
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 16 November 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1063928
This article is part of the Research Topic Neuro-immune Interaction in CNS Injury and Disease View all 14 articles
The central nervous system is the most important nervous system in vertebrates, which is responsible for transmitting information to the peripheral nervous system and controlling the body’s activities. It mainly consists of the brain and spinal cord, which contains rich of neurons, the precision of the neural structures susceptible to damage from the outside world and from the internal factors of inflammation infection, leading to a series of central nervous system diseases, such as traumatic brain injury, nerve inflammation, etc., these diseases may cause irreversible damage on the central nervous or lead to subsequent chronic lesions. After disease or injury, the immune system of the central nervous system will play a role, releasing cytokines to recruit immune cells to enter, and the immune cells will differentiate according to the location and degree of the lesion, and become specific immune cells with different functions, recognize and phagocytose inflammatory factors, and repair the damaged neural structure. However, if the response of these immune cells is not suppressed, the overexpression of some genes can cause further damage to the central nervous system. There is a need to understand the molecular mechanisms by which these immune cells work, and this information may lead to immunotherapies that target certain diseases and avoid over-activation of immune cells. In this review, we summarized several immune cells that mainly play a role in the central nervous system and their roles, and also explained the response process of the immune system in the process of some common neurological diseases, which may provide new insights into the central nervous system.
The immune processes involved in these two types of immunity and the therapeutic regulation of disease and injury by immune cells are not unidirectional in their effects on the CNS, for the reason that the wrong activation or over-response can cause damage to the central nervous system. The developed nervous system of vertebrates is an important sign of high evolution (1, 2). Central Nervous System (CNS) is the main component of the human nervous system, including the brain, which is located in the skull cavity, and the spinal cord, which is located in the spinal canal. CNS can accept incoming information from all over the body, integrate and process it, and finally control the body through outgoing signals (3), or use this information to complete memory and learning, so that the organism can carry out a series of thinking activities.
Because the CNS is the most important vertebrate nervous system, and the brain and spinal cord, the two main components of the CNS, have been shown to be non-renewable (4). Therefore, CNS injury and disease and the subsequent repair of immune responses are crucial for the CNS. Like the immune system of most other tissues, the immune system of CNS is composed of the innate immune system and the specific immune system (5, 6). The innate immune system is mainly composed of congenital macrophages. The main process of specific immunity is the specific immune response produced by the specific combination of antigens and antibodies. The immune process involved in these two immune modes and the therapeutic regulation of immune cells on diseases and injuries have a non-unidirectional effect on the CNS —— incorrect activation or excessive response may cause damage to the central nervous system (7).
At present, there is a relatively clear understanding of the birth and differentiation of immune cells, and more understanding of the possible functions of most immune cells after differentiation. Studies on CNS immunity from 2020 to the present have mainly focused on the molecular mechanism of immune cells entering the CNS and promoting CNS inflammation, and on this basis, how to regulate the response degree of immune cells. For some immune cells, the researchers found other types of differentiation and other effects. Other studies have attempted to explain the link between CNS immunity and other diseases, and have attempted to use CNS immunotherapy as a clinical treatment (8, 9).
In this review, we illustrated the immune components of CNS and their roles, observe the performance of these immune components in different neurological diseases, and judged whether they are beneficial or harmful for CNS diseases. Based on this information, we may find ideas and methods of immunotherapy for these diseases.
The central nervous system of the human body consists of the brain and the spinal cord (Figure 1). The spinal cord is the lowest level of motor center, and it is also the basic reflex center to complete the movement of the organism, under the control of the brain, through the neural circuit signal conduction, so as to achieve motor regulation. The spinal cord is composed of gray and white matter and contains nerve cell bodies and their ascending and descending conduction tracts (10). Classical studies of spinal cord function have focused on well-defined neural pathways that are thought to mediate automatic functions of stereotyping, such as stretch and flexion reflexes, and allocate inputs from sensory and descending fibers to appropriate targets (11).
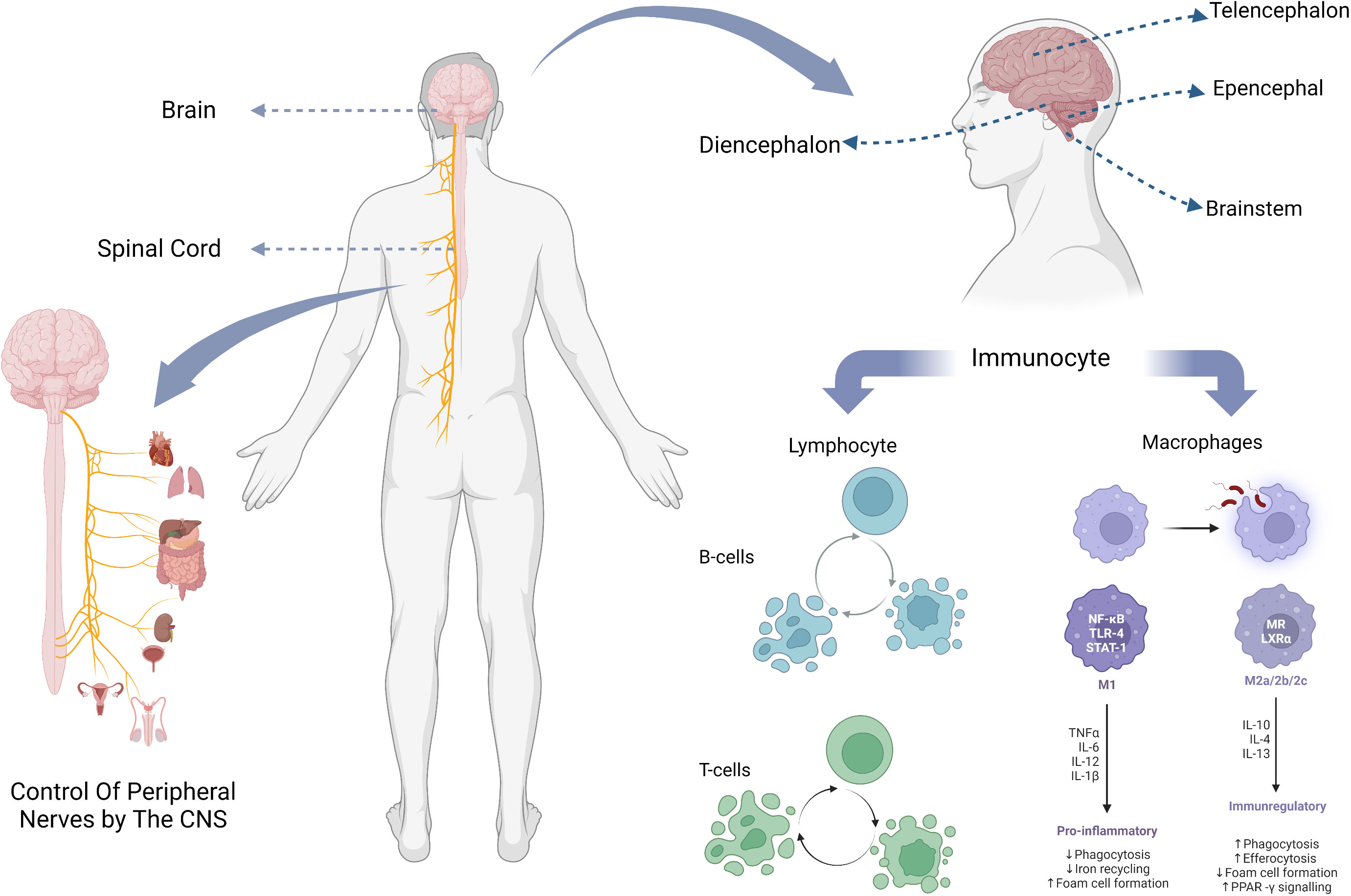
Figure 1 The composition of the CNS, the location of its parts, and the major immune cells it contains.
