- 1Department of Cardiovascular Surgery, China-Japan Friendship Hospital, Beijing, China
- 2Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 3Peking University China-Japan Friendship School of Clinical Medicine, Beijing, China
Introduction: Immune-mediated inflammatory diseases (IMIDs) have been associated with an increased risk of venous thromboembolism (VTE) in multiple observational studies. However, a direct causally relation between IMIDs and VTE remains unclear to date. Here, we used Mendelian randomization (MR) analysis to investigate causal associations between IMIDs and VTE.
Methods: We collected genetic data from published genome-wide association studies (GWAS) for six common IMIDs, specifically inflammatory bowel disease (IBD), Crohn’s disease (CD), ulcerative colitis (UC), rheumatoid arthritis (RA), psoriasis (PSO), and systemic lupus erythematosus (SLE); and summary-level data for VTE, pulmonary embolism (PE), and deep vein thrombosis (DVT) from the FinnGen database. Two-sample MR analysis using inverse variance weighting (IVW) was performed to identify causal associations between IMIDs and VTE/DVT/PE, and sensitivity analyses were implemented for robustness.
Results: IVW analysis showed a causal relationship between genetically predicted UC (one type of IBD) and the risk of VTE (OR = 1.043, 95% CI: 1.013-1.073, p = 0.004) and DVT (OR = 1.088, 95% CI: 1.043-1.136, p < 0.001), but we found no evidence of causality between UC and PE (OR = 1.029, 95% CI: 0.986-1.074, p = 0.19). In addition, no associations were observed between total IBD, CD, RA, SLE, or PSO and VTE/DVT/PE. Sensitivity analysis found no evidence for horizontal pleiotropy.
Conclusion: This MR study provides new genetic evidence for the causal relationship between IMIDs and the risk of VTE. Our findings highlight the importance of active intervention and monitoring to mitigate VTE risk in patients with IBD, in particular those presenting with UC.
1 Introduction
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), affects ~10 million people worldwide every year, representing the third most common cardiovascular disease globally (1, 2). The 30-day case fatality rate after VTE diagnosis is 10.6%, with about 30% to 50% of survivors developing long-term complications that increase the burden of this disease (3–5).
The formation of venous thrombosis involves a complex pathophysiological process that is triggered by the interaction of multiple risk factors. An increasing number of studies suggest that inflammation is closely associated with VTE (6). Specifically, the activation of the immune system induces a process known as immunothrombosis (7), in which activated immune cells (such as neutrophils and monocytes) interact with platelets and the coagulation cascade, which ultimately leads to thrombosis (8).
Immune-mediated inflammatory diseases (IMIDs) comprise a wide range of conditions, such as inflammatory bowel disease (IBD – e.g., Crohn’s disease – CD and ulcerative colitis – UC), rheumatoid arthritis (RA), skin inflammation (e.g., Psoriasis – PSO), and connective tissue disease (e.g., Systemic Lupus erythematosus – SLE) (9–11). Recent cross-sectional studies have found a higher incidence of VTE in the overall IMID population than in the general population (12). In addition, many observational studies have reported a higher incidence of VTE in patients with IBD (UC and CD) (13, 14), RA (15, 16), PSO (17), and SLE (18), than in those without these diseases.
Despite proposing the existence of a relationship between IMIDs and VTE, most of these studies were limited by observational designs and small sample sizes that hamper effective causal inferences and can be easily affected by several confounding factors. In contrast, Mendelian randomization (MR) analysis is an emerging method in epidemiological research that uses genetic variants as instrumental variables (IVs) to help assess the causal effects of exposure factors on outcomes while minimizing the impact of confounding factors and reverse causation (19).
The availability of large-scale genome-wide association studies (GWAS) on IMIDs and cardiovascular disease made it possible to implement MR analysis to investigate the relationship between various diseases. For example, Gao et al. (20) established a causal relationship between SLE and cardiovascular disease, while Li et al. suggested that varicose veins may have a causal role in DVT (21). However, to date, no MR studies focused on the effect caused by IMIDs on VTE risk. To explore this possibility, we implemented a two-sample MR analysis using newly published GWAS data on various IMIDs (including IBD, UC, CD, RA, PSO, and SLE) and VTE (including DVT, and PE).
2 Methods
2.1 Two-sample MR
A two-sample MR study was conducted to assess the existence of a causal relationship between genetic susceptibility to VTE (including subtypes of DVT and PE) and IMIDs (including IBD, UC, CD, RA, SLE, PSO). Multiple single-nucleotide polymorphisms (SNP) representing global human genetic variation were selected as instrumental variables (IVs). Three key hypotheses of classical MR analysis were adopted, as follows (Figure 1): 1. IVs are directly related to exposure; 2. IVs are independent of any confounding variables; 3. IVs only affect the results via exposure (22).
