- 1HLA Laboratory of Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 2Department of Hematology, Jiangsu Institute of Hematology, First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 3Collaborative Innovation Center of Hematology, Soochow University, Suzhou, Jiangsu, China
To examine the production time, type, and MFI of post-transplantation de novo HLA antibodies, and their effects on haplo-HSCT outcomes, we retrospectively included 116 patients who were negative for pre-existing HLA antibodies. In total, 322 serum samples from pre-transplantation to post-transplantation were dynamically tested by Luminex and single-antigen bead reagents. Patients were divided into: HLA antibody persistently negative group (group 1), the de novo HLA antibody transiently positive group (group 2), the de novo HLA antibody non-persistently positive group (group 3), and the de novo HLA antibody persistently positive group (group 4). Group 4 included DSA+non-DSA (NDSA) (group 4a) and NDSA (group 4b) groups. The detection rate of de novo HLA antibodies was 75.9% (88/116). The median MFI for de novo HLA antibodies was 2439 (1033-20162). The incidence of II–IV aGvHD was higher in group 2 than in group 1 (52.6% vs 17.9%, P < 0.01); in group 4a than in group 1 (87.5% vs 17.9%, P < 0.001); and in group 4a than in group 4b (87.5% vs 40.0%, P = 0.001). The DFS (37.5% vs 85.7%, P < 0.01) and OS (37.5% vs 85.7%, P < 0.01) of group 4a were lower than those of group 1. The DFS (48.0% vs 85.7%, P < 0.01) and OS (56.0% vs 85.7%, P = 0.03) of group 4b were lower than those of group 1. Multivariate analysis showed that de novo HLA antibody being transiently positive (HR: 5.30; 95% CI: 1.71–16.42, P = 0.01) and persistently positive (HR: 5.67; 95% CI: 2.00–16.08, P < 0.01) were both associated with a higher incidence of II–IV aGvHD. Persistently positive de novo HLA antibodies were a risk factor for reduced DFS (HR: 6.57; 95% CI: 2.08–20.70, P < 0.01) and OS (HR: 5.51; 95% CI: 1.73–17.53, P < 0.01). DSA and NDSA can be detected since 15 days after haplo-HSCT in patients without pre-existing HLA antibodies, and affect aGvHD, DFS, and OS. Haplo-HSCT patients must be monitored for HLA antibodies changes for appropriate preventive clinical management, and we recommend that 1-month post-transplantation is the best test time point.
1 Introduction
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is an effective treatment for hematologic malignancy patients (1–4). The 2018 guidelines published by the European Society for Blood and Marrow Transplantation included human leukocyte antigen (HLA) antibody testing as routine for haplo-HSCT patients (5). Donor-specific HLA antibodies (DSA) are defined as HLA antibodies in the serum of patients that target donor HLA antigens, and the donor HLA antigens are mismatched with those of the patients. Previous studies have shown that the presence of pre-existing HLA antibodies, particularly DSA, can mediate transplant failure, delay hematopoietic reconstitution, and result in poor graft function, thereby affecting patient outcomes (6–11). It is worth noting that de novo DSA can cause a rejection reaction through antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity in organ transplantation. Therefore, it is widely reported that DSA can mediate post-transplantation rejection reactions (12–14). In haplo-HSCT, the recipient tested negative for pre-existing HLA antibodies may produce de novo HLA antibodies after transplantation when at least one HLA haplotype is mismatched. However, there is no study in China or other countries on the effects of de novo HLA antibodies and their dynamic changes on haplo-HSCT outcomes.
In this study, we included patients who underwent haplo-HSCT and were negative for pre-existing HLA antibodies. All recipients underwent high-resolution typing of HLA alleles. All patients underwent at least one post-transplantation HLA antibody test. We adopted single-antigen bead reagents to detect DSA or NDSA and their MFI values and determined the specificity of de novo antibodies and their dynamic changes. We also analyzed their effects on hematopoietic reconstitution, acute graft-versus-host disease (aGvHD), recurrence, and survival outcomes in haplo-HSCT patients.
2 Materials and methods
2.1 Sample collection and test time points
In this study, we included 216 patients with hematologic malignancy who underwent HLA antibody testing before and after haplo-HSCT in the First Affiliated Hospital of Soochow University from 2016 to October 2020. In total, 100 patients were positive for pre-existing HLA antibodies and 116 patients were negative for pre-existing HLA antibodies. These 116 patients were included in this study, while the other 100 HLA antibody positive patients will be reported in another paper. The inclusion criteria include: 1) A hematologic malignancy, including acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplastic syndrome (MDS), and lymphoma; 2) A negative pre-transplantation HLA antibody test and at least 1 HLA antibody specificity test after transplantation. The post-transplantation test time points include 15 days, 1 month, 3 months, 6 months, and 1 year. A total of 322 serum samples were collected for testing. All enrolled patients were followed up until 30 January 2021.
The studies involving human participants were reviewed and approved by the Institutional Review Board at First Affiliated Hospital of Soochow University. Written informed consent was obtained from the participants and minors’ legal guardian for participation in this study and for the publication of any potentially identifiable images or data included in this article.
2.2 Detection of and judgment standards for HLA antibodies
The collected serum samples were first examined by mixed screening of HLA antibodies (MIX). When the results of mixed screening were positive, HLA-I- or II-specific antibody test was performed to determine the type of HLA antibodies (LABScreen@Single Antigen HLA Class I (LS1 A04) and HLA Class II (LS2A01), One Lambda) and the mean fluorescence intensity (MFI). HLA antibody reactivity was determined using the Luminex platform (Luminex, Canoga Park, CA).
The judgment standards for the MFI values of HLA antibodies were determined according to the standards of the HLA Laboratory of First Affiliated Hospital of Soochow University (15). HLA antibodies with an MFI above 1000 were considered positive. MFI was divided into different groups: between 1000 and 2000, between 2000 and 5000, between 5000 and 8000, and over 8000.
2.3 Definition of HLA antibody groups
Group 1 was the HLA antibody persistently negative group and included patients who were negative before transplantation and had at least one or two negative post-transplantation test results. Group 2 was the de novo HLA antibody transiently positive group: these patients were negative before transplantation, had 1 positive post-transplantation de novo HLA antibody test result, and had at least 1 single-antigen bead (SAB) test during follow-up to show that they converted to negative. Group 3 was the de novo HLA antibody non-persistently positive group: these patients were negative before transplantation, had 1 positive post-transplantation de novo HLA antibody test result, but did not have any HLA antibody test during follow-up to show whether they converted to negative or were persistently positive for HLA antibodies. Group 4 was the de novo HLA antibody persistently positive group: de novo HLA antibodies were detected in at least two follow-up tests and the last follow-up test result was still positive.
2.4 Condition regimen and graft-versus-host disease prophylaxis
All patients underwent myeloablative conditioning. Specifically, 104 patients received the Bu/Cy-based (busulfan/cyclophosphamide) regimen, with MECCNU (250 mg/m2) on day -10, Bu (3.2 mg/kg/day) on days −7 to −5, Ara-C (4 g/m2/day) on days −9 to −8, and CTX (1.8 g/m2/day) on days −4 to −3; 8 patients received TBI/Cy-based (total body irradiation) as the main pretreatment, with MECCNU (250 mg/m2) on day -8, Ara-C (4 g/m2/day) on days −6 to −5, CTX (1.8 g/m2/day) on days −4 to −3, and TBI (with a total dose of 8-8.5 Gy) on days -7 to -6. One patient was treated with a Bu+ATG regimen, one with a Bu+fludarabine (30 mg m2/day) regimen, one with a TBI+fludarabine regimen, and one with a TBI+Bu/Cy regimen.
GvHD prevention included continuous cyclosporine A infusion at 3 mg/kg/day over 24 h from day -10 until patients could switch to oral intake; and mycophenolate mofetil oral formulation at 15 mg/kg/12 h from day −9 to day +30, then gradually tapered until day +60; short-term methotrexate on days +1, +3, +6, and +11 at doses of 15, 10, 10, and 10 mg/kg/day, respectively; and anti-thymocyte globulin on days −5 to −2 at 2.5 mg/kg/day.
