
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 14 November 2022
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1003626
This article is part of the Research Topic Methods in Cancer Immunity and Immunotherapy: 2022 View all 17 articles
Tumor-specific T cells (TSTs) are essential components for the success of personalized tumor-infiltrating lymphocyte (TIL)-based adoptive cellular therapy (ACT). Therefore, the selection of a common biomarker for screening TSTs in different tumor types, followed by ex vivo expansion to clinical number levels can generate the greatest therapeutic effect. However, studies on shared biomarkers for TSTs have not been realized yet. The present review summarizes the similarities and differences of a number of biomarkers for TSTs in several tumor types studied in the last 5 years, and the advantages of combining biomarkers. In addition, the review discusses the possible shortcomings of current biomarkers and highlights strategies to identify TSTs accurately using intercellular interactions. Finally, the development of TSTs in personalized TIL-based ACT for broader clinical applications is explored.
The abundance of antitumor immune cells in tumor-infiltrating lymphocytes (TILs) is positively associated with good prognosis in most tumor types and serves an important role in tumor control (1–4). Analyses of the components of TILs have revealed that the T cells were in different developmental states in the tumor, such as effector T cells, memory T cells, incompetent T cells and exhausted T cells, while only T cells in an activated state were capable of producing actual antitumor effects (5). In fact, activated T cells were classified as tumor-specific T cells [TSTs; recognizing tumor-specific antigens (TSAs), such as cancer/testis antigen 1B and melan-A (MART-1)] and bystander T cells (only recognizing cancer-independent antigens, such as Epstein-Barr virus, human cytomegalovirus or influenza virus antigens) (6, 7). Successful antitumor immune responses after immune checkpoint blockade therapy were considered to require reactivation and clonal expansion of TSTs (8–10). Therefore, efforts have been made to isolate a TST population from TILs for use in adoptive cellular therapy (ACT), although isolating and expanding enough TSTs is another major challenge.
In recent years, the detection and sorting of TSTs using different bioinformatics techniques has stimulated great interest among researchers in identifying biomarkers, and the identification of biomarkers was of great significance in enriching the tumor-specific TIL population, studying endogenous antitumor immune mechanisms, and identifying antigen-specific T-cell receptors (TCRs) or new mutant antigens (11–14). In addition, the observation that lack of clear tumor-specific antigens was associated with the deficiency of TST responses in some tumor types, such as ovarian cancer (15), further emphasizes the necessity of biomarker testing of T cells.
Among the biomarkers of TSTs initially identified in different tumor types, the immunomodulatory suppressor receptor programmed cell death receptor 1(PDCD1), the chronic antigen activation marker CD39 (ENPD1), the tissue-resident marker CD103 (ITGAE) and the costimulatory receptor CD137 (4-1BB, TNFRSF9) were mainly targeted as objects of interest (16–19). CD8+ T cells are recognized as a population with antitumor effects, while CD4+ T cells have the advantage of enhancing the recruitment and effector functions of tumor-specific CD8+ T cells and activating natural killer cells in tumors, which has prompted investigators to screen for TSTs based on both subpopulations combined and individually (Figure 1). At present, there is no clear shared biomarker for TSTs in different tumor types. The present review summarizes several of the most popular biomarkers being studied and assays with potential for clinical biomarker detection in the last 5 years, which is important for the development and clinical application of personalized TIL-based ACT by identifying the common characteristics of different biomarkers to determine the most specific T cell population in different tumor types.
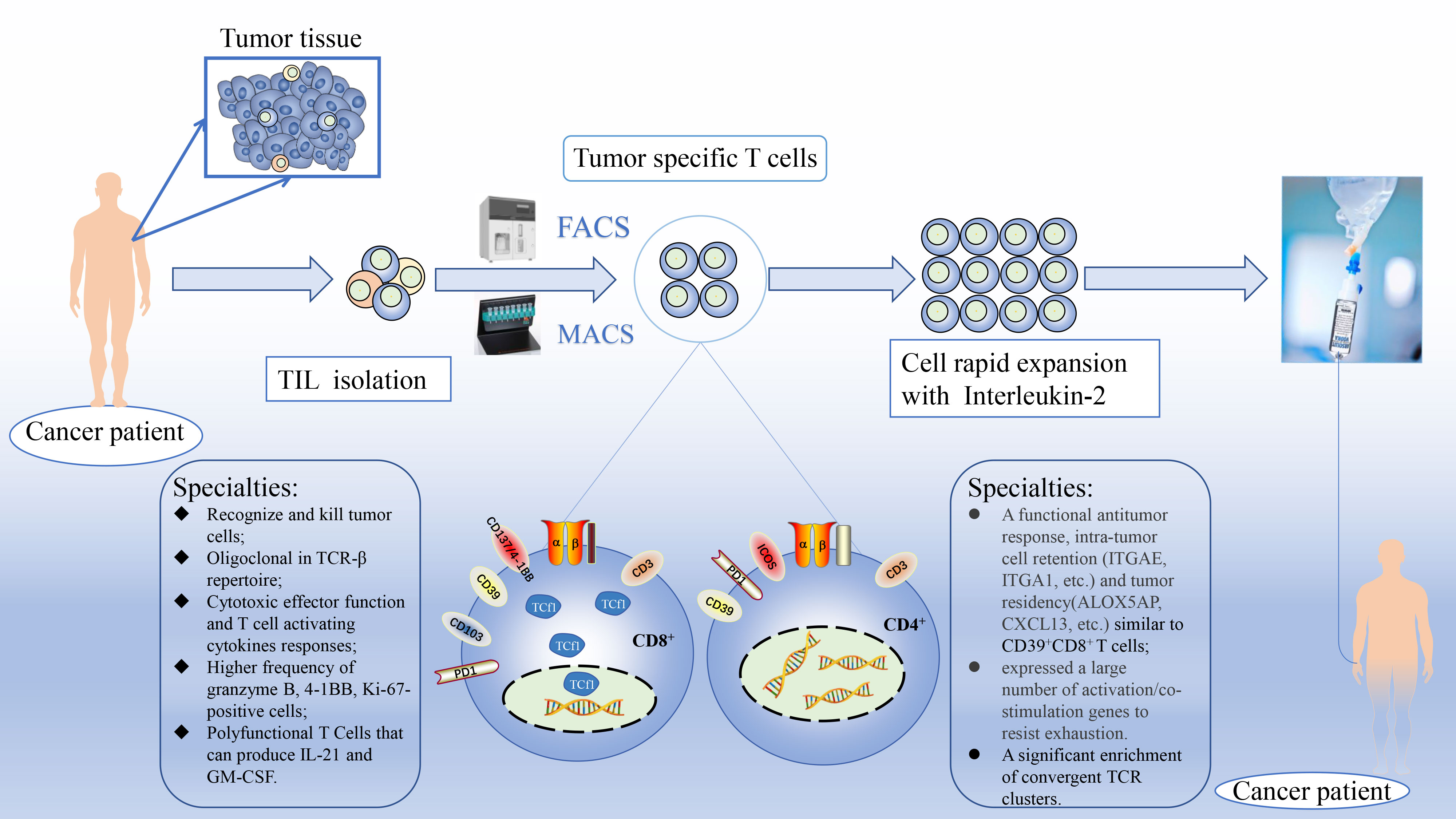
Figure 1 Schematic diagram of personalized enrichment process for TIL therapy. After resect tumor from the patient, the tumor is digested into small fragments or single cell suspensions. Then, sorted out tumor-specific T cells from TIL based on known biomarkers accurately and expanded them in culture with IL-2 rapidly. Finally, TST cultures are expanded to clinically relevant levels and reinfused back into the patient. FACS, fluorescence-activated cell sorting; MACS, magnetic bead-activated cell sorting; Tcf1, T cell factor 1.
