
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 07 January 2022
Sec. Viral Immunology
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.807937
This article is part of the Research Topic Deleterious and Beneficial Humoral Immune Response in Viral Diseases: Two Sides of the Same Coin View all 9 articles
 Qiuling Zang1†
Qiuling Zang1† Yating Wang1†
Yating Wang1† Junshuang Guo1
Junshuang Guo1 Liyang Long2
Liyang Long2 Shuyu Zhang1
Shuyu Zhang1 Can Cui1
Can Cui1 Dandan Song1
Dandan Song1 Boguang Yu3
Boguang Yu3 Fenlan Tang3
Fenlan Tang3 Junfang Teng1
Junfang Teng1 Wang Miao1*
Wang Miao1*A severely comatose female patient was diagnosed with Japanese encephalitis (JE). Her condition was complicated by Hashimoto’s thyroiditis (HT) and Guillain-Barré syndrome (GBS). After antiviral, glucocorticoid, and immunoglobulin treatment, the patient’s consciousness was restored, and she could breathe spontaneously. Following this, new-onset, primarily demyelinating GBS developed, which progressed to demyelination combined with axonal injury. The patient was switched to protein A immunoadsorption (PAIA) therapy, and her Hughes score decreased rapidly, from 4 to 1 after 6 months. This patient is the first to receive PAIA combined with an antiviral-glucocorticoid-immunoglobulin regimen to treat encephalitis, meningitis, HT, and GBS caused by JE infection, thereby reflecting the importance of clinical application of PAIA in the treatment of immunological complications of JE.
Japanese encephalitis virus (JEV) is a major cause of viral encephalitis in Asians. JE primarily presents as fever, seizures, headache, signs of meningeal irritation, and loss of consciousness (1). There is no specific effective treatment, the mortality rate is high, and some survivors have serious sequelae.
JEV produces pathological antibodies resulting in neuroimmunological diseases, such as Guillain-Barré syndrome (GBS) and autoimmune encephalitis (2–4). There are no reports of Hashimoto’s thyroiditis (HT) caused by JEV infection. HT is one of the most common autoimmune diseases and is commonly characterized by elevated thyroid autoimmune antibodies.
Protein A immunoadsorption (PAIA) therapy selectively removes circulating antibodies and immune complexes by binding them to an immobilized ligand (5). It has been shown to be a safe and efficient treatment in several autoimmune diseases (6).
To our knowledge, this is the first reported case of encephalitis, meningitis, HT, and GBS caused by JEV infection and is also the first to be successfully treated with PAIA combined with an antiviral-glucocorticoid-immunoglobulin regimen.
A 43-year-old woman was transferred to the neurological intensive care unit on September 15, 2020, for fever and disturbance of consciousness for 6 days. Body temperature during the fever was 38.5−39.0°C. The patient had intermittent generalized tonic-clonic seizures, which lasted for 1−2 min and then resolved. The patient was previously healthy, had no history of autoimmune diseases or immunosuppressive drugs, no drug abuse, or psychiatric disorders. On the first day of onset, the patient was unresponsive, and on day 2, the patient fell into a light coma and developed a stiff neck. Blood anti-thyroglobulin antibody level was 751.4 IU/mL (Figure 1A), hemoglobin level was 70 g/L, and cerebrospinal fluid (CSF) white blood cell count was 120× 106/L (see Supplementary Table 1). Head magnetic resonance imaging (MRI) indicated symmetrical lesions in the bilateral thalamus, caudate nucleus, lentiform nucleus, and bilateral hippocampus. On day 5, tracheal intubation was performed, CSF white blood cell count was 58 × 106/L (mononuclear cell ratio: 96.6%), and protein level was 1022.4 mg/L. The patient received ganciclovir (0.25 g q. 12 h ivgtt, 2 days), vidarabine (0.4 g q.d. ivgtt, 3 days), and supportive symptomatic treatment at two hospitals and the emergency department of our hospital.
