- 1Department of Psychiatry, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2 The Key Laboratory of Mental Disorder’s Management in Zhejiang Province, Hangzhou, China
- 3Brain Research Institute of Zhejiang University, Hangzhou, China
- 4MOE Frontier Science Center for Brain Science & Brain-Machine Integration, Zhejiang University, Hangzhou, China
- 5Department of Neurology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 6NHC Key Laboratory of Diagnosis and Treatment on Brain Functional Diseases, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 7Institute of Psychiatry, Wenzhou Medical University, Wenzhou, China
- 8Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences and Yunnan Province, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, China
Tetratricopeptide repeat and ankyrin repeat containing 1 (TRANK1) is a robust risk gene of bipolar disorder (BD). However, little is known on the role of TRANK1 in the pathogenesis of BD and whether the gut microbiota is capable of regulating TRANK1 expression. In this study, we first investigated the serum mRNA level of TRANK1 in medication-free patients with a depressive episode of BD, then a mice model was constructed by fecal microbiota transplantation (FMT) to explore the effects of gut microbiota on brain TRANK1 expression and neuroinflammation, which was further verified by in vitro Lipopolysaccharide (LPS) treatment in BV-2 microglial cells and neurons. 22 patients with a depressive episode and 28 healthy individuals were recruited. Serum level of TRANK1 mRNA was higher in depressed patients than that of healthy controls. Mice harboring ‘BD microbiota’ following FMT presented depression-like phenotype. mRNA levels of inflammatory cytokines and TRANK1 were elevated in mice hippocampus and prefrontal cortex. In vitro, LPS treatment activated the secretion of pro-inflammatory factors in BV-2 cells, which was capable of upregulating the neuronal expression of TRANK1 mRNA. Moreover, primary cortical neurons transfected with plasmid Cytomegalovirus DNA (pcDNA3.1(+)) vector encoding human TRANK1 showed decreased dendritic spine density. Together, these findings add new evidence to the microbiota-gut-brain regulation in BD, indicating that microbiota is possibly involved in the neuropathogenesis of BD by modulating the expression of TRANK1.
Introduction
Bipolar disorder (BD) is a recurrent, debilitating mood disorder with a high heritability (1). Although the exact etiology of BD is sophisticated, the dominant role of both gene and environmental factors on the onset and development of BD is widely accepted (1, 2). With the technological advances in molecular biology, susceptible genes related to BD have been increasingly identified (3, 4). For example, several genome-wide association studies (GWAS) and subsequent independent verifications have indicated that tetratricopeptide repeat and ankyrin repeat containing 1 (TRANK1), located on the short arm of chromosome 3 (3p22.2), is a robust risk gene of BD (3–6). In postmortem brains of BD subjects, the expression of TRANK1 was elevated compared to that of healthy individuals (7). TRANK1 protein is secreted mainly by immunocytes and is widely distributed in body tissues, including hippocampus, amygdala and other brain regions. Although the implications of TRANK1 gene in BD remained largely unclear, a newly published large-scale analyses of mRNA co-expression network revealed that genes closely interact with TRANK1, such as glycogen synthase kinase-3 (GSK-3α/β), were engaged in the modulation of synaptic plasticity, neural growth, as well as circadian rhythm (6). More recently, an up-stream regulatory role of GSK-3α/β on TRANK1 transcription has been unraveled (8), partially via the activation the transcription factor, CCAAT/enhancer-binding protein-α (C/EBP-α). Blockage of GSK-3α/β pathways or direct suppression of GSK-3α/β expression significantly led to reduced expression of TRANK1 in U-251 human glioblastoma cells (8). Therefore, further investigations on the interactome of TRANK1 with other genetic and environmental factors relevant to BD help to uncover the pathogenesis of this intractable psychiatric disorder.
Recently, microbiota inhabiting in the human gastrointestinal tract has been recognized as a pivotal environmental factor in regulating physical and mental well-being (9, 10). In previous studies, we have characterized the alterations in diversity and compositions of gut microbiota in patients with a depressive episode of BD when compared to healthy individuals (11, 12). Classification models derived from specific bacterial species not only had the potential to distinguish bipolar depression from health individuals or unipolar depression (11, 12), but also was capable of predicting the efficacy of mood stabilizing treatment (11). Notably, we found that compared to healthy individuals, most bacteria enriched in patients with bipolar depression belonged to the Bacteroides and Flavobacterium (11), both of which were gram-negative bacterial family that could produce lipopolysaccharide (LPS). Interestingly, a negative correlation between gut microbial diversity and methylation of the aryl hydrocarbon receptor nuclear translocator-like gene (ARNTL), a molecular clock gene linked to circadian rhythm, was revealed in BD patients (13). In our recent review, we have systematically discussed a hypothesis that gut microbe-derived LPS could influence the host TRNAK1 expression in BD (14). These preliminary findings indicate the involvement of the microbiota-gut-brain regulation in BD. However, currently available studies on gut microbiota in BD were mainly genetically sequencing-based, or performed correlation analyses between indices of gut microbiota and clinical parameters, thus precluding the further interpretations of findings. Also, animal models focusing on the mechanistic pathways linking the microbiota to the brain are absent in BD studies.
The microbiota-gut-brain axis is known to operate in a bidirectional regulation pattern, which mainly consists of the metabolic, immune, endocrine and automatic nerves pathways (15). Notably, a neuroinflammatory basis underlying the development and progression of BD has been recognized recently (16, 17). Proinflammatory mediators in the peripheral, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), as well as neuroinflammatory markers in the central nervous system (activated microglia), are found to be elevated not only in the acute mood episodes, but also in the remission phase of BD (16, 18). In previous studies, we also found abnormal expression of immune checkpoint inhibitors on peripheral CD8+T cells, such as T cell immunoglobulin and mucin domain 3 (TIM-3), indicating potential disturbances in cellular immunity in BD individuals (19, 20). Moreover, the neuroinflammatory modifications may interact with or be affected by the unbalanced kynurenine pathways and reduction in the expression of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) (16, 21). Kynurenine aminotransferase-2 (KAT-2) is the key role enzyme regulating the production of neuroprotective kynurenic acid (KYNA) from kynurenine (KYN) in the tryptophan metabolism (22). Intriguingly, a most recently published study provided robust evidence that microbial biomolecules from the intestinal tract could be transferred to brain and other organs via outer membrane vesicles (23). Based on these findings, we speculate that the regulatory role of gut microbiota on host genes in BD may link to a complicated neuroinflammatory mechanism.
In this study, we first explored the serum expression of TRANK1 mRNA in patients with BD depression, then constructed a mice model via fecal microbiota transplantation (FMT) to investigate the impact of gut microbiota on TRANK1 expression and neuroinflammation. We further in vitro examined the effects of LPS treatment on microglia, and inflammatory stimuli on the TRANK1 mRNA expression in neurons. The impact of TRANK1 overexpression on the morphology of neurons was also investigated via transfection with plasmid Cytomegalovirus DNA (pcDNA3.1(+)) vector encoding human TRANK1. Collectively, this study helps to reveal that gut microbiota may participate in the BD pathogenesis by interacting with a robust BD risk gene.
Methods
In accordance with the Helsinki Declaration, this study was approved by the Institutional Review Board of the First Affiliated Hospital, Zhejiang University School of Medicine (#2017-397). Written informed consent was obtained from all participants before enrollment.
Participants
22 patients meeting the criteria for a depressive episode of bipolar disorder according to the DSM-IV-TR were recruited from the Psychiatry Department of our hospital. The diagnosis was further confirmed by an experienced clinical psychiatrist using the Mini International Neuropsychiatric Interview (24). 17-item Hamilton Depression Rating Scale (HDRS-17) (25) as used for assessing the severity of depression and Young Mania Rating Scale (YMRS) (26) for assessing mania. In our study, an HDRS-17 score ≥ 14 was considered as a current depressive episode. All BD candidates were required to be treatment-naive or drug-free for ≥ 3 months and had no comorbidity with any other psychiatric disorder. 28 healthy individuals (HCs) without a history of any psychiatric disorder were recruited. Exclusion criteria for all subjects included: a) acute or chronic infection; b) autoimmune diseases or other systematic diseases associated with immune dysregulation; c) severe physical diseases (e.g., cancer, diabetes); d) consumption of prebiotics, probiotics or antibiotics within 1 month prior to recruitment; e) pregnant or lactating females or in menstruation.
