- Department of Environmental Immuno-Dermatology, Graduate School of Medicine, Yokohama City University, Yokohama, Japan
The skin is the outermost layer and largest organ in the human body. Since the skin interfaces with the environment, it has a variety of roles, including providing a protective barrier against external factors, regulating body temperature, and retaining water in the body. It is also involved in the immune system, interacting with immune cells residing in the dermis. Caveolin-1 (CAV-1) is essential for caveolae formation and has multiple functions including endocytosis, lipid homeostasis, and signal transduction. CAV-1 is known to interact with a variety of signaling molecules and receptors and may influence cell proliferation and migration. Several skin-related disorders, especially those of the inflammatory or hyperproliferative type such as skin cancers, psoriasis, fibrosis, and wound healing, are reported to be associated with aberrant CAV-1 expression. In this review, we have explored CAV-1 involvement in skin physiology and skin diseases.
1. Introduction
The skin is the outermost layer and the largest organ in the human body. It consists of three layers: the epidermis, dermis, and skin-associated adipose tissue (1). The skin-associated adipose tissue includes dermal and subcutaneous adipocytes. Moreover, although these two layers are not physically distinguished in humans, they are likely functionally different. The epidermis is the outermost of the three layers, primarily consisting of keratinocytes, melanocytes, immune cells, and Merkel cells (2). The dermis is the layer beneath the epidermis, and its main components are non-cellular connective tissue collagen, elastic fibers, and the extracellular matrix (ECM), as well as cellular components, fibroblasts, macrophages, and mast cells (3). The dermis also contains skin appendages, such as hair follicles, sebaceous glands, and sweat glands, which are important components of the skin (3). Adipocytes are the prominent cells in the adipose tissue and are derived from mesenchymal fibroblast precursor cells known as preadipocytes. Immune cells are the second most common cell type (4).
Since the skin interfaces with the environment, it plays a variety of roles, which include providing a protective barrier against environmental factors such as bacteria or mechanical stress, regulating body temperature, and retaining water in the body (5). In addition, the skin plays a role in immunity by interacting with the immune cells within the dermis (1). Subcutaneous fat infiltrates the dermis and increased adipocytes affect the proliferation of dermal fibroblasts in obese mice (6, 7). Additionally, dermal adipocytes reportedly modulate dermal structure by regulating extracellular matrix production in dermal fibroblasts (8).
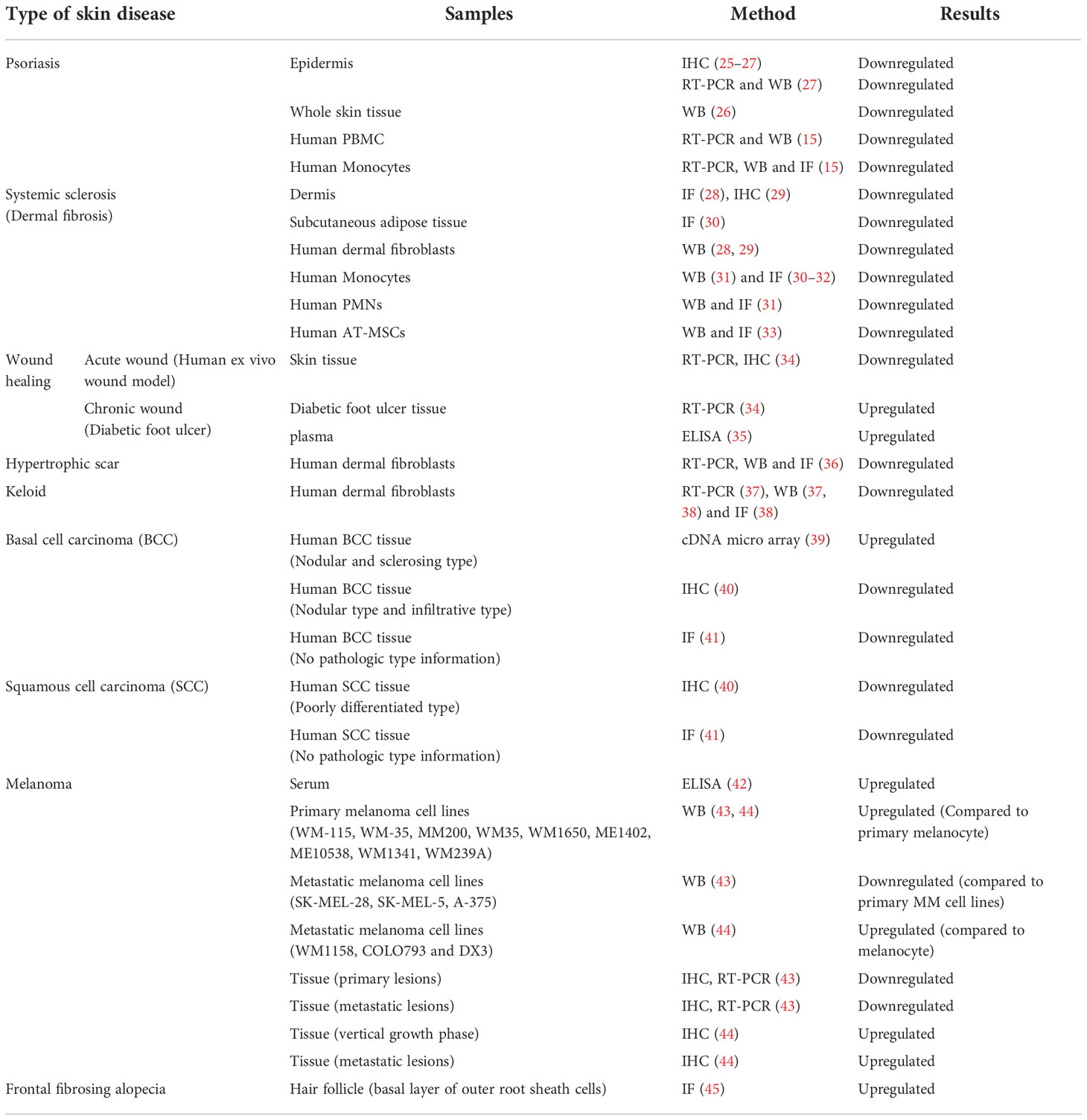
Caveolin-1 (CAV-1) is a 22 kDa membrane protein necessary for caveola formation. Caveola regulates a variety of signaling molecules and receptors that interact with the CAV-1 scaffolding domain (CSD), which corresponds to amino acid 82-101 of CAV-1 (9). CAV-1 has multiple functions, such as endocytosis, lipid homeostasis, and signal transduction; it is involved in cell proliferation and migration by associating with interacting molecules and receptors, such as Src family tyrosine kinases, integrins, epidermal growth factor receptor (EGFR), and transforming growth factor (TGF) receptors (10). Thus, numerous researchers have hypothesized the involvement of CAV-1 in the pathogenesis of inflammatory or hyperproliferative skin disorders, such as skin cancers, psoriasis, fibrosis, and wound healing. Although the contribution of CAV-1 in cell migration remains controversial depending on the cell type and the environment (11–14), CAV-1-regulated cell migration has also been reported to play a key role in skin diseases (15–19). CAV-1 involvement in skin diseases have garnered substantial attention (20–24). In this study, we focused on the latest findings on the role of CAV-1 in various skin diseases. The expression of CAV-1 in skin disorders has been investigated using several techniques (Table 1).
2 Distribution of CAV-1 in the skin
CAV-1 is expressed in most components of the skin, including keratinocytes (46), melanocytes (47), dermal fibroblasts (28), subcutaneous white adipocytes (48) and immune cells (49). CAV-1 expression in the epidermis is most prominent in the basal and granular layers (50). CAV-1 has been suggested to regulate keratinocyte differentiation (46, 50). Decreased CAV-1 levels in keratinocytes and fibroblasts result in enhanced cell proliferation (27, 28). In melanocytes, skin pigmentation induced by UV irradiation may be modulated by CAV-1 through regulation of cyclic adenosine monophosphate (cAMP) levels (47). In adipocytes, caveolae have been reported to regulate insulin signaling (51), fatty acid transportation (52), triacylglycerol synthesis (53) and adiponectin secretion (48), indicating the involvement of CAV-1 in metabolic dysfunction.
CAV-1 is also expressed in immune cells; notably, aberrant CAV-1 expression was found in the monocytes of patients with systemic sclerosis and psoriasis and CAV-1 deficient monocytes are hypermigratory towards disease sites (15, 31).
Moreover, CAV-1 is expressed in the hair follicles (54). In C57B6 mice, CAV-1 was found in the bulge area, in which cells are multipotent and have high proliferative potential, and CAV-1 was expressed during all stages of the hair growth cycle: anagen (growing phase), catagen (transition phase), and telogen (resting phase) (54). CAV-1 expression is upregulated in the bulge area of patients with frontal fibrosing alopecia compared to healthy controls, and it is speculated that CAV-1 upregulation may contribute to the pathogenesis of alopecia (45).
