
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 04 October 2022
Sec. Alloimmunity and Transplantation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1034438
This article is part of the Research TopicThe Role of Hematopoietic and Immune Microenvironment in Hematopoietic Stem Cell TransplantationView all 7 articles
 Guancui Yang1,2,3†
Guancui Yang1,2,3† Xiang Wang1,2†
Xiang Wang1,2† Shiqin Huang1,2†
Shiqin Huang1,2† Ruihao Huang1,2
Ruihao Huang1,2 Jin Wei3
Jin Wei3 Xiaoqi Wang1,2*
Xiaoqi Wang1,2* Xi Zhang1,2,3*
Xi Zhang1,2,3*Allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains the only curative treatment for patients with myeloid malignancies such as myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). However, relapse and graft-versus-host disease (GvHD) still affect the survival of patients who receive allo-HSCT, and more appropriate therapeutic strategies should be applied at all stages of transplantation to prevent these adverse events. The use of epigenetics agents, such as hypomethylating agents (HMAs), has been explored to decrease the risk of relapse by epigenetic modulation, which is especially effective among AML patients with poor mutations in epigenetic regulators. Furthermore, epigenetic agents have also been regarded as prophylactic methods for GvHD management without abrogating graft versus leukemia (GvL) effects. Therefore, the combination of epigenetic therapy and HSCT may optimize the transplantation process and prevent treatment failure. Existing studies have investigated the feasibility and effectiveness of using HMAs in the pretransplant, transplant and posttransplant stages among MDS and AML patients. This review examines the application of HMAs as a bridge treatment to reduce the tumor burden and the determine appropriate dose during allo-HSCT. Within this review, we also examine the efficacy and safety of HMAs alone or HMA-based strategies in posttransplant settings for MDS and AML. Finally, we provide an overview of other epigenetic candidates, which have been discussed in the nontransplant setting.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment for most patients with MDS or AML (1, 2). However, relapse remains the primary cause of mortality in patients receiving allo-HSCT for MDS or AML (3, 4). Epigenetic agents, mainly including hypomethylating agents (HMAs) and histone deacetylase inhibitors (HDACi), have become widely used in the pretransplant, transplant, and posttransplant stages for MDS or AML (5–9) and are regarded as candidates to reduce the tumor burden, as they have tolerable toxicity and mitigate graft-versus-host disease (GvHD) without influencing graft versus leukemia (GvL) (9, 10). Furthermore, HMAs have been proven effective in exerting an antitumor effect by promoting cell differentiation and apoptosis in leukemia cells (11, 12) and by reactivating silent tumor suppressor antigens like MAGE-1 and WT-1 to improve the response to other therapeutic hematologic malignancies (10, 13, 14). In this review, we will summarize the application of epigenetic agents in the pretransplant, transplant and posttransplant stages, including bridging treatment, preconditioning regimens, maintenance, preemptive therapy, and salvage therapy.
Minimal residual disease (MRD) before transplantation significantly increases the probability of relapse after transplantation, which affects patient outcomes (15). Thus, some patients need bridging treatment to control the disease status. HMAs, including azacytidine (AZA) and decitabine (DAC), are widely used in bridge therapy before transplantation.
The safety and efficacy of HMAs has been confirmed by several studies and included in the guideline for HSCT-based treatment of AML (16). One retrospective study reported that compared with a non-DAC-based regimen, patients bridging with DAC showed a higher pretransplant bone marrow complete response (CR) and longer 2-year overall survival (OS) (17). A phase II multicenter study by VOSO et al. enrolled 97 patients with myeloid neoplasia for whom bridging therapy with AZA was administered before transplantation (18). The results showed that the median survival time was 15.2 months, and the overall response rate (ORR) reached 38% (Table 1). In another multivariate analysis, response to AZA was the only independent prognostic factor for survival. Xu et al. retrospectively analyzed the role of HMAs in bridging treatment of intermediate-risk AML. The treatment regimen was DCAG (DAC, cytarabine, aclarubicin hydrochloride and granulocyte colony-stimulating factor) or standard “3+7” regimen with or without HSCT. The results showed that DCAG-bridged HSCT had the best prognosis (5-year OS: 80%, EFS: 85.7%) (23). Therefore, bridging allo-HSCT with HMAs before transplantation can benefit patients. Additionally, the application of HMAs could also increase the safety of transplantation. A retrospective study by Kim et al. demonstrated benefits in NRM after bridging therapy in patients with high bone marrow counts (>5%) (19). However, retrospective trials reported that HMAs could not improve OS/PFS or decrease the relapse rate (21, 22). For elderly individuals, HMAs have been the standard therapy because of their good tolerance (16).
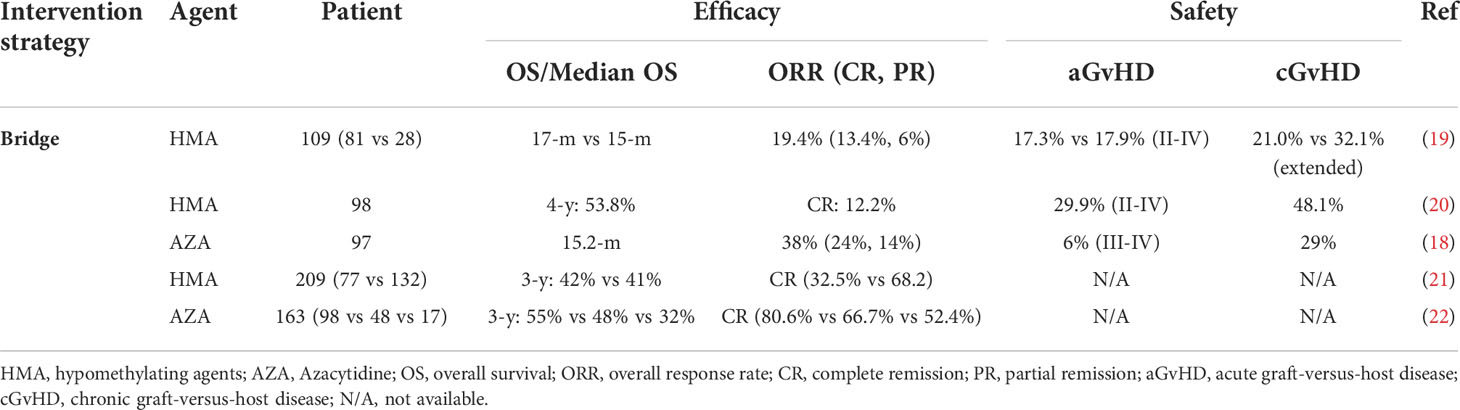
Table 1 The use of hypomethylating agents prior to hematopoietic stem cell transplantation in MDS or AML.
For patients with different disease states, the bridging scheme and the timing of transplantation should be different. For minimal residual disease (MRD) negative patients, HSCT should be conducted as soon as possible. It is currently believed that a shorter disease duration pre-HSCT is associated with improved OS and disease-free survival (DFS) and decreased treatment-related mortality (TRM) (24, 25). Patients with bone marrow counts greater than 5% and those with CR but MRD+ may benefit from bridging treatment (26–28). For this kind of patient, HMAs can reduce the tumor burden, achieving the purpose of “debulking” and improving the prognosis of patients. Yahng et al. conducted a retrospective study of patients with a blast count > 5% who underwent HMA bridging treatment and showed that the prognosis of the bone marrow response group at the time of transplantation was better than that of the no bone marrow response group (20). Sungwoo park’s study showed that some patients with SD also benefited from the use of HMAs (29). Furthermore, numerous studies have shown that even if patients achieve CR, MRD at the time of transplantation is an independent predictor of subsequent relapse (30–33). It has been reported that oral 5-AZA, compared with placebo, can help AML patients to prolong the time of MRD- (11 months vs. 5 months), and the rate of MRD+ to MRD- conversion was higher with oral azacitidine (15). With the continuous development of detection technology for MRD, this indicator is being evaluated before HSCT. However, it remains unclear whether patients who achieved CR but did not achieve MRD- can benefit from bridging treatment before transplantation.
