- 1Zhuang Medical College, Guangxi University of Chinese Medicine, Nanning, Guangxi, China
- 2School of Second Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
As a systemic autoimmune disease, rheumatoid arthritis (RA) usually causes damage not only to joints, but also to other tissues and organs including the heart, kidneys, lungs, digestive system, eyes, skin, and nervous system. Excessive complications are closely related to the prognosis of RA patients and even lead to increased mortality. This article summarizes the serious complications of RA, focusing on its incidence, pathogenesis, clinical features, and treatment methods, aiming to provide a reference for clinicians to better manage the complications of RA.
1 Introduction
Rheumatoid arthritis (RA) is defined as a systemic autoimmune disease associated with a chronic inflammatory process, which gradually leads to joint destruction, deformity, disability, and even death (1). It is a widely distributed disease worldwide, with a prevalence of approximately 0.5% to 2% and a higher prevalence in women, smokers, and those with a family history of it (2). At present, the etiology of RA has not been fully elucidated, but what attracts attention is the immune processes that occur in the joint synovium and synovial fluid (3, 4), during which synovial macrophages release cytokines, such as tumor necrosis factor α (TNF-α), interleukin-1 (IL-1) and interleukin-6 (IL-6), which co-stimulate the activity of osteoclasts with inflammation and fibroblast-like synoviocytes (FLS), thus leading to the progress of bone erosion (5). In addition, activated FLS can produce matrix metalloproteinase (MMP) that leads to cartilage degeneration (6). Nuclear factor-kappa-light-chain-enhancer of activated B cells (NF-κB) is involved in the pathogenesis of chronic inflammatory diseases, and FLS stimulates the NF-κB signaling pathway, allowing T cells to bind to proteins on the surface of osteoclasts, which also leads to further development of bone erosion as it increases osteoclast activity (7). FLS can migrate from one joint to another, resulting in symmetrical joint destruction which is typical in RA (8). In addition, the presence of autoantibodies in the serum of RA patients is a mark of disease, with rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) being the most prominent. These autoantibodies are found in 50-80% of RA patients (9), newly-detected antibodies such as anti-carbamylated protein antibodies and anti-acetylated protein antibodies were also identified in them (10). Antibody production leads to inflammation; citrullination leads to an immune response which indicates the formation of ACPA (11); ACPA may play an important role in the prolonged inflammatory process and its presence directly links bone erosion and pain in RA patients (12). The pathogenesis of rheumatoid arthritis mentioned above is shown in Figure 1.
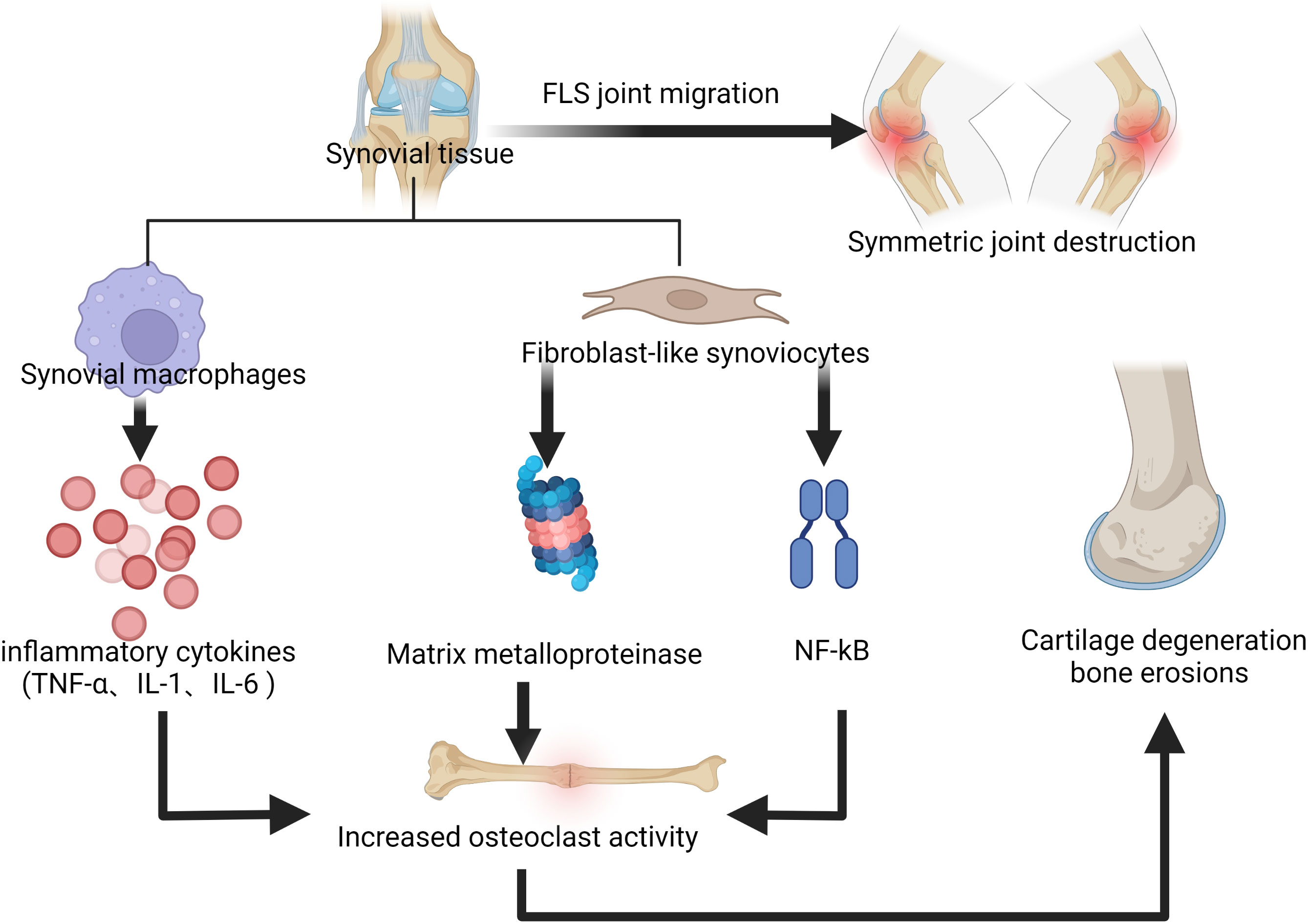
Figure 1 The immune processes that occur in the joint synovium and synovial fluid, leading to the progress of bone erosion and cartilage degeneration.
As a systemic disease, RA usually causes damage to other tissues and organs besides joints, including the heart, kidneys, lungs, digestive system, eyes, skin, and nervous system (13, 14). The results of the study show that about 40% of RA patients suffer from complications, and the incidence of serious complications is 8.3%, among which cardiovascular disease, interstitial lung disease, osteoporosis, and metabolic syndrome are more common (15). The existence of complications seriously reduces the quality of life of RA patients and even leads to increased RA mortality (16). Complications of RA are usually closely related to prognosis and require early diagnosis and active intervention, and the main treatment goals include reducing disease activity and controlling extra-articular damage of RA (17). At present, the treatment methods for RA complications are relatively limited. In this article, we mainly summarize the manifestations of severe extra-articular damage in RA (as shown in Figure 2), and discuss its pathogenesis, incidence, clinical features, and treatment methods, hoping to provide some reference for clinical practice.
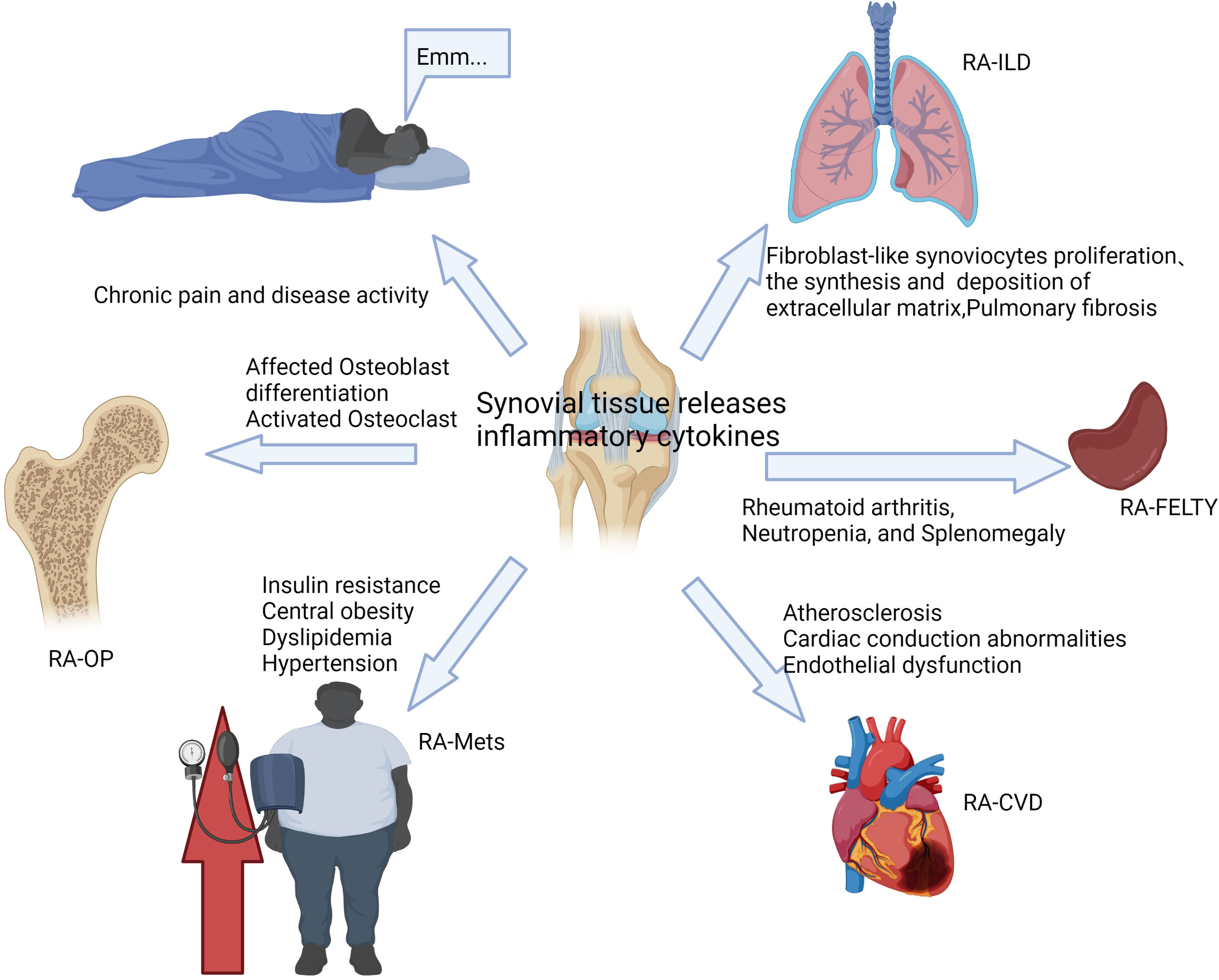
Figure 2 The complications of RA are usually closely related to disease activity and inflammation levels.
