
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 12 September 2022
Sec. Alloimmunity and Transplantation
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1004787
 Yuanfeng Zhang1,2†
Yuanfeng Zhang1,2† Xin Chen1†
Xin Chen1† Donglin Yang1
Donglin Yang1 Aiming Pang1
Aiming Pang1 Rongli Zhang1
Rongli Zhang1 Qiaoling Ma1
Qiaoling Ma1 Weihua Zhai1
Weihua Zhai1 Yi He1
Yi He1 Jialin Wei1
Jialin Wei1 Erlie Jiang1
Erlie Jiang1 Mingzhe Han1
Mingzhe Han1 Sizhou Feng1*
Sizhou Feng1*Whether infections before transplantation impair the survival of patients with severe aplastic anemia (SAA) remains unclear. The aim of this retrospective cohort analysis was to compare survival between patients with SAA who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) with infection (n=66) and patients without infection (n=189) from one medical center. There were no differences in baseline characteristics, except that more patients in the infection group were diagnosed with VSAA (59.09% vs. 30.69%, P<0.001), and their grafts were more peripheral blood stem cells (89.39% vs. 76.72%, P=0.042). In addition, the percentage of patients with multidrug-resistant organism colonization or infection in the infection group was larger (16.7% vs. 0.5%, P<0.001). The median days of engraftment were similar between the two groups; however, the 28-day engraftment rates of neutrophils and platelets were lower in the infection group. No differences were observed in terms of grades II–IV acute graft-versus-host disease (aGVHD) (P=0.418), grades III–IV aGVHD (P=0.075), mild to severe chronic GVHD (cGVHD) (P=0.899), and moderate to severe cGVHD (P=0.342). Patients in the infection group had more bloodstream infections before engraftment (28.8% vs. 15.3%, P=0.016), and the primary cause of death was infection instead of aGVHD in contrast to patients without infection (16.7% vs. 4.2%, P=0.002). Finally, the estimated overall survival (OS), failure-free survival (FFS), and GVHD-free FFS at 5 years were 63% (95% CI, 51–78), 60% (95% CI, 47–74), and 55% (95% CI, 43–70) in patients with infection before transplantation versus 86% (95% CI, 81–92) (P<0.001), 82% (95% CI, 76–88) (P<0.001), and 75% (95% CI, 69–82) (P=0.003) in patients without infection before transplantation, respectively. Multivariate analysis identified haploidentical HSCT and pre-HSCT anti-infection response, defined as partial remission (PR) or stable disease (SD), as adverse factors of OS and FFS. In conclusion, our study demonstrated that SAA patients with infection defined as PR or SD but not complete remission before allo-HSCT showed inferior survival compared with patients without infection. Therefore, more attention should be paid to prophylaxis and complete control of infectious complications before transplantation among SAA patients.
Severe aplastic anemia (SAA) is a life-threatening disease caused mainly by fetal bleeding and recurrent infections due to persistent severe cytopenia. The cornerstone of treatment is considered to reserve normal hematopoiesis by modulating the immune environment through intensive immunosuppressive therapy (IST) or regenerating new blood cells by allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although IST is equal to allo-HSCT in terms of overall survival (OS), its disadvantage is significantly lower failure-free survival (FFS), and treatment failures include no hematological response, relapse, and clonal evolution (1–4); however, the drawbacks of allo-HSCT are high transplant-related mortality (TRM) and potential long-term complications. Patients with previous infections may also have an influence on their choice of therapy. Theoretically, immune and blood count recovery are fundamental treatments for infections. Indeed, compared with IST, allo-HSCT has the advantage of rapid blood recovery, which may outbalance the risk of TRM. Taking advances from supportive care to optimization of conditioning, graft-versus-host disease (GVHD) prophylaxis, and alternative donor transplantation, especially haploidentical donor HSCT (HID-HSCT), SAA patients with infections may be considered a candidate for allo-HSCT. Here, we designed this single-center retrospective study to assess whether infections before allo-HSCT impact the survival of patients with SAA undergoing allo-HSCT.
