
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 17 November 2021
Sec. Autoimmune and Autoinflammatory Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.770852
This article is part of the Research Topic Adhesion Molecules and Autoimmune Diseases View all 11 articles
Autoimmune thyroiditis (AIT) is the most prevalent autoimmune endocrine disease, with a higher incidence in women than in men. Immunological abnormalities may lead to the impairment of ovarian folliculogenesis; however, whether the presence of AIT affects immunological microenvironment in follicles remains controversial. We performed a cross-sectional study including 122 patients, aged 20–40 years, who underwent IVF/ICSI treatment owing to isolated male or tube factor infertility. Patients were divided into AIT and control groups according to clinical presentation, thyroid function, and thyroid autoantibody measurements. Follicular fluid was collected and the distribution of cytokines/chemokines in follicular fluid was measured by flow cytometry using multiplex bead assays between the two groups. Based on differences in levels of intrafollicular chemokines and cytokines between the AIT and control groups, the relevant inflammatory cascade was further demonstrated. Among the 12 chemokines analyzed, three (CXCL9, CXCL10, and CXCL11) showed significantly elevated levels in the follicular fluid of patients with AIT. Among the 11 cytokines detected, compared with those in the control group, significantly higher levels of IFNγ were observed in patients with AIT. IFNγ dose-dependently stimulated the expression and secretion of CXCL9/10/11 in cultured primary granulosa cells. The percentage of CXCR3+ T lymphocytes was significantly elevated in the follicular microenvironment of patients with AIT. We concluded that the IFNγ-CXCL9/10/11-CXCR3+ T lymphocyte inflammatory cascade is activated in the follicular microenvironment of patients with AIT. These findings indicate that a considerable immune imbalance occurred in the follicular microenvironment of patients with AIT.
Autoimmune thyroiditis (AIT) is an organ-specific autoimmune disorder characterized by the production of autoimmune thyroid antibodies and the infiltration of self-reactive lymphocytes into the thyroid, resulting in the destruction of thyrocytes and hypothyroidism. T cell-mediated immune disorders play an important role in the pathogenesis of AIT. In patients with AIT, both in the peripheral blood and in the thyroid gland, the balance between Th1 and Th2 cells is skewed toward Th1 cells and cytotoxic T lymphocytes are considerably activated, which triggers a dramatic activation of the immune response. Meanwhile, pro-inflammatory cytokines such as interferon-gamma (IFNγ) and IL-17 were significantly elevated. Pro-inflammatory chemokines CXCL9, CXCL10, and CXCL11 were also activated in patients with AIT, which further promoted the migration of pro-inflammatory Th1 lymphocytes into the thyroid and aggravated the destruction of the thyroid gland (1–4).
Immunological abnormalities may lead to the impairment of ovarian folliculogenesis; however, whether the presence of AIT affects follicular development and ovulation remains controversial and the relevant studies are limited. Our previous large-scale retrospective cohort study found that after adjusting for thyroid function and ovarian reserve markers, the number of oocytes retrieved during in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment significantly decreased in infertile women with positive thyroid antibodies. This suggests a possible role of AIT in follicular development; however, the mechanism remains unclear (5).
Follicle development and oocyte maturation depend heavily on the follicle fluid, which is derived from blood infiltration and ovarian secretion. An altered follicular microenvironment may directly damage follicle recruitment and growth. A large number of cytokines/chemokines are activated in follicular fluid and interact with each other to form a network that regulates folliculogenesis, oocyte maturation, and luteinization (6). Recent studies have reported an association between abnormal elevation of specific cytokines/chemokines and follicle developmental disorders (7, 8).
This study aimed to investigate the distribution of intrafollicular cytokines/chemokines and the activation of relevant inflammatory pathways in patients with AIT compared to those without AIT.
The study was approved by the Peking University Third Hospital Medical Science Research Ethics Committee.
The study consisted of 122 patients, aged 20–40 years, who underwent IVF/ICSI treatment owing to isolated male or tube factor infertility. Patients were divided into AIT and control groups according to clinical presentation, thyroid function, and thyroid autoantibody measurements. The reference values for serum thyroid-stimulating hormone (TSH) were 0.55–4.78 uIU/mL, and those for serum-free thyroxin (FT4) were 0.89–1.80 ng/dL. The detection range for thyroglobulin antibody (TGAb) was 15–500 IU/mL, and that for thyroid peroxidase antibody (TPOAb) was 28–1300 IU/mL. A value >60 IU/mL was defined as positive for TGAb or TPOAb. Patients positive for TGAb and/or TPOAb were placed in the AIT group. The levels of TSH and FT4 in all patients were required to be within the normal reference range prior to controlled ovarian stimulation; thus, 14 patients that were thyroid antibody-positive received oral levothyroxine owing to abnormal TSH and FT4 levels. Patients with a history of other reproductive diseases, such as polycystic ovarian syndrome or endometriosis; a history of other thyroid diseases, such as hyperthyroidism or thyroid cancer; abnormal results on parental karyotyping; other endocrinological diseases, such as hyperprolactinemia or diabetes; or positive tests for antinuclear antibodies or lupus anticoagulants were excluded.