2 Cardiovascular disease in RA
2.1 Pathogenesis of RA-CVD
It is well known that RA patients may be disabled, but the main cause of their death is cardiovascular disease (CVD) (18). Many studies have shown that the incidence of CVD in RA patients is 30%-60%, mainly involving pericarditis, myocarditis and heart failure, and coronary artery disease (19). Epidemiological investigations suggest that synovial tissue and circulating immune cells in RA release pro-inflammatory cytokines such as TNF-α and IL-6, which directly lead to systemic inflammation and the occurrence of CVD (20, 21). Overactive immune cells, such as T lymphocytes and B lymphocytes, may affect the cardiovascular system through multiple mechanisms (22, 23). Autoantibodies in RA affect the cascade of all structures of the cardiovascular system, from the myocardium to the heart valves, conduction system, and vasculature (24). There is more severe disease activity in ACPA-positive patients, which further leads to atherosclerosis and increases CVD mortality (25). In addition, ACPA is also seen in non-RA patients with cardiovascular disease and has adverse outcomes (26). Imaging methods are essential for the detection and assessment of CVD risk in RA, and carotid ultrasound, aortic pulse wave velocity or arterial enhancement index and ankle-brachial index, echocardiography, and cardiac magnetic resonance can be used to assess the CVD risk of patients with RA in clinical practice (27). Early detection and diagnosis of CVD in RA patients are critical for prognosis and management.
2.1.1 Pericarditis
Pericarditis is one of the common cardiac manifestations of RA. Many patients with early RA can be complicated with pericarditis or develop pericarditis before RA (28). Pericarditis is inflammation and fluid accumulation in the pericardium, and about 15% of RA patients will show corresponding symptoms. However, electrocardiography shows that about 20%-50% of patients have pericardial involvement, clinically manifested as chest pain or dyspnea (29). Therefore, strict physical examination and antibody screening are needed to detect whether RA is complicated by pericarditis as soon as possible. Early diagnosis and effective treatment of pericarditis will significantly improve the prognosis of RA patients.
2.1.2 Myocarditis
Myocarditis is the result of persistent inflammation in the myocardium and is histologically characterized by cellular infiltration composed of lymphocytes, histiocytes, and macrophages, which may form nodular granulomatous lesions (30). The degree of myocardial dysfunction is associated with disease activity of RA because key inflammatory cytokines in RA, such as TNFα, IL-1, and IL-6, may induce myocardial and vascular dysfunction and promote remodeling and fibrosis of the left ventricular (31).
2.1.3 Arrhythmia
Arrhythmia is another common cardiac complication in RA patients, which may be secondary to conduction abnormalities. Its causes include ischemia, rheumatoid nodules, and amyloidosis (32). Recent researches indicate that symptoms and increased sympathetic nerve activity can lead to abnormal heart rhythms, and Holter monitoring can capture latent arrhythmias with higher accuracy (33).
2.1.4 Coronary artery disease
The main etiology of coronary artery disease in RA may be related to atherosclerosis accelerated systemic inflammatory response and abnormal lipids and endothelial dysfunction (34, 35). The chronic inflammation and reactive oxygen species (ROS) response of RA is the core of the pathogenesis of atherosclerosis (36). ROS is a group of small active substances that play a key role in the regulation of biological cellular processes. The balance between ROS and antioxidants is critical for maintaining cellular homeostasis, thus an imbalance between oxidants and antioxidant mechanisms can lead to oxidative stress states (37). Excessive ROS may lead to vascular damage, the result of a complex cascade including oxidative modification of lipoproteins, endothelial activation, and accelerated atherosclerosis by leukocyte migration and differentiation (38). Pro-inflammatory cytokines and chemokines, as well as IL-1 and intercellular and vascular cell adhesion molecules (39), are highly expressed in atherosclerotic lesions, promote leukocyte recruitment, impair vasodilation, and induce oxidation stress and promote coagulation (40).
2.1.5 Heart failure
Heart failure is the main cause of death in RA patients, and the prevalence of heart failure in RA patients is also twice as high as that in the general population, with a higher incidence in women than men in general (41). Studies have found that RA patients are more likely to develop heart failure due to diastolic dysfunction, which may be related to systemic inflammation (42). Elevated levels of c-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), RF, ACPA and inflammatory cytokines may contribute to the progression of heart failure in RA (43).
2.2 Treatment of RA-CVD
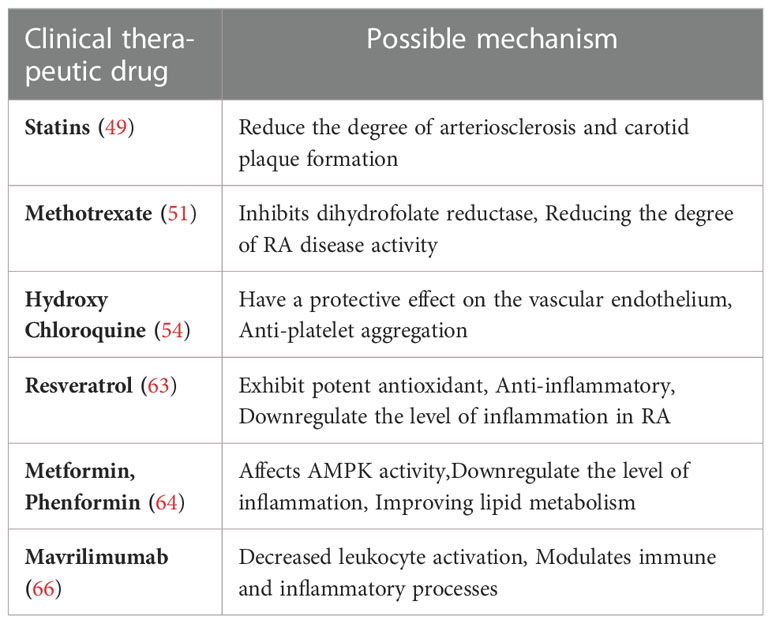
An increasing number of evidence supports that long-term use of NSAIDs has the potential of triggering cardiovascular risks despite reductions in disease activity and some adverse CVD outcomes with conventional RA drugs (44). NSAIDs anti-inflammatory drugs exert therapeutic effects by inhibiting cyclooxygenase isoforms. The drugs inhibit prostacyclin production, leading to vasoconstriction, increased blood pressure, rupture of atherosclerotic plaques, and thrombosis, thus are thought to be the main contributor to CVD in RA (45). Non-steroidal anti-inflammatory drugs (NSAIDs), such as rofecoxib, have been the fundamental treatment for patients with osteoarthritis and other types of pain, but as controlled trials and other meta-analyses indicated an increased risk of cardiovascular problems in RA patients, rofecoxib has withdrawn from the market (46). Glucocorticoids are usually used to treat RA, mainly for short-term control of disease activity. However, glucocorticoids can aggravate hypertension or cause abnormal blood lipid levels and glucose tolerance, insulin resistance, and obesity, and promote the occurrence and development of CVD (47). Studies have shown that the use of statins in RA patients can reduce the degree of arteriosclerosis and carotid plaque formation (48). RA patients treated with at least one disease-modifying Anti-Rheumatic Drugs (DMARDs) and statins at the same time have seen reduced RA-CVD mortality of 21% (49), and disease activity was significantly decreased in those RA patients whose methotrexate (MTX) and corticosteroid regimens are added with statins, and this may indicate a clear positive effect of statins in the control of RA (50).
MTX is the “gold standard” for RA treatment because it has important immunosuppressive and anti-inflammatory effects and inhibits dihydrofolate reductase (51). Many studies have demonstrated the benefits of MTX. Approximately 25-40% of patients receiving MTX alone have seen significant improvement because MTX can play a role in improving microvascular endothelial function by reducing the degree of RA disease activity, reducing the risk of CVD in RA patients, and reducing their mortality (52). In addition, methotrexate appears to have cardioprotective properties on lipids and endothelium, in contrast to patients receiving adalimumab (53). Similarly, Hydroxychloroquine (HCQ) was found to have a protective effect on the vascular endothelium of RA patients (54), and it causes a lower cardiovascular risk in RA patients (55, 56). HCQ treatment can reduce low-density lipoprotein and Triglyceride serum values, and plays an anti-platelet aggregation role, thus it is considered to be cardioprotective (57).Tumor necrosis factor inhibitor (TNFi) therapy in RA reduces CVD risk via inhibition of endothelial dysfunction and slows the progression of atherosclerosis by reducing the expression of pro-inflammatory cytokines and endothelial adhesion molecules (58). In a controlled study, TNFi preparations improved myocardial inflammation and myocardial perfusion in patients with RA-CVD compared with standard disease-modifying antirheumatic drugs (59).
Recently, metabolic modulation therapy has become a research hotspot. Sirtuin 1 (SIRT1) is a sirtuin involved in a wide range of transcriptional and metabolic regulation, which may affect cell proliferation and inflammatory responses and inhibit the activation of NF-κB-dependent inflammation (60). Some SIRT1 activators, such as resveratrol, a polyphenol found in wine, have been extensively studied as SIRT activators and they exhibit potent antioxidant, anti-inflammatory and anti-cancer properties (61). Resveratrol can inhibit NF-κβ-dependent inflammatory response and its effect on RA patients is under evaluation (62). Notably, serum biochemical markers such as CRP, ESR, MMP-3, and IL-6 were also significantly reduced in resveratrol-treated patients (63). In addition, metformin and its analog phenformin are hypoglycemic drugs used in diabetic patients; although the exact mechanism of action remains unclear, their effect on AMPK (Adenosine 5’-monophosphate-activated protein kinase) can be conducive to the beneficial secondary effects of these drugs such as cutting inflammatory markers, improving lipid metabolism, and reducing experimental autoimmune arthritis based on the importance of AMPK on T cells in RA (64). In particular, metformin, as an activator of AMPK, can inhibit the migration of FLS, inhibit the expression of pro-inflammatory cytokines, and downregulate the level of inflammation in RA and its comorbidities (65).
Some pathways involve extracellular targets. Mavrilimumab is a monoclonal antibody against granulocyte–macrophage colony-stimulating factor (GM-CSF), and GM-CSF is expressed at high levels in synovial fluid and plasma as well as synovial tissue cells of RA patients. Phase I and II trials of mavrilimumab in the treatment of RA showed satisfactory safety and efficacy (66). GM-CSF emphasizes the impact of “inflammatory” pathways on arteriosclerosis and endothelial dysfunction. Based on this connection, it is expected that more potential therapeutic targets will be developed to better manage cardiovascular problems in RA patients. Recent clinical studies on RA-CVD are shown in Table 1.
3 Lung disease in RA
3.1 Pathogenesis of lung disease in RA
3.1.1 Interstitial lung disease
ILD is a serious pulmonary complication of RA, resulting in a 10-20% mortality in RA. Pulmonary involvement is common in RA patients, among which the occurrence of pulmonary complications is approximately 60-80% (67, 68). Clinical manifestations include interstitial lung disease, small airway disease, rheumatoid nodules, pleural effusion, pulmonary vasculitis, pulmonary fibrosis, etc (69). Although RA can involve many parts of the respiratory system, such as the airway or pleura, parenchymal lung involvement is associated with the highest morbidity and mortality (70). One diagnostic study showed that approximately 50% of RA patients had interstitial lung disease, of which only 10% had clinically significant symptoms such as cough and progressive exertional dyspnea (71), and that is because cytokine, chemotactic factor, and growth factor-mediated RA inflammatory process can promote FLS proliferation, increase the synthesis and deposition of extracellular matrix, and lead to pulmonary fibrosis (72, 73). The most common patterns of RA-ILD are usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP) (74). There is no universal treatment guideline for RA-ILD, thus accurate screening and diagnosis of the characteristics of ILD development in RA patients is critical for future research and treatment of RA patients (75). Histological biopsy, pulmonary function tests, and high-resolution computed tomography (HRCT) are valuable tools for the diagnosis and evaluation of RA-ILD (76), and HRCT can accurately capture UIP cellular and traction bronchiectasis as well as reticular abnormalities and the “ground glass opacity” in NSIP (77).
