- 1Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, New York City, NY, United States
- 2Mark Lebwohl Center for Neuroinflammation and Sensation, Icahn School of Medicine at Mount Sinai, New York City, NY, United States
- 3Department of Dermatology, Kyoto University Graduate School of Medicine, Kyoto, Japan
- 4Marc and Jennifer Lipschultz Precision Immunology Institute, Icahn School of Medicine at Mount Sinai, New York City, NY, United States
- 5Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York City, NY, United States
Basophils have been implicated in type 2 inflammation and numerous disorders in the skin such as helminth infection, atopic dermatitis, and urticaria. Although similar in form and function to tissue-resident mast cells, classical studies on basophils have centered on those from the hematopoietic compartment. However, increasing studies in tissues like the skin demonstrate that basophils may take on particular characteristics by responding to unique developmental, chemotactic, and activation cues. Herein, we highlight how recent studies in barrier immunology suggest the presence of skin-homing basophils that harbor a unique identity in terms of phenotype, function, and motility. These concepts may uniquely inform how basophils contribute to diseases at multiple epithelial surfaces and our ability to therapeutically target the innate immune system in disease.
Introduction
Basophils are rare granulocytes, accounting for <1% of leukocytes in the peripheral blood, spleen, and bone marrow. Basophils were first described by Paul Ehrlich in 1879. Subsequently, several groups have discovered that basophils in the blood are a source of histamine in the 1950s (1–3). However, it was not until 1972 that basophils were shown to be activated by allergens in an IgE-mediated fashion (4). Given their similarity in form and function to tissue-resident mast cells, basophils have long been considered “circulating mast cells”, although their differences and similarities are often debated. Thus, they have long been studied as a surrogate for mast cells due to their accessibility via the peripheral blood.
Monitoring of human basophils by flow cytometry has revealed changes in cell surface markers and activation of basophils (5). Moreover, a recent study on human basophils by Blom et al. reported unique chemokine receptor expression patterns upon IgE-mediated or non-IgE-mediated activation, strongly suggesting heterogeneous activation manners in human basophils (6). In contrast to human basophils, the characteristics and functions of murine basophils in vivo have come to light with the advent of antibody-mediated cell depletion methods (e.g., anti-FcϵRIa, CD200R3, or Thy1 antibodies) (7–9). However, such methods were not sufficient to distinguish the unique role of murine basophils from mast cells in vivo due to the bystander effects on mast cells. This problem was overcome with the development of unique transgenic mouse technologies and the identification of basophil-specific genes and markers (e.g., Mcpt8-DTR, Mcpt8-Cre, Bas-TRECK Tg, and Basoph8 mice) (10–13). Indeed, these advances have made it possible to directly compare basophils with mast cells, revealing that these two myeloid cell populations differ in surface marker expression, factors required for terminal differentiation, signaling pathways, release of inflammatory mediators, and impact on disease.
Furthermore, it is generally accepted that basophils are effector cells of the innate immune system that promote type 2 immunity and inflammation through the release of a variety of mediators including the type 2 cytokines IL-4 and IL-13. Although residing in the circulation, basophils are rapidly recruited into the tissues such as the intestine, lung, and skin upon inflammation (14). Thus, they have been implicated in promoting the expulsion of helminth parasites from mucosal barriers and in the pathophysiology of a variety of allergic disorders such as asthma, atopic dermatitis (AD), food allergy, and chronic spontaneous urticaria (CSU) (15–19). Further, recent studies have shed light on novel functions of basophils which may even reside in peripheral organs (20–22). However, how basophils are recruited to the tissues upon stimulation and the manner in which they are activated or survive in tissues remain poorly understood. Moreover, the precise contribution of basophils to various allergic disorders such as AD continues to be debated even though many studies have implicated basophils as putative drivers in AD pathogenesis based on both mouse and human studies (17, 23–27).
Herein, we highlight recent advances in basophil biology in peripheral organs such as the skin and how they provoke new hypotheses and theories about basophil function more broadly. We propose revisiting a number of assumptions about the properties of basophils in tissues using new approaches, technology, and therapeutics.
Developmental, maturation, and activation cues from the tissue
Both basophils and mast cells differentiate from hematopoietic stem cells via common myeloid progenitors and granulocyte monocyte progenitors (GMPs). Although similar in terms of granularity, expression of the high affinity IgE receptor (FcϵRI), and shared effector molecules, basophils largely reside in the circulation while mast cells reside in other tissues. Recent studies demonstrate that mast cells arise from the yolk sac and aorta-gonad-mesonephros, and the degree to which they are replenished by bone marrow precursors is variable depending on the organ (28). Skin-resident mast cells, in particular, are devoid of bone marrow-derived mast cells and are mostly seeded in the early phase of embryonic development (29, 30). These findings help to explain, at least in part, why the majority of allergic disorders involving mast cells develop early in life. Furthermore, these findings provoke the hypothesis that dysregulated mast cell development could be one explanation for the heterogeneity of allergic pathologies and therapeutic responses. Notwithstanding these insights into the diversity of mast cell subpopulations, it is largely unknown whether related developmental sophistication underlies basophil heterogeneity.
IL-3 is an important growth factor for both basophils and mast cells (31). For example, IL-3 deficient mice exhibited impaired expansion of basophils and mast cells in a setting of nematode infection despite no obvious abnormality in their number at steady state (32). IL-3 is also capable of promoting basophil differentiation from bone marrow cells and survival in vitro (33, 34). Moreover, IL-3 augments cytokine production from basophils after IgE crosslinking, a canonical activation mechanism in basophils (35). Collectively, many of these early studies established IL-3 as a key regulatory cytokine for basophils as well as mast cell proliferation and function. However, most of these studies centered on studying basophils within the hematopoietic compartment. The precise properties of basophils within barrier tissues have been traditionally poorly understood.
In addition to IL-3, granulocyte–macrophage colony-stimulating factor (GM-CSF), Toll-like receptors (TLRs), and thymic stromal lymphopoietin (TSLP) are also known to regulate basophil development (36–38). Among them, TSLP has been shown to act directly on bone marrow and extramedullary progenitors to promote basophil hematopoiesis independently of IL-3 in mice (20, 36). Furthermore, murine basophils differentiated by TSLP have unique transcriptional profiles and activation states compared to those developed under IL-3-enriched conditions (20). In contrast to murine basophils, human basophils from healthy donors do not respond to TSLP without IL-3 priming (39). However, disease-associated human basophils from patients with asthma were responsive to TSLP alone (40). These findings suggest that inflammatory conditions could affect the responsiveness of the human basophil. In the skin, TSLP is consistently upregulated during AD-associated skin inflammation and has long been pursued as a therapeutic target in humans (41, 42). However, the efficacy of targeting TSLP as a therapy in AD has been brought into question, as the anti-TSLP monoclonal antibody (mAb) tezepelumab has not been successful in treating AD (43).