While there have been no studies investigating the expression of CAV-1 in other skin appendages, sweat glands, and sebaceous glands, Kruglikov et al. speculated that CAV-1 may be involved in sebocyte function because CAV-1 interacts with TGF-β signaling and adiponectin, which regulate lipid production (55, 56).
3 Role of CAV-1 in skin aging
Skin aging is characterized by functional and regenerative potential losses (57). Chronologically-aged skin typically shows decreased numbers of keratinocytes, fibroblasts, and mast cells, resulting in epidermal and dermal atrophy. A significant expansion of the dermal white adipose tissue is also observed in aged skin (58, 59). During skin aging, senescent fibroblasts have impaired growth factor production and activated matrix metalloproteinases (MMPs), leading to decreased cell proliferation and enhanced degradation of the ECM, including collagen (60). Increased production of reactive oxygen species (ROS) (61), mitochondrial dysfunction (62), and DNA and oxidative damage caused by external factors (63) may contribute to age-related skin changes and pathologies.
CAV-1 contributes to cellular senescence, and its upregulation has been observed in senescent cells of several types, such as epithelial cells, fibroblasts, mesenchymal stem cells, and bone marrow stromal cells (64–66). Additionally, CAV-1 knock-out mice showed aging-related phenotypes along with mitochondrial dysfunction (67). The role of CAV-1 in cellular senescence is not fully understood and remains disputed. One study suggested that upregulation of CAV-1 by oxidative stress promotes G1 arrest and activation of the p53/p21 dependent pathway, which induces premature senescence in dermal fibroblasts (68), whereas another study showed that CAV-1 upregulation in human diploid fibroblasts inhibits cell proliferation by directly binding to growth factor receptors, causing senescence-associated growth arrest (69). Furthermore, CAV-1 silencing in senescent human diploid fibroblasts resulted in morphological changes to a young cell-like small spindle shape, probably by altering focal adhesion and actin stress fiber formation due to focal adhesion kinase and Rho family GTPase regulation (70). In contrast, CAV-1 deficiency induces cellular senescence via the p53/p21-dependent pathway along with mitochondrial dysfunction in several cell lines, including human diploid fibroblasts (71, 72).
The expression of CAV-1 is higher in the skin and macrophages from older mice (24 months of age) compared to that of younger mice (8-10 weeks) (73), and aged human skin (70–80 years old) has higher CAV-1 expression levels than those of teenagers (74). A negative correlation was observed between the expression levels of CAV-1 and collagen I in chronologically aged human and mouse skin, and CAV-1 silencing or depletion facilitated collagen production in dermal fibroblast (74). Senescent dermal fibroblasts induced by diabetes show CAV-1 upregulation, which may be due to oxidative stress, while inhibition of CAV-1 prevents diabetes- and oxidative stress-induced premature senescence and enhances wound healing (68).
Impaired cell proliferation is a characteristic of aged tissues. CAV-1 regulates cell proliferation in the epidermis and dermis (18, 25, 75). Keratinocyte proliferation is regulated by CAV-1, likely through the EGFR signaling pathway and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway (25, 75), and fibroblast cell proliferation is regulated by CAV-1, likely through phosphatidylinositol 3-kinase (PI3K)/Akt and Rho-associated kinase (ROCK) (18). Interestingly, the EGFR signaling, JAK/STAT, ROCK, and PI3K/Akt pathways possibly interact with p53-dependent pathways in other cell types (76–79), suggesting that CAV-1 may regulate cell proliferation in senescent keratinocytes and fibroblasts through these signaling pathways by interacting with p53-dependent pathways.
CAV-1 also affects the differentiation of keratinocytes, fibroblasts, and adipocytes. Sando et al. showed that CAV-1 expression is increased during the differentiation of human keratinocytes, possibly associated with protein kinase C (50). CAV-1 is also involved in human adipocyte differentiation, and was upregulated in mature adipocytes derived from human mesenchymal stem cells (hMSC) isolated from subcutaneous adipose tissue along with polymerase I and transcript release factor (PTRF). PTRF is essential for caveolae formation and is highly expressed in adipose tissue. PTRF and CAV-1 upregulation disrupted adipogenesis in a mouse adipocyte cell line (3T3-L1 cells) (80). PTRF upregulation in hMSC resulted in impaired cell proliferation and differentiation into adipocytes, and PTRF silencing promoted new adipocyte formation along with decreased p53 expression levels (81). Taken together, these results suggest that CAV-1 upregulation may play an important role in skin aging by modulating cell proliferation, differentiation, and abnormal regulation of ECM deposition through various pathways via crosstalk with the p53/p21 dependent pathway.
4 Role of CAV-1 in skin diseases
4.1 Role of CAV-1 in psoriasis
Psoriasis is a chronic immune-mediated inflammatory skin disease characterized by scaly skin plaques (82). Its pathogenesis is thought to be by inflammatory cytokines (e.g., tumor necrosis factor [TNF]-α, interleukin [IL]-17, IL-22, and IL-23) produced by immune cells infiltrating the dermis, leading to epidermal hyperproliferation (83). Psoriasis not only affects the skin but is also systemic, which results in an increased risk of comorbidities, such as psoriatic arthritis, cardiovascular disease, diabetes mellitus, obesity, and atherosclerosis, compared with the general population (84, 85).
We and others have reported that CAV-1 expression is decreased in the epidermis of patients with psoriasis (25–27). We found that psoriasis-related cytokines, namely IL-17, IL-22, IL-1β, and TNF-α, reduced CAV-1 expression in human keratinocytes. Reduced CAV-1 expression in keratinocytes showed enhanced cell proliferation via increased STAT-3 activation and enhanced cytokine production, including C-X-C chemokine ligand 8 (CXCL8), CXCL9, C-C chemokine ligand 20 (CCL20), and IL-6 (27). In patients with psoriasis, CAV-1 expression is also reduced in monocytes, and CAV-1 silencing in healthy monocytes enhances the production of cytokines such as IL-1 and IL-6 and migration towards CCL2 (15). Decreased CAV-1 expression in monocytes was reversed when patients were treated with anti-TNF-α antibodies, suggesting that psoriasis-related inflammatory cytokines also reduce CAV-1 expression in monocytes (15). Moreover, circulating monocytes in patients with psoriasis are innately polarized to the M1 phenotype, which contributes to the development of atherosclerosis (86). Silencing of CAV-1 expression in healthy monocytes prompts the polarization of macrophages to the M1 phenotype and might increase the risk of atherosclerosis by increasing macrophage oxidized low-density lipoprotein uptake (86). Leptin, an adipocyte-derived hormone, is correlated with obesity and psoriasis severity (87, 88). CAV-1 and the leptin receptor are co-localized in keratinocytes, and CAV-1 silenced human keratinocytes produce more IL-6 by co-stimulation with leptin and IL-17, suggesting that obesity deteriorates psoriasis by enhancing cytokine production in CAV-1 deficient psoriasis keratinocytes (89).
An in vivo model also revealed the contribution of CAV-1 to the pathogenesis of psoriasis. Imiquimod (IMQ)-induced psoriasis-like inflammation in mice showed reduced CAV-1 expression in the epidermis and monocytes, and restoration of CAV-1 function improved the severity of skin inflammation and monocyte migration into the dermis (15, 27).
This evidence suggests that CAV-1 plays an important role in the development of psoriatic skin inflammation and its comorbidities, and may accelerate chronic inflammation.
4.2 Role of CAV-1 in fibrotic disorders
4.2.1 Systemic sclerosis
Systemic sclerosis (SSc) is an autoimmune-triggered disease characterized by vasculopathy and excessive collagen accumulation in the skin and internal organs with a high mortality (90). The ECM of the skin and internal organs promotes fibrosis (91, 92), and fibroblasts are considered effector cells (93). Several growth factors, such as TGF-β, connective tissue growth factor, and platelet-derived growth factor, can activate the pro-fibrotic response of fibroblasts (94, 95). A reduction of the thickness of dermal adipose layer is observed in SSc (96, 97), and dWAT is also reportedly involved in the development of skin fibrosis through adipocyte-myofibroblast transition (98).
Reduced CAV-1 expression has been reported in SSc-affected skin and dermal fibroblasts isolated from patients with SSc (28). TGF-β1 decreases CAV-1 expression in human skin fibroblasts in a time and dose-dependent manner (37). Restoring CAV-1 function in these fibroblasts suppresses TGF-β1-induced alpha-smooth muscle actin (α-SMA) by inhibiting the phosphorylation of Smad3, indicating that CAV-1 reduction enhances TGF-β signaling in the fibrotic response. In addition, TGF-β receptors may be directly inhibited by caveolae-mediated internalization (99).