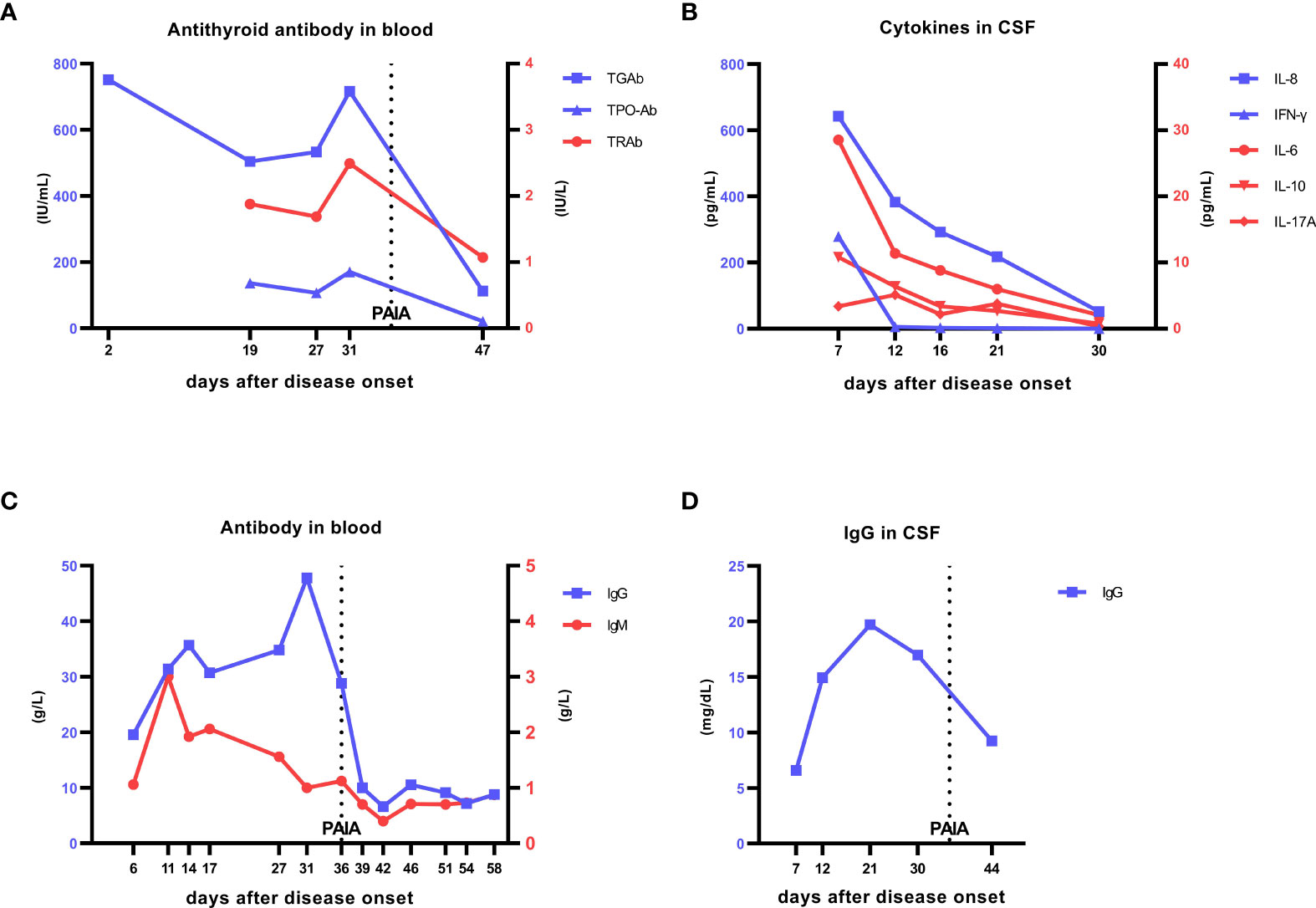
Figure 1 Laboratory data. (A) Changes in anti-thyroid antibody levels in the patient’s blood. On day 2, TGAb was 751.40 IU/mL (0−115), and on re-examination on day 19 of onset, TGAb was 504 IU/mL, TPO-Ab was 136 IU/mL (0−34), and TRAb was 1.88 IU/L (0−1.75). PAIA was started on day 36. On day 47, TGAb was 112 IU/mL, TPO-Ab was 20.8 IU/mL, and TRAb was 1.07 IU/L, all of which were in normal ranges. At the 12-month follow-up after treatment,TGAb was 103 IU/mL, TPO-Ab was 17 IU/mL, and TRAb was 1.41 IU/L, all of which were in normal ranges. (B) Changes in cytokine levels in the patient’s cerebrospinal fluid. On day 7 of onset, IL-8 was 642.09 pg/mL, IFN-γ was 279.12 pg/mL, and IL-6 was 28.55 pg/mL, which were significantly increased. After treatment with antivirals, glucocorticoids, and IVIG, these values were significantly decreased: IL-8 was 51.59 pg/mL, IFN-γ was 0.22 pg/mL, and IL-6 was 2.06 pg/mL, but IL-8 remained at a high level and decreased slowly. (C) Changes in IgG and IgM in the patient’s blood. On day 6 of onset, blood IgG was 19.54 g/L (7−16) and IgM was 1.06 g/L (0.4−2.3). With progression of the disease and after IVIG treatment, IgG continued increasing, and IgM also increased but very rapidly returned to the normal range. On day 31 of onset, IgG was 47.8 g/L. PAIA was started on day 36, and IgG began to decrease and gradually returned to the normal range. On day 58, IgG was 8.8 g/L and IgM was 0.87 g/L. (D) Changes in IgG levels in the cerebrospinal fluid. On day 7 of onset, IgG was 6.59 mg/dL (1−4), which increased to 19.7 mg/dL on day 21, and then decreased on day 30. After PAIA was started, cerebrospinal fluid IgG continued decreasing at a greater rate than before. The line labeled PAIA represents the time of the first PAIA treatment (day 36), the treatment was continued for 5 days, there was an obvious urinary tract infection, and PAIA was suspended; after improvement, PAIA was resumed on day 51,the treatment was continued for 3 days. TGAb, anti-thyroglobulin antibody; TPO-Ab, anti-thyroid peroxidase antibody; TRAb, anti-thyroid stimulating hormone receptor antibody; IVIG, intravenous immunoglobulin; PAIA, protein A immunoadsorption.
