAP10/DAP12 signaling domains demonstrates reduced tonic signaling and increased antitumor activity in b-cell lymphoma models. Clin Cancer Res (2020) 26:3694–706. doi: 10.1158/1078-0432.CCR-19-3417
6. Kotanides H, Sattler RM, Lebron MB, Carpenito C, Shen J, Li J, et al. Characterization of 7A5: A human CD137 (4-1BB) receptor binding monoclonal antibody with differential agonist properties that promotes antitumor immunity. Mol Cancer Ther (2020) 19:988–98. doi: 10.1158/1535-7163.MCT-19-0893
7. Srivastava S, Furlan SN, Jaeger-Ruckstuhl CA, Sarvothama M, Berger C, Smythe KS, et al. Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell (2021) 39:193–208.e10. doi: 10.1016/j.ccell.2020.11.005
8. Abril-Rodriguez G, Ribas A. SnapShot: Immune checkpoint inhibitors. Cancer Cell (2017) 31:848–848.e1. doi: 10.1016/j.ccell.2017.05.010
9. Li B, Chan HL, Chen P. Immune checkpoint inhibitors: Basics and challenges. Curr med Chem (2019) 26. doi: 10.2174/0929867324666170804143706
10. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature (2018) 554:544–8. doi: 10.1038/nature25501
11. Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol (2018) 19:723–32. doi: 10.1038/s41590-018-0132-0
12. Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest (2016) 126:3130–44. doi: 10.1172/JCI83092
13. Daher M, Basar R, Gokdemir E, Baran N, Uprety N, Nunez Cortes AK, et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood (2021) 137:624–36. doi: 10.1182/blood.2020007748
14. Franks SE, Wolfson B, Hodge JW. Natural born killers: NK cells in cancer therapy. Cancers (Basel) (2020) 12:E2131. doi: 10.3390/cancers12082131
15. Della Chiesa M, Setti C, Giordano C, Obino V, Greppi M, Pesce S, et al. NK cell-based immunotherapy in colorectal cancer. Vaccines (Basel) (2022) 10:1033. doi: 10.3390/vaccines10071033
16. Leong JW, Sullivan RP, Fehniger TA. microRNA management of NK-cell developmental and functional programs. Eur J Immunol (2014) 44:2862–8. doi: 10.1002/eji.201444798
17. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol (2001) 22:633–40. doi: 10.1016/s1471-4906(01)02060-9
18. Thorén FB, Romero AI, Hermodsson S, Hellstrand K. The CD16-/CD56bright subset of NK cells is resistant to oxidant-induced cell death. J Immunol (2007) 179:781–5. doi: 10.4049/jimmunol.179.2.781
19. Dubois SP, Miljkovic MD, Fleisher TA, Pittaluga S, Hsu-Albert J, Bryant BR, et al. Short-course IL-15 given as a continuous infusion led to a massive expansion of effective NK cells: Implications for combination therapy with antitumor antibodies. J Immunother Cancer (2021) 9:e002193. doi: 10.1136/jitc-2020-002193
20. Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, et al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol (2001) 31:3121–7. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4
21. Seymour F, Cavenagh JD, Mathews J, Gribben JG. NK cells CD56bright and CD56dim subset cytokine loss and exhaustion is associated with impaired survival in myeloma. Blood Adv (2022) 6:5152–9. doi: 10.1182/bloodadvances.2022007905
22. Kucuksezer UC, Aktas Cetin E, Esen F, Tahrali I, Akdeniz N, Gelmez MY, et al. The role of natural killer cells in autoimmune diseases. Front Immunol (2021) 12:622306. doi: 10.3389/fimmu.2021.622306
23. Fathollahi A, Samimi LN, Akhlaghi M, Jamshidi A, Mahmoudi M, Farhadi E. The role of NK cells in rheumatoid arthritis. Inflammation Res (2021) 70:1063–73. doi: 10.1007/s00011-021-01504-8
24. Lawley PD. Historical origins of current concepts of carcinogenesis. Adv Cancer Res (1994) 65:17–111. doi: 10.1016/s0065-230x(08)60065-2
25. Bai X, Zhou Y, Chen P, Yang M, Xu J. MicroRNA-142-5p induces cancer stem cell-like properties of cutaneous squamous cell carcinoma via inhibiting PTEN. J Cell Biochem (2018) 119:2179–88. doi: 10.1002/jcb.26379
26. Dubrot J, Du PP, Lane-Reticker SK, Kessler EA, Muscato AJ, Mehta A, et al. In vivo CRISPR screens reveal the landscape of immune evasion pathways across cancer. Nat Immunol (2022) 23:1495–506. doi: 10.1038/s41590-022-01315-x
27. Cai Z, Xing R, Liu J, Xing F. Commentary: PIRs mediate innate myeloid cell memory to nonself MHC molecules. Front Immunol (2021) 12:721344. doi: 10.3389/fimmu.2021.721344
