
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 03 November 2022
Sec. Comparative Immunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1031962
A correction has been applied to this article in:
Corrigendum: The effect and underlying mechanism of yeast β-glucan on antiviral resistance of zebrafish against spring viremia of carp virus infection
β-glucan has been used as immunostimulant for fish. However, the effect of yeast β-glucan on viral infections has been less studied in fish. In this study, we investigated the effects of β-glucan on the resistance of zebrafish against spring viraemia of carp virus (SVCV) and elucidated the underlying mechanisms. Zebrafish were fed with a control diet or diet supplemented with 0.01% and 0.025% β-glucan for 2 weeks, and were challenged by SVCV. Zebrafish embryonic fibroblast (ZF4) cells were treated with 5 μg/mL β-glucan and were infected by SVCV. We further investigated the effect of β-glucan on autophagy level post SVCV infection. The intestinal microbiota was evaluated by 16S rRNA gene pyrosequencing. Results showed that dietary supplementation of 0.025% β-glucan significantly increased survival rate of zebrafish compared with control group after SVCV challenge (P < 0.05). Dietary β-glucan significantly increased the expression of genes related to type I IFN antiviral immune pathway in the spleen of zebrafish after viral infection, including type I IFN genes (ifnφ1, ifnφ2, ifnφ3), IFN-stimulated genes (mxb, mxc), as well as other genes involved in the IFN signaling pathway, including tlr7, rig1, mavs, irf3 and irf7. Morpholino knockdown of type I IFN receptors dampened the antiviral effect of β-glucan in zebrafish larvae, indicating that β-glucan-mediated antiviral function was at least partially dependent on IFN immune response. Furthermore, β-glucan can inhibit the replication of SVCV in ZF4 cells. However, β-glucan did not stimulate type I IFN antiviral response in ZF4 cells, and the antiviral effect of β-glucan in ZF4 was independent of Myd88. Interestingly, β-glucan induced autophagy in ZF4 cells after SVCV infection. Inhibition of autophagy blocked the antiviral effect of β-glucan in ZF4 cells. Lastly, dietary β-glucan changed the composition of intestinal microbiota in zebrafish, with reduced abundance of Proteobacteria and an enrichment of Fusobacteria and Firmicutes. To sum up, our results indicate that the β-glucan enhanced resistance of zebrafish against SVCV and the mechanism involved stimulation of type I IFN antiviral immune response of fish after viral infection.
Yeast cell wall consists of β-glucan, mannan, protein, lipid, and chitin, among which the proportion of β -glucan was the highest, about 29% to 64% (1, 2). Previous studies have shown that β-glucan is a natural immune activator that can nonspecifically and specifically enhance the immune system of aquatic animals (3–6). Early studies discovered that β-glucan from yeast cell wall can act on macrophages to stimulate the body’s non-specific immunity (7). β-Glucan can be administered by different routes such as injection, bathing, and dietary supplementation, and the beneficial effects of β-glucan have been reported in various fish species including Atlantic salmon (Salmo salar), common carp (Cyprinus carpio), Nile tilapia (Oreochromis niloticus), etc (8). As a key facet of the benefits, β-glucan can enhance the host’s defenses to improve their resistance to pathogens. For instance, macrophages from β-glucan-treated rainbow trout exhibited increased bactericidal activity against Aeromonas salmonicida (9). Yeast β-glucan was also used as vaccine adjuvant to protect fish against various bacterial pathogens, including Vibrio damsela (10), Edwardsiella tarda (11). In contrast to bacterial diseases, the effect of yeast β-glucan on viral infections has been less studied in fish. Eearly study showed that administration of β-glucans to rainbow trout results in decreased susceptibility to IHNV, presumably as a result of an enhanced innate response (12). β-Glucan treatment improved survival of Olive flounder (Paralichthys olivaceus) after challenge by Viral hemorrhagic septicemia (VHS) (13). Beaulaurier et al. showed that Pacific herring (C. pallasii) fed a β-glucan-containing diet significantly improved survival when challenged with viral haemorrhagic septicaemia rhabdovirus (VHSV) (14). Carp treated with glucan showed enhanced resistance against the infection of grass carp hemorrhage virus (GCHV) (15). Krishnan et al. revealed that β-glucan protected grouper against NNV infection (16). Till now, the mechanism of the antiviral effect of β-glucan has been poorly understood.
Spring viremia of carp (SVC) is caused by spring viremia of carp virus (SVCV) and leads to mass cyprinid mortality and enormous economic losses. In host cells, viral RNAs can be recognized by the innate antiviral immune system, triggering an antiviral response (17). Until now, there is no licensed therapeutic agents available for controlling SVCV infection (18). Type I interferon (IFN) system plays an important role in the antiviral immune response of fish (19). Consistent with the function in mammals, type I IFN in fish can directly inhibit the replication of the virus by inducing the expression of antiviral proteins (19–21). It has been reported that β-glucan can enhance the immune response and resistance of zebrafish against SVCV infection (22). However, the response of type I IFN signaling pathway was less investigated in glucan-treated fish after SVCV infection.
Autophagy is a self-protection ability acquired by the body in long-term evolution. Autophagy can non-specifically degrade pathogenic microorganisms such as bacteria, viruses, and parasites that invade the host (23). Recent studies have shown that autophagy has a double-sided effect on viral infection. On the one hand, autophagy can clear viruses; on the other hand, some viruses can effectively complete their own replication by using autophagy (24). For example, when Sindbis virus invades host cells, autophagy can be induced, and host cells clear the virus through autophagy (25). Infection with vesicular stomatitis virus (VSV) causes autophagy in Drosophila, which in turn inhibits viral replication (26). In contrast, autophagy promoted the replication of polioviruses (27) and dengue virus (28). In terms of fish viruses, previous studies showed that autophagy inhibited the replication of two fish rhabdoviruses, viral hemorrhagic septicemia virus (VHSV) and SVCV in zebrafish embryonic fibroblast (ZF4) cells (29, 30). However, studies also showed that SVCV utilizes the autophagy pathway to promote viral replication in Epithelioma papulosum cyprini (EPC) cells (31). The discrepancy in terms of the effect of autophagy on SVCV infection might be due to different cell models used.
In the present study, we evaluated the effect of dietary β-glucan on antiviral resistance in zebrafish through SVCV infection model, and investigated the underlying mechanisms. Our results showed that dietary β-glucan enhanced the resistance of zebrafish against SVCV infection. Moreover, we found that β-glucan stimulated the antiviral IFN response in zebrafish, and the antiviral effect of β-glucan involved autophagy induction.
Two types of diets were prepared, the basal diet and β-glucan supplemented diet. The β-glucan was obtained from Solarbio (Beijing, China: purity ≥ 80.0%). The composition of basal diet is shown in Table 1. β-Glucan supplemented diet was formulated by adding 0.01% and 0.025% β-glucan into basal diet, which replaced the same amount of bentonite. β-Glucan was dissolved in appropriate amount of sterile water and then mixed with basal diet.
For feeding experiments, we used 2-months-old Tuebingen zebrafish obtained from the China Zebrafish Resource Center (Wuhan, China), with an average initial weight of 0.08 ± 0.005 g. The zebrafish were randomly assigned to 3 L tanks each containing 15 fish, with 3 replicate tanks per group. During 2-week feeding period, zebrafish were fed twice (09:00 and 17:00) a day, with 6% of the total body weight. The water temperature 28°C, pH 6.7-7.0, O2 > 6.0 mg/L, and nitrogen content < 0.50 mg/L. For the experiments with zebrafish larvae, larvae hatched from their chorions at 3 days postfertilization (dpf). Each group had four bottles with 30 fish per bottle. At 4 dpf, the zebrafish larvae were treated with β-glucan by immersion at 0.025% and 0.05% (m/v). At 7 dpf, 0.1 MOI SVCV was added. At 9 dpf, zebrafish larvae were collected for qPCR analysis.
Zebrafish from each experimental group were acclimatized to 22°C during the last week of feeding and then challenged with 106 TCID50/ml SVCV by bath immersion. Zebrafish were not fed during the challenge period and the mortality was monitored daily for 14 days. The survival rate was calculated by the Kaplan-Meier method. Zebrafish were anesthetized with MS222, then the spleen was sampled and RNA was extracted, and the expression of antiviral genes of zebrafish was detected.
ZF4 cells were purchased from the American Type Culture Collection (ATCC number CRL-2050), and cultured in DMEM/F-12 medium containing 10% fatal bovine serum (Gibco, Australia) at 28°C in a 5% CO2 incubator. EPC cells and SVCV (ATCC: VR-1390) were presented by professor Jun-Fa Yuan (Huazhong agricultural university, Wuhan, Hubei, China). EPC cells in MEM medium at 25°C in an incubator with 5% CO2. The SVCV was propagated in EPC cells at 28°C. When cytopathic effect (CPE) was clearly visible in EPC cells, the viral suspensions were divided into aliquots and stored at -80°C.
