- 1Department of Medical Sciences, Uppsala University, Uppsala University Hospital, Uppsala, Sweden
- 2NABAS AS, Ås, Norway
- 3Department of Surgical Sciences, Section of Anaesthesiology and Intensive Care Medicine, Uppsala University, Uppsala, Sweden
- 4NOVA Medical School, New University of Lisbon, Lisboa, Portugal
Antibody-based assays are commonly used in clinical laboratories for analyzing plasma, serum and other samples for particular protein markers. Although such assays have been traditionally based on antibodies raised in mammals (e.g., mice, rabbits, goats), there are several advantages of using avian antibodies (IgY) raised in chickens, including production volumes, costs, and ethical/animal welfare considerations. A further disadvantage of using mammalian IgG in such assays is the potential for agglutination when exposed to rheumatoid factor (RF) in serum. However, when used in the free form the immune complexes formed with avian antibodies have been reported to have less ability than those formed with mammalian antibodies to cause the light scatter which are used for instrument measurement. In addition, when the amount of antigen exceeds the maximum precipitating point in relation to the amount of antibody, there is a rapid decline in the absorbance values of the immune complexes (antigen excess) when IgY is used. However, when avian antibodies are conjugated to a substrate and used in particle enhanced turbidimetric assays (PETIA), these problems are avoided. Here we investigated three clinical assays using chicken antibodies, one using free (unbound) IgY and two with IgY-based PETIA. The IgY PETIA demonstrated a strong scatter response, even at high antigen concentrations in contrast to the steep decline seen with free IgY antibodies. IgY PETIA reagents can provide test results with low coefficient of variation (<1% for duplicate samples). We also investigated the effect of RF on agglutination of mammalian antibodies (IgG from mouse, rabbit, sheep, and human) and chicken antibodies. Whereas agglutination was observed with all the mammalian antibodies in the presence of RF, this was not observed at all with chicken IgY. Our results support the growing body of evidence that chicken egg yolks can thus be a valuable source of antibodies for use in PETIA in clinical laboratories.
Introduction
Turbidimetric and nephelometric assays are widely used in clinical laboratories for quantifying abundant plasma proteins. Turbidimetric methods can easily be applied to the large chemistry analyzers used in such laboratories. These instruments are highly automated and have a capacity of 1000 assays or more per hour. The platforms usually have a broad assay panel (>30 different assays) and are run 24/7. The methods have assay times of around 10 min and measure changes in absorbance due to the scattered light that occurs when an antigen combines with an antibody to form complexes, enabling rapid reporting of patient test results. In most laboratories, including Uppsala University Hospital, Sweden, the test results are reported back to the patients’ electronic files within 40 min after the samples arrive at the laboratory. The initially developed assays used free (unbound) antibodies, often with polyethylene glycol to enhance the precipitation reaction. These assays measure proteins from approximately 0.1 g/L and higher. Assays that use free antibodies quantify, for example, serum/plasma albumin, IgG, IgA, IgM, haptoglobin, fibrinogen, transferrin, antitrypsin, and alpha-acid-glycoprotein. Each of these markers represent at least 100,000 test results per year in Sweden (1).
If the antibodies are coupled to particles (particle enhanced turbidimetric immunoassays; PETIA) (2, 3), rather than being free, the reaction is amplified, and the technology can be used down to concentrations of approximately 0.1 mg/L of analyte. The particles used are latex-based and the antibodies are coupled to the surface. Typical PETIAs measure urine albumin, plasma C-reactive protein, ferritin, soluble transferrin receptor, cystatin C, immunoglobulin free light chains, cerebrospinal fluid IgG, and albumin. Several million PETIAs are performed annually in Sweden (1). The methodology requires more antibodies per test than, for example, a sandwich enzyme-linked immunosorbent assay (ELISA), and the large number of assays means that the total antibody volumes required are much higher than for ELISA methods.
Antibodies for such tests are usually raised in mammals such as mice, rabbits, or goats. However, antibodies for these tests could also be raised in birds. Production of large volumes of antibodies is one of the strengths of using yolk antibodies raised in chickens. One rabbit has been reported to produce 150 mg antibody as compared to 15 g produced by one chicken (4).
Other advantages with using avian antibodies are the lack of interference due to rheumatoid factor (RF) or anti-mammalian IgG antibodies (5, 6). The most well-known of these anti-mammalian IgG antibodies is probably human anti-mouse IgG antibodies (HAMA) (7).
The perspective of animal welfare should not be overlooked in this context. The effectiveness of isolation of IgY from egg yolk enables fewer number of animals to be used, and there is no requirement for blood sampling. This is in agreement with the recommendations of the European Centre for the Validation of Alternative Methods (ECVAM), recommending that egg yolk antibodies should be used instead of mammalian antibodies (8).
The most widely used IgY based PETIAs are for human cystatin C, human fecal calprotectin, human serum/plasma calprotectin and dog CRP. Cystatin C is a low molecular weight protein that is produced by all cells in the body and is removed from the circulation by glomerular filtration in the kidneys. Cystatin C is widely used as an alternative to creatinine to evaluate kidney glomerular filtration rate (GFR). The IgY PETIA is one of the most frequently used serum/plasma cystatin C assays. It has been shown to be superior to creatinine for the prediction of kidney function and as a cardiovascular risk marker (9–13). The IgY PETIA has also been used to detect cystatin C in urine (14), seminal plasma (15) and cerebrospinal fluid (16).
Calprotectin is the most abundant protein in the cytoplasma of neutrophils and it is used as a marker for neutrophil activation. Elevated fecal calprotectin is a marker for migration of neutrophils to the intestinal mucosa, which is caused by inflammatory bowel disease. Fecal calprotectin is today widely used for the detection of inflammatory bowel diseases (17–19). One of the most frequently used tests is fCal Turbo which is an IgY based PETIA (20–23). The assay has also been used for the detection of fecal calprotectin in dogs and cats (24).