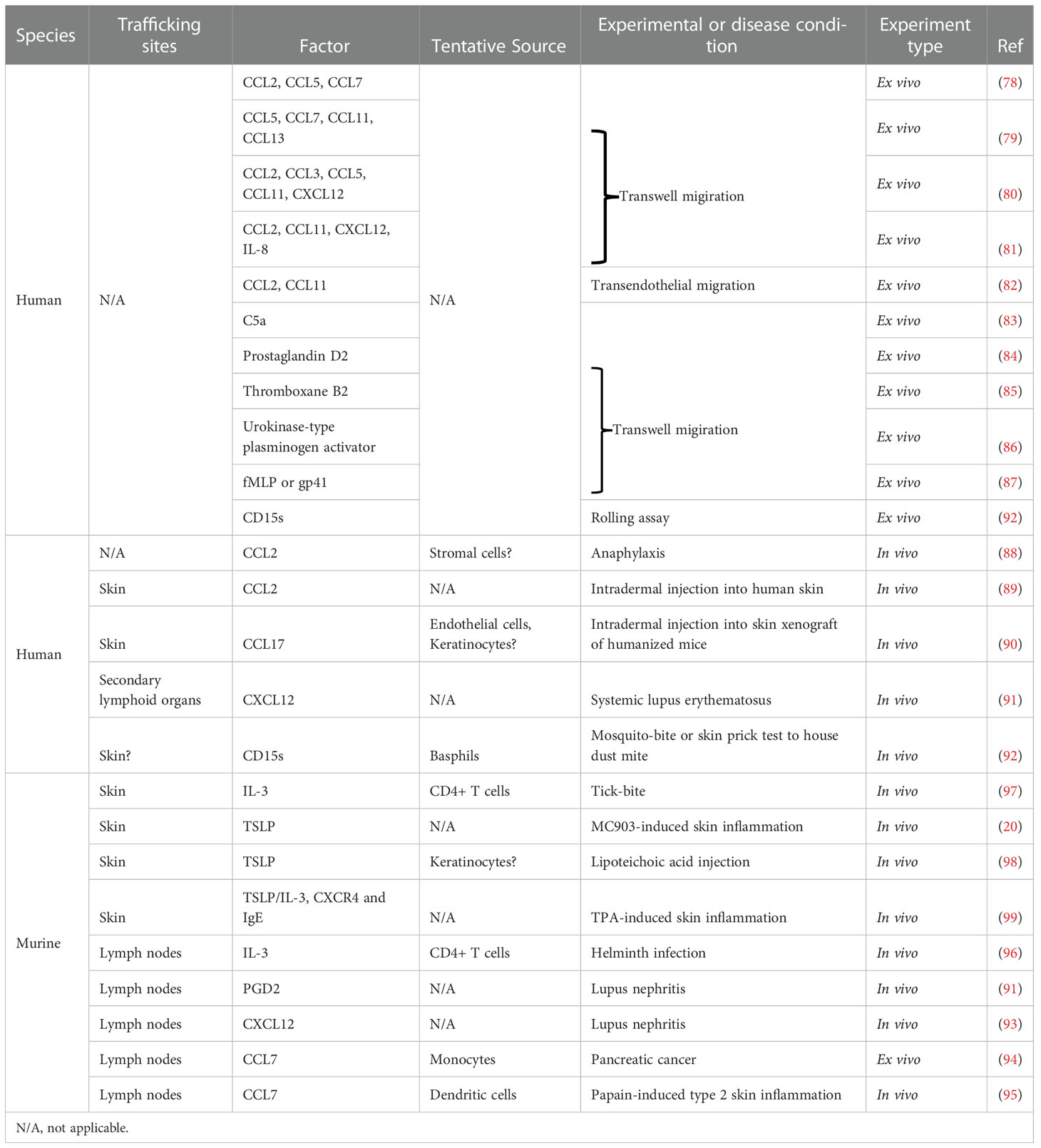
Several studies have implicated TSLP-elicited basophils in murine models of allergic diseases such as AD, food allergy, and eosinophilic esophagitis (15, 16, 20). In addition, TSLP-elicited murine basophils exhibit a highly activated phenotype as evidenced by upregulation of key activating cytokine receptors including those for IL-18 (IL-18R) and IL-33 (ST2, IL-33R) in comparison to IL-3-elicited basophils (20). Both IL-18 and IL-33 are now considered canonical activating cytokines for basophils and strongly implicated in AD-associated inflammation in both mice and humans (44–48). These findings suggest that skin inflammation in AD may skew basophil development via epithelial cell-derived TSLP, creating a reservoir of basophils that can be rapidly activated by skin-associated IL-18 and IL-33. We refer to these basophils as uniquely skin-homing (Figure 1). Similar to IL-33, it is now appreciated that IL-18, in contrast to other organs, acts as an alarmin in the skin to potently promote type 2 immune responses (49). These findings may explain, in part, the failure of tezepelumab in phase 2 clinical trials for AD, as transient blockade of TSLP may not be sufficient to reset the population of basophils that are hyperresponsive to other alarmins in the skin (43). In other words, a typical 12-week clinical trial would likely not be able to capture clinical responses related to such biological effects. Notwithstanding the duration, another possibility is that TSLP blockade alone is no longer sufficient to suppress basophil-mediated skin inflammation after the accumulation of basophils in the skin that exhibit a unique transcriptional signature; such basophils may require simultaneous blockade of IL-18 and/or IL-33 for synergistic therapeutic efficacy. Future studies are warranted to determine the precise array of regulatory cytokines that need to be disrupted to suppress basophils and AD-associated inflammation.
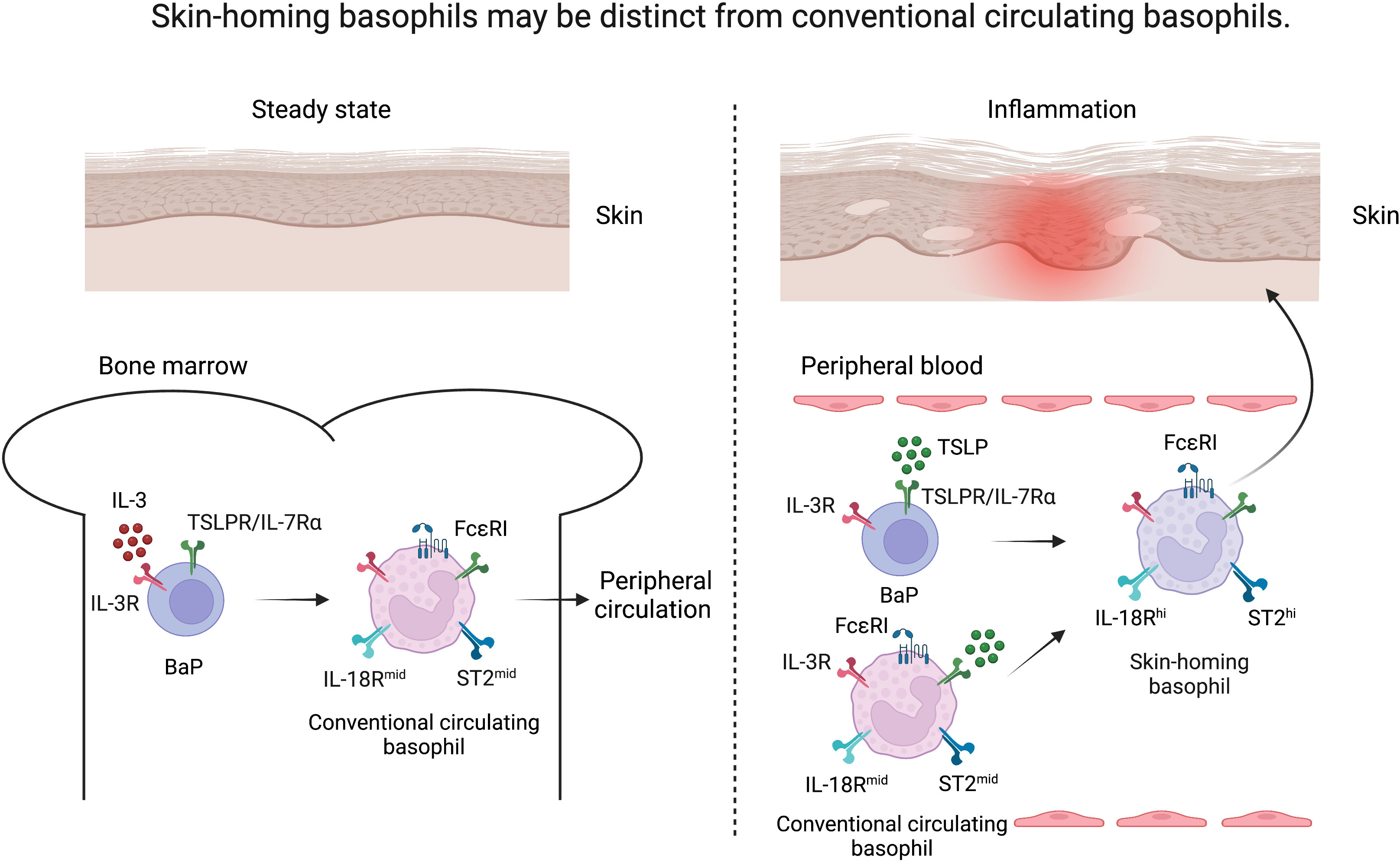
Figure 1 Skin-homing basophils may be distinct from conventional circulating basophils. Under steady state, basophil progenitors (BaP) develop into FcϵRIhi basophils under the influence of IL-3 in the bone marrow (conventional circulating basophils, left). Upon inflammation, TSLP released from the skin drives the maturation of BaPs to exhibit a highly activated phenotype (skin-homing basophils, right) as evidenced by upregulation of IL-18R and ST2 in comparison to IL-3-elicited basophils.