ZF4 cell was seeded on 96-well plates and incubated for 24 h to sub-confluence. Then ZF4 cell was exposed to medium added with β-glucan. At the end of the exposure period, medium with 10% AlarmaBlue cell viability reagent (Invitrogen, Grand Island, NY, USA) was added. After a 1 h incubation, fluorescence was measured with the SynergyH1 microplate reader (Biotek) at excitation and emission wavelengths of 485 nm and 595 nm, respectively. The ratio of cell viability was calculated using the fluorescence readings. Cell survival rate = (value of treatment group - value of blank control group)/(OD value of negative control group - OD value of blank control group) ×100%.
ZF4 cells were treated with β-glucan (1, 2.5, 5, 10, 20 μg/mL) for 24 h. Then 0.1 MOI SVCV was added and incubated at 22°C for 1 h. Infected ZF4 cells monolayers were then washed, fresh medium added, and plates further incubated. At 24 hours after infection, SVCV replication in ZF4 cells was evaluated by qPCR measuring the expression of SVCV N protein. ZF4 cells infected with SVCV but not treated with β-glucan were used as control. For TCID50 assay, EPC cells were maintained in 96 well plates. The supernatants of ZF4 cells were collected at 24 h post SVCV infection. Then, serial 10-fold dilutions of supernatants were performed to incubate with EPC cells. After five days, the wells with CPE were recorded and TCID50/0.1 ml was calculated by the Reed-Muench method (32).
In a 12-well plate, ZF4 cells in the logarithmic growth phase were inoculated. The reagent Lipofectamine RNAiMAX Transfection (Invitrogen) was used to transfect scrambled small interfering RNA (siRNA; negative control) and myd88 siRNA into the plate according to the kit procedure. The sequence of myd88 siRNA is as follows: Sense: GGUCAUCUCUGAUGAUUAUTT; Antisense: AUAAUCAUCAGAGAUG ACCTT. After 24 h, β-glucan was added. SVCV at a concentration of 0.1 MOI was administered after 6 h. qPCR was used to determine the knockdown efficacy of the siRNA as well as the expression of the SVCV N protein.
Vivo-morpholino oligonucleotides (MO) against zebrafish type I IFN receptor subunits CRFB1 and CRFB2 (33) were designed and synthesized by Gene-Tools (Philomath, OR). The sequences of MO used in this study are as follows: CRFB1-vivo-MO (splice blocking), 5′-CGCCAAGATCATACCTGTAAAGTAA-3′; CRFB2-vivo-MO (splice blocking), 5′-AGTTTGTTTTCTCACCTCTGTTCCA-3′; and standard control-vivo-MO, 5′-CCTCTTACCTCAGTTACAATTTATA-3′. Zebrafish larvae (4dpf) were added with 25 nmol/L of CRFB1 and CRFB2 vivo MO (CRFB1 and CRFB2 vivo MO were added together during the experiment), or standard control vivo MO, and then treated with 0.05% β-glucan for 24 h. At 7 dpf, 0.1 MOI SVCV was added. At 9 dpf, zebrafish larvae were collected and SVCV replication in the larvae was measured by qPCR. The knockdown efficiency was evaluated by qPCR quantifying the relative expression of properly spliced transcripts. Related primers are listed in Table 2.
ZF4 cells were treated with 5 μg/mL β-glucan when they reached 80% abundance, SVCV was added after 24 h, cells were trypsinized at 0 h, 6 h and 12 h after challenge. 1 mg AO (acridine orange) was dissolved in 10 mL of PBS with pH = 7.2 to prepare a 100 μg/mL stock solution. 100 μL of cell fluid was added to 4 μL of AO stock solution (acridine orange) staining solution. The fluorescence of stained cells was quantified by flow cytometry.
ZF4 cells were lysed with ice-cold RIPA lysis buffer mixed with 1mM PMSF and phosphatase inhibitors (Abcam, USA). Equivalent amounts of total protein were loaded into a 12% SDS-PAGE for electrophoresis and then transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, USA). After blocking nonspecific binding with 5% non-fat dry milk in PBS, the PVDF membrane was incubated with primary antibodies, i.e., antibodies against β-actin (CMCTAG, AT0544, 1:1000) and LC3A/B (CST, 4108, 1:1000). The blots were developed using horseradish peroxidase (HRP)-conjugated secondary antibodies (GE Health, 1:3000) and the ECL-plus system.
ZF4 cells were treated with autophagy specific inhibitor 3-methyladenine (3-MA) at 5 mM (MedChemExpress) and chloroquine (CQ) at 5 μM (MedChemExpress) for 24 h, and then treated with 5 μg/mL β-glucan for 24 h. At 24 h post-infection SVCV replication in ZF4 cells was evaluated by qPCR.
Total RNA was isolated from spleen tissue of zebrafish and ZF4 cells with Trizol reagent (TaKaRa, Tokyo, Japan) following the manufacturer’s protocol. The extracted RNA was re-suspended in 30 μl RNase-free water then quantified with a BioTek Synergy™2 Multi-detection Microplate Reader (BioTek Instruments, Winooski, VT) and agarose gel electrophoresis. One microgram of total RNA was used for reverse transcription with Revert Aid™ Reverse Transcriptase (TaKaRa, Tokyo, Japan) according to the manufacturer’s instructions. The synthesized cDNA was stored at -20°C.
Experimental methods about RT-qPCR reaction were conducted as previously described (34). The primers used in the experiment were listed in Table 2. Ribosomal protein s11 gene (rps11) was selected and used as the internal reference gene according to our previous work (35, 36), and the data were statistically analyzed by 2-ΔΔCT method.
At the end of the 3-weeks feeding period, the digesta of adult zebrafish were collected 4 h after the last feeding. The digesta were collected under aseptic conditions. The digesta samples from the 6 fish were pooled as a replicate. DNA was extracted from each pooled sample using a Fast DNA SPIN Kit for Soil (MP Biomedicals), according to the manufacturer’s instructions. The 16s V3–V4 region was amplified by using the primers U341F (5′-CGGCAACGAGCGCAACCC-3′) and U806 (5′-CCATTGTAGCACGTGTGTAGCC-3′). 16S rRNA gene sequencing was performed at the Realbio Genomics Institute using the Illumina Miseq platform. Microbiota sequencing data in this study are available from the National Center for Biotechnology Information (NCBI) under accession number PRJNA876409.
All data were performed using GraphPad Prism 8 software (GraphPad Software Inc. CA, USA). All data were expressed as mean ± SEM. Differences between treatments were evaluated by Student’s t-test. The test of Log-rank (Mantel-Cox) was used to analyze survival rate after SVCV challenge. When P values were less than 0.05, the difference was considered significant.
As shown in Figure 1, the survival rate of zebrafish was calculated after challenge with 106 TCID50/ml SVCV. Dietary supplementation of 0.025% β-glucan significantly increased survival rate of fish compared with control group (P < 0.05), while there was no significant difference between the 0.01% β-glucan group and control (P > 0.05). We also evaluated the effect of β-glucan on viral susceptibility of zebrafish larvae. Zebrafish larvae were treated with 0.025% and 0.05% (m/v) β-glucan by immersion and infected with SVCV. Results showed that β-glucan significantly inhibited viral replication in larvae (P < 0.01) (Supplementary Figure 1)
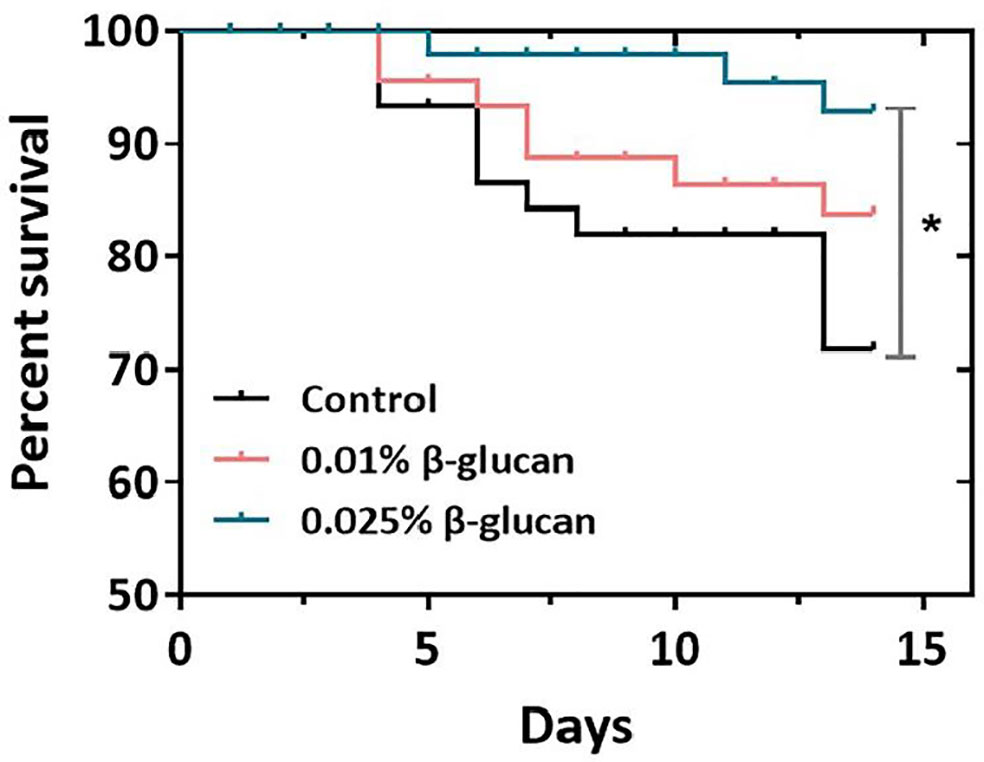
Figure 1 Effect of β-glucan on survival rates of zebrafish post SVCV infection (n = 3) (survival rates (%) over time, days 0-14). The asterisk denotes significant difference (P < 0.05) by Log-rank (Mantel-Cox) test.