Programmed cell death receptor 1 (PD-1) is an important immunosuppressive molecule originally found to be expressed on the surface of T cells, which is highly expressed on all activated T cells. PD-1 induces programmed T-cell death by binding to programmed cell death ligand 1/2 (PD-L1/2) and transducing T-cell failure signals. Therefore, PD-1 has been identified as a molecule that negatively regulates the antitumor immune response of T cells (20). Furthermore, the higher the proportion of PD-1 expression on T cells was, the more terminally exhausted T cells tended to be, and the weaker their proliferative capacity and ability to produce cytotoxic cytokines were, resulting in their impaired or inhibited function in controlling tumor growth (21). High PD-1 expression on the surface of activated T cells in TILs is exploited as a biomarker for the identification of TSTs. Early studies revealed that there was a markedly higher proportion of PD-1+CD8+ T cells than PD-1−CD8+ T cells in melanoma-infiltrating lymphocytes. After sorting and expansion in vitro, the former contained a higher proportion of MART-1-specific T cells, although this cluster was accompanied by impaired effector function, which tentatively demonstrated that tumor-specific CD8+ T cells were predominantly PD-1+ T cells (21, 22). Gros (16) et al. reported that the PD-1+CD8+ TIL population recognized and killed autologous tumor cells compared with their negative controls in six tissues from patients with metastatic melanoma (MM), and the PD-1+CD8+ TIL population with specific TCRβ clonotypes contained clonotypes targeting mutant antigens and reserved the ability to recognize autologous tumor cells, showed highly monoclonal expansion. These findings demonstrated that PD-1 expression on CD8+ TILs also accurately identified a clonally expanded TST repertoire. Further analysis of the TCR-β profiles of both PD-1+ and PD-1− populations in CD8+ T cells revealed that there was hardly any overlap in the TCR profiles of PD-1+ and PD-1−CD8+ T cell subpopulations in at least two human tumor tissues, colorectal cancer (CRC) and breast cancer, thus, it can be concluded that the intrinsic properties of the PD-1+ population determine its ability to recognize tumor antigens specifically (23). A comparison of PD-1+CD8+ TIL and PD-1−CD8+ TIL expansion products in mouse solid tumor models (melanoma and colon cancer) and multiple myeloma models revealed that TSTs existed exclusively in PD-1+CD8+ TIL progeny and inhibited tumor progression. Furthermore, combination with anti-PD-L1 treatment further enhanced the efficacy of TIL therapy, which provided the basis for preclinical experiments on PD-1+CD8+ TIL expansion in ACT (24). High PD-1 expression by TSTs in the peripheral lymphoid organ, the spleen, has also recently been revealed, and peripheral PD-1+CD8+ TSTs were reported to be positively associated with the secretion of IFN-γ in lymphoid tissue after vaccination (25). Likewise, the similarity in tumor antigen specificity and TCR repertoire of PD-1+CD8+ T cells in both circulating peripheral blood and TILs implies that circulating PD-1+CD8+ T lymphocytes could be a window to provide access to TSTs, and thus, PD-1 expression identified diverse patient-specific antitumor T cell responses in the peripheral blood, providing a novel non-invasive strategy for developing personalized therapies for cancer using neoantigen-reactive lymphocytes or TCR-T cells (26–28). In addition to demonstrating the specificity of PD-1+CD8+ TSTs sorted in freshly extracted TILs, a study analyzed the dynamic changes of tumor-specific CD8+ T cells in patients who received CD8+ TIL-transfer therapy and were observed for up to 1 year, and the investigators found that the persisting T cell subpopulations after tumor-specific CD8+ TIL treatment were mostly multifunctional, with a stable partially differentiated phenotype and high expression levels of PD-1 (29). The conclusion suggested that PD-1 can be a stable biomarker for TSTs. Therefore, the aforementioned extensive studies demonstrate the superiority of PD-1 as an individual marker in identifying CD8+ TSTs.
CD39 is an extracellular enzyme encoded by the ectonucleoside triphosphate di phosphohydrolase 1 gene, and early studies found that CD39 participated in a cascade reaction with CD73 for the conversion of ATP to ADP and cAMP, ultimately leading to the production of adenosine, a molecule that produces extracellular immunosuppressive effects (30–32). CD39 expression in T cells has been described as a marker of exhaustion. Early studies of CD39+CD8+ T cells investigated the comparison with their counterpart, CD39−CD8+ T cells, in TILs or metastatic lymph nodes from 33 untreated patients with breast cancer and 4 patients with melanoma, as well as in mouse tumor models (breast cancer and melanoma), the former expressed relatively low levels of TNF-α, IFN-γ and IL-2, and were negatively associated with the secretion of granzyme B and perforin. Additionally, CD39 expression was accompanied by the expression of co-inhibitory receptors (i.e., lymphocyte activating 3, T cell immunoreceptor with Ig and ITIM domains, PD-1, T-cell immunoglobulin mucin family member 3, and 2B4) on T cells and was associated with tumor growth (33, 34). However, in 2018, a study published in Nature reported that the direct distinction between TSTs and bystander T cells within TILs was CD39 expression, which demonstrated that CD39+CD8+ TILs were a population that could recognize tumor neoantigens specifically (7). Based on the publication of the novel insight, a study using whole-exome sequencing algorithms to analyze high-affinity neoantigens (HANs) in tumor tissues from 56 patients with hepatocellular carcinoma revealed that HANs were associated with improved overall survival and the frequency of CD39+CD8+ TILs in patients with hepatocellular carcinoma, and identified HANs− specific CD8+ T cells in CD39+CD8+ TILs, which suggested that HANs triggered antitumor activity by activating tumor-specific CD39+CD8+ T cells, and CD39 could serve as a reliable marker for identifying TSTs (35). During the same year, this team demonstrated that CD39+HBVs− CAR T cells and CD39+ autologous tumor-specific CD8+ T cells induced more apoptosis of tumor cells in the hepatocellular carcinoid organ using CD39 as a marker, which was concluded based on comparisons with the CD39−CD8+ T cell subpopulation (36). In addition, a study of clinical staging of patients with bladder cancer (n=46) revealed that CD39 expression in CD4+/CD8+ T cells was markedly associated with tumor pathological T stage and tumor histology, and CD39+CD8+ T cells presented more potent tumor killing effects by producing higher levels of IFN-γ than other T cell populations (37).
Most recently, Liu et al. (38) revealed that tumor-specific CD4+ T cells differentiated into T helper 1 cells and CD4- T cells, in which CD4- T cells are critical for controlling established tumor metastasis and tumor specific-CD4+ T cells have a synergistic therapeutic effect with PD-L1 blockade. CD39 could alternatively be applied as a marker to identify a population of CD4+ TSTs in three human tissue squamous carcinomas (cervix, vulva and oropharynx). Single-cell RNA sequencing of the CD39+CD4+ conventional T cell cluster revealed the features associated with a functional antitumor response, intra-tumor cell retention (ITGAE, integrin subunit α1, etc.) and tumor residency [arachidonate 5-lipoxygenase, C-X-C motif chemokine ligand 13 (CXCL13), etc.]. CD39+CD4+ TSTs expressed a highly exhausted phenotype but simultaneously expressed a large number of activation/co-stimulation genes that resisted exhaustion to expand in vitro in response to autologous tumor antigens (39). As supported in another high-dimensional profiling study of CD4+ TILs in human cancer tissues, CD39 expression could be used to distinguish tumor-specific CD4+ T cells from diverse CD4+ TILs (e.g., regulatory T cells and bystander CD4+ T cells) (40).
At present, studies have validated the specific antitumor function of CD39+CD8+ T cells in non-small cell lung cancer (NSCLC) tumor tissues, which confirmed that CD39+CD8+ T cells could be the predictor of prognosis in patients with NSCLC after anti-PD-1/PD-L1 therapy (41) and defined the protective prognostic role of CD39high tissue memory CD8+ T cells in luminal-like breast cancer (42). Beyond this, in the latest study on better biomarkers of tumor-specific CD8+ T cells in the peripheral blood circulation, it was determined that three candidate biomarkers, including CD39, might compensate for the low sensitivity of PD-1 that served as a previous marker by single-cell RNA and TCR sequencing, at least in the blood of MC38 tumor-bearing mice and patients with melanoma (43). Therefore, as a chronic local antigen activation marker, the efficacy of CD39 to identify tumor-specific effector T cells deserves in-depth investigation.
