
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 07 January 2022
Sec. T Cell Biology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.811600
Interactions between CD44 and hyaluronan (HA) are crucial for recruiting leukocytes to inflamed tissues. This review summarizes findings from our studies of the roles of CD44-HA interactions in leukocyte trafficking, with a particular focus on airway T helper type 2 (Th2) cells in mouse models of acute asthma. In a mite allergen-induced model of acute asthma, intraperitoneal injection of anti-CD44 monoclonal antibodies blocked lymphocytes and eosinophils from accumulating in the lung, and suppressed both the antigen-induced increase in Th2 cytokines in the bronchoalveolar lavage fluid (BALF) and airway hyperresponsiveness (AHR). CD44 deficiency was associated with decreased mite allergen-induced Th2 cell-mediated airway inflammation and AHR in sensitized mice. Asthmatic responses to antigen-sensitized splenic CD4+ T cells transferred from CD44-deficient mice were weaker than in wild-type mice. Administration of anti-CD44 monoclonal antibodies preferentially suppressed the airway accumulation of antigen-specific Th2 cells induced by antigen challenge, without affecting Th1 and Th17 cells. Increased HA-binding ability of CD44 and expression of Neu1 sialidase were observed on antigen-specific Th2 cells compared with antigen-specific Th1 and Th17 cells. Finally, in a mouse model of acute asthma, neuraminidase 1-deficient SM/J mice exhibited a lower Th2 cytokine concentration and a lower absolute Th2 cell number in the BALF, as well as an attenuated AHR. Our findings indicate that CD44 critically contributes to the antigen challenge-induced airway accumulation of antigen-specific Th2 cells, without affecting Th1 and Th17 cells, in mice. Furthermore, neuraminidase 1 activity is necessary for the interaction between HA and CD44, and Th2 cell-mediated airway inflammation.
The cell surface adhesion receptor cluster of differentiation 44 (CD44) is a heavily glycosylated molecule that regulates the adhesion of lymphocytes to inflamed endothelial cells, T cell activation, tumor metastasis, and many other cellular processes. While hyaluronan (HA) is the principal ligand of CD44, only a few types of cells use CD44 to recognize HA (1, 2). The structural variability of CD44 might affect its ability to recognize HA. Sialic acid is a terminal sugar chain of glycoproteins followed by a β-galactoside, such as CD44, that is catalyzed by neuraminidase. The 4 known mammalian neuraminidases (Neu1, Neu2, Neu3, and Neu4) are involved in variety of physiological processes (3). We demonstrated that CD44 glycosylation, such as by sialic acid, negatively regulates its recognition of HA (4).
Both CD44 and HA critically contribute to leukocyte recruitment to many organs in vivo (5). Disease severity and recruitment of lymphocytes in animal models of acute asthma, arthritis, and graft-versus-host disease are reduced by antibody blockade of CD44 or CD44 deficiency, as well as by enzymatic depletion of endothelial HA (6–8). In addition to the support function of CD44-HA interactions in lymphocyte rolling, direct association between CD44 and integrins enabling high-affinity binding to vascular cell adhesion molecule 1 is necessary for lymphocyte adhesion (9, 10).
Asthma is a condition that presents with reversible airway obstruction, chronic airway inflammation, features of bronchial remodeling, and airway hyperresponsiveness (AHR) (11), and is considered to develop in response to the airway accumulation of antigen-activated CD4+ T cells (12). We investigated how CD44 participates in the airway accumulation of CD4+ T cells by developing an asthmatic phenotype in a mouse model of allergic acute asthma. In this review, we discuss how CD44-HA interactions are involved in CD4+ T cell trafficking. We also explore the mechanisms regulating these interactions, and highlight the importance of T helper type 2 (Th2) cell recruitment by CD44-HA interactions in the pathogenesis of acute allergic asthma in an experimental mouse model (Figure 1).
