- Department of Hematology, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, China
Immunomodulatory drugs (IMiDs) such as thalidomide, lenalidomide and pomalidomide are antitumor compounds that have direct tumoricidal activity and indirect effects mediated by multiple types of immune cells in the tumor microenvironment (TME). IMiDs have shown remarkable therapeutic efficacy in a set of B-cell neoplasms including multiple myeloma, B-cell lymphomas and chronic lymphocytic leukemia. More recently, the advent of immunotherapy has revolutionized the treatment of these B-cell neoplasms. However, the success of immunotherapy is restrained by immunosuppressive signals and dysfunctional immune cells in the TME. Due to the pleiotropic immunobiological properties, IMiDs have shown to generate synergetic effects in preclinical models when combined with monoclonal antibodies, immune checkpoint inhibitors or CAR-T cell therapy, some of which were successfully translated to the clinic and lead to improved responses for both first-line and relapsed/refractory settings. Mechanistically, despite cereblon (CRBN), an E3 ubiquitin ligase, is considered as considered as the major molecular target responsible for the antineoplastic activities of IMiDs, the exact mechanisms of action for IMiDs-based TME re-education remain largely unknown. This review presents an overview of IMiDs in regulation of immune cell function and their utilization in potentiating efficacy of immunotherapies across multiple types of B-cell neoplasms.
1 Introduction
B-cell neoplasms, which stem from distinct stages of B-cell development, are a heterogeneous set of cancers including B-cell lymphomas (BCLs), chronic lymphocytic leukemia (CLL), and plasma cell dyscrasias such as multiple myeloma (MM) (1). Despite great advances have been achieved in diagnosis and treatment, these hematologic disorders still cause significant global morbidity and mortality. The introduction of a safe and more effective new class of drugs, especially the monoclonal antibodies (mAbs) (e.g. anti-CD20 rituximab and anti-CD38 daratumumab), has made remarkable therapeutic progress in the past twenty years. Yet a large number of patients still fail to have response or relapse eventually. More recently, novel immunotherapies including immune checkpoint inhibitors (ICIs) and chimeric antigen receptor (CAR) T-cell therapy have made breakthroughs in treatment of refractory disease (2, 3). However, the success of immunotherapy is impeded by inhibitory signals which reside in cancer cells or that are generated from the tumor microenvironment (TME), which restricts the tumor-suppressive capacity of the immune system (4–6).
TME is a complex network consisting of both cellular and non-cellular compositions, which forms a physical barrier around malignant cells. Increasing evidence has established that components of TME play vital roles in a series of processes of tumor development, including carcinogenesis, progression, metastasis and treatment resistance (6–8). Recognition of the TME has paved the way for exploring novel strategies targeting the microenvironment as well as its interplays with tumor cells (9). Immunomodulatory drugs (IMiDs) are a group of anticancer agents including thalidomide and its analogs lenalidomide and pomalidomide. These compounds show pleiotropic effects in hematologic malignancies including anti-angiogenic, anti-proliferative and immunobiologic properties by direct cytotoxicity towards tumor cells and indirectly interfering with cellular components of the TME (10–12). Herein, we provide a comprehensive review of the immunomodulatory activities of thalidomide analogues towards T cells, tumor-associated macrophages (TAMs), natural killer (NK) cells, dendritic cells (DCs) and stromal cells. In addition, we also discuss the clinical efficacy of IMiDs in combination with the state-of-the-art immunotherapies to shed light on optimal TME–targeted treatment strategy.
2 Development of IMiDs
2.1 Drug repurposing and regeneration
Thalidomide (α-N-phthalimido-glutarimide) (Figure 1A), a synthetic glutamic acid derivative, was once infamous for its potent teratogen causing dysmelia when used for alleviating nausea during pregnancy in the late 1950s and early 1960s. Despite withdrawal from markets that time, thalidomide regained its new life four decades later when immunomodulatory and anti-tumor effects were discovered (10, 13, 14). The first evidence for the immunomodulatory functions of thalidomide was demonstrated that it was effective in the treatment of erythema nodosum leprosum due to its ability to inhibit TNFα secreted by activated monocytes (15, 16). Except for this anti-inflammatory property, thalidomide was subsequently shown to exert other immunomodulatory properties such as co-stimulation of T cells and activation of NK cells (17). Along with these findings, the recognition of thalidomide as an inhibitor of angiogenesis further fueled a surge of interest in repurposing thalidomide as a promising anti-neoplastic therapy (18). As such, a set of formal medicinal chemistry programs were then initiated to discover novel derivatives with enhanced efficacy while less toxicity compared with thalidomide (19). Lenalidomide and pomalidomide (Figure 1A), the two first-in-class IMiDs, are derived by adding an amino group to the fourth carbon of the phthaloyl ring of thalidomide (13).
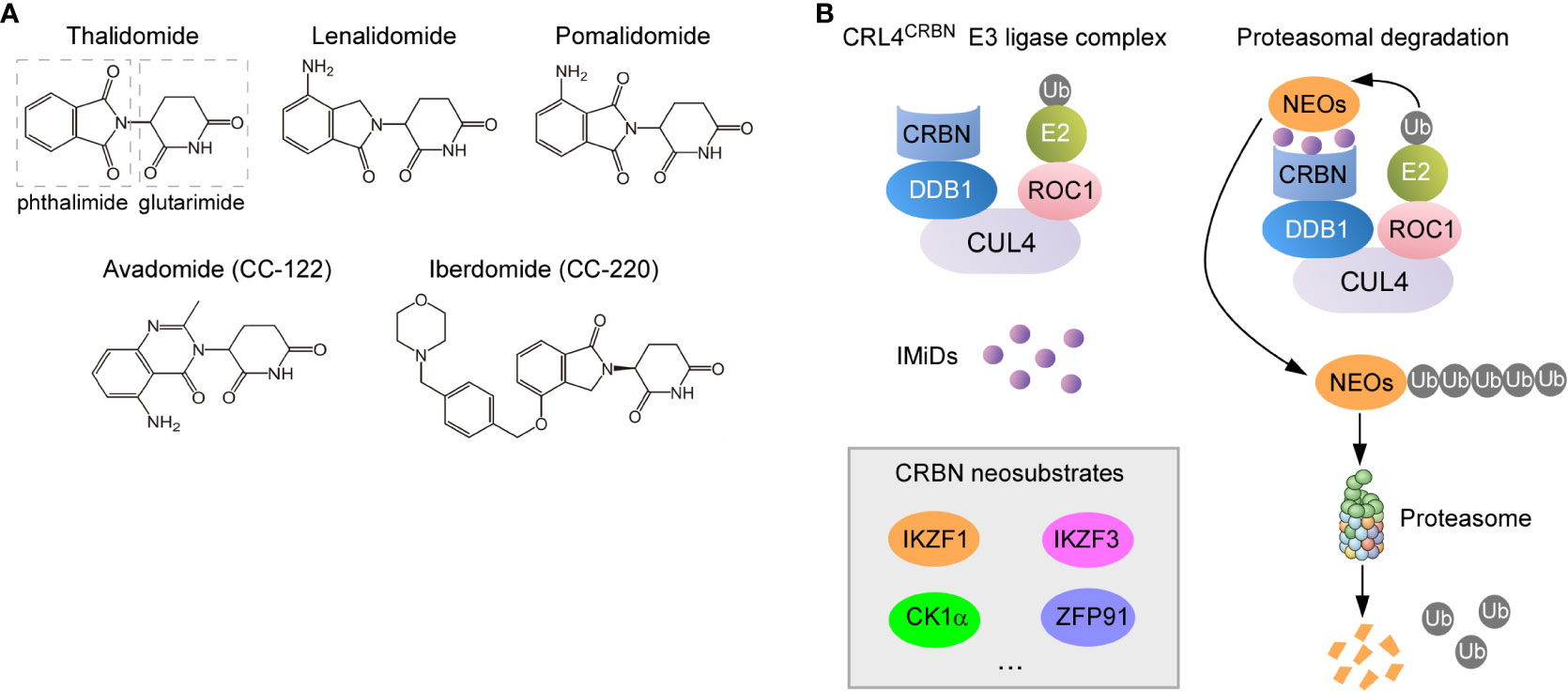
Figure 1 Molecular mechanisms of action for IMiDs. (A) Structure of thalidomide and its analogues. They all share a glutarimide ring that binds to CRBN while vary in the neosubstrate-binding moiety (phthaloyl ring). (B) Proteasomal degradation of CRBN neosubstrates redirected by IMiDs. IMiDs act as the molecular glue to recruit neosubstrate proteins to CRBN receptor component of the CRL4CRBN E3 ligase complex (left), which leads to the sequential ubiquitylation and degradation of neosubstrates (NEOs) (right).
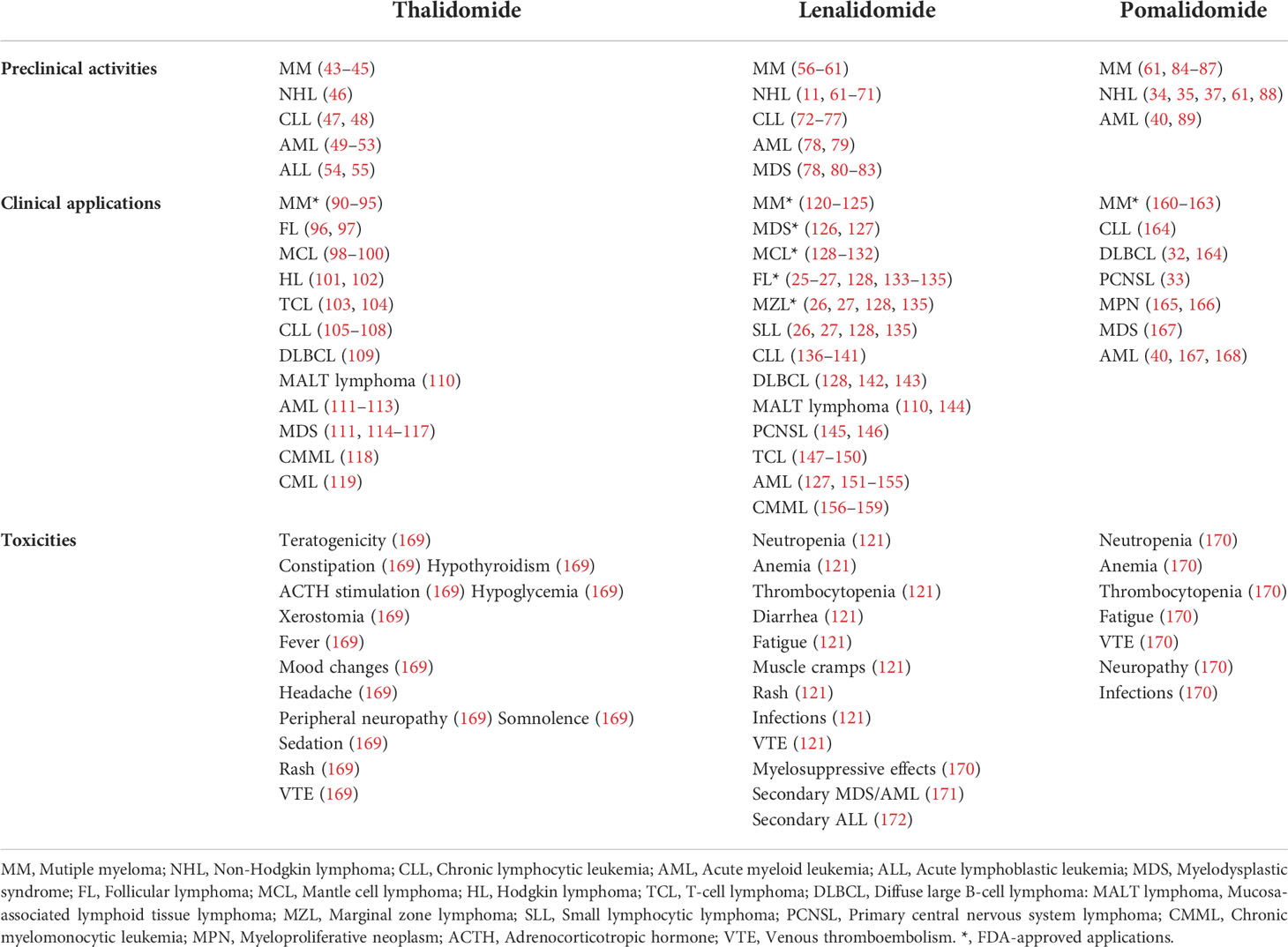
Lenalidomide was the first thalidomide analogue developed, consequently dominating the clinical development in hematologic malignancies (14). Lenalidomide was also the first agent of this group of immunomodulatory drugs approved by US Food and Drug Administration (FDA) for the treatment of MM, relapsed/refractory (R/R) mantle cell lymphoma (MCL), and myelodysplastic syndrome (MDS) with deletion 5q (20–24). Recently, it has been approved for previously treated follicular lymphoma (FL) and marginal zone lymphoma (MZL) in combination with rituximab (25–27). Notably, in 2020, lenalidomide combined with tafasitamab (a CD19 targeting mAb) received accelerated approval for patients with R/R diffuse large B-cell lymphoma (DLBCL) (28).
As the third-generation thalidomide analogue, pomalidomide contains both the phthalimide and the glutarimide moieties like thalidomide but differs in an amino substituent at the four position of the phthalimide ring (Figure 1A) (29). Pomalidomide has been approved for the treatment of MM, which is more powerful than lenalidomide and shows efficacy in cases that are resistant to lenalidomide (30, 31). Furthermore, it is now under extensive exploration in preclinical or clinical studies on aggressive BCLs including DLBCL, primary effusion lymphoma (PEL) and primary central nervous system lymphoma (PCNSL) (32–37). Avadomide (also called CC-122) (Figure 1A), a novel modulator of cereblon E3 ubiquitin ligase (CELMoD) exhibiting potent anti-lymphoma and immunomodulatory activities, is currently in phase I trials (38, 39). Other new CELMoDs such as CC-220 (iberdomide) and CC-885 (Figure 1A) have shown efficacy in the treatment of systemic lupus erythematosus (SLE) and acute myeloid leukemia (AML) (40–42). The established applications and most common side effects of three approved IMiDs (thalidomide, lenalidomide and pomalidomide) are summarized in Table 1.
2.2 Mechanism of action
IMiDs exert their anti-tumor effects by a unique mechanism of action (MOA), not only killing the malignant cells directly, but also modulating nonmalignant immune cells (T cells, NK cells, TAMs, DCs etc.) within the TME, which are believed to contribute to lymphoma progression and survival (10, 11, 13). Due to the pleiotropic effects of IMiDs, their molecular targets were believed to be various. The direct target of IMiDs was unknown until Ito et al. identified cereblon (CRBN) as the sole molecular target underlying thalidomide teratogenicity (173). Thereafter, various studies have focused on elucidating the role of CRBN in the effects of thalidomide analogues, especially for lenalidomide (56, 80, 174–176). As a result, CRBN is currently regarded as a primary direct target for therapeutic activities of all IMiDs (13).
CRBN forms a cullin-4 RING E3 ubiquitin ligase complex (CRL4CRBN) with DNA damage-binding protein 1 (DDB1), cullin 4 (CUL4), and regulator of cullins-1 (ROC1) (Figure 1B) (173, 177, 178). When bound by thalidomide derivatives, CRBN triggers protein ubiquitination and degradation of drug-specific neosubstrates. Substrate selectivity rests with the structure of IMiDs bond to CRBN (13, 179). IMiDs have a conserved glutarimide moiety that directly docks into a tri-tryptophan pocket on the surface of CRBN, which in turn activates its E3 ligase activity, modulates specificity of protein substrate and avoids autoubiquitylation (180, 181). In malignant B cells, IMiDs retarget CRBN-dependent ligase activity to Ikaros (IKZF1) and Aiolos (IKZF3), both of which are zinc finger–containing transcription factors in lymphoid development, resulting in their proteasomal degradation (14, 56, 88, 182, 183) (Figure 1B). The reduced abundance of Ikaros and Aiolos elicits direct anti-proliferative and anti-neoplastic effects against tumor cells. More importantly, a constellation of immunomodulatory effects arising from Ikaros and Aiolos degradation have been proposed to contribute to activities of IMiDs (14, 19), which include improved formation of immune synapse (IS) (184), potentiated co-stimulation of T cells (57), and enhanced release and function of anti-tumor cytokines (185).