Fibroblasts differentiated from monocytes that migrate into the dermis are also involved in SSc pathogenesis (16). Monocytes from patients with SSc have reduced CAV-1 expression, increased C-X-C chemokine receptor 4 expression, and are hypermigratory towards its receptor in lung tissue (31, 100). Circulating and dermal monocytes in patients with SSc expressing C-C chemokine receptor type 5 (CCR5) and its ligands are highly expressed in fibrotic skin tissue. Additionally, complementary CAV-1 inhibit CCR5 expressing monocyte migration, indicating that CAV-1 is involved in dermal fibrosis by modulating monocyte and fibrocyte recruitment (16).
The functional role of CAV-1 in the pathogenesis of SSc was confirmed in an in vivo model. CAV-1 knock-out mice showed a fibrotic skin phenotype (28, 101), and skin fibroblasts from these mice showed significantly increased expression of collagen, α-SMA, and IL-6, and decreased MMP-3 expression compared to those of wild-type mice. Restoring CAV-1 function decreased TGF-β1-induced fibrotic markers and inflammatory cytokines in fibroblasts from CAV-1 knock-out mice, and reduced fibrotic responses in a bleomycin (BLM) -induced skin fibrosis mouse model (16, 28).
4.2.2 Wound healing
Wound healing is the process of skin regeneration in damaged tissue and includes increased cell proliferation, cell adhesion, and cell migration. Optimal wound healing occurs through the following processes (1): coagulation and hemostasis (2); inflammation (3); proliferation; and (4) wound remodeling with scar tissue formation (102, 103). CAV-1 contributes to this process by regulating cell proliferation and migration (34).
CAV-1 overexpression in the corneal epithelium of elderly individuals is associated with delayed wound healing (64). Jozic et al. reported an upregulation of CAV-1 in skin biopsy samples from non-healing chronic wound edges, and downregulation of CAV-1 was observed in acutely healing wounds, especially during the first 48 h of wound healing (34). They also showed that CAV-1 negatively regulates both the proliferation and migration of keratinocytes by associating with the glucocorticoid receptor and EGFR. The same authors also showed that depleting caveolae using a cholesterol-removing agent (methyl-β-cyclodextrin or mevastatin) restored EGF signaling, facilitated keratinocyte migration, and accelerated wound closure (34, 75). Further, they revealed the mechanisms of CAV-1-associated keratinocyte migration in wound healing. Increased cortisol production at wound site leads upregulation of CAV-1, which inhibit a glucocorticoid receptor repressor ArhGAP35, resulting in increased activation of Ras homolog family member A (RhoA) and diminished activation of Cell Division Cycle 42 (Cdc42) promoting keratinocyte migration and wound closure (17).
4.2.3 Scarring (hypertrophic scars and keloids)
Hypertrophic scars (HTS) and keloids are thick raised scars commonly observed during the wound healing process as a result of an abnormal tissue response to injury. HTS and keloids have excess ECM components such as collagen, in the dermis and subcutaneous skin tissue and are sometimes considered to be fibroproliferative skin disorders. Both scar types have a similar disease spectrum, but HTS tends to be milder and does not expand beyond the boundaries of the original skin injury compared with keloids (104, 105). Some proinflammatory factors such as TGF-β, IL-1α, IL-1β, IL-6, and TNF-α, are upregulated in HTS and keloid tissues, making the skin more susceptible to trauma or injury (106, 107). CAV-1 expression was markedly decreased in HTS and keloid-derived human fibroblasts, and reduced CAV-1 expression mediates fibrotic responses by modulating TGF-β signaling, similar to SSc (36, 37).
Microarray results from seven Japanese patients with keloids revealed that Runt-related transcription factor 2 (RUNX2) is an upstream regulator of ECM (38). RUNX2 is a transcription factor (108) known to mediate ECM remodeling and induce aortic fibrosis (109). It regulates cell proliferation, migration, and the expression levels of ECM-related proteins and promotes apoptosis, possibly by suppressing the PI3K/Akt signaling pathway in keloid fibroblasts (18). RUNX2 expression is upregulated in keloid fibroblasts, and silencing of CAV-1 in keloid fibroblasts results in increased RUNX2 expression, which suggests that CAV1 plays a critical role in keloid formation by suppressing RUNX2 (38).
Phosphorylated CAV-1 and ROCK are upregulated in the peripheral skin tissue surrounding keloids, but not in normal skin and keloid sites, and CAV-1/ROCK expression correlates with a high inflammatory and proliferative status (110). The ROCK pathway is associated with phosphorylated CAV-1 (14), and this pathway is reported to contribute to cell proliferation (111), suggesting that the CAV-1/ROCK pathway may contribute to keloid expansion (110).
4.3 Role of CAV-1 in skin cancer
The function of CAV-1 as an oncogene or tumor suppressor in cancer progression remains controversial. Aberrant CAV-1 expression has been reported in several types of cancers and CAV-1 expression level sometimes depends on the tumor’s pathological subtype or clinical staging. For example, downregulation of Cav-1 has been reported in pancreatic cancer cell lines (112), primary and metastatic ovarian cancers (113), metastatic breast cancer cell lines (114), primary laryngeal squamous cell carcinoma cell lines (115), low-grade lung adenocarcinomas (116) and Barrett esophageal adenocarcinoma correlating with poor survival rates (117). In contrast, increased CAV-1 expression has been reported in moderate to severe prostate cancer (118), bladder cancer correlating with tumor grade and metastasis (119), metastatic renal cancer (120), and moderate to severe SCC of the oral cavity (moderate to severe), larynx, oropharynx, hypopharynx, esophagus (metastatic), and cervix (low grade) (121–124). Here in under, we explore the role of CAV-1 in cutaneous cancer.
4.3.1 BCC
Basal cell carcinoma (BCC) is the most common skin cancer and frequently develops in areas of the skin exposed to the sun, such as the face. BCC typically grows slowly and rarely metastasizes (125).
CAV-1 expression in BCC remains controversial. Microarray profiles of 50 BCC samples in one study showed that CAV-1 gene expression was upregulated; they proposed that CAV-1 may play a dynamic role in controlling the slow progression of BCC by decreasing cellular motility since CAV-1 is known to inhibit epidermal growth factor-induced migration in other cell types (11, 39). In contrast, Gheida et al. showed a significant downregulation of CAV-1 in BCC pathological samples compared to healthy controls. Furthermore, CAV-1 expression was significantly reduced in aggressive types (micronodular, infiltrative, and metatypical BCC) compared to non-aggressive types (nodular and superficial BCC) of BCC, suggesting that CAV-1 expression levels could reflect the biological behavior of BCC and aid in the detection of high-risk patients with poor prognosis (40).
4.3.2 SCC
Cutaneous SCC (cSCC) is the second most common type of non-melanoma skin cancer, after BCC. It is characterized by an abnormal, accelerated growth of squamous cells, requires surgical excision in most cases, and may lead to recurrence, metastasis, and death (126).
CAV-1 is significantly decreased in poorly differentiated types of cSCC, compared with moderately and well-differentiated types (40, 41). Trimer et al. showed that CAV-1 overexpression reduced cell growth in mouse and human SCC cell lines (127). In the same report, silencing CAV-1 in a mouse SCC cell line resulted in hyperactivation of the ERK1/2 and mitogen-activated protein kinase (MAPK) signaling pathways and increased activator protein (AP)-1 transcription factor activation. CAV-1 colocalizes and interacts with connexin 43, a known tumor suppressor (41), and it has been speculated that loss of CAV-1 affects the localization of connexin 43 and increases the activation of Ras/AP-1 signaling (40). Taken together, these findings indicate that aberrant CAV-1 expression in cSCC results in uncontrolled cell proliferation, survival, and invasion by altering multiple transduction pathways.
4.3.3 Malignant melanoma
Cutaneous malignant melanoma (cMM) is a skin cancer that develops from melanocytes and melanin-producing cells. cMM is a life-threatening cancer because its proliferative and metastatic status is highly potentiated, and causes approximately 55500 deaths (0.7% of all cancer deaths) worldwide annually (128).
Nakashima et al. reported that CAV-1 overexpression in a human melanoma cell line resulted in decreased cell growth and motility (129), and other groups reported that high CAV-1 expression in stromal cells and melanoma cells was associated with longer survival in cMM patients with lymph node metastasis (130), and CAV-1 overexpression suppressed subcutaneous tumor growth (131). In contrast, some studies have reported that CAV-1 expression enhances metastasis in murine and human melanoma cell lines along with reduced E-cadherin and Rac-1 activation (131, 132). The same group reported that CAV1-enhanced melanoma cell migration, invasion, and metastasis in vivo via tyrosine-14 phosphorylation of CAV-1 by Src family kinases. Moreover, a transient decrease in CAV-1 phosphorylation by these kinase inhibitors prevented the early steps of lung metastasis in a murine melanoma cell line promoted by CAV-1 (132, 133). The same authors also revealed that CAV-1expressing murine melanoma cell lines showed decreased oxygen consumption, and CAV-1 expressing cells had enhanced intracellular ROS, leading to increased cell migration and invasion (19). ROS are reported to be highly expressed in cMM cells and are thought to induce DNA damage, leading to genetic alterations (134).