The expression of antiviral genes after SVCV challenge in zebrafish spleen are shown in Figure 2. Compared with the control group, the expression of type I IFN genes including ifnφ1, ifnφ2, ifnφ3 and the expression of IFN-stimulated genes including mxb and mxc were significantly increased in the 0.025% β-glucan group (Figure 2, P < 0.05). In addition, 0.025% β-glucan group also significantly increased the expression of virus recognition receptors related genes including tlr7, rig1 as well as the expression of downstream genes of IFN pathway such as mavs, irf3 and irf7 (Figure 2, P < 0.05). Similarly, 0.01% β-glucan supplementation also significantly increased the expression of ifnφ1, ifnφ2, ifnφ3, mxb, mxc, irf3, irf7 and rig1 (Figure 2, P < 0.05). Consistently, β-glucan treatment by immersion enhanced the expression of type I IFN pathway-related genes in zebrafish larvae after SVCV infection (Supplementary Figure 2).
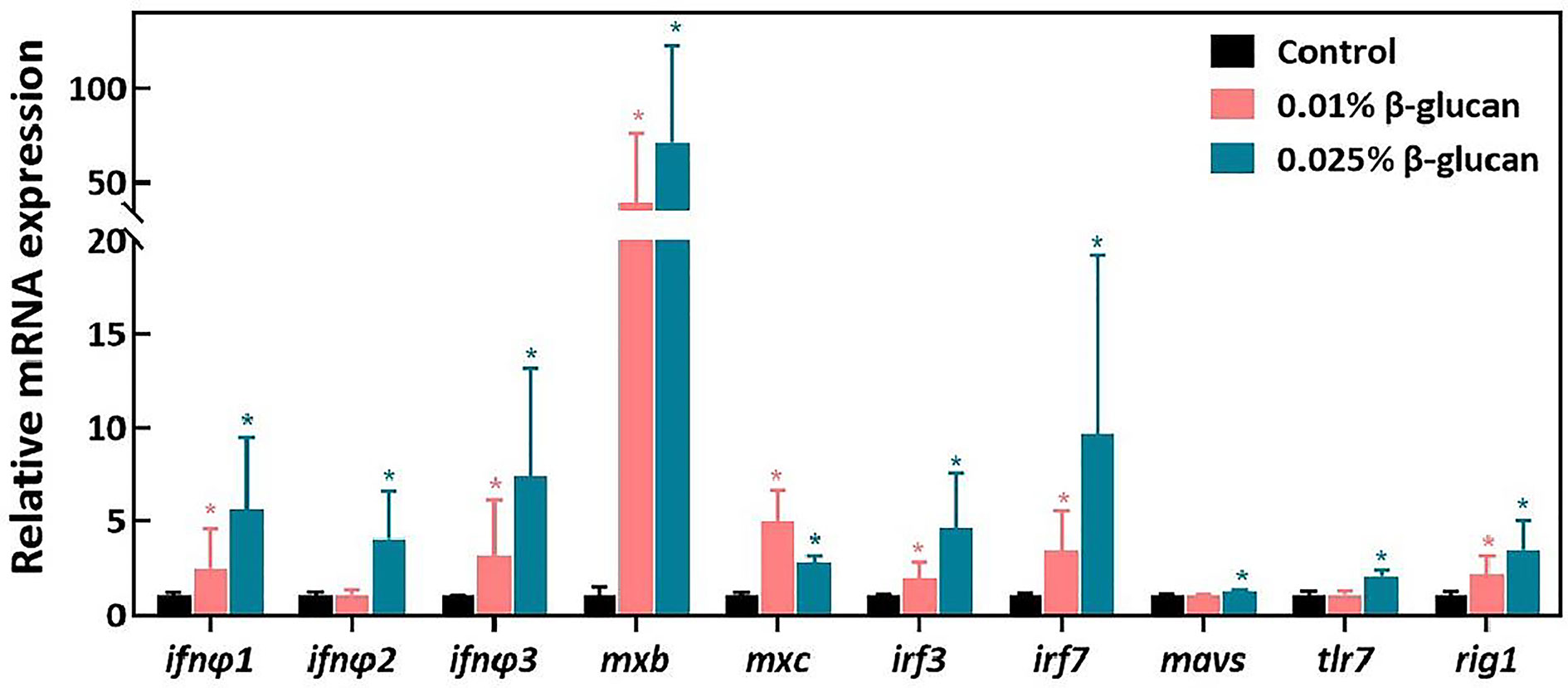
Figure 2 Relative mRNA expression of genes related to antiviral innate immune response in the spleen of zebrafish four days after SVCV challenge (n = 6). The expression of genes was expressed as fold of control group. Values represent the means ± SEM.*P < 0.05.
The stimulation of genes in the IFN pathway by β-glucan in both adult and larval zebrafish suggest the involvement of IFN response in the antiviral function of β-glucan. To investigate the role of type I IFN response in the antiviral effect of β-glucan, the IFN receptor subunits CRFB1 and CRFB2 were knocked down in zebrafish larvae by vivo morpholino (Supplementary Figure 3). The results showed that while β-glucan still inhibited viral replication in zebrafish larvae with deficient IFN receptors, the inhibitory scale was significantly reduced (Figure 3), indicating that the mechanism of antiviral function of β-glucan is at least in part dependent on the stimulation of type I IFN antiviral immune response.
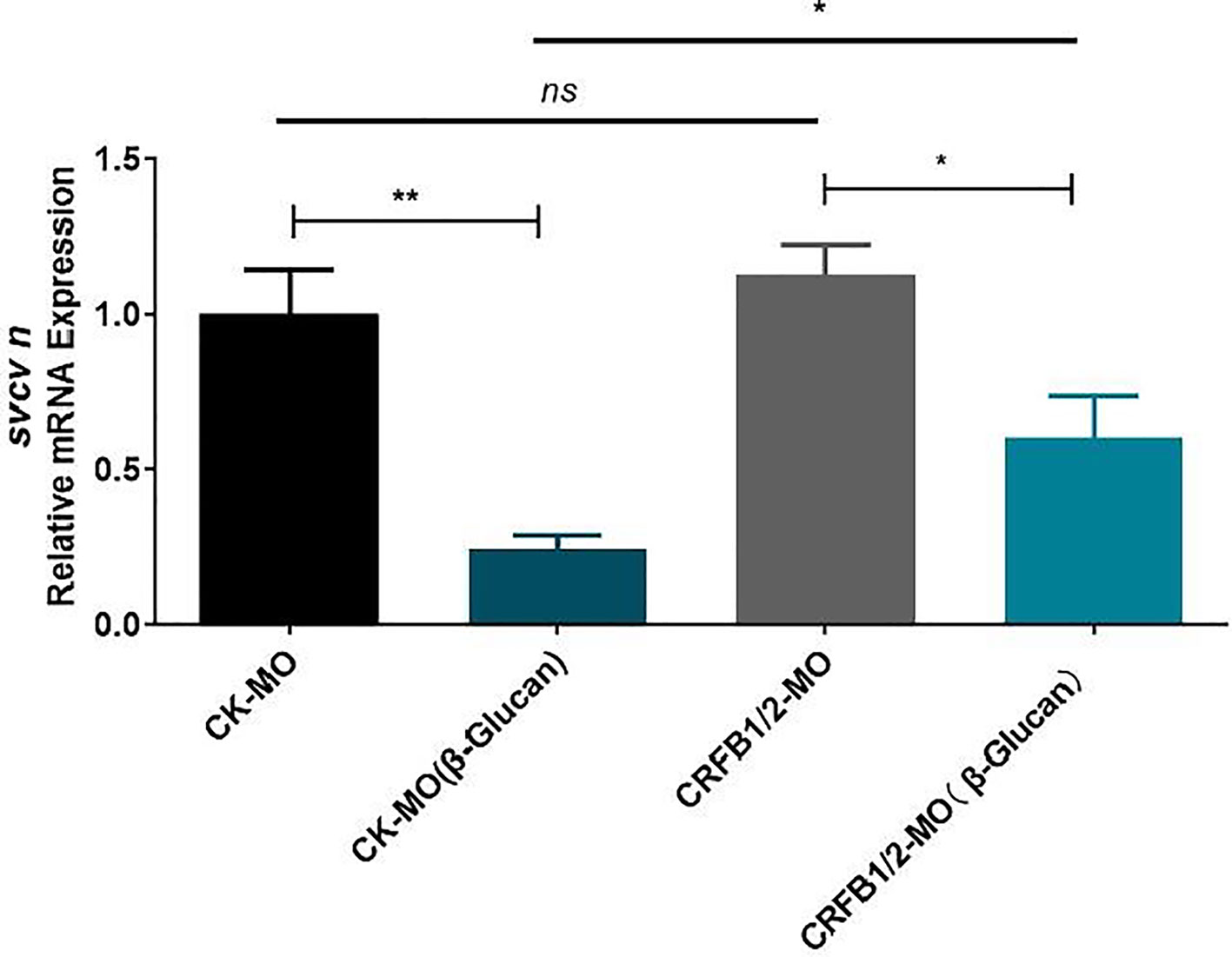
Figure 3 Effects of morpholino-mediated knockdown of IFN receptor subunits (CRFB1 and CRFB2) on the antiviral function of β-glucan in zebrafish larvae (n = 6). Values represent the means ± SEM. *P < 0.05, **P < 0.01, "ns" is a contraction of no significant.