It should be noted that different neosubstrate spectrum that are targeted for proteasomal degradation may account for the distinct activity of each thalidomide derivative (14). For instance, lenalidomide degrades casein kinase 1 alpha (CK1α, encoded by CSNK1A1 gene) more efficiently than thalidomide and pomalidomide in myeloid neoplasms, thus providing a therapeutic window for lenalidomide in del (5q) MDS, where CSNK1A1 haploinsufficiency due to genetic deletion sensitizes tumor cells to lenalidomide (80, 186, 187). A recent study showed that treatment with lenalidomide but not pomalidomide leads to expansion of pre-leukemic Trp53-mutant hematopoietic stem and progenitor cells (HSPCs) due to selective degradation of Ck1α, which offers a potential alternative strategy to mitigate the risk of therapy-related myeloid neoplasms (t-MNs) development (171). Accordingly, the efficacy and toxicity profiles of each IMiD and the precise use of these agents need to be thoroughly investigated.
3 The anti-tumor activities of IMiDs
3.1 Direct effects on malignant B cells
Direct anti-neoplastic activity of IMiDs against malignant B cells has been demonstrated in MM, CLL and aggressive non-Hodgkin lymphoma (NHLs) (12, 188). Degradation of Ikaros and Aiolos by lenalidomide and pomalidomide leads to specific and sequential downregulation of c-Myc followed by interferon regulatory factor 4 (IRF4), which results in subsequent cell death of myeloma cells (189). In addition, lenalidomide can upregulate p21WAF/Cip1 expression and lead to cell cycle arrest in CLL cells (72). In Namalwa CSN.70, a Burkitt’s lymphoma cell line with chromosome 5 deletion, lenalidomide was shown to induce cell cycle arrest and inhibit Akt and Gab1 phosphorylation (190). Moreover, lenalidomide kills activated B cell-like (ABC) DLBCL cells by inhibiting IRF4 and the Ets transcription factor Spi-B while stimulating IFNβ production in a CRBN-dependent manner (191).
3.2 Pleiotropic effects of IMiDs on TME
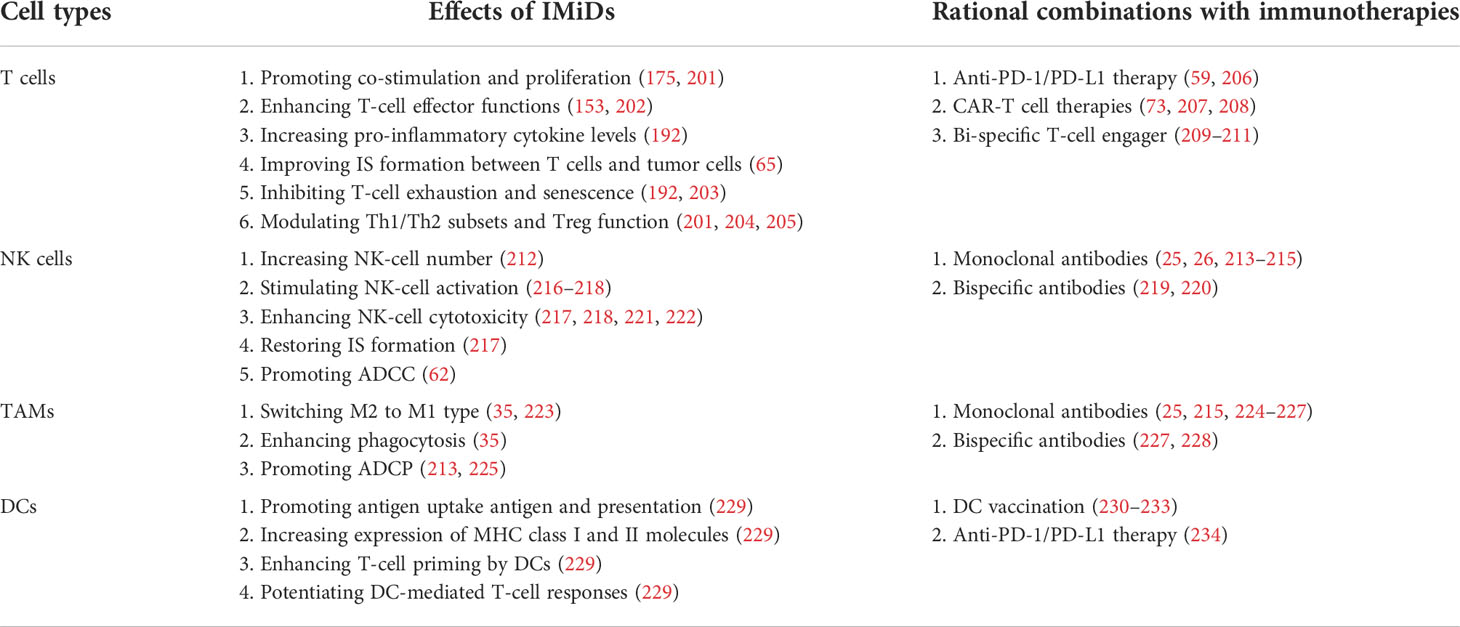
Beyond the direct cytotoxicity towards malignant B cells, recent studies have emphasized the therapeutic implications of IMiDs-remodeled interplay between malignant cells and non-malignant immune cells in the TME within the lymph nodes and bone marrow (11, 12, 192). Despite these nursing cells usually build a supportive network for tumor development and drug resistance, they also have potential to drive antitumor immune responses in specific cases (5, 6). Early studies based on gene expression signature of FL patients found that the length of survival was associated with the molecular features of tumor-infiltrating immune cells at diagnosis, which was independent of clinically prognostic variables (193). This evidence was supported by direct studies demonstrating that TME cells such as follicular dendritic cells (FDCs), CD4+ T cells and bone marrow stromal cells promoted lymphoma cell survival and proliferation (194, 195). In addition, tumor-associated monocytes/macrophages can attract and work in concert with other immune cells (e.g. T cells) by secretion of chemokines CCL3 and CCL4 (196, 197). As a result, TME shields malignant B cells from the immune recognition and elimination. The underlying mechanisms include the dampened expression of molecules (e.g. MHC I and II) required for interactions with immune cells, defected T-cell IS formation, and the recruitment of immunosuppressive cells such as regulatory T cells (Tregs) and TAMs (198–200). The immunomodulatory effects of IMiDs on the TME, especially the immune cells, are summarized in Table 2 and illustrated in Figure 2.
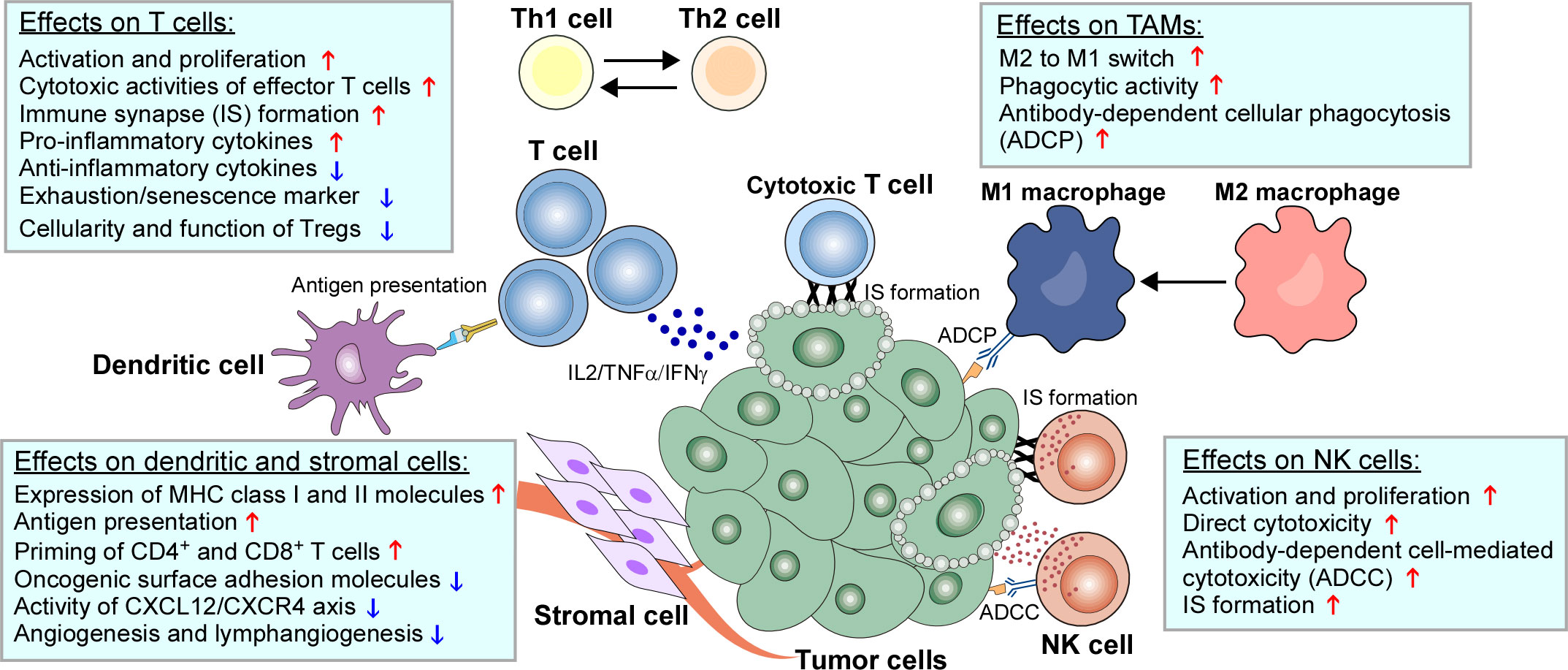
Figure 2 Immunomodulatory effects of IMiDs on the TME of B-cell neoplasms. The biological impacts of IMiDs on T cells, natural killer (NK) cells, tumor-associated macrophages (TAMs), dendritic cells and stromal cells are depicted.
3.2.1 Effects on T cells
Compelling evidence suggests that malignant B cells can induce an immune-suppressed, largely exhausted and senescent T-cell phenotype through numerous mechanisms, such as upregulation of inhibitory ligands, downregulation of co-stimulatory molecules and production of immunosuppressive cytokines, which ultimately results in suppression the T-cell surveillance and immune escape (199, 235–237).
Preclinical studies have shown that treatment with IMiDs enhances co-stimulation and proliferation of T cells by inducing pro-inflammatory cytokine (e.g. IFN-γ, TNF-α and IL-2), decreasing anti-inflammatory cytokines (e.g. IL-6 and IL-10) and potentiating DC-antigen presentation in MM and CLL (12, 192, 238, 239). The degradation of Ikaros and Aiolos by IMiDs relieves the transcriptional repression of Il2 promoter, thus promoting IL-2 production (175). Moreover, IMiDs can reduce immune tolerance of myeloma cells by binding to B7 co-stimulation molecular and activating B7-CD28 pathway (240). IMiDs can also upregulate transcriptional activity of DNA-binding protein AP-1 to increase T-cell cytokine production (212, 240, 241). These mechanisms collectively contribute to a primed T-cell activation (212, 242).
Due to the influence of malignant B cells, tumor-infiltrating CD4+ and CD8+ T cells usually display decreased IS formation and effector function (11). Ex vivo lenalidomide treatment of T cells co-cultured with CLL or FL cells repairs IS formation defect by restoring T-cell actin cytoskeletal signaling and enhancing actin polymerization (184, 198, 202). In addition, lenalidomide was shown to induce actin reorganization and γδT-MCL IS formation, as well as expansion and cytotoxicity of γδT cells against MCL (11). Another study reported that lenalidomide can repair defected T-cell adhesion and migration in CLL by restoring normal levels of Rho-GTPase family (Rho, Rac1 and Cdc42) and rescuing LFA-1 function (243).
Clinical investigations also provided evidence for the positive regulation of IMiDs on T-cell functions. Lenalidomide maintenance therapy after autologous stem-cell transplantation (ASCT) increases CD8+ T-cell numbers, upregulates co-stimulatory molecules and reduce inhibitory checkpoint molecules in MM patients (244). Similarly, Danhof et al. showed that lenalidomide maintenance post ASCT preserves CD8+ T cells and reduces expression of PD-1, enabling synergetic efficacies with ICIs (203). These findings were further validated in patient-derived xenograft (PDX) models showing an enhanced anti-CLL activity by combining avadomide and anti-PD-1 or anti-PD-1 ligand (PD-L1) (245). Moreover, the tumor-promoting Th17/Th1 and Th22 cells and related cytokines (IL-17, IL-6, IL-1β etc.) were decreased in MM patients treated with IMiDs during induction chemotherapy compared to untreated patients, which was associated with a favorable clinical outcome (246). As a result, lenalidomide and obinutuzumab combination was shown to induce an activated T-cell phenotype and reshape gene signatures into effector memory T cell features in FL patients (202). While in vitro studies showed that lenalidomide and pomalidomide strongly inhibit generation, proliferation and function of Tregs possibly due to decreased FOXP3 expression, the impact of IMiDs on the cellularity of Tregs in patients with B-cell neoplasms remains controversial (11, 192). In a post-transplant MM setting, treatment with IMiDs during induction therapy pre-ASCT resulted in decreased Tregs while increased CD8+ T cells in peripheral blood (247). In contrast, another study showed that lenalidomide maintenance after ASCT increased Treg numbers in relapsed MM patients (204). A similar pattern was observed in MCL patients treated with lenalidomide (248).
3.2.2 Effects on NK cells
NK cells are predominant innate lymphocytes that reject types of tumors and clear microbial infections (249), and more importantly, mediate antibody-dependent cell-mediated cytotoxicity (ADCC) against BCLs, which serves as the one of the major cytotoxic mechanisms for anti-CD20 mAb Rituximab (250). Numerous studies have demonstrated that the activity and function of NK cells can be potentiated by IMiDs in B-cell malignancies (212, 251). Lenalidomide treatment can increase NK-cell number, stimulate NK-cell activation, restore IS formation, and enhance direct NK-cell cytotoxicity as well as NK-dependent ADCC (212, 217, 221, 222, 234, 252). Mechanistically, the effect of lenalidomide on NK cells may be mediated indirectly via IL-2 produced by T cells. Either T-cell depletion or IL-2 blockade can completely abrogate NK-cell proliferation and cytotoxicity (212). The increased IL-2 and activation of NK cells correlate to increased IFN-γ synthesis and upregulation of CD69 (253). A recent study by Hideshima et al. demonstrated that pomalidomide directly binds to zeta-chain-associated protein kinase-70 (Zap-70) and triggers its phosphorylation to activate NK cells in a CRBN-independent manner. In addition, they also demonstrated a second mechanism whereby pomalidomide directly triggers granzyme-B and NK cytotoxicity which is mediated by CRBN-IKZF3 axis (218). Consistently, avadomide has shown to promote NK-cell proliferation and cytotoxicity by inducing IL-2 secretion and upregulating granzyme B and NKG2D receptor (254–256).
Lenalidomide was shown to enhance NK-dependent ADCC in BCL cell lines treated with rituximab (62). In this context, the increased expression of granzyme B and Fas ligand (FasL) may account for enhanced ADCC, which could be inhibited by a granzyme B inhibitor or FasL antibody (62). Moreover, lenalidomide lowers NK-cell activation thresholds by rituximab, thus augmenting NK-cell responses (217). On the other hand, lenalidomide synergistically enhances rituximab-induced phosphorylation of JNK and activates the mitochondrial apoptotic pathway in MCL cells (63). In vivo studies using immunodeficient mice inoculated with MCL cells demonstrated that lenalidomide and rituximab combination decreased tumor burden and prolonged animal survival along with the increased number of splenic NK cells (63). These data provide compelling proof-of-concept for the clinical translation of lenalidomide combination with rituximab into B-cell lymphoma treatment.
3.2.3 Effects on TAMs
TAMs are the key cellular components of TME, which can produce chemokines, cytokines and growth factors to recruit immunosuppressive cells and support tumor progression (257–259). TAMs are typically classified into M1-like (anti-tumorigenesis) and M2-like (pro-tumorigenesis) types based on their different surface markers, gene expression signatures and metabolic traits. The conversion between M1 and M2 is a dynamic process named “macrophage polarization” which occurs in response to TME signals (257, 260). Repolarization of M2-like macrophages to M1 phenotype represents a novel promising therapeutic strategy (261).