As an epigenetic agent, DAC has been widely used in a variety of condition regimens due to its low toxicity and antitumor effect. For the application of DAC in condition regimens, the focus of general attention is the dose. This has gone through a process of gradual optimization from high to low doses. As early as 2003, the de Lima team used high-dose DAC (100 mg/m2 every 12 hours×4, 150 mg/m2 every 12 hours×4, 200 mg/m2 every 12 hours×4) combined with a myeloablative condition regimen (34). Twenty-three patients with myeloid tumors were included in this retrospective study, and the median survival reached 17.5 months, but the higher treatment-related mortality rate deserves attention (3 years: 35%). Therefore, a series of clinical trials of low-dose DAC were carried out, including a 10-day regimen and a 5-day regimen. A phase II randomized clinical trial showed that both the 5-day regimen and the 10-day regimen were effective in elderly patients with AML, and there was no significant difference (35) (Table 2). We can observe that the 10-day regimen increases the risk of infection while prolonging survival and reducing relapse, which may be related to the toxicity of DAC (39, 42). The use of DAC doses varies from country to country. For example, in China, considering the patient’s physique, more 5-day DAC schemes are used. Overall, we found that low-dose DAC combined with pretreatment can reduce the proportion of TRM after transplantation, prolong survival and reduce GvHD. A retrospective study from China analyzed 236 AML patients, of whom 59 received DAC combined with the Bucy condition regimen and 177 received the Bucy condition regimen without DAC (37). The results showed that the 2-year OS rate of patients in the DAC group was significantly improved, and the TRM rate within 100 days was 0%. A retrospective study reported by Wang et al. included 76 patients with AML or MDS (38). Forty patients received DAC combined with the Bucy/BuFlu condition regimen, and 36 patients only used the Bucy/BuFlu condition regimen. The results showed that the incidence of grade III-IV acute GvHD decreased significantly. A retrospective study by Li et al. compared the effects of short-term and high-dose DAC (75 mg/m2 on day -9 and 50 mg/m2 on day -8) with short-term and low-dose DAC (15 mg/m2/day on days -10 to -8) on the prognosis of patients (36). The results showed that the three-year overall survival rate of the high-dose group was better than that of the low-dose group, while the incidence rates of relapse, acute GvHD and chronic GvHD in the higher-dose group were lower than those in the low-dose group. Although patients generally benefit from the addition of HMAs to conditioning regimens, the optimal dose is still unclear and needs to be further explored.
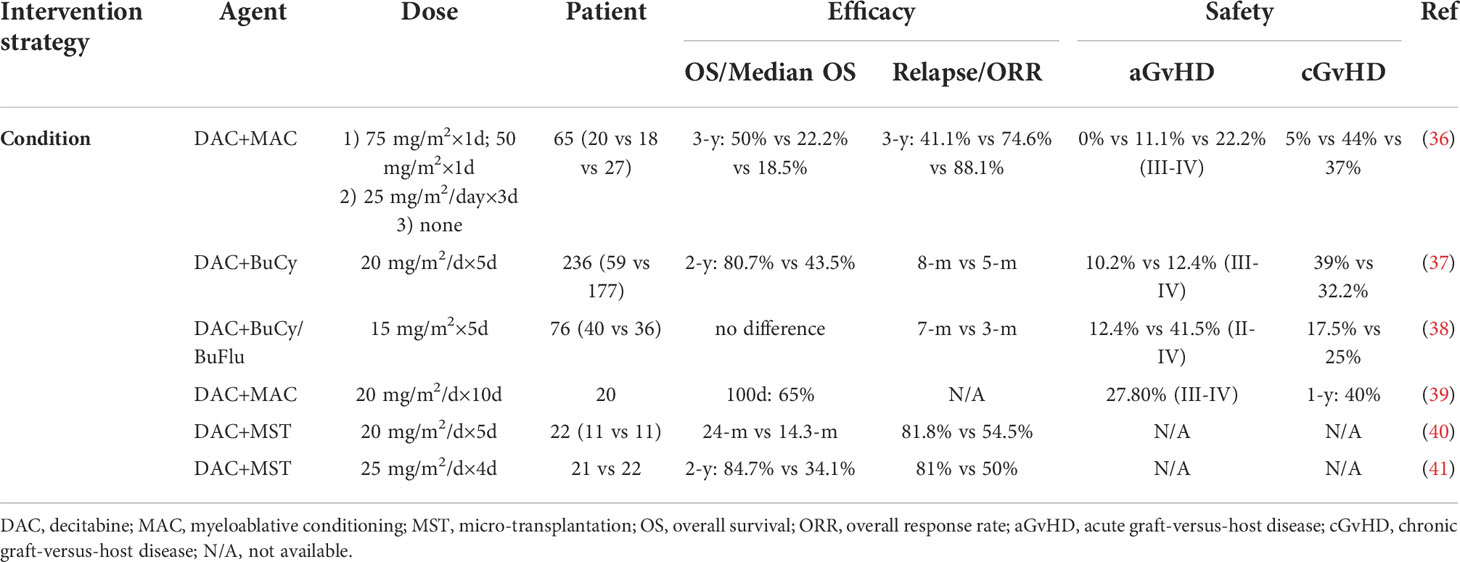
Table 2 The use of hypomethylating agents during hematopoietic stem cell transplantation in MDS or AML.
Huisheng Ai et al. combined DAC with micro transplantation to treat MDS/tAML patients. The overall response rate was 65.1%, and the overall response rate in the MDS group was significantly higher than that in the tAML group (41). The report by Li et al. also showed that DAC combined with MST could increase the overall response rate (40). This is a new scheme of DAC combined with micro-transplantation (MST), which can effectively prolong the survival of patients with high-risk MDS and TAML, especially for MDS patients, and may become an effective bridging method for allogeneic transplantation. Overall, the combination of HMAs with MST results in an increased overall response rate in patients, and the benefit is better in patients with MDS than in those with AML.
Recently, a phase 3, open label, randomized study (43) compared AZA maintenance vs. observation after transplant in high-risk MDS and AML patients. The observations were noteworthy in that both PFS and OS showed no significant difference between the two arms, which differed from the previous encouraging results (44–46). A probable cause for the outcome was that the patient population included in this study was heterogeneous (the majority of enrolled patients had high-risk AML) and that the natural history of AML and MDS was different, with the former having a higher incidence of an adverse prognosis (47–49). Multivariate analyses also suggested that AZA could provide more clinical benefit in patients with MDS than in those with AML. In addition, grade 3 or higher drug-related AEs were observed in the majority of patients in the study, including bone marrow suppression, liver toxicity and infection. Therefore, the toxicity of AZA should be considered. Compared with AZA, DAC might be safer as a posttransplant maintenance treatment for AML or MDS because of its very low nonhematologic toxicity (50). In a phase I/II study (51), the MTD was not reached, but relatively lower doses (10 mg/m2×5 days) seemed to be a better tolerated dose for DAC maintenance in the post-HSCT setting. CC-486, the oral formulation of AZA, is more likely to be associated with better OS and lower GvHD risk (Table 3) (52), which might be related to the means of administration. Oral AZA could lead to extended dosing to prolong AZA activity and better compliance (71, 72). Currently, several studies have suggested that remission duration is one of the strongest predictors of posttransplant survival (73, 74); therefore, a long CC-486 remission duration could reduce the risk of relapse posttransplant. Thus, the use of oral AZA instead of injectable AZA could be explored in future studies in AML and MDS posttransplantation. Overall, MDS/AML patients receiving HMA maintenance after allo-HSCT had better outcomes as well as a reduced incidence of chronic GvHD, consistent with an updated systematic review and meta-analysis (75). Panobinostat (PNB, a histone deacetylase inhibitor), due to its immunomodulatory activity (76) that may enhance leukemia-specific cytotoxicity and mitigate GvHD, was also applied in a transplant setting. The safety and efficacy of PNB was explored in a phase I/II clinical trial (53) in patients with high-risk MDS/AML, and it was well tolerated, with a relatively low relapse rate and incidence of GvHD. Another HDACi, vorinostat, was also effective in GVHD prevention by reducing proinflammatory cytokines and increasing regulatory T-cell number and function after allo-HSCT in MDS and AML patients (9).