2.5 Observed indicators
Platelet reconstitution was defined as the first of 7 consecutive days of platelet counts >20×109/L without transfusion support. Graft rejection was defined as a failure to engraft neutrophils (absolute neutrophil count (ANC) ≤0.5 × 109/L) by day +28 for three consecutive days and the absence of donor hematopoiesis (6). Grades of aGvHD were assessed according to published consensus criteria (16, 17). Overall survival (OS) was defined as survival between transplantation and the end of follow-up or death due to any cause. Disease-free survival (DFS) was calculated from the day of transplantation until death, relapse, primary graft failure, or the last follow-up. Relapse was determined using standard hematological criteria. Non-relapse mortality (NRM) was defined as death from all causes other than relapse.
2.6 Statistical analysis
The nonparametric test (Mann-Whitney test) was employed to compare continuous variables, and the chi-square test (Fisher’s exact test) was utilized to compare categorical variables. Survival analyses were carried out using Kaplan-Meier methods, and curves were compared by the log-rank test using R version 4.0.5 software packages (R Development Core Team, Vienna, Austria). A competitive risk model was applied to calculate the cumulative incidence of GvHD, relapse and NRM. Death was considered a competitive risk for aGvHD, relapse and NRM. Multivariable analyses for aGvHD and relapse were performed using competing risks regression. OS and DFS were determined using the Cox proportional hazards model in SPSS version 25.0 (IBM, Armonk, New York, USA). All test results with two-sided p<0.05 were considered statistically significant.
3 Results
3.1 De novo HLA antibody test result grouping and clinical characteristics of 116 patients
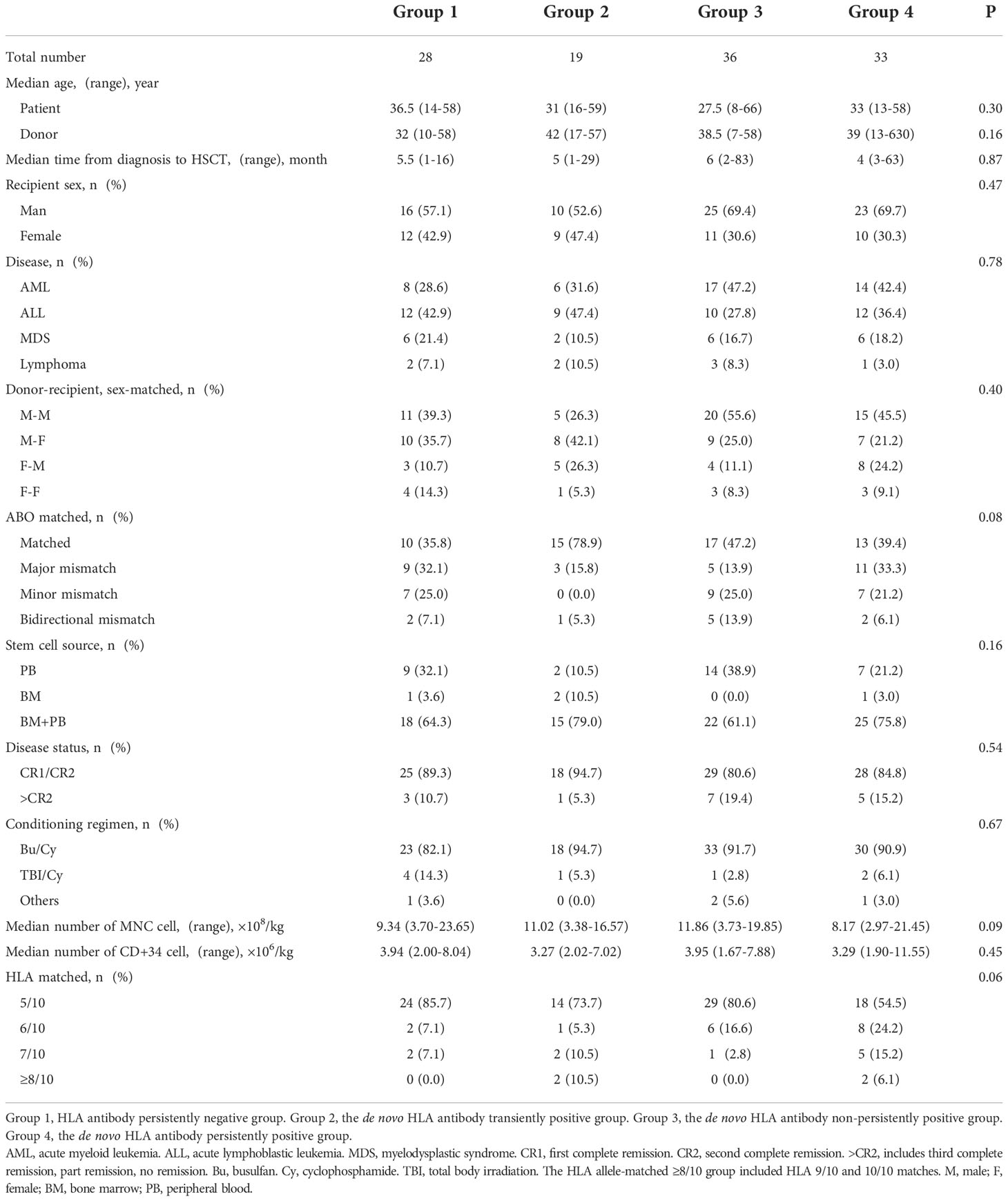
The 116 enrolled patients were classified based on test results into group 1 (n = 28), group 2 (n = 19), group 3 (n = 36), and group 4 (n = 33). There were no significant differences in the clinical characteristics (recipient age, gender, relationship, or diagnosis) of the four groups of patients and their donors (Table 1).
3.2 HLA antibody follow-up results
During the HLA antibody follow-up of 116 patients, 322 samples including 116 pre-transplantation samples, 12 samples at +15 days, 104 samples at 1 months, 74 samples at 3 months, 11 samples at 6 months, and 5 samples at 1 year. In total, 43, 59, 11, and 4 patients were tested once, twice, and three and four times after transplantation, respectively. Table 2 shows the number of samples and test results of different follow-up time points.
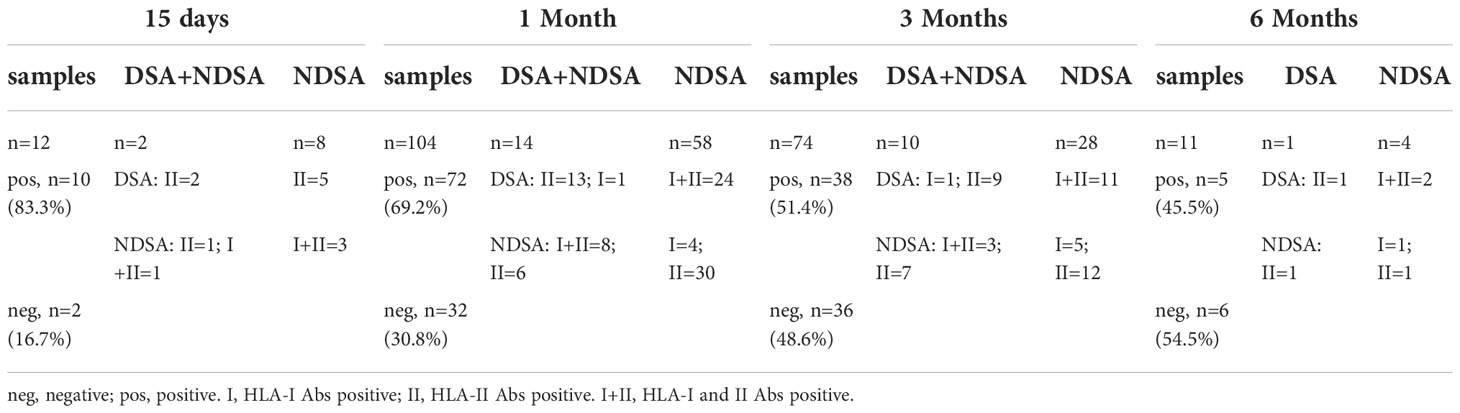
Table 2 Number of samples collected at different follow-up times after transplantation and test results.
