- 1The department of Neurology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2The department of Pathology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Laboratory Medicine, West China Hospital of Sichuan University, Chengdu, Sichuan, China
Background: Appendiceal goblet cell carcinoma (aGCC) is a rare neoplasm with mixed endocrine and exocrine features. No paraneoplastic neurological syndromes or autoantibodies have been identified in cases of aGCC or even appendiceal tumors. Amphiphysin-immunoglobulin G (IgG) autoimmunity was first described in stiff-person syndrome with breast cancer. We firstly described the clinical course and pathological findings of a patient with aGCC-associated amphiphysin-IgG autoimmunity.
Case presentation: A 54-year-old man who developed aGCC was admitted for acute disturbance of consciousness, psychiatric symptoms, cognitive impairment, seizure and hypotension. Amphiphysin-IgG was detected in the patient’s serum and CSF by immunoblotting and tissue-based indirect immunofluorescence assay confirming the diagnosis of definite paraneoplastic amphiphysin-IgG-positive encephalitis. Histopathology revealed amphiphysin protein expression and accompanying immune cell infiltration (predominantly CD20+ B cells, CD3+ and CD8+ T cells) within the tumor tissue, suggesting a possible paraneoplastic origin of amphiphysin-associated paraneoplastic neurological syndromes (PNSs) in this case. Although the patient’s symptoms resolved after high-dose corticosteroid therapy, he experienced recurrence 6 months later, manifesting as paraneoplastic cerebellar dysfunction. Despite treatment with IV cyclophosphamide and oral mycophenolate mofetil, no improvement was noted.
Conclusions: This case suggests that aGCC may trigger amphiphysin-IgG autoimmunity.
Introduction
Amphiphysin-IgG autoimmunity was reported in 1992 by Lichte et al. The clinical presentations associated with amphiphysin antibodies (Abs) include stiff-person syndrome, limbic encephalitis (LE), paraneoplastic cerebellar dysfunction (PCD), myelopathy, and peripheral neuropathies (1).
Malignancies such as small cell lung cancer and breast cancer triggered amphiphysin-Abs associated paraneoplastic neurological syndromes (PNSs) have been reported (1). Appendiceal goblet cell carcinoma (aGCC), recognized by the 2019 World Health Organization classification of digestive system tumors, is a rare neoplasm with mixed endocrine and exocrine features (2). Here, we first report a patient with aGCC who developed LE related to amphiphysin-Abs after tumor removal. Although the patient’s symptoms resolved after immunotherapy, he experienced recurrence of PCD. Histopathology revealed amphiphysin protein expression and accompanying immune cell infiltration within the tumor tissue, suggesting a possible paraneoplastic origin of amphiphysin-associated PNSs in this case.
Case presentation
A 54-year-old male presented with disturbance of consciousness, psychiatric symptoms and behavioral change, cognitive impairment, seizure and hypotension for 11 days. His family history was unremarkable. Exposure to toxic substances was denied. The day before his first symptom, a resection of his right colon was performed in a local hospital, and aGCC was diagnosed by the pathologist.
