
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 05 January 2022
Sec. Immunological Tolerance and Regulation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.791725
This article is part of the Research Topic Immunological Tolerance in Transplantation: More than Deletion View all 10 articles
Chronic rejection and immunosuppression-related toxicity severely affect long-term outcomes of kidney transplantation. The induction of transplantation tolerance – the lack of destructive immune responses to a transplanted organ in the absence of immunosuppression – could potentially overcome these limitations. Immune tolerance to kidney allografts from living donors has been successfully achieved in humans through clinical protocols based on chimerism induction with hematopoietic cell transplantation after non-myeloablative conditioning. Notably, two of these protocols have led to immune tolerance in a significant fraction of HLA-mismatched donor-recipient combinations, which represent the large majority of cases in clinical practice. Studies in mice and large animals have been critical in dissecting tolerance mechanisms and in selecting the most promising approaches for human translation. However, there are several key differences in tolerance induction between these models and humans, including the rate of success and stability of donor chimerism, as well as the relative contribution of different mechanisms in inducing donor-specific unresponsiveness. Kidney allograft tolerance achieved through durable full-donor chimerism may be due to central deletion of graft-reactive donor T cells, even though mechanistic data from patient series are lacking. On the other hand, immune tolerance attained with transient mixed chimerism-based protocols initially relies on Treg-mediated suppression, followed by peripheral deletion of donor-reactive recipient T-cell clones under antigenic pressure from the graft. These conclusions were supported by data deriving from novel high-throughput T-cell receptor sequencing approaches that allowed tracking of alloreactive repertoires over time. In this review, we summarize the most important mechanistic studies on tolerance induction with combined kidney-bone marrow transplantation in humans, discussing open issues that still need to be addressed and focusing on techniques developed in recent years to efficiently monitor the alloresponse in tolerance trials. These cutting-edge methods will be instrumental for the development of immune tolerance protocols with improved efficacy and to identify patients amenable to safe immunosuppression withdrawal.
Renal transplantation is the established treatment of choice for kidney failure, as it confers both the highest survival and the best quality of life compared to other renal replacement therapies (1). Despite continuous advances in the field of solid organ transplantation, long-term outcomes of kidney allografts have only modestly improved in the last decades. Immunosuppressive therapies consistently control acute rejection, but have little effect on chronic rejection, which leads to graft loss in 50% of cases at 10 years (2). In addition, approximately half of the kidney transplants lost are due to death with a functioning graft: the impact of chronic immunosuppression has potentially devastating consequences in terms of cardiovascular disease, infection and malignancy (3–5), and may severely impair recipients’ quality of life.
The induction of tolerance, i.e. the lack of destructive immune responses to a transplanted organ in the absence of immuno-suppression, could potentially overcome both of these limitations. Tolerance in kidney transplantation can be functionally defined by stable renal function and absence of histologic, immune and molecular signs of rejection on a kidney biopsy obtained after complete withdrawal of immunosuppression for at least one year. Spontaneous tolerance is unfortunately a rare and unpredictable event that has been described in a small minority among the patients who choose to discontinue their immunosuppression, who retained graft function despite complete withdrawal of immunosuppression (6).
Among the different methods used to induce tolerance in animal models of kidney transplantation, few have been successfully translated to clinical application. Those protocols that have succeeded in patients entail combined kidney and bone marrow transplantation (CKBMT) as a strategy to induce chimerism, a state wherein donor hematopoietic cells engraft into the recipient bone marrow at a level sufficient to be detected by conventional (as opposed to sensitive PCR-based) methods.
Three centers have developed clinical CKBMT protocols, one of which has so far succeeded in achieving tolerance only in the HLA-identical transplant setting (7). Investigators from Stanford University used total lymphoid irradiation combined with anti-thymocyte globulin to facilitate the engraftment of donor hematopoietic stem cells (HSC), which were infused along with a fixed number of donor T cells after kidney transplantation. Mixed chimerism persisting for at least 6 months was achieved in 83% of the 29 HLA-matched patients treated with this protocol. Mixed chimerism was consistently associated with a tolerant state that allowed safe withdrawal of immunosuppression. Unfortunately, when a similar protocol was applied to haplotype-matched donor-recipient pairs, immunosuppressive drug weaning below therapeutic levels led to loss of chimerism and rejection episodes (8, 9).
Only two strategies have succeeded in effectively inducing operational tolerance across HLA barriers so far. As HLA mismatches are commonly present in solid organ transplantation, in this review we will discuss the features of these regimens and the novel mechanistic insights offered by recent studies in the field.
Animal Studies. More than 60 years ago, Main and Prehn used bone marrow infusion following administration of high-dose, lethal total body irradiation (TBI) to achieve skin allograft tolerance in recipient mice. In this experimental setting, semiallogeneic but not isogenic bone marrow infusion consistently permitted donor-specific skin graft acceptance (10). Subsequent studies from Cobbold and colleagues showed that mice treated with T-cell depleting antibodies along with TBI did not reject MHC-mismatched bone marrow grafts and developed donor-specific tolerance (11). These mice exhibited full donor chimerism, i.e. the entire recipient hematopoietic system was replaced by donor cells (donor cells > 98%), so “self” tolerance of donor T cells was achieved. Later studies suggested that incomplete deletional tolerance of these recipient-reactive donor T cells was achieved, reflecting the absence of a self-renewing source of recipient APCs to ensure complete deletion of host-reactive donor T cells in the thymus. Nevertheless, functional tolerance to the recipient was achieved by a combination of mechanisms that involve thymic stromal cells, which are of recipient origin (12, 13) (Figures 1A, B).
