
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 28 October 2022
Sec. Autoimmune and Autoinflammatory Disorders: Autoinflammatory Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fimmu.2022.1001055
A high prevalence of Epstein–Barr virus (EBV) infection in patients with inflammatory bowel disease (IBD) has been reported in many case reports and studies; thus, the association between EBV and IBD has gained increasing attention. Patients with IBD are at an increased risk of opportunistic EBV infection owing to the common use of immunomodulators. EBV infection in IBD patients can cause various complications, including superimposed viral colitis, which is associated with chronicity, exacerbation, and poor prognosis of refractory IBD, and can induce progression to lymphoproliferative disorders, such as EBV-positive mucocutaneous ulcer (EBVMCU), lymphomatoid granulomatosis (LYG), hemophagocytic lymphohistiocytosis (HLH) and diffuse large B-cell lymphoma (DLBCL). It has been suggested to screen for EBV before initiating immunosuppressive therapy and monitor the status of EBV infection in patients with IBD, especially those who are EBV-seronegative and have a risk of primary EBV infection. Clinicians should also be careful of misdiagnosing IBD and EBV-associated lymphoproliferative diseases due to similarities in both clinical symptoms and endoscopic manifestations. Withdrawal of immunosuppressants has been shown to be an effective strategy to achieve remission of disease at the time of EBV diagnosis, but antiviral therapy remains controversial. The present review aims to describe the characteristics of the complications caused by EBV infection and generalize the recent research progress on and challenges caused by EBV infection in IBD patients. The literature for writing this review was collected from ‘PubMed’ research engine. The keywords ‘inflammatory bowel disease and Epstein–Barr virus’ or ‘ulcerative colitis and Epstein–Barr virus’ or ‘Crohn’s disease and Epstein–Barr virus’ were used to collect the literature and relevant papers were collected to help writing this review.
Inflammatory bowel disease (IBD) is a well-characterized syndrome that includes Crohn’s disease (CD), ulcerative colitis (UC), and inflammatory bowel disease unclassified (IBDU), which have closely related but heterogeneous disease processes and manifest with alternating periods of exacerbation and remission. The pathogenesis of IBD is still unclear; the current leading theory involves uncontrolled immune-mediated chronic inflammation in the intestinal mucosa of genetically predisposed individuals responding to an unknown environmental trigger that interacts with the intestinal gut flora (1).
Epstein–Barr virus (EBV) is a member of the herpesvirus family that successfully infects over 90% of people, mostly in childhood, with lifelong persistence in the latent phase in resting memory B cells. Primary EBV infection during childhood is usually asymptomatic, but when it occurs in adolescence or adulthood, most cases manifest clinically as infectious mononucleosis (IM), which is usually a self-limiting disease (2). However, EBV can be reactivated, especially in immunocompromised people whose immune systems are impaired, resulting in aggressive and even fatal lymphoproliferative diseases such as chronic active EBV infection (CAEBV), hemophagocytic lymphohistiocytosis (HLH), and B- or T/NK-cell lymphomas (2–4). Moreover, other malignant diseases, including nasopharyngeal carcinoma (NPC), post-transplant lymphoproliferative disease (PTLD), gastric adenocarcinoma, and autoimmune diseases, have been reported to be associated with EBV infection (5–8).
An increasing number of studies have verified EBV in the peripheral blood or the intestinal mucosa using the techniques of qPCR and in situ hybridization for EBV-encoded small RNA1 (EBER1) in IBD patients (9–11), and EBV infection may play a role in the exacerbation of the IBD clinical course, resulting in refractory IBD (12–14). In patients with IBD and opportunistic EBV infection, latent EBV can transform into lytic EBV, causing EBV-related colitis, lymphoproliferative diseases, and occasionally malignant lymphomas; this transformation is likely related to the long-term use of immunosuppressants or biologics and chronic inflammation itself (Figure 1) (10, 13, 15, 16). This review aims to describe the characteristics of the complications caused by EBV infection and generalize the recent research progress on and challenges associated with EBV infection in IBD patients.
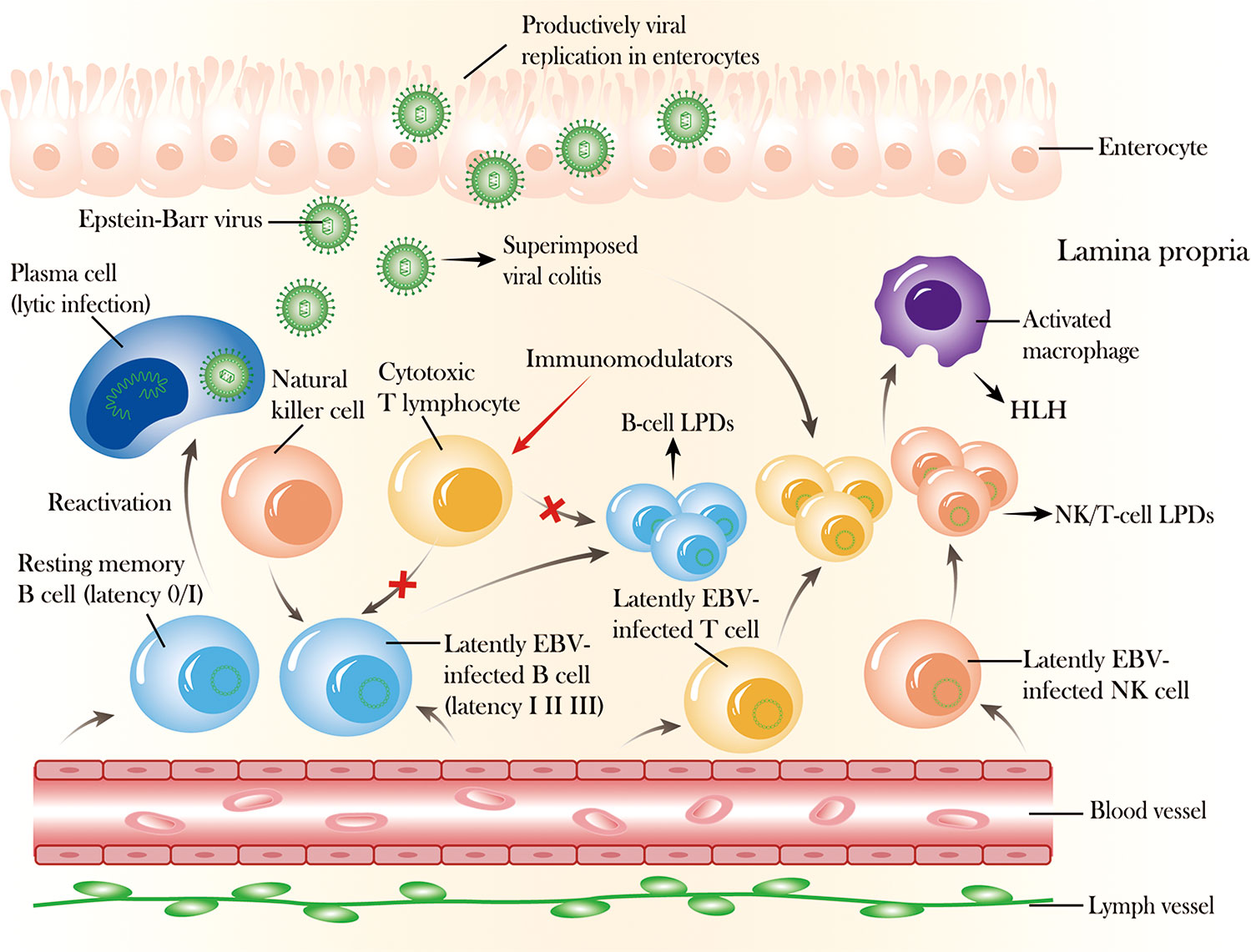
Figure 1 The mechanism and outcome of Epstein–Barr virus (EBV) reactivation in the intestinal mucosa. After primary infection, EBV can express different proteins (latency I, II, III) in latently infected B cells that can be recognized and controlled by cytotoxic T lymphocytes and natural killer (NK) cells, and EBV can exist permanently in resting memory B cells (latency 0/I) in healthy immunocompetent individuals. In addition, EBV can also infect NK or T cells, with prolonged latent infection. However, EBV can be reactivated from latency 0/I to cause lytic infection with active production of virus, and latently infected B or NK/T cells can undergo activation and proliferation due to EBV replication associated with the long-term use of immunosuppressive agents or biologics, which impairs cellular immunity, causing superimposed viral colitis and even B-cell or NK/T-cell lymphoproliferative diseases. Moreover, EBV infection can also spread from lymphocytes to enterocytes, where lytic replication occurs, destroying the mucosal tissue.
During primary infection, EBV infects B lymphocytes in the oropharyngeal mucosa, and possibly, after an initial transient burst of replication in the oropharyngeal epithelium, latency is induced. In most immunocompetent individuals, EBV exists persistently in the latent phase in resting memory B cells and does not cause clinical symptoms because newly infected and differentiating B cells can be controlled by cytotoxic T-cell (CTL) responses. The EBV genome consists of a linear DNA molecule that encodes nearly 100 viral proteins that are expressed during replication, whereas only 10 are expressed in latently infected B cells, including two types of noncoding RNAs (EBER1 and EBER2), six nuclear proteins (EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C and EBNA5) and two membrane proteins (LMP1 and LMP2), which help EBV escape virus-specific, cell-mediated immune responses of the host in immunocompetent individuals. There are four latency patterns (latency 0, I, II, III) of EBV gene expression, and three of them are observed in EBV-associated diseases. Latency I is associated with Burkitt’s lymphoma, and only EBNA-1 and EBER are expressed in this form. Latency II is associated with nasopharyngeal carcinoma, Hodgkin’s lymphoma, and peripheral T-cell lymphoma, and EBNA-1, LMP-1, LMP-2, and EBER are expressed in this second form. Latency III is associated with infectious mononucleosis and X-linked lymphoproliferative disease, and all the genes (EBNA-1, EBNA-2, EBNA-3, LMP-1, LMP-2, and EBER) are expressed in this third form (2). Moreover, EBV can be reactivated and shift to the lytic phase of infection in a subset of immunocompromised individuals, such as patients with cancers or autoimmune diseases, initiated by the immediate early transcription factors BZLF1 and BRLF1 and accompanied by the differentiation of B lymphocytes into plasma cells (17).
