
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 15 December 2022
Sec. Immunological Memory
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1080855
This article is part of the Research TopicThe Characterization and Homeostasis of Allergen-Specific Memory T Cells in the LungView all articles
Memory T cells, which are generated after the primary immune response to cognate antigens, possess unique features compared to naïve or effector T cells. These memory T cells are maintained for a long period of time and robustly reactivate in lymphoid or peripheral tissues where they re-encounter antigens. Environments surrounding memory T cells are importantly involved in the process of the maintenance and reactivation of these T cells. Although memory T cells are generally believed to be formed in response to acute infections, the pathogenesis and persistence of chronic inflammatory diseases, including allergic diseases, are also related to the effector functions of memory CD4 T cells. Thus, the factors involved in the homeostasis of allergen-specific memory CD4 T cells need to be understood to surmount these diseases. Here, we review the characteristics of allergen-specific memory CD4 T cells in allergic diseases and the importance of extrinsic factors for the homeostasis and reactivation of these T cells in the view of mediating persistence, recurrence, and aggravation of allergic diseases. Overall, this review provides a better understanding of memory CD4 T cells to devise effective therapeutic strategies for refractory chronic inflammatory diseases.
Effector CD4 T cells, referred to as helper T (Th) cells, perform protective functions after evasion of distinct types of infectious pathogens, including viruses, bacteria, parasites, and fungi, by enhancing the functions of phagocytes, B cells, or other T cells through the secretion of cytokines (1–3). Th cells, including Th1, Th2, and Th17 cells, differentiate from naïve CD4 T cells after receiving proper cytokines depending on the type of infection or antigen during early immune responses (4, 5). Of particular interest are Th2 cells, because it is known that Th2 cells steer the pathogenesis of diverse allergic disorders by secreting principal type 2 cytokines, including IL-4, IL-5, IL-13, and IL-9 (6–8). These cytokines affect other immune cells, such as B cells, mast cells, eosinophils, as well as non-immune cells, including goblet cells and smooth muscle cells, which drive the pathogenesis of allergic disorders (9, 10).
When antigens are cleared by the immune system, most effector T cells die and only a small portion of these cells survive to serve memory functions. Once these memory T cells re-encounter their antigens, they respond robustly to protect the body by directly killing abnormal cells or further stimulating other immune or non-immune cells. Although these memory functions are generally useful in infectious diseases, the importance of memory CD4 T cells in the pathogenesis of chronic inflammatory diseases, such as allergic or autoimmune diseases, has been documented. For example, the numbers of CD45RO+ memory CD4 T cells in the nasal mucosa of patients with seasonal allergic rhinitis increased during continuous exposure to allergens during the pollen season compared to the non-pollen season (11). In addition, memory Th2 cells enhanced the severity of allergic responses, such as eosinophilic lung inflammation and airway hyperresponsiveness, after re-exposure to allergens in experimental allergic asthma models and atopic patients (12, 13).
In addition to their ability to induce robust recall responses, memory T cells retain the capacity to survive antigen-independently and restore their populations using external cytokines from their surrounding microenvironments (14, 15). This characteristic of memory T cells is referred to as homeostasis and is crucial for the maintenance and exacerbation of allergic diseases. Thus, in this review, we summarize the recent findings on allergen-specific memory CD4 T cells and their external homeostatic microenvironments that mediate the pathology of allergic diseases such as allergic asthma, atopic dermatitis (AD), and food allergies (FA).
Allergic disorders caused by innocuous environmental antigens manifest aberrant immune reactions in accordance with the exposure of allergens to the epithelium, including the lung, airways, skin, and gastrointestinal tract. Although allergic responses vary depending on the tissue, allergen-specific memory CD4 T cells in barrier tissues orchestrate the pathology of these diseases (Figure 1A).
Allergic asthma is a chronic inflammatory disease of the lung that is continuously exposed to inhaled external materials, including allergens. Th2 cells, major players in the pathology of allergic lung inflammatory disease, accumulated in the bronchoalveolar lavage fluid (BALF) of allergic asthma patients (16). In addition, many studies have highlighted the memory responses of Th2 cells in the pulmonary system in experimental animal models and in asthmatic patients (9, 17–21). These studies provide clues that allergen-specific memory Th2 cells are present in the lungs of asthmatic patients. However, the characteristics of memory Th2 cells have directly not been analyzed for their lifespan and migratory capacity until recently. This was probably due to the proposal that the lung does not provide favorable microenvironments for the survival of memory T cells (22).
Previously, our group employed TCR-transgenic mice and measured the longevity of allergen-specific memory CD4 T cells. We found that these cells survived for over 70 days in the murine lung and airways suffering from allergic inflammation (23). Moreover, it was discovered that allergen-specific memory CD4 T cells showed residency in these tissues and induced rapid and enhanced allergic lung inflammation upon allergen re-exposure. Other groups have suggested that resident memory T (TRM) cells in the lung are reactivated by allergens in situ and have transcriptional characteristics distinct from those of circulating memory Th2 cells (17, 18, 24). In human asthmatics, allergen-specific CD4 T cells were discovered in BALF after segmental allergen challenge, and these cells mediated allergic lung inflammation by providing type 2 cytokines and eosinophil recruitment (6, 9, 25). Additionally, the microbiota in the upper airways of humans was correlated with the development of adult asthma (26). Contrary to the original proposal, these studies support the notion that allergic lungs provide favorable microenvironments for the homeostasis of allergen-specific memory CD4 T cells.
Recently, it has also been shown that IL-17 producing cells participate in inducing and maintaining allergic asthma. These IL-17-producing memory CD4 T cells were reported to develop from allergen-specific memory Th2 cells after stimulation by proinflammatory cytokines, and to aggravate allergic asthma upon allergen re-exposure in mice and humans (27). Moreover, dual-positive Th2/Th17 cells differentiated from Th2 cells correlated with asthma severity and decreased responsiveness to dexamethasone in the BALF of asthmatic patients (28). These results may indicate that allergen-specific memory CD4 T cells, particularly Th2 cells, mediate disease relapses and aggravation, including steroid unresponsiveness in the lung.
