
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 30 September 2022
Sec. Mucosal Immunity
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1028209
This article is part of the Research TopicBridging the gap between immunology, virology, genetics, and epigenetics in bronchiolitis: The Multiomics pathway to asthma developmentView all 10 articles
 Conglin Liu1*
Conglin Liu1* Heidi Makrinioti2
Heidi Makrinioti2 Sejal Saglani3,4
Sejal Saglani3,4 Michael Bowman1
Michael Bowman1 Lih-Ling Lin1
Lih-Ling Lin1 Carlos A. Camargo Jr.5
Carlos A. Camargo Jr.5 Kohei Hasegawa5
Kohei Hasegawa5 Zhaozhong Zhu5*
Zhaozhong Zhu5*Asthma is a chronic and heterogeneous respiratory disease with many risk factors that typically originate during early childhood. A complex interplay between environmental factors and genetic predisposition is considered to shape the lung and gut microbiome in early life. The growing literature has identified that changes in the relative abundance of microbes (microbial dysbiosis) and reduced microbial diversity, as triggers of the airway-gut axis crosstalk dysregulation, are associated with asthma development. There are several mechanisms underlying microbial dysbiosis to childhood asthma development pathways. For example, a bacterial infection in the airway of infants can lead to the activation and/or dysregulation of inflammatory pathways that contribute to bronchoconstriction and bronchial hyperresponsiveness. In addition, gut microbial dysbiosis in infancy can affect immune development and differentiation, resulting in a suboptimal balance between innate and adaptive immunity. This evolving dysregulation of secretion of pro-inflammatory mediators has been associated with persistent airway inflammation and subsequent asthma development. In this review, we examine current evidence around associations between the airway and gut microbial dysbiosis with childhood asthma development. More specifically, this review focuses on discussing the integrated roles of environmental exposures, host metabolic and immune responses, airway and gut microbial dysbiosis in driving childhood asthma development.
Asthma is one of the most common chronic respiratory diseases affecting more than 300 million individuals worldwide (1). The prevalence of asthma remains high, mainly due to high incidence in the first years of life (2, 3). Asthma is characterized by symptoms of wheeze, shortness of breath, chest tightness, cough, and expiratory airway limitation (1). Epidemiological studies have suggested many risk factors for asthma development, but mainly representing three domains: demographics (e.g., age, sex, and family history), genetics, and environmental exposures (e.g., bacterial and viral infection, air pollution, and diet) (3–6). The interplay between these risk factors underlies the pathobiological mechanisms of asthma and contributes to the variability in pathogenic mechanisms and in response to treatment. It is accepted that asthma consists of a range of subtypes differing in presentation and pathobiological mechanisms (7), such as allergic vs. non-allergic asthma (8–10) and childhood- vs. adult-onset asthma (9, 11–13). As expected, the effects of risk factors on asthma incidence vary between asthma subtypes. Thus, it is critical to identify asthma subtype-specific risk factors. Of the various risk factors for childhood asthma development, microbial dysbiosis in the airway and gut may play a crucial role given their complex interplay with host genetic susceptibility, environmental exposures, and metabolic and immune response (14–16). For several reasons, such complex interplay becomes the most important for asthma during early-life development. First, early life is a crucial period in which the gene-environmental interaction helps to shape immune development (17). Second, most infections occur in infancy, and often are recurrent episodes and are relatively severe, which have long-term respiratory sequela in later life (18). Third, early life is a critical period of airway development, which is crucial in determining lung function and respiratory diseases in later childhood and adulthood (19). This review will examine the current epidemiological evidence about the relationship between airway and gut microbial dysbiosis and childhood asthma development, their integrated roles with environmental exposures, and the metabolic and immune mechanisms underlying airway and gut microbiota diversity and asthma development in childhood.
The human microbiota contains 10-100 trillion microbes harbored by each person (20). The microbes are distributed across several major body sites, including oral cavity, respiratory system, gastrointestinal system, vagina, and skin (21), with the microbial composition varying depending on the body site. Such distinct microbial composition is determined based on genetic susceptibility and environmental exposures (22). The interplay of these factors underlies the pathobiological mechanisms for diseases, such as asthma (16). Growing evidence suggests that microbiota in the respiratory system consists of four major pathogenic bacterial phyla, including Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria (21). The gastrointestinal system’s microbiota consists of six major bacterial phyla, including Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria, Proteobacteria, and Verrucomicrobia (21). The cross-talk between airway and gut microbiota can have synergistic effects on the development of respiratory diseases. Disturbances in gut microbial composition limit the capacity to modulate adequate immune responses, which not only has been linked to inflammatory conditions in the gastrointestinal tract itself but also in the airway, referred to as the “gut-lung axis” (23–25). The importance of such interaction has become more evident with the identification of microbe-produced metabolites in both the airway and gut. These metabolites can contribute to various respiratory diseases, such as asthma (26–29). Yet, until recently, the mechanisms that underlie the link between microbial dysbiosis in the airway and gut and childhood asthma development remained unclear.
Growing evidence suggests that the airway microbiota in early life is associated with childhood asthma development (29–41). For example, an early study from the Copenhagen Prospective Study on Asthma in Childhood (COPSAC)—a prospective birth cohort—has reported that pathogenic bacterial colonization (e.g., Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae) of the nasopharynx in infants is associated with asthma development by age 5 years (30). Likewise, a study from Childhood Asthma Study (CAS)—a prospective birth cohort from Australia—has also found that Streptococcus colonization in infants’ upper airway is strongly associated with asthma development by age 5 years (32). In addition, a recent study from the 35th Multicenter Airway Research Collaboration (MARC-35)—a prospective cohort of infants with severe bronchiolitis—has used a dual-transcriptome (metatranscriptome and transcriptome) approach and found that a higher abundance of Streptococcus pneumoniae in the nasopharyngeal airway is associated with a greater risk of developing asthma by age 6 years, particularly in infants with non-rhinovirus infection (29). And the major bacterial species drive the asthma-related microbial functional pathways, such as fatty acid metabolism and glycolysis pathways (29), which have been found to play important roles in asthma pathobiology (9, 42–44). The abundance of major bacterial species is also associated with asthma-related host transcripts (e.g., DAGLB) (29). Furthermore, a recent study from the Steps to the Healthy Development and Well-being of Children (STEPS)—a prospective population-based birth cohort study—has reported that infants with persistent Moraxella sparsity have a higher risk of asthma development by age 7 years (34). Finally, a study from COPSAC 2010 (COPSAC2010) has reported that an increased α-diversity (i.e., within-sample measures of similarity or dissimilarity) and higher abundance of Veillonella and Prevotella in the airway at age 1 month are associated with asthma development by age 6 years (33). These studies collectively suggest the important roles of airway microbiota from early life in the development of childhood asthma.