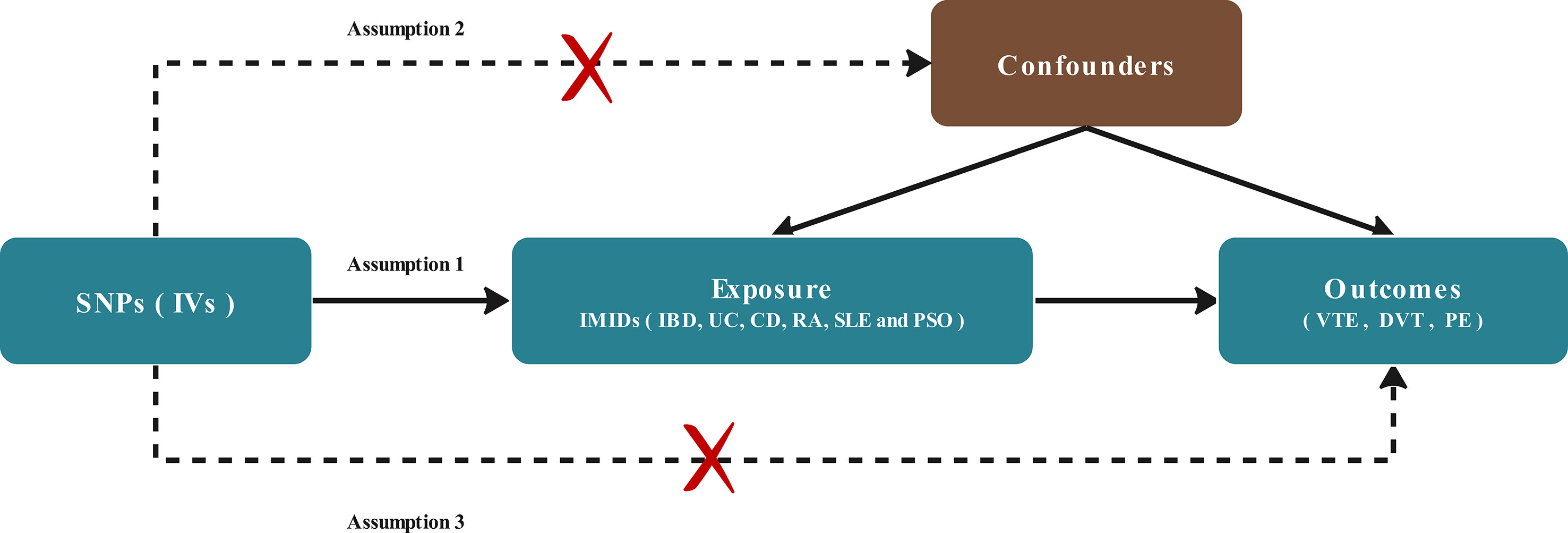
Figure 1 Diagram of the two-sample Mendelian randomization study for the association between IMIDs and risk of VTE/DVT/PE. IVs, instrumental variables; IMIDS, Immune-mediated inflammatory diseases IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; SLE, Systemic Lupus erythematosus; PSO, Psoriasis; VTE, Venous thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism.
2.2 Data sources and study design
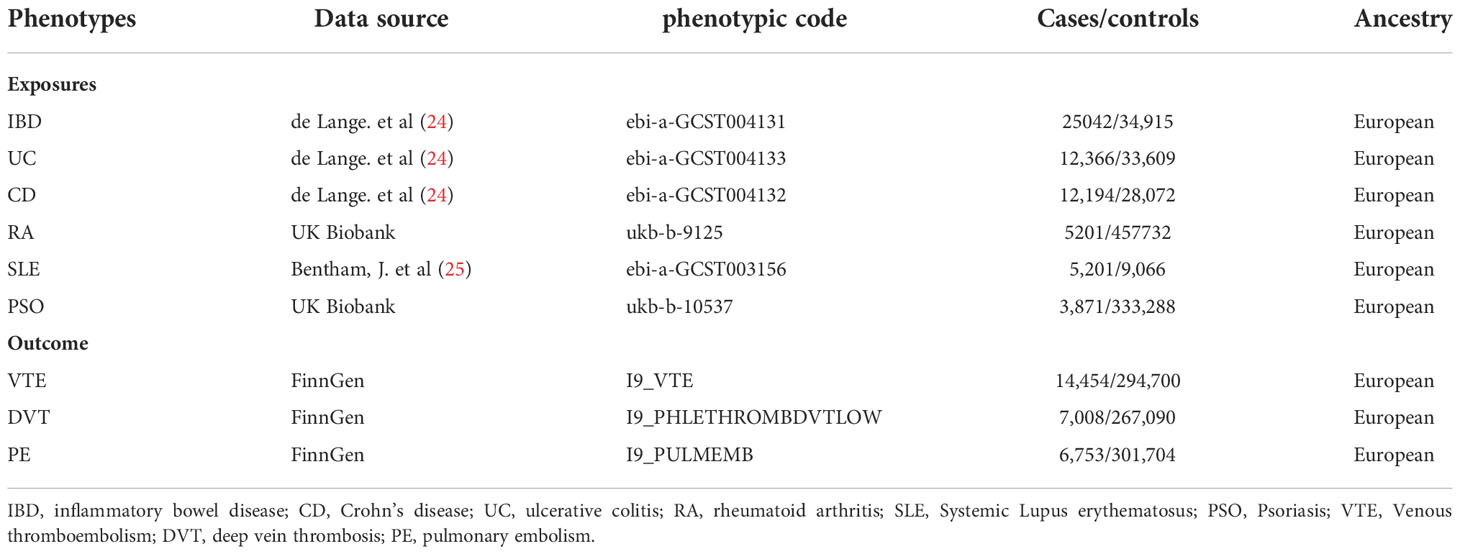
Summary-level statistical data for VTE, DVT, and PE were obtained from the latest R7 release of the FinnGen GWAS results (https://r7.finngen.fi/) (23). The corresponding phenotypic codes obtained were “I9_VTE” (14,454 cases and 294,700 controls), “I9_PHLETHROMBDVTLOW” (7,008 cases and 267,090 controls), and “I9_PULMEMB” (6,753 cases and 301,704 controls), respectively. The diagnosis of these cases was determined by the ICD codes.