CD103 is an integrin molecule, which is expressed mainly on intraepithelial T cells and TILs, and is involved in the migration and residency of T cells in tissues. The latest newly defined tissue-resident memory T cells (TRMs), whose main surface markers are CD103, CD69 and CD49a, are classified as a cluster of memory T cells different from central memory T cells and effector memory T cells, and this population persists in tumor tissues and no longer participates in T cell recirculation (44–47). Intratumor CD8+ TRMs are a subpopulation with identified antitumor functions, in which CD103 is predominantly expressed on the surface, and intra-tumor CD103+ TILs might serve as a prognostic marker for patients with urothelial cell carcinoma of the bladder (48). Therefore, studies on the role of CD103 are mainly based on the CD8+ TRM population. In earlier research, investigators revealed that CD103+CD8+ TRMs expressed high levels of granzyme A, granzyme B, perforin, IFN-γ and TNF-α, as well as the degranulation marker CD107a and the proliferation marker Ki-67 according to the analysis of cytotoxic function-related effector cytokines in ex vivo experiments, suggesting that CD103 was associated with cytotoxic activity in CD8+ T cells (49–52). Since then, several studies have reported that CD103+CD8+ TRMs isolated from TILs of various tumor types were a good prognostic marker for patient survival. Indeed, in immunohistochemistry cohort studies of patients with breast cancer (n=424) and endometrial adenocarcinoma (n=305), the high levels of CD103+ TIL infiltration predicted a good prognosis and prolonged survival (53, 54). In a single-cell analysis of breast cancer-infiltrating T cells, CD8+ TRMs also showed an association with a favorable prognosis in early-stage triple-negative breast cancer (55). In bladder uroepithelial carcinoma, CD103+ TILs have been identified as a predictive marker for overall survival and good prognosis for recurrence-free survival, and the study showed that tumor volume was negatively associated with CD103+ TIL infiltration density (48). A positive association between TRM and good prognosis of hepatocellular carcinoma was also confirmed in a multidimensional analysis of hepatitis B virus-associated hepatocellular carcinoma (56). Analysis of CD103+CD8+ TRMs in a study of NSCLC revealed that their components contained TSTs that recognized TSAs, a subpopulation associated with improved survival and increased intraepithelial lymphocyte infiltration in patients with early-stage NSCLC (57). The higher density of intra-tumoral and stromal CD103+CD8+ TIL cells in oral cancer also predicted an improved prognostic performance, and CD103+CD8+ TILs had the same phenotype as TRMs (58). At present, studies on CD103 as a marker to identify tumor reactive T cells in TILs are getting more and more advanced. On the one hand, given that multiple suppressive immune checkpoint molecules [e.g., PD-1, cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and TIM-3] are highly expressed on the surface of CD103+CD8+ TRMs (59, 60), several studies have identified CD103+CD8+ TRMs as the population serving major antitumor efficacy in patients with different tumor types treated with anti-PD-1/PD-L1 immune checkpoint therapy. It has been demonstrated that a low proportion of CD103+CD8+ TRMs was strongly associated with immunotherapy failure in patients with different tumor types, such as esophageal squamous cell carcinoma, lung cancer and melanoma (18, 61–63), which showed indirectly that the role of CD103+CD8+ TRM subsets in immune checkpoint therapy cannot be ignored. On the other hand, the density of CD103+CD8+ TRMs increased during anti-PD-1 treatment of responsive patients with lung cancer, with the bursting proliferative activity and cytotoxicity (e.g., granzyme B), oligoclonal expansion of the TCR-β profile clonotype and upregulated expression of Aiolos, phosphorylated STAT-3 and IL-17, which were key regulators of T helper 17 cell differentiation (61). Recently, direct evidence was obtained in the transcriptomic analysis of mutation-associated neoantigens (MANA) in lung cancer treated with anti-PD-1 therapy; ~90% of MANA-specific CD8+ T cells had the same signature transcriptional program as TRMs, which indicated the presence of TSTs in the TRM population, and CD103, the marker defining TRMs, might also act as a marker for identifying TSTs (64). In addition to the CD103+CD8+ TRM population, Abd Hamid (65) et al. found that cytotoxic CD8+ T cells (CTLs) maintained CD103 expression by self-secreting TGFβ1 in an activated form, which improved cellular TCR sensitivity as well as cell migration, contributing to the rapid recognition of autologous tumor cell surface antigens and toxic efficacy of CD103+ tumor-specific CTLs in killing tumor cells; however, this population was more susceptible to apoptosis during prolonged exposure to the cancer microenvironment (i.e., the proportion of CD103+ tumor-specific CTLs decreased as the tumor grew). Meanwhile, CD103+ tumor-specific CTLs were found to have a markedly higher basal glycolytic rate and elevated maximal glycolytic capacity in terms of cellular energy metabolism, which also contributed to the induction of more effective and faster antitumor efficacy. Therefore, TSTs, which are a subpopulation of CD103+CD8+ TILs, are essential in the antitumor immune response and favorable prognosis.
CD137 is a member of the tumor necrosis factor receptor family(TNFR) (66) and is expressed on the surface of activated T cells. Binding of CD137 on T cells with its ligand CD137L that expressed on dendritic cells (DCs), activated B cells and macrophages leads to markedly increased T cell proliferation, differentiation and production of effector cytokines. Therefore, CD137 has been described as a surface marker for the identification of activated T cells (67–69). It has been suggested that anti-CD137 treatment could also, promote T cell proliferation independently of CD28 (a costimulatory signaling for T cell activation) and their IL-2 production had similar extent of activation to that shown by the treatment with a combination of anti-CD3 and anti-CD28. However, CD137 could replace the effect of CD28 signaling on T cell activation in certain degree only in the presence of consistent antigenic stimulation (70, 71). Furthermore, it has been demonstrated that CD137L could stimulate human CD28− T cells, leading to the differentiation and proliferation of their cell subpopulation, with subsequent increased expression of cellular inflammatory cytokines IFN-γ, granzyme A and perforin, which ultimately enhanced their cytotoxic effector functions and the expression of the anti-apoptotic protein Bcl-X (72). In vitro enrichment of antigen-specific T cells by three different methods (CD137, CD107a and tetramers) using HLA-A24-restricted CMV pp65 and EBV BRLF1 epitopes as model antigens revealed that CD137-based isolation of antigen-stimulated CD8+ T cells was equivalent to tetramer-based sorting in terms of purity, and superior to the other two methods in terms of subsequent cell expansion (73). As such, tumor-specific effector T cells were successfully isolated from peripheral blood based on the early expression of CD137 on most activated antigen-specific CD8+ T cells without prior knowledge of the specific immunogenic epitopes or HLA-restricted elements identified (74). Similarly, TSTs were present in the population of CD8+ T cells sorted on the basis of CD137 in fresh ovarian cancer tissues, and CD137+CD8+ T cells have been demonstrated to possess specific cell killing ability against autologous tumor cells in vitro and to inhibit the progression of tumors in both melanoma and ovarian cancer NSG mouse models compared with PD-1+ or PD-1−CD137−CD8+ T cells (75). By enriching TILs based on CD137 upregulation of TSTs after in vitro stimulation, 27 tumor-specific TCRs were successfully isolated from 6 patients in a clinical study that identified 14 neoantigens expressed by autologous tumor cells, suggesting that the potential of peripheral blood lymphocytes to become true TSTs by introducing recognized TCRs might be realized in the future (17). After expansion of CD137+ TILs isolated using the magnetic bead sorting technique in vitro, it was found that the expanded cell population showed markedly increased antitumor reactivity and was enriched with T cells that recognized neoantigens as well as shared tumor antigens (76). Interestingly, the expanded products of CD137+ T cells in vitro secreted multiple cellular cytokines, including IFN-γ, TNF-α and IL-2, after co-culture with a mixture of preferentially expressed antigen in melanoma-derived peptides, a specific tumor antigen upregulated in melanoma obtained from the peripheral blood of healthy donors (77). Recently, a systematic analysis of four biomarkers, PD-1, CD39, CD103 and CD137, in ovarian cancer TILs found that PD-1+, CD39+ and CD103+ TILs all contained a subpopulation of CD137+ T cells and CD137+ TILs highly co-expressed the three previous markers, with the highest expression of major histocompatibility complex (MHC)-dependent IFN-γ and other cellular effector cytokines (78). In addition, this subpopulation also uniquely co-expressed the costimulatory molecule CD28, suggesting that CD137+ TILs can recover stronger antitumor potential through combined immune checkpoint blockade (79). Thus, we tentatively hypothesized that CD137 had superior properties as a natural biomarker for identifying tumor-specific effector T cells. In three types of human cancer samples (23 MM, 1 ovarian cancer and 1 sarcoma sample), researchers have recently proposed that application of the combination assays of total intracellular CD137 expression, tumor reactive cytokines (IFN-γ and TNF-α) and scRNA analysis data could obtain the rapid and accurate in vitro identification of a larger proportion of TSTs, such a cell population not only had the specificity to recognize tumor neoantigens but also had immediate tumor responsiveness. Furthermore, in situ detection of the corresponding genes, TNFRSF9, IFNG and TNF, may also be considered as a strategy to rapidly and efficiently identify the functional characteristics of most tumor-specific TILs based on scRNA sequencing data (80). However, TIL expansion in vitro from 16 cases of renal cell carcinoma revealed that, although TILs cultured with autologous tumor single cell suspensions could express a high percentage of CD137, only a small percentage of these cells expressed IFN-γ, TNFα and IL-2 detected by flow cytometry, suggesting that the tumor-specific CD137+ T cell subpopulation might lack tumor responsiveness to some extent (81). In summary, CD137 has the potential to be a natural biomarker for sorting a subset of tumor-specific reactive T cells in vitro, but one also needs to pay attention to possible mechanisms of secretion of dysfunctional cellular effector cytokines by TSTs in different tumor types (Table 1).