Aberrant CAV-1 expression has been implicated in chemotherapy efficacy. The MAPK signaling pathway plays a key role in cMM, and several BRAF and MEK inhibitors (BRAFi and MEKi, respectively) have been approved for patients with cMM harboring BRAFV600 mutations. In BRAF-mutated cMM, the efficacy of treatments targeting the MAPK pathway is high, but can decline because of the development of resistance (135). Das et al. reported the involvement of CAV-1 in BRAF inhibitor-resistant cMM cells, in which the upregulation of CAV-1 in BRAF inhibitor-resistant cMM cells led to overactivation of MAPK signaling and resulting in the suppression of BRAF inhibitors (136).
4.4 Role of CAV-1 in skin infection
Since caveolae directly interact with outer pathogens, such as viruses and bacteria, by modulating endocytosis (137, 138) and CAV-1 is expressed in all types of immune cells (49), CAV-1 is thought to be involved in skin infection. However, only a few studies have reported on CAV-1 and skin infections. Spaan et al. showed that CAV-1 involvement is associated with ovarian tumor deubiquitinase with linear linkage specificity (OTULIN) in severe skin necrosis after S. aureus infection (139). OTULIN is a linear deubiquitinase and a negative regulator of nuclear factor kB (NF-kB) signaling in the context of immunity and inflammation (140, 141), and patients with OTULIN mutations are known to be susceptible to bacterial or viral infections (139, 142). In OTULIN-deficient patients, CAV-1 accumulated in dermal fibroblasts and retained ADAM10, a cell surface receptor of α-toxin, suggesting that CAV-1 accumulation enhances the cytotoxicity of staphylococcal α-toxin (139). These results might explain why staphylococcal scalded skin syndrome (SSSS) is more severe in adults. SSSS is caused by the α -toxin produced by S. aureus, with blisters that spread to a large part of the body. Most patients with SSSS are infants and children, and the mortality rate is approximately 4%; however, when adults develop SSSS, the mortality rate increases to 60% (143). High CAV-1 expression in adult skin (74) may promote sensitivity to staphylococcal α-toxins. For other skin-resident bacteria, such as cutibacterium acnes and staphylococcus epidermidis, no study has reported the involvement of CAV-1 in the diseases caused by these bacteria. However, CAV-1 can possibly play some roles in infection with C. acnes or S. epidermidis since CAV-1 can be involved in the endocytosis pathway of some types of bacteria (144–146). Kruglikov et al. speculated that CAV-1 is involved in C. acnes infection (55).
(blank)For viral infections, several studies have reported on the internalization of the human papilloma virus (HPV) and herpes simplex virus 1 (HSV-1) (147–149). High-risk HPV infections, including HPV types 16, 18, and 31, are linked to Bowen’s disease (epidermal SCC in situ) (150). HPV type 31 enters keratinocytes via caveolae-dependent endocytosis (149, 151), suggesting that CAV-1 may affect tumor development by controlling HPV infection in keratinocytes. In contrast, HPV type 16 and HSV-1 internalization are not CAV-1 dependent (147–149).
5 CAV-1 as a therapeutic target in skin diseases
Various studies have suggested that the loss or gain of CAV-1 function may improve disease phenotype. The depletion of caveola accelerates wound closure by facilitating keratinocyte proliferation and migration (34, 75), and downregulation of CAV-1 in cMM enhances the efficacy of BRAF inhibitor treatment (136). Additionally, restoration of CAV-1 function may improve disorders associated with CAV-1 deficiency. To compensate for CAV-1 function, the CSD peptide (CSD, amino acids 82–101 of CAV-1) was used in several experimental settings. The CSD peptide has a sequence equivalent to CSD and is synthesized as a fusion peptide on the carboxyl-terminus of the antennapedia internalization sequence, which can cross the plasma membrane and functionally complement CAV-1 (152).
To observe the effect of the CSD peptide in psoriasis locally or systemically, we treated mice with IMQ-induced psoriasis-like inflammation by subcutaneous or intraperitoneal injections. Both CSD peptide administration routes in mice with IMQ-induced psoriasis-like inflammation showed a significantly improved phenotype and fewer infiltrating cells in the dermis compared to control mice, suggesting that CSD peptide likely improved inflammation through keratinocytes or monocytes (15, 27).
In SSc, intraperitoneal administration of CSD peptide in mice improved BLM-induced dermal fibrosis and lipodystrophy and attenuated monocyte migration into the dermis (16). In addition, CSD treatment of wild-type mice, which did not receive BLM, promoted thickening of the adipose cell layer (16).
In HTS and keloid-derived fibroblasts, CSD peptide was found to decrease ECM production in a mitogen-activated protein kinase-dependent manner and decrease TGF-β receptor I, suggesting that CSD peptide possibly ameliorates fibrosis in HTS and keloids (36, 37).
CSD subdomains (corresponding to amino acids 82-89, 88-95, and 94-101 of CSD) have also been reported to improve the skin and lung fibrosis phenotypes in BLM-treated mice. Bone marrow monocytes isolated from BLM-treated mice showed greatly enhanced migration in vitro towards CXCL12, and treatment with CSD and its subregions in these mice suppressed enhanced migration (153).
In conclusion, in this review, we have explored the role of CAV-1 in skin diseases. CAV-1 is involved in various skin diseases by regulating cell proliferation, migration, and enhancing proinflammatory cytokine production. Depletion or complementation of CAV-1 may improve hyperproliferative or inflammatory status, indicating that CAV-1 is a good therapeutic target in skin-related diseases.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of interest
YY declares research grants, and/or consulting fees, and/or speaker’s fees from AbbVie, Amgen, Astellas, Boehringer Ingelheim, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Japan.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nguyen AV, Soulika AM. The dynamics of the skin's immune system. Int J Mol Sci (2019) 20(8):1811. doi: 10.3390/ijms20081811
2. Yousef H, Alhaj M, Sharma S. Anatomy, Skin (Integument), Epidermis. (Treasure Island (FL):StatPearls) (2022).
3. Woodley DT. Distinct fibroblasts in the papillary and reticular dermis: Implications for wound healing. Dermatol Clin (2017) 35(1):95–100. doi: 10.1016/j.det.2016.07.004
4. Chen SX, Zhang LJ, Gallo RL. Dermal white adipose tissue: A newly recognized layer of skin innate defense. J Invest Dermatol (2019) 139(5):1002–9. doi: 10.1016/j.jid.2018.12.031
5. Madison KC. Barrier function of the skin: "la raison d'être" of the epidermis. J Invest Dermatol (2003) 121(2):231–41. doi: 10.1046/j.1523-1747.2003.12359.x
6. Ezure T, Amano S, Matsuzaki K. Infiltration of subcutaneous adipose layer into the dermal layer with aging. Skin Res Technol (2022) 28(2):311–6. doi: 10.1111/srt.13133
7. Ezure T, Amano S. Increased subcutaneous adipose tissue impairs dermal function in diet-induced obese mice. Exp Dermatol (2010) 19(10):878–82. doi: 10.1111/j.1600-0625.2009.00970.x
8. Zhang Z, Kruglikov I, Zhao S, Zi Z, Gliniak CM, Li N, et al. Dermal adipocytes contribute to the metabolic regulation of dermal fibroblasts. Exp Dermatol (2021) 30(1):102–11. doi: 10.1111/exd.14181
9. Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem (1998) 273(10):5419–22. doi: 10.1074/jbc.273.10.5419
10. Byrne DP, Dart C, Rigden DJ. Evaluating caveolin interactions: do proteins interact with the caveolin scaffolding domain through a widespread aromatic residue-rich motif? PloS One (2012) 7(9):e44879. doi: 10.1371/journal.pone.0044879
11. Zhang W, Razani B, Altschuler Y, Bouzahzah B, Mostov KE, Pestell RG, et al. Caveolin-1 inhibits epidermal growth factor-stimulated lamellipod extension and cell migration in metastatic mammary adenocarcinoma cells (MTLn3). transformation suppressor effects of adenovirus-mediated gene delivery of caveolin-1. J Biol Chem (2000) 275(27):20717–25. doi: 10.1074/jbc.M909895199