We collected the clinical data of patients diagnosed as acquired AA and consecutively receiving allo-HSCT from the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College from 2005 to 2020. Of the 255 patients enrolled, 189 patients have not experienced infection before transplantation, while 66 patients suffered infection previously. The latter were considered as those experiencing any infection from diagnosis to conditioning. Inclusion criteria included patients diagnosed as SAA, very SAA (VSAA), or transfusion dependence non-SAA (NSAA), and absence of pregnancy. Exclusion criteria included inherited bone marrow failure syndrome and severe organ dysfunction. Owing to time cost in searching a matched unrelated donor (MUD) and great advancement in HID-HSCT in China, few patients received MUD-HSCT during study period and were not enrolled into this research. All patients or their relatives signed the written informed consent. This study was approved by the Ethics Committees of the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
Since the diagnosis of SAA, prophylaxis for fungal infection was not the routine in our center except when IST was administered. When SAA patients have fever, empirical broad-spectrum antibiotics according to the guideline was given (5, 6). Then, modification guided by clinical response, radiographic findings, and microbiologic cultures was performed.
In our center, an SAA patient of age ≤50 years with a donor would receive MSD-HSCT as the first-line therapy, while older patients or patients without an MSD could undergo MSD-HSCT or HSCT from alternative donors as a salvage therapy after IST failure, and sometimes as the first-line therapy according to the intension of the patients.
Conditioning regimens, GVHD prophylaxis, and infection prophylaxis were consistent with our previous studies (4, 7).
Since 2019, we have performed rectal and nasal swabs to screen multidrug-resistant organism (MDRO) colonization before transplantation and then routinely screened for MDRO every week.
A temperature of ≥38.3°C for ≥1 h was considered to be fever (8).The definitions of SAA, VSAA, and NSAA were in accordance with the guideline (9). The duration of administrating antibiotics or antifungal against infection was considered as the course of anti-infection. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were defined according to guidelines (10, 11). The definitions of invasive fungal diseases (IFDs) were in accordance with a consensus, which consisted of proven, probable, and possible IFD (12). Anti-infection responses were classified as complete remission (CR), partial remission (PR), or stable disease (SD) according to the literature (13). CR was defined as the disappearance of all clinical, microbiological, and radiological criteria; PR was defined as an improvement in the above criteria; and SD was defined as no improvement in the criteria above. Cytomegalovirus (CMV) viremia was monitored by plasma CMV DNA testing with real-time PCR every week; it was considered positive when >600 copies/mL CMV in two consecutive tests or >1000 copies/mL CMV in a single test was detected on peripheral blood as previously reported (14), and preemptive therapy was initiated in high-risk patients. The days of neutrophil and platelet engraftment were defined as the first day of three consecutive days with neutrophil count >0.5×109/L unsupported by G-CSF and seven consecutive days with platelet count >20×109/L without transfusion. Primary graft rejection (GR) was defined as failure of neutrophil engraftment until day 28, whereas secondary GR was defined as loss of graft function after initial engraftment. TRM was defined as death without disease progression. OS was defined as survival at the last follow-up. Post-transplantation treatment failures include death, GR, relapse, or clone evolution. FFS was defined as survival with a response. GVHD-free and failure-free survival (GFFS) was defined as survival with response and without grades III–IV aGVHD or moderate to severe cGVHD.
The main objective of this study was to compare the outcomes of patients with SAA with or without infections before allo-HSCT.
The patients included had follow-up through electronic databases, outpatient departments, or telephones. The final follow-up was conducted on February 31, 2022. Groups were compared using the Mann–Whitney U test for continuous variables and the chi-square or Fisher’s exact test when the number of subjects was fewer than 5 for categorical variables. The median follow-up was calculated using the reverse Kaplan–Meier method. Gray’s test was used to calculate the cumulative incidence of GVHD, with death from any cause and GR as competing events. The Kaplan–Meier method was used to estimate the probabilities of OS and FFS, and the groups were compared using the log-rank test. Variables with P values ≤ 0.05 in the univariate analysis were included in a Cox proportional hazards model to identify factors impacting OS and FFS. Data analysis was performed using R software (R 4.1.2), SPSS 20.0, and GraphPad Prism 5. Figures were generated using GraphPad Prism 5. All P values were two-sided, and factors were recognized as independent predictors when the P value was <0.05.
