
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 14 December 2022
Sec. Autoimmune and Autoinflammatory Disorders: Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1044184
This article is part of the Research Topic Pathogenesis and Target-Treatments of Systemic Lupus Erythematosus View all 18 articles
Introduction: This study is aimed to map the clinical and immunological features of active lupus patients with different disease duration.
Methods: For clinical phenotype analysis, we enriched eligible medical records with active SLE (SLEDAI-2k≥8) from the Renji Lupus registry, a single-center database of hospitalized SLE patients with standard care, which covered national-wide patients. Patients with repeated hospitalization records in this enrichment were analyzed longitudinally as validation for the cross-sectional study above.
Results: We enriched a total of 1313 eligible records on active SLE (SLEDAI-2k≥8) for cross-sectional analysis. Stratified into four groups by a 5-year interval of disease duration, these active SLE patients showed a significantly shifting clinical phenotype along with the duration (ascending nephritis, pulmonary hypertension and descending fever, cutaneous symptoms, arthritis, and neuropsychiatric manifestations), especially in stratifications with disease onset age ≤ 45 years old. A longitudinal analysis of 55 patients with repeated hospitalizations for active lupus showed a similar trend. In the cross-sectional study of 222 records with full information on serology and lymphocyte subsets, peripheral B cell proportion, anti-dsDNA antibody, and serum IgG/IgM negatively correlated with duration, while CD8+ T cell proportion was positively correlated (P values, 0.029-4.8×10-17), which were supported by the sensitivity analysis in patient subgroups according to disease onset age and recent treatment. Multivariate linear regression identified duration as the only significant associator with both B cell and CD8+ T cell proportion (P values, 8.9×10-8 and 7.6×10-5, respectively). These duration biased immune phenotypes were highly consistent with the longitudinal observation in 14 patients with repeated hospitalizations.
Conclusions: Both clinical and immunological features of active SLE are significantly duration biased distributed, which merits further investigations in the evolution of SLE pathogenesis.
Systemic Lupus erythematosus (SLE) is a chronic and fluctuant autoimmune disease (1). Present studies on lupus flare pay tremendous attention to the prediction and prevention of flare. In these studies, flares are defined as a general increase in disease activity or hospitalization, with no consideration of specific organ involvement (2–4), which may affect the prevention strategy. Description of organ involvement for established SLE patients remains preliminary and is in short supply.
Pathogenic lymphocyte subsets and their dysfunction are an important part of the pathogenesis study of SLE. However, due to the substantial impact of various treatments on lymphocytes, most in-depth studies have been limited to naive patients (5). For established patients who need therapies demanding more cautious selection, there are few convincing studies to clarify their pathogenic mechanisms. To achieve this goal, the first step would be a comprehensive description of the patient immune features upon different disease courses. In the current study, we tried to make such a description in a population with unified active SLE.
This is a single-center study based on the Renji Lupus registry, a registry study of hospitalized SLE patients with standard care in Renji Hospital, Shanghai Jiaotong university, school of medicine since 2013. This database was constructed via natural language recognition and extraction enhanced by artificial intelligence, with organized information on patient medical history, treatment, and lab tests. Patients would be enrolled if their diagnosis of SLE was confirmed by at least two rheumatology consultant physicians and received standard care (except the hospitalizations specifically for intravenous therapy, e.g., cyclophosphamide or rituximab). Patients with overlap syndromes would not be included in this registry. Written informed consent was obtained from each study participant. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Renji Hospital, Shanghai, China (ID: 2013-126).
We selected records dated from 2013 to 2019, ruling out the impact of COVID-19. A record that fulfilled the following criteria would be defined as eligible: 1) with enough information on the clinical features of 2019 EULAR/ACR SLE criteria, 2) with enough information to assess SLEDAI-2k, and 3) the patient should be clinically active disease defined as a SLEDAI-2k score of ≥ 8. All SLEDAI were scored within the first three days of hospitalization as a routine clinical practice in our center. Records with an obvious infection would be ruled out. Patients with positive microbiological evidence or continuous administration of intravenous antibiotics for more than five days were defined as infected.