Brain, generally refers to three parts which are: cerebellum, brainstem, diencephalon. The brainstem is an important part of the brain responsible for sensing injury and processing pain signals, and transmits and processes signals between the brain, cerebellum and spinal. The conventional wisdom about the cerebellum is that it is essential for motor function and contributes little to cognitive function (12). However, multiple studies by Middleton FA et al. showed that cerebellar output targets involved multiple cortical regions, including not only primary motor cortex (13, 14), but also oculomotor nerve (15, 16), prefrontal lobe and inferior temporal region (17, 18), which indicates that cerebellum plays a role in both motor and cognitive function.
The sensory organs with receptors establish communication with the central nervous system through the afferent nerves, so as to realize the regulation of the central nervous system. Peripheral nerve tissue is composed of afferent sensory fibers and efferent motor fibers. When receptors such as the skin are stimulated by the outside world, they will produce nerve impulses. Nerve synapses release neurotransmitters, which are received by cells, and the resting potential is converted into action potential (19). After receiving the neurotransmitter, the receiver may process it and react on the afferent nerve synapse (20), and intercellular conduction and closed interaction of nerve electrical signals are realized. The electrical signal is transmitted to the CNS along the sensory fibers, and the CNS processes the signal and transmits it along the motor fibers to control the body movement (21). In this way, the CNS is connected to the rest of the nervous system.
Take, for example, the mechanism of regulation of renal afferent sensory nerves in the central nervous system. Kidney is an important sensory organ with abundant baroreceptors and chemoreceptors, as well as a large number of afferent nerves. Renal afferents can directly project to areas of the central nervous system, such as the lateral thalamic area and the paraventricular nucleus (PVN), and indirectly project to other areas of the hypothalamus (22). During stimulation of the afferent renal nerve, the firing frequency of the large-cell neurons in the PVN increased (23). Renal afferents activate the central nervous system and enhance sympathetic activity (24).
In simple terms, the peripheral nervous system gives information to the CNS in the form of electrical stimulation through the afferent nerve, which is processed by the CNS to realize the regulation of the body.
Among all immune cells in the central nervous system, macrophages and glial cells derived from them, as well as lymphocytes including T cells, B cells and self-killing immune cells, play a major role (Table 1).
Microglia are the main cellular component of the innate immune system of the brain. They are distributed in the whole brain at different densities, accounting for 5%-20% of the total brain cells, and are developed from primitive macrophages (25, 26). Microglia are activated and polarized after being exposed to external stimuli (42), which will present two phenotypes M1/M2 with different functional status and markers (27). The M1 phenotype produced by the general activation pathway can exert pro-inflammatory and neurotoxic effects, while the M2 phenotype produced by the selective activation pathway can exert anti-inflammatory and neuroprotective effects (28, 29), promoting medullary regeneration by inhibiting cell differentiation (34). Such a classification of microglia was originally proposed by Mills et al. (30). However, this classification method based on stimuli does not reflect the range and function of phenotypes well, and Mills himself has also shown that the main existence should be a continuous intermediate of M1 and M2 (43), so this once widely used classification method has been questioned by many people (44, 45). Later, Devanney et al. proposed a function-based classification method (27). For the function of microglia, there has been a high similarity between microglia and macrophages due to their structural characteristics (46), researchers have long thought that its function is mainly an immune response, like macrophages, to recognize and take up pathogens and other substances, and analyze their environment (33). However, many subsequent experiments have shown that, especially when there is no need for immunity, the role of microglia is mainly to carry out sensing, information processing and nerve protection in the central nervous system (25), and maintain the homeostasis of the central nervous system (31, 47). It promotes neural development over a period of time (48) and can affect nerve impulses in the adult brain (49).
Lymphocytes are the main cells of the body’s immune response, which are produced by lymphoid organs and can respond to signals such as foreign pathogens or inflammatory stimuli. T cells, B cells and natural killer cells play a significant role in central nervous system immunity. The main reason for the suppression of immune action in the CNS is the blockage of the blood-brain barrier (50, 51). Only a small number of lymphocytes can enter the CNS through the blood-brain barrier, blood-meningeal barrier, and blood-cerebrospinal fluid barrier during brain health and injury, and play an immune function or promote neuroinflammation (52–54).
T cells and B cells together constitute acquired immunity. They can all differentiate into different subtypes (55), release different cytokines or perform different functions, which greatly contributes to their immune specificity (36, 40, 56–58).
B cells travel from the skull to the meninges through blood vessels (59, 60), and their main role is to produce antibodies (61). After receiving antigen presentation, B cells can differentiate into plasma cells and memory cells. The former will produce specific antibodies that bind to the antigen and destroy it[98][99]. B cells can also act as antigen-presenting cells (APCs), processing antigens and presenting them to antigen-specific T cells (38, 39, 62), and promote the development of pro-inflammatory T cells (63). In the treatment of CNS autoimmune diseases, by depleting activated B cells, B cells as APC can be stimulated to target T cells, so that they can differentiate and develop, thus enhancing the immune effect (64, 65).
T lymphocytes, the sentinels of the adaptive immune system, respond to antigen-specific signals by bursting, proliferating, and differentiating into effector subsets to recognize and eliminate threats to the host (66, 67). In addition to the previously mentioned antigen presentation and immune role with B cells, T cells also have the function of promoting cognitive learning in the brain (41, 68, 69), as well as a certain role in maintaining CNS homeostasis. A large number of T cells are used for immune monitoring of the brain barrier (37).
Natural killer immune cell (NK) is a kind of large granular hematopoietic cell derived from bone marrow (70), and its function varies in different individuals due to different genes (35). Like T and B cells, NK also has a variety of subtypes (71), among which the CD56 bright NK cells are the main ones present in CD56 bright NK cells (72). It has been suggested that NK may play a regulatory role in central nervous system diseases (32). NK can repair nerves after CNS damage, coordinate immune responses, and inhibit the development of inflammation (73, 74). It is able to regulate the neurological diseases such as Parkinson disease, and can play a neuroprotective role by killing T cells that promote neurotoxicity (75). In some neuroinflammation, such as autoimmune encephalomyelitis (EAE), the cytotoxicity is reduced and the memory EAE is inhibited (76, 77). In addition, NK can also work with other lymphocytes (78) to cooperate in immune action in neurological diseases such as Alzheimer’s disease (79).
Recent studies have shown that astrocytes play an important role in CNS immunity by associating with other immune cells. For example, the recognition of astrocytes depends on microglia, and the subsequent release of caspases induced by astrocytes promotes apoptosis and reduces cellular inflammation. IF-33 released by astrocytes can recruit twice as many T cells and promote the repair of CNS diseases such as neurodegenerative diseases and spinal cord injuries (80).
The CNS immune system is divided into innate immunity and specific immunity, which make different immune responses in different situations. Some autoimmunity can lead to diseases, but the immune effect after injury helps to recover from injury (81).
After CNS injury, a series of subsequent immune responses are triggered, first innate immunity and then specific immunity. Both types of immunity can expand their number by releasing cytokines and regulating genes from immune cells (82–84). The innate immune system is the first line of defense to help the central nervous system resist foreign pathogens, and the specific immune response is slow, which will gather and play a role after inflammation or injury occurs (85, 86). A correct understanding of the immune system in different CNS diseases at different stages of development can help us find appropriate immunotherapy methods.