3.3 Analysis of production time, type, and MFI level of de novo HLA antibodies
De novo HLA Abs were detected in 88 patients during follow-up and the detection rate was 75.9% (88/116). The median MFI for de novo HLA antibodies was 2439 (1033-20162). Antibodies were detected in 10, 68, 7, and 3 patients on 15 days, 1 month, 3 months, and 6 months after transplantation. Of these patients with de novo HLA antibodies, five patients were positive for HLA-class I antibodies, 48 were positive for HLA-class II antibodies, and 35 were positive for both HLA-class I and II antibodies. Out of all 40 patients with HLA-I antibodies, the detection rates for A, B, and C antibodies were 20.7% (24/116), 31.0% (36/116), and 28.4% (33/116), respectively. Of the 83 patients with HLA-II antibodies, the detection rates for DR, DQ, and DP antibodies were 59.5% (69/116), 56.0% (65/116), and 66.4% (70/116), respectively, and the quantity of de novo antibodies was the highest at 1 month. Figure 1A shows the types of de novo HLA antibodies produced at different follow-up time points. Figure 1B shows the specific types of de novo HLA antibodies at 1 month. The MFI level of de novo specific antibody types in 88 patients were calculated based on the first time when de novo HLA antibodies were detected and 38, 36, 7, and 7 patients had MFI levels of 1,000–2,000, 2,000–5,000, 5,000–8,000, and over 8000, respectively (Figure 1C).
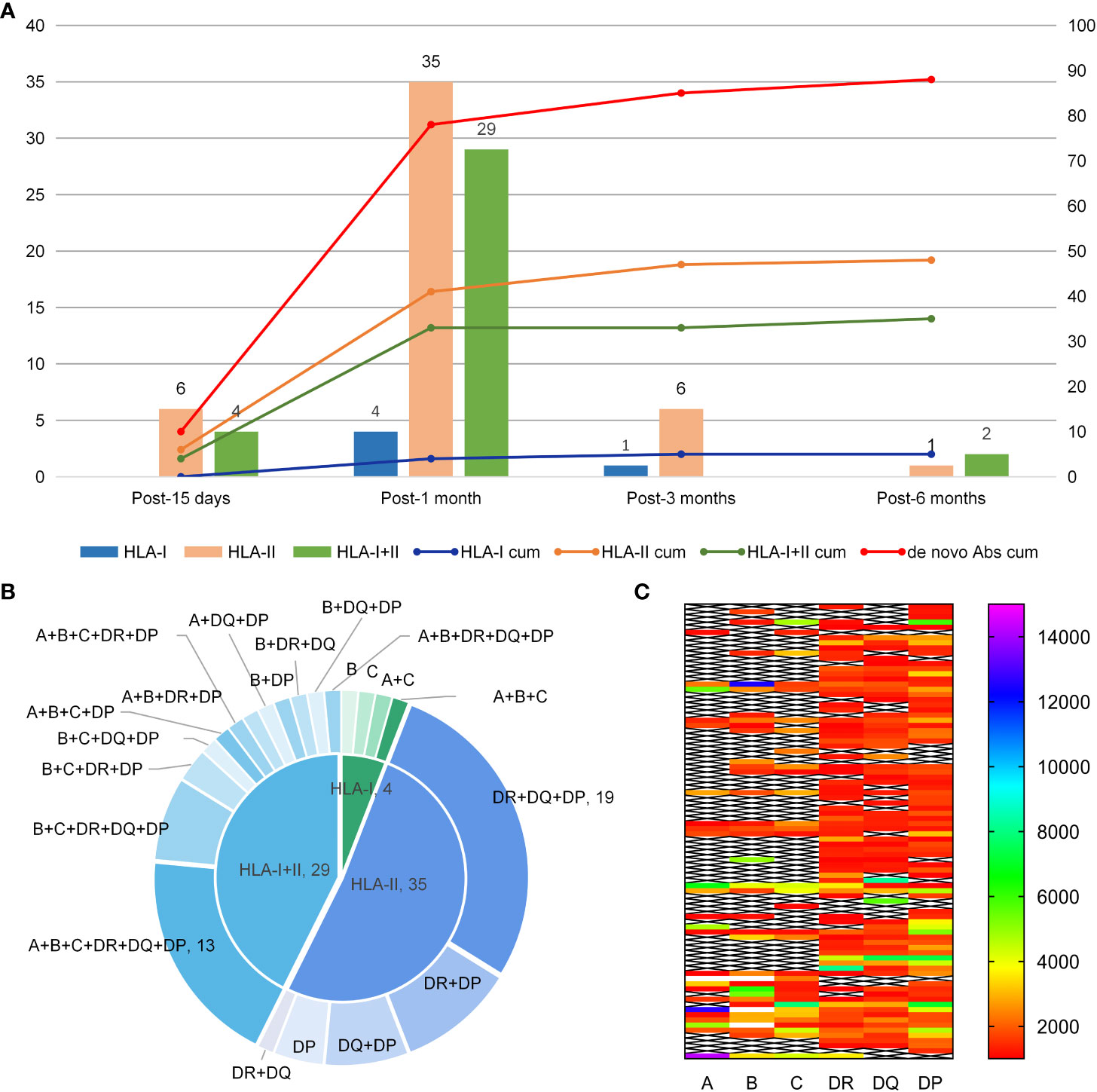
Figure 1 Production time, type, and MFI level of de novo HLA antibodies. (A) Type of de novo HLA antibodies detected at different times after haplo-HSCT. The Y-axis represents the quantity of de novo HLA antibodies. The bars correspond to the left Y-axis; the lines correspond to the right Y-axis and represent the cumulated quantity of antibodies; cum, reprensents accumulation. (B) Specificity of de novo antibodies detected 1 month after transplantation. (C) MFI level distribution of de novo antibodies in 88 patients.
3.4 Analysis of production time, type, and level of persistent de novo HLA antibodies
Out of 33 patients in group 4, de novo HLA antibodies were detected in 8, 24, and 1 patient at 15 days, 1 month, and 3 months after transplantation, respectively. The HLA genotyping results of the donor showed that 8 patients had de novo DSA + NDSA (Table 3, patients 1–8, group 4a) and 25 patients had de novo NDSA (Table 3, patients 9–23, group 4b). Table 3 shows the gender, age, and relationship of group 4 patients and their donors, diagnosis of patients, type of de novo antibodies, MFI level changes, and patient outcomes.
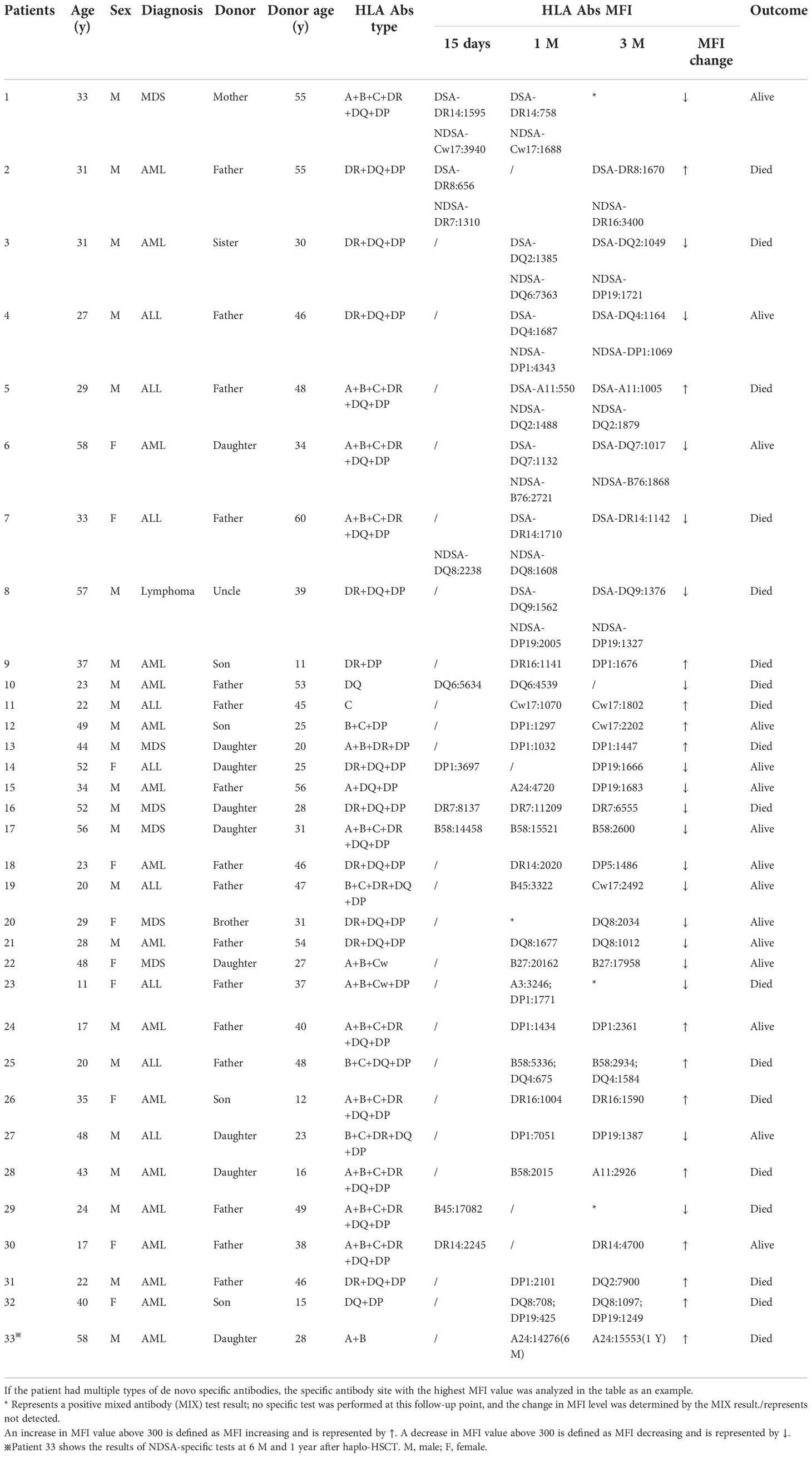
Table 3 Basic clinical information, antibody type and outcomes of patients with persistent de novo HLA antibodies after haplo-HSCT (n=33).