Many studies have confirmed the presence of EBV infection in mucosal inflammatory cells of patients with IBD (Table 1); thus, the relationship between EBV infection and IBD has gained increasing attention (9–11). Various techniques have been developed to diagnose EBV infection, such as blood analysis, EBV viral load (EBV-DNA) measurement by polymerase chain reaction (PCR), serological EBV-specific antibody testing, EBV gene product identification by immunohistochemistry (IHC), EBV-encoded small RNA (EBER) detection by in situ hybridization (ISH) and EBV DNA detection in biopsies of colon mucosa by PCR; among these techniques, EBER-ISH is considered the gold standard due to its high sensitivity and precise cellular localization (10, 11, 25). Although over 90% of the population has positive serology (2), performing serological testing to assess EBV infection has some limitations, as most patients with IBD are serologically IgG positive; however, this implies only prior infection. There are very few cases of IgM positivity, which indicates recent infection, easily leading to a false negative result in immunocompromised patients with IBD (13, 22). PCR is used to identify the presence of EBV in the intestinal samples of patients with IBD but cannot identify the exact location of EBV in the colonic tissue (12). The application of in situ hybridization for EBER in many studies helped identify EBV-infected cells, which were mainly B lymphocytes, in the colonic mucosa in both Western and Asian population (9, 10, 22). Immunohistochemical staining for EBV-specific lytic proteins BMRF1 and BZLF1, indicators of a switch from the EBV latent phase to the lytic phase (22, 25), was performed in some studies and verified the existence of EBV lytic infection in the colonic mucosa of patients with IBD (10, 16, 22). Ciccocioppo et al. used real-time PCR to investigate EBV localization and the viral DNA load in different cell populations in the colonic mucosa of IBD patients for the first time and discovered the presence of EBV DNA in enterocytes isolated from colonic mucosa. This study also found that the lack of ZEBRA (BZLF1) staining in those enterocytes carrying a high viral load cannot rule out the presence of the productive viral replication although it is now accepted that the role of epithelial cells in the EBV life cycle is to support the replication and spread of the virus within the host (16).
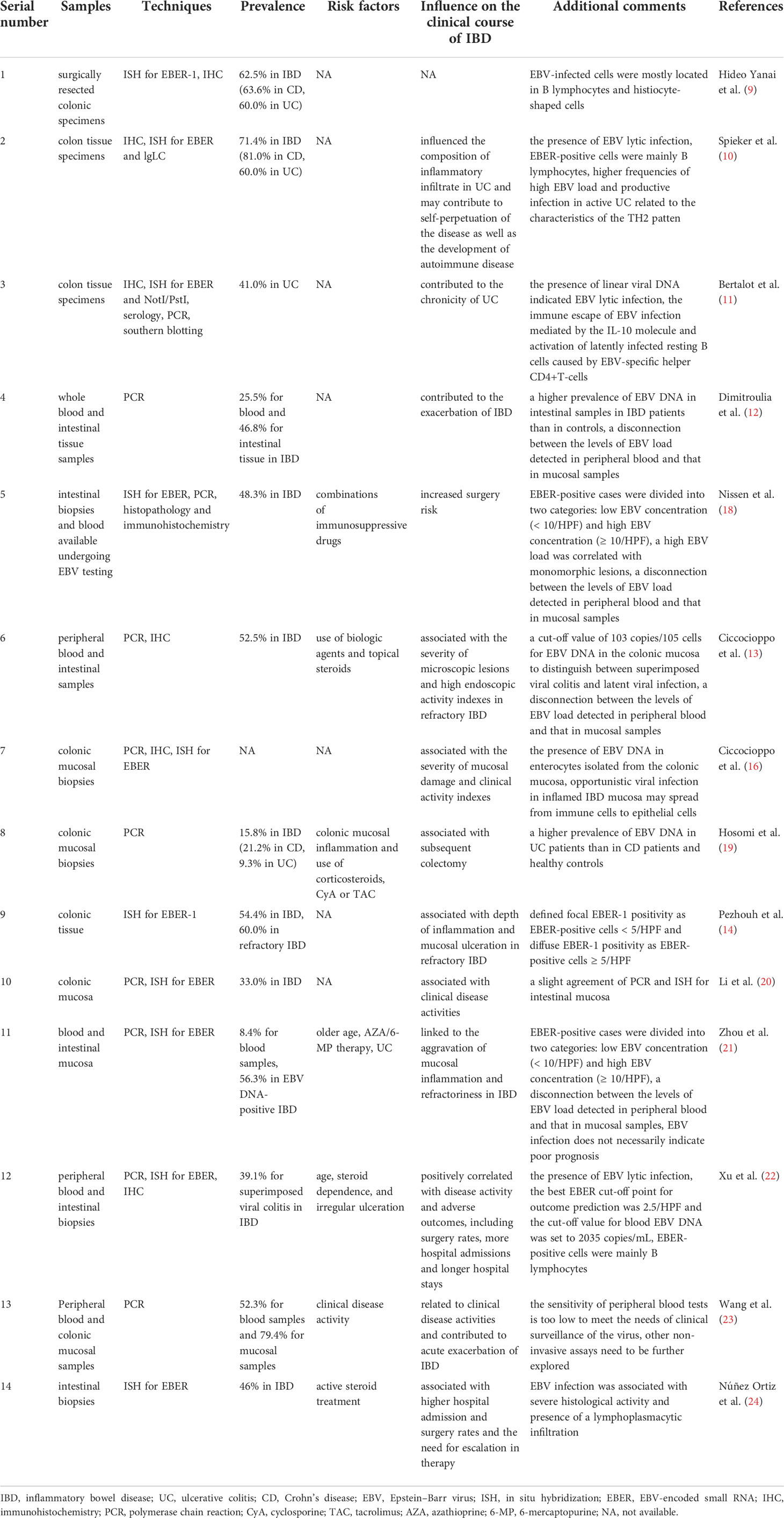
Table 1 Epstein–Barr virus-associated viral colitis in IBD patients and the influence of EBV on the clinical course of IBD.
Although many studies have confirmed the presence of EBV infection in peripheral blood or intestinal tissue of patients with IBD, few studies have attempted to distinguish between latent nonpathogenic EBV infection and superimposed viral colitis, and those that have tended to confuse the two conditions, leading to extremely different outcomes among IBD patients (9–12). Of course, immunohistochemical staining for BZLF1 can help confirm the existence of lytic EBV infection in the intestinal tissues of IBD patients, but BZLF1-positive cells have been detected in very few IBD cases, suggesting a limited value in differentiating lytic infection from latent infection (10, 22). Pezhouh et al. defined focal EBER-1 positivity as EBER-positive cells in < 5/high-power fields (HPF) and diffuse EBER-1 positivity as EBER-positive cells in ≥ 5/HPF (14). Nissen et al. and Zhou et al. divided EBER-positive cases into two groups: cases with low EBV concentrations (< 10/HPF) and high EBV concentrations (≥ 10/HPF) (18, 21). However, a specific cut-off to establish a diagnosis of EBV-related colitis has not been clarified. Accordingly, Shu Xu et al. conducted a study and identified that the best EBER cut-off point for outcome prediction was 2.5/HPF and that the cut-off value for blood EBV DNA was 2035 copies/mL, which was more accurate than the traditional cut-off value of 500 copies/mL, although the sensitivity and specificity were not sufficiently high (22). A prior study by Ciccocioppo et al. suggested a cut-off value of 103 copies/105 cells for EBV DNA in the colonic mucosa to distinguish between superimposed viral colitis and latent viral infection using the highly sensitive RT−qPCR technique (13). These cut-off values may provide better clinician insight for the assessment of the impacts of EBV infection in IBD patients. Although blood testing is a noninvasive and simple method, several studies found a disconnection between the levels of EBV load detected in peripheral blood and that in mucosal samples, suggesting that viral colitis may exist independently of the systemic involvement (12, 13, 18, 21). Moreover, the study by Li et al. identified a slight agreement of PCR and ISH in intestinal mucosa and suggested to using both techniques to detect EBV in clinical practice (20). Further detailed studies are needed to explore the different phases of EBV infection and investigate the best diagnostic methods for EBV-related colitis in IBD patients.
In conclusion, many techniques including PCR, ISH for EBER, and immunohistochemistry have been applied to confirm the presence of EBV infection in the mucosal inflammatory cells of patients with IBD. EBER-ISH is considered as the gold standard to determine EBV infection in intestinal mucosa due to its high sensitivity and precise cellular localization. However, distinguishing between EBV latent infection and superimposed viral colitis remains difficult and the testing of EBER by ISH and EBV-DNA by PCR in intestinal mucosa of IBD seems much more sensitive and accurate than blood testing to some extent.
