- 1Department of Hospital Pharmacy, Erasmus Medical Center, Rotterdam, Netherlands
- 2Center of Tropical Medicine and Travel Medicine, Department of Infectious Diseases, Amsterdam University Medical Centers, Amsterdam, Netherlands
- 3Infection & Immunity, Amsterdam Public Health, University of Amsterdam, Amsterdam, Netherlands
- 4Department of Internal Medicine and Infectious Diseases, University Medical Center Groningen, Groningen, Netherlands
- 5Department of Infectious Diseases, Leiden University Medical Center, Leiden, Netherlands
- 6Department of Viroscience, Erasmus Medical Center, Rotterdam, Netherlands
- 7Department of Internal Medicine, Division of Allergy & Clinical Immunology and Department of Immunology, Erasmus Medical Center, Rotterdam, Netherlands
- 8Department of Experimental Immunology, Amsterdam University Medical Centers, Amsterdam Infection and Immunity Institute, University of Amsterdam, Amsterdam, Netherlands
- 9Department of Medical Microbiology and Infection Prevention, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 10Center for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, Netherlands
- 11Department of Internal Medicine, Erasmus Medical Center, Rotterdam, Netherlands
Vaccination against coronavirus disease 2019 (COVID-19) has contributed greatly to providing protection against severe disease, thereby reducing hospital admissions and deaths. Several studies have reported reduction in vaccine effectiveness over time against the Omicron sub-lineages. However, the willingness to receive regular booster doses in the general population is declining. To determine the need for repeated booster vaccinations in healthy individuals and to aid policymakers in future public health interventions for COVID-19, we aim to gain insight into the immunogenicity of the additional bivalent booster vaccination in a representative sample of the healthy Dutch population. The SWITCH ON study was initiated to investigate three main topics: i) immunogenicity of bivalent vaccines after priming with adenovirus- or mRNA-based vaccines, ii) immunological recall responses and reactivity with relevant variants after booster vaccination, and iii) the necessity of booster vaccinations for the healthy population in the future.
Clinical trial registration: https://clinicaltrials.gov/, identifier NCT05471440.
Background
Vaccination against coronavirus disease 2019 (COVID-19) has contributed greatly to providing protection against severe disease, thereby reducing hospital admissions and deaths (1–3). However, the emergence of the antigenically distinct Omicron sub-lineages, combined with waning antibody levels after vaccination, once again put pressure on public health, healthcare services and the economy of many countries at the end of 2021 (4). The mutated receptor binding domain (RBD) of the spike (S) protein allows Omicron sub-lineages to spread more efficiently and enhances antibody evasion (5–7). Several studies have reported reduction in vaccine-induced neutralizing antibodies cross-reactive with Omicron sub-lineages (8, 9), although T cell immunity remains largely intact (8, 10, 11). Severe acute respiratory distress syndrome coronavirus-2 (SARS-CoV-2) breakthrough infections can occur even after receiving a booster vaccination, or after acquiring infection-induced immune responses. Recent observational studies showed a significant reduction in vaccine effectiveness against the Omicron sub-lineages over time, even after a booster dose (12–14). As a result, there is an increased demand for updated vaccines that can provide better protection against emerging variants.
Bivalent vaccines that contain both the ancestral S protein, to boost previously induced immune responses, and the Omicron BA.1 or BA.5 S protein, to specifically boost antibodies recognizing viruses from the Omicron sub-lineage, have been designed for that reason. A study on one bivalent vaccine reported superiority in the induction of neutralizing antibodies to omicron sub-lineage variant BA.1 compared to the previously approved monovalent vaccine (15). Although the seasonality of SARS-CoV-2 outbreaks has not been determined yet and varies across different climates, more frequent surges have been observed in colder months (16). In preparation for the winter and probable circulation of a SARS-CoV-2 variant from the Omicron sub-lineage, bivalent vaccines have been made rapidly available for upcoming vaccination campaigns.