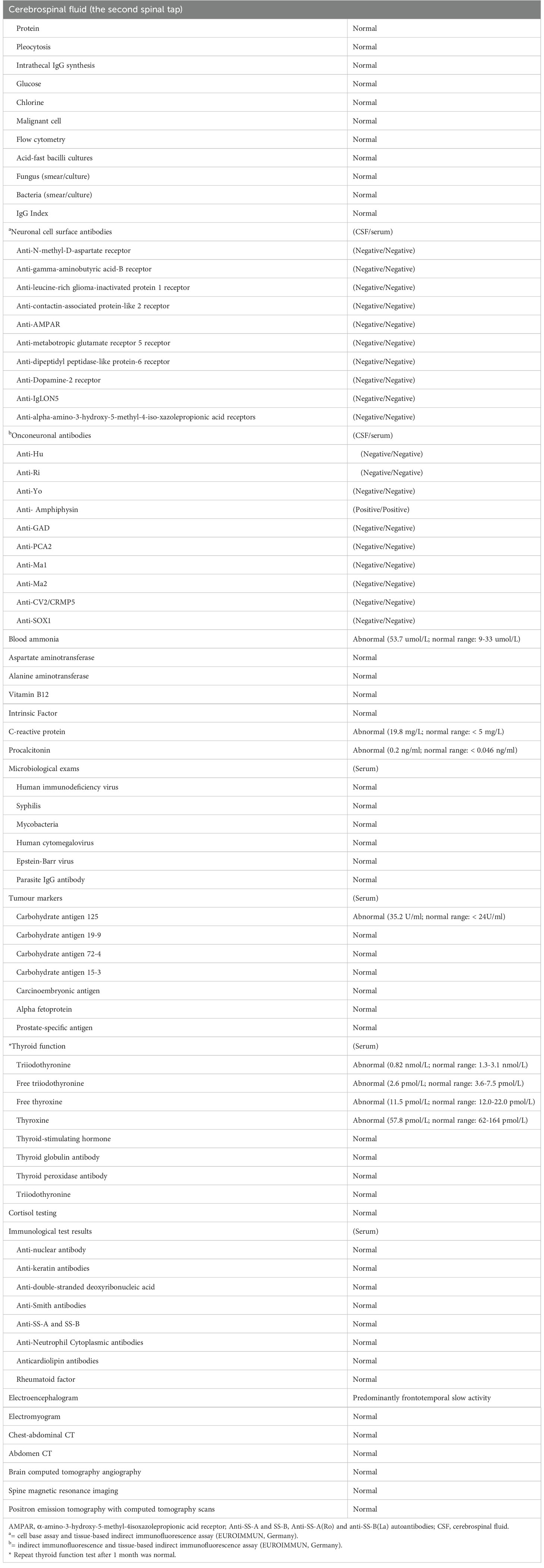
Brain magnetic resonance imaging (MRI; Figure 1A), routine cerebral spinal fluid (CSF) analyses (Supplementary file), and routine laboratory tests were unremarkable (Table 1), except for increased carbohydrate antigen 125 (35.2 U/ml; normal range, < 24 U/ml) and blood ammonia (53.7 µmol/L; normal range, 9-33 µmol/L). The anti-amphiphysin antibody tested by immunoblotting (EUROIMMUN, Lübeck, Germany) was positive in both CSF and serum. The diagnosis of amphiphysin-positive LE associated with aGCC was made. A high dose of methylprednisolone (1000 mg/d for 5 days) was administered, followed by a tapered course of prednisone. The symptoms of psychiatric symptoms and seizures then disappeared one month later, but the patient remained cognitive dysfunction (Mini-mental State Examination score = 9).
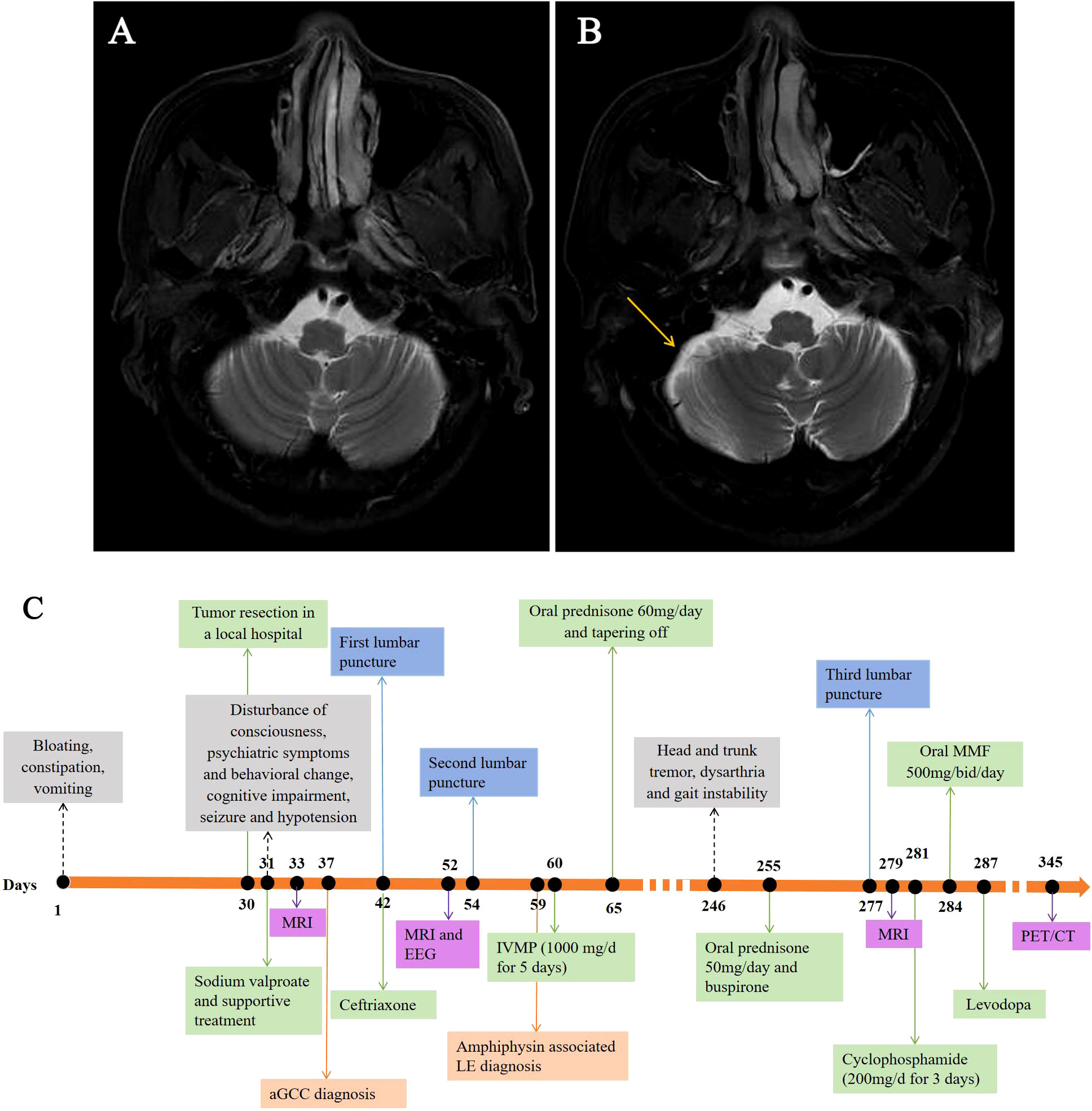
Figure 1 Brain MRI scans obtained from the patient and timeline of his clinical course. Despite there being no specific findings upon an initial T2-weighted MRI (A), mild cerebellar atrophy was observed in the subsequent axial T2-weighted MRI performed seven months later (B, yellow arrow). Timeline of the patient’s clinical course highlighting the manifestation, auxiliary examination, treatment and diagnosis time (C). EEG, electroencephalograph; LE, limbic encephalitis; MMF, mycophenolate mofetil; MRI, magnetic resonance imaging; IVMP, intravenous methylprednisolone; PET/CT, positron emission tomography with computed tomography.
Unfortunately, seven months later, the patient was readmitted to the hospital with progressive head and trunk tremors, dysarthria and gait instability for over 1 month. Neurological examination revealed head and trunk tremors, cognitive dysfunctions, bilateral gaze-evoked nystagmus and cerebellar ataxia (Vedio1). CSF analysis remained unchanged. Brain MRI showed mild cerebellar atrophy (Figure 1B). Amphiphysin-IgG was still present in CSF and serum. Positron emission tomography with computed tomography (PET/CT) scans revealed no tumor metastasis. The patient received IV cyclophosphamide (200 mg/d) and oral mycophenolate mofetil (500 mg/d) therapy. There was no improvement or deterioration of the neurological symptoms 3 months later. The evaluation of PNSs process is summarized in Figure 1C.