3.1.2 Pleurisy and pleural effusion
Pleurisy and pleural effusion are the most common pleural manifestations observed in RA patients, with only 3-5% of patients presenting with clinical symptoms such as cough, dyspnea, chest pain, and fever, which means the majority of RA patients with the pleural disease are with no clinical manifestations (78). In terms of pathogenesis, studies have suggested that IgG, IgE, and other antibodies contribute to the formation of immune complexes to destroy the capillary endothelium and increase the capillary permeability of the pleural cavity (79). Ultrasound-guided thoracentesis can be an important test in RA patients with pleural effusion.
3.1.3 Airway involvement (bronchiolitis, bronchiectasis, and cricoarytenoid arthritis)
The prevalence of airway disease is high in RA as it affects 39% to 60% of RA patients and may involve any part of the airway, including large and distal small airways. The most common manifestations are bronchitis, bronchiectasis, and cricoarytenoid arthritis (80). Pulmonary function tests and HRCT can help diagnose airway-related diseases. Chronic inflammatory infection is the main cause of bronchiectasis in RA patients, and bronchiolitis is characterized by damage to the airway epithelium, which leads to airflow obstruction (81). Because the midline of the vocal folds is adducted, cricoarytenoid arthritis manifests as hoarseness, sore throat, dyspnea, and stridor, which are primarily due to thickening of the synovial membrane of the cricoarytenoid joint and persistent cartilage erosion (82).
3.2 Treatment of lung disease in RA
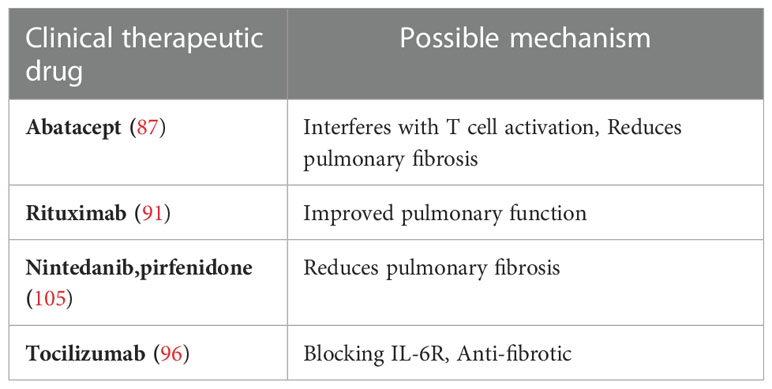
Treatment options for RA-ILD are complicated by the possible pulmonary toxicity of many DMARDs, but their ability to improve lung function and stabilize pulmonary symptoms has been demonstrated (83, 84). Therefore, joint and pulmonary involvement should be assessed independently for therapeutic purposes (85). The Spanish Society of Rheumatology recommends the use of abatacept and rituximab in patients with RA-ILD (86). A retrospective study showed that the use of abatacept, a costimulatory antagonist of T lymphocytes, improved ILD in approximately 88% of cases and reduced their risk of infection (87). In addition, abatacept significantly reduced lung density and fibrotic histology scores and improved ILD (88). Finally, data from a retrospective multicenter study conducted in Italy in 2020 showed that 86.1% and 91.7% of patients with RA-ILD treated with abatacept for at least 6 months had stable or increased forced vital capacity and carbon monoxide diffusing capacity, respectively, while 81.4% of patients had stable or improved chest HRCT (89). Rituximab is considered safe for the treatment of RA-ILD as evidenced by observational studies (90, 91).In addition, a large observational study of patients with RA-ILD showed that the pulmonary function of most ILD patients remained stable or improved after treatment with RTX during long-term follow-up (92). The British College of Rheumatology suggests that doctors be cautious in prescribing TNFi to patients with RA-ILD and recommends RTX for the treatment of refractory ILD (93).
Interstitial lung disease is characterized by alveolar inflammation and interstitial fibrosis, thus anti-fibrotic therapies, such as nintedanib and pirfenidone, have become the spotlight, and in fact, nintedanib and pirfenidone have been proven to slow the disease progression in patients with idiopathic pulmonary fibrosis (94, 95). In addition, tocilizumab as monotherapy can stabilize or even improve ILD (96), and as an IL-6 receptor antagonist, tocilizumab can achieve anti-fibrotic effects by blocking IL-6R, which means this treatment delivers potential benefits in RA-ILD-associated pulmonary fibrosis (97). Although there are still many challenges in practical clinical application, the efficacy and safety of anti-fibrotic agents in RA-ILD patients are still under continuous research (98, 99) for better control over RA-ILD.
In addition, non-drug conservative treatment methods, such as pulmonary rehabilitation and supplemental oxygen, can be used for aged or frail patients or those with multiple comorbidities (100). The role of pulmonary physical rehabilitation in RA-ILD is unclear, but it has beneficial effects on improving dyspnea, functional exercise capacity, and quality of life in idiopathic ILD (101). However, dyspnea and poor joint mobility in patients with ILD limit their pulmonary rehabilitation, thus patients with RA-ILD should take pulmonary rehabilitation in the early course of the disease (102). In addition, supplemental oxygen can be used as primary palliative therapy to improve the quality of life of patients with severe lung disease and reduce respiratory symptoms during daily activities (103). At the same time, smoking is a major risk factor for the progression of RA-ILD, and smoking cessation is important for RA-ILD patients (104).
Lung transplantation may be an option for end-stage RA-ILD, while only a few studies have evaluated post-transplant outcomes in patients with RA-ILD. A recent study reveals that patients with ILD of connective tissue disease (including RA) had similar rates of acute or chronic rejection after lung transplantation compared with patients with idiopathic pulmonary fibrosis, and there was no significant difference in survival (105). Lung transplantation may be an option for younger patients with advanced refractory disease but is not appropriate for patients at risk of advanced age, multiple comorbidities, immobility, and other severe extra-articular damage. Recent clinical studies on pulmonary complications in RA are shown in Table 2.
4. Metabolic syndrome in RA
4.1 Pathogenesis of RA-Mets
The main features of Mets in RA patients are related to inflammation-induced RA disease activity and mainly include insulin resistance (IR), central obesity, dyslipidemia, and hypertension; these manifestations (106). The prevalence of Mets in RA patients varies widely worldwide, ranging from 14.32% to 37.83% according to different criteria (107). In addition, Mets are strongly associated with accelerated atherosclerosis development and increased CVD risk, and are considered to be characteristic pathogenesis of CVD (108). Studies have shown that IR is a fundamental feature of Mets in RA, and is directly related to the levels of IL-6, TNF-α, CRP, and ESR (109). RA-induced IR leads to increased systemic inflammatory responses and directly affects endothelial dysfunction (110). In addition, the continuous increase of macrophages in obese adipose tissue has emerged as a key link to metabolic inflammation (111). Recent studies reveal the heterogeneity of adipose tissue macrophages and their interactions with adipocytes, endothelial cells, and other immune cells in the adipose tissue microenvironment (112). Adipose tissue is a multifunctional organ that, in addition to its central role in storing lipids, secrets a variety of hormones. These various product, collectively referred to as “adipocytokines” or “adipokines”, are responsible for the immune response and mediators of inflammation (113). RA is associated with IR, dyslipidemia, and changes in the adipokines profile (114). In RA, adipocytes and their surrounding macrophages induce innate and adaptive immune cells to release proinflammatory cytokines that cause cartilage degradation and osteoblast dysregulation, thus leading to arthritic disease and Mets (115).
4.2 Treatment of RA-Mets
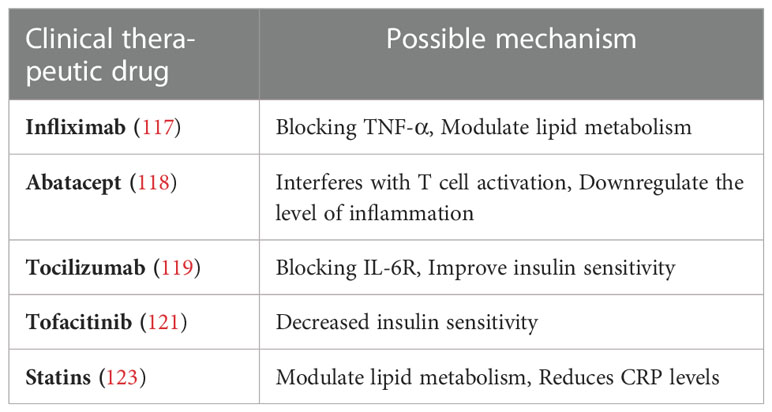
In RA patients, TNF-α is an important mediator of IR; therefore, biological therapies that block proinflammatory cytokines, such as TNF-α antagonists, can reduce CRP levels in RA patients, as well as modulate lipid metabolism and improve IR (116). The majority of patients receiving anti-TNF-α biologic therapy (eg, infliximab) were observed to have significant reductions in serum insulin levels as well as insulin and glucose indices, indicating an improvement in IR (117).
Other non-TNF-α treatments,Such as Abatacept, a novel biologic already approved for the treatment of patients with RA, interferes with T cell activation and prompts the polarization of adipose tissue macrophages from pro-inflammatory M1 to anti-inflammatory M2 phenotype, thereby reducing adipose tissue inflammation to improve insulin sensitivity (118). Based on the close relationship between IR and the levels of inflammatory factors such as IL-6, a study on the IL-6 blocker tocilizumab found that intravenous administration of tocilizumab had a rapid positive effect on IR and insulin sensitivity in RA patients. These findings suggest that IL-6 blocker has a potential beneficial effect on mechanisms associated with Mets and CVD development in RA patients (119).
The Janus kinase and signal transducer and activator of the transcription pathway (JAK-STAT) has an important pathogenic role in the development of low-grade chronic inflammatory responses leading to obesity and type II diabetes (120). Tofacitinib, the first small-molecule oral selective JAK inhibitor approved for the treatment of RA patients in 2018, can reduce IR when used alone as proved by research, which brings the therapeutic potential to the JAK-STAT pathway (121, 122).
Lowering LDL cholesterol with statins is a commonly used treatment in patients with metabolic diseases, and there is evidence that statins have a direct anti-inflammatory effect because they reduce CRP levels (123) to improve RA-Mets. Recent clinical studies on RA-Mets are shown in Table 3.
5 Osteoporosis in RA
5.1 Pathogenesis of RA-OP
Osteoporosis is a common systemic skeletal disease characterized by low bone mass and degeneration of bone tissue microarchitecture that lead to bone fragility and fracture susceptibility (124). A fragility fracture is defined as a spontaneous fracture caused by minimal or no identifiable trauma and is a hallmark of OP (125). Bone erosion and systemic bone loss are typical features of RA. Systemic bone loss leads to the occurrence of OP, which is one of the main complications of RA (126). The incidence rate can reach 30% of RA patients, or even higher (127). Bone fragility in RA is caused by a combination of systemic inflammation, autoantibodies circulation, and the secretion of pro-inflammatory cytokines. Inflammatory cytokines such as TNF-α, IL-6, IL-1, and immune cell-derived cytokines undermine osteoblastogenesis while promoting osteoclastogenesis (128, 129). ACPA is a determinant of bone loss (130) as it has a direct and independent effect on osteoclasts (131). The effect may be mediated by IL-8-dependent osteoclast activation, so the bone loss is more likely to occur around joints of ACPA-positive RA patients. These factors all have a deleterious effect on bone (132).