A recent study showed that lenalidomide altered the M1/M2 polarization in myeloma-associated macrophages (MAMs) from MM patients. Mechanistically, lenalidomide interferes epigenetically with IRF4 and IRF5 via degradation of IKZF1 and shifts M2-like MAMs to a pro-inflammatory and tumoricidal phenotype that resemble M1 cells (223). Similarly, pomalidomide has shown to repolarize macrophages from M2 to M1 and increase their phagocytic activity in mouse models of PCNSL, which is probably mediated by the potentiated STAT1 signaling while inhibited STAT6 signaling (35).
Therapeutically, macrophages possess immense potential of eliciting antibody-dependent cellular phagocytosis (ADCP) to destroy tumor cells (224). Of note, ADCP was demonstrated as one of the driving cytotoxic mechanism for anti-CD20 and anti-CD38 therapeutic antibodies against B-cell neoplasms (224, 262, 263). Thus, harnessing and enhancing macrophage-mediated ADCP through repolarization of M1/M2 macrophages is poised to become a novel and effective strategy for immunotherapy. Lenalidomide was shown to improved MOR202 (an anti-CD38 mAb)-mediated tumoricidal activity of MAMs against primary MM cells by restoring the defective vitamin D pathway in these MAMs with reduced CYP27B1 level (225). In addition, lenalidomide and pomalidomide mediated a substantial CD38 upregulation on MM cell lines, which also contributes to a synergistic enhancement of cytotoxic activity by combining MOR202 with IMiDs (213). Despite the enhanced ADCP of anti-CD20 mAbs by IMiDs has not been fully studied, it deserves further investigation for clinical application especially considering that obinutuzumab, the third-generation type II humanized anti-CD20 mAb (264), has shown to induce stronger ADCP as compared to rituximab, which may be due to the increased activation of FcγRI (CD64) expressed on primary macrophages (226).
3.2.4 Effects on DCs
As the most powerful antigen presenting cells (APCs), DCs are key messengers and link between the innate and adaptive immune systems by capturing and presenting tumor antigens for T-cell recognition (265, 266). Evidence of immunomodulatory activity of IMiDs on DCs was first revealed in mouse, showing that lenalidomide and pomalidomide upregulated MHC class I molecules and CD86 on DCs derived from bone marrow, promoted antigen uptake antigen and presentation of DCs for naive CD8+ T cells (229). Pomalidomide can also increase the expression of MHC class II molecules on DCs, resulting in increasing CD4+ T cell priming (229). Recently, Phan et al. showed that IMiDs have the potential to shift the DC-mediated response from Th1 to Th2 humoral immunity in human. IMiDs potentially enhanced DC-mediated allergic Th2 responses (CCL17 secretion and memory Th2 response) through upregulated STAT6 and IRF4 (267). Interestingly, high CCL17 levels in serum at the onset of rash as a side effect correlate with clinical outcome of lenalidomide treatment, which suggests that DCs immunostimulation inextricably linked side effect and activity of IMiDs (267). These findings also provide evidence for the additional use of IMiDs in dendritic cell–based anti-tumor vaccines (230, 231).
3.2.5 Effects on stromal cells and angiogenesis
In pathological conditions, malignant B cells rely on interactions with nonmalignant stromal cells within bone marrow and secondary lymphoid organs for their survival and proliferation (237). In MM, cytokines derived from bone marrow-derived mesenchymal stromal cells (BMSCs), an integral part of the non-hematopoietic BM microenvironment, are considered important drivers of myeloma pathobiology (268). Treatment with IMiDs significantly abrogates the interaction between MM cells and BMSCs by decreasing the production of IL-6 by stromal cells and downregulating adhesion molecules including LFA-1/ICAM-1 and VLA-4/VCAM-1 (269). In addition, lenalidomide potentially inhibits the pro-survival activity of BMSCs in MCL by inhibiting IL-6-mediated STAT-3 signaling (270). Lenalidomide may also target CXCL12/CXCR4 axis by inhibiting production of CXCL12 by MSCs in NHL (271). To date, the exact impacts of IMiDs on other nonimmune components of TME in B-cell neoplasms such as cancer-associated fibroblasts (CAFs), extracellular matrix (ECM) and pericytes, are still unknown.
Angiogenesis is a constant hallmark from initiation to progression for both MM and BCLs (272, 273). The antiangiogenic activity of IMiDs have been well characterized in MM, which was initially thought as the major MOA of thalidomide analogs against myeloma progression (274). Thalidomide impairs angiogenesis via suppression of vascular endothelial growth factor (VEGF) signaling (275). Similarly, lenalidomide exerts anti-angiogenic activity by downregulating basic fibroblast growth factor (bFGF) and VEGF due at least in part to inhibition of Akt phosphorylation (276). In CLL, lenalidomide was shown to inhibit CLL-mediated pro-angiogenic effect in vitro and modulates angiogenesis-related factors in patients with R/R CLL (277). Moreover, lenalidomide also exhibits inhibitory effects on VEGF-mediated angiogenesis and lymphangiogenesis in mouse models of B-cell lymphoma (64).
4 IMiDs in the era of immunotherapy
4.1 Antibody-based therapies
Due to extensive capacity of antibodies for targeting tumor-specific antigens, antibody-based therapies have become the most frequently used immunotherapeutic method for cancer treatment. The potent anti-tumor activity of rituximab in patients with various lymphoid malignancies has led to its widespread use in most indolent and aggressive CD20+ BCLs (278). As shown in preclinical studies exhibiting synergistic anti-tumor activity, the chemotherapy-free combination of rituximab plus lenalidomide (R2 regimen) proved to be effective in previously untreated indolent lymphoma (FL and MZL) and induced high molecular response (25, 279, 280). Similarly, obinutuzumab plus lenalidomide (GALEN regimen) has also been demonstrated as an active immunomodulatory combination with a manageable safety profile in both front-line and R/R FL (133, 281). Although the MOA of obinutuzumab favors it as a more effective anti-CD20 mAb (264), it remains uncertain whether rituximab or obinutuzumab is the better one when combined with lenalidomide in indolent lymphoma. In CLL, the combination of lenalidomide and ofatumumab was well-tolerated and induced durable responses in the majority of R/R patients with 71% ORR and a long progression-free survival (PFS) of 16 months (282). The ability to augment ADCC and ADCP suggests that lenalidomide should also cooperate with other therapeutic antibodies beyond anti-CD20 mAbs. Daratumumab (an anti-CD38 mAb) is approved as monotherapy or in combination with standard regimens for treatment of newly diagnosed (ND) or R/R MM (214). In RRMM, daratumumab in combination with dexamethasone and lenalidomide led to a significant PFS benefit over dexamethasone and lenalidomide alone (215, 283). The phase 3 MAIA study further demonstrated that daratumumab plus dexamethasone and lenalidomide increased OS and PFS of NDMM patients ineligible for transplantation (120). In addition, the anti-CD19 mAb MOR-28 (Tafasitamab) plus lenalidomide has shown outstanding clinical benefits with durable response rates in a phase 2 trial for R/R DLBCL (28).
Bi-specific T-cell engagers (BiTEs) are a new category of artificial bispecific antibodies (BsAbs) engineered to recognize specific tumor-associated antigen and CD3 at the same time (284, 285). Given the promising clinical efficacy of BiTEs in R/R BCLs (286), the combinations of lenalidomide with BsAbs such as Blinatumomab (a CD19/CD3 BiTE)and Mosunetuzumab (a CD20/CD3 BiTE) are currently being investigated in early-phase 1 clinical trials (209–211).
4.2 ICIs
The use of ICIs targeting PD-1 signaling pathway has ushered in a paradigm shift in cancer due to success in various high-risk solid tumors (287). However, the activity of ICIs in hematologic malignancies is currently restricted to certain subtypes of lymphoma, such as Hodgkin lymphoma (HL) and primary mediastinal B-cell lymphoma (PMBCL) (288). The severe T-cell tolerance and exhaustion within the TME is considered as the major contributor to disappointing clinical results for anti-PD-1 monotherapy in NHLs and CLL (289, 290). A recent study by Geng et al. showed that lenalidomide bypasses the requirement of CD28 for tumor-infiltrating CD8+ T-cell activation and antitumor activity of PD-1 blockade, which suggests that lenalidomide combination is beneficial to overcome PD-1 resistant tumors infiltrated with CD28- exhausted T cells (206). In addition, another preclinical study demonstrated avadomide combination enhanced anti-CLL activity of anti-PD-1/PD-L1 therapy (245). Mechanistically, avadomide stimulated T-cell activation, motility, cytokine production, IS formation, and IFN‐γ‐inducible expression of PD‐L1, thus reshaping a non-T cell-inflamed into a T cell-inflamed TME (245). Moreover, single blockade of PD-1 or dual blockade using anti-PD-1/PD-L1 antibodies plus lenalidomide blocked the cross-talk between myeloma cells and BMSC, thus inducing an anti-myeloma immune response to inhibit cell growth (291). Despite some early-phase 1/2 trials of pembrolizumab (an anti-PD-1 mAb) plus IMiDs and dexamethasone reported a ~50% ORR in patients with RRMM (292–294), however, phase 3 trials (KEYNOTE-183 and KEYNOTE-185) evaluating the combination of pembrolizumab with dexamethasone and an IMiD in RRMM (with pomalidomide) and NDMM (with lenalidomide) was eventually discontinued due to higher risk of death (295, 296). Further studies are needed to determine the mechanism underlying the unexpected toxicity, which will contribute to realize the therapeutic potential of ICIs and IMiDs combination in the clinic.
4.3 CAR-T cell therapy
CAR-T cell therapies have been approved for treatment of R/R B-ALL and aggressive B-NHLs. There are intensive bench-to-bedside studies underway to further improve the efficacy of CAR-T cells, focusing on recently described resistance mechanisms, such as T-cell exhaustion, immunosuppressive TME, defective IS, downregulation of target antigens, among others (297, 298). A strong rationale supports the combination of IMiDs and CAR-T therapy according to the enhanced activity of effector T cells and other cellular components in the TME re-educated by IMiDs. In vivo models have demonstrated that lenalidomide significantly enhances anti-lymphoma functions of CD19 and CD20 CAR-T cells, with decreased tumor burden and increased intratumoral CD8+ T cells (207). Another study showed that lenalidomide improved the efficacy of CS1-directed CAR-T cells against MM by enhancing expansion, cytotoxicity, memory maintenance, Th1 cytokine production, and IS formation of CAR-T cells (208). In addition, lenalidomide has shown to maintain the in vitro activity of CD23 CAR-T cells, preserve functional CAR T-CLL cell immune synapses, and improve the therapeutic efficacy of CD23 CAR-T cells in vivo (73). Despite the evidence of synergistic efficacy, it should be noted that the specific toxicities associated with CAR-T cells plus IMiDs, such as severe cytopenias and cytokine release syndrome (299, 300), will need to be carefully examined. Current ongoing trials have included the combing IMiDs with CD19 or B cell maturation antigen (BCMA) CAR-T cell therapy in DLBCL and MM (301–304).
4.4 Conventional chemotherapy
Despite advances in treatment, conventional chemotherapy is still the mainstay to induce a fast clinical remission of most hematologic cancers in the age of targeted and immune therapies. The introduce of IMiDs to chemotherapy regimen for decades has dramatically increased CR ratio and improved prognosis of NDMM (121, 274). Currently, induction treatments for MM have traditionally relied on a backbone of a combinations of IMiDs (thalidomide, lenalidomide and pomalidomide), proteasome inhibitors, alkylators (or anthracyclines), and/or steroids (274). In this scenario, IMiDs are believed to improve the immune environment beyond direct anti-tumor activity, which ensures persistent minimal residual disease (MRD) negativity through enhanced immunological surveillance against myeloma cells (305). In addition, the recently approved anti-CD38 antibodies have also shown to reshape the MM immune environment via activation of T and NK cells and suppression of Tregs (305). These combined immunogenic chemotherapies are paving a promising way to “cure MM”. Similarly, adding lenalidomide to R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) (R2-CHOP regimen) has recently shown improved outcomes in ABC-type DLBCL (306). As such, a deeper understating of immune dysfunction in B-cell malignancies has already led to the development of a more effective and less toxic immunotherapy-chemotherapy combinations to be given to cancer patients.
5 Conclusions and perspectives
Compelling evidence over last decades has shown the potent immunomodulatory effects of IMiDs on diverse cellular components (T cells, NK cells, TAMs, DCs, etc.) that reside within TMEs of B-cell neoplasms, which repurposes these agents to play a role in the era of immunotherapy (Table 2). The promising outcomes of chemotherapy-free regimen combining IMiDs with mAbs (e.g. rituximab or obinutuzumab) in treatment of both indolent and aggressive NHL types exemplify a shift of paradigm from the standard chemotherapy to a safer and more effective IMiD-intensified immunotherapy. Based on these findings in hematologic cancers, a number of studies have explored the potential applications of IMiDs in solid tumors. For instance, CC-885, a novel CRBN modulator, has shown to induce CRBN- and p97-dependent PLK1 degradation and synergizes with volasertib (PLK1 inhibitor) to suppress lung cancer (307). Moreover, pomalidomide can generate an immune-responsive and anti-tumorigenic environment and provide an ideal combination treatment with chemotherapeutic drugs or other immunotherapies in pancreatic cancer (308). Other studies also reported activities of lenalidomide in breast cancer (309), prostate cancer (310) and colon adenocarcinoma (206). Although IMiDs by themselves exhibit very limited anti-tumor activity against solid tumors in the clinic (311), their broad immunobiological properties revert the immune regulatory milieu of TME and create opportunities for other therapeutics to achieve better responses (206).
Of note, despite a series of preclinical studies have shed novel light on the synergistic effects and MOA, the clinical safety and efficacy of the combination of IMiDs with other novel immunotherapies such as BiTEs, ICIs and CAR-T cell therapy are not yet fully determined. In addition, since all MM patients inevitably develops resistance to IMiDs over time, it is a significant limitation and challenge for clinicians to make decisions about RRMM treatment. From a molecular point of view, IMiD resistance involves downregulation of CRBN expression, IKZF1/3 and CRBN mutations, deregulation of IRF4 expression, abnormal epigenetic mechanisms (CBP/EP300, BRD4 and HDAC) and aberrant signaling pathways (Wnt, STAT3 and MAPK/ERK) (312, 313). Fortunately, recent studies have discovered that some potential novel agents and PROTACs, which target the resistance mechanisms, can increase the sensitivity of MM cells to IMiDs or synergistically enhance the anti-myeloma activity of IMiDs (313). Further studies to verify the safety and efficacy of these strategies in clinic are urgently needed to pave the way for the treatment of R/R settings. Moreover, although the E3 ubiquitin ligase CRBN is now considered as the major target that likely underlies the effects of IMiDs in tumor cells as well as immunomodulation, there are a range of key issues be addressed including: 1) the functions of CRBN in the absence of IMiDs and its physiological significance is still unknown; 2) the common and distinct neosubstrates of CRBN in tumor cells and immune cells are not fully identified; 3) the CRBN-independent mechanisms underlying the anti-tumor and immunomodulatory activities of IMiDs are reported and merit in-depth investigation. Further elucidation of these issues will contribute to optimize IMiDs-based immunotherapeutic combinations and overcome intractable drug resistance.
Author contributions
KZ conceived and designed the review. HG drafted and revised the manuscript. JY and HW helped with the literature collection. XL and YL proofread the manuscript and provided suggestions. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81470336 to KZ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AML, Acute myeloid leukemia; ADCC, Antibody-dependent cell-mediated cytotoxicity; ADCP, Antibody-dependent cellular phagocytosis; APC, Antigen presenting cell; ASCT, Autologous stem cell transplantation; BCL, B-cell lymphoma; BsAb, Bispecific antibody; BiTE, Bi-specific T-cell engager; BMSC, Bone marrow-derived mesenchymal stromal cell; CK1α, Casein kinase 1 alpha; CRBN, Cereblon; CAR, Chimeric antigen receptor; CLL, Chronic lymphocytic leukemia; CR, Complete response; DC, Dendritic cell; DLBCL, Diffuse large B-cell lymphoma; FDC, Follicular dendritic cell; FL, Follicular lymphoma; FDA, Food and Drug Administration; ICI, Immune checkpoint inhibitor; IS, Immune synapse; IMiD, Immunomodulatory drug; IRF4, Interferon regulatory factor 4; MCL, Mantle cell lymphoma; MOA, Mechanism of action; mAb, Monoclonal antibody; MM, Multiple myeloma; MDS, Myelodysplastic syndrome; NK, Natural killer; ND, Newly diagnosed; NHL, Non-Hodgkin lymphoma; ORR, Overall response rate; PDX, Patient-derived xenograft; PCNSL, Primary central nervous system lymphoma; PEL, Primary effusion lymphoma; PFS, Progression-free survival; Treg, Regulatory T cell; R/R, Relapsed/refractory; SLE, Systemic lupus erythematosus; TME, Tumor microenvironment; TAA, Tumor-associated antigen; TAM, Tumor-associated macrophage; VEGF, Vascular endothelial growth factor.
