- 1Department of Maternal, Child & Adolescent Health, School of Public Health, Anhui Medical University, Hefei, China
- 2MOE Key Laboratory of Population Health Across Life Cycle, Hefei, China
- 3NHC Key Laboratory of Study on Abnormal Gametes and Reproductive Tract, Hefei, China
- 4Anhui Provincial Key Laboratory of Population Health and Aristogenics, Hefei, China
- 5Anhui Provincial Institute of Translational Medicine, Hefei, Anhui, China
- 6Department of Obstetrics and Gynecology, the First People’s Hospital of Hefei City, Hefei, China
- 7Department of Obstetrics and Gynecology, the First Affiliated Hospital of Anhui Medical University, Hefei, China
- 8Maternal and Child Health, and Family Planning Service Center, Hefei, China
- 9Department of Obstetrics and Gynecology, Anhui Women and Child Health Care Hospital, Hefei, China
Aim: To estimate the associations of cord meta-inflammatory markers with neurodevelopment, including the potential impact of cord blood vitamin D levels.
Method: The prospective cohort study comprised 7198 participants based on the Maternal & Infants Health in Hefei study. Cord blood C-peptide, high-sensitive C-reactive protein (hsCRP), high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, total cholesterol, triglycerides and 25(OH)D levels were measured. The Gesell Developmental Schedules were used to assess neurodevelopmental outcomes in offspring.
Results: After adjusting potential confounders, per quartile increase in cord blood 25(OH)D concentrations was associated with a decreased risk of neurodevelopmental delay [hazard ratios (HR) 0.65 (95% CI 0.57, 0.74)]. Conversely, significant positive associations with cord blood serum C-peptide levels above the 90th percentile [HR 2.38 (95% CI 1.81, 3.13)] and higher levels of cord hsCRP (per quartile increase) [HR 1.18 (95% CI 1.01, 1.37)] with neurodevelopmental delay were observed. These associations could vary by quartiles of cord blood 25(OH)D levels: the adjusted HRs in neurodevelopmental delay comparing children with vs without hyperinsulinemia were 1.28 (95% CI: 1.03, 1.59) for quartiles 1 (lowest), and 1.06 (95% CI: 0.78, 1.44) for quartile 4 (highest).
Conclusions: Immune activation and metabolic abnormalities in fetal circulation were associated with neurodevelopmental delay in offspring, which could be attenuated by higher cord blood 25(OH)D levels in a dose-response manner.
Introduction
Developing brain during prenatal life is more vulnerable to intrauterine adverse environment like maternal obesity, which contributes to the disruption of neurodevelopmental trajectories (1, 2). Disturbances in fetal circulation in early life may have adverse effects on long-term neurodevelopmental outcomes. Cord blood metabolic markers levels reflect fetal metabolism and the placental transfer of nutrients. However, umbilical cord metabolic markers have not generally predicted neurodevelopment in offspring.
Cord blood C-peptide, which is secreted in equimolar levels with insulin, represents the insulin-secretory activity in the fetus, and fetal hyperinsulinemia or hypoglycemia is characterized by higher levels of cord C-peptide (3). Dysfunction of insulin-secretory activity of pancreatic β-cells could induce a decrease in glucose production and cellular energy sources. Under the condition, infants were inclined to neurological impairment and later neurodevelopmental delay (4, 5). On the other hand, abundant evidence links metabolic dysfunction (such as insulin resistance and obesity) with a chronic low-grade inflammatory state characterized by the recruitment of immune inflammatory cells, abnormal cytokine, acute-phase reactant production, and inflammasome activation, a process collectively known as ‘meta-inflammation’ (6, 7).
Epidemiological and animal studies have demonstrated the detrimental impacts of maternal immune activation on altered brain structure and function in offspring (8–11). Few studies elucidated the associations between cord blood cytokines and later neurodevelopment, while the inflammatory response in the fetal circulation could be triggered by maternal inflammation (12, 13). Moreover, these studies were small-scale and have substantial variability in study design. High-sensitive C-reactive protein (hsCRP) was identified as a biomarker of systemic and low-grade chronic inflammation and was applied in clinical practice, and here cord blood hsCRP was used as a proxy for inflammatory activation in the fetal circulation (14).
Significant associations of vitamin D deficiency with metabolic complications during pregnancy including maternal obesity and gestational diabetes mellitus (GDM) (15, 16). Vitamin D supplementation may further reduce levels of meta-inflammation in obese subjects (17). In addition, vitamin D also has been implicated in the neurodevelopment of offspring and vitamin D concentrations in early life may be associated with an increased risk of neurodevelopmental disorders (18). It is currently unknown whether the association between cord blood meta-inflammatory markers and neurodevelopmental delay differ by vitamin D levels.
Thus, the prospective birth cohort study aims to evaluate the relationships between cord blood meta-inflammatory markers and neurodevelopmental delay and estimate the potential impacts of cord blood vitamin D levels on these relationships.
Methods
Study population
The Maternal & Infants Health in Hefei (MIH-Hefei) study is a prospective birth cohort study in three centers including Anhui Women and Child Health Care, Hospital, the First People’s Hospital of Hefei City, and the First Affiliated Hospital of Anhui Medical University. The women in the MIH-Hefei study were recruited from March 2015 to June 2021. Eligible women for the study were aged 18 to 44 years, lived in Hefei city, had no communication problems, and planned to deliver at specific participating hospitals.
Women suffered from major disorders [preexisting diabetes or hypertension (n=178), thyroid dysfunction (n=183) and heart failure (n=23)], with assisted reproductive technology (n=63) and with multiple gestations (n=206) were excluded. Moreover, newborns with birth defects (n=38), stillbirth (n=28) and/or infants without breastfeeding data (n=80) were also excluded. At the postpartum follow-up, the Denver Developmental Screening Test-II (DDST-II) and subsequent the Gesell Developmental Schedules (GDS) were used for the assessment of children’s neurodevelopmental delay aged 6-36 mon. Finally, a total of 7198 mother-infant pairs were included in the analysis (Supplemental Figure 1). Mothers provided written informed consent for themselves and their children before enrollment. The study was approved by the Ethics Committee of Anhui Medical University (no. 2015002).