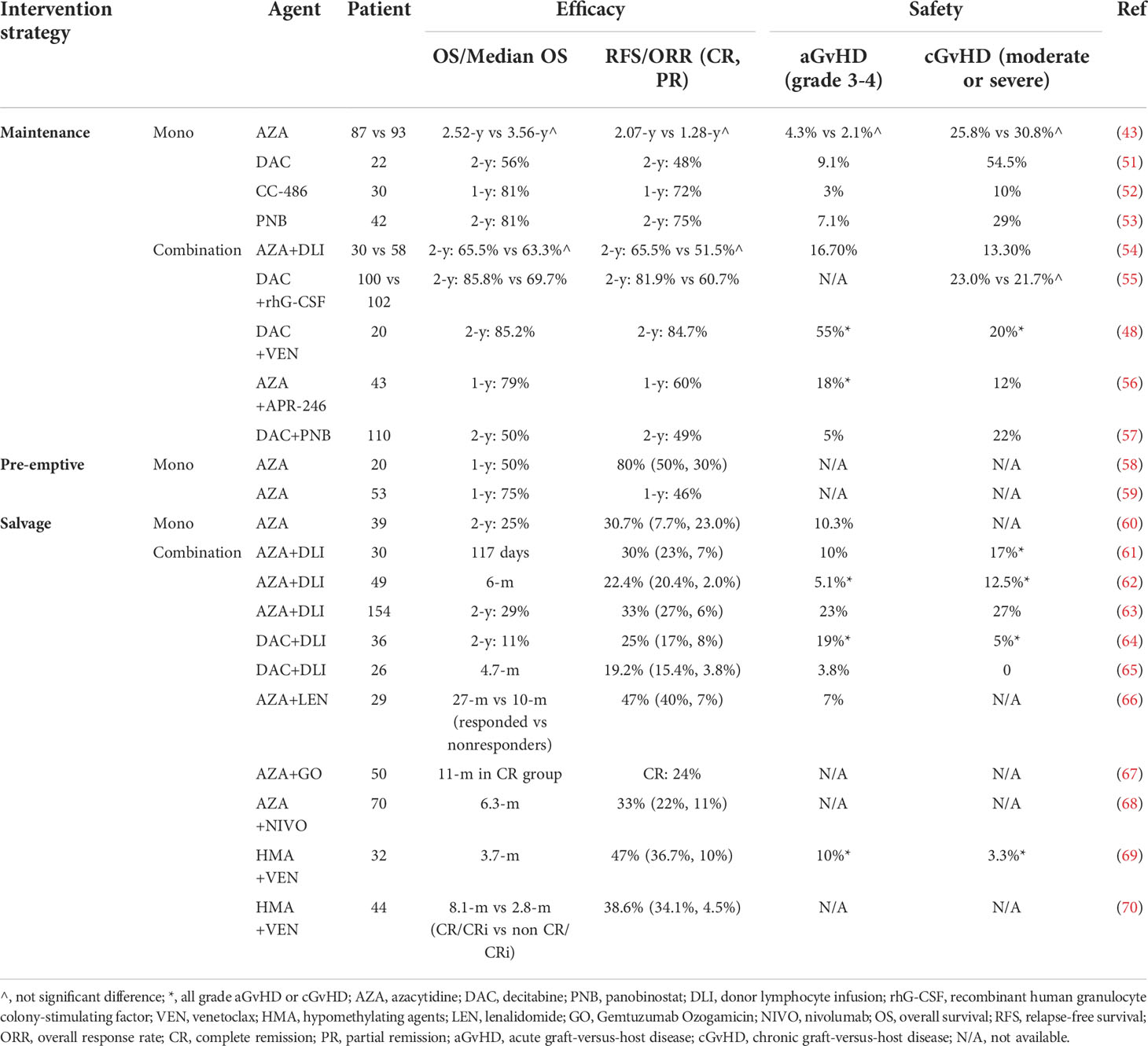
Table 3 The use of hypomethylating agents after hematopoietic stem cell transplantation in MDS or AML.
For combined therapies, a phase II randomized controlled trial (RCT) from China evaluated the efficacy of low-dose DAC (5 mg/m2×5 days) maintenance combined with recombinant human granulocyte colony-stimulating factor (rhG-CSF) after allo-HSCT in AML or MDS patients (55). The results showed that this treatment reduced the relapse rate due to the GvL effect, which was generated by the increased numbers of NK and CD8+ T cells, and controlled the progression of GvHD due to the increased numbers of Treg cells. Another single-arm study by Burak Kalin et al. (57) showed that PNB after allo-HSCT alone or in combination with low-dose DAC (10 mg/m2×3 days) was feasible in AML patients, especially in low-risk AML patients. Low-dose DAC (15 mg/m2×3 days) plus venetoclax (BCL-2i) has also shown promising efficacy in maintenance therapy after HSCT (48). Together with an acceptable toxicity profile, these data suggest that low-dose DAC in combination with traditional therapies may exert additive or synergistic effects in AML or MDS maintenance therapy posttransplantation. Furthermore, HMAs could also be combined with targeted drugs as maintenance therapy posttransplant. A phase II trial presented at the 2021 annual meeting of the American Society of Hematology (ASH) suggested that a novel targeted agent, eprenetapopt (APR-246, a small molecule p53 stabilizer), in combination with AZA as maintenance therapy for TP53-mutated AML or MDS after allo-HSCT was relatively favorable and well tolerated (56). APR-246/AZA was proven to reactivate the p53 pathway and induce apoptosis in tumor cells (77); thus, TP53-mutated MDS/AML may be better targeted by this combination to conventional treatments. Moreover, AZA in conjunction with gemtuzumab ozogamicin (GO), which is associated with a long survival (>700 days), could be safely administered as maintenance therapy in MDS and AML patients after HSCT (78).
The prognosis of AML and MDS patients who relapse after allo-HSCT is usually poor, with a 3-year OS of 19% (79). Thus, relapse is a major cause of treatment failure in patients receiving allografts (80). Only 20% of patients respond to traditional salvage chemotherapy or DLI (81, 82), and even after receiving a second transplant, the 5-year OS for these patients is lower than 30% (83). HMAs have also emerged in recent years as salvage treatment modalities in patients with MDS or AML. The outcome of HMA monotherapy was dismal in relapsed MDS or AML patients after allo-HSCT, with a 2-y OS of 25% (60), and combination therapies are often needed to effectively treat these patients.
Combined HMAs/DLI can not only reduce the incidence of GvHD, the major complication of DLI in the treatment of relapsed AML after allo-HSCT (approximately 40% of patients develop GvHD after DLI) (82), but also enhance the immunomodulatory role, such as increasing the response of CD8+ T cells (45, 84). A phase II study (61) of 30 patients demonstrated the feasibility and clinical efficacy of AZA in combination with DLI as salvage therapy for relapse after allo-HSCT. However, another recent prospective study of 49 patients showed that the administration of AZA and DLI had no significant impact on either response or survival (62). We evaluated the regimens of two different studies, and the latter administered DLI one day after AZA. Due to the cytotoxicity of AZA (85), the administration of DLI a relatively short period after AZA might exert a toxic effect on lymphocytes and decrease the GvL effect. Thus, it might be inappropriate to administer DLI immediately after AZA. Regarding DAC-DLI, a retrospective study (64) examined 36 AML and MDS patients who were treated with DAC and DLI for relapse after HSCT. More than half of the patients received other salvage therapies prior to DAC. As a result, 6 (17%) patients achieved CR, including one-third of patients with previous AZA-DLI failure due to AZA intolerability. Therefore, DAC-DLI could be an alternative to AZA-DLI or even a second choice after AZA-DLI failure. Another retrospective study (86) also agreed with the conclusion that DAC could result in durable survival in patients with more aggressive hematologic relapse when combined with DLI in AML or MDS after HSCT. Taken together, additional multicenter or prospective research with a larger population should be conducted in the future to compare AZA and DAC as salvage therapy in combination with DLI for relapse after HSCT, and multivariate analyses should be conducted to identify patients who may benefit most from the combination of DLI. Another combination agent was lenalidomide (LEN), which is known to cause myelosuppression and might eliminate the abnormal MDS/AML clone (87). Additionally, Len often causes acute GvHD after allo-SCT (88, 89), and thus, combined AZA/Len might theoretically improve clinical activity in relapsed AML or MDS without increasing the risk of severe GvHD. In the VIOLA trial, Charles Craddock et al. (66) found that AZA/Len therapy was well tolerated, and no severe GvHD was observed. Interestingly, the authors also found that this combination did not change the T-cell phenotype, which was previously reported to be associated with relapsed AML (90, 91). As a result, AZA in combination with Len could become a preemptive strategy in the post-allo-HSCT setting since it may not affect the results of the MRD assay. First, when combined with DLI/LEN, it has been shown to mitigate GvHD without sacrificing GvL. Second, HMAs have also been applied posttransplantation with some targeted drugs, such as Ven, GO, ICI (immune checkpoint inhibitor) and APR-246. AZA and Ven displayed combinatorial antitumor activity in vivo through transcriptional induction of the proapoptotic BH3-only protein NOXA (92). GO/HMA was associated with enhanced GO and anti-CD33-mediated inhibition of leukemia cell growth (93). HMAs increase the expression of PD-1 and PD-L1, especially in AML. APR-246/HMAs was demonstrated to reactivate the p53 pathway (77).