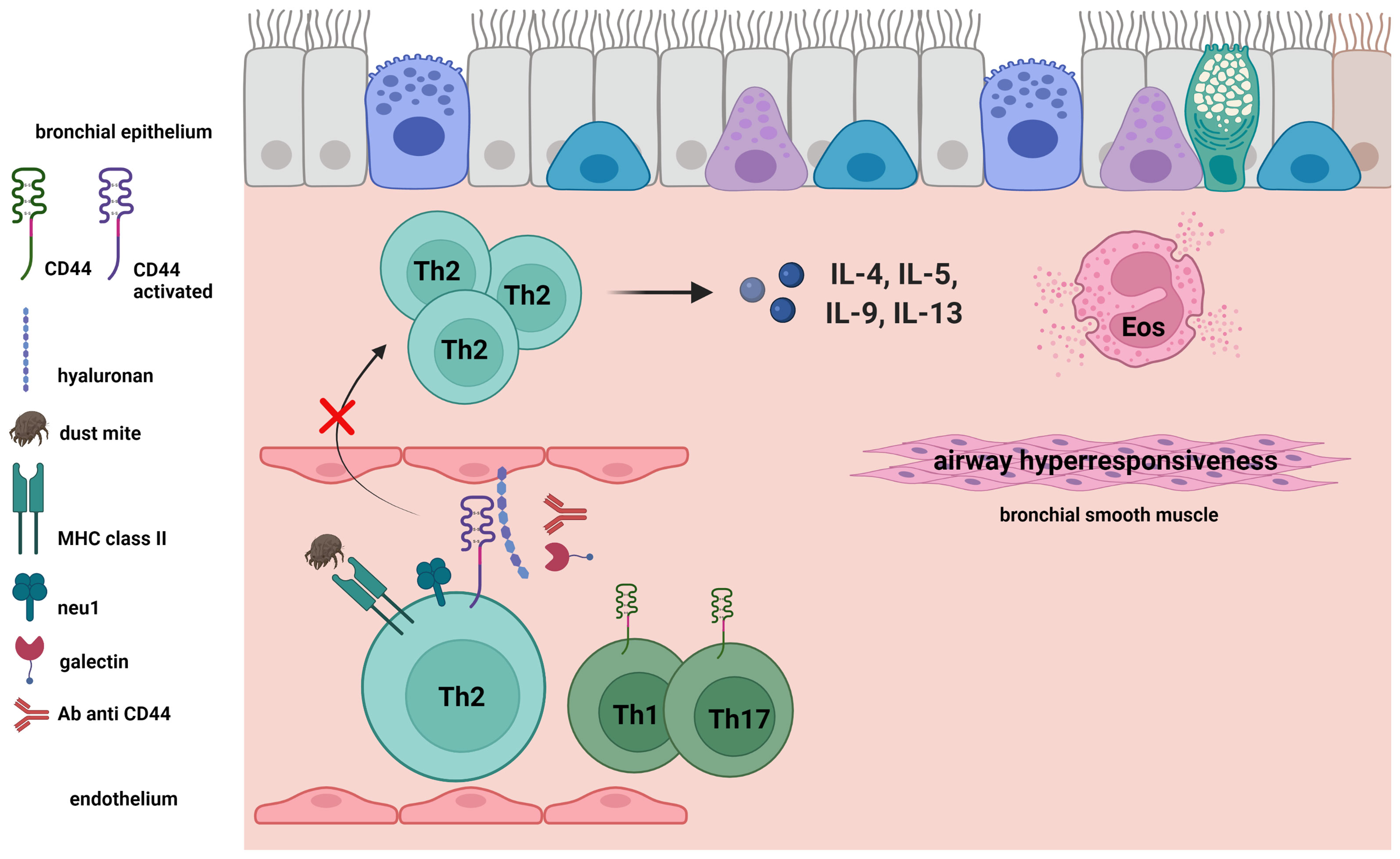
Figure 1 Antigen-induced activation of CD44 expressed on Th2 cells. CD44 receptor activity for hyaluronan (HA) and expression of neuraminidase 1 (Neu1) on Th2 cells were induced by antigen challenge in a mouse model of acute asthma. We demonstrated the importance of Th2 cell recruitment in the bronchus by CD44-HA interactions in the pathogenesis of acute allergic asthma in an experimental mouse model. Galectin-9 and anti-CD44 mAb inhibit the CD44-HA interaction, as well as the following Th2-mediated eosinophilic airway inflammation and airway hyperresponsiveness.
CD44 is a heavily glycosylated cell adhesion molecule deriving from alternative splicing of a single gene and modifications of the emerging protein. Lymphocytes are the best known cell type that uses CD44 to recognize and bind its ligand HA through their activation (13, 14). We demonstrated that protein glycosylation disruption in some cell types may increase their HA recognition. Flow cytometry to analyze the HA-binding ability of CD44 alone was performed using a purified CD44-immunoglobulin fusion protein and fluorescein-conjugated HA. Enhanced HA binding ability was observed when the CD44 fusion protein was treated with neuraminidase (4). These findings indicate that modifications of carbohydrates in CD44 may prevent its recognition of abundant HA in the body.