Prior studies found that the prevalence of EBV in the intestinal mucosa of patients ranged from 16% to 79.4% (10, 11, 13, 14, 19–23). Differences in the enrolled patient populations, regions, means of detection, and study designs may explain the inconsistencies among the results. A recent study by Zhou et al. (21) found that the prevalence of detectable levels of EBV DNA in the blood of patients with IBD was 8.4%, while much higher prevalences of EBV DNA in peripheral blood were found in prior studies [20% in the study by Ciccocioppo et al. (13) and 35% in the study by Magro et al. (26)]. It is worth noting that these studies used different blood samples. Magro et al. (26) used whole blood to detect EBV DNA, while Zhou et al. (21), who reported a much lower prevalence, tested plasma, which may explain the discrepancy in the results. Blood samples used to detect EBV DNA include whole blood, plasma, and mononuclear cells, but plasma is tested more frequently and is more precise for adult patients. However, all these studies concluded that infection with EBV may have a role in the pathogenesis of IBD, exacerbation of symptoms, and the poor clinical course leading to refractory IBD (Table 1).
The study by Spieker et al. demonstrated that EBV infection may influence the composition of inflammatory infiltrate in UC and may contribute to self-perpetuation of the disease as well as the development of autoimmune disease (10). Subsequently, the study by Bertalot et al. suggested that EBV infection may be associated with chronic UC (11). A large cross-sectional study comprising 94 patients with IBD identified that EBV genetic material was detected more frequently in patients with IBD than in controls and was detected significantly more frequently in intestinal tissue in patients with disease exacerbation than in patients with remission (12). Moreover, studies by Ciccocioppo et al. revealed a higher prevalence of EBV-DNA in refractory IBD patients than in nonrefractory IBD patients and controls, and the EBV DNA load was positively correlated with the severity of mucosal damage and clinical indexes of activity (13, 16). In addition, they innovatively discovered the potential mechanism of EBV-associated IBD refractory to conventional therapies, in which opportunistic viral particles in inflamed IBD mucosal cells spread from immune cells to epithelial cells, thus resulting in productive viral replication and significantly contributing to tissue damage (16). In a case−control retrospective study using ISH for the detection of EBER1, a much higher proportion of EBER-positive lymphocytes was found in the colectomy specimens of patients with refractory IBD than in controls, and a positive correlation between EBER positivity and the depth of inflammation and mucosal ulceration in patients with refractory IBD was identified (14). Likewise, recent studies formed the same conclusions in cohorts of Chinese patients with IBD (20–23). In addition to these clinical studies, a recent study using an IBD mouse model, namely, the dextran sodium sulfate (DSS) mouse colitis model, detected that the presence of EBV DNA exacerbated colitis by aggravating colonic disease activity and increasing damage to the colon histologic architecture; this result indicates the possibility of potential therapeutic approaches targeting endosomal Toll-like receptor (TLR) signaling, which needs to be tested in large proportions of patients with IBD (27).
The presence of EBV infection in the intestinal mucosa also seems to contribute to the poor prognosis of IBD. A study by Nissen et al. found that high EBV concentrations were more likely to occur in EBV-positive patients undergoing colectomy than in those without colectomy and were associated with the presence of atypical inflammatory infiltration and B-lymphocytes (18). Coinfection with EBV was discovered to be a risk factor for subsequent colectomy in patients with UC in the study by Hosomi et al. (19). A study in a cohort of 92 UC patients by Xu et al. revealed that UC patients with high EBV concentrations had a higher risk of adverse outcomes, including surgery, hospital admission, and a prolonged hospital stay (22). A very recent study aiming to explore the impacts of EBV infection on IBD outcomes also reported that EBV positivity was associated with higher hospital admission and surgery rates and a greater need for therapy escalation (24). However, a study by Zhou et al. found that EBV infection was not associated with a poor prognosis. This discrepancy may result from the small number of patients enrolled since this study included only 27 EBER-1-positive IBD patients (28).
Among these studies, age; irregular ulceration; and therapy comprising steroids, biological agents or immunosuppressants were possible risk factors for EBV infection in patients with IBD (13, 18, 21, 22) (Table 1). In addition, the study by Zhou et al. found a higher prevalence of EBV infection in UC patients than CD patients and considered UC to be a risk factor for EBER positivity in IBD patients (21). This conclusion is consistent with that of the study by Spieker et al., who discovered higher frequencies of a high EBV load and productive infection in active UC patients than in CD patients and controls, probably related to the characteristics of the TH2 pattern and elevated serum levels of soluble CD30 protein in UC patients (10). Recently, several studies have explored possible mechanisms of the role of EBV infection in the pathogenesis of UC. A study by Wyss et al. indicated that EBV infection may contribute to the severity of colonic inflammation through the EBI2-7α,25-dihydroxycholesterol axis in patients with UC and mice with colitis (29). Another study in 76 UC patients identified that EBV loads were positively correlated with the disease activity of UC and that EBV infection had a potential influence on the decrease in Helios+ FoxP3+ Tregs in severe active UC patients (30).
In conclusion, a high prevalence of EBV infection in the intestinal mucosa is identified in patients with IBD, especially in those who are refractory to traditional therapy. The co-infection of EBV is probably associated with the exacerbation and poor prognosis including surgery rates and more hospital admissions in the clinical course of IBD under the possible risk of age, therapy of steroids, biological agents or immunosuppressants.
EBV infection is not only associated with the complication of superimposed viral colitis, but can also lead to lymphoplasmacytic infiltration and a heterogeneous spectrum of lymphoproliferative diseases in patients with IBD due to the extensive use of immunomodulators (18, 31–35). According to the 2016 World Health Organization (WHO) classification of lymphoid neoplasms, EBV-associated lymphoproliferative diseases can be divided into the following categories: B-cell lymphoproliferative diseases (B-cell LPDs) and NK/T-cell lymphoproliferative diseases (NK/T-cell LPDs) (Table 2) (36, 37). In patients with IBD, cases of EBV-positive mucocutaneous ulcer (EBVMCU), lymphomatoid granulomatosis (LYG), HLH, B-cell lymphoma, and very rare NK/T-cell lymphoma have been reported and well described (Table 2) (33, 35, 38–41). Ohshima et al. classified EBV-associated T/NK LPD based on pathological evaluation and molecular data into the following categories: A1 cases, which are polymorphic and polyclonal; A2 cases, which are polymorphic and monoclonal; A3 cases, which are monomorphic and monoclonal; and B cases, which are monomorphic and monoclonal and have a fulminant clinical course (42). This classification applies to EBV-associated lymphoproliferative diseases in patients with IBD and helps both pathologists and clinicians better define and differentiate these diseases. The specific threshold of EBV load to predict the onset of EBV-associated lymphoproliferative diseases has not been determined, but Nissen et al. found that high EBV concentrations with EBER-positive cells in ≥ 10/HPF were significantly more frequent in the monomorphic groups (18); this can provide a reference for clinicians.
EBVMCU, as a new entity recognized in the 2016 review of the WHO classification of lymphoid neoplasms (36), was first described in a study by Dojcinov et al. in 2010 that included 26 patients receiving different types of immunosuppression, including one patient with UC (43). In general, EBVMCU is a very rare B-cell lymphoproliferative disease associated with age-related immunosenescence or iatrogenic immunosuppression. It is morphologically characterized by shallow, sharply circumscribed ulcers involving the oropharyngeal mucosa, skin, or gastrointestinal tract, and it is pathologically characterized by a polymorphous infiltrate of small lymphocytes, histiocytes, plasma cells, eosinophils, and atypical large B-cell blasts, often with Hodgkin/Reed-Sternberg (HRS) cell-like morphology. It is immunologically characterized by a B-cell immunophenotype in the lesional immunoblasts with positive staining of CD30, CD15, CD20, CD79a, CD45, MUM1, PAX5, OCT-2, and BCL-6 and genetically characterized by EBER positivity in the infiltrating cells on ISH and the presence of clonal Ig gene rearrangements and monoclonal or clonally restricted T-cell patterns compatible with the restricted T-cell response against EBV infection on PCR in a subset of cases (43, 44). Very few cases have been reported in patients with IBD who mostly received therapy comprising immunosuppressive agents, including methotrexate (MTX), azathioprine (AZA) or 6-mercaptopurine (6-MP), or combination therapy with anti-TNF drugs, including infliximab, adalimumab or golimumab (31, 40, 44–47), though some cases have possibly been misdiagnosed or are unpublished. The clinical course of EBVMCU is mostly self-limiting and indolent, and the lesions tend to regress spontaneously after the withdrawal of immunosuppressants and respond well to conservative management (40, 43). However, with the increase in the number of cases reported, scientists found that EBVMCU can exhibit an aggressive course and may require aggressive therapy to prevent disease progression (44). Moran NR et al. first reported a case of EBVMCU progressing to widespread Hodgkin lymphoma in a patient with CD who underwent emergency colectomy and subsequent urgent chemotherapy (45). There are also reports of cases that required CD20- and CD30-directed antibody therapy, such as rituximab and brentuximab, to achieve clinical recovery; providing new therapeutic strategies for some severe cases of EBVMCU (31, 47).
LYG is also a rare EBV-driven B-cell lymphoproliferative disease (LPD) clinically characterized by universal involvement of the lungs as well as other common extranodal sites, including the skin, central nervous system, liver, and kidneys. It is pathologically characterized by infiltrate composed of EBV-positive atypical B cells with different numbers and densities on a graded basis, angioinvasive/angiodestructive reactive T-cell infiltrate, and various degrees of necrosis. Treatment methods for LYG include immune modulation of interferon-α2b in low-grade disease, immunochemotherapy in high-grade disease, and crossover treatment in some cases of relapse or progression (48). LYG rarely occurs in patients with IBD. Subramaniam et al. reported a case of EBV-associated lymphoproliferative disease resembling LYG based on histopathological examination of tissue obtained from a large necrotic gastric ulcer and a necrotic pulmonary nodule subjected to immunochemotherapy in a 42-year-old woman with CD, but this patient suffered from various severe complications and finally died from refractory disease involving the central nervous system (41). Thus, the detailed management of LYG in patients with IBD remains unclear because of the rarity of this disease.