References
1. Cuenca M, Peperzak V. Advances and perspectives in the treatment of b-cell malignancies. Cancers (2021) 13(9):2266. doi: 10.3390/cancers13092266
2. Ayyappan S, Maddocks K. Novel and emerging therapies for b cell lymphoma. J Hematol Oncol (2019) 12(1):82. doi: 10.1186/s13045-019-0752-3
3. Wang L, Qin W, Huo Y-J, Li X, Shi Q, Rasko JEJ, et al. Advances in targeted therapy for malignant lymphoma. Signal Transduct Target Ther (2020) 5(1):15. doi: 10.1038/s41392-020-0113-2
4. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: A milieu hindering and obstructing antitumor immune responses. Front Immunol (2020) 11:940. doi: 10.3389/fimmu.2020.00940
5. Fowler NH, Cheah CY, Gascoyne RD, Gribben J, Neelapu SS, Ghia P, et al. Role of the tumor microenvironment in mature b-cell lymphoid malignancies. Haematologica (2016) 101(5):531–40. doi: 10.3324/haematol.2015.139493
6. Liu Y, Zhou X, Wang X. Targeting the tumor microenvironment in b-cell lymphoma: challenges and opportunities. J Hematol Oncol (2021) 14(1):1–17. doi: 10.1186/s13045-021-01134-x
7. Höpken UE, Rehm A. Targeting the tumor microenvironment of leukemia and lymphoma. Trends Cancer (2019) 5(6):351–64. doi: 10.1016/j.trecan.2019.05.001
8. Qu Y, Dou B, Tan H, Feng Y, Wang N, Wang D. Tumor microenvironment-driven non-cell-autonomous resistance to antineoplastic treatment. Mol Cancer (2019) 18(1):69. doi: 10.1186/s12943-019-0992-4
9. Jin M-Z, Jin W-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther (2020) 5(1):1–16. doi: 10.1038/s41392-020-00280-x
10. Yamshon S, Ruan J. IMiDs new and old. Curr Hematol Malignancy Rep (2019) 14(5):414–25. doi: 10.1007/s11899-019-00536-6
11. Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in b-cell non-Hodgkin lymphoma. J Clin Oncol (2015) 33(25):2803. doi: 10.1200/JCO.2014.59.5363
12. Ioannou N, Jain K, Ramsay AG. Immunomodulatory drugs for the treatment of b cell malignancies. Int J Mol Sci (2021) 22(16):8572. doi: 10.3390/ijms22168572
13. Ito T, Handa H. Molecular mechanisms of thalidomide and its derivatives. Proc Japan Academy Ser B (2020) 96(6):189–203. doi: 10.2183/pjab.96.016
14. Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation–lessons learned with lenalidomide. Nat Rev Clin Oncol (2021) 18(7):401–17. doi: 10.1038/s41571-021-00479-z
15. Sampaio EP, Kaplan G, Miranda A, Nery JA, Miguel CP, Viana SM, et al. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J Infect Diseases (1993) 168(2):408–14. doi: 10.1093/infdis/168.2.408
16. Sampaio E, Hernandez M, Carvalho D, Sarno E. Management of erythema nodosum leprosum by thalidomide: thalidomide analogues inhibit m. leprae-induced TNFα production in vitro. Biomed Pharmacother (2002) 56(1):13–9. doi: 10.1016/S0753-3322(01)00147-0
17. Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med (1998) 187(11):1885–92. doi: 10.1084/jem.187.11.1885
18. D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci (1994) 91(9):4082–5. doi: 10.1073/pnas.91.9.4082
19. Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer (2004) 4(4):314–22. doi: 10.1038/nrc1323
20. List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. New Engl J Med (2005) 352(6):549–57. doi: 10.1056/NEJMoa041668
21. List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. New Engl J Med (2006) 355(14):1456–65. doi: 10.1056/NEJMoa061292
22. Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau J-L, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. New Engl J Med (2007) 357(21):2123–32. doi: 10.1056/NEJMoa070594
23. Habermann TM, Lossos IS, Justice G, Vose JM, Wiernik PH, McBride K, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol (2009) 145(3):344–9. doi: 10.1111/j.1365-2141.2009.07626.x
24. Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, et al. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. New Engl J Med (2015) 373(19):1835–44. doi: 10.1056/NEJMoa1505237
25. Morschhauser F, Fowler NH, Feugier P, Bouabdallah R, Tilly H, Palomba ML, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. New Engl J Med (2018) 379(10):934–47. doi: 10.1056/NEJMoa1805104
26. Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, et al. AUGMENT: A phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol: Off J Am Soc Clin Oncol (2019) 37(14):1188–99. doi: 10.1200/JCO.19.00010
27. Andorsky DJ, Coleman M, Yacoub A, Melear JM, Fanning SR, Kolibaba K, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. Am Soc Clin Oncol (2019) 37(15_suppl):7513. doi: 10.1016/j.htct.2020.10.372
28. Salles G, Duell J, Barca EG, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large b-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol (2020) 21(7):978–88. doi: 10.1016/S1470-2045(20)30225-4
29. Yeung AJ-T, Ling SC. “Pomalidomide”. In: Ling SC, Trieu S. editors. Resistance to Targeted Therapies in Multiple Myeloma. Resistance to Targeted Anti-Cancer Therapeutics, Cham, Switzerland: Springer (2021) 22, 31–7. doi: 10.1007/978-3-030-73440-4_3
30. Richardson PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(6):781–94. doi: 10.1016/S1470-2045(19)30152-4
31. Siegel DS, Schiller GJ, Song KW, Agajanian R, Stockerl-Goldstein K, Kaya H, et al. Pomalidomide plus low-dose dexamethasone in relapsed refractory multiple myeloma after lenalidomide treatment failure. Br J Haematol (2020) 188(4):501–10. doi: 10.1111/bjh.16213
32. Xu P, Wang L, Cheng S, Zhao WL. Trial in progress: Pomalidomide plus rituximab, ifosfamide, carboplatin, and etoposide for relapsed or refractory diffuse Large b-cell lymphoma (PRIDE). Blood (2021) 138:4562. doi: 10.1182/blood-2021-146851
33. Tun HW, Johnston PB, DeAngelis LM, Atherton PJ, Pederson LD, Koenig PA, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood J Am Soc Hematol (2018) 132(21):2240–8. doi: 10.1182/blood-2018-02-835496
34. Park S, Jo S-H, Kim J-H, Kim S-Y, Ha JD, Hwang JY, et al. Combination treatment with GSK126 and pomalidomide induces b-cell differentiation in EZH2 gain-of-Function mutant diffuse Large b-cell lymphoma. Cancers (2020) 12(9):2541. doi: 10.3390/cancers12092541
35. Li Z, Qiu Y, Personett D, Huang P, Edenfield B, Katz J, et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PloS One (2013) 8(8):e71754. doi: 10.1371/journal.pone.0071754
36. Tun HW, Johnston PB, Grommes C, Reeder CB, Omuro AMP, Menke DM, et al. Phase I clinical trial on pomalidomide and dexamethasone in treating patients with relapsed/refractory primary central nervous system lymphoma (PCNSL) or primary vitreoretinal lymphoma (PVRL). Am Soc Clin Oncol (2017) 35(15_suppl):7516. doi: 10.1200/JCO.2017.35.15_suppl.7516
37. Shrestha P, Davis DA, Jaeger HK, Stream A, Aisabor AI, Yarchoan R. Pomalidomide restores immune recognition of primary effusion lymphoma through upregulation of ICAM-1 and B7-2. PloS Pathogens (2021) 17(1):e1009091. doi: 10.1371/journal.ppat.1009091
38. Hagner PR, Man H-W, Fontanillo C, Wang M, Couto S, Breider M, et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood J Am Soc Hematol (2015) 126(6):779–89. doi: 10.1182/blood-2015-02-628669
39. Rasco DW, Papadopoulos KP, Pourdehnad M, Gandhi AK, Hagner PR, Li Y, et al. A first-in-human study of novel cereblon modulator avadomide (CC-122) in advanced malignancies. Clin Cancer Res (2019) 25(1):90–8. doi: 10.1158/1078-0432.CCR-18-1203
40. Piccolomo A, Schifone CP, Strafella V, Specchia G, Musto P, Albano F. Immunomodulatory drugs in acute myeloid leukemia treatment. Cancers (2020) 12(9):2528. doi: 10.3390/cancers12092528
41. Merrill JT, Werth VP, Furie R, van Vollenhoven R, Dörner T, Petronijevic M, et al. Phase 2 trial of iberdomide in systemic lupus erythematosus. New Engl J Med (2022) 386(11):1034–45. doi: 10.1056/NEJMoa2106535
42. Schafer PH, Ye Y, Wu L, Kosek J, Ringheim G, Yang Z, et al. Cereblon modulator iberdomide induces degradation of the transcription factors ikaros and aiolos: immunomodulation in healthy volunteers and relevance to systemic lupus erythematosus. Ann Rheum Diseases (2018) 77(10):1516–23. doi: 10.1136/annrheumdis-2017-212916
43. Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood J Am Soc Hematol (2002) 99(12):4525–30. doi: 10.1182/blood.V99.12.4525
44. Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai Y-T, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood J Am Soc Hematol (2000) 96(9):2943–50. doi: 10.1182/blood.V96.9.2943
45. Drucker L, Uziel O, Tohami T, Shapiro H, Radnay J, Yarkoni S, et al. Thalidomide down-regulates transcript levels of GC-rich promoter genes in multiple myeloma. Mol Pharmacol (2003) 64(2):415–20. doi: 10.1124/mol.64.2.415
46. Schlenzka J, Moehler TM, Kipriyanov SM, Kornacker M, Benner A, Bähre A, et al. Combined effect of recombinant CD19× CD16 diabody and thalidomide in a preclinical model of human b cell lymphoma. Anti Cancer Drugs (2004) 15(9):915–9. doi: 10.1097/00001813-200410000-00013
47. Skórka K, Bhattacharya N, Własiuk P, Kowal M, Mertens D, Dmoszyńska A. Thalidomide regulation of NF-κB proteins limits tregs activity in chronic lymphocytic leukemia. Adv Clin Exp Med (2014) 23(1):25–32. doi: 10.17219/acem/37018
48. Pointon JC, Eagle G, Bailey J, Evans P, Allsup D, Greenman J. Thalidomide enhances cyclophosphamide and dexamethasone-mediated cytotoxicity towards cultured chronic lymphocytic leukaemia cells. Oncol Rep (2010) 24(5):1315–21. doi: 10.3892/or_00000988
49. Salemi M, Mohammadi S, Ghavamzadeh A, Nikbakht M. Anti-vascular endothelial growth factor targeting by curcumin and thalidomide in acute myeloid leukemia cells. Asian Pacific J Cancer Prevention: APJCP (2017) 18(11):3055. doi: 10.22034/APJCP.2017.18.11.3055
50. Kian MM, Salemi M, Bahadoran M, Haghi A, Dashti N, Mohammadi S, et al. Curcumin combined with thalidomide reduces expression of STAT3 and bcl-xL, leading to apoptosis in acute myeloid leukemia cell lines. Drug Design Dev Ther (2020) 14:185. doi: 10.2147/DDDT.S228610
51. Noman ASM, Koide N, Khuda II-E, Dagvadorj J, Tumurkhuu G, Naiki Y, et al. Thalidomide inhibits epidermal growth factor-induced cell growth in mouse and human monocytic leukemia cells via ras inactivation. Biochem Biophys Res Commun (2008) 374(4):683–7. doi: 10.1016/j.bbrc.2008.07.090
52. Girgis E, Mahoney J, Darling-Reed S, Soliman M. Arsenic trioxide enhances the cytotoxic effect of thalidomide in a KG-1a human acute myelogenous leukemia cell line. Oncol Lett (2010) 1(3):473–9. doi: 10.3892/ol_00000083
53. Liu P, Li J, Lu H, Xu B. Thalidomide inhibits leukemia cell invasion and migration by upregulation of early growth response gene 1. Leukemia Lymphoma (2009) 50(1):109–13. doi: 10.1080/10428190802588352
54. Czyżewski K, Zaborowska A, Styczyński J. Thalidomide increases in vitro sensitivity of childhood acute lymphoblastic leukemia cells to prednisolone and cytarabine. Archivum Immunologiae Therapiae Experimentalis (2006) 54(5):341–5. doi: 10.1007/s00005-006-0036-9
55. Styczynski J, Czyzewski K, Wysocki M. Ex vivo activity of thalidomide in childhood acute leukemia. Leukemia Lymphoma (2006) 47(6):1123–8. doi: 10.1080/10428190500467891
56. Krönke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science (2014) 343(6168):301–5. doi: 10.1126/science.1244851
57. Luptakova K, Rosenblatt J, Glotzbecker B, Mills H, Stroopinsky D, Kufe T, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother (2013) 62(1):39–49. doi: 10.1007/s00262-012-1308-3
58. Breitkreutz I, Raab M, Vallet S, Hideshima T, Raje N, Mitsiades C, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia (2008) 22(10):1925–32. doi: 10.1038/leu.2008.174
59. Görgün G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple MyelomaLenalidomide in combination with checkpoint blockade in multiple myeloma. Clin Cancer Res (2015) 21(20):4607–18. doi: 10.1158/1078-0432.CCR-15-0200
60. Van Der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica (2011) 96(2):284–90. doi: 10.3324/haematol.2010.030759
61. Escoubet-Lozach L, Lin I-L, Jensen-Pergakes K, Brady HA, Gandhi AK, Schafer PH, et al. Pomalidomide and lenalidomide induce p21WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res (2009) 69(18):7347–56. doi: 10.1158/0008-5472.CAN-08-4898
62. Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res (2008) 14(14):4650–7. doi: 10.1158/1078-0432.CCR-07-4405
63. Zhang L, Qian Z, Cai Z, Sun L, Wang H, Bartlett JB, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol (2009) 84(9):553–9. doi: 10.1002/ajh.21468
64. Song K, Herzog BH, Sheng M, Fu J, McDaniel JM, Ruan J, et al. Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell LymphomaAnti-lymphangiogenesis by lenalidomide in MCL. Cancer Res (2013) 73(24):7254–64. doi: 10.1158/0008-5472.CAN-13-0750
65. Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, MacDougall F, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood J Am Soc Hematol (2009) 114(21):4713–20. doi: 10.1182/blood-2009-04-217687
66. Qian Z, Zhang L, Cai Z, Sun L, Wang H, Yi Q, et al. Lenalidomide synergizes with dexamethasone to induce growth arrest and apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia Res (2011) 35(3):380–6. doi: 10.1016/j.leukres.2010.09.027
67. Verhelle D, Corral LG, Wong K, Mueller JH, Moutouh-de Parseval L, Jensen-Pergakes K, et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant b cells while expanding normal CD34+ progenitor cells. Cancer Res (2007) 67(2):746–55. doi: 10.1158/0008-5472.CAN-06-2317
68. Sakamaki I, Kwak LW, Cha S, Yi Q, Lerman B, Chen J, et al. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. Leukemia (2014) 28(2):329–37. doi: 10.1038/leu.2013.177
69. Zhang L-H, Schafer PH, Muller G, Stirling D, Bartlett B. Direct inhibitory effects of lenalidomide on the proliferation and VEGF production of non-Hodgkin lymphoma cells are associated with increased SPARC expression. Am Soc Hematol (2008) 112(11):2612. doi: 10.1182/blood.V112.11.2612.2612
70. Gunnellini M, Falchi L. Therapeutic activity of lenalidomide in mantle cell lymphoma and indolent non-hodgkin’s lymphomas. Adv Hematol (2012) 2012:523842. doi: 10.1155/2012/523842.