To investigate a possible paraneoplastic origin of amphiphysin-PNSs in this patient, tissue blocks of the aGCC were examined pathologically. Immunohistochemical staining for CK20, CDX-2, carcinoembryonic antigen (CEA) and neuroendocrine markers (synaptophysin and chromogranin) were positive (Figures 2A–F), which meets the diagnostic pathological diagnostic criteria of Agcc according to the 2019 WHO.2 The expression of Ki67 protein was about 10%. MUC1 and MUC2 were also positive. Immunohistochemistry revealed positive CD3+ T cells, CD8+ T cells and CD20+ B cells in the tumor tissue (Figures 2G–I). However, CK7, CD138+ plasma cells, CD68+ macrophages, and IgG were negative in the tissue. Tumor cells showed reactivity with amphiphysin-Abs (Figure 2H), which indicates that the aGCC might play a role in triggering amphiphysin-IgG autoimmunity. Tissue-based indirect immunofluorescence assay (TBA) of the patient’s serum revealed IgG antibody to Purkinje cells of the monkey cerebellum and dentate gyrus glia cells of the monkey hippocampus (Figures 2K, L).
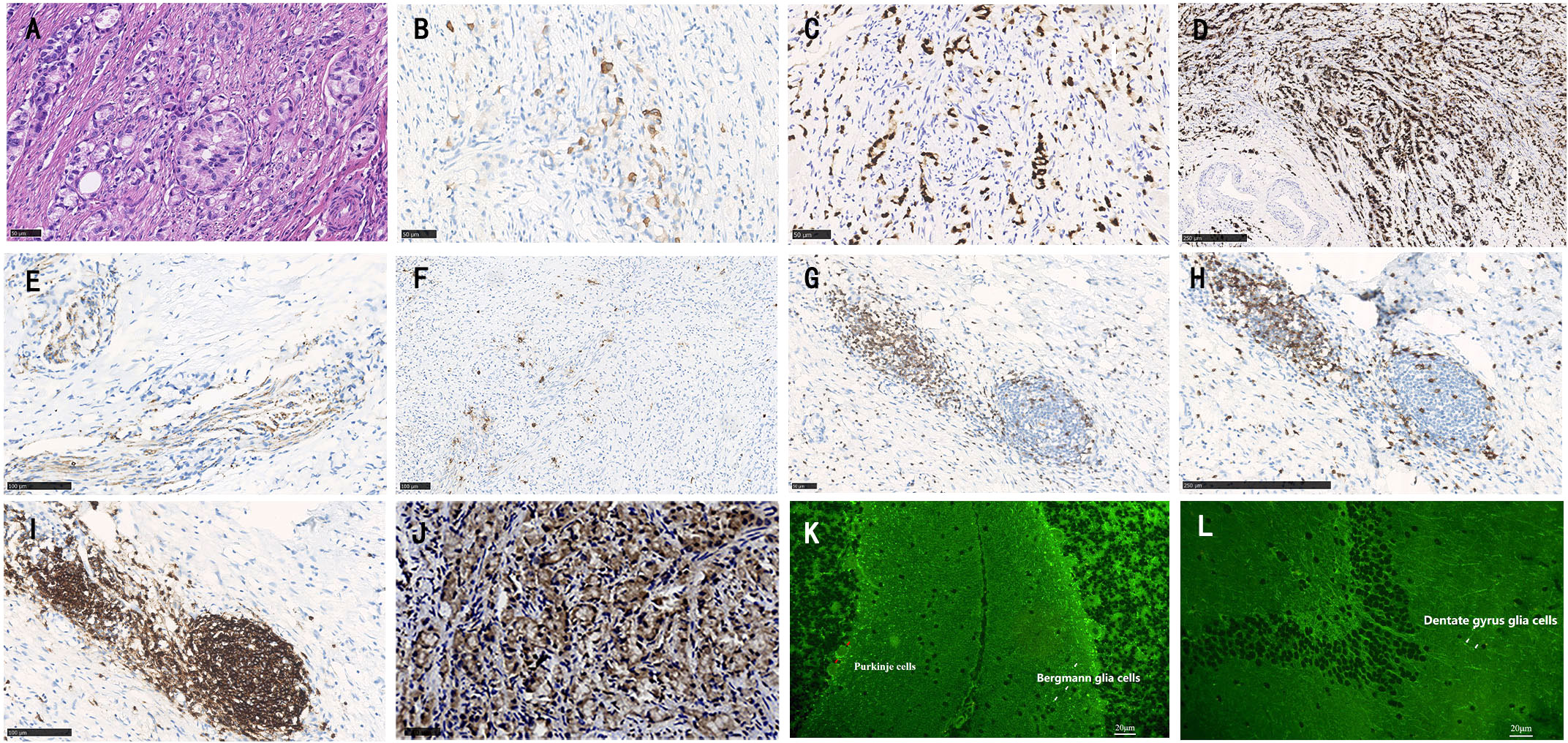
Figure 2 Histopathology of appendiceal goblet cell carcinoma of the patient. Tumor cells exhibit a goblet cell morphology and characteristically form small tight clusters (A). The immunohistochemical staining shows moderate positivity for CK20 (B), CDX-2 (C), and strong positivity for carcinoembryonic antigen (CEA, D). Staining for synaptophysin (Syn, E), and chromogranin (F) is mild. Varying degrees of positivity are also shown in lymphocytes such as CD3+ T cells (G), CD8+ T cells (H) and CD20+ B cells (I). Immunohistochemistry shows positive staining for amphiphysin-IgG (J). Tissue-based indirect immunofluorescence assay (TBA) of the patient’s serum detected fluorescence responses, with specific fluorescence responses in Purkinje cells (red arrows) from the monkey cerebellum brain section (K) as well as dentate gyrus glial cells (white arrows) from the monkey hippocampal tissue. (A,–C, G, J) bar = 50 µm; D, H bar = 250 µm; (E, F, O) bar = 100 µm, (K, L) bar = 20 µm).
Diagnostic assessment