12. Katsuno-Kambe H, Parton RG, Yap AS, Teo JL. Caveolin-1 influences epithelial collective cell migration via FMNL2 formin. Biol Cell (2021) 113(2):107–17. doi: 10.1111/boc.202000116
13. Lin F, Pei L, Zhang Q, Han W, Jiang S, Lin Y, et al. Ox-LDL induces endothelial cell apoptosis and macrophage migration by regulating caveolin-1 phosphorylation. J Cell Physiol (2018) 233(10):6683–92. doi: 10.1002/jcp.26468
14. Grande-García A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, et al. Caveolin-1 regulates cell polarization and directional migration through src kinase and rho GTPases. J Cell Biol (2007) 177(4):683–94. doi: 10.1083/jcb.200701006
15. Takamura N, Yamaguchi Y, Watanabe Y, Asami M, Komitsu N, Aihara M. Downregulated caveolin-1 expression in circulating monocytes may contribute to the pathogenesis of psoriasis. Sci Rep (2019) 9(1):125. doi: 10.1038/s41598-018-36767-5
16. Lee R, Perry B, Heywood J, Reese C, Bonner M, Hatfield CM, et al. Caveolin-1 regulates chemokine receptor 5-mediated contribution of bone marrow-derived cells to dermal fibrosis. Front Pharmacol (2014) 5:140. doi: 10.3389/fphar.2014.00140
17. Jozic I, Abujamra BA, Elliott MH, Wikramanayake TC, Marjanovic J, Stone RC, et al. Glucocorticoid-mediated induction of caveolin-1 disrupts cytoskeletal organization, inhibits cell migration and re-epithelialization of non-healing wounds. Commun Biol (2021) 4(1):757. doi: 10.1038/s42003-021-02298-5
18. Lv W, Wu M, Ren Y, Luo X, Hu W, Zhang Q, et al. Treatment of keloids through Runx2 siRNA−induced inhibition of the PI3K/AKT signaling pathway. Mol Med Rep (2021) 23(1):55. doi: 10.3892/mmr.2020.11693
19. Díaz-Valdivia N, Simón L, Díaz J, Martinez-Meza S, Contreras P, Burgos-Ravanal R, et al. Mitochondrial dysfunction and the glycolytic switch induced by caveolin-1 phosphorylation promote cancer cell migration, invasion, and metastasis. Cancers (Basel) (2022) 14(12):2862. doi: 10.3390/cancers14122862
20. Egger AN, Rajabiestarabadi A, Williams NM, Resnik SR, Fox JD, Wong LL, et al. The importance of caveolins and caveolae to dermatology: Lessons from the caves and beyond. Exp Dermatol (2020) 29(2):136–48. doi: 10.1111/exd.14068
21. Kruglikov IL, Scherer PE. Caveolin as a universal target in dermatology. Int J Mol Sci (2019) 21(1):80. doi: 10.3390/ijms21010080
22. Kruglikov IL, Scherer PE. Caveolin-1 as a pathophysiological factor and target in psoriasis. NPJ Aging Mech Dis (2019) 5:4. doi: 10.1038/s41514-019-0034-x
23. Kruglikov IL, Scherer PE. Caveolin-1 as a target in prevention and treatment of hypertrophic scarring. NPJ Regener Med (2019) 4:9. doi: 10.1038/s41536-019-0071-x
24. Kruglikov IL, Zhang Z, Scherer PE. Caveolin-1 in skin aging - from innocent bystander to major contributor. Ageing Res Rev (2019) 55:100959. doi: 10.1016/j.arr.2019.100959
25. Campbell L, Gumbleton M. Aberrant caveolin-1 expression in psoriasis: a signalling hypothesis. IUBMB Life (2000) 50(6):361–4. doi: 10.1080/713803750
26. Ma WY, Zhuang L, Cai DX, Zhong H, Zhao C, Sun Q. Inverse correlation between caveolin-1 expression and clinical severity in psoriasis vulgaris. J Int Med Res (2012) 40(5):1745–51. doi: 10.1177/030006051204000513
27. Yamaguchi Y, Watanabe Y, Watanabe T, Komitsu N, Aihara M. Decreased expression of caveolin-1 contributes to the pathogenesis of psoriasiform dermatitis in mice. J Invest Dermatol (2015) 135(11):2764–74. doi: 10.1038/jid.2015.249
28. Del Galdo F, Sotgia F, de Almeida CJ, Jasmin JF, Musick M, Lisanti MP, et al. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheumatol (2008) 58(9):2854–65. doi: 10.1002/art.23791
29. Liakouli V, Elies J, El-Sherbiny YM, Scarcia M, Grant G, Abignano G, et al. Scleroderma fibroblasts suppress angiogenesis via TGF-β/caveolin-1 dependent secretion of pigment epithelium-derived factor. Ann Rheum Dis (2018) 77(3):431–40. doi: 10.1136/annrheumdis-2017-212120
30. Lee R, Reese C, Carmen-Lopez G, Perry B, Bonner M, Zemskova M, et al. Deficient adipogenesis of scleroderma patient and healthy African American monocytes. Front Pharmacol (2017) 8:174. doi: 10.3389/fphar.2017.00174
31. Tourkina E, Richard M, Oates J, Hofbauer A, Bonner M, Gööz P, et al. Caveolin-1 regulates leucocyte behaviour in fibrotic lung disease. Ann Rheum Dis (2010) 69(6):1220–6. doi: 10.1136/ard.2009.117580
32. Lee R, Reese C, Perry B, Heywood J, Bonner M, Zemskova M, et al. Enhanced chemokine-receptor expression, function, and signaling in healthy African American and scleroderma-patient monocytes are regulated by caveolin-1. Fibrogenesis Tissue Repair. (2015) 8:11. doi: 10.1186/s13069-015-0028-7
33. Lee R, Del Papa N, Introna M, Reese CF, Zemskova M, Bonner M, et al. Adipose-derived mesenchymal stromal/stem cells in systemic sclerosis: Alterations in function and beneficial effect on lung fibrosis are regulated by caveolin-1. J Scleroderma Relat Disord (2019) 4(2):127–36. doi: 10.1177/2397198318821510
34. Jozic I, Sawaya AP, Pastar I, Head CR, Wong LL, Glinos GD, et al. Pharmacological and genetic inhibition of caveolin-1 promotes epithelialization and wound closure. Mol Ther (2019) 27(11):1992–2004. doi: 10.1016/j.ymthe.2019.07.016
35. Sheng Q, Li G, Liu H, Liu P. Clinical evidence for elevated levels of caveolin-1 in circulation of patients with diabetic foot ulcers. Wound Repair Regen. (2022) 30(1):107–16. doi: 10.1111/wrr.12983
36. Zhang GY, He B, Liao T, Luan Q, Tao C, Nie CL, et al. Caveolin 1 inhibits transforming growth factor-β1 activity via inhibition of smad signaling by hypertrophic scar derived fibroblasts in vitro. J Dermatol Sci (2011) 62(2):128–31. doi: 10.1016/j.jdermsci.2010.10.018
37. Zhang GY, Yu Q, Cheng T, Liao T, Nie CL, Wang AY, et al. Role of caveolin-1 in the pathogenesis of tissue fibrosis by keloid-derived fibroblasts in vitro. Br J Dermatol (2011) 164(3):623–7. doi: 10.1111/j.1365-2133.2010.10111.x
38. Hsu CK, Lin HH, Harn HI, Ogawa R, Wang YK, Ho YT, et al. Caveolin-1 controls hyperresponsiveness to mechanical stimuli and fibrogenesis-associated RUNX2 activation in keloid fibroblasts. J Invest Dermatol (2018) 138(1):208–18. doi: 10.1016/j.jid.2017.05.041
39. Howell BG, Solish N, Lu C, Watanabe H, Mamelak AJ, Freed I, et al. Microarray profiles of human basal cell carcinoma: insights into tumor growth and behavior. J Dermatol Sci (2005) 39(1):39–51. doi: 10.1016/j.jdermsci.2005.02.004
40. Gheida SF, Neinaa YME-H, El-Aziz Mohammed DA. Caveolin-1 expression in hyperproliferative skin disorders: A potential predictive marker of disease severity and progression. Dermatologica Sinica. (2018) 36(4):179–84. doi: 10.1016/j.dsi.2018.06.002
41. Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1. Cancer Res (2010) 70(10):4222–32. doi: 10.1158/0008-5472.CAN-09-3281
42. Tas F, Karabulut S, Tilgen Yasasever C, Duranyildiz D. Clinical significance of serum caveolin-1 levels in melanoma patients. Int J Dermatol (2016) 55(5):558–62. doi: 10.1111/ijd.12862
43. Trimmer C, Whitaker-Menezes D, Bonuccelli G, Milliman JN, Daumer KM, Aplin AE, et al. CAV1 inhibits metastatic potential in melanomas through suppression of the integrin/Src/FAK signaling pathway. Cancer Res (2010) 70(19):7489–99. doi: 10.1158/0008-5472.CAN-10-0900
44. Lobos-Gonzalez L, Aguilar-Guzmán L, Fernandez JG, Muñoz N, Hossain M, Bieneck S, et al. Caveolin-1 is a risk factor for postsurgery metastasis in preclinical melanoma models. Melanoma Res (2014) 24(2):108–19. doi: 10.1097/CMR.0000000000000046
45. Jozic I, Chéret J, Abujamra BA, Miteva M, Gherardini J, Paus R. A cell membrane-level approach to cicatricial alopecia management: Is caveolin-1 a viable therapeutic target in frontal fibrosing alopecia? Biomedicines (2021) 9(5):572. doi: 10.3390/biomedicines9050572