We further evaluated the antiviral effect of β-glucan in ZF4 cell model. ZF4 cells were treated with 1, 2.5, 5, 10, 20 μg/mL β-glucan for 24 h and then were infected by SVCV (Supplementary Figure 4). Viral replication in ZF4 cells was evaluated at 24 hpi. Results showed that 5 μg/mL β-glucan inhibited the replication of SVCV in ZF4 cells (Figure 4A, P < 0.05), which is consistent with the in vivo results. Moreover, compared with the control group, β-glucan significantly reduced the SVCV titer in the supernatant of ZF4 cells (Figure 4B, P < 0.05). We also evaluated the effect of β-glucan on cell viability. The results showed that 5 μg/mL β-glucan treatment for 24 and 48 h did not affect the survival of ZF4 cells (Figure 4C, P > 0.05).
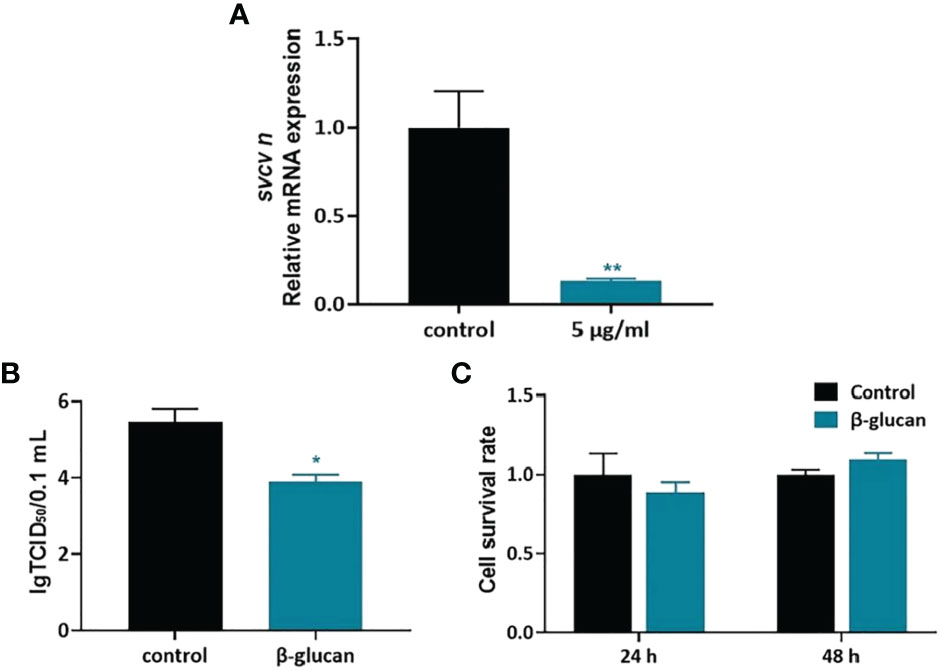
Figure 4 Antiviral effect of β-glucan in ZF4 cells. (A) Effects of β-glucan in the mRNA expression of SVCV N protein (n = 6); (B) Effects of β-glucan on the TCID50 of SVCV (n = 6); (C) Effects of β-glucan on cell viability (n = 6). Values represent the means ± SEM. *P < 0.05; **P < 0.01.
To eliminate the influence of viral replication on antiviral gene expression, viral RNA mimics polyriboinosinic polyribocytidylic acid (poly (I:C) was transfected into ZF4 cells after β-glucan treatment. The expression of genes related to the type I IFN signaling pathway was detected. Results showed that the expression of ifnφ1, ifnφ2, ifnφ3, mxb, and mxc after poly (I:C) stimulation was not significantly different in 5 μg/mL β-glucan compared with control (Supplementary Figure 5, P > 0.05), suggesting that β-glucan does not stimulate type I IFN antiviral response in ZF4 cells. The discrepancy of results in terms of IFN response in ZF4 cells and in vivo might be due to cell specific stimulation of IFN pathway by β-glucan in zebrafish.
LC3 is conjugated to phosphatidylethanolamine to form LC3-II after autophagy induction, which is conserved among vertebrates including fish. LC3-II/LC3-I ratio is commonly used to evaluate autophagy activation. In order to investigate the effect of β-glucan on autophagy formation in ZF4 cells, western blot for LC3 was performed. The results showed that β-glucan did not affect LC3-II/LC3-I ratio in non-infected cells, but significantly increased the ratio after SVCV infection (Figures 5A, B), indicating that β-glucan can enhance autophagy induction after viral infection.
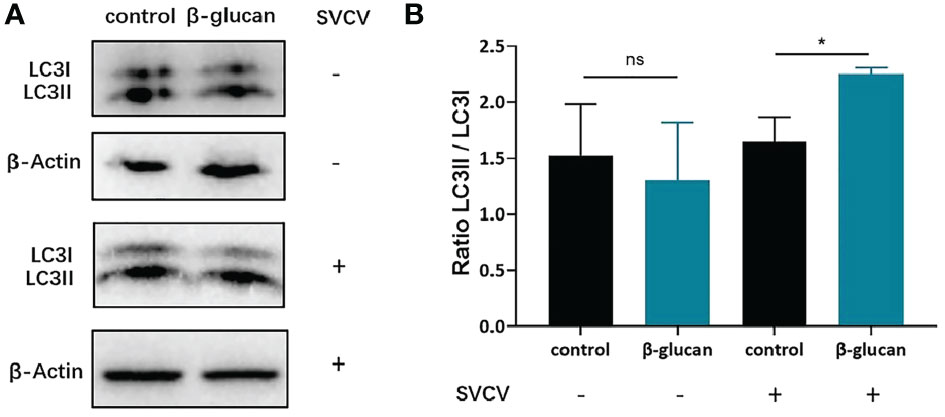
Figure 5 Effects of β-glucan on the LC3II/LC3I ratio post SVCV infection in ZF4 cells. (A) Western blot analysis of LC3 in ZF4 cells (n = 3); (B) LC3II/LC3I ratio in ZF4 cells were calculated by Image J software (n = 3). Values represent the means ± SEM. *P < 0.05, "ns" is a contraction of no significant.
Furthermore, autophagy was quantified by flow cytometry after AO staining. AO fluoresces green in whole cell but fluoresces red in autophagic vacuoles. The results showed that β-glucan significantly enhanced the autophagy levels of ZF4 cells 6 h after SVCV infection (Figures 6B, D, P < 0.05), while there was no significant difference in autophagy between β-glucan-treated and control cells at 0 h and 12 h post infection (Figures 6A, C, D, P > 0.05). This indicated that the β-glucan-mediated autophagy activation was transient and occurred in the early stage after viral infection.
