
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol., 27 September 2021
Sec. Inflammation
Volume 12 - 2021 | https://doi.org/10.3389/fimmu.2021.756920
This article is part of the Research TopicHexose Uptake and Metabolism in Immune Homeostasis and InflammationView all 8 articles
High glucose and fructose intake have been proven to display pro-inflammatory roles during the progression of inflammatory diseases. However, mannose has been shown to be a special type of hexose that has immune regulatory functions. In this review, we trace the discovery process of the regulatory functions of mannose and summarize some past and recent studies showing the therapeutic functions of mannose in inflammatory diseases. We conclude that treatment with mannose can suppress inflammation by inducing regulatory T cells, suppressing effector T cells and inflammatory macrophages, and increasing anti-inflammatory gut microbiome. By summarizing all the important findings, we highlight that mannose treatment is a safe and promising novel strategy to suppress inflammatory diseases, including autoimmune disease and allergic disease.
Sugar intake, mainly glucose and fructose, within generic diets has increased dramatically during the past century. Hexose, especially glucose, is the most important energy source in living organisms. However, it has been well documented that consuming too much sugar can raise the incidence of many health problems, including diabetes and obesity. Recently, more studies have demonstrated the harmful effects of sugar. For example, one study reported that glucose-fructose syrup (HFCS) enhances intestinal tumor growth in mice via activation of glycolysis and increased synthesis of fatty acids in tumor cells that support tumor growth (1); Another study found that high fructose intake is associated with increased hepatic fatty acid synthesis and marked insulin resistance (2), and a final study showed that dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate (3). More than that, one clinical study among US adults found that consumption of sugar-sweetened beverages (SSBs) is positively associated with total mortality (4).
In addition, high glucose intake and high fructose intake have also been proven to have pro-inflammatory roles for inflammatory diseases. Zhang et al. revealed that high glucose intake exacerbates autoimmunity in mouse models of colitis and experimental autoimmune encephalomyelitis (EAE) by promoting T helper-17 (Th17) cell differentiation (5). Jones et al. found that fructose reprograms glutamine-dependent oxidative metabolism in mononuclear phagocytes to support LPS-induced inflammation (6). Another study found that high-fructose diet (HFrD) elicited endotoxemia, could activate toll-like receptor (TLR) signaling in liver macrophages, and induce liver inflammation (7). Another two studies also reported that dietary simple sugars alter microbial ecology in the gut and promote colitis and EAE in mice (8, 9).
Surprisingly, not all hexoses are pathogenic or pro-inflammatory. During the past few years, the immune regulatory functions of mannose, a C-2 epimer of glucose, have been revealed (10). Although mannose has been shown to be effective in the treatment of bacterial urinary tract infections by blocking the adhesion of enteric bacteria to uroepithelial cells (11–14), it was thought for a long time that the key function of mannose was to glycosylate certain proteins (15, 16), and that a mannose supplement must be given to the individuals with congenital disorders of glycosylation type Ib to support their survival (17, 18). Recently, quite a few studies have highlighted the fact that mannose is an effective suppressor of inflammation and autoimmunity (10, 19–21). Mannose has been shown to suppress numerous inflammatory diseases, including Type I diabetes (T1D) (10), asthma (10), colitis (19), obesity (20), osteoarthritis (22), chronic graft-versus- host disease (cGVHD) and lupus (21). In this review, we summarize the mechanisms of immunomodulatory effects mediated by mannose treatment, highlight mannose treatment as a promising strategy to suppress inflammation, and point to the remaining key questions that need to be addressed urgently in further studies.