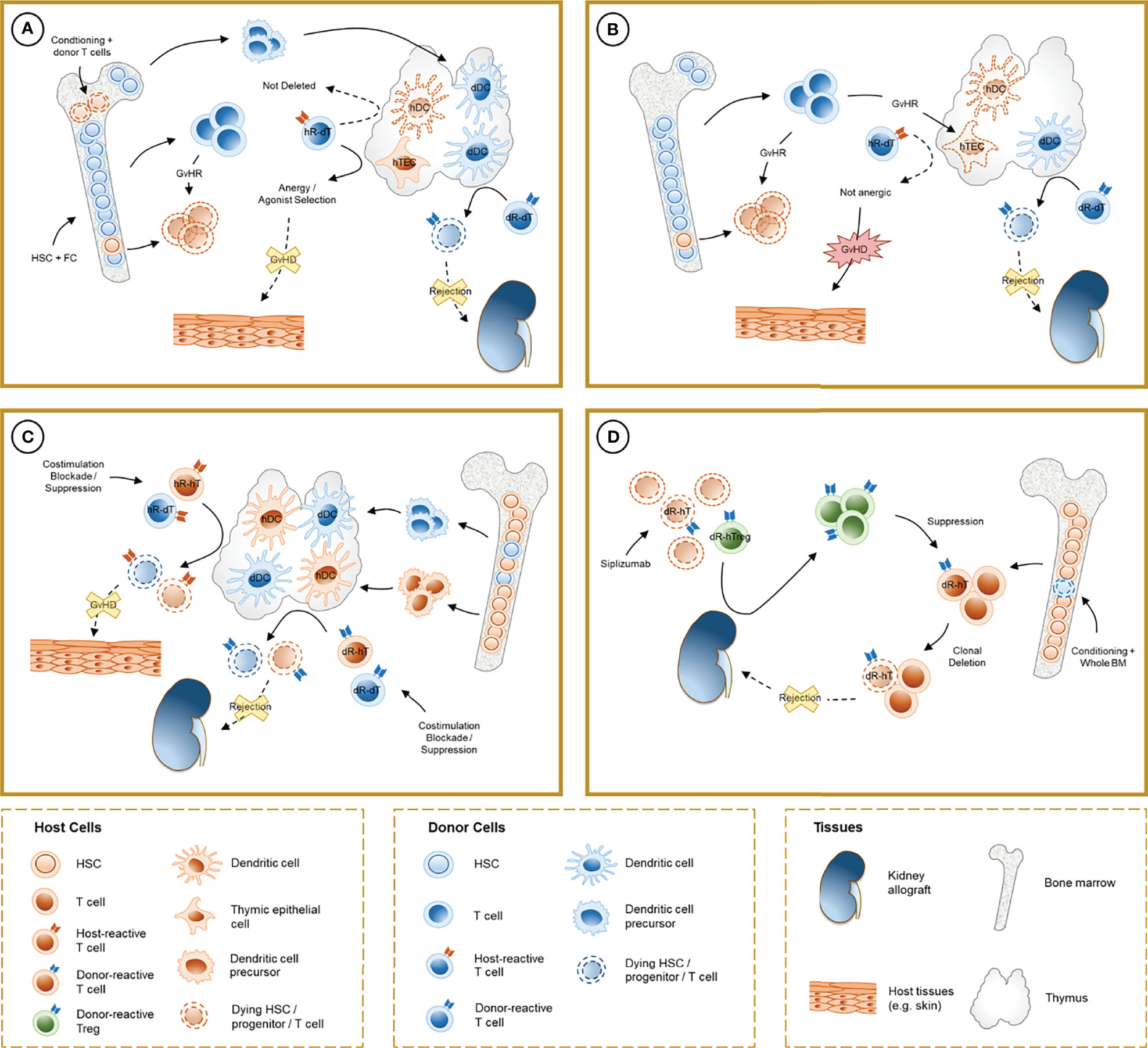
Figure 1 Schematic representation of the mechanisms involved in chimerism-based tolerance to kidney allografts. (A) The induction of full-donor chimerism through hematopoietic stem cell (HSC) infusion along with facilitating cells (FC) after non-myeloablative conditioning results in destruction of host HSC, presumably by graft-versus-host reaction (GvHR) from infused donor T cells, and durable engraftment of donor hematopoietic precursors. After thymic repopulation by donor-derived dendritic cells (dDC), donor-reactive T cells from the donor (dR-dT) undergo clonal deletion in the thymus (central tolerance). Host-reactive donor T cells (hR-dT) are incompletely deleted, reflecting the absence of a self-renewing source of recipient APCs, but functional tolerance to the recipient may be achieved by a combination of mechanisms (anergy and selection of host-specific Tregs) that involve recipient thymic epithelial cells (hTEC). (B) Destruction of hTEC and thymic structure by GvHR may cause failure of negative selection and precipitate graft-versus-host disease (GVHD). (C) In durable mixed chimerism, donor-derived precursors populate the host thymus and differentiate into DC (dDC) without depletion of their host-derived counterparts (hDC). Donor- and host-reactive T cells from both the donor and the host undergo negative selection, allowing allograft tolerance without GVHD. Treg-mediated suppression may also play a role in experimental regimens where clonal deletion is incomplete. (D) In CKBMT patients receiving a siplizumab-based conditioning regimen and unprocessed bone marrow, transient mixed chimerism promotes peripheral tolerance. Host Tregs are relatively spared from global T cell depletion, and donor-reactive host Tregs (dR-hTreg) are expanded by antigenic pressure from the graft. Emerging donor-reactive T cells, which are not subjected to central deletion, are suppressed by dR-hTreg and ultimately undergo peripheral deletion over time.
Several strategies have been studied to reduce the risk of bone marrow engraftment failure and to curtail the impact of myeloablative conditioning regimens that were initially necessary to allow the engraftment of allogeneic bone marrow stem cells. Ildstad et al. reported the engraftment-promoting effects of a cell product termed “facilitating cells” (FC) in mice treated with high TBI doses. Murine FC include a population of CD8α+ TCR-, but paradoxically CD3+, plasmacytoid-precursor dendritic cells and also seem to include populations of B cells, NK cells, granulocytes and monocytes. Murine FCs have been reported to provide survival and homing signals to HSC, induce antigen-specific regulatory T cells (Tregs) and expand IL-10-producing Tr1 cells (14–17). These cells were also reported to be present in human bone marrow (18) and have served as the basis for the proprietary product used in the Northwestern University clinical protocol described below.
Clinical Protocols. Investigators from Northwestern University utilized a non-myeloablative conditioning regimen that achieved durable full donor chimerism in humans, attempting to exploit the engraftment-promoting and immunosuppressive effect of FC (19, 20). This regimen builds on the Hopkins protocol that uses post-transplant cyclophosphamide to inhibit GVHD across HLA barriers (21) and includes pre-transplant fludarabine, cyclophosphamide and TBI, which “make space” for HSC engraftment and control anti-donor responses that would otherwise lead to graft rejection (Table 1). Kidney transplantation is followed by infusion of a G-CSF +/- plerixafor-mobilized apheresis product treated to retain HSC and FC, as well as a controlled number of donor T cells. While the proprietary method for apheresis product treatment has not been disclosed, the full chimerism achieved in most of these patients, despite non-myeloablative conditioning, suggests a major role for GVH-reactive donor T cells in destroying recipient hematopoietic cells in the bone marrow.
Out of the 37 patients transplanted, 26 exhibited durable donor chimerism (23 developed full-donor chimerism) and were successfully weaned off immunosuppression after one year from transplant. These subjects showed significantly better kidney function compared to matched controls receiving conventional immunosuppression. Two graft losses due to opportunistic infections were recorded in the first year after transplantation, and one tolerant recipient died due to sepsis. Studies in mice have highlighted that full donor chimeras are somewhat immunoincompetent (22, 23) due to the absence of recipient APCs in the periphery, which are needed to optimally present antigens to T cells that are positively selected by recipient thymic epithelium. Indeed, cytotoxic T cells generated in chimeric mice lacking shared MHC alleles between the donor and recipient are unable to clear virally infected donor cells (24), which thereby serve as a viral reservoir that can result in chronic illness (23). While viral reactivation and other opportunistic infections occurred quite frequently in patients on this study, patients with full donor chimerism nevertheless could be successfully vaccinated after immune cell reconstitution, likely reflecting, at least in part, persistence of immune memory and immunity carried by donor T cells in the hematopoietic cell transplant (25). Additional complications included acute rejection in two patients with transient chimerism that were non-compliant with medications, and one death due to lung cancer. A potentially alarming toxic effect was recorded after a longer observation period: despite the use of post-transplantation cyclophosphamide, two subjects ultimately developed graft-versus-host disease (GVHD). One patient was diagnosed with grade 3 intestinal GVHD and CMV infection that led to a fatal outcome. Although relatively limited in frequency (5% of treated patients), the risk of GVHD in our view outweighs the benefits obtained with approaches based on full donor chimerism for tolerance induction.