Another rare complication, HLH, has been increasingly reported in IBD patients with EBV infection who are exposed to immunosuppressants or biologics (3, 31, 38, 49–52). HLH is a life-threatening clinical syndrome with symptoms of prolonged fever, hepatosplenomegaly and pancytopenia. Characteristic clinical findings include increased ferritin, triglyceride, transaminase, bilirubin, lactate dehydrogenase, and soluble interleukin-2 receptor α-chain levels; decreased fibrinogen levels; characteristic bone marrow aspirate results; increased numbers of macrophages; and evidence of hemophagocytosis (53). Many cases of HLH complicated with IBD have been reported in pediatric patients or adolescents who are more prone to suffer from primary EBV infection (31, 38, 49, 51, 54), and a respective study revealed that pediatric patients with CD and thiopurine administration had a 100-fold higher risk for the development of HLH (39). However, it is worth noting that some cases of HLH occur in adult patients with IBD (3, 50, 52, 55). CD seemed to be a risk factor for the development of HLH among the reported cases; this result was also found in Li et al.’s review, and Thompson et al. suspected that there may be underlying disease-specific factors contributing to HLH due to the importance of the Th1-driven cytokine response in both CD and HLH (52, 56, 57). Very few reports analyzed the function of NK cells. Two case reports identified NK cell deficiency in a teenage patient and an adult patient, respectively, and they were successfully treated with only rituximab therapy, which eliminates EBV-infected B cells and is more specific and less toxic than traditional chemotherapy (51, 52). Thus, early recognition, diagnosis, and treatment of HLH in susceptible IBD patients are of vital importance to prevent disease progression since HLH tends to be associated with a poor outcome, and several cases are complicated by EBV-associated lymphoproliferation and even malignant lymphomas (3, 50, 54). In addition, the diagnosis of X-linked proliferative (XLP) syndrome should also be taken into consideration when HLH occurs in a young patient with IBD associated with primary EBV infection, as reported by Hügle et al. (58).
An increasing number of EBV-associated lymphoproliferative diseases and lymphomas have been identified as complications of IBD, including Hodgkin lymphoma (34, 41, 59–61), diffuse large B-cell lymphoma, NK/T-cell lymphoma (33, 35, 62–65), and some unclassifiable B-cell lymphoproliferative disorders (3, 66–69). Since the clinical symptoms of intestinal lymphoproliferative diseases are diverse and tend to be similar to those of IBD and the final diagnosis mainly relies on histopathological and immunophenotypic examinations of intestinal biopsy or surgical resection tissue, scientists have recommended examining deeper intestinal tissue to observe atypical infiltration of B or T/NK lymphocytes and the specific immunophenotypes of different lymphomas to avoid misdiagnosis and treatment delay, especially when IBD becomes severe and refractory (34, 63). It is challenging to elucidate the exact role of EBV in the pathogenesis of lymphomas in patients with IBD, and EBV possibly contributes differently to different types of lymphomas. EBV can have an antiapoptotic function, such as c-myc deregulation or loss of B-cell antigen receptor (BCR) function, in B-cell lymphomas, while the mechanism of EBV in NK/T-cell lymphomas remains unknown. Similar to that of in PTLD, the pathogenesis of lymphoma in patients with IBD may be attributed to immunosuppression by immunosuppressive therapy, which impairs the immunosurveillance system and CTL immune activity, reactivates EBV latent infection and promotes oncogenic function, possibly related to the chronic inflammatory microenvironment in IBD (4, 70). Future studies are needed to explore the molecular mechanisms of EBV-associated lymphomas in patients with IBD exposed and unexposed to immunosuppressants to better explain the relationship among EBV, lymphomas and IBD. Although lymphoproliferative diseases in IBD patients share similar clinicopathological characteristics with PTLDs, the prognosis of lymphoproliferative diseases in IBD patients seems to be better than that of PTLD after stopping the use of immunosuppressive therapy and receiving chemotherapy when necessary (18, 71).
It is still unclear whether there is an association between lymphoma and IBD, and prior studies found no significantly increased risk of lymphomas in patients with IBD compared with the general population (72, 73). However, the association between lymphoma and immunosuppressive therapy in patients with IBD has been clarified in many studies (15, 32, 74–78) on the basis of the common use of azathioprine and 6-mercaptopurine for the treatment of steroid-dependent or steroid-refractory IBD (79) (Table 3). The study by Dayharsh et al. reported a slight association between immunosuppressant (azathioprine, 6-mercaptopurine) use and EBV-positive lymphoma in patients with IBD (15). The first large prospective study, the CESAME cohort study (32), revealed that patients with IBD receiving thiopurines had a higher risk of development of lymphoproliferative diseases than those who had never used the drugs, and the overall multivariate hazard ratio was 5.28 (95% confidence interval [CI], 2.01-13.9). Although this study did not specify the EBV involvement, most cases of lymphoproliferative diseases in this cohort were post-transplant lymphoproliferative disorder-like diseases of B-cell origin and were associated with EBV infection. This study also suggested that older age, male sex, and a longer course of IBD were additional risk factors for lymphomas in IBD (32), which was in accordance with the results of a case−control study (77). Interestingly, an increased risk of EBV-positive lymphoma was also observed in younger adults (<50 years) with IBD in the study by Vos et al. (75). In this nationwide study involving a cohort of 17,834 IBD patients, 92% of the patients complicated with EBV-positive lymphoma were exposed to azathioprine or 6-mercaptopurine, compared with 19% of the patients with EBV-negative lymphoma, implying a strong association between EBV-positive lymphoma in IBD and thiopurine use (75).
The risk of incident lymphoma in patients with IBD receiving anti-TNF therapy remains controversial because few patients receive anti-TNF monotherapy; most of them are treated with thiopurines alone or combined with immunosuppressants and anti-TNF agents (73, 80). Therefore, Lemaitre et al. conducted a nationwide cohort study to assess the risk of lymphoma in adult patients with IBD exposed to thiopurine monotherapy, anti-TNF (infliximab, adalimumab) monotherapy and combination therapy and discovered that thiopurine monotherapy, anti-TNF monotherapy and combination therapy were all associated with a significantly increased risk of lymphoma in IBD patients compared with unexposed patients. Moreover, the risk associated with combination therapy was higher than those associated with thiopurine monotherapy and anti-TNF monotherapy (81). Unlike that for infliximab and adalimumab, which are widely used in IBD patients, no data on the risk associated with therapy with other anti-TNF agents, such as golimumab and certolizumab pegol, in IBD patients complicated with lymphomas have been available. In addition, the association between the use of anti-TNF agents and EBV-associated lymphomas in IBD patients remains unclear because EBV status and the association with EBV infection were not detected in IBD patients with lymphomas in prior studies.
Schwartz et al. suggested that the increased risk of lymphomas in IBD patients was associated with a high dose and long use duration of immunosuppressants, but the exact dose and duration remain unclear (62); additionally, studies found that there tended to be a long duration between the onset of IBD and the occurrence of lymphoma (32, 76). No studies have proven the association between chronic inflammation or disease activity and lymphoma in patients with IBD. In contrast, it has been clearly shown that there is an increased risk of lymphoma in rheumatoid arthritis patients; and this risk is strongly associated with disease activity (82, 83).
In summary, EBV infection predisposes patients with IBD to develop lymphoproliferative diseases, including EBV-positive mucocutaneous ulcer (EBVMCU), lymphomatoid granulomatosis (LYG), hemophagocytic lymphohistiocytosis (HLH), B-cell lymphoma, and very rare NK/T-cell lymphoma which is usually associated with therapy of immunosuppressants or biologics. In addition, old age, male sex, and longer duration of IBD are also associated with increased risk for lymphomas in IBD. The pathogenesis of EBV-associated LDs in IBD has not been clarified and is probably related to the impairment of CTL function caused by immunosuppression.
To date, no international guidelines for the diagnosis of and therapy for complications caused by EBV infection in patients with IBD exist, and many questions remain to be answered and explored in clinical practice.
Whether and when to identify EBV infection status are controversial topics. A prospective cohort study in Canada conducted serological testing for VCA-IgM, VCA-IgG, and EBNA-IgG in 263 patients, and the results showed that the prevalence of EBV seronegativity in the IBD population aged 18-25 years was 29%, which was similar to that in the general population; however, EBV seropositivity reached 100% in those older than 25 years, and seropositivity was associated with thiopurine use (84). This supports that younger patients are at increased risk for primary EBV infection, which may result in fatal outcomes such as hemophagocytic lymphohistiocytosis. A recent study by Jennifer Bachmann et al. also confirmed that children with IBD treated with thiopurines had a higher risk of primary EBV infection and developing HLH and suggested to offer functional or genetic testing for XIAP to male patients with EBV-related complications and those with therapy refractory severe CD-like disease during follow-up in a large single center cohort of children with IBD (85). Therefore, EBV testing in younger patients with IBD before the initiation of immunosuppressive drugs is suggested (38). However, the largest study to date on the seroprevalence of EBV infection in adult patients with IBD found that the overall seroprevalence of EBV infection was 97.4%, which suggested the existence of a risk of primary EBV infection in a small percentage of adults with IBD (86). Moreover, a recent multicenter, cross-sectional, observational study in Japan revealed that the average age at the time of primary EBV infection was older than previously reported, with a significant number of uninfected patients in their 20s (87). The latest European Crohn’s and Colitis Organisation (ECCO) guidelines suggest that the use of thiopurines in EBV-IgG-negative patients should be carefully considered (88). Thus, it is of vital importance to screen for EBV infection when considering the administration of immunosuppressants in patients with IBD at any age, not only younger patients. Of course, as discussed above, most adult patients with IBD suffer from complications including superimposed EBV colitis and lymphoproliferative diseases caused by EBV reactivation from latent infection associated with immunosuppressive therapy (22, 32). If the symptoms in IBD patients receiving immunosuppressants become more severe or refractory to traditional drugs or present frequent relapse and endoscopic examination identifies irregular ulcerations, EBV detection in intestinal biopsy or surgical specimens by PCR or EBER-ISH should be considered (14, 22). In addition, considering that the EBER-ISH technique is costly and infrequently performed in clinical practice, it is recommended determining the EBV load in the intestinal mucosa of patients with IBD when the histopathological findings show atypical inflammatory infiltration and/or B-lymphocytes (18). It is also worth noting that opportunistic EBV infection in patents with IBD can develop into malignant lymphoma, and the diagnosis can be a major challenge for clinicians (75). Further studies are needed to explore whether and when to screen for EBV infection and monitor the status of EBV infection and the best testing method to determine the cut-off point of the EBV load to predict the development of EBV-associated complications in patients with IBD.