The emergence of skin-homing basophils
Classically considered short-lived, both murine and human basophils have been shown to rapidly lose their viability in a matter of a few days in vivo and in vitro, respectively (9, 50). However, these survival assays were performed on bulk populations of basophils from the bone marrow, blood, or spleen. It is increasingly appreciated that when basophils traffick into the skin (or possibly the lung), they can acquire distinct transcriptional and functional properties (21, 22). We have long observed that while basophils are generally absent in healthy skin, upon the induction of AD-like inflammation, they traffick into the skin as early as day 4 and persist stably through day 12, and likely well beyond (17, 51). Further, it has recently been shown that basophils in AD-like skin are distinct from splenic basophils and persist in the skin beyond the acute inflammatory phase to aid in the resolution of inflammation. Strikingly, these late-phase basophils promote the expansion of M2-like macrophages via cooperative production of IL-4 and monocyte colony-stimulating factor (M-CSF) (21). It has recently been shown that basophils which reside in the lung at early developmental stages imprint a unique developmental program in alveolar macrophages. Indeed, these lung-associated basophils demonstrated a distinct transcriptional profile from those in circulation (22). In addition to transcriptional differences, basophils in the skin could show morphologically distinct characteristics compared to circulating basophils. For example, Cheng et al. found that basophils accumulated in antigen-sensitized skin close to blood vessels, while those in non-sensitized skin were more widely distributed upon antigen challenge (52). Similarly, basophils have been shown to exhibit unique motility and apparent contacts with sensory neurons upon the antigen challenge as well in the setting of AD-like disease (53). Taken together, these studies provoke the hypothesis that basophils, upon entry into the skin, acquire a distinct transcriptional program leading to the distinct morphological changes and unique survival and effector programs not observed from traditional studies in the hematopoietic compartments, which likely focused more on conventional circulating basophils. However, it is important to note that these studies on basophil heterogeneity used Mcpt8 as a basophil-specific marker for transcriptional studies and cell-depletion. Recent studies have suggested that integrinβ7+ mast cells also express Mcpt8 both in the skin and the lung under allergic inflammation (54, 55). Nevertheless, how basophils could acquire distinct identities in peripheral organs remains to be fully clarified and addressed.
While it is increasingly appreciated that there is developmental and functional heterogeneity of basophils, it has only recently come to light how diversity of basophil function can influence different aspects of a single disease (56, 57). For example, it is well-recognized that basophils are associated with human AD and promote the pathogenesis of AD-like disease in mice (17, 21, 23, 24, 27). By contrast, as described above, it has been observed that in the resolution phase of AD-like disease in mice, basophils in the skin also promote restoration of barrier function and disease resolution (21). In a context of itch sensation, basophils appear to be dispensable for chronic itch, while they are known to be essential for allergen-mediated acute itch (25, 27, 53). Indeed, it has been shown that TSLP promotes a program that is also highly enriched for the arachidonic acid pathway which leads to the production of leukotrienes and other bioactive lipids that serve as key effector molecules of murine basophils (20). One such leukotriene, namely LTC4 is now recognized as a very potent pruritogen (53, 58, 59). Taken together, these findings demonstrate the sophisticated array of effector functions orchestrated by basophils.
CSU exemplifies how skin-homing basophils can help to explain disease pathogenesis. CSU is an itchy, immune-mediated skin disorder that afflicts 1% of the population and has a profoundly negative impact on quality of life. It is defined by both hives and itch; these processes are mediated, in part, by activation of IgE and release of histamine from mast cells. Notwithstanding the role of mast cells, it is also appreciated that basophils accumulate in the lesions of urticaria, and that blood basophil deficiency is a feature of CSU (60). Thus, it is hypothesized that basophils recruited to the skin could contribute to the pathogenesis of CSU. The role of basophils in CSU is further suggested by a report that the number of basophils in the blood of CSU patients increases after anti-IgE mAb (omalizumab) treatment (60). Another study has revealed that the surface expression of FcϵRI on basophils was lower in CSU patients who showed better response to omalizumab (61). In addition, it is known that IgE and FcϵRI trigger the migration of murine mast cells toward antigens and that IgE and FcϵRI also mediate human basophil migration in vitro (62–64). Thus, these studies suggest that omalizumab may inhibit IgE-mediated activation in basophils, resulting in decreased motility into the skin. Future studies will be required to clarify this possibility.
However, the activation of basophils in CSU does not seem to be exclusively explained by IgE- and FcϵRI-mediated pathways. Antihistamines are the first-line therapy for CSU; however, even high doses are insufficient in 54% of patients (65). Anti-IgE therapy is the second-line strategy to which 40% of patients with CSU are refractory as well (66). These therapeutic gaps strongly suggest that other histamine- and IgE-independent pathways are operative. In 2015, a seminal paper by McNeil et al. identified that Mas-related G protein-coupled receptor B2 (MrgprB2), and its human ortholog, MRGPRX2 are key receptors that respond to a host of cationic neuropeptides and drugs that induce IgE-independent mast cell activation or allergic-like reactions (67). Indeed, MRGPRX2 has been identified as a possible biomarker in CSU (68). Although the expression and function of MRGPRX2 were mainly studied in mast cells, it has recently been reported that human basophils also express MRGPRX2 (69, 70). Given the potential role of MRGPRX2 on both mast cells and basophils, the heterogeneity of the therapeutic response in CSU may be explained, in part, by the overall composition of IgE-reactive vs. MRGPRX2-reactive mast cells and basophils, respectively, in CSU. This remains a major area of investigation to inform new pathways for treatment.