Animal Studies. Mixed chimerism defines a state wherein, unlike full donor chimerism, the host hematopoietic system is not completely destroyed and replaced by the donor’s, and hematopoietic cells of both the recipient and the donor coexist in the bone marrow.
Sharabi and Sachs demonstrated that durable mixed chimerism and tolerance could be induced in mice conditioned with T cell-depleting antibodies, low-dose TBI and thymic irradiation (TI) (26). This method overcomes peripheral and intra-thymic rejection (27) of donor HSCs and facilitates bone marrow engraftment, which in turn provides a durable supply of progenitors that migrate to the thymus, differentiating into lymphocytes and dendritic cells. Tolerance to donor and recipient in these models is achieved via intra-thymic negative selection of alloreactive T cell clones, mediated by both donor- and recipient-derived antigen-presenting cells (28, 29) and regulatory mechanisms are notably absent in the long-term tolerance maintenance phase (30). Durable mixed chimerism can also be achieved through co-stimulation blockade combined with bone marrow transplantation, which resulted in anergy and peripheral deletion of donor-reactive clones (31–33). Peripheral tolerance of donor-reactive CD4 and CD8 T cells relied on distinct mechanisms, with a role for NFAT, LAG3, TGFβ, PD1 and recipient CD4 T cells, B cells and MHC class II for the CD8 T-cell anergy followed by deletion (34–38) and a pathway involving CD4 T cell-intrinsic CTLA4 and recipient CD80 and CD86 without regulatory mechanisms, leading to peripheral CD4 cell deletion (32, 39). The caspase 9-dependent intrinsic and cell-extrinsic Fas-FasL apoptosis pathways have both been implicated in clonal deletion in these models (40, 41). Notably, alternative mixed chimerism-based regimens that do not achieve complete deletion of donor-reactive T cells also rely on alloreactive Treg-mediated suppression to induce donor-specific tolerance (42, 43) (Figure 1C).
Before human application, non-myeloablative conditioning regimens for the induction of allograft tolerance were tested in non-human primates [extensively reviewed in (44)], a key step to assess the safety and efficacy of these protocols. These experiments underscored that the rate of success and the stability of chimerism induction in primates is considerably lower compared to rodents, partly due to the higher abundance of memory T cells in the former, which are more resistant to conventional T cell-depleting agents (45). The addition of splenectomy (or co-stimulation blockade) and a short course of cyclosporine could partially overcome this barrier in a significant fraction of animals, but mixed chimerism was only transient in all of them (46, 47). Contrary to initial assumptions, tolerance to renal allografts developed in more than 60% of recipients, providing the first proof of principle that durable chimerism is not essential for tolerance induction in primates, thus paving the road to human translation.
Clinical Protocols. Mixed chimerism-based approaches to induce tolerance to kidney allografts have been tested at the Massachusetts General Hospital (MGH) in patients with and without hematologic malignancies. Differences between these regimens have been reviewed in detail elsewhere (48), and we will focus our current discussion on patients without malignancy, as these protocols have the highest potential for translation to routine clinical practice in the future.
Initial studies used a non-myeloablative conditioning regimen that included cyclophosphamide, the anti-CD2 T cell-depleting monoclonal antibody siplizumab and thymic irradiation (TI) (Table 1) (49, 50). Unprocessed donor bone marrow was infused on the day of kidney transplantation, and subjects also received calcineurin inhibitors and a short course of corticosteroids postoperatively. Pre- and peri-transplant rituximab doses were introduced after evidence of antibody-mediated rejection in one patient and de-novo DSA development in 2 additional patients. After this modification, all patients remained immunosuppression-free for the duration of the study. Transient mixed chimerism for up to 3 weeks was induced in all recipients, without evidence of GVHD. Maintenance immunosuppressive drugs were slowly tapered after 6 months in patients with normal protocol biopsy, and the primary endpoint of 24-month immunosuppression-free kidney allograft survival was achieved in 7 of the 10 patients enrolled. Three of these subjects later (at 4 to 7 years post-transplant) experienced chronic rejection or glomerulonephritis recurrence, which led to reintroduction of immunosuppressive drugs and ultimately resulted in graft loss more than 10 years after transplantation. Of note, these patients were successfully retransplanted with conventional immunosuppression, and there were no significant opportunistic infections in any of them.
In parallel with early host T cell recovery, 9 patients unexpectedly developed severe acute kidney injury. Renal histology was consistent with engraftment syndrome, entailing capillary endothelial injury with vascular leak and lympho-monocytic infiltrating cells in peritubular and glomerular capillaries. Renal function normalized in all but 2 recipients, one of whom experienced graft loss due to acute humoral rejection as a consequence of preformed DSA that were undetectable on a pre-transplant ELISA, but were subsequently confirmed by Luminex. In the other patient, acute kidney injury was initially misdiagnosed as rejection and was treated with higher doses of tacrolimus, which triggered thrombotic microangiopathy. Finally, one patient developed severe cellular rejection after a pyelonephritis episode following immunosuppression withdrawal. Protocol biopsies (at 2-8 years) in tolerant subjects showed either completely normal histology or minimal alterations, including focal glomerular basement membrane duplication and mild podocyte foot process effacement (50).
An additional protocol was tested at MGH based on further observations from studies conducted in non-human primates (46, 51). Compared to previous regimens, cyclophosphamide was substituted with TBI to prevent engraftment syndrome. Renal function remained stable in the two patients enrolled, but one did not develop sufficient chimerism to allow immunosuppression weaning. Immunosuppressive drugs were successfully discontinued in the other patient, but were resumed after more than 4 years due to evidence of humoral rejection on a protocol biopsy (52).
Investigators at the Samsung Medical Center initially used a nearly identical protocol to those outlined above, but the anti-CD2 monoclonal antibody siplizumab was substituted with ATG due to local unavailability (52, 53). To curtail the risk of engraftment syndrome, fludarabine and an additional dose of ATG were added in a second protocol iteration, which allowed reduction of the dose of cyclophosphamide. Due to development of BK nephritis, ATG and fludarabine dose was subsequently decreased, and tacrolimus was substituted with sirolimus one month after transplantation. Overall, mixed chimerism was achieved transiently (at least 3 weeks) in all 8 enrolled subjects. Immunosuppression was successfully discontinued for more than one year in 5 patients, even though one of them experienced acute cellular rejection after a respiratory tract infection, which led to reintroduction of tacrolimus.
The mechanism that underlies tolerance to kidney allografts associated with full-donor chimerism hypothetically involves central tolerance of donor T cells to donor antigens, with donor progenitor cells migrating to the recipient thymus, differentiating into antigen-presenting cells and finally mediating negative selection of “self”-reactive donor T cell clones. Bulk functional assays, including mixed-lymphocyte reactions (MLR) and cell-mediated lympholysis (CML), demonstrated donor-specific hyporesponsiveness in tolerant patients. However, the same effect was observed in recipients who exhibited only transient chimerism and developed rejection after immunosuppression withdrawal (54), suggesting that these assays cannot be relied upon to infer a tolerant state. On the other hand, development of full donor chimerism was the single most accurate predictor of tolerance in these patients (54). An intra-graft signature of tolerance was also described for these patients, which was characterized by upregulation of genes involved in B cell regulation and pro-tolerogenic plasmacytoid DC enrichment, as well as the induction of regulatory pathways involved in the control of inflammation and maintenance of tissue homeostasis (55). Overall, however, studies elucidating the mechanism of tolerance in these subjects are currently lacking.