Peripheral Expression of TRANK1
Peripheral venous blood (1ml) was collected from all participants in a fasting state at 7:00~8:00 a.m. and immediately used for detecting mRNA expression. Total RNA from human blood was isolated using Spin Column Blood Total RNA Purification Kit according to the manufacturer’s instruction (Sangon Biotech; Order No. B518653). Reverse transcription was performed using 4×EZscript Reverse Transcription Mix II (EZBioscience; Cat. No.EZB-RT2GQ) in a 20 ul reaction volume. TRANK1 mRNA was detected by QuantStudio 5DX Real-Time PCR System using SYBR Green Fast qPCR Mix (ABclonal, Wuhan, China). Each sample was tested three times and GAPDH was used as an internal control. Forward primer (5’-3’) for TRANK1 (human) was CAGCACTCCACATCTTTCTAGA and reverse primer (5’-3’) was TTGAGGTAGTCGAATTCAGTGG.
FMT and Behavioral Tests
To determine whether gut microbiota from patients with a depressive episode of BD was sufficient to cause depression-like behaviors in mice, FMT from HCs and untreated patients were performed. The animal experiment protocol was approved by the Hospital Animal Ethical Committee (Approval No. #2019-6).
FMT Procedures
At baseline, fecal samples were collected from all participants. Fecal samples from untreated patients with a depressive episode of BD (n = 10, age 16–43 years) and HCs (n = 10, age 16–40 years) were randomly chosen to colonize the guts of mice. The demographic and clinical profiles of these participants were shown in Supplemental Table 1. Fecal samples for FMT were handled under anaerobic conditions and detailed procedures for preparing feces were previously described.[81] Each fecal sample (0.1 g) was suspended with 1.5 ml of reduced sterile phosphate-buffered saline, and pools were made from equal volumes of donor suspensions. Adult (6-8-week-old) male Kunming mice were colonized with pooled samples derived from either BD patients or HCs. Different groups of recipient mice were separately bred in different gnotobiotic isolators to prevent contamination of gut microbiota. Within each individual gnotobiotic isolator, either ‘BD microbiota’ or ‘healthy microbiota’ recipient mice were bred in different cages (five mice per cage). Mice were weighted at the beginning of FMT experimentation and immediately prior to sacrifice of the mice. The behavioral tests (including OFT and FST) were performed on week 1 and 2 after fecal transplantation. Brain samples were collected immediately when the mice were sacrificed and snap-frozen in liquid N2 and stored at -80°C.
Mice Behavioral Tests
Before initiation of the experiments, mice were kept in flexible film gnotobiotic isolators. Mice were fed the same chow and water with autoclaved treatment under a 12-h light-dark cycle (lights on at 07:30 AM), a constant temperature of 21-22°C and humidity of 50-60%. Before each behavioral test, mice were transferred to the specialized experimental room for acclimation ≥ 1 h prior to the test. Experimenters who carried out these tests were blind to the animal groupings between 08:00 and 17:00. A video-computerized tracking system (SMART, Panlab, Barcelona, Spain) was used to videotape and quantify the process of behavioral tests.
Open-Field Test (OFT) and Forced Swimming Test (FST)
The detailed procedures for OFT and FST were previously described.[13] In OFT, the total motion distance was considered to reflect the degree of locomotor activity, while the proportion of distance spent in the center (inner 25% part of the total surface area) was considered as an index of anxiety-like behavior. In FST, immobility was defined as the absence of all motion with the exception of movements to keep the mouse’s head over water surface. Test sessions lasted for 6 minutes and the latter 5 minutes scored for immobility, which was considered as a proxy of depression-like behavior.
Expression of Molecules of Interest in Mice Brain Following FMT
Total RNAs from mice brain tissues were isolated using Trizol reagent according to the manufacturer’s instructions (Invitrogen, USA). Reverse transcription was performed using an ABScript II cDNA First Strand Synthesis Kit (ABclonal, Wuhan, China) in a 20 ml reaction volume. The genes of interest were detected by QuantStudio 5DXReal-Time PCR System using SYBR Green Fast qPCR Mix (ABclonal, Wuhan, China). Each sample was tested three times and GAPDH was used as an internal control. Primers for GAPDH, IL-1β, IL-6, IFN-1β, TIM-3, KAT-2, TRANK1 and BDNF are listed in Supplemental Table 2.
Effects of LPS Treatment on the TRANK1 Expression in Neurons
BV-2 culture and Treatment
The mice BV-2 cell line (Invitrogen, USA) was cultured in six-well plates at 37°C with 5% CO2 incubator, supplemented by high glucose DMEM medium with 10% FBS. BV-2 were stimulated by LPS (100ng/ml) for 24h when cells proliferated to 70~80%. Then, cell supernatant was collected for stimulating primary CNS neuron, and BV-2 were used to extract RNA for examining inflammatory cytokines levels (IL-1β, IL-6 and TNF-α).
CNS Neuron Cultures and Treatment
Sprague Dawley rats were anesthetized and euthanized at E18 days. Hippocampus of fetal rats was immediately dissected and digested by trypsin into a single-cell suspension. Hippocampus neurons were counted (0.02*106 cells/cm2) and seeded in six-well plates coated with poly-D-lysine (10 μg/mL), supplemented by neuro-basal medium with 2% B27 (Invitrogen, USA), 2.0 mM glutamax and 2.5% FBS. The neurons were cultured at 37°C with 5% CO2 to day 21 and then were stimulated by the previously LPS-treated BV-2 suspension for 24h. TRANK1 gene expression in hippocampus neurons after treatment were measured by RT-PCR.
RT-PCR
The steps of RT-PCR in BV-2 cells and hippocampus neurons were in accordance with the section “Expression of molecules of interest in mice brain following FMT”. Each sample was tested three times and β-actin was used as an internal control. Primers for IL-1β, IL-6, TNF-α and TRANK1 are given in Supplemental Table 3.
TRANK1 Overexpression in Primary Cortical Neurons
TRANK1 Transfection
To determine the impact of TRANK1 overexpression on neuronal morphology, pcDNA3.1(+) vector encoding human TRANK1 with a C-terminus FLAG-tag was constructed. Integrity of the recombinant was verified by Sanger sequencing. Given that signals of FLAG fluorophore alone failed to provide ideal resolution for analyzing dendritic spine structures under our experimental condition, the Venus vector encoding the EGFP protein was co-transfected in all groups. Briefly, the recombinant constructs for TRANK1 or control vectors (i.e., empty pcDNA3.1) were respectively transfected into rat neurons together with Venus at days in vitro (DIV) 14 using Lipofectamine 3000 (Life Technologies) according to the manufacturer’s protocol. Confocal analyses were performed 72 hours after the transfection.
Neuronal Morphology Analysis
The transfected neurons were fixed with PBS (4% paraformaldehyde plus 4% sucrose) at room temperature and stained with antibodies against FLAG (Rabbit monoclonal FLAG antibody, CST, #14793S) and GFP (Chicken polyclonal GFP antibody, Abcam, #ab13970). Fluorescence positive neurons were randomly selected for images captured with an LSM 880 Basic Operation (Carl Zeiss) using consistent acquisition parameters. The dendritic spine analyses were then carried out as previously described. In brief, Neuron Studio was used to semi-automatically analyze spines on secondary and tertiary dendrites. Each experimental group contained at least 15 neurons with satisfactory demonstration of at least two dendrites for averaged analyses.
Statistical Analysis
Clinical and experimental data generated in this study were analyzed with SPSS 20.0 Statistical Package (IBM, IL, USA). Categorical data was conducted with chi-square test, while measurement data was calculated with independent sample t-test (two-tailed). To analyze the neuronal morphology following TRANK1 transfection, two-tailed t-test and two-way ANOVA (multiple comparisons using Fisher’s LSD) were conducted to calculate the statistical differences between two experimental conditions. P < 0.05 was set as statistically significant.
