
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 30 September 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1028282
Objective: To report a case of autoimmune nodopathy (AN) with concurrent serum and CSF immunoglobulin (Ig)G4 anti-neurofascin 155 (NF155) and anti-GD1b antibodies.
Methods: A 20-year-old male presented distal weakness of the 4 limbs, hypoesthesia, absent tendon reflexes and sensory ataxia. Nerve conduction studies (NCS), MRI, and autoantibody tests were performed.
Results: NCS revealed a diffuse demyelinating neuropathy in the peripheral nerve with motor and sensory involvement. MRI of the cervical and lumbar plexus showed diffuse enlargement. IgG4 anti-NF155 antibodies in both serum and CSF and IgG anti-GD1b antibodies in serum were positive. After treatment with IVIg, rituximab, and plasma exchange, the titer of the patient’s anti-NF155 antibodies decreased, but symptoms did not significantly improve.
Discussion: This patient presented a typical clinical feature of AN with serum and CSF anti-NF155 antibodies and serum anti-GD1b antibodies coexistent but poor response to IVIg, rituximab and plasma exchange. Early detection of antibodies may be helpful in both diagnosis and therapy of the disease. And prospective studies are necessary to demonstrate the potential role of anti-NF155 antibodies in CSF and help further understand this complex and heterogeneous disease.
Chronic inflammatory demyelination polyneuropathy (CIDP) is a clinically and pathologically diverse autoimmune syndrome of the peripheral nervous system, which could cause significant disability (1, 2). Autoantibodies against the node and paranode of Ranvier, such as neurofascin 155 (NF155) (3), have been described in small subsets of patients with CIDP, sharing immunopathologic mechanisms, clinical features, and treatment response, which differ from those of typical CIDP (4, 5). The existence of anti-paranodal axo–glial junctional molecules leads to different morphological features of the peripheral nerves in this group of patients from conventional CIDP patients, characterized by paranodal dissection and the absence of classical macrophage-mediated demyelination (6). This has led to the appearance of the autoimmune nodopathy (AN) diagnostic category in the recent update of the European Academy of Neurology/Peripheral Nerve Society CIDP diagnostic guidelines (7). Herein we report a case of anti-NF155 positive AN presenting with distal weakness, hypoesthesia, absent tendon reflexes and sensory ataxia. Diffuse demyelinating neuropathy and enlargement of cervical and lumbar plexus were found. Especially, IgG4 anti-NF155 antibodies in both serum and CSF and IgG anti-GD1b antibodies in serum were positive.
A 20-year-old male with no medical history was admitted to our department with progressive distal muscle weakness and unstable walking for 5 weeks. He had vomiting and diarrhea initially, and 10 days later, weakness developed. Physical examination on the first hospital day (H1) showed distal weakness of the 4 limbs (the muscle strength of the distal limbs was grade 4), hypoesthesia, absent tendon reflexes and sensory ataxia. No muscle atrophy or fasciculation were observed. The modified Rankin Scale (mRS) rated 2 out of 5.
Routine blood tests were normal, but serum Epstein-Barr virus (EBV) viral capsid antigen IgG and nuclear antigen IgG antibodies were positive. Nerve conduction studies (NCS) revealed a diffuse demyelinating neuropathy in the peripheral nerve with motor and sensory involvement (Tables 1, 2). Cerebral MRI and visual evoked potential (VEP) were normal. MRI of the cervical and lumbar plexus showed diffuse enlargement (Figure 1). CSF analysis showed significantly elevated protein levels (4.178 g/L, average <0.45) and a white blood cell count of 2x106/L (within normal limits). Tests for antibodies showed positive IgG4 anti-NF155 antibodies in both serum and CSF (titers of 1:320 and 1:32, respectively) and seropositive IgG anti-GD1b antibodies (Figure 1).