Cord blood metabolic biomarkers measurement
Cord blood samples were collected at delivery and were stored at -80°C until assayed. Cord metabolic markers included C-peptide, HDL-cholesterol, LDL- cholesterol, and TG. The levels of cord C-peptide were detected using an immunoassay (AutoDELFIA, PerkinElmer). Cord blood high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), total cholesterol (TC) and triglycerides (TG) were measured by an automatic analyzer (Beckman Coulter, Brea, CA, USA). Cord blood hsCRP levels, reflecting fetal immune activation, were measured using a Beckman Coulter immunoturbidometric assay (Beckman Coulter, Brea, CA, USA). Both intra- and inter-coefficients of variation were <10%.
Cord blood 25(OH)D measurement
Cord blood vitamin D levels (total 25(OH)D) including the concentrations of 25(OH)D2 and 25(OH)D3 in cord blood plasma, were measured using the Electrochemical Luminescence Detection Kit for Roche E601 (Sandhofer, Mannheim, Germany). Both intra- and inter-coefficients of variation were <10%.
Assessment of neurodevelopmental delay
All the children underwent the assessment of neurodevelopmental delay using DDST-II and GDS by a specially trained examiner. The DDST-II were applied in evaluating children’s development regarding their ability to perform tasks organized in four domains: gross motor, fine motor, personal-social and language (19). Each domain was scored and evaluated as follows: pass or fail. In terms of the overall developmental assessment, children were considered as “Developmental delay” if they failed two or more domains that 75 to 90% of children of their age could pass or if they failed one or more domains that more than 90% of children younger than their age could pass. Otherwise, the development of the children was considered “Normal”.
The GDS was performed for the infants with “Developmental delay” after the assessment of DDST-II by a trained pediatrician. The GDS was designed to diagnose the neurologic and intellectual development of infants aged 4 weeks to 3 y (20). The test included 5 domains for the evaluation of the developmental quotient (DQ): adaptability (i.e., cognitive), gross motor, fine motor, language (i.e., communication), and personal-social domains (20). The mean score with SD for the overall DQ was 100 ± 15. Infants were considered as “average development” based on their scores below −1 SD from the mean score (≥ 85); “borderline development” between −1 and −2 SD from the mean score (70–85); “developmental delay” below −2 SD from the mean score (< 70). In the study, neurodevelopmental delay for infants was defined as failing more than two domains of the GDS.
Confounding variables
Potential confounders in the study included both characteristics of mothers and infants. The information on maternal age (<30 and ≥30 years), education (≤12 and >12 years), husband’s income (<4000 and ≥4000 yuan), multipara (yes/no), and pregnancy lifestyle (the supplement of folic acid as well as iron during pregnancy and physical activity) were reported using a standardized questionnaire. Prepregnancy body mass index (BMI) (≥24 and <24 kg/m2) and GDM (yes and no) were obtained from medical records. At the follow-up, infants’ characteristics included mode of delivery, gestational age as well as weight at birth, prematurity status, gender and the pattern of infant feeding (exclusive breastfeeding, partial breastfeeding, and formula feeding) at 6 months via questionnaires.
Statistical analyses
MIH-Hefei data by child’s sex were summarized with means (SDs) or median (interquartile range, IQR) for continuous variables and counts (frequencies) for categorical variables. Pearson’s correlation was used to evaluate the correlation between cord blood metabolic markers.
The associations of cord blood metabolic biomarkers with offspring neurodevelopmental delay were estimated utilizing Cox regression models to calculate hazard ratios (HRs). Fetal hyperinsulinemia was defined as cord blood C-peptide level above the 90th percentile (P90) in clinical practice and was used as a dichotomous variable. Cord blood hsCRP and 25(OH)D levels were used as categorical variables (by quartiles). The model adjusted several confounders including maternal age, education, husband’s income, parity, prepregnancy BMI, GDM, the supplement of folic acid as well as iron during pregnancy, physical activity, delivery mode, gestational week and the pattern of infant feeding. We further explored these relationships stratified by sex.
We examined whether the associations of cord blood C-peptide level above P90 and CRP levels (per quartile increase) varied by cord blood 25(OH)D level [used as a categorical variable (by quartiles)]. The model adjusted several confounders including maternal age, education, husband’s income, parity, prepregnancy BMI, GDM, the supplement of folic acid as well as iron during pregnancy, physical activity, delivery mode, gestational week and the pattern of infant feeding. All analyses were performed using SPSS version 22.0 software (IBM Corp).