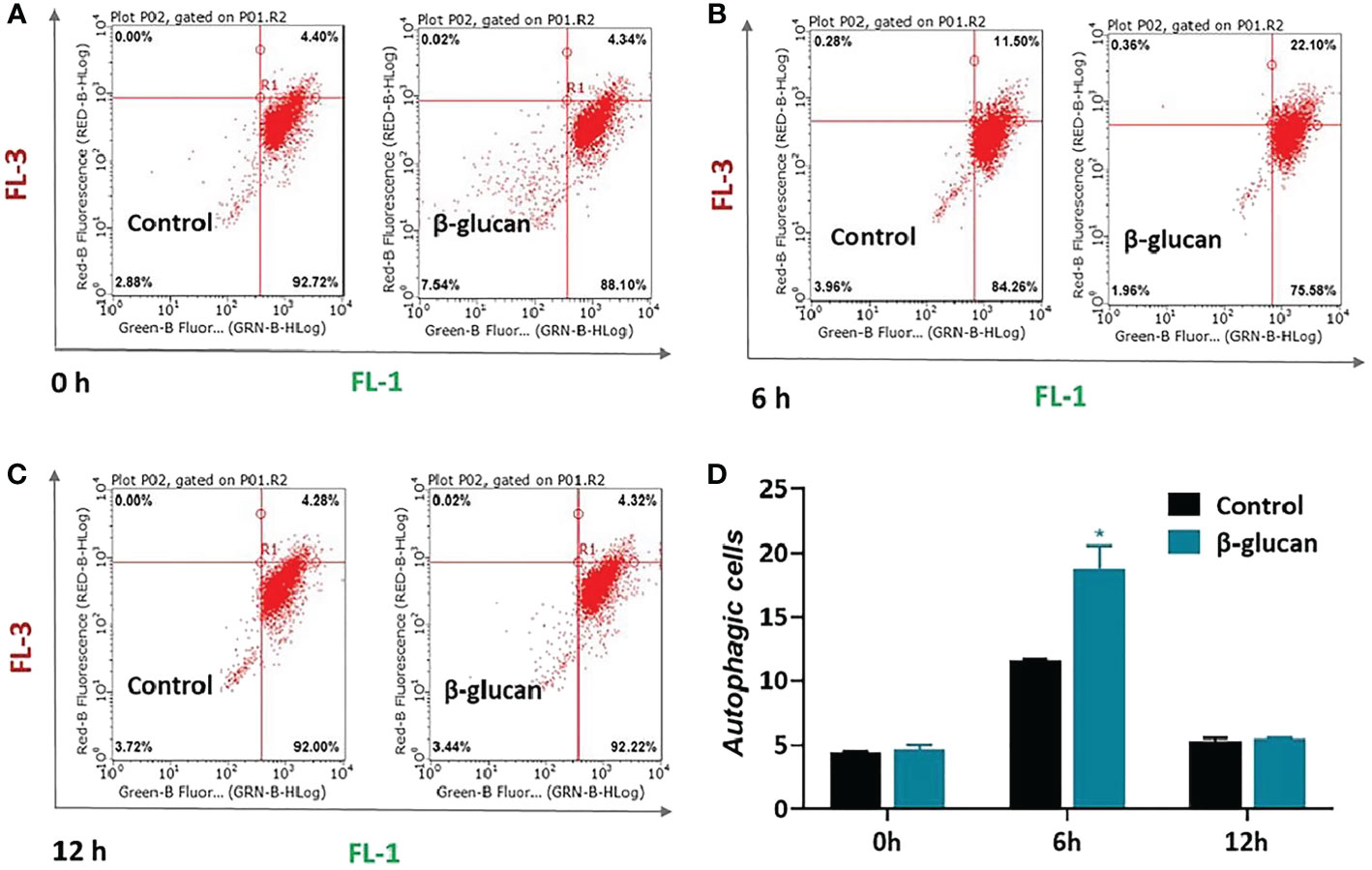
Figure 6 Effects of β-glucan on autophagy level in ZF4 cells. Autophagy was quantified by flow cytometry after AO staining. The flow cytometry results of control or β-glucan-treated ZF4 cells at 0 h (A), 6 h (B), and 12 h (C) post SVCV infection were exhibited; (D) Percentage of control or β-glucan-treated ZF4 cells positive for autophagosomes at different time points after viral infection. Values represent the means ± SEM. *P < 0.05 (n = 6).
To investigate the role of autophagy in β-glucan-induced antiviral effect, we used 3-MA and CQ to inhibit autophagy. The results showed that pretreatment with both autophagy inhibitors blocked the antiviral effect of β-glucan in ZF4 cells (Figures 7A, B, Supplementary Figures 6A, B).
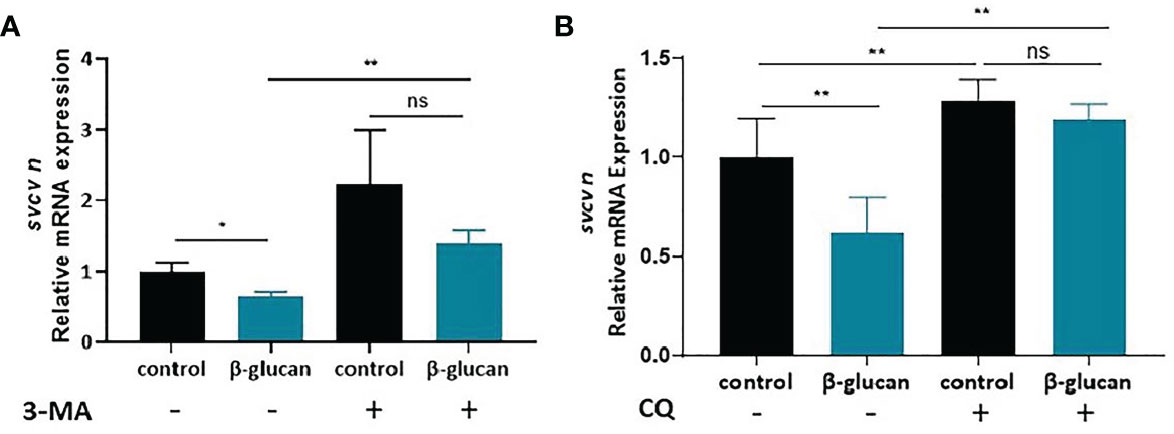
Figure 7 Effects of 3-MA (A) and CQ (B) on the antiviral effect of β-glucan in ZF4 cells (n = 6). Values represent the means ± SEM. *P < 0.05, **P < 0.01, "ns" is a contraction of no significant..
To gain insights into the potential receptors of β-glucan, we evaluated the role of Myd88 in the antiviral effect. siRNA of myd88 was used to knock down myd88 gene in ZF4 cells. The myd88 gene was successfully knocked down by siRNA (Figure 8A). However, the viral replication was still significantly reduced in cells with deficient Myd88 (Figure 8B, P < 0.05), indicating that Myd88 was not required for β-glucan-mediated antiviral effect.
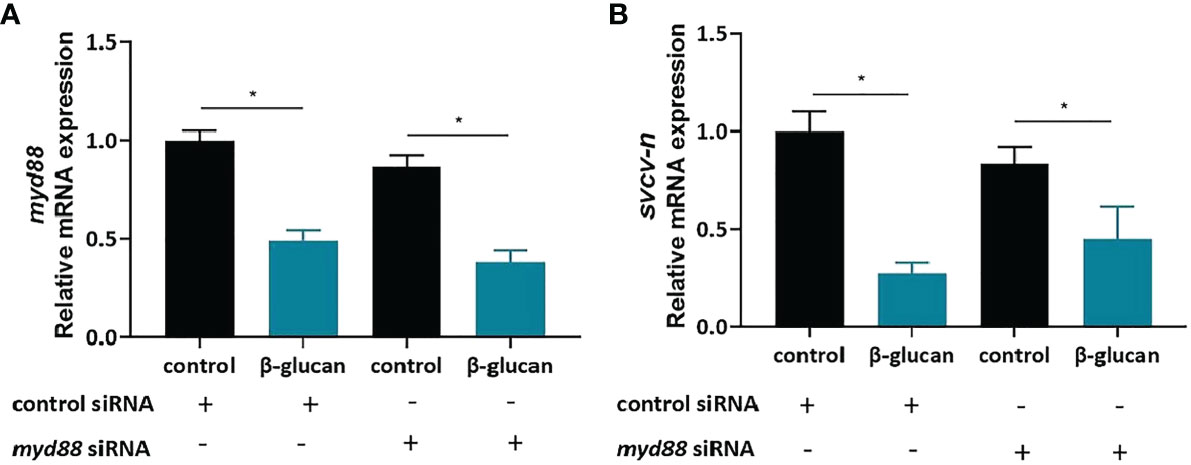
Figure 8 Myd88 is not required for β-glucan to protect against SVCV infection. (A) The knockdown efficiency of myd88 (n = 6); (B) Effects of β-glucan on the mRNA expression of SVCV N protein after siRNA knockdown of myd88 (n = 6). Values represent the means ± SEM. *P < 0.05.
The result of α- diversity of gut microbiota was shown in Supplementary Table 1. The α-diversity of gut microbiota showed no significant difference between control group and β-glucan group. Compared to the control group, dietary β-glucan decreased the relative abundance of Actinobacteria and Proteobacteria while increased Fusobacteriota and Firmicutes abundance at the phylum level (Figure 9A). At the genus level, the addition of β-glucan increased the abundance of Cetobacterium and Lactobacillus (Figure 9B, n = 6). PCoA analysis showed significant difference in the composition of gut microbiota between the control group and the 0.025% β-glucan group at the phylum and genus level (Figures 9C, D, n = 6).