Given the full donor chimerism achieved in these patients, the achievement and mechanism of GVH tolerance is also worthy of investigation. Studies in mouse models discussed above would suggest that de novo GVH tolerance might be characterized by a combination of clonal deletion, anergy and regulatory T cell-mediated mechanisms. However, GVH tolerance has not been demonstrated in these patients and the inclusion in the infused product of mature donor T cells that eliminate host hematopoiesis suggests that an ongoing GVH reaction may occur, which has culminated in GVHD in several patients. Whether or not GVH reactions in patients without overt GVHD results in thymic injury and failure to negatively select host-reactive T cells, as reported in murine models (56–59), has not been investigated.
The mechanisms of tolerance in protocols based on transient mixed chimerism have been the topic of extensive studies in recent years. Central tolerance is unlikely to be the main mechanism operating in these CKBMT patients, since transient chimerism is likely insufficient to allow long-term thymic repopulation with donor antigen-presenting cells.
Preliminary studies with bulk functional assays were partly inconclusive, since a lack of post-transplant donor-specific responses was observed both in tolerant patients and in the patient who developed acute rejection after immunosuppression withdrawal in the MGH trial (60). Several mechanisms, including T cell anergy and peripheral deletion, could underlie the observed donor-specific hyporesponsiveness, but these assays could not discriminate between them. Nonetheless, these results were extremely informative when compared with those from recipients of bone marrow transplantation conditioned with a similar regimen but without kidney transplantation. In these subjects, donor-specific reactivity reappeared after chimerism was lost, indicating that the kidney allograft is likely to play a pivotal role in tolerance development in CKBMT recipients (61).
The advent of platforms to perform high-throughput sequencing of the TCRβ CDR3 hypervariable region led to the development of novel tools to analyze the T cell alloresponse. We hypothesized that a significant fraction of the donor-reactive repertoire could be identified in a pre-transplant MLR, by sequencing sorted recipient T cells that divided in response to donor stimulation. These sequences were compared with those of sorted unstimulated recipient CD4+ and CD8+ T cells to define a fingerprint of the anti-donor T cell repertoire. Thresholds for detection were based on a uniform clonal frequency (to normalize for sample size variability over time) and on a minimal fold-expansion (to avoid capturing highly abundant but not specifically donor-reactive clones), while computational methods were used to account for sorting errors (62). This fingerprint was then longitudinally compared with samples obtained at different post-transplant time points to track circulating donor-reactive clones over time. Both tolerant and non-tolerant patients, as well as kidney transplant recipients under conventional immunosuppression, had considerable repertoire turnover, reflecting the use of T cell depleting agents in the conditioning regimens. However, all tolerant patients analyzed displayed a progressive and specific reduction in both donor-reactive CD4+ and CD8+ T cell clones, whereas no significant change was identified in the non-tolerant patient (60), and conventional kidney transplant recipients showed expansion of CD4+ T clones. These results suggest that clonal deletion is involved in the development of tolerance and may serve as a marker to identify patients amenable to safe immunosuppression weaning. Conversely, T cells in the non-tolerant patient were probably anergic, but were re-activated after immunosuppression withdrawal by the infective episode, thus precipitating acute rejection.
The existence of a suppressive mechanism in these patients was initially suggested by re-emergence of anti-donor responses in bulk functional assays performed with Treg-depleted samples from the first post-transplant year. However, samples obtained at later time points failed to show a similar response, suggesting that suppression could be relevant only as an early mechanism (63). Consistent with this hypothesis, limiting dilution assays conducted after the first post-transplant year failed to show an increase in response at higher dilution, which usually indicates the presence of suppressive cells at a lower frequency than responder cells (60).
Phenotypic analysis of circulating mononuclear cells in tolerant patients identified an early expansion of Tregs (80% of CD4+ T cells during the first week) with evidence of peripheral proliferation, possibly recent thymic emigration and, in one patient, conversion from conventional T cells (64). Expression of CD45RA declined after two weeks from transplant (64), suggesting that previously resting Tregs acquired an activated phenotype (65). The presence of a highly demethylated FoxP3 Treg specific region, an epigenetic hallmark of stable Tregs, confirmed the results from phenotypic data.
Subsequent studies demonstrated that the anti-CD2 monoclonal antibody siplizumab could induce costimulation blockade and T cell depletion, but selectively spared Tregs and promoted the expansion of alloreactive Tregs in vitro (66). In vivo, this process may be further amplified by the lymphopenia-driven expansion state that follows global T cell depletion. Interestingly, siplizumab predominantly reduced the frequency of effector memory T cells, which express the highest CD2 levels among T cell subsets (66, 67). This additional effect may be relevant for tolerance induction, since cross-reactive memory T cells are abundant in humans, and constitute a barrier to the establishment of chimerism and tolerance. Indeed, these cells are more resistant to depletion with ATG, depend less on costimulatory signals and are less susceptible to Treg-mediated suppression (68).
By using the same sequencing approach detailed above, we interrogated donor-reactive sequences that mapped to the unstimulated sorted Treg pool, but these sequences were detected at a very low frequency due to the low numbers of Tregs in the circulation. The method was therefore optimized by expanding the donor-reactive Treg pool with activated donor B cells instead of performing a conventional MLR. Expansion of donor-specific Tregs with activated donor B cells greatly increased the number of unique donor-specific Treg sequences identified and the specificity and potency of these cells in suppressing anti-donor responses was markedly increased, demonstrating that truly donor-specific Tregs were enriched in this repertoire. Using this method of pre-transplant donor-specific Treg repertoire identification, tolerant patients were found to display significant expansion of donor-specific Tregs at 6 months from transplantation, while the single non-tolerant subject did not (69). This study also showed that the majority of expanded Tregs in tolerant subjects mapped to the pre-transplant unstimulated Treg pool rather than conventional T cells, suggesting that expansion of pre-existing Tregs rather than induction of donor-specific Tregs was the major mechanism for increased donor-specific Tregs in these patients.
Overall, these data indicate a central role for early Treg-mediated suppression in the development of tolerance in combined kidney bone marrow transplantation. It could be speculated that prolonged stimulation of donor-reactive T cell clones by graft antigens under constant restraint by Tregs might mediate anergy and subsequent peripheral deletion of these cells. This suppressive effect loses potency over time as gradual clonal deletion of donor-reactive T cells eliminates the alloresponse needed to maintain expanded donor-specific Treg populations (Figure 1D).
Even though tolerance induction has been achieved in humans through chimerism development, these regimens still need to be refined before they can be translated to routine clinical practice. We believe that the ultimate aim will be to develop a protocol capable of reproducibly inducing tolerance through durable mixed chimerism.