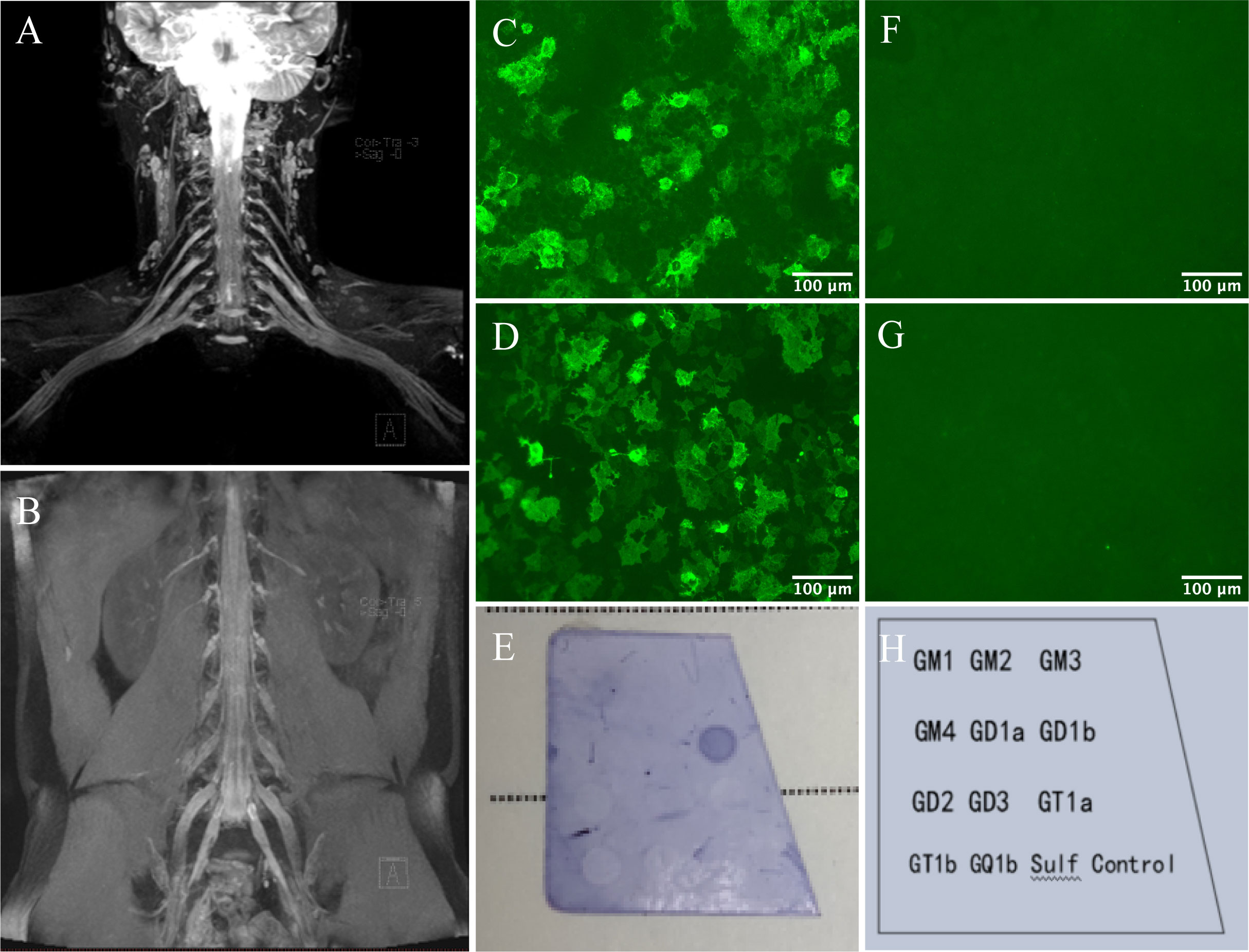
Figure 1 Nerve imaging and autoantibodies against NF155 and GD1b. Symmetrical bilateral enlarged cervical and lumbar plexus on MIP Thin Rage (A, B). The presence of anti-NF155 antibodies was confirmed with NF155-transfected Human Embryonic Kidney (HEK) 293 cells using a cell-based assay (CBA). Reactivity was analyzed by immunofluorescence. Serum (C) and CSF (D) anti-NF155 antibodies were positive (×400), while negative in the control group of serum (F) and CSF (G). Scale bar = 100 μm. The presence of anti-GD1b antibodies was confirmed by dot immunoassay. A clearly discernible color spot was shown in the GD1b antigen-coated area of the test strip (E, H).