Allergic skin diseases, such as AD and atopic eczema, induced by protein allergens or chemicals, are multifactorial and complex diseases (29). Similar to asthma, several reports have raised a point that these allergic skin diseases are also considered to be initiated and maintained by CD4 T cells (30–33). Upon allergic stimulations, CD4 T cells were shown to migrate massively into the epidermis of itchy sites and induce type 2 immune responses to mediate allergic skin diseases (34, 35). When allergic eczema patients were rechallenged with allergens, the numbers of allergen-reactive Th2 cells increased, mainly due to the proliferation of these cells in the skin lesions (36). Another study using AD patients presented that allergen-specific Th2 cells persisted in the skin for more than four years (37).
In the case of allergic contact dermatitis and contact hypersensitivity, hapten-specific CD4 TRM cells accumulated even after the skin has healed (38). Further, these cells expanded in the dermis and were sufficient to mediate skin inflammation after re-exposure to the same hapten. Thus, in this type of allergic disease, skin-resident allergen-specific memory CD4 T cells are crucial for mediating local skin inflammation. Altogether, allergen-specific memory CD4 T cells steer the recurrence and exacerbation of allergic inflammation through their persistence and reactivation in the skin.
FA triggers diverse symptoms by mediating type 2 inflammation in various tissues, including the cutaneous and respiratory systems, as well as the gastrointestinal system. In underlying pathologic mechanisms of FA, allergen-specific IgE and Th2 cells are considered important mediators (39–42). In accordance with the development of research tools, recent studies have indicated that allergen-specific CD4 T cells, in particular, IL-13-producing follicular helper T cells, play a role in the production of high affinity IgE rather than Th2 effector cells (43). These findings highlight that allergen-specific CD4 T cells are also involved in the pathology of FA.
Another important aspect of FA is the breakdown of oral tolerance. Oral tolerance to food antigens is mainly maintained by antigen-specific regulatory T (Treg) cells (39, 44). The activation of STAT6 in these Treg cells stimulated by IL-4 reprogrammed and converted them into a Th2-like phenotype, resulting in an imbalance in oral tolerance (Figure 1B) (45). Recently, a greater proportion of activated Tregs and memory-like Treg cells were observed in peripheral blood mononuclear cells (PBMCs) of peanut-allergic patients compared to non-allergic controls (46). Since severe manifestations, such as anaphylaxis, are induced after re-exposure to food antigens, it was speculated that food allergen-specific memory CD4 T cells are present in the gastrointestinal system (47). Although the presence and characteristics of allergen-specific memory CD4 T cells in the gut are yet to be defined, these diseases appear to represent memory responses to food allergens.
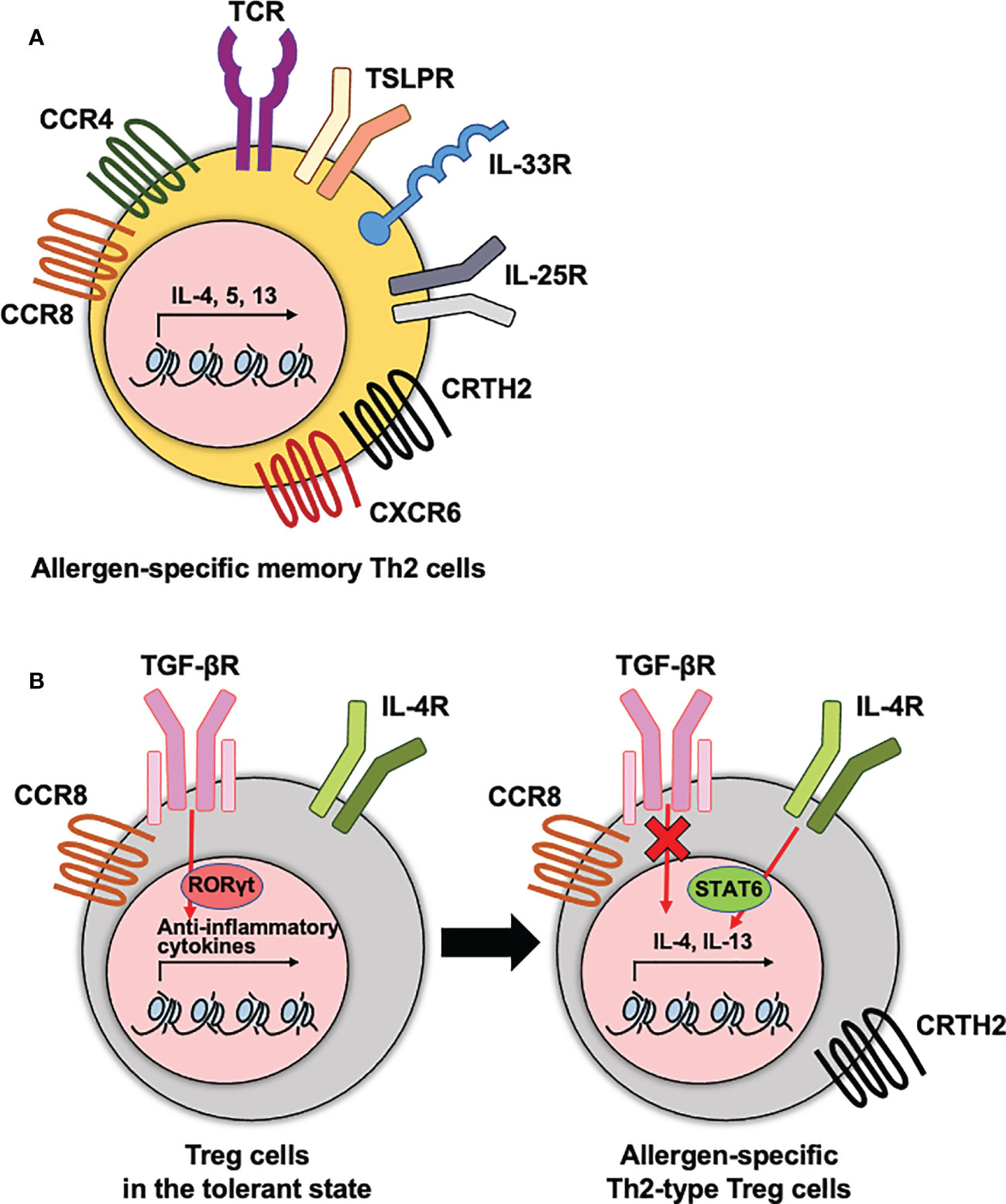
Figure 1 The phenotypes of allergen-specific memory CD4 T cells and Treg cells. (A) Allergen-specific memory Th2 cells express diverse cytokine and chemokine receptors that are correlated with the pathogenesis of allergic diseases. (B) Treg cells express diverse cytokine and chemokine receptors. To maintain the immune tolerance, TGF-βR needs to be expressed on Treg cells. This receptor signaling mediates RORγt transcription factor for anti-inflammatory functions of Treg cells. In dysfunctional Treg cells, hyperactive IL-4-STAT6 axis and no TGF-β signaling enhances type 2 cytokine production. These receptors contribute to the type 2 cytokine production by Th2 cells and dysfunctional Th2-type Treg cells during the pathogenesis of allergic diseases.