According to the clinical manifestations, tumor, positive anti-amphiphysin antibody in CSF and serum, and well response to immunotherapy, aGCC associated amphiphysin-IgG autoimmunity was considered in this patient.
Discussion
This case presents several novelties. This is the first case report of aGCC associated PNSs to date. Although the etiology of PNSs remains unknown, it is proposed that the expression of neuronal antigens within tumors could initiate an autoimmune response that leads to the production of autoantibodies (3). Immunopathological analysis of amphiphysin-IgG autoimmunity may help to elucidate the underlying pathophysiological mechanism of the disease, but unfortunately, published data are scarce, to date, brain and/or spinal cord immunopathological reactions have only been studied in 4 patients (4–7). In those cases, biopsies revealed marked inflammatory responses in perivascular and parenchymal regions. Both T (especially CD3+ and CD8+ T cells) and B lymphocytes (especially CD20+ B cells) were present in the lesions, and CD68+ microglial cells were abundant in the pons in one previous patient. In addition, only 14 cases reported pathological findings coming from concomitant neoplasms (small cell lung cancer, breast cancer and non-small cell lung cancer) in which amphiphysin-IgG was present in patients’ tumor tissues, which correlated with the patients’ antibody specificity (8, 9). In this case, immunohistochemical amphiphysin-Abs positivity was shown in appendiceal tumor tissue, confirming that the syndrome was triggered by the tumor.
In addition, in the literature, most of autoimmune cerebellar dysfunction is characterized by limb or trunk tremors, and only a few cases of autoimmune cerebellar dysfunction associated head tremor have been reported (10–12). Head tremor had not been reported in amphiphysin-Abs associated PNSs. From the largest series reported by Pittock et al., a minority of patients with amphiphysin-IgG presented with involuntary movement, including mandibular involuntary movement, chorea and truncal tremor (4). In this case, considering the progressive cerebellar atrophy shown by the brain MRI, tremors in this patient were considered of cerebellar origin.