We retrieved the summary IMIDs GWAS dataset from the IEU OpenGWAS Database Project (https://gwas.mrcieu.ac.uk/). SNPs associated with IBD were obtained from a GWAS study involving 25,042 patients and 34,915 controls, which were further subdivided into secondary outcomes of either UC (12,366 patients and 33,609 controls) or CD (12,194 patients and 28,072 controls) (24). The summary statistics for RA (5,201 patients and 462,933 controls) and PSO (5,314 patients and 462,933 controls) of European descent were obtained from the UK Biobank (http://www.nealelab.is/uk-biobank/). Finally, summary statistics for SLE were derived from a GWAS including 5,201 cases and 9,066 controls (25). The details of the data source and definition are listed in Table 1.
2.3 Instrumental variable selection
SNPs associated with each IMID at the genome-wide significance threshold p < 5.0 × 10-8 were selected as potential IVs. To ensure independence between the genetic variants used as IVs, we set the linkage disequilibrium (LD) threshold for grouping to r2 < 0.001 and a window size of 1000 kb. The SNP showing the lowest p-value at each locus was retained for analyses. The software PhenoScanner (26) was used to examine the associated phenotypes of each genetic variant. SNPs corresponding to the phenotypes associated with VTE were removed to prevent potential pleiotropic effects. In addition, we used the MR residual and outliers (MR-PRESSO) test to detect potential horizontal pleiotropy, and effectively controlled for pleiotropic effects by removing outliers.
Variance (R2) and F-statistics were used to estimate the strength of the selected IVs (27). R2 was calculated as follows: 2×(1-MAF)×MAF×β2 (EAF, effect allele frequency; β, effect size on the exposure). The F-statistic was calculated as follows: F=R2(N-K-1)/[K(1-R2)], where R2 refers to the portion of exposure variance explained by the IVs, N represents the effective sample size, and K represents the number of variants included in the IV model. An F-statistic >10 indicates a strong correlation between the IVs and exposure (28).
2.4 Statistical analyses
The “TwoSampleMR” and “MR-PRESSO” packages of the R software (version 4.13) were used to perform MR analysis. We used multiple MR methods to infer causal relationships between a total of six IMIDs and three VTE phenotypes, including inverse variance weighting (IVW) (29), median weighting (30), MR-Egger (31), and MR-pleiotropy residual sum and outlier (MR-PRESSO) (32).
Each method makes different assumptions on the validity of IVs, but the IVW method is generally regarded as the most reliable, so in this study, IVW was used as the primary method to identify the causal relationship between exposure and outcome, while the other methods were used as complementary or to provide other information. IVW is essentially a meta-analysis method combining cumulative causal estimates of Wald ratios for each IV, while MR-PRESSO can automatically detect and remove outliers in IVW linear regression to provide corrected MR estimates (32).
2.5 Sensitivity analyses
The Cochrane’s Q test was used to assess the heterogeneity of selected SNPs (P< 0.05), in which case the random effects IVW test was used to provide more conservative and robust estimates. Funnel plots were generated using the mr_funnel_plot function for visualizing the heterogeneity of IVs. In addition, directional pleiotropy was assessed and corrected based on the intercept obtained from the MR-Egger regression model analysis. Finally, we performed a leave-one-out sensitivity analysis to test whether the stability of the results was affected by a single SNP and generated a forest plot to illustrate the results.
3 Results
3.1 Selection of instrumental variables
SNPs associated with IMIDs were selected as IVs based on established quality control criteria (95 SNPs for IBD, 51 SNPs for UC, 76 SNPs for CD, 5 SNPs for RA, 40 SNPs for SLE, 20 SNPs for PSO). The F-statistics of the vast majority of these SNPs were above the threshold of 10, which indicated that they strongly represent IMIDs in the MR analysis. The detailed characteristics of these IVs are displayed in Supplementary Table S1.
3.2 Causal estimates of genetic susceptibility to IMIDs and VTE risk
We performed MR analysis on six IMIDs diseases, including IBD and two of its subtypes (UC and CD), RA, SLE, and PSO; with VTE, DVT and PE. This allowed us to test a total of 18 causality pairs, of which two were statistically significant. As shown in Figure 2, the IVW model indicated that genetically predicted UC is associated with a higher risk of VTE (OR = 1.043, 95% CI: 1.013-1.073, p = 0.004) and DVT (OR = 1.088, 95% CI: 1.043-1.136, p < 0.001). The MR-Egger regression, Weighted median, and MR-PRESSO analyses showed that the IVW association pattern remained directionally consistent in most statistical models, demonstrating the robustness of the inferred causal relationships between UC and VTE/DVT (Figure 2B). In addition, the risk of DVT in genetically predicted IBD patients had an increasing trend with marginal statistical effect in the IVW analysis (OR = 1.032, 95% CI: 0.999-1.066, p = 0.055) (Figure 2A).