28. Magro A, Magro A, Shrestha S, Brundage K, Rankin G. Metalloproteinase dependent reduction of cell surface cluster determinants upon the induction of apoptosis. Int J Oncol (2014) 44:1539–50. doi: 10.3892/ijo.2014.2344
29. Belizário JE, Neyra JM, Setúbal Destro Rodrigues MF. When and how NK cell-induced programmed cell death benefits immunological protection against intracellular pathogen infection. Innate Immun (2018) 24:452–65. doi: 10.1177/1753425918800200
30. Krzewski K, Gil-Krzewska A, Nguyen V, Peruzzi G, Coligan JE. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood (2013) 121:4672–83. doi: 10.1182/blood-2012-08-453738
31. Prager I, Liesche C, van Ooijen H, Urlaub D, Verron Q, Sandström N, et al. NK cells switch from granzyme b to death receptor-mediated cytotoxicity during serial killing. J Exp Med (2019) 216:2113–27. doi: 10.1084/jem.20181454
32. Du L, Deng W, Zeng S, Xu P, Huang L, Liang Y, et al. Single-cell transcriptome analysis reveals defective decidua stromal niche attributes to recurrent spontaneous abortion. Cell Prolif (2021) 54:e13125. doi: 10.1111/cpr.13125
33. Turchinovich G, Ganter S, Bärenwaldt A, Finke D. NKp46 calibrates tumoricidal potential of type 1 innate lymphocytes by regulating TRAIL expression. J Immunol (2018) 200:3762–8. doi: 10.4049/jimmunol.1701333
34. De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell (2008) 14:299–311. doi: 10.1016/j.ccr.2008.09.004
35. Zhuang L, Fulton RJ, Rettman P, Sayan AE, Coad J, Al-Shamkhani A, et al. Activity of IL-12/15/18 primed natural killer cells against hepatocellular carcinoma. Hepatol Int (2019) 13:75–83. doi: 10.1007/s12072-018-9909-3
36. Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev (2002) 13:143–54. doi: 10.1016/s1359-6101(01)00033-8
37. Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell (2018) 172:1022–1037.e14. doi: 10.1016/j.cell.2018.01.004
38. Zhu H, Blum RH, Bjordahl R, Gaidarova S, Rogers P, Lee TT, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood (2020) 135:399–410. doi: 10.1182/blood.2019000621
39. Cózar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating natural killer cells. Cancer Discovery (2021) 11:34–44. doi: 10.1158/2159-8290.CD-20-0655
40. Ou Z-L, Luo Z, Wei W, Liang S, Gao T-L, Lu Y-B. Hypoxia-induced shedding of MICA and HIF1A-mediated immune escape of pancreatic cancer cells from NK cells: role of circ_0000977/miR-153 axis. RNA Biol (2019) 16:1592–603. doi: 10.1080/15476286.2019.1649585
41. Badrinath S, Dellacherie MO, Li A, Zheng S, Zhang X, Sobral M, et al. A vaccine targeting resistant tumours by dual T cell plus NK cell attack. Nature (2022) 606:992–8. doi: 10.1038/s41586-022-04772-4
42. Huang J, Song P, Hang K, Chen Z, Zhu Z, Zhang Y, et al. Sleep deprivation disturbs immune surveillance and promotes the progression of hepatocellular carcinoma. Front Immunol (2021) 12:727959. doi: 10.3389/fimmu.2021.727959
43. Li H, Zhai N, Wang Z, Song H, Yang Y, Cui A, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut (2018) 67:2035–44. doi: 10.1136/gutjnl-2017-314098
44. Ghaddar B, Biswas A, Harris C, Omary MB, Carpizo DR, Blaser MJ, et al. Tumor microbiome links cellular programs and immunity in pancreatic cancer. Cancer Cell (2022) 40:1240–1253.e5. doi: 10.1016/j.ccell.2022.09.009
45. Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol (2022). doi: 10.1038/s41571-022-00689-z
46. Ribas A, Haining WN, Schumancher TNM. When cancer cells become the enablers of an antitumor immune response. Cancer Discovery (2022) 12. doi: 10.1158/2159-8290.CD-22-0706
47. Cooley S, Parham P, Miller JS. Strategies to activate NK cells to prevent relapse and induce remission following hematopoietic stem cell transplantation. Blood (2018) 131:1053–62. doi: 10.1182/blood-2017-08-752170
48. Simonetta F, Lohmeyer JK, Hirai T, Maas-Bauer K, Alvarez M, Wenokur AS, et al. Allogeneic CAR invariant natural killer T cells exert potent antitumor effects through host CD8 T-cell cross-priming. Clin Cancer Res (2021) 27:6054–64. doi: 10.1158/1078-0432.CCR-21-1329
49. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
50. Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol (2013) 10:230–52. doi: 10.1038/cmi.2013.10
51. Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett (2020) 472:175–80. doi: 10.1016/j.canlet.2019.11.033
52. Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol (2019) 10:2278. doi: 10.3389/fimmu.2019.02278
53. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol (2021) 18:842–59. doi: 10.1038/s41423-020-00565-9
54. Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell (2019) 36:471–82. doi: 10.1016/j.ccell.2019.09.006
55. Wang H, Kaur G, Sankin AI, Chen F, Guan F, Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J Hematol Oncol (2019) 12:59. doi: 10.1186/s13045-019-0746-1
56. Yilmaz A, Cui H, Caligiuri MA, Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J Hematol Oncol (2020) 13:168. doi: 10.1186/s13045-020-00998-9
57. Valentine M, Li L, Zhou H, Xu S, Sun J, Liu C, et al. Transferrin epitope-CD19-CAR-T cells effectively kill lymphoma cells in vitro and in vivo. Front Biosci (Landmark Ed) (2020) 25:270–82. doi: 10.2741/4806
58. Qin JS, Johnstone TG, Baturevych A, Hause RJ, Ragan SP, Clouser CR, et al. Antitumor potency of an anti-CD19 chimeric antigen receptor T-cell therapy, lisocabtagene maraleucel in combination with ibrutinib or acalabrutinib. J Immunother (2020) 43:107–20. doi: 10.1097/CJI.0000000000000307
59. Pan K, Farrukh H, Chittepu VCSR, Xu H, Pan C-X, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res (2022) 41:119. doi: 10.1186/s13046-022-02327-z
60. Jan C-I, Huang S-W, Canoll P, Bruce JN, Lin Y-C, Pan C-M, et al. Targeting human leukocyte antigen G with chimeric antigen receptors of natural killer cells convert immunosuppression to ablate solid tumors. J Immunother Cancer (2021) 9:e003050. doi: 10.1136/jitc-2021-003050
61. Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in b-cell malignancies. J Cell Mol Med (2016) 20:1287–94. doi: 10.1111/jcmm.12810
62. Soldierer M, Bister A, Haist C, Thivakaran A, Cengiz SC, Sendker S, et al. Genetic engineering and enrichment of human NK cells for CAR-enhanced immunotherapy of hematological malignancies. Front Immunol (2022) 13:847008. doi: 10.3389/fimmu.2022.847008
63. Hughes-Parry HE, Cross RS, Jenkins MR. The evolving protein engineering in the design of chimeric antigen receptor T cells. Int J Mol Sci (2019) 21:E204. doi: 10.3390/ijms21010204
64. Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol (2021) 14:73. doi: 10.1186/s13045-021-01083-5
65. Daher M, Rezvani K. Outlook for new CAR-based therapies with a focus on CAR NK cells: What lies beyond CAR-engineered T cells in the race against cancer. Cancer Discovery (2021) 11:45–58. doi: 10.1158/2159-8290.CD-20-0556
66. Wingert S, Reusch U, Knackmuss S, Kluge M, Damrat M, Pahl J, et al. Preclinical evaluation of AFM24, a novel CD16A-specific innate immune cell engager targeting EGFR-positive tumors. MAbs (2021) 13:1950264. doi: 10.1080/19420862.2021.1950264
67. Wang J, Lupo KB, Chambers AM, Matosevic S. Purinergic targeting enhances immunotherapy of CD73+ solid tumors with piggyBac-engineered chimeric antigen receptor natural killer cells. J Immunother Cancer (2018) 6:136. doi: 10.1186/s40425-018-0441-8
68. Leivas A, Valeri A, Córdoba L, García-Ortiz A, Ortiz A, Sánchez-Vega L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J (2021) 11:146. doi: 10.1038/s41408-021-00537-w
69. Du Z, Ng YY, Zha S, Wang S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol Ther Methods Clin Dev (2021) 23:582–96. doi: 10.1016/j.omtm.2021.10.014
70. Christodoulou I, Ho WJ, Marple A, Ravich JW, Tam A, Rahnama R, et al. Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities. J Immunother Cancer (2021) 9:e003894. doi: 10.1136/jitc-2021-003894
71. Töpfer K, Cartellieri M, Michen S, Wiedemuth R, Müller N, Lindemann D, et al. DAP12-based activating chimeric antigen receptor for NK cell tumor immunotherapy. J Immunol (2015) 194:3201–12. doi: 10.4049/jimmunol.1400330
72. Lu C, Guo C, Chen H, Zhang H, Zhi L, Lv T, et al. A novel chimeric PD1-NKG2D-41BB receptor enhances antitumor activity of NK92 cells against human lung cancer H1299 cells by triggering pyroptosis. Mol Immunol (2020) 122:200–6. doi: 10.1016/j.molimm.2020.04.016
73. Zhuang X, Long EO. NK cells equipped with a chimeric antigen receptor that overcomes inhibition by HLA class I for adoptive transfer of CAR-NK cells. Front Immunol (2022) 13:840844. doi: 10.3389/fimmu.2022.840844