Results
Demographic and Clinical Characteristics of Participants
22 patients with a depressive episode of BD and 28 HCs were included in this study. No significant difference was found in age, sex or BMI between these two groups (all P > 0.05). BD patients scored averagely at 26.64 for HDRS-17 and 20.05 for HAMA. The detailed demographic and clinical characteristics for participants were displayed in Table 1.
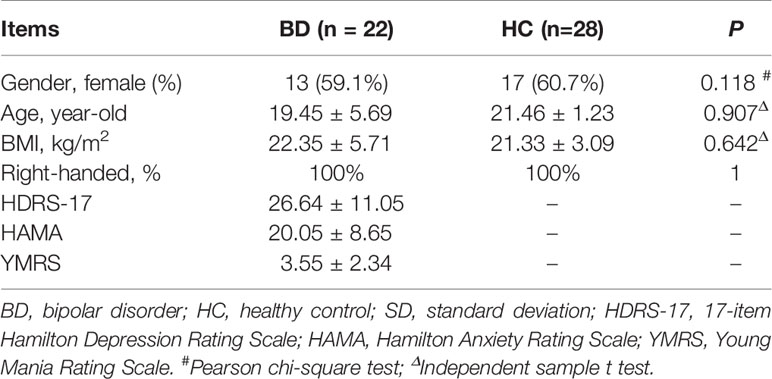
Table 1 Demographic and clinical characteristics of participants enrolled for TRANK1 mRNA test (mean ± SD).
Elevated Serum TRANK1 mRNA Expression in Untreated BD Patients
Compared to HCs, patients with BD depression showed an elevated level of TRANK1 mRNA in peripheral blood (P = 0.002, Figure 1).
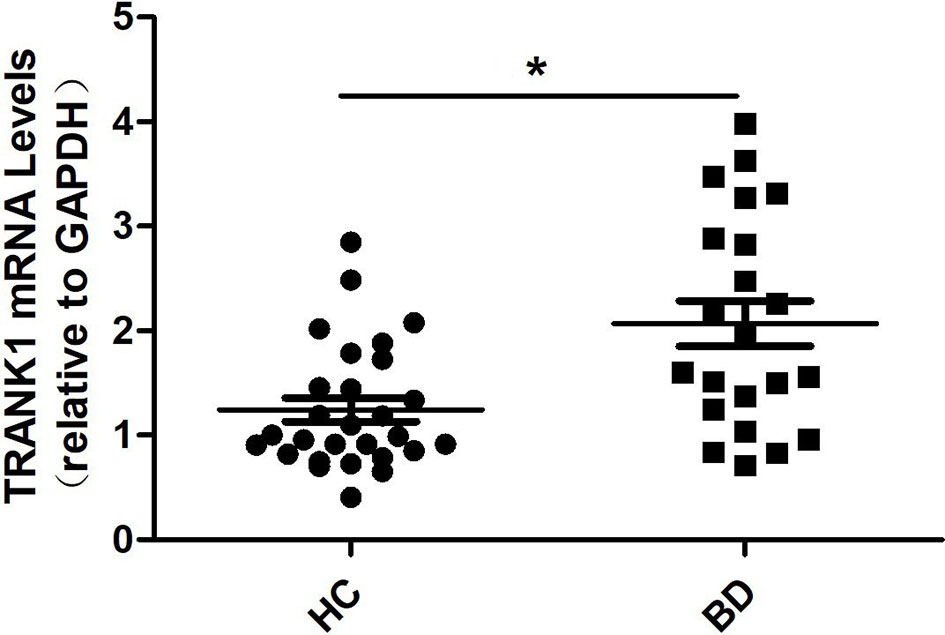
Figure 1 Peripheral blood levels of TRANK1 mRNA by qRT-PCR in patients with a depressive episode of BD (n = 22) and healthy controls (n = 28). *p = 0.002.
Depression-Like Behavior in “BD Microbiota” Recipient Mice
Mice were transplanted with feces from either unmedicated patients with BD depression or healthy controls to verify behavioral consequences. Although no significant differences were found in OFT central distance (P = 0.289), central distance (P = 0.336), central time (P = 0.422) or percentage (P = 0.375) between the two groups (Figure 2), FST immobility time (P < 0.001) and percentage (P < 0.001) were significantly increased in “BD microbiota” recipient mice than “healthy microbiota” recipient mice (Figure 2).
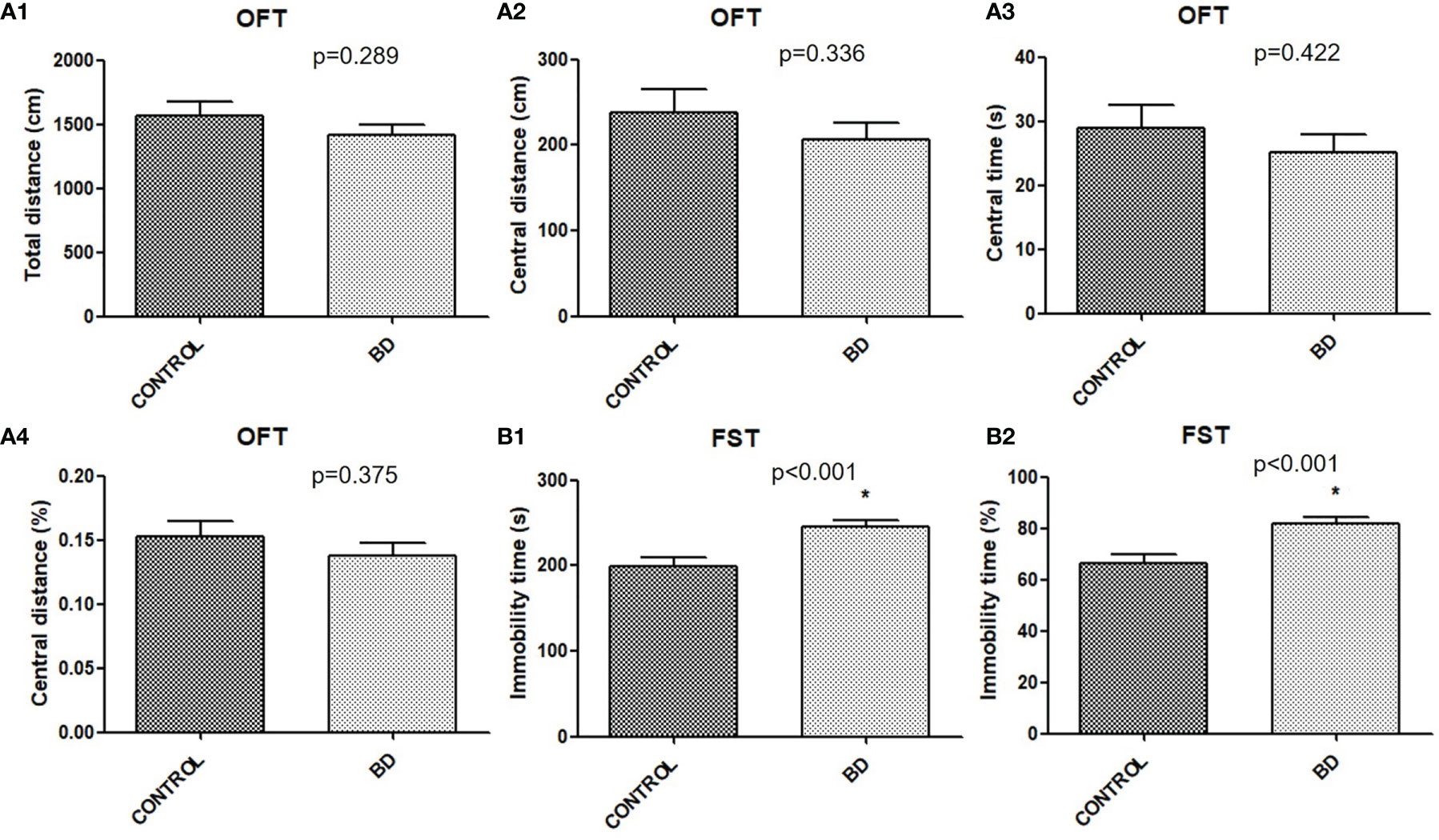
Figure 2 Behavioral consequences of mice transplanted with fecal microbiota from untreated BD patients or healthy controls (BD mice, n=40; control mice, n=22). (A) OFT. The total distance (cm), central distance (cm), central time (s) and central time percentage (%), were measured (A1-A4). (B)FST. The immobility time (s), and immobility time percentage (%), were measured (B1, B2). All data were presented as means ± SEM. *p < 0.001 using independent t tests. FST, forced swimming test; OFT, open field test.