At the time of transfer, her Glasgow Coma Scale (GCS) was E1TM1, and neck stiffness was present. Brain stem reflexes were present, tendon reflexes of both lower limbs were positive, and no pathological signs were elicited. Blood lymphocyte count and the number of each subgroup decreased (see Supplementary Table 1). T2WI head MRI indicated hyperintense signals in the thalamus and caudate nucleus, and new-onset lesions in the bilateral cerebral peduncles (substantia nigra) (Figures 2A, B). Ultrasonography showed diffuse echo changes of the thyroid. JEV-immunoglobulin M (IgM) antibody was positive in the serum and CSF. We tested the patient’s blood and CSF for other viruses, bacteria, fungi, mycobacterium tuberculosis, markers of the tumor and paraneoplastic, antibodies to autoimmune encephalitis, and infectious disease tests (including antibodies to HIV and treponema pallidum, etc.), all of which were negative. The patient was diagnosed with JE, HT, and iron deficiency anemia. Intravenous infusion of penciclovir 5mg/kg and glucocorticoid 80 mg every 12 h, intravenous immunoglobulin (IVIG) provided at 0.4g/kg/d for 5 days, and palliative treatment were administered (Figure 3). On day 7, the patient was treated with mechanical ventilation. Interleukin (IL)-8 and interferon gamma (IFN-γ) in the CSF were significantly elevated (Figure 1B). Free triiodothyronine, free thyroxine, and thyroid stimulating hormone in the blood were 2.79 pmol/L, 7.84 pmol/L, and 0.08µlU/mL. On day 12, fever and epilepsy disappeared, consciousness gradually recovered, muscle tone in the extremities increased (the upper extremities exhibited cogwheel rigidity), and the muscle strength of the extremities was grade 0. Over the next few days, the patient was weaned from ventilation for short periods of time. On day 17, the right forehead creases became shallower, the right eye was incompletely closed, the extremities exhibited low muscle tone, and the patient required continuous ventilation. Electromyography (EMG) indicated primarily demyelinating lesions in the peripheral nerves of the extremities (see Supplementary Table 2). On day 19, the patient suddenly became unconscious, and head computed tomography (CT) indicated hemorrhage in the right thalamus (Figure 2C). On day 24, the patient regained consciousness, but the cranial nerve and extremity symptoms did not improve. On day 27, IVIG 0.4g/kg/d was resumed for five consecutive days. On day 31, GCS was E3TM1, the patient was breathing spontaneously without ventilation, cytokines decreased during the treatment (Figure 1B). However, EMG showed the amplitude of compound muscle action potential was low, indicating axonal injury in partial peripheral nerve. She exhibited an involuntary smacking motion and limb muscle strength was grade 0. Symptoms did not improve over the next week. On day 36, GCS was E3TM1, and the Hughes score was 4. Considering the EMG and clinical symptoms of the patient, we believed that the disease was progressing and treatment strategies need to be changed (Figure 3).