As shown in Table 1, no differences were observed between the two groups in the donor type, patient age, patient sex, donor sex, presence of paroxysmal nocturnal hemoglobinuria clones, previous IST, interval from diagnosis to transplantation, donor age, anti-thymocyte globulin source, conditioning regimen, years of transplantation, and amounts of mononuclear cells and CD34+ cells infused. However, more patients in the infection group were diagnosed with VSAA (59.09% vs. 30.69%, P<0.001) and received peripheral blood stem cells as grafts (89.39% vs. 76.72%, P=0.042). In addition, the percentage of patients with MDRO colonization or infection in the infection group was larger (16.7% vs. 0.5%, P<0.001). The details of the patients with previous infections are shown in Table 2. Before transplantation, 18, 42, and 6 patients had CR, PR, and SD, respectively, according to the anti-infection response. Invasive pulmonary fungal diseases, bacterial pneumonia, and bloodstream infections (BSIs) account for the majority of infections. Among the 51 evaluable patients, 45 were administered with carbapenem antibiotics, and the median course of anti-infection was 54 days, ranging from 3 to 324 days. One patient with VSAA presented with invasive pulmonary and paranasal sinus fungal diseases and soft tissue and gingival infection at diagnosis, and his anti-infection course persisted for nearly 1 year before allo-HSCT. At the last follow-up 769 days post-HSCT, the patient was alive without active infection.
No differences were observed between the two groups with regard to the GR. One and two patients experienced primary GR, while two and eight patients experienced secondary GR in the infection and non-infection groups, respectively (P=0.89). On day 28 post-transplantation, neutrophil and platelet engraftment was achieved in 61 (92.42%) and 48 (72.73%) of 68 patients in the infection group versus 188 (99.47%) (P=0.005) and 162 (85.71%) (P=0.028) of 188 patients in the non-infection group. The median time for neutrophil and platelet recovery was 12 (range, 9–23) and 15 (range, 9–201) days in the infection group compared with 12 (range, 8–23) and 13 (range, 7–83) days in the non-infection group, respectively, which showed no statistically significant difference.
More patients in the infection group were subjected to BSIs before engraftment (28.8% vs. 15.3%, P=0.016), while there was no statistical difference between the two groups with regard to CMV viremia post-transplantation (41.8% vs. 27.27%, P=0.052).
At 100 days post-HSCT, the cumulative incidences of grades II–IV and grades III–IV aGVHD were 26% (95% confidence interval [CI], 7–41) and 15% (95% CI, 0–28) in the infection group versus 21% (95% CI, 10–30) (P=0.418) (Figure 1A) and 8% (95% CI, 0–14) (P=0.075) (Figure 1B) in the non-infection group. At 5 years post-HSCT, the cumulative incidences of mild-to-severe cGVHD and moderate-to-severe cGVHD were 18% (95% CI, 0–33) and 10% (95% CI, 0–19) in the infection group versus 18% (95% CI, 5–28) (P=0.899) (Figure 1C) and 6% (95% CI, 0–11) (P=0.342) (Figure 1D) in the non-infection group, respectively.