Compared with the airway microbiota, it seems less intuitive that the gut microbiota would be connected with respiratory diseases since gut and lungs differ anatomically. Yet, alterations in gut microbial composition may have a notable effect on respiratory diseases, such as asthma, by shaping microbial communities and modulating the metabolic and immune response, a so-called “gut-lung axis” concept (23–25). Growing evidence suggests that gut microbiota in early life is associated with childhood asthma development (26–28, 36, 45–50). For example, a study from COPSAC2010 has found that immature gut microbial composition (measured by microbiota age) in children at age 1 year is associated with a higher risk of asthma development by age 5 years (47). Such association is stronger in children who have mothers with asthma, indicating the synergistic effect between genetic predisposition and inadequate gut microbial stimulation on asthma development (47). Additionally, a study from the Wayne County Health, Environment, Allergy and Asthma Longitudinal Study (WHEALS)—a prospective birth cohort—has found that lower relative abundance of beneficial bacteria in neonatal gut (e.g., Akkermansia, Bifidobacterium, and Faecalibacterium) are associated with a higher risk of asthma by age 4 years (26). Furthermore, a recent study from the Vitamin D Antenatal Asthma Reduction Trial (VDAART)—a randomized trial on the effects of prenatal vitamin D supplementation on asthma in offspring—has found that a higher level of Veillonella and histidine pathway metabolites or a lower level of Oscillospiraceae UCG-005 in gut of children at age 3 years are associated with an increased wheeze frequency between ages 3 and 5 years (28). In contrast, a mature gut microbial composition in early life may help to lower the risk of childhood asthma development. For example, a recent study from the Protection against Allergy-Study in Rural Environments (PASTURE)—a prospective birth cohort—has found that a mature gut microbial composition in infants from age 2 to 12 months—consisting of Bacteroides, Coprococcus, Roseburia, and Turicibacter—can produce short-chain fatty acids (SCFAs), such as butyrate, which have a protective effect on asthma development by 6 years (48). Likewise, a recent study from the Canadian Healthy Infant Longitudinal Development (CHILD)—a prospective birth cohort—has found that an increased α-diversity in gut microbiota due to decreased antibiotic use in infancy is associated with a reduced risk of asthma development by age 5 years (49). Taken together, these diverse pieces of evidence provide support for the relation of gut microbiota in early life and development of childhood asthma.
The complex interplay between environmental exposures and microbiota underlies the pathobiological mechanisms for asthma, especially in early life (Figure 1). One prominent example of environmental exposure affecting asthma development is the farm effect. Many epidemiological studies have found living on a farm in early life is associated with reduced risk of childhood asthma development (48, 51–54). For example, an early study has investigated two farm populations (Amish and Hutterites) with similar genetic backgrounds but different farming practices and found a distinct risk for childhood asthma development (52). The Amish follow traditional farming practices (i.e., high microbial exposures to animals) whereas the Hutterites use industrialized farming practices. The study has found the environment from Amish farms protects children against asthma development (52). On the other hand, a recent study from COPSAC2010 found that an airway and gut microbial signature due to urbanization (i.e., no farm effect) in infancy increases the risk of asthma, eczema, and allergic sensitization at age 6 years (55). Collectively, these interesting results suggest that early-life microbial farm animal exposures may lower the risk of childhood asthma development, perhaps by shaping the innate immune response.
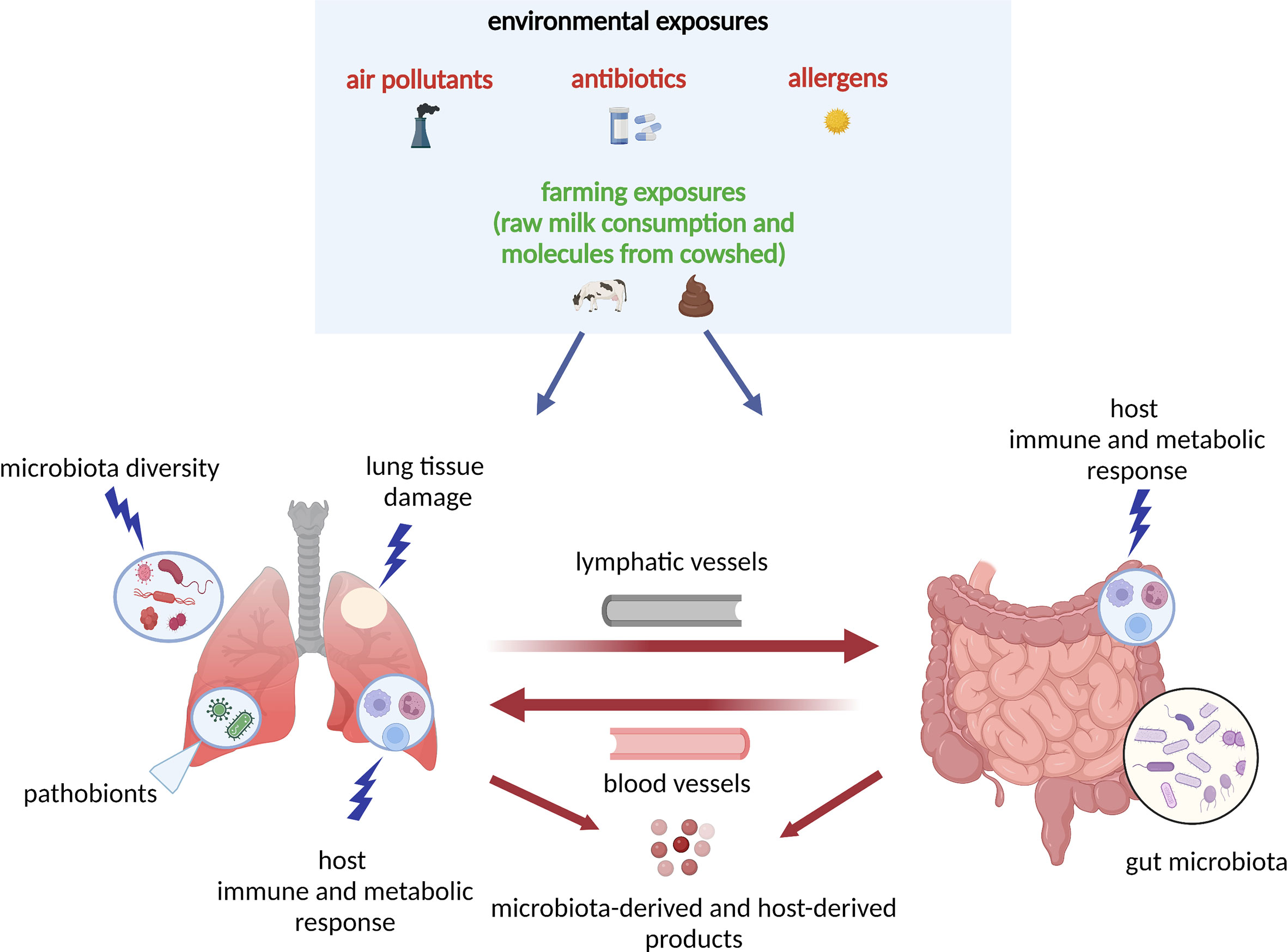
Figure 1 Model of the host–microbiota interaction during dysbiosis as a pathogenetic mechanism underlying asthma development: the “gut-lung axis”. A variety of environmental exposures, either protective (green color) or non-protective (red color) can trigger microbial dysbiosis (i.e., the alteration of the function and composition of the microbiota). In this model, in response to the non-protective environmental exposures (air pollutants, antibiotics, allergens), there is lung tissue damage and release of molecules that interact with host immune and metabolic pathways. The lung and gut microbiota diversity is perturbed, and there is increase in pathobionts (i.e., potentially disease-causing organisms that under normal circumstances act as symbionts) in both lung and gut tissues. In response to microbial dysbiosis, there is also increased activation of leukocytes and other innate-mediated soluble factors, which circulate through blood and lymphatic vessels and trigger adaptive immune responses with TH1, TH2, TH17, Treg differentiation. All host- and microbiota‐derived (e.g., SCFAs, cytokines and chemokines) products act at the local (lung) or distal (gut) levels via circulation through blood and lymphatic vessels. The “gut-lung axis” refers to bidirectional crosstalk between these two mucosal sites of the body as described above. SCFAs, short-chain fatty acids; TH2, T helper 2; TH17, T helper 17; Treg, regulatory T cell.