5.2 Treatment of RA-OP
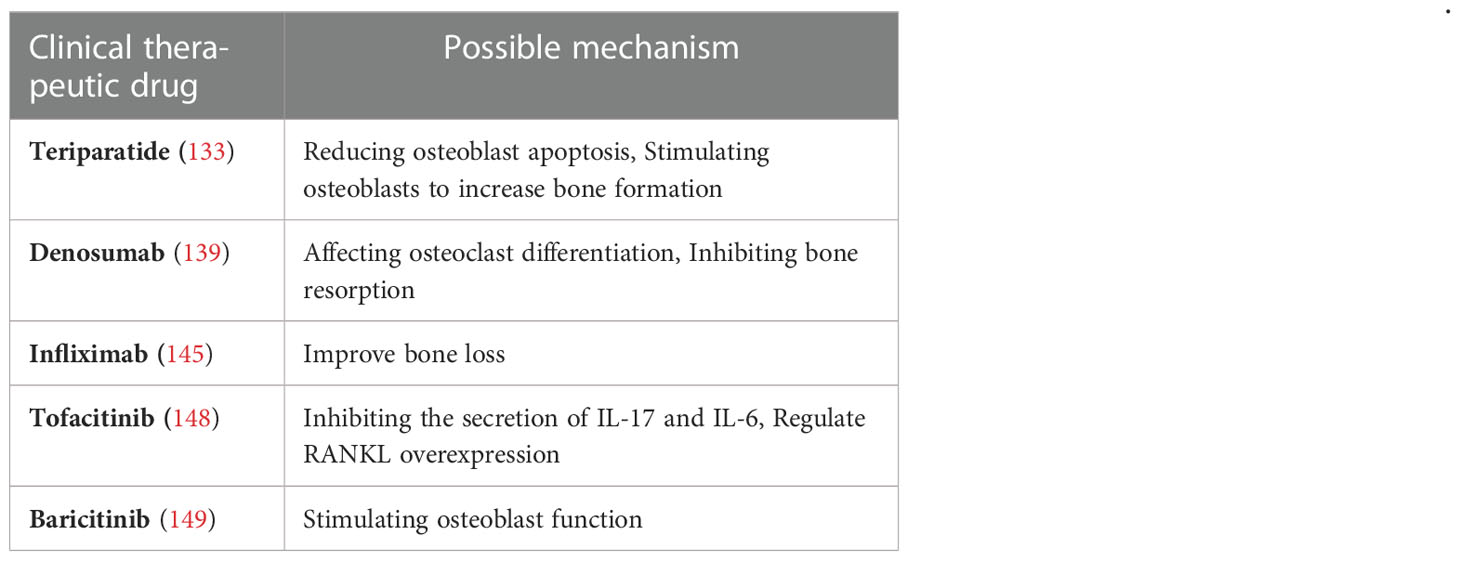
Teriparatide, a parathyroid hormone analog, can act as an anabolic drug by reducing osteoblast apoptosis and stimulating osteoblasts to increase bone formation with subcutaneous administration (133). The study showed that teriparatide resulted in a significantly greater increase in bone mineral density levels and a significant reduction in spinal fractures, compared with the active comparator and the anti-resorptive drug alendronate, and that was confirmed in clinical practice (134). Another study showed a significant reduction in spinal fractures in RA patients treated with teriparatide (135). Furthermore, in cases of high fracture risk, calcium and vitamin D should be supplemented with anti-osteoporotic therapy (136).
The receptor activator of NF-κB ligand (RANKL) is a key molecule in osteoclast differentiation and activation and is a potential therapeutic target for osteolytic diseases (137). Denosumab is a RANKL-specific human monoclonal antibody currently used to treat osteoporosis, osteosarcoma, multiple myeloma, and bone metastases (138). RANKL is expressed at moderate and high levels in the inflammatory state of RA patients, while denosumab can prevent the receptor activator of RANKL from binding to RANK on osteoclasts, thereby inhibiting bone resorption (139). In a phase II randomized controlled trial, the result of the combined use of methotrexate and denosumab in the treatment of RA was a significant increase in bone mineral density at the lumbar spine and hip of RA patients (140), suggesting that the combination of methotrexate and denosumab can prevent the development of bone erosions in RA (141).
TNFi is the first biological agent for RA treatment and is a key drug for inhibiting inflammation (142). Inflammatory cytokines induce osteoclast maturation and inhibit osteoblast activation to perturb bone homeostasis, thus, anti-TNF therapy can improve bone homeostasis in RA patients (143, 144). Infliximab has beneficial effects on bone metabolism in RA patients, studies on the effect of TNFi on bone loss have demonstrated that the use of infliximab can improve bone loss in RA patients (145). Another observational study indicated a lower incidence of vertebral fractures in RA patients treated with TNFi, suggesting that TNFi plays a bone-protective role in RA patients (146).
Janus kinases are a family of protein tyrosine kinases JAK1, JAK2, JAK3, and TYK2, which act on signal transducers and activators of transcription, and JAK inhibitors are approved for the treatment of RA (147). Tofacitinib, a JAK inhibitor, can regulate RANKL overexpression in the synovium by inhibiting the secretion of IL-17 and IL-6 to reduce the damage to joints caused by RA inflammation as proved by research (148). It is also proved that baricitinib can improve bone loss in RA by stimulating osteoblast function (149). The above results demonstrate that JAK inhibitors are effective therapeutics to increase osteoblast function and bone formation. Recent clinical studies on RA-OP are shown in Table 4.
6 Felty syndrome in RA
6.1 Pathogenesis of Felty syndrome in RA
Felty syndrome is a rare and severe extra-articular manifestation of RA, with an incidence of approximately 1% of RA patients. Typical manifestations are unexplained RA-complicated neutropenia and splenomegaly (150), and due to long-term granulocyte deficiency, patients are more prone to opportunistic infections, which results in increased mortality (151). Felty syndrome is common in RA patients with a disease history of more than 10 years while it is not uncommon that patients with short onset and atypical clinical symptoms are not diagnosed or misdiagnosed (152). The cause of peripheral blood cytopenia in Felty syndrome is not fully understood, and neutropenia is the most common symptom, which may be related to the presence of granulocyte-specific antinuclear factors (GS-ANF). It has been reported that the positive rate of GS-ANF in patients with Felty syndrome is as high as 75%, while that in RA patients is only 25% to 30% (153). At the same time, the presence of IgG-like granulocyte antibodies in the peripheral blood of patients with Felty syndrome can further destroy granulocytes and reduce their ability to phagocytose immune complexes, while T cell activation can inhibit granulocyte production (154). In addition, splenomegaly can cause thrombocytopenia, and the mechanism may be related to factors such as decreased platelet production, spleen retention, peripheral platelet depletion, and peripheral immune-mediated platelet destruction (155).
6.2 Treatment of Felty syndrome in RA
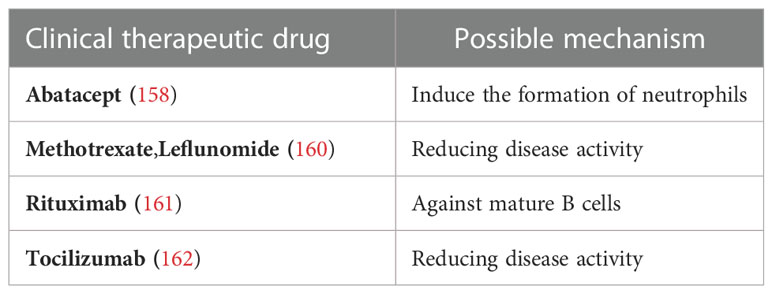
Treatment of Felty syndrome is supportive and is aimed at controlling underlying RA while improving neutropenia to prevent life-threatening infections (156). However, due to the lack of evidence-based medicine, most drugs are empirical (157). Granulocyte colony-stimulating factor ameliorates neutropenia by inducing the production of neutrophils and has good efficacy and tolerance by patients (158). It has been reported that a patient with a 38-year history of RA and Felty syndrome had a significant increase in absolute neutrophil counts after treatment with abatacept (159). Both MTX and leflunomide can improve joint and vascular inflammation in patients with Felty syndrome (160). Currently, the most widely used drug is rituximab, an anti-CD20 monoclonal antibody that acts against mature B cells and has been approved for the treatment of complex RA. In addition, rituximab has also been reported to successfully treat refractory neutropenia in Felty syndrome (161). Another report of Felty syndrome told that the patient’s clinical symptoms has been resolved after tocilizumab treatment, and his spleen had returned to normal size, the absolute neutrophil count had stabilized, and joint erosions had not continued to worsen (162). These case reports suggest new options for the treatment of Felty syndrome. Recent clinical studies on RA-Felty are shown in Table 5.
7 Sleep disorders in RA
7.1. Pathogenesis of sleep disorders in RA
Sleep disorder is closely related to the development of chronic disease. In the long course of RA, chronic pain and disease activity may be the main factors related to sleep disorders in RA (163, 164). Sleep disorder is multifactorial thus the degree of disease activity increases the risk of depression and anxiety in RA patients, while depression can affect the quality of life and treatment compliance of RA patients. The above factors, which are underestimated or even ignored, all contribute to sleep disorders caused by disease activities and emotional problems (165). In fact, the incidence of sleep disorder in RA patients is as high as 50% (166), and poor sleep quality severely undermines the physical function of patients. Therefore, it is necessary to pay attention to the treatment of sleep disorders in RA patients because of their crucial impact on patients’ quality of life.
7.2. Treatment of sleep disorders in RA
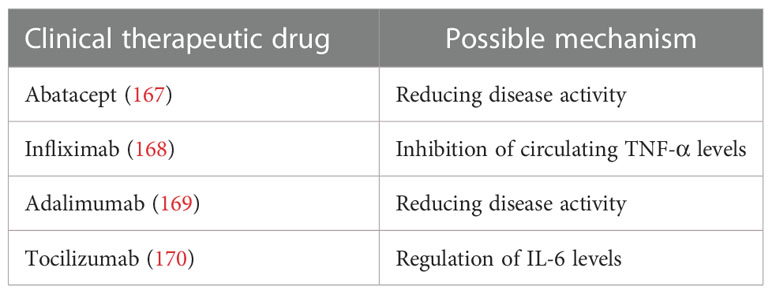
Studies have shown that anti-TNF and other biologics can improve the sleep quality of RA patients. Abatacept significantly improves sleep disorders in RA patients as the MOS-Sleep Scale demonstrated its validity, reliability, and sensitivity to changes (167). Infliximab improves sleep quality and relieves vigilance disorders in RA patients, possibly a result of central effects by suppressing TNF-α circulation (168). In addition, adalimumab was proven to be beneficial in improving sleep disorder in RA patients for it reduces disease activity while improving sleep problems in RA patients (169). Another study has shown that the IL-6 antagonist tocilizumab improved sleep quality in RA patients, yet patients’ disease activity was not significantly reduced, which deserves further study as it seems to indicate a potential role of IL-6 in sleep regulation (170). Recent clinical studies on sleep disorders in patients with RA are shown in Table 6.
8 Conclusion
RA complications are a major scientific issue worthy of attention. However, the current international research on the pathological mechanism of RA complications remains unclear, and safe and effective clinical drugs and methods are limited. Given that much of the extra-articular damage in RA is related to disease activity and disease severity, control of disease activity in RA should be the optimal treatment, and earlier and more aggressive management of RA can reduce the impact of complications on prognosis. Although there exist some guidelines on the management of RA-related complications, the range of recommendations including ILD and CVD is still limited. In this review, we discuss the pathogenesis, morbidity, and updated management guidelines of serious complications such as cardiovascular problems and pulmonary involvement in patients with RA. We hope that the recommendations reviewed in this article can provide clinicians with a better reference to treatment options for RA complications.