Albeit progressively refined over the course of the last decades, conditioning regimens still bear potentially significant systemic toxicity, which results in both short- and long-term clinically relevant complications. The development of costimulation blockers and other novel drugs targeting specific cell populations and molecular moieties could help to refine conditioning regimens further, thus limiting side effects. Avoidance of engraftment syndrome observed in current regimens represents a realistic short-term goal, which may be achieved with revised protocols in the near future. Studies in animal models and humans have outlined that several mechanisms for tolerance coexist, and future strategies may exploit this knowledge to induce a more robust tolerant state. A future, intriguing possibility to promote durable chimerism without increasing the risk of GVHD is represented by peri-transplant infusion of ex-vivo expanded recipient Tregs. Administration of polyclonal Tregs was able to induce mixed chimerism in mice in the absence of cytoreductive therapy (70), and promoted more durable mixed chimerism and tolerance, that permitted delayed kidney transplantation without immunosuppression, in primates treated with non-myeloablative conditioning (71).
Reproducibility in humans remains a key issue of translational research in transplantation, especially in the context of tolerance trials, where a universally accepted and validated biomarker of the tolerant state has been lacking so far. The newly developed methods based on TCR sequencing to track donor-reactive T cell/Treg clones deserve further exploration as a tool that may be useful for the identification of patients amenable to safe immunosuppression withdrawal in a personalized manner. Furthermore, this approach has considerable potential to further identify the role of and elucidate mechanisms of host-vs-graft and graft-vs-host reactivity and tolerance, respectively, in recipients of hematopoietic cell transplantation for the purpose of allograft tolerance induction.
Tolerance studies will be also pivotal to pave the way to clinical xenotransplantation, considered to be the next frontier in solid organ transplantation due to its potential to overcome the severe shortage of human organs. Murine models have shown that mixed chimerism induction can promote tolerance to xenografts through several concomitant mechanisms, including deletion of xenoreactive B cells (72–75) with disappearance of natural antibodies to xenoantigens, as well as tolerization of xenoreactive T (76) and NK cells (77). These results have been replicated by induction of porcine mixed chimerism in immunodeficient mice with human immune systems (78–81). However, immune barriers to xenogeneic mixed chimerism induction are considerably greater than those to allogeneic chimerism, particularly due to the rapid destruction of porcine cells by human macrophages (82, 83), which can be at least partially overcome by the introduction of a human CD47 transgene into the pig (84–86). Current protocols will need to be optimized before clinical translation can be safely attempted.
MP and MS jointly wrote the first draft of the manuscript and revised its content critically. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the American Society of Transplantation Research Network. The work was also supported in part by NIH grants (ROI #AI084074), Immune Tolerance Network contract (N01 AI15416) and Immune Tolerance Network Award (UM1 AI109565).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Dr. Federica Casiraghi for her helpful comments on the manuscript and Julissa Cabrera for assistance with its submission.
1. Hariharan S, Israni AK, Danovitch G. Long-Term Survival After Kidney Transplantation. N Engl J Med (2021) 385:729–43. doi: 10.1056/NEJMra2014530
2. Gondos A, Döhler B, Brenner H, Opelz G. Kidney Graft Survival in Europe and the United States: Strikingly Different Long-Term Outcomes. Transplantation (2013) 95:267–74. doi: 10.1097/TP.0b013e3182708ea8
3. Ying T, Shi B, Kelly PJ, Pilmore H, Clayton PA, Chadban SJ. Death After Kidney Transplantation: An Analysis by Era and Time Post-Transplant. JASN (2020) 31:2887–99. doi: 10.1681/ASN.2020050566
4. Rama I, Grinyó JM. Malignancy After Renal Transplantation: The Role of Immunosuppression. Nat Rev Nephrol (2010) 6:511–9. doi: 10.1038/nrneph.2010.102
5. Karuthu S, Blumberg EA. Common Infections in Kidney Transplant Recipients. CJASN (2012) 7:2058–70. doi: 10.2215/CJN.04410512
6. Newell KA, Adams AB, Turka LA. Biomarkers of Operational Tolerance Following Kidney Transplantation – The Immune Tolerance Network Studies of Spontaneously Tolerant Kidney Transplant Recipients. Hum Immunol (2018) 79:380–7. doi: 10.1016/j.humimm.2018.02.007
7. Scandling JD, Busque S, Lowsky R, Shizuru J, Shori A, Engleman E, et al. Macrochimerism and Clinical Transplant Tolerance. Hum Immunol (2018) 79:266–71. doi: 10.1016/j.humimm.2018.01.002
8. Scandling JD, Busque S, Shizuru JA, Lowsky R, Hoppe R, Dejbakhsh-Jones S, et al. Chimerism, Graft Survival, and Withdrawal of Immunosuppressive Drugs in HLA Matched and Mismatched Patients After Living Donor Kidney and Hematopoietic Cell Transplantation. Am J Transplant (2015) 15:695–704. doi: 10.1111/ajt.13091
9. Busque S, Scandling JD, Lowsky R, Shizuru J, Jensen K, Waters J, et al. Mixed Chimerism and Acceptance of Kidney Transplants After Immunosuppressive Drug Withdrawal. Sci Transl Med (2020) 12:eaax8863. doi: 10.1126/scitranslmed.aax8863
10. Main JM, Prehn RT. Successful Skin Homografts After the Administration of High Dosage X Radiation and Homologous Bone Marrow. JNCI: J Natl Cancer Instit (1955) 15:1023–9. doi: 10.1093/jnci/15.4.1023
11. Cobbold SP, Martin G, Qin S, Waldmann H. Monoclonal Antibodies to Promote Marrow Engraftment and Tissue Graft Tolerance. Nature (1986) 323:164–6. doi: 10.1038/323164a0
12. Ramsdell F, Lantz T, Fowlkes BJ. A Nondeletional Mechanism of Thymic Self Tolerance. Science (1989) 246:1038–41. doi: 10.1126/science.2511629
13. Gao EK, Lo D, Sprent J. Strong T Cell Tolerance in Parent—-F1 Bone Marrow Chimeras Prepared With Supralethal Irradiation. Evidence for Clonal Deletion and Anergy. J Exp Med (1990) 171:1101–21. doi: 10.1084/jem.171.4.1101
14. Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic Characterization of a Novel Bone Marrow-Derived Cell That Facilitates Engraftment of Allogeneic Bone Marrow Stem Cells. Blood (1994) 84:2436–46. doi: 10.1182/blood.V84.8.2436.2436
15. Fugier-Vivier IJ, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, et al. Plasmacytoid Precursor Dendritic Cells Facilitate Allogeneic Hematopoietic Stem Cell Engraftment. J Exp Med (2005) 201:373–83. doi: 10.1084/jem.20041399
16. Huang Y, Bozulic LD, Miller T, Xu H, Hussain L-R, Ildstad ST. Cd8α+ Plasmacytoid Precursor DCs Induce Antigen-Specific Regulatory T Cells That Enhance HSC Engraftment In Vivo. Blood (2011) 117:2494–505. doi: 10.1182/blood-2010-06-291187