MRGPRX2 is now emerging as a therapeutic target in the field of allergy. However, MRGPRX2 expression on basophils either at steady state or upon activation remains a major area of controversy (71). It is hypothesized that MRGPRX2 is often internalized in basophils but could be exposed upon activation (71). In support of this, it has been shown that MRGPRX2 expression on human basophils was upregulated by cross-linking of IgE, complement component 5a (C5a), natural N-formyl peptide (fMLP) or IL-3 stimulation in vitro (69, 70). In relation to mast cells, MRGPRX2 function was promoted by TSLP but was dampened by SCF or IL-4 (72–74). Therefore, we speculate that maturation and/or activating factors for basophils including IL-18, IL-33, or TSLP could modulate MRGPRX2 expression on human basophils, contributing to their functional heterogeneity (Figure 2). To this end, future studies are required to determine the precise ligands and their effects on non-canonical basophil activation and function. We hypothesize that, given MRGPRX2’s close association with skin-resident mast cells, its expression on basophils likely marks their identity as also being more skin-associated or homing.
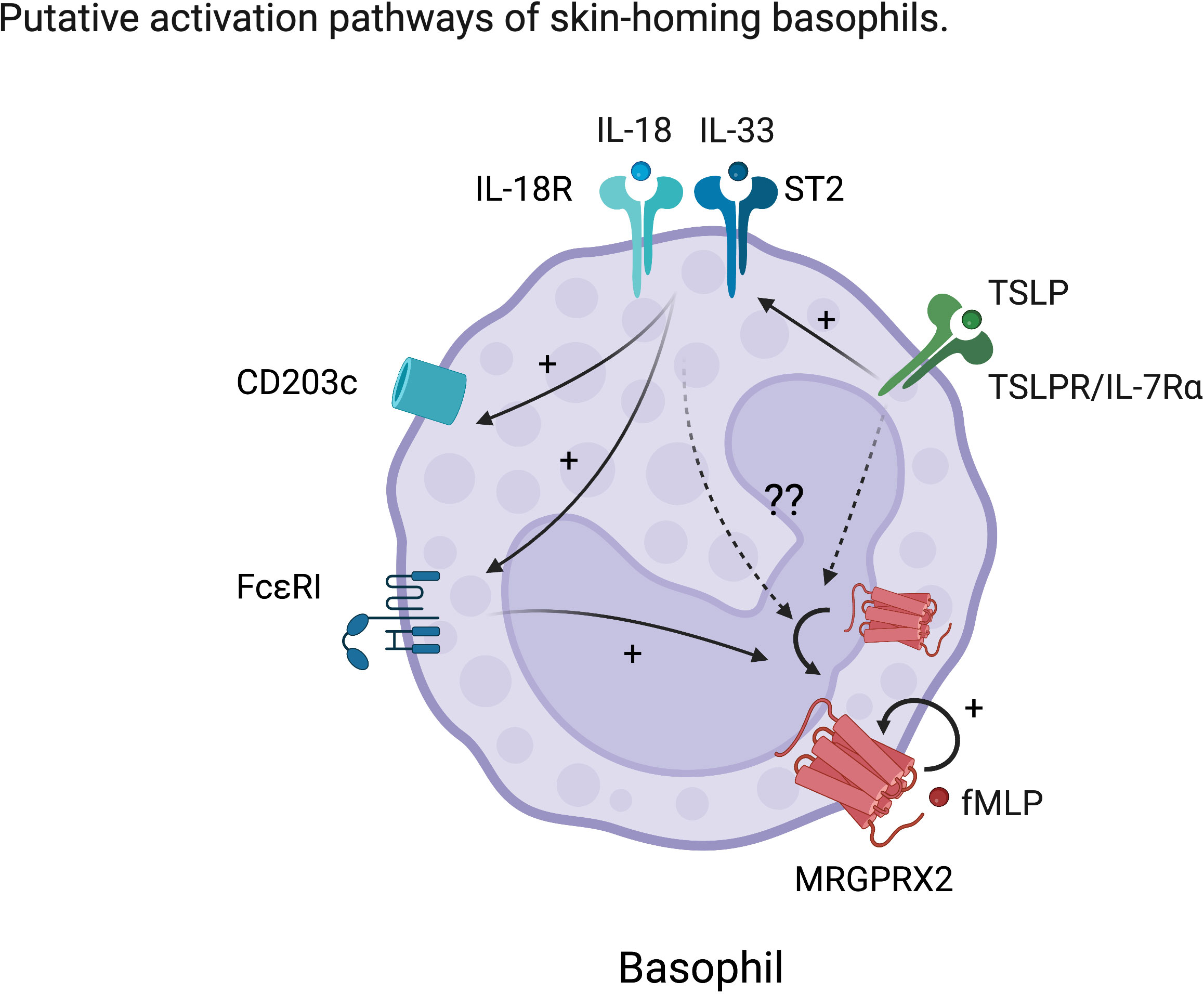
Figure 2 Hypothetic characteristic activation of skin-homing basophils. TSLP enhances the response to IL-18 and IL-33 in basophils. IL-18 and IL-33 further activate basophils resulting in upregulation of conventional activation markers such as CD203c and enhancement of FcϵRI expression. However, the effects of these skin-derived cues on MRGPRX2 expression in basophils are still unknown. We hypothesize that skin-derived cues also upregulate or externalize MRGPRX2, which is considered to be internalized at steady state on human basophils.
Trafficking of basophils into the skin
Although we have discussed how immune dysregulation may promote the emergence of a unique population of basophils that is capable of responding to skin-derived signals, how basophils are recruited into the skin remains a mystery. It is well-known that basophils rapidly accumulate into peripheral tissues including the skin in a variety of settings such as helminth infection, tick bite, or allergic inflammation (11, 19, 75–77). However, there is still a paucity of evidence on the specific chemokines or cellular processes involved in basophil trafficking. Human studies ex vivo have revealed that basophils can migrate toward numerous chemokines (e.g., CCL2/3/5/7/11/13, and CXCL12/13), C5a, Prostaglandin D2 (PGD2), Thromboxane B2, urokinase-type plasminogen activator, and bacterial/viral peptides (fMLP and gp41) (78–87). Notably, serum levels of CCL2 were found to be elevated in a setting of venom- or food-induced anaphylaxis, which correlated a decrease in circulating basophil numbers (88). In addition, basophil accumulation in human skin or xenografted skin was observed after intradermal injection of CCL2 or CCL17, respectively (89, 90). Another study revealed increased migration of basophils in patients with systemic lupus erythematosus toward CXCL12 compared to those from healthy controls (91). A recent study by Blom et al. revealed that human basophils activated by IgE, C5a, or fMLP express various types of chemokine receptors including CCR4, CCR10, CCR6, CCR8, XCR1 and CCX-CKR, some of which are known as skin-homing receptors (6). In this study, they also found a bimodal expression of certain chemokine receptors such as XCR1, cutaneous lymphocyte antigen (CLA), or CXCR4 even among the CD63+ activated subset, further supporting phenotypic heterogeneity of human basophils upon activation. Puan et al. revealed that FUT6 is essential to sialyl-Lewis x (CD15s) expression on human basophils and its deficiency severely reduces their rolling capacity on E-selectins and cutaneous allergic symptoms (92). In mice, both PGD2 and CXCL12 have also been shown to be important in basophil trafficking to secondary lymphoid organs in a murine lupus model, while other studies showed CCL7-dependent migration to the draining lymph nodes in a context of pancreatic tumor or type 2 skin inflammation (91, 93–95). The accumulation of basophils in the lymph nodes after helminth infection depends on IL-3 from CD4+ T cells (96), while IL-3 supplied by skin-resident CD4+ memory T cells is essential for their recruitment to the skin in the setting of tick bite (97). In the setting of AD-associated inflammation, basophil recruitment to the skin is uniquely dependent on TSLP (20); similar dependence on TSLP has also been observed with intradermal injection of lipoteichoic acid (LTA), a cell wall component of bacteria (98). Moreover, under TPA-induced chronic skin inflammation, TSLP and IL-3 externalize CXCR4 expression on basophils and their recruitment to the skin depends on CXCL12-CXCR4 axis and IgE (99). Taken together, these studies demonstrate that a number of factors have been implicated in basophil trafficking in the past (Table 1). However, future studies will have to be aimed at understanding the tissue-specific signals that drive basophil migration into various organs and their unique interactions in the context of disease.