74. Ma R, Lu T, Li Z, Teng K-Y, Mansour AG, Yu M, et al. An oncolytic virus expressing IL15/IL15Rα combined with off-the-Shelf EGFR-CAR NK cells targets glioblastoma. Cancer Res (2021) 81:3635–48. doi: 10.1158/0008-5472.CAN-21-0035
75. Wang X, Jasinski DL, Medina JL, Spencer DM, Foster AE, Bayle JH. Inducible MyD88/CD40 synergizes with IL-15 to enhance antitumor efficacy of CAR-NK cells. Blood Adv (2020) 4:1950–64. doi: 10.1182/bloodadvances.2020001510
76. Rafei H, Daher M, Rezvani K. Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: Leveraging the power of innate immunity. Br J Haematol (2021) 193:216–30. doi: 10.1111/bjh.17186
77. Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer: Implications for immunotherapy. Front Oncol (2021) 11:610303. doi: 10.3389/fonc.2021.610303
78. Ueda T, Kumagai A, Iriguchi S, Yasui Y, Miyasaka T, Nakagoshi K, et al. Non-clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti-glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci (2020) 111:1478–90. doi: 10.1111/cas.14374
79. Li Y, Basar R, Wang G, Liu E, Moyes JS, Li L, et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat Med (2022) 28:2133–44. doi: 10.1038/s41591-022-02003-x
80. Park J-E, Kim S-E, Keam B, Park H-R, Kim S, Kim M, et al. Anti-tumor effects of NK cells and anti-PD-L1 antibody with antibody-dependent cellular cytotoxicity in PD-L1-positive cancer cell lines. J Immunother Cancer (2020) 8:e000873. doi: 10.1136/jitc-2020-000873
81. Zhu H, Kaufman DS. Engineered human pluripotent stem cell-derived natural killer cells: the next frontier for cancer immunotherapy. Blood Sci (2019) 1:4–11. doi: 10.1097/BS9.0000000000000023
82. Morgan MA, Büning H, Sauer M, Schambach A. Use of cell and genome modification technologies to generate improved “Off-the-Shelf” CAR T and CAR NK cells. Front Immunol (2020) 11:1965. doi: 10.3389/fimmu.2020.01965
83. Bateman RM, Sharpe MD, Jagger JE, Ellis CG, Solé-Violán J, López-Rodríguez M, et al. 36th international symposium on intensive care and emergency Medicine : Brussels, belgium. 15-18 march 2016. Crit Care (2016) 20:94. doi: 10.1186/s13054-016-1208-6
84. Voynova E, Hawk N, Flomerfelt FA, Telford WG, Gress RE, Kanakry JA, et al. Increased activity of a NK-specific CAR-NK framework targeting CD3 and CD5 for T-cell leukemias. Cancers (Basel) (2022) 14:524. doi: 10.3390/cancers14030524
85. Grossenbacher SK, Aguilar EG, Murphy WJ. Leveraging natural killer cells for cancer immunotherapy. Immunotherapy (2017) 9:487–97. doi: 10.2217/imt-2017-0013
86. Mata MM, Mahmood F, Sowell RT, Baum LL. Effects of cryopreservation on effector cells for antibody dependent cell-mediated cytotoxicity (ADCC) and natural killer (NK) cell activity in (51)Cr-release and CD107a assays. J Immunol Methods (2014) 406:1–9. doi: 10.1016/j.jim.2014.01.017
87. Marofi F, Rahman HS, Thangavelu L, Dorofeev A, Bayas-Morejón F, Shirafkan N, et al. Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy. Stem Cell Res Ther (2021) 12:200. doi: 10.1186/s13287-021-02251-7
88. Kloess S, Oberschmidt O, Dahlke J, Vu X-K, Neudoerfl C, Kloos A, et al. Preclinical assessment of suitable natural killer cell sources for chimeric antigen receptor natural killer-based “Off-the-Shelf” acute myeloid leukemia immunotherapies. Hum Gene Ther (2019) 30:381–401. doi: 10.1089/hum.2018.247
89. Gurney M, O’Reilly E, Corcoran S, Brophy S, Krawczyk J, Otto NM, et al. Concurrent transposon engineering and CRISPR/Cas9 genome editing of primary CLL-1 chimeric antigen receptor-natural killer cells. Cytotherapy (2022) 24:1087–94. doi: 10.1016/j.jcyt.2022.07.008
90. Zhu Y-G, Xiao B-F, Zhang J-T, Cui X-R, Lu Z-M, Wu N. Genetically modified T cells for esophageal cancer therapy: A promising clinical application. Front Oncol (2021) 11:763806. doi: 10.3389/fonc.2021.763806
91. Müller S, Bexte T, Gebel V, Kalensee F, Stolzenberg E, Hartmann J, et al. High cytotoxic efficiency of lentivirally and alpharetrovirally engineered CD19-specific chimeric antigen receptor natural killer cells against acute lymphoblastic leukemia. Front Immunol (2019) 10:3123. doi: 10.3389/fimmu.2019.03123
92. Roex G, Campillo-Davo D, Flumens D, Shaw PAG, Krekelbergh L, De Reu H, et al. Two for one: Targeting BCMA and CD19 in b-cell malignancies with off-the-shelf dual-CAR NK-92 cells. J Transl Med (2022) 20:124. doi: 10.1186/s12967-022-03326-6
93. Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, et al. Adoptive transfer of NKG2D CAR mRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther (2019) 27:1114–25. doi: 10.1016/j.ymthe.2019.03.011
94. Oberschmidt O, Morgan M, Huppert V, Kessler J, Gardlowski T, Matthies N, et al. Development of automated separation, expansion, and quality control protocols for clinical-scale manufacturing of primary human NK cells and alpharetroviral chimeric antigen receptor engineering. Hum Gene Ther Methods (2019) 30:102–20. doi: 10.1089/hgtb.2019.039
95. Klöß S, Oberschmidt O, Morgan M, Dahlke J, Arseniev L, Huppert V, et al. Optimization of human NK cell manufacturing: Fully automated separation, improved ex vivo expansion using IL-21 with autologous feeder cells, and generation of anti-CD123-CAR-Expressing effector cells. Hum Gene Ther (2017) 28:897–913. doi: 10.1089/hum.2017.157
96. Jennifer Zhang Q. Donor selection based on NK alloreactivity for patients with hematological malignancies. Hum Immunol (2022) 83:695–703. doi: 10.1016/j.humimm.2022.07.006
97. Gemelli M, Noonan DM, Carlini V, Pelosi G, Barberis M, Ricotta R, et al. Overcoming resistance to checkpoint inhibitors: Natural killer cells in non-small cell lung cancer. Front Oncol (2022) 12:886440. doi: 10.3389/fonc.2022.886440
98. Pockley AG, Vaupel P, Multhoff G. NK cell-based therapeutics for lung cancer. Expert Opin Biol Ther (2020) 20:23–33. doi: 10.1080/14712598.2020.1688298
99. Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res (2017) 23:2255–66. doi: 10.1158/1078-0432.CCR-16-1300
100. Chen J, López-Moyado IF, Seo H, Lio C-WJ, Hempleman LJ, Sekiya T, et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature (2019) 567:530–4. doi: 10.1038/s41586-019-0985-x
101. Adusumilli PS, Zauderer MG, Rivière I, Solomon SB, Rusch VW, O’Cearbhaill RE, et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discovery (2021) 11:2748–63. doi: 10.1158/2159-8290.CD-21-0407
102. Chauvin J-M, Ka M, Pagliano O, Menna C, Ding Q, DeBlasio R, et al. IL15 stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin Cancer Res (2020) 26:5520–33. doi: 10.1158/1078-0432.CCR-20-0575
103. Liu L, You X, Han S, Sun Y, Zhang J, Zhang Y. CD155/TIGIT, a novel immune checkpoint in human cancers (Review). Oncol Rep (2021) 45:835–45. doi: 10.3892/or.2021.7943
104. Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol (2009) 10:48–57. doi: 10.1038/ni.1674
105. Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol (2020) 200:108–19. doi: 10.1111/cei.13407
106. Song D, Yan F, Fu H, Li L, Hao J, Zhu Z, et al. A cellular census of human peripheral immune cells identifies novel cell states in lung diseases. Clin Transl Med (2021) 11:e579. doi: 10.1002/ctm2.579
107. Freed-Pastor WA, Lambert LJ, Ely ZA, Pattada NB, Bhutkar A, Eng G, et al. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen-expressing pancreatic cancer. Cancer Cell (2021) 39:1342–1360.e14. doi: 10.1016/j.ccell.2021.07.007
108. Arruga F, Gyau BB, Iannello A, Vitale N, Vaisitti T, Deaglio S. Immune response dysfunction in chronic lymphocytic leukemia: Dissecting molecular mechanisms and microenvironmental conditions. Int J Mol Sci (2020) 21:E1825. doi: 10.3390/ijms21051825
109. Szereday L, Nagy DU, Csiszar B, Kevey D, Feik T, Meggyes M. Examination of the TIGIT, CD226, CD112, and CD155 immune checkpoint molecules in peripheral blood mononuclear cells in women diagnosed with early-onset preeclampsia. Biomedicines (2021) 9:1608. doi: 10.3390/biomedicines9111608
110. Kawashima S, Inozume T, Kawazu M, Ueno T, Nagasaki J, Tanji E, et al. TIGIT/CD155 axis mediates resistance to immunotherapy in patients with melanoma with the inflamed tumor microenvironment. J Immunother Cancer (2021) 9:e003134. doi: 10.1136/jitc-2021-003134
111. He Y, Peng H, Sun R, Wei H, Ljunggren H-G, Yokoyama WM, et al. Contribution of inhibitory receptor TIGIT to NK cell education. J Autoimmun (2017) 81:1–12. doi: 10.1016/j.jaut.2017.04.001
112. Sun H, Huang Q, Huang M, Wen H, Lin R, Zheng M, et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology (2019) 70:168–83. doi: 10.1002/hep.30347