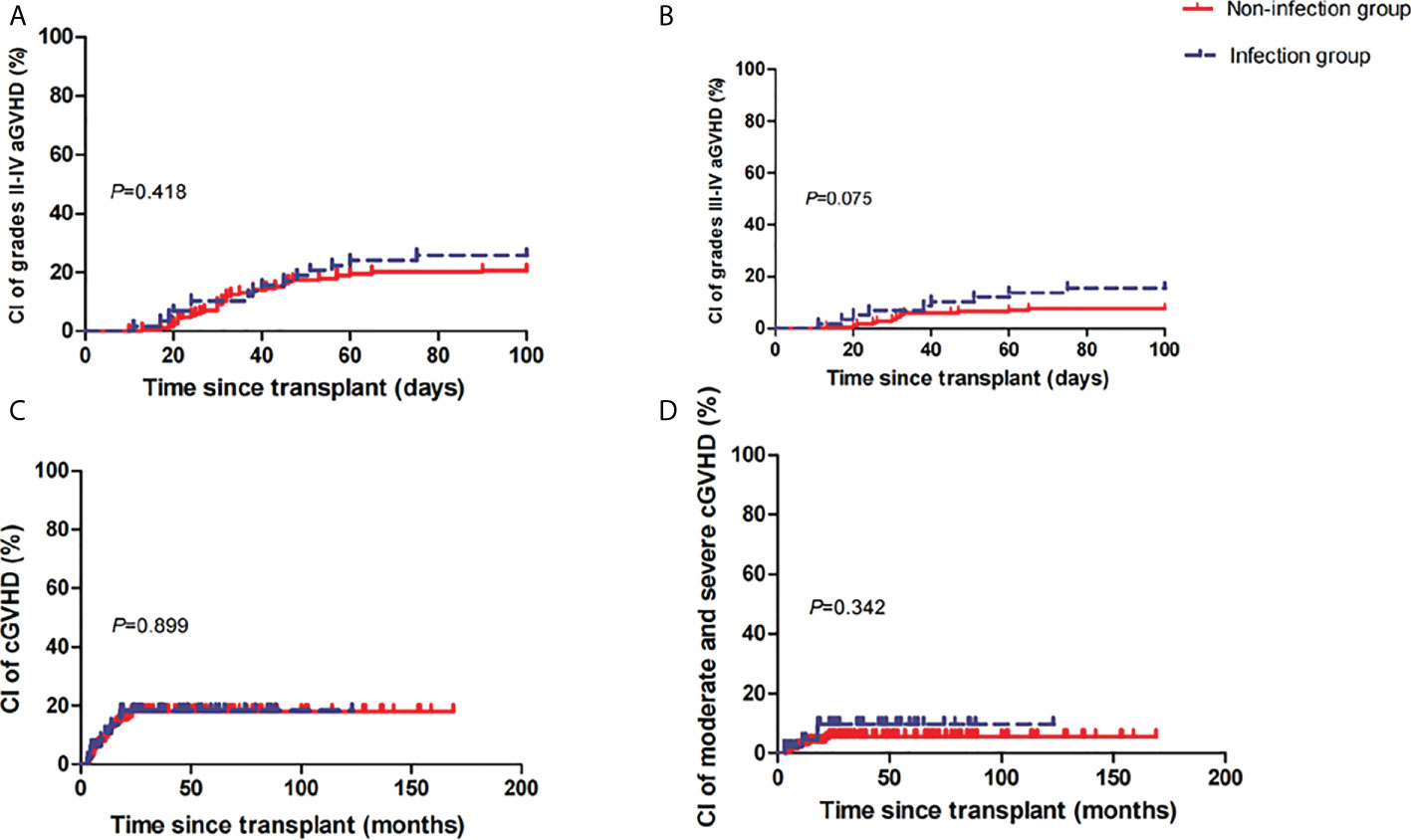
Figure 1 Cumulative incidences (CIs) of grades II–IV acute graft-versus-host disease (aGVHD) (A), grades III–IV aGVHD (B), mild-to-severe chronic GVHD (cGVHD) (C), and moderate-to-severe cGVHD (D) between the two groups.
In total, 22 and 25 patients died in the infection and non-infection groups, respectively, and the leading primary causes of death in the two groups were infection (n=11) and aGVHD (n=11), respectively (Table 3). More patients in the infection group died due to infection (16.7% vs. 4.2%, P=0.002). Strikingly, there were six early deaths before engraftment in the infection group compared with none of the patients in the non-infection group (P<0.001, Table 1). Among the 11 patients with MDRO colonization or infection before transplantation in the infection group, 4 patients developed BSIs caused by the same pathogen. Eventually, six patients died: infection in four patients, aGVHD in one patient, and GR in one patient.