There have been many attempts to identify allergen-specific memory CD4 T cells regarding to their functions or disease severities in allergic disorders. Among the markers used to define the populations, cytokine or chemokine receptors expressed on CD4 T cells have well been elucidated as characteristics of allergen-specific memory CD4 T cells and Treg cells that mediate their recruitment and regulate their functions in the tissue (Figure 1). These receptors include C-C chemokine receptor 4 (CCR4), a chemoattractant receptor-homologous molecule expressed on Th2 (CRTH2), C-C chemokine receptor type 8 (CCR8), and C-X-C Motif Chemokine Receptor 6 (CXCR6).
CCR4 is highly expressed on Th2 cells and contributes to the migration of antigen-specific Th2 cells into inflamed tissue sites (48). In asthmatics, the frequency of CCR4+ CD4 T cells was higher in the BALF than in children without any airway diseases (49). Moreover, it has been shown that the frequencies of CCR4+ CD45RO+ CD4 T cells representing the memory phenotype were higher in allergic patients than in healthy subjects, and that these increased frequencies were positively correlated with AD disease severity (30, 50). In the case of FA, allergen-specific memory CD4 T cells indicated a CCR4+ phenotype in the PBMC of shrimp-allergic individuals (51).
The role of CRTH2, which has been used to identify human Th2 cells, includes the production of IL-4, IL-5, and IL-13, chemotaxis of Th2 cells in response to prostaglandin D2, and prevention of apoptosis (52–54). A greater number of CRTH2+ CD4 T cells was observed in patients with allergic diseases and was positively correlated with the severity of allergic airway or gastrointestinal inflammation (55–57). CRTH2+ allergen-specific memory CD4 T cells are associated with poor compliance to corticosteroids in allergic pulmonary inflammation (58). In addition, a recent study showed that CRTH2 expression on Tregs was also correlated with the malfunction of them as well as enhanced function of Th2 cells in allergic asthmatics (59).
The expression of CCR8 has a potential for driving the migration of CD4 T cells including Th2 cells and Foxp3+ CD4 T cells into allergic inflammatory sites (60). In atopic asthmatics and dermatitis patients, CCR8-expressing Th2 cells infiltrated in the airways and skin after allergen challenge, respectively (61, 62). Among the compartments of T cells in the skin, CCR8+ CD4 T cells are associated with a resident memory phenotype, CD69 and CD103, and greater proliferation capacity in in vitro stimulation (63).
CXCR6 is required for the localization of TRM cells in various infection models (64, 65). The level of this gene was highly expressed in the lung CD4 TRM cells than in circulating memory CD4 T cells after allergic lung inflammation (17). Moreover, recent research reported that the CXCR6 expression on Th2 cells correlated with the severity of atopic dermatitis (66).
Rechallenge of allergens increased the expression of diverse cytokine receptors on allergen-specific CD4 T cells. Type 2-associated innate immune receptors, including ST2 (IL-33 receptor) and IL17RB (IL-25 receptor), were highly expressed on allergen-specific CD4 T cells from the BALF of patients with allergic asthma after an allergen challenge (9). In the case of allergic rhinitis, the expression of IL17RB on CD4 T cells also increased in the blood of patients with seasonal allergic rhinitis after exposure to natural allergens (67). Moreover, ST2 expressing lung CD4 TRM cells contribute to the induction of excessive steroid-resistant eosinophilic inflammation in the lung (68).
In conclusion, diverse expressions of chemokine and cytokine receptors on allergen-specific memory CD4 T cells are implicated in the pathogenesis of allergic disorders. Thus, identification of the precise phenotype of allergen-specific memory CD4 T cells will provide a better tool for studying the roles of these cells.
The niches of the immune system regulate the survival, differentiation, and maintenance of immune cells under immune homeostatic or pathological conditions. Memory T cells are antigen-independently maintained, and reactivated by the interactions with neighboring cells, providing exogenous stimuli such as co-stimulatory signals and cytokine signals. In this section, we discuss the importance of exogenous regulatory mechanisms for the reactivation and homeostasis of allergen-specific memory CD4 T cells in allergic disorders (Figure 2).