All patients underwent standardized protocols for controlled ovarian stimulation (COS) and oocyte retrieval. The individualized dose of recombinant follicle-stimulating hormones for inducing COS was decided based on the patient’s age, body mass index (BMI), antral follicle count, and anti-Mullerian hormone levels. Two hundred and fifty micrograms of recombinant human chorionic gonadotropin (HCG; Eiser, Serono, Germany) was administered to trigger oocyte maturation when at least two follicles reached 18 mm. Oocyte retrieval was performed 34–36 h after HCG administration. Follicular fluid without visible blood contamination was aspirated from the first and largest follicle, collected separately and centrifuged at 800 g for 10 min. Supernatants were collected and stored at −80°C until further analysis.
Concentrations of cytokines/chemokines in the follicle fluid were measured using flow cytometry with multiplex bead assays, and the procedures were performed using the Legendplex Multi-Analyte Flow Assay Kit according to the manufacturer’s instructions (BioLegend). Cytokine plexes include IL-2, IL-4, IL-6, IL-9, IL-10, IL13, IL-17A, IL-17F, IL-22, IFNγ, and TNFα. The chemokine plexes included CCL2, CCL3, CCL4, CCL5, CCL11, CCL17, CXCL1, CXCL5, CXCL8, CXCL9, CXCL10, and CXCL11.
Primary granulosa cells were collected from follicular aspirates and purified via centrifugation at 600 g for 30 min using the Ficoll gradient method. For chemokine secretion assays, primary granulosa cells were seeded onto six-well plates in growth medium (Dulbecco’s modified Eagle’s medium/Ham’s F-12 nutrient mixture containing 10% fetal bovine serum). The growth medium containing leukocytes was removed after 24 h, granulosa cells were washed in PBS, and incubated in serum-free growth medium. Cells were incubated for 24 h with different doses of IFNγ (R&D Systems; 500, 1000, 5000, and 10,000 IU/mL). The supernatant was removed after 24 h and stored at −20°C until required for the chemokine assay.
Total RNA was extracted from cultured primary granulosa cells using TRIzol reagent (Life Technologies) according to the manufacturer’s instructions. RNA was reverse transcribed into first-strand cDNA using a cDNA synthesis kit (Thermo Fisher Scientific). Reverse transcription quantitative RT-PCR was performed on an Applied Biosystems Quant Studio3 Real-Time PCR System in 96 well optical reaction plates. The following primers were used: β-actin F, TGCCCATCTACGAGGGGTAT; β-actin R, CTTAATGTCACGCACGATTTCC; CXCL9 F, CATCATCTTGCTGGTTCTGATTG; CXCL9 R, GATTGTAGGTGGATAGTCCCTTG; CXCL10 F, GCTGCCTTATCTTTCTGACTCT; CXCL10 R, GACCTTGGATTAACAGGTTGATTAC; CXCL11 F, ATGAGTGTGAAGGGCATGG; CXCL11 R, TATGCAAAGACAGCGTCCTC.
The percentage of intrafollicular CXCR3+ T lymphocytes was detected in 14 patients with AIT and in the 12 control patients. The remaining follicle fluid in each patient was collected after the oocytes were removed and centrifugated at 800 g for 10 min. Cells were aspirated and washed with Dulbecco’s PBS. The cells were treated with lysis buffer (BD Biosciences) to remove red blood cells. Approximately 2 × 106 cells were filtered through a 70-μm mesh to remove clumps of cells. Cells were incubated with 100 μL PBS containing CD45 microbeads and CD45 antibody. CD45+ cells were isolated using a MACS separator (Miltenyi Biotec). Cells were further phenotyped by staining with CD3, CD4, and CD183 (CXCR3) and counted using a flow cytometer (BD FACSCelesta™).
Statistical analysis was performed using SPSS version 24.0 software (SPSS Inc, Chicago, IL, USA) and GraphPad Prism 6.0 (La Jolla, CA, USA). Comparisons of continuous data among groups were performed using ANOVA or non-parametric tests. A two-tailed P value of <0.05 was defined as statistically significant.
The baseline characteristics of patients with AIT and the control group are shown in Table 1. The distributions of age and BMI were similar between the two groups. No significant difference was observed in FT4 and TSH levels in serum or follicular fluid of patients with or without AIT. Significantly higher levels of TGAb and TPOAb in serum and follicular fluid were detected in patients with AIT than in the control group (P <0.001). Thyroid antibodies in serum and follicular fluid were absent in the control group.
We analyzed the distribution of chemokines and cytokines in the follicular fluid between the AIT and control groups (Figure 1). Among the 12 chemokines analyzed, only the concentrations of three, CXCL9, CXCL10, and CXCL11, were significantly elevated in the follicular fluid of patients with AIT. There was no significant difference in the levels of other chemokines between the two groups. Among the 11 cytokines analyzed, significantly higher levels of IFNγ were observed in patients with AIT than in the control group (P <0.01). Marginal yet significantly higher levels of IL4, IL-6, and IL-10 were also observed in patients with AIT than in the control group (P <0.05). There was no significant difference in the levels of other cytokines between the two groups.