The estimated OS rates at 5 years in the infection group were 63% (95% CI, 51–78) and 86% (95% CI, 81–92) in the non-infection group (P<0.001) (Figure 2A). Accordingly, the estimated FFS and GFFS rates at 5 years were 60% (95% CI, 47–74) and 55% (95% CI, 43–70), and 82% (95% CI, 76–88) (P<0.001) (Figure 2B) and 75% (95% CI, 69–82) (P=0.003) (Figure 2C), respectively. Moreover, the estimated OS rate at 5 years among VSAA patients with infection (n=39) was 56% (95% CI, 36–76), which was lower than SAA or NSAA patients with infection (n=27) (74%, [95% CI, 61–94]); however, the P value was not significant (0.332). In multivariate analysis, HID-HSCT and anti-infection response, defined as PR or SD, respectively, were adverse factors of OS and FFS (Table 4).
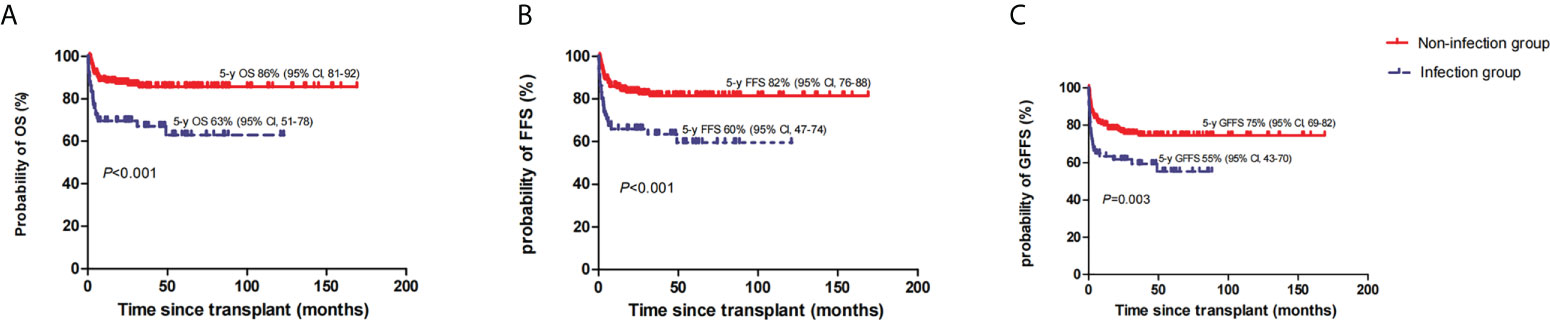
Figure 2 The estimated 5-year overall survival (OS) (A), failure-free survival (FFS) (B), and GVHD-free, failure-free survival (GFFS) (C) between the two groups.
Whether and when to perform allo-HSCT for patients with SAA with infection remains uncertain. Sometimes, it is difficult to control infections in an immunocompromised state, and recurrent infection is an intractable issue. In contrast to IST, allo-HSCT can rapidly restore hematopoiesis. Nevertheless, we suggest that patients with SAA and incompletely controlled infections before transplantation experienced inferior survival.
In our study, we noticed that patients with VSAA were more likely to have infections before transplantation. This finding is in line with the results of Liu et al. (15). In addition, the absolute neutrophil count may represent the potential residual hematopoiesis of bone marrow, and VSAA patients may exhibit a poor response to IST (16, 17). Therefore, prompt infection prophylaxis and timely effective therapy for patients with VSAA may reduce the risk of infection and improve survival. Alternative donors HSCT, including HID-HSCT, can be considered for patients without an MSD (2, 3, 18, 19). It has the advantages of universal and immediate availability of donors. In our previous study, we showed that HID-HSCT had a comparable OS but superior FFS compared with IST (4). Xu et al. (2) and Liu et al. (3) also reported similar results, demonstrating that HID-HSCT was superior to IST in terms of FFS. However, in the current study, we identified HID-HSCT as a risk factor for survival, which was different from that reported by Xu et al. (18). This difference may be ascribed to HID-HSCT as salvage therapy and a longer interval from diagnosis to therapy in our patients (5 months vs. 1.5 months) (4).