We now know that sterile immunity to SARS-CoV-2 infection is not achieved by vaccination, and that SARS-CoV-2 continues to circulate among humans. However, booster vaccinations remain important to prevent severe disease, especially in immunocompromised risk groups and the elderly. The willingness to receive regular booster doses in the general population is declining, partly because of the reduced disease severity observed after the emergence of the Omicron sub-lineages. Therefore, questions have arisen about future vaccination policies. Over 70% of the European adult population has completed the basic series of COVID-19 vaccination, yet just over 50% has received an additional booster dose (17). To determine the need for repeated booster vaccinations in healthy individuals and to aid policymakers in future public health interventions for COVID-19, we aim to gain insight into the immunogenicity of the additional bivalent booster vaccination in a representative sample of the healthy Dutch population. The SWITCH ON study was initiated to investigate three main topics: i) immunogenicity of bivalent vaccines after priming with adenovirus- or mRNA-based vaccines, ii) immunological recall responses and reactivity with relevant variants after booster vaccination, and iii) the necessity of booster vaccinations for the healthy population in the future.
SWITCH ON study design
SWITCH ON is a multi-center, open labelled, randomized, controlled trial. A total of 400 participants, between 18 and 65 years old, will be recruited from healthcare workers across four university medical centers (UMC) in the Netherlands (i.e., Amsterdam UMC, Erasmus MC Rotterdam, UMC Groningen, and Leiden UMC). Participants will be predominantly, but not exclusively, recruited from previously published SWITCH (9, 18–20) and healthcare worker (HCW) studies (7, 8, 21). Extensive records on immune response to vaccinations against COVID-19 and breakthrough infections are available for participants from those two studies. Prior infection with SARS-CoV-2 is accepted, but not in the three months before the start of the study. Some additional exclusion criteria will be applied, such as pregnancy, immunosuppressive medication consumption, receiving cancer therapy, or allergic reactions to ingredients of the bivalent vaccine. A full overview of inclusion and exclusion criteria can be found in Supplementary File 1, page 17. Half of the participants of SWITCH ON were primed with an adeno-based vaccine (Ad26.COV2.S), while the other half were primed with an mRNA-based vaccine (either BNT162b2 or mRNA-1273). The trial starts recruiting in September 2022, vaccinations will start in October 2022.
Participants will be equally divided into two study arms: direct boost (DB) or postponed boost (PPB). The DB arm consists of two branches: one with 100 Ad26.COV2.S-primed participants and the other with 100 mRNA-based-primed participants. The PPB arm has the same structure (Figure 1). We refer to Supplementary File 1, page 18, for the sample size calculation. Participants in the DB arm will receive the bivalent (ancestral/OMI BA.1) booster dose in the first week of October 2022, as per Dutch policy. Participants in the PPB group will receive the booster 3 months later than those in the DB arm; it is expected that by that time the novel bivalent ancestral/BA.5 vaccines will be used. Block randomization with 1:1 ratio was used between DB and PPB groups with stratification for priming vaccine type. By randomizing over the different groups, we assume that prior infections are equally distributed. Participants will be asked by questionnaire about prior COVID-19 (whether and when a participant had a prior SARS-CoV-2 infection). To confirm prior infection, or to identify missed sub-clinical COVID-19 cases, N-specific antibodies will be retrospectively measured in the pre-boost sample from all participants. A record of breakthrough infections after booster vaccination will be compiled via questionnaires and retrospective assessment of N-specific antibodies in serum collected 3 months after booster vaccination. To measure vaccine immunogenicity, SARS-CoV-2 specific antibody and T-cell responses will be measured in all participants at day 0, 7 and 28, and 3 months after booster administration. Neutralizing antibodies against variants encoded by the vaccine (ancestral SARS-CoV-2, and the Omicron BA.1 and BA.5 variants), and circulating variants (e.g. Omicron BQ.1.1 and XBB) will be measured by an in-house developed plaque reduction neutralization test (PRNT) in a random selection of participants. The PRNT has been extensively validated but is RUO as there is no comparable IVD registered alternative. SARS-CoV-2-specific T-cell responses will be assessed via two assays (1): a CE-IVD whole-blood interferon gamma release assay (IGRA, QuantiFERON SARS-CoV-2, QIAGEN) on all participants, and (2) a RUO activation induced marker (AIM) flow cytometry assay. This assay will use overlapping peptide pools from the ancestral S protein, and the Omicron BA.1, and BA.5 S proteins to stimulate peripheral blood mononuclear cells (PBMCs) isolated from 25% of participants in each branch of the trial arms. Cryopreserved PBMCs will additionally be used for the quantification and phenotyping of virus-specific B-cells, by staining with the ancestral, BA.1, or BA.5 S protein. The data will be analyzed on an intention-to-treat basis.