46. Qin H, Bollag WB. The caveolin-1 scaffolding domain peptide decreases phosphatidylglycerol levels and inhibits calcium-induced differentiation in mouse keratinocytes. PloS One (2013) 8(11):e80946. doi: 10.1371/journal.pone.0080946
47. Domingues L, Hurbain I, Gilles-Marsens F, Sirés-Campos J, André N, Dewulf M, et al. Coupling of melanocyte signaling and mechanics by caveolae is required for human skin pigmentation. Nat Commun (2020) 11(1):2988. doi: 10.1038/s41467-020-16738-z
48. Brännmark C, Kay EI, Örtegren Kugelberg U, Chanclón B, Shrestha MM, Wernstedt Asterholm I, et al. Adiponectin is secreted via caveolin 1-dependent mechanisms in white adipocytes. J Endocrinol (2020) 247(1):25–38. doi: 10.1530/JOE-20-0078
49. Harris J, Werling D, Hope JC, Taylor G, Howard CJ. Caveolae and caveolin in immune cells: distribution and functions. Trends Immunol (2002) 23(3):158–64. doi: 10.1016/S1471-4906(01)02161-5
50. Sando GN, Zhu H, Weis JM, Richman JT, Wertz PW, Madison KC. Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J Invest Dermatol (2003) 120(4):531–41. doi: 10.1046/j.1523-1747.2003.12051.x
51. Fagerholm S, Ortegren U, Karlsson M, Ruishalme I, Strålfors P. Rapid insulin-dependent endocytosis of the insulin receptor by caveolae in primary adipocytes. PloS One (2009) 4(6):e5985. doi: 10.1371/journal.pone.0005985
52. Ortegren U, Yin L, Ost A, Karlsson H, Nystrom FH, Strålfors P. Separation and characterization of caveolae subclasses in the plasma membrane of primary adipocytes; segregation of specific proteins and functions. FEBS J (2006) 273(14):3381–92. doi: 10.1111/j.1742-4658.2006.05345.x
53. Ost A, Ortegren U, Gustavsson J, Nystrom FH, Strålfors P. Triacylglycerol is synthesized in a specific subclass of caveolae in primary adipocytes. J Biol Chem (2005) 280(1):5–8. doi: 10.1074/jbc.C400429200
54. Selleri S, Arnaboldi F, Palazzo M, Hussein U, Balsari A, Rumio C. Caveolin-1 is expressed on multipotent cells of hair follicles and might be involved in their resistance to chemotherapy. Br J Dermatol (2005) 153(3):506–13. doi: 10.1111/j.1365-2133.2005.06746.x
55. Kruglikov IL, Scherer PE. Caveolin-1 as a possible target in the treatment for acne. Exp Dermatol (2020) 29(2):177–83. doi: 10.1111/exd.14063
56. Jung YR, Lee JH, Sohn KC, Lee Y, Seo YJ, Kim CD, et al. Adiponectin signaling regulates lipid production in human sebocytes. PloS One (2017) 12(1):e0169824. doi: 10.1371/journal.pone.0169824
57. Csekes E, Račková L. Skin aging, cellular senescence and natural polyphenols. Int J Mol Sci (2021) 22(23):12641. doi: 10.3390/ijms222312641
58. Rodríguez SA, Grochová D, McKenna T, Borate B, Trivedi NS, Erdos MR, et al. Global genome splicing analysis reveals an increased number of alternatively spliced genes with aging. Aging Cell (2016) 15(2):267–78. doi: 10.1111/acel.12433
59. Salzer MC, Lafzi A, Berenguer-Llergo A, Youssif C, Castellanos A, Solanas G, et al. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell (2018) 175(6):1575–90.e22. doi: 10.1016/j.cell.2018.10.012
60. Lee H, Hong Y, Kim M. Structural and functional changes and possible molecular mechanisms in aged skin. Int J Mol Sci (2021) 22(22):12489. doi: 10.3390/ijms222212489
61. Hensley K, Floyd RA. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophys (2002) 397(2):377–83. doi: 10.1006/abbi.2001.2630
62. Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab (2016) 23(2):303–14. doi: 10.1016/j.cmet.2015.11.011
63. Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: A review. Int J Cosmet Sci (2008) 30(2):87–95. doi: 10.1111/j.1468-2494.2007.00415.x
64. Rhim JH, Kim JH, Yeo EJ, Kim JC, Park SC. Caveolin-1 as a novel indicator of wound-healing capacity in aged human corneal epithelium. Mol Med (2010) 16(11-12):527–34. doi: 10.2119/molmed.2010.00046
65. Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, et al. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem (2000) 275(27):20847–52. doi: 10.1074/jbc.M908162199
66. Sun C, Wang N, Huang J, Xin J, Peng F, Ren Y, et al. Inhibition of phosphatidylcholine-specific phospholipase c prevents bone marrow stromal cell senescence in vitro. J Cell Biochem (2009) 108(2):519–28. doi: 10.1002/jcb.22282
67. Asterholm IW, Mundy DI, Weng J, Anderson RG, Scherer PE. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab (2012) 15(2):171–85. doi: 10.1016/j.cmet.2012.01.004
68. Bitar MS, Abdel-Halim SM, Al-Mulla F. Caveolin-1/PTRF upregulation constitutes a mechanism for mediating p53-induced cellular senescence: implications for evidence-based therapy of delayed wound healing in diabetes. Am J Physiol Endocrinol Metab (2013) 305(8):E951–63. doi: 10.1152/ajpendo.00189.2013
69. Cho KA, Ryu SJ, Park JS, Jang IS, Ahn JS, Kim KT, et al. Senescent phenotype can be reversed by reduction of caveolin status. J Biol Chem (2003) 278(30):27789–95. doi: 10.1074/jbc.M208105200
70. Cho KA, Ryu SJ, Oh YS, Park JH, Lee JW, Kim HP, et al. Morphological adjustment of senescent cells by modulating caveolin-1 status. J Biol Chem (2004) 279(40):42270–8. doi: 10.1074/jbc.M402352200
71. Kraus S, Bunsen T, Schuster S, Cichoń MA, Tacke M, Reinheckel T, et al. Cellular senescence induced by cathepsin X downregulation. Eur J Cell Biol (2011) 90(8):678–86. doi: 10.1016/j.ejcb.2011.03.008
72. Yu DM, Jung SH, An HT, Lee S, Hong J, Park JS, et al. Caveolin-1 deficiency induces premature senescence with mitochondrial dysfunction. Aging Cell (2017) 16(4):773–84. doi: 10.1111/acel.12606
73. Lim JS, Nguyen KC, Nguyen CT, Jang IS, Han JM, Fabian C, et al. Flagellin-dependent TLR5/caveolin-1 as a promising immune activator in immunosenescence. Aging Cell (2015) 14(5):907–15. doi: 10.1111/acel.12383
74. Lee JA, Choi DI, Choi JY, Kim SO, Cho KA, Lee JB, et al. Methyl-β-cyclodextrin up-regulates collagen I expression in chronologically-aged skin via its anti-caveolin-1 activity. Oncotarget (2015) 6(4):1942–53. doi: 10.18632/oncotarget.3039
75. Sawaya AP, Jozic I, Stone RC, Pastar I, Egger AN, Stojadinovic O, et al. Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight (2019) 4(23):e129320. doi: 10.1172/jci.insight.129320
76. Khanal P, Lee KY, Kang KW, Kang BS, Choi HS. Tpl-2 kinase downregulates the activity of p53 and enhances signaling pathways leading to activation of activator protein 1 induced by EGF. Carcinogenesis (2009) 30(4):682–9. doi: 10.1093/carcin/bgp040
77. Goyal H, Chachoua I, Pecquet C, Vainchenker W, Constantinescu SN. A p53-JAK-STAT connection involved in myeloproliferative neoplasm pathogenesis and progression to secondary acute myeloid leukemia. Blood Rev (2020) 42:100712. doi: 10.1016/j.blre.2020.100712
78. Guo F, Zheng Y. Involvement of rho family GTPases in p19Arf- and p53-mediated proliferation of primary mouse embryonic fibroblasts. Mol Cell Biol (2004) 24(3):1426–38. doi: 10.1128/MCB.24.3.1426-1438.2004
79. Qiu W, Leibowitz B, Zhang L, Yu J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene (2010) 29(11):1622–32. doi: 10.1038/onc.2009.451
80. Perez-Diaz S, Garcia-Rodriguez B, Gonzalez-Irazabal Y, Valero M, Lagos-Lizan J, Arbones-Mainar JM. Knockdown of PTRF ameliorates adipocyte differentiation and functionality of human mesenchymal stem cells. Am J Physiol Cell Physiol (2017) 312(1):C83–91. doi: 10.1152/ajpcell.00246.2016
81. Perez-Diaz S, Johnson LA, DeKroon RM, Moreno-Navarrete JM, Alzate O, Fernandez-Real JM, et al. Polymerase I and transcript release factor (PTRF) regulates adipocyte differentiation and determines adipose tissue expandability. FASEB J (2014) 28(8):3769–79. doi: 10.1096/fj.14-251165
82. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet (2007) 370(9583):263–71. doi: 10.1016/S0140-6736(07)61128-3
83. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature (2007) 445(7130):866–73. doi: 10.1038/nature05663
84. Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol (2018) 36(1):21–8. doi: 10.1016/j.clindermatol.2017.09.005
85. Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: A comprehensive review. Adv Ther (2020) 37(5):2017–33. doi: 10.1007/s12325-020-01346-6