The IgY PETIA for serum/plasma calprotectin has been used as a marker for inflammatory conditions including bacterial infections such as sepsis (25, 26). It has been shown to be a superior sepsis and infectious disease marker to e.g. procalcitonin which is a widely used marker for bacterial infections (27–31).
There is also a IgY PETIA specific for dog CRP (32, 33). CRP is a valuable inflammation marker in humans. The crossreactivity between human and dog CRP varies. Thus, veterinarians screened human CRP reagent lots to find a lot that showed a strong crossreactivity to dog CRP and used that lot until it expired and then they searched for a new lot. The development of the specific dog CRP PETIA ensured a better lot to lot consistency and eliminated the search process to find suitable lots.
This report describes the use of avian antibodies in PETIA methods that are suitable for routine laboratory assays.
Materials and methods
Turbidimetric assays
Three different turbidimetric assays in which IgY-based immunoassays were used to assess the relative content of specific analyte antigens are summarized below. See Table 1 for an overview of the assay parameters. The samples used were Li-heparin samples sent to the Department of Clinical Chemistry and Pharmacology. The blood samples were collected in vacutainer plasma separation tubes. The use of surplus patient samples without patient identities was approved by the Uppsala University Ethical committee (approval 01/367).
Assay 1: turbidimetric assay using unbound chicken IgY antibodies for fibrinogen
The reaction between free chicken anti-fibrinogen antibodies (IgY Lab Systems, Uppsala, Sweden) and fibrinogen in plasma samples was analyzed using a Beckman Array system (Beckman Coulter, Brea, CA, USA), with 42 µl of samples prediluted in 0.9% NaCl added to the measuring cuvette. The avian anti-fibrinogen antibody vial was placed in one of the instrument’s antibody reagent positions and the assay was performed with the buffers and settings provided by Beckman Coulter.
Assay 2: PETIA using chicken IgY for kappa immunoglobulin free light chains
IgY Production: Chicken antibodies specific for human kappa free light chain (FLC) were generated at NABAS AS (ÅS, Norway) by immunizing chickens with kappa FLC that had been purified from urine samples containing Bence Jones (BJ) proteins (kindly provided by A. Grubb, Lund University Hospital, Sweden). IgY was isolated from egg yolks using the water dilution method of Akita & Nakai (34), followed by salt precipitation. It was then affinity purified using the kappa FLC antigen immobilized onto HiTrap NHS-activated crosslinked agarose (Cytiva 17-0716-01), and adsorbed free of whole molecule kappa light chains, in a procedure as described by Bradwell et al. (35).
PETIA reagent 1 (R1): Kappa FLC antibodies were covalently conjugated to 194 nm latex particles (IKERLAT Polymers S.L., Gipuzkoa, Spain) and stabilized in a 25 mM tris buffer, pH 8.2. The PETIA reaction buffer, reagent 2 (R2), was a 3-(N-morpholino) propanesulfonic acid buffer, pH 6.9.
Kappa FLC measurements were performed on an Alcor analyser (Edif Instruments s.r.l., Italy) using the following instrument settings: primary wavelength 600 nm; 180 µl R1 and 3 µl sample mixed with 60 µl R2, and the endpoint measurement recorded after 544 seconds (just under 10 minutes).
A single 9-point calibration curve (range 0 – 231 mg/L) was prepared using serial dilutions of a purified polyclonal kappa FLC antigen isolated from pooled human urine (Advy Chemical Pvt Mumbai, India). Kappa FLC values were assigned from measurements obtained using the Binding Site (The Binding Site Group, Birmingham, UK) kappa FLC assay.
Assay 3: PETIA using chicken IgY for cystatin C
The cystatin C method was introduced as a routine method at the Department of Clinical Chemistry and Pharmacology in 2007. Since the introduction we have produced close to one million test results using the IgY based PETIA. Immunoparticles coated with purified chicken antibodies to cystatin C were obtained from Gentian AS (Moss, Norway). Plasma cystatin-C measurements were performed on an Architect c8000 analyzer (Abbott Laboratories, Abbott Park, IL, USA) using the following instrument settings: primary wavelength 548 nm, 7-point spline calibration; 220 µl reagent 1 (R1), 3 µl plasma sample, and 45 µl reagent 2 (R2). The analyzer measured the turbidimetry in the samples every eighteenth second. At time zero, the buffer was added (R1) and then the plasma samples were added to the R1 and mixed before adding the immunoparticles (R2) at reading point 16 and recording the sample blank at reading point 18. The increase in absorbance was calculated as endpoint readings (difference between results at reading points 18 and 33) or as rate readings. A rate reading was defined as the steepest slope value around time points 19 to 24. The total assay time is 33 x 18 seconds (c.a. 10 min). The measuring range for the cystatin C method was 0.3-8.1 mg/L. Assay precision was investigated for both rate and endpoint measurements by calculating the coefficient of variation (%CV) from duplicate measurements.
The effect of rheumatoid factor on immunoparticle agglutination when using mammalian or avian antibodies
Immunoparticles were coated with various purified antibodies: chicken IgY, and IgG from mouse, rabbit, sheep, and human using standard methods. The effect of RF on the particles was investigated by mixing 20 µl of the different coated particles with samples of serum known to contain RF from ten patients and with serum samples from ten healthy blood donors. The serum was diluted 1:4 in saline prior to investigation. Agglutination of the particles was determined visually.
Results
Turbidimetric assays
Assay 1: turbidimetric assay using unbound chicken IgY antibodies for fibrinogen
The absorbance increased with increasing amounts of fibrinogen in the samples until it reached a peak around 500 mg/L. After the peak, there was a very rapid decline in absorbance as the amount of antigen further increased (Figure 1).