Activated Neuroinflammation and Enhanced Expression of TRANK1 mRNA in Mice Brain After FMT
We further examined the influence of FMT on brain mRNA expressions of molecules linking to neuroinflammation, neurotransmitter production and neural growth. Compared to control mice, “BD” mice exhibited increased mRNA expressions of IL-6 in the corpus striatum (P < 0.05), IL-1β and IFN-1β in the prefrontal cortex and corpus striatum (all P < 0.05), and KAT-2 in hippocampus (P < 0.01). Increased mRNA level of BDNF was observed in the hippocampus of BD mice (P < 0.001). Of particular note, TRANK1 mRNA expression in the hippocampus (P < 0.01) and prefrontal cortex (P < 0.05) was elevated in “BD” mice (Figure 3).
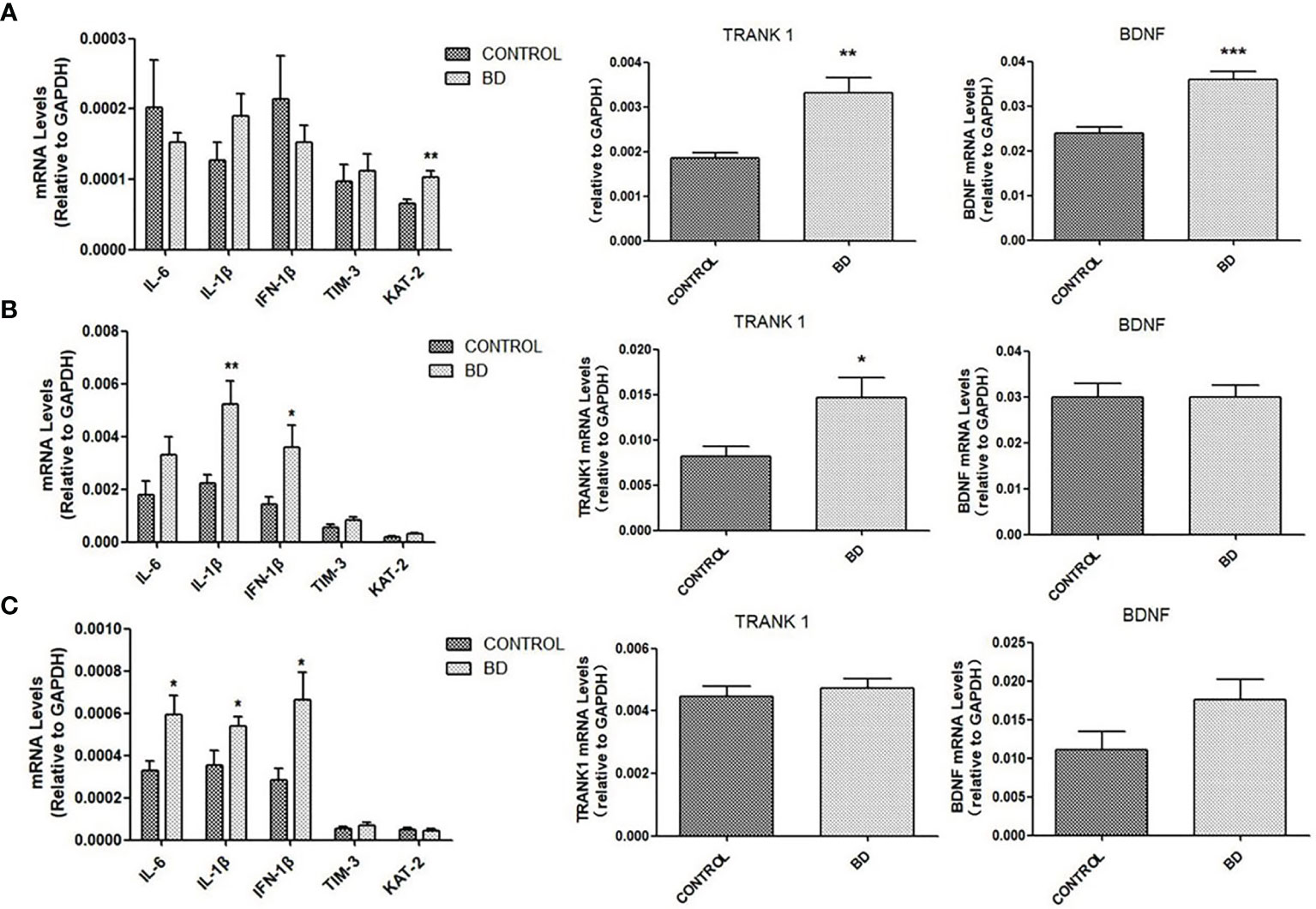
Figure 3 mRNA levels of IL-6, IL-1β, IFN-1β, TIM-3, KAT-2, TRANK1 and BDNF measured by qRT-PCR in hippocampus (A), prefrontal cortex (B) and corpus striatum (C) of mice colonized with fecal microbiota from healthy controls or untreated patients with a depressive episode of BD (n=10 mice in each group) at the 4th week after fecal microbiota transplantation. All data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 using independent sample t-test.
LPS Treatment Activates Neuroinflammation and Upregulates TRANK1 Expression in Neurons
To verify the effects of gut microbe-derived components on microglia and neurons, we performed a study in vitro with LPS, a typical component of gram-negative bacteria. Following LPS treatment, the mRNA levels of proinflammatory factors in BV-2 cells, including IL-1β, IL-6 and TNF-α, were significantly promoted (all P < 0.05, Figure 4A). When stimulated by LPS-treated BV-2 cell supernatant, hippocampus neurons of rats displayed an increased level of TRANK1 mRNA (P < 0.05, Figure 4B).