Of these patients with persistent de novo HLA antibodies, three patients were positive for HLA-class I antibodies, 13 were positive for HLA-class II antibodies, and 17 were positive for both HLA-class I and II antibodies. The distribution of the different specific antibody types detected in DR, DQ, and DP antibodies is shown in Figure 2.
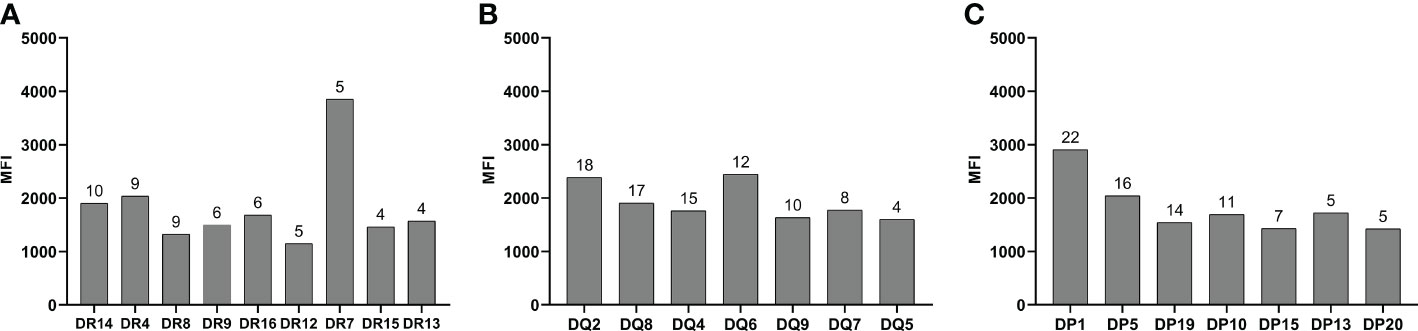
Figure 2 Type of HLA-DR, DQ, and DP specific antibodies. (A) DR antibody specificity type. (B) DQ antibody specificity type. (C) DR antibody specificity type. The numbers on the bars represent the number of patients positive for the specific antibodies.
3.5 Therapy and dynamic detection of persistent de novo HLA antibodies
Three of the patients with persistent de novo HLA antibodies received HLA antibody therapy. The first patient showed de novo HLA-II NDSA at 1-month post-transplantation with a maximum MFI value of 1114. This patient developed severe aGvHD at 1.8 months and was treated with plasmapheresis combined with rituximab at 2 months. SAB tests showed NDSA levels at 3 months with a maximum MFI of 1676. This patient had persistent low platelets and died at 14 months due to disease relapse.
In the second patient, HLA-II NDSA was detected at 15 days post-transplantation with a maximum MFI value of 8137. This patient had persistently low platelet counts, and the MFI level increased to 11209 at 1 month. After treatment with plasmapheresis combined with rituximab at 2 months, the platelet count gradually increased and the MFI decreased to 6555 at 3 months. This patient eventually died at 9 months due to multiple organ dysfunction syndrome.
In the third patient, HLA-I+II NDSA was detected at 15 days and 3 months post-transplantation, with the highest MFI values of 2245 and 4700, respectively. The patient developed low platelets and was treated with plasmapheresis combined with rituximab. The MFI decreased to 3821 at 6 months, but platelet count continued to be low. The patient survived to the follow-up endpoint.
4 Effects of de novo HLA antibody on haplo-HSCT outcomes
4.1 Hematopoietic reconstitution
4.1.1 Platelet reconstitution
The median platelet reconstitution time was 15 (10–36) days, 16 (10–98) days, 16 (10–70) days, and 14 (10–154) days in groups 1, 2, 3, and 4, respectively (P = 0.49). The median platelet reconstitution time was 12 (10–21) and 15 (10–154) days in group 4a and group 4b, respectively. There were no statistical differences between the two groups and group 1 (P=0.32).
4.1.2 Incidence of platelet transfusion dependence
Platelet transfusion dependence was observed in 33 patients and total incidence was 28.4% (33/116). The total incidence was 17.9% (5/28), 21.1% (4/19), 27.8% (10/36), and 42.4% (14/33) in groups 1, 2, 3, and 4, respectively (P = 0.16). However, the total incidence of group 4 was significantly higher than that of group 1 (P = 0.04).
4.1.3 Graft rejection
Only 1 out of 116 enrolled patients developed graft rejection. In this patient, neutrophil and platelet counts observed at 28 days after transplantation did not reach the standard for hematopoietic recovery, and short tandem repeat chimerism analysis showed neither the appearance nor complete loss of donor-derived neutrophils. The pre-transplantation and post-transplantation HLA antibody test results of this patient were both negative and grafts were successfully transplanted in other patients.
4.2 aGvHD
4.2.1 Incidence of aGvHD in the four groups
The cumulative incidence of grade II–IV aGvHD within 100 days in all enrolled patients was 17.9% (5/28), 52.6% (10/19), 27.8% (10/36), 51.5% (17/33), respectively, in groups 1, 2, 3, and 4 (P = 0.01, Figure 3A). The incidence of grade II–IV aGvHD in group 2 was significantly higher than in group 1 (HR: 4.47, 95% CI: 1.52–13.12; P < 0.01), and it was significantly higher in group 4 than in group 1 (HR: 3.30, 95% CI: 1.42–7.68; P = 0.01).