Author contributions
DW, YL designed the study together, equal contribution, Listed as co-first author. AH, YP as co-corresponding author, TLi, XZ, TLv, PO, HL, XL were all involved in the revision of the manuscript, GF, AH, YP made final critical revisions. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Natural Science Foundation of China project Study of the Mechanism for Regulating the Th17/Treg Balance along Gut-immune-joint Axis in Treating Rheumatoid Arthritis by the Component Compatibility of the Zhuang Medicine Longzuan Tongbi Formula (Number 81973976), the Guipai Chinese Medicine Inheritance and Innovation Team of Guangxi University of Chinese Medicine -- Clinical Medicine Research and Application Innovation Team of Zhuang Medicine Poison Disease (2022A003), the Guipai Xinglin Young Talents of Guangxi University of Chinese Medicine (2022C035), and the Qihuang Scholar Cultivation Program of Guangxi (YP).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1051082/full#supplementary-material
References
1. Lin YJ, Anzaghe M, Schülke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells (2020) 9(4):880. doi: 10.3390/cells9040880
2. Myasoedova E, Davis J, Matteson EL, Crowson CS. Is the epidemiology of rheumatoid arthritis changing? results from a population-based incidence study, 1985-2014. Ann Rheum Dis (2020) 79(4):440–4. doi: 10.1136/annrheumdis-2019-216694
3. Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol (2020) 16(6):316–33. doi: 10.1038/s41584-020-0413-5
4. Hu XX, Wu YJ, Zhang J, Wei W. T-Cells interact with b cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. Int Immunopharmacol (2019) 70:428–34. doi: 10.1016/j.intimp.2019.03.008
5. Yoshitomi H. Regulation of immune responses and chronic inflammation by fibroblast-like synoviocytes. Front Immunol (2019) 10:1395. doi: 10.3389/fimmu.2019.01395
6. Hu Q, Ecker M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int J Mol Sci (2021) 22(4):1742. doi: 10.3390/ijms22041742
7. Xia ZB, Meng FR, Fang YX, Wu X, Zhang CW, Liu Y, et al. Inhibition of NF-κB signaling pathway induces apoptosis and suppresses proliferation and angiogenesis of human fibroblast-like synovial cells in rheumatoid arthritis. Med (Baltimore) (2018) 97(23):e10920. doi: 10.1097/MD.0000000000010920
8. Køster D, Egedal JH, Lomholt S, Hvid M, Jakobsen MR, Müller-Ladner U, et al. Phenotypic and functional characterization of synovial fluid-derived fibroblast-like synoviocytes in rheumatoid arthritis. Sci Rep (2021) 11(1):22168. doi: 10.1038/s41598-021-01692-7
9. Conforti A, Di Cola I, Pavlych V, Ruscitti P, Berardicurti O, Ursini F, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev (2021) 20(2):102735. doi: 10.1016/j.autrev.2020.102735
10. Juarez M, Bang H, Hammar F, Reimer U, Dyke B, Sahbudin I, et al. Identification of novel antiacetylated vimentin antibodies in patients with early inflammatory arthritis. Ann Rheum Dis (2016) 75(6):1099–107. doi: 10.1136/annrheumdis-2014-206785
11. Darrah E, Andrade F. Rheumatoid arthritis and citrullination. Curr Opin Rheumatol (2018) 30(1):72–8. doi: 10.1097/BOR.0000000000000452
12. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet (2010) 376(9746):1094–108. doi: 10.1016/S0140-6736(10)60826-4
13. Derksen VFAM, Huizinga TWJ, van der Woude D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol (2017) 39(4):437–46. doi: 10.1007/s00281-017-0627-z
14. Giles JT. Extra-articular manifestations and comorbidity in rheumatoid arthritis: Potential impact of pre-rheumatoid arthritis prevention. Clin Ther (2019) 41(7):1246–55. doi: 10.1016/j.clinthera.2019.04.018
15. Taylor PC, Atzeni F, Balsa A, Gossec L, Müller-Ladner U, Pope J. The key comorbidities in patients with rheumatoid arthritis: A narrative review. J Clin Med (2021) 10(3):509. doi: 10.3390/jcm10030509
16. Kronzer VL, Crowson CS, Sparks JA, Myasoedova E, Davis JM 3rd. Comorbidities as risk factors for rheumatoid arthritis and their accrual after diagnosis. Mayo Clin Proc (2019) 94(12):2488–98. doi: 10.1016/j.mayocp.2019.08.010
17. Nagy G, Roodenrijs NMT, Welsing PMJ, Kedves M, Hamar A, van der Goes MC, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis (2022) 81(1):20–33. doi: 10.1136/annrheumdis-2021-220973
18. Blum A, Adawi M. Rheumatoid arthritis (RA) and cardiovascular disease. Autoimmun Rev (2019) 18(7):679–90. doi: 10.1016/j.autrev.2019.05.005
19. Rezuş E, Macovei LA, Burlui AM, Cardoneanu A, Rezuş C. Ischemic heart disease and rheumatoid arthritis-two conditions, the same background. Life (Basel) (2021) 11(10):1042. doi: 10.3390/life11101042
20. Chen J, Norling LV, Cooper D. Cardiac dysfunction in rheumatoid arthritis: The role of inflammation. Cells (2021) 10(4):881. doi: 10.3390/cells10040881
21. Błyszczuk P, Szekanecz Z. Pathogenesis of ischaemic and non-ischaemic heart diseases in rheumatoid arthritis. RMD Open (2020) 6(1):e001032. doi: 10.1136/rmdopen-2019-001032
22. Schwartz DM, Burma AM, Kitakule MM, Luo Y, Mehta NN. T Cells in autoimmunity-associated cardiovascular diseases. Front Immunol (2020) 11:588776. doi: 10.3389/fimmu.2020.588776
23. Wu R, Gao W, Yao K, Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol (2019) 10:648. doi: 10.3389/fimmu.2019.00648
24. Amaya-Amaya J, Montoya-Sánchez L, Rojas-Villarraga A. Cardiovascular involvement in autoimmune diseases. BioMed Res Int (2014) 2014:367359. doi: 10.1155/2014/367359
25. Dijkshoorn B, Raadsen R, Nurmohamed MT. Cardiovascular disease risk in rheumatoid arthritis anno 2022. J Clin Med (2022) 11(10):2704. doi: 10.3390/jcm11102704
26. Hermans MPJ, van der Velden D, Montero Cabezas JM, Putter H, Huizinga TWJ, Kuiper J, et al. Long-term mortality in patients with ST-segment elevation myocardial infarction is associated with anti-citrullinated protein antibodies. Int J Cardiol (2017) 240:20–4. doi: 10.1016/j.ijcard.2017.04.046
27. Jamthikar AD, Gupta D, Puvvula A, Johri AM, Khanna NN, Saba L, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis using carotid ultrasound b-mode imaging. Rheumatol Int (2020) 40(12):1921–39. doi: 10.1007/s00296-020-04691-5
28. Castañeda S, González-Juanatey C, González-Gay MA. Sex and cardiovascular involvement in inflammatory joint diseases. Clin Rev Allergy Immunol (2019) 56(3):278–92. doi: 10.1007/s12016-017-8635-2
29. Maiuolo J, Muscoli C, Gliozzi M, Musolino V, Carresi C, Paone S, et al. Endothelial dysfunction and extra-articular neurological manifestations in rheumatoid arthritis. Biomolecules (2021) 11(1):81. doi: 10.3390/biom11010081
30. Makavos G, Varoudi M, Papangelopoulou K, Kapniari E, Plotas P, Ikonomidis I, et al. Echocardiography in autoimmune rheumatic diseases for diagnosis and prognosis of cardiovascular complications. Medicina (Kaunas) (2020) 56(9):445. doi: 10.3390/medicina56090445
31. Pascale V, Finelli R, Giannotti R, Coscioni E, Izzo R, Rozza F, et al. Cardiac eccentric remodeling in patients with rheumatoid arthritis. Sci Rep (2018) 8(1):5867. doi: 10.1038/s41598-018-24323-0
32. Patel KHK, Jones TN, Sattler S, Mason JC, Ng FS. Proarrhythmic electrophysiological and structural remodeling in rheumatoid arthritis. Am J Physiol Heart Circ Physiol (2020) 319(5):H1008–20. doi: 10.1152/ajpheart.00401.2020
33. Plastiras SC, Moutsopoulos HM. Arrhythmias and conduction disturbances in autoimmune rheumatic disorders. Arrhythm Electrophysiol Rev (2021) 10(1):17–25. doi: 10.15420/aer.2020.43
34. Cavalli G, Favalli EG. Cardiovascular disease in patients with rheumatoid arthritis: impact of classic and disease-specific risk factors. Ann Transl Med (2018) 6(Suppl 1):S82. doi: 10.21037/atm.2018.10.72
35. Agca R, Blanken AB, van Sijl AM, Smulders YM, Voskuyl AE, van der Laken C, et al. Arterial wall inflammation is increased in rheumatoid arthritis compared with osteoarthritis, as a marker of early atherosclerosis. Rheumatol (Oxford) (2021) 60(7):3360–8. doi: 10.1093/rheumatology/keaa789
36. Salem HR, Zahran ES. Vascular cell adhesion molecule-1 in rheumatoid arthritis patients: Relation to disease activity, oxidative stress, and systemic inflammation. Saudi Med J (2021) 42:620–8. doi: 10.15537/smj.2021.42.6.20200753
37. da Fonseca LJS, Nunes-Souza V, Goulart MOF, Rabelo LA. Oxidative stress in rheumatoid arthritis: what the future might hold regarding novel biomarkers and add-on therapies. Oxid Med Cell Longevity (2019) 2019(7):16. doi: 10.1155/2019/7536805.7536805
38. Wang X, Fan D, Cao X, Ye Q, Wang Q, Zhang M, et al. The role of reactive oxygen species in the rheumatoid arthritis-associated synovial microenvironment. Antioxid (Basel) (2022) 11(6):1153. doi: 10.3390/antiox11061153
39. Davies R, Williams J, Sime K, Jin HS, Thompson C, Jordan L, et al. The role of interleukin-6 trans-signalling on cardiovascular dysfunction in inflammatory arthritis. Rheumatology (2021) 60:2852–61. doi: 10.1093/rheumatology/keaa725
40. Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J (2018) 39(22):2063–9. doi: 10.1093/eurheartj/ehy128
41. Ferreira MB, Fonseca T, Costa R, Marinhoc A, Carvalho HC, Oliveira JC, et al. Prevalence, risk factors and proteomic bioprofiles associated with heart failure in rheumatoid arthritis: The RA-HF study. Eur J Intern Med (2021) 85:41–9. doi: 10.1016/j.ejim.2020.11.002
42. Patel RB, Shah SJ. Drug targets for heart failure with preserved ejection fraction: A mechanistic approach and review of contemporary clinical trials. Annu Rev Pharmacol Toxicol (2019) 59:41–63. doi: 10.1146/annurev-pharmtox-010818-021136
43. DeMizio DJ, Geraldino-Pardilla LB. Autoimmunity and inflammation link to cardiovascular disease risk in rheumatoid arthritis. Rheumatol Ther (2020) 7(1):19–33. doi: 10.1007/s40744-019-00189-0
44. Rane MA, Gitin A, Fiedler B, Fiedler L, Hennekens CH. Risks of cardiovascular disease and beyond in prescription of nonsteroidal anti-inflammatory drugs. J Cardiovasc Pharmacol Ther (2020) 25(1):3–6. doi: 10.1177/1074248419871902