71. Moros A, Bustany S, Cahu J, Saborit-Villarroya I, Martínez A, Colomer D, et al. Antitumoral activity of lenalidomide in In vitro and In vivo models of mantle cell lymphoma involves the destabilization of cyclin D1/p27KIP1 ComplexesLenalidomide targets cyclin D1/p27KIP1 in MCL. Clin Cancer Res (2014) 20(2):393–403. doi: 10.1158/1078-0432.CCR-13-1569
72. Fecteau J-F, Corral LG, Ghia EM, Gaidarova S, Futalan D, Bharati IS, et al. Lenalidomide inhibits the proliferation of CLL cells via a cereblon/p21WAF1/Cip1-dependent mechanism independent of functional p53. Blood J Am Soc Hematol (2014) 124(10):1637–44. doi: 10.1182/blood-2014-03-559591
73. Tettamanti S, Rotiroti MC, Giordano Attianese GMP, Arcangeli S, Zhang R, Banerjee P, et al. Lenalidomide enhances CD23.CAR T cell therapy in chronic lymphocytic leukemia. Leukemia Lymphoma (2022) 63(7):1566–79. doi: 10.1080/10428194.2022.2043299
74. Lapalombella R, Andritsos L, Liu Q, May SE, Browning R, Pham LV, et al. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal b cells through a PI3-kinase–dependent pathway. Blood J Am Soc Hematol (2010) 115(13):2619–29. doi: 10.1182/blood-2009-09-242438
75. Schulz A, Dürr C, Zenz T, Döhner H, Stilgenbauer S, Lichter P, et al. Lenalidomide reduces survival of chronic lymphocytic leukemia cells in primary cocultures by altering the myeloid microenvironment. Blood J Am Soc Hematol (2013) 121(13):2503–11. doi: 10.1182/blood-2012-08-447664
76. Fiorcari S, Martinelli S, Bulgarelli J, Audrito V, Zucchini P, Colaci E, et al. Lenalidomide interferes with tumor-promoting properties of nurse-like cells in chronic lymphocytic leukemia. Haematologica (2015) 100(2):253. doi: 10.3324/haematol.2014.113217
77. Lapalombella R, Yu B, Triantafillou G, Liu Q, Butchar JP, Lozanski G, et al. Lenalidomide down-regulates the CD20 antigen and antagonizes direct and antibody-dependent cellular cytotoxicity of rituximab on primary chronic lymphocytic leukemia cells. Blood J Am Soc Hematol (2008) 112(13):5180–9. doi: 10.1182/blood-2008-01-133108
78. He X, Dou A, Feng S, Roman-Rivera A, Hawkins C, Lawley L, et al. Cyclosporine enhances the sensitivity to lenalidomide in MDS/AML in vitro. Exp Hematol (2020) 86:21–7. e2. doi: 10.1016/j.exphem.2020.05.001
79. Hickey CJ, Schwind S, Radomska HS, Dorrance AM, Santhanam R, Mishra A, et al. Lenalidomide-mediated enhanced translation of C/EBPα-p30 protein up-regulates expression of the antileukemic microRNA-181a in acute myeloid leukemia. Blood J Am Soc Hematol (2013) 121(1):159–69. doi: 10.1182/blood-2012-05-428573
80. Krönke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, et al. Lenalidomide induces ubiquitination and degradation of CK1α in del (5q) MDS. Nature (2015) 523(7559):183–8. doi: 10.1038/nature14610
81. Stahl M, Zeidan AM. Lenalidomide use in myelodysplastic syndromes: Insights into the biologic mechanisms and clinical applications. Cancer (2017) 123(10):1703–13. doi: 10.1002/cncr.30585
82. Matsuoka A, Tochigi A, Kishimoto M, Nakahara T, Kondo T, Tsujioka T, et al. Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia (2010) 24(4):748–55. doi: 10.1038/leu.2009.296
83. Fink EC, Krönke J, Hurst SN, Udeshi ND, Svinkina T, Schneider RK, et al. Lenalidomide induces ubiquitination and degradation of CSNK1A1 in MDS with del (5q). Blood (2014) 124(21):4. doi: 10.1182/blood.V124.21.4.4
84. Yamamoto J, Suwa T, Murase Y, Tateno S, Mizutome H, Asatsuma-Okumura T, et al. ARID2 is a pomalidomide-dependent CRL4CRBN substrate in multiple myeloma cells. Nat Chem Biol (2020) 16(11):1208–17. doi: 10.1038/s41589-020-0645-3
85. Vo M-C, Yang S, Jung S-H, Chu T-H, Lee H-J, Lakshmi TJ, et al. Synergistic antimyeloma activity of dendritic cells and pomalidomide in a murine myeloma model. Front Immunol (2018) 9:1798. doi: 10.3389/fimmu.2018.01798
86. Rychak E, Mendy D, Shi T, Ning Y, Leisten J, Lu L, et al. Pomalidomide in combination with dexamethasone results in synergistic anti-tumour responses in pre-clinical models of lenalidomide-resistant multiple myeloma. Br J Haematol (2016) 172(6):889–901. doi: 10.1111/bjh.13905
87. Bolzoni M, Storti P, Bonomini S, Todoerti K, Guasco D, Toscani D, et al. Immunomodulatory drugs lenalidomide and pomalidomide inhibit multiple myeloma-induced osteoclast formation and the RANKL/OPG ratio in the myeloma microenvironment targeting the expression of adhesion molecules. Exp Hematol (2013) 41(4):387–97. e1. doi: 10.1016/j.exphem.2012.11.005
88. Gopalakrishnan R, Matta H, Tolani B, Triche T, Chaudhary PM. Immunomodulatory drugs target IKZF1-IRF4-MYC axis in primary effusion lymphoma in a cereblon-dependent manner and display synergistic cytotoxicity with BRD4 inhibitors. Oncogene (2016) 35(14):1797–810. doi: 10.1038/onc.2015.245
89. Le Roy A, Prebet T, Castellano R, Goubard A, Riccardi F, Fauriat C, et al. Immunomodulatory drugs exert anti-leukemia effects in acute myeloid leukemia by direct and immunostimulatory activities. Front Immunol (2018) 9:977. doi: 10.3389/fimmu.2018.00977
90. Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. New Engl J Med (1999) 341(21):1565–71. doi: 10.1056/NEJM199911183412102
91. Richardson P, Anderson K. Thalidomide and dexamethasone: a new standard of care for initial therapy in multiple myeloma. J Clin Oncol: Off J Am Soc Clin Oncol (2005) 24(3):334–6. doi: 10.1200/jco.2005.03.8851
92. Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, Van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. New Engl J Med (2006) 354(10):1021–30. doi: 10.1056/NEJMoa053583
93. Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern cooperative oncology group. J Clin Oncol (2006) 24(3):431–6. doi: 10.1200/JCO.2005.03.0221
94. Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 study. J Clin Oncol (2010) 28(19):3160–6. doi: 10.1200/JCO.2009.26.1610
95. Rosiñol L, Oriol A, Teruel A, de la Guía A, Blanchard M, de la Rubia J, et al. Bortezomib and thalidomide maintenance after stem cell transplantation for multiple myeloma: a PETHEMA/GEM trial. Leukemia (2017) 31(9):1922–7. doi: 10.1038/leu.2017.35
96. Smith SM, Grinblatt D, Johnson JL, Niedzwiecki D, Rizzieri D, Bartlett NL, et al. Thalidomide has limited single-agent activity in relapsed or refractory indolent non-Hodgkin lymphomas: a phase II trial of the cancer and leukemia group b. Br J Haematol (2008) 140(3):313–9. doi: 10.1111/j.1365-2141.2007.06937.x
97. Grinblatt DL, Johnson J, Niedzwicki D, Rizzieri DA, Bartlett N, Cheson BD. Phase II study of thalidomide in escalating doses for follicular (F-NHL) and small lymphocytic lymphoma (Sll): CALGB study 50002. Blood (2004) 104(11):3284. doi: 10.1182/blood.V104.11.3284.3284
98. Damaj G, Lefrere F, Delarue R, Varet B, Furman R, Hermine O. Thalidomide therapy induces response in relapsed mantle cell lymphoma. Leukemia (2003) 17(9):1914–5. doi: 10.1038/sj.leu.2403058
99. Drach J, Kaufmann H, Woehrer S, Chott A, Zielinski C, Raderer M. Durable remissions after rituximab plus thalidomide for relapsed/refractory mantle cell lymphoma. J Clin Oncol (2004) 22(14_suppl):6583–. doi: 10.1200/jco.2004.22.90140.6583
100. Ruan J, Martin P, Coleman M, Furman RR, Cheung K, Faye A, et al. Durable responses with the metronomic rituximab and thalidomide plus prednisone, etoposide, procarbazine, and cyclophosphamide regimen in elderly patients with recurrent mantle cell lymphoma. Cancer: Interdiscip Int J Am Cancer Society (2010) 116(11):2655–64. doi: 10.1002/cncr.25055
101. Kuruvilla J, Song K, Mollee P, Panzarella T, McCrae J, Nagy T, et al. A phase II study of thalidomide and vinblastine for palliative patients with hodgkin’s lymphoma. Hematology (2006) 11(1):25–9. doi: 10.1080/10245330500276592
102. García-Sanz R, González-López T, Vázquez L, Hermida G, Graciani I, San Miguel J. The combination of thalidomide, cyclophosphamide and dexamethasone is potentially useful in highly resistant hodgkin’s lymphoma. Eur J Haematol (2010) 84(3):266–70. doi: 10.1111/j.1600-0609.2009.01375.x
103. Wu H, Zhao C, Gu K, Jiao Y, Hao J, Sun G. Thalidomide plus chemotherapy exhibit enhanced efficacy in the clinical treatment of T-cell non−Hodgkin’s lymphoma: A prospective study of 46 cases. Mol Clin Oncol (2014) 2(5):695–700. doi: 10.3892/mco.2014.307
104. Oxberry S, Johnson M. Response to thalidomide in chemotherapy-resistant cutaneous T-cell lymphoma. Clin Oncol (2006) 18(1):86–7. doi: 10.1016/j.clon.2005.08.006
105. Giannopoulos K, Dmoszynska A, Kowal M, Wąsik-Szczepanek E, Bojarska-Junak A, Rolinski J, et al. Thalidomide exerts distinct molecular antileukemic effects and combined thalidomide/fludarabine therapy is clinically effective in high-risk chronic lymphocytic leukemia. Leukemia (2009) 23(10):1771–8. doi: 10.1038/leu.2009.98
106. Chanan-Khan A, Miller KC, Takeshita K, Koryzna A, Donohue K, Bernstein ZP, et al. Results of a phase 1 clinical trial of thalidomide in combination with fludarabine as initial therapy for patients with treatment-requiring chronic lymphocytic leukemia (CLL). Blood (2005) 106(10):3348–52. doi: 10.1182/blood-2005-02-0669
107. Giannopoulos K, Mertens D, Stilgenbauer S. Treating chronic lymphocytic leukemia with thalidomide and lenalidomide. Expert Opin Pharmacother (2011) 12(18):2857–64. doi: 10.1517/14656566.2011.635644
108. Furman R, Leonard J, Allen S, Coleman M, Rosenthal T, Gabrilove J. Thalidomide alone or in combination with fludarbabine are effective treatments for patients with fludarabine-relapsed and refractory CLL. J Clin Oncol (2005) 23(16_suppl):6640–. doi: 10.1200/jco.2005.23.16_suppl.6640
109. Tueger S, Chen F, Ahsan G, McDonald V, Andrews V, Madrigal J, et al. Thalidomide induced remission of refractory diffuse large b-cell lymphoma post-allogeneic SCT. Haematologica (2006) 91(6_Suppl):ECR16–ECR. doi: 10.3324/%25x
110. Kiesewetter B, Raderer M. Immunomodulatory treatment for mucosa-associated lymphoid tissue lymphoma (MALT lymphoma). Hematol Oncol (2020) 38(4):417–24. doi: 10.1002/hon.2754
111. Raza A, Mehdi M, Mumtaz M, Ali F, Lascher S, Galili N. Combination of 5-azacytidine and thalidomide for the treatment of myelodysplastic syndromes and acute myeloid leukemia. Cancer: Interdiscip Int J Am Cancer Society (2008) 113(7):1596–604. doi: 10.1002/cncr.23789
112. Steins MB, Padró T, Bieker R, Ruiz S, Kropff M, Kienast J, et al. Efficacy and safety of thalidomide in patients with acute myeloid leukemia. Blood J Am Soc Hematol (2002) 99(3):834–9. doi: 10.1182/blood.V99.3.834
113. Thomas DA, Estey E, Giles FJ, Faderl S, Cortes J, Keating M, et al. Single agent thalidomide in patients with relapsed or refractory acute myeloid leukaemia. Br J Haematol (2003) 123(3):436–41. doi: 10.1046/j.1365-2141.2003.04639.x
114. Strupp C, Germing U, Aivado M, Misgeld E, Haas R, Gattermann N. Thalidomide for the treatment of patients with myelodysplastic syndromes. Leukemia (2002) 16(1):1–6. doi: 10.1038/sj.leu.2402330
115. Raza A, Meyer P, Dutt D, Zorat F, Lisak L, Nascimben F, et al. Thalidomide produces transfusion independence in long-standing refractory anemias of patients with myelodysplastic syndromes. Blood J Am Soc Hematol (2001) 98(4):958–65. doi: 10.1182/blood.V98.4.958
116. Chung C-Y, Lin S-F, Chen P-M, Chang M-C, Kao W-Y, Chao T-Y, et al. Thalidomide for the treatment of myelodysplastic syndrome in Taiwan: results of a phase II trial. Anticancer Res (2012) 32(8):3415–9.