To improve tumor reactivity of sorted TIL products in various solid tumors, several researchers have proposed the combination of identified markers to determine tumor-specific effector T cells rather than single molecules to obtain a subpopulation of TILs that recognize tumor antigens more accurately, promoting the widespread clinical application of personalized TIL-based ACT. Therefore, it is meaningful to summarize representative research findings constantly (Table 2).
A study of pulmonary metastatic dormancy in patients with breast cancer revealed that adoptive transferred monoclonal CD39+PD-1+CD8+ T cells prevented distant metastases successively, although the cancer cells were not cleared completely. Notably, CD39+PD-1+CD8+ T cells from the primary tumor and metastatic sites rather than overall CD8+ T cells mediated pulmonary metastatic dormancy, raising the possibility that this cell subpopulation might have better tumor specificity and was positively associated with increased disease-free survival in patients undergoing breast cancer resection (82). A Korean research team reported that CD39+PD-1highCD8+ T cells were highly expressed in epithelial ovarian cancer primary tissues and metastatic sites (n=65). Not only was high PD-1 expression associated with a highly exhausted phenotype, but it was also associated with high activation of this subpopulation and tumor specificity (83). Based on evidence from a publicly available database of melanoma tumor antigen-specific TCR sequences, a previous study determined TCR clonotype clusters and found a marked enrichment of convergent TCR clusters in the CD39+PD-1+ subpopulation of CD4+ and CD8+ TILs (84). In addition to defining TSTs using CD39+PD-1+ in human cancer tissues, researchers also focused on circulating CD39+PD-1+CD4+ T cells in patients with human papillomavirus (HPV)-induced tumors, a cell subpopulation enriched with activated HLA-DR+ and inducible T cell costimulator (ICOS)+ and proliferating KI67+ cells in the peripheral circulation, as well as a high proportion of HPV-specific T cells. Overall, the proportion of circulating CD39+PD-1+CD4+ T cells in patients with HPV-induced tumors treated by immune checkpoint blockade may serve as a predictor of clinical response (85).
In 2018, Duhen (19) et al. reported that CD39+CD103+CD8+ TILs in the primary and metastatic sites of six malignant solid tumors had a unique TCR profile and killed autologous tumor cells effectively in an MHC-I-dependent manner in vitro, which demonstrated that this subpopulation was enriched in tumor-specific effector T cells. Furthermore, the number of infiltrating CD39+CD103+CD8+ T cells in human head and neck squamous cell carcinoma (HNSCC) was positively associated with a good survival prognosis. In addition, auto-neoantigen-specific T cells could be detected in the CD39+CD103+CD8+ TILs in 3 of 7 patients with low mutation burden CRC through whole-exome and transcriptome sequencing of inferred neo-antigenic epitopes from tumor and normal tissues (86). Transcriptional activity and transcript stability of CD39+CD103+CD8+ TRMs in situ in human high-grade endometrial cancer indicated that this cell subpopulation had favorable transcriptional activity and expressed tissue-resident transcriptional profiles in the resting state, and the expression levels of markers of T cell activation were upregulated, and cytolytic activity and cytokine production were increased in the activated state. These results revealed that CD39+CD103+CD8+ TRMs in high-grade endometrial cancer were a multifunctional T cell population with a reactive response pool that included a high degree of tumor responsiveness. Furthermore, highly stable PMA-reactive immune and mitochondrial genes suggested such differential regulation enhanced the rapid responses of this subpopulation upon reactivation (87). Since then, the combination of double positive (referred to as DP here; CD39+CD103+) has been well accepted by more researchers. For example, analysis of the aggregation of TSTs in metastatic lesions of patients with CRC after chemotherapy was performed based on the DP CD8+ T cell subpopulation, and this reaffirmed that DP CD8+ T cells were a cell subpopulation capable of reacting to mutant antigens in primary and metastatic tumors (88). In a phase Ib clinical trial (NCT02274155), researchers applied an anti-OX40 (a costimulatory molecule) agonist antibody (MEDI6469) as an adjuvant therapy prior to surgical resection in 16 patients with HNSCC and evaluated the phenotype of patients’ peripheral blood lymphocytes 2 weeks after administration. The results revealed increased activation (ICOS and CD38) and proliferative capacity (Ki67) in the CD4+ and CD8+ T cells. Tumor biopsies pre- and post-treatment showed increased frequency of activated conventional CD4+ TILs in the majority of patients, which was accompanied by higher oligoclonality in TCRβ sequencing. CD8+ TIL analysis revealed elevated proliferative capacity, and activation as well as the maintenance of the ability to identify tumor antigens specifically of tumor-specific DP T cells in patients (N=4/16) with evaluable tumor tissues, and all patients stayed disease-free (89).
The results of a study evaluating single cell characteristics of TILs in human high-grade plasmacytoid ovarian cancer revealed that triple-positive (CD39+CD103+PD-1+) CD8+ TILs exhibited a highly activating/exhausted phenotype and reduced TCR diversity, and genes involved in lysis cytotoxicity and humoral immunity by comparing the cellular phenotype, clonality and prognostic significance of various combinations of three markers (CD39, CD103 and PD-1). Triple-positive CD8+ TILs could upregulate the expression of the cytokine CXCL13 to generate a tumor microenvironment adapted to coordinate antitumor B-cell responses. Compared with CD39+CD103+ TILs, PD-1 in triple-positive CD8+ TILs was associated with a higher tumor responsiveness and had the most positive impact on good patient prognosis (90). Therefore, it is necessary to define TILs with high tumor responsiveness by combining the three markers to obtain better prognostic significance.
Recent in vitro tumor specificity analysis of PD-1highCD8+ T cells in ovarian cancer tissues revealed a higher frequency of CD137+ T cells in the cell population, despite the low quantity of this subpopulation (91). CD137, a costimulatory molecule in CD8+ TILs in hepatocellular carcinoma tissues, exhibited exclusive expression in highly exhausted PD-1highCD8+ T cells. CD137+PD-1highCD8+ T cells exhibited higher levels of tumor reactivity and T cell activation markers, and were markedly enriched in T cell inflammatory genetic features, including active IFN-γ responses, cytotoxic effector functions and activated cytokines associated with anti-PD-1 treatment responses (92). Ye (75) et al. showed directly that ovarian cancer TSTs existed exclusively in the PD-1+CD137+CD8+ T cell subpopulation rather than the PD-1+CD8+ T cells or PD-1−CD137+CD8+ T cell subpopulation. However, this result is inconsistent with a study of melanoma TILs, which concluded that TSTs existed in both cell populations (16). Therefore, the use of PD-1 as a single marker to screen for TSTs may be controversial and it is not possible to identify universally applicable markers due to differences in tumor types, whereas PD-1+CD137+CD8+ T cells are supposed to be a highly tumor-specific responsive T cell population.
Alspach (93) et al. demonstrated that CD4+ Th cells positive for PD-1 and ICOS were neoantigen-specific in a murine sarcoma tumor model. Recently, when analyzing the expression patterns of PD-1 and ICOS on CD4+ Th TILs in HNSCC and CRC tissues, researchers found that PD-1+ICOS+CD4+ Th TILs exhibited a tissue-resident memory phenotype with an oligoclonal expansion of their TCR repertoire, which occurred in tumors but was present at a low frequency in the periphery. Finally, PD-1+ICOS+CD4+ Th TILs were demonstrated to recognize both tumor-associated antigens and tumor-specific neoantigens (94).