CD4+CD25+Foxp3+ regulatory T cells (Treg cells) are the most important cell population to maintain tolerance to self-antigens (23) and harmless antigens (24) (e.g. pollen) by suppressing responses of effector T cells (Teff cells) and other immune cell responses (25–28). Interestingly, Zhang et al. reported that supraphysiological levels of mannose supplemented orally through drinking water could induce Treg cells and suppress Type I diabetes and OVA-induced airway inflammation in mouse models (10). They found that mannose induced Treg cells both in vivo and in vitro through the activation of transforming growth factor beta (TGF-β) from its latent form, and they further found that the activation of TGF-β induced by mannose was mediated by increased reactive oxygen species (ROS) and integrin αvβ8. Interestingly, the increased ROS production induced by mannose was from fatty acid oxidation (FAO), as mannose treatment increased FAO, but suppressed aerobic glycolysis in CD4+ T cells. Moreover, mannose could also suppress type 1 helper T cells (Th1 cells) and type 2 helper T cells (Th2 cells) in a Treg cell and TGF-β independent mechanism, as they found that mannose could suppress Th1 cell cytokines (Ifng and Il2) and Th2 cell cytokines (Il4 and Il13) during CD4+ T cell activation (before the generation of Treg cells), and they also showed that Treg cell depletion in vivo did not increase Th1 cell frequency in NOD mice treated with mannose (10). Overall, this encompassing work revealed the immune regulatory functions of mannose by inducing Treg cells and suppressing Teff cells, and provided a fascinating new insight into the beneficial effects of this unique sugar (Figure 1) (10). These findings suggested that mannose could be a safe dietary supplement to promote immune tolerance and to treat/prevent human diseases associated with autoimmunity and allergy (29). In the meantime, mannose treatment did not affect Il17 expression, suggesting that mannose may specifically induce Treg cells without affecting other protective responses such as Th17 cell-mediated gut integrity (30).
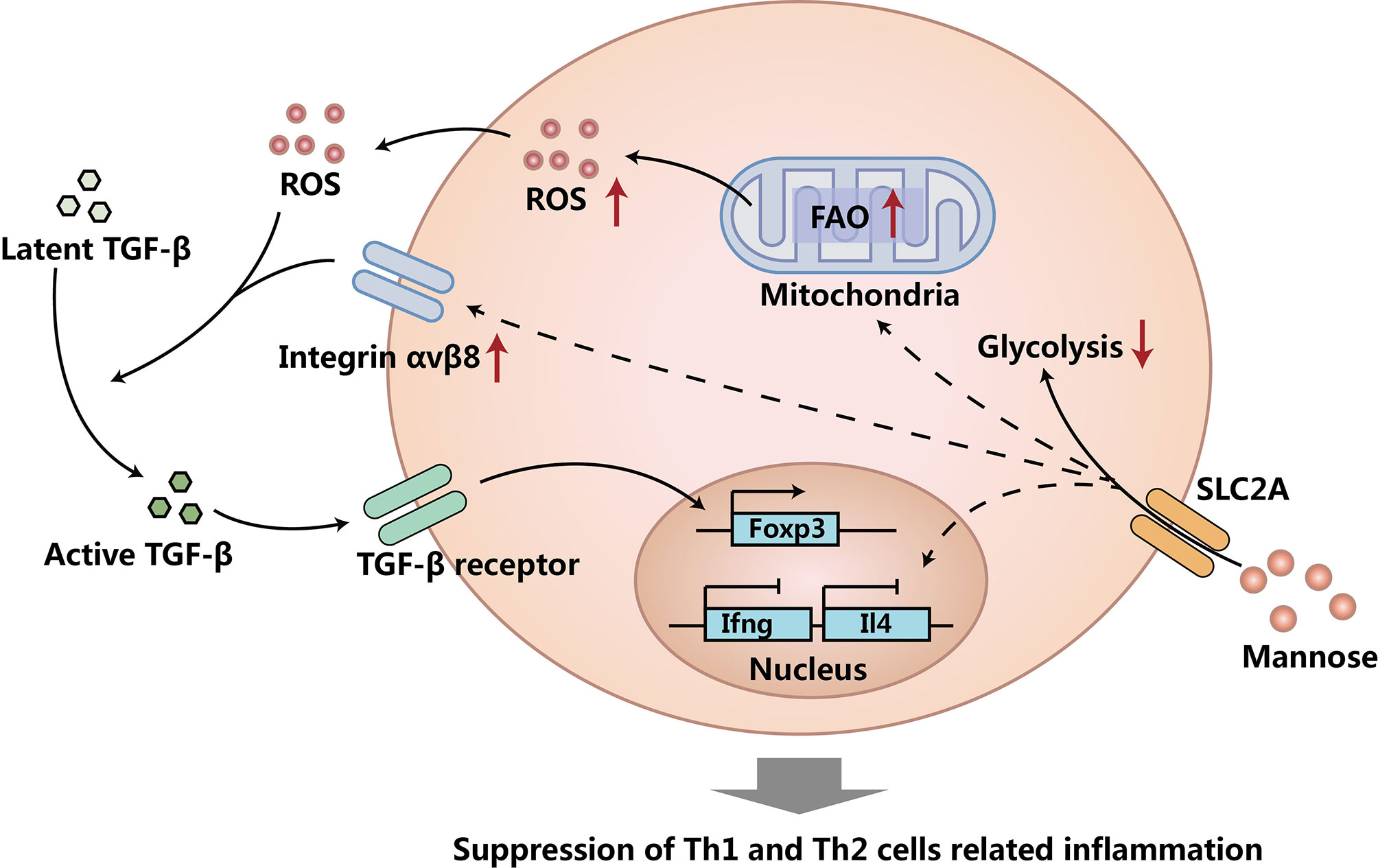
Figure 1 Mannose induces Treg cells and suppresses Teff cells. Mannose suppresses glycolysis, increases fatty acid oxidation (FAO), and up-regulates integrin αvβ8 in CD4+ T cells. Increased FAO caused more reactive oxygen species (ROS) production. The increased ROS and up-regulated integrin αvβ8 activates more transforming growth factor beta (TGF-β) from its latent form. TGF-β induces more Treg cells, suppresses Th1 and Th2 cells, and causes the suppression of Th1 and Th2 cells related to inflammation. Mechanisms of Treg cell and TGF-β independent Th1 and Th2 cell suppression caused by mannose treatment needs to be further investigated.