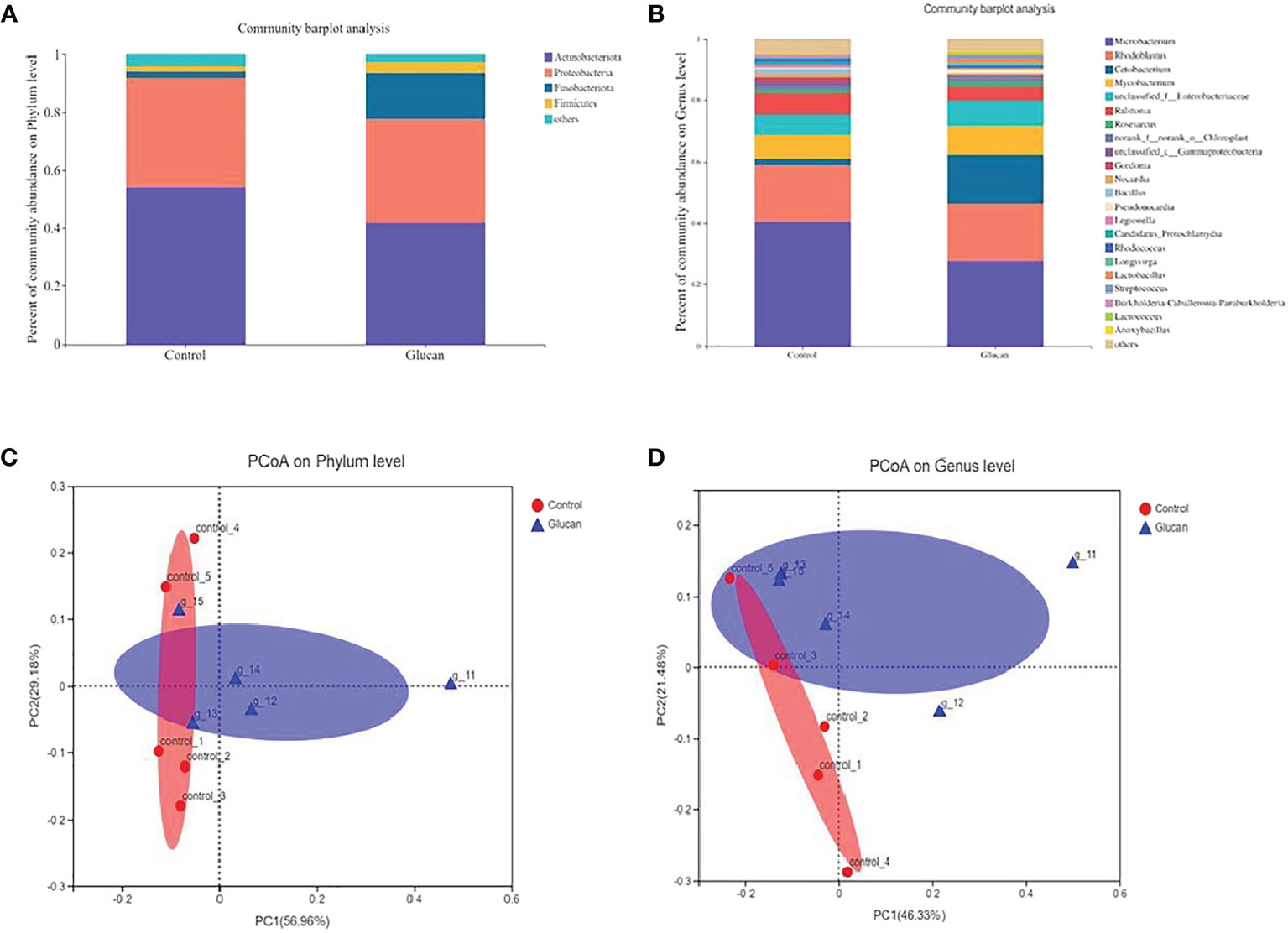
Figure 9 The composition of gut microbiota in zebrafish fed with or without 0.025% b-glucan diets. The composition of gut microbiota in zebrafish was analyzed using 16S rRNA sequencing analysis. (A) Relative abundance at the phylum level between the two groups; (B) Relative abundance at the genus level between the two groups; (C, D) Principal coordinate analysis (PCoA) of all samples by weighted UniFrac distance.
Glucans play a very important role in aquaculture as immunostimulant to improve the performance and health of farmed animals. β-Glucan has been found to induce immunity in various cultured fish species and resistance against different pathogens has been reported (8, 37, 38). Research have shown that β-glucan dosages, quality, route, and time of administration and duration of treatments may influence the its beneficial effects on growth, survival, and immunity of fish (39). Medina-Gali et al. reported that injection of yeast β-glucan enhanced zebrafish immune responses against SVCV and increased zebrafish survival after challenge with SVCV (22). However, the effect of dietary β-glucan on antiviral resistance of fish has not been reported yet, and the underlying mechanism was largely unknown. In the present study we investigated the antiviral potential of dietary β-glucan in zebrafish. Survival rate was significantly increased in zebrafish fed diet supplemented with β-glucan compared with control. Moreover, dietary β-glucan enhanced the expression of genes related to the type I IFN pathway, including IFNs, IFN-stimulated genes, virus recognition receptors, and downstream genes of IFN pathway. To confirm the involvement of type I IFN response in the antiviral effect of β-glucan, we knocked down the IFN receptors to block the IFN antiviral immunity. Zebrafish has two groups of type I IFNs: group I (IFNφ1 and IFNφ4) and group II (IFNφ2 and IFNφ3), which were recognized by different heterodimeric receptors, CRFB1/CRFB5 and CRFB2/CRFB5, respectively (33). Therefore, simultaneous knockdown of CRFB1 and CRFB2 can block the IFN antiviral immunity in zebrafish. We found that knockdown of the two IFN receptor subunits partially blocked the antiviral effect of β-glucan in zebrafish larvae, suggesting that the mechanism of antiviral function of β-glucan at least in part involves IFN response. The partial blockage of antiviral phenotype by the morpholinos might be due to incomplete deletion of the IFN receptors (Supplementary Figure 3). Alternatively, it suggests that in addition to type I IFN, other antiviral pathways are involved in β-glucan-mediated antiviral effect, which deserves further investigation.
We found that 5 μg/mL β-glucan can significantly reduce SVCV replication in ZF4 cells, which is consistent with the in vivo results. We further investigated the activation of type I interferon signaling pathway by β-glucan in ZF4 cells. We used poly (I:C) to avoid the confounding factors associated with viral replication that can affect the expression of antiviral genes. To our surprise, the results showed that β-glucan did not induce IFN signaling in ZF4 cells. In contrast with results in ZF4 cells, β-glucan enhanced type I interferon signaling pathway in vivo, both in the spleen of adult zebrafish (dietary supplementation) and in zebrafish larvae (immersion). This discrepancy might be due to that β-glucan stimulates IFN signaling in specific cell types of zebrafish, which does not include fibroblast cells such as ZF4 cell line.
In mammalian studies, several receptors have been reported to be involved in β-glucan recognition and binding, including TLR2, Dectin-1, and CR3 (complement receptor 3) (8, 40–42). Dectin-1 is a member of the C-type lectin family. It has been suggested that the pattern recognition receptors of β-glucan in fish may be other members of C-type lectin family (43). β-glucan enhances tlr2 gene expression in both european eel (Anguilla anguilla) and zebrafish (44), and enhanced carp (Cyprinus carpio) tlr3 gene expression (45). This suggests that the zebrafish TLR2 receptor might be a receptor for β-glucan. CR3 receptor is a promiscuous pattern recognition receptor recognizing many ligands including β-glucan. The involvement of CR3 as β-glucan receptor in fish has been less studied. Increased expression of CR3 gene was observed in the intestine of Atlantic salmon after oral intake of purified β-glucan, suggesting its involvement in the recognition of glucan (46). We used siRNA to knock down Myd88, which is a key adaptor protein for TLR receptors. Knockdown of Myd88 did not affect the antiviral effect of β-glucan in ZF4 cells, suggesting that β-glucan receptor is not a TLR in zebrafish. The recognition receptor(s) of β-glucan in fish, which mediates the downstream antiviral effects, deserves further investigation.