17. Wen Y, Elliott MJ, Huang Y, Miller TO, Corbin DR, Hussain L-R, et al. DOCK2 Is Critical for CD8(+) TCR(-) Graft Facilitating Cells to Enhance Engraftment of Hematopoietic Stem and Progenitor Cells. Stem Cells (2014) 32:2732–43. doi: 10.1002/stem.1780
18. Huang Y, Elliott MJ, Yolcu ES, Miller TO, Ratajczak J, Bozulic LD, et al. Characterization of Human CD8(+)TCR(-) Facilitating Cells In Vitro and In Vivo in a NOD/SCID/Il2rγ(Null) Mouse Model. Am J Transplant (2016) 16:440–53. doi: 10.1111/ajt.13511
19. Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, et al. Chimerism and Tolerance Without GVHD or Engraftment Syndrome in HLA-Mismatched Combined Kidney and Hematopoietic Stem Cell Transplantation. Sci Transl Med (2012) 4:124ra28. doi: 10.1126/scitranslmed.3003509
20. Tantisattamo E, Leventhal JR, Mathew JM, Gallon L. Chimerism and Tolerance: Past, Present and Future Strategies to Prolong Renal Allograft Survival. Curr Opin Nephrol Hypertens (2021) 30:63–74. doi: 10.1097/MNH.0000000000000666
21. Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable Engraftment of Major Histocompatibility Complex-Incompatible Cells After Nonmyeloablative Conditioning With Fludarabine, Low-Dose Total Body Irradiation, and Posttransplantation Cyclophosphamide. Blood (2001) 98:3456–64. doi: 10.1182/blood.v98.12.3456
22. Singer A, Hathcock KS, Hodes RJ. Self Recognition in Allogeneic Radiation Bone Marrow Chimeras. A Radiation-Resistant Host Element Dictates the Self Specificity and Immune Response Gene Phenotype of T-Helper Cells. J Exp Med (1981) 153:1286–301. doi: 10.1084/jem.153.5.1286
23. Koehn BH, Williams MA, Borom K, Gangappa S, Pearson TC, Ahmed R, et al. Fully MHC-Disparate Mixed Hemopoietic Chimeras Show Specific Defects in the Control of Chronic Viral Infections. J Immunol (2007) 179:2616–26. doi: 10.4049/jimmunol.179.4.2616
24. Rüedi E, Sykes M, Ildstad ST, Chester CH, Althage A, Hengartner H, et al. Antiviral T Cell Competence and Restriction Specificity of Mixed Allogeneic (P1 + P2—-P1) Irradiation Chimeras. Cell Immunol (1989) 121:185–95. doi: 10.1016/0008-8749(89)90016-6
25. Leventhal JR, Elliott MJ, Yolcu ES, Bozulic LD, Tollerud DJ, Mathew JM, et al. Immune Reconstitution/Immunocompetence in Recipients of Kidney Plus Hematopoietic Stem/Facilitating Cell Transplants. Transplantation (2015) 99:288–98. doi: 10.1097/TP.0000000000000605
26. Sharabi Y, Sachs DH. Mixed Chimerism and Permanent Specific Transplantation Tolerance Induced by a Nonlethal Preparative Regimen. J Exp Med (1989) 169:493–502. doi: 10.1084/jem.169.2.493
27. Tomita Y, Khan A, Sykes M. Mechanism by Which Additional Monoclonal Antibody (mAB) Injections Overcome the Requirement for Thymic Irradiation to Achieve Mixed Chimerism in Mice Receiving Bone Marrow Transplantation After Conditioning With Anti-T Cell mABs and 3-Gy Whole Body Irradiation. Transplantation (1996) 61:477–85. doi: 10.1097/00007890-199602150-00028
28. Tomita Y, Khan A, Sykes M. Role of Intrathymic Clonal Deletion and Peripheral Anergy in Transplantation Tolerance Induced by Bone Marrow Transplantation in Mice Conditioned With a Nonmyeloablative Regimen. J Immunol (1994) 153:1087–98.
29. Tomita Y, Sachs DH, Khan A, Sykes M. Additional Monoclonal Antibody (mAB) Injections Can Replace Thymic Irradiation to Allow Induction of Mixed Chimerism and Tolerance in Mice Receiving Bone Marrow Transplantation After Conditioning With Anti-T Cell mABs and 3-Gy Whole Body Irradiation. Transplantation (1996) 61:469–77. doi: 10.1097/00007890-199602150-00027
30. Khan A, Tomita Y, Sykes M. Thymic Dependence of Loss of Tolerance in Mixed Allogeneic Bone Marrow Chimeras After Depletion of Donor Antigen. Peripheral Mechanisms Do Not Contribute to Maintenance of Tolerance. Transplantation (1996) 62:380–7. doi: 10.1097/00007890-199608150-00014
31. Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic Bone Marrow Transplantation With Co-Stimulatory Blockade Induces Macrochimerism and Tolerance Without Cytoreductive Host Treatment. Nat Med (2000) 6:464–9. doi: 10.1038/74731
32. Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of Early Peripheral CD4 T-Cell Tolerance Induction by Anti-CD154 Monoclonal Antibody and Allogeneic Bone Marrow Transplantation: Evidence for Anergy and Deletion But Not Regulatory Cells. Blood (2004) 103:4336–43. doi: 10.1182/blood-2003-08-2642
33. Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, et al. Extrathymic T Cell Deletion and Allogeneic Stem Cell Engraftment Induced With Costimulatory Blockade Is Followed by Central T Cell Tolerance. J Exp Med (1998) 187:2037–44. doi: 10.1084/jem.187.12.2037
34. Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T Cell Tolerance Requires Recipient B Cells, Dendritic Cells and MHC Class II. J Immunol (2008) 181:165–73. doi: 10.4049/jimmunol.181.1.165
35. Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early Regulation of CD8 T Cell Alloreactivity by CD4+CD25- T Cells in Recipients of Anti-CD154 Antibody and Allogeneic BMT Is Followed by Rapid Peripheral Deletion of Donor-Reactive CD8+ T Cells, Precluding a Role for Sustained Regulation. Eur J Immunol (2005) 35:2679–90. doi: 10.1002/eji.200526190
36. Fehr T, Wang S, Haspot F, Kurtz J, Blaha P, Hogan T, et al. Rapid Deletional Peripheral CD8 T Cell Tolerance Induced by Allogeneic Bone Marrow: Role of Donor Class II MHC and B Cells. J Immunol (2008) 181:4371–80. doi: 10.4049/jimmunol.181.6.4371
37. Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T, et al. Peripheral Deletional Tolerance of Alloreactive CD8 But Not CD4 T Cells Is Dependent on the PD-1/PD-L1 Pathway. Blood (2008) 112:2149–55. doi: 10.1182/blood-2007-12-127449
38. Lucas CL, Workman CJ, Beyaz S, LoCascio S, Zhao G, Vignali DAA, et al. LAG-3, TGF-β, and Cell-Intrinsic PD-1 Inhibitory Pathways Contribute to CD8 But Not CD4 T-Cell Tolerance Induced by Allogeneic BMT With Anti-CD40L. Blood (2011) 117:5532–40. doi: 10.1182/blood-2010-11-318675
39. Kurtz J, Raval F, Vallot C, Der J, Sykes M. CTLA-4 on Alloreactive CD4 T Cells Interacts With Recipient CD80/86 to Promote Tolerance. Blood (2009) 113:3475–84. doi: 10.1182/blood-2008-01-133736
40. Cippà PE, Gabriel SS, Chen J, Bardwell PD, Bushell A, Guimezanes A, et al. Targeting Apoptosis to Induce Stable Mixed Hematopoietic Chimerism and Long-Term Allograft Survival Without Myelosuppressive Conditioning in Mice. Blood (2013) 122:1669–77. doi: 10.1182/blood-2012-09-453944
41. Wekerle T, Kurtz J, Sayegh MH, Ito H, Wells AD, Bensinger S, et al. Peripheral Deletion After Bone Marrow Transplantation With Costimulatory Blockade Has Features of Both Activation-Induced Cell Death and Passive Cell Death. J Immunol (2001) 166:2311–6. doi: 10.4049/jimmunol.166.4.2311
42. Bemelman F, Honey K, Adams E, Cobbold S, Waldmann H. Bone Marrow Transplantation Induces Either Clonal Deletion or Infectious Tolerance Depending on the Dose. J Immunol (1998) 160:2645–8.