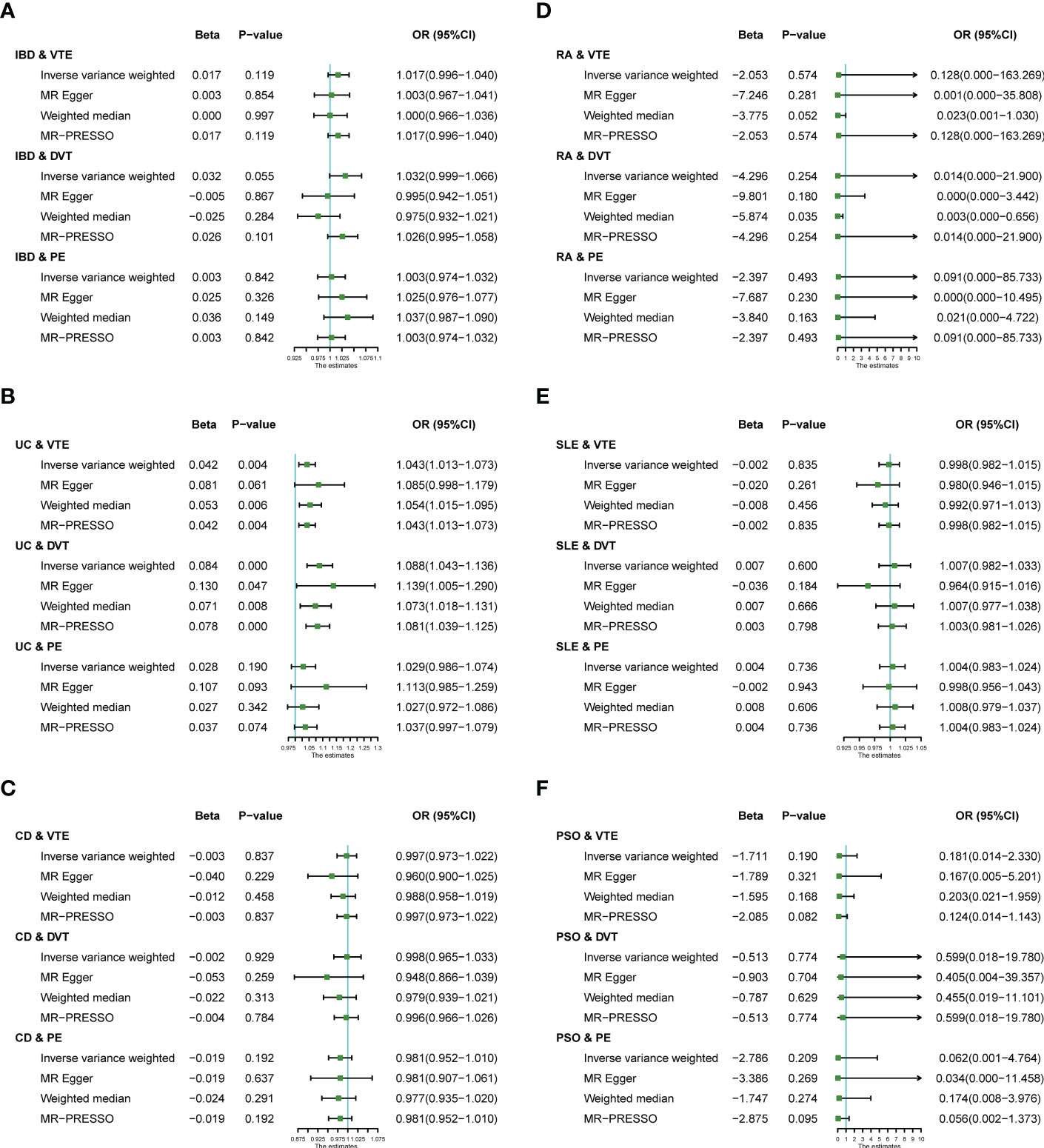
Figure 2 Estimates from Mendelian randomization analysis of IMIDs [(A) IBD; (B) UC; (C) CD; (D) RA; (E) SLE; (F) PSO] and risk of VTE/DVT/PE. OR, Odd Ratio; IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; SLE, Systemic Lupus erythematosus; PSO, Psoriasis; VTE, Venous thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism; CI, confidence interval.
Otherwise, there was no evidence of a causal relationship between UC and PE (OR = 1.029, 95% CI: 0.986-1.074), IBD and VTE (OR = 1.017, 95% CI: 0.996-1.040), IBD and PE (OR = 1.003, 95% CI: 0.974-1.032), CD and VTE (OR = 0.997, 95% CI: 0.973-1.022), CD and DVT (OR = 0.998, 95% CI: 0.965-1.033), CD and PE (OR = 0.981, 95% CI: 0.952-1.010), RA and VTE (OR = 0.128, 95% CI: 0.000-163.269), RA and DVT (OR = 1.014, 95% CI: 0.000-21.900), RA and PE (OR = 0.091, 95% CI: 0.000-85.733), SLE and VTE (OR = 0.998, 95% CI: 0.982-1.015), SLE and DVT (OR = 1.007, 95% CI: 0.982-1.033), SLE and PE (OR = 1.004, 95% CI: 0.983-1.024), PSO and VTE (OR = 1.181, 95% CI: 0.014-2.330), PSO and DVT (OR = 0.599, 95% CI: 0.018-19.780), PSO and PE (OR = 0.062, 95% CI: 0.001-4.764) in the IVW analysis results (Figures 2A-F).