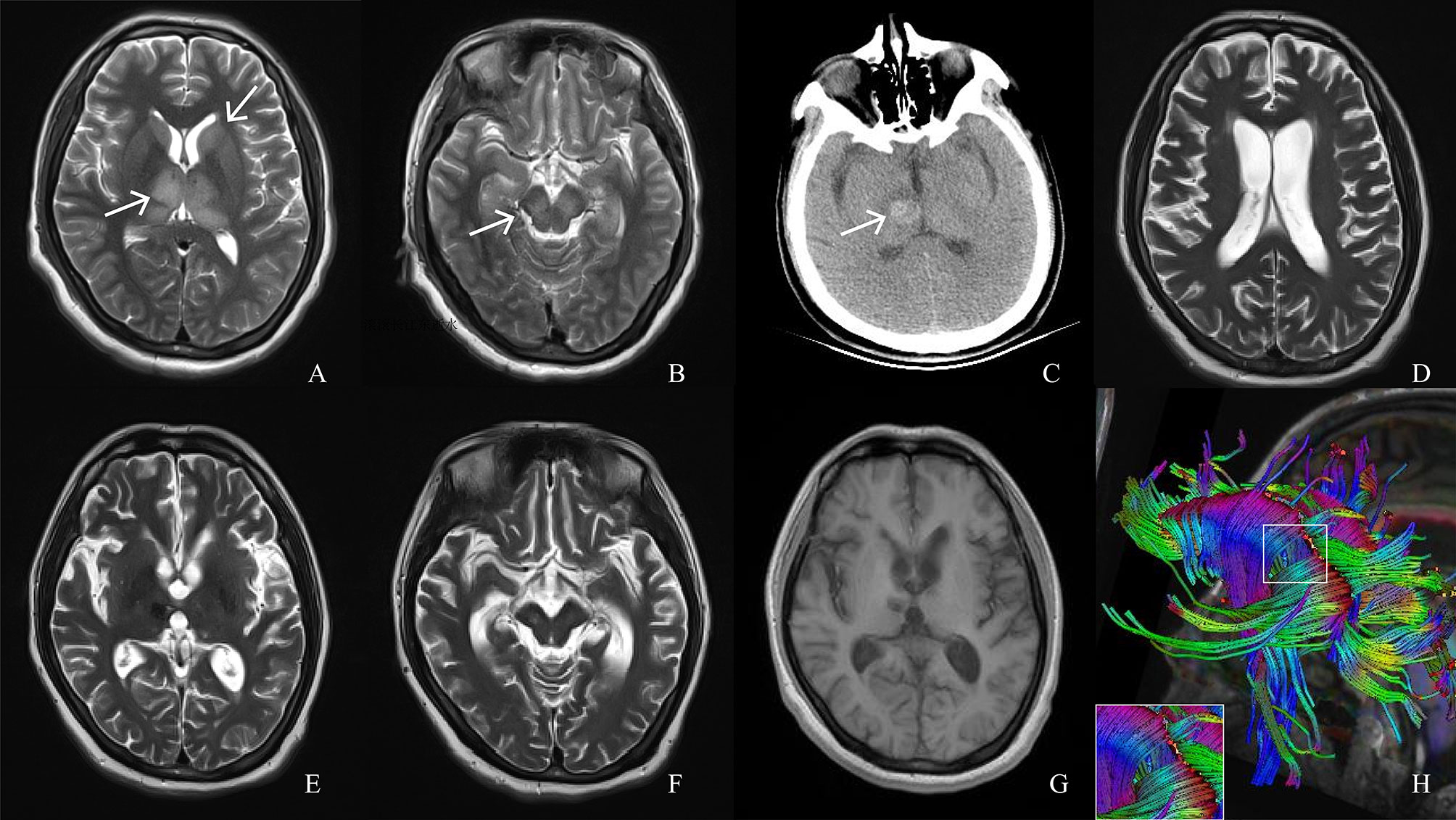
Figure 2 Magnetic resonance imaging (MRI), computed tomography (CT) and diffusion tensor imaging (DTI) scans of the patient. (A, B) T2 MRI on day 6 of onset shows hyperintense signals in the thalamus and caudate nucleus (white arrows) and cerebral peduncles (white arrows). (C) CT performed on day 19 of onset, when the patient fell back into a coma, shows right thalamus hemorrhage (white arrow). (D–F) T2 MRI re-examination at month 6 of the course of disease shows extensive brain atrophy and the lesion area was smaller than before. (G) T1MRI examination at month 14 of the course of disease shows brain atrophy. (H) DTI at month 14 of the course of disease shows partially broken corpus callosum tract.
The patient was started on PAIA treatment (adsorption column model: KCIA08; KONPIA®; Koncen Bioscience, Guangzhou, China). The plasma regenerated in each treatment was approximately 1.5-2 times the circulating plasma volume. Blood IgG level was monitored after PAIA treatment, and when it was lower than 4 g/L, IVIG 5 g was supplemented to maintain some level of resistance in the body. The treatment was continued for 5 days.
On day 41, GCS was E4TM3, and the best limb muscle strength was grade 2. Blood IgG and IgM were significantly lower than that before treatment, and thyroid antibodies were normal. There was an obvious urinary tract infection, and PAIA was suspended. After improvement, PAIA was resumed on day 51 and the treatment was continued for 3 days. Blood IgG and IgM levels are shown in Figure 1C. On day 55, GCS was E4V2M4, the muscle strength of the distal left upper limb and both lower limbs was grade 3, the muscle strength of the distal right upper limb was grade 2, and the muscle strength of both proximal upper limbs was grade 1. On day 56, EMG showed partial nerve conduction velocities and amplitudes improved, but abnormal spontaneous electric potentials were present. The patient was discharged after undergoing physical therapy and gait rehabilitation training for 1.5 months. At the time of discharge, she was able to walk with support from her family, and exhibited flat affect, slow speech, increased muscle tone, and a Hughes score of 3.