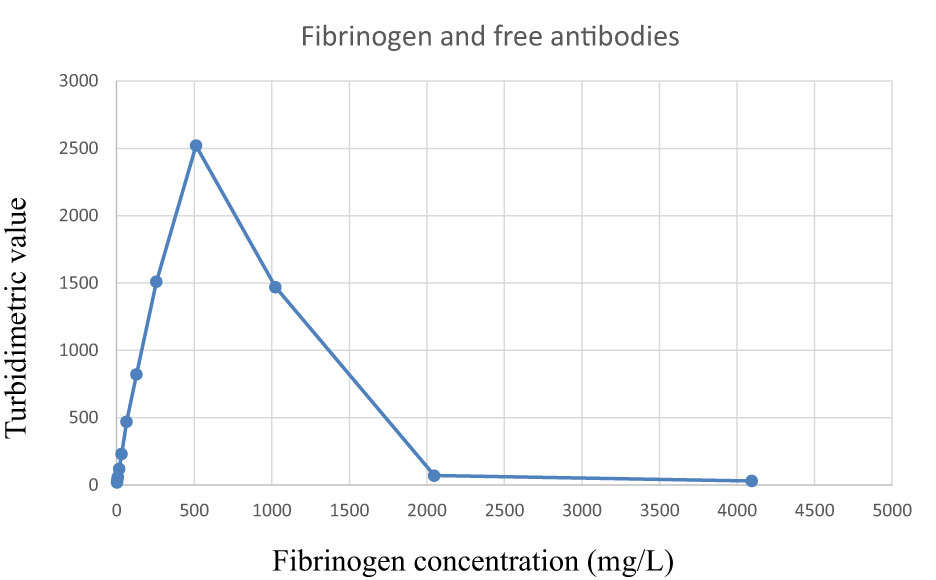
Figure 1 Reaction curve when using free avian anti-fibrinogen antibodies to detect fibrinogen in plasma samples. Observe the rapid decrease when the fibrinogen concentrations exceed the precipitation maximum (right hand side of the plot). The samples were analyzed as singletons.
Assay 2: PETIA using chicken IgY for kappa immunoglobulin free light chains
When the concentration of kappa FLC increased in the samples, an antigen excess situation was reached, and the absorbance values started to decrease. Although kappa FLC values were in excess of 200 mg/L, there was still a strong reaction in excess of OD 0.78 (Figure 2).
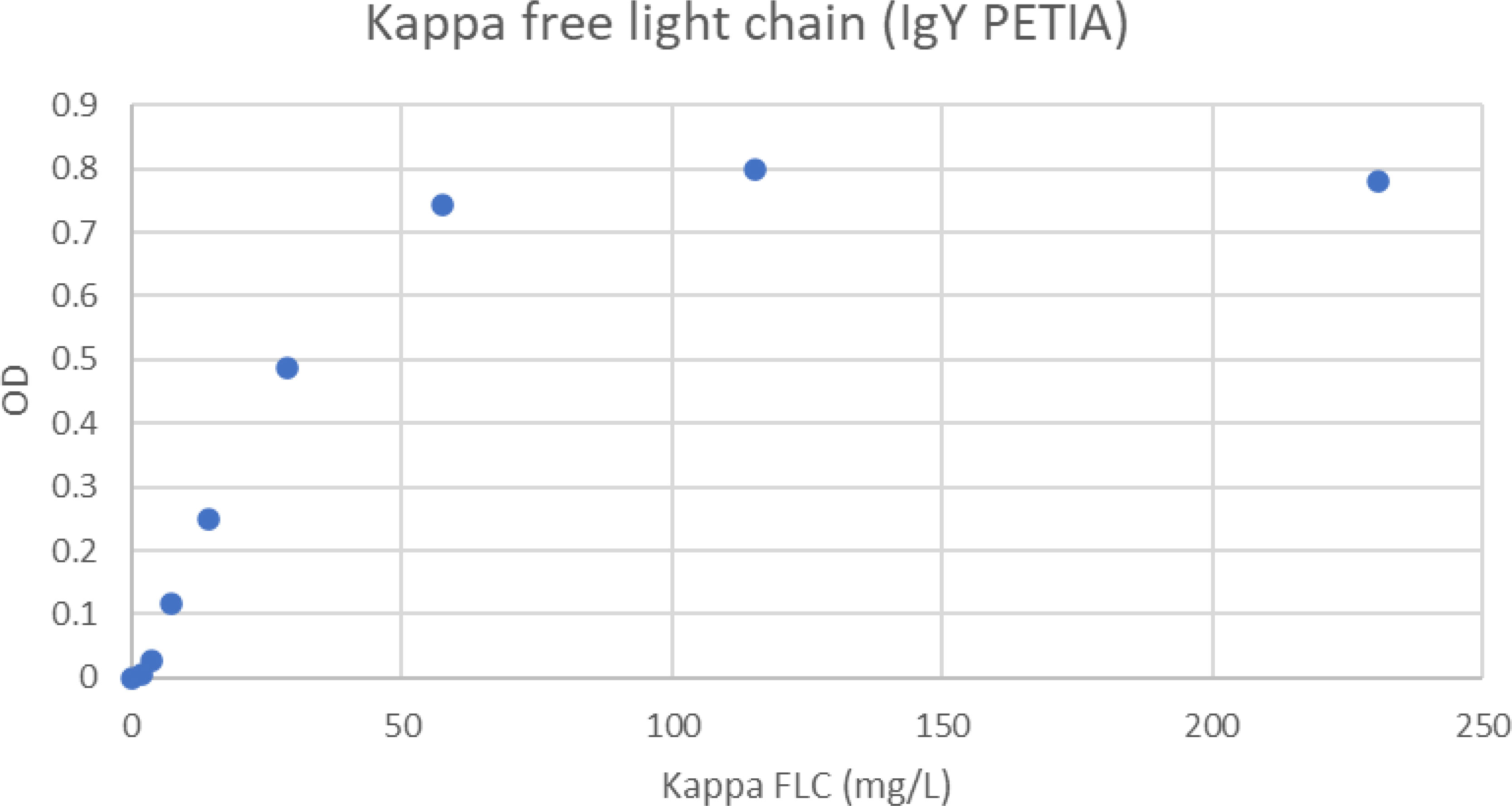
Figure 2 Antigen excess curve for kappa FLC PETIA. The figure shows the free kappa chain concentration in the samples versus the absorbance readings. The samples were analyzed as singletons. The plot shows that antigen excess is not present below 230 mg/L free kappa chain.