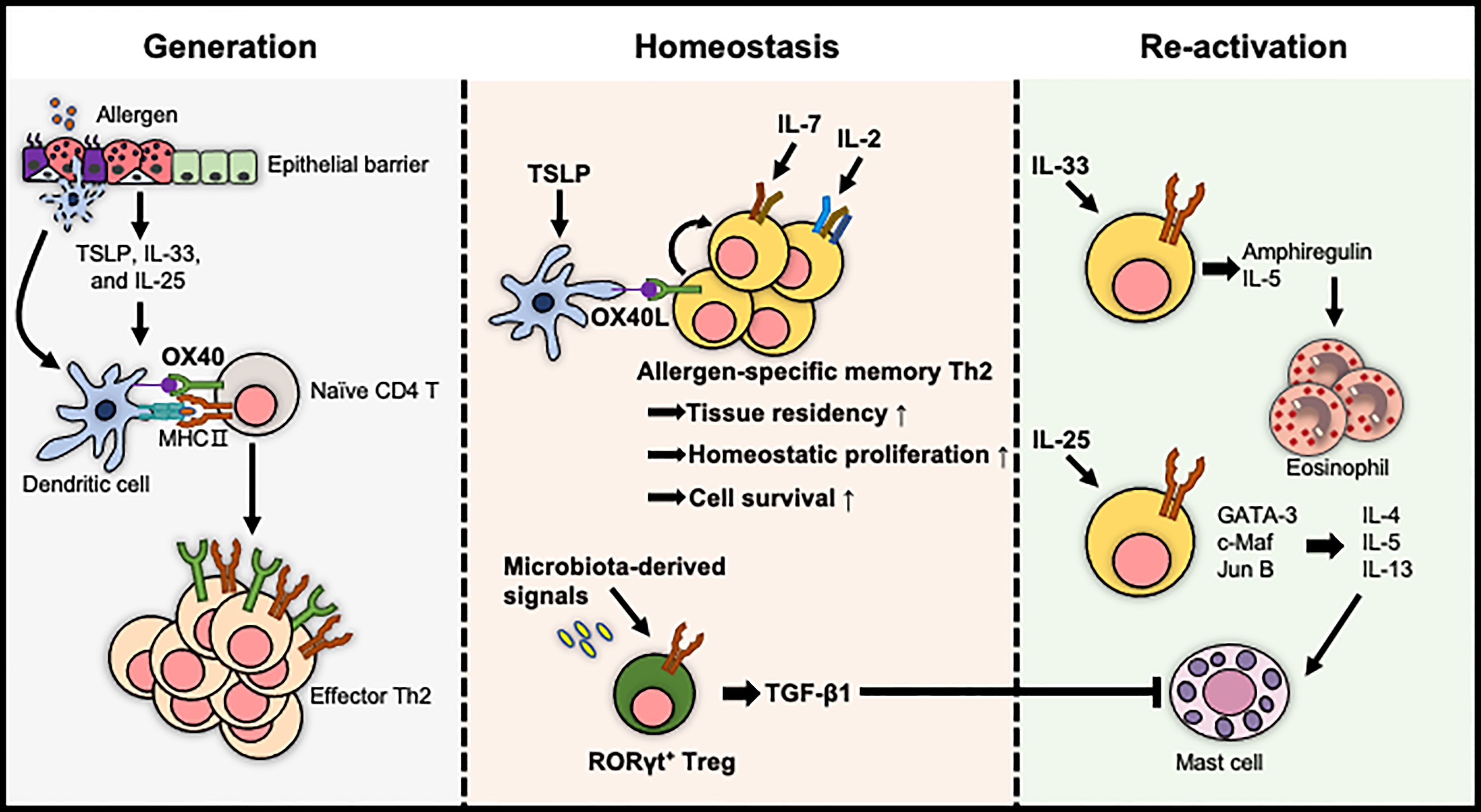
Figure 2 The extrinsic factors for the regulation of allergen-specific memory CD4 T cells. The generation of effector Th2 cells is proposed to be enhanced by the tissue-derived cytokine/DC axis. The pool of allergen-specific memory Th2 cells and their tissue residency are maintained either by cytokines such as IL-2 and IL-7 or by OX40L expressed on TSLP-activated DCs. In contrast, TGF-β1 produced by RORγt+ Tregs, which are differentiated by microbiota-derived signals, induces immune tolerance. The reactivation of allergen-specific memory Th2 cells is mediated by IL-33 and IL-25, exacerbating allergic diseases in an allergen-independent manner.
OX40L (CD252), a co-stimulator provided by DCs or IgE-activated mast cells, stimulates allergen-specific CD4 T cells to promote their clonal expansion and survival during the primary response and induces the development of allergen-specific memory CD4 T cells (69–72). Consistent with this, OX40 expressing memory CD4 T cells co-localized with OX40L+ DCs or OX40L+ mast cells to enhance allergic inflammation in the lung and skin upon re-exposure to allergens or epithelial cell-derived cytokines (73–75). Moreover, a blockade of OX40L induced the tolerogenic state of allergen-specific memory CD4 T cells, resulting in the reduced severity of airway inflammation (75). Altogether, OX40L derived from immune cells, including DCs and mast cells, is crucial for the development, maintenance, and reactivation of allergen-specific memory CD4 T cells to exacerbate allergic diseases.
Local tissue-derived cytokines, particularly epithelial cell-derived cytokines called alarmins, such as TSLP, IL-33, and IL-25, are positively correlated with the severity of allergic diseases and are crucial for inducing Th2 polarization (76–79). Furthermore, recent studies have shown that these epithelium-derived signals influence the survival and reactivation of memory CD4 T cells (80–82).
Epigenetic modifications of chromatin structure including DNA methylation and post-translational modifications of histones are well known to determine and maintain the differentiation of cells for regulating the gene expression patterns (83). Moreover, IL-4 in memory Th2 cells is rapidly expressed by this type of epigenetic modification upon antigen re-stimulation (84). Thus, epigenetic changes are crucial strategy for enhancing the function of memory Th2 cells. These alarmins have been proposed to mediate chromatin modification of allergen-specific memory CD4 T cells resulting in the enhancement of type 2 cytokine expressions. For example, TSLP stably programmed the effector state of memory Th2 cells by hyperacetylation within Th2 cytokine locus (81). Additionally, allergen-specific memory T cells expressing ST2 produced high levels of IL-5 by selective remodeling of chromatin structure at the Il5 locus with a challenge of IL-33 without allergen (85).
Recent research reported another role of IL-33 in allergic response. ST2hi memory CD4 T cells stimulated by IL-33 produced epidermal growth factors, particularly amphiregulin. This growth factor reprogrammed eosinophils toward an inflammatory state and then induced fibrosis of nasal polyps in allergic rhinitis (86).
Although individual alarmins have been intensively studied, these cytokines are also known to be released and to cooperate in the same organ to develop a memory Th2 response. DCs activated by TSLP enhanced the expression of IL-17RB on memory Th2 cells by providing OX40L. Finally, memory Th2 cells, which were stimulated by IL-25, upregulated transcription factors including GATA-3, c-Maf, and Jun B, and type 2 cytokines in the absence of TCR triggering (87). Altogether, these results imply that tissue-derived cytokines have many aspects to reactivate allergen-specific memory CD4 T cells for the pathology of allergic diseases.
Increasing efforts to identify homeostatic microenvironments for the formation, survival, and maintenance of allergen-specific memory CD4 T cells have led to a better understanding of the mechanisms underlying allergic disease relapses.
First, it was reported that DCs and IL-2 signalings, which are known to be critical to the activation and proliferation of naïve T cells, contributed to the formation of memory Th2 cells. In a mouse model of allergic asthma, IL-2 signalings provided in the allergic lung microenvironments importantly facilitate the early residency of memory Th2 cells (88). DCs, particularly TSLP-activated DCs, also induced the homeostatic proliferation of allergen-specific central memory Th2 cells to maintain the pool of these cells without antigens (72). Thus, these findings suggest that IL-2- and DC-mediated signalings are crucial for the homeostasis of allergen-specific memory CD4 T cells.