Unless otherwise specified, the following clinical features were defined according to the 2019 EULAR/ACR criteria for SLE (6). All manifestations needed to be present currently or no more than one month before the recording time. Cutaneous manifestations included acute cutaneous lupus, oral ulcer, non-scarring alopecia, and subacute cutaneous or discoid rash. Serositis included pleuritis and pericarditis. Nephritis needed a current proteinuria >0.5 g per day. Hematologic manifestations included hemolytic anemia, leukopenia (<4000/mm3), and thrombocytopenia (<100,000/mm3). The definitions of other clinical features were defined as follows: NP lupus was assessed according to the ACR nomenclature and case definitions for neuropsychiatric lupus (7). Pulmonary hypertension (PH) was diagnosed based on right heart catheterization or echocardiography (peak tricuspid regurgitation velocity >3.4 m/s). Gastrointestinal manifestations included protein-losing gastroenteropathy, mesenteric vasculitis, and colitis.
The disease duration was calculated from the diagnosis date for each case. Naive patients were defined as those cases within three months since SLE diagnosis and having no current or historical use of any systemic steroids, hydroxychloroquine, immunosuppressants, or targeted therapy.
For immunological analysis, we required hospitalization records additionally on top of active SLE: the eligible records needed to have complete test results of anti-dsDNA antibody, serum complements, and lymphocyte subtype counting. All these tests were performed by the clinical laboratory of Renji hospital. All tests were clinical routine and, in most cases, performed within the first two days after admission. Anti-dsDNA antibody was detected by radioimmunoassay. The lymphocyte subset test was detected by a commercial kit (BD 340503) based on flow cytometry. It classified B cell, CD4+ T cell, CD8+ T cell, and NK cell from the total lymphocyte by the antigen combination of CD3 FITC, CD16 PE + CD56 PE, CD45 PerCP, and CD19 APC.
The Cochran-Armitage trend test and Cochran-Mantel-Haenszel test were employed to compare the presence of each clinical manifestation among groups with different disease duration. Serological test results/lymphocyte phenotype with disease duration was tested by the Spearman correlation. Multivariate linear regression was used to identify associators with lymphocyte phenotype. Wilcoxon test was used for comparison between parameters of repeated hospitalizations of one patient. A p-value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed by SPSS (SPSS Inc., Chicago, IL, USA).
From 2013 to 2019, a total of 2032 patients with 2841 hospitalization records were enrolled in our registry. We enriched a total of 1313 eligible records on active SLE (SLEDAI-2k≥8) from the Renji Lupus registry, a registry study of hospitalized SLE patients with standard care (Figure 1). 92% of patients were female. The median age was 34 years old (IQR: 26-43). There were no statistical discrepancies among study populations. 65% of patients had recent conventional immunosuppressants (IS), and 4.4% of the patients received recent rituximab (Table 1). The study population was national-wide and covered 27/34 provinces of China. (Supplementary Table 1)
Divided by a 5-year interval of disease duration, the presence of fever, cutaneous symptoms, arthritis, hemocytopenia, neuropsychiatric (NP), and gastrointestinal manifestations descended in the long-duration groups, while nephritis and pulmonary hypertension ascended significantly (Cochran-Armitage trend test for each clinical dimension was significant, P values are illustrated in Figure 2A). Of the 239 active patients with SLE for more than 10 years, 79.1% had active nephritis, 21.3% had hemocytopenia, and other symptoms were 10% or less. In the subgroup analysis stratified with disease onset age, the trend of fever, mucocutaneous, arthritis, and hematological impairment were still significant in patients with younger onset ages (≤ 45 years old). The trend of nephritis was significant in all onset groups (Supplementary Table 2).