At the 4-months follow-up after PAIA treatment (month 6 of the disease course), the patient’s Hughes score was 2, and she exhibited a smiling facial expression, slow speech, alleviated high muscle tone, and a Montreal Cognitive Assessment score of 20. MRI indicated extensive brain atrophy, but the lesion area was smaller than before (Figures 2D–F, 4). On a telephonic follow-up 6 months after the treatment, the patient’s Hughes score was 1 (month 8 of the disease course), the muscle strength of the left upper limb was grade 4+, and the muscle strength of the remaining regions was grade 5. At the 12-months follow-up after treatment (month 14 of the disease course), the patient’s Hughes score was 1, lower limbs muscle tone was slightly higher, EMG indicated greater improvement in peripheral nerve injury (see Supplementary Table 2), the Montreal Cognitive Assessment score was 21, thyroid function and thyroid antibodies were normal (see Supplementary Table 1). The patient exhibited natural facial expression and could speak normally. However, MRI still indicated brain atrophy (Figure 2G) and DTI showed that the patient’s corpus callosum tract was partially broken (Figure 2H).
JEV can cause GBS, anti-N-methyl-D-aspartate receptor encephalitis, acute transverse myelitis, and other neuroimmunological diseases (2–4). However, there are no reports of HT caused by JEV infection or immunological damage to multiple systems due to JEV. In this case, JEV caused multiple lesions simultaneously. When the patient was transferred to our hospital, IL-8 and IFN-γ were significantly elevated in the CSF (Figure 1B). Studies have shown that a significant elevation of IL-8 in the CSF can cause GBS (7), and a significant elevation of IFN-γ in blood is associated with the development of HT (8). There was no elevation of IFN-γ in the blood of this patient at the time of onset, but few studies have shown that HT can be caused by viral infection (9, 10).
JEV is an enveloped, single-stranded, positive-sense RNA virus. The following could be the possible mechanisms of various pathological changes in this patient. First, viral replication could have directly caused neuronal injury and dysfunction (11). Second, a large number of inflammatory and proinflammatory cytokines were released, producing an inflammatory response and further aggravating the injury (12, 13). Third, humoral immunity was stimulated, self-reactive antibodies against the nervous system or other tissues were produced, and antibody-mediated damage may have occurred (3). The patient’s fever, seizures, and disturbance in consciousness at the time of onset may have been primarily associated with the direct injury caused by the virus and a large number of inflammatory cytokine storms (7, 11–14), whereas HT and GBS may have been associated with the damage caused by pathological antibodies produced through humoral immunity (2, 7, 8). Therefore, treatment requires multi-targeted therapy against multiple pathological mechanisms.
A recent study has found that ganciclovir triphosphate can inhibit RNA virus replication by interfering with the RNA polymerase reaction (15), penciclovir could convert into the ganciclovir triphosphate analog, penciclovir triphosphate. This may be the mechanism of its anti-JEV activity. The elevated IL-8, IFN-γ, and IL-6 levels in the CSF of this patient at the early stages of onset indicate a significant cytokine inflammatory storm (7, 8, 12, 13). This may not only be the reason for the fever, seizures, disorders of consciousness, and meningeal irritation in the early stage, but also the reason for the increased permeability of the blood-brain barrier and increased white blood cells and proteins leaking into the CSF. Glucocorticoid is an immunosuppressive agent that can reduce proinflammatory signals and gene expression and suppress cytokine storms, thereby reducing inflammatory reactions (16). After treatment, the patient regained consciousness and fever and seizures disappeared; the return of IFN–γ and IL–6 to normal levels also supports these observations.
During the course of the disease, the patient developed early protective anti-JEV-IgM antibodies and pathological JEV-IgG and thyroid peroxidase antibodies, as well as antibodies against peripheral nerve myelin and axons that may not have been detected at later stages (only antiganglioside antibodies were tested). This indicates obvious activation of the patient’s humoral immune response with a wide extent of organ involvement (in the brain tissue, thyroid, and peripheral nerves) and high antibody burden for prolonged long duration. The virus was suppressed by a combination of antiviral-glucocorticoid-IVIG therapy, which suppresses the immune response and neutralizes pathogenic antibodies (17). However, the appearance and progression of GBS could not be prevented. There may be two reasons for this. First, IL-8 decreased gradually and continued to be at an extremely high level (Figure 1B), which is prone to progress to the acute inflammatory demyelinating polyneuropathic form of GBS (7). The clinical manifestations of this patient are consistent with the manifestations of acute inflammatory demyelinating polyneuropathy. Second, when IVIG is used to neutralize pathological antibodies but is not eliminated from the body immediately, the possibility of immune complex damage cannot be excluded, and a different strategy is necessary. In PAIA, pathological antibodies and immune complexes are eliminated. As plasma and CSF immunoglobulins decreased rapidly (Figures 1A, C, D), the patient rapidly recovered limb muscle strength. From the results of the follow-up, the patient’s Montreal Cognitive Assessment score was lower than normal, probably because the patient had brain atrophy (Figure 4) and nerve fiber bundle damage (Figure 2H) (18), which may be a long-term process.