45. Khan S, Andrews KL, Chin-Dusting JPF. Cyclo-oxygenase (COX) inhibitors and cardiovascular risk: Are non-steroidal anti-inflammatory drugs really anti-inflammatory? Int J Mol Sci (2019) 20(17):4262. doi: 10.3390/ijms20174262
46. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA (2006) 296(13):1633–44. doi: 10.1001/jama.296.13.jrv60011
47. Kerola AM, Rollefstad S, Semb AG. Atherosclerotic cardiovascular disease in rheumatoid arthritis: Impact of inflammation and antirheumatic treatment. Eur Cardiol (2021) 16:e18. doi: 10.15420/ecr.2020.44
48. Chhibber A, Hansen S, Biskupiak J. Statin use and mortality in rheumatoid arthritis: an incident user cohort study. J Manag Care Spec Pharm (2021) 27(3):296–305. doi: 10.18553/jmcp.2021.27.3.296
49. de Jong HJI, Cohen Tervaert JW, Lalmohamed A, de Vries F, Vandebriel RJ, van Loveren H, et al. Pattern of risks of rheumatoid arthritis among patients using statins: A cohort study with the clinical practice research datalink. PloS One (2018) 13(2):e0193297. doi: 10.1371/journal.pone.0193297
50. Myasoedova E, Karmacharya P, Duarte-Garcia A, Davis JM 3rd, Murad MH, Crowson CS. Effect of statin use on the risk of rheumatoid arthritis: A systematic review and meta-analysis. Semin Arthritis Rheumatol (2020) 50(6):1348–56. doi: 10.1016/j.semarthrit.2020.03.008
51. Friedman B, Cronstein B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine (2019) 86(3):301–7. doi: 10.1016/j.jbspin.2018.07.004
52. Johnson TM, Sayles HR, Baker JF, George MD, Roul P, Zheng C, et al. Investigating changes in disease activity as a mediator of cardiovascular risk reduction with methotrexate use in rheumatoid arthritis. Ann Rheum Dis (2021) 80(11):1385–92. doi: 10.1136/annrheumdis-2021-220125
53. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ (2018) 361:k1036. doi: 10.1136/bmj.k1036
54. Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med (2020) 12(8):e12476. doi: 10.15252/emmm.202012476
55. Dos Reis Neto ET, Kakehasi AM, de Medeiros Pinheiro M, Ferreira GA, Marques CDL, da Mota LMH, et al. Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv Rheumatol (2020) 60(1):32. doi: 10.1186/s42358-020-00134-8
56. Lo CH, Wei JC, Wang YH, Tsai CF, Chan KC, Li LC, et al. Hydroxychloroquine does not increase the risk of cardiac arrhythmia in common rheumatic diseases: A nationwide population-based cohort study. Front Immunol (2021) 12:631869. doi: 10.3389/fimmu.2021.631869
57. Rempenault C, Combe B, Barnetche T, Gaujoux-Viala C, Lukas C, Morel J, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis (2018) 77(1):98–103. doi: 10.1136/annrheumdis-2017-211836
58. Meyer PW, Anderson R, Ker JA, Ally MT. Rheumatoid arthritis and risk of cardiovascular disease. Cardiovasc J Afr (2018) 29(5):317–21. doi: 10.5830/CVJA-2018-018
59. Ntusi NAB, Francis JM, Sever E, Liu A, Piechnik SK, Ferreira VM, et al. Anti-TNF modulation reduces myocardial inflammation and improves cardiovascular function in systemic rheumatic diseases. Int J Cardiol (2018) 270:253–9. doi: 10.1016/j.ijcard.2018.06.099
60. Wang G, Xie X, Yuan L, Qiu J, Duan W, Xu B, et al. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. Biofactors (2020) 46(3):441–53. doi: 10.1002/biof.1599
61. Rubio-Ruiz ME, Guarner-Lans V, Cano-Martínez A, Díaz-Díaz E, Manzano-Pech L, Gamas-Magaña A, et al. Resveratrol and quercetin administration improves antioxidant DEFENSES and reduces fatty liver in metabolic syndrome rats. Molecules (2019) 24(7):1297. doi: 10.3390/molecules24071297
62. Lu J, Zheng Y, Yang J, Zhang J, Cao W, Chen X, et al. Resveratrol alleviates inflammatory injury and enhances the apoptosis of fibroblast−like synoviocytes via mitochondrial dysfunction and ER stress in rats with adjuvant arthritis. Mol Med Rep (2019) 20(1):463–72. doi: 10.3892/mmr.2019.10273
63. Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules (2021) 26(1):229. doi: 10.3390/molecules26010229
64. Lu Q, Li X, Liu J, Sun X, Rousselle T, Ren D, et al. AMPK is associated with the beneficial effects of antidiabetic agents on cardiovascular diseases. Biosci Rep (2019) 39:BSR20181995. doi: 10.1042/BSR20181995
65. Chen Y, Qiu F, Yu B, Chen Y, Zuo F, Zhu X, et al. Metformin, an AMPK activator, inhibits activation of FLSs but promotes HAPLN1 secretion. Mol Ther Methods Clin Dev (2020) 17:1202–14. doi: 10.1016/j.omtm.2020.05.008
66. Cook AD, Hamilton JA. Investigational therapies targeting the granulocyte macrophage colony-stimulating factor receptor-α in rheumatoid arthritis: focus on mavrilimumab. Ther Adv Musculoskelet Dis (2018) 10(2):29–38. doi: 10.1177/1759720X17752036
67. Wang N, Zhang Q, Jing X, Guo J, Huang H, Xu Z. The association between MUC5B mutations and clinical outcome in patients with rheumatoid arthritis-associated interstitial lung disease: A retrospective exploratory study in China. Med Sci Monit (2020) 26:e920137. doi: 10.12659/MSM.920137
68. Huang S, Kronzer VL, Dellaripa PF, Deane KD, Bolster MB, Nagaraja V, et al. Rheumatoid arthritis-associated interstitial lung disease: Current update on prevalence, risk factors, and pharmacologic treatment. Curr Treatm Opt Rheumatol (2020) 6(4):337–53. doi: 10.1007/s40674-020-00160-z
69. Yu KH, Chen HH, Cheng TT, Jan YJ, Weng MY, Lin YJ, et al. Consensus recommendations on managing the selected comorbidities including cardiovascular disease, osteoporosis, and interstitial lung disease in rheumatoid arthritis. Med (Baltimore) (2022) 101(1):e28501. doi: 10.1097/MD.0000000000028501
70. Florescu A, Gherghina FL, Muşetescu AE, Pădureanu V, Roşu A, Florescu MM, et al. Novel biomarkers, diagnostic and therapeutic approach in rheumatoid arthritis interstitial lung disease-a narrative review. Biomedicines (2022) 10(6):1367. doi: 10.3390/biomedicines10061367
71. Kronzer VL, Huang W, Dellaripa PF, Huang S, Feathers V, Lu B, et al. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J Rheumatol (2021) 48(5):656–63. doi: 10.3899/jrheum.200863
72. Jönsson E, Ljung L, Norrman E, Freyhult E, Ärlestig L, Dahlqvist J, et al. Pulmonary fibrosis in relation to genetic loci in an inception cohort of patients with early rheumatoid arthritis from northern Sweden. Rheumatol (Oxford) (2022) 61(3):943–52. doi: 10.1093/rheumatology/keab441
73. Lai NL, Jia W, Wang X, Luo J, Liu GY, Gao C, et al. Risk factors and changes of peripheral NK and T cells in pulmonary interstitial fibrosis of patients with rheumatoid arthritis. Can Respir J (2019) 2019:7262065. doi: 10.1155/2019/7262065
74. McDermott GC, Doyle TJ, Sparks JA. Interstitial lung disease throughout the rheumatoid arthritis disease course. Curr Opin Rheumatol (2021) 33(3):284–91. doi: 10.1097/BOR.0000000000000787
75. England BR, Hershberger D. Management issues in rheumatoid arthritis-associated interstitial lung disease. Curr Opin Rheumatol (2020) 32(3):255–63. doi: 10.1097/BOR.0000000000000703
76. Ekici M, Baytar Y, Kardas RC, Sari A, Akdogan A, Durhan G, et al. Predictors of mortality in rheumatoid arthritis-associated lung disease: A retrospective study on ten years. Joint Bone Spine (2021) 88(3):105133. doi: 10.1016/j.jbspin.2021.105133
77. Ebner L, Christodoulidis S, Stathopoulou T, Geiser T, Stalder O, Limacher A, et al. Meta-analysis of the radiological and clinical features of usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP). PloS One (2020) 15(1):e0226084. doi: 10.1371/journal.pone.0226084
78. Laria A, Lurati AM, Zizzo G, Zaccara E, Mazzocchi D, Re KA, et al. Interstitial lung disease in rheumatoid arthritis: A practical review. Front Med (Lausanne) (2022) 9:837133. doi: 10.3389/fmed.2022.837133
79. Liao KM, Lin CL, Shen TC. Rheumatoid arthritis increases the risk of pleural empyema. Open Med (Wars) (2020) 15(1):1012–8. doi: 10.1515/med-2020-0225
80. Kadura S, Raghu G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur Respir Rev (2021) 30(160):210011. doi: 10.1183/16000617.0011-2021
81. Yang JA, Lee JS, Park JK, Lee EB, Song YW, Lee EY. Clinical characteristics associated with occurrence and poor prognosis of interstitial lung disease in rheumatoid arthritis. Korean J Intern Med (2019) 34(2):434–41. doi: 10.3904/kjim.2016.349
82. Azam AT, Odeyinka O, Alhashimi R, Thoota S, Ashok T, Palyam V, et al. Rheumatoid arthritis and associated lung diseases: A comprehensive review. Cureus (2022) 14(2):e22367. doi: 10.7759/cureus.22367
83. Kiely P, Busby AD, Nikiphorou E, Sullivan K, Walsh DA, Creamer P, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open (2019) 9(5):e028466. doi: 10.1136/bmjopen-2018-028466
84. Bes C. Comprehensive review of current diagnostic and treatment approaches to interstitial lung disease associated with rheumatoid arthritis. Eur J Rheumatol (2018) 6(3):146–9. doi: 10.5152/eurjrheum.2019.19036
85. Kremer JM. Methotrexate pulmonary toxicity: Deep inspiration. Arthritis Rheumatol (2020) 72(12):1959–62. doi: 10.1002/art.41451
86. Holroyd CR, Seth R, Bukhari M, Malaviya A, Holmes C, Curtis E, et al. The British society for rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatol (Oxford) (2019) 58(2):e3–e42. doi: 10.1093/rheumatology/key208
87. Tardella M, Di Carlo M, Carotti M, Giovagnoni A, Salaffi F. Abatacept in rheumatoid arthritis-associated interstitial lung disease: short-term outcomes and predictors of progression. Clin Rheumatol (2021) 40(12):4861–7. doi: 10.1007/s10067-021-05854-w
88. Kurata I, Tsuboi H, Terasaki M, Shimizu M, Toko H, Honda F, et al. Effect of biological disease-modifying anti-rheumatic drugs on airway and interstitial lung disease in patients with rheumatoid arthritis. Intern Med (2019) 58(12):1703–12. doi: 10.2169/internalmedicine.2226-18