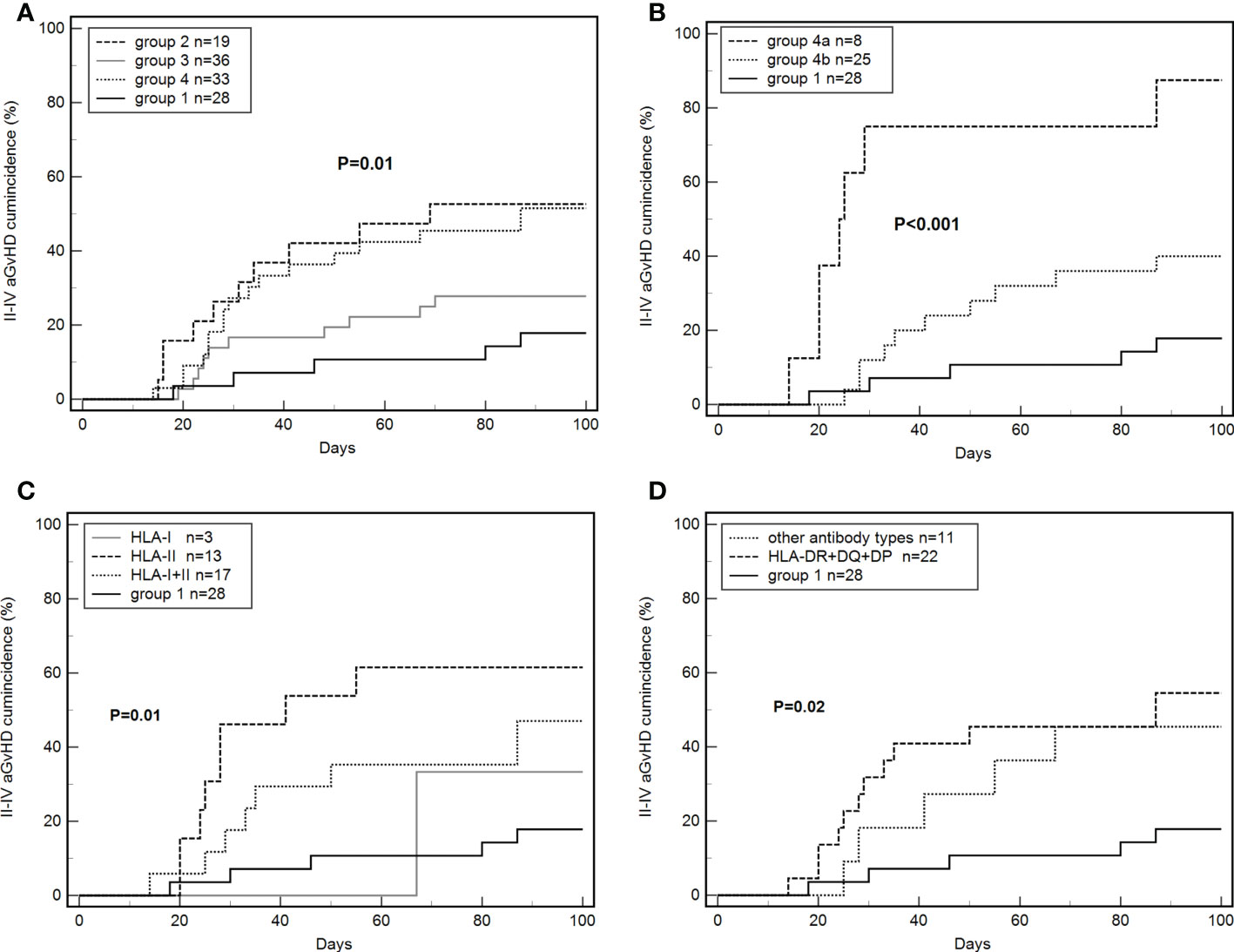
Figure 3 Effects of de novo antibodies on grade II–IV aGvHD. (A) De novo HLA antibodies in four groups. (B) Persistent de novo antibodies. (C) Different de novo HLA antibody types. (D) De novo HLA-DR+DQ+DP antibodies.
4.2.2 Effects of persistent de novo antibodies on aGvHD
4.2.2.1 De novo DSA+NDSA and NDSA
The incidence of grade II–IV aGvHD was 87.5% (7/8) and 40.0% (10/25) in groups 4a and 4b, respectively. The differences between groups 4a, 4b, and 1 were significant (P < 0.001, Figure 3B). The incidence of grade II–IV aGvHD in group 4a was significantly higher than in group 1 (87.5% vs 17.9%; HR: 60.44, 95% CI: 10.97–332.89; P < 0.001). Group 4b tended to have higher values than group 1 but the difference was not significant (40.0% vs 17.9%, P = 0.07). Group 4a was significantly higher than group 4b (87.5% vs 40.0%, HR: 12.64, 95% CI: 3.02–52.94; P = 0.001).
The cumulative incidence of grade III–IV aGvHD within 100 days in all of the enrolled patients was 17.24% (20/116). The incidence of grade III–IV aGvHD was 62.5% (5/8), 20.0% (5/25), and 7.1% (2/28) in groups 4a, 4b, and 1, respectively. Specifically, the incidence of grade III–IV aGvHD was significantly higher in group 4a than in group 1 (HR: 78.01, 95% CI: 9.84–618.27; P < 0.001) and group 4b (HR: 11.93, 95% CI: 2.2–64.65; P = 0.004).
4.2.2.2 MFI level changes
The 33 patients with persistent de novo antibodies were divided into the MFI increasing group (n=14) and MFI decreasing group (n=19) according to the results in Table 3. The incidence of grade II–IV aGvHD was 42.9% (6/14) and 57.9% (11/19) in the increasing and decreasing groups, respectively (P = 0.33).
4.2.2.3 HLA antibody type
The cumulative incidence of grade II–IV aGvHD was 33.3% (1/3), 61.5% (8/13), and 47.1% (8/17) in patients with de novo HLA-I, HLA-II, and HLA-I+II antibodies, respectively. The three groups had significant differences when compared with group 1 (P=0.01, Figure 3C).
A total of 22 patients were positive for HLA-DR+DQ+DP antibodies and 11 patients for other antibodies. The incidence of grade II–IV aGvHD was 54.6% (12/22) and 45.4% (5/11) in the two groups, with significant differences from group 1 (P=0.02, Figure 3D).
4.3 Relapse
By the end of the follow-up, 16 patients relapsed, and the relapse rate was 13.8%. The cumulative relapse rates of groups 1, 2, 3, and 4 were 10.7% (3/28), 0.0% (0/19), 13.9% (5/36), and 24.2% (8/33), respectively (P=0.11). The relapse rate of group 4 tended to be higher than that of group 1 but the difference was not significant (24.2% vs 10.7%, P = 0.13).
The cumulative relapse rates of groups 4a, 4b, and 1 were 12.5%, 28.0%, 10.7% (P = 0.22). The relapse rate of the MFI increasing group and MFI decreasing group was 35.7% (5/14) and 15.8% (3/19), respectively (P = 0.27).
4.4 NRM
The cumulative incidence of NRM was 10.7% (3/28), 26.3% (5/19), 16.7% (6/36), and 30.3% (10/33) in groups 1, 2, 3, and 4, respectively (P = 0.31). The incidence of NRM in group 4 tended to be higher than that in group 1 (P = 0.08).
The incidence of NRM was 50.0% (4/8), 24.0% (6/25), and 10.7% (3/28) in groups 4a, 4b, and 1, respectively (P = 0.04). The incidence of NRM was significantly higher in group 4a than in group 1 (HR: 4.40; 95% CI: 1.22–5.08; P = 0.01), and tended to be higher in group 4b than in group 1 (P = 0.25) and in group 4a than in group 4b (P = 0.11).
4.5 DFS and OS
4.5.1 DFS and OS of the four groups
By the end of the follow-up, 35 out of 116 patients died. The DFS of groups 1, 2, 3, and 4 were 85.7% (24/28), 73.7% (14/19), 69.4% (25/36), 45.5% (15/33), respectively (P = 0.02, Figure 4A). The total OS was 69.8%. The OS of groups 1, 2, 3, and 4 were 85.7% (24/28), 73.7% (14/19), 72.2% (26/36), 51.5% (17/33), respectively (P = 0.05, Figure 4B). The DFS (HR: 3.54, 95% CI: 1.53-8.17; P < 0.01) and OS (HR: 3.25; 95% CI: 1.35-7.81; P < 0.01) of group 4 were significantly lower than those of group 1. The DFS (P = 0.09) and OS (P = 0.13) of group 3 tended to be lower than those of group 1 but these differences were not significant. The DFS (33.3% vs. 80.7%; HR: 9.59, 95% CI: 4.37-21.06; P < 0.001) and OS (36.4% vs. 83.1%; HR: 10.02, 95% CI: 4.46-22.55; P < 0.001) of platelet-transfusion-dependent patients were significantly lower than those of patients who were not platelet transfusion dependent.