3.3 MR sensitivity analysis
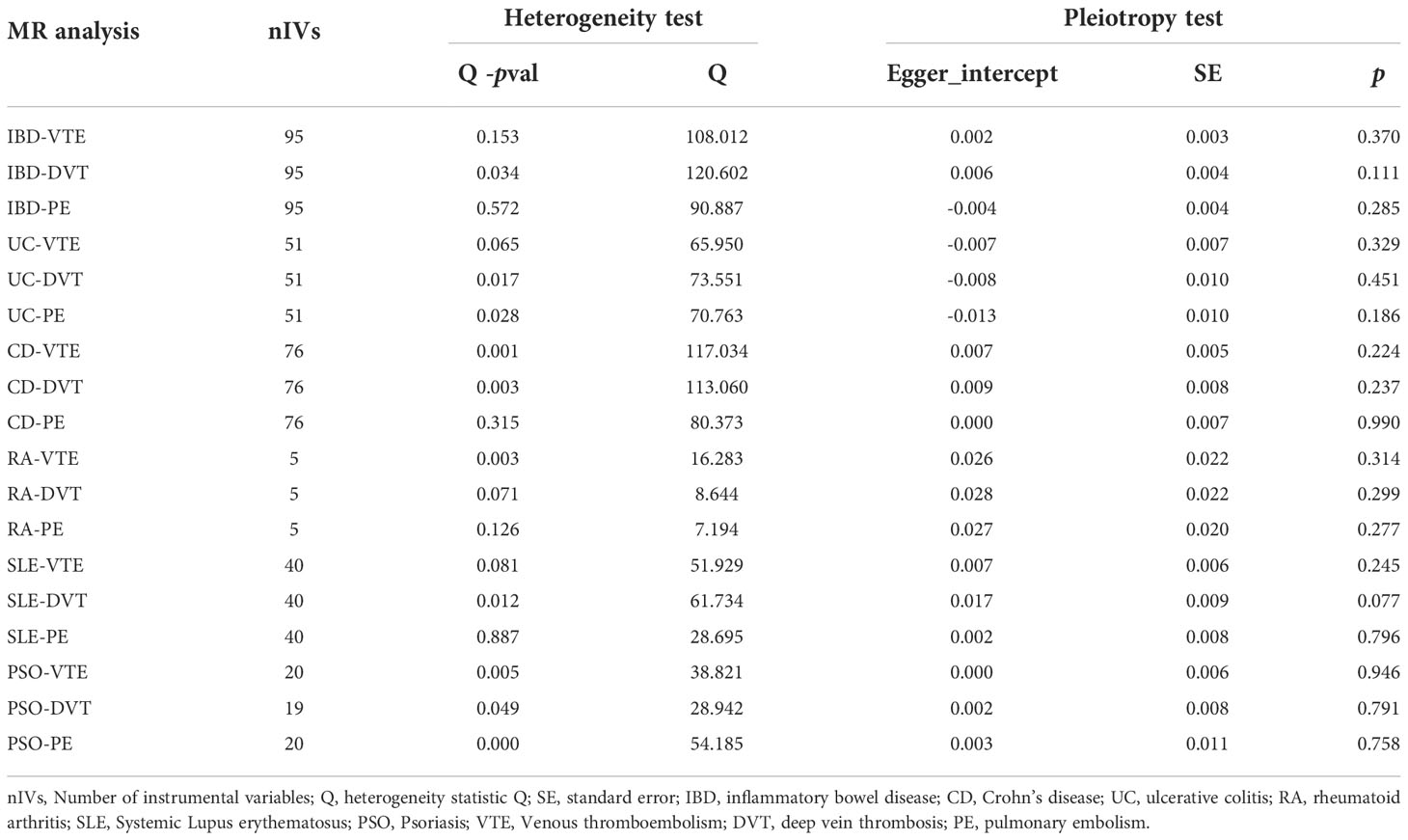
In the Cochran’s Q test, p-values of Q statistics in the IBD-DVT, UC-DVT, UC-PE, CD-VTE, CD-DVT, RA-VTE, SLE-DVT, PSO-VTE, PSO-DVT, PSO-PE analyses were lower than 0.05, indicating IVs heterogeneity (Table 2, Supplementary Figure S1) and justifying the use of a random effects model in these cases. The remaining IVW analyses were performed using a fixed-effects model instead. We note that no MR-Egger regression intercepts deviated from zero (Supplementary Figure S2), and no evidence for horizontal pleiotropy in the IMIDs’ IVs with VTEs (all intercept p > 0.05) (Table 2). Finally, the leave-one-out analysis confirmed no causal associations were driven by a specific IV (Supplementary Figure S3).
4 Discussion
The association between inflammatory diseases and the risk of venous thromboembolism has received increasing attention from the scientific community (6, 12, 33), but to our knowledge, this is the first study to systematically explore potential causal relationships between IMIDs and VTE risk using MR methods. Our findings suggest that genetic predisposition to UC (one subtype of IBD) is associated with an increased risk of VTE and DVT. However, no MR evidence supports potential causality between genetic predisposition to CD, RA, SLE, and PSO, and the risk of VTE/DVT/PE.
IMIDs constitute a diverse and pervasive spectrum of diseases driven by immune and genetic pathways and characterized by alterations in the cellular homeostasis of the body (9). In addition to affecting various parts of the body, systemic involvement is common in different IMIDs due to abnormal activation of the immune system and inflammatory pathways, which reportedly increases the risk of venous thrombosis. However, a lack of evidence from high-quality RCT studies based on the association between IMIDs and VTE risk and poor consistency across observational epidemiological studies make these relationships unclear.