In addition to farm environmental exposures, other environmental exposures (e.g., antibiotics use, diet) may also have an effect on childhood asthma development by modulating the airway and gut microbiota in children’s early life. For example, a recent study from the STEPS study has found that exposures to antibiotics within the first year of life are associated with increased risk of asthma development by age 7 years; and such effect is partially mediated by longitudinal changes in the nasal airway microbiota characterized as a low asthma risk profile with persistent Moraxella dominance vs a high asthma risk profile with early Moraxella sparsity (56). Also, a study from the Urban Environment and Childhood Asthma (URECA)—a longitudinal birth cohort—has reported that the exposure to both allergens and the bacterial species (primarily from Bacteroidetes and Firmicutes phyla) within the first year of life can protect from atopy and recurrent wheeze development by age 3 years (57). Additionally, a study from the Infant Susceptibility to Pulmonary Infections and Asthma Following Respiratory Syncytial Virus Exposure (INSPIRE)—a longitudinal observational birth cohort—has found that breastfeeding during infancy has several beneficial effects on childhood asthma development by reducing the dose-response effect of the feeding on the ⍺-diversity of the early-life upper airway and gut microbiota, and protects children from the development of lower respiratory tract infections in infancy, and asthma at age 4 years (41). Moreover, a recent study from COPSAC2010 has found that if the gut microbial signature at age 1 year is retained from a cesarean section delivery period, there is a higher risk of asthma development by age 6 years, but not at a higher risk if the gut microbial signature became mature (e.g., a higher abundance of Akkermansia, Bacteroides, and Ruminococcus) (50). This finding indicates the maturation of gut microbial composition may mitigate the effect of cesarean section delivery on childhood asthma development. These studies collectively suggest that it is crucial to consider the integrated roles of environmental exposures and microbiota from early life in the childhood asthma development.
Microorganisms can present in host (e.g., airway) without interaction with the host, i.e., colocalization. Once there is an imbalance in the disease-related microbial community and interaction with host (i.e., microbial dysbiosis), host immune system can respond to the pathogenic microbes and lead to the local or/and systematic inflammation (14). Underlying mechanisms of the airway microbial dysbiosis and asthma link warrant further clarification. Figure 2A illustrates the major host immune mechanisms for airway epithelial cell signaling in response to bacterial pathogens. First, many studies have reported that innate immune responses play direct roles in host defense during the early stages of a respiratory infection, and they also exert a profound influence on the generation of the adaptive immune responses that ensue and on driving long-term respiratory sequela (most commonly asthma) (58, 59). The airway epithelium is a physical barrier and the first point of contact for inhaled pathogens (60). Notably, the airway epithelium contains a wide variety of pattern recognition receptors and antimicrobial compounds (e.g., mucins) that establish innate immunity (60). The pattern recognition receptors in the airway epithelium, such as Toll-like receptors (TLRs), are activated by invading pathogens, bacterial virulence factors, and endogenous mediators released due to airway tissue damage (61). The innate immune recognition of Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae, mainly involves TLR2 and TLR4. These TLRs activate the transcription factor nuclear factor kappa B (NF-кB) and interferon regulatory factor 3 (IRF3) (61), which regulates the expression of inflammatory genes and the release of cytokines and chemokines related to asthma (62, 63), such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α. For example, an in vivo study using an ovalbumin (OVA)-induced mouse model of allergic asthma has demonstrated that dysfunction of TLR4-positive innate immune cells including neutrophils and macrophages during Haemophilus influenzae infection promotes bacterial persistence and leads to the development of steroid-resistant neutrophilic asthma (64). In addition, an in vitro study has also shown that chronic colocalization with Haemophilus influenzae can induce neutrophil extracellular trap formation (NETosis) and releases soluble IL-6 receptor (sIL-6R) and IL-6 from neutrophils, which also associated with higher lung epithelium expression of TLR2 and TLR4 (65). The causal relationship between sIL-6R and childhood asthma is also supported by a recent Mendelian randomization study (66).