Assay 3: PETIA using chicken IgY for cystatin C
Figure 3 shows typical turbidimetric progress curves for the IgY cystatin C PETIA on the Architect c8000. The increase in turbidimetric absorbance is an effect of the amount of antigen in the sample and the concentration of cystatin C was calculated using the standard curve generated using the calibrator set.
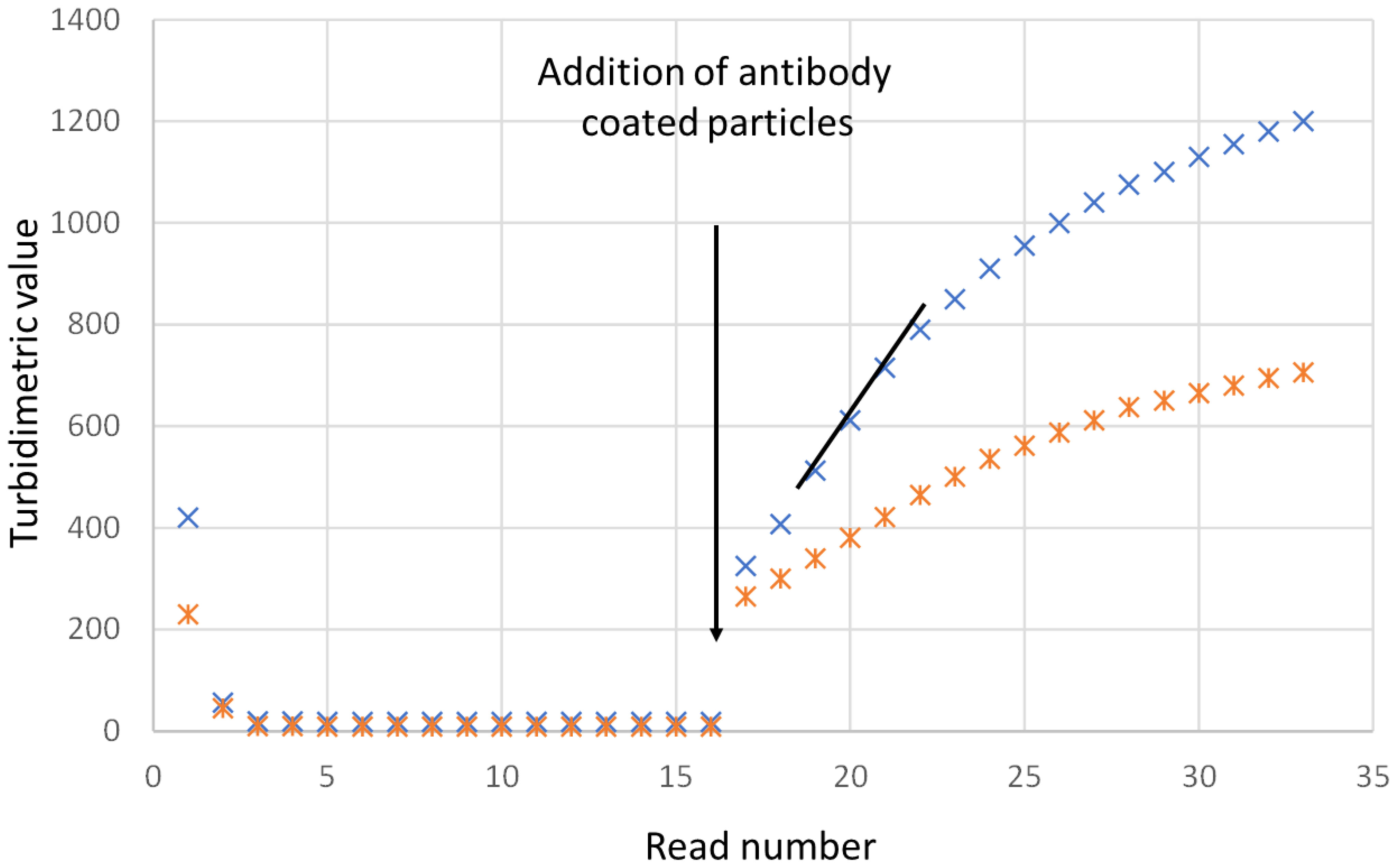
Figure 3 Presentation of the turbidimetric readings when analyzing cystatin C on an Architect c8000. The samples were analyzed as singletons. The instrument measures the turbidimetric readings every eighteenth second. The readings are shown for two samples, one sample with a cystatin C concentration of 7 mg/L (upper curve) and one sample with a cystatin C concentration of 3.5 mg/L. The antibody coated particles are added after position 16. The reaction between the particles and the sample can be measured as endpoint or rate readings. Rate is the steepest slope of the readings after position 18 and is represented in the figure as a black line.
The mean %CV of the calculated cystatin C (in mg/L) for duplicate samples was 0.58% (n=179) for endpoint measurements and 0.50% (n=118) for rate determinations.
The effect of rheumatoid factor on immunoparticle agglutination when using mammalian or avian antibodies
All immunoparticles coated with mouse, rabbit, sheep, and human IgG agglutinated when mixed with RF-positive sera (Figure 4). The immunoparticles coated with chicken IgY did not agglutinate when mixed with RF-positive sera. None of the particles agglutinated when mixed with the blood donor samples, regardless of the type and source of antibody.