Venetoclax (VEN), a selective inhibitor of the antiapoptotic protein Bcl-2, has been demonstrated to have the potential to sensitize AML cells to HMAs (94). Recent studies have shown encouraging treatment effects in relapsed/refractory (R/R) AML and elderly patients with combined HMAs/VEN (95, 96), while the clinical value for HMAs/Ven in the transplantation setting has been explored preliminarily, and a phase 3, randomized clinical study of HMAs/VEN in AML posttransplantation (NCT04161885) is now ongoing. In a recent multicenter retrospective study from China (70), TP53 mutation (HR = 17.339, p =.033) and relapse within 1 year posttransplantation (HR = 6.261, p =.026) were identified as independent risk factors when HMAs/VEN was used in relapsed AML or MDS patients. The results from Esther Schuler et al. (69) suggested that HMAs/VEN combinations for molecular relapse or as first salvage therapy conferred better benefit.
There is now substantial evidence showing that patients who are molecular relapsed/hematological relapse with lower blast count or a longer interval from HSCT to relapse might be associated with better survival when HMAs-based therapies are used in relapsed AML or MDS (61–63, 69, 70, 97). Thus, for molecular relapsed (bone blast<5%) MDS or AML according to MRD determination, intervention strategies should be recommended and implemented as early as possible. Monotherapy with AZA has been suggested to delay relapse with a 1-y PFS of approximately 50% (59) as a preemptive treatment in higher-risk MDS or AML patients with MRD positivity after HSCT.
It has been shown that disease stage at the time of transplantation is one of the most important factors that influences outcome after allogeneic HSCT (32, 98), and a blast count greater than 5% is associated with relapse-related poor prognosis. Furthermore, it can be a timely process to find a human leukocyte antigen-matched donor after diagnosis. Disease progression and complications may be an obstacle to HSCT without bridging treatment. Therefore, it is feasible and essential to conduct bridge therapy prior to transplantation to reduce the tumor burden, but the trial results were not always good and remain to be further explored (99). In addition, the use of AZA can influence the mutation spectrum and evolution of new subclones after transplantation (100).
As a condition regimen, there are relatively few studies on the application of HMAs during transplantation. Patients may benefit more from conditioning regimens using HMAs, especially at a lower dose. Two regimens (5 days and 10 days) of low-dose commonly used therapies show fair efficacy. Compared with the 10-day regimen, a low incidence of infection was reported in the 5-day regimen, which calls attention to infection screening and antibiotic prophylaxis in the 10-day regimen.
Relapse after allo-HSCT for AML or MDS is the main cause of treatment failure and is associated with a very poor prognosis and a short survival (101). Reductions of immunosuppressive therapy, chemotherapy with or without infusion of DLI or second allo-HSCT are regularly proposed, but the results remain disappointing, and the incidence of GvHD after the use of DLI or Len is approximately 40% (82). Compared with traditional treatments after HSCT, novel agents need to have a good response, good tolerance and low toxicity, including HMAs. Data on preventing relapse of using single HMAs posttransplant after allo-HSCT are limited, as the antitumor effects tend to be weak. Although no direct comparison was performed, HMA-based therapies showed advantages in reducing GvHD over other traditional therapies, such as DLI, Len and some targeted drugs (Table 3).
The efficacy and safety of HMAs alone or HMA-based strategies to prevent and treat recurrence after allo-HSCT are different. HMAs alone have been proven to cause cell apoptosis in leukemia cells and improve Tregs conversion and expansion in vitro. When combined with other therapies, HMAs could potentially reverse some epigenetic silencing genes like MAGE-1 and WT-1 to exert an antitumor effect (Figure 1). Therefore, scientists and clinicians need to use HMAs to prevent and treat recurrence after allo-HSCT according to the following rules. 1) Patients should be administered HMAs as early as possible after allo-HSCT to maximize the efficacy, especially in patients with a relatively low leukemic burden as well as slow-growing tumors. 2) Regarding safety, HMAs have been reported to have direct cellular toxicity, especially at high doses, and it might be inappropriate to administer DLI immediately after HMAs. Oral AZA CC-486 showed beneficial effects on prolonged administration and reduced injection-site reactions (102). 3) To increase the clinical effectiveness, the combination of HMAs with synergistic therapies was more feasible than monotherapy, especially in the salvage setting.
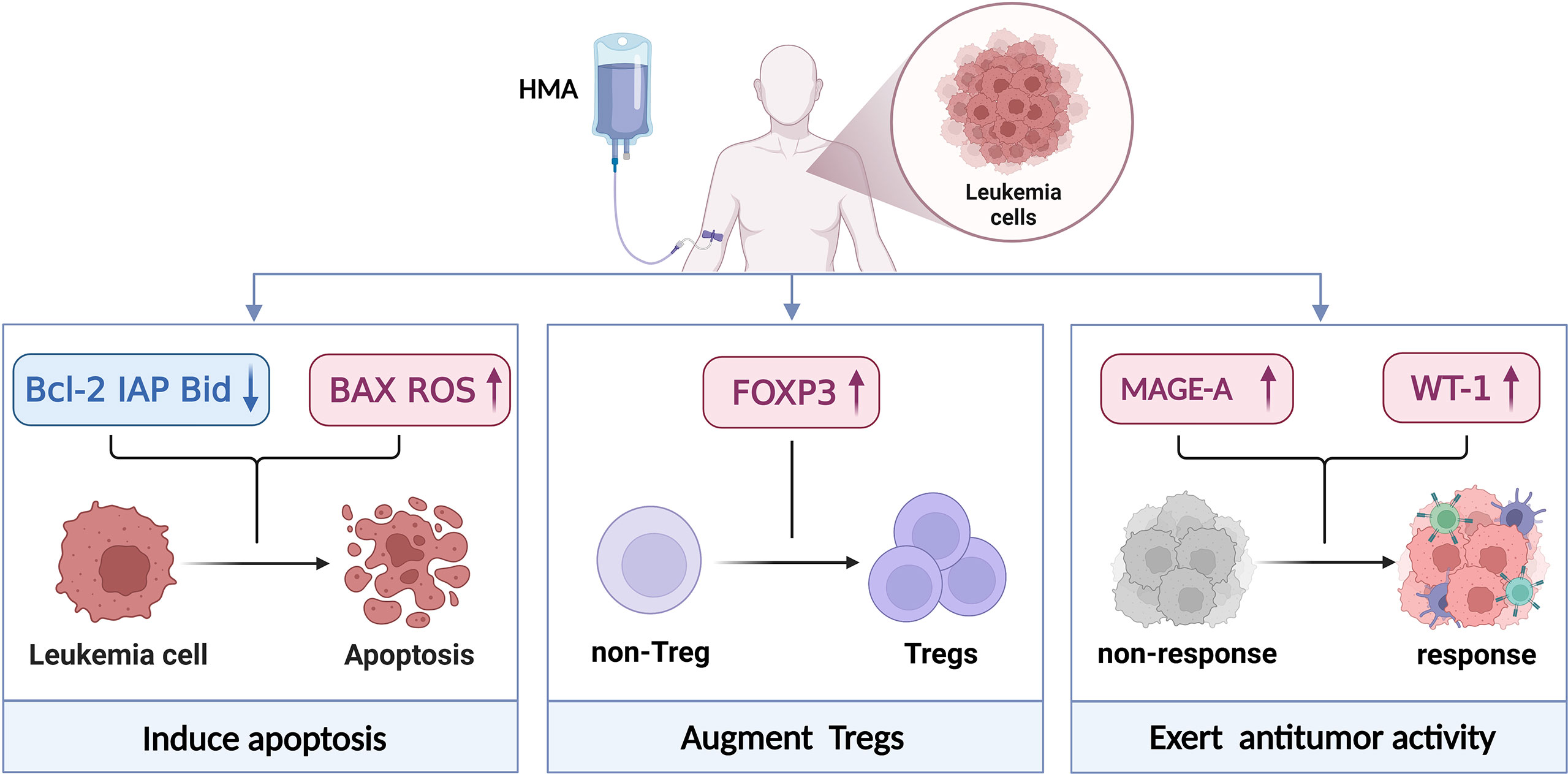
Figure 1 Mechanism of action of HMAs. HMAs induced apoptosis by engaging extrinsic and intrinsic apoptosis pathways and intracellular reactive oxygen species generation. This process was correlated with the downregulation of anti-apoptotic Bcl-2, IAP protein levels, the cleavage of Bid proteins, BAX activation and ROS upregulation which contributes to cell death. HMAs could mitigate GvHD by increasing the number and function (CD4+CD25- non-Tregs convert to CD4+CD25-FOXP3+Tregs) of Tregs after allo-HSCT. And their suppressor function is dependent on direct contact, partially dependent on perforin 1 (Prf1). When combined with other drugs, HMAs could enhance the immunotherapy responses and sensitise immunologically recalcitrant tumours to immunotherapy. Created with BioRender.com.