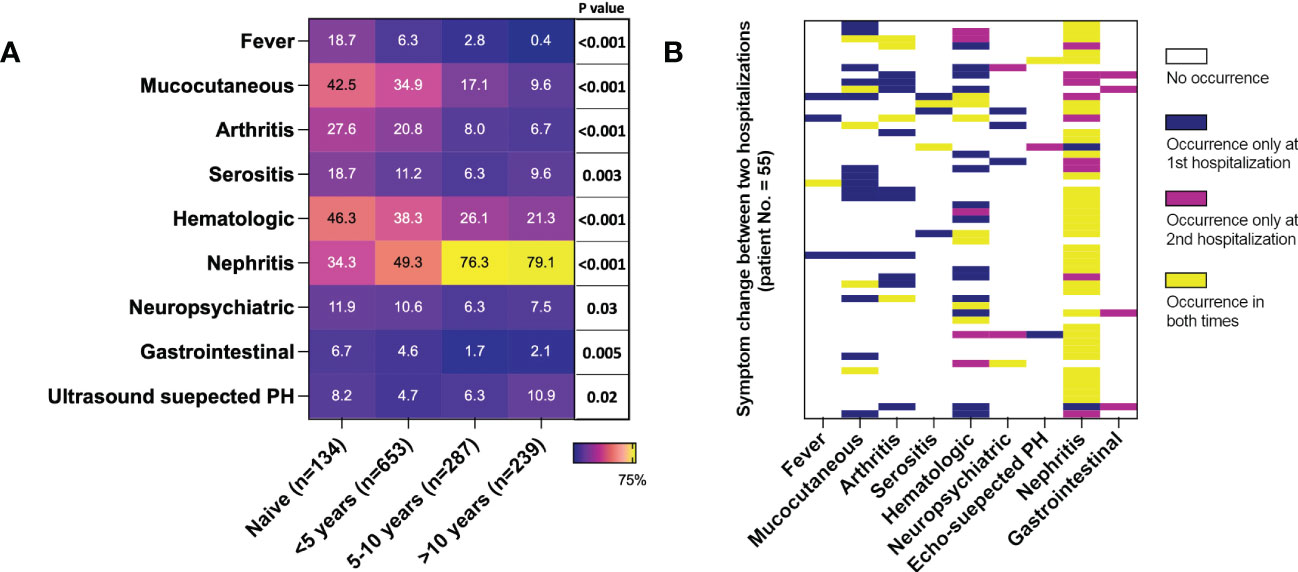
Figure 2 Duration biased clinical phenotype distribution in active SLE. 1313 cases were included. (A) Presence of SLE manifestations in groups of different disease durations. Each cell represents the occurrence of one symptom in the duration group in percentage. P values were calculated by the Cochran-Armitage trend test for each manifestation. (B) Changes in clinical manifestations of 55 patients with repeated hospitalizations due to active lupus (both hospitalizations with SLEDAI≥8). PH, pulmonary hypertension.
55 patients had repeated records with a median interval of 425 days (IQR: 233-834 days), and their longitudinal data supported the cross-sectional findings. Symptoms, including fever, arthritis, serositis, and cutaneous symptoms, were present on the first visit, but most of them did not occur in the second hospitalization. Besides, among patients without these symptoms during the first time, none of them had a new onset until the second hospitalization. On the contrary, most nephritis (44/46) was a flare or new onset in the second visit. Hematological impairment was mixed; 14 patients had it only the first time, and 12 patients only the second time. (Figure 2B)
222 records contained full results of serology and lymphocyte subset. The total number of lymphocytes was not correlated with duration. However, we observed a strong negative correlation between disease duration and peripheral B cell proportion and a simultaneously moderate positive correlation between disease duration and peripheral CD8+ T cell proportion. Moderate negative correlations with IgG and IgM were observed as well. Although a ceiling effect of anti-dsDNA was present in 39.2% of cases over the upper limit of radioimmunoassay detection, The negative correlation of dsDNA antibody with duration was still significant. The CD4+ T cells or NK cells were not correlated with disease duration. (Table 2) Sensitivity analysis showed consistent correlations in most subgroups stratified according to disease onset age or recent treatment (Figure 3A). Disease duration, age of disease onset, presence of recent steroids/IS, and recent use of CNI was associated with both B cell and CD8+ T cell proportions in the univariate analysis. (Supplementary Table 3) Only disease duration was significantly associated with both B and CD8+ T cell proportion in the multivariate analysis (Table 3 and Supplementary Table 4).