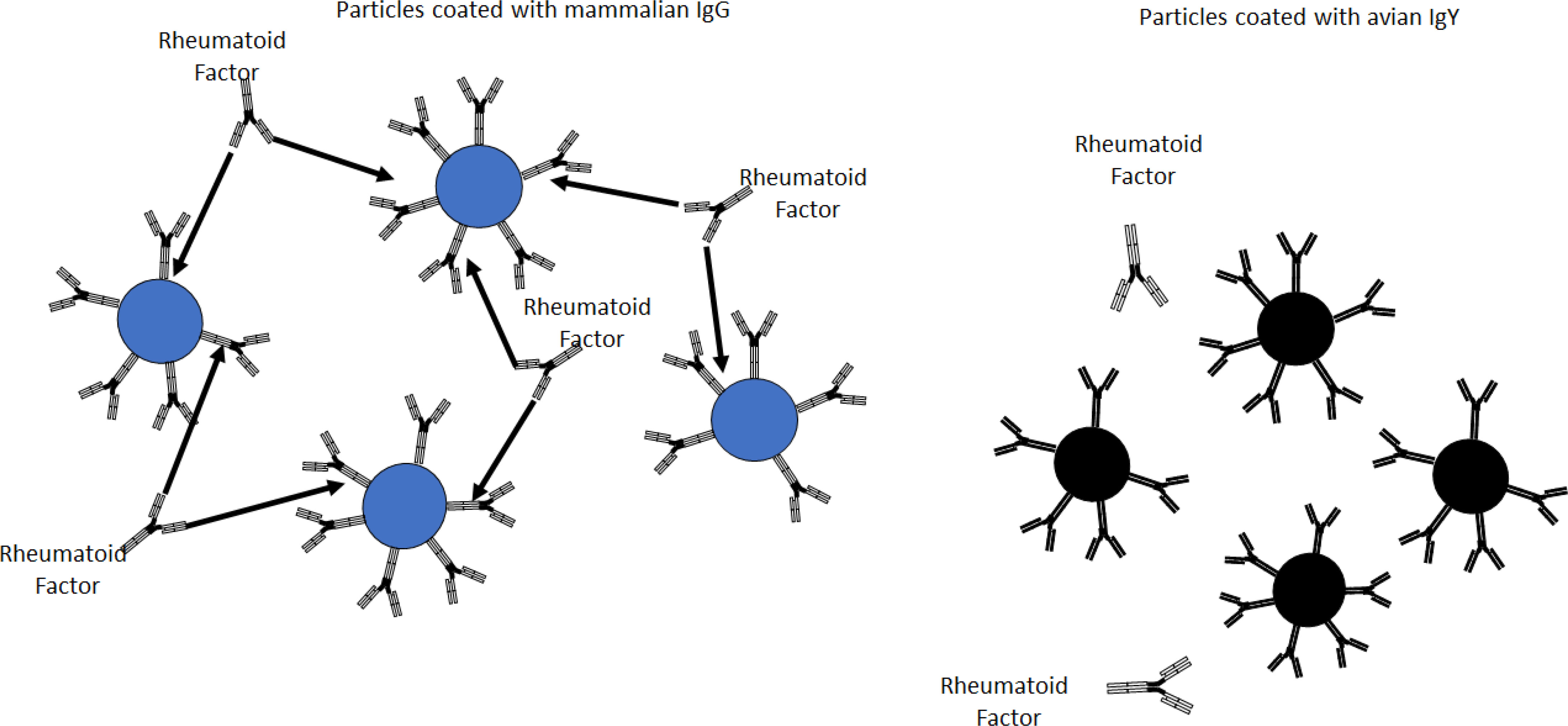
Figure 4 Rheumatoid factor-positive sera agglutinated particles coated with mammalian IgG (left) but not particles coated with chicken IgY (right).
Discussion
The main finding from this study was that whereas there is a rapid decrease in absorbance when reaching the antigen excess area in assays using avian antibodies in solution, for IgY PETIAs there is a very favorable antigen excess curve. In addition, IgY-based PETIA reagents demonstrate high absorbance values and high precision (low coefficients of variation).
There are several benefits from using IgY purified from egg yolk in clinical PETIA: firstly, purification is simple and relatively inexpensive (36); secondly, there is a cost-effective perspective on IgY, as one hen may produce up to a quantity of 2 g of IgY monthly in egg yolk, which corresponds to approximately 600 ml of whole blood; more than 100 mg of purified IgY may be isolated from just one egg (37); thirdly, the immunological response is more potent with increasing phylogenetic distance between the antigen source and the responding immune system (38–40). Thus, the greater evolutionary distance provides an advantage in using IgY rather than IgG as the primary antibody in some immunoassays. Using IgY, means that the risk of IgG binding to the antigenic site of an epitope, identified through a cross-reaction with a secondary mammalian antibody, minimized (41, 42). Furthermore, chicken IgY does not cross-react with mammalian IgG, but mammalian epitopes are recognized more readily by chicken IgY than by mammalian antibodies (41, 43, 44).
Polyclonal IgY antibodies have a very good storage stability. When IgY antibodies, originating from egg yolk, were stored at + 4°C in saline buffered with phosphate and mixed with 0.02% NaN3, there was no significant loss of antibody titer after 30 years of storage. The activity of affinity-purified and biotinylated IgY antibodies of avian origin was well preserved after 5 years of storage at + 4°C (7). After 6 months of storage the paratope of these purified antibodies was adequately retained.
Chicken IgY is thus an interesting alternative for large-scale production of antibodies for diagnostic purposes. As with mammalian IgG antibodies, IgY has two antigen-binding sites and should precipitate multivalent antigens. Even in the presence of polyethylene glycol 6000, chicken antibodies form antibody-antigen complexes that result in less scatter than a corresponding complex containing mammalian antibodies. Chicken antibodies lack the hinge region of mammalian antibodies and are thus more rigid. This possibly contributes to the lower light-scattering ability of the complexes when chicken antibodies are used free (not bound to particles). Another aspect to consider is that, in the unbound form, avian antibodies have a much steeper decline in the antigen-excess part of the precipitation curve. In principle, sample results should all be on the increasing part of the precipitation curve (left-hand side). If the concentration of analyte in a patient sample is so high that the result is on the right-hand side of the curve, then the result will be similar to that from a sample with a value on the left-hand side, and the instrument will report the lower value. Biomarkers for which patients may have very high values are, for instance, tumor markers, IgG, IgA, IgM, FLC (myeloma patients), and urine albumin. To avoid erroneous results, the methods have a safety margin that ensures that a test result does not surpass the peak value of the precipitation curve. The rapid decline when free (unbound) antibodies are used means that this safety margin is narrow. In order to increase the safety margin, the amounts of antibodies need to be increased. The problems associated with antigen excess have been particularly noted for FLC assays (45). When using IgY, the assay can be used with an automatic rerun at higher sample dilutions when the absorbance value is above 0.75. This will give a good safety margin and minimize the risk of erroneously reporting low values for antigen-excess samples. However, when avian antibodies are used in PETIA, they behave very differently to free antibodies; they give higher turbidimetric readings and have a broader antigen-excess margin. As IgY PETIA assays have a very slow decline from peak value, thereby providing a much broader safety margin, this is clearly a better format for such assays.