Due to the particularity of the CNS, the BBB still acts as a barrier, making the CNS an immune privileged organ. Its carrier and receptor proteins are the only way for outside proteins to enter the CNS, ensuring precise regulation of what enters the brain (87). This barrier creates an important difference between CNS immunity and peripheral nervous system immunity. There are only a small number of protoimmune cells in the CNS. These immune cells and some immune progenitors release chemokines and cytokines to recruit peripheral immune cells after brain injury, thus amplifying the immune effect (88). For example, fatty acids can activate peripheral macrophages through TLR4-mediated mechanism. Such recruitment needs to be carefully regulated, and the process will be discussed later (89).
However, the CNS has connections to all surrounding organs (90). There is an external barrier to immune cells, but the immune system of CNS is not isolated. Cerebrospinal fluid is used as the medium to exchange immune cells with the outside world through the meninges. Compartmentalized immune cells are recruited to cross the blood-brain barrier when the brain needs immune repair (91). These barriers used to be thought to insulate the CNS from its immune system, but recent research suggests that these barriers facilitate communication between the CNS and the outside world. Some of them also act as immune hubs, such as the dura mater, which associates with the brain through the lymphatic system, metabolizing waste and maintaining brain homeostasis (92).
Central nervous system diseases include central neurodegenerative diseases, nervous system infections, brain tumors and other types of diseases, including brain injury, spinal cord injury and peripheral nerve injury from the point of view of injury and disease location. These diseases are related to the immune system to some extent. Here we focus on central neurodegenerative neurological diseases, CNS tumors, traumatic brain injury, and spinal cord injury.
Brain tumors are generally divided into two categories, namely those from external sources, also known as metastases, and those arising from the central nervous system, such as gliomas (93). We focus on tumors arising spontaneously in the central nervous system. When tumors occur, tumor associated macrophages (TAMs) are the main immune cells in the tumor microenvironment (TME) in the CNS. Studies have shown that the incidence of some primary tumors is very low after immunosuppression (94, 95), which indicates that TAM targeted inhibition plays an important role in tumor therapy (96). Take glioma as an example, it is a tumor developed by glial cells, among which glioblastoma (GBM) is the most common glial tumor and one of the highest mortality among all cancers (97). The average overall survival time is not more than 15 years, and only seven percent of patients can be cured (98, 99). Some other subgroups of low-grade gliomas have molecular features similar to glioblastoma, with an invasive process (100). Glial cells will release CSF-1 and other cytokines and recruit TAM (101). Studies have shown that TAM can promote the growth rate and morphological size of glioma cells (102, 103), and a small number of TAMs and microglia will lead to a larger volume of tumors (104). In fact, in GBM, the number of TAMs is positively correlated with tumor severity, and there are very few in patients who do not relapse after recovery (105). According to this characteristic of TAM, the development of tumor therapy targeting TAM has become a new direction for brain tumor treatment (106, 107).
CNS tumor immune process after the basic follow - tumor immune system cycle, this is Chen et al. Applied to inducing tumor immune response rule (108), after the tumor cells results in the release, antigen precursor release activating factor to activate T cells, as effector cells infiltrating tumor, T cell contact and identify the antibody, to destroy, then the immune cells apoptosis (109). T cells can track and eliminate cancer by recognizing specific exogenous factors to track and damage host cells (79). In fact, the development of tumor immunotherapy based on this principle has been widely carried out and achieved remarkable results (110, 111), and the application in brain tumors is also an example to follow.
In 2019, there were about 50 million people living with neurodegenerative diseases worldwide, and this number is expected to rise to 152 million by 2060 (112). In his study, Richard Armstrong listed a series of factors that lead to degenerative neurological diseases and pointed out that age is the most important risk factor (113). The two diseases with the highest incidence of degenerative neurological diseases are Alzheimer’s disease (AD) (114) and Parkinson’s disease (PD) (115, 116), which are also a hot research topic in CNS diseases.
An important component of neurodegenerative diseases is the triggering of innate immune mechanisms. Microglia and other cell types in the brain can be activated by misfolded proteins or abnormally localized nucleic acids. This detents microglia from their physiological and beneficial functions and leads to a sustained release of their proinflammatory mediators (25). In the process of Parkinson’s disease, for example, after the activation of microglia as the main cells of immune function, may cause nerve nutrition through compound, such as brain derived neurotrophic factor, nerve protective effect, but there are also likely to produce neurotoxicity of proinflammatory cytokines (such as tumor necrosis factor (TNF), interleukins) (117, 118). For these two functions, which are almost opposite to the development of the disease, the current view is that at the beginning, the cytokines of activated microglia may have neuroprotective effects, but then the activated microglia undergo toxic degeneration, leading to the progression of Parkinson’s disease to neurotoxicity (119). Sawada showed in his experimental studies that activated microglia may be neuroprotective in neonatal mice but neurotoxic in aged mice. This conclusion is consistent with the fact that age is a major risk factor in the pathogenesis of degenerative neurological diseases, because the performance of two subsets of activated microglia (with toxic and neuroprotective functions, respectively) is strongly correlated with age.
Spinal cord injury (SCI) refers to the injury of the spinal cord and the pathological changes such as sensory and motor disorders after the body encounters direct or indirect external interference. Spinal cord injury may lead to loss of limb perception, incontinence and even complete paralysis, which may not recover for a lifetime or even be life-threatening (120).
After SCI, to ensure the regeneration of damaged axons (121), the CNS begins to recruit peripheral neutrophils into the CNS (122) and begins to clear axons and myelin debris nerve remnants (123), within the first hour after injury, playing a major clearing role. Subsequently, the recruited neutrophils begin to apoptosis and release attractant factors that attract and recruit monocytes, and at the same time can also recruit certain macrophages. After reaching the injury site, monocytes differentiate into macrophages according to the chemokines at the injury site (124–126), and the macrophages produced at this time will phagocytize the apoptotic neutrophils and other tissue debris (127). Similarly, microglia are also involved in injury repair (128). So similar to the white blood cells, neutrophils and other related immune cells in the spinal cord damage happens after apoptosis, part of the nerve ending structure will be destroyed in the process of damage (120). Experiments have shown that there are many repair factors in the central nervous system that promote regeneration of biological factors in nerve and immune cells (Ramer, Ramer et al., 2005), but regeneration does not represent full functional recovery. Therefore, after the immune system deals with spinal cord injury, the body will have reduced immunity and be prone to infection (129). Moreover, although the immune system responds to traumatic stimuli, it drastically changes as the injury worsens, which may exacerbate the injury and inflammation (130, 131).
Traumatic brain injury (TBI) is an injury to the brain caused by external forces or external shocks, which may lead to a reduction or change in the state of consciousness and easily cause biochemical cascade damage, so it may be accompanied by long-term sequelae (132). After the occurrence of TBI, symptoms such as intracranial hemorrhage, brain contusion, concussion, and damage to nerve synapses are first caused (133), and a cascade of damage occurs within minutes to months. TBI has a high incidence among both military personnel and the general public (134).
Neuroinflammation and peripheral neuropathy are easily triggered after TBI (135). There are many factors that cause this situation, such as purines, heat shock proteins, and receptors of pathogen associated endogenous molecules (PAMPs) and damage associated endogenous molecules (DAMPs), which may promote inflammation (136–138). The latter can combine with proteins to form inflammasomes, release and infiltrate microglia, astrocytes and other cell populations, and produce proinflammatory factors after activation (139). Proinflammatory factors can further lead to neuroinflammation, and more severe inflammation can even transform TBI into chronic neurological diseases (140).