Figure 2 Metabolic and immune mechanisms for the link between airway or gut microbiota and asthma. (A) shows immune mechanisms for the airway epithelial cell signaling in response to bacterial pathogens. Various bacterial pathogens bind to innate sensors TLR2 and TLR4 and further activate MyD88- and TRIF-dependent pathways. MyD88 recruits TRAF6, which further activates the IKK complex, allows NF-кB to translocate into the nucleus, and leads to the overall production of inflammatory cytokines and chemokines, and activation of T cells. Additionally, recognition of various bacterial pathogens activates the TRIF-dependent signaling pathway, which involves the recruitment of TRIF, that leads to subsequent activation of TBK1 and IKKϵ along with induction of transcription factor IRF3. This signaling pathway results in interferon-related cytokines, and can potentiate NF-κB gene transcription. Enhanced neutrophil TLR2 and TLR4 signaling by bacterial pathogens promote neutrophils cytokine production and NETosis, a program for formation of NETs. Bacterial pathogens can also be recognized by TLR2 and TLR4 on macrophages, leading to activation of NF-κB and IRF3 signaling pathway and secretion of inflammatory mediators. Macrophages can also function as APC and regulate T cell activation. The T cell is presented an antigen with MHC II by APC. The recognition of the antigen-MHC II complex and the co-stimulatory molecules activates the T cell and leads downstream to differentiation into TH2 and TH17 cells, that can release various cytokines such as IL-4, IL-5 and IL-13, which lead to eosinophilic inflammation. APC, antigen-presenting cell; IKK, inhibitory kappa B kinases; IL, interleukin; IRF3, interferon regulatory factor 3; MHC, major histocompatibility complex; MyD88, myeloid differentiation primary response protein 88; NETosis, neutrophil extracellular trap formation; NETs, neutrophil extracellular traps; NF-кB, nuclear factor kappa B; TBK1, TANK-binding kinase 1; TH2, T helper 2; TH17, T helper 17; TLR, Toll-like receptor; TRAF6, tumor necrosis factor receptor associated factor 6; TRIF, TIR-domain-containing adapter-inducing interferon-β. (B) shows metabolic and immune mechanisms for the link between gut microbiota and asthma. PSA from Bacteroides fragilis induces and ligates TLR2 on pDC, which stimulate anti-inflammatory cytokine IL-10 secretion by CD4+ T cells. cDC and macrophage bound by gut microbiota show impaired ability to promote TH2- and TH17-type responses and tend to promote TH1-type responses and Treg proliferation, which lead to decreased eosinophilic inflammation. IL-10 dependent reprogramming of tissue macrophages is also essential for resolving inflammation by promoting M2 macrophage polarization. Bacteroides and Bifidobacterium can digest the fiber and produce SCFAs, such as butyrate. Faecalibacterium prausnitzii increases SCFAs level, such as butyrate and propionate, which leads to reduced levels of IL-4, IL-5 and IL-13, and elevated level of Tregs. cDC, classical dendritic cell; IFN, interferon; IL, interleukin; pDC, plasmacytoid dendritic cell; PSA, polysaccharide A; SCFAs, short-chain fatty acids; TH2, T helper 2; TH17, T helper 17; TLR, Toll-like receptor; Treg, regulatory T cell.
Second, in addition to the innate immunity, growing evidence suggests that adaptive immunity plays a pivotal role in the underlying mechanisms of the airway microbiota and asthma link (14, 67). For example, a recent in vivo study using a house dust mite (HDM)-challenged mouse model of the allergic airway inflammation has found that airway infection of Streptococcus pneumoniae leads to increased number of activated T helper 2 (TH2) cells and elevated level of TH2 cytokines, such as IL-4, IL-5 and IL-13 (68). Additionally, an in vivo study using HDM-challenged mouse model has found that airway infection with Moraxella catarrhalis triggers a strong inflammatory response with neutrophilic infiltrates, such as high amounts of IL-6 and TNF-α and moderate levels of CD4+ T-cell-derived interferon (IFN)-γ and IL-17. If bacterial infection occurs during HDM allergen sensitization, the allergic airway response is exacerbated, particularly by the expansion of T helper 17 (TH17) cells and increased TNF-α levels (69). In addition, a study using human bronchoalveolar lavage samples has demonstrated that a high bacterial load and supraglottic predominant taxa (e.g., Prevotella and Veillonella) is associated with an increased number of CD4+ IL-17+ T cells, and cytokines (IL-1β and IL-6) or chemokine (fractalkine) related to TH17 differentiation (70). Finally, an in vivo study using a HDM-challenged neonatal mouse model has demostrated that a dynamic change in lung microbiota in the first 2 weeks of life, from a dominance of Gammaproteobacteria and Firmicutes towards Bacteroidetes, is associated with an increased level of regulatory T cells (Tregs) and reduced aeroallergen responsiveness (71). Such findings indicate that the maturation of airway microbiota in early life is crucial to reduce the risk of developing allergic airway inflammation in later life. Notwithstanding the complexity of these mechanisms, the identification of the airway microbiome-host immune response interaction and its contribution to asthma pathobiology will likely prove important to future efforts to prevent childhood asthma.