43. Domenig C, Sanchez-Fueyo A, Kurtz J, Alexopoulos SP, Mariat C, Sykes M, et al. Roles of Deletion and Regulation in Creating Mixed Chimerism and Allograft Tolerance Using a Nonlymphoablative Irradiation-Free Protocol. J Immunol (2005) 175:51–60. doi: 10.4049/jimmunol.175.1.51
44. Sasaki H, Oura T, Spitzer TR, Chen Y-B, Madsen JC, Allan J, et al. Preclinical and Clinical Studies for Transplant Tolerance via the Mixed Chimerism Approach. Hum Immunol (2018) 79:258–65. doi: 10.1016/j.humimm.2017.11.008
45. Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous Immunity Provides a Potent Barrier to Transplantation Tolerance. J Clin Invest (2003) 111:1887–95. doi: 10.1172/JCI17477
46. Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed Allogeneic Chimerism and Renal Allograft Tolerance in Cynomolgus Monkeys. Transplantation (1995) 59:256–62. doi: 10.1097/00007890-199501000-00018
47. Yamada Y, Ochiai T, Boskovic S, Nadazdin O, Oura T, Schoenfeld D, et al. Use of CTLA4Ig for Induction of Mixed Chimerism and Renal Allograft Tolerance in Nonhuman Primates. Am J Transplant (2014) 14:2704–12. doi: 10.1111/ajt.12936
48. Sykes M. Immune Monitoring of Transplant Patients in Transient Mixed Chimerism Tolerance Trials. Hum Immunol (2018) 79:334–42. doi: 10.1016/j.humimm.2017.12.011
49. Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-Mismatched Renal Transplantation Without Maintenance Immunosuppression. N Engl J Med (2008) 358:353–61. doi: 10.1056/NEJMoa071074
50. Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, et al. Long-Term Results in Recipients of Combined HLA-Mismatched Kidney and Bone Marrow Transplantation Without Maintenance Immunosuppression. Am J Transplant (2014) 14:1599–611. doi: 10.1111/ajt.12731
51. Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, et al. Overcoming Memory T-Cell Responses for Induction of Delayed Tolerance in Nonhuman Primates. Am J Transplant (2012) 12:330–40. doi: 10.1111/j.1600-6143.2011.03795.x
52. Issa F, Strober S, Leventhal JR, Kawai T, Kaufman DB, Levitsky J, et al. The Fourth International Workshop on Clinical Transplant Tolerance. Am J Transplant (2021) 21:21–31. doi: 10.1111/ajt.16139
53. Lee KW, Park JB, Park H, Kwon Y, Lee JS, Kim KS, et al. Inducing Transient Mixed Chimerism for Allograft Survival Without Maintenance Immunosuppression With Combined Kidney and Bone Marrow Transplantation: Protocol Optimization. Transplantation (2020) 104:1472–82. doi: 10.1097/TP.0000000000003006
54. Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, Elliott MJ, et al. Tolerance Induction in HLA Disparate Living Donor Kidney Transplantation by Donor Stem Cell Infusion: Durable Chimerism Predicts Outcome. Transplantation (2013) 95:169–76. doi: 10.1097/TP.0b013e3182782fc1
55. Gallon L, Mathew JM, Bontha SV, Dumur CI, Dalal P, Nadimpalli L, et al. Intragraft Molecular Pathways Associated With Tolerance Induction in Renal Transplantation. J Am Soc Nephrol (2018) 29:423–33. doi: 10.1681/ASN.2017030348
56. Holländer GA, Widmer B, Burakoff SJ. Loss of Normal Thymic Repertoire Selection and Persistence of Autoreactive T Cells in Graft vs Host Disease. J Immunol (1994) 152:1609–17.
57. Hauri-Hohl MM, Keller MP, Gill J, Hafen K, Pachlatko E, Boulay T, et al. Donor T-Cell Alloreactivity Against Host Thymic Epithelium Limits T-Cell Development After Bone Marrow Transplantation. Blood (2007) 109:4080–8. doi: 10.1182/blood-2006-07-034157
58. Krenger W, Blazar BR, Holländer GA. Thymic T-Cell Development in Allogeneic Stem Cell Transplantation. Blood (2011) 117:6768–76. doi: 10.1182/blood-2011-02-334623
59. Na I-K, Lu SX, Yim NL, Goldberg GL, Tsai J, Rao U, et al. The Cytolytic Molecules Fas Ligand and TRAIL Are Required for Murine Thymic Graft-Versus-Host Disease. J Clin Invest (2010) 120:343–56. doi: 10.1172/JCI39395
60. Morris H, DeWolf S, Robins H, Sprangers B, LoCascio SA, Shonts BA, et al. Tracking Donor-Reactive T Cells: Evidence for Clonal Deletion in Tolerant Kidney Transplant Patients. Sci Transl Med (2015) 7:272ra10. doi: 10.1126/scitranslmed.3010760
61. Shaffer J, Villard J, Means TK, Alexander S, Dombkowski D, Dey BR, et al. Regulatory T-Cell Recovery in Recipients of Haploidentical Nonmyeloablative Hematopoietic Cell Transplantation With a Humanized Anti-CD2 mAb, MEDI-507, With or Without Fludarabine. Exp Hematol (2007) 35:1140–52. doi: 10.1016/j.exphem.2007.03.018
62. DeWolf S, Sykes M. Alloimmune T Cells in Transplantation. J Clin Invest (2017) 127:2473–81. doi: 10.1172/JCI90595
63. Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, et al. Mechanisms of Donor-Specific Tolerance in Recipients of Haploidentical Combined Bone Marrow/Kidney Transplantation. Am J Transplant (2011) 11:1236–47. doi: 10.1111/j.1600-6143.2011.03566.x
64. Sprangers B, DeWolf S, Savage TM, Morokata T, Obradovic A, LoCascio SA, et al. Origin of Enriched Regulatory T Cells in Patients Receiving Combined Kidney-Bone Marrow Transplantation to Induce Transplantation Tolerance. Am J Transplant (2017) 17:2020–32. doi: 10.1111/ajt.14251
65. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity (2009) 30:899–911. doi: 10.1016/j.immuni.2009.03.019
66. Podestà MA, Binder C, Sellberg F, DeWolf S, Shonts B, Ho S-H, et al. Siplizumab Selectively Depletes Effector Memory T Cells and Promotes a Relative Expansion of Alloreactive Regulatory T Cells In Vitro. Am J Transplant (2020) 20:88–100. doi: 10.1111/ajt.15533