In the absence of complications caused by EBV infection in IBD patients, it can be difficult to differentiate IBD from rare EBV-associated intestinal lymphoproliferative diseases such as EBVMCU, HLH, systemic CAEBV involving the gastrointestinal tract, and very rare lymphomas, including EBV-positive diffuse large B-cell lymphoma (EBV+ DLBCL), nasal type extranodal NK/T-cell lymphoma, intestinal T-cell LPD, Hodgkin lymphoma, Burkitt lymphoma, and extracavitary primary effusion lymphoma (EPEL), because of their overlapping and nonspecific clinical symptoms and endoscopic manifestations (3, 34, 89–92). CAEBV involving the gastrointestinal tract is most commonly misdiagnosed as refractory IBD due to overlapping clinical symptoms, laboratory findings, and endoscopic manifestations. In addition to the common gastrointestinal symptoms, including diarrhea, abdominal pain, and hematochezia, patients with CAEBV often have systemic symptoms, such as intermittent fever, hepatomegaly, splenomegaly, and lymphadenopathy, which are rare in IBD. Other characteristics to help differentiate CAEBV from IBD include extremely high levels of ferritin associated with EBV infection and atypical endoscopic manifestations (91, 93). It is also of vital significance to detect the EBV load by qPCR or EBER-ISH in both peripheral blood and intestinal mucosa because the EBV load in patients with CAEBV is much higher than that in patients with IBD. Although no international criteria for the threshold of EBV load to determine active EBV infection have been established, prior CAEBV cases series found that patients often had more than 105 copies/mL of EBV DNA in peripheral blood, more than 100 EBV+ cells/HPF in surgery samples, and more than 30 EBV+ cells/HPF in biopsy samples, which provides support for clinicians for a correct diagnosis (91, 93). Because CAEBV usually has a poor prognosis and has the potential to progress to hemophagocytic lymphohistiocytosis or malignant NK/T lymphoma, especially when involving T or NK cells in Asian populations, early diagnosis of the disease is important, and the only proven curative treatment for the disease is hematopoietic stem cell transplantation (94, 95). Intestinal lymphoma is a common differential diagnosis for IBD in clinical practice, but misdiagnosis in cases of EBV-positive primary intestinal lymphoma is not uncommon, especially primary EBV-positive intestinal NK/T-cell lymphomas (NKTCLs). The gastrointestinal tract is the most common primary site of extranodal lymphomas, and intestinal NKTCL is a relatively rare type with a high degree of malignancy that most commonly involves the colon and part of the small intestine (96). The symptoms that overlap with IBD include fever, abdominal pain, diarrhea, and complications such as perforation and fistula. However, the intermittent pattern of fever in patients with primary intestinal NKTCL can help differentiate it from IBD. The endoscopic manifestations (Figure 2) and pathological findings of primary intestinal NKTCL are atypical and resemble those of IBD. However, primary intestinal NKTCL possesses some distinctive immunophenotypic features, including NK/T cells [CD3 positive] expressing EBER, CD56 [NK-cell type] and monoclonal TCRγ [T-cell type] genes, and negative expression of CD5 (97). Thus, repeat deep endoscopic biopsy combined with examination of the immunophenotype, detection of EBV infection, and exploratory laparotomy should be considered to help establish the early correct diagnosis when intestinal malignant lymphoma is highly suspected (89, 90, 97, 98).
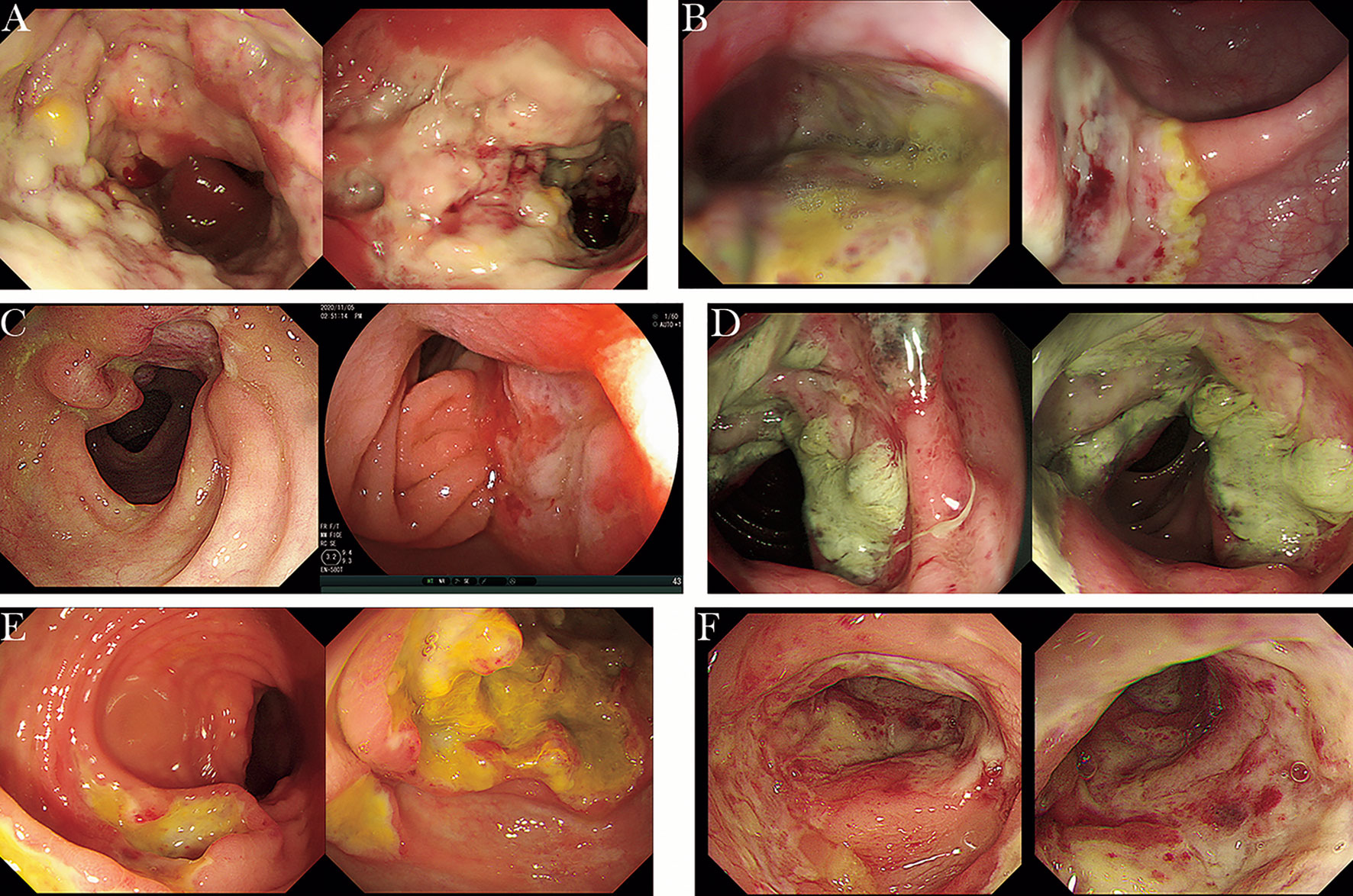
Figure 2 The endoscopic manifestations of intestinal NKTCL (cases from Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine). (A) Extensive and irregular ulcers in the sigmoid colon, accompanied by mucosal hyperplasia and covered with yellow and white adhesive material. (B) Multiple large, irregular ulcers covered with white adhesive material in the transverse colon and sigmoid colon. (C) A large bulging lesion involving one half of the lumen with a sunken ulcer in the center covered with pus in the ileum and large ulcerative foci in the colon (30 cm from the anus). (D) Annular mucosal proliferative lesion with mucosal hyperemia and edema in the transverse colon. (E) Circumferential large proliferative lesion in the ileocecum and numerous circular ulcers in the transverse colon. (F) Multiple, irregular ulcers with rigid, narrow and congestive lumen in the sigmoid colon.
The therapeutic schedule for patients with IBD complicated with EBV infection is controversial. In most cases, a reduction in or withdrawal of immunosuppressants successfully aided in achieving remission of disease when EBV infection exacerbated the severity of inflammation and resulted in atypical inflammatory infiltration or lymphocytes in the intestinal mucosa of patients with IBD (18). However, therapeutic modifications, including immunosuppressant changes at EBV diagnosis, can also be complex in clinical practice (24). Prophylactic antiviral use has been proven to be an effective strategy to reduce the risk of PTLD (99), but controversy remains about antiviral therapy in patients with IBD complicated with EBV infection. Two case reports showed that the addition of ganciclovir or acyclovir helped improve symptoms in patients with IBD and EBV-associated complications (31, 100), while the study by Ciccocioppo et al. indicated that antiviral therapy was ineffective in refractory IBD patients with EBV-related colitis (13). More prospective studies are needed to specify the role of antiviral therapy for EBV infection and explore potential effective therapeutic measures.