The mechanisms underlying a link between gut microbiota and asthma involve complex interactions between microbes, metabolites, and host immune responses (14). Figure 2B illustrates the major metabolic and immune mechanisms for the link between gut commensal bacteria and asthma. In the gut, commensal bacteria help to shape the cellular and physical maturation of both innate and adaptive immunity in early life and have profound effects on asthma pathobiology (72, 73). More specifically, the presence of specific microbial species helps maintain the gut barrier function by preserving tight junction formation at the gut epithelium, and by modulating immune responses to allergens (74). For example, an early in vivo study of a mouse model showed polysaccharide A from Bacteroides fragilis induces and ligates TLR2 on plasmacytoid dendritic cells, which is priming IL-10 producing T cells with potential anti-inflammatory properties (75). Additionally, another in vivo study using an HDM-challenged mouse model has found that high-fiber diet increases the abundance of Bacteroides and Bifidobacterium, which can digest the fiber and produce SCFAs, such as butyrate (76). Butyrate can decrease excessive inflammation through downregulating the secretion of pro-inflammatory mediators (e.g., IL-6, TNF-a) and activating IL-10 producing T cells and macrophages (77). Consequently, the study has found that mice fed a high-fiber diet have increased concentration of circulating SCFAs and decreased airway hyperresponsiveness (AHR) and allergic airway inflammation (76). A recent study has also demonstrated that infants who are breastfed and given Bifidobacterium infantis EVC001, has reduced intestinal TH2 and TH17 cytokines and increased IFN-β level (78). Furthermore, HDM-challenged mice given with Faecalibacterium prausnitzii have increased SCFAs (e.g., butyrate, propionate) level, which leads to reduced levels of IL-4, IL-5 and IL-13, and elevated level of Tregs, consequently alleviating the symptoms of allergic asthma (79). Finally, intervention studies have provided mechanistic evidence on gut commensal bacteria and immunoregulation. For example, a recent in vivo study of OVA-induced murine allergic airways disease has showed that probiotic administration (e.g., Akkermansia) alleviate the airway inflammation in mice with a genetic predisposition for airway inflammation (80). The study has found that probiotic bacteria—such as Akkermansia—can produce acetate, another SCFA that can inhibit NF-кB activity (80). The inhibition of NF-кB is known to alleviate airway inflammation in asthma by reducing TH2 cytokine production, such as IL-5 and IL-13 (81). The biodiversity intervention (i.e., more nature-oriented environment) may modify the gut microbiota in children (e.g., Faecalibacterium), which was associated with changes in plasma cytokine and Tregs levels (82). This finding suggests the biodiversity intervention improved immunoregulatory pathways in children and can potentially lower the risk of immune-mediated diseases (e.g., asthma) in urban societies (82).
In this review, we have examined a broad range of major epidemiological and mechanistic studies and summarized the current evidence for the link between airway or gut microbiota and childhood asthma development. We also discussed some of the metabolic and immunological mechanisms underlying the link between microbiome exposure in early life and childhood asthma development. Nearly all microbiome-asthma studies have investigated either airway or gut microbiota – but not both. Thus, the synergistic effect of the airway and gut microbiota on childhood asthma development remains largely unclear (36, 83). We suggest future research shall examine the integrated effect of airway and gut microbiota on childhood asthma development in the same individual. Also, majority of mechanistic studies are in murine models, and often not early life models. The next step is to consider intervention studies with mechanisms built in. Additionally, we believe that integrating microbiome with other omics data—such as genomics, epigenomics, transcriptomics, proteomics, and metabolomics—will provide further insights into the pathobiology of asthma and its subtypes. Taken together, these efforts will facilitate the development of an early life microbiome-targeted prevention and intervention strategies for the primary prevention of childhood asthma (84).
CL conducted the literature review, drafted the initial manuscript, designed the figure, and approved the final manuscript as submitted. HM designed the figure, reviewed the initial manuscript, and approved the final manuscript as submitted. SS, MB, and L-LL reviewed the initial manuscript and approved the final manuscript as submitted. CC and KH conceptualized the study, reviewed the initial manuscript, and approved the final manuscript as submitted. ZZ conceptualized and supervised the study, obtained funding, conducted the literature review, drafted the initial manuscript, designed the figure, and approved the final manuscript as submitted.
This study was supported by the following grants: the National Institutes of Health: K01 AI-153558 (ZZ); Massachusetts General Hospital Department of Emergency Medicine Fellowship/Eleanor and Miles Shore Faculty Development Awards Program (ZZ); and the Harvard University William F. Milton Fund (ZZ).
CL, MB, and L-LL are employees of Sanofi US and may hold shares and/or stock options in the company. CC and KH report grants from National Institutes of Health outside the submitted work. ZZ reports grants from National Institutes of Health and Harvard University during the conduct of the study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. The Global Initiative for Asthma. 2022 gina report, global strategy for asthma management and prevention. (2022).
2. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr (2019) 7:246. doi: 10.3389/fped.2019.00246
3. Johnson CC, Havstad SL, Ownby DR, Joseph CLM, Sitarik AR, Biagini Myers J, et al. Pediatric asthma incidence rates in the united states from 1980 to 2017. J Allergy Clin Immunol (2021) 148(5):1270–80. doi: 10.1016/j.jaci.2021.04.027
4. Subbarao P, Mandhane PJ, Sears MR. Asthma: Epidemiology, etiology and risk factors. CMAJ (2009) 181(9):E181–190. doi: 10.1503/cmaj.080612
5. Zhu Z, Hasegawa K, Camargo CA Jr., Liang L. Investigating asthma heterogeneity through shared and distinct genetics: Insights from genome-wide cross-trait analysis. J Allergy Clin Immunol (2021) 147(3):796–807. doi: 10.1016/j.jaci.2020.07.004
6. Miller RL, Grayson MH, Strothman K. Advances in asthma: New understandings of asthma's natural history, risk factors, underlying mechanisms, and clinical management. J Allergy Clin Immunol (2021) 148(6):1430–41. doi: 10.1016/j.jaci.2021.10.001
7. Borish L, Culp JA. Asthma: A syndrome composed of heterogeneous diseases. Ann Allergy Asthma Immunol (2008) 101(1):1–9. doi: 10.1016/S1081-1206(10)60826-5
8. Zhu Z, Lee PH, Chaffin MD, Chung W, Loh PR, Lu Q, et al. A genome-wide cross-trait analysis from uk biobank highlights the shared genetic architecture of asthma and allergic diseases. Nat Genet (2018) 50(6):857–64. doi: 10.1038/s41588-018-0121-0
9. Zhu Z, Guo Y, Shi H, Liu CL, Panganiban RA, Chung W, et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in uk biobank. J Allergy Clin Immunol (2020) 145(2):537–49. doi: 10.1016/j.jaci.2019.09.035
10. Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA Jr., Liang L. Association of asthma and its genetic predisposition with the risk of severe covid-19. J Allergy Clin Immunol (2020) 146(2):327–329 e324. doi: 10.1016/j.jaci.2020.06.001
11. Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic architectures of childhood- and adult-onset asthma are partly distinct. Am J Hum Genet (2019) 104(4):665–84. doi: 10.1016/j.ajhg.2019.02.022
12. Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: Genome-wide and transcriptome-wide studies. Lancet Respir Med (2019) 7(6):509–22. doi: 10.1016/S2213-2600(19)30055-4
13. Zhu Z, Zhu X, Liu CL, Shi H, Shen S, Yang Y, et al. Shared genetics of asthma and mental health disorders: A large-scale genome-wide cross-trait analysis. Eur Respir J (2019) 2019:54(6). doi: 10.1183/13993003.01507-2019
14. Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity (2020) 52(2):241–55. doi: 10.1016/j.immuni.2020.01.007
15. Hufnagl K, Pali-Scholl I, Roth-Walter F, Jensen-Jarolim E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin Immunopathol (2020) 42(1):75–93. doi: 10.1007/s00281-019-00775-y
16. Tang HHF, Teo SM, Sly PD, Holt PG, Inouye M. The intersect of genetics, environment, and microbiota in asthma-perspectives and challenges. J Allergy Clin Immunol (2021) 147(3):781–93. doi: 10.1016/j.jaci.2020.08.026
17. Saglani S, Custovic A. Childhood asthma: Advances using machine learning and mechanistic studies. Am J Respir Crit Care Med (2019) 199(4):414–22. doi: 10.1164/rccm.201810-1956CI
18. Glynn JR, Moss PAH. Systematic analysis of infectious disease outcomes by age shows lowest severity in school-age children. Sci Data (2020) 7(1):329. doi: 10.1038/s41597-020-00668-y
19. Saglani S, Bush A. The early-life origins of asthma. Curr Opin Allergy Clin Immunol (2007) 7(1):83–90. doi: 10.1097/ACI.0b013e32801297e6
20. Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature (2007) 449(7164):804–10. doi: 10.1038/nature06244
21. Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and diseases. Signal Transduct Target Ther (2022) 7(1):135. doi: 10.1038/s41392-022-00974-4
22. Chung KF. Airway microbial dysbiosis in asthmatic patients: A target for prevention and treatment? J Allergy Clin Immunol (2017) 139(4):1071–81. doi: 10.1016/j.jaci.2017.02.004
23. Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol (2019) 12(4):843–50. doi: 10.1038/s41385-019-0160-6
24. Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y. The cross-talk between gut microbiota and lungs in common lung diseases. Front Microbiol (2020) 11:301. doi: 10.3389/fmicb.2020.00301
25. Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, et al. The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol (2020) 10:9. doi: 10.3389/fcimb.2020.00009
26. Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and t cell differentiation. Nat Med (2016) 22(10):1187–91. doi: 10.1038/nm.4176
27. Levan SR, Stamnes KA, Lin DL, Panzer AR, Fukui E, McCauley K, et al. Elevated faecal 12,13-dihome concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat Microbiol (2019) 4(11):1851–61. doi: 10.1038/s41564-019-0498-2
28. Lee-Sarwar K, Dedrick S, Momeni B, Kelly RS, Zeiger RS, O’Connor GT, et al. Association of the gut microbiome and metabolome with wheeze frequency in childhood asthma. J Allergy Clin Immunol (2022) 150(2):325–36. doi: 10.1016/j.jaci.2022.02.005
29. Zhu Z, Camargo CA Jr., Raita Y, Freishtat RJ, Fujiogi M, Hahn A, et al. Nasopharyngeal airway dual-transcriptome of infants with severe bronchiolitis and risk of childhood asthma: A multicenter prospective study. J Allergy Clin Immunol (2022) 150(4):806–16. doi: 10.1016/j.jaci.2022.04.017
30. Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med (2007) 357(15):1487–95. doi: 10.1056/NEJMoa052632
31. Bisgaard H, Hermansen MN, Bonnelykke K, Stokholm J, Baty F, Skytt NL, et al. Association of bacteria and viruses with wheezy episodes in young children: Prospective birth cohort study. BMJ (2010) 341:c4978. doi: 10.1136/bmj.c4978
32. Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe (2015) 17(5):704–15. doi: 10.1016/j.chom.2015.03.008