117. Leitch HA, Buckstein R, Shamy A, Storring JM. The immunomodulatory agents lenalidomide and thalidomide for treatment of the myelodysplastic syndromes: a clinical practice guideline. Crit Rev Oncology/hematology. (2013) 85(2):162–92. doi: 10.1016/j.critrevonc.2012.07.003
118. Kenealy M, Patton N, Filshie R, Nicol A, Ho S-J, Hertzberg M, et al. Results of a phase II study of thalidomide and azacitidine in patients with clinically advanced myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML) and low blast count acute myeloid leukemia (AML). Leukemia Lymphoma (2017) 58(2):298–307. doi: 10.1080/10428194.2016.1190971
119. Monroy RH, Vargas-Viveros P, Ceballos EC, Velazquez JC, Munos SC. Imatinib (IM) plus thalidomide (Thali), a effective combination for the treatment of chronic myeloid leukemia (CML) Philadelphia chromosomepositive (Ph+) in IM-resistant disease. Report of 14 new cases from a single center in Mexico. Blood (2013) 122(21):5172. doi: 10.1182/blood.V122.21.5172.5172
120. Facon T, Cook G, Usmani SZ, Hulin C, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia (2022) 36(4):1066–77. doi: 10.1038/s41375-021-01488-8
121. Holstein SA, Suman VJ, McCarthy PL. Update on the role of lenalidomide in patients with multiple myeloma. Ther Adv Hematol (2018) 9(7):175–90. doi: 10.1177/2040620718775629
122. Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. New Engl J Med (2012) 366(19):1782–91. doi: 10.1056/NEJMoa1114138
123. Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. New Engl J Med (2012) 366(19):1759–69. doi: 10.1056/NEJMoa1112704
124. McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. New Engl J Med (2012) 366(19):1770–81. doi: 10.1056/NEJMoa1114083
125. Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in north America. New Engl J Med (2007) 357(21):2133–42. doi: 10.1056/NEJMoa070596
126. List A, Bennett J, Sekeres M, Skikne B, Fu T, Shammo J, et al. Extended survival and reduced risk of AML progression in erythroid-responsive lenalidomide-treated patients with lower-risk del (5q) MDS. Leukemia (2014) 28(5):1033–40. doi: 10.1038/leu.2013.305
127. Ossenkoppele G, Breems D, Stuessi G, van Norden Y, Bargetzi M, Biemond B, et al. Lenalidomide added to standard intensive treatment for older patients with AML and high-risk MDS. Leukemia (2020) 34(7):1751–9. doi: 10.1038/s41375-020-0725-0
128. Witzig TE, Nowakowski G, Habermann T, Goy A, Hernandez-Ilizaliturri F, Chiappella A, et al. A comprehensive review of lenalidomide therapy for b-cell non-Hodgkin lymphoma. Ann Oncol (2015) 26(8):1667–77. doi: 10.1093/annonc/mdv102
129. Ruan J, Martin P, Christos P, Cerchietti L, Tam W, Shah B, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood J Am Soc Hematol (2018) 132(19):2016–25. doi: 10.1182/blood-2018-07-859769
130. Wang M, Fayad L, Wagner-Bartak N, Zhang L, Hagemeister F, Neelapu SS, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol (2012) 13(7):716–23. doi: 10.1016/S1470-2045(12)70200-0
131. Goy A, Sinha R, Williams ME, Besisik SK, Drach J, Ramchandren R, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol (2013) 31(29):3688. doi: 10.1200/JCO.2013.49.2835
132. Yamshon S, Martin P, Shah B, Schuster SJ, Christos PJ, Rodriguez A, et al. Initial treatment with lenalidomide plus rituximab for mantle cell lymphoma (MCL): 7-year analysis from a multi-center phase II study. Blood (2020) 136:45–6. doi: 10.1182/blood-2020-138731
133. Morschhauser F, Le Gouill S, Feugier P, Bailly S, Nicolas-Virelizier E, Bijou F, et al. Obinutuzumab combined with lenalidomide for relapsed or refractory follicular b-cell lymphoma (GALEN): a multicentre, single-arm, phase 2 study. Lancet Haematol (2019) 6(8):e429–e37. doi: 10.1016/S2352-3026(19)30089-4
134. Flowers CR, Leonard JP, Fowler NH. Lenalidomide in follicular lymphoma. Blood (2020) 135(24):2133–6. doi: 10.1182/blood.2019001751
135. Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-hodgkin’s lymphoma. J Clin Oncol (2009) 27(32):5404–9. doi: 10.1200/JCO.2008.21.1169
136. Ferrajoli A, Lee B-N, Schlette EJ, O’Brien SM, Gao H, Wen S, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood J Am Soc Hematol (2008) 111(11):5291–7. doi: 10.1182/blood-2007-12-130120
137. Badoux XC, Keating MJ, Wen S, Lee B-N, Sivina M, Reuben J, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood J Am Soc Hematol (2011) 118(13):3489–98. doi: 10.1182/blood-2011-03-339077
138. Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol (2011) 29(9):1175. doi: 10.1200/JCO.2010.29.8133
139. Shanafelt TD, Ramsay AG, Zent CS, Leis JF, Tun HW, Call TG, et al. Long-term repair of T-cell synapse activity in a phase II trial of chemoimmunotherapy followed by lenalidomide consolidation in previously untreated chronic lymphocytic leukemia (CLL). Blood J Am Soc Hematol (2013) 121(20):4137–41. doi: 10.1182/blood-2012-12-470005
140. Itchaki G, Brown JR. Lenalidomide in the treatment of chronic lymphocytic leukemia. Expert Opin Investigational Drugs (2017) 26(5):633–50. doi: 10.1080/13543784.2017.1313230
141. Strati P, Keating MJ, Wierda WG, Badoux XC, Calin S, Reuben JM, et al. Lenalidomide induces long-lasting responses in elderly patients with chronic lymphocytic leukemia. Blood J Am Soc Hematol (2013) 122(5):734–7. doi: 10.1182/blood-2013-04-495341
142. Zinzani PL, Rodgers T, Marino D, Frezzato M, Barbui AM, Castellino C, et al. RE-MIND: comparing tafasitamab+ lenalidomide (L-MIND) with a real-world lenalidomide monotherapy cohort in relapsed or refractory diffuse large b-cell lymphoma. Clin Cancer Res (2021) 27(22):6124–34. doi: 10.1158/1078-0432.CCR-21-1471
143. Thieblemont C, Delfau-Larue M-H, Coiffier B. Lenalidomide in diffuse large b-cell lymphoma. Adv Hematol (2012) 2012:861060. doi: 10.1155/2012/861060
144. Kiesewetter B, Troch M, Dolak W, Müllauer L, Lukas J, Zielinski CC, et al. A phase II study of lenalidomide in patients with extranodal marginal zone b-cell lymphoma of the mucosa associated lymphoid tissue (MALT lymphoma). Haematologica (2013) 98(3):353. doi: 10.3324/haematol.2012.065995
145. Houillier C, Choquet S, Touitou V, Martin-Duverneuil N, Navarro S, Mokhtari K, et al. Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology (2015) 84(3):325–6. doi: 10.1212/WNL.0000000000001158
146. Ghesquieres H, Chevrier M, Laadhari M, Chinot O, Choquet S, Molucon-Chabrot C, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective ‘proof of concept’phase II study of the French oculo-cerebral lymphoma (LOC) network and the lymphoma study association (LYSA). Ann Oncol (2019) 30(4):621–8. doi: 10.1093/annonc/mdz032
147. Hopfinger G, Nösslinger T, Lang A, Linkesch W, Melchardt T, Weiss L, et al. Lenalidomide in combination with vorinostat and dexamethasone for the treatment of relapsed/refractory peripheral T cell lymphoma (PTCL): report of a phase I/II trial. Ann Hematol (2014) 93(3):459–62. doi: 10.1007/s00277-014-2009-0
148. Dueck G, Chua N, Prasad A, Stewart D, White D, Vanderjagt R, et al. Activity of lenalidomide in a phase II trial for T-cell lymphoma: Report on the first 24 cases. J Clin Oncol (2009) 27(15_suppl):8524–. doi: 10.1200/jco.2009.27.15_suppl.8524
149. Sakamoto H, Itonaga H, Sawayama Y, Furumoto T, Fujioka M, Chiwata M, et al. Treatment with mogamulizumab or lenalidomide for relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: the Nagasaki transplant group experience. Hematol Oncol (2020) 38(2):162–70. doi: 10.1002/hon.2712
150. Ruan J, Zain JM, Palmer B, Jovanovic B, Mi X, Swaroop A, et al. Multicenter phase II study of romidepsin plus lenalidomide for patients with previously untreated peripheral T-cell lymphoma (PTCL). Wolters Kluwer Health (2021) 39(S2):98–9. doi: 10.1002/hon.55_2879
151. Fehniger TA, Byrd JC, Marcucci G, Abboud CN, Kefauver C, Payton JE, et al. Single-agent lenalidomide induces complete remission of acute myeloid leukemia in patients with isolated trisomy 13. Blood J Am Soc Hematol (2009) 113(5):1002–5. doi: 10.1182/blood-2008-04-152678
152. Pollyea D, Kohrt H, Gallegos L, Figueroa M, Abdel-Wahab O, Zhang B, et al. Safety, efficacy and biological predictors of response to sequential azacitidine and lenalidomide for elderly patients with acute myeloid leukemia. Leukemia (2012) 26(5):893–901. doi: 10.1038/leu.2011.294
153. Govindaraj C, Madondo M, Kong YY, Tan P, Wei A, Plebanski M. Lenalidomide-based maintenance therapy reduces TNF receptor 2 on CD4 T cells and enhances immune effector function in acute myeloid leukemia patients. Am J Hematol (2014) 89(8):795–802. doi: 10.1002/ajh.23746
154. Pollyea DA, Zehnder J, Coutre S, Gotlib JR, Gallegos L, Abdel-Wahab O, et al. Sequential azacitidine plus lenalidomide combination for elderly patients with untreated acute myeloid leukemia. Haematologica (2013) 98(4):591. doi: 10.3324/haematol.2012.076414
155. Fehniger TA, Uy GL, Trinkaus K, Nelson AD, Demland J, Abboud CN, et al. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood J Am Soc Hematol (2011) 117(6):1828–33. doi: 10.1182/blood-2010-07-297143
156. Buckstein R, Kerbel R, Cheung M, Shaked Y, Chodirker L, Lee CR, et al. Lenalidomide and metronomic melphalan for CMML and higher risk MDS: A phase 2 clinical study with biomarkers of angiogenesis. Leukemia Res (2014) 38(7):756–63. doi: 10.1016/j.leukres.2014.03.022
157. Sekeres MA, Othus M, List AF, Odenike O, Stone RM, Gore SD, et al. Randomized phase II study of azacitidine alone or in combination with lenalidomide or with vorinostat in higher-risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American intergroup study SWOG S1117. J Clin Oncol (2017) 35(24):2745. doi: 10.1200/JCO.2015.66.2510
158. Kunacheewa C, Thongthang P, Ungprasert P, Utchariyaprasit E, Owattanapanich W. A systematic review and meta-analysis of the efficacy and adverse events of azacitidine-plus-lenalidomide treatment for patients with acute myeloid leukemia, myelodysplastic syndromes and chronic myelomonocytic leukemia. Hematology (2019) 24(1):498–506. doi: 10.1080/16078454.2019.1631425
159. Burgstaller S, Stauder R, Kuehr T, Lang A, Machherndl-Spandl S, Mayrbaeurl B, et al. A phase I study of lenalidomide in patients with chronic myelomonocytic leukemia (CMML)–AGMT_CMML-1. Leukemia Lymphoma (2018) 59(5):1121–6. doi: 10.1080/10428194.2017.1369070
160. Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood J Am Soc Hematol (2014) 123(12):1826–32. doi: 10.1182/blood-2013-11-538835
161. San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol (2013) 14(11):1055–66. doi: 10.1016/S1470-2045(13)70380-2
162. Chanan-Khan A, Swaika A, Paulus A, Kumar S, Mikhael J, Rajkumar S, et al. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J (2013) 3(9):e143–e. doi: 10.1038/bcj.2013.38
163. Offidani M, Corvatta L, Caraffa P, Leoni P, Pautasso C, Larocca A, et al. Pomalidomide for the treatment of relapsed–refractory multiple myeloma: a review of biological and clinical data. Expert Rev Anticancer Ther (2014) 14(5):499–510. doi: 10.1586/14737140.2014.906904
164. Mato AR, Schuster SJ, Foss FM, Isufi I, Ding W, Brander DM, et al. A phase Ia/Ib study exploring the synthetic lethality of the orally administered novel BTK inhibitor, dtrmwxhs-12 (DTRM-12), in combination with everolimus and pomalidomide in patients with relapsed/refractory CLL, DLBCL or other b-cell lymphomas. Blood (2019) 134:810. doi: 10.1182/blood-2019-126192
165. Begna K, Mesa R, Pardanani A, Hogan W, Litzow M, McClure R, et al. A phase-2 trial of low-dose pomalidomide in myelofibrosis. Leukemia (2011) 25(2):301–4. doi: 10.1038/leu.2010.254
166. Gowin KL, Mesa RA. Profile of pomalidomide and its potential in the treatment of myelofibrosis. Ther Clin Risk Management (2015) 11:549. doi: 10.2147/TCRM.S69211
167. Zeidner JF, Knaus HA, Zeidan AM, Blackford AL, Montiel-Esparza R, Hackl H, et al. Immunomodulation with pomalidomide at early lymphocyte recovery after induction chemotherapy in newly diagnosed AML and high-risk MDS. Leukemia (2020) 34(6):1563–76. doi: 10.1038/s41375-019-0693-4
168. Montiel-Esparza R, Knaus HA, Zeidner JF, Vulic A, Prince GT, Smith BD, et al. Immune modulation with pomalidomide after induction chemotherapy in newly diagnosed acute myeloid leukemia (AML). Blood (2015) 126(23):1351. doi: 10.1182/blood.V126.23.1351.1351
169. Grogan DP, Winston NR. Thalidomide. In: StatPearls. StatPearls Publishing (2022). Treasure Island (FL)
170. Lacy MQ, McCurdy AR. Pomalidomide. Blood (2013) 122(14):2305–9. doi: 10.1182/blood-2013-05-484782
171. Sperling AS, Guerra VA, Kennedy JA, Yan Y, Hsu JI, Wang F, et al. Lenalidomide promotes the development of TP53-mutated therapy-related myeloid neoplasms. Blood (2022) blood.2021014956. Online ahead of print. doi: 10.1182/blood.2021014956
172. Khan SR, Tariq M, Fayyaz SM, Soomar SM, Moosajee M. Lenalidomide induced secondary acute lymphoblastic leukemia in a multiple myeloma patient: A case-report. Leukemia Res Rep (2022) 17:100315. doi: 10.1016/j.lrr.2022.100315
173. Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. science (2010) 327(5971):1345–50. doi: 10.1126/science.1177319
174. Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi A, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia (2012) 26(11):2326–35. doi: 10.1038/leu.2012.119
175. Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors I karos and a iolos via modulation of the e 3 ubiquitin ligase complex CRL 4 CRBN. Br J Haematol (2014) 164(6):811–21. doi: 10.1111/bjh.12708
176. Zhu YX, Braggio E, Shi C-X, Bruins LA, Schmidt JE, Van Wier S, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood J Am Soc Hematol (2011) 118(18):4771–9. doi: 10.1182/blood-2011-05-356063
177. Petroski MD, Deshaies RJ. Function and regulation of cullin–RING ubiquitin ligases. Nat Rev Mol Cell Biol (2005) 6(1):9–20. doi: 10.1038/nrm1547
178. Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1–CUL4A ubiquitin ligase machinery. Nature (2006) 443(7111):590–3. doi: 10.1038/nature05175
179. Chamberlain PP, Cathers BE. Cereblon modulators: Low molecular weight inducers of protein degradation. Drug Discovery Today: Technologies (2019) 31:29–34. doi: 10.1016/j.ddtec.2019.02.004
180. Collins I, Wang H, Caldwell JJ, Chopra R. Chemical approaches to targeted protein degradation through modulation of the ubiquitin-proteasome pathway. Biochem J (2017) 474(7):1127–47. doi: 10.1042/BCJ20160762
181. Fischer ES, Böhm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature (2014) 512(7512):49–53. doi: 10.1038/nature13527
182. Krönke J, Hurst SN, Ebert BL. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology (2014) 3(7):e941742. doi: 10.4161/21624011.2014.941742
183. Krönke J, Udeshi N, Narla A, Grauman P, Hurst S, McConkey M, et al. Lenalidomide promotes CRBN-mediated ubiquitination and degradation of IKZF1 and IKZF3. Blood (2013) 122(21):LBA–5. doi: 10.1182/blood.V122.21.LBA-5.LBA-5
184. Ramsay AG, Johnson AJ, Lee AM, Gorgün G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest (2008) 118(7):2427–37. doi: 10.1172/JCI35017