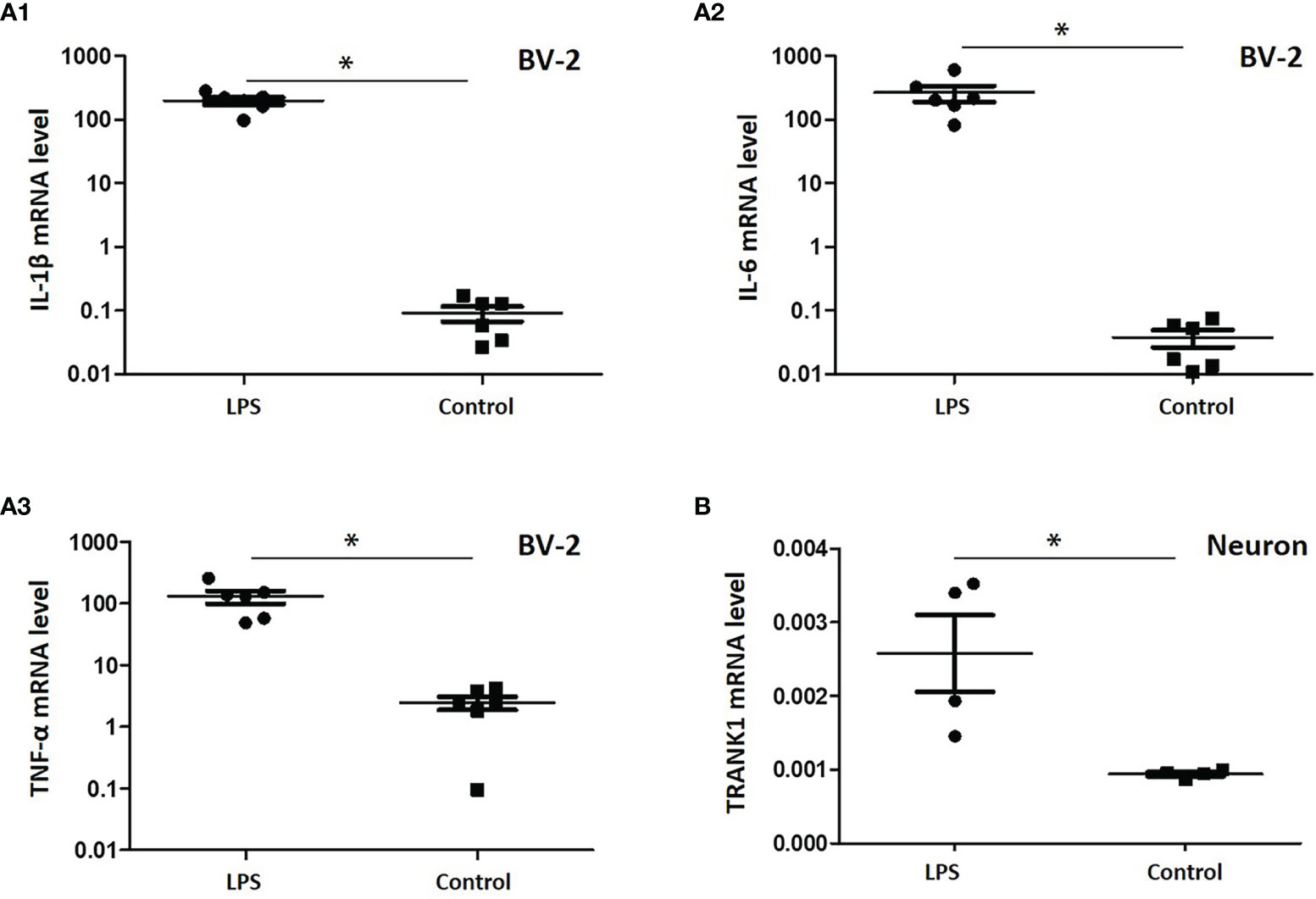
Figure 4 Expression of proinflammatory factors in BV-2 cells treated with LPS and the effects of neuroinflammation on TRANK1 expression in neurons. (A) A1, A2, A3 represented the mRNA levels of IL-1β, IL-6 and TNF-α, respectively; (B) The effects of LPS-treated BV-2 supernatant on the expression of TRANK1 mRNA in neurons. *p < 0.05 using independent sample t-test.
Overexpression of TRANK1 in Neurons Leads to Decreased Spine Density
Our results indicated that higherTRANK1 expression was possibly linked to depressive episodes in BD. To identify whether the increased expression of TRANK1 causes any functional consequences, we examined its effects on the morphology and density of dendritic spines, a potential endophenotype for BD [43]. Primary cortical neurons transfected with TRANK1-overexpression vector exhibited significantly altered densities of dendritic spines compared with those transfected with the control vector (Figure 5A). In brief, the density of total dendritic spines significantly decreased in neurons overexpressing TRANK1 (P < 0.001, two-way t-test, Figure 5B), probably due to the significant reduction of the densities of thin and mushroom spines compared with neurons in the control group (both P < 0.01, two-way ANOVA, Figure 5B), whereas stubby spine did not alter obviously. This result is consistent with the previously reported decrease of dendritic spine density in the postmortem brains of BD patients (27), suggesting that TRANK1 might participate in the pathogenesis of BD (depressive episodes) via modulating this pivotal physiological feature.
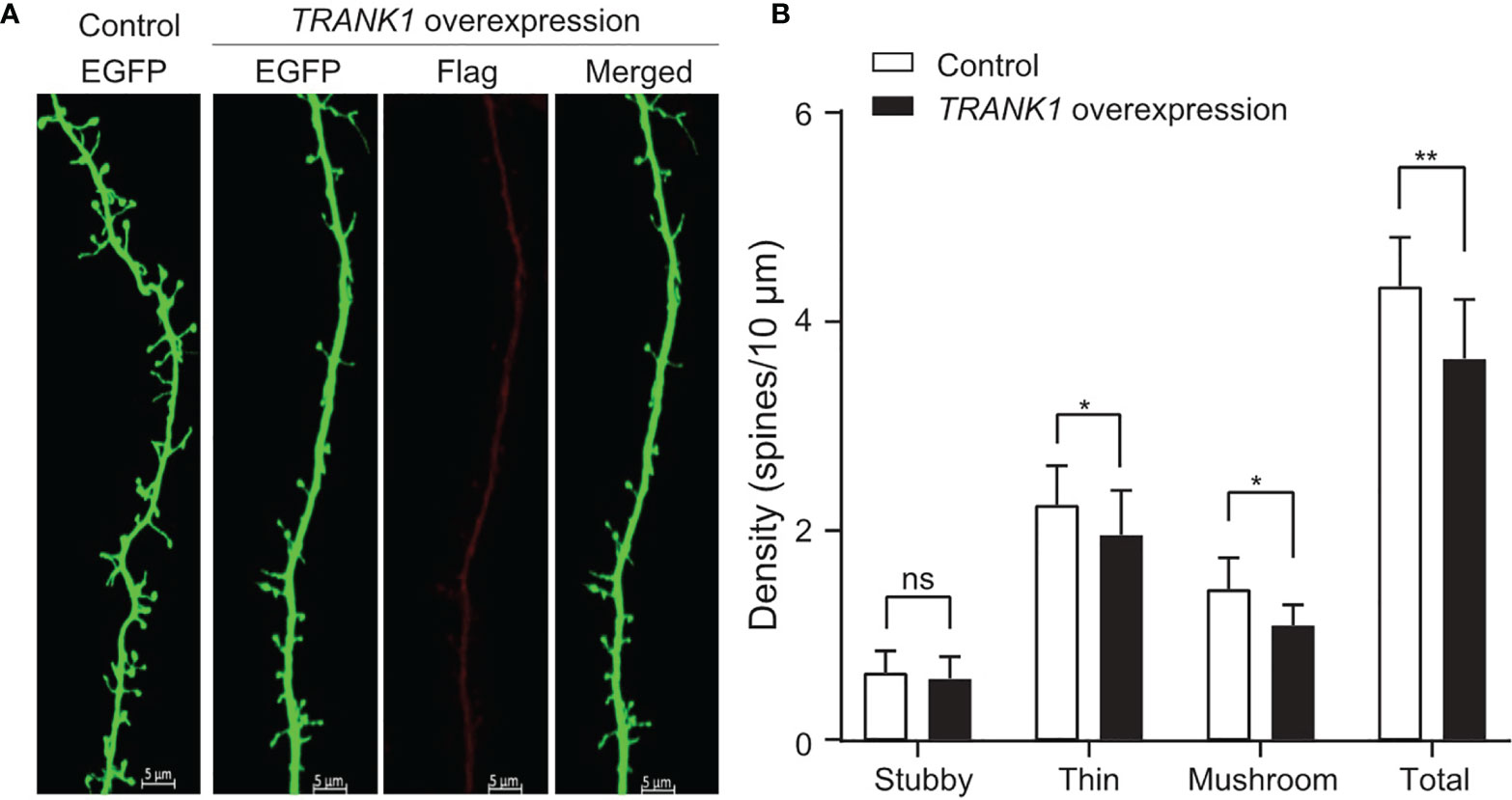
Figure 5 TRANK1 overexpression decreases dendritic spine density in rat primary cortical neurons. (A) Representative microphotographs of dendrites from neurons transfected with either control or TRANK1 overexpression vectors plus the Venus vector (which encodes EGFP protein) at DIV14. Scale bar: 5 µm. (B) Density of total, stubby-shaped, thin-shaped, and mushroom-shaped spines in neurons overexpressing TRANK1 compared with control neurons. Dendritic spine density was quantified as the number of spines normalized to 10 μm of dendritic length (**p < 0.001, *p < 0.01, n=15/16 neurons per condition). ns, not significant.