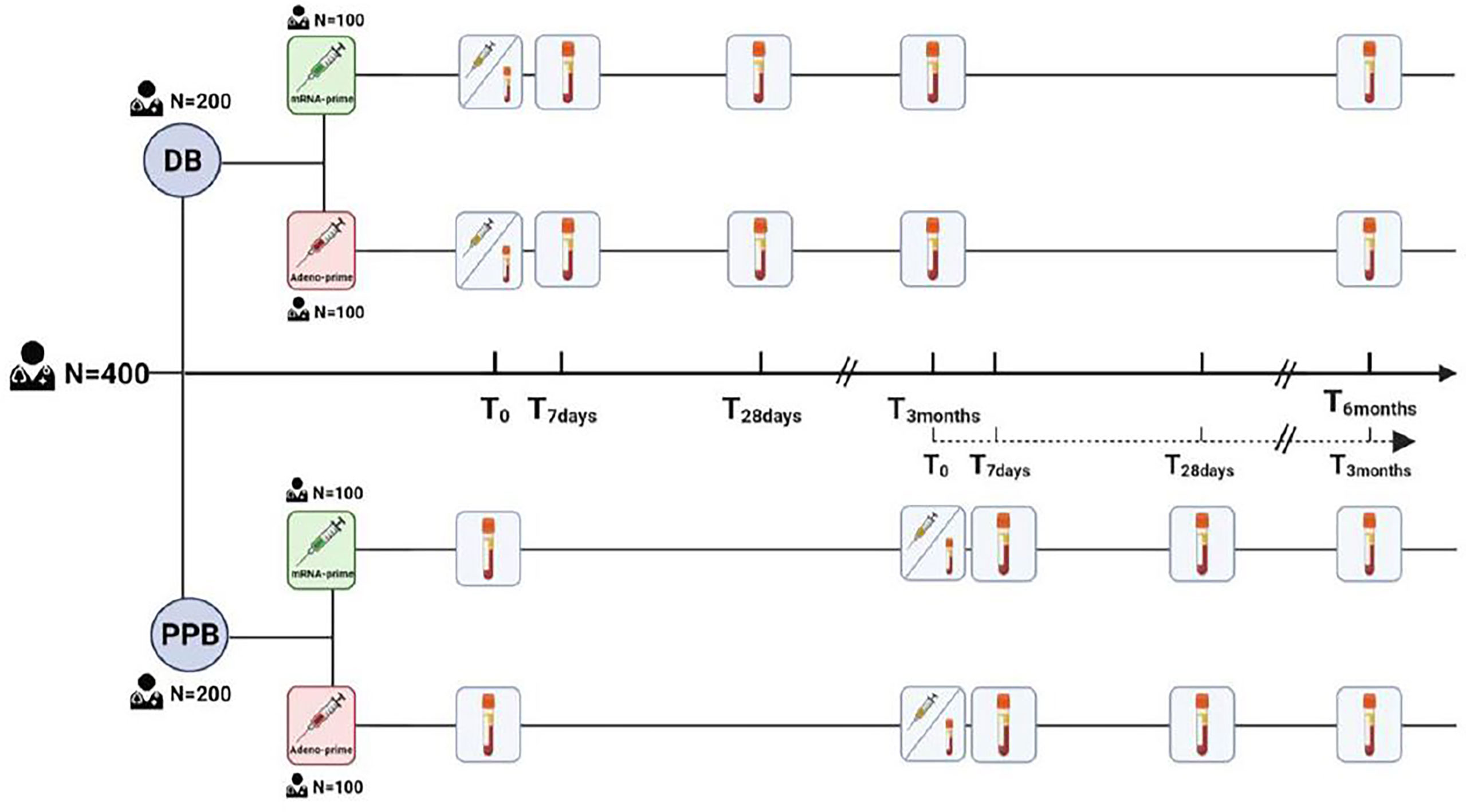
Figure 1 The 400 participants will be split into two groups, each consisting of 100 participants primed with Ad26.COV2.S (red) and 100 participants primed with an mRNA-based vaccine (green). The immunological response will be measured on day 0, 7, 28, and at 3 and 6 months after vaccination.