86. Asami M, Ototake Y, Takamura N, Watanabe Y, Aihara M, Yamaguchi Y. Abnormal inflammatory traits and downregulated caveolin-1 expression in monocytes of psoriasis patients may be associated with psoriatic inflammation and atherosclerosis. J Dermatol Sci (2022) 107(2):65–74. doi: 10.1016/j.jdermsci.2022.07.003
87. Johnston A, Arnadottir S, Gudjonsson JE, Aphale A, Sigmarsdottir AA, Gunnarsson SI, et al. Obesity in psoriasis: leptin and resistin as mediators of cutaneous inflammation. Br J Dermatol (2008) 159(2):342–50. doi: 10.1111/j.1365-2133.2008.08655.x
88. Cerman AA, Bozkurt S, Sav A, Tulunay A, Elbaşi MO, Ergun T. Serum leptin levels, skin leptin and leptin receptor expression in psoriasis. Br J Dermatol (2008) 159(4):820–6. doi: 10.1111/j.1365-2133.2008.08742.x
89. Watanabe Y, Yamaguchi Y, Takamura N, Komitsu N, Aihara M. Leptin induces interleukin-6 production in keratinocytes via decreased expression of caveolin-1: a possible link between obesity and psoriatic inflammation. Br J Dermatol (2020) 183(4):768–70. doi: 10.1111/bjd.19133
90. Denton CP, Khanna D. Systemic sclerosis. Lancet (2017) 390(10103):1685–99. doi: 10.1016/S0140-6736(17)30933-9
91. Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of systemic sclerosis. Front Immunol (2015) 6:272. doi: 10.3389/fimmu.2015.00272
92. Hinchcliff M, O'Reilly S. Current and potential new targets in systemic sclerosis therapy: a new hope. Curr Rheumatol Rep (2020) 22(8):42. doi: 10.1007/s11926-020-00918-3
93. Garrett SM, Baker Frost D, Feghali-Bostwick C. The mighty fibroblast and its utility in scleroderma research. J Scleroderma Relat Disord (2017) 2(2):69–134. doi: 10.5301/jsrd.5000240
94. Ihn H. Autocrine TGF-beta signaling in the pathogenesis of systemic sclerosis. J Dermatol Sci (2008) 49(2):103–13. doi: 10.1016/j.jdermsci.2007.05.014
95. Sonnylal S, Xu S, Jones H, Tam A, Sreeram VR, Ponticos M, et al. Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro. J Cell Sci (2013) 126(Pt 10):2164–75. doi: 10.1242/jcs.111302
96. Fleischmajer R, Damiano V, Nedwich A. Alteration of subcutaneous tissue in systemic scleroderma. Arch Dermatol (1972) 105(1):59–66. doi: 10.1001/archderm.1972.01620040031005
97. Watanabe T, Nishimoto T, Mlakar L, Heywood J, Malaab M, Hoffman S, et al. Optimization of a murine and human tissue model to recapitulate dermal and pulmonary features of systemic sclerosis. PloS One (2017) 12(6):e0179917. doi: 10.1371/journal.pone.0179917
98. Liu SY, Wu JJ, Chen ZH, Zou ML, Teng YY, Zhang KW, et al. Insight into the role of dermal white adipose tissue loss in dermal fibrosis. J Cell Physiol (2022) 237(1):169–77. doi: 10.1002/jcp.30552
99. Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol (2003) 5(5):410–21. doi: 10.1038/ncb975
100. Tourkina E, Bonner M, Oates J, Hofbauer A, Richard M, Znoyko S, et al. Altered monocyte and fibrocyte phenotype and function in scleroderma interstitial lung disease: reversal by caveolin-1 scaffolding domain peptide. Fibrogenesis Tissue Repair. (2011) 4(1):15. doi: 10.1186/1755-1536-4-15
101. Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem (2001) 276(41):38121–38. doi: 10.1074/jbc.M105408200
102. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res (2009) 37(5):1528–42. doi: 10.1177/147323000903700531
103. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol (2020) 10(9):200223. doi: 10.1098/rsob.200223
104. Betarbet U, Blalock TW. Keloids: A review of etiology, prevention, and treatment. J Clin Aesthet Dermatol (2020) 13(2):33–43.
105. Ekstein SF, Wyles SP, Moran SL, Meves A. Keloids: a review of therapeutic management. Int J Dermatol (2021) 60(6):661–71. doi: 10.1111/ijd.15159
106. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci (2017) 18(3):606. doi: 10.3390/ijms18030606
107. Sun Q, Guo S, Wang CC, Sun X, Wang D, Xu N, et al. Cross-talk between TGF-β/Smad pathway and wnt/β-catenin pathway in pathological scar formation. Int J Clin Exp Pathol (2015) 8(6):7631–9.
108. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa: A transcriptional activator of osteoblast differentiation. Cell (1997) 89(5):747–54. doi: 10.1016/S0092-8674(00)80257-3
109. Raaz U, Schellinger IN, Chernogubova E, Warnecke C, Kayama Y, Penov K, et al. Transcription factor Runx2 promotes aortic fibrosis and stiffness in type 2 diabetes mellitus. Circ Res (2015) 117(6):513–24. doi: 10.1161/CIRCRESAHA.115.306341
110. Dohi T, Padmanabhan J, Akaishi S, Than PA, Terashima M, Matsumoto NN, et al. The interplay of mechanical stress, strain, and stiffness at the keloid periphery correlates with increased caveolin-1/ROCK signaling and scar progression. Plast Reconstr Surg (2019) 144(1):58e–67e. doi: 10.1097/PRS.0000000000005717
111. Samarakoon R, Chitnis SS, Higgins SP, Higgins CE, Krepinsky JC, Higgins PJ. Redox-induced src kinase and caveolin-1 signaling in TGF-β1-initiated SMAD2/3 activation and PAI-1 expression. PloS One (2011) 6(7):e22896. doi: 10.1371/journal.pone.0022896
112. Han F, Gu D, Chen Q, Zhu H. Caveolin-1 acts as a tumor suppressor by down-regulating epidermal growth factor receptor-mitogen-activated protein kinase signaling pathway in pancreatic carcinoma cell lines. Pancreas (2009) 38(7):766–74. doi: 10.1097/MPA.0b013e3181b2bd11
113. Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, et al. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol (2001) 159(5):1635–43. doi: 10.1016/S0002-9440(10)63010-6
114. Sloan EK, Stanley KL, Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene (2004) 23(47):7893–7. doi: 10.1038/sj.onc.1208062
115. Gu D, Li H, Wang Z, Chen Q, Jiang J, Zhu H. Caveolin-1 inhibits the growth of human laryngeal squamous cell carcinoma and down regulates EGFR-MAPKs signaling pathway. Laryngoscope (2007) 117(10):1782–9. doi: 10.1097/MLG.0b013e31811edd31
116. Kato T, Miyamoto M, Kato K, Cho Y, Itoh T, Morikawa T, et al. Difference of caveolin-1 expression pattern in human lung neoplastic tissue. atypical adenomatous hyperplasia, adenocarcinoma and squamous cell carcinoma. Cancer Lett (2004) 214(1):121–8. doi: 10.1016/j.canlet.2004.04.017
117. Prade E, Tobiasch M, Hitkova I, Schäffer I, Lian F, Xing X, et al. Bile acids down-regulate caveolin-1 in esophageal epithelial cells through sterol responsive element-binding protein. Mol Endocrinol (2012) 26(5):819–32. doi: 10.1210/me.2011-1140
118. Yang G, Goltsov AA, Ren C, Kurosaka S, Edamura K, Logothetis R, et al. Caveolin-1 upregulation contributes to c-myc-induced high-grade prostatic intraepithelial neoplasia and prostate cancer. Mol Cancer Res (2012) 10(2):218–29. doi: 10.1158/1541-7786.MCR-11-0451
119. Liang W, Hao Z, Han JL, Zhu DJ, Jin ZF, Xie WL. CAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transition. Urol Oncol (2014) 32(6):855–63. doi: 10.1016/j.urolonc.2014.01.005
120. Campbell L, Al-Jayyoussi G, Gutteridge R, Gumbleton N, Griffiths R, Gumbleton S, et al. Caveolin-1 in renal cell carcinoma promotes tumour cell invasion, and in co-operation with pERK predicts metastases in patients with clinically confined disease. J Transl Med (2013) 11:255. doi: 10.1186/1479-5876-11-255
121. Xue J, Chen H, Diao L, Chen X, Xia D. Expression of caveolin-1 in tongue squamous cell carcinoma by quantum dots. Eur J Histochem (2010) 54(2):e20. doi: 10.4081/ejh.2010.e20
122. Nohata N, Hanazawa T, Kikkawa N, Mutallip M, Fujimura L, Yoshino H, et al. Caveolin-1 mediates tumor cell migration and invasion and its regulation by miR-133a in head and neck squamous cell carcinoma. Int J Oncol (2011) 38(1):209–17. doi: 10.3892/ijo_00000840
123. Ando T, Ishiguro H, Kimura M, Mitsui A, Mori Y, Sugito N, et al. The overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol Rep (2007) 18(3):601–9. doi: 10.3892/or.18.3.601
124. Sun J, Gao J, Hu JB, Fan LF, Zhu XB, Subahan R, et al. Expression of cav-1 in tumour cells, rather than in stromal tissue, may promote cervical squamous cell carcinoma proliferation, and correlates with high-risk HPV infection. Oncol Rep (2012) 27(6):1733–40. doi: 10.3892/or.2012.1703