In contrast to other studies (15, 20), we found that infection, defined as PR or SD, was an adverse factor of OS and FFS. Xu et al. showed that 51 SAA patients with PR or SD before transplantation had similar results in terms of 3-year OS (85.4% vs. 92.9%, P=0.530) and 3-year FFS (82.7% vs. 92.9%, P=0.458) compared with 14 patients with CR before transplantation. MDRO information was not available in this study, and the only factor impacting OS in the multivariate analysis was an ECOG score of 3 pre-HSCT (20). Liu et al. also reported comparable survival of 130 SAA patients with grade I to IV infections, defined by the Common Terminology Criteria for Adverse Events Version 4.0, versus 300 SAA patients without infection. They also found that more patients died from infection in the infection group (14.29% vs. 5.33%, P=0.002), as demonstrated in our study (15). The reason for these variances may be that more patients had severe and complex infections in our study. A total of 11 patients had MDRO colonization or infection, and 12 patients had greater than or equal to three sites of infection. In addition, our patients with infection had a longer interval from diagnosis to transplantation (median of 3 months). From another point of view, more patients in our study encountered pre-engraftment BSIs than in the study by Liu et al (28.8% vs. 20.3%) (15).
There were several reasons for the higher mortality rate in the infection group. First, more attention should be paid to patients with MDRO colonization or infection. As reported, among acute myeloid leukemia patients undergoing allo-HSCT, the 5-year OS was inferior for those with MDRO colonization compared with non-colonized patients (43.3 vs. 65.5%, P=0.002), mainly due to higher non-relapse mortality (33.9 vs. 9.4%, P<0.001) related to infections (15.5% vs. 4.9%) (21). Girmenia et al. (22) also reported that colonization with gram-negative MDRO is significantly associated with an increased incidence in BSIs caused by the same pathogen. Studies have also demonstrated that broad-spectrum antibiotic exposure is a risk factor for MDRO colonization, leading to MDRO infections (23–25). In our research, we noticed that 4 out of 11 patients with MDRO colonization or infection developed BSIs caused by the same pathogen, and 3 patients died. Second, patients in the infection group had more pre-engraftment BSIs, which is related to a higher mortality rate (26). Moreover, Girmenia et al. revealed that pre-engraftment gram-negative BSIs were an adverse factor related to death 4 months after allo-HSCT, and gram-negative BSIs accounted for most of our positive cases (15 out of 19 patients). Third, a higher proportion of patients in the infection group experienced grades III–IV aGVHD, but without significant differences between the two groups (P=0.075) and deaths due to aGVHD (P=0.392). Loss of intestinal diversity caused by broad-spectrum antibiotic exposure has been associated with increased aGVHD (27, 28).
Our study had several limitations. First, as this was a retrospective study, selection bias was unavoidable. The higher number of peripheral blood stem cell recipients in the infection group may have led to a higher cumulative incidence of GVHD. Even so, we did not observe statistical difference of grades III–IV aGVHD between the two groups. Second, the advent of drugs for fungal prophylaxis and treatment has contributed to the reduced mortality associated with fungal infections in the past decade. In our study, four patients died before 2010 due to invasive pulmonary (n=3) or cerebral fungal diseases (n=1). However, we could not perform further analyses because of the small sample size.
In conclusion, our study demonstrated that SAA patients with infection defined as PR or SD but not CR before allo-HSCT showed inferior survival compared with patients without infection. Therefore, more attention should be paid to prophylaxis and complete control of infectious complications before transplantation. However, there are no defined recommendations for the prophylactic antibiotic or antimycotic drugs in SAA patients after diagnosis (9, 29). In the future, prospective studies to access the optimal strategy for infection prophylaxis of SAA patients should be explored.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was reviewed and approved by Ethics Committees of Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
SF contributed to the study design and manuscript review. YZ and XC contributed to the data collection and analysis. YZ wrote the manuscript and performed the statistical analyses. XC, DY, AP, RZ, QM, WZ, YH, JW, EJ, MH, and SF contributed to the disease treatment and data collection. All authors contributed to the manuscript and approved the submitted version.
This work was supported by the Haihe Laboratory of Cell Ecosystem Innovation Fund (grant number HH22KYZX0036) and the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant numbers 2021-I2M-1-017 and 2021-I2M-C&T-B-080).