Second, IL-7 and IL-15 are well documented to be important for the survival and homeostatic proliferation of memory CD8 T cells (89). The roles of these cytokines in the maintenance of memory CD4 T cells have also been studied. Between these two cytokines, IL-7 enhanced the survival of memory Th2 cells by increasing the expression of anti-apoptotic protein Bcl-2 (90). This cytokine, produced by Thy1+ lymphatic endothelial cells, provided a survival environment for allergen-specific memory Th2 cells in the inducible bronchus-associated lymphoid tissue of the lung, which is formed after allergic lung inflammations in humans and mice (90). IL-7 is also crucial for the development of memory CD4 T cells by mediating the transition of effector T cells into memory T cells (91). In the lung and airways suffered from allergic inflammation, the generation of allergen-specific memory CD4 T cells was affected by IL-7Rα-mediated signals (23). Moreover, in a skin hypersensitivity model, hair follicle-derived IL-7 was required to maintain memory CD4 TRM cells (92). Accordingly, it has been suggested that IL-7 plays an important role in the homeostasis of allergen-specific memory CD4 T cells in barrier tissues.
Our bodies have evolved immune homeostasis mechanisms that need to decide between protection against pathogens and tolerance to innocuous substances, such as food, commensal bacteria, and inhaled environmental particles (93, 94). Among these mechanisms, it has extensively examined that Treg cells play a crucial role in preventing the development and aggravation of hypersensitivity against harmless environmental substances (95). The underlying mechanisms include the development of Treg cells in the thymus by TGF-β signaling, which mediates the suppressive functions of these cells in certain peripheral tissues (96, 97). In an experimental model of FA, TGF-β1-deficient allergen-specific Treg cells did not develop into oral tolerance-promoting RORγt+ Treg cells, resulting in an increased incidence of FA. Furthermore, the regulation of oral tolerance mediated by RORγt+ Tregs was affected by microbiota-derived signals (98). These findings suggest that extrinsic factors contributing to immune tolerance by stimulating allergen-specific CD4 T cells are required to modulate allergic disorders.
Allergic diseases diminish the patient’s quality of life and incur enormous personal and social costs to maintain their lives (99, 100). The roles of CD4 T cells in the steering pathogenesis of allergic disorders have been studied for many decades. In this review, we summarize the characteristics and regulation of extrinsic factors for allergen-specific memory CD4 T cells in hypersensitivity, particularly allergic disorders. Since much lights have been shed to understand the roles of microenvironments in the homeostasis and effector function of allergen-specific memory CD4 T cells, we propose to investigate how these microenvironments can alter the longevity of memory T cells to regulate these diseases. This future direction will guide us to develop a novel strategy for the discovery of therapeutics for allergic diseases.
Conceptualization: AC, YWJ, HC. Data curation: AC, YWJ, HC. Funding acquisition: YWJ. Supervision: YWJ, HC. Visualization: AC, YWJ, HC. Writing - original draft: AC, YWJ, HC. Writing - review & editing: AC, YWJ, HC. All authors contributed to the article and approved the submitted version.
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2021R1A2C2004279 and 2019R1A6A1A03031807).
The authors are grateful to Sang-Hoon Kim, Heonju Song, and Yeaji Kim for their useful and constructive suggestions on this review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol (1989) 7:145–73. doi: 10.1146/annurev.iy.07.040189.001045
2. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol (2005) 6(11):1123–32. doi: 10.1038/ni1254
3. Kobayashi T, Iijima K, Checkel JL, Kita H. IL-1 family cytokines drive Th2 and Th17 cells to innocuous airborne antigens. Am J Respir Cell Mol Biol (2013) 49(6):989–98. doi: 10.1165/rcmb.2012-0444OC
4. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol (2010) 28:445–89. doi: 10.1146/annurev-immunol-030409-101212
5. Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature (1996) 383(6603):787–93. doi: 10.1038/383787a0
6. Seumois G, Ramírez-Suástegui C, Schmiedel BJ, Liang S, Peters B, Sette A, et al. Single-cell transcriptomic analysis of allergen-specific T cells in allergy and asthma. Sci Immunol (2020) 5(48):eaba6087. doi: 10.1126/sciimmunol.aba6087
7. Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med (2019) 25(7):1153–63. doi: 10.1038/s41591-019-0468-5
8. León B, Ballesteros-Tato A. Modulating Th2 cell immunity for the treatment of asthma. Front Immunol (2021) 12:637948. doi: 10.3389/fimmu.2021.637948
9. Cho JL, Ling MF, Adams DC, Faustino L, Islam SA, Afshar R, et al. Allergic asthma is distinguished by sensitivity of allergen-specific CD4+ T cells and airway structural cells to type 2 inflammation. Sci Transl Med (2016) 8(359):359ra132. doi: 10.1126/scitranslmed.aag1370
10. Bosnjak B, Stelzmueller B, Erb KJ, Epstein MM. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res (2011) 12(1):114. doi: 10.1186/1465-9921-12-114
11. Karlsson MG, Davidsson A, Hellquist HB. Increase in CD4+ and CD45RO+ memory T cells in the nasal mucosa of allergic patients. Apmis (1994) 102(10):753–8. doi: 10.1111/j.1699-0463.1994.tb05230.x
12. Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-lived Th2 memory in experimental allergic asthma. J Immunol (2002) 169(9):4788–96. doi: 10.4049/jimmunol.169.9.4788
13. Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet (1999) 353(9148):196–200. doi: 10.1016/S0140-6736(98)05104-6