Tcf1 is an intranuclear transcription factor expressed in stem-like CD8+ T cells. Tcf1+CD8+ T cells show a highly proliferative and differentiation potential and are recognized as the most self-renewing population in TILs until now. PD-1 has been identified as a specific molecule that is upregulated on the surface of tumor reactive T cells. The results of a melanoma study identified Tcf1+PD-1+CD8+ T cells as a cluster of tumor reactive TILs with an exhausted phenotype and central memory characteristics by combining the aforementioned two molecules(Tcf1 and PD-1), suggesting that immune checkpoint blockade therapy results not in the functional recovery of highly exhausted cells but in promoting the strong proliferation of Tcf1+PD-1+CD8+ T cells to produce antitumor effects (95). Therefore, future efforts should be devoted to improving the approach to expand this subpopulation during ex vivo rapid expansion to enhance the potential of personalized TIL-based ACT. To further isolate and expand this subset of specific T cells with durable differentiation in vitro, researchers have focused on screening for cell surface markers co-expressed with Tcf1 transcription factors in different tumor types for further exploration. Previous studies have identified surface markers such as C-X-C motif chemokine receptor 5, CD28, Slamf6 (Ly108) and C-C motif chemokine receptor 4 co-expressed with Tcf1 in TILs of gastric, lung, kidney, colorectal, liver and ovarian cancer to screen for stem-like exhausted (Tcf1+PD-1+) CD8+ T cells to some extent. A positive association between this cell cluster and the persistent and durable effectiveness of ACT in patients has also been demonstrated (96–101). In 2020, an article published in Science reported that complete and durable control of MM requires infusion of tumor-specific CD8+T cells with stem cell-like profiles, which were mainly CD39−CD69−CD8+ T cells that co-expressed Tcf1 (102). Therefore, the innovative finding may contribute to the long-term effectiveness of personalized TIL-based ACT in oncology patients.
Liu (103) et al. recently developed the first inter-cellular interaction glycosyltransferase-mediated labeling approach (referred to here as the FucoID strategy), where glycosyltransferase-induced transfer of a fucosylatedbiotin (Fuc-Bio)-based tag to the surface of T cells interacting with DCs accurately identified a TSA-reactive cell population in TILs in mouse tumor models, and this cell population (i.e., PD-1+Bio+CD8+ T cells) with a dysfunctional phenotype (almost all expressing PD-1) also showed marked proliferative and tumor-killing capacity, while a subset of PD-1+Bio+CD8+ T cells (4-18%) had a stem cell-like exhausted phenotype (Tcf1+TIM-3−). In addition, they identified a novel population of bystander T cells (PD-1+Bio− T cells) with completely different transcriptional characteristics compared with the previously defined bystander T cells (PD-1−CD8+T cells), which demonstrated that not all PD-1+ TILs are TSA-responsive T cells. The PD-1+Bio+CD8+ T cells can be directly enriched for expansion and the corresponding TCRs can be isolated to construct TCR-engineered T cells for functional assays. Thus, the FucoID strategy represents genetically manipulation-free protocols and rapid turnaround cycles compared with techniques that depend on bioinformatics-assisted TSA identification.
TSTs have been demonstrated to inhibit tumor progression effectively in several clinical applications. To better sort out the cluster of T cells, researchers profit from the following observations. Firstly, some phenotypes are activated and expressed by T cells undergoing tumor antigen recognition, such as PD-1, CD39, CD103 and CD137, which have been demonstrated to be markers associated with TSTs; secondly, considering tumor reactivity and tumor responsiveness (secretion of antitumor cytokines, release of cytotoxic granules and upregulation of activation markers), some reports suggested that the combination of intracellular CD137-specific activation marker and cytokines, including IFN-γ and TNF-α, could identify tumor-specific responsive T cells more accurately (80). However, the application of TSTs defined by the aforementioned markers has exposed numerous drawbacks: The cells are mostly in a state of high exhausted and impaired effector function, the population still includes some bystander T cells and misses a low proportion of the TSA-specific effector T cell population. Extensive papers have identified a cluster of stem-cell like exhausted T cells in TILs, the main marker of which is the transcription factor Tcf1, with the combination of tumor responsive surface molecule PD-1 can recognize a population of TILs with sustained proliferative and differentiation potential. The high proliferative activity, high differentiation potential and dysfunctional T cells can produce durable tumor suppressive ability after in vivo transfusion. Furthermore, de Vries (104) et al. revealed immune characteristics of local tumor tissues and the systemic environment in patients with CRC by high-dimensional analysis and identified a population of innate lymphocytes (ILCs) with the exclusion of CD4+/CD8+ T cells. ILCs were defined as Lineage(Lin)−CD7+CD127−CD56+CD45RO+, a population that was enriched in CRC tissues and exhibited marked antitumor activity with a tumor-resident profile (CD103+CD69+). The results also indicated the diversity of tumor-specific cell populations, which should be enriched and sorted out as much as possible to achieve optimal therapeutic effects.
As all aforementioned potential biomarkers, a single marker may not meet the need for accurate sorting of specific TILs. Therefore, combined markers may be more valuable for application. However, different tumor types have different TIL components, and the tumor immune microenvironment may be the crucial factor affecting the ratio of TSTs to bystander T cells in TILs. It increases challenges to sort out the most specific tumor reactive T cells in different tumor types accurately. In the present review, it is necessary to summarize the biomarkers of TILs from different tumor types (Table 3). Furthermore, moving beyond these empirical cell phenotypes, the FucoID strategies that employ biochemical labeling and capture tumor antigen-specific T cells hold more promise for clinical applications. In addition, the straightforward approach to estimate TIL enrichment with tumor responsive clones based on the TCR repertoire, at the R&D phase, could greatly facilitate the selection of optimal TIL subsets and the optimization of TIL culture conditions and downstream enrichment procedures, and in clinical applications, it should be possible to estimate tumor-specific TIL abundance at the level of independent tumor samples, before and after culture and/or enrichment (84, 105, 106). In the future, the precise isolation of TSTs from different tumor microenvironments remains a major challenge to be overcome. The combination of other therapeutic approaches to further enhance the antitumor capacity of sorted TSTs, such as adding specific agonist antibodies (4-1BB, OX40, etc.), immune checkpoint inhibitors (PD-1, CTLA-4, etc.) and related cytokines (IL-2, IL-15, etc.), provides advantages for clinical application. In conclusion, TSTs remain the most active antitumor cell component in the clinical application of personalized TIL-based ACT and there is a great necessity for them to receive close attention.
WG performed the analyses and wrote the manuscript. YQD, YD, LC contributed to the conception of the paper. JC, ML, JW contributed to analysis. WW, XM helped perform the analysis with constructive discussions. All authors contributed to the article and approved the submitted version.
This project was supported by grants from the National key research and development program (Grant No: 2019YFA0110703),Science and Technology Innovation Foundation of Hunan Province (Grant No: 2020SK53614) and Natural Science Foundation of Hunan Province, China (Grant No: 2021JJ31018; 2020JJ4841) .