This patient was initially diagnosed with Guillain-Barré Syndrome. A three-day course of IVIg (0.4 g/kg·d) started on H3. However, he responded poorly to it, for no symptoms improved. Moreover, sensory ataxia worsened, suggesting the disease was progressing. Combined with the subacute onset, nearly two-month duration of the disease, and the positive anti-NF155 antibodies, the final diagnosis was anti-NF155 positive AN. He received a one-time dose of 500mg rituximab on H10, leading to a slight improvement in limb weakness. The muscle strength of the distal limbs of both upper limbs recovered to grade 4+, and the distal limbs of both lower limbs recovered to grade 5. Nevertheless, the mRS remained at 2. One month after discharge, we followed up with him by telephone and learned that walking instability still existed. He was admitted to another hospital and was treated with plasma exchange, after which the anti-NF155 titers of serum decreased from 1:320 to 1:10. But the symptoms did not relieve. The second telephone follow-up was made in the third month after his discharge from our hospital, and his symptoms as well as mRS score, remained stable.
The clinical features of patients with anti-NF155 antibodies AN differ from typical CIDP: younger at onset, a subacute or chronic disease course, distal weakness, sensory ataxia, tremor, and no or poor response to IVIg treatment (3, 8, 9). And the clinical feature of the case we report here is consistent with the prior studies but lacks tremor.
There have been few reports of AN with IgG4 anti-NF155 antibodies positive in CSF. One recent study on AN identified 3 patients with anti-NF155 antibodies positive in CSF. Subtype analysis and titration revealed that the antibodies were IgG4, and their titers were significantly lower than in serum (10). These findings are in agreement with the case we report. The presence of anti-NF155 antibodies in CSF of our case is confirmed by cell-based assay at titers much lower than the serum antibodies. No evidence of central demyelination was found, as his cerebral MRI and VEP were normal. Combined with the high protein content in CSF and hypertrophy of cervical and lumbar plexus, it is reasonable to assume that anti-NF155 antibodies appear in the CSF due to an inflammatory response leading to disruption of the blood-brain barrier and thus the entry of serum antibodies into the CNS. The possibility of intrathecal synthesis also cannot be excluded. But regrettably, the detection of oligoclonal bands was not performed. On the other hand, IgG anti-GD1b antibodies also seem to play a role in this patient’s clinical presentation, especially in ataxia, as it has been discovered that it may induce ataxia (11). The EBV antibodies may suggest a previous infection. However, this association with the current disease is unclear. Because we have no more evidence to confirm it and we are unable to follow up on the patient’s EBV antibodies.
A few points presented in our case may be instructive. First, the clinical features of this patient were mostly consistent with previous reports, except for the absence of tremors, which reflects the heterogeneity of this disease. The symptoms were partially relieved after treatment, implying that the utility of therapeutic strategies may require more extensive and dedicated studies. But also, it could be because of our not long enough follow-ups, as one study mentioned that the rituximab effect was not clearly seen until the third month of the disease (10). Second, the presentation of our case may suggest that anti-NF155 antibodies in CSF indicate more severe inflammation of the peripheral nerves than in an adverse patient, leading to more severe clinical features. Additional studies are needed to clarify whether antibodies in CSF are associated with a worse response to treatment. Third, given the pathogenicity of anti-GD1b antibodies (11), it seems reasonable to consider this as another cause of severe clinical features in this patient.