14. Jung YW, Kim HG, Perry CJ, Kaech SM. CCR7 expression alters memory CD8 T-cell homeostasis by regulating occupancy in IL-7- and IL-15-dependent niches. Proc Natl Acad Sci U S A (2016) 113(29):8278–83. doi: 10.1073/pnas.1602899113
15. Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity (2008) 29(6):848–62. doi: 10.1016/j.immuni.2008.11.002
16. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med (1992) 326(5):298–304. doi: 10.1056/NEJM199201303260504
17. Rahimi RA, Nepal K, Cetinbas M, Sadreyev RI, Luster AD. Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease. J Exp Med (2020) 217(9):e20190865. doi: 10.1084/jem.20190865
18. Bošnjak B, Kazemi S, Altenburger LM, Mokrović G, Epstein MM. Th2-T(RMs) maintain life-long allergic memory in experimental asthma in mice. Front Immunol (2019) 10:840. doi: 10.3389/fimmu.2019.00840
19. Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW, et al. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. J Immunol (2011) 187(1):462–71. doi: 10.4049/jimmunol.1100259
20. Nakagome K, Dohi M, Okunishi K, To Y, Sato A, Komagata Y, et al. Antigen-sensitized CD4+CD62Llow memory/effector T helper 2 cells can induce airway hyperresponsiveness in an antigen free setting. Respir Res (2005) 6(1):46. doi: 10.1186/1465-9921-6-46
21. Epstein MM. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol Ther (2006) 109(1-2):107–36. doi: 10.1016/j.pharmthera.2005.06.006
22. Cauley LS, Cookenham T, Miller TB, Adams PS, Vignali KM, Vignali DA, et al. Cutting edge: virus-specific CD4+ memory T cells in nonlymphoid tissues express a highly activated phenotype. J Immunol (2002) 169(12):6655–8. doi: 10.4049/jimmunol.169.12.6655
23. Yeon SM, Halim L, Chandele A, Perry CJ, Kim SH, Kim SU, et al. IL-7 plays a critical role for the homeostasis of allergen-specific memory CD4 T cells in the lung and airways. Sci Rep (2017) 7(1):11155. doi: 10.1038/s41598-017-11492-7
24. Turner DL, Goldklang M, Cvetkovski F, Paik D, Trischler J, Barahona J, et al. Biased generation and In situ activation of lung tissue-resident memory CD4 T cells in the pathogenesis of allergic asthma. J Immunol (2018) 200(5):1561–9. doi: 10.4049/jimmunol.1700257
25. Ling MF, Luster AD. Allergen-specific CD4(+) T cells in human asthma. Ann Am Thorac Soc (2016) 13 Suppl 1(Suppl 1):S25–30. doi: 10.1513/AnnalsATS.201507-431MG
26. Losol P, Choi JP, Kim SH, Chang YS. The role of upper airway microbiome in the development of adult asthma. Immune Netw (2021) 21(3):e19. doi: 10.4110/in.2021.21.e19
27. Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med (2010) 207(11):2479–91. doi: 10.1084/jem.20101376
28. Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol (2014) 134(5):1175–86.e7. doi: 10.1016/j.jaci.2014.05.038
29. David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: Pathophysiology. Adv Exp Med Biol (2017) 1027:21–37. doi: 10.1007/978-3-319-64804-0_3
30. Nakatani T, Kaburagi Y, Shimada Y, Inaoki M, Takehara K, Mukaida N, et al. CCR4 memory CD4+ T lymphocytes are increased in peripheral blood and lesional skin from patients with atopic dermatitis. J Allergy Clin Immunol (2001) 107(2):353–8. doi: 10.1067/mai.2001.112601
31. Leung DY. Pathogenesis of atopic dermatitis. J Allergy Clin Immunol (1999) 104(3 Pt 2):S99–108. doi: 10.1016/S0091-6749(99)70051-5
32. Grewe M, Bruijnzeel-Koomen CA, Schöpf E, Thepen T, Langeveld-Wildschut AG, Ruzicka T, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today (1998) 19(8):359–61. doi: 10.1016/S0167-5699(98)01285-7
33. Nakazawa M, Sugi N, Kawaguchi H, Ishii N, Nakajima H, Minami M. Predominance of type 2 cytokine-producing CD4+ and CD8+ cells in patients with atopic dermatitis. J Allergy Clin Immunol (1997) 99(5):673–82. doi: 10.1016/S0091-6749(97)70030-7
35. Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol (2016) 138(2):336–49. doi: 10.1016/j.jaci.2016.06.010
36. Eyerich S, Onken AT, Weidinger S, Franke A, Nasorri F, Pennino D, et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. N Engl J Med (2011) 365(3):231–8. doi: 10.1056/NEJMoa1104200
37. Bohle B, Schwihla H, Hu HZ, Friedl-Hajek R, Sowka S, Ferreira F, et al. Long-lived Th2 clones specific for seasonal and perennial allergens can be detected in blood and skin by their TCR-hypervariable regions. J Immunol (1998) 160(4):2022–7. doi: 10.4049/jimmunol.160.4.2022
38. Murata A, Hayashi SI. CD4(+) resident memory T cells mediate long-term local skin immune memory of contact hypersensitivity in BALB/c mice. Front Immunol (2020) 11:775. doi: 10.3389/fimmu.2020.00775
39. Chiang D, Chen X, Jones SM, Wood RA, Sicherer SH, Burks AW, et al. Single-cell profiling of peanut-responsive T cells in patients with peanut allergy reveals heterogeneous effector T(H)2 subsets. J Allergy Clin Immunol (2018) 141(6):2107–20. doi: 10.1016/j.jaci.2017.11.060
40. Cardoso CR, Provinciatto PR, Godoi DF, Ferreira BR, Teixeira G, Rossi MA, et al. IL-4 regulates susceptibility to intestinal inflammation in murine food allergy. Am J Physiol Gastrointest Liver Physiol (2009) 296(3):G593–600. doi: 10.1152/ajpgi.90431.2008
41. Prussin C, Lee J, Foster B. Eosinophilic gastrointestinal disease and peanut allergy are alternatively associated with IL-5+ and IL-5(-) T(H)2 responses. J Allergy Clin Immunol (2009) 124(6):1326–32.e6. doi: 10.1016/j.jaci.2009.09.048
42. Chu DK, Mohammed-Ali Z, Jiménez-Saiz R, Walker TD, Goncharova S, Llop-Guevara A, et al. T Helper cell IL-4 drives intestinal Th2 priming to oral peanut antigen, under the control of OX40L and independent of innate-like lymphocytes. Mucosal Immunol (2014) 7(6):1395–404. doi: 10.1038/mi.2014.29
43. Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science (2019) 365(6456):eaaw6433. doi: 10.1126/science.aaw6433