Results
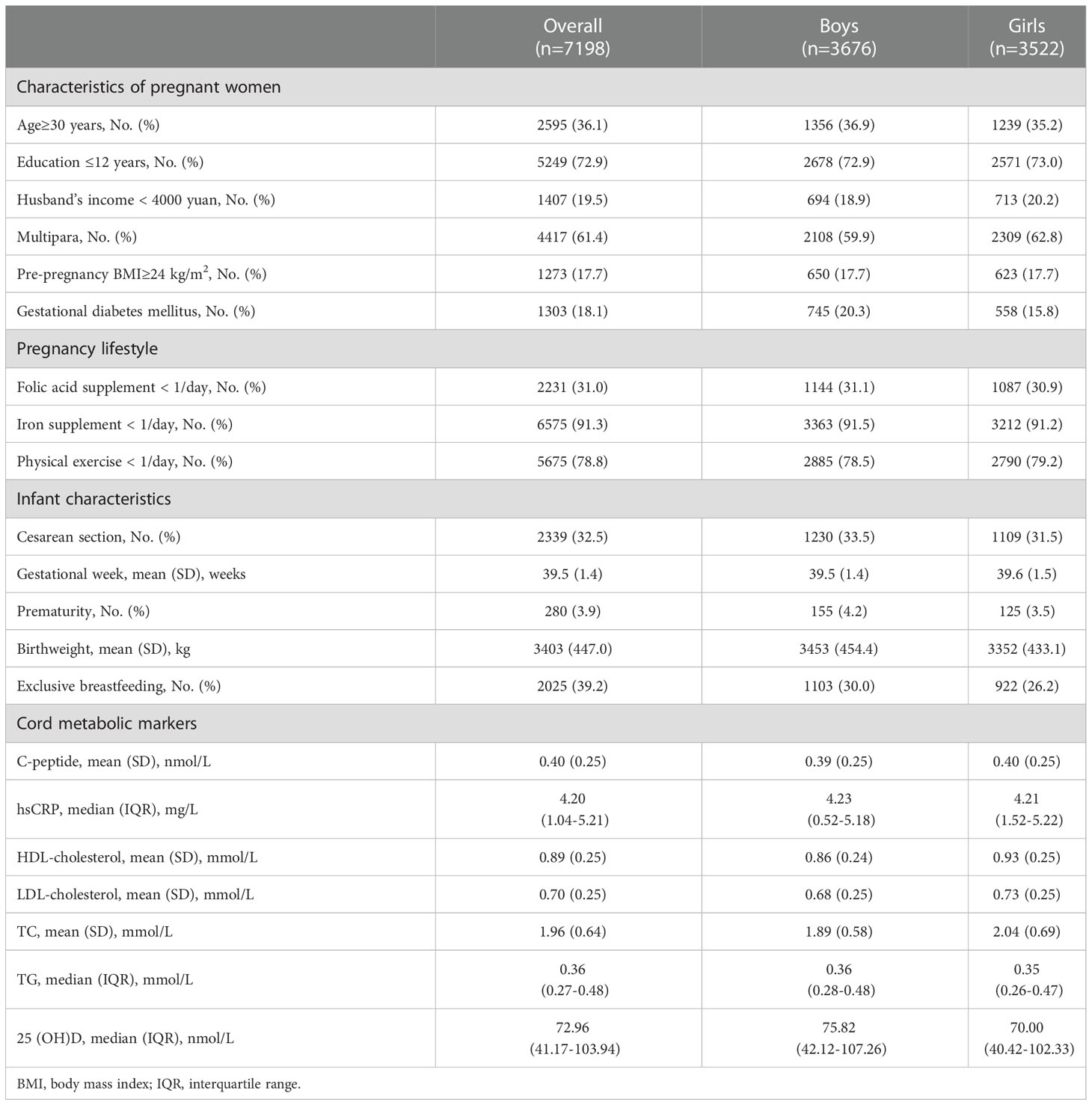
Participant characteristics
The primary analytic data included 7198 participants and their characteristics were summarized in Table 1. In the present study, 36.1% of the mothers were more than 30 y at conception and 72.9% had less than 12 years of education. 17.7% of the women were overweight/obese before conception (pre-pregnancy BMI≥24 kg/m2) and 18.1% of the women suffered from GDM. In terms of the infants’ characteristics, the mean (SD) gestational age and birth weight at born were 39.5 (1.4) weeks and 3403 (447.0) g, respectively. The mean (SD) cord C-peptide was 0.40(0.25) nmol/L. The median cord hsCRP and 25(OH)D level was 4.20 mg/L (IQR: 1.04-5.21) and 72.96 nmol/L (IQR: 41.17-103.94), respectively.
Supplemental Figure 2 showed summary statistics and Pearson correlation coefficients between cord blood metabolic markers. hsCRP levels were correlated to C-peptide, LDL-C, TC, TG and 25(OH)D (r: -0.16, 0.14); C-peptide levels were correlated to hsCRP, HDL-C, TG and 25(OH)D (r: -0.22, 0.10); 25(OH)D levels were correlated to other metabolic markers (r: -0.22, 0.23).
Cord blood metabolic markers and neurodevelopmental delay
The associations of cord blood metabolic markers and risks of neurodevelopmental delay in offspring were exhibited in Figure 1. The significant associations of cord blood serum C-peptide levels above P90 with higher risks of neurodevelopmental delay were observed [HR with 95% CI: 2.38(1.81, 3.13)]. Similarly, our analysis demonstrated the significant relationship between cord hsCRP levels by quartile increase and neurodevelopmental delay in offspring [HR with 95% CI: 1.18(1.01, 1.37)]. In addition, cord 25(OH)D levels by quartile increase were associated with decreased risk of neurodevelopmental delay [HR with 95% CI: 0.65(0.57, 0.74)]. However, these significant associations with other cord blood metabolic markers were not observed.