Discussion
In the current study, an upregulated circulating TRANK1 expression was firstly reported in patients with BD depression. Accumulating large-scale GWAS studies have provided adequate evidence that TRANK1 is a robust susceptible gene of BD (3–6). Our finding further supports the involvement of TRANK1 in the BD pathogenesis. The FMT findings in our study revealed that fecal microbiota from patients with BD depression were sufficient to elicit depression-like behavior in mice, which may link to activated neuroinflammation and an elevated expression of TRANK1. It is noteworthy that TRANK1 overexpression resulted in decreased dendritic density in primary neurons, which may comprise neuronal morphological basis of depressive episodes in BD.
In recent years, the implications of the microbiota-gut-brain axis in the neuropsychiatric disorders have been gradually uncovered (28). The use of prebiotics, probiotics and FMT as adjuvant therapies for mentally ill patients is becoming a research hotspot (28). Some researchers even have proposed the study protocol for verifying the efficacy and safety of FMT in bipolar patients during depressive episodes (29). Nonetheless, most of the explorations are still limited to animal experiments. Previous studies have shown gut microbiota from mentally ill patients were sufficient to elicit disease-specific behavioral patterns in recipient mice (30–33). However, no study has ever explored this phenomenon in BD patients. To address the effects of BD microbiota on brain function and behavior, “BD microbiota” or “healthy microbiota” recipient mice were designed to observe the behavioral consequences in our study. Compared to “healthy microbiota” recipient mice, increased immobility time in FST was observed in “BD microbiota” recipient mice, which was interpreted as depression-like behaviors.
To further determine the brain molecular mechanisms underlying the behavioral changes, mRNA expression levels of inflammatory factors, BDNF, KAT-2 and TRANK1 were measured in the hippocampus, prefrontal cortex and corpus striatum of recipient mice. We found an up-regulation of immune mediators, such as IL-6, IL-1β and IFN-1β. Neuroinflammation is recently considered to be involved in the pathogenesis of major psychiatric disorders, including schizophrenia, BD and major depressive disorder (MDD) (34–36). Similarities in the pattern of cytokine networks indicate a common underlying immune dysfunction of these psychiatric disorders (36). Although no inflammatory biomarker was independently capable of differentiating mood phases of BD, a combination of high-sensitivity C-reactive protein/IL-6, BDNF/TNF-α or soluble TNF-α receptor 1 was identified to be mood phase-specific in BD (37). In our study, an increased level of IL-6 and IL-1β indicated the activation of neuroinflammation due to fecal microbiota transplantation from patients with a depressive episode of BD. The actions of IFN-1β, however, include induction of regulatory mediators, decreasing the secretion of proinflammatory cytokines and modulating cell trafficking across the blood–brain barrier (BBB) (38). Therefore, a parallel activation of both proinflammatory and immuno-suppressive processes reflect a compensatory mechanism of neuroinflammation in BD. In addition, increased mRNA levels of BDNF and KAT-2 were detected in “BD microbiota” recipient mice. BDNF is the dominant neurotrophin in brain, and the BDNF/TrkB signaling pathway plays an important role in synaptic maturation, plasticity, neuronal growth and survival (39). Available findings in relevant to peripheral BDNF levels amongst BD patients were inconsistent (40–42). In particular, longer duration of illness was associated with high serum BDNF level (40). Acute stress treatment may upregulate the expression of hippocampal BDNF mRNA (43). Therefore, increased hippocampal BDNF transcripts in recipient mice were possibly caused by the acute colonization of “BD microbiota”. However, some researchers pointed out that the neurodevelopmental trait of BDNF could be attenuated by the underlying neuroinflammation processes (42). KAT-2 is a key enzyme catalyzing the synthesis of KYNA from KYN, which was known as the tryptophan metabolic pathway (44). The tryptophan catabolism can be activated upon inflammatory stimuli, such as viral invasion, bacterial LPS, and IFN stimulation (45, 46). Therefore, in “BD microbiota” recipient mice, CNS inflammatory status caused by LPS-induced immune activation and enhanced IFN-1β stimulation, eventually promoted the expression of KAT-2 and increased the concentration of KYNA. Abnormal KYNA level is implicated in various neuropsychiatric disorders, including schizophrenia (31, 44), Alzheimer’s disease and other illnesses (44). In patients with BD, an increased CSF level of KYNA was also reported in previous studies and was associated with manic episodes and psychotic features (47, 48). These findings indicated the dysregulation of KYN-KYNA metabolic pathway was possibly linked to BD pathophysiology.
The interplay between gut microbiota and host genes expression has been rarely investigated in the microbiota-gut-brain regulation. Inspiringly, our study is the first to report that gut microbiota can influence the CNS expression of TRANK1 in the hippocampus and prefrontal cortex. Expression of TRANK1 was observed under different pathological conditions, such as neuroinflammation, disrupted formation and functioning of blood vessels in the BBB (49). In a rat model of psychosis, one-week social isolation led to increased mRNA expression of TRANK1 in the prefrontal cortex and other specific genes involved in neuroinflammation, formation and integrity of the BBB, and cerebral blood vessel morphogenesis (49). This is consistent with our findings that CNS inflammatory profiles are elevated in “BD microbiota” recipient mice. Moreover, gut microbial dysbiosis was associated with impaired integrity and increased permeability of the BBB (50, 51). Loss of BBB integrity was implicated in neuropsychiatric diseases, such as depression, bipolar disorder and schizophrenia (51). A compromised BBB may facilitate the entrance of gut microbes-derived components, such as LPS, into the CNS and activate the neuroinflammation. In accordance with previous studies (52), we found that in vitro LPS stimuli could promote the transformation of microglia into the M1 phenotype and the secretion of proinflammatory factors (IL-1, IL-6 and TNF-α), which was capable of upregulating the expression of TRANK1 in neurons. Furthermore, we showed that overexpression of TRANK1 resulted in loss of dendritic spine density in cortical primary neurons. In patients with severe mental illnesses, dendritic spine abnormalities in the prefrontal cortex have been considered to be neurobiological mechanisms underlying these diseases (53, 54). Impairment of the dendritic spine development could lead to cognitive deficits and behavioral abnormalities in BD (53, 55). Deficits in dendritic spine morphogenesis link to synaptic dysfunction and synapse loss (56). In other words, overexpression of TRANK1 is possibly associated with brain dysfunction by hampering the growth of dendritic spines and disrupting synaptic functions.
Several limitations in this study need to be mentioned. First, although we found that circulating TRANK1 mRNA level was elevated in patients with BD depression, no patients with manic episodes or other major psychiatric disorders, such as schizophrenia and MDD, were included in this study. Therefore, we cannot conclude that the upregulated expression of TRANK1 was an exclusive trait of BD depression. Another weakness of our study is the absence of manic/hypomanic and remissive participants, we thus are unable to compare the impacts of different conditions on the gut microbiota, as well as the behavioral patterns following FMT. Third, lack of examining the gut microbiota in mice weakened the stringency of this study, though different conditions in the mice brain existed. Fourth, although we observed a regulatory role of LPS-induced pro-inflammatory environment on TRANK1, further explorations via inhibiting the LPS-toll-like receptors interactions and its effects on TRANK1 expression are also needed.
Taken together, the current study revealed an upregulated circulating TRANK1 expression in patients with BD depression and a potential regulatory role of gut microbiota on neuroinflammation and TRANK1 expression in the brain. Based on the gene × microbiota perspective, our study helps to better understand the role of TRANK1 in the BD pathogenesis and its involvement in the gut-microbiota-brain regulation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the First Affiliated Hospital, Zhejiang University School of Medicine. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. The animal study was reviewed and approved by Animal Ethical Committee of First Affiliated Hospital, Zhejiang University School of Medicine.