Additionally, it appears that basophils can exhibit heterogeneous behavior even within the same tissue under the same inflammatory condition. We have found that in vivo stimulation with allergen in the skin results in the emergence of two distinct populations of basophils - one that is enlarged and immotile and another that is small and highly motile in the setting of AD-associated acute itch flares (53). Although why such heterogeneity of motility exists within the skin remains unknown, these findings support the hypothesis that there are likely numerous different subsets of basophils across tissues that respond differentially to even the same signals. Thus, basophil trafficking could be regulated in a subset-dependent manner, indicating increasing complexity in terms of their regulation.
As noted above, there is significant evidence that basophils imprint unique transcriptional and functional programs onto macrophages in the skin and lung (21, 22). However, whether skin-resident macrophages recruit basophils into the skin via reciprocal interactions remain to be shown. There is a large body of work that suggests that other circulating granulocytes like neutrophils are heavily influenced by tissue-resident macrophage-derived signals upon tissue damage or pathogen entry (100–102). Indeed, macrophages are capable of producing various types of mediators which have been implicated in basophil chemotaxis in vitro (e.g., CCL2, CXCL1, CXCL2, C5a) (103, 104). Thus, we speculate that homologous mechanisms likely underlie basophil recruitment as well in the context of helminth parasite invasion or allergic barrier disruption. Future studies will be required to determine the full range of cellular and molecular cues that aid in the homing of basophils into the skin.
Finally, whether specific populations of basophils go back into the circulation and travel distally also remains poorly understood. In the setting of helminth infection, it is reported that group 2 innate lymphoid cells (ILC2s) in the tissue are extruded to the circulation to disseminate type 2 inflammation (105). Both skin-homing basophils and ILC2s receive similar activation cues from the skin (e.g., IL-18 or IL-33) to critically mediate type 2 inflammation, despite being rare populations. In light of our speculation that skin-homing basophils acquire the ability to survive much longer, it is possible that basophils could also move from the skin into the circulation and on to other distal sites. However, future studies will be required to fully understand the importance of basophil movement into and out of the skin.
Conclusion
The unique characteristics of basophils have been greatly informed in the last decade due the development of unique tools. Studies using animal models have revealed their critical involvement in a number of disease states in the skin including helminth infection, tick bites, and AD (15–17, 106). However, in addition to their ability to promote allergy, basophils are increasingly appreciated for their dynamic ability to respond to allergen, cytokines, and exhibit both proinflammatory and restorative properties. By understanding how specific subsets of basophils may have unique proinflammatory, survival, and survival properties, we speculate that selectively targeting such basophils may represent a highly effective therapeutic strategy for a variety of skin diseases such as AD, or CSU.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
RS contributed to writing manuscript and crating Figures under supervision of BK. All authors contributed to the article and approved the submitted version.
Conflict of interest
BK has served as a consultant for 23andMe, AbbVie, ABRAX Japan, Almirall, Amagma Therapeutics, Arcutis Biotherapetuics, Arena Pharmaceuticals, argenx BV, AstraZeneca, Bellus Health, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squib, Cara Therapeutics, Clexio Biosciences, Cymabay Therapeutics, Daewoong Pharmaceutical Company, Eli Lilly and Company, Escient Pharmaceuticals, Evommune, Galderma, Genentech, GlaxoSmithKline, Granular Therapeutics, Incyte Corporation, Janssen, Kiniksa, LEO Pharma, Maruho Co., Medicxi, Menlo Therapeutics, Novartis,OM Pharma, Pfizer, Recens Medical, Regeneron, Sanofi, Septerna, Third Harmonic, Trevi Therapeutics, Veradermics. He holds stock in Locus Biosciences, Recens Medical, and ABRAX Japan.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Graham HT, Lowry OH, Wahl N, Priebat MK. Mast cells as sources of tissue histamine. J Exp Med (1955) 102:307–18. doi: 10.1084/jem.102.3.307
2. Valentine WN, Lawrence JS, Pearce ML, Beck WS. The relationship of the basophil to blood histamine in man. Blood (1955) 10:154–9. doi: 10.1182/blood.V10.2.154.154
3. Code CF, Mitchell RG. Histamine, eosinophils and basophils in the blood. J Physiol (1957) 136:449–68. doi: 10.1113/jphysiol.1957.sp005773
4. Ishizaka T, De Bernardo R, Tomioka H, Lichtenstein LM, Ishizaka K. Identification of basophil granulocytes as a site of allergic histamine release. J Immunol (1972) 108:1000–8.
5. Bochner BS, Sterbinsky SA. Altered surface expression of CD11 and leu 8 during human basophil degranulation. J Immunol (1991) 146:2367–73.