89. Cassone G, Manfredi A, Atzeni F, Venerito V, Vacchi C, Picerno V, et al. Safety of abatacept in Italian patients with rheumatoid arthritis and interstitial lung disease: A multicenter retrospective study. J Clin Med (2020) 9:E277. doi: 10.3390/jcm9010277
90. Vacchi C, Manfredi A, Cassone G, Erre GL, Salvarani C, Sebastiani M. Efficacy and safety of rituximab in the treatment of connective tissue disease-related interstitial lung disease. Drugs Context (2021) 10:2020–8–7. doi: 10.7573/dic.2020-8-7
91. Atienza-Mateo B, Remuzgo-Martínez S, Prieto-Peña D, Mora Cuesta VM, Iturbe-Fernández D, Llorca J, et al. Rituximab in the treatment of interstitial lung disease associated with autoimmune diseases: Experience from a single referral center and literature review. J Clin Med (2020) 9(10):3070. doi: 10.3390/jcm9103070
92. Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, et al. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years' experience at a single centre. Rheumatol (Oxford) (2017) 56(8):1348–57. doi: 10.1093/rheumatology/kex072
93. Bukhari M, Abernethy R, Deighton C, Ding T, Hyrich K, Lunt M, et al. BSR and BHPR standards, guidelines and audit working group. BSR and BHPR guidelines on the use of rituximab in rheumatoid arthritis. Rheumatol (Oxford) (2011) 50(12):2311–3. doi: 10.1093/rheumatology/ker106a
94. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med (2019) 381(18):1718–27. doi: 10.1056/NEJMoa1908681
95. Teng F, Peng JM, Wang Q, Tian XL, Huo Z, Weng L. Successful treatment with tocilizumab in a patient with rapidly progressive interstitial lung disease with positive anti-melanoma differentiation-associated gene-5 antibody. Chin Med J (2020) 134(8):999–1000. doi: 10.1097/CM9.0000000000001235
96. Manfredi A, Cassone G, Furini F, Gremese E, Venerito V, Atzeni F, et al. Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: a multicentre retrospective study. Intern Med J (2020) 50(9):1085–90. doi: 10.1111/imj.14670
97. Shao T, Shi X, Yang S, Zhang W, Li X, Shu J, et al. Interstitial lung disease in connective tissue disease: A common lesion with heterogeneous mechanisms and treatment considerations. Front Immunol (2021) 12:684699. doi: 10.3389/fimmu.2021.684699
98. Liang M, Matteson EL, Abril A, Distler JHW. The role of antifibrotics in the treatment of rheumatoid arthritis-associated interstitial lung disease. Ther Adv Musculoskelet Dis (2022) 14:1759720X221074457. doi: 10.1177/1759720X221074457
99. Dowman L, Hill CJ, May A, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev (2021) 2(2):CD006322. doi: 10.1002/14651858.CD006322.pub4
100. Hanada M, Kasawara KT, Mathur S, Rozenberg D, Kozu R, Hassan SA, et al. Aerobic and breathing exercises improve dyspnea, exercise capacity and quality of life in idiopathic pulmonary fibrosis patients: systematic review and meta-analysis. J Thorac Dis (2020) 12(3):1041–55. doi: 10.21037/jtd.2019.12.27
101. Kozu R, Shingai K, Hanada M, Oikawa M, Nagura H, Ito H, et al. Respiratory impairment, limited activity, and pulmonary rehabilitation in patients with interstitial lung disease. Phys Ther Res (2021) 24(1):9–16. doi: 10.1298/ptr.R0012
102. Ora J, Coppola A, Perduno A, Manzetti GM, Puxeddu E, Rogliani P. Acute effect of oxygen therapy on exercise tolerance and dyspnea perception in ILD patients. Monaldi Arch Chest Dis (2021) 92(2). doi: 10.4081/monaldi.2021.1925
103. Raimundo K, Solomon JJ, Olson AL, Kong AM, Cole AL, Fischer A, et al. Rheumatoid arthritis-interstitial lung disease in the united states: Prevalence, incidence, and healthcare costs and mortality. J Rheumatol (2019) 46(4):360–9. doi: 10.3899/jrheum.171315
104. Yang X, Wei D, Liu M, Wu B, Zhang J, Xu H, et al. Survival and outcomes after lung transplantation for connective tissue disease-associated interstitial lung diseases. Clin Rheumatol (2021) 40(9):3789–95. doi: 10.1007/s10067-021-05704-9
105. Saito S, Alkhatib A, Kolls JK, Kondoh Y, Lasky JA. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac Dis (2019) 11(Suppl 14):S1740–54. doi: 10.21037/jtd.2019.04.62
106. Cioffi G, Viapiana O, Tarantini L, Orsolini G, Idolazzi L, Sonographer FO, et al. Clinical profile and outcome of patients with chronic inflammatory arthritis and metabolic syndrome. Intern Emerg Med (2021) 16(4):863–74. doi: 10.1007/s11739-020-02520-y
107. Cai W, Tang X, Pang M. Prevalence of metabolic syndrome in patients with rheumatoid arthritis: An updated systematic review and meta-analysis. Front Med (Lausanne) (2022) 9:855141. doi: 10.3389/fmed.2022.855141
108. Bhattacharya PK, Barman B, Jamil M, Bora K. Metabolic syndrome and atherogenic indices in rheumatoid arthritis and their relationship with disease activity: A hospital-based study from northeast India. J Transl Int Med (2020) 8(2):99–105. doi: 10.2478/jtim-2020-0015
109. Guin A, Sinhamahapatra P, Misra S, Choudhury Mazumder SR, Chatterjee S, Ghosh A. Incidence and effect of insulin resistance on progression of atherosclerosis in rheumatoid arthritis patients of long disease duration. BioMed J (2019) 42(6):394–402. doi: 10.1016/j.bj.2019.01.007
110. Verma AK, Bhatt D, Goyal Y, Dev K, Beg MMA, Alsahli MA, et al. Association of rheumatoid arthritis with diabetic comorbidity: Correlating accelerated insulin resistance to inflammatory responses in patients. J Multidiscip Healthc (2021) 14:809–20. doi: 10.2147/JMDH.S285469
111. Manrique-Arija S, Mena-Vazquez N, Ureña I, Rioja J, Valdivielso P, Ginel-Mendoza L, et al. Cumulative inflammatory burden and obesity as determinants of insulin resistance in patients with established rheumatoid arthritis: cross-sectional study. BMJ Open (2021) 11(2):e044749. doi: 10.1136/bmjopen-2020-044749
112. Kochumon S, Al Madhoun A, Al-Rashed F, Thomas R, Sindhu S, Al-Ozairi E, et al. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci Rep (2020) 10(1):16364. doi: 10.1038/s41598-020-73347-y
113. Francisco V, Ruiz-Fernández C, Pino J, Mera A, González-Gay MA, Gómez R, et al. Adipokines: Linking metabolic syndrome, the immune system, and arthritic diseases. Biochem Pharmacol (2019) 165:196–206. doi: 10.1016/j.bcp.2019.03.030
114. Neumann E, Hasseli R, Ohl S, Lange U, Frommer KW, Müller-Ladner U. Adipokines and autoimmunity in inflammatory arthritis. Cells (2021) 10(2):216. doi: 10.3390/cells10020216
115. Turgunova LG, Shalygina AA, Zalkalns JP, Klyuyev DA, Akhmaltdinova LL, Dosmagambetova RS. Assessment of adipokines, CXCL16 chemokine levels in patients with rheumatoid arthritis combined with metabolic syndrome. Clin Med Insights Arthritis Musculoskelet Disord (2021) 14:1179544120985860. doi: 10.1177/1179544120985860
116. Lillegraven S, Greenberg JD, Reed GW, Saunders K, Curtis JR, Harrold L, et al. Immunosuppressive treatment and the risk of diabetes in rheumatoid arthritis. PloS One (2019) 14(1):e0210459. doi: 10.1371/journal.pone.0210459
117. Wang CR, Tsai HW. Anti- and non-tumor necrosis factor-α-targeted therapies effects on insulin resistance in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. World J Diabetes (2021) 12(3):238–60. doi: 10.4239/wjd.v12.i3.238
118. Virone A, Bastard JP, Fellahi S, Capeau J, Rouanet S, Sibilia J, et al. Comparative effect of tumour necrosis factor inhibitors versus other biological agents on cardiovascular risk-associated biomarkers in patients with rheumatoid arthritis. RMD Open (2019) 5(2):e000897. doi: 10.1136/rmdopen-2019-000897
119. Favalli EG. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: A comprehensive review of IL-6 inhibition for the management of rheumatoid arthritis. Rheumatol Ther (2020) 7(3):473–516. doi: 10.1007/s40744-020-00219-2
120. Bako HY, Ibrahim MA, Isah MS, Ibrahim S. Inhibition of JAK-STAT and NF-κB signalling systems could be a novel therapeutic target against insulin resistance and type 2 diabetes. Life Sci (2019) 239:117045. doi: 10.1016/j.lfs.2019.117045
121. Hosseini A, Gharibi T, Marofi F, Javadian M, Babaloo Z, Baradaran B. Janus kinase inhibitors: A therapeutic strategy for cancer and autoimmune diseases. J Cell Physiol (2020) 235(9):5903–24. doi: 10.1002/jcp.29593
122. Cohen SB, Greenberg JD, Harnett J, Madsen A, Smith TW, Gruben D, et al. Real-world evidence to contextualize clinical trial results and inform regulatory decisions: Tofacitinib modified-release once-daily vs immediate-release twice-daily for rheumatoid arthritis. Adv Ther (2021) 38(1):226–48. doi: 10.1007/s12325-020-01501-z
123. Lillich FF, Imig JD, Proschak E. Multi-target approaches in metabolic syndrome. Front Pharmacol (2021) 11:554961. doi: 10.3389/fphar.2020.554961
124. Sobh MM, Abdalbary M, Elnagar S, Nagy E, Elshabrawy N, Abdelsalam M, et al. Secondary osteoporosis and metabolic bone diseases. J Clin Med (2022) 11(9):2382. doi: 10.3390/jcm11092382
125. Llorente I, García-Castañeda N, Valero C, González-Álvaro I, Castañeda S. Osteoporosis in rheumatoid arthritis: Dangerous liaisons. Front Med (Lausanne) (2020) 7:601618. doi: 10.3389/fmed.2020.601618
126. Yan X, Xu Z, Li S, Yan L, Lyu G, Wang Z. Establishment and verification of an osteoporosis risk model in patients with rheumatoid arthritis: a valuable new model. Arch Osteoporos (2021) 16(1):3. doi: 10.1007/s11657-020-00867-5
127. Lindner L, Callhoff J, Alten R, Krause A, Ochs W, Zink A, et al. Osteoporosis in patients with rheumatoid arthritis: trends in the German national database 2007-2017. Rheumatol Int (2020) 40(12):2005–12. doi: 10.1007/s00296-020-04593-6
128. Fang Q, Zhou C, Nandakumar KS. Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediators Inflamm (2020) 2020:3830212. doi: 10.1155/2020/3830212
129. Steffen U, Schett G, Bozec A. How autoantibodies regulate osteoclast induced bone loss in rheumatoid arthritis. Front Immunol (2019) 10:1483. doi: 10.3389/fimmu.2019.01483