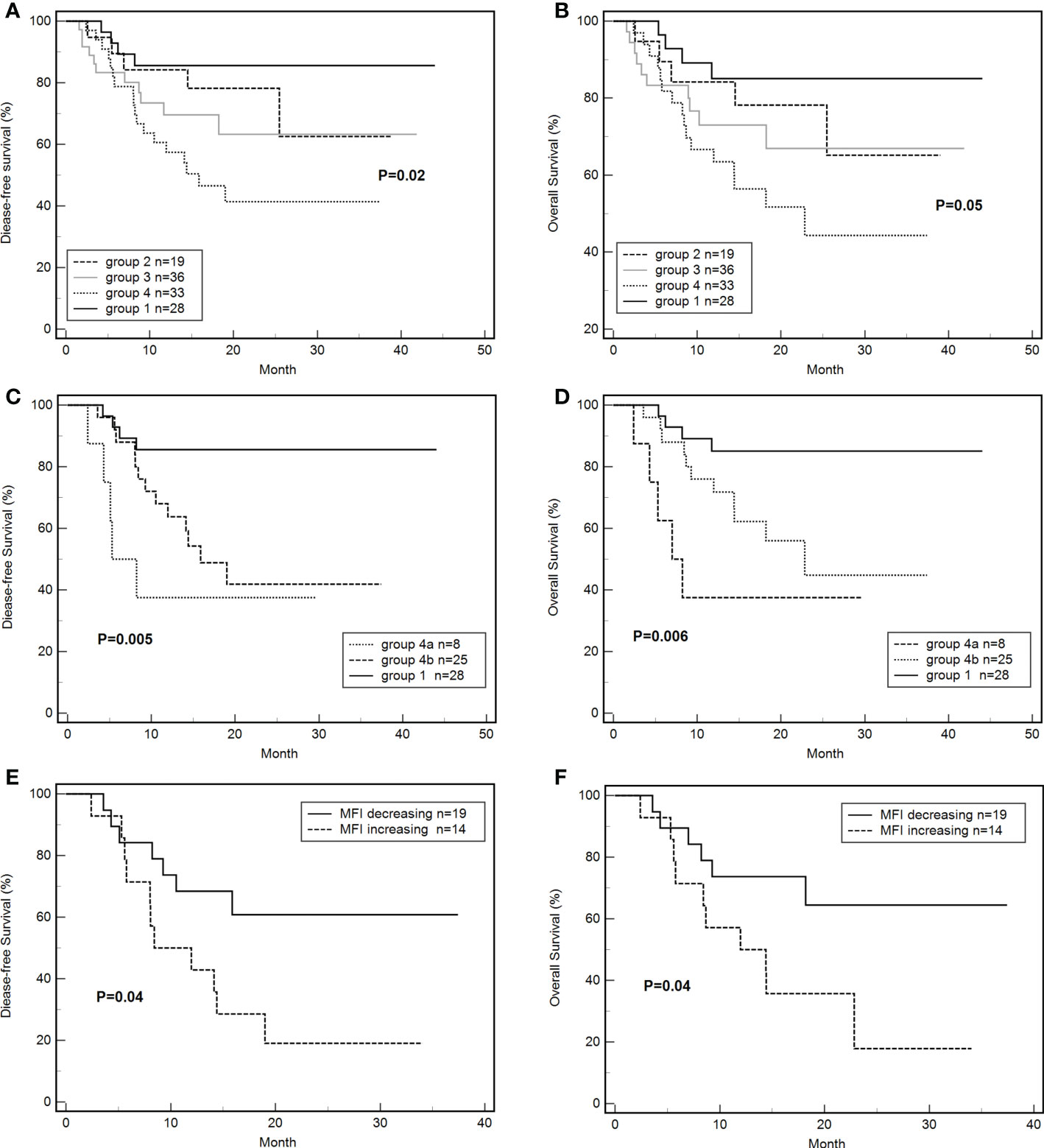
Figure 4 Effects of de novo HLA antibody, persistent de novo HLA antibody, and changes in MFI on DFS and OS. (A) Effects of de novo HLA antibodies in four groups on DFS. (B) Effects of de novo HLA antibodies in four groups on OS. (C) Effects of persistent de novo antibodies on DFS. (D) Effects of persistent de novo antibodies on OS. (E) Effects of MFI level changes of de novo antibodies on DFS. (F) Effects of MFI level changes of de novo antibodies on OS. Tables.
4.5.2 Effects of persistent de novo antibodies on DFS and OS
4.5.2.1 De novo DSA + NDSA and NDSA
The differences in DFS (37.5% vs 48.0% vs 85.7%, P < 0.01, Figure 4C) and OS (37.5% vs 56.0% vs 85.7%, P < 0.01, Figure 4D) of groups 4a, 4b, and 1 were significant. The DFS (HR: 15.85, 95% CI: 2.76-91.02; P < 0.01) and OS (HR: 17.58; 95% CI: 2.30-103.06; P < 0.01) of group 4a were significantly lower than those of group 1. The DFS (HR: 3.59, 95% CI: 1.38-9.37; P < 0.01) and OS (HR: 3.11; 95% CI: 1.12-8.62; P = 0.03) of group 4b were significantly lower than those of group 1. The differences of DFS (P =0.27) and OS (P=0.17) between group 4a and 4b were not significant.
4.5.2.2 MFI level changes
The DFS of the MFI increasing group was 21.4% (3/14), which was significantly lower than that of the MFI decreasing group (63.2%,12/19) (HR: 2.77, 95% CI: 1.07-7.22; P = 0.04, Figure 4E). Similarly, the OS of the MFI increasing group was 28.6% (3/14), which was significantly lower than that of the MFI decreasing group (68.4%, 13/19) (HR: 2.81, 95% CI: 1.03-7.75; P = 0.04, Figure 4F).
4.6 Multivariate analysis
Multivariate analysis showed that de novo HLA antibody being transiently positive (HR: 5.30; 95% CI: 1.71–16.42, P = 0.01) and persistently positive (HR: 5.67; 95% CI: 2.00–16.08, P < 0.01) were both associated with a higher cumulative incidence of II–IV aGvHD. Donor-recipient HLA compatibility greater than 5/10 was a protective factor for grade II–IV aGvHD (HR: 0.34; 95% CI: 0.14-0.80, P = 0.01). Persistently positive de novo HLA antibodies were a risk factor for reduced DFS (HR: 6.57; 95% CI: 2.08–20.70, P < 0.01) and OS (HR: 5.51; 95% CI: 1.73–17.53, P < 0.01) in patients (Table 4).
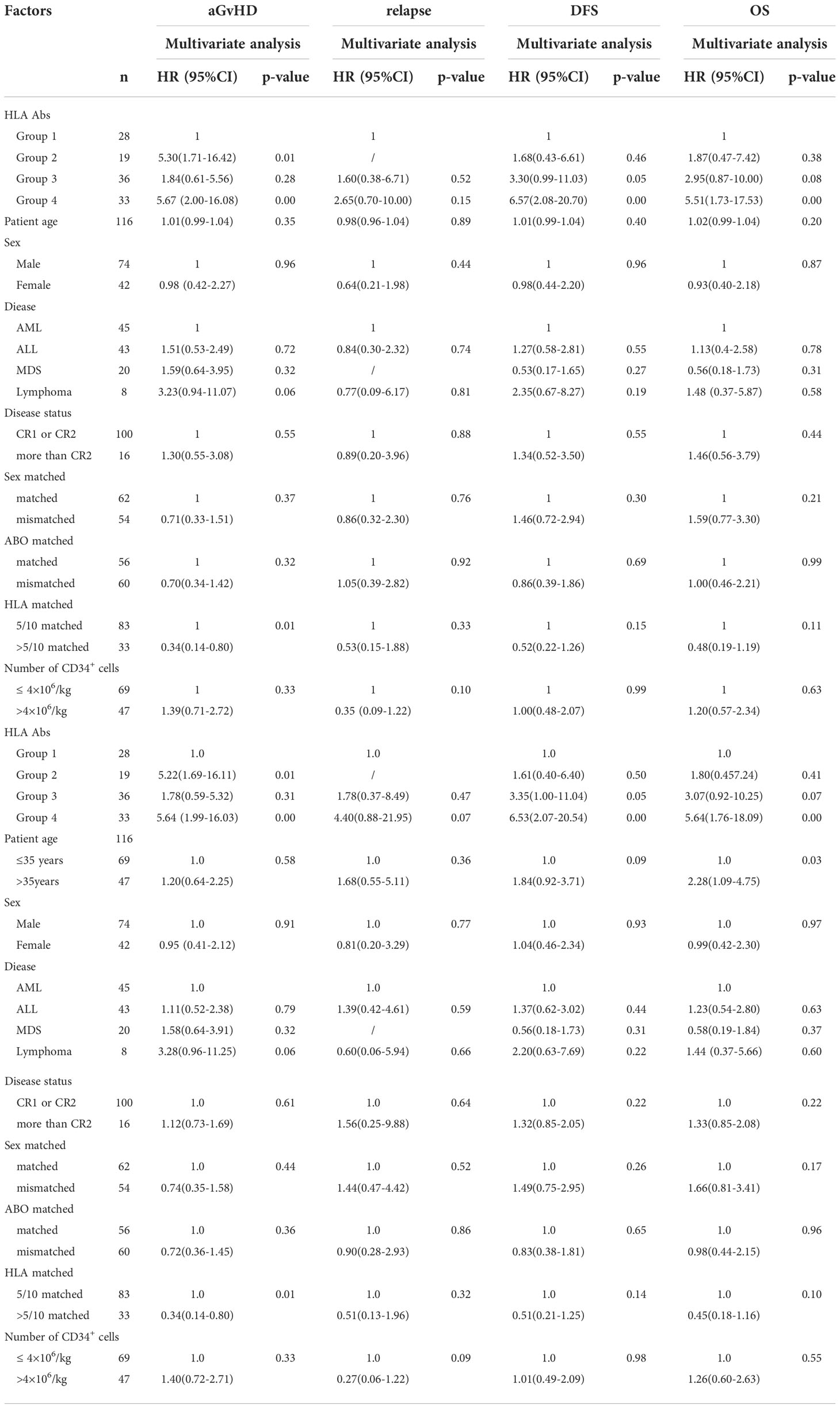
Table 4 Multivariate analysis of aGvHD, relapse, DFS and OS after haplo-HSCT in 116 patients with malignant hematological diseases.