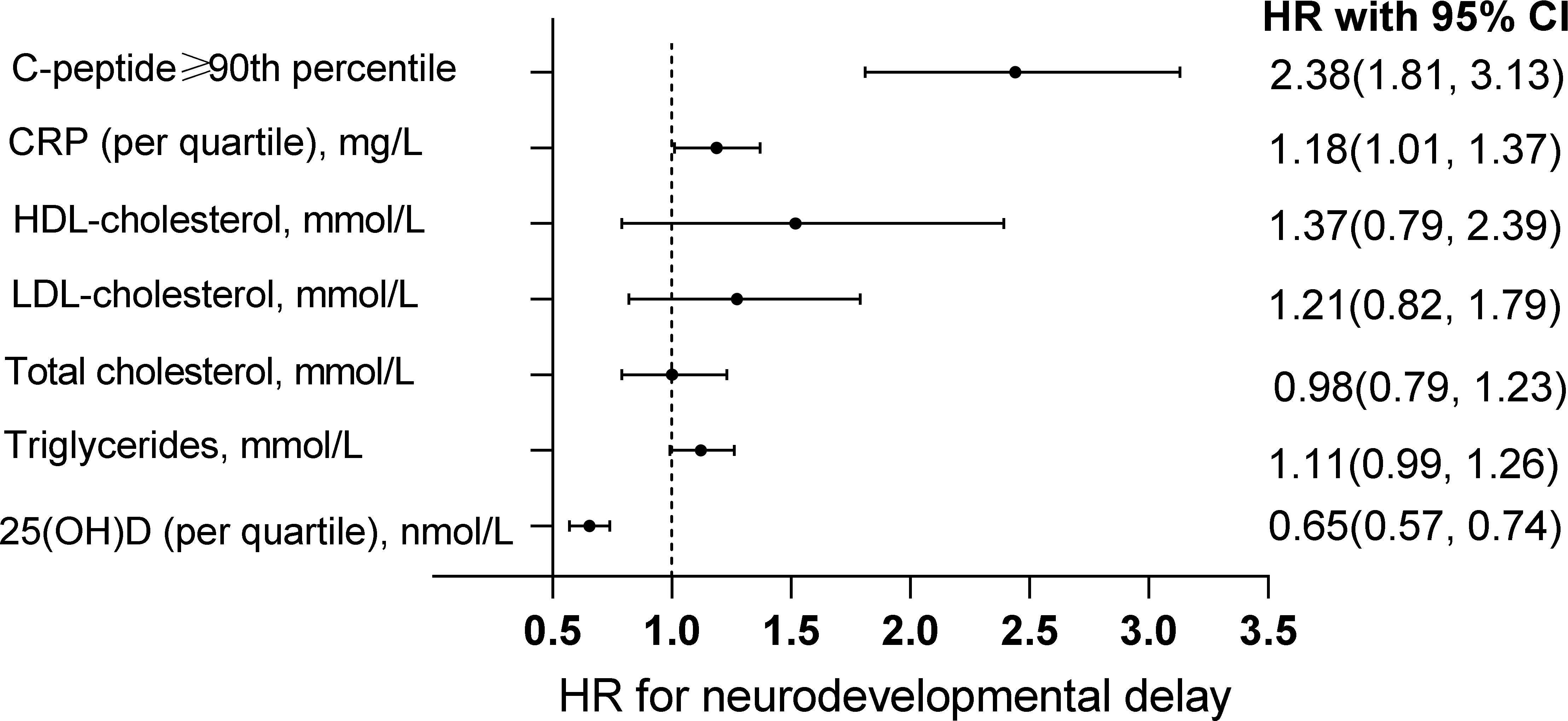
Figure 1 Association between cord blood markers and neurodevelopmental delay in offspring. The associations of cord blood C-peptide level above the 90th percentile, hsCRP by quartile increase, HDL-cholesterol, LDL-cholesterol, total cholesterol, triglycerides and 25(OH)D with neurodevelopmental delay in offspring. The models adjusted maternal age, education, husband’s income, parity, depressed mood, the supplement of folic acid, multivitamin as well as iron during pregnancy, physical activity, delivery mode, gestational week, sex and the pattern of infant feeding.
Sex-specific effects
We further explored the sex-specific effects on the relationships between cord blood metabolic markers and neurodevelopmental delay. The stratified analysis indicated that cord hsCRP levels by quartile increase were associated with higher risks of neurodevelopmental delay in boys [HR with 95% CI: 1.29(1.02, 1.63)] but not girls (Table 2). In addition, compared with girls, boys exposed to high levels of cord blood TG have a higher neurodevelopmental delay risk [HR with 95% CI: 1.17(1.03, 1.33)].
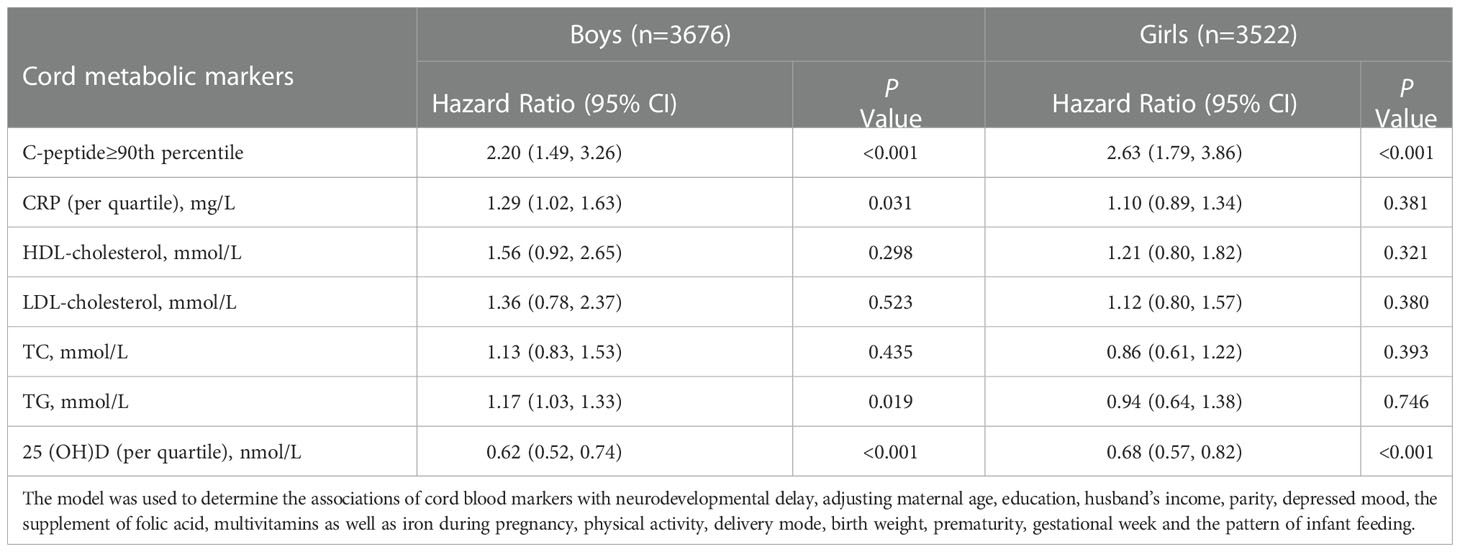
Table 2 Sex-specific effects on the associations of cord blood markers with neurodevelopmental delay.