185. Muller GW, Corral LG, Shire MG, Wang H, Moreira A, Kaplan G, et al. Structural modifications of thalidomide produce analogs with enhanced tumor necrosis factor inhibitory activity. J Medicinal Chem (1996) 39(17):3238–40. doi: 10.1021/jm9603328
186. Petzold G, Fischer ES, Thomä NH. Structural basis of lenalidomide-induced CK1α degradation by the CRL4CRBN ubiquitin ligase. Nature (2016) 532(7597):127–30. doi: 10.1038/nature16979
187. Schneider RK, Ademà V, Heckl D, Järås M, Mallo M, Lord AM, et al. Role of casein kinase 1A1 in the biology and targeted therapy of del (5q) MDS. Cancer Cell (2014) 26(4):509–20. doi: 10.1016/j.ccr.2014.08.001
188. Fuchs O. Role of immunomodulatory drugs in the treatment of lymphoid and myeloid malignancies. Front Clin Drug Res Hematol (2018) 3:73. doi: 10.2174/9781681083674118030005
189. Bjorklund C, Lu L, Kang J, Hagner P, Havens C, Amatangelo M, et al. Rate of CRL4CRBN substrate ikaros and aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-myc and IRF4. Blood Cancer J (2015) 5(10):e354–e. doi: 10.1038/bcj.2015.66
190. Gandhi AK, Kang J, Naziruddin S, Parton A, Schafer PH, Stirling DI. Lenalidomide inhibits proliferation of namalwa CSN.70 cells and interferes with Gab1 phosphorylation and adaptor protein complex assembly. Leukemia Res (2006) 30(7):849–58. doi: 10.1016/j.leukres.2006.01.010
191. Zhang LH, Kosek J, Wang M, Heise C, Schafer PH, Chopra R. Lenalidomide efficacy in activated b-cell-like subtype diffuse large b-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol (2013) 160(4):487–502. doi: 10.1111/bjh.12172
192. D’Souza C, Prince HM, Neeson PJ. Understanding the role of T-cells in the antimyeloma effect of immunomodulatory drugs. Front Immunol (2021) 12:632399. doi: 10.3389/fimmu.2021.632399
193. Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med (2004) 351(21):2159–69. doi: 10.1056/NEJMoa041869
194. Küppers R. Mechanisms of b-cell lymphoma pathogenesis. Nat Rev Cancer (2005) 5(4):251–62. doi: 10.1038/nrc1589
195. Dogan A, Du M-Q, Aiello A, Diss TC, Ye H-T, Pan L-X, et al. Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic b-cell population in the interfollicular zone. Blood J Am Soc Hematol (1998) 91(12):4708–14. doi: 10.1182/blood.V91.12.4708
196. Zucchetto A, Tripodo C, Benedetti D, Deaglio S, Gaidano G, Del Poeta G, et al. Monocytes/macrophages but not T lymphocytes are the major targets of the CCL3/CCL4 chemokines produced by CD38(+)CD49d(+) chronic lymphocytic leukaemia cells. Br J Haematol (2010) 150(1):111–3. doi: 10.1111/j.1365-2141.2010.08152.x
197. Burger JA, Quiroga MP, Hartmann E, Bürkle A, Wierda WG, Keating MJ, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia b cells in nurselike cell cocultures and after BCR stimulation. Blood (2009) 113(13):3050–8. doi: 10.1182/blood-2008-07-170415
198. Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood J Am Soc Hematol (2012) 120(7):1412–21. doi: 10.1182/blood-2012-02-411678
199. Scott DW, Gascoyne RD. The tumour microenvironment in b cell lymphomas. Nat Rev Cancer (2014) 14(8):517–34. doi: 10.1038/nrc3774
200. Upadhyay R, Hammerich L, Peng P, Brown B, Merad M, Brody JD. Lymphoma: immune evasion strategies. Cancers (2015) 7(2):736–62. doi: 10.3390/cancers7020736
201. Aue G, Sun C, Liu D, Park J-H, Pittaluga S, Tian X, et al. Activation of Th1 immunity within the tumor microenvironment is associated with clinical response to lenalidomide in chronic lymphocytic leukemia. J Immunol (2018) 201(7):1967–74. doi: 10.4049/jimmunol.1800570
202. Ménard C, Rossille D, Dulong J, Nguyen T-T, Papa I, Latour M, et al. Lenalidomide triggers T-cell effector functions in vivo in patients with follicular lymphoma. Blood Advances (2021) 5(8):2063–74. doi: 10.1182/bloodadvances.2020003774
203. Danhof S, Schreder M, Knop S, Rasche L, Strifler S, Löffler C, et al. Expression of programmed death-1 on lymphocytes in myeloma patients is lowered during lenalidomide maintenance. Haematologica (2018) 103(3):e126. doi: 10.3324/haematol.2017.178947
204. Minnema M, van der Veer M, Aarts T, Emmelot M, Mutis T, Lokhorst H. Lenalidomide alone or in combination with dexamethasone is highly effective in patients with relapsed multiple myeloma following allogeneic stem cell transplantation and increases the frequency of CD4+ Foxp3+ T cells. Leukemia (2009) 23(3):605–7. doi: 10.1038/leu.2008.247
205. Nguyen-Pham T-N, Jung S-H, Vo M-C, Thanh-Tran H-T, Lee Y-K, Lee H-J, et al. Lenalidomide synergistically enhances the effect of dendritic cell vaccination in a model of murine multiple myeloma. J Immunother (2015) 38(8):330–9. doi: 10.1097/CJI.0000000000000097
206. Geng CL, Chen JY, Song TY, Jung JH, Long M, Song MF, et al. Lenalidomide bypasses CD28 co-stimulation to reinstate PD-1 immunotherapy by activating notch signaling. Cell Chem Biol (2022) 29(8):1260–1272.e8. doi: 10.1016/j.chembiol.2022.05.012
207. Otáhal P, Průková D, Král V, Fabry M, Vočková P, Latečková L, et al. Lenalidomide enhances antitumor functions of chimeric antigen receptor modified T cells. Oncoimmunology (2016) 5(4):e1115940. doi: 10.1080/2162402X.2015.1115940
208. Wang X, Walter M, Urak R, Weng L, Huynh C, Lim L, et al. Lenalidomide enhances the function of CS1 chimeric antigen receptor-redirected T cells against multiple myeloma. Clin Cancer Research: an Off J Am Assoc Cancer Res (2018) 24(1):106–19. doi: 10.1158/1078-0432.CCR-17-0344
209. Poh C, Frankel P, Ruel C, Abedi M, Schwab E, Costello CL, et al. Blinatumomab/Lenalidomide in Relapsed/Refractory non-hodgkin’s lymphoma: A phase I California cancer consortium study of safety, efficacy and immune correlative analysis. Blood (2019) 134(Supplement_1):760–. doi: 10.1182/blood-2019-124254
210. Robinson HR, Qi J, Cook EM, Nichols C, Dadashian EL, Underbayev C, et al. A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood (2018) 132(5):521–32. doi: 10.1182/blood-2018-02-830992
211. Martens AWJ, Janssen SR, Derks IAM, Adams Iii HC, Izhak L, van Kampen R, et al. CD3xCD19 DART molecule treatment induces non-apoptotic killing and is efficient against high-risk chemotherapy and venetoclax-resistant chronic lymphocytic leukemia cells. J Immunother Cancer (2020) 8(1):e000218. doi: 10.1136/jitc-2019-000218
212. Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol (2005) 128(2):192–203. doi: 10.1111/j.1365-2141.2004.05286.x
213. Boxhammer R, Steidl S, Endell J. Effect of IMiD compounds on CD38 expression on multiple myeloma cells: MOR202, a human CD38 antibody in combination with pomalidomide. Am Soc Clin Oncol (2015) 33(15_suppl):8588. doi: 10.1200/jco.2015.33.15_suppl.8588
214. Offidani M, Corvatta L, Morè S, Nappi D, Martinelli G, Olivieri A, et al. Daratumumab for the management of newly diagnosed and relapsed/refractory multiple myeloma: current and emerging treatments. Front Oncol (2021) 10:624661. doi: 10.3389/fonc.2020.624661
215. Kaufman JL, Usmani SZ, San-Miguel J, Bahlis N, White DJ, Benboubker L, et al. Four-year follow-up of the phase 3 pollux study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in relapsed or refractory multiple myeloma (RRMM). Blood (2019) 134:1866. doi: 10.1182/blood-2019-123483
216. Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood (2006) 108(2):618–21. doi: 10.1182/blood-2005-10-4184
217. Lagrue K, Carisey A, Morgan DJ, Chopra R, Davis DM. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood J Am Soc Hematol (2015) 126(1):50–60. doi: 10.1182/blood-2015-01-625004
218. Hideshima T, Ogiya D, Liu J, Harada T, Kurata K, Bae J, et al. Immunomodulatory drugs activate NK cells via both zap-70 and cereblon-dependent pathways. Leukemia (2021) 35(1):177–88. doi: 10.1038/s41375-020-0809-x
219. Roider T, Brinkmann BJ, Kim V, Knoll M, Kolb C, Roessner PM, et al. An autologous culture model of nodal b-cell lymphoma identifies ex vivo determinants of response to bispecific antibodies. Blood Advances (2021) 5(23):5060–71. doi: 10.1182/bloodadvances.2021005400
220. Wang Z, Yin C, Lum LG, Simons A, Weiner GJ. Bispecific antibody-activated T cells enhance NK cell-mediated antibody-dependent cellular cytotoxicity. J Hematol Oncol (2021) 14(1):1–4. doi: 10.1186/s13045-021-01216-w
221. Acebes-Huerta A, Huergo-Zapico L, Gonzalez-Rodriguez AP, Fernandez-Guizan A, Payer AR, López-Soto A, et al. Lenalidomide induces immunomodulation in chronic lymphocytic leukemia and enhances antitumor immune responses mediated by NK and CD4 T cells. BioMed Res Int (2014) 2014:265840. doi: 10.1155/2014/265840
222. Chiu H, Trisal P, Bjorklund C, Carrancio S, Toraño EG, Guarinos C, et al. Combination lenalidomide-rituximab immunotherapy activates anti-tumour immunity and induces tumour cell death by complementary mechanisms of action in follicular lymphoma. Br J Haematol (2019) 185(2):240–53. doi: 10.1111/bjh.15797
223. Mougiakakos D, Bach C, Böttcher M, Beier F, Röhner L, Stoll A, et al. The IKZF1-IRF4/IRF5 axis controls polarization of myeloma-associated macrophages. Cancer Immunol Res (2021) 9(3):265–78. doi: 10.1158/2326-6066.CIR-20-0555
224. Cao X, Chen J, Li B, Dang J, Zhang W, Zhong X, et al. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy. Sci Adv (2022) 8(11):eabl9171. doi: 10.1126/sciadv.abl9171
225. Busch L, Mougiakakos D, Büttner-Herold M, Müller MJ, Volmer DA, Bach C, et al. Lenalidomide enhances MOR202-dependent macrophage-mediated effector functions via the vitamin d pathway. Leukemia (2018) 32(11):2445–58. doi: 10.1038/s41375-018-0114-0
226. Elias S, Kahlon S, Kotzur R, Kaynan N, Mandelboim O. Obinutuzumab activates FcγRI more potently than other anti-CD20 antibodies in chronic lymphocytic leukemia (CLL). Oncoimmunology (2018) 7(6):e1428158. doi: 10.1080/2162402X.2018.1428158
227. Chen S, Lai SWT, Brown CE, Feng M. Harnessing and enhancing macrophage phagocytosis for cancer therapy. Front Immunol (2021) 12:635173. doi: 10.3389/fimmu.2021.635173
228. Weiskopf K, Weissman IL. Macrophages are critical effectors of antibody therapies for cancer. MAbs (2015) 7(2):303–10. doi: 10.1080/19420862.2015.1011450
229. Henry JY, Labarthe MC, Meyer B, Dasgupta P, Dalgleish AG, Galustian C. Enhanced cross-priming of naive CD8+ T cells by dendritic cells treated by the IMiDs® immunomodulatory compounds lenalidomide and pomalidomide. Immunology (2013) 139(3):377–85. doi: 10.1111/imm.12087
230. Palma M, Hansson L, Mulder TA, Adamson L, Näsman-Glaser B, Eriksson I, et al. Lenalidomide as immune adjuvant to a dendritic cell vaccine in chronic lymphocytic leukemia patients. Eur J Haematol (2018) 101(1):68–77. doi: 10.1111/ejh.13065
231. Lapenta C, Donati S, Spadaro F, Lattanzi L, Urbani F, Macchia I, et al. Lenalidomide improves the therapeutic effect of an interferon-α-dendritic cell-based lymphoma vaccine. Cancer Immunol Immunother (2019) 68(11):1791–804. doi: 10.1007/s00262-019-02411-y
232. Vo M-C, Jung S-H, Chu T-H, Lee H-J, Lakshmi TJ, Park H-S, et al. Lenalidomide and programmed death-1 blockade synergistically enhances the effects of dendritic cell vaccination in a model of murine myeloma. Front Immunol (2018) 9:1370. doi: 10.3389/fimmu.2018.01370
233. Luptakova K, Glotzbecker B, Mills H, Stroopinsky D, Vasir B, Rosenblatt J, et al. Lenalidomide decreases PD-1 expression, depletes regulatory T-cells and improves cellular response to a multiple myeloma/dendritic cell fusion vaccine in vitro. Blood (2010) 116(21):492. doi: 10.1182/blood.V116.21.492.492
234. Giuliani M, Janji B, Berchem G. Activation of NK cells and disruption of PD-L1/PD-1 axis: two different ways for lenalidomide to block myeloma progression. Oncotarget (2017) 8(14):24031–44. doi: 10.18632/oncotarget.15234
235. Crespo J, Sun H, Welling TH, Tian Z, Zou W. T Cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol (2013) 25(2):214–21. doi: 10.1016/j.coi.2012.12.003
236. Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8+ T cell fates in the antitumor immune response. J Immunol Res (2016) 2016:8941260. doi: 10.1155/2016/8941260
237. Nicholas NS, Apollonio B, Ramsay AG. Tumor microenvironment (TME)-driven immune suppression in b cell malignancy. Biochim Biophys Acta (BBA) - Mol Cell Res (2016) 1863(3):471–82. doi: 10.1016/j.bbamcr.2015.11.003
238. Kater AP, Tonino SH, Egle A, Ramsay AG. How does lenalidomide target the chronic lymphocytic leukemia microenvironment? Blood (2014) 124(14):2184–9. doi: 10.1182/blood-2014-05-578286
239. Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia (2010) 24(1):22–32. doi: 10.1038/leu.2009.236
240. LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood (2004) 103(5):1787–90. doi: 10.1182/blood-2003-02-0361
241. Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man H-W, Schnetkamp PP, et al. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther (2003) 305(3):1222–32. doi: 10.1124/jpet.102.048496
242. McDaniel JM, Pinilla-Ibarz J, Epling-Burnette P. Molecular action of lenalidomide in lymphocytes and hematologic malignancies. Adv Hematol (2012) 2012:513702. doi: 10.1155/2012/513702
243. Ramsay AG, Evans R, Kiaii S, Svensson L, Hogg N, Gribben JG. Chronic lymphocytic leukemia cells induce defective LFA-1-directed T-cell motility by altering rho GTPase signaling that is reversible with lenalidomide. Blood (2013) 121(14):2704–14. doi: 10.1182/blood-2012-08-448332
244. Fostier K, Caers J, Meuleman N, Broos K, Corthals J, Thielemans K, et al. Impact of lenalidomide maintenance on the immune environment of multiple myeloma patients with low tumor burden after autologous stem cell transplantation. Oncotarget (2018) 9(29):20476. doi: 10.18632/oncotarget.24944
245. Ioannou N, Hagner PR, Stokes M, Gandhi AK, Apollonio B, Fanous M, et al. Triggering interferon signaling in T cells with avadomide sensitizes CLL to anti-PD-L1/PD-1 immunotherapy. Blood (2021) 137(2):216–31. doi: 10.1182/blood.2020006073
246. Di Lullo G, Marcatti M, Heltai S, Tresoldi C, Paganoni AM, Bordignon C, et al. Immunomodulatory drugs in the context of autologous hematopoietic stem cell transplantation associate with reduced pro-tumor T cell subsets in multiple myeloma. Front Immunol (2019) 9:3171. doi: 10.3389/fimmu.2018.03171
247. Chung DJ, Pronschinske KB, Shyer JA, Sharma S, Leung S, Curran SA, et al. T-Cell exhaustion in multiple myeloma relapse after autotransplant: optimal timing of immunotherapy. Cancer Immunol Res (2016) 4(1):61–71. doi: 10.1158/2326-6066.CIR-15-0055
248. Eve HE, Carey S, Richardson SJ, Heise CC, Mamidipudi V, Shi T, et al. Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: results from a UK phase II study suggest activity and possible gender differences. Br J Haematol (2012) 159(2):154–63. doi: 10.1111/bjh.12008
249. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol (2008) 9(5):503–10. doi: 10.1038/ni1582
250. Weiner GJ. Rituximab: mechanism of action. seminars in hematology. Semin Hematol (2010) 47(2):115–23. doi: 10.1053/j.seminhematol.2010.01.011.