It is unusual that neurological symptoms occur shortly after tumor resection, which might be due to the immune trigger from antigen exposure at the time of resection (13). In this case, the onset of PCD seven months after tumor resection is also extremely rare. Since whole body PET/CT was negative, and the patient is still stable on regular follow-up after the occurrence of cerebellar dysfunction, the evidence of tumor recurrence or metastasis is insufficient. To the best of our knowledge, there are only a few published cases describe the recurrence of paraneoplastic neurologic syndromes after tumor resection, but without evidence of tumor recurrence (14–16). However, owing to their rarity, the mechanism of this condition is still unknown. Moreover, the case showed a different phenotype and treatment response during disease onset compared to relapse, which is unusual in previously reported autoimmune encephalitis (17). He improved during the first stage of the disease when limbic encephalitis was predominant. Nevertheless, during relapse characterized by cerebellar dysfunction despite aggressive treatment regarding second-line immunotherapy administration, no improvement was noted. Only stabilization of the clinical symptoms was achieved. We suggested for the patient to receive intravenous immunoglobulin and other immunotherapy. Unfortunately, due to economic reasons, the patient refused.
In conclusion, this case suggests that aGCC may trigger amphiphysin-IgG autoimmunity, and amphiphysin-IgG autoimmunity could relapse with a different clinical phenotype months after the initial episode, so long-term follow-up is needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JFL and TY drafted and revised the manuscript. MW and JW collected and interpreted the data. JML designed the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Supported by the National Natural Science Foundation of China (grants 82071459) and the Institute of Brain science and Brain-inspired technology of West China Hospital, Sichuan University (grants ZYJC21001).
Acknowledgments
The authors are indebted to our patient and his family for their support and permission. We also would like to thank Li Li, Fei Chen and Chunjuan Bao (Laboratory of Pathology, West China Hospital, Sichuan University) for helping with processing the histological sections and staining.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1001264/full#supplementary-material
Supplementary Video 1 | Neurological examination revealed head and trunk tremors, bilateral gaze-evoked nystagmus and cerebellar ataxia.
Abbreviations
aGCC, appendiceal goblet cell carcinoma; CSF, cerebral spinal fluid; LE, limbic encephalitis; MRI, magnetic resonance imaging; PNSs, paraneoplastic neurological syndromes; TBA, tissue-based indirect immunofluorescence assay.
References
1. Dubey D, Jitprapaikulsan J, Bi H, Do Campo RV, McKeon A, Pittock SJ, et al. Amphiphysin-IgG autoimmune neuropathy: A recognizable clinicopathologic syndrome. Neurology (2019) 93:e1873–80. doi: 10.1212/WNL.0000000000008472
2. Sinno SAJ, Jurdi NMH. Goblet cell tumors of the appendix: A review. Ann Diagn Pathol (2019) 43:151401. doi: 10.1016/j.anndiagpath.2019.151401
3. Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med (2018) 378:840–51. doi: 10.1056/NEJMra1708712
4. Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol (2005) 58:96–107. doi: 10.1002/ana.20529
5. Poh MQ, Simon NG, Buckland ME, Salisbury E, Watson S. Evidence of T-cell mediated neuronal injury in stiff-person syndrome with anti-amphiphysin antibodies. J Neurol Sci (2014) 337:235–7. doi: 10.1016/j.jns.2013.12.015