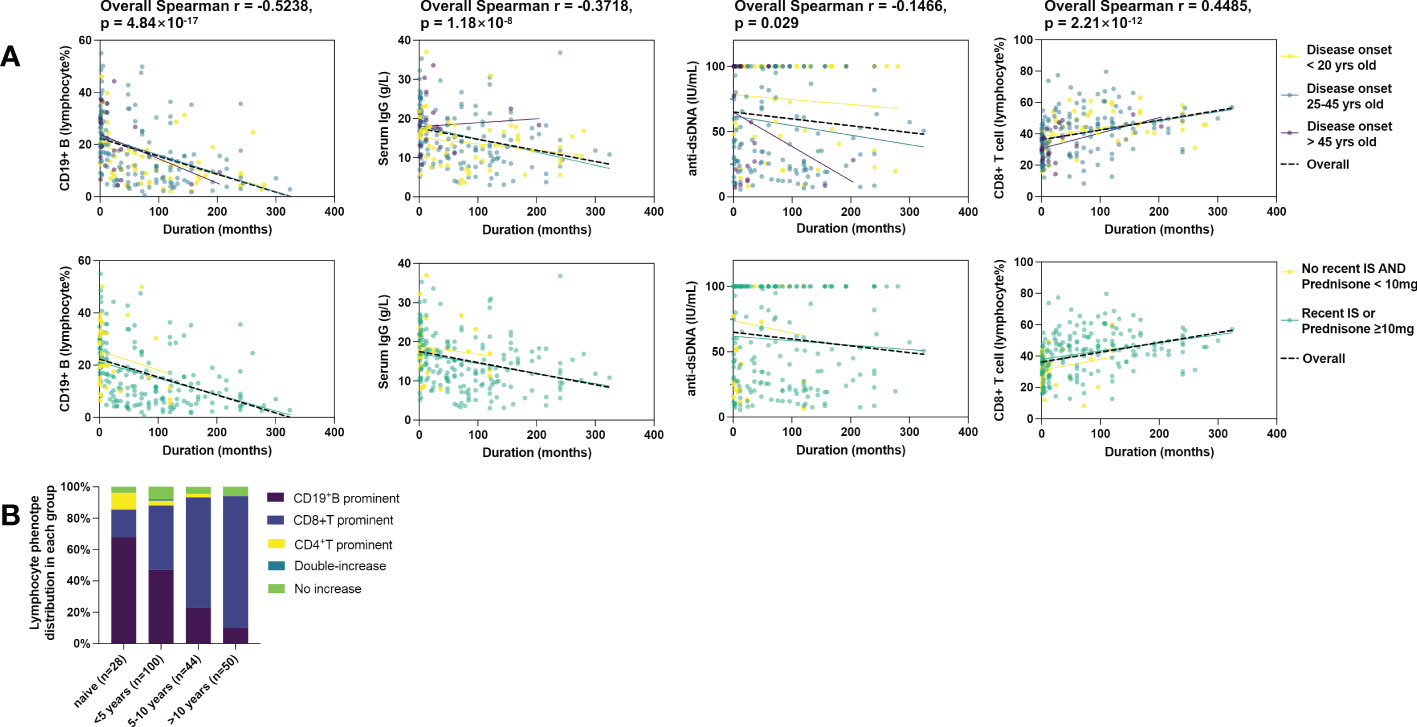
Figure 3 Correlation of immunological phenotypes of active SLE with disease duration. 222 cases were included. (A) Correlation of B cell%, serum IgG, serum anti-dsDNA, and CD8+ T cell% with disease duration. The black lines represent fit plots for the whole study population (n=222), and colored lines represent fit plots for subgroups by disease onset age (top) or recent treatment (bottom). (B) Duration biased distribution of lymphocyte balance in active SLE. The definition of lymphocyte types is shown in Supplementary Table 5.
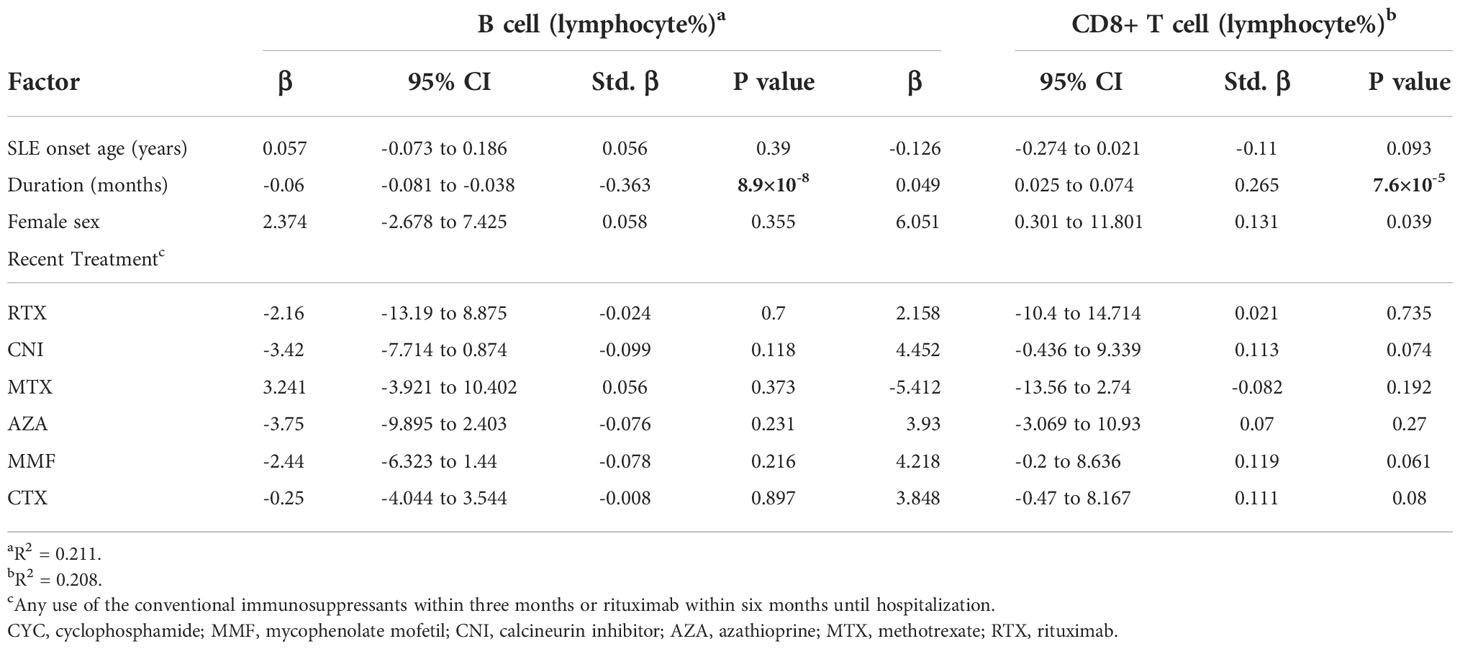
Table 3 Multivariate analysis of associators for peripheral B cell and CD8+ T cell proportion in active SLE cases (n=222, Model 1).
The balance of lymphocyte subsets was defined by the reference intervals of each subset for the Chinese population (Detailed definitions are in Supplementary Table 5) (8). As shown in Figure 3B, the B cell prominent and CD8+T cell prominent were the two major phenotypes, while other types together less than 15.3%. No NK-prominent type was found. In consistency with the correlation findings, the frequency of the B prominent type declined in duration groups, and the CD8 prominent type arose opposite to the B type with disease duration (Fisher’s exact probability=7.76×10-9). In the patients with a duration of over ten years, only 10% of patients still had B cell elevated, and up to 84% of patients were CD8 type.
Fourteen patients had repeated hospitalizations with lymphocyte subset records. The median interval was 306 days (range: 84-672 days). Compared to the first hospitalization, B cell proportion (Figure 4A), anti-dsDNA (Figure 4B), and serum IgG (Figure 4C) decreased significantly while the CD8+ T population (Figure 4D) and serum C3 (Figure 4E) increased in the second visit.