Autophagy can play either antiviral or proviral roles, depending on the virus (47). Previous studies showed that SVCV infection induces autophagy in both ZF4 cells and zebrafish larvae, and autophagy inhibits SVCV infection (29, 30). Consistently, our results showed that SVCV infection induced autophagy in ZF4 cells. On top of it, we found that β-glucan enhanced autophagy after SVCV infection, and the antiviral effect of β-glucan was blocked in vitro by autophagy inhibitors, implicating the involvement of autophagy induction in the antiviral effect of β-glucan in ZF4 cells. The influence of β-glucan on autophagy has been reported previously. Yeast β-glucan inhibited autophagy of liver cancer cells (48), while β-glucan derived from Agaricus bisporus showed autophagy induction activity in zebrafish (49). The effect of β-glucan on autophagy might depend on the source, doses, and the cells. Notably, β-glucan treatment only enhanced autophagy of ZF4 cells after SVCV infection, but did not induce autophagy in non-infected cells, suggesting a synergistic effect of SVCV infection and β-glucan on autophagy induction. This was different from previous studies (48, 49), in which the effect on autophagy was observed at basal condition. The relationship between autophagy and type I IFN antiviral immunity has been studied recently in mammalian virus infection model (50), but related knowledge is largely unknown about fish viruses. The link between autophagy and IFN response in SVCV-zebrafish infection model deserves further investigation.
Reports reveal that the gut microbiota plays an important role in regulating the human immune system and improving gut health (51). Recently, studies have shown that the gut microbiota also regulates viral infection (52, 53). The intestinal microbiota may exert antiviral function by regulating the antiviral immunity of host. Ichinohe et al. (2011) (54) found that the microbiota is crucial for the immune response against influenza virus in mice. To evaluate the response of intestinal microbiota to the intake of β-glucan, the composition of intestinal microbiota in zebrafish was analyzed by sequencing the 16S rRNA gene. Dietary 0.025% β-glucan decreased the relative abundance of Actinobacteria and Proteobacteria while increased Fusobacteriota and Firmicutes abundance at the phylum level. At the genus level, the addition of β-glucan increased the abundance of Cetobacterium and Lactobacillus. The bacterial taxa in the microbiota responsible for the antiviral function deserve further investigation. Notably, we observed that β-glucan can improve the expression of intestinal HIF-1α (hypoxia-inducible factor-1α) (Supplementary Figure 7). HIF has been recognized as an important regulator of intestinal homeostasis (55). In our previous study, stimulation of intestinal HIF-1α was proved to have beneficial effect on the microbiota composition (34). Therefore, the influence of β-glucan on intestinal microbiota of zebrafish might be attributable to its effect on HIF-1α.
In summary, the present study revealed that the dietary supplementation of β-glucan can enhance the antiviral ability of zebrafish. β-glucan stimulated the type I IFN signaling in both adult and larval zebrafish after SVCV infection. Mechanistic study by morpholino-mediated knockdown of IFN receptors confirmed the involvement of type I IFN pathway in the antiviral effect of β-glucan. Furthermore, we found that β-glucan inhibited SVCV infection in ZF4 cells. While β-glucan did not affect antiviral immune response in ZF4 cells, it induced autophagy of the cells after viral infection. Lastly, we found that dietary β-glucan altered the intestinal microbiota of zebrafish, and the β-glucan-associated microbiota might also contribute to the antiviral function.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
All experimental procedures were performed in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China). The study was reviewed and approved by the Feed Research Institute of the Chinese Academy of Agricultural Sciences Animal Care Committee under the auspices of the China Council for Animal Care. All efforts were made to minimize suffering.
HL and YL: formal analysis, data curation, writing original draft preparation and revising the draft. ML, WZ and JC: investigation, interpretation of data. ZZ: analysis and resources. YLY: analysis and resources. CR: research design, data curation, funding acquisition, revising the draft, revised and edited the manuscript. ZGZ: research design, data curation, funding acquisition and revising the draft. All authors contributed to the article and approved the submitted version.
This work was funded by grants from the National Key R&D Program of China (2018YFD0900400) and National Natural Science Foundation of China (31925038, 32122088).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1031962/full#supplementary-material
1. Bacon J, Farmer VC, Jones D, Taylor IF. The glucan components of the cell wall of baker’s yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem J (1969) 114:557–67. doi: 10.1042/bj1140557
2. Fleet GH, Manners DJ. Isolation and composition of an alkali-soluble glucan from the cell walls of saccharomyces cerevisiae. J Gen Microbiol (1976) 94:180–92. doi: 10.1099/00221287-94-1-180
3. Korolenko TA, Bgatova NP, Vetvicka V. Glucan and mannan-two peas in a pod. Int J Mol Sci (2019) 20:3189. doi: 10.3390/ijms20133189
4. Brogden G, Krimmling T, Adamek M, Naim HY, Steinhagen D, Köckritz-Blickwede M. The effect of β-glucan on formation and functionality of neutrophil extracellular traps in carp (Cyprinus carpio l. ) Dev Comp Immunol (2014) 44:280–5. doi: 10.1016/j.dci.2014.01.003
5. Falco A, Miest JJ, Pionnier N, Pietretti D, Forlenza M, Wiegertjes GF, et al. β-Glucan-Supplemented diets increase poly (I:C)-induced gene expression of mx, possibly Via Tlr3-mediated recognition mechanism in common carp (Cyprinus carpio). Fish Shellfish Immunol (2014) 36:494–502. doi: 10.1016/j.fsi.2013.12.005
6. Burrells C, Williams PD, Forno PF. Dietary nucleotides: A novel supplement in fish feeds: 1. effects on resistance to disease in salmonids. Aquaculture (2001) 199:159–69. doi: 10.1016/S0044-8486(01)00577-4
7. Vetvicka V, Vannucci L, Sima P. The effects of β-glucan on fish immunity. N Am J Med Sci (2013) 5:580–8. doi: 10.4103/1947-2714.120792
8. Rodrigues MV, Zanuzzo FS, Fernando J, Koch A, Vetvicka V. Development of fish immunity and the role of β-glucan in immune responses. Molecules (2020) 25:5378. doi: 10.3390/molecules25225378
9. Jørgensen JB, Sharp G, Secombes CJ, Robertsen B. Effect of a yeast-Cell-Wall glucan on the bactericidal activity of rainbow trout macrophages. Fish Shellfish Immunol (1993) 3:267–77. doi: 10.1006/fsim.1993.1026
10. Figueras A, Santarem MM, Nov B. Influence of the sequence of administration of β−Glucans and a vibrio damsela vaccine on the immune response of turbot (Scophthalmus maximus l.). Vet Immunol Immunopathol (1998) 64:59–68. doi: 10.1016/S0165-2427(98)00114-7
11. Ashida T, Okimasu E, Ui M, Heguri M, Oyama Y, Amemura A. Protection of Japanese flounder Paralichthys olivaceus against experimental edwardsiellosis by Formalin−Killed Edwardsiella tarda in combination with oral administration of immunostimulants. Fish Sci (1999) 65:527–30. doi: 10.2331/fishsci.65.527
12. LaPatra SE, Lauda KA, Jones GR, Shewmaker WS, Bayne CJ. Resistance to IHN virus infection in rainbow trout is increased by glucan while subsequent production of serum neutralizing activity is decreased. Fish Shellfish Immunol (1998) 8:435–46. doi: 10.1006/fsim.1998.0151
13. Lee JH, Kim JW, Kang YJ. Effects of β-1, 3-glucan on innate immunity responses and mortality induced by vibrio harveyi, hemorrhagic septicemia virus, or miamiensis avidus in the olive flounder paralichthys olivaceus. Aquacult Int (2018) 26:743–56. doi: 10.1007/s10499-018-0248-0
14. Beaulaurier J, Bickford N, Gregg JL, Grady CA, Gannam AL, Winton JR. Susceptibility of pacific herring to viral hemorrhagic septicemia is influenced by diet. J Aquat Anim Health (2012) 24:43–8. doi: 10.1080/08997659.2012.668511
15. Kim YS, Ke F, Zhang QY. Effect of β−Glucan on activity of antioxidant enzymes and mx gene expression in vitus infected grass carp. Fish Shellfish Immunol (2009) 27:336–40. doi: 10.1016/j.fsi.2009.06.006