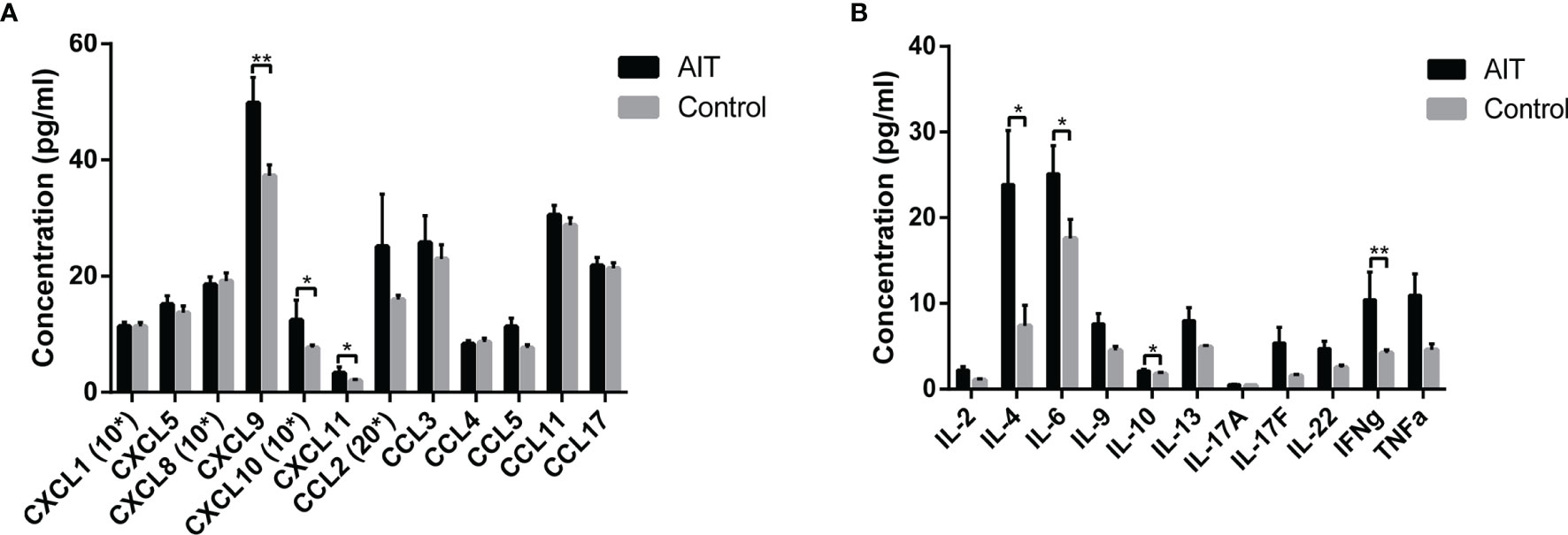
Figure 1 Distribution of cytokines and chemokines in follicle fluid obtained from patients with and without AIT. Among the 12 chemokines analyzed, concentrations of CXCL9, CXCL10, and CXCL11 were significantly higher in patients with AIT than those in the control group (A). Bars represent the mean ± SEM. *P < 0.05 and **P < 0.01 by the Mann-Whitney U test. Among the 11 cytokines analyzed, IL4, IL-6, IL-10, and IFNγ concentrations were significantly higher in patients with AIT than in the control group (B). Bars represent the mean ± SEM. *P < 0.05 and **P < 0.01 by the Mann-Whitney U test. *10, data needed to be multiplied by 10; *20, data needed to be multiplied by 20.
IFNγ has been evidenced as an effective inducer for the activation of CXCL9/10/11 in thyrocytes, however, the role of IFNγ in intrafollicular granulosa cells remains unclear. Based on differences in distribution of intrafollicular chemokines and cytokines between the AIT and control groups, elevated CXCL9/10/11 secretion may be induced by increased IFNγ in the follicular microenvironment of patients with AIT. To investigate whether intrafollicular IFNγ stimulates the secretion of CXCL9/10/11 in granulosa cells, primary granulosa cells were cultured in vitro and treated with different doses of IFNγ. As shown in Figure 2, IFNγ dose-dependently induced the expression and secretion of CXCL9/10/11 in primary granulosa cells.