EBV infection is very common in patients with IBD and can cause various complications, including superimposed EBV-related colitis and lymphoproliferative disorders. Thus, it is important to screen for EBV and monitor the status of EBV infection in patients with IBD, especially those who are EBV-seronegative and have a risk of primary EBV infection. Clinicians should recognize the presence of EBV infection to make a correct diagnosis as early as possible and avoid misdiagnosis in patients with refractory IBD.
HZ (First Author): Conceptualization, Writing-Original Draft; SZ (Corresponding Author): Conceptualization, Funding Acquisition, Supervision, Writing-Review & Editing; ZC (Corresponding Author): Conceptualization, Funding Acquisition, Supervision, Writing-Review & Editing. All authors contributed to the article and approved the submitted version.
Establishment and application of inflammatory bowel disease cohort database, No. SHDC2020CR6020, Scientific Research Project of Shanghai Science and Technology Commission, No.22Y11907900 and the National Natural Science Foundation of China (Grant No. 81972655).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Malik TA. Inflammatory bowel disease: Historical perspective, epidemiology, and risk factors. Surg Clin North Am (2015) 95(6). doi: 10.1016/j.suc.2015.07.006
2. Cohen JI. Epstein-Barr Virus infection. N Engl J Med (2000) 343(7):481–92. doi: 10.1056/NEJM200008173430707
3. N’Guyen Y, Andreoletti L, Patey M, Lecoq-Lafon C, Cornillet P, Léon A, et al. Fatal Epstein-Barr virus primo infection in a 25-Year-Old man treated with azathioprine for crohn’s disease. J Clin Microbiol (2009) 47(4):1252–4. doi: 10.1128/JCM.02052-08
4. Vockerodt M, Yap L-F, Shannon-Lowe C, Curley H, Wei W, Vrzalikova K, et al. The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol (2015) 235(2):312–22. doi: 10.1002/path.4459
5. Paya CV, Fung JJ, Nalesnik MA, Kieff E, Green M, Gores G, et al. Epstein-Barr Virus-induced posttransplant lymphoproliferative disorders. Asts/Astp ebv-ptld task force and the Mayo clinic organized international consensus development meeting. Transplantation (1999) 68(10):1517–25. doi: 10.1097/00007890-199911270-00015
6. Toussirot E, Roudier J. Epstein-Barr Virus in autoimmune diseases. Best Pract Res Clin Rheumatol (2008) 22(5):883–96. doi: 10.1016/j.berh.2008.09.007
7. Tsao SW, Tsang CM, Lo KW. Epstein-Barr Virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci (2017) 372(1732). doi: 10.1098/rstb.2016.0270
8. Sasaki S, Nishikawa J, Sakai K, Iizasa H, Yoshiyama H, Yanagihara M, et al. Ebv-associated gastric cancer evades T-cell immunity by pd-1/Pd-L1 interactions. Gastric Cancer (2019) 22(3):486–96. doi: 10.1007/s10120-018-0880-4
9. Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K. Epstein-Barr Virus infection of the colon with inflammatory bowel disease. Am J Gastroenterol (1999) 94(6):1582–6. doi: 10.1111/j.1572-0241.1999.01148.x
10. Spieker T, Herbst H. Distribution and phenotype of Epstein-Barr virus-infected cells in inflammatory bowel disease. Am J Pathol (2000) 157(1):51–7. doi: 10.1016/S0002-9440(10)64516-6
11. Bertalot G, Villanacci V, Gramegna M, Orvieto E, Negrini R, Saleri A, et al. Evidence of Epstein-Barr virus infection in ulcerative colitis. Dig Liver Dis (2001) 33(7):551–8. doi: 10.1016/S1590-8658(01)80106-7
12. Dimitroulia E, Pitiriga VC, Piperaki E-T, Spanakis NE, Tsakris A. Inflammatory bowel disease exacerbation associated with Epstein-Barr virus infection. Dis Colon Rectum (2013) 56(3):322–7. doi: 10.1097/DCR.0b013e31827cd02c
13. Ciccocioppo R, Racca F, Paolucci S, Campanini G, Pozzi L, Betti E, et al. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: Need for mucosal viral load measurement. World J Gastroenterol (2015) 21(6):1915–26. doi: 10.3748/wjg.v21.i6.1915
14. Pezhouh MK, Miller JA, Sharma R, Borzik D, Eze O, Waters K, et al. Refractory inflammatory bowel disease: Is there a role for Epstein-Barr virus? a case-controlled study using highly sensitive Epstein-Barr virus-encoded small Rna1 in situ hybridization. Hum Pathol (2018) 82:187–92. doi: 10.1016/j.humpath.2018.08.001
15. Dayharsh GA, Loftus EV, Sandborn WJ, Tremaine WJ, Zinsmeister AR, Witzig TE, et al. Epstein-Barr Virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology (2002) 122(1):72–7. doi: 10.1053/gast.2002.30328
16. Ciccocioppo R, Racca F, Scudeller L, Piralla A, Formagnana P, Pozzi L, et al. Differential cellular localization of Epstein-Barr virus and human cytomegalovirus in the colonic mucosa of patients with active or quiescent inflammatory bowel disease. Immunol Res (2016) 64(1):191–203. doi: 10.1007/s12026-015-8737-y
17. Münz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol (2019) 17(11):691–700. doi: 10.1038/s41579-019-0249-7
18. Nissen LHC, Nagtegaal ID, de Jong DJ, Kievit W, Derikx LAAP, Groenen PJTA, et al. Epstein-Barr Virus in inflammatory bowel disease: The spectrum of intestinal lymphoproliferative disorders. J Crohns Colitis (2015) 9(5):398–403. doi: 10.1093/ecco-jcc/jjv040
19. Hosomi S, Watanabe K, Nishida Y, Yamagami H, Yukawa T, Otani K, et al. Combined infection of human herpes viruses: A risk factor for subsequent colectomy in ulcerative colitis. Inflammation Bowel Dis (2018) 24(6):1307–15. doi: 10.1093/ibd/izy005
20. Li X, Chen N, You P, Peng T, Chen G, Wang J, et al. The status of Epstein-Barr virus infection in intestinal mucosa of Chinese patients with inflammatory bowel disease. Digestion (2019) 99(2):126–32. doi: 10.1159/000489996
21. Zhou J-Q, Zeng L, Zhang Q, Wu X-Y, Zhang M-L, Jing X-T, et al. Clinical features of Epstein-Barr virus in the intestinal mucosa and blood of patients with inflammatory bowel disease. Saudi J Gastroenterol (2020). doi: 10.4103/sjg.SJG_30_20
22. Xu S, Chen H, Zu X, Hao X, Feng R, Zhang S, et al. Epstein-Barr Virus infection in ulcerative colitis: A clinicopathologic study from a Chinese area. Therap Adv Gastroenterol (2020) 13:1756284820930124. doi: 10.1177/1756284820930124
23. Wang W, Chen X, Pan J, Zhang X, Zhang L. Epstein-Barr Virus and human cytomegalovirus infection in intestinal mucosa of Chinese patients with inflammatory bowel disease. Front Microbiol (2022) 13:915453. doi: 10.3389/fmicb.2022.915453
24. Núñez Ortiz A, Rojas Feria M, de la Cruz Ramírez MD, Gómez Izquierdo L, Trigo Salado C, Herrera Justiniano JM, et al. Impact of Epstein-Barr virus infection on inflammatory bowel disease (Ibd) clinical outcomes. Rev Esp Enferm Dig (2021). doi: 10.17235/reed.2021.7915/2021
25. Gulley ML, Tang W. Laboratory assays for Epstein-Barr virus-related disease. J Mol Diagn (2008) 10(4):279–92. doi: 10.2353/jmoldx.2008.080023
26. Magro F, Santos-Antunes J, Albuquerque A, Vilas-Boas F, Macedo GN, Nazareth N, et al. Epstein-Barr Virus in inflammatory bowel disease-correlation with different therapeutic regimens. Inflammation Bowel Dis (2013) 19(8):1710–6. doi: 10.1097/MIB.0b013e318281f31c
27. Andari S, Hussein H, Fadlallah S, Jurjus AR, Shirinian M, Hashash JG, et al. Epstein-Barr Virus DNA exacerbates colitis symptoms in a mouse model of inflammatory bowel disease. Viruses (2021) 13(7). doi: 10.3390/v13071272
28. Wang S-Z, Dai Y-H, Zhang J, Lu F-G, Yan L-M, Wu S. Clinical features of Nk/T-cell ebv-associated lpd manifested as gastrointestinal symptoms in patients with normal immunity: A case report and literature review. BMC Gastroenterol (2021) 21(1):254. doi: 10.1186/s12876-021-01718-4
29. Wyss A, Raselli T, Perkins N, Ruiz F, Schmelczer G, Klinke G, et al. The Ebi2-oxysterol axis promotes the development of intestinal lymphoid structures and colitis. Mucosal Immunol (2019) 12(3):733–45. doi: 10.1038/s41385-019-0140-x
30. Long Y, Zhao X, Xia C, Liu X, Fan C, Liu C. Infection of Epstein-Barr virus is associated with the decrease of Heliosfoxp3regulatory T cells in active ulcerative colitis patients. Immunol Invest (2021) 50(1):23–36. doi: 10.1080/08820139.2020.1723021
31. Goetgebuer RL, van der Woude CJ, de Ridder L, Doukas M, de Vries AC. Clinical and endoscopic complications of Epstein-Barr virus in inflammatory bowel disease: An illustrative case series. Int J Colorectal Dis (2019) 34(5):923–6. doi: 10.1007/s00384-019-03257-7
32. Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: A prospective observational cohort study. Lancet (2009) 374(9701):1617–25. doi: 10.1016/S0140-6736(09)61302-7
33. Hiyama K, Terashima H, Nakano Y, Kamiga M, Harada K, Horiguchi H, et al. Primary rectal diffuse Large b-cell lymphoma associated with ulcerative colitis: A case report. Clin Case Rep (2015) 3(3):150–5. doi: 10.1002/ccr3.185
34. Rasmussen SL, Thomsen C. Rectal Hodgkin lymphoma in a patient with ulcerative colitis: A case study. Diagn Pathol (2015) 10:25. doi: 10.1186/s13000-015-0271-7
35. Kakimoto K, Inoue T, Nishikawa T, Ishida K, Kawakami K, Kuramoto T, et al. Primary Cd56+ Nk/T-cell lymphoma of the rectum accompanied with refractory ulcerative colitis. J Gastroenterol (2008) 43(7):576–80. doi: 10.1007/s00535-008-2192-7
36. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world health organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
37. Rezk SA, Zhao X, Weiss LM. Epstein-Barr Virus (Ebv)-associated lymphoid proliferations, a 2018 update. Hum Pathol (2018) 79:18–41. doi: 10.1016/j.humpath.2018.05.020
38. Posthuma EF, Westendorp RG, van der Sluys Veer A, Kluin-Nelemans JC, Kluin PM, Lamers CB. Fatal infectious mononucleosis: A severe complication in the treatment of crohn’s disease with azathioprine. Gut (1995) 36(2):311–3. doi: 10.1136/gut.36.2.311
39. Biank VF, Sheth MK, Talano J, Margolis D, Simpson P, Kugathasan S, et al. Association of crohn’s disease, thiopurines, and primary Epstein-Barr virus infection with hemophagocytic lymphohistiocytosis. J Pediatr (2011) 159(5):808–12. doi: 10.1016/j.jpeds.2011.04.045
40. Matnani R, Peker D. Azathioprine induced Epstein Barr virus-positive mucocutaneous ulcer arising in perianal fistula and abscess associated with crohn’s disease. J Crohns Colitis (2014) 8(12):1747–8. doi: 10.1016/j.crohns.2014.08.010
41. Subramaniam K, Cherian M, Jain S, Latimer M, Corbett M, D’Rozario J, et al. Two rare cases of Epstein-Barr virus-associated lymphoproliferative disorders in inflammatory bowel disease patients on thiopurines and other immunosuppressive medications. Intern Med J (2013) 43(12):1339–42. doi: 10.1111/imj.12287
42. Ohshima K, Kimura H, Yoshino T, Kim CW, Ko YH, Lee S-S, et al. Proposed categorization of pathological states of ebv-associated T/Natural killer-cell lymphoproliferative disorder (Lpd) in children and young adults: Overlap with chronic active ebv infection and infantile fulminant ebv T-lpd. Pathol Int (2008) 58(4):209–17. doi: 10.1111/j.1440-1827.2008.02213.x
43. Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. Ebv positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol (2010) 34(3):405–17. doi: 10.1097/PAS.0b013e3181cf8622
44. Roberts TK, Chen X, Liao JJ. Diagnostic and therapeutic challenges of ebv-positive mucocutaneous ulcer: A case report and systematic review of the literature. Exp Hematol Oncol (2015) 5:13. doi: 10.1186/s40164-016-0042-5
45. Moran NR, Webster B, Lee KM, Trotman J, Kwan Y-L, Napoli J, et al. Epstein Barr Virus-positive mucocutaneous ulcer of the colon associated Hodgkin lymphoma in crohn’s disease. World J Gastroenterol (2015) 21(19):6072–6. doi: 10.3748/wjg.v21.i19.6072
46. Teixeira Mendes LS, McCaul J, Wotherspoon A, Attygalle AD. Epstein-Barr Virus-positive mucocutaneous ulcer with a background of crohn’s disease and waldenström macroglobulinaemia: A case report highlighting diagnostic pitfalls. Histopathology (2018) 72(5):874–7. doi: 10.1111/his.13420
47. Montes L, Tredez E, Yzet C, Delette C, Chatelain D, Lebon D, et al. Epstein-Barr Virus-related mucocutaneous ulcer lymphoma associated with crohn’s disease, treated with monoclonal antibody anti-Cd30. Clin Case Rep (2020) 8(6):958–61. doi: 10.1002/ccr3.2721
48. Melani C, Jaffe ES, Wilson WH. Pathobiology and treatment of lymphomatoid granulomatosis, a rare ebv-driven disorder. Blood (2020) 135(16):1344–52. doi: 10.1182/blood.2019000933
49. Francolla KA, Altman A, Sylvester FA. Hemophagocytic syndrome in an adolescent with crohn disease receiving azathioprine and infliximab. J Pediatr Gastroenterol Nutr (2008) 47(2):193–5. doi: 10.1097/MPG.0b013e31816a30b9
50. Serrate C, Silva-Moreno M, Dartigues P, Poujol-Robert A, Sokol H, Gorin NC, et al. Epstein-Barr Virus-associated lymphoproliferation awareness in hemophagocytic syndrome complicating thiopurine treatment for crohn’s disease. Inflammation Bowel Dis (2009) 15(10):1449–51. doi: 10.1002/ibd.20823
51. Fitzgerald MP, Armstrong L, Hague R, Russell RK. A case of ebv driven haemophagocytic lymphohistiocytosis complicating a teenage crohn’s disease patient on azathioprine, successfully treated with rituximab. J Crohns Colitis (2013) 7(4):314–7. doi: 10.1016/j.crohns.2012.05.002
52. Thompson G, Pepperell D, Lawrence I, McGettigan BD. Crohn’s disease complicated by Epstein-Barr virus-driven haemophagocytic lymphohistiocytosis successfully treated with rituximab. BMJ Case Rep (2017) 2017. doi: 10.1136/bcr-2016-218578
53. Janka GE, Lehmberg K. Hemophagocytic syndromes–an update. Blood Rev (2014) 28(4):135–42. doi: 10.1016/j.blre.2014.03.002
54. Virdis F, Tacci S, Messina F, Varcada M. Hemophagocytic lymphohistiocytosis caused by primary Epstein-Barr virus in patient with crohn’s disease. World J Gastrointest Surg (2013) 5(11):306–8. doi: 10.4240/wjgs.v5.i11.306
55. Salado CT, Gallego AG, Carnerero EL, de la Cruz Ramírez D, Justiniano JMH, Galán JLM, et al. Hemophagocytic lymphohistiocytosis in crohn’s disease associated with primary infection by Epstein-Barr virus. Inflammation Bowel Dis (2011) 17(11):E143–E4. doi: 10.1002/ibd.21827
56. Takada H, Ohga S, Mizuno Y, Nomura A, Hara T. Increased il-16 levels in hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol (2004) 26(9):567–73. doi: 10.1097/01.mph.0000134465.86671.2e
57. Fuss IJ, Becker C, Yang Z, Groden C, Hornung RL, Heller F, et al. Both il-12p70 and il-23 are synthesized during active crohn’s disease and are down-regulated by treatment with anti-Il-12 P40 monoclonal antibody. Inflammation Bowel Dis(2006) 12(1). doi: 10.1097/01.MIB.0000194183.92671.b6
58. Hügle B, Astigarraga I, Henter J-I, Porwit-MacDonald A, Meindl A, Schuster V. Simultaneous manifestation of fulminant infectious mononucleosis with haemophagocytic syndrome and b-cell lymphoma in X-linked lymphoproliferative disease. Eur J Pediatr (2007) 166(6):589–93. doi: 10.1007/s00431-006-0290-1
59. Kumar S, Fend F, Quintanilla-Martinez L, Kingma DW, Sorbara L, Raffeld M, et al. Epstein-Barr Virus-positive primary gastrointestinal hodgkin’s disease: Association with inflammatory bowel disease and immunosuppression. Am J Surg Pathol (2000) 24(1):66–73. doi: 10.1097/00000478-200001000-00008
60. Li S, Borowitz MJ. Primary Epstein-Barr virus-associated Hodgkin disease of the ileum complicating crohn disease. Arch Pathol Lab Med (2001) 125(3):424–7. doi: 10.5858/2001-125-0424-PEBVAH
61. Bai M, Katsanos KH, Economou M, Kamina S, Balli C, Briasoulis E, et al. Rectal Epstein-Barr virus-positive hodgkin’s lymphoma in a patient with crohn’s disease: Case report and review of the literature. Scand J Gastroenterol (2006) 41(7):866–9. doi: 10.1080/00365520500529629
62. Schwartz LK, Kim MK, Coleman M, Lichtiger S, Chadburn A, Scherl E. Case report: Lymphoma arising in an ileal pouch anal anastomosis after immunomodulatory therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol (2006) 4(8):1030–4. doi: 10.1016/j.cgh.2006.05.024
63. Zheng S, Xu H, Ouyang Q, Xue L, Zhang Y, Cui D. A case of rapid growing colonic Nk/T cell lymphoma complicated by crohn’s disease. Chin J Cancer Res (2013) 25(1):119–23. doi: 10.3978/j.issn.1000-9604.2012.12.06
64. Allen PB, Laing G, Connolly A, O’Neill C. Ebv-associated colonic b-cell lymphoma following treatment with infliximab for ibd: A new problem? BMJ Case Rep (2013) 2013. doi: 10.1136/bcr-2013-200423
65. Wong NACS, Herbst H, Herrmann K, Kirchner T, Krajewski AS, Moorghen M, et al. Epstein-Barr Virus infection in colorectal neoplasms associated with inflammatory bowel disease: Detection of the virus in lymphomas but not in adenocarcinomas. J Pathol (2003) 201(2):312–8. doi: 10.1002/path.1442