Although the clear mechanism of mannose induced Th1 and Th2 cell suppression is still unknown, it is very likely that the possible mechanism of the Treg cell and TGF-β independent suppression of Th1 cells and Th2 cells might be through the suppression of aerobic glycolysis in CD4+ T cells, as it has been well-proven that both differentiations and functions of Th1 cells and Th2 cells largely rely on aerobic glycolysis (31, 32). Besides suppressing Th1 and Th2 cells via a TGF-β activation independent mechanism, mannose might also have other mechanisms to promote Treg cell generation. One study reported that mannose treated mesenchymal stem cells (MSCs) could induce more Treg cells via the suppression of Interleukin 6 (IL-6) produced by MSCs (33). All these findings show that mannose can suppress inflammation via induction of Treg cells and suppression of Teff cells.
Macrophages are a critical immune cell population that are important for innate immunity (34). Besides suppressing Th1 cells and Th2 cells, mannose has also been found to suppress inflammatory macrophages (19). First of all, Torretta et al. proved that mannose could limit the production of Interleukin 1β (IL-1β) and suppress the activation of lipopolysaccharide (LPS)-induced macrophages in vitro. On the other hand, mannose could also promote the survival of LPS-treated mice in vivo. Next, they found that mannose suppressed macrophage-derived IL-1β production by reducing glycolysis, tricarboxylic acid (TCA) cycle, and suppressing succinate-mediated HIF-1α activation in macrophages. Since mannose cannot be used for glycolysis efficiently, these findings further showed that mannose reduced glycolysis by competing glucose transporter (SLC2A, also called GLUT) and hexokinase (HK) with glucose, and the reduced glycolysis caused the reduction of TCA cycle. Moreover, they also demonstrated that mannose could suppress dextran sulfate sodium (DSS) induced colitis in mice via limiting glycolysis, TCA cycle, and IL-1β production in macrophages. Taken together, this fantastic work revealed the immune regulatory function of mannose by suppressing IL-1β production of inflammatory macrophages (Figure 2) (19).