In this study, we showed a causal relationship between genetically predicted UC and VTE/DVT, as well as an elevated DVT risk in total IBD. Previous observational studies reported an increased risk of thrombosis in IBD patients (34), in accordance with our findings based on MR analysis. Furthermore, two recent cohort studies based on Asian populations reported that IBD patients are 1.80-, 1.98- and 2-fold more likely to develop PE, DVT (35), and VTE (36), respectively. A meta-analysis summarizing data from 11 observational studies covering 3,175,012 IBD patients and 920,144,253 controls showed an RR of 2.03 (95% CI: 1.72-2.39) for the occurrence of VTE in the former group (37). However, these observational studies reported significant heterogeneity, as demonstrated by stratified analysis based on sample size showing lower VTE risk in larger samples (RR 1.77, 95% CI 1.48-2.13 in large sample size studies; versus RR 2.67, 95% CI 1.97-2.93 in smaller sample sizes). These observations highlight how observational findings are susceptible to sample size and confounding factors that can be overcome using MR analysis.
The IBD GWAS dataset used in this study combines UC and CD, whereby we performed separate MR analyses on total IBD, UC, and CD. Unlike previous studies which reported that the risk of VTE was increased in both CD and UC patients (35–37), the MR evidence provided by this study only showed a causal relationship between UC and VTE. Notably, the meta-analysis results published by Fumery, M et al. showed a significantly higher risk of VTE in UC inpatients than in CD inpatients (P = 0.0029) (14), which together with our findings suggested that UC patients may have a greater risk of VTE compared with CD patients.
Our separate PE and DVT analyses showed that genetically predicted UC was causally associated with DVT only, despite evidence for increased risk for both DVT and PE in IBD patients (DVT risk is nearly twice that of PE) (38, 39). Since PE is mostly a secondary event following DVT, it is expected that there is no direct causal association between IBD and PE, which may explain this seemingly contradictory result (40).
While previous controversial associations were found for other common IMIDs (i.e., RA, SLE, PSO) and VTE/PE/DVT risk, such relationships were not apparent in our study. Galloway et al. reported an increased risk of VTE in RA patients compared to matched controls (AHR 1.54, 95%CI 1.40-1.70), but found no evidence for an association between psoriasis and VTE risk (AHR 1.21, 95%CI 0.96 to 1.52) (12). Similarly, another large RA cohort study from North America reported associations with increased risk of VTE, PE, and DVT, but this and multiple studies noted that two different JAK inhibitors used to treat RA (i.e., baricitinib and tofacitinib) were also associated with an increased risk of thromboembolism, making this an important confounder (15, 41). In addition, a long-term cohort study found a higher risk of VTE in SLE patients compared with matched control subjects. Specifically, during a median follow-up of 8.5 years, the incidence of VTE was 6.03% (95%CI: 5.17%-6.98%) in SLE patients and 1.68% (95%CI: 1.44%-1.95%) in controls (42). However, results from an MR study of SLE and cardiovascular disease conducted by Gao et al. demonstrated no causal associations exist between SLE and VTE risk (OR=1.001, 95% CI [1.000-1.002]), which is consistent with our findings (20).
Despite extensive evidence from observational studies on the relationship between IMIDs and VTE risk, these approaches have obvious limitations and confounding factors, such as an inability to evaluate causal relationships between exposure and outcome. In addition, with glucocorticoids, immunosuppressants, and antithrombotic drugs widely used in the treatment of IMIDs, the role of these drugs in promoting or inhibiting thrombosis has become an important confounder in determining the relationship between IMIDs and the risk of VTE (9). For example, high doses of glucocorticoids significantly increase the risk of atherosclerosis and thrombosis (43, 44); immunosuppressants including azathioprine, cyclophosphamide, and anti-tumor necrosis factor drugs may help suppress thrombosis by controlling systemic inflammation and disease activity (43); interestingly, some immunosuppressive agents such as JAK inhibitors have been reported to have some adverse risk of thrombosis in RA treatment (45). The use of these drugs to inhibit or promote VTE in IMIDs has inevitably resulted in confounding bias in observational studies. By calculating the correlation between the genetically predicted IMIDs risk and the genetically predicted VTE risk, MR Study cleverly reduced the influence of confounding deviation in the intermediate process. This stresses the importance of using Mendelian randomization strategies to explore the risk of VTE in patients with IMIDs.
Our study is the first MR analysis to explore potential causal relationships between multiple IMIDs diseases (IBD, UC, CD, RA, SLE, PSO) and VTE risk (including DVT and PE). When compared to previous observational studies, our MR design circumvents traditional confounding factors and problems associated with reverse causation. Moreover, we tested these relationships on populations with the same ethnicity using large-scale GWAS data, which provides strong and reliable IVs and strengthens inferences of causality that were confirmed by sensitivity analyses. However, a lack of demographic data (e.g., sex and age) in the original study hampered further subgroup analyses. In addition, MR analyses were not performed in disease subcategories – for example, psoriasis included psoriasis-associated arthritis, which was not evaluated separately for an association with VTE risk. Due to the small number of available GWAS for our study subjects, we were unable to perform multi-database validation. Finally, it should be noted that GWAS data was used for people of European ancestry, whereby extrapolations to other ethnic groups are limited.