Currently, some other epigenetic drugs have been explored in the nontransplant setting. Guadecitabine, a next-generation HMA, can reduce the degradation of DAC and prolong its exposure in vivo, which has been used in myeloid neoplasia and has shown promising efficacy in elderly patients with AML (103). Current studies suggested that HDACi could reduce the incidence of GvHD by inhibiting the production of proinflammatory cytokines, regulating the function of alloreactive T cells, and upregulating the function and number of regulatory T cells (76). Both preclinical and clinical studies have shown that combinations of HMAs and HDACi may exert additive or synergistic antitumor activity (57, 104–106). The limited understanding of HDACi in the transplant setting is due to a relatively small number of studies. In addition, isocitrate dehydrogenase (IDH) and bromodomain and extraterminal (BET) inhibitors have also been reported in the treatment of myeloid neoplasms (107, 108), and further studies should be carried out to elucidate the role of IDH and BET inhibitors in the HSCT setting.
In summary, residual disease before HSCT, GvHD and relapse after HSCT remain three challenges for MDS and AML patients receiving allografts and our study highlight epigenetic therapy could be a generalist strategy in transplant-setting due to its immunologic activity and safety profile (Figure 2).
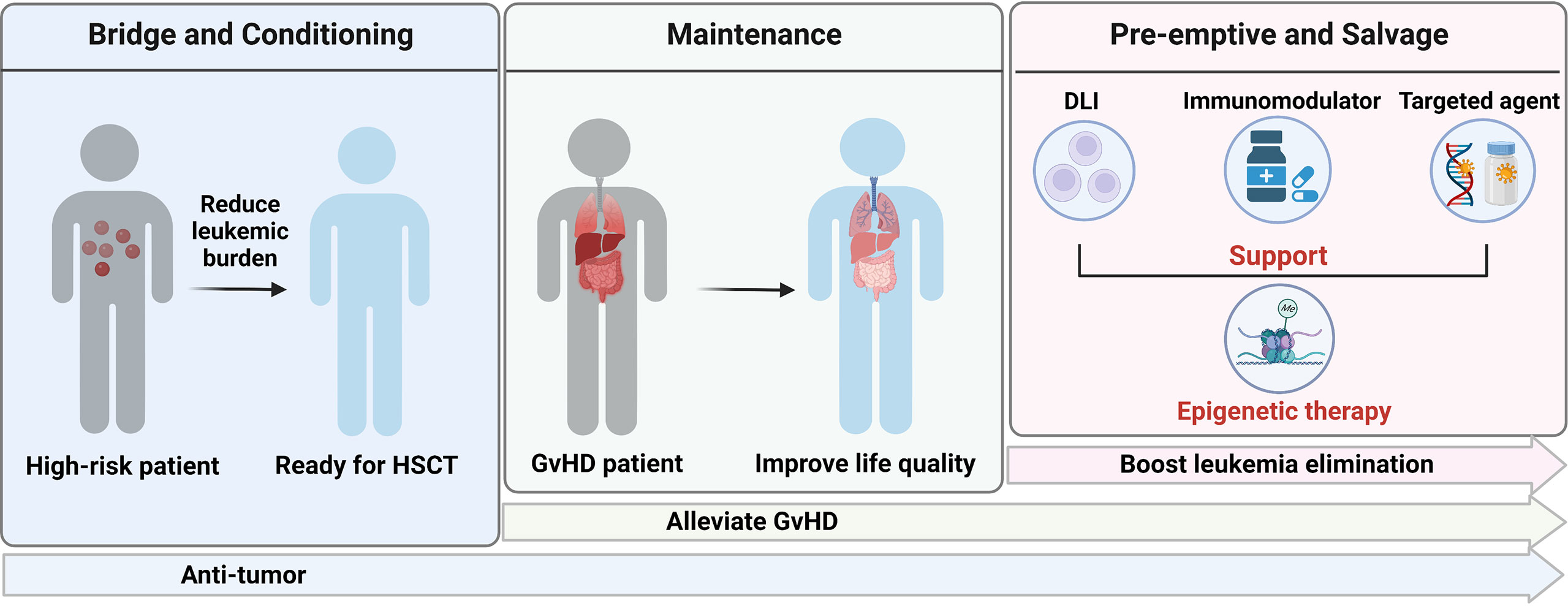
Figure 2 Application of epigenetic therapy in HSCT. Created with BioRender.com.