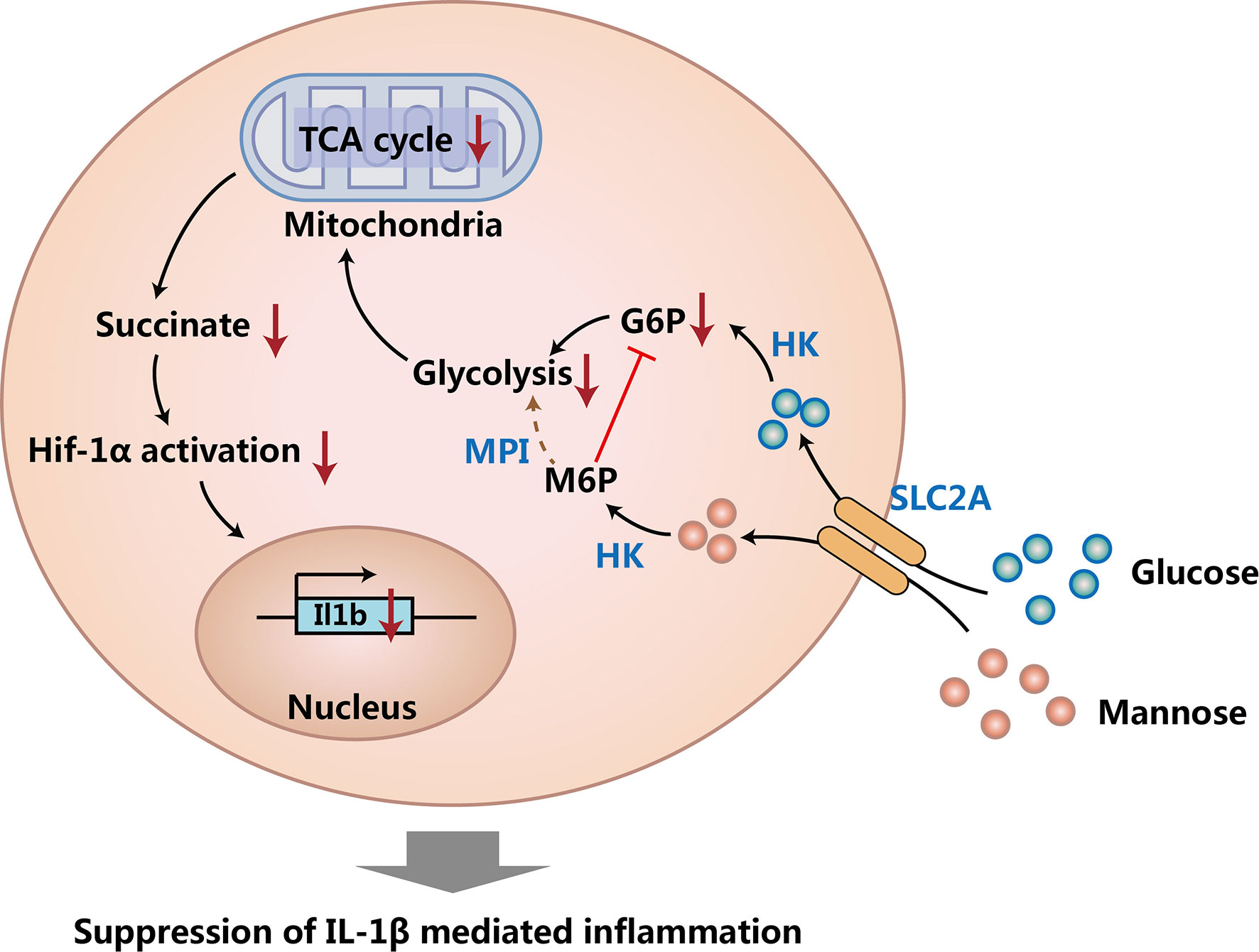
Figure 2 Mannose suppresses macrophage IL-1β production. Mannose and glucose share the same transporter (SLC2A) and can be converted to mannose 6-phosphate (M6P) and glucose 6-phosphate (G6P) respectively by hexokinase (HK). The process of M6P generation suppresses glycolysis by reducing G6P, and M6P cannot be used for glycolysis efficiently due to the low expression of phosphomannose isomerase (MPI) in macrophages. Suppressed glycolysis causes reduced tricarboxylic acid (TCA) cycle and decreases the production of succinate. Decreased succinate reduces succinate-mediated HIF-1α activation, and then causes the decreased expression of IL-1β in macrophages.
Interestingly, the suppression of macrophage IL-1β production completely relies on the low expression of phosphomannose isomerase (MPI) in macrophages, as overexpression of MPI in macrophages can overcome the suppression function of mannose (19). Since MPI could convert mannose-6-phosphate to fructose-6-phophate and then use fructose-6-phophate for glycolysis (34), these findings suggest that mannose can suppress glycolysis in cells expressing low amounts of MPI by competing hexokinase (HK) with glucose; whereas in cells expressing high amounts of MPI, mannose-6-phosphate could be converted to fructose-6-phophate efficiently by MPI, therefore mannose could support glycolysis quite efficiently in these cells. Since it was found that mannose could suppress glycolysis in CD4+ T cells (10), we can presume that the expression of MPI in CD4+ T cells should also be quite low. Besides suppressing macrophage IL-1β production, one study found that mannose treatment promoted proliferation, enhanced autophagy, and reduced apoptosis of IL-1β-treated rat chondrocytes, and therefore suppressed the progression of osteoarthritis (OA) (22). All of these findings show that mannose not only suppresses IL-1β production of macrophages, but also protects bodies from IL-1β induced degeneration.