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QC declared a shared affiliation, with no collaboration, with the authors to the handling editor at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. van den Berg JH, Heemskerk B, van Rooij N, Gomez-Eerland R, Michels S, van Zon M, et al. Tumor infiltrating lymphocytes (Til) therapy in metastatic melanoma: Boosting of neoantigen-specific T cell reactivity and long-term follow-up. J Immunother Cancer (2020) 8(2):e000848. doi: 10.1136/jitc-2020-000848
2. Zhang D, He W, Wu C, Tan Y, He Y, Xu B, et al. Scoring system for tumor-infiltrating lymphocytes and its prognostic value for gastric cancer. Front Immunol (2019) 10:71. doi: 10.3389/fimmu.2019.00071
3. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med (2003) 348(3):203–13. doi: 10.1056/NEJMoa020177
4. Zhou X, Wu J, Duan C, Liu Y. Retrospective analysis of adoptive til therapy plus anti-Pd1 therapy in patients with chemotherapy-resistant metastatic osteosarcoma. J Immunol Res (2020) 2020:7890985. doi: 10.1155/2020/7890985
5. Shi H, Qi X, Ma B, Cao Y, Wang L, Sun L, et al. The status, limitation and improvement of adoptive cellular immunotherapy in advanced urologic malignancies. Chin J Cancer Res (2015) 27(2):128–37. doi: 10.3978/j.issn.1000-9604.2014.12.15
6. Scheper W, Kelderman S, Fanchi LF, Linnemann C, Bendle G, de Rooij MAJ, et al. Low and variable tumor reactivity of the intratumoral tcr repertoire in human cancers. Nat Med (2019) 25(1):89–94. doi: 10.1038/s41591-018-0266-5
7. Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander Cd8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature (2018) 557(7706):575–9. doi: 10.1038/s41586-018-0130-2
8. McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (2016) 351(6280):1463–9. doi: 10.1126/science.aaf1490
9. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature (2017) 547(7662):217–21. doi: 10.1038/nature22991
10. Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized rna mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature (2017) 547(7662):222–6. doi: 10.1038/nature23003
11. Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer (2008) 8(4):299–308. doi: 10.1038/nrc2355
12. Yossef R, Tran E, Deniger DC, Gros A, Pasetto A, Parkhurst MR, et al. Enhanced detection of neoantigen-reactive T cells targeting unique and shared oncogenes for personalized cancer immunotherapy. JCI Insight (2018) 3(19):e122467. doi: 10.1172/jci.insight.122467
13. Aydin AM, Bunch BL, Beatty M, Hajiran A, Dhillon J, Sarnaik AA, et al. The factors affecting expansion of reactive tumor infiltrating lymphocytes (Til) from bladder cancer and potential therapeutic applications. Front Immunol (2021) 12:628063. doi: 10.3389/fimmu.2021.628063
14. Leung W, Heslop HE. Adoptive immunotherapy with antigen-specific T cells expressing a native tcr. Cancer Immunol Res (2019) 7(4):528–33. doi: 10.1158/2326-6066.Cir-18-0888
15. Matsuda T, Leisegang M, Park JH, Ren L, Kato T, Ikeda Y, et al. Induction of neoantigen-specific cytotoxic T cells and construction of T-cell receptor-engineered T cells for ovarian cancer. Clin Cancer Res (2018) 24(21):5357–67. doi: 10.1158/1078-0432.Ccr-18-0142
16. Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, et al. Pd-1 identifies the patient-specific Cd8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest (2014) 124(5):2246–59. doi: 10.1172/jci73639
17. Parkhurst M, Gros A, Pasetto A, Prickett T, Crystal JS, Robbins P, et al. Isolation of T-cell receptors specifically reactive with mutated tumor-associated antigens from tumor-infiltrating lymphocytes based on Cd137 expression. Clin Cancer Res (2017) 23(10):2491–505. doi: 10.1158/1078-0432.Ccr-16-2680
18. Han L, Gao QL, Zhou XM, Shi C, Chen GY, Song YP, et al. Characterization of Cd103(+) Cd8(+) tissue-resident T cells in esophageal squamous cell carcinoma: May be tumor reactive and resurrected by anti-Pd-1 blockade. Cancer Immunol Immunother (2020) 69(8):1493–504. doi: 10.1007/s00262-020-02562-3
19. Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, et al. Co-Expression of Cd39 and Cd103 identifies tumor-reactive Cd8 T cells in human solid tumors. Nat Commun (2018) 9(1):2724. doi: 10.1038/s41467-018-05072-0
20. Flemming A. Cancer: Pd1 makes waves in anticancer immunotherapy. Nat Rev Drug Discovery (2012) 11(8):601. doi: 10.1038/nrd3806
21. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific Cd8 T cells infiltrating the tumor express high levels of pd-1 and are functionally impaired. Blood (2009) 114(8):1537–44. doi: 10.1182/blood-2008-12-195792
22. Inozume T, Hanada K, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, et al. Selection of Cd8+Pd-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother (2010) 33(9):956–64. doi: 10.1097/CJI.0b013e3181fad2b0
23. Sukegawa K, Shitaoka K, Hamana H, Kobayashi E, Miyahara Y, Fujii K, et al. Relationship between T cell receptor clonotype and pd-1 expression of tumor-infiltrating lymphocytes in colorectal cancer. Eur J Immunol (2020) 50(10):1580–90. doi: 10.1002/eji.201948399
24. Fernandez-Poma SM, Salas-Benito D, Lozano T, Casares N, Riezu-Boj JI, Mancheño U, et al. Expansion of tumor-infiltrating Cd8(+) T cells expressing pd-1 improves the efficacy of adoptive T-cell therapy. Cancer Res (2017) 77(13):3672–84. doi: 10.1158/0008-5472.Can-17-0236
25. Wang Z, Chen T, Lin W, Zheng W, Chen J, Huang F, et al. Functional tumor specific Cd8 + t cells in spleen express a high level of pd-1. Int Immunopharmacol (2020) 80:106242. doi: 10.1016/j.intimp.2020.106242
26. Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med (2016) 22(4):433–8. doi: 10.1038/nm.4051
27. Li T, Zhao L, Yang Y, Wang Y, Zhang Y, Guo J, et al. T Cells expanded from pd-1(+) peripheral blood lymphocytes share more clones with paired tumor-infiltrating lymphocytes. Cancer Res (2021) 81(8):2184–94. doi: 10.1158/0008-5472.Can-20-2300
28. Gros A, Tran E, Parkhurst MR, Ilyas S, Pasetto A, Groh EM, et al. Recognition of human gastrointestinal cancer neoantigens by circulating pd-1+ lymphocytes. J Clin Invest (2019) 129(11):4992–5004. doi: 10.1172/jci127967
29. Donia M, Kjeldsen JW, Andersen R, Westergaard MCW, Bianchi V, Legut M, et al. Pd-1(+) polyfunctional T cells dominate the periphery after tumor-infiltrating lymphocyte therapy for cancer. Clin Cancer Res (2017) 23(19):5779–88. doi: 10.1158/1078-0432.Ccr-16-1692
30. Zimmermann H. 5'-nucleotidase: Molecular structure and functional aspects. Biochem J (1992) 285(Pt 2):345–65. doi: 10.1042/bj2850345
31. Ohta A, Sitkovsky M. Role of G-Protein-Coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature (2001) 414(6866):916–20. doi: 10.1038/414916a
32. Timperi E, Barnaba V. Cd39 regulation and functions in T cells. Int J Mol Sci (2021) 22(15):8068. doi: 10.3390/ijms22158068
33. Canale FP, Ramello MC, Núñez N, Araujo Furlan CL, Bossio SN, Gorosito Serrán M, et al. Cd39 expression defines cell exhaustion in tumor-infiltrating Cd8(+) T cells. Cancer Res (2018) 78(1):115–28. doi: 10.1158/0008-5472.Can-16-2684
34. Gallerano D, Ciminati S, Grimaldi A, Piconese S, Cammarata I, Focaccetti C, et al. Genetically driven Cd39 expression shapes human tumor-infiltrating Cd8(+) T-cell functions. Int J Cancer (2020) 147(9):2597–610. doi: 10.1002/ijc.33131
35. Liu T, Tan J, Wu M, Fan W, Wei J, Zhu B, et al. High-affinity neoantigens correlate with better prognosis and trigger potent antihepatocellular carcinoma (Hcc) activity by activating Cd39(+)Cd8(+) T cells. Gut (2021) 70(10):1965–77. doi: 10.1136/gutjnl-2020-322196
36. Zou F, Tan J, Liu T, Liu B, Tang Y, Zhang H, et al. The Cd39(+) hbv surface protein-targeted car-T and personalized tumor-reactive Cd8(+) T cells exhibit potent anti-hcc activity. Mol Ther (2021) 29(5):1794–807. doi: 10.1016/j.ymthe.2021.01.021
37. Zhu W, Zhao Z, Feng B, Yu W, Li J, Guo H, et al. Cd8+Cd39+ T cells mediate anti-tumor cytotoxicity in bladder cancer. Onco Targets Ther (2021) 14:2149–61. doi: 10.2147/ott.S297272
38. Liu Q, Wang L, Lin H, Wang Z, Wu J, Guo J, et al. Tumor-specific Cd4(+) T cells restrain established metastatic melanoma by developing into cytotoxic Cd4(-) T cells. Front Immunol (2022) 13:875718. doi: 10.3389/fimmu.2022.875718
39. Kortekaas KE, Santegoets SJ, Sturm G, Ehsan I, van Egmond SL, Finotello F, et al. Cd39 identifies the Cd4(+) tumor-specific T-cell population in human cancer. Cancer Immunol Res (2020) 8(10):1311–21. doi: 10.1158/2326-6066.Cir-20-0270
40. Li S, Simoni Y, Zhuang S, Heit A, Tan I, Tan WD, et al. High-dimensional profiling of tumor-infiltrating Cd4+T cells in human cancer: Are they all tumor-specific? J Immunother Cancer (2020) 204(1 Supplement):243.22.