Cord blood metabolic markers and neurodevelopmental delay by 25(OH)D levels
Potential relationships between cord hsCRP levels by quartile increase, C-peptide levels above P90 and neurodevelopmental delay risk were performed in a dose-dependent manner by cord blood 25(OH)D concentration (Figure 2). Our results showed that by quartiles of cord blood 25(OH)D, the adjusted HRs in neurodevelopmental delay comparing children with vs without hyperinsulinemia were 1.28 (95% CI: 1.03, 1.59) for quartiles 1 (lowest), 1.26 (95% CI: 0.91, 1.74) for quartile 2, 1.16 (95% CI: 0.86, 1.56) for quartile 3, and 1.06 (95% CI: 0.78, 1.44) for quartile 4 (highest). Similarly, the adjusted HRs in neurodevelopmental delay comparing children with vs without higher levels inflammation (per quartile increase in CRP) were 2.67 (95% CI: 1.69, 4.21) for quartiles 1 (lowest), 2.56 (95% CI: 1.54, 4.25) for quartile 2, 1.76 (95% CI: 0.84, 3.72) for quartile 3, and 1.63 (95% CI: 0.48, 5.59) for quartile 4 (highest). Specifically, sex-stratified cord hsCRP associations were stronger among boys (Supplemental Figures 2A, B).
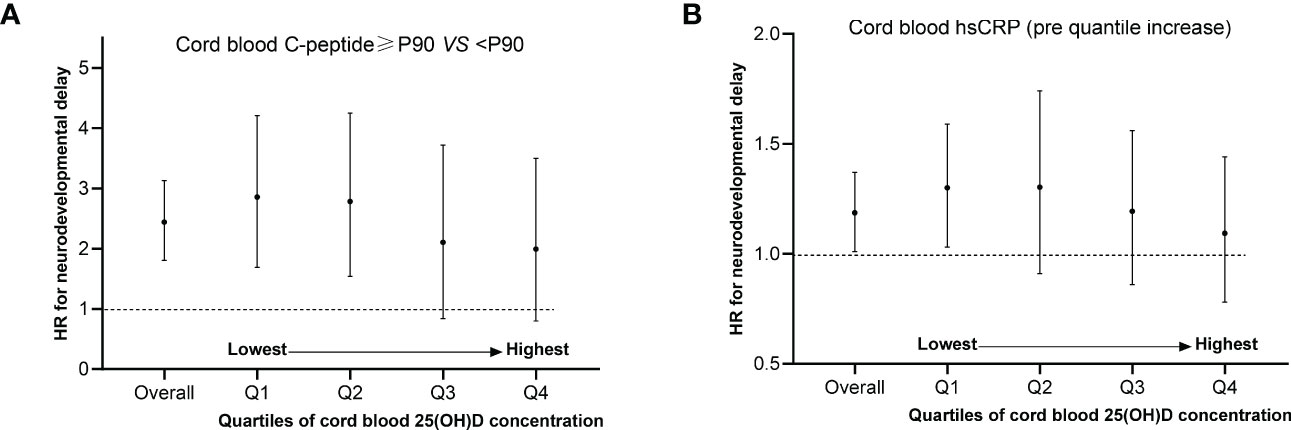
Figure 2 Potential relationships between C-peptide levels above P90, cord hsCRP levels by quartile increase and neurodevelopmental delay risk stratified by level of cord blood 25(OH)D concentration. (A, B) Adjusted maternal age, education, husband’s income, parity, depressed mood, the supplement of folic acid, multivitamin as well as iron during pregnancy, physical activity, delivery mode, gestational week, sex and the pattern of infant feeding. The no. of quartile 1 (n=1809), quartile 2 (n=1882), quartile 3 (n=1742) and quartile 4 (n=1763) for cord blood 25(OH)D concentration was analyzed.
Discussion
In the prospective birth cohort study, after adjusting a series of confounders, we found that offspring exposed to higher levels of cord blood serum C-peptide level above P90 or cord blood hsCRP had an increased risk of neurodevelopmental delay. This risk could be attenuated by higher cord blood 25(OH)D levels. Overall, our results suggested that meta-inflammatory markers in the fetal circulation could be implicated in later neurodevelopmental delay and addressed the potentially protective impacts of vitamin D levels.
The current analysis reported the significance of cord serum C-peptide levels above P90 (defined as fetal hyperinsulinemia) to increased risks of neurodevelopmental delay. Increasing evidence has indicated that cord blood C-peptide was associated with maternal insulin sensitivity, fetal hyperinsulinemia and/or neonatal hypoglycemia (21, 22). There have been few studies to date on the impact of cord serum C-peptide on neurodevelopmental delay. Moreover, possible biological mechanisms linking cord blood C-peptide with brain development remain elusive. Alternatively, cord blood C-peptide could cross the placental barrier, resulting in dysregulated, uncoupled glucose and fuel metabolism and a subsequent decrease in glucose production. Under these circumstances, fetuses and infants are disposed to brain injury or later neurological impairment. Another possible explanation was that the higher levels of C-peptide induced the upregulation of nitric oxide synthase underlying the nitric oxide signaling pathway (23). Excessive nitric oxide was detrimental to cognitive dysfunction and neurological changes, especially for fetal and infant brain development (24). Thus, the present study provided new data that higher cord C-peptide was associated with neurodevelopment in offspring. Our results suggested that metabolic disorders in maternal-fetal circulation could be implicated in the neurodevelopment of offspring.