The immunopathology of allergic respiratory inflammation may be due in part to the airway accumulation of CD4+ T cells and eosinophils following antigen activation (12). When an antigen is administered in a mouse model of acute asthma, the lungs begin to accumulate CD44-highly expressing CD4+ T cells (15). Therefore, we investigated the contribution of CD44 to allergen-induced acute respiratory inflammation in a mouse model of allergic asthma induced by intranasal administration of Ascaris sum extract and mite antigens followed by treatment with 2 anti-CD44 monoclonal antibodies (mAbs), and analyzed the bronchoalveolar lavage fluid (BALF) contents and AHR. The mAb KM201, directly prevents CD44-HA binding (16), whereas the mAb IM7 promotes receptor shedding from the cell surface (17). Injection of anti-CD44 antibodies to prevent CD44-HA binding abolished eosinophil and lymphocyte infiltration into the airways and reduced Th2 cytokine, interleukin (IL)-4, and IL-5 levels. Anti-CD44 treatment, however reduced the allergen-induced AHR. These findings suggest that CD44 is critically involved in the progression of acute allergic respiratory inflammation (6).
Galectin-9 (Gal-9) is a β-galactoside-binding protein that has roles in cell adhesion, chemoattraction, activation, and apoptosis (18). Hirashima et al. observed that Gal-9 induces the apoptosis of activated T cells in humans (19). Zhu et al. revealed that Gal-9 promotes Th1, but not in Th2, cell death in mice via a Tim-3–dependent pathway (20). We unexpectedly found that Gal-9 directly binds CD44, which blocks the CD44-HA interaction. To investigate the involvement of Gal-9 in the pathogenesis of allergic airway inflammation, we administered stable human Gal-9 (21) in a mouse model of acute asthma induced by intranasal administration of mite allergen. Intravenous injection of Gal-9 reduced both AHR and Th2 cell-associated airway inflammation induced by the mite allergen in sensitized mice. In addition, administration of Gal-9 impeded the airway infiltration of peripheral blood Th2 cells (22). Taken together, these findings indicate that Gal-9 inhibits allergen-induced airway inflammation and AHR by regulating the CD44-mediated leukocyte recognition of HA.
We studied the contribution of CD44 expressed on CD4+ T cells to the airway accumulation of Th2 cells using CD44-deficient mice and anti-CD44 mAbs. The CD44-deficency was associated with decreased mite allergen-induced Th2 cell-mediated airway inflammation in sensitized mice. Asthmatic responses to antigen-sensitized splenic CD4+ T cells transferred from CD44-deficient mice were weaker than in wild-type mice. We then assessed CD44 receptor activity for HA and expression of Neu1 sialidase on ovalbumin (OVA)-specific Th1, Th2, and Th17 cells in vitro, as previously described (23). OVA-specific Th2 cells more highly expressed Neu1 sialidase and exhibited higher CD44 HA receptor activity than OVA-specific Th1 and Th17 cells. Anti-CD44 mAbs preferentially suppressed the antigen challenge-induced accumulation of these Th2 cells in the airway, as compared with Th1 and Th17 cells in a mouse Th cell-transfer model (24, 25). Together, these findings demonstrated that CD44-expressing CD4+ T cells are critical for the airway accumulation of antigen-specific Th2 cells, but not Th1 or Th17 cells (Figure 1).
Sialic acid residues in CD44 negatively regulate the function of CD44, and CD44 is critically involved in the airway accumulation of Th2 cells in a mouse model of acute asthma (15, 24, 25). We therefore investigated how sialidase is involved in CD44-HA interactions on CD4+ T cells, and how it contributes to mite allergen-induced acute asthma in a mouse model. In splenic CD4+ T cells obtained from the model mice, the HA receptor activity of CD44 and Neu1 sialidase expression were increased after culture with the antigen. The antigen-induced HA binding ability of CD44 was markedly suppressed by a sialidase inhibitor. Binding of HA to CD44, however, was not observed in Neu1-deficient SM/J mice with a partial deficiency of lysosomal sialidase (26, 27). Further, the Neu1-deficient SM/J mice also exhibited a lower Th2 cytokine concentration and a lower absolute Th2 cell number in the BALF (27). These findings together indicate that Neu1 sialidase is required for the CD44-HA interaction and the development of acute asthmatic inflammation. It may be that enzyme activity remodels the cell surface CD44 expressed on CD4+ T cells, thereby altering the ability of CD44 to interact with HA.