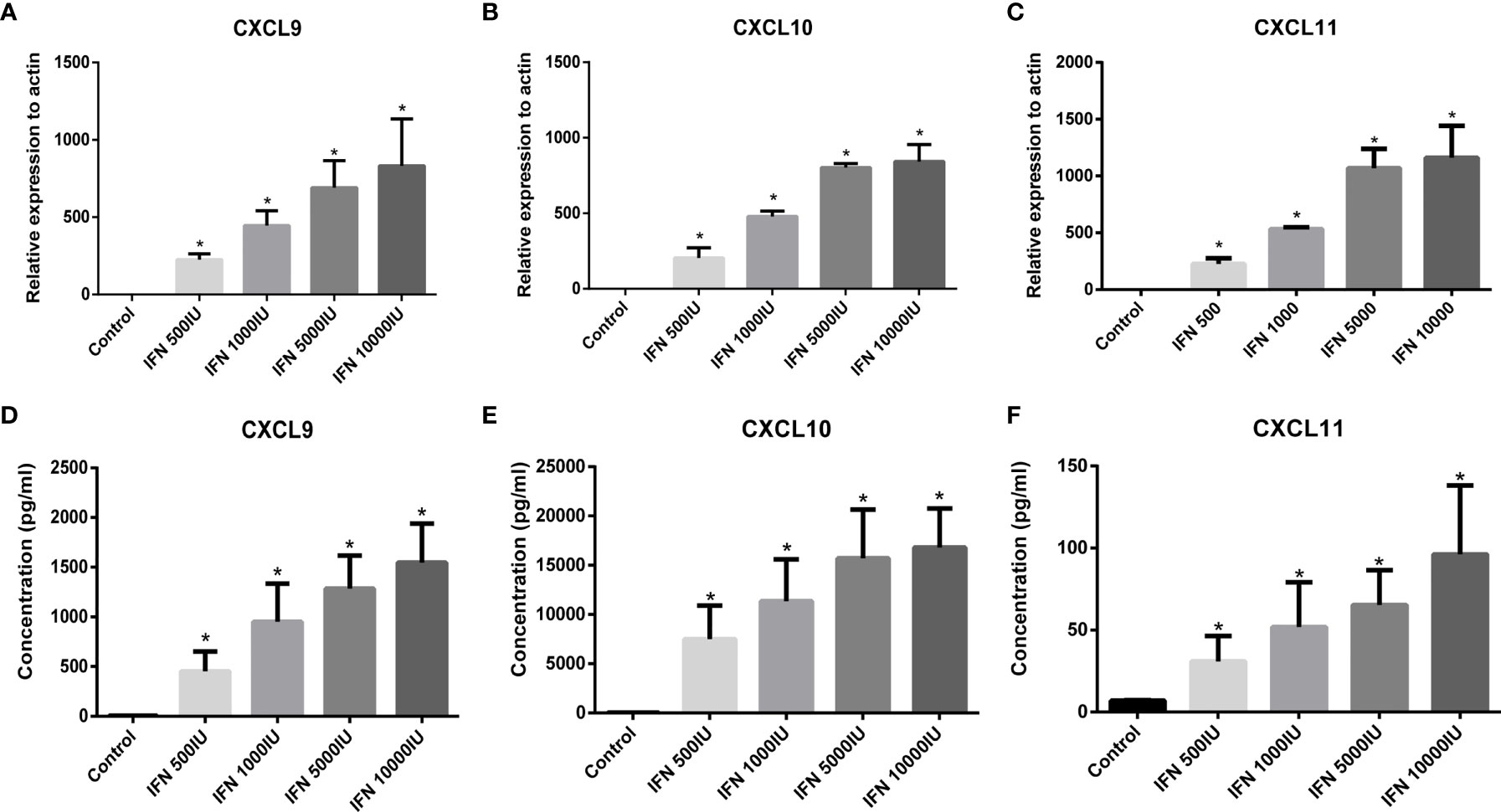
Figure 2 Expression of CXCL9 (A), CXCL10 (B), and CXCL11 (C) in primary granulosa cells stimulated by IFNγ (IFN-g) was detected by RT-PCR. Expression of CXCL9/10/11 in granulosa cells was absent under basal conditions (control) and was significantly stimulated by increasing doses of IFNγ (ANOVA, P < 0.05). Bars represent the mean ± SEM. *P < 0.05 vs control by the Bonferroni-Dunn test. Concentration of CXCL9 (D), CXCL10 (E), and CXCL11 (F) in the culture supernatant from granulosa cells stimulated by IFNγ. The release of CXCL9/10/11 from granulosa cells was absent under basal conditions (control) and was significantly stimulated by increasing doses of IFNγ (ANOVA, P <0.05). Bars represent the mean ± SEM. *P < 0.05 vs control by the Bonferroni-Dunn test.
CXCL9/10/11 shares the common receptor CXCR3 (CD183), which is primarily expressed in T lymphocytes (9, 10). To investigate whether elevated intrafollicular CXCL9/10/11 in patients with AIT promoted the infiltration of T lymphocytes into follicle fluid, we measured the percentage of T lymphocytes expressing CXCR3 in follicular fluid from 14 patients with AIT and compared it with 12 control patients using flow cytometry. As shown in Figure 3, of the CD45+CD3+ T lymphocytes, the proportions of CD4+CXCR3+ and CD4−CXCR3+ T lymphocytes in the AIT group were significantly higher than those in the control group.