Conclusion
This MR study provides new genetic evidence for the relationship between IMIDs and VTE risk. We found the existence of a causal association between UC and VTE/DVT risk, but no evidence for causal associations with other IMIDs, including CD, RA, SLE, and PSO. Our findings highlight the importance of active intervention and monitoring to mitigate VTE risk in patients with IBD, especially those presenting with ulcerative colitis. Future longitudinal clinical studies and experimental analyses are needed to confirm our findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JW designed, guided, and funded the study. XL conducted most of the MR analysis and draft of the manuscript. XG draft the manuscript and prepare the figure and Table. JL, QN and YD conducted data acquisition and provided technical support. XF, ZY and PL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 82270443, 81670275, 81670443, 82170066) and the International S&T cooperation program (2013DFA31900).
Acknowledgments
We want to acknowledge the participants and investigators of the FinnGen and UK Biobank studies, and we thank the IEU Open GWAS project for providing summary data publicly.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LW declared a shared parent affiliation with the authors XL and XG to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1042751/full#supplementary-material
Supplementary Table 1 | The detailed characteristics of IVs.
Abbreviations
MR, mendelian randomization; IMIDs, immune-mediated inflammatory diseases; VTE, venous thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism; IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; SLE, systemic Lupus erythematosus; PSO, psoriasis.
References
1. Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet (London England) (2021) 398(10294):64–77. doi: 10.1016/s0140-6736(20)32658-1
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol (2015) 12(8):464–74. doi: 10.1038/nrcardio.2015.83
3. Horner D, Goodacre S, Davis S, Burton N, Hunt BJ. Which is the best model to assess risk for venous thromboembolism in hospitalised patients? BMJ (2021) 373:n1106. doi: 10.1136/bmj.n1106
4. Yamashita Y, Morimoto T, Kimura T. Venous thromboembolism: Recent advancement and future perspective. J Cardiol (2022) 79(1):79–89. doi: 10.1016/j.jjcc.2021.08.026
5. Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE. European Union-28: An annualised cost-of-Illness model for venous thromboembolism. Thromb Haemostasis (2016) 115(4):800–8. doi: 10.1160/th15-08-0670
6. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol (2021) 18(9):666–82. doi: 10.1038/s41569-021-00552-1
7. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol (2013) 13(1):34–45. doi: 10.1038/nri3345
8. Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in covid-19. Nat Rev Immunol (2021) 21(5):319–29. doi: 10.1038/s41577-021-00536-9
9. McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: Past, present and future. Nat Rev Immunol (2021) 21(10):680–6. doi: 10.1038/s41577-021-00603-1
10. Agca R, Smulders Y, Nurmohamed M. Cardiovascular disease risk in immune-mediated inflammatory diseases: Recommendations for clinical practice. Heart (British Cardiac Society) (2022) 108(1):73–9. doi: 10.1136/heartjnl-2019-316378
11. Faye AS, Lee KE, Dodson J, Chodosh J, Hudesman D, Remzi F, et al. Increasing rates of venous thromboembolism among hospitalised patients with inflammatory bowel disease: A nationwide analysis. Aliment Pharmacol Ther (2022) 56(7):1157–67. doi: 10.1111/apt.17162
12. Galloway J, Barrett K, Irving P, Khavandi K, Nijher M, Nicholson R, et al. Risk of venous thromboembolism in immune-mediated inflammatory diseases: A uk matched cohort study. RMD Open (2020) 6(3):e001392. doi: 10.1136/rmdopen-2020-001392
13. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: A cohort study. Lancet (London England) (2010) 375(9715):657–63. doi: 10.1016/s0140-6736(09)61963-2
14. Fumery M, Xiaocang C, Dauchet L, Gower-Rousseau C, Peyrin-Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: A meta-analysis of observational studies. J Crohn's Colitis (2014) 8(6):469–79. doi: 10.1016/j.crohns.2013.09.021
15. Li L, Lu N, Avina-Galindo AM, Zheng Y, Lacaille D, Esdaile JM, et al. The risk and trend of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: A general population-based study. Rheumatol (Oxford England) (2021) 60(1):188–95. doi: 10.1093/rheumatology/keaa262
16. Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C, Knight EL. Risk of venous thromboembolism in patients with rheumatoid arthritis: A systematic review and meta-analysis. Clin Rheumatol (2014) 33(3):297–304. doi: 10.1007/s10067-014-2492-7
17. Ungprasert P, Sanguankeo A, Upala S, Suksaranjit P. Psoriasis and risk of venous thromboembolism: A systematic review and meta-analysis. QJM Monthly J Assoc Phys (2014) 107(10):793–7. doi: 10.1093/qjmed/hcu073
18. Sciascia S, Radin M, Cecchi I, Rubini E, Foddai SG, Barinotti A, et al. Incidence of a first thrombo-embolic event in patients with systemic lupus erythematosus and anti-Phosphatidylserine/Prothrombin antibodies: A prospective study. Front Med (2021) 8:621590. doi: 10.3389/fmed.2021.621590
19. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
20. Gao N, Kong M, Li X, Wei D, Zhu X, Hong Z, et al. Systemic lupus erythematosus and cardiovascular disease: A mendelian randomization study. Front Immunol (2022) 13:908831. doi: 10.3389/fimmu.2022.908831