Author Contributions
Designed the experiments: SH. Collected the clinical samples: JL, PFZ, CX, LW, XG, DZ, YC, and HH. Performed the animal and in vitro cell study: PFZ, ML, JJ, YL, HL, XC, and PZ. Performed the analysis and drafted the manuscript: JL, PZF, JJ, and TM. Revised the manuscript for intellectual content: SH. All authors contributed to the article and approved the submitted version.
Funding
This study was granted by the National Natural Science Foundation of China (81971271), and the Natural Science Foundation of Zhejiang Province (LQ20H090013). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.789647/full#supplementary-material
References
1. McIntyre RS, Berk M, Brietzke E, Goldstein BI, Lopez-Jaramillo C, Kessing LV, et al. Bipolar Disorders. Lancet (2020) 396(10265):1841–56. doi: 10.1016/S0140-6736(20)31544-0
2. Strejilevich SA, Quiroz D, Bitran JA. Bipolar Disorder. N Engl J Med (2020) 383(14):1398. doi: 10.1056/NEJMc2026462
3. Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-Wide Association Study Identifies 30 Loci Associated With Bipolar Disorder. Nat Genet (2019) 51(5):793–803. doi: 10.1038/s41588-019-0397-8
4. Mullins N, Forstner AJ, O'Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-Wide Association Study of More Than 40,000 Bipolar Disorder Cases Provides New Insights Into the Underlying Biology. Nat Genet (2021) 53(6):817–29. doi: 10.1038/s41588-021-00857-4
5. Zhang YL, Liang W, Chen ZM, Zhang HM, Zhang JH, Weng XQ, et al. Validity and Reliability of Patient Health Questionnaire-9 and Patient Health Questionnaire-2 to Screen for Depression Among College Students in China. Asia Pac Psychiatry (2013) 5(4):268–75. doi: 10.1111/appy.12103
6. Li W, Cai X, Li HJ, Song M, Zhang CY, Yang Y, et al. Independent Replications and Integrative Analyses Confirm TRANK1 as a Susceptibility Gene for Bipolar Disorder. Neuropsychopharmacology (2021) 46(6):1103–12. doi: 10.1038/s41386-020-00788-4
7. Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S, et al. Transcriptome-Wide Isoform-Level Dysregulation in ASD, Schizophrenia, and Bipolar Disorder. Science (2018) 362(6420):eaat8127. doi: 10.1126/science.aat8127
8. Chang H, Cai X, Yang ZH, Xiao X, Li M. Regulation of TRANK1 by GSK-3 in the Brain: Unexpected Interactions. Mol Psychiatry (2021). doi: 10.1038/s41380-021-01120-2
9. Morais LH, Schreiber H, Mazmanian SK. The Gut Microbiota-Brain Axis in Behaviour and Brain Disorders. Nat Rev Microbiol (2021) 19(4):241–55. doi: 10.1038/s41579-020-00460-0
10. Gentile CL, Weir TL. The Gut Microbiota at the Intersection of Diet and Human Health. Science (2018) 362(6416):776–80. doi: 10.1126/science.aau5812
11. Hu S, Li A, Huang T, Lai J, Li J, Sublette ME, et al. Gut Microbiota Changes in Patients With Bipolar Depression. Adv Sci (Weinh) (2019) 6(14):1900752. doi: 10.1002/advs.201900752
12. Zheng P, Yang J, Li Y, Wu J, Liang W, Yin B, et al. Gut Microbial Signatures Can Discriminate Unipolar From Bipolar Depression. Adv Sci (Weinh) (2020) 7(7):1902862. doi: 10.1002/advs.201902862
13. Bengesser SA, Morkl S, Painold A, Dalkner N, Birner A, Fellendorf FT, et al. Epigenetics of the Molecular Clock and Bacterial Diversity in Bipolar Disorder. Psychoneuroendocrinology (2019) 101:160–6. doi: 10.1016/j.psyneuen.2018.11.009
14. Lai J, Jiang J, Zhang P, Xi C, Wu L, Gao X, et al. Impaired Blood-Brain Barrier in the Microbiota-Gut-Brain Axis: Potential Role of Bipolar Susceptibility Gene TRANK1. J Cell Mol Med (2021) 25(14):6463–9. doi: 10.1111/jcmm.16611
15. Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol (2018) 6(2):133–48. doi: 10.1016/j.jcmgh.2018.04.003
16. Benedetti F, Aggio V, Pratesi ML, Greco G, Furlan R. Neuroinflammation in Bipolar Depression. Front Psychiatry (2020) 11:71. doi: 10.3389/fpsyt.2020.00071
17. Naaldijk YM, Bittencourt MC, Sack U, Ulrich H. Kinins and Microglial Responses in Bipolar Disorder: A Neuroinflammation Hypothesis. Biol Chem (2016) 397(4):283–96. doi: 10.1515/hsz-2015-0257
18. Pereira AC, Oliveira J, Silva S, Madeira N, Pereira CMF, Cruz MT. Inflammation in Bipolar Disorder (BD): Identification of New Therapeutic Targets. Pharmacol Res (2021) 163:105325. doi: 10.1016/j.phrs.2020.105325
19. Wu W, Zheng YL, Tian LP, Lai JB, Hu CC, Zhang P, et al. Circulating T Lymphocyte Subsets, Cytokines, and Immune Checkpoint Inhibitors in Patients With Bipolar II or Major Depression: A Preliminary Study. Sci Rep (2017) 7:40530. doi: 10.1038/srep40530
20. Lu J, Ma L, Jiang J, Huang B, Mou T, Huang T, et al. Linking Peripheral CD8(+) Single-Cell Transcriptomic Characteristics of Mood Disorders Underlying With the Pathological Mechanism. Clin Transl Med (2021) 11(7):e489. doi: 10.1002/ctm2.489
21. Lai J, Jiang J, Zhang P, Xi C, Wu L, Gao X, et al. Gut Microbial Clues to Bipolar Disorder: State-Of-the-Art Review of Current Findings and Future Directions. Clin Transl Med (2020) 10(4):e146. doi: 10.1002/ctm2.146
22. Zhang P, Huang H, Gao X, Jiang J, Xi C, Wu L, et al. Involvement of Kynurenine Metabolism in Bipolar Disorder: An Updated Review. Front Psychiatry (2021) 12:677039. doi: 10.3389/fpsyt.2021.677039
23. Bittel M, Reichert P, Sarfati I, Dressel A, Leikam S, Uderhardt S, et al. Visualizing Transfer of Microbial Biomolecules by Outer Membrane Vesicles in Microbe-Host-Communication In Vivo. J Extracell Vesicles (2021) 10(12):e12159. doi: 10.1002/jev2.12159
24. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J Clin Psychiatry (1998) 59(Suppl 20):22–33;quiz 4-57.