The manuscript was conceptualized by XZ and XiaoW. GY wrote the majority of the manuscript. GY, XianW and SH contributed equally to the manuscript. The figures were designed by JW and RH and drawn by GY and SH. GY, XianW, and SH summarized the tables. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81873424 and 82100235), Natural Science Foundation of Chongqing Innovation Group Science Program (No. cstc2021jcyj-cxttX0001) and Clinical Medical Research Project of Army Medical University (Major Project A, No. 2018XLC1006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med (2015) 373:12. doi: 10.1056/NEJMra1406184
2. Sureda A, Bader P, Cesaro S. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant (2015) 50:8. doi: 10.1038/bmt.2015.6
3. Piemontese S, Boumendil A, Labopin M. Leukemia relapse following unmanipulated haploidentical transplantation: A risk factor analysis on behalf of the ALWP of the EBMT. J Hematol Oncol (2019) 12:1. doi: 10.1186/s13045-019-0751-4
4. Schmid C, Labopin M, Nagler A. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood (2012) 119:6. doi: 10.1182/blood-2011-08-375840
5. Ciotti G, Marconi G, Martinelli G. Hypomethylating agent-based combination therapies to treat post-hematopoietic stem cell transplant relapse of acute myeloid leukemia. Front Oncol (2021) 11:810387. doi: 10.3389/fonc.2021.810387
6. Schroeder T, Rautenberg C, Haas R. Hypomethylating agents after allogeneic blood stem cell transplantation. Stem Cell Investig (2016) 3. doi: 10.21037/sci.2016.11.04
7. Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol (2021) 14:1. doi: 10.1186/s13045-020-01017-7
8. Choi SW, Braun T, Henig I. Vorinostat plus tacrolimus/methotrexate to prevent GVHD after myeloablative conditioning, unrelated donor HCT. Blood (2017) 130:15. doi: 10.1182/blood-2017-06-790469
9. Choi SW, Gatza E, Hou G. Histone deacetylase inhibition regulates inflammation and enhances tregs after allogeneic hematopoietic cell transplantation in humans. Blood (2015) 125:5. doi: 10.1182/blood-2014-10-605238
10. Goodyear OC, Dennis M, Jilani NY. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood (2012) 119:14. doi: 10.1182/blood-2011-09-377044
11. Shin DY, Park Y-S, Yang K. Decitabine, a DNA methyltransferase inhibitor, induces apoptosis in human leukemia cells through intracellular reactive oxygen species generation. Int J Oncol (2012) 41:3. doi: 10.3892/ijo.2012.1546
12. Dittmann J, Haydn T, Metzger P. Next-generation hypomethylating agent SGI-110 primes acute myeloid leukemia cells to IAP antagonist by activating extrinsic and intrinsic apoptosis pathways. Cell Death Differ (2020) 27:6. doi: 10.1038/s41418-019-0465-8
13. Goodyear O, Agathanggelou A, Novitzky-Basso I. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood (2010) 116:11. doi: 10.1182/blood-2009-11-249474
14. Weber J, Salgaller M, Samid D. Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2'-deoxycytidine. Cancer Res (1994) 54(7):1766–71.
15. Roboz GJ, Ravandi F, Wei AH. Oral azacitidine prolongs survival of patients with AML in remission independently of measurable residual disease status. Blood (2022) 139:14. doi: 10.1182/blood.2021013404
16. Greenberg PL, Stone RM, Al-Kali A. NCCN guidelines® insights: Myelodysplastic syndromes, version 3.2022. J Natl Compr Canc Netw (2022) 20:2. doi: 10.6004/jnccn.2022.0009
17. Zheng H, Wang J, Zhou J. [Retrospective efficacy analysis of decitabine bridging allogeneic hematopoietic stem cell transplantation on the treatment of myelodysplastic syndrome]. Zhonghua Xue Ye Xue Za Zhi (2015) 36:2. doi: 10.3760/cma.j.issn.0253-2727.2015.02.008
18. Voso MT, Leone G, Piciocchi A. Feasibility of allogeneic stem-cell transplantation after azacitidine bridge in higher-risk myelodysplastic syndromes and low blast count acute myeloid leukemia: Results of the BMT-AZA prospective study. Ann Oncol (2017) 28:7. doi: 10.1093/annonc/mdx154
19. Kim Y, Kim IH, Kim HJ. Multicenter study evaluating the impact of hypomethylating agents as bridging therapy to hematopoietic stem cell transplantation in myelodysplastic syndromes. Int J Hematol (2014) 99:5. doi: 10.1007/s12185-014-1549-3
20. Yahng SA, Kim M, Kim TM. Better transplant outcome with pre-transplant marrow response after hypomethylating treatment in higher-risk MDS with excess blasts. Oncotarget (2017) 8:7. doi: 10.18632/oncotarget.12511
21. Potter VT, Iacobelli S, van Biezen A. Comparison of intensive chemotherapy and hypomethylating agents before allogeneic stem cell transplantation for advanced myelodysplastic syndromes: A study of the myelodysplastic syndrome subcommittee of the chronic malignancies working party of the European society for blood and marrow transplant research. Biol Blood Marrow Transplant (2016) 22:9. doi: 10.1016/j.bbmt.2016.05.026
22. Damaj G, Duhamel A, Robin M. Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: A study by the société française de greffe de moelle et de thérapie-cellulaire and the groupe-francophone des myélodysplasies. J Clin Oncol (2012) 30:36. doi: 10.1200/jco.2012.44.3499
23. Xu Q, Li Y, Jing Y. Epigenetic modifier gene mutations-positive AML patients with intermediate-risk karyotypes benefit from decitabine with CAG regimen. Int J Cancer (2020) 146:5. doi: 10.1002/ijc.32593
24. Bartenstein M, Deeg HJ. Hematopoietic stem cell transplantation for MDS. Hematol Oncol Clin North Am (2010) 24:2. doi: 10.1016/j.hoc.2010.02.003
25. Runde V, de Witte T, Arnold R. Bone marrow transplantation from HLA-identical siblings as first-line treatment in patients with myelodysplastic syndromes: Early transplantation is associated with improved outcome. chronic leukemia working party of the European group for blood and marrow transplantation. Bone Marrow Transplant (1998) 21:3. doi: 10.1038/sj.bmt.1701084
26. Anthias C, Dignan FL, Morilla R. Pre-transplant MRD predicts outcome following reduced-intensity and myeloablative allogeneic hemopoietic SCT in AML. Bone Marrow Transplant (2014) 49:5. doi: 10.1038/bmt.2014.9
27. Walter RB, Gyurkocza B, Storer BE. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia (2015) 29:1. doi: 10.1038/leu.2014.173
28. Warlick ED, Cioc A, Defor T. Allogeneic stem cell transplantation for adults with myelodysplastic syndromes: importance of pretransplant disease burden. Biol Blood Marrow Transplant (2009) 15:1. doi: 10.1016/j.bbmt.2008.10.012
29. Park S, Baek DW, Sohn SK. Favorable outcomes with tumor burden reduction following administration of hypomethylating agents before allogeneic hematopoietic cell transplantation in patients with higher risk myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk (2019) 19:7. doi: 10.1016/j.clml.2019.03.016
30. Leung W, Campana D, Yang J. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood (2011) 118:2. doi: 10.1182/blood-2011-01-333070
31. Leung W, Pui C-H, Coustan-Smith E. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood (2012) 120:2. doi: 10.1182/blood-2012-02-409813
32. Kebriaei P, Kline J, Stock W. Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant (2005) 35:10. doi: 10.1038/sj.bmt.1704938
33. Walter RB, Gooley TA, Wood BL. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol (2011) 29:9. doi: 10.1200/JCO.2010.31.8121
34. de Lima M, Ravandi F, Shahjahan M. Long-term follow-up of a phase I study of high-dose decitabine, busulfan, and cyclophosphamide plus allogeneic transplantation for the treatment of patients with leukemias. Cancer (2003) 97:5. doi: 10.1002/cncr.11184
35. Short NJ, Kantarjian HM, Loghavi S. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomised phase 2 trial. Lancet Haematol (2019) 6:1. doi: 10.1016/s2352-3026(18)30182-0
36. Li Y, Cheng L, Xu C. A retrospective observation of treatment outcomes using decitabine-combined standard conditioning regimens before transplantation in patients with relapsed or refractory acute myeloid leukemia. Front Oncol (2021) 11:702239. doi: 10.3389/fonc.2021.702239
37. Tang X, Valdez BC, Ma Y. Low-dose decitabine as part of a modified bu-cy conditioning regimen improves survival in AML patients with active disease undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant (2021) 56:7. doi: 10.1038/s41409-021-01238-5
38. Wang QY, Li Y, Liang ZY. Decitabine-containing conditioning regimen for allogeneic hematopoietic stem cell transplantation in patients with intermediate- and high-risk myelodysplastic Syndrome/Acute myeloid leukemia: Potential decrease in the incidence of acute graft versus host disease. Cancer Manag Res (2019) 11:10195–203. doi: 10.2147/CMAR.S229768
39. D'Angelo CR, Hall A, Woo KM. Decitabine induction with myeloablative conditioning and allogeneic hematopoietic stem cell transplantation in high-risk patients with myeloid malignancies is associated with a high rate of infectious complications. Leuk Res (2020) 96. doi: 10.1016/j.leukres.2020.106419
40. Li M, Li C, Geng S. Decitabine with or without micro-transplantation for the treatment of intermediate or high-risk myelodysplastic syndrome: A Chinese single-center retrospective study of 22 patients. Front Oncol (2021) 11:628127. doi: 10.3389/fonc.2021.628127
41. Hu KX, Sun QY, Guo M. A study of human leukocyte antigen mismatched cellular therapy (Stem cell microtransplantation) in high-risk myelodysplastic syndrome or transformed acute myelogenous leukemia. Stem Cells Transl Med (2016) 5:4. doi: 10.5966/sctm.2015-0196
42. Cruijsen M, Hobo W, van der Velden W. Addition of 10-day decitabine to Fludarabine/Total body irradiation conditioning is feasible and induces tumor-associated antigen-specific T cell responses. Biol Blood Marrow Transplant (2016) 22:6. doi: 10.1016/j.bbmt.2016.02.003
43. Oran B, de Lima M, Garcia-Manero G. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv (2020) 4:21. doi: 10.1182/bloodadvances.2020002544
44. Ali N, Tomlinson B, Metheny L. Conditioning regimen intensity and low-dose azacitidine maintenance after allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Leuk Lymphoma (2020) 61:12. doi: 10.1080/10428194.2020.1789630
45. Craddock C, Jilani N, Siddique S. Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial. Biol Blood Marrow Transplant (2016) 22:2. doi: 10.1016/j.bbmt.2015.09.004
46. Vij R, Le-Rademacher J, Laumann K. A phase II multicenter study of the addition of azacitidine to reduced-intensity conditioning allogeneic transplant for high-risk myelodysplasia (and older patients with acute myeloid leukemia): Results of CALGB 100801 (Alliance). Biol Blood Marrow Transplant (2019) 25:10. doi: 10.1016/j.bbmt.2019.06.007
47. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: acute myeloid leukemia (AML) . Available at: https://seer.cancer.gov/statfacts/html/amyl.html (Accessed Aug 31, 2022).