Outcome
The primary outcome is defined as the fold-change in SARS-CoV-2-specific binding antibodies between day 0 and 28 for the adenovirus-primed and mRNA-based-primed participants, measured by quantitative IgG assay with the ancestral and Omicron BA.1 S protein. The secondary outcome focuses on recall responses induced by booster vaccination. To this end, S-specific antibodies and T-cells will be measured and compared on day 7 and 28 post boost. Clinical trials involving bivalent vaccines thus far only measured neutralizing antibodies at day 28 after the booster (15, 22). The analysis of the response at day 7 has two advantages: i) rapid recall responses after vaccination could be a proxy for recall upon infection, indicative of whether booster vaccinations are required or not, and ii) early immunogenicity evaluation can accelerate the interpretation of future studies. Finally, the breadth of immunological responses will be studied in depth by comparing antibody and T-cell reactivity to the relevant SARS-CoV-2 variants (similar to our previous study (20)). With respect to safety, we will analyze the reactogenicity during the first 7 days after booster vaccination. We strive to publish the results as soon as possible to inform the policymakers and aid individual decision making regarding the booster dose.
Implications
The COVID-19 pandemic is currently in a transition phase towards endemic circulation, with reduced morbidity and mortality at the population level compared to the initial waves of circulation. Given the growing societal aversion towards repeated booster vaccinations, an important question is whether, and for how long, time-intervals between subsequent booster vaccinations can be prolonged. Differences in recall responses depending on the initial priming schedule (mRNA-based or vector-based) can guide the direction of future booster strategies, whereas the speed and breath of recall cellular memory after booster vaccination will provide important information for the timing of future booster vaccinations. The impact of intercurrent COVID-19 infections despite earlier vaccinations needs to be factored in when assessing these immune responses.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by METC Erasmus MC. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors were involved in the design and execution of the study and writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The trial is funded by Netherlands Organization for Health Research and Development ZonMw in the COVID-19 Vaccine program (project grant number: 10430072110001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1067749/full#supplementary-material
References
1. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Diseases. (2022) 22(9):1293–302. doi: 10.1016/S1473-3099(22)00320-6
2. Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet (2021) 398(10317):2126–8. doi: 10.1016/S0140-6736(21)02758-6
3. Suthar AB, Wang J, Seffren V, Wiegand RE, Griffing S, Zell E. Public health impact of covid-19 vaccines in the US: observational study. BMJ (2022) 377:e069317. doi: 10.1136/bmj-2021-069317
4. Care DoHS. Direct and indirect health impacts of COVID-19 in England: emerging omicron impacts (2022). Available at: https://www.gov.uk/government/publications/direct-and-indirect-health-impacts-of-covid-19-in-england-emerging-omicron-impacts/direct-and-indirect-health-impacts-of-covid-19-in-england-emerging-omicron-impacts.
5. Cameroni E, Bowen JE, Rosen LE, Saliba C, Zepeda SK, Culap K, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 omicron antigenic shift. Nature (2022) 602(7898):664–70. doi: 10.1038/s41586-021-04386-2
6. Gobeil SMC, Henderson R, Stalls V, Janowska K, Huang X, May A, et al. Structural diversity of the SARS-CoV-2 omicron spike. Mol Cell (2022) 8(11):2050–86.e6. doi: 10.1101/2022.01.25.477784
7. Mykytyn AZ, Rissmann M, Kok A, Rosu ME, Schipper D, Breugem TI, et al. Antigenic cartography of SARS-CoV-2 reveals that omicron BA. 1 and BA. 2 are antigenically distinct. Sci Immunol (2022) 7(75):eabq4450. doi: 10.1126/sciimmunol.abq4450
8. GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S, et al. Divergent SARS CoV-2 omicron-reactive T- and b cell responses in COVID-19 vaccine recipients. Sci Immunol (2022) 7(69):eabo2202. doi: 10.1126/sciimmunol.abo2202
9. Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, Visser LG, Schmitz KS, et al. Durability of immune responses after boosting in Ad26.COV2.S-primed healthcare workers. Clin Infect Diseases. (2022). doi: 10.1093/cid/ciac495
10. Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from alpha to omicron. Cell (2022) 185(5):847–59.e11. doi: 10.1101/2021.12.28.474333
11. Gao Y, Cai C, Grifoni A, Müller TR, Niessl J, Olofsson A, et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the omicron variant. Nat Med (2022) 28:472–6. doi: 10.1038/s41591-022-01700-x
12. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B. 1.1. 529) variant. New Engl J Med (2022) 386(16):1532–46. doi: 10.1056/NEJMoa2119451
13. Malhotra S, Mani K, Lodha R, Bakhshi S, Mathur VP, Gupta P, et al. COVID-19 infection, and reinfection, and vaccine effectiveness against symptomatic infection among health care workers in the setting of omicron variant transmission in new Delhi, India. Lancet Regional Health-Southeast Asia. (2022) 3:100023. doi: 10.1016/j.lansea.2022.100023
14. Patalon T, Saciuk Y, Peretz A, Perez G, Lurie Y, Maor Y, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun (2022) 13(1):1–7. doi: 10.1038/s41467-022-30884-6
15. Chalkias S, Harper C, Vrbicky K, Walsh SR, Essink B, Brosz A, et al. A bivalent omicron-containing booster vaccine against covid-19. N Engl J Med (2022) 387(14):1279–91. doi: 10.21203/rs.3.rs-2239682/v1
16. D’Amico F, Marmiere M, Righetti B, Scquizzato T, Zangrillo A, Puglisi R, et al. COVID-19 seasonality in temperate countries. Environ Res (2022) 206:112614. doi: 10.1016/j.envres.2021.112614
17. Control ECfDPa. COVID-19 vaccine tracker: European centre for disease prevention and control (ECDC) (2021). Available at: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab.