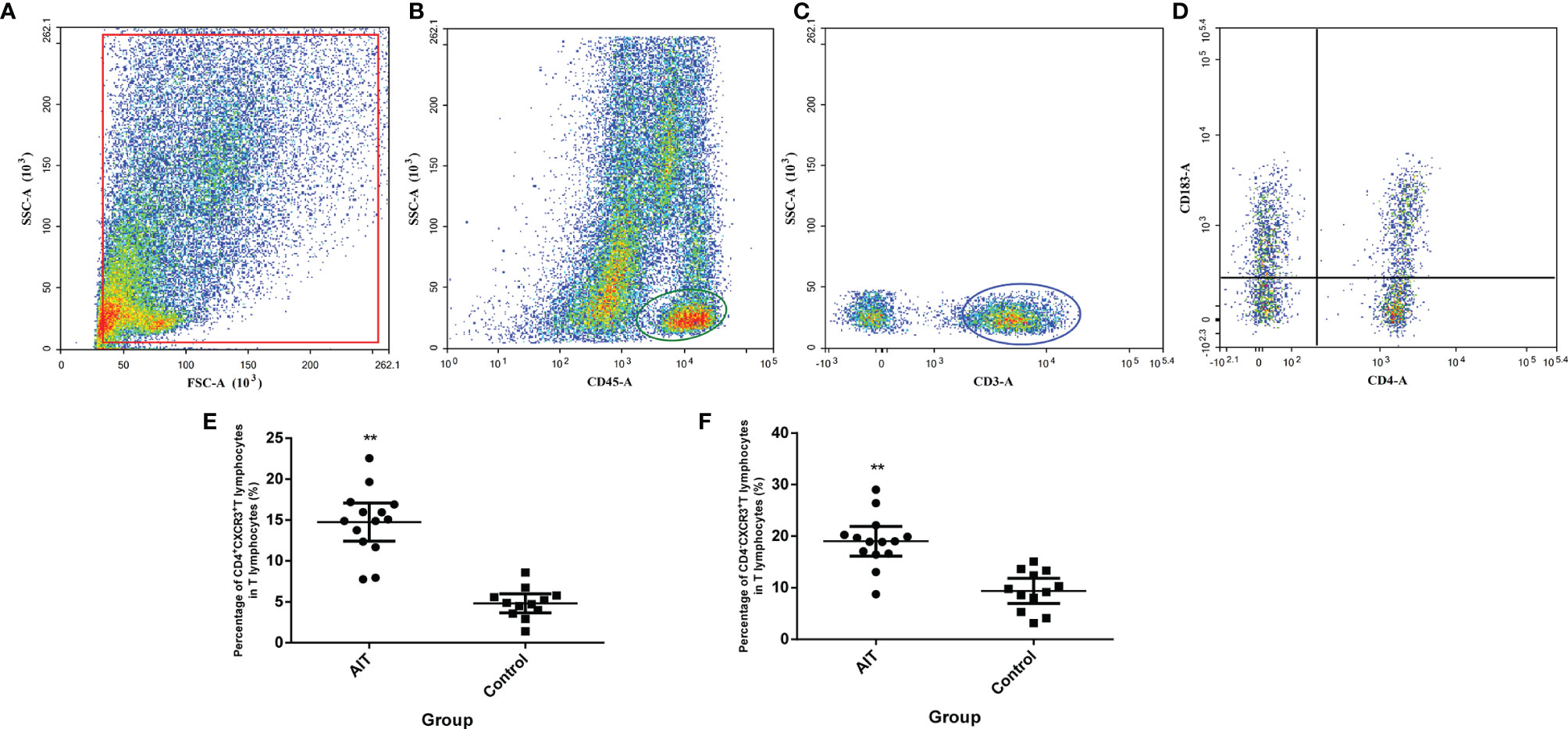
Figure 3 Detection of CXCR3+ T lymphocytes in the follicular microenvironment. Cells were enriched from follicular fluid, and CD45+ cells were positively selected by MACS cell separation. Separated cells were directly incubated with conjugated anti-CD3, CD4, and CXCR3 antibodies and counted by flow cytometry (A). CD45+ cells were gated (B) and CD3+ cells were gated from CD45+ cells (C), the expressions of CXCR3 in CD4+ and CD4-T cells were gated from CD3+ cells (D). (E, F) The percentage of CD4+CXCR3+ and CD4- CXCR3+ T lymphocytes. **P < 0.01 by the Mann-Whitney U test.
Our study is the first to demonstrate the substantial activation of IFNγ-CXCL9/10/11-CXCR3+ T lymphocytes in the ovarian microenvironment of patients with AIT. This cascade included a classical inflammatory response: IFNγ secreted by T lymphocytes stimulated the release of CXCL9/10/11 from granulosa cells. CXCL9, CXCL10, and CXCL11 belong to the chemokine family and share the common receptor CXCR3, which is particularly expressed in T lymphocytes. Elevation of CXCL9/10/11 in follicle fluid attracts CXCR3+ T lymphocytes to infiltrate the follicle. T lymphocytes that remained in the follicle further increased the secretion of IFNγ, which constitutes an intact response loop in the follicular microenvironment (Figure 4).