On the other hand, increasing evidence has demonstrated the relationships between maternal immune activation and altered brain development in neonates and toddlers (10, 11). Recently, data from the Generation R study found significant associations of maternal continuous CRP levels with lower cerebellar volume in late childhood (25). Few studies explored the associations between cord blood cytokines and later neurodevelopment, while the inflammatory response in the fetal circulation could be triggered by maternal inflammation (12, 13). A longitudinal study inborn small-for-gestational-age and preterm birth found that higher levels of cord blood tumor necrosis factor-α (TNF-α) were associated with a decrease in verbal intelligence quotients (13). Conversely, another nested case-control analysis indicated no significant relationship between cord serum inflammatory cytokine levels (like TNF-α and interleukin 8) and neurodevelopmental delay in children (12). These studies were small-scale and the conclusion from the retrospective studies remained inconsistent. In the present prospective cohort study, cord blood hsCRP used as a proxy for fetal inflammatory activation was associated with increased risks of neurodevelopmental delay in children. Mechanistically, microglia play a critical role in regulating neuronal differentiation and neural circuit formation during the developing brain (26–29). Activated microglia by fetal immune activation induces the release of pro-inflammatory cytokines, resulting in neurodevelopmental delays in children. Therefore, our results suggested a positive association between fetal neuroinflammation and poorer neurodevelopment. Considering the interactions of immune activation in the maternal-fetal circulation, maternal inflammation was likely to be a potential intervention target for the prevention of abnormal neurodevelopment and adhering to the higher anti-inflammatory potential of maternal diet pattern was associated with lower risks of neurodevelopmental abnormalities (30).
The potential effects of vitamin D on immune function and cellular metabolic pathways have been recognized (31). The activated form of Vitamin D (1,25(OH)2D3) or its analogs bind to vitamin D receptor (VDR) and further induce VDR physiological functions (32). Macrophage VDR activation might inhibit inhibitor of kappa β kinase (IKKβ)-induced inflammation activity via suppressing nuclear factor-κB (NF-κB) signaling activation (33). NF-κB signaling plays a pivotal role in inflammatory responses and energy homeostasis metabolic diseases such as obesity and type 2 diabetes (34). Anti-inflammatory inhibition of NF-κB signaling by VDR activation improves insulin resistance in obese mice (35). A meta-analysis including five randomized controlled trials (RCT) involving 310 women found that GDM women with vitamin D supplement may lead to an improvement in serum metabolic a (36)nd inflammatory markers such as TC and hs-CRP (36). In addition, vitamin D is directly involved in the physiological process of the developing brain such as neurotransmitter synthesis and calcium (37). Results from a register-based cohort study showed that early life vitamin D status was associated with autism spectrum disorders (18). However, no study reported that early life vitamin D could modify the relationships between cord blood meta-inflammation and neurodevelopment. Hence, our results suggested that early life vitamin D exposure to fetal immune responses and metabolic disorders might represent a plausible mechanism linking early life meta-inflammation to neurodevelopmental delay in humans.
In line with the literature, we found the sex-specific effect of fetal immune activation on later neurodevelopment in males. Growing evidence has demonstrated that male offspring may be more vulnerable to maternal metabolic disorders and immune activation during pregnancy, which could lead to later poorer brain development (38–40). Our results provided new evidence on the sex-specific effect on the relationship between intrauterine exposure to meta-inflammation and later neurodevelopmental delay.
The current analysis has some strengths. First, it is the first time to investigate the impacts of cord meta-inflammatory markers on neurodevelopmental outcomes in a large-scale prospective birth cohort study. Our study has demonstrated that the associations of cord C-peptide and hs-CRP with neurodevelopmental delay may be attenuated by higher levels of vitamin D. Third, the current analysis included a series of potential confounders related to mothers and infants such as characteristics of pregnancy lifestyle. However, the present study also has several limitations. First, information on maternal diet during pregnancy was not detailed in our study. Gestational diet has been demonstrated to be related to both cord blood metabolic biomarkers and offspring neurodevelopment (41, 42). Second, a single marker for fetal immune activation (cord blood hsCRP) is one of the current analyses, while hsCRP was identified as a biomarker of systemic and low-grade chronic inflammation, and was applied in clinical practice. Data on the measurements of multiple inflammatory cytokines were not available in the study. In this large-scale prospective cohort study, several conventional cord metabolic markers (such as C-peptide, and high-density lipoprotein-cholesterol) were measured due to the limited research funding. More inflammatory measures and metabolomics in the future studies would add in future studies. Third, although the positive relationships between cord meta-inflammatory markers and neurodevelopmental delay in offspring were observed, the assessment of subsequent neurodevelopmental trajectories in childhood and even adolescence were required.
Conclusion
In the prospective birth cohort study, we found that the higher levels of cord blood C-peptide and hsCRP were associated with increased risks of neurodevelopmental delay, which might be modified by adequate cord blood 25(OH)D levels. Our results suggest that metabolic disorders and immune activation in fetal circulation may adversely affect the programming of the brain development in offspring and optimal vitamin D levels could prevent them from the later neurodevelopmental delay. Further clinical trials on the effect of vitamin D supplementation during pregnancy on neurodevelopmental outcomes are necessary to confirm this benefit.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Anhui Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PW designed the study, interpreted the data and wrote the manuscript. PW, LW and W-JY conducted data analysis and wrote the manuscript. R-XT and YZ advised on statistical methods and participated in the acquisition of the data. W-JY, P-PL and Z-YS designed the study, and interpreted the data, X-MJ and PZ were the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by National Natural Science Foundation of China (82173531, 81872631), Foundation for Scientific Research Improvement of Anhui Medical University (2021xkjT009), the National Key R&D Program of China (2022YFC2702901) and Anhui Provincial Key Research and Development Plan (201904a07020008).