Within the past decade, the gut microbiome has been proven to have critical functions in immune homeostasis and inflammation (35, 36). Sharma et al. showed that mannose treatment by drinking-water supplementation prevented weight gain, lowered adiposity, reduced liver steatosis, and improved glucose tolerance during the induction of obesity in high-fat diet (HFD) mice (20). Interestingly, these beneficial effects of mannose were observed only when initiated early in life, but not when provided later. These changes in HFD mice with different ages, coupled with the fact that continuous mannose supplementation is required, made authors postulate that mannose treatment might change gut microbiome in HFD mice. Indeed, they proved that mannose treatment initiated early in life increased the Bacteroidetes to Firmicutes ratio in the gut microbiota of HFD mice, showing an association between gut microbiota composition and the timing of mannose introduction (20). The decrease of the Bacteroidetes to Firmicutes ratio in the gut microbiota has been shown to cause obesity in both mice and human, and the increase of the ratio is positively associated with weight loss (37–39). These findings show that mannose suppressed obesity by increasing the Bacteroidetes to Firmicutes ratio in the gut microbiota.
Importantly, Bacteroides are the most abundant members of Bacteroidetes in the intestinal tract of mammals, and the immunomodulatory activities of Bacteroides have been identified (40–43). Lower levels of Bacteroides in the gut microbiota have been shown to be associated with Inflammatory Bowel Disease (IBD) (40), and polysaccharide A expressed by Bacteroides can induce Treg cell growth and suppress immunity (42). Consistent with these findings, mannose treatment increased Bacteroidetes and also reduced gene expression of inflammatory markers (Tnfa and Ifng) in adipocytes of HFD mice (20). Another study also reported that mannose treatment attenuated bone loss induced by senility and estrogen deficiency in mice, via the increase of Treg cells and Bacteroidetes (44). These findings suggest mannose treatment has gut microbiota-dependent anti-inflammatory effects.
However, an increase in Bacteroides is not necessarily always beneficial for anti-inflammation (45). For example, one comparative study reported that Bacteroides vulgatus (a genera of Bacteroides) in the outer membrane might be implicated in the pathogenesis of ulcerative colitis (46), another study found the growth inhibition of the Bacteroides vulgatus may repress exacerbation of intestinal inflammation (47). So mannose associated increases in Bacteroidetes might not necessarily be the main mechanism in which chronic inflammation is suppressed. Taken together, mannose treatment may have gut microbiota-dependent anti-inflammatory effects, but the clear mechanisms need to be investigated further.
High sugar intake has long been shown to have pathogenic roles in a variety of diseases, including diabetes and obesity. Recently, functions of high-sugar intake in autoimmunity were also revealed (5, 8, 48). Unlike glucose and fructose, mannose is a special hexose that suppresses inflammation (10, 49). Also, the therapeutic concentration of circulating mannose can be reached in mice and humans (50, 51), and even a very low concentration of mannose supplemented in drinking water has a therapeutic function both in vitro and in vivo (10, 20). More than that, mannose treatment is supposed to be a safe treatment, as long-term mannose ingestion was well tolerated and did not show any adverse effect in mice and humans (50, 51).
On the other hand, there are still many important questions that need to be answered in the future. For example, it is necessary to calculate the ideal dose of mannose treatment for human inflammation, determine how mannose increases FAO in CD4+ T cells, and whether or not mannose can affect functions of CD8+ T cell and other immune cells. Moreover, since it was also reported that mannose treatment could enhance cancer chemokine therapy (52, 53), whether mannose mediated immune responses are involved during this process is totally unknown. Nevertheless, although there are still a number of key questions need to be figured out, the great therapeutic promise of mannose treatment has been disclosed.
In conclusion, mannose treatment is a promising novel strategy to suppress inflammatory diseases, including autoimmune disease and allergic disease. More intense study and research would greatly benefit patients with inflammatory disease.
WZ and HC wrote the manuscript. YG, QZ, SL, and WQ edited the manuscript. AT designed the layout of manuscript and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (NO. 82073404), and Major Subject of the Science and Technology Department of Sichuan Province (2020YFS0251).