48. Wei Y, Xiong X, Li X. Low-dose decitabine plus venetoclax is safe and effective as post-transplant maintenance therapy for high-risk acute myeloid leukemia and myelodysplastic syndrome. Cancer Sci (2021) 112:9. doi: 10.1111/cas.15048
49. Kantarjian H, Beran M, Cortes J. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer (2006) 106:5. doi: 10.1002/cncr.21699
50. Ruter B, Wijermans PW, Lubbert M. Superiority of prolonged low-dose azanucleoside administration? results of 5-aza-2'-deoxycytidine retreatment in high-risk myelodysplasia patients. Cancer (2006) 106:8. doi: 10.1002/cncr.21796
51. Pusic I, Choi J, Fiala MA. Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant (2015) 21:10. doi: 10.1016/j.bbmt.2015.05.026
52. de Lima M, Oran B, Champlin RE. CC-486 maintenance after stem cell transplantation in patients with acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transplant (2018) 24:10. doi: 10.1016/j.bbmt.2018.06.016
53. Bug G, Burchert A, Wagner EM. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial). Leukemia (2017) 31:11. doi: 10.1038/leu.2017.242
54. Guillaume T, Malard F, Magro L. Prospective phase II study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant (2019) 54:11. doi: 10.1038/s41409-019-0536-y
55. Gao L, Zhang Y, Wang S. Effect of rhG-CSF combined with decitabine prophylaxis on relapse of patients with high-risk MRD-negative AML after HSCT: An open-label, multicenter, randomized controlled trial. J Clin Oncol (2020) 38:36. doi: 10.1200/jco.19.03277
56. Mishra A, Tamari R, DeZern AE. Phase II trial of eprenetapopt (APR-246) in combination with azacitidine (AZA) as maintenance therapy for TP53 mutated AML or MDS following allogeneic stem cell transplantation (SCT). Blood (2021) 138. doi: 10.1182/blood-2021-147962
57. Kalin B, van Norden Y, van Gelder M. Panobinostat and decitabine prior to donor lymphocyte infusion in allogeneic stem cell transplantation. Blood Adv (2020) 4:18. doi: 10.1182/bloodadvances.2020002074
58. Platzbecker U, Wermke M, Radke J. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia (2012) 26:3. doi: 10.1038/leu.2011.234
59. Platzbecker U, Middeke JM, Sockel K. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol (2018) 19:12. doi: 10.1016/s1470-2045(18)30580-1
60. Woo J, Deeg HJ, Storer B. Factors determining responses to azacitidine in patients with myelodysplastic syndromes and acute myeloid leukemia with early post-transplantation relapse: A prospective trial. Biol Blood Marrow Transplant (2017) 23:1. doi: 10.1016/j.bbmt.2016.10.016
61. Schroeder T, Czibere A, Platzbecker U. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia (2013) 27:6. doi: 10.1038/leu.2013.7
62. Poire X, Graux C, Ory A. Sequential administration of low dose 5-azacytidine (AZA) and donor lymphocyte infusion (DLI) for patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) in relapse after allogeneic stem cell transplantation (SCT): A prospective study from the Belgian hematology society (BHS). Bone Marrow Transplant (2022) 57:1. doi: 10.1038/s41409-021-01464-x
63. Schroeder T, Rachlis E, Bug G. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions–a retrospective multicenter analysis from the German cooperative transplant study group. Biol Blood Marrow Transplant (2015) 21:4. doi: 10.1016/j.bbmt.2014.12.016
64. Schroeder T, Rautenberg C, Krüger W. Treatment of relapsed AML and MDS after allogeneic stem cell transplantation with decitabine and DLI-a retrospective multicenter analysis on behalf of the German cooperative transplant study group. Ann Hematol (2018) 97:2. doi: 10.1007/s00277-017-3185-5
65. Sommer S, Cruijsen M, Claus R. Decitabine in combination with donor lymphocyte infusions can induce remissions in relapsed myeloid malignancies with higher leukemic burden after allogeneic hematopoietic cell transplantation. Leuk Res (2018) 72:20–26. doi: 10.1016/j.leukres.2018.07.005
66. Craddock C, Slade D, De Santo C. Combination lenalidomide and azacitidine: A novel salvage therapy in patients who relapse after allogeneic stem-cell transplantation for acute myeloid leukemia. J Clin Oncol (2019) 37:7. doi: 10.1200/jco.18.00889
67. Medeiros BC, Tanaka TN, Balaian L. A phase I/II trial of the combination of azacitidine and gemtuzumab ozogamicin for treatment of relapsed acute myeloid leukemia. Clin Lymphoma Myeloma Leuk (2018) 18:5. doi: 10.1016/j.clml.2018.02.017
68. Daver N, Garcia-Manero G, Basu S. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in Relapsed/Refractory acute myeloid leukemia: A nonrandomized, open-label, phase II study. Cancer Discovery (2019) 9:3. doi: 10.1158/2159-8290.Cd-18-0774
69. Schuler E, Wagner-Drouet EM, Ajib S. Treatment of myeloid malignancies relapsing after allogeneic hematopoietic stem cell transplantation with venetoclax and hypomethylating agents-a retrospective multicenter analysis on behalf of the German cooperative transplant study group. Ann Hematol (2021) 100:4. doi: 10.1007/s00277-020-04321-x
70. Gao F, Gao Y, Luo Y. Venetoclax plus hypomethylating agent for the salvage treatment of relapsing myeloid malignancies after hematopoietic stem cell transplantation: A multicenter retrospective study on behalf of the zhejiang cooperative group for blood and marrow transplantation. Am J Hematol (2022) 97:2. doi: 10.1002/ajh.26405
71. Garcia-Manero G, Gore SD, Kambhampati S. Efficacy and safety of extended dosing schedules of CC-486 (oral azacitidine) in patients with lower-risk myelodysplastic syndromes. Leukemia (2016) 30:4. doi: 10.1038/leu.2015.265
72. Laille E, Shi T, Garcia-Manero G. Pharmacokinetics and pharmacodynamics with extended dosing of CC-486 in patients with hematologic malignancies. PloS One (2015) 10:8. doi: 10.1371/journal.pone.0135520
73. Arellano ML, Langston A, Winton E. Treatment of relapsed acute leukemia after allogeneic transplantation: A single center experience. Biol Blood Marrow Transplant (2007) 13:1. doi: 10.1016/j.bbmt.2006.09.005
74. Bejanyan N, Weisdorf DJ, Logan BR. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: A center for international blood and marrow transplant research study. Biol Blood Marrow Transplant (2015) 21:3. doi: 10.1016/j.bbmt.2014.11.007
75. Kungwankiattichai S, Ponvilawan B, Roy C. Maintenance with hypomethylating agents after allogeneic stem cell transplantation in acute myeloid leukemia and myelodysplastic syndrome: A systematic review and meta-analysis. Front Med (Lausanne) (2022) 9:801632. doi: 10.3389/fmed.2022.801632
76. Xu X, Li X, Zhao Y. Immunomodulatory effects of histone deacetylation inhibitors in graft-vs.-Host disease after allogeneic stem cell transplantation. Front Immunol (2021) 12:641910. doi: 10.3389/fimmu.2021.641910