CD44-HA interactions critically contribute to the airway accumulation of allergen-specific Th2 cells in allergen-induced acute asthma mouse models. Neu1 sialidase activity in Th2 cells is a mechanism of CD44 receptor activation for binding HA (Figure 1). These findings suggest that CD44 and Neu1 sialidase could be candidate treatment targets for Th2 cell-mediated acute airway inflammation. Additional studies are needed to clarify the detailed role of CD44 in the development of chronic airway inflammation, such as human asthma, and to clarify the possible involvement of CD44 in other immune cell mechanisms underlying asthma pathophysiology.
The author confirms being the sole contributor of this manuscript and approved it for publication.
This work was supported in part by Core Research for Evolutional Science and Technology of the Japan Science and Technology Agency and Grants-in-Aid for Scientific Research, Japan.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The author would like to thank Dr. Karin Mesches for her critical review of this manuscript.
1. Lesley J, Hyman R, Kincade PW. CD44 and its Interaction With Extracellular Matrix. Adv Opin Immunol (1993) 54:271–335. doi: 10.1016/s0065-2776(08)60537-4
2. Kincade PW, Zheng Z, Katoh S, Hanson L. The Importance of Cellular Environment to Function of the CD44 Matrix Receptor. Curr Opin Cell Biol (1997) 9:635–42. doi: 10.1016/s0955-0674(97)80116-0
3. Miyagi T, Yamaguchi K. Mammalian Sialidase: Physiological and Pathological Roles in Cellular Functions. Glycobiology (2012) 22:880–96. doi: 10.1093/glycob/cws057
4. Katoh S, Zheng Z, Oritani K, Shimozato T, Kincade PW. Glycosylation of CD44 Negatively Regulates its Recognition of Hyaluronan. J Exp Med (1995) 182:419–29. doi: 10.1084/jem.182.2.419
5. DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in Activated T Cell Extravasation Into an Inflammatory Site. Science (1997) 278:672–5. doi: 10.1126/science.278.5338.672
6. Katoh S, Matsumoto M, Kawakita K, Tominaga A, Kincade PW, Matsukura S. A Role for CD44 in an Antigen-Induced Murine Model of Pulmonary Eosinophilia. J Clin Invest (2003) 111:1563–70. doi: 10.1172/JCI16583
7. Mikecz K, Brennan FR, Kim JH, Glant TT. Anti-CD44 Treatment Abrogates Tissue Oedema and Leukocyte Infiltration in Murine Arthritis. Nat Med (1995) 1:558–63. doi: 10.1038/nm0695-558
8. Milinkovic M, Antin JH, Hergrueter CA, Underhill CB, Sackstein R. CD44-Hyaluronic Acid Interactions Mediate Shear-Resistant Binding of Lymphocytes to Dermal Endothelium in Acute Cutaneous GVHD. Blood (2004) 103:740–2. doi: 10.1182/blood-2003-05-1500
9. DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its Ligand Hyaluronate Mediate Rolling Under Physiologic Flow: A Novel Lymphocyte-Endothelial Cell Primary Adhesion Pathway. J Exp Med (1996) 183:1119–30. doi: 10.1084/jem.183.3.1119
10. Nandi A, Estess P, Siegelman M. Bimolecular Complex Between Rolling and Firm Adhesion Receptors Required for Cell Arrest; CD44 Association With VLA-4 in T Cell Extravasation. Immunity (2004) 20:455–65. doi: 10.1016/s1074-7613(04)00077-9
11. Busse WW, Lemanske RF Jr. Asthma. N Engl J Med (2001) 344:350–62. doi: 10.1056/NEJM200102013440507
12. Wills-Karp M. Immunologic Basis of Antigen-Induced Airway Hyperresponsiveness. Annu Rev Immunol (1999) 17:255–81. doi: 10.1146/annurev.immunol.17.1.255
13. Murakami S, Miyake K, June CH, Kincade PW, Hodes RJ. IL-5 Induces a Pgp-1 (CD44) Bright B Cell Subpopulation That is Highly Enriched in Proliferative and Ig Secretory Activity and Binds to Hyaluronate. J Immunol (1990) 145:3618–27.