Similar to other neurological diseases, in TBI, immune cells can both promote recovery and aggravate injury (141). TBI can instantly induce cell death, after which the damaged cells release DAMPs, signal to immune cells, recruit microglia, astrocytes, etc. After they are activated, they will further recruit peripheral immune cells to pass through the damaged blood-brain barrier (142). Microglia respond immediately after injury and accumulate in large numbers in the injured area (33, 143), removing cell debris through phagocytosis (44). It can also release trophic factors to protect nerves (133) and is involved in remodeling injured nerves (144). However, microglial activation will produce excessive inflammatory mediators, recruit peripheral immune cells, produce a large number of pro-inflammatory factors and cytotoxic substances, hinder the repair of the central nervous system, and even lead to cell death and neuronal dysfunction (145, 146). Similarly, although neutrophils can participate in the immune regulation of TBI, they will also release some acute inflammatory cytokines to aggravate brain injury. The protective and injury effects largely depend on the location, type and stage of injury (147). Which more complicated is that the immune function and immune effect after TBI are related to gender (148) and age (149). Therefore, studying the relationship between these influencing factors and immune effect is an important entry point for the treatment of TBI (Table 2).
Now the treatment of some kinds of new ideas are mainly concentrated in cell therapy (151), because the immune cells such as macrophages have different phenotypes, their functions have significant difference, so can adjust the balance between different phenotypes, as far as possible avoid inflammation cause of neurodegenerative diseases, lower immune cell toxicity effect (152). In a study of the effects of Anakinra, a recombinant human IL-1 receptor antagonist, the treatment group had a higher cure rate than the control group (153). In addition, some small particles, such as zinc ions, have also been shown to be involved in neuroprotection and nerve recovery after injury (150). TBI ZnT3-KO mice were more severely injured when compared with juvenile wild-type mice.
In each of these diseases, the brain is damaged to a degree that releases specific substances that activate the innate immune system, which in turn activates and recruits the peripheral immune system. The immune system plays an important role in the anti-inflammatory response to disease injury and subsequent damage repair, but some immune responses also lead to the deterioration of the disease, so it is believed that CNS diseases and CNS immune system have an important relationship (Figure 2).
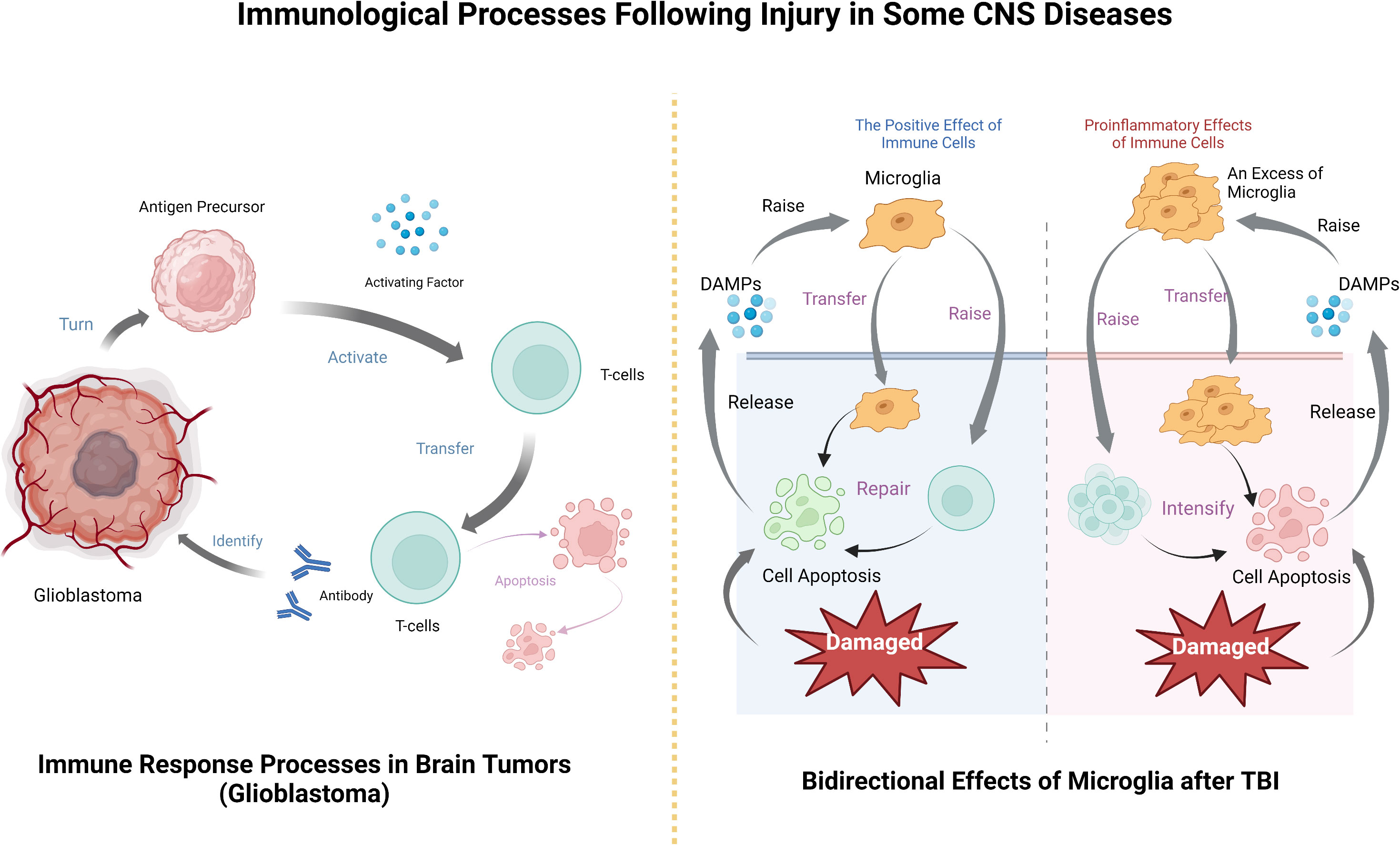
Figure 2 The processes by which immune cells exert their effects after CNS tumors and TBI, including their bidirectional effects on the disease.
Although some immune cells in the CNS have been discovered and their functions have been relatively obvious, a large part of the CNS is still unknown. Previous studies have shown that the molecular pattern and molecular reason for these apparent phenomena are still unclear after addressing the expression role of the immune system. For example, do NK cells maintain or regulate repair functions in the central nervous system and how do they do it? What is the mechanism of macrophage phagocytosis and foam cell formation after SCI? How do macrophages recognize and internalize specific molecules in apoptotic neutrophils? All these problems now seem difficult to solve (154, 155).
In addition, some immune cells were surprisingly found to be present in specific disease contexts, which could not be explained by current understanding. Some CNS diseases currently have no suitable treatment (156, 157), and some therapies that appear to be effective have substantial limitations. For example, although checkpoint inhibitors have been relatively successful in many solid tumor types, they have been difficult to succeed in CNS tumors. Even some serious CNS diseases have not received enough attention (158).
In the future studies, more neurological diseases will be taken into account, and the immune connection between the CNS and the peripheral nervous system may become an important consideration of immune effects.