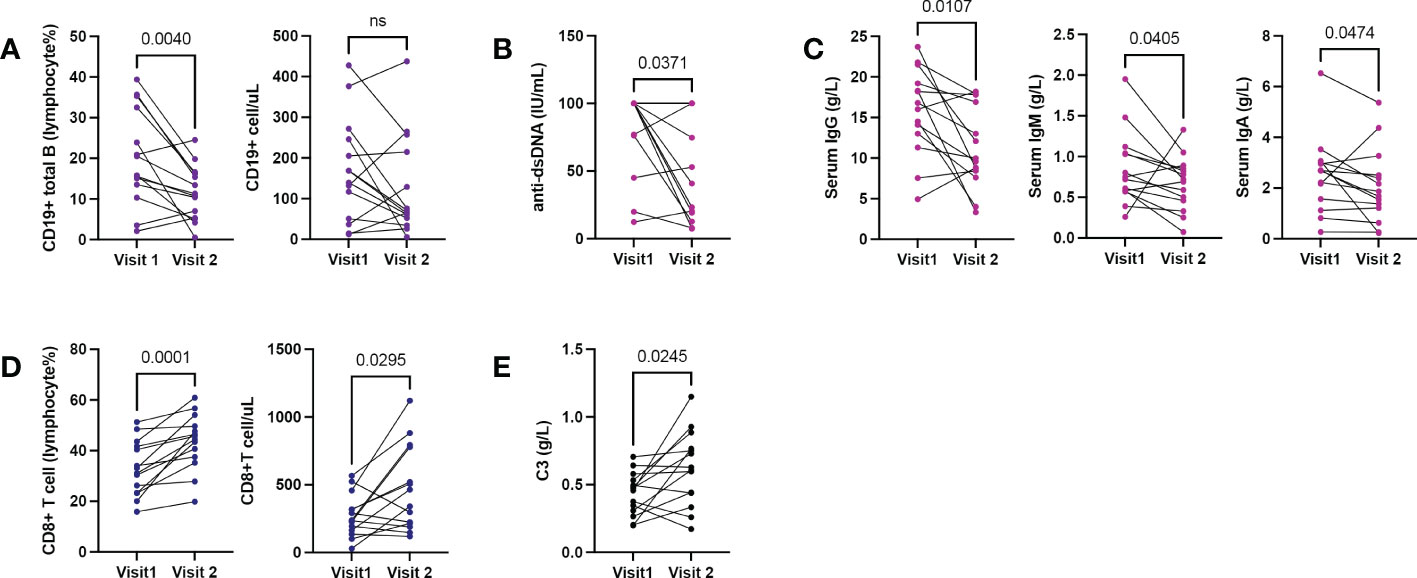
Figure 4 Shifting immune phenotypes in longitudinal records of active SLE. 14 patients with repeated hospitalizations due to active lupus (each hospitalization with SLEDAI≥8). (A) Changes in B cell counts and proportions. (B, C) Changes in serum anti-dsDNA and serum immunoglobulin levels. (D) Changes in CD8+T cell counts and proportions. (E) Changes in serum C3. Wilcoxon-test for all comparisons.
To our knowledge, this study is the first systematic description of clinical and immune features of SLE patients in long-term duration. Catching clinical and immunological features of active disease for established patients (i.e., flares or refractory activity) in a large number is not easy for patients usually with low activity in long-term follow-up, even in a large cohort (9). Our study somehow overcame this difficulty and provided a large number of patients with active disease and long-duration via enriched patients with SLEDA-2k≥8 from a big registry. Moreover, we extracted longitudinal datasets from the whole study population as confirmations, which we believed to the largest extent compensated the shortness of a cross-sectional study.
Previous reports on the shifting clinical phenotypes were fragmented (10). Among these, lupus nephritis was the most reported organ involvement increased with disease duration (9, 11–13). Recently, a decrease in NP events with lupus duration was observed (14). In consistence with previous observations that hematological impairment was a common flare (11–13), we for the first time show the mixed manner of hematological impairment (Figure 2B), which could be led by different pathogenetic ways. Arthritis and cutaneous flare were common in short follow-up (12, 13), but this study