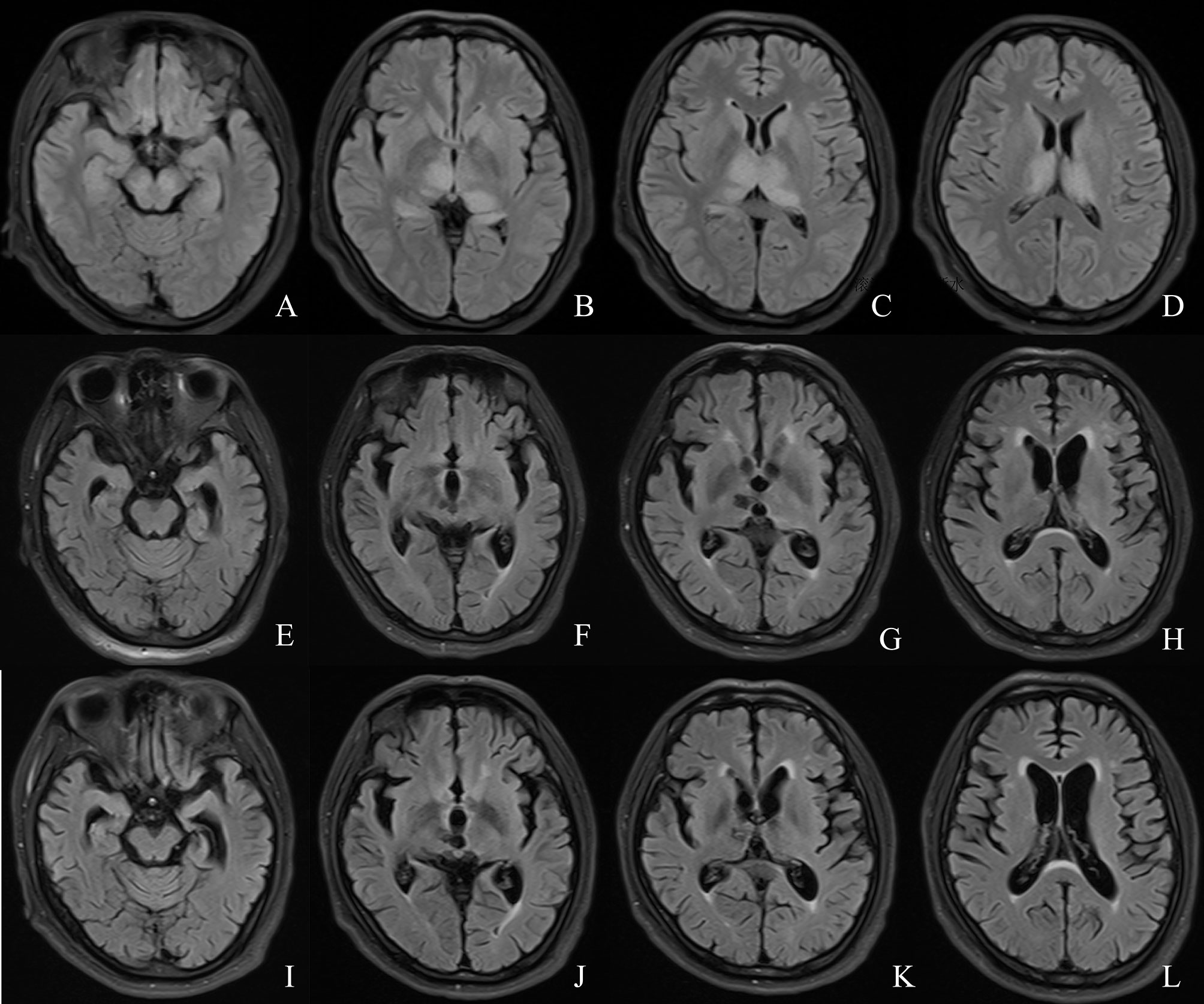
Figure 4 Comparison of the patient’s MRI. (A–D) MRI on day 6 of onset. (E–H) MRI re-examination at month 6 of the course of disease. (I–L) MRI examination at month 14 of the course of disease.