25. Hamilton M. Development of a Rating Scale for Primary Depressive Illness. Br J Soc Clin Psychol (1967) 6(4):278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x
26. Young RC, Biggs JT, Ziegler VE, Meyer DA. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br J Psychiatry (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
27. Konopaske GT, Lange N, Coyle JT, Benes FM. Prefrontal Cortical Dendritic Spine Pathology in Schizophrenia and Bipolar Disorder. JAMA Psychiatry (2014) 71(12):1323–31. doi: 10.1001/jamapsychiatry.2014.1582
28. Generoso JS, Giridharan VV, Lee J, Macedo D, Barichello T. The Role of the Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders. Braz J Psychiatry (2021) 43(3):293–305. doi: 10.1590/1516-4446-2020-0987
29. Cooke NCA, Bala A, Allard JP, Hota S, Poutanen S, Taylor VH. The Safety and Efficacy of Fecal Microbiota Transplantation in a Population With Bipolar Disorder During Depressive Episodes: Study Protocol for a Pilot Randomized Controlled Trial. Pilot Feasibility Stud (2021) 7(1):142. doi: 10.1186/s40814-021-00882-4
30. Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, et al. Human Gut Microbiota From Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell (2019) 177(6):1600–18 e17. doi: 10.1016/j.cell.2019.05.004
31. Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of Microbiota From Drug-Free Patients With Schizophrenia Causes Schizophrenia-Like Abnormal Behaviors and Dysregulated Kynurenine Metabolism in Mice. Mol Psychiatry (2020) 25(11):2905–18. doi: 10.1038/s41380-019-0475-4
32. Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, et al. The Gut Microbiome From Patients With Schizophrenia Modulates the Glutamate-Glutamine-GABA Cycle and Schizophrenia-Relevant Behaviors in Mice. Sci Adv (2019) 5(2):eaau8317. doi: 10.1126/sciadv.aau8317
33. Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut Microbiome Remodeling Induces Depressive-Like Behaviors Through a Pathway Mediated by the Host's Metabolism. Mol Psychiatry (2016) 21(6):786–96. doi: 10.1038/mp.2016.44
34. Giridharan VV, Sayana P, Pinjari OF, Ahmad N, da Rosa MI, Quevedo J, et al. Postmortem Evidence of Brain Inflammatory Markers in Bipolar Disorder: A Systematic Review. Mol Psychiatry (2020) 25(1):94–113. doi: 10.1038/s41380-019-0448-7
35. Isgren A, Sellgren C, Ekman CJ, Holmen-Larsson J, Blennow K, Zetterberg H, et al. Markers of Neuroinflammation and Neuronal Injury in Bipolar Disorder: Relation to Prospective Clinical Outcomes. Brain Behav Immun (2017) 65:195–201. doi: 10.1016/j.bbi.2017.05.002
36. Goldsmith DR, Rapaport MH, Miller BJ. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder and Depression. Mol Psychiatry (2016) 21(12):1696–709. doi: 10.1038/mp.2016.3
37. Rowland T, Perry BI, Upthegrove R, Barnes N, Chatterjee J, Gallacher D, et al. Neurotrophins, Cytokines, Oxidative Stress Mediators and Mood State in Bipolar Disorder: Systematic Review and Meta-Analyses. Br J Psychiatry (2018) 213(3):514–25. doi: 10.1192/bjp.2018.144
38. Kieseier BC. The Mechanism of Action of Interferon-Beta in Relapsing Multiple Sclerosis. CNS Drugs (2011) 25(6):491–502. doi: 10.2165/11591110-000000000-00000
39. Autry AE, Monteggia LM. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharmacol Rev (2012) 64(2):238–58. doi: 10.1124/pr.111.005108
40. Munkholm K, Vinberg M, Kessing LV. Peripheral Blood Brain-Derived Neurotrophic Factor in Bipolar Disorder: A Comprehensive Systematic Review and Meta-Analysis. Mol Psychiatry (2016) 21(2):216–28. doi: 10.1038/mp.2015.54
41. Schroter K, Brum M, Brunkhorst-Kanaan N, Tole F, Ziegler C, Domschke K, et al. Longitudinal Multi-Level Biomarker Analysis of BDNF in Major Depression and Bipolar Disorder. Eur Arch Psychiatry Clin Neurosci (2020) 270(2):169–81. doi: 10.1007/s00406-019-01007-y
42. Poletti S, Aggio V, Hoogenboezem TA, Ambree O, de Wit H, Wijkhuijs AJ, et al. Brain-Derived Neurotrophic Factor (BDNF) and Gray Matter Volume in Bipolar Disorder. Eur Psychiatry (2017) 40:33–7. doi: 10.1016/j.eurpsy.2016.06.008
43. Shi SS, Shao SH, Yuan BP, Pan F, Li ZL. Acute Stress and Chronic Stress Change Brain-Derived Neurotrophic Factor (BDNF) and Tyrosine Kinase-Coupled Receptor (TrkB) Expression in Both Young and Aged Rat Hippocampus. Yonsei Med J (2010) 51(5):661–71. doi: 10.3349/ymj.2010.51.5.661
44. Han Q, Cai T, Tagle DA, Li J. Structure, Expression, and Function of Kynurenine Aminotransferases in Human and Rodent Brains. Cell Mol Life Sci (2010) 67(3):353–68. doi: 10.1007/s00018-009-0166-4
45. Mellor AL, Munn DH. IDO Expression by Dendritic Cells: Tolerance and Tryptophan Catabolism. Nat Rev Immunol (2004) 4(10):762–74. doi: 10.1038/nri1457
46. Taylor MW, Feng GS. Relationship Between Interferon-Gamma, Indoleamine 2,3-Dioxygenase, and Tryptophan Catabolism. FASEB J (1991) 5(11):2516–22. doi: 10.1096/fasebj.5.11.1907934
47. Wang AK, Miller BJ. Meta-Analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr Bull (2018) 44(1):75–83. doi: 10.1093/schbul/sbx035
48. Olsson SK, Sellgren C, Engberg G, Landen M, Erhardt S. Cerebrospinal Fluid Kynurenic Acid is Associated With Manic and Psychotic Features in Patients With Bipolar I Disorder. Bipolar Disord (2012) 14(7):719–26. doi: 10.1111/bdi.12009
49. Schiavone S, Mhillaj E, Neri M, Morgese MG, Tucci P, Bove M, et al. Early Loss of Blood-Brain Barrier Integrity Precedes NOX2 Elevation in the Prefrontal Cortex of an Animal Model of Psychosis. Mol Neurobiol (2017) 54(3):2031–44. doi: 10.1007/s12035-016-9791-8
50. Parker A, Fonseca S, Carding SR. Gut Microbes and Metabolites as Modulators of Blood-Brain Barrier Integrity and Brain Health. Gut Microbes (2020) 11(2):135–57. doi: 10.1080/19490976.2019.1638722
51. Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry - Novel Perspectives on Brain Disorders. Nat Rev Neurol (2019) 15(6):317–28. doi: 10.1038/s41582-019-0174-4
52. Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 Polarization and Metabolic States. Br J Pharmacol (2016) 173(4):649–65. doi: 10.1111/bph.13139
53. Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar Disorders. Nat Rev Dis Primers (2018) 4:18008. doi: 10.1038/nrdp.2018.8
54. Yang Z, Zhou D, Li H, Cai X, Liu W, Wang L, et al. The Genome-Wide Risk Alleles for Psychiatric Disorders at 3p21.1 Show Convergent Effects on mRNA Expression, Cognitive Function, and Mushroom Dendritic Spine. Mol Psychiatry (2020) 25(1):48–66. doi: 10.1038/s41380-019-0592-0
55. Ng E, Georgiou J, Avila A, Trought K, Mun HS, Hodgson M, et al. Mice Lacking Neuronal Calcium Sensor-1 Show Social and Cognitive Deficits. Behav Brain Res (2020) 381:112420. doi: 10.1016/j.bbr.2019.112420
Keywords: bipolar disorder, gut microbiota, TRANK1, neuroinflammation, fecal microbiota transplantation
Citation: Lai J, Zhang P, Jiang J, Mou T, Li Y, Xi C, Wu L, Gao X, Zhang D, Chen Y, Huang H, Li H, Cai X, Li M, Zheng P and Hu S (2021) New Evidence of Gut Microbiota Involvement in the Neuropathogenesis of Bipolar Depression by TRANK1 Modulation: Joint Clinical and Animal Data. Front. Immunol. 12:789647. doi: 10.3389/fimmu.2021.789647
Received: 05 October 2021; Accepted: 06 December 2021;
Published: 21 December 2021.
Edited by:
Danielle S Macedo, Federal University of Ceara, BrazilReviewed by:
Swapna Mahurkar-Joshi, University of California, Los Angeles, United StatesMilica Milovan Borovcanin, University of Kragujevac, Serbia
Copyright © 2021 Lai, Zhang, Jiang, Mou, Li, Xi, Wu, Gao, Zhang, Chen, Huang, Li, Cai, Li, Zheng and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohua Hu, dorhushaohua@zju.edu.cn
†These authors have contributed equally to this work
 Jianbo Lai
Jianbo Lai Peifen Zhang1†
Peifen Zhang1† Yifan Li
Yifan Li Ming Li
Ming Li Shaohua Hu
Shaohua Hu