offered an opposite view of long-established SLE activation. PH of SLE varies from association with disease activity in SLE, response to immunosuppressive therapy, and presence in early or established patients (15, 16). The ascending trend of PH is reported for the first time in this study and is worthy of further investigation.
This work highlights the impact of disease duration on lupus immune phenotype. Along with disease evolution, SLE activates with a seemly dampened humoral immune response, with a parallel decrease in peripheral B cell, anti-dsDNA, and serum IgG and IgM. Lower anti-dsDNA antibody level in late-onset lupus nephritis patients (17), and a seemly decrease in ANA positive rate in a 5-year follow-up of SLE were reported as secondary results previously (9).
This duration-associated dampened humoral immune response may further lead to a discrepant response to B-cell-targeted therapies. Recently, a small study found that SLE patients with longer durations had difficulties achieving complete depletion of peripheral B cells (18), and a small observation shows that a shorter duration of LN responds better to rituximab (19). Our findings merits more detailed profiling of B cell in duration courses, especially the balance of physiological/pathological B cells, which may give a better interpretation of this phenomenon.
Consistent with a recent finding that circulating cytotoxic CD8+ T cell expended in SLE patients (20), a duration-associated relative increase of CD8+ T cells is observed for the first time in our study. The previous functional studies on CD8+T cells in SLE focused on their impaired cytotoxicity, maintenance of autoimmunity, and the damaging role of tissue CD8+T cells (21). Whether the CD8+ T increase is secondary or pathological needs further investigation.