We are grateful to the patients and their family members for their trust in our study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yoshida N, Kobayashi R, Yabe H, Kosaka Y, Yagasaki H, Watanabe K, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica (2014) 99(12):1784–91. doi: 10.3324/haematol.2014.109355
2. Xu ZL, Zhou M, Jia JS, Mo WJ, Zhang XH, Zhang YP, et al. Immunosuppressive therapy versus haploidentical transplantation in adults with acquired severe aplastic anemia. Bone Marrow Transplant (2019) 54(8):1319–26. doi: 10.1038/s41409-018-0410-3
3. Liu L, Zhang Y, Jiao W, Zhou H, Wang Q, Jin S, et al. Comparison of efficacy and health-related quality of life of first-line haploidentical hematopoietic stem cell transplantation with unrelated cord blood infusion and first-line immunosuppressive therapy for acquired severe aplastic anemia. Leukemia (2020) 34(12):3359–69. doi: 10.1038/s41375-020-0933-7
4. Zhang Y, Huo J, Liu L, Shen Y, Chen J, Zhang T, et al. Comparison of hematopoietic stem cell transplantation outcomes using matched sibling donors, haploidentical donors, and immunosuppressive therapy for patients with acquired aplastic anemia. Front Immunol (2022) 13:837335. doi: 10.3389/fimmu.2022.837335
5. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis (2011) 52(4):e56–93. doi: 10.1093/cid/cir073%JClinicalInfectiousDiseases
6. Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, et al. European Guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European conference on infections in leukemia. Haematologica (2013) 98(12):1826–35. doi: 10.3324/haematol.2013.091025
7. Chen X, Wei J, Huang Y, He Y, Yang D, Zhang R, et al. Effect of antithymocyte globulin source on outcomes of HLA-matched sibling allogeneic hematopoietic stem cell transplantation for patients with severe aplastic anemia. Biol Blood Marrow Transplant (2018) 24(1):86–90. doi: 10.1016/j.bbmt.2017.10.007
8. Torres HA, Bodey GP, Rolston KV, Kantarjian HM, Raad II, Kontoyiannis DP. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer-am Cancer Soc (2003) 98(1):86–93. doi: 10.1002/cncr.11478
9. Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol (2016) 172(2):187–207. doi: 10.1111/bjh.13853
10. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant (1995) 15(6):825–8.
11. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-Host disease: I. the 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant (2015) 21(3):389–401:e381. doi: 10.1016/j.bbmt.2014.12.001
12. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis (2020) 71(6):1367–76. doi: 10.1093/cid/ciz1008
13. Quillen K, Wong E, Scheinberg P, Young NS, Walsh TJ, Wu CO, et al. Granulocyte transfusions in severe aplastic anemia: an eleven-year experience. Haematologica (2009) 94(12):1661–8. doi: 10.3324/haematol.2009.010231
14. Liu J, Kong J, Chang YJ, Chen H, Chen YH, Han W, et al. Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoietic stem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin Microbiol Infect (2015) 21(12):1121.e1129–1115. doi: 10.1016/j.cmi.2015.06.009
15. Liu L, Miao M, Chen X, Zhang Y, Lei M, Li B, et al. Outcomes of severe aplastic anemia patients with infection proceeding with allogeneic hematopoietic stem cell transplantation, versus patients without infection. Bone Marrow Transplant (2021) 56(10):2591–4. doi: 10.1038/s41409-021-01398-4
16. Chang MH, Kim KH, Kim HS, Jun HJ, Kim DH, Jang JH, et al. Predictors of response to immunosuppressive therapy with antithymocyte globulin and cyclosporine and prognostic factors for survival in patients with severe aplastic anemia. Eur J Haematol (2010) 84(2):154–9. doi: 10.1111/j.1600-0609.2009.01378.x
17. Valdez JM, Scheinberg P, Nunez O, Wu CO, Young NS, Walsh TJ. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis (2011) 52(6):726–35. doi: 10.1093/cid/ciq245