16. Krishnan R, Jang YS, Oh MJ. Beta glucan induced immune priming protects against nervous necrosis virus infection in sevenband grouper. Fish Shellfish Immunol (2022) 121:163–71. doi: 10.1016/j.fsi.2022.01.005
17. Stark GR. How cells respond to interferons revisited: from early history to current complexity. Cytokine Growth Factor Rev (2007) 18:419–23. doi: 10.1016/j.cytogfr.2007.06.013
18. Song DW, Liu L, Shan LP, Qiu TX, Chen JP. Therapeutic potential of phenylpropanoid-based small molecules as anti-SVCV agents in aquaculture. Aquaculture (2020) 526:735349. doi: 10.1016/j.aquaculture.2020.735349
19. Robertsen B. The interferon system of teleost fish. Fish Shellfish Immunol (2006) 20:172–91. doi: 10.1016/j.fsi.2005.01.010
20. Gan Z, Chen SN, Huang B, Zou J, Nie P. Fish type I and type II interferons: Composition, receptor usage, production and function. Rev Aquac (2020) 12:773–804. doi: 10.1111/raq.12349
21. Langevin C, Aleksejeva E, Passoni G, Palha N, Levraud JP, Boudinot P. The antiviral innate immune response in fish: Evolution and conservation of the IFN system. J Mol Biol (2013) 425:4904–20. doi: 10.1016/j.jmb.2013.09.033
22. Medina-Gali RM, Ortega-Villaizan M, Mercado L, Novoa B, Coll J, Perez L. Beta-glucan enhances the response to SVCV infection in zebrafish. Dev Comp Immunol (2018) 84:307–14. doi: 10.1016/j.dci.2018.02.019
23. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature (2008) 451:1069. doi: 10.1038/nature06639
24. Kim HJ, Lee S, Jung JU. When autophagy meets viruses: A double-edged sword with functions in defense and offense. Semin Immunopathol (2010) 32:323–41. doi: 10.1007/s00281-010-0226-8
25. Orvedahl A, Alexander D, Tallóczy Z, Sun Q, Wei Y, Wei Z. Hsv-1 Icp34.5 confers neurovirulence by targeting the beclin 1 autophagy protein. Cell Host Microbe (2007) 1:23–35. doi: 10.1016/j.chom.2006.12.001
26. Shelly S, Lukinova N, Bambina S, Berman A, Cherry S. Autophagy is an essential component of drosophila immunity against vesicular stomatitis virus. Immunity (2009) 30:588–98. doi: 10.1016/j.immuni.2009.02.009
27. Jackson WT, Giddings TH, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR. Subversion of cellular autophagosomal machinery by RNA viruses. PloS Biol (2005) 3:e156. doi: 10.1371/journal.pbio.0030156
28. Heaton NS, Randall G. Dengue virus and autophagy. Viruses (2011) 3:1332–41. doi: 10.3390/v3081332
29. García-Valtanen P, Ortega-Villaizán MM, Martínez-López A. Autophagy-inducing peptides from mammalian VSV and fish VHSV rhabdoviral G glycoproteins (G) as models for the development of new therapeutic molecules. Autophagy (2014) 10:1666–80. doi: 10.4161/auto.29557
30. Espín-Palazón R, Martínez-López A, Roca FJ, López-Muoz A, Mulero V. Tnfα impairs rhabdoviral clearance by inhibiting the host autophagic antiviral response. PloS Pathog (2016) 12:e1005699. doi: 10.1371/journal.ppat.1005699
31. Liu L, Zhu B, Wu S, Lin L, Liu G, Zhou Y, et al. Autophagy and SVCV replication. Cell Microbiol (2015) 17:595–605. doi: 10.1111/cmi.12387
32. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg (1938) 27:493–7. doi: 10.1093/oxfordjournals.aje.a118408
33. Aggad D, Mazel M, Boudinot P, Mogensen KE, Hamming OJ, Hartmann R, et al. The two groups of zebrafish virus-induced interferons signal Via distinct receptors with specific and shared chains. J Immunol (2009) 183(6):3924–31. doi: 10.4049/jimmunol.0901495
34. Zhang Z, Ran C, Ding QW, Liu HL, Xie MX, Yang YL, et al. Ability of prebiotic polysaccharides to activate a HIF1A-antimicrobial peptide axis determines liver injury risk in zebrafish. Commun Biol (2019) 2:274. doi: 10.1038/s42003-019-0526-z
35. Ding Q, Zhang Z, Li Y, Liu H, Hao Q, Yang Y, et al. Propionate induces intestinal oxidative stress Via Sod2 propionylation in zebrafish. iScience (2021) 24:102515. doi: 10.1016/j.isci.2021.102515
36. Ran C, Xie M, Li J, Xie Y, Ding Q, Li Y, et al. Dietary nucleotides alleviate hepatic lipid deposition via exogenous AMP-mediated AMPK activation in zebrafish. J Nutr (2021) 151:2986–96. doi: 10.1093/jn/nxab232
37. Pogue R, Murphy EJ, Fehrenbach GW, Rezoagli E, Rowan NJ. Exploiting immunomodulatory properties of β-glucans derived from natural products for improving health and sustainability in aquaculture-farmed organisms: Concise review of existing knowledge, innovation and future opportunities. Curr Opin Environ Sci Health (2021) 21:100248. doi: 10.1016/j.coesh.2021.100248
38. Gupta A, Gupta SK, Priyam M, Siddik MAB, Pattanayak A. Immunomodulation by dietary supplements: A preventive health strategy for sustainable aquaculture of tropical freshwater fish, labeo rohita (Hamilton, 1822). Rev Aquac (2021) 13:2364–94. doi: 10.1111/raq.12581
39. Meena DK, Das P, Kumar S. Beta-glucan: An ideal immunostimulant in aquaculture (A review). Fish Physiol Biochem (2013) 39:431–57. doi: 10.1007/s10695-012-9710-5
41. Willment JA, Gordon S, Brown GD. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J Biol Chem (2001) 276:43818–23. doi: 10.1074/jbc.M107715200
42. Dennehy KM, Brown GD. The role of the β-glucan receptor dectin-1 in control of fungal infection. J Leukoc Biol (2007) 82:253–8. doi: 10.1189/jlb.1206753
43. Petit J, Bailey EC, Wheeler RT, De Oliveira CAF, Forlenza M, Wiegertjes GF. Studies into β-glucan recognition in fish suggests a key role for the c-type lectin pathway. Front Immunol (2019) 10:280. doi: 10.3389/fimmu.2019.00280
44. Rodríguez I, Chamorro R, Novoa B, Figueras A. β-glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio). Fish Shellfish Immunol (2009) 27:369–73. doi: 10.1016/j.fsi.2009.02.007
45. Selvaraj V, Sampath K, Sekar V. Administration of yeast glucan enhances survival and some non-specific and specific immune parameters in carp (Cyprinus carpio) infected with aeromonas hydrophila. Fish Shellfish Immunol (2005) 19:293–306. doi: 10.1016/j.fsi.2005.01.001
46. Kiron V, Kulkarni A, Dahle D, Vasanth G, Lokesh J, Elvebo O. Recognition of purified beta 1,3/1,6 glucan and molecular signalling in the intestine of Atlantic salmon. Dev Comp Immunol (2016) 56:57–66. doi: 10.1016/j.dci.2015.11.007
47. Keller MD, Torres VJ, Cadwell K. Autophagy and microbial pathogenesis. Cell Death Differ (2020) 27:872–86. doi: 10.1038/s41418-019-0481-8
48. Wang N, Liu H, Liu G, Li M, He X, Yin C, et al. Yeast β-d-glucan exerts antitumour activity in liver cancer through impairing autophagy and lysosomal function, promoting reactive oxygen species production and apoptosis. Redox Biol (2020) 32:101495. doi: 10.1016/j.redox.2020.101495
49. Li X, Xue Y, Pang L, Len B, Lin Z, Huang J, et al. Agaricus bisporus-derived β-glucan prevents obesity through PPAR Γ downregulation and autophagy induction in zebrafish fed by chicken egg yolk. Int J Biol Macromol (2019) 125:820–8. doi: 10.1016/j.ijbiomac.2018.12.122
50. Hui X, Zhang L, Cao L, Huang K, Zhao Y, Zhang Y, et al. SARS-CoV-2 promote autophagy to suppress type I interferon response. Signal Transduct Target Ther (2021) 6:180. doi: 10.1038/s41392-021-00574-8
51. Monedero V, Collado MC, Rodríguez-Díaz J. Therapeutic opportunities in intestinal microbiota–virus interactions. Trends Biotechnol (2018) 36:645–8. doi: 10.1016/j.tibtech.2017.12.009
52. Shi Z, Zou J, Zhang Z, Zhao X, Noriega J, Zhang B. Segmented filamentous bacteria prevent and cure rotavirus infection. Cell (2019) 179:644–58. doi: 10.1016/j.cell.2019.09.028
53. Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza a virus infection. Proc Natl Acad Sci USA (2011) 108:5354–9. doi: 10.1073/pnas.1019378108
54. Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. a review in the theme: Cellular responses to hypoxia. Am J Physiol Cell Physiol (2015) 309:350–60. doi: 10.1152/ajpcell.00191.2015
Keywords: β-glucan, zebrafish, SVCV, antiviral immunity, gut microbiota
Citation: Liang H, Li Y, Li M, Zhou W, Chen J, Zhang Z, Yang Y, Ran C and Zhou Z (2022) The effect and underlying mechanism of yeast β-glucan on antiviral resistance of zebrafish against spring viremia of carp virus infection. Front. Immunol. 13:1031962. doi: 10.3389/fimmu.2022.1031962
Received: 30 August 2022; Accepted: 14 October 2022;
Published: 03 November 2022.
Edited by:
Jorge Galindo-Villegas, Nord University, NorwayReviewed by:
Patricia Pereiro, Spanish National Research Council (CSIC), SpainCopyright © 2022 Liang, Li, Li, Zhou, Chen, Zhang, Yang, Ran and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Ran, cmFuY2hhb0BjYWFzLmNu; Zhigang Zhou, emhvdXpoaWdhbmcwM0BjYWFzLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.