The highlighted disease duration in this study implies two sub-factors, treatment and aging, both of which are well-known impactors on the immune system. To analyze the impact of treatment, we separated groups of naive patients from those with disease duration < 5 years as a reference. Besides, the overall immunosuppressive treatment, as well as some single agents, were associated with peripheral B cell% and CD8 T cell% in univariate analysis. Regarding aging, we used the age of SLE onset to adjust its impact on disease duration, and onset age was also associated with peripheral B cell% and CD8 T cell% in univariate analysis. However, in multivariate analysis, neither overall treatment nor onset age was significant. Therefore, we believe it is reasonable to consider duration as a single factor when assessing the pathogenesis evolution in SLE.
The limitations of this study are the following: 1) This study is limited to hospitalized patients, which may underestimate some symptoms routinely treated in the clinics, such as chronic cutaneous lupus and arthritis (22, 23). 2) We did not have the information in this registry on exactly when each patient fulfilled the SLE classification criteria. Therefore, we calculated the disease duration alternatively from the date of SLE diagnosis, which could underestimate the disease duration. 3) There was a large number of missing cases in the immune type analysis. This was because we required patients with lymphocyte subtype test results, as well as other serum markers if they could be enrolled, for we wanted the serological test matched with cell types. However, the lymphocyte subtype test (BTNK) was not a clinical routine in our hospital until about 2017, and we had to exclude most cases before 2017.
This study reveals a comprehensive picture of clinical and immunological features of active SLE patients, which is significantly duration biased. Disease duration should be considered as an important confounder in future investigations on SLE.
The data analyzed in this study is subject to the following licenses/restrictions: All data are from the Renji Lupus Registry, an internal medical system of Renji Hospital, Shanghai Jiao Tong University School of Medicine. Requests to access these datasets should be directed to QY, eWFucWluZ3JhbkByZW5qaS5jb20=. We can provide data excel sheets, but the data is not uploaded, all without links or websites.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Renji Hospital, Shanghai, China (ID: 2013-126). The patients/participants provided their written informed consent to participate in this study.
QY, BL, MY contributed equally to this work and share the first authorship. QT, BL, and MY carried out the study with support from QL and BL. QY and LL initiated and designed the project. TL built the registrydataset. JW provided statistical support. QY wrote the manuscript. All authorsdiscussed the results and contributed to the final manuscript. All authors read and approved the final manuscript.
This work is supported by Shanghai Municipal Commission of Health and Family Planning (20204Y0088) and the National Natural Science Foundation of China (81974251).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1044184/full#supplementary-material
1. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update on the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis (2021) 80(1):14–25. doi: 10.1136/annrheumdis-2020-218272
2. Almeida-Brasil CC, Hanly JG, Urowitz M, Clarke AE, Ruiz-Irastorza G, Gordon C, et al. Flares after hydroxychloroquine reduction or discontinuation: results from the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis (2022) 81(3):370. doi: 10.1136/annrheumdis-2021-221295
3. Furie R, Morand EF, Askanase AD, Vital EM, Merrill JT, Kalyani RN, et al. Anifrolumab reduces flare rates in patients with moderate to severe systemic lupus erythematosus. Lupus. (2021) 30(8):1254–63. doi: 10.1177/09612033211014267
4. Petri MA, van Vollenhoven RF, Buyon J, Levy RA, Navarra SV, Cervera R, et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheumatol (2013) 65(8):2143–53. doi: 10.1002/art.37995
5. Wu C, Fu Q, Guo Q, Chen S, Goswami S, Sun S, et al. Lupus-associated atypical memory b cells are mTORC1-hyperactivated and functionally dysregulated. Ann Rheum Dis (2019) 78(8):1090–100. doi: 10.1136/annrheumdis-2019-215039
6. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. European League against Rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol (2019) 71(9):1400–12.
7. The American college of rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum (1999) 42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F
8. Xu K, Miao L, Chen W, Wu H, Gong Y, Tu X, et al. Establishment of the reference intervals of lymphocyte subsets for healthy Chinese han adults and its influencing factors. Ann Trans Med (2021) 9(19):1495–. doi: 10.21037/atm-21-4031
9. Choi MY, Clarke AE, Urowitz M, Hanly J, St-Pierre Y, Gordon C, et al. Longitudinal analysis of ANA in the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis (2022) 81(8):1143–50.