33. Thorsen J, Rasmussen MA, Waage J, Mortensen M, Brejnrod A, Bonnelykke K, et al. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat Commun (2019) 10(1):5001. doi: 10.1038/s41467-019-12989-7
34. Toivonen L, Karppinen S, Schuez-Havupalo L, Waris M, He Q, Hoffman KL, et al. Longitudinal changes in early nasal microbiota and the risk of childhood asthma. Pediatrics (2020) 2020:146(4). doi: 10.1542/peds.2020-0421
35. Mansbach JM, Luna PN, Shaw CA, Hasegawa K, Petrosino JF, Piedra PA, et al. Increased moraxella and streptococcus species abundance after severe bronchiolitis is associated with recurrent wheezing. J Allergy Clin Immunol (2020) 145(2):518–527.e518. doi: 10.1016/j.jaci.2019.10.034
36. Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P. Microbiome composition and its impact on the development of allergic diseases. Front Immunol (2020) 11:700. doi: 10.3389/fimmu.2020.00700
37. Tang HHF, Lang A, Teo SM, Judd LM, Gangnon R, Evans MD, et al. Developmental patterns in the nasopharyngeal microbiome during infancy are associated with asthma risk. J Allergy Clin Immunol (2021) 147(5):1683–91. doi: 10.1016/j.jaci.2020.10.009
38. Raita Y, Camargo CA Jr., Bochkov YA, Celedon JC, Gern JE, Mansbach JM, et al. Integrated-omics endotyping of infants with rhinovirus bronchiolitis and risk of childhood asthma. J Allergy Clin Immunol (2021) 147(6):2108–17. doi: 10.1016/j.jaci.2020.11.002
39. Raita Y, Perez-Losada M, Freishtat RJ, Harmon B, Mansbach JM, Piedra PA, et al. Integrated omics endotyping of infants with respiratory syncytial virus bronchiolitis and risk of childhood asthma. Nat Commun (2021) 12(1):3601. doi: 10.1038/s41467-021-23859-6
40. Raita Y, Perez-Losada M, Freishtat RJ, Hahn A, Castro-Nallar E, Ramos-Tapia I, et al. Nasopharyngeal metatranscriptome profiles of infants with bronchiolitis and risk of childhood asthma: A multicentre prospective study. Eur Respir J (2022) 2022:60(1). doi: 10.1183/13993003.02293-2021
41. Rosas-Salazar C, Shilts MH, Tang Z-Z, Hong Q, Turi KN, Snyder BM, et al. Exclusive breast-feeding, the early-life microbiome and immune response, and common childhood respiratory illnesses. J Allergy Clin Immunol (2022) 150(3):612–21. doi: 10.1016/j.jaci.2022.02.023
42. Zhu Z, Camargo CA Jr., Hasegawa K. Metabolomics in the prevention and management of asthma. Expert Rev Respir Med (2019) 13(12):1135–8. doi: 10.1080/17476348.2019.1674650
43. Zhu Z, Camargo CA Jr., Raita Y, Fujiogi M, Liang L, Rhee EP, et al. Metabolome subtyping of severe bronchiolitis in infancy and risk of childhood asthma. J Allergy Clin Immunol (2022) 149(1):102–12. doi: 10.1016/j.jaci.2021.05.036
44. Fujiogi M, Zhu Z, Raita Y, Ooka T, Celedon JC, Freishtat R, et al. Nasopharyngeal lipidomic endotypes of infants with bronchiolitis and risk of childhood asthma: A multicentre prospective study. Thorax (2022). doi: 10.1136/thorax-2022-219016
45. Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Muller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol (2011) 128(3):646–652.e641-645. doi: 10.1016/j.jaci.2011.04.060
46. Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med (2015) 7(307):307ra152. doi: 10.1126/scitranslmed.aab2271
47. Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun (2018) 9(1):141. doi: 10.1038/s41467-017-02573-2
48. Depner M, Taft DH, Kirjavainen PV, Kalanetra KM, Karvonen AM, Peschel S, et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med (2020) 26(11):1766–75. doi: 10.1038/s41591-020-1095-x
49. Patrick DM, Sbihi H, Dai DLY, Al Mamun A, Rasali D, Rose C, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: Evidence from population-based and prospective cohort studies. Lancet Respir Med (2020) 8(11):1094–105. doi: 10.1016/S2213-2600(20)30052-7
50. Stokholm J, Thorsen J, Blaser MJ, Rasmussen MA, Hjelmso M, Shah S, et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci Transl Med (2020) 2020:12(569). doi: 10.1126/scitranslmed.aax9929
51. von Mutius E, Vercelli D. Farm living: Effects on childhood asthma and allergy. Nat Rev Immunol (2010) 10(12):861–8. doi: 10.1038/nri2871
52. Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med (2016) 375(5):411–21. doi: 10.1056/NEJMoa1508749
53. House JS, Wyss AB, Hoppin JA, Richards M, Long S, Umbach DM, et al. Early-life farm exposures and adult asthma and atopy in the agricultural lung health study. J Allergy Clin Immunol (2017) 140(1):249–256.e214. doi: 10.1016/j.jaci.2016.09.036
54. Strieker S, Weinmann T, Gerlich J, von Mutius E, Nowak D, Radon K, et al. Farm living and allergic rhinitis from childhood to young adulthood - prospective results of the gabriel study. J Allergy Clin Immunol (2022). doi: 10.1016/j.jaci.2022.05.027
55. Lehtimaki J, Thorsen J, Rasmussen MA, Hjelmso M, Shah S, Mortensen MS, et al. Urbanized microbiota in infants, immune constitution, and later risk of atopic diseases. J Allergy Clin Immunol (2021) 148(1):234–43. doi: 10.1016/j.jaci.2020.12.621
56. Toivonen L, Schuez-Havupalo L, Karppinen S, Waris M, Hoffman KL, Camargo CA, et al. Antibiotic treatments during infancy, changes in nasal microbiota, and asthma development: Population-based cohort study. Clin Infect Dis (2021) 72(9):1546–54. doi: 10.1093/cid/ciaa262
57. Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol (2014) 134(3):593–601.e512. doi: 10.1016/j.jaci.2014.04.018
58. Diacovich L, Gorvel JP. Bacterial manipulation of innate immunity to promote infection. Nat Rev Microbiol (2010) 8(2):117–28. doi: 10.1038/nrmicro2295
59. Paludan SR, Pradeu T, Masters SL, Mogensen TH. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat Rev Immunol (2021) 21(3):137–50. doi: 10.1038/s41577-020-0391-5
60. Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol (2011) 45(2):189–201. doi: 10.1165/rcmb.2011-0011RT
61. Koppe U, Suttorp N, Opitz B. Recognition of streptococcus pneumoniae by the innate immune system. Cell Microbiol (2012) 14(4):460–6. doi: 10.1111/j.1462-5822.2011.01746.x
62. Kawai T, Akira S. Signaling to nf-kappab by toll-like receptors. Trends Mol Med (2007) 13(11):460–9. doi: 10.1016/j.molmed.2007.09.002
63. Gubernatorova EO, Namakanova OA, Gorshkova EA, Medvedovskaya AD, Nedospasov SA, Drutskaya MS. Novel anti-cytokine strategies for prevention and treatment of respiratory allergic diseases. Front Immunol (2021) 12:601842. doi: 10.3389/fimmu.2021.601842
64. Essilfie AT, Simpson JL, Dunkley ML, Morgan LC, Oliver BG, Gibson PG, et al. Combined haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax (2012) 67(7):588–99. doi: 10.1136/thoraxjnl-2011-200160
65. Winslow S, Odqvist L, Diver S, Riise R, Abdillahi S, Wingren C, et al. Multi-omics links il-6 trans-signalling with neutrophil extracellular trap formation and haemophilus infection in copd. Eur Respir J (2021) 2021:58(4). doi: 10.1183/13993003.03312-2020
66. Raita Y, Zhu Z, Camargo CA Jr., Freishtat RJ, Ngo D, Liang L, et al. Relationship of soluble interleukin-6 receptors with asthma: A mendelian randomization study. Front Med (Lausanne) (2021) 8:665057. doi: 10.3389/fmed.2021.665057
67. Lira-Lucio JA, Falfan-Valencia R, Ramirez-Venegas A, Buendia-Roldan I, Rojas-Serrano J, Mejia M, et al. Lung microbiome participation in local immune response regulation in respiratory diseases. Microorganisms (2020) 2020:8(7). doi: 10.3390/microorganisms8071059
68. Peng D, Shi Y, Pang J, Cui L, Xu Y, Meng H, et al. Early-life infection of the airways with streptococcus pneumoniae exacerbates hdm-induced asthma in a murine model. Cell Immunol (2022) 376:104536. doi: 10.1016/j.cellimm.2022.104536
69. Alnahas S, Hagner S, Raifer H, Kilic A, Gasteiger G, Mutters R, et al. Il-17 and tnf-alpha are key mediators of moraxella catarrhalis triggered exacerbation of allergic airway inflammation. Front Immunol (2017) 8:1562. doi: 10.3389/fimmu.2017.01562
70. Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a th17 phenotype. Nat Microbiol (2016) 1:16031. doi: 10.1038/nmicrobiol.2016.31
71. Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via pd-l1. Nat Med (2014) 20(6):642–7. doi: 10.1038/nm.3568
72. Khan R, Petersen FC, Shekhar S. Commensal bacteria: An emerging player in defense against respiratory pathogens. Front Immunol (2019) 10:1203. doi: 10.3389/fimmu.2019.01203
73. Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol (2011) 8(2):110–20. doi: 10.1038/cmi.2010.67
74. Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci U.S.A. (2014) 111(36):13145–50. doi: 10.1073/pnas.1412008111
75. Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, Kasper DL. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe (2014) 15(4):413–23. doi: 10.1016/j.chom.2014.03.006
76. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med (2014) 20(2):159–66. doi: 10.1038/nm.3444
77. Chen J, Vitetta L. The role of butyrate in attenuating pathobiont-induced hyperinflammation. Immune Netw (2020) 20(2):e15. doi: 10.4110/in.2020.20.e15
78. Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell (2021) 184(15):3884–3898.e3811. doi: 10.1016/j.cell.2021.05.030
79. Hu W, Lu W, Li L, Zhang H, Lee YK, Chen W, et al. Both living and dead faecalibacterium prausnitzii alleviate house dust mite-induced allergic asthma through the modulation of gut microbiota and short-chain fatty acid production. J Sci Food Agric (2021) 101(13):5563–73. doi: 10.1002/jsfa.11207
80. Casaro MB, Thomas AM, Mendes E, Fukumori C, Ribeiro WR, Oliveira FA, et al. Correction to: A probiotic has differential effects on allergic airway inflammation in a/j and c57bl/6 mice and is correlated with the gut microbiome. Microbiome (2021) 9(1):159. doi: 10.1186/s40168-021-01116-8
81. Shimizu K, Konno S, Ozaki M, Umezawa K, Yamashita K, Todo S, et al. Dehydroxymethylepoxyquinomicin (dhmeq), a novel nf-kappab inhibitor, inhibits allergic inflammation and airway remodelling in murine models of asthma. Clin Exp Allergy (2012) 42(8):1273–81. doi: 10.1111/j.1365-2222.2012.04007.x
82. Roslund MI, Puhakka R, Gronroos M, Nurminen N, Oikarinen S, Gazali AM, et al. Group ar. biodiversity intervention enhances immune regulation and health-associated commensal microbiota among daycare children. Sci Adv (2020) 2020:6(42). doi: 10.1126/sciadv.aba2578
83. Yagi K, Asai N, Huffnagle GB, Lukacs NW, Fonseca W. Early-life lung and gut microbiota development and respiratory syncytial virus infection. Front Immunol (2022) 13:877771. doi: 10.3389/fimmu.2022.877771
Keywords: microbial dysbiosis, airway microbiome, gut microbiome, immune mechanism, metabolic mechanism, childhood asthma
Citation: Liu C, Makrinioti H, Saglani S, Bowman M, Lin L-L, Camargo CA Jr, Hasegawa K and Zhu Z (2022) Microbial dysbiosis and childhood asthma development: Integrated role of the airway and gut microbiome, environmental exposures, and host metabolic and immune response. Front. Immunol. 13:1028209. doi: 10.3389/fimmu.2022.1028209
Received: 25 August 2022; Accepted: 20 September 2022;
Published: 30 September 2022.
Edited by:
Susetta Finotto, University Hospital Erlangen, GermanyReviewed by:
Adam Collison, The University of Newcastle, AustraliaCopyright © 2022 Liu, Makrinioti, Saglani, Bowman, Lin, Camargo, Hasegawa and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Conglin Liu, Y29uZ2xpbi5saXVAc2Fub2ZpLmNvbQ==; Zhaozhong Zhu, enpodTVAbWdoLmhhcnZhcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.