14. Lesley J, Howes N, Perschl A, Hyman R. Hyaluronan Binding Function of CD44 is Transiently Activated on T Cells During an In Vivo Immune Response. J Exp Med (1994) 180:383–7. doi: 10.1084/jem.180.1.383
15. Kennedy JD, Hatfield CA, Fidler SF, Winterrowd GE, Haas JV, Chin JE, et al. Phenotypic Characterization of T Lymphocytes Emigrating Into Lung Tissue and the Airway Lumen After Antigen Inhalation in Sensitized Mice. Am J Respir Cell Mol Biol (1995) 12:613–23. doi: 10.1165/ajrcmb.12.6.7766426
16. Zheng Z, Katoh S, He Q, Oritani K, Miyake K, Lesley J, et al. Monoclonal Antibodies to CD44 and Their Influence on Hyaluronan Recognition. J Cell Biol (1995) 130:485–95. doi: 10.1083/jcb.130.2.485
17. Camp RL, Scheynius A, Johansson C, Pure E. CD44 is Necessary for Optimal Contact Allergic Responses But is Not Required for Normal Leukocyte Extravasation. J Exp Med (1993) 178:497–507. doi: 10.1084/jem.178.2.497
18. Hirashima M, Kashio Y, Nishi N, Yamauchi A, Imaizumi T, Kageshita T, et al. Galectin-9 in Physiological and Pathological Conditions. Glycoconj J (2002) 19:593–600. doi: 10.1023/B:GLYC.0000014090.63206.2f
19. Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, et al. Galectin-9 Induces Apoptosis Through the Calcium-Calpain-Caspase-1 Pathway. J Immunol (2003) 170:3631–6. doi: 10.4049/jimmunol.170.7.3631
20. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury S, et al. The Tim-3 Ligand Galectin-9 Negatively Regulates T Helper Type 1 Immunity. Nat Immunol (2005) 6:1245–52. doi: 10.1038/ni1271
21. Nishi N, Itoh A, Fujiyama A, Yoshida N, Araya S-I, Hirashima M, et al. Development of Highly Stable Galectins: Truncation of the Linker Peptide Confers Protease-Resistance on Tandem-Repeat Type Galectins. FEBS Lett (2005) 579:2058–64. doi: 10.1016/j.febslet.2005.02.054
22. Katoh S, Ishii N, Nobuumoto A, Takeshita K, Dai SY, Shinonaga R, et al. Galectin-9 Inhibits CD44-Hyaluronan Interaction and Suppresses a Murine Model of Allergic Asthma. Am J Respir Crit Care Med (2007) 176:27–35. doi: 10.1164/rccm.200608-1243OC
23. Mori A, Ogawa K, Someya K, Kunori Y, Nagakubo D, Yoshie O, et al. Selective Suppression of Th2-Mediated Airway Eosinophil Infiltration by Low-Molecular Weight CCR3 Antagonists. Int Immunol (2007) 19:913–21. doi: 10.1093/intimm/dxm049
24. Katoh S, Kaminuma O, Hiroi T, Mori A, Ohtomo T, Maeda S, et al. CD44 is Critical for Airway Accumulation of Antigen-Specific Th2, But Not Th1, Cells Induced by Antigen Challenge in Mice. Eur J Immunol (2011) 41:3198–207. doi: 10.1002/eji.201141521
25. Nishimura T, Katoh S, Mori A, Ohtomo T, Saeki M, Hiroi T, et al. Critical Role of CD44 in Antigen-Induced Th2- But Not Th17-Mediated Murine Airway Inflammation. Allergol Int (2016) 65:S59–61. doi: 10.1016/j.alit.2016.04.010
26. d’Azzo A, Bonten E, Rottier RJ. A Point Mutation in the Neu-1 Locus Causes the Neuraminidase Defect in the SM/J Mouse. Hum Mol Genet (1998) 7:313–21. doi: 10.1093/hmg/7.2.313d
Keywords: acute asthma, CD44, CD44-deficient mice, hyaluronan, Th2 cell, Neu1 sialidase
Citation: Katoh S (2022) Critical Involvement of CD44 in T Helper Type 2 Cell-Mediated Eosinophilic Airway Inflammation in a Mouse Model of Acute Asthma. Front. Immunol. 12:811600. doi: 10.3389/fimmu.2021.811600
Received: 09 November 2021; Accepted: 15 December 2021;
Published: 07 January 2022.
Edited by:
Takashi MaruYama, National Institutes of Health (NIH), United StatesReviewed by:
Benoit Allard, Université de la Réunion, FranceCopyright © 2022 Katoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigeki Katoh, a3NoaWdla2lAbWVkLmthd2FzYWtpLW0uYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.