66. Calaminici MR, Sheaff MT, Norton AJ, Feakins RM. Ileocaecal Epstein-Barr virus-positive lymphoproliferative disorder complicating crohn’s disease. Histopathology (1999) 35(4):388–90. doi: 10.1046/j.1365-2559.1999.0747b.x
67. Daniel F, Damotte D, Moindrot H, Molina T, Berger A, Cellier C. A steroid-refractory ulcerative colitis revealing Epstein-Barr Virus/Cytomegalovirus-positive colonic lymphoma. Int J Colorectal Dis (2006) 21(3):288–90. doi: 10.1007/s00384-004-0719-9
68. Akamatsu T, Watanabe N, Chiba T. Epstein-Barr Virus-associated lymphoma developed shortly after immunosuppressive treatment for ulcerative colitis. Clin Gastroenterol Hepatol (2007) 5(4):521. doi: 10.1016/j.cgh.2007.02.003
69. Courby S, Fabre B, Salameire D, Gaulard P, Hincky-Vitrat V, Gressin R, et al. Multifocal polyclonal Epstein-Barr virus-associated b-cell lymphoproliferative disorder secondary to azathioprine therapy successfully treated with rituximab. Leuk Lymphoma (2010) 51(1):174–7. doi: 10.3109/10428190903402029
70. Pietersma F, Piriou E, van Baarle D. Immune surveillance of ebv-infected b cells and the development of non-Hodgkin lymphomas in immunocompromised patients. Leuk Lymphoma (2008) 49(6):1028–41. doi: 10.1080/10428190801911662
71. Severyns T, Kirchgesner J, Lambert J, Thieblemont C, Amiot A, Abitbol V, et al. Prognosis of lymphoma in patients with known inflammatory bowel disease: A French multicentre cohort study. J Crohns Colitis (2020) 14(9):1222–30. doi: 10.1093/ecco-jcc/jjaa048
72. Lewis JD, Bilker WB, Brensinger C, Deren JJ, Vaughn DJ, Strom BL. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology (2001) 121(5):1080–7. doi: 10.1053/gast.2001.28703
73. Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-tnf therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol (2011) 106(12):2146–53. doi: 10.1038/ajg.2011.283
74. Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut (2005) 54(8):1121–5. doi: 10.1136/gut.2004.049460
75. Vos ACW, Bakkal N, Minnee RC, Casparie MK, de Jong DJ, Dijkstra G, et al. Risk of malignant lymphoma in patients with inflammatory bowel diseases: A Dutch nationwide study. Inflammation Bowel Dis (2011) 17(9):1837–45. doi: 10.1002/ibd.21582
76. Sokol H, Beaugerie L, Maynadié M, Laharie D, Dupas J-L, Flourié B, et al. Excess primary intestinal lymphoproliferative disorders in patients with inflammatory bowel disease. Inflammation Bowel Dis (2012) 18(11):2063–71. doi: 10.1002/ibd.22889
77. Afif W, Sandborn WJ, Faubion WA, Rahman M, Harmsen SW, Zinsmeister AR, et al. Risk factors for lymphoma in patients with inflammatory bowel disease: A case-control study. Inflammation Bowel Dis (2013) 19(7):1384–9. doi: 10.1097/MIB.0b013e318281325e
78. Khan N, Abbas AM, Lichtenstein GR, Loftus EV, Bazzano LA. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: A nationwide retrospective cohort study. Gastroenterology (2013) 145(5). doi: 10.1053/j.gastro.2013.07.035
79. Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American Gastroenterological association institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology (2006) 130(3):935–9. doi: 10.1053/j.gastro.2006.01.047
80. Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of crohn’s disease: A meta-analysis. Clin Gastroenterol Hepatol (2009) 7(8):874–81. doi: 10.1016/j.cgh.2009.01.004
81. Lemaitre M, Kirchgesner J, Rudnichi A, Carrat F, Zureik M, Carbonnel F, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA (2017) 318(17):1679–86. doi: 10.1001/jama.2017.16071
82. Baecklund E, Ekbom A, Sparén P, Feltelius N, Klareskog L. Disease activity and risk of lymphoma in patients with rheumatoid arthritis: Nested case-control study. BMJ (1998) 317(7152):180–1. doi: 10.1136/bmj.317.7152.180
83. Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum (2006) 54(3):692–701. doi: 10.1002/art.21675
84. Linton MS, Kroeker K, Fedorak D, Dieleman L, Fedorak RN. Prevalence of Epstein-Barr virus in a population of patients with inflammatory bowel disease: A prospective cohort study. Aliment Pharmacol Ther (2013) 38(10):1248–54. doi: 10.1111/apt.12503
85. Bachmann J, Le Thi G, Brückner A, Kalteis A-L, Schwerd T, Koletzko S, et al. Epstein-Barr Virus prevalence at diagnosis and seroconversion during follow-up in pediatric inflammatory bowel disease. J Clin Med (2021) 10(21). doi: 10.3390/jcm10215187
86. de Francisco R, Castaño-García A, Martínez-González S, Pérez-Martínez I, González-Huerta AJ, Morais LR, et al. Impact of Epstein-Barr virus serological status on clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther (2018) 48(7):723–30. doi: 10.1111/apt.14933
87. Miura M, Shimizu H, Saito D, Miyoshi J, Matsuura M, Kudo T, et al. Multicenter, cross-sectional, observational study on Epstein-Barr viral infection status and thiopurine use by age group in patients with inflammatory bowel disease in Japan (Ebisu study). J Gastroenterol (2021). doi: 10.1007/s00535-021-01832-w
88. Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, et al. Ecco guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis (2021) 15(6):879–913. doi: 10.1093/ecco-jcc/jjab052
89. Li H, Lyu W. Intestinal Nk/T cell lymphoma: A case report. World J Gastroenterol (2020) 26(27):3989–97. doi: 10.3748/wjg.v26.i27.3989
90. Wang S, Dai Y, Zhang J, Ou D, Ouyang C, Lu F. Intestinal ulcers as an initial finding in ebv-associated lymphoproliferative disorder: A case report. Med (Baltimore) (2020) 99(3):e18764. doi: 10.1097/MD.0000000000018764
91. Xu W, Jiang X, Chen J, Mao Q, Zhao X, Sun X, et al. Chronic active Epstein-Barr virus infection involving gastrointestinal tract mimicking inflammatory bowel disease. BMC Gastroenterol (2020) 20(1):257. doi: 10.1186/s12876-020-01395-9
92. Volaric AK, Singh K, Gru AA. Rare ebv-associated b cell neoplasms of the gastrointestinal tract. Semin Diagn Pathol (2021) 38(4):38–45. doi: 10.1053/j.semdp.2021.04.004
93. Liu R, Wang M, Zhang L, Zhou W, Huang Y, Guo H, et al. The clinicopathologic features of chronic active Epstein-Barr virus infective enteritis. Mod Pathol (2019) 32(3):387–95. doi: 10.1038/s41379-018-0144-1
94. Kimura H, Cohen JI. Chronic active Epstein-Barr virus disease. Front Immunol (2017) 8:1867. doi: 10.3389/fimmu.2017.01867
95. Aihara Y, Moriya K, Shimozato N, Nagamatsu S, Kobayashi S, Uejima M, et al. Chronic active ebv infection in refractory enteritis with longitudinal ulcers with a cobblestone appearance: An autopsied case report. BMC Gastroenterol (2021) 21(1):6. doi: 10.1186/s12876-020-01589-1
96. Jiang M, Chen X, Yi Z, Zhang X, Zhang B, Luo F, et al. Prognostic characteristics of gastrointestinal tract Nk/T-cell lymphoma: An analysis of 47 patients in China. J Clin Gastroenterol (2013) 47(8):e74–e9. doi: 10.1097/MCG.0b013e31829e444f
97. Wang Z, Zhang W, Luo C, Zhu M, Zhen Y, Mu J, et al. Primary intestinal Epstein-Barr virus-associated natural Killer/T-cell lymphoproliferative disorder: A disease mimicking inflammatory bowel disease. J Crohns Colitis (2018) 12(8):896–904. doi: 10.1093/ecco-jcc/jjy043
98. Wan Ahmad Kammal WS, Mohd Rose I, Md Zin RR, Raja Ali RA, Masir N. Extranodal Nk/T-cell lymphoma mimicking crohn’s colitis. Malays J Pathol (2019) 41(2):195–9.
99. Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant (2005) 5(12):2894–900. doi: 10.1111/j.1600-6143.2005.01115.x
Keywords: inflammatory bowel disease, Epstein–Barr virus, viral colitis, lymphoproliferative diseases, immunosuppression
Citation: Zhang H, Zhao S and Cao Z (2022) Impact of Epstein–Barr virus infection in patients with inflammatory bowel disease. Front. Immunol. 13:1001055. doi: 10.3389/fimmu.2022.1001055
Received: 22 July 2022; Accepted: 07 October 2022;
Published: 28 October 2022.
Edited by:
Massimo Martinelli, University of Naples Federico II, ItalyReviewed by:
Valeria Dipasquale, University of Messina, ItalyCopyright © 2022 Zhang, Zhao and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijun Cao, Y2FvempfcmVuamlAMTYzLmNvbQ==; Shuliang Zhao, c2h1bGlhbmd6aGFvQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.