In conclusion, this patient presented a typical clinical feature of AN with serum and CSF anti-NF155 antibodies coexistent but poor response to IVIg, rituximab and plasma exchange. Prospective studies are necessary to demonstrate the potential role of anti-NF155 antibodies in CSF and help further understand this complex and heterogeneous disease.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WW, QM contributed to conception and design of the study. WW organized the database and wrote the first draft of the manuscript. WW, LL, QM revised the manuscript. MZ, RY, DL, SY contributed the statistical analysis. All authors contributed to manuscript revision, read, and approved the submitted version.
Yunnan Health Training Project of High-level Talents. Grant number: L-2017013.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lehmann HC, Burke D, Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry (2019) 90(9):981–7. doi: 10.1136/jnnp-2019-320314
2. Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry (2015) 86(9):973–85. doi: 10.1136/jnnp-2014-309697
3. Querol L, Nogales-Gadea G, Rojas-Garcia R, Diaz-Manera J, Pardo J, Ortega-Moreno A, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology (2014) 82(10):879–86. doi: 10.1212/wnl.0000000000000205
4. Pascual-Goñi E, Martín-Aguilar L, Querol L. Autoantibodies in chronic inflammatory demyelinating polyradiculoneuropathy. Curr Opin Neurol (2019) 32(5):651–7. doi: 10.1097/wco.0000000000000725
5. Querol L, Devaux J, Rojas-Garcia R, Illa I. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol (2017) 13(9):533–47. doi: 10.1038/nrneurol.2017.84
6. Koike H, Kadoya M, Kaida KI, Ikeda S, Kawagashira Y, Iijima M, et al. Paranodal dissection in chronic inflammatory demyelinating polyneuropathy with anti-neurofascin-155 and anti-contactin-1 antibodies. J Neurol Neurosurg Psychiatry (2017) 88(6):465–73. doi: 10.1136/jnnp-2016-314895
7. Van den Bergh PYK, van Doorn PA, Hadden RDM, Avau B, Vankrunkelsven P, Allen JA, et al. European Academy of Neurology/Peripheral nerve society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force-second revision. Eur J Neurol (2021) 28(11):3556–83. doi: 10.1111/ene.14959
8. Ogata H, Yamasaki R, Hiwatashi A, Oka N, Kawamura N, Matsuse D, et al. Characterization of IgG4 anti-neurofascin 155 antibody-positive polyneuropathy. Ann Clin Transl Neurol (2015) 2(10):960–71. doi: 10.1002/acn3.248
9. Devaux JJ, Miura Y, Fukami Y, Inoue T, Manso C, Belghazi M, et al. Neurofascin-155 IgG4 in chronic inflammatory demyelinating polyneuropathy. Neurology (2016) 86(9):800–7. doi: 10.1212/wnl.0000000000002418
10. Martín-Aguilar L, Lleixà C, Pascual-Goñi E, Caballero-Ávila M, Martínez-Martínez L, Díaz-Manera J, et al. Clinical and laboratory features in anti-NF155 autoimmune nodopathy. Neurol Neuroimmunol Neuroinflamm (2022) 9(1):e1098. doi: 10.1212/nxi.0000000000001098
Keywords: autoimmune nodopathy (AN), chronic inflammatory demyelination polyneuropathy (CIDP), anti-neurofascin 155 antibodies, anti-GD1b antibodies, sensory ataxia, case report
Citation: Wang W, Liu L, Zhang M, Yang R, Liu D, Yang S and Meng Q (2022) Case report: Autoimmune nodopathy with concurrent serum and CSF IgG4 anti-neurofascin 155 antibodies. Front. Immunol. 13:1028282. doi: 10.3389/fimmu.2022.1028282
Received: 25 August 2022; Accepted: 20 September 2022;
Published: 30 September 2022.
Edited by:
Lidia Sabater, Institut de Recerca Biomèdica August Pi i Sunyer (IDIBAPS), SpainReviewed by:
Koike Haruki, Nagoya University, JapanCopyright © 2022 Wang, Liu, Zhang, Yang, Liu, Yang and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Meng, bXEzMDFAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.