41. Yeong J, Suteja L, Simoni Y, Lau KW, Tan AC, Li HH, et al. Intratumoral Cd39(+)Cd8(+) T cells predict response to programmed cell death protein-1 or programmed death ligand-1 blockade in patients with nsclc. J Thorac Oncol (2021) 16(8):1349–58. doi: 10.1016/j.jtho.2021.04.016
42. Losurdo A, Scirgolea C, Alvisi G, Brummelman J, Errico V, Di Tommaso L, et al. Single-cell profiling defines the prognostic benefit of Cd39(High) tissue resident memory Cd8+ T cells in luminal-like breast cancer. Commun Biol (2021) 4(1):1117. doi: 10.1038/s42003-021-02595-z
43. Pauken KE, Shahid O, Lagattuta KA, Mahuron KM, Luber JM, Lowe MM, et al. Single-cell analyses identify circulating anti-tumor Cd8 T cells and markers for their enrichment. J Exp Med (2021) 218(4). doi: 10.1084/jem.20200920
44. Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science (2001) 291(5512):2413–7. doi: 10.1126/science.1058867
45. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol (2009) 10(5):524–30. doi: 10.1038/ni.1718
46. Woodberry T, Suscovich TJ, Henry LM, August M, Waring MT, Kaur A, et al. Alpha e beta 7 (Cd103) expression identifies a highly active, tonsil-resident effector-memory ctl population. J Immunol (2005) 175(7):4355–62. doi: 10.4049/jimmunol.175.7.4355
47. Mami-Chouaib F, Blanc C, Corgnac S, Hans S, Malenica I, Granier C, et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer (2018) 6(1):87. doi: 10.1186/s40425-018-0399-6
48. Wang B, Wu S, Zeng H, Liu Z, Dong W, He W, et al. Cd103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J Urol (2015) 194(2):556–62. doi: 10.1016/j.juro.2015.02.2941
49. Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Annu Rev Immunol (2019) 37:521–46. doi: 10.1146/annurev-immunol-042617-053214
50. Komdeur FL, Prins TM, van de Wall S, Plat A, Wisman GBA, Hollema H, et al. Cd103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial Cd8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology (2017) 6(9):e1338230. doi: 10.1080/2162402x.2017.1338230
51. Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol (2017) 18(8):940–50. doi: 10.1038/ni.3775
52. Wang P, Huang B, Gao Y, Yang J, Liang Z, Zhang N, et al. Cd103(+)Cd8(+) T lymphocytes in non-small cell lung cancer are phenotypically and functionally primed to respond to pd-1 blockade. Cell Immunol (2018) 325:48–55. doi: 10.1016/j.cellimm.2018.02.002
53. Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. Cd103 and intratumoral immune response in breast cancer. Clin Cancer Res (2016) 22(24):6290–7. doi: 10.1158/1078-0432.Ccr-16-0732
54. Workel HH, Komdeur FL, Wouters MC, Plat A, Klip HG, Eggink FA, et al. Cd103 defines intraepithelial Cd8+ Pd1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur J Cancer (2016) 60:1–11. doi: 10.1016/j.ejca.2016.02.026
55. Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med (2018) 24(7):986–93. doi: 10.1038/s41591-018-0078-7
56. Lim CJ, Lee YH, Pan L, Lai L, Chua C, Wasser M, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis b virus-related hepatocellular carcinoma. Gut (2019) 68(5):916–27. doi: 10.1136/gutjnl-2018-316510
57. Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, et al. Cd8+Cd103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol (2015) 194(7):3475–86. doi: 10.4049/jimmunol.1402711
58. Xiao Y, Li H, Mao L, Yang QC, Fu LQ, Wu CC, et al. Cd103(+) T and dendritic cells indicate a favorable prognosis in oral cancer. J Dent Res (2019) 98(13):1480–7. doi: 10.1177/0022034519882618
59. Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for Cd103(+)Cd8+ tissue-resident memory T cells of skin. Nat Immunol (2013) 14(12):1294–301. doi: 10.1038/ni.2744
60. Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, et al. The molecular signature of tissue resident memory Cd8 T cells isolated from the brain. J Immunol (2012) 189(7):3462–71. doi: 10.4049/jimmunol.1201305
61. Corgnac S, Malenica I, Mezquita L, Auclin E, Voilin E, Kacher J, et al. Cd103(+)Cd8(+) T(Rm) cells accumulate in tumors of anti-Pd-1-Responder lung cancer patients and are tumor-reactive lymphocytes enriched with Tc17. Cell Rep Med (2020) 1(7):100127. doi: 10.1016/j.xcrm.2020.100127
62. Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, et al. Cd103(+) tumor-resident Cd8(+) T cells are associated with improved survival in immunotherapy-naïve melanoma patients and expand significantly during anti-Pd-1 treatment. Clin Cancer Res (2018) 24(13):3036–45. doi: 10.1158/1078-0432.Ccr-17-2257
63. Banchereau R, Chitre AS, Scherl A, Wu TD, Patil NS, de Almeida P, et al. Intratumoral Cd103+ Cd8+ T cells predict response to pd-L1 blockade. J Immunother Cancer (2021) 9(4):e002231. doi: 10.1136/jitc-2020-002231
64. Caushi JX, Zhang J, Ji Z, Vaghasia A, Zhang B, Hsiue EH, et al. Transcriptional programs of neoantigen-specific til in anti-Pd-1-Treated lung cancers. Nature (2021) 596(7870):126–32. doi: 10.1038/s41586-021-03752-4
65. Abd Hamid M, Colin-York H, Khalid-Alham N, Browne M, Cerundolo L, Chen JL, et al. Self-maintaining Cd103(+) cancer-specific T cells are highly energetic with rapid cytotoxic and effector responses. Cancer Immunol Res (2020) 8(2):203–16. doi: 10.1158/2326-6066.Cir-19-0554
66. Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1bb ligand, a member of the tnf family, is important for the generation of antiviral Cd8 T cell responses. J Immunol (1999) 163(9):4859–68.
67. Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, et al. 4-1bb ligand induces cell division, sustains survival, and enhances effector function of Cd4 and Cd8 T cells with similar efficacy. J Immunol (2001) 167(3):1313–24. doi: 10.4049/jimmunol.167.3.1313
68. Gramaglia I, Cooper D, Miner KT, Kwon BS, Croft M. Co-Stimulation of antigen-specific Cd4 T cells by 4-1bb ligand. Eur J Immunol (2000) 30(2):392–402. doi: 10.1002/1521-4141(200002)30:2<392::Aid-immu392>3.0.Co;2-h
69. Wen T, Bukczynski J, Watts TH. 4-1bb ligand-mediated costimulation of human T cells induces Cd4 and Cd8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol (2002) 168(10):4897–906. doi: 10.4049/jimmunol.168.10.4897
70. Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of Cd137 broadens primary antiviral Cd8+ T cell responses. Nat Immunol (2002) 52(1):96–108. doi: 10.1038/ni798
71. Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial apcs expressing ligands for the T-cell receptor, Cd28 and 4-1bb. Nat Biotechnol (2002) 20(2):143–8. doi: 10.1038/nbt0202-143
72. Bukczynski J, Wen T, Watts TH. Costimulation of human Cd28- T cells by 4-1bb ligand. Eur J Immunol (2003) 33(2):446–54. doi: 10.1002/immu.200310020
73. Watanabe K, Suzuki S, Kamei M, Toji S, Kawase T, Takahashi T, et al. Cd137-guided isolation and expansion of antigen-specific Cd8 cells for potential use in adoptive immunotherapy. Int J Hematol (2008) 88(3):311–20. doi: 10.1007/s12185-008-0134-z
74. Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, et al. Activation-induced expression of Cd137 permits detection, isolation, and expansion of the full repertoire of Cd8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood (2007) 110(1):201–10. doi: 10.1182/blood-2006-11-056168
75. Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, et al. Cd137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res (2014) 20(1):44–55. doi: 10.1158/1078-0432.Ccr-13-0945
76. Seliktar-Ofir S, Merhavi-Shoham E, Itzhaki O, Yunger S, Markel G, Schachter J, et al. Selection of shared and neoantigen-reactive T cells for adoptive cell therapy based on Cd137 separation. Front Immunol (2017) 8:1211. doi: 10.3389/fimmu.2017.01211
77. Lee KH, Gowrishankar K, Street J, McGuire HM, Luciani F, Hughes B, et al. Ex vivo enrichment of prame antigen-specific T cells for adoptive immunotherapy using Cd137 activation marker selection. Clin Transl Immunol (2020) 9(10):e1200. doi: 10.1002/cti2.1200