Chicken IgY have several biochemical advantages when used for the detection of mammalian or bacterial antigens. Egg yolk antibodies could thus replace mammalian serum antibodies in many immunoassays. Still there is a very limited use of avian antibodies. This is most likely due to tradition. There are a lot more rabbit or mouse antibodies commercially available that can be combined with secondary antibodies labelled with a broad range of detector molecules. The number of publications on the use of chicken antibodies is much more limited compared to mammalian antibodies.
One explanation could be that most researchers are used to bleeding rabbits and handling serum samples. It is much more difficult to draw blood from a hen or a rooster. Chickens have very fragile veins, so a venous puncture usually results in a large haemorrhage in the subcutaneous tissue which blocks the view. Also, chicken have very limited clot retraction. The amount of serum is therefore usually less than 10% of the total blood volume. This can be circumvented by adding an anticoagulant and collect plasma instead of serum. Most researchers are not familiar with the use of yolk as a source of antibodies. The purification of yolk antibodies requires different methodologies to the purification of mammalian antibodies from serum. Other obstacles are that commonly used methods for purification of mammalian antibodies such as protein A and protein G can not be used for purification of avian antibodies.
We have previously shown that IgY-based PETIA reagents gave a stronger turbidimetric reaction than rabbit antibody-based PETIA reagents (46). The strong reaction is probably an important factor for the low CV obtained with the IgY-based PETIA for analyzing for cystatin C. Despite the low plasma sample volumes (3 µl) used in the current study, we observed %CVs of 0.58% for endpoint measurements and 0.50% for rate determinations. The CV for rate measurements is lower than for endpoint measurements. Thus, it is not certain that the best results are always obtained with rate measurements.
Yolk antibodies are usually pooled over time and from several animals. This should, theoretically, reduce batch-to-batch variation in the antibodies produced. In our experience, batch-to-batch variation of the cystatin C immunoparticles was below 3% over several years (47). Furthermore, storage of eggs at +4°C for up to 180 days did not significantly lower antibody activity in egg yolk as compared to fresh eggs (48).
The lack of reactivity between RF positive sera and IgY-coated immunoparticles observed in our study is supported by previous publications reporting that neither RF nor human anti-mouse IgG antibodies do not react with avian antibodies (7, 49).
In conclusion, using IgY in PETIA assays provides stable results (very low %CV), and IgY-PETIA are preferable to free-IgY immunoprecipitation methods when the analyte antigen is in excess.
Thus, IgY is very well suited to use in PETIA and has several advantages over assays based on mammalian antibodies with respect to performance, RF reactivity, economic factors, and animal welfare considerations.
Data availability statement
The original contributions presented in the study are included in the article/ supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the ethical committee at Uppsala University (01–367). The patients/participants provided their written informed consent to participate in this study.
Author contributions
AL and ME conceptualized the study. AL and AC designed the experiments, collated the assay results, and performed the data analysis. AL, AC, and ME wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The project was supported by funding from the Uppsala University Research Fund. The kappa free light chain study was supported by Eurostars (grant number 114334).
Acknowledgments
We are grateful to scientific staff at NABAS and Uppsala University Hospital for assistance in running the assays. Lucy J. Robertson is acknowledged for critical comment and revision in preparation of this manuscript.
Conflict of interest
AC is an employee of NABAS, a chicken IgY manufacturer and supplier, and AL is a board member of IgY Lab Systems AB, a chicken IgY manufacturer.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Bargnoux AS, Cavalier E, Cristol JP, Simon N, Dupuy AM, Garrigue V, et al. Cystatin c is a reliable marker for estimation of glomerular filtration rate in renal transplantation: Validation of a new turbidimetric assay using monospecific sheep antibodies. Clin Chem Lab Med (2011) 49(2):265–70. doi: 10.1515/cclm.2011.043
3. Grubb A, Nyman U, Björk J, Lindström V, Rippe B, Sterner G, et al. Simple cystatin c-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the counahan-barratt prediction equations for children. Clin Chem (2005) 51(8):1420–31. doi: 10.1373/clinchem.2005.051557
4. Sheng Y-J, Ni H-J, Zhang H-Y, Li Y-H, Wen K, Wang Z-H. Production of chicken yolk igy to sulfamethazine: Comparison with rabbit antiserum igg. Food Agric Immunol (2015) 26(3):305–16. doi: 10.1080/09540105.2014.914468
5. Larsson A, Sjöquist J. Chicken antibodies: A tool to avoid false positive results by rheumatoid factor in latex fixation tests. J Immunol Methods (1988) 108(1-2):205–8. doi: 10.1016/0022-1759(88)90420-6