125. McDaniel B, Badri T, Steele RB. Basal cell carcinoma. (Treasure Island (FL):StatPearls) (2022).
126. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol (2018) 78(2):237–47. doi: 10.1016/j.jaad.2017.08.059
127. Trimmer C, Bonuccelli G, Katiyar S, Sotgia F, Pestell RG, Lisanti MP, et al. Cav1 suppresses tumor growth and metastasis in a murine model of cutaneous SCC through modulation of MAPK/AP-1 activation. Am J Pathol (2013) 182(3):992–1004. doi: 10.1016/j.ajpath.2012.11.008
128. Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet (2018) 392(10151):971–84. doi: 10.1016/S0140-6736(18)31559-9
129. Nakashima H, Hamamura K, Houjou T, Taguchi R, Yamamoto N, Mitsudo K, et al. Overexpression of caveolin-1 in a human melanoma cell line results in dispersion of ganglioside GD3 from lipid rafts and alteration of leading edges, leading to attenuation of malignant properties. Cancer Sci (2007) 98(4):512–20. doi: 10.1111/j.1349-7006.2007.00419.x
130. Wu KN, Queenan M, Brody JR, Potoczek M, Sotgia F, Lisanti MP, et al. Loss of stromal caveolin-1 expression in malignant melanoma metastases predicts poor survival. Cell Cycle (2011) 10(24):4250–5. doi: 10.4161/cc.10.24.18551
131. Lobos-González L, Aguilar L, Diaz J, Diaz N, Urra H, Torres VA, et al. E-cadherin determines caveolin-1 tumor suppression or metastasis enhancing function in melanoma cells. Pigment Cell Melanoma Res (2013) 26(4):555–70. doi: 10.1111/pcmr.12085
132. Urra H, Torres VA, Ortiz RJ, Lobos L, Díaz MI, Díaz N, et al. Caveolin-1-enhanced motility and focal adhesion turnover require tyrosine-14 but not accumulation to the rear in metastatic cancer cells. PloS One (2012) 7(4):e33085. doi: 10.1371/journal.pone.0033085
133. Ortiz R, Díaz J, Díaz-Valdivia N, Martínez S, Simón L, Contreras P, et al. Src-family kinase inhibitors block early steps of caveolin-1-enhanced lung metastasis by melanoma cells. Biochem Pharmacol (2020) 177:113941. doi: 10.1016/j.bcp.2020.113941
134. Fruehauf JP, Meyskens FL. Reactive oxygen species: a breath of life or death? Clin Cancer Res (2007) 13(3):789–94. doi: 10.1158/1078-0432.CCR-06-2082
135. Torres-Collado AX, Knott J, Jazirehi AR. Reversal of resistance in targeted therapy of metastatic melanoma: Lessons learned from vemurafenib (BRAF. Cancers (Basel). (2018) 10(6):157. doi: 10.3390/cancers10060157
136. Das I, Gad H, Bräutigam L, Pudelko L, Tuominen R, Höiom V, et al. AXL and CAV-1 play a role for MTH1 inhibitor TH1579 sensitivity in cutaneous malignant melanoma. Cell Death Differ (2020) 27(7):2081–98. doi: 10.1038/s41418-019-0488-1
137. Hoffmann C, Berking A, Agerer F, Buntru A, Neske F, Chhatwal GS, et al. Caveolin limits membrane microdomain mobility and integrin-mediated uptake of fibronectin-binding pathogens. J Cell Sci (2010) 123(Pt 24):4280–91. doi: 10.1242/jcs.064006
138. Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A. Caveolar endocytosis of simian virus 40 is followed by brefeldin a-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J Virol (2002) 76(10):5156–66. doi: 10.1128/JVI.76.10.5156-5166.2002
139. Spaan AN, Neehus AL, Laplantine E, Staels F, Ogishi M, Seeleuthner Y, et al. Human OTULIN haploinsufficiency impairs cell-intrinsic immunity to staphylococcal α-toxin. Science (2022) 376(6599):eabm6380. doi: 10.1126/science.abm6380
140. Keusekotten K, Elliott PR, Glockner L, Fiil BK, Damgaard RB, Kulathu Y, et al. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell (2013) 153(6):1312–26. doi: 10.1016/j.cell.2013.05.014
141. Damgaard RB, Walker JA, Marco-Casanova P, Morgan NV, Titheradge HL, Elliott PR, et al. The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell (2016) 166(5):1215–30.e20. doi: 10.1016/j.cell.2016.07.019
142. Zhou Q, Yu X, Demirkaya E, Deuitch N, Stone D, Tsai WL, et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc Natl Acad Sci U S A. (2016) 113(36):10127–32. doi: 10.1073/pnas.1612594113
143. Patel GK, Finlay AY. Staphylococcal scalded skin syndrome: diagnosis and management. Am J Clin Dermatol (2003) 4(3):165–75. doi: 10.2165/00128071-200304030-00003
144. Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN. Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem (2004) 279(18):18944–51. doi: 10.1074/jbc.M400769200
145. Abraham SN, Duncan MJ, Li G, Zaas D. Bacterial penetration of the mucosal barrier by targeting lipid rafts. J Investig Med (2005) 53(6):318–21. doi: 10.2310/6650.2005.53609
146. Bajmoczi M, Gadjeva M, Alper SL, Pier GB, Golan DE. Cystic fibrosis transmembrane conductance regulator and caveolin-1 regulate epithelial cell internalization of pseudomonas aeruginosa. Am J Physiol Cell Physiol (2009) 297(2):C263–77. doi: 10.1152/ajpcell.00527.2008
147. Devadas D, Koithan T, Diestel R, Prank U, Sodeik B, Döhner K. Herpes simplex virus internalization into epithelial cells requires Na+/H+ exchangers and p21-activated kinases but neither clathrin- nor caveolin-mediated endocytosis. J Virol (2014) 88(22):13378–95. doi: 10.1128/JVI.03631-13
148. Praena B, Bello-Morales R, López-Guerrero JA. Hsv-1 endocytic entry into a human oligodendrocytic cell line is mediated by clathrin and dynamin but not caveolin. Viruses (2020) 12(7):734. doi: 10.3390/v12070734
149. Smith JL, Campos SK, Wandinger-Ness A, Ozbun MA. Caveolin-1-dependent infectious entry of human papillomavirus type 31 in human keratinocytes proceeds to the endosomal pathway for pH-dependent uncoating. J Virol (2008) 82(19):9505–12. doi: 10.1128/JVI.01014-08
150. Grundmeier N, Hamm H, Weissbrich B, Lang SC, Bröcker EB, Kerstan A. High-risk human papillomavirus infection in bowen's disease of the nail unit: report of three cases and review of the literature. Dermatology (2011) 223(4):293–300. doi: 10.1159/000335371
151. Smith JL, Campos SK, Ozbun MA. Human papillomavirus type 31 uses a caveolin 1- and dynamin 2-mediated entry pathway for infection of human keratinocytes. J Virol (2007) 81(18):9922–31. doi: 10.1128/JVI.00988-07
152. Tourkina E, Richard M, Gööz P, Bonner M, Pannu J, Harley R, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol (2008) 294(5):L843–61. doi: 10.1152/ajplung.00295.2007
Keywords: caveolin-1, skin, aging, psoriasis, fibrosis, skin cancer, skin infection
Citation: Takamura N and Yamaguchi Y (2022) Involvement of caveolin-1 in skin diseases. Front. Immunol. 13:1035451. doi: 10.3389/fimmu.2022.1035451
Received: 02 September 2022; Accepted: 17 October 2022;
Published: 30 November 2022.
Edited by:
Cecilia Jacques G. de Almeida, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Ivan Jozic, University of Miami, United StatesIlja L. Kruglikov, Wellcomet GmbH, Germany
Copyright © 2022 Takamura and Yamaguchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yukie Yamaguchi, eXVpMTc4M0B5b2tvaGFtYS1jdS5hYy5qcA==
 Naoko Takamura
Naoko Takamura Yukie Yamaguchi
Yukie Yamaguchi