The removal of pathogenic antibodies can be achieved by Immunoadsorption (IA) and plasma exchange. Previous studies have shown the benefits of plasma exchange in treating GBS (19–21). IA has the advantage of exerting little influence on blood composition and plasma volume. It does not need plasma infusion, and thereby, it reduces the risk of infection or anaphylactic reactions to allogeneic proteins (5). In addition, IA clears antibodies faster and more specifically, it might be an effective treatment for patients with GBS who respond poorly to plasma exchange or IVIG therapy. IA has been used in the treatment of GBS (22). In the past, tryptophan columns were used as adsorption columns. PAIA involves the use of bioengineered recombinant Staphylococcus aureus protein A, which binds to the antibodies with biological affinity and has higher specificity. Unlike tryptophan-dependent hydrophobic interaction binding antibodies, PAIA reduces the occurrence of severe hypofibrinogenemia (5). PAIA has high removal efficiency, with IgG and IgM removal rates of approximately 86% and 57% after three treatments, respectively (5). It adsorbs antibodies rapidly and efficiently, and removes antibodies and immune complexes after elution, thereby preventing injury caused by pathogenic antibodies and promoting nerve repair. The PAIA adsorption column for each patient can be reused after elution, which reduces cost. PAIA has demonstrated advantages in the treatment of some diseases (6, 23).
Our case report has several limitations. First, this a clinical case and the effect of PAIA needs to be confirmed in larger studies. Second, the mechanism of HT caused by JE deserves further exploration.
In conclusion, our case report provides several key points: JEV can damage the central nervous system, peripheral nervous system, and glands of patients through a variety of mechanisms, and clinicians should be aware of these changes. This patient is the first to receive PAIA combined with an antiviral-glucocorticoid-IVIG regimen for treating encephalitis, meningitis, HT, and GBS caused by JEV infection, thus reflecting the importance of the clinical application of PAIA in the treatment of immunological complications of JE. The efficacy of PAIA in disease treatment may be associated with the elimination of antibodies or immune complexes.
The patient and her family were satisfied with the improvement of her clinical condition.
The original data presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (approval no. 2020-KY-077). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WM conceived and designed the study. QZ and YW performed data analysis and wrote the paper. JG performed data collection. LL, SZ, CC, and DS participated in patient management. BY and FT provided some technical guidance for immunoadsorption. JT contributed imaging interpretation. All authors contributed to the article and approved the submitted version.
Joint Co-construction Project of Henan Medical Science and Technology Research Plan (No.: LHGJ20190087); Key Scientific Research Project of Henan Province Colleges and Universities (No. 17A320067).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all members of the research team, as well as the patient and her family, and we would like to thank Editage (www.editage.cn) for English language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.807937/full#supplementary-material
1. Kakoti G, Dutta P, Ram Das B, Borah J, Mahanta J. Clinical Profile and Outcome of Japanese Encephalitis in Children Admitted With Acute Encephalitis Syndrome. BioMed Res Int (2013) 2013:152656. doi: 10.1155/2013/152656
2. Wang G, Li H, Yang X, Guo T, Wang L, Zhao Z, et al. Guillain-Barré Syndrome Associated With JEV Infection. N Engl J Med (2020) 383(12):1188–90. doi: 10.1056/NEJMc1916977
3. Ma J, Han W, Jiang L. Japanese Encephalitis-Induced Anti-N-Methyl-D-Aspartate Receptor Encephalitis: A Hospital-Based Prospective Study. Brain Dev (2020) 42(2):179–84. doi: 10.1016/j.braindev.2019.09.003
4. Verma R, Praharaj HN, Patil TB, Giri P. Acute Transverse Myelitis Following Japanese Encephalitis Viral Infection: An Uncommon Complication of a Common Disease. BMJ Case Rep (2012) 5:bcr2012007094. doi: 10.1136/bcr-2012-007094
5. Süfke S, Lehnert H, Uhlenbusch-Körwer I, Gebauer F. Safety Aspects of Immunoadsorption in IgG Removal Using a Single-Use, Multiple-Pass Protein A Immunoadsorber (LIGASORB): Clinical Investigation in Healthy Volunteers. Ther Apher Dial (2017) 21(4):405–13. doi: 10.1111/1744-9987.12532