6. Petzold GC, Marcucci M, Butler MH, van Landeghem FK, Einhäupl KM, Solimena M, et al. Rhabdomyolysis and paraneoplastic stiff-man syndrome with amphiphysin autoimmunity. Ann Neurol (2004) 55:286–90. doi: 10.1002/ana.10841
7. Wessig C, Klein R, Schneider MF, Toyka KV, Naumann M, Sommer C. Neuropathology and binding studies in anti-amphiphysin-associated stiff-person syndrome. Neurology (2003) 61:195–8. doi: 10.1212/01.WNL.0000073143.53337.DD
8. Dropcho EJ. Antiamphiphysin antibodies with small-cell lung carcinoma and paraneoplastic encephalomyelitis. Ann Neurol (1996) 39:659–67. doi: 10.1002/ana.410390516
9. Nakashima K, Fujii Y, Sato M, Igarashi K, Kobayashi M, Ishizuka T. A case of non-small cell lung cancer presenting anti-amphiphysin antibody-positive paraneoplastic neurological syndrome. Respir Med Case Rep (2021) 34:101525. doi: 10.1016/j.rmcr.2021.101525
10. Wallwitz U, Brock S, Schunck A, Wildemann B, Jarius S, Hoffmann F, et al. From dizziness to severe ataxia and dysarthria: New cases of anti-Ca/ARHGAP26 autoantibody-associated cerebellar ataxia suggest a broad clinical spectrum. J Neuroimmunol (2017) 309:77–81. doi: 10.1016/j.jneuroim.2017.05.011
11. Painous C, López-Pérez MÁ, Illa I, Querol L. Head and voice tremor improving with immunotherapy in an anti-NF155 positive CIDP patient. Ann Clin Transl Neurol (2018) 5(4):499–501. doi: 10.1002/acn3.539
12. Farhat E, Zouari M, Abdelaziz IB, Drissi C, Beyrouti R, Hammouda MB, et al. Progressive cerebellar degeneration revealing primary sjögren syndrome: a case report. Cerebellum Ataxias (2016) 3:18. doi: 10.1186/s40673-016-0056-0
13. Jones AA, Chen T. Delayed opsoclonus-myoclonus syndrome after ovarian teratoma resection. J Neuroophthalmol (2021) 42(1):e450–1. doi: 10.1212/NXI.0000000000000433
14. Pranzatelli MR, McGee NR. Neuroimmunology of OMS and ANNA-1/anti-Hu paraneoplastic syndromes in a child with neuroblastoma. Neurol Neuroimmunol Neuroinflamm (2017) 5(2):e433. doi: 10.1212/NXI.0000000000000433
15. Shaulov A, Rottenstreich M, Peleg H, Spiegel M, Shichman B, Argov Z, et al. Myasthenia gravis appearing 18 years after resection of benign thymoma with subsequent limbic encephalitis. J Neurol Sci (2012) 317(1-2):146–7. doi: 10.1016/j.jns.2012.03.007
16. Wallwitz U, Brock S, Schunck A, Wildemann B, Jarius S, Hoffmann F, et al. From dizziness to severe ataxia and dysarthria: New cases of anti-Ca/ARHGAP26 autoantibody-associated cerebellar ataxia suggest a broad clinical spectrum. J Neuroimmunol (2017) 309:77–81. doi: 10.1016/j.jneuroim.2017.05.011
Keywords: amphiphysin, appendiceal goblet cell carcinoma, paraneoplastic neurological syndromes, tremors, pathological findings
Citation: Lin J, Yu T, Wang M, Wang J and Li J (2023) Case report: Amphiphysin-IgG autoimmunity: a paraneoplastic presentation of appendiceal goblet cell carcinoma. Front. Immunol. 13:1001264. doi: 10.3389/fimmu.2022.1001264
Received: 23 July 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Harry Alexopoulos, National and Kapodistrian University of Athens, GreeceReviewed by:
Sudhakar Tummala, University of Texas MD Anderson Cancer Center, United StatesYuri Matteo Falzone, San Raffaele Scientific Institute (IRCCS), Italy
Copyright © 2023 Lin, Yu, Wang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinmei Li, bGlqaW5tZWlAd2Noc2N1LmNu
†These authors have contributed equally to this work
‡ORCID: Jingfang Lin, orcid.org/0000-0002-4545-460X
Jinmei Li, orcid.org/0000-0002-7411-8269
 Jingfang Lin
Jingfang Lin Tianping Yu2†
Tianping Yu2† Minjin Wang
Minjin Wang Jinmei Li
Jinmei Li