10. Minowa K, Amano H, Ando S, Watanabe T, Ogasawara M, Kawano S, et al. Disease flare patterns and predictors of systemic lupus erythematosus in a monocentric cohort of 423 Japanese patients during a long-term follow-up: The JUDE study. Mod Rheumatol (2017) 27(1):72–6. doi: 10.1080/14397595.2016.1192745
11. Arnaud L, Tektonidou MG. Long-term outcomes in systemic lupus erythematosus: trends over time and major contributors. Rheumatol (Oxford) (2020) 59(Suppl5):v29–38. doi: 10.1093/rheumatology/keaa382
12. Adamichou C, Bertsias G. Flares in systemic lupus erythematosus: diagnosis, risk factors and preventive strategies. Mediterr J Rheumatol (2017) 28(1):4–12. doi: 10.31138/mjr.28.1.4
13. Inês L, Duarte C, Silva RS, Teixeira AS, Fonseca FP, da Silva JAP. Identification of clinical predictors of flare in systemic lupus erythematosus patients: a 24-month prospective cohort study. Rheumatology. (2014) 53(1):85–9. doi: 10.1093/rheumatology/ket322
14. Hanly JG, Urowitz MB, Gordon C, Bae SC, Romero-Diaz J, Sanchez-Guerrero J, et al. Neuropsychiatric events in systemic lupus erythematosus: a longitudinal analysis of outcomes in an international inception cohort using a multistate model approach. Ann Rheum Dis (2020) 79(3):356–62. doi: 10.1136/annrheumdis-2019-216150
15. Sun F, Lei Y, Wu W, Guo L, Wang K, Chen Z, et al. Two distinct clinical phenotypes of pulmonary arterial hypertension secondary to systemic lupus erythematosus. Ann Rheum Dis (2019) 78(1):148–50. doi: 10.1136/annrheumdis-2018-214197
16. Qian J, Li M, Zhao J, Wang Q, Tian Z, Zeng X. Inflammation in SLE-PAH: good news or not? Ann Rheum Dis (2019) 78(12):e135. doi: 10.1136/annrheumdis-2018-214605
17. Ichinose K, Kitamura M, Sato S, Fujikawa K, Horai Y, Matsuoka N, et al. Comparison of complete renal response and mortality in early- and late-onset lupus nephritis: a multicenter retrospective study of a Japanese cohort. Arthritis Res Ther (2020) 22(1):175. doi: 10.1186/s13075-020-02271-3
18. Reddy VR, Pepper RJ, Shah K, Cambridge G, Henderson SR, Klein C, et al. Disparity in peripheral and renal b-cell depletion with rituximab in systemic lupus erythematosus: an opportunity for obinutuzumab? Rheumatol (Oxford) (2022) 61(7):2894–904. doi: 10.1093/rheumatology/keab827
19. Lindholm C, Börjesson-Asp K, Zendjanchi K, Sundqvist AC, Tarkowski A, Bokarewa M. Longterm clinical and immunological effects of anti-CD20 treatment in patients with refractory systemic lupus erythematosus. J Rheumatol (2008) 35(5):826–33.
20. Perez RK, Gordon MG, Subramaniam M, Kim MC, Hartoularos GC, Targ S, et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science (2022) 376(6589):eabf1970. doi: 10.1126/science.abf1970
21. Chen PM, Tsokos GC. The role of CD8+ T-cell systemic lupus erythematosus pathogenesis: an update. Curr Opin Rheumatol (2021) 33(6):586–91. doi: 10.1097/BOR.0000000000000815
22. Jacobsen S, Petersen J, Ullman S, Junker P, Voss A, Rasmussen JM, et al. A multicentre study of 513 Danish patients with systemic lupus erythematosus. i. disease manifestations and analyses of clinical subsets. Clin Rheumatol (1998) 17(6):468–77. doi: 10.1007/BF01451282
Keywords: long-established lupus, disease duration, B cell, CD8+ T cell, serological markers
Citation: Yan Q, Liu B, Yang M, Li Q, Wang J, Li T and Lu L (2022) Duration biased distribution of clinical and immunological phenotypes in active SLE. Front. Immunol. 13:1044184. doi: 10.3389/fimmu.2022.1044184
Received: 14 September 2022; Accepted: 29 November 2022;
Published: 14 December 2022.
Edited by:
Dong-Qing Ye, Anhui Medical University, ChinaReviewed by:
Coziana Ciurtin, University College London, United KingdomCopyright © 2022 Yan, Liu, Yang, Li, Wang, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangjing Lu, bHVfbGlhbmdqaW5nQDE2My5jb20=; Ting Li, bGVldGluZzAwN0AxNjMuY29t; Qingran Yan, eWFucWluZ3JhbkByZW5qaS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.