6. Zhang B, Yu D, Zhu Q, Ruan H, Yu B, Cui C, et al. Protein A Immunoadsorption for the Treatment of Refractory Anti-N-Methyl-D-Aspartate Receptor Encephalitis: A Single-Center Prospective Study. J Neurol Sci (2021) 428:117568. doi: 10.1016/j.jns.2021.117568
7. Breville G, Lascano AM, Roux-Lombard P, Vuilleumier N, Lalive PH. Interleukin 8, a Biomarker to Differentiate Guillain-Barré Syndrome From CIDP. Neurol Neuroimmunol Neuroinflamm (2021) 8:e1031. doi: 10.1212/nxi.0000000000001031
8. Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 Serum Cytokine Profiles Characterize Patients With Hashimoto's Thyroiditis (Th1) and Graves' Disease (Th2). Neuroimmunomodulation (2004) 11(4):209–13. doi: 10.1159/000078438
9. Thomas D, Liakos V, Michou V, Kapranos N, Kaltsas G, Tsilivakos V, et al. Detection of Herpes Virus DNA in Post-Operative Thyroid Tissue Specimens of Patients With Autoimmune Thyroid Disease. Exp Clin Endocrinol Diabetes (2008) 116(1):35–9. doi: 10.1055/s-2007-956171
10. Virot E, Duclos A, Adelaide L, Miailhes P, Hot A, Ferry T, et al. Autoimmune Diseases and HIV Infection: A Cross-Sectional Study. Med (Baltimore) (2017) 96(4):e5769. doi: 10.1097/md.0000000000005769
11. Guo F, Yu X, Xu A, Xu J, Wang Q, Guo Y, et al. Japanese Encephalitis Virus Induces Apoptosis by Inhibiting Foxo Signaling Pathway. Vet Microbiol (2018) 220:73–82. doi: 10.1016/j.vetmic.2018.05.008
12. Yu SP, Ong KC, Perera D, Wong KT. Neuronal Transcriptomic Responses to Japanese Encephalitis Virus Infection With a Special Focus on Chemokine CXCL11 and Pattern Recognition Receptors RIG-1 and MDA5. Virology (2019) 527:107–15. doi: 10.1016/j.virol.2018.10.015
13. Winter PM, Dung NM, Loan HT, Kneen R, Wills B, Thu le T, et al. Proinflammatory Cytokines and Chemokines in Humans With Japanese Encephalitis. J Infect Dis (2004) 190(9):1618–26. doi: 10.1086/423328
14. Turtle L, Solomon T. Japanese Encephalitis - the Prospects for New Treatments. Nat Rev Neurol (2018) 14(5):298–313. doi: 10.1038/nrneurol.2018.30
15. Jockusch S, Tao C, Li X, Anderson TK, Chien M, Kumar S, et al. A Library of Nucleotide Analogues Terminate RNA Synthesis Catalyzed by Polymerases of Coronaviruses That Cause SARS and COVID-19. Antiviral Res (2020) 180:104857. doi: 10.1016/j.antiviral.2020.104857
16. Timmermans S, Souffriau J, Libert C. A General Introduction to Glucocorticoid Biology. Front Immunol (2019) 10:1545. doi: 10.3389/fimmu.2019.01545
17. Miao W, Guo J, Zhang S, Shen N, Shang X, Liu F, et al. The Effect of a Combined Ganciclovir, Methylprednisolone, and Immunoglobulin Regimen on Survival and Functional Outcomes in Patients With Japanese Encephalitis. Front Neurol (2021) 12:711674. doi: 10.3389/fneur.2021.711674
18. Saar-Ashkenazy R, Veksler R, Guez J, Jacob Y, Shelef I, Shalev H, et al. Breakdown of Inter-Hemispheric Connectivity Is Associated With Posttraumatic Symptomatology and Memory Impairment. PloS One (2016) 11(2):e0144766. doi: 10.1371/journal.pone.0144766
19. Verboon C, Doets AY, Galassi G, Davidson A, Waheed W, Péréon Y, et al. Current Treatment Practice of Guillain-Barré Syndrome. Neurology (2019) 93(1):e59–76. doi: 10.1212/wnl.0000000000007719
20. Lehmann HC, Hartung HP. Plasma Exchange and Intravenous Immunoglobulins: Mechanism of Action in Immune-Mediated Neuropathies. J Neuroimmunol (2011) 231(1-2):61–9. doi: 10.1016/j.jneuroim.2010.09.015
21. Lehmann HC, Hartung HP, Hetzel GR, Stüve O, Kieseier BC. Plasma Exchange in Neuroimmunological Disorders: Part 2. Treatment of Neuromuscular Disorders. Arch Neurol (2006) 63(8):1066–71. doi: 10.1001/archneur.63.8.1066
22. Galldiks N, Dohmen C, Neveling M, Fink GR, Haupt WF. Selective Immune Adsorption Treatment of Severe Guillain Barré Syndrome in the Intensive Care Unit. Neurocrit Care (2009) 11(3):317–21. doi: 10.1007/s12028-009-9252-6
Keywords: Japanese encephalitis, Guillain-Barré syndrome, Hashimoto’s thyroiditis, protein A immunoadsorption, case report
Citation: Zang Q, Wang Y, Guo J, Long L, Zhang S, Cui C, Song D, Yu B, Tang F, Teng J and Miao W (2022) Treatment of Severe Japanese Encephalitis Complicated With Hashimoto’s Thyroiditis and Guillain-Barré Syndrome With Protein A Immunoadsorption: A Case Report. Front. Immunol. 12:807937. doi: 10.3389/fimmu.2021.807937
Received: 02 November 2021; Accepted: 15 December 2021;
Published: 07 January 2022.
Edited by:
Feng-Liang Liu, Kunming Institute of Zoology, ChinaReviewed by:
Jianping Ma, National Institutes of Health (NIH), United StatesCopyright © 2022 Zang, Wang, Guo, Long, Zhang, Cui, Song, Yu, Tang, Teng and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Miao, bWlhb3dhbmc3MjExQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.