Acknowledgments
The authors thank the Department of Obstetrics and Gynecology, Hefei Maternal and Child Health Hospital, Hefei First People’s Hospital and The First Affiliated Hospital of Anhui Medical University, Hefei, China, for assistance and support in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1078340/full#supplementary-material
References
1. Huang L, Yu X, Keim S, Li L, Zhang L, Zhang J. Maternal prepregnancy obesity and child neurodevelopment in the collaborative perinatal project. Int J Epidemiol (2014) 43(3):783–92. doi: 10.1093/ije/dyu030
2. Kong L, Chen X, Liang Y, Forsell Y, Gissler M, Lavebratt C. Association of preeclampsia and perinatal complications with offspring neurodevelopmental and psychiatric disorders. JAMA Netw Open (2022) 5(1):e2145719. doi: 10.1001/jamanetworkopen.2021.45719
3. Kuhl C, Andersen GE, Hertel J, Molsted-Pedersen L. Metabolic events in infants of diabetic mothers during first 24 hours after birth. i. changes in plasma glucose, insulin and glucagon. Acta Paediatr Scand (1982) 71(1):19–25. doi: 10.1111/j.1651-2227.1982.tb09366.x
4. Lord K, Radcliffe J, Gallagher PR, Adzick NS, Stanley CA, De Leon DD. High risk of diabetes and neurobehavioral deficits in individuals with surgically treated hyperinsulinism. J Clin Endocrinol Metab (2015) 100(11):4133–9. doi: 10.1210/jc.2015-2539
5. Wickstrom R, Skiold B, Petersson G, Stephansson O, Altman M. Moderate neonatal hypoglycemia and adverse neurological development at 2-6 years of age. Eur J Epidemiol (2018) 33(10):1011–20. doi: 10.1007/s10654-018-0425-5
6. Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol (2016) 12(1):15–28. doi: 10.1038/nrendo.2015.189
7. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature (2017) 542(7640):177–85. doi: 10.1038/nature21363
8. Crum WR, Sawiak SJ, Chege W, Cooper JD, Williams SCR, Vernon AC. Evolution of structural abnormalities in the rat brain following in utero exposure to maternal immune activation: A longitudinal in vivo MRI study. Brain Behav Immun (2017) 63:50–9. doi: 10.1016/j.bbi.2016.12.008
9. Antonson AM, Balakrishnan B, Radlowski EC, Petr G, Johnson RW. Altered hippocampal gene expression and morphology in fetal piglets following maternal respiratory viral infection. Dev Neurosci (2018) 40(2):104–19. doi: 10.1159/000486850
10. Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, et al. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol Psychiatry (2018) 83(2):109–19. doi: 10.1016/j.biopsych.2017.05.027
11. Rasmussen JM, Graham AM, Entringer S, Gilmore JH, Styner M, Fair DA, et al. Maternal interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage (2019) 185:825–35. doi: 10.1016/j.neuroimage.2018.04.020
12. Liu J, Feng ZC. Increased umbilical cord plasma interleukin-1 beta levels was correlated with adverse outcomes of neonatal hypoxic-ischemic encephalopathy. J Trop Pediatr (2010) 56(3):178–82. doi: 10.1093/tropej/fmp098
13. von Ehrenstein OS, Neta GI, Andrews W, Goldenberg R, Goepfert A, Zhang J. Child intellectual development in relation to cytokine levels in umbilical cord blood. Am J Epidemiol (2012) 175(11):1191–9. doi: 10.1093/aje/kwr393
14. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med (1999) 340(6):448–54. doi: 10.1056/NEJM199902113400607
15. Pereira-Santos M, Bispo Pereira LL, Santana de Oliveira D. Obesity, asthma, and vitamin d deficiency in pregnancy: Cause or consequence? J Allergy Clin Immunol (2018) 141(2):828–9. doi: 10.1016/j.jaci.2017.08.036
16. Makgoba M, Nelson SM, Savvidou M, Messow CM, Nicolaides K, Sattar N. First-trimester circulating 25-hydroxyvitamin d levels and development of gestational diabetes mellitus. Diabetes Care (2011) 34(5):1091–3. doi: 10.2337/dc10-2264
17. Lotfi-Dizaji L, Mahboob S, Aliashrafi S, Vaghef-Mehrabany E, Ebrahimi-Mameghani M, Morovati A. Effect of vitamin d supplementation along with weight loss diet on meta-inflammation and fat mass in obese subjects with vitamin d deficiency: A double-blind placebo-controlled randomized clinical trial. Clin Endocrinol (Oxf) (2019) 90(1):94–101. doi: 10.1111/cen.13861
18. Lee BK, Eyles DW, Magnusson C, Newschaffer CJ, McGrath JJ, Kvaskoff D, et al. Developmental vitamin d and autism spectrum disorders: findings from the Stockholm youth cohort. Mol Psychiatry (2021) 26(5):1578–88. doi: 10.1038/s41380-019-0578-y
19. Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: a major revision and restandardization of the Denver developmental screening test. Pediatrics (1992) 89(1):91–7. doi: 10.1542/peds.89.1.91
20. Ball RS. The gesell developmental schedules: Arnold gesell (1880-1961). J Abnorm Child Psychol (1977) 5(3):233–9. doi: 10.1007/BF00913694
21. Chen J, Yang X, Huang L, Zhang Z, Yao J, Liang H, et al. Insulin resistance biomarkers in small-for-gestational-age infants born to mothers with gestational diabetes mellitus. J Matern Fetal Neonatal Med (2021) 16:1–5. doi: 10.1080/14767058.2021.2014449
22. Saber AM, Mohamed MA, Sadek AA, Mahmoud RA. Role of umbilical cord c-peptide levels in early prediction of hypoglycemia in infants of diabetic mothers. BMC Pediatr (2021) 21(1):85. doi: 10.1186/s12887-021-02547-w