130. Bemis EA, Norris JM, Seifert J, Frazer-Abel A, Okamoto Y, Feser ML, et al. Complement and its environmental determinants in the progression of human rheumatoid arthritis. Mol Immunol (2019) 112:256–65. doi: 10.1016/j.molimm.2019.05.012
131. Sun M, Rethi B, Krishnamurthy A, Joshua V, Circiumaru A, Hensvold AH, et al. Anticitrullinated protein antibodies facilitate migration of synovial tissue-derived fibroblasts. Ann Rheum Dis (2019) 78(12):1621–31. doi: 10.1136/annrheumdis-2018-214967
132. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med (2011) 365(23):2205–19. doi: 10.1056/NEJMra1004965
133. Azuaga-Piñango AB, Peris P. Effect of antiresorptive and bone forming treatments in bone erosions in rheumatoid arthritis. Med Clin (Barc) (2020) 154(9):358–65. doi: 10.1016/j.medcli.2019.12.005
134. Ebina K, Hirao M, Hashimoto J, Hagihara K, Kashii M, Kitaguchi K, et al. Assessment of the effects of switching oral bisphosphonates to denosumab or daily teriparatide in patients with rheumatoid arthritis. J Bone Miner Metab (2018) 36(4):478–87. doi: 10.1007/s00774-017-0861-4
135. Langdahl BL, Silverman S, Fujiwara S, Saag K, Napoli N, Soen S, et al. Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone (2018) 116:58–66. doi: 10.1016/j.bone.2018.07.013
136. De Martinis M, Allegra A, Sirufo MM, Tonacci A, Pioggia G, Raggiunti M, et al. Vitamin d deficiency, osteoporosis and effect on autoimmune diseases and hematopoiesis: A review. Int J Mol Sci (2021) 22(16):8855. doi: 10.3390/ijms22168855
137. Fessler J, Husic R, Schwetz V, Lerchbaum E, Aberer F, Fasching P, et al. Senescent T-cells promote bone loss in rheumatoid arthritis. Front Immunol (2018) 9:95. doi: 10.3389/fimmu.2018.00095
138. Huang SY, Yoon SS, Shimizu K, Chng WJ, Chang CS, Wong RS, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, randomized controlled phase 3 study-Asian subgroup analysis. Adv Ther (2020) 37(7):3404–16. doi: 10.1007/s12325-020-01395-x
139. Lewiecki EM. New and emerging concepts in the use of denosumab for the treatment of osteoporosis. Ther Adv Musculoskelet Dis (2018) 10(11):209–23. doi: 10.1177/1759720X18805759
140. Takeuchi T, Tanaka Y, Soen S, Yamanaka H, Yoneda T, Tanaka S, et al. Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): a randomised, double-blind, placebo-controlled phase 3 trial. Ann Rheum Dis (2019) 78(7):899–907. doi: 10.1136/annrheumdis-2018-214827
141. Raterman HG, Lems WF. Pharmacological management of osteoporosis in rheumatoid arthritis patients: A review of the literature and practical guide. Drugs Aging (2019) 36(12):1061–72. doi: 10.1007/s40266-019-00714-4
142. George MD, Baker JF, Ogdie A. Comparative persistence of methotrexate and tumor necrosis factor inhibitors in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol (2020) 47(6):826–34. doi: 10.3899/jrheum.190299
143. Yao Z, Getting SJ, Locke IC. Regulation of TNF-induced osteoclast differentiation. Cells (2021) 11(1):132. doi: 10.3390/cells11010132
144. Jura-Półtorak A, Szeremeta A, Olczyk K, Zoń-Giebel A, Komosińska-Vassev K. Bone metabolism and RANKL/OPG ratio in rheumatoid arthritis women treated with TNF-α inhibitors. J Clin Med (2021) 10(13):2905. doi: 10.3390/jcm10132905
145. Maeda K, Yoshida K, Nishizawa T, Otani K, Yamashita Y, Okabe H, et al. Inflammation and bone metabolism in rheumatoid arthritis: Molecular mechanisms of joint destruction and pharmacological treatments. Int J Mol Sci (2022) 23(5):2871. doi: 10.3390/ijms23052871
146. Chen MH, Yu SF, Chen JF, Chen WS, Liou TL, Chou CT, et al. Different effects of biologics on systemic bone loss protection in rheumatoid arthritis: An interim analysis of a three-year longitudinal cohort study. Front Immunol (2021) 12:783030. doi: 10.3389/fimmu.2021.783030
147. Sanpaolo ER, Rotondo C, Cici D, Corrado A, Cantatore FP. JAK/STAT pathway and molecular mechanism in bone remodeling. Mol Biol Rep (2020) 47(11):9087–96. doi: 10.1007/s11033-020-05910-9
148. Yokota K, Sato K, Miyazaki T, Aizaki Y, Tanaka S, Sekikawa M, et al. Characterization and function of tumor necrosis factor and interleukin-6-Induced osteoclasts in rheumatoid arthritis. Arthritis Rheumatol (2021) 73(7):1145–54. doi: 10.1002/art.41666
149. Adam S, Simon N, Steffen U, Andes FT, Scholtysek C, Müller DIH, et al. JAK inhibition increases bone mass in steady-state conditions and ameliorates pathological bone loss by stimulating osteoblast function. Sci Transl Med (2020) 12(530):eaay4447. doi: 10.1126/scitranslmed.aay4447
150. Gupta A, Abrahimi A, Patel A. Felty syndrome: a case report. J Med Case Rep (2021) 15(1):273. doi: 10.1186/s13256-021-02802-9
151. Hoshina Y, Teaupa S, Chang D. Infective endocarditis-like presentation of felty syndrome: A case report. Cureus (2021) 13(12):e20713. doi: 10.7759/cureus.20713
152. Ruffer N, Tomas NM, Schmiedel S, Jordan S, Kötter I. Imitation eines felty-syndroms durch eine viszerale leishmaniasis bei rheumatoider arthritis unter therapie mit methotrexat und etanercept [Visceral leishmaniasis mimicking felty's syndrome in rheumatoid arthritis treated with methotrexate and etanercept]. Z Rheumatol (2022) 81(3):240–3. doi: 10.1007/s00393-021-01105-0
153. Wu P, Sun W, Li J. Rheumatoid arthritis patients with peripheral blood cell reduction should be evaluated for latent felty syndrome: A case report. Med (Baltimore) (2020) 99(51):e23608. doi: 10.1097/MD.0000000000023608
154. Serrano Santiago VE, Morgan Z. The diagnosis felt(y) right: A case report of felty syndrome with limited articular involvement. Cureus (2022) 14(4):e24593. doi: 10.7759/cureus.24593
155. Savola P, Brück O, Olson T, Kelkka T, Kauppi MJ, Kovanen PE, et al. Somatic STAT3 mutations in felty syndrome: an implication for a common pathogenesis with large granular lymphocyte leukemia. Haematologica (2018) 103(2):304–12. doi: 10.3324/haematol.2017.175729
156. Rodrigues L, da Silva GN, de Lacerda AP. Felty's syndrome - a rare case of febrile neutropenia. Arch Clin Cases (2021) 6(2):48–52. doi: 10.22551/2019.23.0602.10153
157. Patel R, Akhondi H. Felty syndrome. 2022 jul 4. In: StatPearls. Treasure Island (FL: StatPearls Publishing (2022).
158. Yazıcı A, Uçar A, Mehtap Ö, Gönüllü EÖ, Tamer A. Presentation of three cases followed up with a diagnosis of felty syndrome. Eur J Rheumatol (2014) 1(3):120–2. doi: 10.5152/eurjrheumatol.2014.026
159. Kimura Y, Yoshida S. Successful abatacept treatment for felty's syndrome in a patient with rheumatoid arthritis. Mod Rheumatol Case Rep (2020) 4(2):168–70. doi: 10.1080/24725625.2020.1717740
160. Gorodetskiy VR, Sidorova YV, Kupryshina NA, Vasilyev VI, Probatova NA, Ryzhikova NV, et al. Analysis of a single-institution cohort of patients with felty's syndrome and T-cell large granular lymphocytic leukemia in the setting of rheumatoid arthritis. Rheumatol Int (2021) 41(1):147–56. doi: 10.1007/s00296-020-04757-4
161. Wang CR, Chiu YC, Chen YC. Successful treatment of refractory neutropenia in felty's syndrome with rituximab. Scand J Rheumatol (2018) 47(4):340–1. doi: 10.1080/03009742.2017.1334816
162. Li R, Wan Q, Chen P, Mao S, Wang Q, Li X, et al. Tocilizumab treatment in felty's syndrome. Rheumatol Int (2020) 40(7):1143–9. doi: 10.1007/s00296-020-04588-3
163. Austad C, Kvien TK, Olsen IC, Uhlig T. Sleep disturbance in patients with rheumatoid arthritis is related to fatigue, disease activity, and other patient-reported outcomes. Scand J Rheumatol (2017) 46(2):95–103. doi: 10.3109/03009742.2016.1168482
164. Abad VC, Sarinas PS, Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev (2008) 12(3):211–28. doi: 10.1016/j.smrv.2007.09.001
165. Guo G, Fu T, Yin R, Zhang L, Zhang Q, Xia Y, et al. Sleep quality in Chinese patients with rheumatoid arthritis: contributing factors and effects on health-related quality of life. Health Qual Life Outcomes (2016) 14(1):151. doi: 10.1186/s12955-016-0550-3
166. Sariyildiz MA, Batmaz I, Bozkurt M, Bez Y, Cetincakmak MG, Yazmalar L, et al. Sleep quality in rheumatoid arthritis: relationship between the disease severity, depression, functional status and the quality of life. J Clin Med Res (2014) 6(1):44–52. doi: 10.4021/jocmr1648w
167. Wells G, Li T, Tugwell P. Investigation into the impact of abatacept on sleep quality in patients with rheumatoid arthritis, and the validity of the MOS-sleep questionnaire sleep disturbance scale. Ann Rheum Dis (2010) 69(10):1768–73. doi: 10.1136/ard.2009.119727
168. Zamarrón C, Maceiras F, Mera A, Gómez-Reino JJ. Effect of the first infliximab infusion on sleep and alertness in patients with active rheumatoid arthritis. Ann Rheum Dis (2004) 63(1):88–90. doi: 10.1136/ard.2003.007831
169. Tektonidou MG, Katsifis G, Georgountzos A, Theodoridou A, Koukli EM, Kandili A, et al. Real-world evidence of the impact of adalimumab on work productivity and sleep measures in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Ther Adv Musculoskelet Dis (2020) 12:1759720X20949088. doi: 10.1177/1759720X20949088
Keywords: rheumatoid arthritis, complications, incidence, treatment, prospects
Citation: Wu D, Luo Y, Li T, Zhao X, Lv T, Fang G, Ou P, Li H, Luo X, Huang A and Pang Y (2022) Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front. Immunol. 13:1051082. doi: 10.3389/fimmu.2022.1051082
Received: 22 September 2022; Accepted: 09 December 2022;
Published: 22 December 2022.
Edited by:
Raphaela Goldbach-Mansky, National Institute of Allergy and Infectious Diseases (NIH), United StatesReviewed by:
Devis Benfaremo, Marche Polytechnic University, ItalyMaryam Masoumi, Qom University of Medical Sciences, Iran
Copyright © 2022 Wu, Luo, Li, Zhao, Lv, Fang, Ou, Li, Luo, Huang and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: An Huang, Mjk1NTMzOTg0OUBxcS5jb20=; Yuzhou Pang, cGFuZ3l6QGd4dGNtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Di Wu
Di Wu Yehao Luo2†
Yehao Luo2† Hongyi Li
Hongyi Li