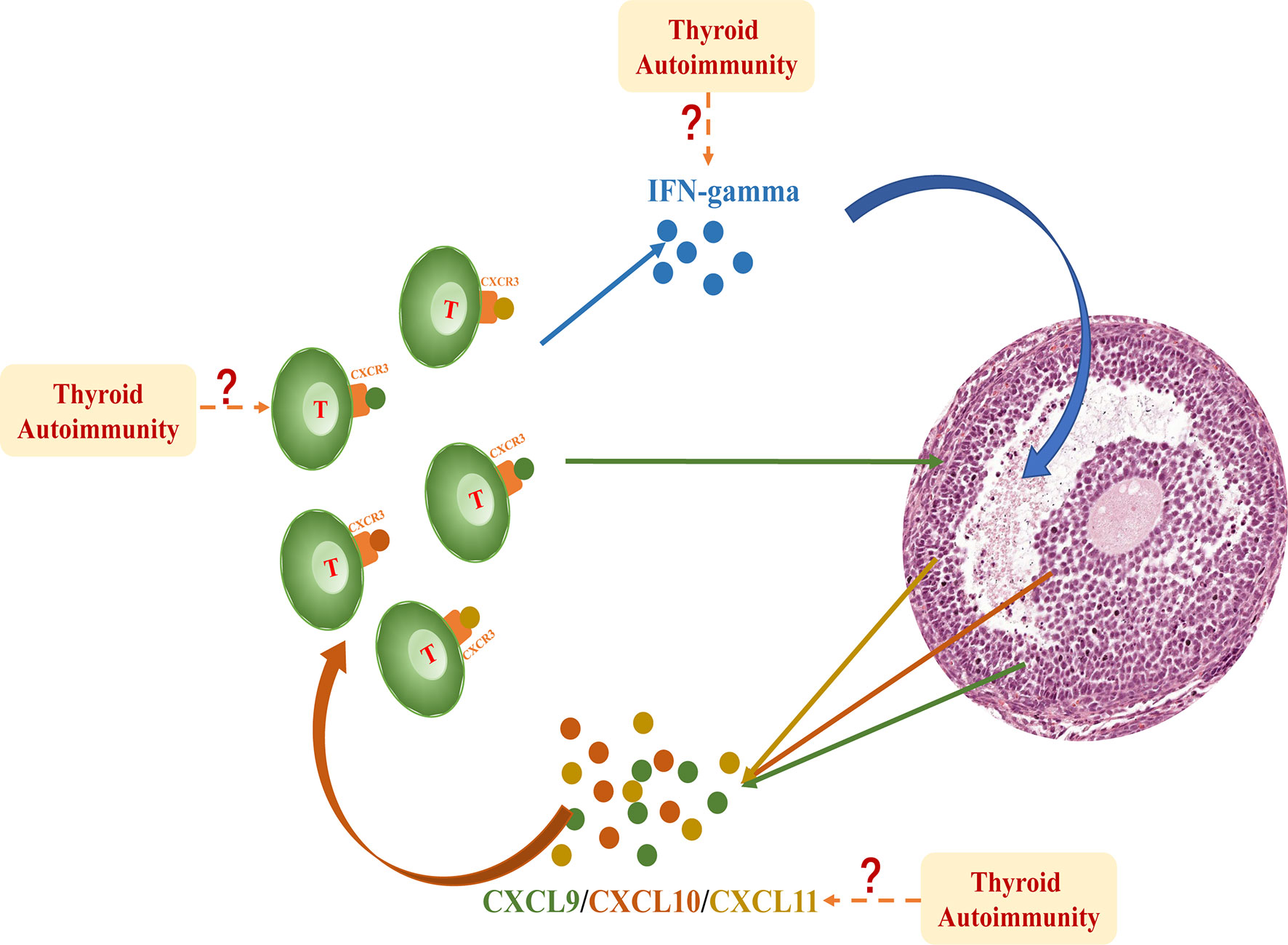
Figure 4 IFNγproduced by T lymphocytes dose-dependently induced the expression and secretion of CXCL9/10/11 in primary granulosa cells. These chemokines bind to their receptor CXCR3 and further promote the migration of T lymphocytes into the follicle, thereby perpetuating an inflammatory cascade in the follicular microenvironment. This intrafollicular response is significantly activated in patients with AIT; however, the initiation of this cascade is still unclear. The excess influx of inflammatory cells and factors into the follicle fluid may be involved in this mechanism.
There is increasing evidence to demonstrate the role of intrafollicular inflammation in the pathogenesis of reproductive diseases. A previous study analyzed the association between follicular pro-inflammatory cytokines/chemokines and infertility related diseases and showed that different types of cytokines/chemokines were activated in patients with different reproductive diseases. For example, higher follicular MIP-1α (CCL3) and CD44 were correlated with polycystic ovary syndrome and IL-23, IFN-γ, and TNF-α correlated with endometriosis (11). An imbalance between pro-inflammatory cytokines such as IFNγ and TNFα and anti-inflammatory cytokines such as IL-10 has also been reported to play a critical role in the pathological mechanism of premature ovarian insufficiency (8). Our study showed a significant difference in the distribution of intrafollicular chemokines and cytokines between the AIT and control groups, suggesting the presence of an activated abnormal immune response in the follicular microenvironment in patients with AIT.