113. Maas RJ, Hoogstad-van Evert JS, van der Meer JM, Mekers V, Rezaeifard S, Korman AJ, et al. TIGIT blockade enhances functionality of peritoneal NK cells with altered expression of DNAM-1/TIGIT/CD96 checkpoint molecules in ovarian cancer. Oncoimmunology (2020) 9:1843247. doi: 10.1080/2162402X.2020.1843247
114. Chan IS, Knútsdóttir H, Ramakrishnan G, Padmanaban V, Warrier M, Ramirez JC, et al. Cancer cells educate natural killer cells to a metastasis-promoting cell state. J Cell Biol (2020) 219:e202001134. doi: 10.1083/jcb.202001134
115. Chiu DK, Yuen VW, Cheu JW, Wei LL, Ting V, et al. Hepatocellular carcinoma cells up-regulate PVRL1, stabilizing PVR and inhibiting the cytotoxic T-cell response via TIGIT to mediate tumor resistance to PD1 inhibitors in mice. Gastroenterology (2020) 159. doi: 10.1053/j.gastro.2020.03.074
116. Guillerey C, Harjunpää H, Carrié N, Kassem S, Teo T, Miles K, et al. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood (2018) 132:1689–94. doi: 10.1182/blood-2018-01-825265
117. André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell (2018) 175:1731–1743.e13. doi: 10.1016/j.cell.2018.10.014
118. Kusumi M, Yamashita T, Fujii T, Nagamatsu T, Kozuma S, Taketani Y. Expression patterns of lectin-like natural killer receptors, inhibitory CD94/NKG2A, and activating CD94/NKG2C on decidual CD56bright natural killer cells differ from those on peripheral CD56dim natural killer cells. J Reprod Immunol (2006) 70:33–42. doi: 10.1016/j.jri.2005.12.008
119. Plougastel B, Jones T, Trowsdale J. Genomic structure, chromosome location, and alternative splicing of the human NKG2A gene. Immunogenetics (1996) 44:286–91. doi: 10.1007/BF02602558
120. Yabe T, McSherry C, Bach FH, Fisch P, Schall RP, Sondel PM, et al. A multigene family on human chromosome 12 encodes natural killer-cell lectins. Immunogenetics (1993) 37:455–60. doi: 10.1007/BF00222470
121. Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J Immunol (2006) 177:3590–6. doi: 10.4049/jimmunol.177.6.3590
122. Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest (2019) 129:2094–106. doi: 10.1172/JCI123955
123. Shreeve N, Depierreux D, Hawkes D, Traherne JA, Sovio U, Huhn O, et al. The CD94/NKG2A inhibitory receptor educates uterine NK cells to optimize pregnancy outcomes in humans and mice. Immunity (2021) 54:1231–1244.e4. doi: 10.1016/j.immuni.2021.03.021
124. Lentz RW, Colton MD, Mitra SS, Messersmith WA. Innate immune checkpoint inhibitors: The next breakthrough in medical oncology? Mol Cancer Ther (2021) 20:961–74. doi: 10.1158/1535-7163.MCT-21-0041
125. Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, et al. Senescent cells evade immune clearance via HLA-e-mediated NK and CD8+ T cell inhibition. Nat Commun (2019) 10:2387. doi: 10.1038/s41467-019-10335-5
126. Le Dréan E, Vély F, Olcese L, Cambiaggi A, Guia S, Krystal G, et al. Inhibition of antigen-induced T cell response and antibody-induced NK cell cytotoxicity by NKG2A: Association of NKG2A with SHP-1 and SHP-2 protein-tyrosine phosphatases. Eur J Immunol (1998) 28:264–76. doi: 10.1002/(SICI)1521-4141(199801)28:01<264::AID-IMMU264>3.0.CO;2-O
127. Carretero M, Palmieri G, Llano M, Tullio V, Santoni A, Geraghty DE, et al. Specific engagement of the CD94/NKG2-a killer inhibitory receptor by the HLA-e class ib molecule induces SHP-1 phosphatase recruitment to tyrosine-phosphorylated NKG2-a: Evidence for receptor function in heterologous transfectants. Eur J Immunol (1998) 28:1280–91. doi: 10.1002/(SICI)1521-4141(199804)28:04<1280::AID-IMMU1280>3.0.CO;2-O
128. Lin Chua H, Brahmi Z. Expression of p58.2 or CD94/NKG2A inhibitory receptors in an NK-like cell line, YTINDY, leads to HLA class I-mediated inhibition of cytotoxicity in the p58.2- but not the CD94/NKG2A-expressing transfectant. Cell Immunol (2002) 219:57–70. doi: 10.1016/s0008-8749(02)00578-6
129. Vacca P, Pietra G, Tumino N, Munari E, Mingari MC, Moretta L. Exploiting human NK cells in tumor therapy. Front Immunol (2019) 10:3013. doi: 10.3389/fimmu.2019.03013
130. Berrien-Elliott MM, Cashen AF, Cubitt CC, Neal CC, Wong P, Wagner JA, et al. Multidimensional analyses of donor memory-like NK cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discovery (2020) 10:1854–71. doi: 10.1158/2159-8290.CD-20-0312
131. Wang X, Xiong H, Ning Z. Implications of NKG2A in immunity and immune-mediated diseases. Front Immunol (2022) 13:960852. doi: 10.3389/fimmu.2022.960852