6. Larsson A, Mellstedt H. Chicken antibodies: A tool to avoid interference by human anti-mouse antibodies in Elisa after in vivo treatment with murine monoclonal antibodies. Hybridoma (1992) 11(1):33–9. doi: 10.1089/hyb.1992.11.33
7. Carlander D, Stålberg J, Larsson A. Chicken antibodies: A clinical chemistry perspective. Ups J Med Sci (1999) 104(3):179–89. doi: 10.3109/03009739909178961
8. Schade R, Calzado EG, Sarmiento R, Chacana PA, Porankiewicz-Asplund J, Terzolo HR. Chicken egg yolk antibodies (Igy-technology): A review of progress in production and use in research and human and veterinary medicine. Altern Lab Anim (2005) 33(2):129–54. doi: 10.1177/026119290503300208
9. Aldenbratt A, Lindberg C, Johannesson E, Hammarsten O, Svensson MK. Estimation of kidney function in patients with primary neuromuscular diseases: Is serum cystatin c a better marker of kidney function than creatinine? J Nephrol (2022) 35(2):493–503. doi: 10.1007/s40620-021-01122-x
10. Helmersson-Karlqvist J, Lipcsey M, Ärnlöv J, Bell M, Ravn B, Dardashti A, et al. Addition of cystatin c predicts cardiovascular death better than creatinine in intensive care. Heart (2022) 108(4):279–84. doi: 10.1136/heartjnl-2020-318860
11. Wallentin L, Eriksson N, Olszowka M, Grammer TB, Hagström E, Held C, et al. Plasma proteins associated with cardiovascular death in patients with chronic coronary heart disease: A retrospective study. PloS Med (2021) 18(1):e1003513. doi: 10.1371/journal.pmed.1003513
12. Hijazi Z, Granger CB, Hohnloser SH, Westerbergh J, Lindbäck J, Alexander JH, et al. Association of different estimates of renal function with cardiovascular mortality and bleeding in atrial fibrillation. J Am Heart Assoc (2020) 9(18):e017155. doi: 10.1161/jaha.120.017155
13. Shlipak MG, Coca SG, Wang Z, Devarajan P, Koyner JL, Patel UD, et al. Presurgical serum cystatin c and risk of acute kidney injury after cardiac surgery. Am J Kidney Dis (2011) 58(3):366–73. doi: 10.1053/j.ajkd.2011.03.015
14. Soveri I, Helmersson-Karlqvist J, Fellström B, Larsson A. Day-to-Day variation of the kidney proximal tubular injury markers urinary cystatin c, Kim1, and ngal in patients with chronic kidney disease. Ren Fail (2020) 42(1):400–4. doi: 10.1080/0886022x.2020.1757463
15. Carlsson L, Ronquist G, Ronquist G, Eliasson R, Egberg N, Larsson A. Association of cystatin c with prostasomes in human seminal plasma. Int J Androl (2011) 34(4):363–8. doi: 10.1111/j.1365-2605.2010.01090.x
16. Nilsson E, Bodolea C, Gordh T, Larsson A. Cerebrospinal fluid cathepsin b and s. Neurol Sci (2013) 34(4):445–8. doi: 10.1007/s10072-012-1022-0
17. Dulai PS, Battat R, Barsky M, Nguyen NH, Ma C, Narula N, et al. Incorporating fecal calprotectin into clinical practice for patients with moderate-to-Severely active ulcerative colitis treated with biologics or small-molecule inhibitors. Am J Gastroenterol (2020) 115(6):885–94. doi: 10.14309/ajg.0000000000000596
18. Lee JM, Jang JH, Ryu JH, Yoo J, Lee BI, Kim SJ, et al. A comparison of diagnostic performance between two quantitative rapid fecal calprotectin assays in detecting active inflammatory bowel disease. PloS One (2021) 16(8):e0255974. doi: 10.1371/journal.pone.0255974
19. Kittanakom S, Shajib MS, Garvie K, Turner J, Brooks D, Odeh S, et al. Comparison of fecal calprotectin methods for predicting relapse of pediatric inflammatory bowel disease. Can J Gastroenterol Hepatol (2017) 2017:1450970. doi: 10.1155/2017/1450970
20. Nilsen T, Sunde K, Hansson LO, Havelka AM, Larsson A. A novel turbidimetric immunoassay for fecal calprotectin optimized for routine chemistry analyzers. J Clin Lab Anal (2017) 31(4):e22061. doi: 10.1002/jcla.22061
21. Pelkmans LPJ, de Groot MJM, Curvers J. Analytical performance and clinicopathologic correlation of four fecal calprotectin methods. Am J Clin Pathol (2019) 152(3):392–8. doi: 10.1093/ajcp/aqz051
22. Garnett E, Pagaduan J, Rajapakshe D, Tam E, Kellermayer R, Devaraj S. Validation of the newly fda-approved buhlmann fcal turbo assay for measurement of fecal calprotectin in a pediatric population. Pract Lab Med (2020) 22:e00178. doi: 10.1016/j.plabm.2020.e00178
23. Lin L, Wyness SP, Jensen R, Bird J, Norgyal T, Jensen G, et al. Comparison of next-generation assays for fecal calprotectin vs the phical assay. Am J Clin Pathol (2022) 157(2):252–6. doi: 10.1093/ajcp/aqab114
24. Enderle LL, Köller G, Heilmann RM. Verification of the fcal turbo immunoturbidimetric assay for measurement of the fecal calprotectin concentration in dogs and cats. J Vet Diagn Invest (2022) 34(5):813–24. doi: 10.1177/10406387221114031
25. Åsberg A, Løfblad L, Felic A, Hov GG. Measuring calprotectin in plasma and blood with a fully automated turbidimetric assay. Scand J Clin Lab Invest (2019) 79(1-2):50–7. doi: 10.1080/00365513.2018.1550810
26. Udegbune M, Sharrod-Cole H, Townsend S, Allen B, Brookes MJ, Ford C, et al. Diagnostic performance of serum calprotectin in discriminating active from inactive ulcerative colitis in an outpatient setting. Ann Clin Biochem (2022), 45632221116830. doi: 10.1177/00045632221116830
27. García de Guadiana-Romualdo L, Rodríguez Rojas C, Morell-García D, Andaluz-Ojeda D, Rodríguez Mulero MD, Rodríguez-Borja E, et al. Circulating levels of calprotectin, a signature of neutrophil activation in prediction of severe respiratory failure in covid-19 patients: A multicenter, prospective study (Calcov study). Inflamm Res (2022) 71(1):57–67. doi: 10.1007/s00011-021-01516-4