18. Sablerolles RSG, Goorhuis A, GeurtsvanKessel CH, de Vries RD, Huckriede ALW, Koopmans MPG, et al. Heterologous Ad26.COV2.S prime and mRNA-based boost COVID-19 vaccination regimens: The SWITCH trial protocol. Front Immunol (2021) 12:753319. doi: 10.3389/fimmu.2021.753319
19. Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, Visser LG, Geers D, et al. Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S priming. N Engl J Med (2022) 386:951–63. doi: 10.1101/2021.10.18.21264979
20. Geers D, Sablerolles R, van Baarle D, Kootstra N, Rietdijk W, Schmitz K, et al. Ad26. COV2. s priming provides a solid immunological base for mRNA-based COVID-19 booster vaccination. medRxiv (2022). doi: 10.1101/2022.07.15.22277639
21. Geers D, Shamier MC, Bogers S, Den Hartog G, Gommers L, Nieuwkoop NN, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol (2021) 6(59):eabj1750. doi: 10.1126/sciimmunol.abj1750
22. BioNTech. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals: Clinicaltrials.gov (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT04368728.
Keywords: COVID-19, immune memory, mRNA vaccine, adenovirus-based vaccine, immune response
Citation: Tan NH, Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, Visser LG, Bogers S, Geers D, Zaeck LM, Koopmans MPG, Dalm VASH, Kootstra NA, Huckriede ALW, van Baarle D, Lafeber M, GeurtsvanKessel CH, de Vries RD and van der Kuy P-HM (2022) Analyzing the immunogenicity of bivalent booster vaccinations in healthcare workers: The SWITCH ON trial protocol. Front. Immunol. 13:1067749. doi: 10.3389/fimmu.2022.1067749
Received: 12 October 2022; Accepted: 15 November 2022;
Published: 29 November 2022.
Edited by:
Else Bijker, University of Oxford, United KingdomReviewed by:
Kapil Bahl, Moderna Therapeutics, United StatesSimon Jochum, Roche Diagnostics GmbH, Germany
Copyright © 2022 Tan, Sablerolles, Rietdijk, Goorhuis, Postma, Visser, Bogers, Geers, Zaeck, Koopmans, Dalm, Kootstra, Huckriede, van Baarle, Lafeber, GeurtsvanKessel, de Vries and van der Kuy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul-Hugo Marie van der Kuy, aC52YW5kZXJrdXlAZXJhc211c21jLm5s
 Ngoc H. Tan1
Ngoc H. Tan1 Roos S. G. Sablerolles
Roos S. G. Sablerolles Wim J. R. Rietdijk
Wim J. R. Rietdijk Douwe F. Postma
Douwe F. Postma Leo G. Visser
Leo G. Visser Marion P. G. Koopmans
Marion P. G. Koopmans Virgil A. S. H. Dalm
Virgil A. S. H. Dalm Neeltje A. Kootstra
Neeltje A. Kootstra Anke L. W. Huckriede
Anke L. W. Huckriede Corine H. GeurtsvanKessel
Corine H. GeurtsvanKessel Rory D. de Vries
Rory D. de Vries Paul-Hugo Marie van der Kuy
Paul-Hugo Marie van der Kuy