23. Shah P, Rahman SA, Demirbilek H, Guemes M, Hussain K. Hyperinsulinaemic hypoglycaemia in children and adults. Lancet Diabetes Endocrinol (2017) 5(9):729–42. doi: 10.1016/S2213-8587(16)30323-0
24. Bourgognon JM, Spiers JG, Robinson SW, Scheiblich H, Glynn P, Ortori C, et al. Inhibition of neuroinflammatory nitric oxide signaling suppresses glycation and prevents neuronal dysfunction in mouse prion disease. Proc Natl Acad Sci U.S.A. (2021) 118(10). doi: 10.1073/pnas.2009579118
25. Suleri A, Blok E, Durkut M, Rommel AS, Witte L, Jaddoe V, et al. The long-term impact of elevated c-reactive protein levels during pregnancy on brain morphology in late childhood. Brain Behav Immun (2022) 103:63–72. doi: 10.1016/j.bbi.2022.03.018
26. Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol (2014) 10(11):643–60. doi: 10.1038/nrneurol.2014.187
27. Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection - maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol (2018) 299(Pt A):241–51. doi: 10.1016/j.expneurol.2017.07.002
28. Gumusoglu SB, Stevens HE. Maternal inflammation and neurodevelopmental programming: A review of preclinical outcomes and implications for translational psychiatry. Biol Psychiatry (2019) 85(2):107–21. doi: 10.1016/j.biopsych.2018.08.008
29. Han VX, Patel S, Jones HF, Nielsen TC, Mohammad SS, Hofer MJ, et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry (2021) 11(1):71. doi: 10.1038/s41398-021-01198-w
30. Polanska K, Kaluzny P, Aubert AM, Bernard JY, Duijts L, El Marroun H, et al. Dietary quality and dietary inflammatory potential during pregnancy and offspring emotional and behavioral symptoms in childhood: An individual participant data meta-analysis of four European cohorts. Biol Psychiatry (2021) 89(6):550–9. doi: 10.1016/j.biopsych.2020.10.008
31. Vanherwegen AS, Gysemans C, Mathieu C. Vitamin d endocrinology on the cross-road between immunity and metabolism. Mol Cell Endocrinol (2017) 453:52–67. doi: 10.1016/j.mce.2017.04.018
32. Gascon-Barre M, Demers C, Mirshahi A, Neron S, Zalzal S, Nanci A. The normal liver harbors the vitamin d nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology (2003) 37(5):1034–42. doi: 10.1053/jhep.2003.50176
33. Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin d receptor inhibits nuclear factor kappaB activation by interacting with IkappaB kinase beta protein. J Biol Chem (2013) 288(27):19450–8. doi: 10.1074/jbc.M113.467670
34. Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa b signaling pathway: integrating metabolism with inflammation. Trends Cell Biol (2012) 22(11):557–66. doi: 10.1016/j.tcb.2012.08.001
35. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med (2005) 11(2):191–8. doi: 10.1038/nm1185
36. Jahanjoo F, Farshbaf-Khalili A, Shakouri SK, Dolatkhah N. Maternal and neonatal metabolic outcomes of vitamin d supplementation in gestational diabetes mellitus: A systematic review and meta-analysis. Ann Nutr Metab (2018) 73(2):145–59. doi: 10.1159/000491643
37. Eyles DW, Burne TH, McGrath JJ, Vitamin D. Effects on brain development, adult brain function and the links between low levels of vitamin d and neuropsychiatric disease. Front Neuroendocrinol (2013) 34(1):47–64. doi: 10.1016/j.yfrne.2012.07.001
38. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol (2010) 22(3):330–5. doi: 10.1002/ajhb.20995
39. Li S, Zhu Y, Yeung E, Chavarro JE, Yuan C, Field AE, et al. Offspring risk of obesity in childhood, adolescence and adulthood in relation to gestational diabetes mellitus: a sex-specific association. Int J Epidemiol (2017) 46(6):2104. doi: 10.1093/ije/dyx211
40. Hunter SK, Hoffman MC, D'Alessandro A, Noonan K, Wyrwa A, Freedman R, et al. Male Fetus susceptibility to maternal inflammation: C-reactive protein and brain development. Psychol Med (2021) 51(3):450–9. doi: 10.1017/S0033291719003313
41. Wang X, Cong P, Wang X, Liu Y, Wu L, Li H, et al. Maternal diet with sea urchin gangliosides promotes neurodevelopment of young offspring via enhancing NGF and BDNF expression. Food Funct (2020) 11(11):9912–23. doi: 10.1039/d0fo01605e
Keywords: inflammation, immune activation, fetal hyperinsulinemia, vitamin D, neurodevelopment
Citation: Wang P, Wu L, Yin W-j, Tao R-x, Zhang Y, Li P-p, Jiang X-m, Shao Z-y and Zhu P (2023) Associations of cord blood meta-inflammation and vitamin D with neurodevelopmental delay: A prospective birth cohort study in China. Front. Immunol. 13:1078340. doi: 10.3389/fimmu.2022.1078340
Received: 25 October 2022; Accepted: 14 December 2022;
Published: 04 January 2023.
Edited by:
Reinaldo B. Oria, Federal University of Ceara, BrazilReviewed by:
Chunyue Wang, Jilin Agricultural University, ChinaDarryl W. Eyles, The University of Queensland, Australia
Copyright © 2023 Wang, Wu, Yin, Tao, Zhang, Li, Jiang, Shao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Zhu, cGVuZ3podUBhaG11LmVkdS5jbg==; Zi-yu Shao, c2hhb3ppeXUxMjM0QDE2My5jb20=
 Peng Wang1,2,3
Peng Wang1,2,3 Wan-jun Yin
Wan-jun Yin Peng Zhu
Peng Zhu