28. Bauer W, Diehl-Wiesenecker E, Ulke J, Galtung N, Havelka A, Hegel JK, et al. Outcome prediction by serum calprotectin in patients with covid-19 in the emergency department. J Infect (2021) 82(4):84–123. doi: 10.1016/j.jinf.2020.11.016
29. Luis García de Guadiana R, Mulero MDR, Olivo MH, Rojas CR, Arenas VR, Morales MG, et al. Circulating levels of gdf-15 and calprotectin for prediction of in-hospital mortality in covid-19 patients: A case series. J Infect (2021) 82(2):e40–e2. doi: 10.1016/j.jinf.2020.08.010
30. Havelka A, Sejersen K, Venge P, Pauksens K, Larsson A. Calprotectin, a new biomarker for diagnosis of acute respiratory infections. Sci Rep (2020) 10(1):4208. doi: 10.1038/s41598-020-61094-z
31. Larsson A, Tydén J, Johansson J, Lipcsey M, Bergquist M, Kultima K, et al. Calprotectin is superior to procalcitonin as a sepsis marker and predictor of 30-day mortality in intensive care patients. Scand J Clin Lab Invest (2020) 80(2):156–61. doi: 10.1080/00365513.2019.1703216
32. Hillström A, Hagman R, Söder J, Häggström J, Ljungvall I, Kjelgaard-Hansen M. Validation and application of a canine-specific automated high-sensitivity c-reactive protein assay. J Vet Diagn Invest (2015) 27(2):182–90. doi: 10.1177/1040638715575751
33. Löfqvist K, Kjelgaard-Hansen M, Nielsen MBM. Usefulness of c-reactive protein and serum amyloid a in early detection of postoperative infectious complications to tibial plateau leveling osteotomy in dogs. Acta Vet Scand (2018) 60(1):30. doi: 10.1186/s13028-018-0385-5
34. Akita EM, Nakai S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic e. Coli Strain. J Immunol Methods (1993) 160(2):207–14. doi: 10.1016/0022-1759(93)90179-b
35. Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem (2001) 47(4):673–80. doi: 10.1093/clinchem/47.4.673
36. Bhanushali JK, Gilbert JM, McDougald LR. Simple method to purify chicken immunoglobulin G. Poult Sci (1994) 73(7):1158–61. doi: 10.3382/ps.0731158
37. Tressler RL, Roth TF. Igg receptors on the embryonic chick yolk sac. J Biol Chem (1987) 262(32):15406–12. doi: 10.1016/S0021-9258(18)47740-X
38. Olovsson M, Larsson A. Biotin labelling of chicken antibodies and their subsequent use in Elisa and immunohistochemistry. Comp Immunol Microbiol Infect Dis (1993) 16(2):145–52. doi: 10.1016/0147-9571(93)90007-r
39. Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem (1999) 45(7):942–56. doi: 10.1093/clinchem/45.7.942
40. Larsson A. Peroxidase-labelling of chicken antibodies. Food Agric Immunol (1999) 11(1):43–9. doi: 10.1080/09540109999906
41. Svendsen Bollen L, Crowley A, Stodulski G, Hau J. Antibody production in rabbits and chickens immunized with human igg. A comparison of titre and avidity development in rabbit serum, chicken serum and egg yolk using three different adjuvants. J Immunol Methods (1996) 191(2):113–20. doi: 10.1016/0022-1759(96)00010-5
42. Larsson A, Bålöw RM, Lindahl TL, Forsberg PO. Chicken antibodies: Taking advantage of evolution-a review. Poult Sci (1993) 72(10):1807–12. doi: 10.3382/ps.0721807
43. Song CS, Yu JH, Bai DH, Hester PY, Kim KH. Antibodies to the alpha-subunit of insulin receptor from eggs of immunized hens. J Immunol (1985) 135(5):3354–9.
44. Hädge D, Ambrosius H. Evolution of low molecular weight immunoglobulins–iv. igy-like immunoglobulins of birds, reptiles and amphibians, precursors of mammalian iga. Mol Immunol (1984) 21(8):699–707. doi: 10.1016/0161-5890(84)90022-1
45. Bosmann M, Kössler J, Stolz H, Walter U, Knop S, Steigerwald U. Detection of serum free light chains: The problem with antigen excess. Clin Chem Lab Med (2010) 48(10):1419–22. doi: 10.1515/cclm.2010.283
46. Hansson LO, Flodin M, Nilsen T, Caldwell K, Fromell K, Sunde K, et al. Comparison between chicken and rabbit antibody based particle enhanced cystatin c reagents for immunoturbidimetry. J Immunoassay Immunochem (2008) 29(1):1–9. doi: 10.1080/15321810701734644
47. Larsson A, Hansson LO, Flodin M, Katz R, Shlipak MG. Calibration of the Siemens cystatin c immunoassay has changed over time. Clin Chem (2011) 57(5):777–8. doi: 10.1373/clinchem.2010.159848
48. Nilsson E, Stålberg J, Larsson A. Igy stability in eggs stored at room temperature or at +4°C. Br Poult Sci (2012) 53(1):42–6. doi: 10.1080/00071668.2011.646951
Keywords: chicken IgY, cystatin C, free light chains, immunoassays, particle enhanced turbidimetric assay, PETIA
Citation: Larsson A, Campbell A and Eriksson M (2022) Chicken antibodies are highly suitable for particle enhanced turbidimetric assays. Front. Immunol. 13:1016781. doi: 10.3389/fimmu.2022.1016781
Received: 11 August 2022; Accepted: 27 September 2022;
Published: 11 October 2022.
Edited by:
Marcin Sienczyk, Wrocław University of Science and Technology, PolandReviewed by:
Zachary R. Stromberg, Pacific Northwest National Laboratory (DOE), United States;Salomo Hutahaean, Universitas Sumatera Utara, IndonesiaCopyright © 2022 Larsson, Campbell and Eriksson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anders Larsson, QW5kZXJzLkxhcnNzb25AYWthZGVtaXNrYS5zZQ==
 Anders Larsson
Anders Larsson Andrew Campbell2
Andrew Campbell2