78. Eiva MA, Omran DK, Chacon JA, Powell DJ Jr. Systematic analysis of Cd39, Cd103, Cd137, and pd-1 as biomarkers for naturally occurring tumor antigen-specific tils. Eur J Immunol (2021) 52(1):96–108. doi: 10.1002/eji.202149329
79. Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted Cd8 T cells by pd-1-Targeted therapies is Cd28-dependent. Science (2017) 355(6332):1423–7. doi: 10.1126/science.aaf0683
80. Draghi A, Chamberlain CA, Khan S, Papp K, Lauss M, Soraggi S, et al. Rapid identification of the tumor-specific reactive til repertoire Via combined detection of Cd137, tnf, and ifnγ, following recognition of autologous tumor-antigens. Front Immunol (2021) 12:705422. doi: 10.3389/fimmu.2021.705422
81. van Asten SD, de Groot R, van Loenen MM, Castenmiller SM, de Jong J, Monkhorst K, et al. T Cells expanded from renal cell carcinoma display tumor-specific Cd137 expression but lack significant ifn-Γ, tnf-A or il-2 production. Oncoimmunology (2021) 10(1):1860482. doi: 10.1080/2162402x.2020.1860482
82. Tallón de Lara P, Castañón H, Vermeer M, Núñez N, Silina K, Sobottka B, et al. Cd39(+)Pd-1(+)Cd8(+) T cells mediate metastatic dormancy in breast cancer. Nat Commun (2021) 12(1):769. doi: 10.1038/s41467-021-21045-2
83. Leem G, Park J, Jeon M, Kim ES, Kim SW, Lee YJ, et al. 4-1bb Co-stimulation further enhances anti-Pd-1-Mediated reinvigoration of exhausted Cd39(+) Cd8 T cells from primary and metastatic sites of epithelial ovarian cancers. J Immunother Cancer (2020) 8(2):e001650. doi: 10.1136/jitc-2020-001650
84. Goncharov MM, Bryushkova EA, Sharaev NI, Skatova VD, Baryshnikova AM, Sharonov GV, et al. Pinpointing the tumor-specific T cells Via tcr clusters. Elife (2022) 11:e77274. doi: 10.7554/eLife.77274
85. Martinez-Gomez C, Michelas M, Scarlata CM, Salvioni A, Gomez-Roca C, Sarradin V, et al. Circulating exhausted pd-1(+)Cd39(+) helper Cd4 T cells are tumor-Antigen-Specific and predict response to pd-1/Pd-L1 axis blockade. Cancers (Basel) (2022) 14(15):3679. doi: 10.3390/cancers14153679
86. van den Bulk J, Verdegaal EME, Ruano D, Ijsselsteijn ME, Visser M, van der Breggen R, et al. Neoantigen-specific immunity in low mutation burden colorectal cancers of the consensus molecular subtype 4. Genome Med (2019) 11(1):87. doi: 10.1186/s13073-019-0697-8
87. Workel HH, van Rooij N, Plat A, Spierings DCJ, Fehrmann RSN, Nijman HW, et al. Transcriptional activity and stability of Cd39+Cd103+Cd8+ T cells in human high-grade endometrial cancer. Int J Mol Sci (2020) 21(11):3770. doi: 10.3390/ijms21113770
88. Rajamanickam V, Ballesteros-Merino C, Samson K, Ross D, Bernard B, Fox BA, et al. Robust antitumor immunity in a patient with metastatic colorectal cancer treated with cytotoxic regimens. Cancer Immunol Res (2021) 9(6):602–11. doi: 10.1158/2326-6066.Cir-20-1024
89. Duhen R, Ballesteros-Merino C, Frye AK, Tran E, Rajamanickam V, Chang SC, et al. Neoadjuvant anti-Ox40 (Medi6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat Commun (2021) 12(1):1047. doi: 10.1038/s41467-021-21383-1
90. Laumont CM, Wouters MCA, Smazynski J, Gierc NS, Chavez EA, Chong LC, et al. Single-cell profiles and prognostic impact of tumor-infiltrating lymphocytes coexpressing Cd39, Cd103, and pd-1 in ovarian cancer. Clin Cancer Res (2021) 27(14):4089–100. doi: 10.1158/1078-0432.Ccr-20-4394
91. Salas-Benito D, Conde E, Tamayo-Uria I, Mancheño U, Elizalde E, Garcia-Ros D, et al. The mutational load and a T-cell inflamed tumour phenotype identify ovarian cancer patients rendering tumour-reactive T cells from pd-1(+) tumour-infiltrating lymphocytes. Br J Cancer (2021) 124(6):1138–49. doi: 10.1038/s41416-020-01218-4
92. Kim HD, Park S, Jeong S, Lee YJ, Lee H, Kim CG, et al. 4-1bb delineates distinct activation status of exhausted tumor-infiltrating Cd8(+) T cells in hepatocellular carcinoma. Hepatology (2020) 71(3):955–71. doi: 10.1002/hep.30881
93. Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, et al. Mhc-ii neoantigens shape tumour immunity and response to immunotherapy. Nature (2019) 574(7780):696–701. doi: 10.1038/s41586-019-1671-8
94. Duhen R, Fesneau O, Samson KA, Frye AK, Beymer M, Rajamanickam V, et al. Pd-1 and icos coexpression identifies tumor-reactive Cd4+ T cells in human solid tumors. J Clin Invest (2022) 132(12):e156821. doi: 10.1172/jci156821
95. Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, et al. Intratumoral Tcf1(+)Pd-1(+)Cd8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity (2019) 50(1):195–211.e10. doi: 10.1016/j.immuni.2018.12.021
96. Wang J, Li R, Cao Y, Gu Y, Fang H, Fei Y, et al. Intratumoral Cxcr5(+)Cd8(+)T associates with favorable clinical outcomes and immunogenic contexture in gastric cancer. Nat Commun (2021) 12(1):3080. doi: 10.1038/s41467-021-23356-w
97. Brummelman J, Mazza EMC, Alvisi G, Colombo FS, Grilli A, Mikulak J, et al. High-dimensional single cell analysis identifies stem-like cytotoxic Cd8(+) T cells infiltrating human tumors. J Exp Med (2018) 215(10):2520–35. doi: 10.1084/jem.20180684
98. Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, et al. An intra-tumoral niche maintains and differentiates stem-like Cd8 T cells. Nature (2019) 576(7787):465–70. doi: 10.1038/s41586-019-1836-5
99. Gao Y, You M, Fu J, Tian M, Zhong X, Du C, et al. Intratumoral stem-like Ccr4+ regulatory T cells orchestrate the immunosuppressive microenvironment in hcc associated with hepatitis b. J Hepatol (2022) 76(1):148–59. doi: 10.1016/j.jhep.2021.08.029
100. Baharom F, Ramirez-Valdez RA, Tobin KKS, Yamane H, Dutertre CA, Khalilnezhad A, et al. Intravenous nanoparticle vaccination generates stem-like Tcf1(+) neoantigen-specific Cd8(+) T cells. Nat Immunol (2021) 22(1):41–52. doi: 10.1038/s41590-020-00810-3
101. Martinez-Usatorre A, Carmona SJ, Godfroid C, Yacoub Maroun C, Labiano S, Romero P. Enhanced phenotype definition for precision isolation of precursor exhausted tumor-infiltrating Cd8 T cells. Front Immunol (2020) 11:340. doi: 10.3389/fimmu.2020.00340
102. Krishna S, Lowery FJ, Copeland AR, Bahadiroglu E, Mukherjee R, Jia L, et al. Stem-like Cd8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science (2020) 370(6522):1328–34. doi: 10.1126/science.abb9847
103. Liu Z, Li JP, Chen M, Wu M, Shi Y, Li W, et al. Detecting tumor antigen-specific T cells Via interaction-dependent fucosyl-biotinylation. Cell (2020) 183(4):1117–33.e19. doi: 10.1016/j.cell.2020.09.048
104. de Vries NL, van Unen V, Ijsselsteijn ME, Abdelaal T, van der Breggen R, Farina Sarasqueta A, et al. High-dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. Gut (2020) 69(4):691–703. doi: 10.1136/gutjnl-2019-318672
105. Shugay M, Bagaev DV, Zvyagin IV, Vroomans RM, Crawford JC, Dolton G, et al. Vdjdb: A curated database of T-cell receptor sequences with known antigen specificity. Nucleic Acids Res (2018) 46(D1):D419–d27. doi: 10.1093/nar/gkx760
Keywords: tumor infiltration lymphocyte, tumor specific T cell, biomarker, adoptive cellular therapy, cancer treatment
Citation: Ge W, Dong Y, Deng Y, Chen L, Chen J, Liu M, Wu J, Wang W and Ma X (2022) Potential biomarkers: Identifying powerful tumor specific T cells in adoptive cellular therapy. Front. Immunol. 13:1003626. doi: 10.3389/fimmu.2022.1003626
Received: 26 July 2022; Accepted: 27 October 2022;
Published: 14 November 2022.
Edited by:
Magdalena Plebanski, RMIT University, AustraliaReviewed by:
Ekaterina A. Bryushkova, Lomonosov Moscow State University, RussiaCopyright © 2022 Ge, Dong, Deng, Chen, Chen, Liu, Wu, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqian Ma, bXhxODkzM0Bjc3UuZWR1LmNu
‡ORCID: Xiaoqian Ma, orcid.org/0000-0002-8315-9927
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.