5 Discussion
This study showed that post-transplantation dynamic testing of patients without preexisting HLA antibodies will detect de novo HLA antibodies after HSCT. In this study, we employed HLA antibody specificity testing to dynamically detect de novo HLA antibodies and found that the proportion of patients with de novo HLA antibodies was 75.9% and the proportion of patients with persistent de novo antibodies was 28.4%. De novo HLA antibodies could be detected at 15 days and 1 month after transplantation and the quantity and number of types of de novo antibodies was the highest at 1 month. These persistent de novo antibodies increased the incidence of post-transplantation aGvHD and severely affected patient survival. Our study results showed that HLA-II antibodies were the main de novo HLA antibody, which is consistent with the results of some organ transplantation studies (18–20). This finding is also consistent with our previous study on post-kidney transplantation de novo HLA antibodies, showing that post-transplantation de novo DSA antibodies were mainly HLA-II antibodies (21). Previous studies in organ transplantation (19, 20, 22, 23) and our study have shown that de novo HLA antibodies will affect graft rejection and survival. Therefore, HLA antibodies should be monitored after transplantation, and we recommend that 1-month post-transplantation is the best test time point. Previous studies also showed that HLA antibodies are associated with platelet transfusion refractoriness (24, 25). HLA antibody testing should be carried out when thrombocytopenia, transfusion dependence, or transfusion refractoriness occurs.
A potential explanation why post-HSCT HLA antibodies could contribute to GVHD may be that HLA is expressed on the surface of endothelial cells. In the setting of haplo-identical transplantation, due to donor–recipient HLA mismatch, specific T cells differentiate into effector cells that promote B cells into plasma B cells, which produce HLA antibodies. HLA antibodies crosslinking of HLA molecules on endothelial cells stimulates intracellular signaling, including FAK/Src, ERK, PIK, and mTORC1. HLA molecules trigger these signaling pathways by physically associating with integrin β4 or TLR4, thereby leading to activation of endothelial cells and recruitment of leukocytes, which manifests as transplant vasculopathy (26, 27).
The main reason why de novo HLA antibodies are HLA-II antibodies may be that HLA-II molecules can directly recognize foreign antigens and genes encoding the α and β chains of HLA-II antigen are polymorphic (28) and are associated with the intensity of HLA-II antigens. These results suggest that we not only need to carry out HLA antibody specificity testing and HLA high-resolution typing, but donors also need to undergo HLA allele analysis to determine the type of de novo antibody specificity and DSA.
In this study, single-antigen beads were used for dynamic testing of HLA antibodies. Single-antigen bead reagents have high antigen density and stronger antibody binding capacity. Additionally, they not only can detect HLA antibody specificity in patients and the type of corresponding HLA allele, thereby identifying DSA, but can also dynamically analyze antigen–antibody interaction strength as measured by MFI level. Thus, they can improve identification of antibody reactivity and specificity, and are widely used in organ transplantation and HSCT (29). The value of dynamic testing of HLA antibody is not limited to discovery of de novo HLA antibodies; the types of antibodies and changes in MFI level can also be discovered. This study also demonstrated that de novo HLA antibodies and their changes can affect the outcomes of patients who underwent haplo-HSCT. Therefore, dynamic testing of HLA antibodies is extremely important in haplo-HSCT.
There are some potential limitations and unanswered questions in this study. Considering that this research retrospectively analyzed HLA antibodies of patients after transplantation, the uncertainly of sample collection at different time points might have led to the low cumulative incidence of DSA positivity. Our subsequent study based on a prospective design and a larger sample size may compensate for the shortage and allow to explore the potential mechanism. In addition, some studies have focused on donor versus recipient antibodies (RSA). Taniguchi et al. (30) showed that donor-derived HLA antibodies may be present in patients, and Delbos et al. (31) suggested that RSA may be a risk factor for the development of GvHD. RSA testing can be performed on female or older donors if necessary.
In summary, all patients in this study underwent pre-transplantation and post-transplantation HLA antibody dynamic testing using single-antigen bead reagents. We found that patients who were negative for pre-existing HLA antibodies could produce de novo HLA antibodies after transplantation, and de novo antibodies were mostly detected 1 month after transplantation. Persistent de novo HLA antibodies affected the occurrence of aGvHD and long-term survival. Therefore, specific method must be used to detect HLA antibodies after haplo-HSCT in patients who are negative for HLA antibodies before transplantation. We recommend that testing should be carried out 1 month after transplantation. In addition, HLA antibody testing should also be carried out when thrombocytopenia, transfusion dependence, or rejection occurs in the patient.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board at First Affiliated Hospital of Soochow University. Written informed consent was obtained from the participants and minors’ legal guardian for participation in this study and for the publication of any potentially identifiable images or data included in this article.
Author contributions
JH contributed to the design of the project and statistical analysis plan, oversaw drafting of the manuscript and provided feedback on the manuscript; LW participated in recruitment of patients and collection of data, analyzed the clinical data and interpretation, and draft the manuscript; KJ participated in recruitment of patients and collection of data; YL and WZ participated in clinical and experimental data analysis; LC, XY and XB participated in the detection and interpretation of HLA antibodies data; XW provided feedback on the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82070180) and Jiangsu Provincial Key Research and Development Program (BE2019656).