251. Vogler M, Shanmugalingam S, Särchen V, Reindl LM, Grèze V, Buchinger L, et al. Unleashing the power of NK cells in anticancer immunotherapy. J Mol Med (2022) 100(3):337–49. doi: 10.1007/s00109-021-02120-z
252. Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase II study. J Clin Oncol (2006) 24(34):5343–9. doi: 10.1200/JCO.2005.05.0401
253. Payvandi F, Wu L, Naziruddin SD, Haley M, Parton A, Schafer PH, et al. Immunomodulatory drugs (IMiDs) increase the production of IL-2 from stimulated T cells by increasing PKC-θ activation and enhancing the DNA-binding activity of AP-1 but not NF-κB, OCT-1, or NF-AT. J Interferon Cytokine Res (2005) 25(10):604–16. doi: 10.1089/jir.2005.25.604
254. Gandhi AK, Mendy D, Parton A, Wu L, Kosek J, Zhang L-H, et al. CC-122 is a novel pleiotropic pathway modifier with potent in vitro immunomodulatory and anti-angiogenic properties and in vivo anti-tumor activity in hematological cancers. Blood (2012) 120(21):2963–. doi: 10.1182/blood.V120.21.2963.2963
255. Carpio C, Bouabdallah R, Ysebaert L, Sancho J-M, Salles G, Cordoba R, et al. Avadomide monotherapy in relapsed/refractory DLBCL: safety, efficacy, and a predictive gene classifier. Blood (2020) 135(13):996–1007. doi: 10.1182/blood.2019002395
256. Cubillos-Zapata C, Cordoba R, Avendaño-Ortiz J, Arribas-Jiménez C, Hernández-Jiménez E, Toledano V, et al. CC-122 immunomodulatory effects in refractory patients with diffuse large b-cell lymphoma. Oncoimmunology (2016) 5(12):e1231290. doi: 10.1080/2162402X.2016.1231290
257. Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol (2020) 11:583084. doi: 10.3389/fimmu.2020.583084
258. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol (2019) 12(1):76. doi: 10.1186/s13045-019-0760-3
259. Zhu S, Yi M, Wu Y, Dong B, Wu K. Roles of tumor-associated macrophages in tumor progression: implications on therapeutic strategies. Exp Hematol Oncol (2021) 10(1):1–17. doi: 10.1186/s40164-021-00252-z
260. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci (2021) 22(13):6995. doi: 10.3390/ijms22136995
261. Li C, Xu X, Wei S, Jiang P, Xue L, Wang J. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer (2021) 9(1):e001341. doi: 10.1136/jitc-2020-001341
262. van de Donk N, Usmani SZ. CD38 antibodies in multiple myeloma: Mechanisms of action and modes of resistance. Front Immunol (2018) 9:2134. doi: 10.3389/fimmu.2018.02134
263. VanDerMeid KR, Elliott MR, Baran AM, Barr PM, Chu CC, Zent CS. Cellular cytotoxicity of next-generation CD20 monoclonal AntibodiesCellular cytotoxicity of CD20 monoclonal antibodies. Cancer Immunol Res (2018) 6(10):1150–60. doi: 10.1158/2326-6066.CIR-18-0319
264. Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with b-cell malignancies. Adv Ther (2017) 34(2):324–56. doi: 10.1007/s12325-016-0451-1
265. Wang Y, Xiang Y, Xin VW, Wang X-W, Peng X-C, Liu X-Q, et al. Dendritic cell biology and its role in tumor immunotherapy. J Hematol Oncol (2020) 13(1):107. doi: 10.1186/s13045-020-00939-6
266. Patente TA, Pinho MP, Oliveira AA, Evangelista GC, Bergami-Santos PC, Barbuto JA. Human dendritic cells: their heterogeneity and clinical application potential in cancer immunotherapy. Front Immunol (2019) 9:3176. doi: 10.3389/fimmu.2018.03176
267. Phan V, Ito T, Inaba M, Azuma Y, Kibata K, Inagaki-Katashiba N, et al. Immunomodulatory drugs suppress Th1-inducing ability of dendritic cells but enhance Th2-mediated allergic responses. Blood Adv (2020) 4(15):3572–85. doi: 10.1182/bloodadvances.2019001410
268. Maiso P, Mogollón P, Ocio EM, Garayoa M. Bone marrow mesenchymal stromal cells in multiple myeloma: Their role as active contributors to myeloma progression. Cancers (2021) 13(11):2542. doi: 10.3390/cancers13112542
269. Chang X, Zhu Y, Shi C, Stewart AK. Mechanism of immunomodulatory drugs’ action in the treatment of multiple myeloma. Acta Biochim Biophys Sinica (2014) 46(3):240–53. doi: 10.1093/abbs/gmt142
270. Zhang L, Yang J, Qian J, Li H, Romaguera JE, Kwak LW, et al. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood (2012) 120(18):3783–92. doi: 10.1182/blood-2012-04-424630
271. Wobus M, Benath G, Ferrer RA, Wehner R, Schmitz M, Hofbauer LC, et al. Impact of lenalidomide on the functional properties of human mesenchymal stromal cells. Exp Hematol (2012) 40(10):867–76. doi: 10.1016/j.exphem.2012.06.004
272. Ribatti D, Vacca A. New insights in anti-angiogenesis in multiple myeloma. Int J Mol Sci (2018) 19(7):2031. doi: 10.3390/ijms19072031
273. Menzel L, Höpken UE, Rehm A. Angiogenesis in lymph nodes is a critical regulator of immune response and lymphoma growth. Front Immunol (2020) 11:591741. doi: 10.3389/fimmu.2020.591741
274. Raza S, Safyan RA, Lentzsch S. Immunomodulatory drugs (IMiDs) in multiple myeloma. Curr Cancer Drug Targets (2017) 17(9):846–57. doi: 10.2174/1568009617666170214104426
275. Mercurio A, Adriani G, Catalano A, Carocci A, Rao L, Lentini G, et al. A mini-review on thalidomide: Chemistry, mechanisms of action, therapeutic potential and anti-angiogenic properties in multiple myeloma. Curr Medicinal Chem (2017) 24(25):2736–44. doi: 10.2174/0929867324666170601074646
276. Dredge K, Horsfall R, Robinson SP, Zhang L-H, Lu L, Tang Y, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and akt phosphorylation in vitro. Microvasc Res (2005) 69(1):56–63. doi: 10.1016/j.mvr.2005.01.002
277. Maffei R, Fiorcari S, Bulgarelli J, Rizzotto L, Martinelli S, Rigolin GM, et al. Endothelium-mediated survival of leukemic cells and angiogenesis-related factors are affected by lenalidomide treatment in chronic lymphocytic leukemia. Exp Hematol (2014) 42(2):126–36.e1. doi: 10.1016/j.exphem.2013.10.007
278. Pierpont TM, Limper CB, Richards KL. Past, present, and future of rituximab-the world’s first oncology monoclonal antibody therapy. Front Oncol (2018) 8:163. doi: 10.3389/fonc.2018.00163
279. Delfau-Larue MH, Boulland ML, Beldi-Ferchiou A, Feugier P, Maisonneuve H, Casasnovas RO, et al. Lenalidomide/rituximab induces high molecular response in untreated follicular lymphoma: LYSA ancillary RELEVANCE study. Blood Adv (2020) 4(14):3217–23. doi: 10.1182/bloodadvances.2020001955
280. Becnel MR, Nastoupil LJ, Samaniego F, Davis RE, You MJ, Green M, et al. Lenalidomide plus rituximab (R(2)) in previously untreated marginal zone lymphoma: subgroup analysis and long-term follow-up of an open-label phase 2 trial. Br J Haematol (2019) 185(5):874–82. doi: 10.1111/bjh.15843
281. Bachy E, Houot R, Feugier P, Bouabdallah K, Bouabdallah R, Virelizier EN, et al. Obinutuzumab plus lenalidomide in advanced, previously untreated follicular lymphoma in need of systemic therapy: a LYSA study. Blood (2022) 139(15):2338–46. doi: 10.1182/blood.2021013526
282. Vitale C, Falchi L, Ten Hacken E, Gao H, Shaim H, Van Roosbroeck K, et al. Ofatumumab and lenalidomide for patients with relapsed or refractory chronic lymphocytic leukemia: Correlation between responses and immune characteristics. Clin Cancer Research: an Off J Am Assoc Cancer Res (2016) 22(10):2359–67. doi: 10.1158/1078-0432.CCR-15-2476
283. Dimopoulos MA, San-Miguel J, Belch A, White D, Benboubker L, Cook G, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica (2018) 103(12):2088–96. doi: 10.3324/haematol.2018.194282
284. Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbüse R, Schlereth B, et al. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiology (2009) 214(6):441–53. doi: 10.1016/j.imbio.2008.11.014
285. Pishko A, Nasta SD. The role of novel immunotherapies in non-Hodgkin lymphoma. Trans Cancer Res (2017) 6(1):93–103. doi: 10.21037/tcr.2017.01.08
286. Goebeler M-E, Bargou RC. T Cell-engaging therapies — BiTEs and beyond. Nat Rev Clin Oncol (2020) 17(7):418–34. doi: 10.1038/s41571-020-0347-5
287. Hatic H, Sampat D, Goyal G. Immune checkpoint inhibitors in lymphoma: challenges and opportunities. Ann Trans Med (2021) 9(12):1037. doi: 10.21037/atm-20-6833
288. Salik B, Smyth MJ, Nakamura K. Targeting immune checkpoints in hematological malignancies. J Hematol Oncol (2020) 13(1):1–19. doi: 10.1186/s13045-020-00947-6
289. Nagasaki J, Togashi Y. A variety of ‘exhausted’ T cells in the tumor microenvironment. Int Immunol (2022) dxac013. Online ahead of print. doi: 10.1093/intimm/dxac013
290. Ansell SM, Lin Y. Immunotherapy of lymphomas. J Clin Invest (2020) 130(4):1576–85. doi: 10.1172/JCI129206
291. Görgün G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Research: an Off J Am Assoc Cancer Res (2015) 21(20):4607–18. doi: 10.1158/1078-0432.CCR-15-0200
292. Badros AZ, Kocoglu MH, Ma N, Rapoport AP, Lederer E, Philip S, et al. A phase II study of anti PD-1 antibody pembrolizumab, pomalidomide and dexamethasone in patients with Relapsed/Refractory multiple myeloma (RRMM). Blood (2015) 126(23):506. doi: 10.1182/blood.V126.23.506.506
293. San Miguel J, Mateos M-V, Shah JJ, Ocio EM, Rodriguez-Otero P, Reece D, et al. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone for Relapsed/Refractory multiple myeloma (RRMM): Keynote-023. Blood (2015) 126(23):505–. doi: 10.1182/blood.V126.23.505.505
294. Mateos MV, Orlowski RZ, Ocio EM, Rodríguez-Otero P, Reece D, Moreau P, et al. Pembrolizumab combined with lenalidomide and low-dose dexamethasone for relapsed or refractory multiple myeloma: phase I KEYNOTE-023 study. Br J Haematol (2019) 186(5):e117–e21. doi: 10.1111/bjh.15946
295. Mateos MV, Blacklock H, Schjesvold F, Oriol A, Simpson D, George A, et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): a randomised, open-label, phase 3 trial. Lancet Haematol (2019) 6(9):e459–e69. doi: 10.1016/S2352-3026(19)30110-3
296. Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol (2019) 6(9):e448–e58. doi: 10.1016/S2352-3026(19)30109-7
297. Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol (2019) 16(6):372–85. doi: 10.1038/s41571-019-0184-6
298. Gumber D, Wang LD. Improving CAR-T immunotherapy: Overcoming the challenges of T cell exhaustion. EBioMedicine (2022) 77:103941. doi: 10.1016/j.ebiom.2022.103941
299. Neelapu SS. Managing the toxicities of car T-cell therapy. Hematol Oncol (2019) 37:48–52. doi: 10.1002/hon.2595
300. Pan B, Lentzsch S. The application and biology of immunomodulatory drugs (IMiDs) in cancer. Pharmacol Ther (2012) 136(1):56–68. doi: 10.1016/j.pharmthera.2012.07.004
301. Thieblemont C, Chevret S, Allain V, Di Blasi R, Morin F, Vercellino L, et al. Lenalidomide enhance CAR T-cells response in patients with Refractory/Relapsed Large b cell lymphoma experiencing progression after infusion. Blood (2020) 136:16–7. doi: 10.1182/blood-2020-136279
302. Neelapu SS, Kharfan-Dabaja MA, Oluwole OO, Krish P, Reshef R, Riedell PA, et al. A phase 2, open-label, multicenter study evaluating the safety and efficacy of axicabtagene ciloleucel in combination with either rituximab or lenalidomide in patients with refractory Large b-cell lymphoma (ZUMA-14). Blood (2019) 134:4093. doi: 10.1182/blood-2019-126369
303. Lemoine J, Morin F, Di Blasi R, Vercellino L, Cuffel A, Benlachgar N, et al. Lenalidomide exposure at time of CAR T-cells expansion enhances response of Refractory/Relapsed aggressive Large b-cell lymphomas. Blood (2021) 138:1433. doi: 10.1182/blood-2021-151109
304. Zhao G, Wei R, Feng L, Wu Y, He F, Xiao M, et al. Lenalidomide enhances the efficacy of anti-BCMA CAR-T treatment in relapsed/refractory multiple myeloma: A case report and revies of the literature. Cancer Immunol Immunother (2022) 71(1):39–44. doi: 10.1007/s00262-021-02959-8
305. Suzuki K, Nishiwaki K, Yano S. Treatment strategy for multiple myeloma to improve immunological environment and maintain MRD negativity. Cancers (2021) 13(19):4867. doi: 10.3390/cancers13194867
306. Nowakowski GS, Hong F, Scott DW, Macon WR, King RL, Habermann TM, et al. Addition of lenalidomide to r-CHOP improves outcomes in newly diagnosed diffuse Large b-cell lymphoma in a randomized phase II US intergroup study ECOG-ACRIN E1412. J Clin Oncol: Off J Am Soc Clin Oncol (2021) 39(12):1329–38. doi: 10.1200/JCO.20.01375
307. Li L, Xue W, Shen Z, Liu J, Hu M, Cheng Z, et al. A cereblon modulator CC-885 induces CRBN- and p97-dependent PLK1 degradation and synergizes with volasertib to suppress lung cancer. Mol Ther - Oncolytics (2020) 18:215–25. doi: 10.1016/j.omto.2020.06.013
308. Bastea LI, Liou GY, Pandey V, Fleming AK, von Roemeling CA, Doeppler H, et al. Pomalidomide alters pancreatic macrophage populations to generate an immune-responsive environment at precancerous and cancerous lesions. Cancer Res (2019) 79(7):1535–48. doi: 10.1158/0008-5472.CAN-18-1153
309. Brosseau C, Colston K, Dalgleish AG, Galustian C. The immunomodulatory drug lenalidomide restores a vitamin d sensitive phenotype to the vitamin d resistant breast cancer cell line MDA-MB-231 through inhibition of BCL-2: potential for breast cancer therapeutics. Apoptosis (2012) 17(2):164–73. doi: 10.1007/s10495-011-0670-5
310. Dahut WL, Aragon-Ching JB, Woo S, Tohnya TM, Gulley JL, Arlen PM, et al. Phase I study of oral lenalidomide in patients with refractory metastatic cancer. J Clin Pharmacol (2009) 49(6):650–60. doi: 10.1177/0091270009335001
311. Semeraro M, Vacchelli E, Eggermont A, Galon J, Zitvogel L, Kroemer G, et al. Trial watch: Lenalidomide-based immunochemotherapy. Oncoimmunology (2013) 2(11):e26494. doi: 10.4161/onci.26494
312. Barrio S, Munawar U, Zhu YX, Giesen N, Shi C-X, Da Viá M, et al. IKZF1/3 and CRL4CRBN E3 ubiquitin ligase mutations and resistance to immunomodulatory drugs in multiple myeloma. Haematologica (2020) 105(5):e237. doi: 10.3324/haematol.2019.217943
Keywords: Immunomodulatory drug, B-cell lymphoma, Multiple myeloma, Tumor microenvironment, Immunotherapy, CRBN
Citation: Guo H, Yang J, Wang H, Liu X, Liu Y and Zhou K (2022) Reshaping the tumor microenvironment: The versatility of immunomodulatory drugs in B-cell neoplasms. Front. Immunol. 13:1017990. doi: 10.3389/fimmu.2022.1017990
Received: 12 August 2022; Accepted: 27 September 2022;
Published: 12 October 2022.
Edited by:
Yangqiu Li, Jinan University, ChinaReviewed by:
Xiaofang Wang, Guangzhou Medical University, ChinaLiang Lin, Cytovia Therapeutics, United States
Copyright © 2022 Guo, Yang, Wang, Liu, Liu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keshu Zhou, ZHJ6aG91a3M3N0AxNjMuY29t
 Hao Guo
Hao Guo Jingyi Yang
Jingyi Yang Haoran Wang
Haoran Wang Keshu Zhou
Keshu Zhou