Author SL is employed by HitGen Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, Murphy CJ, et al. High-Fructose Corn Syrup Enhances Intestinal Tumor Growth in Mice. Science (2019) 363(6433):1345–9. doi: 10.1126/science.aat8515
2. Softic S, Gupta MK, Wang GX, Fujisaka S, O’Neill BT, Rao TN, et al. Divergent Effects of Glucose and Fructose on Hepatic Lipogenesis and Insulin Signaling. J Clin Invest (2017) 127(11):4059–74. doi: 10.1172/JCI94585
3. Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, et al. Dietary Fructose Feeds Hepatic Lipogenesis via Microbiota-Derived Acetate. Nature (2020) 579(7800):586–91. doi: 10.1038/s41586-020-2101-7
4. Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, et al. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation (2019) 139(18):2113–25. doi: 10.1161/CIRCULATIONAHA.118.037401
5. Zhang D, Jin W, Wu R, Li J, Park SA, Tu E, et al. High Glucose Intake Exacerbates Autoimmunity Through Reactive-Oxygen-Species-Mediated TGF-Beta Cytokine Activation. Immunity (2019) 51(4):671–81.e5. doi: 10.1016/j.immuni.2019.08.001
6. Jones N, Blagih J, Zani F, Rees A, Hill DG, Jenkins BJ, et al. Fructose Reprogrammes Glutamine-Dependent Oxidative Metabolism to Support LPS-Induced Inflammation. Nat Commun (2021) 12(1):1209. doi: 10.1038/s41467-021-21461-4
7. Todoric J, Di Caro G, Reibe S, Henstridge DC, Green CR, Vrbanac A, et al. Fructose Stimulated De Novo Lipogenesis Is Promoted by Inflammation. Nat Metab (2020) 2(10):1034–45. doi: 10.1038/s42255-020-0261-2
8. Khan S, Waliullah S, Godfrey V, Khan MAW, Ramachandran RA, Cantarel BL, et al. Dietary Simple Sugars Alter Microbial Ecology in the Gut and Promote Colitis in Mice. Sci Transl Med (2020) 12(567):eaay6218. doi: 10.1126/scitranslmed.aay6218
9. Cao G, Wang Q, Huang W, Tong J, Ye D, He Y, et al. Long-Term Consumption of Caffeine-Free High Sucrose Cola Beverages Aggravates the Pathogenesis of EAE in Mice. Cell Discov (2017) 3:17020. doi: 10.1038/celldisc.2017.20
10. Zhang D, Chia C, Jiao X, Jin W, Kasagi S, Wu R, et al. D-Mannose Induces Regulatory T Cells and Suppresses Immunopathology. Nat Med (2017) 23(9):1036–45. doi: 10.1038/nm.4375
11. Michaels EK, Chmiel JS, Plotkin BJ, Schaeffer AJ. Effect of D-Mannose and D-Glucose on Escherichia Coli Bacteriuria in Rats. Urol Res (1983) 11(2):97–102. doi: 10.1007/BF00256954
12. Schaeffer AJ, Chmiel JS, Duncan JL, Falkowski WS. Mannose-Sensitive Adherence of Escherichia Coli to Epithelial Cells From Women With Recurrent Urinary Tract Infections. J Urol (1984) 131(5):906–10. doi: 10.1016/s0022-5347(17)50706-5
13. Kranjcec B, Papes D, Altarac S. D-Mannose Powder for Prophylaxis of Recurrent Urinary Tract Infections in Women: A Randomized Clinical Trial. World J Urol (2014) 32(1):79–84. doi: 10.1007/s00345-013-1091-6
14. Lenger SM, Bradley MS, Thomas DA, Bertolet MH, Lowder JL. Sutcliffe S. D-Mannose vs Other Agents for Recurrent Urinary Tract Infection Prevention in Adult Women: A Systematic Review and Meta-Analysis. Am J Obstet Gynecol (2020) 223(2):265.e1–13. doi: 10.1016/j.ajog.2020.05.048
15. Alton G, Hasilik M, Niehues R, Panneerselvam K, Etchison JR, Fana F, et al. Direct Utilization of Mannose for Mammalian Glycoprotein Biosynthesis. Glycobiology (1998) 8(3):285–95. doi: 10.1093/glycob/8.3.285
16. Freeze HH, Sharma V. Metabolic Manipulation of Glycosylation Disorders in Humans and Animal Models. Semin Cell Dev Biol (2010) 21(6):655–62. doi: 10.1016/j.semcdb.2010.03.011
17. Schneider A, Thiel C, Rindermann J, DeRossi C, Popovici D, Hoffmann GF, et al. Successful Prenatal Mannose Treatment for Congenital Disorder of Glycosylation-Ia in Mice. Nat Med (2011) 18(1):71–3. doi: 10.1038/nm.2548