18. Xu LP, Jin S, Wang SQ, Xia LH, Bai H, Gao SJ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol (2017) 10(1):25. doi: 10.1186/s13045-017-0398-y
19. Zhang XH, Chen J, Han MZ, Huang H, Jiang EL, Jiang M, et al. The consensus from the Chinese society of hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol (2021) 14(1):145. doi: 10.1186/s13045-021-01159-2
20. Xu S, Wu L, Zhang Y, Mo W, Zhou M, Li Y, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of severe aplastic anemia patients with infection: A single-center retrospective study. Biol Blood Marrow Transplant (2018) 24(12):2532–9. doi: 10.1016/j.bbmt.2018.07.018
21. Scheich S, Lindner S, Koenig R, Reinheimer C, Wichelhaus TA, Hogardt M, et al. Clinical impact of colonization with multidrug-resistant organisms on outcome after allogeneic stem cell transplantation in patients with acute myeloid leukemia. Cancer (2018) 124(2):286–96. doi: 10.1002/cncr.31045
22. Girmenia C, Bertaina A, Piciocchi A, Perruccio K, Algarotti A, Busca A, et al. Incidence, risk factors and outcome of pre-engraftment gram-negative bacteremia after allogeneic and autologous hematopoietic stem cell transplantation: An Italian prospective multicenter survey. Clin Infect Dis (2017) 65(11):1884–96. doi: 10.1093/cid/cix690
23. Cattaneo C, Di Blasi R, Skert C, Candoni A, Martino B, Di Renzo N, et al. Bloodstream infections in haematological cancer patients colonized by multidrug-resistant bacteria. Ann Hematol (2018) 97(9):1717–26. doi: 10.1007/s00277-018-3341-6
24. Zhang L, Zhai W, Lin Q, Zhu X, Xiao Z, Yang R, et al. Carbapenem-resistant enterobacteriaceae in hematological patients: Outcome of patients with carbapenem-resistant enterobacteriaceae infection and risk factors for progression to infection after rectal colonization. Int J Antimicrob Agents (2019) 54(4):527–9. doi: 10.1016/j.ijantimicag.2019.06.023
25. Kaundal S, Jandial A, Singh H, Chopra M, Kasudhan KS, Khaire N, et al. Impact of broad-spectrum antibiotic exposures and multidrug-resistant gram-negative bacteremia on hematopoietic cell transplantation outcomes. Transpl Infect Dis (2021) 23(5):e13717. doi: 10.1111/tid.13717
26. Yan CH, Wang Y, Mo XD, Sun YQ, Wang FR, Fu HX, et al. Incidence, risk factors, microbiology and outcomes of pre-engraftment bloodstream infection after hap loidentical hematopoietic stem cell transplantation and comparison with HLA-identical sibling transp lantation. Clin Infect Dis (2018) 67(Suppl 2):S162–s173. doi: 10.1093/cid/ciy658
27. Staffas A, Burgos da Silva M, van den Brink MR. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood (2017) 129(8):927–33. doi: 10.1182/blood-2016-09-691394
28. Han L, Zhang H, Chen S, Zhou L, Li Y, Zhao K, et al. Intestinal microbiota can predict acute graft-versus-Host disease following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2019) 25(10):1944–55. doi: 10.1016/j.bbmt.2019.07.006
Keywords: aplastic anemia, infection, stem cell transplantation, comparison, survival
Citation: Zhang Y, Chen X, Yang D, Pang A, Zhang R, Ma Q, Zhai W, He Y, Wei J, Jiang E, Han M and Feng S (2022) The prognostic impact of previously infectious complications on allogeneic hematopoietic stem cell transplantation for patients with severe aplastic anemia: A single-center, retrospective study. Front. Immunol. 13:1004787. doi: 10.3389/fimmu.2022.1004787
Received: 27 July 2022; Accepted: 22 August 2022;
Published: 12 September 2022.
Edited by:
Tomomi Toubai, Yamagata University, JapanCopyright © 2022 Zhang, Chen, Yang, Pang, Zhang, Ma, Zhai, He, Wei, Jiang, Han and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sizhou Feng, ZG9jdG9yX3N6aGZlbmdAMTYzLmNvbQ==; c3pmZW5nQGloY2Ftcy5hYy5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.