77. Maslah N, Salomao N, Drevon L. Synergistic effects of PRIMA-1(Met) (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica (2020) 105:6. doi: 10.3324/haematol.2019.218453
78. Oshikawa G, Kakihana K, Saito M. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br J Haematol (2015) 169:5. doi: 10.1111/bjh.13248
79. Thanarajasingam G, Kim HT, Cutler C. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2013) 19:12. doi: 10.1016/j.bbmt.2013.09.011
80. Cui JK, Xiao Y, You Y. Decitabine for relapsed acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation. J Huazhong Univ Sci Technol Med Sci (2017) 37:5. doi: 10.1007/s11596-017-1790-0
81. Pavletic SZ, Kumar S, Mohty M. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: Report from the committee on the epidemiology and natural history of relapse following allogeneic cell transplantation. Biol Blood Marrow Transplant (2010) 16:7. doi: 10.1016/j.bbmt.2010.04.004
82. Schmid C, Labopin M, Nagler A. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: A retrospective risk factors analysis and comparison with other strategies by the EBMT acute leukemia working party. J Clin Oncol (2007) 25:31. doi: 10.1200/JCO.2007.11.6053
83. Eapen M, Giralt SA, Horowitz MM. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant (2004) 34:8. doi: 10.1038/sj.bmt.1704645
84. Ueda M, El-Jurdi N, Cooper B. Low-dose azacitidine with DNMT1 level monitoring to treat post-transplantation acute myelogenous leukemia or myelodysplastic syndrome relapse. Biol Blood Marrow Transplant (2019) 25:6. doi: 10.1016/j.bbmt.2018.12.764
85. Sullivan M, Hahn K, Kolesar JM. Azacitidine: a novel agent for myelodysplastic syndromes. Am J Health Syst Pharm (2005) 62:15. doi: 10.2146/ajhp040385
86. Sommer S, Claus R, Bertz H. Decitabine (DAC) in combination with donor lymphocyte infusions (DLIs) can induce remissions of overt aml relapses after allogeneic transplantation. Blood (2016) 128:22. doi: 10.1182/blood.V128.22.2247.2247
87. Fehniger TA, Uy GL, Trinkaus K. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood (2011) 117:6. doi: 10.1182/blood-2010-07-297143
88. Kneppers E, van der Holt B, Kersten MJ. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 trial. Blood (2011) 118:9. doi: 10.1182/blood-2011-04-348292
89. Sockel K, Bornhaeuser M, Mischak-Weissinger E. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica (2012) 97:9. doi: 10.3324/haematol.2012.067629
90. Williams P, Basu S, Garcia-Manero G. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer (2019) 125:9. doi: 10.1002/cncr.31896
91. Bewersdorf JP, Stahl M, Zeidan AM. One plus one does not always equal two, especially with regard to hypomethylating agents: the question of synergy of azacitidine and lenalidomide for treatment of relapsed acute myeloid leukemia and myelodysplastic syndromes post allogeneic hematopoietic stem cell transplant. Expert Rev Hematol (2019) 12:8. doi: 10.1080/17474086.2019.1635005
92. Jin S, Cojocari D, Purkal JJ. 5-azacitidine induces NOXA to prime AML cells for venetoclax-mediated apoptosis. Clin Cancer Res (2020) 26:13. doi: 10.1158/1078-0432.Ccr-19-1900
93. Balaian L, Ball ED. Cytotoxic activity of gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia correlates with the expression of protein kinase syk. Leukemia (2006) 20:12. doi: 10.1038/sj.leu.2404437
94. Bogenberger JM, Kornblau SM, Pierceall WE. BCL-2 family proteins as 5-azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia (2014) 28:8. doi: 10.1038/leu.2014.44
95. Aldoss I, Yang D, Aribi A. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica (2018) 103:9. doi: 10.3324/haematol.2018.188094
96. DiNardo CD, Pratz K, Pullarkat V. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood (2019) 133:1. doi: 10.1182/blood-2018-08-868752
97. Craddock C, Labopin M, Robin M. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica (2016) 101:7. doi: 10.3324/haematol.2015.140996
98. Lim Z, Brand R, Martino R. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol (2010) 28:3. doi: 10.1200/JCO.2009.21.8073
99. Kröger N, Sockel K, Wolschke C. Comparison between 5-azacytidine treatment and allogeneic stem-cell transplantation in elderly patients with advanced MDS according to donor availability (VidazaAllo study). J Clin Oncol (2021) 39:30. doi: 10.1200/jco.20.02724
100. Jacoby MA, Duncavage EJ, Chang GS. Subclones dominate at MDS progression following allogeneic hematopoietic cell transplant. JCI Insight (2018) 3:5. doi: 10.1172/jci.insight.98962
101. Tsirigotis P, Byrne M, Schmid C. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. a review from the ALWP of the EBMT. Bone Marrow Transplant (2016) 51:11. doi: 10.1038/bmt.2016.167
102. Wei AH, Dohner H, Pocock C. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med (2020) 383:26. doi: 10.1056/NEJMoa2004444
103. Roboz GJ, Kantarjian HM, Yee KWL. Dose, schedule, safety, and efficacy of guadecitabine in relapsed or refractory acute myeloid leukemia. Cancer (2018) 124:2. doi: 10.1002/cncr.31138
104. Kirschbaum M, Gojo I, Goldberg SL. A phase 1 clinical trial of vorinostat in combination with decitabine in patients with acute myeloid leukaemia or myelodysplastic syndrome. Br J Haematol (2014) 167:2. doi: 10.1111/bjh.13016
105. How J, Minden MD, Brian L. A phase I trial of two sequence-specific schedules of decitabine and vorinostat in patients with acute myeloid leukemia. Leuk Lymphoma (2015) 56:10. doi: 10.3109/10428194.2015.1018248
106. Zhu WG, Lakshmanan RR, Beal MD. DNA Methyltransferase inhibition enhances apoptosis induced by histone deacetylase inhibitors. Cancer Res (2001) 61:4:1327–33.
107. Chen NC, Borthakur G, Pemmaraju N. Bromodomain and extra-terminal (BET) inhibitors in treating myeloid neoplasms. Leuk Lymphoma (2021) 62:3. doi: 10.1080/10428194.2020.1842399
Keywords: hematopoietic stem cell transplantation, epigenetic therapy, graft-versus-host disease, myelodysplastic syndrome, acute myeloid leukemia
Citation: Yang G, Wang X, Huang S, Huang R, Wei J, Wang X and Zhang X (2022) Generalist in allogeneic hematopoietic stem cell transplantation for MDS or AML: Epigenetic therapy. Front. Immunol. 13:1034438. doi: 10.3389/fimmu.2022.1034438
Received: 01 September 2022; Accepted: 20 September 2022;
Published: 04 October 2022.
Edited by:
Xiao-Dong Mo, Peking University People’s Hospital, ChinaCopyright © 2022 Yang, Wang, Huang, Huang, Wei, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xi Zhang, emhhbmd4eGlAc2luYS5jb20=; Xiaoqi Wang, eGlhb3Fpd2FuZzI3QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.