JX and CM had the idea for the article. MH and JL performed the literature search and data analysis. JW and ZX drafted and critically revised the work. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Albuixech-Crespo B, Herrera-Úbeda C, Marfany G, Irimia M, Garcia-Fernàndez J. Origin and evolution of the chordate central nervous system: Insights from amphioxus genoarchitecture. Int J Dev Biol (2017) 61(10-11-12):655–64. doi: 10.1387/ijdb.170258jg
2. Albuixech-Crespo B, López-Blanch L, Burguera D, Maeso I, Sánchez-Arrones L, Moreno-Bravo JA, et al. Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PloS Biol (2017) 15(4):e2001573. doi: 10.1371/journal.pbio.2001573
3. Osadchiy V, Martin CR, Mayer EA. Gut microbiome and modulation of cns function. Compr Physiol (2019) 10(1):57–72. doi: 10.1002/cphy.c180031
4. Shinozaki M, Nagoshi N, Nakamura M, Okano H. Mechanisms of stem cell therapy in spinal cord injuries. Cells (2021) 10(10):2676. doi: 10.3390/cells10102676
5. Wubben R, Efstathiou C, Stevenson NJ. The interplay between the immune system and viruses. Vitam Horm (2021) 117:1–15. doi: 10.1016/bs.vh.2021.06.011
6. Colgan TJ, Moran PA, Archer LC, Wynne R, Hutton SA, McGinnity P, et al. Evolution and expression of the immune system of a facultatively anadromous salmonid. Front Immunol (2021) 12:568729. doi: 10.3389/fimmu.2021.568729
7. Hagan N, Kane JL, Grover D, Woodworth L, Madore C, Saleh J, et al. Csf1r signaling is a regulator of pathogenesis in progressive Ms. Cell Death Dis (2020) 11(10):904. doi: 10.1038/s41419-020-03084-7
8. Stepp MA, Menko AS. Immune responses to injury and their links to eye disease. Transl Res (2021) 236:52–71. doi: 10.1016/j.trsl.2021.05.005
9. Lebrun A, Kean RB, Hooper DC. Brain tissue-resident immune memory cells are required for long-term protection against cns infection with rabies virus. Future Virol (2020) 15(11):755–61. doi: 10.2217/fvl-2020-0132
10. Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou X, et al. Microenvironment imbalance of spinal cord injury. Cell Transplant (2018) 27(6):853–66. doi: 10.1177/0963689718755778
11. Boyce VS, Mendell LM. Neurotrophins and spinal circuit function. Front Neural Circuits (2014) 8:59. doi: 10.3389/fncir.2014.00059
12. Baizer JS. Unique features of the human brainstem and cerebellum. Front Hum Neurosci (2014) 8:202. doi: 10.3389/fnhum.2014.00202
13. Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science (1994) 266(5184):458–61. doi: 10.1126/science.7939688
14. Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev (2000) 31(2-3):236–50. doi: 10.1016/s0165-0173(99)00040-5
15. Middleton FA, Strick PL. Basal ganglia and cerebellar output influences non-motor function. Mol Psychiatry (1996) 1(6):429–33.
16. Middleton FA, Strick PL. Cerebellar output channels. Int Rev Neurobiol (1997) 41:61–82. doi: 10.1016/s0074-7742(08)60347-5
17. Middleton FA, Strick PL. Dentate output channels: Motor and cognitive components. Prog Brain Res (1997) 114:553–66. doi: 10.1016/s0079-6123(08)63386-5
18. Middleton FA, Strick PL. Cerebellar output: Motor and cognitive channels. Trends Cognit Sci (1998) 2(9):348–54. doi: 10.1016/s1364-6613(98)01220-0
19. Sulkowski G, Dąbrowska-Bouta B, Salińska E, Strużyńska L. Modulation of glutamate transport and receptor binding by glutamate receptor antagonists in eae rat brain. PloS One (2014) 9(11):e113954. doi: 10.1371/journal.pone.0113954
20. Shen J, Rothman DL, Behar KL, Xu S. Determination of the glutamate-glutamine cycling flux using two-compartment dynamic metabolic modeling is sensitive to astroglial dilution. J Cereb Blood Flow Metab (2009) 29(1):108–18. doi: 10.1038/jcbfm.2008.102
21. Catala M, Kubis N. Gross anatomy and development of the peripheral nervous system. Handb Clin Neurol (2013) 115:29–41. doi: 10.1016/b978-0-444-52902-2.00003-5
22. Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in the cat. J Auton Nerv Syst (1981) 3(2-4):311–20. doi: 10.1016/0165-1838(81)90072-2
23. Ciriello J. Afferent renal inputs to paraventricular nucleus vasopressin and oxytocin neurosecretory neurons. Am J Physiol (1998) 275(6):R1745–54. doi: 10.1152/ajpregu.1998.275.6.R1745
24. Wang Y, Liu S, Liu Q, Lv Y. The interaction of central nervous system and acute kidney injury: Pathophysiology and clinical perspectives. Front Physiol (2022) 13:826686. doi: 10.3389/fphys.2022.826686
25. Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res (1999) 117(2):145–52. doi: 10.1016/s0165-3806(99)00113-3
26. Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience (1990) 39(1):151–70. doi: 10.1016/0306-4522(90)90229-w
27. Devanney NA, Stewart AN, Gensel JC. Microglia and macrophage metabolism in cns injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol (2020) 329:113310. doi: 10.1016/j.expneurol.2020.113310
28. Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res (2015) 1619:1–11. doi: 10.1016/j.brainres.2014.12.045
29. Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol (2016) 53(2):1181–94. doi: 10.1007/s12035-014-9070-5
30. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol (2000) 164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166
31. Wright-Jin EC, Gutmann DH. Microglia as dynamic cellular mediators of brain function. Trends Mol Med (2019) 25(11):967–79. doi: 10.1016/j.molmed.2019.08.013
32. Takahashi K, Aranami T, Endoh M, Miyake S, Yamamura T. The regulatory role of natural killer cells in multiple sclerosis. Brain (2004) 127(Pt 9):1917–27. doi: 10.1093/brain/awh219
33. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science (2005) 308(5726):1314–8. doi: 10.1126/science.1110647
34. Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during cns remyelination. Nat Neurosci (2013) 16(9):1211–8. doi: 10.1038/nn.3469
35. Boudreau JE, Hsu KC. Natural killer cell education and the response to infection and cancer therapy: Stay tuned. Trends Immunol (2018) 39(3):222–39. doi: 10.1016/j.it.2017.12.001
36. Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol (2009) 9(11):811–6. doi: 10.1038/nri2654
37. Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the cns: Anatomical sites and molecular mechanisms. Trends Immunol (2005) 26(9):485–95. doi: 10.1016/j.it.2005.07.004
38. Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming Cd4+ T cells to protein antigens in vivo. J Immunol (1995) 155(8):3734–41.
39. Finkelman FD, Lees A, Morris SC. Antigen presentation by b lymphocytes to Cd4+ T lymphocytes in vivo: Importance for b lymphocyte and T lymphocyte activation. Semin Immunol (1992) 4(4):247–55.