Whether increased levels of intrafollicular CXCL9/10/11 affect follicle development is still unclear; however, CXCL9/10/11 has been reported to inhibit angiogenesis. Several studies have reported that increased levels of CXCL9/10/11 significantly inhibit blood vessel formation and endothelial cell motility (12, 13). In the ovary, angiogenesis is essential for folliculogenesis and ovulation. A rich vascular network surrounding the theca cell layer provides sufficient nutrients and oxygen to support follicle growth, and suppression of normal thecal vasculature leads to considerably reduced thecal thickness, decreased granulosa cell proliferation, prevention of ovulation, and increased rate of follicle atresia (14, 15). Elevated levels of intrafollicular CXCL9/10/11 in patients with AIT may indirectly block follicle development by inhibiting ovarian angiogenesis.
CXCL9/10/11 belongs to a family of chemokines with the capacity to attract circulating leukocytes to inflammatory sites by binding to their specific receptor, CXCR3. Prior to ovulation, only a few resident immune cells were present in vessels around the thecal cells. Upon LH surge or stimulation by HCG, the basal lamina is disrupted and several capillaries form and extend into the granulosa cell compartment, with numerous infiltrating leukocytes and a large number of cytokines are secreted into the follicle fluid (16). The association between the infiltration of leukocytes and ovulation is not completely clear, and the complexity of the inflammatory response and leukocyte types makes it difficult to investigate the role that a specific type of leukocyte plays in follicle fluid. A previous study reported that perfused LH and leukocytes in rat ovaries produced a higher number of ovulations; however, perfused leukocytes alone failed to induce ovulation, suggesting that the influx of leukocytes plays a synergetic, yet not necessary, role in ovulation (17). In our study, elevated CXCL9/10/11 levels enhanced the migration of T lymphocytes via CXCR3 and led to a higher proportion of intrafollicular T lymphocytes in patients with AIT, which may aggravate the regional inflammatory response.
In our study, the initiation of the IFNγ-CXCL9/10/11-CXCR3+ T lymphocyte cascade in the follicular microenvironment remains elusive. We provided two possible hypotheses. First, excess infiltration of circulating CXCL9/10/11 or IFNγ from blood in patients with AIT may affect the cascade. Several studies have demonstrated that CXCL9/10/11 is dramatically increased in the development of AIT. Kimura et al. established a transgenic mouse model with features similar to those of human AIT, which aberrantly expresses IFNγ within the thyroid gland. Compared with wild-type littermates, a transgenic mouse model exclusively expressed CXCL9 and CXCL11, and showed increased expression of CXCL10, which verified that IFNγ stimulated the secretion of CXCL9/10/11 from the thyroid gland (3). A study showed that circulating CXCL9 and CXCL10 levels were significantly elevated in patients with AIT compared with normal controls or patients with multinodular goiter (1). Another study found that circulating CXCL9 and CXCL11 levels were significantly elevated in patients with AIT compared with euthyroid controls or patients with multinodular goiter (18). Circulating IFNγ, the upstream stimulator of CXCL9/10/11, also increased in patients with AIT (4).
Second, local activation of the inflammatory response may be another hypothesis for the initiation of this cascade. A previous study identified thyroid peroxidase using immunocytochemistry in granulosa cells of the human ovarian follicle and provided a hypothesis that the human ovarian follicle may be an independent thyroid-hormone-producing unit (19). Here, thyroid antibodies were detected in the follicular fluid of patients with AIT, with a similar concentration in the serum (Table 1). The presence of thyroid peroxidase and thyroid antibodies in follicular fluid may provide a hypothesis for activation of the intrafollicular antibody-mediated immune response.
Although our findings demonstrate an immunological alteration occurred in follicles of patients with AIT, whether the substantial activation of inflammatory cascade affect folliculogenesis requires further study.