Acknowledgments
The authors thank the Sample Bank of the First Affiliated Hospital of Soochow University, for assistance with the sample retrieval and the Department of Hematology for providing clinical data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood (2015) 125(25):3956–62. doi: 10.1182/blood-2015-02-627786
2. Chang YJ, Huang XJ. Haploidentical stem cell transplantation: Anti-thymocyte globulin-based experience. Semin Hematol (2016) 53(2):82–9. doi: 10.1053/j.seminhematol.2016.01.004
3. Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune escape of relapsed AML cells after allogeneic transplantation. New Engl J Med (2018) 379(24):2330–41. doi: 10.1056/NEJMoa1808777
4. Schmitz N, Lenz G, Stelljes M. Allogeneic hematopoietic stem cell transplantation for T-cell lymphomas. Blood (2018) 132(3):245–53. doi: 10.1182/blood-2018-01-791335
5. Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Malki MA, Fuchs E, et al. The European society for blood and marrow transplantation (EBMT) consensus guidelines for the detection and treatment of donor-specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone marrow Transplant (2018) 53(5):521–34. doi: 10.1038/s41409-017-0062-8
6. Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol (2015) 8:84. doi: 10.1186/s13045-015-0182-9
7. Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood marrow Transplant (2015) 21(8):1392–8. doi: 10.1016/j.bbmt.2015.05.001
8. Zhao X, Zhao X, Huo M, Fan Q, Pei X, Wang Y, et al. Donor-specific anti-human leukocyte antigen antibodies predict prolonged isolated thrombocytopenia and inferior outcomes of haploidentical hematopoietic stem cell transplantation. J Immunol Res (2017) 2017:1043836. doi: 10.1155/2017/1043836
9. Bramanti S, Calafiore V, Longhi E, Mariotti J, Crespiatico L, Sarina B, et al. Donor-specific anti-HLA antibodies in haploidentical stem cell transplantation with post-transplantation cyclophosphamide: Risk of graft failure, poor graft function, and impact on outcomes. Biol Blood marrow Transplant (2019) 25(7):1395–406. doi: 10.1016/j.bbmt.2019.02.020
10. Chang YJ, Xu LP, Wang Y, Zhang XH, Chen H, Chen YH, et al. Rituximab for desensitization during HLA-mismatched stem cell transplantation in patients with a positive donor-specific anti-HLA antibody. Bone Marrow Transplant (2020) 55(7):1326–36. doi: 10.1038/s41409-020-0928-z
11. Zhang X, Wang J, Zhou Z, Zhang Y, Liu H, Tong C, et al. The role of HLA antibodies in HLA mismatched allogeneic hematopoietic stem cell transplantation. Clin Transpl (2014) 31:245–50.
12. Senev A, Lerut E, Van Sandt V, Coemans M, Callemeyn J, Sprangers B, et al. Specificity, strength, and evolution of pretransplant donor-specific HLA antibodies determine outcome after kidney transplantation. Am J Transplant (2019) 19(11):3100–13. doi: 10.1111/ajt.15414
13. Guidicelli G, Guerville F, Lepreux S, Wiebe C, Thaunat O, Dubois V, et al. Non-Complement-Binding De novo donor-specific anti-HLA antibodies and kidney allograft survival. J Am Soc Nephrol (2016) 27(2):615–25. doi: 10.1681/ASN.2014040326
14. Senev A, Coemans M, Lerut E, Van Sandt V, Kerkhofs J, Daniels L, et al. Eplet mismatch load and De novo occurrence of donor-specific anti-HLA antibodies, rejection, and graft failure after kidney transplantation: An observational cohort study. J Am Soc Nephrol (2020) 31(9):2193–204. doi: 10.1681/ASN.2020010019
15. Chen LY, Yuan XN, Bao XJ, He J. Using experimental results of international proficiency testing for establishing the decision limit value of mean fluorescent intensity of anti-human leukocyte antigen antibody and its clinical application. Chin J Organ Transplant (2021) 42(8):468–74. doi: 10.3760/cma.j.cn421203-20201116-00388
16. Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR task force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant (2018) 53(11):1401–15. doi: 10.1038/s41409-018-0204-7
17. Chinese Medical Association, Stem Cell Application Group HB. Chinese Consensus of allogeneic hematopoietic stem cell transplantation for hematological disease (III) —acute graft-versus-host disease (2020). Chin J Hematol (2020) 41(7):529–36. doi: 10.3760/cma.j.issn.0253-2727.2020.07.001
18. Schmitzer M, Winter H, Kneidinger N, Meimarakis G, Dick A, Schramm R, et al. Persistence of de novo donor specific HLA-antibodies after lung transplantation: A potential marker of decreased patient survival. Hla (2018) 92:24–32. doi: 10.1111/tan.13306
19. Kovandova B, Slavcev A, Honsova E, Erhartova D, Skibova J, Viklicky O, et al. De novo HLA class II antibodies are associated with the development of chronic but not acute antibody-mediated rejection after liver transplantation - a retrospective study. Transplant Int (2020) 33(12):1799–806. doi: 10.1111/tri.13763
20. Liu W, Zhao J, Kang ZY, Xiao YL, Yang L, Liu C, et al. De novo donor-specific HLA antibodies reduce graft survival rates and increase the risk of kidney transplant rejection: A single-center retrospective study. Transpl Immunol (2021) 68:101430. doi: 10.1016/j.trim.2021.101430
21. Wei Xd, He J, Yuan Xn, Pan Zj, Pu Jx, Hou Jq. The clinical features of the pre-formed and de novo human leucocyte antigen antibodies post-kidney transplantation: A single center analysis of the eight-year follow-up data. Chin J Exp Surg (2017) 34(12):2071–4. doi: 10.3760/cma.j.issn.1001-9030.2017.12.018
22. Yamamoto T, Watarai Y, Takeda A, Tsujita M, Hiramitsu T, Goto N, et al. De novo anti-HLA DSA characteristics and subclinical antibody-mediated kidney allograft injury. Transplantation (2016) 100(10):2194–202. doi: 10.1097/TP.0000000000001012
23. Tang C, Unterrainer C, Fink A, Cinca S, Ruhenstroth A, Scherer S, et al. Analysis of de novo donor-specific HLA-DPB1 antibodies in kidney transplantation. Hla (2021) 98(5):423–30. doi: 10.1111/tan.14422
24. Stanworth SJ, Navarrete C, Estcourt L, Marsh J. Platelet refractoriness–practical approaches and ongoing dilemmas in patient management. Br J Haematol (2015) 171(3):297–305. doi: 10.1111/bjh.13597
25. Schiffer CA, Bohlke K, Delaney M, Hume H, Magdalinski AJ, McCullough JJ, et al. Platelet transfusion for patients with cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol (2018) 36(3):283–99. doi: 10.1200/jco.2017.76.1734
26. McCaughan JA, Tinckam KJ. Donor specific HLA antibodies & allograft injury: mechanisms, methods of detection, manifestations and management. Transplant Int (2018) 31(10):1059–70. doi: 10.1111/tri.13324
27. Jin YP, Nevarez-Mejia J, Terry AQ, Sosa RA, Heidt S, Valenzuela NM, et al. Cross-talk between HLA class I and TLR4 mediates p-selectin surface expression and monocyte capture to human endothelial cells. J Immunol (2022) 209(7):1359–69. doi: 10.4049/jimmunol.2200284
28. Klein J, Sato A. The HLA system. First two parts New Engl J Med (2000) 343(10):702–9. doi: 10.1056/nejm200009073431006
29. Gladstone DE, Bettinotti MP. HLA donor-specific antibodies in allogeneic hematopoietic stem cell transplantation: Challenges and opportunities. Hematol Am Soc Hematol Educ Program (2017) 2017(1):645–50. doi: 10.1182/asheducation-2017.1.645
30. Taniguchi K, Yoshihara S, Maruya E, Ikegame K, Kaida K, Hayashi K, et al. Donor-derived HLA antibody production in patients undergoing SCT from HLA antibody-positive donors. Bone Marrow Transplant (2012) 47(10):1338–42. doi: 10.1038/bmt.2012.28
Keywords: de novo human leukocyte antigen antibody (de novo HLA antibody), donor-specific anti-HLA antibody (DSA), acute graft-versus-host disease (aGvHD), haploidentical hematopoietic stem cell transplantation (haplo-HSCT), outcomes
Citation: Wang L, Ji K, Chen L, Li Y, Zhu W, Yuan X, Bao X, Wu X and He J (2022) Posttransplant de novo DSA and NDSA affect GvHD, OS, and DFS after haplo-HSCT in patients without pre-existing HLA antibodies of hematological malignancies. Front. Immunol. 13:1047200. doi: 10.3389/fimmu.2022.1047200
Received: 17 September 2022; Accepted: 08 November 2022;
Published: 01 December 2022.
Edited by:
Rongqian Wu, Xi’an Jiaotong University, ChinaReviewed by:
Ying-Jun Chang, Peking University People’s Hospital, ChinaMeng Lv, Peking University People’s Hospital, China
Erlie Jiang, Chinese Academy of Medical Sciences & Peking Union Medical College, China
Copyright © 2022 Wang, Ji, Chen, Li, Zhu, Yuan, Bao, Wu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun He, anVuaGUxOTY0QDE2My5jb20=
†ORCID: Lan Wang, orcid.org/0000-0002-3942-2935
 Lan Wang
Lan Wang Kai Ji1
Kai Ji1 Xiaojin Wu
Xiaojin Wu Jun He
Jun He