40. Pistoia V. Production of cytokines by human b cells in health and disease. Immunol Today (1997) 18(7):343–50. doi: 10.1016/s0167-5699(97)01080-3
41. Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T Cell deficiency leads to cognitive dysfunction: Implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U.S.A. (2004) 101(21):8180–5. doi: 10.1073/pnas.0402268101
42. Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization–new prospects for brain repair. Nat Rev Neurol (2015) 11(1):56–64. doi: 10.1038/nrneurol.2014.207
43. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep (2014) 6:13. doi: 10.12703/p6-13
44. Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: Time for a paradigm shift. Neuron (2017) 95(6):1246–65. doi: 10.1016/j.neuron.2017.07.010
45. Morganti JM, Riparip LK, Rosi S. Call off the Dog(Ma): M1/M2 polarization is concurrent following traumatic brain injury. PloS One (2016) 11(1):e0148001. doi: 10.1371/journal.pone.0148001
46. Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol (2018) 18(4):225–42. doi: 10.1038/nri.2017.125
47. Bohlen CJ, Friedman BA, Dejanovic B, Sheng M. Microglia in brain development, homeostasis, and neurodegeneration. Annu Rev Genet (2019) 53:263–88. doi: 10.1146/annurev-genet-112618-043515
48. Frost JL, Schafer DP. Microglia: Architects of the developing nervous system. Trends Cell Biol (2016) 26(8):587–97. doi: 10.1016/j.tcb.2016.02.006
49. Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol (2014) 14(7):463–77. doi: 10.1038/nri3705
50. Hickey WF. Leukocyte traffic in the central nervous system: The participants and their roles. Semin Immunol (1999) 11(2):125–37. doi: 10.1006/smim.1999.0168
51. Pachter JS, de Vries HE, Fabry Z. The blood-brain barrier and its role in immune privilege in the central nervous system. J Neuropathol Exp Neurol (2003) 62(6):593–604. doi: 10.1093/jnen/62.6.593
52. Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, et al. Ifn-Γ-Dependent activation of the brain’s choroid plexus for cns immune surveillance and repair. Brain (2013) 136(Pt 11):3427–40. doi: 10.1093/brain/awt259
53. Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest (2017) 127(9):3210–9. doi: 10.1172/jci90603
54. Raper D, Louveau A, Kipnis J. How do meningeal lymphatic vessels drain the cns? Trends Neurosci (2016) 39(9):581–6. doi: 10.1016/j.tins.2016.07.001
55. Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, et al. Reciprocal regulation of polarized cytokine production by effector b and T cells. Nat Immunol (2000) 1(6):475–82. doi: 10.1038/82717
56. Ware CF, VanArsdale TL, Crowe PD, Browning JL. The ligands and receptors of the lymphotoxin system. Curr Top Microbiol Immunol (1995) 198:175–218. doi: 10.1007/978-3-642-79414-8_11
57. Cui D, Tang Y, Jiang Q, Jiang D, Zhang Y, Lv Y, et al. Follicular helper T cells in the immunopathogenesis of sars-Cov-2 infection. Front Immunol (2021) 12:731100. doi: 10.3389/fimmu.2021.731100
58. Wang G, Xiang Z, Wang W, Chen Z. Seasonal coronaviruses and sars-Cov-2: Effects of preexisting immunity during the covid-19 pandemic. J Zhejiang University-Sci B (2022) 23(6):451–60. doi: 10.1631/jzus.B2200049
59. Brioschi S, Wang WL, Peng V, Wang M, Shchukina I, Greenberg ZJ, et al. Heterogeneity of meningeal b cells reveals a lymphopoietic niche at the cns borders. Science (2021) 373(6553). doi: 10.1126/science.abf9277
60. Schafflick D, Wolbert J, Heming M, Thomas C, Hartlehnert M, Börsch AL, et al. Single-cell profiling of cns border compartment leukocytes reveals that b cells and their progenitors reside in non-diseased meninges. Nat Neurosci (2021) 24(9):1225–34. doi: 10.1038/s41593-021-00880-y
61. Ankeny DP, Popovich PG. B cells and autoantibodies: Complex roles in cns injury. Trends Immunol (2010) 31(9):332–8. doi: 10.1016/j.it.2010.06.006
62. Weber MS, Prod’homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, et al. B-cell activation influences T-cell polarization and outcome of anti-Cd20 b-cell depletion in central nervous system autoimmunity. Ann Neurol (2010) 68(3):369–83. doi: 10.1002/ana.22081
63. Cervenak L, Magyar A, Boja R, László G. Differential expression of Gl7 activation antigen on bone marrow b cell subpopulations and peripheral b cells. Immunol Lett (2001) 78(2):89–96. doi: 10.1016/s0165-2478(01)00239-5
64. Lehmann-Horn K, Schleich E, Hertzenberg D, Hapfelmeier A, Kümpfel T, von Bubnoff N, et al. Anti-Cd20 b-cell depletion enhances monocyte reactivity in neuroimmunological disorders. J Neuroinflamm (2011) 8:146. doi: 10.1186/1742-2094-8-146
65. Martinez-Pasamar S, Abad E, Moreno B, Velez de Mendizabal N, Martinez-Forero I, Garcia-Ojalvo J, et al. Dynamic cross-regulation of antigen-specific effector and regulatory T cell subpopulations and microglia in brain autoimmunity. BMC Syst Biol (2013) 7:34. doi: 10.1186/1752-0509-7-34
66. Cantrell D. Signaling in lymphocyte activation. Cold Spring Harb Perspect Biol (2015) 7(6). doi: 10.1101/cshperspect.a018788
67. Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: Insights into metabolism and lymphocyte function. Science (2013) 342(6155):1242454. doi: 10.1126/science.1242454
68. Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun (2008) 22(6):861–9. doi: 10.1016/j.bbi.2007.12.008
69. Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, et al. Regulation of learning and memory by meningeal immunity: A key role for il-4. J Exp Med (2010) 207(5):1067–80. doi: 10.1084/jem.20091419
70. Hazenberg MD, Spits H. Human innate lymphoid cells. Blood (2014) 124(5):700–9. doi: 10.1182/blood-2013-11-427781
71. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol (2008) 9(5):503–10. doi: 10.1038/ni1582
72. Han S, Lin YC, Wu T, Salgado AD, Mexhitaj I, Wuest SC, et al. Comprehensive immunophenotyping of cerebrospinal fluid cells in patients with neuroimmunological diseases. J Immunol (2014) 192(6):2551–63. doi: 10.4049/jimmunol.1302884
73. Liu Q, Sanai N, Jin WN, La Cava A, Van Kaer L, Shi FD. Neural stem cells sustain natural killer cells that dictate recovery from brain inflammation. Nat Neurosci (2016) 19(2):243–52. doi: 10.1038/nn.4211
74. Zhang L, Zhang Y, Fan D. Pathological role of natural killer cells in parkinson’s disease: A systematic review. Front Aging Neurosci (2022) 14:890816. doi: 10.3389/fnagi.2022.890816
75. Earls RH, Lee JK. The role of natural killer cells in parkinson’s disease. Exp Mol Med (2020) 52(9):1517–25. doi: 10.1038/s12276-020-00505-7
76. Antel J, Owens T. Multiple sclerosis and immune regulatory cells. Brain (2004) 127(Pt 9):1915–6. doi: 10.1093/brain/awh272
77. Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, et al. Role of natural killer cells and tcr gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol (1998) 28(5):1681–8. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T
78. Campagnolo DI, Dixon D, Schwartz J, Bartlett JA, Keller SE. Altered innate immunity following spinal cord injury. Spinal Cord (2008) 46(7):477–81. doi: 10.1038/sc.2008.4
79. Uddin I, Joshi K, Oakes T, Heather JM, Swanton C, Chain B. An economical, quantitative, and robust protocol for high-throughput T cell receptor sequencing from tumor or blood. Methods Mol Biol (2019) 1884:15–42. doi: 10.1007/978-1-4939-8885-3_2