In conclusion, our study showed that a considerable immune imbalance occurred in the follicular microenvironment of patients with AIT. Furthermore, to the best of our knowledge, this is the first demonstration of the activation of the intrafollicular IFNγ-CXCL9/10/11-CXCR3+ T lymphocyte cascade in patients with AIT.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Peking University Third Hospital Medical Science Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
NH, HC, and JQ developed the conception of the study, and all authors contributed to the research discussion. NH, DL, and YL collected the samples. NH and HC performed the experiment and contributed to data analysis. NH wrote the initial draft of the paper, and all authors contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 81871212) and The Second Tibetan Plateau Scientific Expedition and Research (Grand No. 2019QZKK0607).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Antonelli A, Ferrari SM, Frascerra S, Galetta F, Franzoni F, Corrado A, et al. Circulating Chemokine (CXC Motif) Ligand (CXCL)9 Is Increased in Aggressive Chronic Autoimmune Thyroiditis, in Association With CXCL10. Cytokine (2011) 55(2):288–93. doi: 10.1016/j.cyto.2011.04.022
2. Antonelli A, Ferrari SM, Mancusi C, Mazzi V, Pupilli C, Centanni M, et al. Interferon-α, -β and -γ Induce CXCL11 Secretion in Human Thyrocytes: Modulation by Peroxisome Proliferator-Activated Receptor γ Agonists. Immunobiology (2013) 218(5):690–5. doi: 10.1016/j.imbio.2012.08.267
3. Kimura H, Kimura M, Rose NR, Caturegli P. Early Chemokine Expression Induced by Interferon-Gamma in a Murine Model of Hashimoto's Thyroiditis. Exp Mol Pathol (2004) 77(3):161–7. doi: 10.1016/j.yexmp.2004.08.004
4. Qin Q, Liu P, Liu L, Wang R, Yan N, Yang J, et al. The Increased But Non-Predominant Expression of Th17- and Th1-Specific Cytokines in Hashimoto's Thyroiditis But Not in Graves' Disease. Braz J Med Biol Res (2012) 45(12):1202–8. doi: 10.1590/s0100-879x2012007500168
5. Huang N, Chen L, Lian Y, Wang H, Li R, Qiao J, et al. Impact of Thyroid Autoimmunity on In Vitro Fertilization/Intracytoplasmic Sperm Injection Outcomes and Fetal Weight. Front Endocrinol (Lausanne) (2021) 12:698579. doi: 10.3389/fendo.2021.698579
6. Field SL, Dasgupta T, Cummings M, Orsi NM. Cytokines in Ovarian Folliculogenesis, Oocyte Maturation and Luteinisation. Mol Reprod Dev (2014) 81(4):284–314. doi: 10.1002/mrd.22285
7. Mao XD, Hu CY, Zhu MC, Ou HL, Qian YL. Immunological Microenvironment Alterations in Follicles of Women With Proven Severe Endometriosis Undergoing In Vitro Fertilization. Mol Biol Rep (2019) 46(5):4675–84. doi: 10.1007/s11033-019-04753-3
8. Huang Y, Hu C, Ye H, Luo R, Fu X, Li X, et al. Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. J Immunol Res (2019) 2019:8069898. doi: 10.1155/2019/8069898
9. Charo IF, Ransohoff RM. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N Engl J Med (2006) 354(6):610–21. doi: 10.1056/NEJMra052723
10. Karin N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front Immunol (2020) 11:976. doi: 10.3389/fimmu.2020.00976
11. Sarapik A, Velthut A, Haller-Kikkatalo K, Faure GC, Béné MC, de Carvalho Bittencourt M, et al. Follicular Proinflammatory Cytokines and Chemokines as Markers of IVF Success. Clin Dev Immunol (2012) 2012:606459. doi: 10.1155/2012/606459
12. Bodnar RJ, Yates CC, Wells A. IP-10 Blocks Vascular Endothelial Growth Factor-Induced Endothelial Cell Motility and Tube Formation via Inhibition of Calpain. Circ Res (2006) 98(5):617–25. doi: 10.1161/01.Res.0000209968.66606.10
13. Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, et al. An Alternatively Spliced Variant of CXCR3 Mediates the Inhibition of Endothelial Cell Growth Induced by IP-10, Mig, and I-TAC, and Acts as Functional Receptor for Platelet Factor 4. J Exp Med (2003) 197(11):1537–49. doi: 10.1084/jem.20021897
14. Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of Thecal Angiogenesis, Antral Follicular Growth, and Ovulation in the Primate by Treatment With Vascular Endothelial Growth Factor Trap R1r2. Endocrinology (2002) 143(7):2797–807. doi: 10.1210/endo.143.7.8886
15. Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and Vascular Function in the Ovary. Reproduction (2009) 138(6):869–81. doi: 10.1530/rep-09-0283
16. Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: Parallels With Inflammatory Processes. Endocr Rev (2019) 40(2):369–416. doi: 10.1210/er.2018-00075
17. Hellberg P, Thomsen P, Janson PO, Brännström M. Leukocyte Supplementation Increases the Luteinizing Hormone-Induced Ovulation Rate in the In Vitro-Perfused Rat Ovary. Biol Reprod (1991) 44(5):791–7. doi: 10.1095/biolreprod44.5.791
18. Antonelli A, Ferrari SM, Frascerra S, Di Domenicantonio A, Nicolini A, Ferrari P, et al. Increase of Circulating CXCL9 and CXCL11 Associated With Euthyroid or Subclinically Hypothyroid Autoimmune Thyroiditis. J Clin Endocrinol Metab (2011) 96(6):1859–63. doi: 10.1210/jc.2010-2905
Keywords: autoimmune thyroiditis, ovarian microenvironment, follicle development, IFNγ-CXCL9/10/11-CXCR3+ T lymphocyte, follicular fluid
Citation: Huang N, Liu D, Lian Y, Chi H and Qiao J (2021) Immunological Microenvironment Alterations in Follicles of Patients With Autoimmune Thyroiditis. Front. Immunol. 12:770852. doi: 10.3389/fimmu.2021.770852
Received: 05 September 2021; Accepted: 25 October 2021;
Published: 17 November 2021.
Edited by:
Jianfeng Chen, Shanghai Institute of Biochemistry and Cell Biology (CAS), ChinaReviewed by:
Bin Li, Shanghai Jiao Tong University School of Medicine, ChinaCopyright © 2021 Huang, Liu, Lian, Chi and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongbin Chi, Y2hpaGJAMTYzLmNvbQ==; Jie Qiao, amllLnFpYW9AMjYzLm5ldA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.