18. de Lonlay P, Seta N. The Clinical Spectrum of Phosphomannose Isomerase Deficiency, With an Evaluation of Mannose Treatment for CDG-Ib. Biochim Biophys Acta (2009) 1792(9):841–3. doi: 10.1016/j.bbadis.2008.11.012
19. Torretta S, Scagliola A, Ricci L, Mainini F, Di Marco S, Cuccovillo I, et al. D-Mannose Suppresses Macrophage IL-1beta Production. Nat Commun (2020) 11(1):6343. doi: 10.1038/s41467-020-20164-6
20. Sharma V, Smolin J, Nayak J, Ayala JE, Scott DA, Peterson SN, et al. Mannose Alters Gut Microbiome, Prevents Diet-Induced Obesity, and Improves Host Metabolism. Cell Rep (2018) 24(12):3087–98. doi: 10.1016/j.celrep.2018.08.064
21. Wang H, Teng X, Abboud G, Li W, Ye S. Morel L. D-Mannose Ameliorates Autoimmune Phenotypes in Mouse Models of Lupus. BMC Immunol (2021) 22(1):1. doi: 10.1186/s12865-020-00392-7
22. Lin Z, Miao J, Zhang T, He M, Zhou X, Zhang H, et al. D-Mannose Suppresses Osteoarthritis Development In Vivo and Delays IL-1beta-Induced Degeneration In Vitro by Enhancing Autophagy Activated via the AMPK Pathway. BioMed Pharmacother (2021) 135:111199. doi: 10.1016/j.biopha.2020.111199
23. Zhang D, Tu E, Kasagi S, Zanvit P, Chen Q, Chen W. Manipulating Regulatory T Cells: A Promising Strategy to Treat Autoimmunity. Immunotherapy (2015) 7(11):1201–11. doi: 10.2217/imt.15.79
24. Akdis M, Blaser K, Akdis CA. T Regulatory Cells in Allergy: Novel Concepts in the Pathogenesis, Prevention, and Treatment of Allergic Diseases. J Allergy Clin Immunol (2005) 116(5):961–8; quiz 969. doi: 10.1016/j.jaci.2005.09.004
25. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J Immunol (1995) 155(3):1151–64.
26. Hori S, Nomura T, Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science (2003) 299(5609):1057–61. doi: 10.1126/science.1079490
27. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat Immunol (2003) 4(4):330–6. doi: 10.1038/ni904
28. Khattri R, Cox T, Yasayko SA, Ramsdell F. An Essential Role for Scurfin in CD4+CD25+ T Regulatory Cells. Nat Immunol (2003) 4(4):337–42. doi: 10.1038/ni909
29. Shi YB, Yin D. A Good Sugar, D-Mannose, Suppresses Autoimmune Diabetes. Cell Biosci (2017) 7:48. doi: 10.1186/s13578-017-0175-1
30. Villa M, Qiu J. Pearce EL. A Sweet Deal for Diabetes. Trends Endocrinol Metab (2018) 29(1):1–2. doi: 10.1016/j.tem.2017.10.006
31. Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, et al. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J Immunol (2011) 186(6):3299–303. doi: 10.4049/jimmunol.1003613
32. MacIver NJ, Michalek RD, Rathmell JC. Metabolic Regulation of T Lymphocytes. Annu Rev Immunol (2013) 31:259–83. doi: 10.1146/annurev-immunol-032712-095956
33. Guo L, Hou Y, Song L, Zhu S, Lin F. Bai Y. D-Mannose Enhanced Immunomodulation of Periodontal Ligament Stem Cells via Inhibiting IL-6 Secretion. Stem Cells Int (2018) 2018:7168231. doi: 10.1155/2018/7168231
34. Sharma V, Ichikawa M, Freeze HH. Mannose Metabolism: More Than Meets the Eye. Biochem Biophys Res Commun (2014) 453(2):220–8. doi: 10.1016/j.bbrc.2014.06.021
35. Belkaid Y, Hand TW. Role of the Microbiota in Immunity and Inflammation. Cell (2014) 157(1):121–41. doi: 10.1016/j.cell.2014.03.011
36. Zheng D, Liwinski T, Elinav E. Interaction Between Microbiota and Immunity in Health and Disease. Cell Res (2020) 30(6):492–506. doi: 10.1038/s41422-020-0332-7
37. Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity Alters Gut Microbial Ecology. Proc Natl Acad Sci USA (2005) 102(31):11070–5. doi: 10.1073/pnas.0504978102
38. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial Ecology: Human Gut Microbes Associated With Obesity. Nature (2006) 444(7122):1022–3. doi: 10.1038/4441022a
39. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut Microbiota From Twins Discordant for Obesity Modulate Metabolism in Mice. Science (2013) 341(6150):1241214. doi: 10.1126/science.1241214
40. Zhou Y, Zhi F. Lower Level of Bacteroides in the Gut Microbiota Is Associated With Inflammatory Bowel Disease: A Meta-Analysis. BioMed Res Int (2016) 2016:5828959. doi: 10.1155/2016/5828959
41. Mazmanian SK, Round JL, Kasper DL. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature (2008) 453(7195):620–5. doi: 10.1038/nature07008
42. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll-Like Receptor 2 Pathway Establishes Colonization by a Commensal of the Human Microbiota. Science (2011) 332(6032):974–7. doi: 10.1126/science.1206095
43. Delday M, Mulder I, Logan ET, Grant G. Bacteroides Thetaiotaomicron Ameliorates Colon Inflammation in Preclinical Models of Crohn’s Disease. Inflammation Bowel Dis (2019) 25(1):85–96. doi: 10.1093/ibd/izy281
44. Liu H, Gu R, Zhu Y, Lian X, Wang S, Liu X, et al. D-Mannose Attenuates Bone Loss in Mice via Treg Cell Proliferation and Gut Microbiota-Dependent Anti-Inflammatory Effects. Ther Adv Chronic Dis (2020) 11:2040622320912661. doi: 10.1177/2040622320912661
45. Zhang SL, Wang SN, Miao CY. Influence of Microbiota on Intestinal Immune System in Ulcerative Colitis and Its Intervention. Front Immunol (2017) 8:1674. doi: 10.3389/fimmu.2017.01674
46. Bamba T, Matsuda H, Endo M, Fujiyama Y. The Pathogenic Role of Bacteroides Vulgatus in Patients With Ulcerative Colitis. J Gastroenterol (1995) 30 Suppl 8:45–7.
47. Setoyama H, Imaoka A, Ishikawa H, Umesaki Y. Prevention of Gut Inflammation by Bifidobacterium in Dextran Sulfate-Treated Gnotobiotic Mice Associated With Bacteroides Strains Isolated From Ulcerative Colitis Patients. Microbes Infect (2003) 5(2):115–22. doi: 10.1016/s1286-4579(02)00080-1
48. Galgani M, Matarese G. The Sweet Kiss Breaching Immunological Self-Tolerance. Trends Mol Med (2019) 25(10):819–20. doi: 10.1016/j.molmed.2019.08.003
49. Wei Z, Huang L, Cui L, Zhu X. Mannose: Good Player and Assister in Pharmacotherapy. BioMed Pharmacother (2020) 129:110420. doi: 10.1016/j.biopha.2020.110420
50. Mayatepek E, Schroder M, Kohlmuller D, Bieger WP, Nutzenadel W. Continuous Mannose Infusion in Carbohydrate-Deficient Glycoprotein Syndrome Type I. Acta Paediatr (1997) 86(10):1138–40. doi: 10.1111/j.1651-2227.1997.tb14825.x
51. Davis JA, Freeze HH. Studies of Mannose Metabolism and Effects of Long-Term Mannose Ingestion in the Mouse. Biochim Biophys Acta (2001) 1528(2-3):116–26. doi: 10.1016/s0304-4165(01)00183-0
52. Gonzalez PS, O’Prey J, Cardaci S, Barthet VJA, Sakamaki JI, Beaumatin F, et al. Mannose Impairs Tumour Growth and Enhances Chemotherapy. Nature (2018) 563(7733):719–23. doi: 10.1038/s41586-018-0729-3
Keywords: mannose, inflammation, hexose, inflammatory diseases, mannose treatment
Citation: Zhang W, Cheng H, Gui Y, Zhan Q, Li S, Qiao W and Tong A (2021) Mannose Treatment: A Promising Novel Strategy to Suppress Inflammation. Front. Immunol. 12:756920. doi: 10.3389/fimmu.2021.756920
Received: 11 August 2021; Accepted: 09 September 2021;
Published: 27 September 2021.
Edited by:
Chaohong Liu, Huazhong University of Science and Technology, ChinaReviewed by:
Philip Brandon Busbee, University of South Carolina, United StatesCopyright © 2021 Zhang, Cheng, Gui, Zhan, Li, Qiao and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aiping Tong, aipingtong@scu.edu.cn
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.