80. Han RT, Kim RD, Molofsky AV, Liddelow SA. Astrocyte-immune cell interactions in physiology and pathology. Immunity (2021) 54(2):211–24. doi: 10.1016/j.immuni.2021.01.013
81. Schwartz M, Raposo C. Protective autoimmunity: A unifying model for the immune network involved in cns repair. Neuroscientist (2014) 20(4):343–58. doi: 10.1177/1073858413516799
82. Buck MD, O’Sullivan D, Pearce EL. T Cell metabolism drives immunity. J Exp Med (2015) 212(9):1345–60. doi: 10.1084/jem.20151159
83. Wu J, Guo Y, Lu X, Huang F, Lv F, Wei D, et al. Th1/Th2 cells and associated cytokines in acute hepatitis e and related acute liver failure. J Immunol Res (2020) 2020. doi: 10.1155/2020/6027361
84. Li P, Zhang Y, Xu Y, Cao H, Li L. Characteristics of Cd8+ and Cd4+ tissue-resident memory lymphocytes in the gastrointestinal tract. Adv Gut Microbiome Res (2022) 2022. doi: 10.1155/2022/9157455
85. Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, et al. Cns-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U.S.A. (2013) 110(6):2264–9. doi: 10.1073/pnas.1211270110
86. Zorzella-Pezavento SF, Chiuso-Minicucci F, França TG, Ishikawa LL, da Rosa LC, Colavite PM, et al. Downmodulation of peripheral mog-specific immunity by Pvaxhsp65 treatment during eae does not reach the cns. J Neuroimmunol (2014) 268(1-2):35–42. doi: 10.1016/j.jneuroim.2013.12.015
87. Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci Ther (2021) 27(1):36–47. doi: 10.1111/cns.13569
88. Ramaglia V, Florescu A, Zuo M, Sheikh-Mohamed S, Gommerman JL. Stromal cell-mediated coordination of immune cell recruitment, retention, and function in brain-adjacent regions. J Immunol (2021) 206(2):282–91. doi: 10.4049/jimmunol.2000833
89. Avraham O, Feng R, Ewan EE, Rustenhoven J, Zhao G, Cavalli V. Profiling sensory neuron microenvironment after peripheral and central axon injury reveals key pathways for neural repair. Elife (2021) 10. doi: 10.7554/eLife.68457
90. Ludwig PE, Reddy V, Varacallo M. Neuroanatomy, central nervous system (Cns). In: Statpearls. Treasure Island (FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC (2022).
91. Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol (2021) 22(9):1083–92. doi: 10.1038/s41590-021-00994-2
92. Chen Z, Liu P, Xia X, Wang L, Li X. Living on the border of the cns: Dural immune cells in health and disease. Cell Immunol (2022) 377:104545. doi: 10.1016/j.cellimm.2022.104545
93. Friebel E, Kapolou K, Unger S, Núñez NG, Utz S, Rushing EJ, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell (2020) 181(7):1626–42.e20. doi: 10.1016/j.cell.2020.04.055
94. Coté TR, Manns A, Hardy CR, Yellin FJ, Hartge P. Epidemiology of brain lymphoma among people with or without acquired immunodeficiency syndrome. Aids/Cancer Study Group J Natl Cancer Inst (1996) 88(10):675–9. doi: 10.1093/jnci/88.10.675
95. Gandhi MK, Hoang T, Law SC, Brosda S, O’Rourke K, Tobin JWD, et al. Ebv-associated primary cns lymphoma occurring after immunosuppression is a distinct immunobiological entity. Blood (2021) 137(11):1468–77. doi: 10.1182/blood.2020008520
96. Zhai H, Heppner FL, Tsirka SE. Microglia/Macrophages promote glioma progression. Glia (2011) 59(3):472–85. doi: 10.1002/glia.21117
97. Lukas RV, Wainwright DA, Ladomersky E, Sachdev S, Sonabend AM, Stupp R. Newly diagnosed glioblastoma: A review on clinical management. Oncol (Williston Park) (2019) 33(3):91–100.
98. Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol (2019) 15(7):405–17. doi: 10.1038/s41582-019-0220-2
99. Vargas López AJ. Glioblastoma in adults: A society for neuro-oncology (Sno) and European society of neuro-oncology (Eano) consensus review on current management and future directions. Neuro Oncol (2021) 23(3):502–3. doi: 10.1093/neuonc/noaa287
100. Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, et al. Cimpact-now update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, idh-wildtype, with molecular features of glioblastoma, who grade iv”. Acta Neuropathol (2018) 136(5):805–10. doi: 10.1007/s00401-018-1913-0
101. Badie B, Schartner J, Klaver J, Vorpahl J. In vitro modulation of microglia motility by glioma cells is mediated by hepatocyte growth Factor/Scatter factor. Neurosurgery (1999) 44(5):1077–82. doi: 10.1097/00006123-199905000-00075
102. Bettinger I, Thanos S, Paulus W. Microglia promote glioma migration. Acta Neuropathol (2002) 103(4):351–5. doi: 10.1007/s00401-001-0472-x
103. Carvalho da Fonseca AC, Wang H, Fan H, Chen X, Zhang I, Zhang L, et al. Increased expression of stress inducible protein 1 in glioma-associated Microglia/Macrophages. J Neuroimmunol (2014) 274(1-2):71–7. doi: 10.1016/j.jneuroim.2014.06.021
104. Wei J, Gabrusiewicz K, Heimberger A. The controversial role of microglia in malignant gliomas. Clin Dev Immunol (2013) 2013:285246. doi: 10.1155/2013/285246
105. Dorward IG, Luo J, Perry A, Gutmann DH, Mansur DB, Rubin JB, et al. Postoperative imaging surveillance in pediatric pilocytic astrocytomas. J Neurosurg Pediatr (2010) 6(4):346–52. doi: 10.3171/2010.7.Peds10129
106. Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol (2019) 16(8):509–20. doi: 10.1038/s41571-019-0177-5
107. Lu-Emerson C, Snuderl M, Kirkpatrick ND, Goveia J, Davidson C, Huang Y, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol (2013) 15(8):1079–87. doi: 10.1093/neuonc/not082
108. Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012
109. Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ (2008) 15(1):3–12. doi: 10.1038/sj.cdd.4402269
110. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without braf mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
111. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med (2013) 369(2):122–33. doi: 10.1056/NEJMoa1302369
112. Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr (2019) 19(1):181. doi: 10.1186/s12877-019-1175-2
113. Armstrong R. What causes neurodegenerative disease? Folia Neuropathol (2020) 58(2):93–112. doi: 10.5114/fn.2020.96707
114. Ownby RL. Quetiapine and rivastigmine for agitation in alzheimer’s disease. Curr Psychiatry Rep (2006) 8(1):10. doi: 10.1007/s11920-006-0075-2
115. Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The incidence of parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology (2016) 46(4):292–300. doi: 10.1159/000445751
116. Vinters HV. Emerging concepts in alzheimer’s disease. Annu Rev Pathol (2015) 10:291–319. doi: 10.1146/annurev-pathol-020712-163927
117. Sawada M, Sawada H, Nagatsu T. Effects of aging on neuroprotective and neurotoxic properties of microglia in neurodegenerative diseases. Neurodegener Dis (2008) 5(3-4):254–6. doi: 10.1159/000113717
118. Wu J, Lu WY, Cui LL. Clinical significance of Stat3 and mapk phosphorylation, and the protein expression of cyclin D1 in skin squamous cell carcinoma tissues. Mol Med Rep (2015) 12(6):8129–34. doi: 10.3892/mmr.2015.4460
119. Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in parkinson’s disease. J Neural Transm Suppl (2006) 70):373–81. doi: 10.1007/978-3-211-45295-0_57