132. Antonioli L, Fornai M, Pellegrini C, Blandizzi C. NKG2A and COVID-19: another brick in the wall. Cell Mol Immunol (2020) 17:672–4. doi: 10.1038/s41423-020-0450-7
133. Klümper N, Ralser DJ, Bawden EG, Landsberg J, Zarbl R, Kristiansen G, et al. LAG3 (LAG-3, CD223) DNA methylation correlates with LAG3 expression by tumor and immune cells, immune cell infiltration, and overall survival in clear cell renal cell carcinoma. J Immunother Cancer (2020) 8:e000552. doi: 10.1136/jitc-2020-000552
134. Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, et al. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci U.S.A. (1997) 94:5744–9. doi: 10.1073/pnas.94.11.5744
135. Huard B, Tournier M, Triebel F. LAG-3 does not define a specific mode of natural killing in human. Immunol Lett (1998) 61:109–12. doi: 10.1016/s0165-2478(97)00170-3
136. Bruniquel D, Borie N, Triebel F. Genomic organization of the human LAG-3/CD4 locus. Immunogenetics (1997) 47:96–8. doi: 10.1007/s002510050332
137. Workman CJ, Vignali DAA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol (2003) 33:970–9. doi: 10.1002/eji.200323382
138. Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell (2019) 176:334–347.e12. doi: 10.1016/j.cell.2018.11.010
139. Sahir F, Mateo JM, Steinhoff M, Siveen KS. Development of a 43 color panel for the characterization of conventional and unconventional T-cell subsets, b cells, NK cells, monocytes, dendritic cells, and innate lymphoid cells using spectral flow cytometry. Cytometry A (2020). doi: 10.1002/cyto.a.24288
140. Bauché D, Joyce-Shaikh B, Jain R, Grein J, Ku KS, Blumenschein WM, et al. LAG3+ regulatory T cells restrain interleukin-23-Producing CX3CR1+ gut-resident macrophages during group 3 innate lymphoid cell-driven colitis. Immunity (2018) 49:342–352.e5. doi: 10.1016/j.immuni.2018.07.007
141. Huang C-T, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity (2004) 21:503–13. doi: 10.1016/j.immuni.2004.08.010
142. Maruhashi T, Sugiura D, Okazaki I-M, Shimizu K, Maeda TK, Ikubo J, et al. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity (2022) 55:912–924.e8. doi: 10.1016/j.immuni.2022.03.013
143. Andreae S, Piras F, Burdin N, Triebel F. Maturation and activation of dendritic cells induced by lymphocyte activation gene-3 (CD223). J Immunol (2002) 168:3874–80. doi: 10.4049/jimmunol.168.8.3874
144. Johnson DB, Nixon MJ, Wang Y, Wang DY, Castellanos E, Estrada MV, et al. Tumor-specific MHC-II expression drives a unique pattern of resistance to immunotherapy via LAG-3/FCRL6 engagement. JCI Insight (2018) 3:e120360. doi: 10.1172/jci.insight.120360
145. Huang L, Xu W, Liu H, Xue M, Liu X, Zhang K, et al. African Swine fever virus pI215L negatively regulates cGAS-STING signaling pathway through recruiting RNF138 to inhibit K63-linked ubiquitination of TBK1. J Immunol (2021) 207:2754–69. doi: 10.4049/jimmunol.2100320
146. Marçais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol (2014) 15:749–57. doi: 10.1038/ni.2936
147. Cao Y, Wang X, Jin T, Tian Y, Dai C, Widarma C, et al. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct Target Ther (2020) 5:250. doi: 10.1038/s41392-020-00348-8
148. Zhao L, Cheng S, Fan L, Zhang B, Xu S. TIM-3: An update on immunotherapy. Int Immunopharmacol (2021) 99:107933. doi: 10.1016/j.intimp.2021.107933
149. Meggyes M, Miko E, Polgar B, Bogar B, Farkas B, Illes Z, et al. Peripheral blood TIM-3 positive NK and CD8+ T cells throughout pregnancy: TIM-3/galectin-9 interaction and its possible role during pregnancy. PloS One (2014) 9:e92371. doi: 10.1371/journal.pone.0092371
150. Zhao L, Yu G, Han Q, Cui C, Zhang B. TIM-3: An emerging target in the liver diseases. Scand J Immunol (2020) 91:e12825. doi: 10.1111/sji.12825
151. Zhao D, Guo M, Liu B, Lin Q, Xie T, Zhang Q, et al. Frontline science: Tim-3-mediated dysfunctional engulfment of apoptotic cells in SLE. J Leukoc Biol (2017) 102:1313–22. doi: 10.1189/jlb.3HI0117-005RR
152. Zhou J, Yu X, Hou L, Zhao J, Zhou F, Chu X, et al. Epidermal growth factor receptor tyrosine kinase inhibitor remodels tumor microenvironment by upregulating LAG-3 in advanced non-small-cell lung cancer. Lung Cancer (2021) 153:143–9. doi: 10.1016/j.lungcan.2021.01.010