67. Binder C, Sellberg F, Cvetkovski F, Berglund E, Berglund D. Siplizumab, an Anti-CD2 Monoclonal Antibody, Induces a Unique Set of Immune Modulatory Effects Compared to Alemtuzumab and Rabbit Anti-Thymocyte Globulin In Vitro. Front Immunol (2020) 11:592553. doi: 10.3389/fimmu.2020.592553
68. Zuber J, Sykes M. Mechanisms of Mixed Chimerism-Based Transplant Tolerance. Trends Immunol (2017) 38:829–43. doi: 10.1016/j.it.2017.07.008
69. Savage TM, Shonts BA, Obradovic A, DeWolf S, Lau S, Zuber J, et al. Early Expansion of Donor-Specific Tregs in Tolerant Kidney Transplant Recipients. JCI Insight (2018) 3(22):e124086. doi: 10.1172/jci.insight.124086
70. Pilat N, Baranyi U, Klaus C, Jaeckel E, Mpofu N, Wrba F, et al. Treg-Therapy Allows Mixed Chimerism and Transplantation Tolerance Without Cytoreductive Conditioning. Am J Transplant (2010) 10:751–62. doi: 10.1111/j.1600-6143.2010.03018.x
71. Duran-Struuck R, Sondermeijer HP, Bühler L, Alonso-Guallart P, Zitsman J, Kato Y, et al. Effect of Ex Vivo-Expanded Recipient Regulatory T Cells on Hematopoietic Chimerism and Kidney Allograft Tolerance Across MHC Barriers in Cynomolgus Macaques. Transplantation (2017) 101:274–83. doi: 10.1097/TP.0000000000001559
72. Ohdan H, Yang YG, Swenson KG, Kitamura H, Sykes M. T Cell and B Cell Tolerance to GALalpha1,3GAL-Expressing Heart Xenografts Is Achieved in Alpha1,3-Galactosyltransferase-Deficient Mice by Nonmyeloablative Induction of Mixed Chimerism. Transplantation (2001) 71:1532–42. doi: 10.1097/00007890-200106150-00009
73. Ohdan H, Yang YG, Shimizu A, Swenson KG, Sykes M. Mixed Chimerism Induced Without Lethal Conditioning Prevents T Cell- and Anti-Gal Alpha 1,3Gal-Mediated Graft Rejection. J Clin Invest (1999) 104:281–90. doi: 10.1172/JCI6656
74. Shimizu I, Kawahara T, Haspot F, Bardwell PD, Carroll MC, Sykes M. B-Cell Extrinsic CR1/CR2 Promotes Natural Antibody Production and Tolerance Induction of anti-alphaGAL-Producing B-1 Cells. Blood (2007) 109:1773–81. doi: 10.1182/blood-2006-02-002386
75. Kawahara T, Shimizu I, Ohdan H, Zhao G, Sykes M. Differing Mechanisms of Early and Late B Cell Hyporesponsiveness Induced by Mixed Chimerism. Am J Transplant (2005) 5:2821–9. doi: 10.1111/j.1600-6143.2005.01121.x
76. Nikolic B, Lei H, Pearson DA, Sergio JJ, Swenson KG, Sykes M. Role of Intrathymic Rat Class II+ Cells in Maintaining Deletional Tolerance in Xenogeneic Rat–>Mouse Bone Marrow Chimeras. Transplantation (1998) 65:1216–24. doi: 10.1097/00007890-199805150-00013
77. Kawahara T, Rodriguez-Barbosa J-I, Zhao Y, Zhao G, Sykes M. Global Unresponsiveness as a Mechanism of Natural Killer Cell Tolerance in Mixed Xenogeneic Chimeras. Am J Transplant (2007) 7:2090–7. doi: 10.1111/j.1600-6143.2007.01905.x
78. Waffarn EE, Khosravi-Maharlooei M, Vecchione A, Shao S, Vishwasrao P, HÖlzl MA, et al. Mixed Xenogeneic Porcine Chimerism Tolerizes Human Anti-Pig Natural Antibody-Producing Cells in a Humanized Mouse Model. Xenotransplantation (2021) 28:e12691. doi: 10.1111/xen.12691
79. Li HW, Vishwasrao P, Hölzl MA, Chen S, Choi G, Zhao G, et al. Impact of Mixed Xenogeneic Porcine Hematopoietic Chimerism on Human NK Cell Recognition in a Humanized Mouse Model. Am J Transplant (2017) 17:353–64. doi: 10.1111/ajt.13957
80. Sykes M, Sachs DH. Transplanting Organs From Pigs to Humans. Sci Immunol (2019) 4:eaau6298. doi: 10.1126/sciimmunol.aau6298
81. Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R, et al. Induction of Human T-Cell Tolerance to Porcine Xenoantigens Through Mixed Hematopoietic Chimerism. Blood (2004) 103:3964–9. doi: 10.1182/blood-2003-10-3697
82. Abe M, Cheng J, Qi J, Glaser RM, Thall AD, Sykes M, et al. Elimination of Porcine Hemopoietic Cells by Macrophages in Mice. J Immunol (2002) 168:621–8. doi: 10.4049/jimmunol.168.2.621
83. Basker M, Alwayn IP, Buhler L, Harper D, Abraham S, Kruger Gray H, et al. Clearance of Mobilized Porcine Peripheral Blood Progenitor Cells Is Delayed by Depletion of the Phagocytic Reticuloendothelial System in Baboons. Transplantation (2001) 72:1278–85. doi: 10.1097/00007890-200110150-00017
84. Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha Signaling in Xenograft Rejection by Macrophages. Proc Natl Acad Sci USA (2007) 104:5062–6. doi: 10.1073/pnas.0609661104
85. Tena AA, Sachs DH, Mallard C, Yang Y-G, Tasaki M, Farkash E, et al. Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human Cd47. Transplantation (2017) 101:316–21. doi: 10.1097/TP.0000000000001267
86. Watanabe H, Sahara H, Nomura S, Tanabe T, Ekanayake-Alper DK, Boyd LK, et al. GalT-KO Pig Lungs Are Highly Susceptible to Acute Vascular Rejection in Baboons, Which May Be Mitigated by Transgenic Expression of Hcd47 on Porcine Blood Vessels. Xenotransplantation (2018) 25:e12391. doi: 10.1111/xen.12391
Keywords: chimerism and tolerance, kidney, transplantation, mixed chimerism, clinical protocol
Citation: Podestà MA and Sykes M (2022) Chimerism-Based Tolerance to Kidney Allografts in Humans: Novel Insights and Future Perspectives. Front. Immunol. 12:791725. doi: 10.3389/fimmu.2021.791725
Received: 08 October 2021; Accepted: 15 December 2021;
Published: 05 January 2022.
Edited by:
Thomas Wekerle, Medical University of Vienna, AustriaReviewed by:
Gerald Brandacher, Johns Hopkins University, United StatesCopyright © 2022 Podestà and Sykes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Megan Sykes, bWVnYW4uc3lrZXNAY29sdW1iaWEuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.