120. Popovich P, McTigue D. Damage control in the nervous system: Beware the immune system in spinal cord injury. Nat Med (2009) 15(7):736–7. doi: 10.1038/nm0709-736
121. Vargas ME, Barres BA. Why is wallerian degeneration in the cns so slow? Annu Rev Neurosci (2007) 30:153–79. doi: 10.1146/annurev.neuro.30.051606.094354
122. Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci (2006) 26(39):9841–50. doi: 10.1523/jneurosci.1993-06.2006
123. Lindborg JA, Mack M, Zigmond RE. Neutrophils are critical for myelin removal in a peripheral nerve injury model of wallerian degeneration. J Neurosci (2017) 37(43):10258–77. doi: 10.1523/jneurosci.2085-17.2017
124. Alexander JK, Popovich PG. Neuroinflammation in spinal cord injury: Therapeutic targets for neuroprotection and regeneration. Prog Brain Res (2009) 175:125–37. doi: 10.1016/s0079-6123(09)17508-8
125. Hawthorne AL, Popovich PG. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics (2011) 8(2):252–61. doi: 10.1007/s13311-011-0032-6
126. Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun L, et al. Macrophages in spinal cord injury: Phenotypic and functional change from exposure to myelin debris. Glia (2015) 63(4):635–51. doi: 10.1002/glia.22774
127. Milich LM, Ryan CB, Lee JK. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol (2019) 137(5):785–97. doi: 10.1007/s00401-019-01992-3
128. Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, et al. Deficient Cx3cr1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6clo/Inos+ macrophages. J Neurosci (2011) 31(27):9910–22. doi: 10.1523/jneurosci.2114-11.2011
129. García-Alías G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase abc treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci (2009) 12(9):1145–51. doi: 10.1038/nn.2377
130. Campagnolo DI, Bartlett JA, Keller SE, Sanchez W, Oza R. Impaired phagocytosis of staphylococcus aureus in complete tetraplegics. Am J Phys Med Rehabil (1997) 76(4):276–80. doi: 10.1097/00002060-199707000-00005
131. Campagnolo DI, Keller SE, DeLisa JA, Glick TJ, Sipski ML, Schleifer SJ. Alteration of immune system function in tetraplegics. a pilot study. Am J Phys Med Rehabil (1994) 73(6):387–93. doi: 10.1097/00002060-199411000-00003
132. Griffin GD. The injured brain: Tbi, mtbi, the immune system, and infection: Connecting the dots. Mil Med (2011) 176(4):364–8. doi: 10.7205/milmed-d-10-00021
133. Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature (2014) 505(7482):223–8. doi: 10.1038/nature12808
134. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol (2008) 7(8):728–41. doi: 10.1016/s1474-4422(08)70164-9
135. Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Front Biosci (Landmark Ed) (2009) 14(10):3795–813. doi: 10.2741/3489
136. Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol (2014) 193(12):5765–71. doi: 10.4049/jimmunol.1401417
137. Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. Atp mediates rapid microglial response to local brain injury in vivo. Nat Neurosci (2005) 8(6):752–8. doi: 10.1038/nn1472
138. Gold M, El Khoury J. β-amyloid, microglia, and the inflammasome in alzheimer’s disease. Semin Immunopathol (2015) 37(6):607–11. doi: 10.1007/s00281-015-0518-0
139. Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, et al. Expression of the Nlrp3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res (2013) 38(10):2072–83. doi: 10.1007/s11064-013-1115-z
140. VanItallie TB. Traumatic brain injury (Tbi) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (Cte). Metabolism (2019) 100s:153943. doi: 10.1016/j.metabol.2019.07.007
141. Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, et al. Final results of mrc crash, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet (2005) 365(9475):1957–9. doi: 10.1016/s0140-6736(05)66552-x
142. Loane DJ, Kumar A. Microglia in the tbi brain: The good, the bad, and the dysregulated. Exp Neurol (2016) 275 Pt 3(03):316–27. doi: 10.1016/j.expneurol.2015.08.018
143. Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Grimmig B, Diamond DM, et al. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PloS One (2013) 8(1):e53376. doi: 10.1371/journal.pone.0053376
144. Elkabes S, DiCicco-Bloom EM, Black IB. Brain Microglia/Macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci (1996) 16(8):2508–21. doi: 10.1523/jneurosci.16-08-02508.1996
145. David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci (2011) 12(7):388–99. doi: 10.1038/nrn3053
146. Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol (2010) 6(7):393–403. doi: 10.1038/nrneurol.2010.74
147. Liu YW, Li S, Dai SS. Neutrophils in traumatic brain injury (Tbi): Friend or foe? J Neuroinflamm (2018) 15(1):146. doi: 10.1186/s12974-018-1173-x
148. Caplan HW, Cox CS, Bedi SS. Do microglia play a role in sex differences in tbi? J Neurosci Res (2017) 95(1-2):509–17. doi: 10.1002/jnr.23854
149. Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell (2011) 10(2):263–76. doi: 10.1111/j.1474-9726.2010.00660.x
150. Doering P, Stoltenberg M, Penkowa M, Rungby J, Larsen A, Danscher G. Chemical blocking of zinc ions in cns increases neuronal damage following traumatic brain injury (Tbi) in mice. PloS One (2010) 5(4):e10131. doi: 10.1371/journal.pone.0010131
151. Aertker BM, Bedi S, Cox CS Jr. Strategies for cns repair following tbi. Exp Neurol (2016) 275 Pt 3:411–26. doi: 10.1016/j.expneurol.2015.01.008
152. Ma SF, Chen YJ, Zhang JX, Shen L, Wang R, Zhou JS, et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun (2015) 45:157–70. doi: 10.1016/j.bbi.2014.11.007
153. Nuki G, Bresnihan B, Bear MB, McCabe D. Long-term safety and maintenance of clinical improvement following treatment with anakinra (Recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: Extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum (2002) 46(11):2838–46. doi: 10.1002/art.10578
154. Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx (2004) 1(4):472–81. doi: 10.1602/neurorx.1.4.472
155. Michel-Monigadon D, Brachet P, Neveu I, Naveilhan P. Immunoregulatory properties of neural stem cells. Immunotherapy (2011) 3(4 Suppl):39–41. doi: 10.2217/imt.11.49
156. Goyal M, Avery JA. Paroxysmal disorders and the autonomic nervous system in pediatrics. Am J Electroneurodiagn Technol (2005) 45(4):240–7. doi: 10.1080/1086508X.2005.11079541
157. Popugaev KA, Lubnin AY, Zabelin MV, Samoylov AS. Autonomic nervous system and its imbalance in neuro intensive care unit. Anesteziol Reanimatol (2016) 61(2):137–42.
Keywords: central nervous system (CNS) injury, immunocytes, immune system, neuro-immune interaction, role
Citation: Xu J, Ma C, Hua M, Li J, Xiang Z and Wu J (2022) CNS and CNS diseases in relation to their immune system. Front. Immunol. 13:1063928. doi: 10.3389/fimmu.2022.1063928
Received: 07 October 2022; Accepted: 31 October 2022;
Published: 16 November 2022.
Edited by:
Bo Li.Department of Orthopedics, Sun Yat-sen University, ChinaReviewed by:
Gang Chen, Tongji University School of Medicine, ChinaCopyright © 2022 Xu, Ma, Hua, Li, Xiang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wu, d3VqaWFuZ2xpbnhpbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.