21. Li R, Chen Z, Gui L, Wu Z, Miao Y, Gao Q, et al. Varicose veins and risk of venous thromboembolic diseases: A two-Sample-Based mendelian randomization study. Front Cardiovasc Med (2022) 9:849027. doi: 10.3389/fcvm.2022.849027
22. Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ (2018) 362:k601. doi: 10.1136/bmj.k601
23. Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv (2022). doi: 10.1101/2022.03.03.22271360
24. de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet (2017) 49(2):256–61. doi: 10.1038/ng.3760
25. Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet (2015) 47(12):1457–64. doi: 10.1038/ng.3434
26. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. Phenoscanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinf (Oxford England) (2019) 35(22):4851–3. doi: 10.1093/bioinformatics/btz469
27. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol (2011) 40(3):740–52. doi: 10.1093/ije/dyq151
28. Burgess S, Thompson SG. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol (2011) 40(3):755–64. doi: 10.1093/ije/dyr036
29. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat Med (2008) 27(8):1133–63. doi: 10.1002/sim.3034
30. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
31. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through egger regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
32. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
33. Chopard R, Albertsen IE, Piazza G. Diagnosis and treatment of lower extremity venous thromboembolism: A review. Jama (2020) 324(17):1765–76. doi: 10.1001/jama.2020.17272
34. Olivera PA, Zuily S, Kotze PG, Regnault V, Al Awadhi S, Bossuyt P, et al. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol (2021) 18(12):857–73. doi: 10.1038/s41575-021-00492-8
35. Chung WS, Lin CL, Hsu WH, Kao CH. Inflammatory bowel disease increases the risks of deep vein thrombosis and pulmonary embolism in the hospitalized patients: A nationwide cohort study. Thromb Res (2015) 135(3):492–6. doi: 10.1016/j.thromres.2014.12.025
36. Kim SY, Cho YS, Kim HS, Lee JK, Kim HM, Park HJ, et al. Venous thromboembolism risk in Asian patients with inflammatory bowel disease: A population-based nationwide inception cohort study. Gut Liver (2022) 16(4):555–66. doi: 10.5009/gnl210190
37. Arvanitakis KD, Arvanitaki AD, Karkos CD, Zintzaras E, Germanidis GS. The risk of venous thromboembolic events in patients with inflammatory bowel disease: A systematic review and meta-analysis. Ann Gastroenterol (2021) 34(5):680–90. doi: 10.20524/aog.2021.0631
38. Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (Vte) in europe. the number of vte events and associated morbidity and mortality. Thromb Haemostasis (2007) 98(4):756–64. doi: 10.1160/TH07-03-0212
39. Anderson FA Jr., Zayaruzny M, Heit JA, Fidan D, Cohen AT. Estimated annual numbers of us acute-care hospital patients at risk for venous thromboembolism. Am J Hematol (2007) 82(9):777–82. doi: 10.1002/ajh.20983
40. Wenger N, Sebastian T, Engelberger RP, Kucher N, Spirk D. Pulmonary embolism and deep vein thrombosis: Similar but different. Thromb Res (2021) 206:88–98. doi: 10.1016/j.thromres.2021.08.015
41. Harigai M. Growing evidence of the safety of jak inhibitors in patients with rheumatoid arthritis. Rheumatol (Oxford England) (2019) 58(Suppl 1):i34–42. doi: 10.1093/rheumatology/key287
42. Yafasova A, Fosbøl EL, Schou M, Baslund B, Faurschou M, Docherty KF, et al. Long-term cardiovascular outcomes in systemic lupus erythematosus. J Am Coll Cardiol (2021) 77(14):1717–27. doi: 10.1016/j.jacc.2021.02.029
43. Silvestri E, Scalera A, Emmi G, Squatrito D, Ciucciarelli L, Cenci C, et al. Thrombosis in autoimmune diseases: A role for immunosuppressive treatments? Semin Thromb Hemost (2016) 42(6):650–61. doi: 10.1055/s-0036-1579642
44. Simion C, Campello E, Bensi E, Bellio A, Pontarin A, Spiezia L, et al. Use of glucocorticoids and risk of venous thromboembolism: A narrative review. Semin Thromb Hemost (2021) 47(6):654–61. doi: 10.1055/s-0040-1722270
45. Berthe P, Scailteux LM, Lescoat A, Staumont D, Coiffier G, Guéret P, et al. Oral janus kinase inhibitors and venous thromboembolic events in atopic dermatitis: Protocols for a case-time control study and a nested case-control study based on the French national health insurance (Snds) cohort. BMJ Open (2022) 12(9):e059979. doi: 10.1136/bmjopen-2021-059979
Keywords: venous thromboembolism, immune-mediated inflammatory diseases, Mendelian randomization, inflammatory bowel disease, ulcerative colitis
Citation: Lv X, Gao X, Liu J, Deng Y, Nie Q, Fan X, Ye Z, Liu P and Wen J (2022) Immune-mediated inflammatory diseases and risk of venous thromboembolism: A Mendelian randomization study. Front. Immunol. 13:1042751. doi: 10.3389/fimmu.2022.1042751
Received: 13 September 2022; Accepted: 29 November 2022;
Published: 13 December 2022.
Edited by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TurkeyReviewed by:
Laiyuan Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaShizheng Qiu, Harbin Institute of Technology, China
Ariela Hoxha, University Hospital of Padua, Italy
Copyright © 2022 Lv, Gao, Liu, Deng, Nie, Fan, Ye, Liu and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianyan Wen, amlhbnlhbndlbkBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiaoshuo Lv1,2†
Xiaoshuo Lv1,2† Jingwen Liu
Jingwen Liu Jianyan Wen
Jianyan Wen