44. Berin MC, Mayer L. Can we produce true tolerance in patients with food allergy? J Allergy Clin Immunol (2013) 131(1):14–22. doi: 10.1016/j.jaci.2012.10.058
45. Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity (2015) 42(3):512–23. doi: 10.1016/j.immuni.2015.02.004
46. Neeland MR, Andorf S, Dang TD, McWilliam VL, Perrett KP, Koplin JJ, et al. Altered immune cell profiles and impaired CD4 T-cell activation in single and multi-food allergic adolescents. Clin Exp Allergy (2021) 51(5):674–84. doi: 10.1111/cea.13857
47. Koenig JFE, Bruton K, Phelps A, Grydziuszko E, Jiménez-Saiz R, Jordana M. Memory generation and re-activation in food allergy. Immunotargets Ther (2021) 10:171–84. doi: 10.2147/ITT.S284823
48. Mikhak Z, Fukui M, Farsidjani A, Medoff BD, Tager AM, Luster AD. Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. J Allergy Clin Immunol (2009) 123(1):67–73.e3. doi: 10.1016/j.jaci.2008.09.049
49. Hartl D, Griese M, Nicolai T, Zissel G, Prell C, Konstantopoulos N, et al. Pulmonary chemokines and their receptors differentiate children with asthma and chronic cough. J Allergy Clin Immunol (2005) 115(4):728–36. doi: 10.1016/j.jaci.2004.11.049
50. Wakugawa M, Nakamura K, Kakinuma T, Onai N, Matsushima K, Tamaki K. CC chemokine receptor 4 expression on peripheral blood CD4+ T cells reflects disease activity of atopic dermatitis. J Invest Dermatol (2001) 117(2):188–96. doi: 10.1046/j.0022-202x.2001.01430.x
51. Renand A, Newbrough S, Wambre E, DeLong JH, Robinson D, Kwok WW. Arginine kinase pen m 2 as an important shrimp allergen recognized by TH2 cells. J Allergy Clin Immunol (2014) 134(6):1456–9.e7. doi: 10.1016/j.jaci.2014.07.048
52. Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol (2005) 175(10):6531–6. doi: 10.4049/jimmunol.175.10.6531
53. Xue L, Barrow A, Pettipher R. Novel function of CRTH2 in preventing apoptosis of human Th2 cells through activation of the phosphatidylinositol 3-kinase pathway. J Immunol (2009) 182(12):7580–6. doi: 10.4049/jimmunol.0804090
54. Iwasaki M, Nagata K, Takano S, Takahashi K, Ishii N, Ikezawa Z. Association of a new-type prostaglandin D2 receptor CRTH2 with circulating T helper 2 cells in patients with atopic dermatitis. J Invest Dermatol (2002) 119(3):609–16. doi: 10.1046/j.1523-1747.2002.01862.x
55. Campos Alberto E, Maclean E, Davidson C, Palikhe NS, Storie J, Tse C, et al. The single nucleotide polymorphism CRTh2 rs533116 is associated with allergic asthma and increased expression of CRTh2. Allergy (2012) 67(11):1357–64. doi: 10.1111/all.12003
56. Fajt ML, Gelhaus SL, Freeman B, Uvalle CE, Trudeau JB, Holguin F, et al. Prostaglandin D2 pathway upregulation: relation to asthma severity, control, and TH2 inflammation. J Allergy Clin Immunol (2013) 131(6):1504–12. doi: 10.1016/j.jaci.2013.01.035
57. Mitson-Salazar A, Yin Y, Wansley DL, Young M, Bolan H, Arceo S, et al. Hematopoietic prostaglandin d synthase defines a proeosinophilic pathogenic effector human T(H)2 cell subpopulation with enhanced function. J Allergy Clin Immunol (2016) 137(3):907–18.e9. doi: 10.1016/j.jaci.2015.08.007
58. Palikhe NS, Laratta C, Nahirney D, Vethanayagam D, Bhutani M, Vliagoftis H, et al. Elevated levels of circulating CD4(+) CRTh2(+) T cells characterize severe asthma. Clin Exp Allergy (2016) 46(6):825–36. doi: 10.1111/cea.12741
59. Boonpiyathad T, Capova G, Duchna HW, Croxford AL, Farine H, Dreher A, et al. Impact of high-altitude therapy on type-2 immune responses in asthma patients. Allergy (2020) 75(1):84–94. doi: 10.1111/all.13967
60. Soler D, Chapman TR, Poisson LR, Wang L, Cote-Sierra J, Ryan M, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol (2006) 177(10):6940–51. doi: 10.4049/jimmunol.177.10.6940
61. Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, et al. The c-c chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest (2001) 107(11):1357–64. doi: 10.1172/JCI12655
62. Islam SA, Chang DS, Colvin RA, Byrne MH, McCully ML, Moser B, et al. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat Immunol (2011) 12(2):167–77. doi: 10.1038/ni.1984
63. McCully ML, Ladell K, Andrews R, Jones RE, Miners KL, Roger L, et al. CCR8 expression defines tissue-resident memory T cells in human skin. J Immunol (2018) 200(5):1639–50. doi: 10.4049/jimmunol.1701377
64. Wein AN, McMaster SR, Takamura S, Dunbar PR, Cartwright EK, Hayward SL, et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med (2019) 216(12):2748–62. doi: 10.1084/jem.20181308
65. Obata-Ninomiya K, Ishiwata K, Nakano H, Endo Y, Ichikawa T, Onodera A, et al. CXCR6(+)ST2(+) memory T helper 2 cells induced the expression of major basic protein in eosinophils to reduce the fecundity of helminth. Proc Natl Acad Sci U S A (2018) 115(42):E9849–e58. doi: 10.1073/pnas.1714731115
66. Zhang B, Roesner LM, Traidl S, Koeken V, Xu CJ, Werfel T, et al. Single-cell profiles reveal distinctive immune response in atopic dermatitis in contrast to psoriasis. Allergy (2022) 1–15. doi: 10.1111/all.15486
67. Matsumoto Y, Noguchi E, Imoto Y, Nanatsue K, Takeshita K, Shibasaki M, et al. Upregulation of IL17RB during natural allergen exposure in patients with seasonal allergic rhinitis. Allergol Int (2011) 60(1):87–92. doi: 10.2332/allergolint.10-OA-0230
68. Mato N, Hirahara K, Ichikawa T, Kumagai J, Nakayama M, Yamasawa H, et al. Memory-type ST2(+)CD4(+) T cells participate in the steroid-resistant pathology of eosinophilic pneumonia. Sci Rep (2017) 7(1):6805. doi: 10.1038/s41598-017-06962-x
69. Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol (2000) 165(6):3043–50. doi: 10.4049/jimmunol.165.6.3043
70. Croft M. Co-Stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol (2003) 3(8):609–20. doi: 10.1038/nri1148
71. Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes bcl-xL and bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity (2001) 15(3):445–55. doi: 10.1016/S1074-7613(01)00191-1
72. Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity (2006) 24(6):827–38. doi: 10.1016/j.immuni.2006.03.019
73. Elsner JS, Carlsson M, Stougaard JK, Nygaard U, Buchner M, Fölster-Holst R, et al. The OX40 axis is associated with both systemic and local involvement in atopic dermatitis. Acta Derm Venereol (2020) 100(6):adv00099. doi: 10.2340/00015555-3452
74. Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PT, et al. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int (2014) 63(3):443–55. doi: 10.2332/allergolint.13-OA-0672
75. Gracias DT, Sethi GS, Mehta AK, Miki H, Gupta RK, Yagita H, et al. Combination blockade of OX40L and CD30L inhibits allergen-driven memory T(H)2 cell reactivity and lung inflammation. J Allergy Clin Immunol (2021) 147(6):2316–29. doi: 10.1016/j.jaci.2020.10.037
76. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity (2001) 15(6):985–95. doi: 10.1016/S1074-7613(01)00243-6
77. Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol (2002) 169(1):443–53. doi: 10.4049/jimmunol.169.1.443
78. Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol (2009) 123(5):1047–54. doi: 10.1016/j.jaci.2009.02.026
79. Li Y, Wang W, Lv Z, Li Y, Chen Y, Huang K, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics In vivo: A potential biomarker of severe refractory disease. J Immunol (2018) 200(7):2253–62. doi: 10.4049/jimmunol.1701455
80. Calise J, Garabatos N, Bajzik V, Farrington M, Robinson D, Jeong D, et al. Optimal human pathogenic T(H)2 cell effector function requires local epithelial cytokine signaling. J Allergy Clin Immunol (2021) 148(3):867–75.e4. doi: 10.1016/j.jaci.2021.02.019
81. Rochman Y, Dienger-Stambaugh K, Richgels PK, Lewkowich IP, Kartashov AV, Barski A, et al. TSLP signaling in CD4(+) T cells programs a pathogenic T helper 2 cell state. Sci Signal (2018) 11(521):eaam8858. doi: 10.1126/scisignal.aam8858
82. Iinuma T, Okamoto Y, Yamamoto H, Inamine-Sasaki A, Ohki Y, Sakurai T, et al. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Ann Allergy Asthma Immunol (2015) 114(4):289–98. doi: 10.1016/j.anai.2015.01.013
83. Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (2010) 105(1):4–13. doi: 10.1038/hdy.2010.54
84. Tykocinski LO, Hajkova P, Chang HD, Stamm T, Sözeri O, Löhning M, et al. A critical control element for interleukin-4 memory expression in T helper lymphocytes. J Biol Chem (2005) 280(31):28177–85. doi: 10.1074/jbc.M502038200
85. Endo Y, Hirahara K, Iinuma T, Shinoda K, Tumes DJ, Asou HK, et al. The interleukin-33-p38 kinase axis confers memory T helper 2 cell pathogenicity in the airway. Immunity (2015) 42(2):294–308. doi: 10.1016/j.immuni.2015.01.016
86. Morimoto Y, Hirahara K, Kiuchi M, Wada T, Ichikawa T, Kanno T, et al. Amphiregulin-producing pathogenic memory T helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity (2018) 49(1):134–50.e6. doi: 10.1016/j.immuni.2018.04.023
87. Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med (2007) 204(8):1837–47. doi: 10.1084/jem.20070406
88. Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, et al. Interleukin-2-Dependent allergen-specific tissue-resident memory cells drive asthma. Immunity (2016) 44(1):155–66. doi: 10.1016/j.immuni.2015.11.004
89. Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev (2006) 211:154–63. doi: 10.1111/j.0105-2896.2006.00401.x
90. Shinoda K, Hirahara K, Iinuma T, Ichikawa T, Suzuki AS, Sugaya K, et al. Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. Proc Natl Acad Sci U S A (2016) 113(20):E2842–51. doi: 10.1073/pnas.1512600113
91. Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med (2003) 198(12):1797–806. doi: 10.1084/jem.20030735
92. Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, et al. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med (2015) 21(11):1272–9. doi: 10.1038/nm.3962
93. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature (2016) 535(7610):75–84. doi: 10.1038/nature18848
94. Jain N, Walker WA. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol (2015) 12(1):14–25. doi: 10.1038/nrgastro.2014.153
95. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol (2008) 8(7):523–32. doi: 10.1038/nri2343
96. Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol (2008) 9(6):632–40. doi: 10.1038/ni.1607
97. Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, et al. Transforming growth factor-β signaling in regulatory T cells controls T helper-17 cells and tissue-specific immune responses. Immunity (2017) 46(4):660–74. doi: 10.1016/j.immuni.2017.03.015
98. Turner JA, Stephen-Victor E, Wang S, Rivas MN, Abdel-Gadir A, Harb H, et al. Regulatory T cell-derived TGF-β1 controls multiple checkpoints governing allergy and autoimmunity. Immunity (2020) 53(6):1202–14.e6. doi: 10.1016/j.immuni.2020.10.002
99. Stróżek J, Samoliński BK, Kłak A, Gawińska-Drużba E, Izdebski R, Krzych-Fałta E, et al. The indirect costs of allergic diseases. Int J Occup Med Environ Health (2019) 32(3):281–90. doi: 10.13075/ijomeh.1896.01275
Keywords: allergen-specific memory CD4 T cells, homeostasis, chronic inflammatory diseases, microenvironments, extrinsic factors
Citation: Choi A, Jung YW and Choi H (2022) The extrinsic factors important to the homeostasis of allergen-specific memory CD4 T cells. Front. Immunol. 13:1080855. doi: 10.3389/fimmu.2022.1080855
Received: 26 October 2022; Accepted: 30 November 2022;
Published: 15 December 2022.
Edited by:
Scheherazade Sadegh-Nasseri, Johns Hopkins University, United StatesReviewed by:
Kiyoshi Hirahara, Chiba University, JapanCopyright © 2022 Choi, Jung and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanbyeul Choi, Ymlnc3RhQGtvcmVhLmFjLmty; Yong Woo Jung, eWp1bmdAa29yZWEuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.