6. Blom LH, Bartko EA, Møller TKR, Poulsen LK, Jensen BM. FcϵRI-activated basophils express CCR4, CCR8, CCR9, CCX-CKR and XCR1. Allergy (2022). doi: 10.1111/all.15488
7. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol (2008) 9:310–8. doi: 10.1038/ni1558
8. Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood (2007) 110:913–20. doi: 10.1182/blood-2007-01-068718
9. Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood (2009) 113:2816–25. doi: 10.1182/blood-2008-05-154773
10. Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol (2012) 188:1809–18. doi: 10.4049/jimmunol.1101746
11. Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity (2010) 33:364–74. doi: 10.1016/j.immuni.2010.08.011
12. Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest (2010) 120:2867–75. doi: 10.1172/JCI42680
13. Sullivan BM, Liang H-E, Bando JK, Wu D, Cheng LE, McKerrow JK, et al. Genetic analysis of basophil function in vivo. Nat Immunol (2011) 12:527–35. doi: 10.1038/ni.2036
14. Sasaki H, Kurotaki D, Tamura T. Regulation of basophil and mast cell development by transcription factors. Allergol Int (2016) 65:127–34. doi: 10.1016/j.alit.2016.01.006
15. Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin–basophil axis. J Allergy Clin Immunol (2014) 133:1390–1399.e6. doi: 10.1016/j.jaci.2014.01.021
16. Noti M, Wojno EDT, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin–elicited basophil responses promote eosinophilic esophagitis. Nat Med (2013) 19:1005–13. doi: 10.1038/nm.3281
17. Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol (2014) 193:3717–25. doi: 10.4049/jimmunol.1401307
18. Gauvreau GM, JM L, RM W, AM I, LB S, O’Byrne PM. Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am J Respir Crit Care Med (2000) 161:1473–8. doi: 10.1164/ajrccm.161.5.9908090
19. Giacomin PR, Siracusa MC, Walsh KP, Grencis RK, Kubo M, Comeau MR, et al. Thymic stromal lymphopoietin-dependent basophils promote Th2 cytokine responses following intestinal helminth infection. J Immunol (2012) 189:4371–8. doi: 10.4049/jimmunol.1200691
20. Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature (2011) 477:229–33. doi: 10.1038/nature10329
21. Pellefigues C, Naidoo K, Mehta P, Schmidt AJ, Jagot F, Roussel E, et al. Basophils promote barrier dysfunction and resolution in the atopic skin. J Allergy Clin Immunol (2021) 148:799–812.e10. doi: 10.1016/j.jaci.2021.02.018
22. Cohen M, Giladi A, Gorki A-D, Solodkin DG, Zada M, Hladik A, et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell (2018) 175:1031–1044.e18. doi: 10.1016/j.cell.2018.09.009
23. Yamanishi Y, Mogi K, Takahashi K, Miyake K, Yoshikawa S, Karasuyama H. Skin-infiltrating basophils promote atopic dermatitis-like inflammation via IL-4 production in mice. Allergy (2020) 75:2613–22. doi: 10.1111/all.14362
24. Imai Y, Yasuda K, Nagai M, Kusakabe M, Kubo M, Nakanishi K, et al. IL-33–induced atopic dermatitis–like inflammation in mice is mediated by group 2 innate lymphoid cells in concert with basophils. J Invest Dermatol (2019) 139:2185–2194.e3. doi: 10.1016/j.jid.2019.04.016
25. Mitchell EB, Crow J, Chapman MD, Jouhal SS, Pope FM, Platts-Mills TA. Basophils in allergen-induced patch test sites in atopic dermatitis. Lancet (1982) 1:127–30. doi: 10.1016/S0140-6736(82)90379-8
26. Mashiko S, Mehta H, Bissonnette R, Sarfati M. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J Dermatol Sci (2017) 88:167–74. doi: 10.1016/j.jdermsci.2017.07.003
27. James JM, Kagey-Sobotka A, Sampson HA. Patients with severe atopic dermatitis have activated circulating basophils. J Allergy Clin Immunol (1993) 91:1155–62. doi: 10.1016/0091-6749(93)90318-A
28. St John AL, Rathore APS, Ginhoux F. New perspectives on the origins and heterogeneity of mast cells. Nat Rev Immunol (2022). doi: 10.1038/s41577-022-00731-2
29. Gentek R, Ghigo C, Hoeffel G, Bulle MJ, Msallam R, Gautier G, et al. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity (2018) 48:1160–1171.e5. doi: 10.1016/j.immuni.2018.04.025
30. Li Z, Liu S, Xu J, Zhang X, Han D, Liu J, et al. Adult connective tissue-resident mast cells originate from late erythro-myeloid progenitors. Immunity (2018) 49:640–653.e5. doi: 10.1016/j.immuni.2018.09.023
31. Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol (2002) 2:773–86. doi: 10.1038/nri914
32. Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature (1998) 392:90–3. doi: 10.1038/32190
33. Ohmori K, Luo Y, Jia Y, Nishida J, Wang Z, Bunting KD, et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol (2009) 182:2835–41. doi: 10.4049/jimmunol.0802870
34. Chen Y-H, Bieneman AP, Creticos PS, Chichester KL, Schroeder JT. IFN-alpha inhibits IL-3 priming of human basophil cytokine secretion but not leukotriene C4 and histamine release. J Allergy Clin Immunol (2003) 112:944–50. doi: 10.1016/j.jaci.2003.08.027
35. Gibbs BF, Haas H, Falcone FH, Albrecht C, Vollrath IB, Noll T, et al. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol (1996) 26:2493–8. doi: 10.1002/eji.1830261033
36. Siracusa MC, Saenz SA, Wojno EDT, Kim BS, Osborne LC, Ziegler CG, et al. Thymic stromal lymphopoietin-mediated extramedullary hematopoiesis promotes allergic inflammation. Immunity (2013) 39:1158–70. doi: 10.1016/j.immuni.2013.09.016
37. Reece P, Baatjes AJ, Cyr MM, Sehmi R, Denburg JA. Toll-like receptor-mediated eosinophil-basophil differentiation: autocrine signalling by granulocyte-macrophage colony-stimulating factor in cord blood haematopoietic progenitors. Immunology (2013) 139:256–64. doi: 10.1111/imm.12078
38. Denburg JA, Woolley M, Leber B, Linden M, O'Byrne P Basophil and eosinophil differentiation in allergic reactions. J Allergy Clin Immunol (1994) 94:1135–41. doi: 10.1016/0091-6749(94)90321-2
39. Salter BM, Oliveria JP, Nusca G, Smith SG, Watson RM, Comeau M, et al. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. J Allergy Clin Immunol (2015) 136:1636–44. doi: 10.1016/j.jaci.2015.03.039
40. Salabert-Le Guen N, Hémont C, Delbove A, Poli C, Braudeau C, Fantou A, et al. Thymic stromal lymphopoietin does not activate human basophils. J Allergy Clin Immunol (2018) 141:1476–1479.e6. doi: 10.1016/j.jaci.2017.11.012
41. Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat Immunol (2002) 3:673–80. doi: 10.1038/ni805
42. Nygaard U, Hvid M, Johansen C, Buchner M, Fölster-Holst R, Deleuran M, et al. IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol (2016) 30:1930–8. doi: 10.1111/jdv.13679
43. Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti–thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J Am Acad Dermatol (2019) 80:1013–21. doi: 10.1016/j.jaad.2018.11.059
44. Tanaka T, Tsutsui H, Yoshimoto T, Kotani M, Matsumoto M, Fujita A, et al. Interleukin-18 is elevated in the sera from patients with atopic dermatitis and from atopic dermatitis model mice, NC/Nga. Int Arch Allergy Immunol (2001) 125:236–40. doi: 10.1159/000053821
45. Hon KLE, Leung TF, Ma KC, Wong CK, Wan H, Lam CWK. Serum concentration of IL-18 correlates with disease extent in young children with atopic dermatitis. Pediatr Dermatol (2004) 21:619–22. doi: 10.1111/j.0736-8046.2004.21600.x
46. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med (2013) 210:2939–50. doi: 10.1084/jem.20130351
47. Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA (2013) 110:13921–6. doi: 10.1073/pnas.1307321110
48. Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimäki S, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol (2012) 132:1392–400. doi: 10.1038/jid.2011.446
49. Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang H-E, et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol (2018) 19:1093–9. doi: 10.1038/s41590-018-0201-4
50. Yamaguchi M, Hirai K, Morita Y, Takaishi T, Ohta K, Suzuki S, et al. Hemopoietic growth factors regulate the survival of human basophils in vitro. Int Arch Allergy Immunol (1992) 97:322–9. doi: 10.1159/000236140
51. Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons Co-opt classical immune signaling pathways to mediate chronic itch. Cell (2017) 171:217–228.e13. doi: 10.1016/j.cell.2017.08.006
52. Cheng LE, Sullivan BM, Retana LE, Allen CDC, Liang H-E, Locksley RM. IgE-activated basophils regulate eosinophil tissue entry by modulating endothelial function. J Exp Med (2015) 212:513–24. doi: 10.1084/jem.20141671
53. Wang F, Trier AM, Li F, Kim S, Chen Z, Chai JN, et al. A basophil-neuronal axis promotes itch. Cell (2021) 184:422–440.e17. doi: 10.1016/j.cell.2020.12.033
54. Keith YH, Honda T, Ono S, Lee B, Shibuya R, Hanakawa S, et al. Infiltration and local differentiation of bone marrow-derived integrinβ7-positive mast cell progenitors in atopic dermatitis-like skin. J Allergy Clin Immunol (2022). doi: 10.1016/j.jaci.2022.09.011
55. Derakhshan T, Samuchiwal SK, Hallen N, Bankova LG, Boyce JA, Barrett NA, et al. Lineage-specific regulation of inducible and constitutive mast cells in allergic airway inflammation. J Exp Med (2021) 218. doi: 10.1084/jem.20200321
56. Siracusa MC, Wojno EDT, Artis D. Functional heterogeneity in the basophil cell lineage. Adv Immunol (2012) 115:141–59. doi: 10.1016/B978-0-12-394299-9.00005-9
57. Oetjen LK, Noti M, Kim BS. New insights into basophil heterogeneity. Semin Immunopathol (2016) 38:549–61. doi: 10.1007/s00281-016-0567-z
58. Solinski HJ, Kriegbaum MC, Tseng P-Y, Earnest TW, Gu X, Barik A, et al. Nppb neurons are sensors of mast cell-induced itch. Cell Rep (2019) 26:3561–3.e4. doi: 10.1016/j.celrep.2019.02.089
59. Voisin T, Perner C, Messou M-A, Shiers S, Ualiyeva S, Kanaoka Y, et al. The CysLT2R receptor mediates leukotriene C4-driven acute and chronic itch. Proc Natl Acad Sci (2021) 118:e2022087118. doi: 10.1073/pnas.2022087118
60. Saini SS, Omachi TA, Trzaskoma B, Hulter HN, Rosén K, Sterba PM, et al. Effect of omalizumab on blood basophil counts in patients with chronic Idiopathic/Spontaneous urticaria. J Invest Dermatol (2017) 137:958–61. doi: 10.1016/j.jid.2016.11.025
61. Deza G, Bertolín-Colilla M, Pujol RM, Curto-Barredo L, Soto D, García M, et al. Basophil FcϵRI expression in chronic spontaneous urticaria: A potential immunological predictor of response to omalizumab therapy. Acta Derm Venereol (2017) 97:698–704. doi: 10.2340/00015555-2654
62. Orida N, Feldman JD, Katz DH, Liu FT. IgE-mediated chemotaxis of rat basophilic leukemia cells towards specific antigen. J Exp Med (1983) 157:2166–71. doi: 10.1084/jem.157.6.2166
63. Ishizuka T, Okajima F, Ishiwara M, Iizuka K, Ichimonji I, Kawata T, et al. Sensitized mast cells migrate toward the antigen: a response regulated by p38 mitogen-activated protein kinase and rho-associated coiled-coil-forming protein kinase. J Immunol (2001) 167:2298–304. doi: 10.4049/jimmunol.167.4.2298
64. Suzukawa M, Hirai K, Iikura M, Nagase H, Komiya A, Yoshimura-Uchiyama C, et al. IgE- and FcepsilonRI-mediated migration of human basophils. Int Immunol (2005) 17:1249–55. doi: 10.1093/intimm/dxh301
65. van den Elzen MT, van Os-Medendorp H, van den Brink I, van den Hurk K, Kouznetsova OI, Lokin ASHJ, et al. Effectiveness and safety of antihistamines up to fourfold or higher in treatment of chronic spontaneous urticaria. Clin Transl Allergy (2017) 7:4. doi: 10.1186/s13601-017-0141-3
66. Kaplan A, Ferrer M, Bernstein JA, Antonova E, Trzaskoma B, Raimundo K, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol (2016) 137:474–81. doi: 10.1016/j.jaci.2015.08.023
67. McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature (2015) 519:237–41. doi: 10.1038/nature14022
68. Cao TBT, Cha HY, Yang EM, Ye YM. Elevated MRGPRX2 levels related to disease severity in patients with chronic spontaneous urticaria. Allergy Asthma Immunol Res (2021) 13:498–506. doi: 10.4168/aair.2021.13.3.498
69. Wedi B, Gehring M, Kapp A. The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy (2020) 75:2229–42. doi: 10.1111/all.14213
70. Sabato V, Van Gasse A, Cop N, Claesen K, Decuyper II, Faber MA, et al. The mas-related G protein-coupled receptor MRGPRX2 is expressed on human basophils and up-regulated upon activation. J Allergy Clin Immunol (2017) 139:AB168. doi: 10.1016/j.jaci.2016.12.550
71. Sabato V, Elst J, Van Houdt M, Bridts C, Mertens C, Ebo DG. Surface expression of MRGPRX2 on resting basophils: An area of controversy. Allergy (2020) 75:2421–2. doi: 10.1111/all.14252
72. Babina M, Wang Z, Franke K, Zuberbier T. Thymic stromal lymphopoietin promotes MRGPRX2-triggered degranulation of skin mast cells in a STAT5-dependent manner with further support from JNK. Cells (2021) 10. doi: 10.3390/cells10010102
73. Babina M, Guhl S, Artuc M, Zuberbier T. Allergic FcϵRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy (2018) 73:256–60. doi: 10.1111/all.13301
74. Babina M, Wang Z, Artuc M, Guhl S, Zuberbier T. MRGPRX2 is negatively targeted by SCF and IL-4 to diminish pseudo-allergic stimulation of skin mast cells in culture. Exp Dermatol (2018) 27:1298–303. doi: 10.1111/exd.13762
75. Obata-Ninomiya K, Ishiwata K, Tsutsui H, Nei Y, Yoshikawa S, Kawano Y, et al. The skin is an important bulwark of acquired immunity against intestinal helminths. J Exp Med (2013) 210:2583–95. doi: 10.1084/jem.20130761
76. McLaren DJ, Worms MJ, Brown SJ, Askenase PW. Ornithodorus tartakovskyi: quantitation and ultrastructure of cutaneous basophil responses in the guinea pig. Exp Parasitol (1983) 56:153–68. doi: 10.1016/0014-4894(83)90058-9
77. Irani AM, Huang C, Xia HZ, Kepley C, Nafie A, Fouda ED, et al. Immunohistochemical detection of human basophils in late-phase skin reactions. J Allergy Clin Immunol (1998) 101:354–62. doi: 10.1016/S0091-6749(98)70248-9
78. Dahinden CA, Geiser T, Brunner T, von Tscharner V, Caput D, Ferrara P, et al. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med (1994) 179:751–6. doi: 10.1084/jem.179.2.751
79. Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, et al. High expression of the chemokine receptor CCR3 in human blood basophils. role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest (1997) 100:1137–43. doi: 10.1172/JCI119624
80. Jinquan T, Jacobi HH, Jing C, Reimert CM, Quan S, Dissing S, et al. Chemokine stromal cell-derived factor 1alpha activates basophils by means of CXCR4. J Allergy Clin Immunol (2000) 106:313–20. doi: 10.1067/mai.2000.108108
81. Iikura M, Miyamasu M, Yamaguchi M, Kawasaki H, Matsushima K, Kitaura M, et al. Chemokine receptors in human basophils: inducible expression of functional CXCR4. J Leukocyte Biol (2001) 70:113–20. doi: 10.1189/jlb.70.1.113
82. Iikura M, Ebisawa M, Yamaguchi M, Tachimoto H, Ohta K, Yamamoto K, et al. Transendothelial migration of human basophils. J Immunol (2004) 173:5189–95. doi: 10.4049/jimmunol.173.8.5189
83. Lett-Brown MA, Boetcher DA, Leonard EJ. Chemotactic responses of normal human basophils to C5a and to lymphocyte-derived chemotactic factor. J Immunol (1976) 117:246–52.
84. Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med (2001) 193:255–61. doi: 10.1084/jem.193.2.255
85. Böhm E, Sturm GJ, Weiglhofer I, Sandig H, Shichijo M, McNamee A, et al. 11-dehydro-thromboxane B2, a stable thromboxane metabolite, is a full agonist of chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) in human eosinophils and basophils. J Biol Chem (2004) 279:7663–70. doi: 10.1074/jbc.M310270200
86. de Paulis A, Montuori N, Prevete N, Fiorentino I, Rossi FW, Visconte V, et al. Urokinase induces basophil chemotaxis through a urokinase receptor epitope that is an endogenous ligand for formyl peptide receptor-like 1 and -like 2. J Immunol (2004) 173:5739–48. doi: 10.4049/jimmunol.173.9.5739
87. Paulis A, de Paulis A, Florio G, Prevete N, Triggiani M, Fiorentino I, et al. HIV-1 envelope gp41 peptides promote migration of human FcϵRI cells and inhibit IL-13 synthesis through interaction with formyl peptide receptors. J Immunol (2002) 169:4559–67. doi: 10.4049/jimmunol.169.8.4559
88. Korosec P, Turner PJ, Silar M, Kopac P, Kosnik M, Gibbs BF, et al. Basophils, high-affinity IgE receptors, and CCL2 in human anaphylaxis. J Allergy Clin Immunol (2017) 140:750–758.e15. doi: 10.1016/j.jaci.2016.12.989
89. Gaga M, Ong Y-E, Benyahia F, Aizen M, Barkans J, Kay AB. Skin reactivity and local cell recruitment in human atopic and nonatopic subjects by CCL2/MCP-1 and CCL3/MIP-1α. Allergy (2008) 63:703–11. doi: 10.1111/j.1398-9995.2007.01578.x
90. Gilet J, Chang Y, Chenivesse C, Legendre B, Vorng H, Duez C, et al. Role of CCL17 in the generation of cutaneous inflammatory reactions in hu-PBMC-SCID mice grafted with human skin. J Invest Dermatol (2009) 129:879–90. doi: 10.1038/jid.2008.333
91. Pellefigues C, Dema B, Lamri Y, Saidoune F, Chavarot N, Lohéac C, et al. Prostaglandin d amplifies lupus disease through basophil accumulation in lymphoid organs. Nat Commun (2018) 9:725. doi: 10.1038/s41467-018-03129-8
92. Puan KJ, San Luis B, Yusof N, Kumar D, Andiappan AK, Lee W, et al. FUT6 deficiency compromises basophil function by selectively abrogating their sialyl-Lewis x expression. Commun Biol (2021) 4:832. doi: 10.1038/s42003-021-02295-8
93. Pellefigues C, Tchen J, Saji C, Lamri Y, Charles N. AMG853, a bispecific prostaglandin d receptor 1 and 2 antagonist, dampens basophil activation and related lupus-like nephritis activity in Lyn-deficient mice. Front Immunol (2022) 13:824686. doi: 10.3389/fimmu.2022.824686
94. De Monte L, Wörmann S, Brunetto E, Heltai S, Magliacane G, Reni M, et al. Basophil recruitment into tumor-draining lymph nodes correlates with Th2 inflammation and reduced survival in pancreatic cancer patients. Cancer Res (2016) 76:1792–803. doi: 10.1158/0008-5472.CAN-15-1801-T
95. Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, et al. The T helper type 2 response to cysteine proteases requires dendritic cell–basophil cooperation via ROS-mediated signaling. Nat Immunol (2010) 11:608–17. doi: 10.1038/ni.1883
96. Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol (2010) 184:1143–7. doi: 10.4049/jimmunol.0902447
97. Ohta T, Yoshikawa S, Tabakawa Y, Yamaji K, Ishiwata K, Shitara H, et al. Skin CD4+ memory T cells play an essential role in acquired anti-tick immunity through interleukin-3-Mediated basophil recruitment to tick-feeding sites. Front Immunol (2017) 0:1348. doi: 10.3389/fimmu.2017.01348
98. Brauweiler AM, Goleva E, Leung DYM. Staphylococcus aureus lipoteichoic acid initiates a TSLP-Basophil-IL4 axis in the skin. J Invest Dermatol (2020) 140:915–7.e2. doi: 10.1016/j.jid.2019.09.004
99. Hayes MD, Ward S, Crawford G, Seoane RC, Jackson WD, Kipling D, et al. Inflammation-induced IgE promotes epithelial hyperplasia and tumour growth. Elife (2020) 9. doi: 10.7554/eLife.51862
100. Li W, Hsiao H-M, Higashikubo R, Saunders BT, Bharat A, Goldstein DR, et al. Heart-resident CCR2 macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling. JCI Insight (2016) 1. doi: 10.1172/jci.insight.87315
101. Karasawa K, Asano K, Moriyama S, Ushiki M, Monya M, Iida M, et al. Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J Am Soc Nephrol (2015) 26:896–906. doi: 10.1681/ASN.2014020195
102. Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, et al. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol (2014) 15:45–53. doi: 10.1038/ni.2769
103. Huber-Lang M, Younkin EM, Sarma JV, Riedemann N, McGuire SR, Lu KT, et al. Generation of C5a by phagocytic cells. Am J Pathol (2002) 161:1849–59. doi: 10.1016/S0002-9440(10)64461-6
104. David BA, Kubes P. Exploring the complex role of chemokines and chemoattractants in vivo on leukocyte dynamics. Immunol Rev (2019) 289:9–30. doi: 10.1111/imr.12757
105. Ricardo-Gonzalez RR, Schneider C, Liao C, Lee J, Liang H-E, Locksley RM. Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. J Exp Med (2020) 217. doi: 10.1084/jem.20191172
Keywords: basophil, CysLTR2, histamine, IL-33 and ST2, leukotriene C4, mast cell, MRGPRX2
Citation: Shibuya R and Kim BS (2022) Skin-homing basophils and beyond. Front. Immunol. 13:1059098. doi: 10.3389/fimmu.2022.1059098
Received: 30 September 2022; Accepted: 12 December 2022;
Published: 22 December 2022.
Edited by:
Christophe Pellefigues, CNRS EMR8252 Centre de Recherche sur l’Inflammation, FranceReviewed by:
Bernhard F. Gibbs, University of Oldenburg, GermanyAdrian Piliponsky, Seattle Children’s Research Institute, United States
Copyright © 2022 Shibuya and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian S. Kim, aXRjaGRvY3RvckBtb3VudHNpbmFpLm9yZw==
 Rintaro Shibuya
Rintaro Shibuya Brian S. Kim
Brian S. Kim