153. Dixon KO, Tabaka M, Schramm MA, Xiao S, Tang R, Dionne D, et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature (2021) 595:101–6. doi: 10.1038/s41586-021-03626-9
154. Schultheiß C, Paschold L, Simnica D, Mohme M, Willscher E, von Wenserski L, et al. Next-generation sequencing of T and b cell receptor repertoires from COVID-19 patients showed signatures associated with severity of disease. Immunity (2020) 53:442–455.e4. doi: 10.1016/j.immuni.2020.06.024
155. Yang R, Sun L, Li C-F, Wang Y-H, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun (2021) 12:832. doi: 10.1038/s41467-021-21099-2
156. Saresella M, Piancone F, Marventano I, La Rosa F, Tortorella P, Caputo D, et al. A role for the TIM-3/GAL-9/BAT3 pathway in determining the clinical phenotype of multiple sclerosis. FASEB J (2014) 28:5000–9. doi: 10.1096/fj.14-258194
157. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol (2005) 6:1245–52. doi: 10.1038/ni1271
158. Yu X, Lang B, Chen X, Tian Y, Qian S, Zhang Z, et al. The inhibitory receptor Tim-3 fails to suppress IFN-γ production via the NFAT pathway in NK-cell, unlike that in CD4+ T cells. BMC Immunol (2021) 22:25. doi: 10.1186/s12865-021-00417-9
159. So EC, Khaladj-Ghom A, Ji Y, Amin J, Song Y, Burch E, et al. NK cell expression of Tim-3: First impressions matter. Immunobiology (2019) 224:362–70. doi: 10.1016/j.imbio.2019.03.001
160. Albini A, Gallazzi M, Palano MT, Carlini V, Ricotta R, Bruno A, et al. TIMP1 and TIMP2 downregulate TGFβ induced decidual-like phenotype in natural killer cells. Cancers (Basel) (2021) 13:4955. doi: 10.3390/cancers13194955
161. Xu L-Y, Chen D-D, He J-Y, Lu C-C, Liu X-G, Le H-B, et al. Tim-3 expression by peripheral natural killer cells and natural killer T cells increases in patients with lung cancer–reduction after surgical resection. Asian Pac J Cancer Prev (2014) 15:9945–8. doi: 10.7314/apjcp.2014.15.22.9945
162. Buckle I, Guillerey C. Inhibitory receptors and immune checkpoints regulating natural killer cell responses to cancer. Cancers (Basel) (2021) 13:4263. doi: 10.3390/cancers13174263
163. Zheng Y, Li Y, Lian J, Yang H, Li F, Zhao S, et al. TNF-α-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J Transl Med (2019) 17:165. doi: 10.1186/s12967-019-1917-0
164. Chauvin J-M, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest (2015) 125:2046–58. doi: 10.1172/JCI80445\
165. Jiang W, Li F, Jiang Y, Li S, Liu X, Xu Y, et al. Tim-3 blockade elicits potent anti-multiple myeloma immunity of natural killer cells. Front Oncol (2022) 12:739976. doi: 10.3389/fonc.2022.739976
166. Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother (2010) 59:1739–44. doi: 10.1007/s00262-010-0896-z
167. Gang M, Marin ND, Wong P, Neal CC, Marsala L, Foster M, et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood (2020) 136:2308–18. doi: 10.1182/blood.2020006619
168. Menon T, Gopal S, Rastogi Verma S. Targeted therapies in non-small cell lung cancer and the potential role of AI interventions in cancer treatment. Biotechnol Appl Biochem (2022). doi: 10.1002/bab.2356
169. Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine (2020) 59:102975. doi: 10.1016/j.ebiom.2020.102975
170. Wrona E, Borowiec M, Potemski P. CAR-NK cells in the treatment of solid tumors. Int J Mol Sci (2021) 22:5899. doi: 10.3390/ijms22115899
Keywords: CAR-NK cell, tumor immunotherapy, immune checkpoint inhibitors, clinical, prospect
Citation: Yang K, Zhao Y, Sun G, Zhang X, Cao J, Shao M, Liang X and Wang L (2023) Clinical application and prospect of immune checkpoint inhibitors for CAR-NK cell in tumor immunotherapy. Front. Immunol. 13:1081546. doi: 10.3389/fimmu.2022.1081546
Received: 27 October 2022; Accepted: 20 December 2022;
Published: 19 January 2023.
Edited by:
Marina Cella, Washington University in St. Louis, United StatesReviewed by:
Jiacheng Bi, Chinese Academy of Sciences (CAS), ChinaXiaoping Chen, Guangzhou Institutes of Biomedicine and Health (CAS), China
Copyright © 2023 Yang, Zhao, Sun, Zhang, Cao, Shao, Liang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xijun Liang, bGlhbmd4al8wNzExQDE2My5jb20=; Lina Wang, cmVuYTEwMjJAMTYzLmNvbQ==
†These authors share first authorship
 Kangdi Yang
Kangdi Yang Yuze Zhao2†
Yuze Zhao2† Xijun Liang
Xijun Liang